Abdominal aortic aneurysm (AAA) is a common degenerative vascular disease with a prevalence up to 8% in males over 60 years of age.1 This is a complex disorder characterized by permanent and focal dilation of abdominal aorta that exceeds at least 50% of the normal diameter. In these patients, the aortic diameter progressively expands boosted by smoking and aging as major risk factors. Unfortunately, aneurysm growth increases the risk of aortic rupture, a life-threatening emergency that carries a mortality rate of 80%. Despite the increasing effort of the scientific community to identify therapeutic strategies for AAA, there are currently no pharmacological tools that ameliorate aneurysm expansion, while the underlying mechanisms involved in this disease are not completely understood.
In the last decades, Wnt signaling has captivated the attention of several researchers due to its high therapeutic potential. In adults, this pathway is reactivated in several pathological scenarios, including cardiovascular diseases, and, despite certain controversy, it is generally considered that the excessive activation of Wnt signaling in the cardiovascular system has detrimental consequences.2 However, whether an aberrant activation of the Wnt signal transduction pathway could underlie AAA development and the potential of this route as a pharmacological target for this disease remains virtually unexplored.
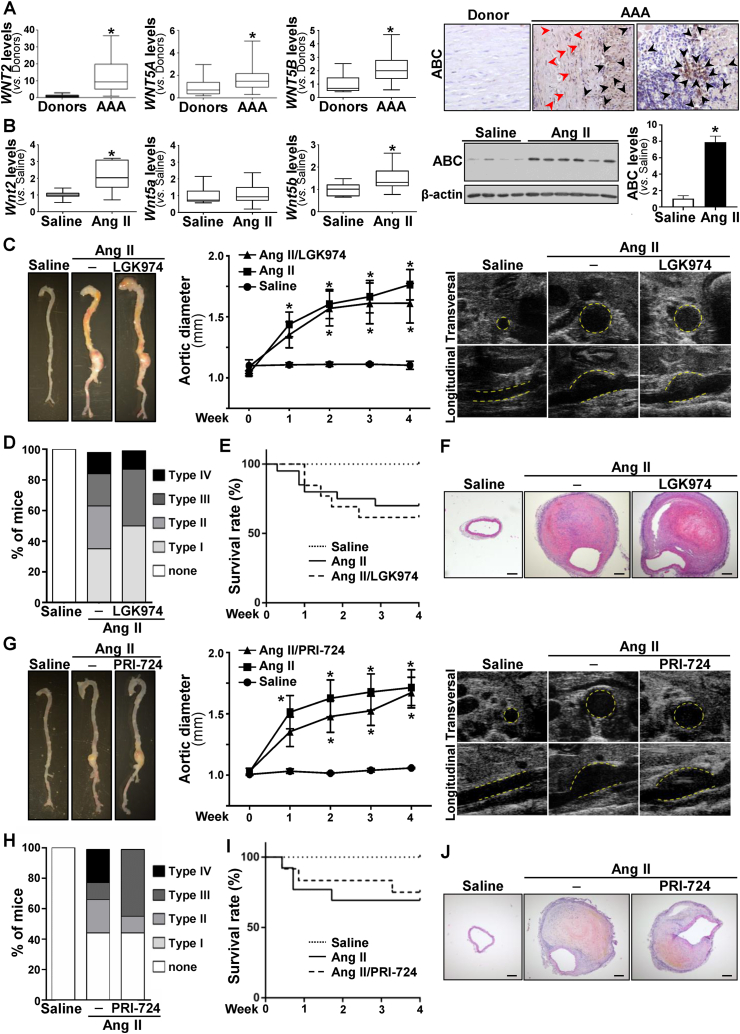
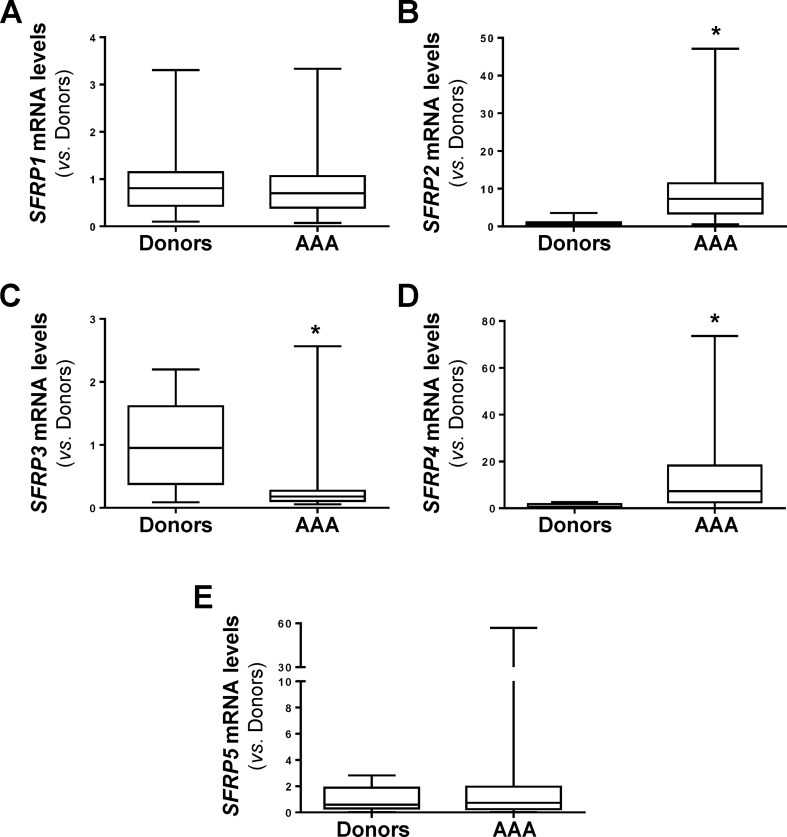
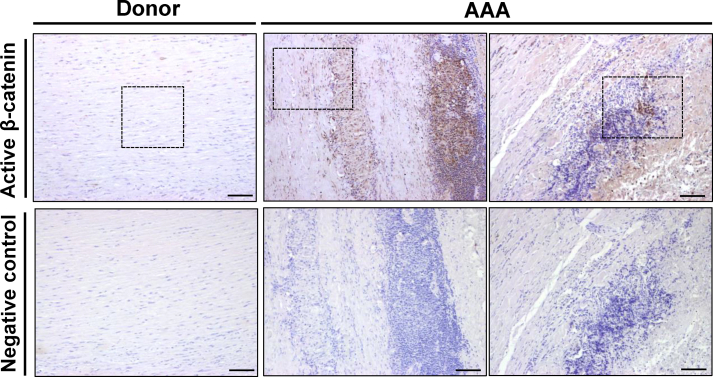
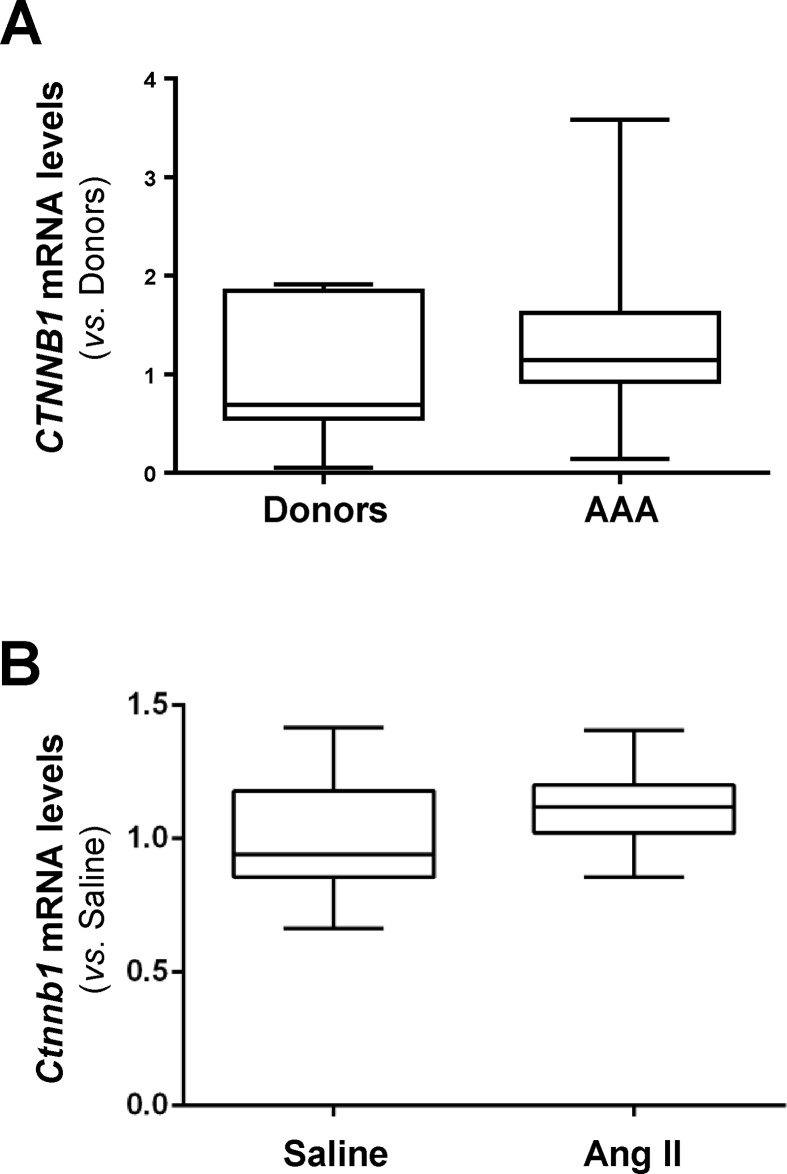
In this study we analyzed whether the Wnt signaling pathway is deregulated in human AAA. Compared to healthy donor aortas, WNT2 mRNA levels were strikingly increased in human aneurysmal samples, those of WNT5A and WNT5B, showed a moderate increment, and the expression of WNT1 and WNT3A fall below the detection limit (Fig. 1A). Similarly, SFRPs expression was disturbed in the aneurysmal aorta (Fig. S1). Enhanced mRNA levels for SFRP2 and SFRP4 were detected in patients with AAA, while the expression of SFRP3 was significantly decreased and that of SFRP1 and SFRP5 remained unchanged. In view of this complex scenario, we aimed to establish the activation status of the pathway. Consequently, we assessed the expression of the transcriptionally active form of β-catenin that is dephosphorylated at serine 37 (Ser37) and threonine 41 (Thr41). In human aneurysmal lesions, a strong immunostaining for active β-catenin was primarily detected at the inflammatory infiltrate and to a lesser extent in VSMC, while it was almost absent in healthy aortas (Fig. 1A, right panels; Fig. S2), thus supporting the activation of the Wnt/β-catenin pathway (or canonical route) in human AAA. Previous studies suggested the activation of canonical Wnt signaling in human AAA based on the enhanced β-catenin mRNA levels detected in a very small number of patient samples.3 Of note, this response was of borderline significance and could not be confirmed in either our more extensive AAA cohort (Fig. S3A) or in aneurysms from ApoE−/− mice challenged with AngII (Fig. S3B). Therefore, our study is the first one providing solid evidence about the activation of Wnt/β-catenin signaling in human AAA.
Figure 1.
Activation of Wnt signaling in AAA and effect of LGK974 and PRI-724 on the formation of Ang II-induced AAA. (A-B, left panels), Boxplots showing mRNA levels of WNT2, WNT5A and WNT5B (A), and their homologues in mice (B) analyzed by real-time PCR in abdominal aorta from healthy donors and patients with AAA [Donors (n = 9), AAA (n = 48); ∗P < 0.02 vs. Donors. Mann–Whitney test] or abdominal aorta from ApoE−/− mice infused with saline solution or AngII (1000 ng/kg/min for 28 days) (at least n = 9; ∗P < 0.01 vs. Saline. t-test). The box extends from the 25th to the 75th percentile, the median is indicated in horizontal line, and the whiskers represent the maximum and minimum values. (A-B, right panels), in A representative immunohistochemical analysis for active β-catenin (ABC) in abdominal aortas from AAA patients and healthy donors (black and red arrow heads indicate positive inflammatory cells and VSMC, respectively); in B representative immunoblot images showing ABC levels in abdominal aortas from animals as indicated above (levels of β-actin are shown as a loading control), and histogram showing the results (mean ± SEM) of the densitometric analysis [Saline (n = 4), AngII (n = 6); ∗P < 0.01 vs. Saline. Mann–Whitney test]. (C–F) ApoE−/− mice were infused with saline solution or AngII (1000 ng/kg/min for 28 days) and were treated or not with LGK974 (5 mg/kg/day). (C, left panel) representative images of aortas from each experimental group; (C, middle panel) data (mean ± SEM) from echocardiography analysis of the abdominal aortic diameter [Saline (n = 5), AngII (n = 14) and AngII/LGK974 (n = 8); ∗P < 0.05 vs. Saline. Two-way ANOVA]; (C, right panels) representative ultrasonographic frames after 28 days of AngII infusion. Transverse and longitudinal images taken at the level of the suprarenal aorta are shown. The aortic perimeter is indicated with a yellow line. (D) severity of AAA by experimental group (n as indicated in C middle panel). (E) Graph showing the survival rate of the experimental groups (Chi-square test). (F) Representative hematoxylin-eosin staining of abdominal aortic sections for each experimental group (bars: 200 μm). (G–J) ApoE−/− mice were infused with saline solution or AngII (1000 ng/kg/min for 28 days) and were treated or not with PRI-724 (15 mg/kg/day). (G, left panel) representative images of aortas from each experimental group; (G, middle panel) data (mean ± SEM) from echocardiography analysis of the abdominal aortic diameter [Saline (n = 5), AngII (n = 9) and AngII/PRI-724 (n = 9); ∗P < 0.05 vs. Saline. Two-way ANOVA]; (G, right panels) representative ultrasonographic frames after 28 days of AngII infusion as indicated above. D, Severity of AAA by experimental group (n as indicated in G middle panel). E, Graph showing the survival rate of the experimental groups (Chi-square test). F, Representative hematoxylin-eosin staining of abdominal aortic sections for each experimental group (bars: 200 μm).
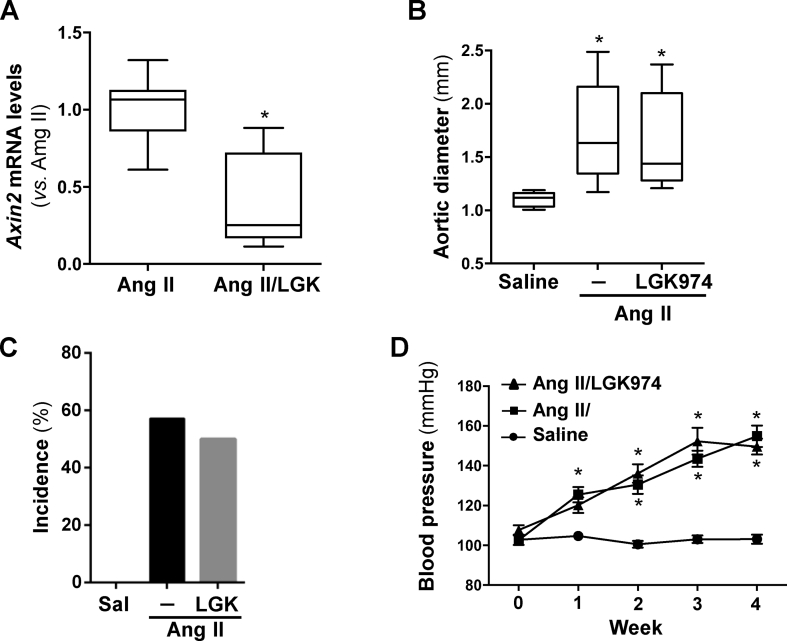
Notably, we found that aneurysmal aortas from AngII-infused ApoE−/− mice, a well-established model for AAA, essentially resembles the deregulation of Wnt signaling detected in humans (Fig. 1B), being active β-catenin protein levels drastically increased (Fig. 1B, right panel). Considering these data, we hypothesized that targeting the Wnt pathway might be a valuable therapeutic approach for this disease. LGK974, a potent, safe and specific porcupine inhibitor,2 was administered to AngII-infused ApoE−/− mice. This drug reduced the vascular expression of the Wnt/β-catenin/TCF signaling target gene Axin2, confirming the effectiveness of the treatment (Fig. S4A). However, the progressive enlargement of aortic diameter induced by AngII was not significantly affected by LGK974 (Fig. 1C; Fig. S4B). After AngII infusion, about 60% of mice developed aneurysms and those treated with LGK974 exhibit a comparable incidence (Fig. S4C). Additionally, no essential differences in disease severity (Fig. 1D), survival (Fig. 1E) and blood pressure were found (Fig. S4D). Further, porcupine inhibition did not affect the exacerbated vascular remodeling triggered by AngII (Fig. 1F). Of note, LGK974 limited the increase in LV mass induced by AngII infusion (Table S1), consistent with a previous study evidencing that another porcupine inhibitor limited cardiac hypertrophy induced by transverse aortic constriction.4
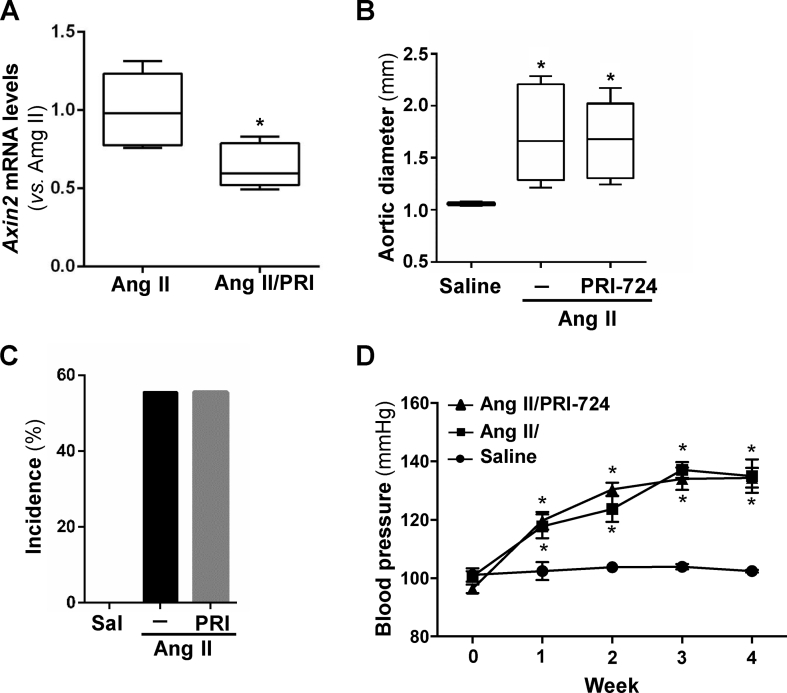
Next, we aimed to determine whether the selective inhibitor of β-catenin/CBP interaction, PRI-724,2 could ameliorate experimental AAA. The systemic administration of PRI-724, which effectively blocked canonical Wnt signaling (Fig. S5A), slightly delayed the AngII-induced enlargement of aortic diameter, although this effect was not significant (Fig.1G; Fig. S5B). The incidence of AAA in the group treated with PRI-724 was comparable to that of mice receiving AngII alone (Fig. S5C). Interestingly, PRI-724 improved aneurysm severity, since type IV aneurysmal lesions were absent (Fig. 1H). However, the administration of PRI-724 did not induce a clear benefit on survival (Fig. 1I) or blood pressure levels (Fig. S5D), nor ameliorated vascular remodeling (Fig. 1J). It should be noted that AngII was unable to increase LV mass in PRI-724-treated mice (Table S1); therefore, the favorable cardiac outcome provided by porcupine inhibition could be extended to the blockade of CBP-β-catenin interaction.
Pharmacological strategies aiming to regress established AAA or ameliorate aneurysm progression are urgently needed and still awaited. It has been previously reported that the Wnt/β-catenin inhibitor sclerostin and the blockade of WISP1, a β-catenin regulated gene, ameliorate AAA in AngII-challenged ApoE−/− mice,3,5 thus suggesting that the inhibition of the canonical Wnt signaling could be a valuable therapeutical approach for this disease. Several Wnt modulators have entered the clinical trial phase for tumoral diseases. However, none of the Wnt inhibitors tested here show a clear benefit on experimental AAA, although a favourable response on cardiac hypertrophy was detected.
In summary, our study has provided evidence about the striking activation of the Wnt/β-catenin pathway in human and experimental AAA. Unfortunately, neither porcupine inhibition nor the disruption of CBP-β-catenin interaction have shown any beneficial response on AAA other than a slight reduction in aneurysm severity induced by PRI-724, excluding that these anti-tumoral drugs could be repurposed for aneurysmal diseases. However, in view of the upregulation of Wnt/β-catenin signaling in this disease, further research is warranted to determine whether targeting specific elements of this route could ameliorate aneurysm development.
Conflict of interests
There are no conflicts of interest.
Funding
This work was supported by Instituto de Salud Carlos III (ISCIII) (No. PI21/01048, PI20/01649), the European Regional Development Fund (ERDF-FEDER, a way to build Europe), Ministerio de Ciencia e Innovación (No. RTI2018-094727-B-100), AGAUR (No. 2017-SGR-00333, 2017-SGR-1807) and Consejo Superior de Investigaciones Científicas (No. 2021AEP073). L. P. is supported by a PFIS contract (ISCIII), C.B–S by a FPU fellowship and A.R.-S. and M.G. were funded by the Miguel Servet Program.
Acknowledgements
We thank Ma Ángeles Velasco for her technical assistance.
Footnotes
Peer review under responsibility of Chongqing Medical University.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.gendis.2022.05.017.
Contributor Information
José Martínez-González, Email: jose.martinez@iibb.csic.es.
Cristina Rodríguez, Email: crodriguezs@santpau.cat.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
figs1.
SFRPs expression is altered in human AAA.SFRP1 (A), SFRP2 (B), SFRP3 (C), SFRP4 (D) and SFRP5 (E) mRNA levels were analyzed by real-time PCR in abdominal aorta samples from healthy donors and patients with AAA (Donors, n= 9; AAA, n= 48). Data are expressed as boxplots. The box extends from the 25th to the 75th percentile, and the median is indicated in horizontal line. The whiskers represent the maximum and minimum values. ∗P < 0.02 vs. Donors. Mann-Whitney test.
figs2.
Active β-catenin expression in human AAA. Representative immunohistochemical analysis for active β-catenin assessed in abdominal aorta samples from patients with AAA and healthy donors. Samples were counterstained with haematoxylin. The indicated areas are magnified and shown in Figure 1A. Negative controls in which the primary antibody against active β-catenin was omitted were performed in consecutive serial sections. Bars: 100 µm.
figs3.
The expression of β-catenin is not altered in human and experimental AAA.CTNNB1 mRNA levels were analyzed by real-time PCR in abdominal aorta samples from healthy donors and patients with AAA (A; Donors, n = 9; AAA, n = 48) and in the aortic wall from ApoE-/- mice infused with Ang II or Saline (B; Saline, n = 9; Ang, n = 31). Data are expressed as boxplots. The box extends from the 25th to the 75th percentile, and the median is indicated in horizontal line. The whiskers represent the maximum and minimum values; No significant differences were detected.
figs4.
LGK974 blocks Wnt signaling but does not alter AAA development or blood pressure in AngII-infused ApoE-/- mice. ApoE-/- mice were infused with saline solution or AngII (1000 ng/kg/min) for 28 days. The mice challenged with AngII were treated or not with LGK974 (LGK; 5 mg/kg/day). A)Axin2 mRNA levels were analyzed by real-time PCR in abdominal aorta samples (AngII-infused mice non-treated with LGK974, n = 14; AngII-infused mice treated with LKG974, n = 8). ∗P < 0.0001 vs. AngII-infused mice. t-test. B) Quantitative analysis of the aortic diameter at the end of the experimental period (28 days). The results are expressed as boxplots. The box extends from the 25th to the 75th percentile, and the median is indicated by a horizontal line. The whisker represents the maximum and minimum values (saline-infused mice, n = 5; AngII-infused animals, n= 14; AngII-infused mice treated with LGK974, n = 8). ∗P < 0.05 vs. saline. Kruskal-Wallis test. C) Incidence of AAA in each group (Chi-square test). D) Blood pressure levels. Data are mean ± SEM ∗P < 0.05 vs. saline. Two-way ANOVA with repeated measures.
figs5.
PRI-724 inhibits Wnt signaling but does not ameliorate aortic dilation, AAA incidence or blood pressure in AngII-infused ApoE-/- mice. ApoE-/- mice were infused with saline solution or AngII (1000 ng/kg/min) for 28 days. The mice challenged with AngII were treated or not with PRI-724 (PRI; 15 mg/kg/day). A)Axin2 mRNA levels were analyzed by real-time PCR in abdominal aorta samples (AngII-infused mice non-treated with PRI-724, n = 5; AngII-infused mice treated with PRI-724, n = 6) B) Quantitative analysis of the aortic diameter at the end of the experimental period (28 days). The results are expressed as boxplots. The box extends from the 25th to the 75th percentile, and the median is indicated by a horizontal line. The whisker represents the maximum and minimum values (saline-infused mice, n = 5; AngII-infused animals, n = 9; AngII-infused mice treated with PRI-724, n = 9). ∗P < 0.01 vs. saline. Kruskal-Wallis test. C) Incidence of AAA in each experimental group. Chi-square test. D) Blood pressure levels. Data are mean ± SEM; ∗P < 0.05 vs. saline. Two-way ANOVA with repeated measures.
References
- 1.Nordon I.M., Hinchliffe R.J., Loftus I.M., et al. Pathophysiology and epidemiology of abdominal aortic aneurysms. Nat Rev Cardiol. 2011;8(2):92–102. doi: 10.1038/nrcardio.2010.180. [DOI] [PubMed] [Google Scholar]
- 2.Foulquier S., Daskalopoulos E.P., Lluri G., et al. WNT signaling in cardiac and vascular disease. Pharmacol Rev. 2018;70(1):68–141. doi: 10.1124/pr.117.013896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Krishna S.M., Seto S.W., Jose R.J., et al. Wnt signaling pathway inhibitor sclerostin inhibits angiotensin II-induced aortic aneurysm and atherosclerosis. Arterioscler Thromb Vasc Biol. 2017;37(3):553–566. doi: 10.1161/ATVBAHA.116.308723. [DOI] [PubMed] [Google Scholar]
- 4.Jiang J., Lan C., Li L., et al. A novel porcupine inhibitor blocks WNT pathways and attenuates cardiac hypertrophy. Biochim Biophys Acta (BBA) - Mol Basis Dis. 2018;1864(10):3459–3467. doi: 10.1016/j.bbadis.2018.07.035. [DOI] [PubMed] [Google Scholar]
- 5.Williams H., Wadey K.S., Frankow A., et al. Aneurysm severity is suppressed by deletion of CCN4. J Cell Commun Signal. 2021;15(3):421–432. doi: 10.1007/s12079-021-00623-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.