The discovery of clinically useful targets to treat specific pathological conditions can provide novel therapeutic approaches. To unbiasedly identify key genes related to tumor metastasis, we developed a random gene perturbation method using a piggyBac transposon system under the control of a doxycycline regulated promoter. Using this random gene perturbation method and utilizing a mouse model of metastatic pancreatic cancer, we identified genes dysregulated in metastasized cells from a random mutagenesis library after multiple rounds of in vivo selection. Analysis of these metastasized clones revealed the downregulation of ARPC1B gene. Our further mechanistic studies revealed that ARPC1B gene and its closely related gene ARPC1A worked in a regulatory loop to control tumor metastasis. These findings validate that piggyBac transposon mediated random gene perturbation is a powerful tool to investigate the functional relevance of novel genes and the ARPC1A/B axis is a potential key regulator of tumor metastasis.
Despite significant advancement in our understanding and development of novel therapeutics, mortality due to cancers, especially those metastasize to the secondary organs, remains high.1 Although both forward and reverse genetics have identified many genes that are associated with the metastatic process,2 our understanding of cellular and molecular pathways that contribute to or limit the metastatic process is far from complete.
The development of novel approaches that insert random gene mutations has provided an unbiased approach to discovering specific gene functions relevant to specific pathologic conditions.3 Here we used a novel random gene perturbation method to generate a library of cells with insertion mutations, in combination with a mouse model of cancer metastasis to identify key genes dysregulated in the metastasized tumor compared to the parent cell population.
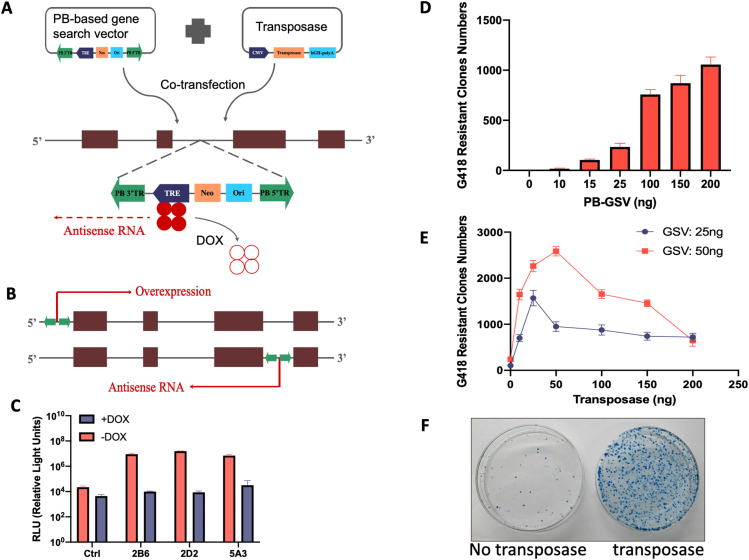
To develop a functional genetic screen, we combined piggyBac transposon mutagenesis,4 Tet-inducible expression system and antisense RNA technology. The piggyBac transposon-based gene search vector (GSV) contains a tetracycline-regulated element (TRE) regulated promoter, a G418 selection marker and a plasmid replication origin, all of which are flanked by the PB terminal DNA repeats (Fig. S1A). With the help of transposase, the GSV can integrate into the cellular genome. The gain-of-function or loss-of-function mutagenesis, initiated by the sense or antisense RNA, was determined by the integration orientation of GSV and regulated by tTA. In the absence of tetracycline, the tetracycline transactivator (tTA) binds to TRE and initiates the sense or antisense transcription, then enhances or blocks the target gene expression (Fig. S1B). The addition of doxycycline will reverse the production of either sense or antisense RNA by detaching tTA from TRE, thus reverse the target gene expression and phenotypes. To establish a Tet-off AsPC-1 human pancreatic cell line, we used luciferase reporter assay as the screening tool. Clone 2D2 possessed high transactivator activity, which was effectively suppressed by doxycycline (Fig. S1C). To generate a gene mutagenesis library, the amount of GSV and transposase were tittered (Fig. S1D, E) and the cells were selected in medium containing G418 for 2 weeks. In the absence of transposase, very few G418 resistant colonies were generated, potentially resulting from the random insertions of GSV in the genome. In contrast, co-transfection of GSV with transposase generated thousands of G418 resistant colonies (Fig. S1F). These mutated clones were injected into the BALB/c nude mice by intravenous (i.v.) injection and the cells that metastasized to the lung were isolated in vivo by primary cell culture using G418 selection (Fig. 1A). Multiple rounds of in vivo selection were performed, and the metastasized clones were obtained for subsequent analysis.
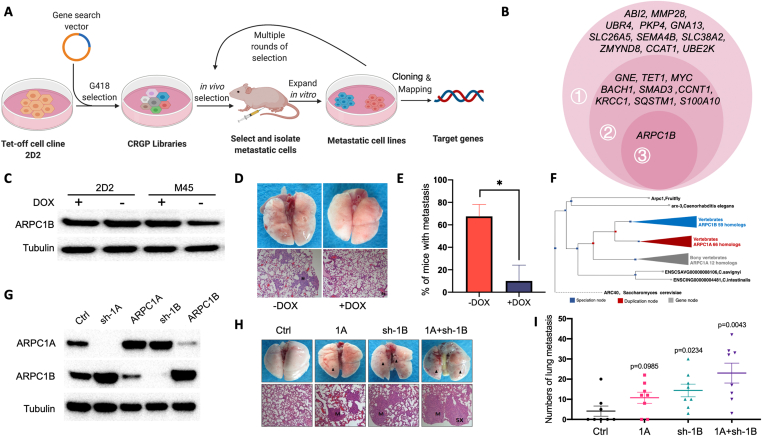
Figure 1.
Identification of ARPC1B gene as a key regulator in cancer metastasis. (A) Flow chart of the experimental approach. (B) Genes identified from lung metastasized G418 resistant clones by Splinkerette PCR in three rounds of screening. (C) Decreased levels of ARPC1B in clone M45 and its negative regulation by doxycycline (DOX). This regulation was absent in parent clone 2D2. (D, E) Lung metastasis of clone M45 was inhibited by doxycycline. Representative gross lung pathology and histological staining are shown (D). The quantification of mice with metastatic lung tumor in the presence or absence of doxycycline is shown (E). (F) The gene tree of ARPC1B was constructed using ENSEMBLE, genetic evolutionary tree analysis identifying gene bifurcation resulted in ARPC1A and ARPC1B in mammals. (G) Silencing of ARPC1B increased the expression of ARPC1A and vice versa in pancreatic cancer cell line ASPC-1. (H, I)In vivo experiments confirmed that the overexpression of ARPC1A and the low expression of ARPC1B could significantly increase the number of pulmonary metastases (H). The quantification of mice with metastatic lung tumor in ORF-ARPC1A group, sh-ARPC1B group and ORF-ARPC1A + sh-ARPC1B (I).
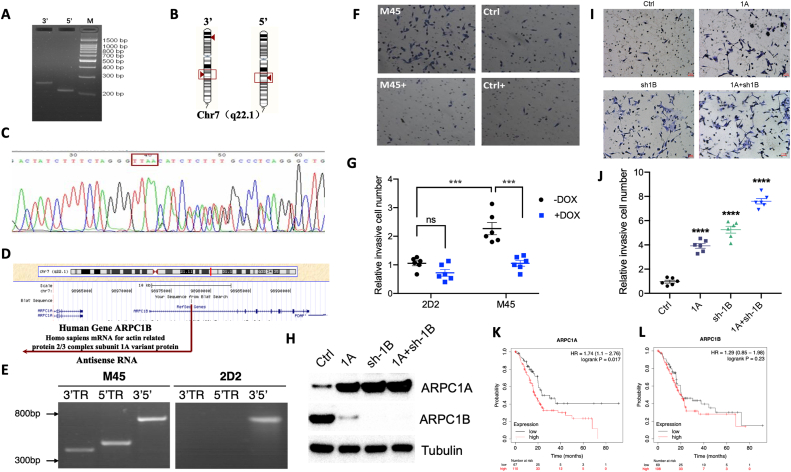
In the first round, 28 metastasized clones were identified, the genomic DNA was extracted, and Splinkerette PCR was performed to clone the candidate genes, in total 21 candidate genes were obtained. In the second round, 10 candidate genes were identified and in the third round, only actin-related protein 2/3 complex subunit 1B (ARPC1B) gene was obtained (Fig. 1B). We then investigated the location of the candidate gene to identify the insertion site of GSV. GSV in the last round of clones was found to be integrated into the intron of ARPC1B gene and initiated the transcription to produce antisense RNA, thus blcoking ARPC1B expression. The Splinkerette PCR products in the process of cloning ARPC1B were found to be located in the same location (Fig. S2A–D). To confirm the integration site of GSV, the genomic PCR was performed (Fig. S2E). Interestingly, many of the other clones found in this screen (SMAD3, S100A, MYC, SQSTM, BACH1, etc.) have been reported to be related to cancer metastasis, supporting the validity of our screening method. Insertion sites of GSV in these genes are indicated in Table S1.
To validate the differential expression of ARPC1B gene in metastasized tumor cells, we measured the protein levels of ARPC1B and its regulation by doxycycline in metastasized clone M45 and parent cell line 2D2. We found that decreased expression of ARPC1B in clone M45 was reversed by doxycycline treatment (Fig. 1C). To validate the casual relation of ARPC1B gene to the metastatic phenotype, the nude mice were injected with clone M45 i.v. and then divided into two groups with or without oral doxycycline administration. We observed that doxycycline treatment significantly decreased the lung metastasis of clone M45 (Fig. 1D, E). Moreover, in vitro cell migration assay demonstrated that doxycycline treatment decreased the migration ability of clone M45 (Fig. S2F, G).
Actin-related protein 2/3 complex subunit 1A and 1B are alternative subunits of ARP2/3 complex.5 Blast analysis showed that human ARPC1B has 68% homology with human SOP2L (ARPC1A). In Saccharomyces cerevisiae, Caenorhabditis elegans, Drosophila melanogaster, and other lower organisms which have ARP2/3 complex homolog, ARPC1A and ARPC1B orthologous genes exist as a single gene (Fig. 1F).
Next, we investigated potential interaction between ARPC1B and ARPC1A and how this interaction affects the metastasis phenotype in cancer cells. We constructed overexpression and knockdown plasmids of ARPC1A and ARPC1B using the lentivirus packaging system LV-EF1α-ORF and PLL3.7, respectively. The results showed that ARPC1B overexpression downregulated the level of ARPC1A, and ARPC1B down-regulation led to increased expression of ARPC1A in ASPC-1 cells, and vice versa, indicating a regulatory loop between ARPC1A and ARPC1B (Fig. 1G). To understand the contribution of ARPC1A in cancer metastasis, we constructed three groups of cells including ORF-ARPC1A group, sh-ARPC1B group and ORF-ARPC1A + sh-ARPC1B group along with the control group using ASPC-1 cell line (Fig. S2H). The cells were then injected into the nude mice. Histological analysis demonstrated that ARPC1A overexpression promoted lung metastasis, and overexpression of ARPC1A along with knockdown of ARPC1B further increased the disease severity. In addition, either overexpression of ARPC1A or knockdown of ARPC1B aggravated lung metastasis, which was further increased in ORF-ARPC1A + sh-ARPC1B group (Fig. 1H, I). To further explore the mechanism of ARPC1A contributing to metastasis, we used a Transwell system to detect cell migration ability across the Transwell chamber. The results showed that both overexpression of ARPC1A and knockdown of ARPC1B significantly enhanced the invasiveness of cells in vitro as manifested by increased migration ability (Fig. S2I, J) indicating potential mechanisms by which ARPC1A promotes tumor metastasis.
Finally, to determine the clinical relevance of ARPC1A gene expression level for prognosis of cancer, we extracted expression data of 177 pancreatic adenocarcinoma (PAAD) patients from PanCancer Atlas in The Cancer Genome Atlas (TCGA) consortium and plotted Kaplan-Meier curves with a web-based survival analysis tool (http://kmplot.com/analysis/index.php?p=background). Our data showed that high expression of ARPC1A was inversely correlated with the survival time of PAAD patients validating the role of ARPC1A in the development and progression of pancreatic cancer (Fig. S2K, L).
Together, this study not only establishes and validates the use of the piggyBac system to generate random mutagenesis to identify novel therapeutic targets in specific pathogenic conditions, but also provides a conceptual framework for identifying and functionally dissecting the master regulators in human diseases including cancer.
Conflict of interests
The authors declare that there is no conflict of interests.
Funding
This study was supported by funding from The National Key Research and Development Program of China (No. 2021YFC2302300), Beijing Nova Program Interdisciplinary Cooperation Project (DC; No. Z191100001119021), Chinese PLA General Hospital Youth Project (DC; No. QNF19074), Beijing Nova Program Project (DC; No. Z171100001117012), China 13th Five-year National Key Grant (LXX; No. 2018ZX09201013), and the Fundamental Research Funds for the Central Universities (KL; No. BMU2021YJ073).
Acknowledgements
We would like to express our great appreciation to Dr. Limin Li from Peking Union Medical College for his valuable and constructive suggestions during the planning and development of this research work.
Footnotes
Peer review under responsibility of Chongqing Medical University.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.gendis.2022.06.006.
Contributor Information
Lixin Xie, Email: 2464277169@qq.com.
Lokesh Sharma, Email: lokeshkumar.sharma@yale.edu.
Kailong Li, Email: kailongli@bjmu.edu.cn.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
figs1.
Construction of the controlled random gene perturbation system. Antisense transcription initiated at TRE leads to loss of target gene function and is regulated by doxycycline (A). Sense transcription initiated at TRE lead to gain of target gene function (B). Doxycycline regulated gene expression in candidate clones. Gene expression was measured by luciferase reporter activities in the presence or absence of doxycycline (C). Titration of the transfection efficiency of the gene search vector (D). Titration of the gene search vector and transposase for co-transfection (E). Representative G-418 clones after transfected with the gene search vector, with or without transposase (F).
figs2.
Cloning of ARPC1B gene and validation of its role in cancer metastasis. Cloning of perturbated gene using Splinkerette PCR (A). The chromosomal location of GSV insertion in M45 clone (obtained from lung metastasis) was determined by ENSEMBLE (B). The PCR product was sequenced to identify the chromosome sequence flanked by GSV (C). The insertion site of GSV was identified using UCSC genome browser (D). The identity of the gene was validated by genomic PCR (E). The reversal of the migration ability by doxycycline in M45 clone but not 2D2 cells (F). The quantification of cell migration in M45 and 2D2 clone in presence or absence of doxycycline (G). Cell models with overexpression of ARPC1A, low expression of ARPC1B, and overexpression of ARPC1A and low expression of ARPC1B were established (H). In vitro experiments confirmed that overexpression of ARPC1A and low expression of ARPC1B promote the invasion and migration ability of tumor cells (I). The quantification of cell migration in ORF-ARPC1A group, sh-ARPC1B group and ORF- ARPC1A + sh-ARPC1B(J). Correlation between ARPC1A or ARPC1B expression and survival of patients with pancreatic cancer (K, I).
References
- 1.Fares J., Fares M.Y., Khachfe H.H., et al. Molecular principles of metastasis: a hallmark of cancer revisited. Signal Transduct Target Ther. 2020;5(1):28. doi: 10.1038/s41392-020-0134-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Quinn J.J., Jones M.G., Okimoto R.A., et al. Single-cell lineages reveal the rates, routes, and drivers of metastasis in cancer xenografts. Science. 2021;371(6532) doi: 10.1126/science.abc1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beckmann P.J., Largaespada D.A. Transposon insertion mutagenesis in mice for modeling human cancers: critical insights gained and new opportunities. Int J Mol Sci. 2020;21(3):1172. doi: 10.3390/ijms21031172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang W., Bradley A., Huang Y. A piggyBac transposon-based genome-wide library of insertionally mutated Blm-deficient murine ES cells. Genome Res. 2009;19(4):667–673. doi: 10.1101/gr.085621.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Molinie N., Rubtsova S.N., Fokin A., et al. Cortical branched actin determines cell cycle progression. Cell Res. 2019;29(6):432–445. doi: 10.1038/s41422-019-0160-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.