Abstract
Plasmacytoma variant translocation 1 (PVT1) is a long non-coding RNA (lncRNA) gene identified as a recurrent breakpoint of Burkitt’s lymphomas. Human PVT1 gene is located on region 8q24.21, a well-known cancer risk region, and encodes at least 26 linear ncRNA isoforms and 26 circular RNA isoforms, as well as 6 microRNAs. Several PVT1 functioning models have been reported recently such as competing endogenous RNA (ceRNA) activity and regulating protein stability of oncogenes, especially MYC oncogene. The promoter of PVT1 gene is a boundary element of tumor-suppressor DNA. CircPVT1 derived from PVT1 gene is also a critical non-coding oncogenic RNA. Although substantial advancements have been made in understanding the roles of PVT1 in cancer recently, the detailed mechanisms underlying its functions remain unclear. Herein, we summarize the recent progressions on the mechanisms underlying PVT1 regulated gene expression at different levels. We also discuss the interaction between lncRNA and protein, RNA and DNA, as well as the potential cancer therapy strategy by targeting these networks.
Keywords: Cancer, ceRNA, CircPVT1, Long non-coding RNAs, MicroRNAs, MYC, PVT1, Regulatory mechanism
Introduction
Long non-coding RNAs (lncRNAs), the RNAs containing more than 200 nucleotides while lacking extended open reading frames, are involved in the regulation of cell survival, proliferation, metabolism, differentiation, as well as other functions.1 With the development of genome-wide sequencing technology, hundreds of types of functional lncRNAs have been identified, and their biological functions have been elucidated. A variety of lncRNAs exist throughout the genome.2 LncRNAs exhibit a significant temporal and spatial specificity during histological development and differentiation. During the differentiation process, different splicing manners and/or dynamic expression may happen. Numerous lncRNAs have been demonstrated to function in tumorigenesis and organ development,3 and many of them can regulate carcinogenesis through impacting the oncogenes and/or tumor suppressing genes.4
Accumulating evidence highlights that PVT1 is a critical gene expression regulator in differentiation, development, heart diseases and cancers. Previous studies have revealed that PVT1 plays critical roles in the generation, growth, and metastasis of human cancers, and it is frequently upregulated in various cancers including lung, pancreas, colorectal, breast, gastric, and cervical cancer.5 Therefore, PVT1 is a potential diagnostic and therapeutic biomarker for some cancers. Studies have identified specific regulatory functions and mechanisms of PVT1 in a series of crucial biological processes, including cell survival, differentiation, proliferation, and chromatin. Via crosstalk with other RNA species and/or through different chromatin-based mechanisms, PVT1 participates in remodeling of chromatin, and regulation of transcriptional and/or post-transcriptional events. PVT1 can function as scaffolds, decoys, guides, or enhancer RNA.6
As supported by increasing evidence, PVT1 may have competing endogenous RNA (ceRNA) activity.7 Moreover, PVT1 gene promoter may compete with protein coding gene promoter. Cho et al reported that the promoter of PVT1 gene can inhibit the MYC gene expression by competing with the MYC promoter.8 More and more studies have demonstrated that PVT1 can bind to DNAs, mRNAs, microRNAs (miRNAs), and/or proteins, thereby regulating a variety of cellular processes. This review summarizes the characteristics of PVT1, including their biological functions and the related mechanisms, which was the most comprehensive to date.
LncRNA PVT1
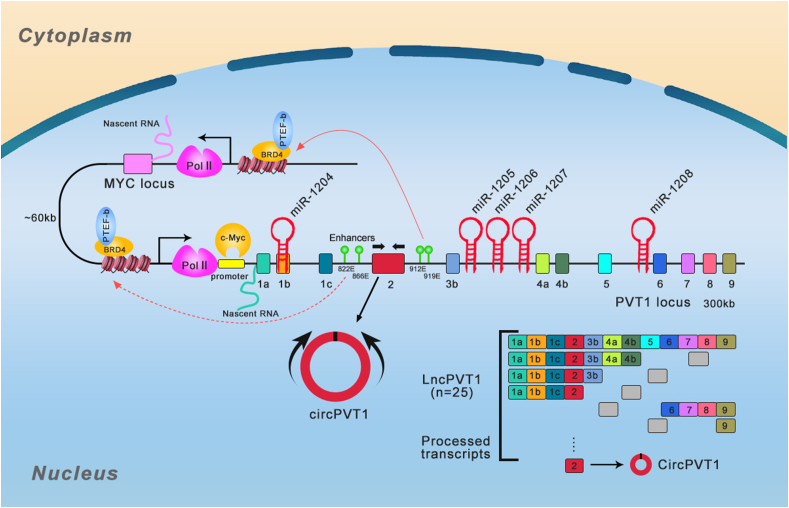
PVT1 is encoded by a gene that resides in a cancer-associated region: a long arm of mouse chromosome 15 (qD1) and human chromosome 8 (8q24). In 1992, for the first time, Huppi K et al5 found PVT1 transcripts accompanies chromosomal translocation and amplification in murine lymphocytic-B neoplasms. The PVT1 gene locus was determined as a cluster of T(8;22) and T(2;8) variant MYC-activating chromosomal translocation breakpoints, localized 400 kb downstream of MYC.5 Human PVT1 genomic locus is localized 54 kb downstream of proto-oncogene MYC6 (Fig. 1). These two genes have some interactions, including feedback regulation, and they synergistically drive tumorigenesis as the oncogenic function of MYC relies on the expression of PVT1.9,10 The PVT1 locus contains at least 12 exons, which gives rise to multiple alternatively spliced non-protein coding transcripts, and encodes at least 52 ncRNA variants with oncogenic functions, including 26 circular and 26 linear RNA isoforms and 6 miRNAs: miR-1208, miR-1207-5p, miR-1207-3p, miR-1205, and miR-12047 (Fig. 1). Recent studies have identified several functioning models of PVT1, such as protein stability regulation for important oncogenes especially MYC oncogene and competing endogenous RNA activity.
Figure 1.
Schematic view of human PVT1 gene locus and alternative splicing. PVT1 located in the region of 8q24.21 harboring MYC/PVT1 loci and PVT1-encoded five miRNAs and probable mode of biogenesis of circPVT1. The positive feedback and promoter-enhancer competition between PVT1 and MYC were shown. The rectangles represent the exons.
Alternative splicing from one primary transcript to generate multiple protein isoforms represents a major proteomic diversity driver in human cells.11 PVT1 is a lncRNA with multiple alternatively spliced transcripts polyadenylated at 3′ end and capped at 5′ end.7,12 PVT1 isoforms exhibit significantly different expression manners across different human tissues: heart and adrenal gland have the highest expression levels, while white blood cells and lymph nodes have the lowest expression levels.7 PVT1 has been shown to play crucial roles in cancers as well as other diseases. Different PVT1 exons could be expressed differentially and might have different functions. Ilboudo A et al found that the expression manners of most PVT1 exons are consistent in prostate cancer (PCa). More specifically, PVT1 exon 9 was consistently overexpressed in the aggressive PCa cell lines compared to their non-tumorigenic mother cell lines. Among all the 12 PVT1 exons, PVT1 exon 9 was the only one exhibiting a very consistent expression, suggesting that the overexpression of PVT1 exon 9 is closely correlated with the aggressiveness in this PCa model.13,14 Pal G et al utilized data from 1000 genomes to analyze the PVT1 locus and found there was a significant difference in chromosomal region spanning PVT1 exons 4A and 4B between African and non-African populations, and the gene expression of PVT1 exons 4A and 4B was dramatically enhanced in PCa tissues compared to benign prostatic hyperplasia and normal prostate tissues. These results suggest that PVT1 exons 4A and 4B might have clinical implications in PCa.15 A novel splicing variant transcript of PVT1 lacking exon 4 (PVT1ΔE4) has also been reported in clear cell renal cell carcinoma (ccRcc), which has a higher endogenous expression level than full-length transcript but can similarly enhance cell invasion and proliferation as full-length transcript does. In addition, study showed that SRSF1 decreased the inclusion of full-length transcript exon 4 while enhanced the expression of PVT1ΔE4 in ccRcc.16 Mfossa A et al investigated the expression patterns of possible circRNA variants for numerous genes including PVT1 in mouse utero and primary cortical neurons and found three (circ-0005724, circ-0005721 and circ-0000604) among five circular transcripts were significantly amplified. Two circular variants (circ-0005724 and circ-0000604) were more stable than the linear cognates in primary cortical neurons. Moreover, the circular and linear transcripts were highly enriched in brain and liver, respectively, and the expression of PVT1 transcripts elevated during the development of embryonic brain.17
The role of PVT1 in pretranscription regulation
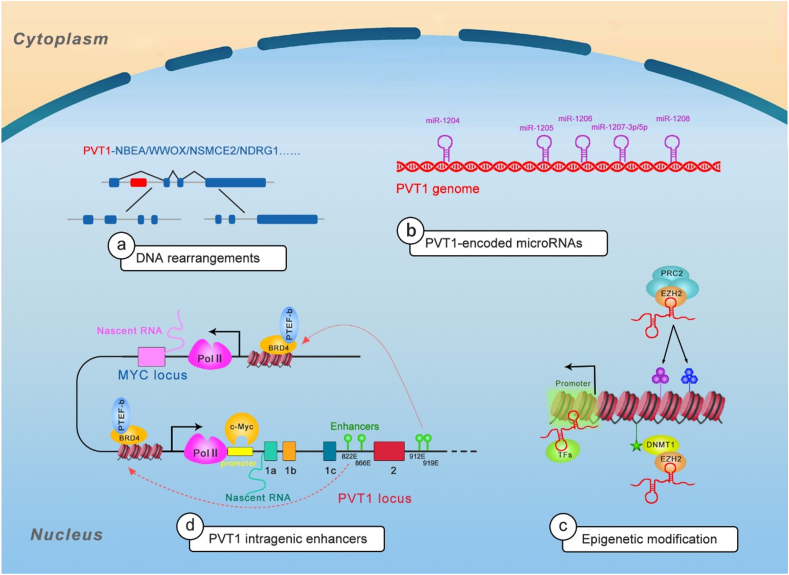
PVT1 influences all the stages of gene life cycle—from chromatin remodeling and epigenetic regulation to transcriptional and posttranscriptional control to protein metabolism (Fig. 2).
Figure 2.
The mechanisms of PVT1 in pretranscription regulation. a Listed are a few examples of PVT1 gene rearrangements and fusion transcripts. b Listed are five PVT1-encoded miRNAs. c showed the mechanisms of PVT1 in epigenetic modification. d showed schematic representing the model of promoter-enhancer competition between PVT1 and MYC. PVT1 promoter regulates MYC expression in cis manner.
PVT1 can act as a fusion partner
With the development of sequencing technologies, a total of 98 different PVT1 fusion transcripts have been identified in both solid tumors and hematological malignancies, which arise from rearrangements of the 8q24 chromosomal region and induce abnormalities of this region.18 The roles of lncRNA gene fusions in joining two protein-coding genes have not been sufficiently investigated. PVT1 is a well-known fusion partner, and PVT1 fusion transcripts are powerful driver in cancers. Most PVT1 fusions are characterized by amplification of genomic locus (8q24.21).18 Analysis of Reactome pathways reveals that some PVT1 fusion partners converge in carcinogenic events, like NOTCH1 signaling, TP53 regulation, AP transcription factor family activity, and UB-specific processing proteases.19
The PVT1-MYC/MYC-PVT1 fusion was fully studied in cancers. PVT1 fusion genes were first described when PVT1 displaced intron 1 and exon 1 in colorectal adenocarcinoma, which resulted in rearrangement of DNA.20 Later, two different PVT1-MYC fusions were identified, both of which involved PVT1 the 5′ end (with exon 1 or exon 1 and 3) fused to MYC (exons 2 and 3).21 PVT1-MYC fusion is present in 0.18% of Genomics Evidence Neoplasia Information Exchange (GENIE) cases in American Association for Cancer Research (AACR) projects with breast invasive ductal carcinoma, endometrial endometrioid adenocarcinoma, Burkitt lymphoma, diffuse large B-cell lymphoma, and colon adenocarcinoma, not otherwise specified having the greatest prevalence.22 In a multiple myeloma study, two highly expressed chimeric genes, PVT1-WWOX harboring der(16)t(16;22)ins(16;8) (q23;q24) and PVT1-NBEA harboring t(8;13) (q24;q13), were identified. The PVT1-WWOX in which PVT1 exon 1 was fused to WWOX exon 9 and PVT1-NBEA chimera in which PVT1 exon 1 was fused to NBEA exon 2 were correlated with the high expression of abnormal WWOX and NBEA, respectively. Both WWOX and NBEA are tumor suppressors in multiple myeloma.23 In a study on acute myelogenous leukemia, the genomic junctions of PVT1-NSMCE2 were found to be located within intron 1 of PVT1 and in a region upstream of exon 1 of NSMCE2, and PVT1-NSMCE2 was also accompanied by amplification of 8q24. NSMCE2 rearrangement can induce chromosomal breakage and loss.24 PVT1-NDRG1, a highly recurrent fusion gene in which PVT1 exon 1 is fused to the 3′ end of NDRG1, has been identified in medulloblastoma,21 breast cancer25 and hepatocellular carcinoma.26 However, the functions of PVT1-NDRG1 need to be further elucidated. EVI1 is a proto-oncogene associated with human myeloid leukemia. The aberrant expression of EVI1 in acute myeloid leukemia (AML) is correlated with the t (3;8) (q26;q24). Further study showed that the breakpoints in t (3;8) (q26;q24) are located at EV1/MDS1 on chromosome 3 and distal to PVT1 on chromosome 8, and this rearrangement can induce aberrant expression of EVI1, thus increasing tumorigenesis.27 Notably, all of these fusion genes contain PVT1 exon 1. Using whole transcriptome analysis, Kim et al identified six different inter-chromosomal fusion genes (PVT1-PPAPDC1A, PVT1-ATE1, PVT1-APIP, PDHX-PVT1, and PVT1-PDHX) in human gastric cancer28; two variants were identified for PVT1/PDHX, with PVT1 exon 1 being fused to PDHX exon 9 or 2, and two PVT1/APIP variants were identified with PVT1 exon 1 being fused to APIP exon 2 or 6. All the identified fusions involved PVT1 exon 1 except PDHX/PVT1, where PDHX exon 3 is fused to PVT1 exon 4. The PVT1 fusion partners APIP and ATE1 were found to be amplified, indicative of important role for PVT1 in DNA rearrangement.
These studies suggest that PVT1 exon 1 is frequently involved in DNA rearrangement, and PVT1 can thereby act as a fusion partner in cancers, impact regulation of oncogenes and tumor suppressors, and promote tumorigenesis.
PVT1 can encode miRNAs
Increasing evidence has shown that the miRNAs residing in PVT1 locus are the key driver for the oncogenic roles of PVT1. Reports have shown that miRNAs can act as tumor suppressors or oncogenes due to their frequent deregulation in cancers. Critically, miRNAs can be applied as cancer therapeutic targets or for cancer phenotype evaluation. As mentioned above, PVT1 encodes a series of non-coding RNAs, including a group of six annotated miRNAs: miR-1208, miR-1207-3p, miR-1207-5p, miR-1206, miR-1205, and miR-1204.5,29
Residing in PVT1 exon (1b), mir-1204 is fused to the light chain of immunoglobulin in a subset of Burkitt's lymphoma and highly present in tumors with amplified PVT1-MYC fusion.5 The miR-1204 expression level is higher in PVT1-MYC fusion-positive than in -negative medulloblastomas, which has a correlation with malignant phenotypes.21 Studies showed that P53 can induce the expression of the PVT1 locus, thereby activating miR-1204 transcription. Reversely, ectopic expression of miR-1204 can enhance P53 level and induce cell death, indicative of a positive feedback loop between them.30,31 Mir-1205, which is encoded on the PVT1 intron 3 locus, can increase growth of castration-resistant PCa by targeting Egl-9 family hypoxia inducible factor 3 (EGLN3)32 and fry-like (FRYL).33
PVT1 has been demonstrated to have a positively correlated expression pattern with its encoded miRNAs. However, paradoxical conclusions were reported between mir-1207 pair and PVT1. Takahashi Y et al showed that different from other PVT1 encoded miRNAs, miR-1207-5p is the only miRNA inhibited by PVT1. MiR-1207-5p can decrease CASP9 and suppress apoptosis in colorectal cancer.34 Cui M et al demonstrated that PVT1 could strengthen cancer stem cell-like properties in nasopharyngeal carcinoma (NPC) through activating PI3K/AKT signal pathway and inhibiting miR-1207.35 Das DK et al found that miR-1207-3p, but not miR-1207-5p, is remarkably downregulated in PCa and promotes tumorigenesis by targeting fibronectin type III domain containing 1 (FNDC1).36 Moreover, Yan C et al demonstrated that miR-1207-5p and PVT1 were consistently induced by estrogen and promote breast cancer tumorigenesis by targeting STAT6.37 You L et al showed that gemcitabine increased the miR-1207 pair (miR-1207-3p and miR-1207-5p) and decreased PVT1 levels through upregulating microprocessor complex subunit DGCR8 and ribonuclease III Drosha and targeting RhoA (by miR-1207-3p) and SRC (by miR-1207-5p).38 Alvarez ML et al showed that miR-1207-5p up-regulation induced by TGFβ1 and glucose is independent of PVT1. Similar with PVT1, miR-1207-5p upregulates FN1, PAI-1, and TGF-β1 independent of host gene in diabetic nephropathy.12 The functions of miR-1206 and miR-1208 associated with PVT1 have not been reported yet. However, circPVT1 knockdown enhances radiosensitivity in NSCLC by sponging miR-1208.39 The functions of PVT1-encoded miRNAs have not been elucidated sufficiently, and further studies are required and may provide novel insights into the PVT1 functions (Fig. 2B).
PVT1 can act as an epigenetic modulator
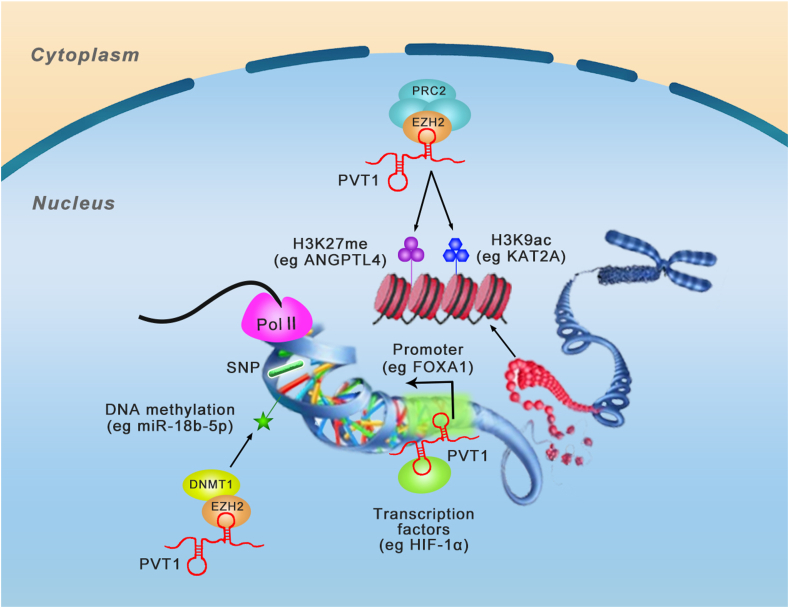
A hallmark function of PVT1 is its ability to interact with histone-modifying complexes, mediating epigenetic regulation (Fig. 3). Studies showed that PVT1 can act at chromatin level as signal, guide, or scaffold to modulate gene expression. Enhancer of zeste homolog 2 (EZH2) is an enzymatic subunit and histone methyltransferase of polycomb repressive complex 2 (PRC2), which is an epigenetic multiprotein complex and plays critical roles in the progression of cancers and other diseases. Histone methyltransferase enhancer of EZH2 is responsible to catalyze mono-, di-, and tri-methylate lysine 27 of histone H3 (H3K27me1/2/3) and induces silencing of the involved genes.40
Figure 3.
The mechanisms of PVT1 in epigenetic modification. PVT1 can act as scaffold binds to chromatin and epigenetic modifier, guide the epigenetic modifier to gene promotor. PVT1 can facilitate enhancer and promoter element interactions, critical for gene activation. Listed are a few examples of PVT1 regulation from epigenetic regulation and chromatin remodeling to transcriptional control.
Epigenetic modification complex PRC2 was found to communicate with PVT1. PVT1 combines with EZH2 and recruits EZH2 to anti-tumor gene promoter regions, then enhances histone H3K27 trimethylation and decreases gene transcription levels.41 PVT1 increases proliferation of gastric cancer cells through silencing p15INK4a and p16INK4b by the occupancy of EZH2, which is recruited by PVT1.41 Similarly, PVT1 binds EZH2, and down-regulates P57 expression, and changes the biology of ovarian cancer cells.42 PVT1 can regulate histone methylation of angiopoietin-like 4 (ANGPTL4), thereby promoting cholangiocarcinoma (CCA) oncogenesis and development by binding to PRC2.43 Similar mechanism targeting protein ANGPTL4 was also reported in preeclampsia patients.44 Similarly, PVT1 can also downregulate large tumor suppressor kinase 2 (LATS2) in non-small cell lung cancer (NSCLC) through methylation of the LATS2 promoter and recruitment of EZH2.45
PVT1 suppresses cell apoptosis and enhances cell proliferation by stabilizing expression of murine double minute 2 (MDM2), suppressing expression of P53 and recruiting EZH2 in hepatocellular carcinoma (HCC).46 PVT1 regulates proliferation of thyroid cancer cells by modulating thyroid-stimulating hormone receptor (TSHR) and recruiting EZH2.47 PVT1 can also recruit EZH2 to the mir-200b promoter and inhibit its expression via increasing the trimethylation level of its histone H3K27. The cell proliferation and migration promoting effects of PVT1 in cervical cancer relies on the silencing of miR-200b.48 PVT1 can also regulate miR-195 through promoting the methylation of H3K27me3 in the promoter region via recruiting EZH2.49 A study on diabetic nephropathy (DN) patients revealed that substantial C-phosphate-G (CpG) island sites are localized at the promoter region of forkhead box A1 (FOXA1), where PVT1 recruits EZH2 to accumulate H3K27me3, thereby suppressing the level of FOXA1. Reversely, the silencing of PVT1 attenuated the damage and suppressed apoptosis of podocytes in DN through increasing FOXA1.50 Apart from regulating the methylation of H3K27, Wang et al51 found that PVT1 can act as a scaffold for KAT2A, a chromatin modification factor, induce acetylation of H3K9, recruiting TIF1β to activate the transcription of NF90, thereby activating the KAT2A acetyltransferase, stabilizing HIF-1α, and enhancing malignant phenotype in NPC. Notably, different from inhibiting target genes, PVT1 can also bind to EZH2 and inhibit its recruitment to MYC promoter, thereby enhancing the expression of MYC by altering the status of H3K37me3 in hepatitis B virus-positive liver cancer.52
DNA methylation as an epigenetic mechanism is also involved in the function of PVT1. PVT1 can recruit DNA methyltransferase 1 (DNMT1) to the mir-18b-5p promoter through EZH2 and suppress miR-18b-5p transcription via DNA methylation. Moreover, PVT1 can regulate proliferation of gallbladder cancer (GBC) cells via HIF-1α.53 PVT1 also induces expression of miR-146a in prostate cancer by promoting DNA methylation of CpG island in its promoter. The functions of PVT1 in prostate cancer tumorigenesis relies on miR-146a, and overexpression of miR-146a can eliminate the effects of PVT1 knockdown.54 In addition, it is worth mentioning that PVT1 itself is regulated by DNA methylation.55,56
The role of PVT1 in transcription regulation
The transcription stages include initiation, elongation, and termination. Transcription initiates from binding of RNA polymerase II (RNA Pol II) to a gene’s promoter region supported by general transcription factors (TFs), and other TFs accelerate transcription by binding to enhancer region. When the polymerase meets the terminator, the transcription is terminated. PVT1 can affect gene expression through direct binding to TFs and impacting binding of polymerase to promotor. Liu SJ et al conducted a CRISPR interference (CRISPRi)-based genome-scale identification with an sgRNA library along the PVT1 locus in ENCODE (ENCyclopedia of DNA Elements Project) cancer cell lines. The sgRNAs outside of 1 kb window around the TSSs had no effect on the transcription of major PVT1 isoform, suggesting the observed pro-growth phenotype is mediated by transcriptional interference.57 Pyfrom SC et al performed a novel computational method PLAIDOH to calculate the transcript cis-regulatory predictive scores of cancer-specific lncRNAs and found that PVT1 has a dramatical high enhancer cis-regulatory score,58 which was validated to suppress MYC in cis via promoter competing for common intragenic enhancer elements.8 These results suggest that PVT1 promoter has a PVT1 gene independent tumor suppressing function. The regulation of MYC in cis by PVT1 locus might be attributed to the MYC gene body that functions independently of the transcribed RNA, or DNA within PVT1 promoter or sequence-specific functions of mature PVT1 transcript. Guo L et al found a strong enrichment of RNA Pol II at the place where the MYC promoter interacts with the BET inhibitor (BETi) resistance-specific PVT1 enhancer in leukemia cells, raising the possibility that combination therapy may block the loading of RNA Pol II at this place. CDK7 inhibitor can block loading of RNA Pol II at PVT1 enhancer, thereby inhibiting MYC transcription, thereby exerting a synergistic effect on BETi-resistant leukemia.59 Moreover, our previous work demonstrated that PVT1 can bind to the HIF-1α promoter and induce the transcription, thereby driving progression of pancreatic cancer (PC), indicating that PVT1 can regulate unlinked gene expression through with enhancers and/or promoters.60 However, the mechanisms underlying the functions of PVT1 in elongation and termination processes require further research.
The role of PVT1 in posttranscription regulation
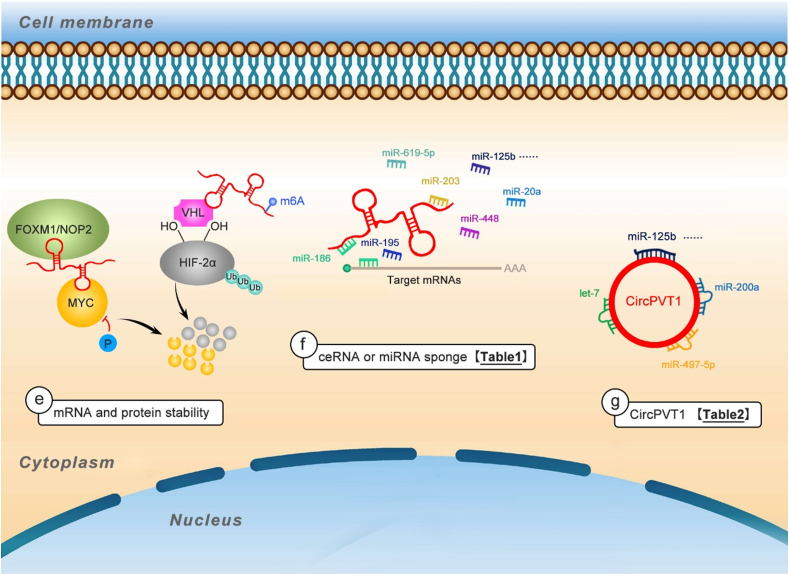
Following transcription, a variety of RNA-binding proteins (RBPs) regulate the pre-mRNAs, including capping, polyadenylating, splicing, editing, and transferring them from nucleus to cytoplasm. The mRNA stability is very important for translation. PVT1 plays critical roles in mRNA splicing, stability, and translation, and it can also indirectly regulate mRNA expression by competing endogenous RNA or acting as a miRNA sponge (Fig. 4).
Figure 4.
The mechanisms of PVT1 in posttranscription regulation. e listed PVT1 can interact with proteins and/or mRNAs to form RNP complexes, or act as scaffold for two or more proteins, thus stabilizing and protecting mRNAs from degradation, which regulate posttranscriptional gene regulation. f listed PVT1 acting as miRNA sponges attenuates the miRNA′ effect on downregulating mRNA expression (Table 1). g listed CircPVT1 acting as molecular sponges for miRNAs (Table 2).
PVT1 can enhance the mRNA and protein stability
Modulation of mRNA degradation is a control step in gene expression for regulation of protein synthesis, and the combination between PVT1 and MYC has been well studied. As a tumorigenesis driver, MYC regulates a large number of genes involved in multiple oncogenic processes. PVT1 promotes MYC stability via protecting it against proteasome-dependent degradation and decreasing its phosphorylation at threonine 58 (Thr58).9,20 Not only in cancers, in skeletal muscle cells, during muscle atrophy, PVT1 also interacts with MYC, and the up-regulated PVT1 blocks the phosphorylation and degradation of MYC.61 Notably, m6A modification of PVT1 transcripts enhances its interaction with MYC and stabilizes the MYC protein in epidermal progenitor cells.62 Besides PVT1-MYC pair, the positive feedback loop between PVT1 and other TFs has also been reported in a variety of tumors. PVT1 can block protein degeneration and ubiquitination via direct binding to proteins. For example, PVT1 directly binds to FOXM1 and stabilizes FOXM1, thereby enhancing gastric cancer cell proliferation and invasion.63 PVT1 binds to the VHL region of HIF-1α60 and HIF-2α64 proteins and enhances their stability by blocking ubiquitination-dependent degradation in PC and ccRcc, respectively. In turn, these TFs can also bind to the enhancer of PVT1 to transactivate its expression.60,63,64 Wang F et al found that PVT1 can bind to NOP2 and enhance the stability of NOP2; moreover, PVT1 functions depending on the presence of NOP2 in hepatocellular carcinoma.65
PVT1 can act as miRNA sponges
Competing endogenous RNAs (ceRNAs), also called miRNA decoys or miRNA sponges, have been identified as drivers in many diseases, especially cancers. The ceRNAs encompass different RNAs (lncRNAs, circRNAs, or pseudogenes) competing with each other to attract miRNAs. PVT1 can act as a miRNA sponge to suppress miRNAs binding to their mRNA targets, thereby promoting the stability of the target mRNAs, and regulating protein expression. Table 1 and Table S1 list the related diseases and miRNAs sponged by PVT1.
Table 1.
Related diseases and miRNAs sponged by PVT1.
| Related diseases | miRNAs | Target genes or signaling pathways | Function | References |
|---|---|---|---|---|
| Acute kidney injury (AKI) | miR-20a-5p | NLRP3 | Modulate NLRP3-mediated pyroptosis | 66 |
| Atrial fibrillation | miR-145-5p | IL-16 | Boost the extracellular matrix remodeling of atrial fibroblasts | 67 |
| Bladder cancer | miR-194-5p | BCLAF1 | Increase malignant phenotypes | 68 |
| Cardiac hypertrophy | miR-196b | OSMR | Knockdown attenuates the myocardial hypertrophy | 69 |
| Cardiotoxicity | miR-187-3p | AGO1 | Aggravate doxorubicin-induced cardiomyocyte apoptosis | 70 |
| Cervical cancer | miR-140-5p | SMAD3 | Promote the proliferation and metastasis | 71 |
| Clear cell renal cell carcinoma (ccRcc) | miR-328-3p | FAM193B | Promote the proliferation of ccRCC cells | 72 |
| Diabetic cataract (DC) | miR-214-3p | MMP2, regulated by SP1 | Modulate the proliferation and apoptosis of lens epithelial cells | 73 |
| Diabetic nephropathy (DN) | miR-23b-3p | WT1 | Knockdown alleviates high glucose-induced proliferation and fibrosis in human mesangial cells | 74 |
| Diabetic osteoarthritis (DOA) | miR-146a | TGF-β/SMAD4 pathway | Promote cartilage degradation | 75 |
| Esophageal squamous cell carcinoma (ESCC) | miR-203 | LASP1 | Promote ESCC progression | 76 |
| Gallbladder cancer (GBC) | miR-143 | HK2 | Knockdown inhibits cell proliferation, migration, and invasion | 77 |
| Gastric cancer | miR-186 | HIF-1α | promoted the GC cell proliferation and invasion | 78 |
| Glioma | miR-128-3p | GREM1 and BMP Signaling Pathway | Promote tumorigenesis and progression | 79 |
| Ischemic stroke | miR-24-3p | STAT3, regulated by SOX2 | Depleting improves ischemic stroke | 80 |
| Myocardial ischemia/reperfusion (I/R) injury | miR-186 | Beclin-1 | Knockdown protects cardiomyocytes apoptosis and autophagy | 81 |
| Neuropathic pain | miR-186-5p | CXCL13/CXCR5 | Depletion alleviates neuropathic pain, astrocytic activation and reduced the expression of neuroinflammatory factors and proteins | 82 |
| Non-small cell lung cancer (NSCLC) | miR-200a and miR-200b | MMP9 | Promote the invasive ability of NSCLC cells | 83 |
| Osteoarthritis (OA) | miR-27b-3p | TRAF3 | Inhibit IL-1β-induced injury in chondrocytes | 84 |
| Osteosarcoma (OS) | miR-497 | HK2 | Contribute to OS cell glucose metabolism, cell proliferation, and motility | 85 |
| Papillary thyroid carcinoma (PTC) | miR-30a | IGF1R | Enhance the viability and invasion | 86 |
| PM2.5-exposed lung cancer | miR-199a-5p | Caveolin 1 | Affect the role of lentinan in PM2.5-exposed lung cancer cells | 87 |
| Pulpitis | miR-455-5p | SOCS3 and PLXNC1 | Involve in the pathogenesis of pulpitis | 88 |
| Retinoblastoma (RB) | miR-488-3p | NOTCH2 | Silencing inhibits cell proliferation, migration, invasion, and cell cycle progression and induces cell apoptosis | 89 |
| Rheumatoid arthritis (RA) | miR-543 | SCUBE2 | Regulate apoptosis of fibroblast-like synoviocytes | 90 |
| RSV-infected asthma | miR-203a | E2F3 | Involve in the mechanism of α-asarone in treating RSV-induced asthma | 91 |
| Temporomandibular joint osteoarthritis (TMJ OA) | miR-211-3p | TNF-α | Induce chondrocyte apoptosis | 92 |
First, the researchers analyzed their concerned disease-related lncRNAs by microarray analysis or in the public databases TCGA or GEO and determined the research target PVT1. Then, the structure and function of PVT1 were analyzed, and the expression pattern of PVT1 in cells as well as the correlation between PVT1 levels and clinical prognosis was investigated. The results showed that PVT1 was closely related to the concerned disease (often upregulated in cancers or other diseases). PVT1 was identified as an oncogenic lncRNA that could act as a prognostic or prognostic biomarker in cancers. Furthermore, gain- and loss-of-function assays revealed that PVT1 could enhance proliferation, migration, and invasion of cancer cells, inhibit apoptosis, promote tumor growth and angiogenesis of tumor tissues in mice, and impair sensitivity to antineoplastic drugs in vitro and in vivo, suggesting that PVT1 affects the progression of cancer. Finally, subcellular localization was conducted for subsequent analysis from the perspective of ceRNA.
After the target lncRNA-PVT1 was identified, the miRNA docking site of PVT1 was predicted with RegRNA, miRDB, miRWalk, miRBase, miRPathDB, miRanda, TargetScan, or starBase bioinformatics data resources.93,94 Then, the sequence and function of target miRNA were analyzed, and the expression pattern of miRNA in cells as well as the correlation between PVT1 levels and clinical prognosis was investigated. To confirm the ceRNA relationship between PVT1 and the targeted miRNA, PVT1 was silenced/overexpressed in vitro and in vivo, followed by analysis using luciferase assay and RNA immunoprecipitation (RIP) assay. Furthermore, the biological functions of miRNA were also analyzed using gain- and loss-of-function assays. Functional experiments showed that miRNA could inhibit cell proliferation, migration, and invasion, while promote cell apoptosis. In addition, functional rescue experiments further demonstrated that there was an antagonistic effect between PVT1 and the target miRNA.
After PVT1 and miRNA were identified, the final mRNA needs to be determined. The above bioinformatics data resources were used to predict the target genes of miRNA. TCGA and GEO data or RT-PCR, Western blot and/or immunohistochemical assay were used again to analyze the expression level of target mRNA, and the clinical prognosis, binding relationship, and the correlation between PVT1 and miRNA were analyzed. The target mRNA was proved to function in carcinogenesis. After finding the target mRNA of miRNA, the lncRNA-miRNA-mRNA were correlated, and it was accurately demonstrated that this ceRNA network did affect the occurrence and development of cancers or other diseases. Thus, a relatively complete ceRNA regulatory network was basically formed. This ceRNA network involving PVT1 and miRNA in cancers characterizes the signaling pathways mediating tumorigenesis, tumor metastasis and chemoresistance in a variety of cancers.95 More importantly, though identified as an oncogenic lncRNA at the very beginning, PVT1 was demonstrated to play critical roles in non-cancer diseases, such as diabetes and its complications, osteoarthritis, and myocardial infarction, among others (Table 1 and Table S1).
The role of PVT1 in relation with circPVT1
CircPVT1, located on chromosome 8q24, is generated by circularization from exon 2 of the PVT1 gene, the same genetic locus encoding for PVT1 (Fig. 1). However, current reports showed that PVT1 and circPVT1 are independently transcripted by different promoters. PVT1 primarily localizes in the nucleus, while circPVT1 generally localizes in the cytoplasm, indicative of different post-transcriptional regulation for circPVT1 and PVT1.96 Both circRNAs and circPVT1 have significant importance in pathological processes like cancer. Circular RNA profile allows circPVT1 to act as a prognostic marker and proliferative factor in many cancers. Studies focusing on circPVT1 showed that circPVT1 can enhance proliferation migration, and invasion of cancer cells, as well as drug resistance.97 CircPVT1 was also identified as a senescence-associated circRNA (SAC-RNA). Several proliferative proteins such as IGF2BP1, KRAS and HMGA2, encoded by let-7 target mRNAs, can prevent senescence, and the levels of these proteins can be decreased by circPVT1 silencing.98
The interaction between circPVT1 and PVT1 has been highlighted in a few studied malignancies. Current reports indicated their cooperation with an undifferentiated and/or more aggressive cell phenotype, thereby promoting cancer progression. Martina Ghetti et al found that upregulation of circPVT1 and PVT1 through genomic rearrangements or amplification and/or promoted transcription can enhance proliferation of malignant cells in acute lymphoblastic leukemia (circular PVT1), Burkitt lymphoma, acute promyelocytic leukemia, acute myeloid leukemia, and multiple myeloma (linear PVT1).7
Numerous studies showed that circRNAs function as miRNA sponges or ceRNAs. The role of circPVT1 in oncogenesis has also been correlated with their function as ceRNA. CircPVT1 and miRNAs could act as sponges for RNA-binding proteins through binding to tumor suppressors. Table 2 and Table S2 list the related diseases and miRNAs sponged by circPVT1.
Table 2.
Related diseases and miRNAs sponged by circPVT1.
| Related diseases | miRNAs | Target genes or signaling pathways | Function | References |
|---|---|---|---|---|
| Adenomyosis (ADS) | miR-145 | TALIN1 | Promote eutopic endometrial cell proliferation and invasion | 99 |
| Breast cancer (BCa) | MiR-29a-3p | AGR2-HIF-1α Pathway | Promote the progression of breast cancer | 100 |
| Clear cell renal cell carcinoma (ccRcc) | miR-145-5p | TBX15 | Promote ccRCC growth and metastasis | 101 |
| Colorectal cancer (CRC) | let-7 | NRAS | Drive cancer cells towards oncogenicity | 102 |
| Epithelial ovarian cancer (EOC) | miR-149 | NR | Enhance cell proliferation but inhibits apoptosis | 103 |
| Esophageal carcinoma (EC) | miR-4663 | PAXs and PPARs | Promote cell invasive ability | 104 |
| Gastric cancer (GC) | miR-124-3p | ZEB1 | Contribute to paclitaxel resistance of gastric cancer cells | 105 |
| Hepatocellular carcinoma (HCC) | miR-3666 | SIRT7 | Promote HCC cell growth | 106 |
| Lung adenocarcinoma (LADC) | miR-429 | FOXK1 | Interference inhibits the progression of lung ADC and enhances its sensitivity to DDP | 107 |
| Lung squamous cell carcinoma (LUSC) | miR-30d and miR-30e | CCNF, regulated by HuR | Promote the proliferation of LUSC cells | 108 |
| Myocardial infarction (MI) | miR-125b,miR-200a | p53/Traf6, Sirt7, Keap 1/Nrf 2, and PDCD4 | Apoptotic signaling | 109 |
| Neck squamous cell carcinoma (HNSCC) | miR-497-5p | P53/YAP/TEAD | Increase the malignant phenotype | 110 |
| Non-small cell lung cancer (NSCLC) | miR-125b | E2F2 | Promote NSCLC cell growth and invasion | 111 |
| Oral squamous cell carcinoma (OSCC) | miR-125b | STAT3 | Regulate cell proliferation | 112 |
| Osteosarcoma (OS) | miR-137 | TRIAP1 | Boosted doxorubicin (DXR) resistance | 113 |
| Senescence | let-7 | IGF2BP1, KRAS and HMGA2 | Silencing promotes cell senescence and reverses the proliferative phenotype | 98 |
| Steroid-induced osteonecrosis of the femoral head (SIONFH) | miR-21-5p | SMAD7/TGFβ signaling pathway | Attenuate the apoptosis and cell viability inhibition | 114 |
| T cell acute lymphoblastic leukemia (T-ALL) | miR-30e | DLL4-NOTCH signaling | Knockdown inhibits the cell proliferation and increase the cell apoptosis | 115 |
The role of SNPs at PVT1
The role of single nucleotide polymorphisms (SNP) at PVT1 associated with diseases was also explored. A pooling-based genome-wide SNP association study identified PVT1 as a candidate gene for end-stage renal disease (ESRD) in type 2 diabetes and found rs2720709 at PVT1 might contribute to ESRD susceptibility in diabetes.116 Similarly, SNP was also found to be associated with diabetic nephropathy.117 Zhou S et al118 found that the combined SNP actions in mir-146a rs2910164 and PVT1 rs13281615 impacted the function of lung in chronic obstructive pulmonary disease (COPD) smokers. An analysis of breast cancer susceptibility loci potential targets by capture Hi-C identified more than 70 variants correlated with risk of breast cancer, including the risk loci targeting PVT1.119 An intronic variant in PVT1 rs10505506 was associated with chest radiotherapy-induced breast cancer risk after Hodgkin lymphoma (HL) in female.120 In addition, the risk allele (G) of SNP rs13281615 was observed to function in breast cancer via affecting the expression of PVT1.121 Moreover, the GG genotype of rs378854 was identified as a new risk variant for prostate cancer by increasing the expression of PVT1, but not MYC.122 SNP array data obtained from 52 ovarian tumors showed that PVT1, but not MYC, was significantly upregulated when compared with normal ovary, as well as tumors lacking copy number alterations.123 A genome-wide association study (GWAS) showed that PVT1 rs2114358∗A was associated with event-free phenotype during an at least two-year GA treatment.124 A GWAS on kidney transplants in 532 African American (AA) deceased donors (DDs) found that several SNPs in PVT1 affected renal allograft survival following transplantation independently or via interaction with apolipoprotein L1 gene (APOL1).125 Moreover, the SNP-SNP interactions in region of CASC11-MYC-PVT1 have a more significant influence than individual effects of SNP on risk of prostate cancer in AA men.126 These studies consistently point to an important role of PVT1 in diseases.
Conclusions and perspectives
PVT1 has been demonstrated as the most potent long noncoding RNA with oncogenic functions. Recent studies have been performed on all aspects of PVT1, including its potential oncogenic roles, molecular alteration, biogenesis, as well as its bioactivities including regulating protein interactions, modulating miRNA expression, forming fusion genes, targeting regulatory genes, interacting with MYC and its circular transcripts—circPVT1, functioning as a ceRNA, among others. In this review, we summarized recent progress in PVT1 functions and metabolism. We speculate that the modes of action of PVT1 may also be applied to many other lncRNAs such as HOTAIR, H19, NEAT, MALAT1, HOTTIP, among others; thus, our summary of PVT1 may provide hints for studies of other lncRNAs.
Another research hotspot is the therapeutic potential of lncRNAs. A comprehensive inquiry on 9972 cancer cases of 21 types has demonstrated that PVT1 may act as a potential human cancer biomarker correlated with cancer prognosis and progression.127 Besides its critical roles in diagnostic and prognostic process, PVT1 also has potential therapeutic roles. PVT1 can act either as a biomarker to make the existing cancer therapeutics more effective or as a potential target for designing novel therapeutic candidates. However, there will be a long way to go to be able to fully exploit PVT1 for cancer therapy and prognostication, which will be the next focus and research direction.
Conflict of interests
The authors declare that they have no conflict of interests.
Footnotes
Peer review under responsibility of Chongqing Medical University.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.gendis.2022.05.037.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Chen H., Shan G. The physiological function of long-noncoding RNAs. Noncoding RNA Res. 2020;5(4):178–184. doi: 10.1016/j.ncrna.2020.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fu L., Ding Z., Tan D., et al. Genome-wide discovery and functional prediction of salt-responsive lncRNAs in duckweed. BMC Genom. 2020;21(1):212. doi: 10.1186/s12864-020-6633-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ye M., Zhang J., Wei M., et al. Emerging role of long noncoding RNA-encoded micropeptides in cancer. Cancer Cell Int. 2020;20:506. doi: 10.1186/s12935-020-01589-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fang Y., Fullwood M.J. Roles, functions, and mechanisms of long non-coding RNAs in cancer. Dev Reprod Biol. 2016;14(1):42–54. doi: 10.1016/j.gpb.2015.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huppi K., Volfovsky N., Runfola T., et al. The identification of microRNAs in a genomically unstable region of human chromosome 8q24. Mol Cancer Res. 2008;6(2):212–221. doi: 10.1158/1541-7786.MCR-07-0105. [DOI] [PubMed] [Google Scholar]
- 6.Huppi K., Siwarski D., Goodnight J., et al. Alternative splicing of pvt-1 transcripts in murine lymphocytic-B neoplasms accompanies amplification and chromosomal translocation. Int J Oncol. 1992;1(5):525–532. doi: 10.3892/ijo.1.5.525. [DOI] [PubMed] [Google Scholar]
- 7.Ghetti M., Vannini I., Storlazzi C.T., et al. Linear and circular PVT1 in hematological malignancies and immune response: two faces of the same coin. Mol Cancer. 2020;19(1):69. doi: 10.1186/s12943-020-01187-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cho S.W., Xu J., Sun R., et al. Promoter of lncRNA gene PVT1 is a tumor-suppressor DNA boundary element. Cell. 2018;173(6):1398–1412. doi: 10.1016/j.cell.2018.03.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tseng Y.Y., Moriarity B.S., Gong W., et al. PVT1 dependence in cancer with MYC copy-number increase. Nature. 2014;512(7512):82–86. doi: 10.1038/nature13311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jin K., Wang S., Zhang Y., et al. Long non-coding RNA PVT1 interacts with MYC and its downstream molecules to synergistically promote tumorigenesis. Cell Mol Life Sci. 2019;76(21):4275–4289. doi: 10.1007/s00018-019-03222-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ule J., Blencowe B.J. Alternative splicing regulatory networks: functions, mechanisms, and evolution. Mol Cell. 2019;76(2):329–345. doi: 10.1016/j.molcel.2019.09.017. [DOI] [PubMed] [Google Scholar]
- 12.Alvarez M.L., Khosroheidari M., Eddy E., et al. Role of microRNA 1207-5P and its host gene, the long non-coding RNA Pvt1, as mediators of extracellular matrix accumulation in the kidney: implications for diabetic nephropathy. PLoS One. 2013;8(10) doi: 10.1371/journal.pone.0077468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ilboudo A., Chouhan J., McNeil B.K., et al. PVT1 exon 9: a potential biomarker of aggressive prostate cancer? Int J Environ Res Publ Health. 2015;13(1) doi: 10.3390/ijerph13010012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pal G., Huaman J., Levine F., et al. Long noncoding RNA from PVT1 exon 9 is overexpressed in prostate cancer and induces malignant transformation and castration resistance in prostate epithelial cells. Genes. 2019;10(12):964. doi: 10.3390/genes10120964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pal G., Di L., Orunmuyi A., et al. Population differentiation at the PVT1 gene locus: implications for prostate cancer. G3 (Bethesda) 2020;10(7):2257–2264. doi: 10.1534/g3.120.401291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang T., Zhou H., Liu P., et al. lncRNA PVT1 and its splicing variant function as competing endogenous RNA to regulate clear cell renal cell carcinoma progression. Oncotarget. 2017;8(49):85353–85367. doi: 10.18632/oncotarget.19743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mfossa A.C.M., Thekkekara Puthenparampil H., Inalegwu A., et al. Exposure to ionizing radiation triggers prolonged changes in circular RNA abundance in the embryonic mouse brain and primary neurons. Cells. 2019;8(8):778. doi: 10.3390/cells8080778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tolomeo D., Agostini A., Visci G., et al. PVT1: a long non-coding RNA recurrently involved in neoplasia-associated fusion transcripts. Gene. 2021;779 doi: 10.1016/j.gene.2021.145497. [DOI] [PubMed] [Google Scholar]
- 19.Croft D., O'Kelly G., Wu G., et al. Reactome: a database of reactions, pathways and biological processes. Nucleic Acids Res. 2011;39(Database issue):D691–D697. doi: 10.1093/nar/gkq1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shtivelman E., Bishop J.M. The PVT gene frequently amplifies with MYC in tumor cells. Mol Cell Biol. 1989;9(3):1148–1154. doi: 10.1128/mcb.9.3.1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Northcott P.A., Shih D.J., Peacock J., et al. Subgroup-specific structural variation across 1,000 medulloblastoma genomes. Nature. 2012;488(7409):49–56. doi: 10.1038/nature11327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.AACR Project GENIE Consortium AACR project GENIE: powering precision medicine through an international consortium. Cancer Discov. 2017;7(8):818–831. doi: 10.1158/2159-8290.CD-17-0151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nagoshi H., Taki T., Hanamura I., et al. Frequent PVT1 rearrangement and novel chimeric genes PVT1-NBEA and PVT1-WWOX occur in multiple myeloma with 8q24 abnormality. Cancer Res. 2012;72(19):4954–4962. doi: 10.1158/0008-5472.CAN-12-0213. [DOI] [PubMed] [Google Scholar]
- 24.Chinen Y., Sakamoto N., Nagoshi H., et al. 8q24 amplified segments involve novel fusion genes between NSMCE2 and long noncoding RNAs in acute myelogenous leukemia. J Hematol Oncol. 2014;7:68. doi: 10.1186/s13045-014-0068-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mishra A., Bonello M., Byron A., et al. Evaluation of gene expression data from cybrids and tumours highlights elevated NDRG1-driven proliferation in triple-negative breast cancer. Breast Cancer. 2020;14 doi: 10.1177/1178223420934447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhao K., Zhao Y., Zhu J.Y., et al. A panel of genes identified as targets for 8q24.13-24.3 gain contributing to unfavorable overall survival in patients with hepatocellular carcinoma. Curr Med Sci. 2018;38(4):590–596. doi: 10.1007/s11596-018-1918-x. [DOI] [PubMed] [Google Scholar]
- 27.Lennon P.A., Abruzzo L.V., Medeiros L.J., et al. Aberrant EVI1 expression in acute myeloid leukemias associated with the t(3;8)(q26;q24) Cancer Genet Cytogenet. 2007;177(1):37–42. doi: 10.1016/j.cancergencyto.2007.05.007. [DOI] [PubMed] [Google Scholar]
- 28.Kim H.P., Cho G.A., Han S.W., et al. Novel fusion transcripts in human gastric cancer revealed by transcriptome analysis. Oncogene. 2014;33(47):5434–5441. doi: 10.1038/onc.2013.490. [DOI] [PubMed] [Google Scholar]
- 29.Beck-Engeser G.B., Lum A.M., Huppi K., et al. Pvt 1-encoded microRNAs in oncogenesis. Retrovirology. 2008;5:4. doi: 10.1186/1742-4690-5-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barsotti A.M., Beckerman R., Laptenko O., et al. p53-Dependent induction of PVT1 and miR-1204. J Biol Chem. 2012;287(4):2509–2519. doi: 10.1074/jbc.M111.322875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bisio A., De Sanctis V., Del Vescovo V., et al. Identification of new p53 target microRNAs by bioinformatics and functional analysis. BMC Cancer. 2013;13:552. doi: 10.1186/1471-2407-13-552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang Y., Li X., Liu W., et al. MicroRNA-1205, encoded on chromosome 8q24, targets EGLN3 to induce cell growth and contributes to risk of castration-resistant prostate cancer. Oncogene. 2019;38(24):4820–4834. doi: 10.1038/s41388-019-0760-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Naidoo M., Levine F., Gillot T., et al. MicroRNA-1205 regulation of FRYL in prostate cancer. Front Cell Dev Biol. 2021;9 doi: 10.3389/fcell.2021.647485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Takahashi Y., Sawada G., Kurashige J., et al. Amplification of PVT-1 is involved in poor prognosis via apoptosis inhibition in colorectal cancers. Br J Cancer. 2014;110(1):164–171. doi: 10.1038/bjc.2013.698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cui M., Chang Y., Fang Q.G., et al. Non-coding RNA Pvt 1 promotes cancer stem cell-like traits in nasopharyngeal cancer via inhibiting miR-1207. Pathol Oncol Res. 2019;25(4):1411–1422. doi: 10.1007/s12253-018-0453-1. [DOI] [PubMed] [Google Scholar]
- 36.Das D.K., Ogunwobi O.O. A novel microRNA-1207-3p/FNDC1/FN1/AR regulatory pathway in prostate cancer. RNA Dis. 2017;4(1) [PMC free article] [PubMed] [Google Scholar]
- 37.Yan C., Chen Y., Kong W., et al. PVT1-derived miR-1207-5p promotes breast cancer cell growth by targeting STAT6. Cancer Sci. 2017;108(5):868–876. doi: 10.1111/cas.13212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.You L., Wang H., Yang G., et al. Gemcitabine exhibits a suppressive effect on pancreatic cancer cell growth by regulating processing of PVT1 to miR 1207. Mol Oncol. 2018;12(12):2147–2164. doi: 10.1002/1878-0261.12393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Huang M., Li T., Wang Q., et al. Silencing circPVT1 enhances radiosensitivity in non-small cell lung cancer by sponging microRNA-1208. Cancer Biomarkers. 2021;31(3):263–279. doi: 10.3233/CBM-203252. [DOI] [PubMed] [Google Scholar]
- 40.Tan J.Z., Yan Y., Wang X.X., et al. EZH2: biology, disease, and structure-based drug discovery. Acta Pharmacol Sin. 2014;35(2):161–174. doi: 10.1038/aps.2013.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kong R., Zhang E.B., Yin D.D., et al. Long noncoding RNA PVT1 indicates a poor prognosis of gastric cancer and promotes cell proliferation through epigenetically regulating p15 and p16. Mol Cancer. 2015;14:82. doi: 10.1186/s12943-015-0355-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li T., Yang J., Yang B., et al. Ketamine inhibits ovarian cancer cell growth by regulating the lncRNA-PVT1/EZH2/p57 axis. Front Genet. 2021;11 doi: 10.3389/fgene.2020.597467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yu Y., Zhang M., Liu J., et al. Long non-coding RNA PVT1 promotes cell proliferation and migration by silencing ANGPTL4 expression in cholangiocarcinoma. Mol Ther Nucleic Acids. 2018;13:503–513. doi: 10.1016/j.omtn.2018.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xu Y., Lian Y., Zhang Y., et al. The long non-coding RNA PVT1 represses ANGPTL4 transcription through binding with EZH2 in trophoblast cell. J Cell Mol Med. 2018;22(2):1272–1282. doi: 10.1111/jcmm.13405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wan L., Sun M., Liu G.J., et al. Long noncoding RNA PVT1 promotes non-small cell lung cancer cell proliferation through epigenetically regulating LATS2 expression. Mol Cancer Therapeut. 2016;15(5):1082–1094. doi: 10.1158/1535-7163.MCT-15-0707. [DOI] [PubMed] [Google Scholar]
- 46.Guo J., Hao C., Wang C., et al. Long noncoding RNA PVT1 modulates hepatocellular carcinoma cell proliferation and apoptosis by recruiting EZH2. Cancer Cell Int. 2018;18:98. doi: 10.1186/s12935-018-0582-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhou Q., Chen J., Feng J., et al. Long noncoding RNA PVT1 modulates thyroid cancer cell proliferation by recruiting EZH2 and regulating thyroid-stimulating hormone receptor (TSHR) Tumour Biol. 2016;37(3):3105–3113. doi: 10.1007/s13277-015-4149-9. [DOI] [PubMed] [Google Scholar]
- 48.Zhang S., Zhang G., Liu J. Long noncoding RNA PVT1 promotes cervical cancer progression through epigenetically silencing miR-200b. APMIS. 2016;124(8):649–658. doi: 10.1111/apm.12555. [DOI] [PubMed] [Google Scholar]
- 49.Shen C.J., Cheng Y.M., Wang C.L. LncRNA PVT1 epigenetically silences miR-195 and modulates EMT and chemoresistance in cervical cancer cells. J Drug Target. 2017;25(7):637–644. doi: 10.1080/1061186X.2017.1307379. [DOI] [PubMed] [Google Scholar]
- 50.Liu D.W., Zhang J.H., Liu F.X., et al. Silencing of long noncoding RNA PVT1 inhibits podocyte damage and apoptosis in diabetic nephropathy by upregulating FOXA1. Exp Mol Med. 2019;51(8):1–15. doi: 10.1038/s12276-019-0259-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang Y., Chen W., Lian J., et al. The lncRNA PVT1 regulates nasopharyngeal carcinoma cell proliferation via activating the KAT2A acetyltransferase and stabilizing HIF-1α. Cell Death Differ. 2020;27(2):695–710. doi: 10.1038/s41418-019-0381-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jiang B., Yang B., Wang Q., et al. lncRNA PVT1 promotes hepatitis B virus-positive liver cancer progression by disturbing histone methylation on the c-Myc promoter. Oncol Rep. 2020;43(2):718–726. doi: 10.3892/or.2019.7444. [DOI] [PubMed] [Google Scholar]
- 53.Jin L., Cai Q., Wang S., et al. Long noncoding RNA PVT1 promoted gallbladder cancer proliferation by epigenetically suppressing miR-18b-5p via DNA methylation. Cell Death Dis. 2020;11(10):871. doi: 10.1038/s41419-020-03080-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liu H.T., Fang L., Cheng Y.X., et al. LncRNA PVT1 regulates prostate cancer cell growth by inducing the methylation of miR-146a. Cancer Med. 2016;5(12):3512–3519. doi: 10.1002/cam4.900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Li Z., Tan H., Yu H., et al. DNA methylation and gene expression profiles characterize epigenetic regulation of lncRNAs in colon adenocarcinoma. J Cell Biochem. 2020;121(3):2406–2415. doi: 10.1002/jcb.29463. [DOI] [PubMed] [Google Scholar]
- 56.Ahmed M., Soares F., Xia J.H., et al. CRISPRi screens reveal a DNA methylation-mediated 3D genome dependent causal mechanism in prostate cancer. Nat Commun. 2021;12(1):1781. doi: 10.1038/s41467-021-21867-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Liu S.J., Horlbeck M.A., Cho S.W., et al. CRISPRi-based genome-scale identification of functional long noncoding RNA loci in human cells. Science. 2017;355(6320):aah7111. doi: 10.1126/science.aah7111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pyfrom S.C., Luo H., Payton J.E. PLAIDOH: a novel method for functional prediction of long non-coding RNAs identifies cancer-specific LncRNA activities. BMC Genom. 2019;20(1):137. doi: 10.1186/s12864-019-5497-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Guo L., Li J., Zeng H., et al. A combination strategy targeting enhancer plasticity exerts synergistic lethality against BETi-resistant leukemia cells. Nat Commun. 2020;11(1):740. doi: 10.1038/s41467-020-14604-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhu Y., Wu F., Gui W., et al. A positive feedback regulatory loop involving the lncRNA PVT1 and HIF-1α in pancreatic cancer. J Mol Cell Biol. 2021;13(9):676–689. doi: 10.1093/jmcb/mjab042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Alessio E., Buson L., Chemello F., et al. Single cell analysis reveals the involvement of the long non-coding RNA Pvt 1 in the modulation of muscle atrophy and mitochondrial network. Nucleic Acids Res. 2019;47(4):1653–1670. doi: 10.1093/nar/gkz007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lee J., Wu Y., Harada B.T., et al. N6 -methyladenosine modification of lncRNA Pvt 1 governs epidermal stemness. EMBO J. 2021;40(8) doi: 10.15252/embj.2020106276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Xu M.D., Wang Y., Weng W., et al. A positive feedback loop of lncRNA- PVT1 and FOXM1 facilitates gastric cancer growth and invasion. Clin Cancer Res. 2017;23(8):2071–2080. doi: 10.1158/1078-0432.CCR-16-0742. [DOI] [PubMed] [Google Scholar]
- 64.Zhang M.X., Zhang L.Z., Fu L.M., et al. Positive feedback regulation of lncRNA PVT1 and HIF2α contributes to clear cell renal cell carcinoma tumorigenesis and metastasis. Oncogene. 2021;40(37):5639–5650. doi: 10.1038/s41388-021-01971-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wang F., Yuan J.H., Wang S.B., et al. Oncofetal long noncoding RNA PVT1 promotes proliferation and stem cell-like property of hepatocellular carcinoma cells by stabilizing NOP2. Hepatology. 2014;60(4):1278–1290. doi: 10.1002/hep.27239. [DOI] [PubMed] [Google Scholar]
- 66.Deng L.T., Wang Q.L., Yu C., et al. lncRNA PVT1 modulates NLRP3-mediated pyroptosis in septic acute kidney injury by targeting miR-20a-5p. Mol Med Rep. 2021;23(4):271. doi: 10.3892/mmr.2021.11910. [DOI] [PubMed] [Google Scholar]
- 67.Cao F., Li Z., Ding W., et al. Angiotensin II-treated cardiac myocytes regulate M1 macrophage polarization via transferring exosomal PVT1. J Immunol Res. 2021;2021 doi: 10.1155/2021/1994328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chen M., Zhang R., Lu L., et al. LncRNA PVT1 accelerates malignant phenotypes of bladder cancer cells by modulating miR-194-5p/BCLAF1 axis as a ceRNA. Aging (Albany NY) 2020;12(21):22291–22312. doi: 10.18632/aging.202203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wu Q., Chen Q., Wang J., et al. Long non-coding RNA Pvt 1 modulates the pathological cardiac hypertrophy via miR-196b-mediated OSMR regulation. Cell Signal. 2021;86 doi: 10.1016/j.cellsig.2021.110077. [DOI] [PubMed] [Google Scholar]
- 70.Zhan J., Hu P., Wang Y. lncRNA PVT1 aggravates doxorubicin-induced cardiomyocyte apoptosis by targeting the miR-187-3p/AGO1 axis. Mol Cell Probes. 2020;49 doi: 10.1016/j.mcp.2019.101490. [DOI] [PubMed] [Google Scholar]
- 71.Chang Q.Q., Chen C.Y., Chen Z., et al. LncRNA PVT1 promotes proliferation and invasion through enhancing Smad 3 expression by sponging miR-140-5p in cervical cancer. Radiol Oncol. 2019;53(4):443–452. doi: 10.2478/raon-2019-0048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Xie G., Zheng X., Zheng Z., et al. The ceRNA PVT1 inhibits proliferation of ccRCC cells by sponging miR-328-3p to elevate FAM193B expression. Aging (Albany NY) 2021;13(17):21712–21728. doi: 10.18632/aging.203514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yang J., Zhao S., Tian F. SP1-mediated lncRNA PVT1 modulates the proliferation and apoptosis of lens epithelial cells in diabetic cataract via miR-214-3p/MMP2 axis. J Cell Mol Med. 2020;24(1):554–561. doi: 10.1111/jcmm.14762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhong W., Zeng J., Xue J., et al. Knockdown of lncRNA PVT1 alleviates high glucose-induced proliferation and fibrosis in human mesangial cells by miR-23b-3p/WT1 axis. Diabetol Metab Syndrome. 2020;12:33. doi: 10.1186/s13098-020-00539-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wang Y.Z., Yao-Li, Liang S.K., et al. LncPVT1 promotes cartilage degradation in diabetic OA mice by downregulating miR-146a and activating TGF-β/SMAD4 signaling. J Bone Miner Metabol. 2021;39(4):534–546. doi: 10.1007/s00774-020-01199-7. [DOI] [PubMed] [Google Scholar]
- 76.Li P.D., Hu J.L., Ma C., et al. Upregulation of the long non-coding RNA PVT1 promotes esophageal squamous cell carcinoma progression by acting as a molecular sponge of miR-203 and LASP1. Oncotarget. 2017;8(21):34164–34176. doi: 10.18632/oncotarget.15878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chen J., Yu Y., Li H., et al. Long non-coding RNA PVT1 promotes tumor progression by regulating the miR-143/HK2 axis in gallbladder cancer. Mol Cancer. 2019;18(1):33. doi: 10.1186/s12943-019-0947-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Huang T., Liu H.W., Chen J.Q., et al. The long noncoding RNA PVT1 functions as a competing endogenous RNA by sponging miR-186 in gastric cancer. Biomed Pharmacother. 2017;88:302–308. doi: 10.1016/j.biopha.2017.01.049. [DOI] [PubMed] [Google Scholar]
- 79.Fu C., Li D., Zhang X., et al. LncRNA PVT1 facilitates tumorigenesis and progression of glioma via regulation of miR-128-3p/GREM1 axis and BMP signaling pathway. Neurotherapeutics. 2018;15(4):1139–1157. doi: 10.1007/s13311-018-0649-9. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 80.Chen Z., Fan T., Zhao X., et al. Depleting SOX2 improves ischemic stroke via lncRNA PVT1/microRNA-24-3p/STAT3 axis. Mol Med. 2021;27(1):107. doi: 10.1186/s10020-021-00346-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ouyang M., Lu J., Ding Q., et al. Knockdown of long non-coding RNA PVT1 protects human AC16 cardiomyocytes from hypoxia/reoxygenation-induced apoptosis and autophagy by regulating miR-186/Beclin-1 axis. Gene. 2020;754 doi: 10.1016/j.gene.2020.144775. [DOI] [PubMed] [Google Scholar]
- 82.Zhang P., Sun H., Ji Z. Downregulating lncRNA PVT1 relieves astrocyte overactivation induced neuropathic pain through targeting miR-186-5p/CXCL13/CXCR5 axis. Neurochem Res. 2021;46(6):1457–1469. doi: 10.1007/s11064-021-03287-0. [DOI] [PubMed] [Google Scholar]
- 83.Chen W., Zhu H., Yin L., et al. lncRNA-PVT1 facilitates invasion through upregulation of MMP9 in nonsmall cell lung cancer cell. DNA Cell Biol. 2017;36(9):787–793. doi: 10.1089/dna.2017.3725. [DOI] [PubMed] [Google Scholar]
- 84.Lu X., Yu Y., Yin F., et al. Knockdown of PVT1 inhibits IL-1β-induced injury in chondrocytes by regulating miR-27b-3p/TRAF3 axis. Int Immunopharm. 2020;79 doi: 10.1016/j.intimp.2019.106052. [DOI] [PubMed] [Google Scholar]
- 85.Song J., Wu X., Liu F., et al. Long non-coding RNA PVT1 promotes glycolysis and tumor progression by regulating miR-497/HK2 axis in osteosarcoma. Biochem Biophys Res Commun. 2017;490(2):217–224. doi: 10.1016/j.bbrc.2017.06.024. [DOI] [PubMed] [Google Scholar]
- 86.Feng K., Liu Y., Xu L.J., et al. Long noncoding RNA PVT1 enhances the viability and invasion of papillary thyroid carcinoma cells by functioning as ceRNA of microRNA-30a through mediating expression of insulin like growth factor 1 receptor. Biomed Pharmacother. 2018;104:686–698. doi: 10.1016/j.biopha.2018.05.078. [DOI] [PubMed] [Google Scholar]
- 87.Qi H., Liu Y., Wang N., et al. Lentinan attenuated the PM2.5 exposure-induced inflammatory response, epithelial-mesenchymal transition and migration by inhibiting the PVT1/miR-199a-5p/caveolin 1 pathway in lung cancer. DNA Cell Biol. 2021;40(5):683–693. doi: 10.1089/dna.2020.6338. [DOI] [PubMed] [Google Scholar]
- 88.Lei F., Zhang H., Xie X. Comprehensive analysis of an lncRNA-miRNA-mRNA competing endogenous RNA network in pulpitis. PeerJ. 2019;7 doi: 10.7717/peerj.7135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wu X.Z., Cui H.P., Lv H.J., et al. Knockdown of lncRNA PVT1 inhibits retinoblastoma progression by sponging miR-488-3p. Biomed Pharmacother. 2019;112 doi: 10.1016/j.biopha.2019.108627. [DOI] [PubMed] [Google Scholar]
- 90.Wang J., Kong X., Hu H., et al. Knockdown of long non-coding RNA PVT1 induces apoptosis of fibroblast-like synoviocytes through modulating miR-543-dependent SCUBE2 in rheumatoid arthritis. J Orthop Surg Res. 2020;15(1):142. doi: 10.1186/s13018-020-01641-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Yu X., Zhe Z., Tang B., et al. α-Asarone suppresses the proliferation and migration of ASMCs through targeting the lncRNA-PVT1/miR-203a/E2F3 signal pathway in RSV-infected rats. Acta Biochim Biophys Sin. 2017;49(7):598–608. doi: 10.1093/abbs/gmx048. [DOI] [PubMed] [Google Scholar]
- 92.Xu K., Meng Z., Xian X.M., et al. LncRNA PVT1 induces chondrocyte apoptosis through upregulation of TNF-α in synoviocytes by sponging miR-211-3p. Mol Cell Probes. 2020;52 doi: 10.1016/j.mcp.2020.101560. [DOI] [PubMed] [Google Scholar]
- 93.Shaker F., Nikravesh A., Arezumand R., et al. Web-based tools for miRNA studies analysis. Comput Biol Med. 2020;127 doi: 10.1016/j.compbiomed.2020.104060. [DOI] [PubMed] [Google Scholar]
- 94.Mortazavi S.S., Bahmanpour Z., Daneshmandpour Y., et al. An updated overview and classification of bioinformatics tools for MicroRNA analysis, which one to choose? Comput Biol Med. 2021;134 doi: 10.1016/j.compbiomed.2021.104544. [DOI] [PubMed] [Google Scholar]
- 95.Ogunwobi O.O., Kumar A. Chemoresistance mediated by ceRNA networks associated with the PVT1 locus. Front Oncol. 2019;9:834. doi: 10.3389/fonc.2019.00834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Panda A.C., De S., Grammatikakis I., et al. High-purity circular RNA isolation method (RPAD) reveals vast collection of intronic circRNAs. Nucleic Acids Res. 2017;45(12):e116. doi: 10.1093/nar/gkx297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Adhikary J., Chakraborty S., Dalal S., et al. Circular PVT1: an oncogenic non-coding RNA with emerging clinical importance. J Clin Pathol. 2019;72(8):513–519. doi: 10.1136/jclinpath-2019-205891. [DOI] [PubMed] [Google Scholar]
- 98.Panda A.C., Grammatikakis I., Kim K.M., et al. Identification of senescence-associated circular RNAs (SAC-RNAs) reveals senescence suppressor CircPVT1. Nucleic Acids Res. 2017;45(7):4021–4035. doi: 10.1093/nar/gkw1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wang Y.Y., Duan H., Wang S., et al. Elevated circular RNA PVT1 promotes eutopic endometrial cell proliferation and invasion of adenomyosis via miR-145/Talin 1 axis. BioMed Res Int. 2021;2021 doi: 10.1155/2021/8868700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wang J., Huang K., Shi L., et al. CircPVT1 promoted the progression of breast cancer by regulating miR-29a-3p-mediated AGR2-HIF-1α pathway. Cancer Manag Res. 2020;12:11477–11490. doi: 10.2147/CMAR.S265579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zheng Z., Chen Z., Zhong Q., et al. CircPVT1 promotes progression in clear cell renal cell carcinoma by sponging miR-145-5p and regulating TBX15 expression. Cancer Sci. 2021;112(4):1443–1456. doi: 10.1111/cas.14814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Danac J.M.C., Garcia R.L. CircPVT1 attenuates negative regulation of NRAS by let-7 and drives cancer cells towards oncogenicity. Sci Rep. 2021;11(1):9021. doi: 10.1038/s41598-021-88539-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Sun X., Luo L., Gao Y. Circular RNA PVT1 enhances cell proliferation but inhibits apoptosis through sponging microRNA-149 in epithelial ovarian cancer. J Obstet Gynaecol Res. 2020;46(4):625–635. doi: 10.1111/jog.14190. [DOI] [PubMed] [Google Scholar]
- 104.Zhong R., Chen Z., Mo T., et al. Potential Role of circPVT1 as a proliferative factor and treatment target in esophageal carcinoma. Cancer Cell Int. 2019;19:267. doi: 10.1186/s12935-019-0985-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Liu Y.Y., Zhang L.Y., Du W.Z. Circular RNA circ-PVT1 contributes to paclitaxel resistance of gastric cancer cells through the regulation of ZEB1 expression by sponging miR-124-3p. Biosci Rep. 2019;39(12) doi: 10.1042/BSR20193045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Li Y., Shi H., Yuan J., et al. Downregulation of circular RNA circPVT1 restricts cell growth of hepatocellular carcinoma through downregulation of Sirtuin 7 via microRNA-3666. Clin Exp Pharmacol Physiol. 2020;47(7):1291–1300. doi: 10.1111/1440-1681.13273. [DOI] [PubMed] [Google Scholar]
- 107.Cao L., Zhou X., Ding X., et al. Knockdown of circ-PVT1 inhibits the progression of lung adenocarcinoma and enhances the sensitivity to cisplatin via the miR-429/FOXK1 signaling axis. Mol Med Rep. 2021;24(4):684. doi: 10.3892/mmr.2021.12323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Shi J., Lv X., Zeng L., et al. CircPVT1 promotes proliferation of lung squamous cell carcinoma by binding to miR-30d/e. J Exp Clin Cancer Res. 2021;40(1):193. doi: 10.1186/s13046-021-01976-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Luo C., Ling G.X., Lei B.F., et al. Circular RNA PVT1 silencing prevents ischemia-reperfusion injury in rat by targeting microRNA-125b and microRNA-200a. J Mol Cell Cardiol. 2021;159:80–90. doi: 10.1016/j.yjmcc.2021.05.019. [DOI] [PubMed] [Google Scholar]
- 110.Verduci L., Ferraiuolo M., Sacconi A., et al. The oncogenic role of circPVT1 in head and neck squamous cell carcinoma is mediated through the mutant p53/YAP/TEAD transcription-competent complex. Genome Biol. 2017;18(1):237. doi: 10.1186/s13059-017-1368-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Li X., Zhang Z., Jiang H., et al. Circular RNA circPVT1 promotes proliferation and invasion through sponging miR-125b and activating E2F2 signaling in non-small cell lung cancer. Cell Physiol Biochem. 2018;51(5):2324–2340. doi: 10.1159/000495876. [DOI] [PubMed] [Google Scholar]
- 112.He T., Li X., Xie D., et al. Overexpressed circPVT1 in oral squamous cell carcinoma promotes proliferation by serving as a miRNA sponge. Mol Med Rep. 2019;20(4):3509–3518. doi: 10.3892/mmr.2019.10615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Li D., Huang Y., Wang G. Circular RNA circPVT1 contributes to doxorubicin (DXR) resistance of osteosarcoma cells by regulating TRIAP1 via miR-137. BioMed Res Int. 2021;2021 doi: 10.1155/2021/7463867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Hao Y., Lu C., Zhang B., et al. CircPVT1 up-regulation attenuates steroid-induced osteonecrosis of the femoral head through regulating miR-21-5p-mediated Smad 7/TGFβ signalling pathway. J Cell Mol Med. 2021;25(10):4608–4622. doi: 10.1111/jcmm.16294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Jia Y., Gu W. Up-regulation of circPVT1 in T cell acute lymphoblastic leukemia promoted cell proliferation via miR-30e/DLL4 induced activating NOTCH signaling. Pathol Res Pract. 2021;224 doi: 10.1016/j.prp.2021.153536. [DOI] [PubMed] [Google Scholar]
- 116.Hanson R.L., Craig D.W., Millis M.P., et al. Identification of PVT1 as a candidate gene for end-stage renal disease in type 2 diabetes using a pooling-based genome-wide single nucleotide polymorphism association study. Diabetes. 2007;56(4):975–983. doi: 10.2337/db06-1072. [DOI] [PubMed] [Google Scholar]
- 117.Alwohhaib M., Alwaheeb S., Alyatama N., et al. Single nucleotide polymorphisms at erythropoietin, superoxide dismutase 1, splicing factor, arginine/serin-rich 15 and plasmacytoma variant translocation genes association with diabetic nephropathy. Saudi J Kidney Dis Transpl. 2014;25(3):577–581. doi: 10.4103/1319-2442.132190. [DOI] [PubMed] [Google Scholar]
- 118.Zhou S., Liu Y., Li M., et al. Combined effects of PVT1 and miR-146a single nucleotide polymorphism on the lung function of smokers with chronic obstructive pulmonary disease. Int J Biol Sci. 2018;14(10):1153–1162. doi: 10.7150/ijbs.25420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Dryden N.H., Broome L.R., Dudbridge F., et al. Unbiased analysis of potential targets of breast cancer susceptibility loci by Capture Hi-C. Genome Res. 2014;24(11):1854–1868. doi: 10.1101/gr.175034.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Opstal-van Winden A.W.J., de Haan H.G., Hauptmann M., et al. Genetic susceptibility to radiation-induced breast cancer after Hodgkin lymphoma. Blood. 2019;133(10):1130–1139. doi: 10.1182/blood-2018-07-862607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Zhang Z., Zhu Z., Zhang B., et al. Frequent mutation of rs13281615 and its association with PVT1 expression and cell proliferation in breast cancer. J Genet Genomics. 2014;41(4):187–195. doi: 10.1016/j.jgg.2014.03.006. [DOI] [PubMed] [Google Scholar]
- 122.Meyer K.B., Maia A.T., O'Reilly M., et al. A functional variant at a prostate cancer predisposition locus at 8q24 is associated with PVT1 expression. PLoS Genet. 2011;7(7) doi: 10.1371/journal.pgen.1002165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Haverty P.M., Hon L.S., Kaminker J.S., et al. High-resolution analysis of copy number alterations and associated expression changes in ovarian tumors. BMC Med Genom. 2009;2:21. doi: 10.1186/1755-8794-2-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kulakova O., Bashinskaya V., Kiselev I., et al. Pharmacogenetics of glatiramer acetate therapy for multiple sclerosis: the impact of genome-wide association studies identified disease risk loci. Pharmacogenomics. 2017;18(17):1563–1574. doi: 10.2217/pgs-2017-0058. [DOI] [PubMed] [Google Scholar]
- 125.Divers J., Ma L., Brown W.M., et al. Genome-wide association study for time to failure of kidney transplants from African American deceased donors. Clin Transplant. 2020;34(6) doi: 10.1111/ctr.13827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Lin H.Y., Callan C.Y., Fang Z., et al. Interactions of PVT1 and CASC11 on prostate cancer risk in African Americans. Cancer Epidemiol Biomarkers Prev. 2019;28(6):1067–1075. doi: 10.1158/1055-9965.EPI-18-1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.He R.Q., Qin M.J., Lin P., et al. Prognostic significance of LncRNA PVT1 and its potential target gene network in human cancers: a comprehensive inquiry based upon 21 cancer types and 9972 cases. Cell Physiol Biochem. 2018;46(2):591–608. doi: 10.1159/000488627. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.