Fasting induces mammalian metabolic switch from glucose to fatty acid-derived ketones, resulting in a marked change in blood glucose, triglyceride, free fatty acid and β-hydroxybutyrate levels and a phenotype of transient hepatic steatosis. However, the underlying mechanism remains incompletely understood. This study aimed to investigate the role of 3-hydroxy-3-methylglutaryl-CoA synthase 2 (HMGCS2), a rate-limiting enzyme in ketogenesis, in fasting-induced ketogenesis and hepatic lipid accumulation. Hepatocyte-specific HMGCS2 knockout (LKO) mice were generated and used to compare the difference in liver metabolic response to fasting between wild-type (WT) and LKO mice. Our findings demonstrate that HMGCS2 is essential in enhanced hepatic ketogenesis during fasting. Dysfunction of HMGCS2 leads to excessive hepatic triglyceride accumulation and severe liver injury possibly via increased liver fatty acid uptake by CD36. Therefore, liver HMGCS2 may represent a key enzyme in the maintenance of liver lipid homeostasis during fasting.
After 24 h fasting, mouse body weight was significantly decreased (Fig. S1A). However, blood β-OH levels were increased ∼2-folds (Fig. S1B). The mRNA expression of HMGCS2 was markedly upregulated in the livers of the fasting mice (Fig. S1C). Consistently, Western blot assay revealed that the protein expression of hepatic HMGCS2 was also significantly elevated in the fasting mice (Fig. S1D). To determine the role of HMGCS2 in liver lipid homeostasis during fasting, we generated a liver-specific HMGCS2 gene knockout mouse line (L-HMGCS2−/−, LKO) by crossing an HMGCS2flox/flox mouse with an albumin gene promoter-driven Cre transgenic mouse (Alb-cre) (Fig. S2A, B). As expected, the expression of hepatic HMGCS2 was almost undetectable in the LKO mice as assessed by Western blot and immunohistochemistry assays (Fig. S2C, D). Consistently, hepatic HMGCS2 mRNA expression in the LKO mice was almost undetectable (Fig. S2E). However, the levels of other enzymes involved in hepatic ketogenesis including ACAT1, HMGCL and BDH1 were not altered in the LKO mice (Fig. S2F–H). Together, these results indicate that hepatocyte-specific HMGCS2 knockout mice were successfully generated.
Under basal condition, blood β-OH levels were slightly lower in the LKO mice than those in wild-type (WT) mice. After 24-h fasting, WT mice exhibited a significant increase in blood β-OH levels. However, the LKO mice failed to develop hyperketonemia (Fig. S3A), suggesting that liver HMGCS2 is important for blood ketone levels in the fed-state, but is essential in the development of hyperketonemia during fasting. Consistently, it has been recently reported that although HMGCS2 is induced in both liver and kidney after starvation, the liver is the main ketogenic organ for circulating ketones during fasting.1 Similarly, 24-h fasting induced a significant elevation in blood triglyceride and free fatty acid levels in WT mice, which was almost completely abolished in the LKO mice (Fig. S3B, C). Little difference was found in blood triglyceride, free fatty acid, glucose and insulin levels between WT and LKO mice at the basal condition (Fig. S3B–E). Upon 24-h starvation, both genotypes exhibited a significant decrease in blood glucose levels, with slightly higher levels in the LKO mice than in WT mice (Fig. S3D). Although 24-h fasting resulted in a marked decrease in plasma insulin levels in both WT and LKO mice, but no difference was observed between two genotypes (Fig. S3E). Unlike other metabolic parameters, blood total cholesterol levels were similar between WT and LKO mice at both basal and fasting conditions (Fig. S3F). Together, these findings suggest that liver HMGCS2 deficiency mainly affects fatty acid and triglyceride metabolism with little effect on glucose and cholesterol homeostasis.
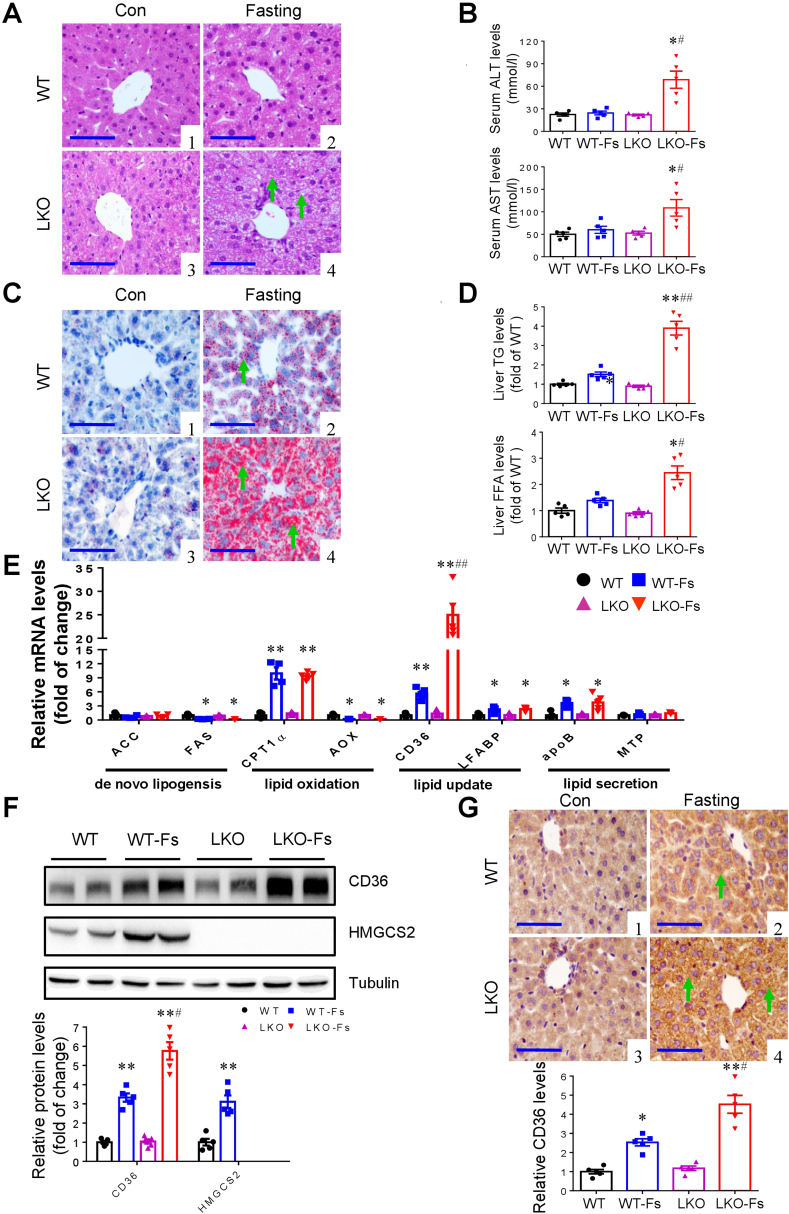
Histological examination and functional analysis showed that the LKO mice exhibited more severe liver injury and function damage than that in WT mice after 24-h fasting (Fig. 1A–C). Consistent with a previous report,2 upon 24-h fasting WT mice showed normal liver enzymatic activity and a minor fatty liver phenotype characterized by a slight increase in the numbers and sizes of lipid droplets (LDs) and the contents of neutral lipids especially triglycerides as assessed by histological examination, Oil Red O staining and biochemical analysis (Fig. 1A–D). However, compared with WT mice, 24-h fasted LKO mice showed a significant increase in hepatic triglyceride and free fatty acid contents with a dramatic elevation in serum ALT and AST levels (Fig. 1A–D). To explore the underlying mechanisms, we used real-time PCR assay to determine the mRNA expression levels of genes involved in hepatic lipogenesis (ACC and FAS), lipid β-oxidation (CPT1α and AOX), lipid secretion (apoB and MTP), and fatty acid uptake (CD36 and LFABP) (Fig. 1E). The result showed that although no difference was found in the pathways of hepatic lipogenesis, lipid β-oxidation and VLDL excretion between two genotypes, CD36 expression in the LKO mice was ∼4-fold higher than that in WT mice. Consistently, Western blot analysis and immunohistochemistry study further confirmed a marked increase in hepatic CD36 protein expression in the LKO mice compared to WT mice (Fig. 1F, G). Moreover, in vitro results showed that the starvation medium (SM) (the HBBS medium with 5.5 mM glucose and without fetal bovine serum) treatment for 24 h significantly increased the protein levels of both HMGCS2 and CD36 and lipid accumulation in the BRL cells, a hepatocyte cell line (Fig. S4A–D). However, SM-induced CD36 expression and triglyceride contents were significantly increased by siRNA-mediated HMGCS2 knockdown (Fig. S4A–D). Together, these findings demonstrate that increased fatty acid uptake via CD36 is associated with worsened lipid accumulation observed in the livers of the LKO mice subject to 24 h fasting.
Figure 1.
Liver-specific deletion of HMGCS2 worsens liver injury and hepatic lipid accumulation after 24 h fasting. (A) HE staining demonstrated significant hepatic injuries (arrows) in 24 h fasting LKO mice. Representative images: (1) WT; (2) WT-Fasting; (3) LKO; 4) LKO-Fasting (Scale bar = 100 μm). (B) The LKO mice exhibited a significant increase in serum ALT and AST levels after fasting compared with WT mice. (C) Oil Red O staining showed a significant increase in 24 h fasting-induced lipid accumulation (arrows) in the livers of the LKO mice. (D) Biochemical assays showed that hepatic TG and FFA levels were significantly higher in 24 h fasting LKO mice than those in WT mice with fasting. (E) Quantitative RT-PCR analysis of mRNA levels of genes involved in hepatic lipid metabolism. (F) Changes of the protein levels of hepatic CD36 and HMGCS2 in fasting-induced WT and LKO mice. Representative Western blot are presented. Western blot assay showed that CD36 protein expression was induced in the livers of both genotypes after fasting. However, CD36 protein levels were markedly higher in the LKO mice than those in WT mice. (G) Representative immunostaining for CD36 by immunohistochemistry: (1) WT; (2) WT-Fasting; (3) LKO; (4) LKO-Fasting. Semi-quantification of CD36 protein immunoreactivity was performed. After fasting for 24 h, hepatic CD36 expression was markedly increased, which was further increased in the LKO mice. Data are presented as mean ± SEM. ∗P < 0.05, ∗∗P < 0.01 vs. WT; #P < 0.05, ##P < 0.01 vs. WT-Fs, n = 5–6. Note: Fs means 24 h fasting. Scale bar = 100 μm.
The main finding of the present study was that the LKO mice had impaired adaptation to fasting-induced hepatic accumulation of LDs. The maladaptation to fasting-induced hepatic accumulation of LDs appears to result from enhanced hepatic uptake of free fatty acids, since hepatic expression of CD36 was markedly induced in WT mice and further increased in the LKO mice. CD36 (also known as scavenger receptor B2) is a multifunctional receptor that mediates cellular uptake of long-chain fatty acids and is ubiquitously present on the surface of many cell types.3 Therefore, induction of CD36 expression is very likely responsible for fasting-induced severe fatty liver in mice with liver-specific deletion of HMGCS2. In support, siRNA-mediated CD36 knockdown markedly decreased CD36 expression in the BRL cells (Fig. S5A, B). Moreover, CD36 knockdown abolished SM-induced lipid droplet and TG accumulation in SM-treated cells in the presence or absence of HMGCS2 siRNA treatment (Fig. S5C, D). These findings suggest that fasting-induced hepatocellular lipid accumulation is likely dependent on the upregulation of CD36 expression. However, the exact underlying mechanism by which hepatic HMGCS2 deficiency enhances liver CD36 expression remains unknown.
HMGCS2 as a well-known rate-limiting enzyme in ketogenesis may be involved in both adaptive fatty liver during fasting and non-alcoholic fatty liver disease. Knockdown of mouse liver HMGCS2 with specific antisense oligonucleotides abolished high-fat diet (HFD)-induced hyperketonemia.4 Ketogenesis-insufficient mice exhibited worsened HFD-elicited fatty liver, which can be reversed by the supplementation of the precursors of ketone bodies, suggesting that insufficient ketogenesis contributes to HFD-induced fatty liver.4 It has also been reported that neonatal mice with global HMGCS2 gene knockout developed hepatosteatosis, suggesting that neonatal ketogenesis protects the energy-producing capacity of mitochondria possibly by preventing the hyperacetylation of mitochondrial proteins.5 The present study further provides direct evidence that liver-specific HMGCS2 ablation completely abolished fasting-induced hyperketonemia and markedly worsened adaptive fatty liver during fasting. Together, these findings highlight the important role of ketogenesis in preserving mitochondrial function and in the prevention of fatty liver with various etiologies. Thus, liver HMGCS2 might represent a potential therapeutic target for the treatment of fatty liver.
In summary, we found that hepatic specific deletion of HMGCS2 completely abolished fasting-induced hyperketonemia and significantly attenuated fasting-induced elevation of blood triglyceride and free fatty acid levels. Liver-specific ablation of HMGCS2 markedly worsened fasting-elicited hepatic lipid accumulation and liver injury. The underlying mechanism may be due to upregulated expression of hepatic fatty acid translocase CD36. Together, our data demonstrate that hepatic HMGCS2-mediated ketogenesis is critical in maintaining liver fatty acid and triglyceride homeostasis during nutrient deprivation or fasting. Modulation of HMGCS2 activity may represent a potential therapeutic strategy for the treatment of fatty liver.
Author contributions
Y. Z. and Y. G. designed the study. Y. Z., T. G., L. Z., H. L., and Y. C. performed experiments; Y. Z., Z. L., W. J., and S. H. analyzed the data. Y. Z. drafted the manuscript. B. L., and X. Z. performed the critical review. Y. Z., and Y. G. edited the manuscript. All authors approved the final version of the manuscript.
Conflict of interests
Authors declare no conflict of interests.
Funding
This work was supported by the National Natural Science Foundation of China (No. 81970606, 81970595, and 81970636); the Guangdong Medical Research Foundation (No. A2020260), China; the Shenzhen Basic Research Project (No. JCYJ20210324095005015), China; and the SZU Top Ranking Project of China (No. 86000000210).
Footnotes
Peer review under responsibility of Chongqing Medical University.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.gendis.2022.08.004.
Contributor Information
Yunfeng Zhou, Email: zhouyf1980@szu.edu.cn.
Youfei Guan, Email: guanyf@dmu.edu.cn.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Venable A.H., Lee L.E., Feola K., et al. Fasting-induced HMGCS2 expression in the kidney does not contribute to circulating ketones. Am J Physiol Ren Physiol. 2022;322(4):F460–F467. doi: 10.1152/ajprenal.00447.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li Y., Chao X., Yang L., et al. Impaired fasting-induced adaptive lipid droplet biogenesis in liver-specific Atg5-deficient mouse liver is mediated by persistent nuclear factor-like 2 activation. Am J Pathol. 2018;188(8):1833–1846. doi: 10.1016/j.ajpath.2018.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yang X., Okamura D.M., Lu X., et al. CD36 in chronic kidney disease: novel insights and therapeutic opportunities. Nat Rev Nephrol. 2017;13(12):769–781. doi: 10.1038/nrneph.2017.126. [DOI] [PubMed] [Google Scholar]
- 4.Cotter D.G., Ercal B., Huang X., et al. Ketogenesis prevents diet-induced fatty liver injury and hyperglycemia. J Clin Invest. 2014;124(12):5175–5190. doi: 10.1172/JCI76388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arima Y., Nakagawa Y., Takeo T., et al. Murine neonatal ketogenesis preserves mitochondrial energetics by preventing protein hyperacetylation. Nat Metab. 2021;3(2):196–210. doi: 10.1038/s42255-021-00342-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.