Abstract
The endometrium is the inner mucosal lining of the uterus that undergoes extensive cyclic growth, regeneration, differentiation, and shedding throughout the menstrual cycle in response to steroid hormones. It repeatedly undergoes approximately 450 cycles of degeneration and regeneration in a woman's lifetime. Endometrial abnormalities can be associated with repeated embryo implantation failure, recurrent spontaneous abortion, and other physiological features responsible for female infertility. This significant regenerative capacity may occur as a result of tissue-resident stem cell populations within the endometrium. Indeed, the existence of endometrial stem cells was only observed in humans and rodents through several isolation and characterization methods in the last few years. Although endometrial stem cells share various biological characteristics with other types of mesenchymal stem cells, they also show some differences in phenotype, self-renewal, and multilineage differentiation potential. Extensive studies over many years on endometrial stem cells will provide new insights into the physiology and mechanisms underlying various gynaecological diseases related to endometrial abnormalities such as female infertility, endometriosis, and endometrial cancer. Here we summarized recent studies about cellular origins and biological characteristics of endometrial stem cells. We also reviewed various recent studies to improve our understanding of their physiological roles. Many preclinical studies on their potential therapeutic applications to various endometrial diseases that could lead to reproductive dysfunction were also reviewed.
Keywords: CD146, LGR5, OCT-4, Side population, SOX9, SSEA-1, SUSD2, Wnt/β-catenin
Introduction
The endometrium is a unique and dynamic tissue that undergoes regeneration, differentiation, and shedding of the functional layer under the control of ovarian hormones, estrogen, and progesterone, during each menstrual cycle.1,2 The deeper endometrium basalis adjacent to the myometrium is not shed during menstruation. The functional endometrial layer is cyclically regenerated from this basalis.3 This remarkable regenerative capacity of endometrium is crucial for endometrial receptivity and successful embryo implantation. Endometrial abnormality can increase the risk of repeated implantation failure, spontaneous miscarriage, and other pathologies known to cause female infertility.4,5
Tissue-specific resident stem cells have notably been identified and isolated from various adult tissues such as stomach, intestine, and liver. They are essential for tissue regeneration and maintenance following injury.6,7 Although mechanisms regulating this remarkable regenerative capacity remain largely unclear, pluripotent tissue-resident endometrial stem cell populations in a dormant state are likely to play an important role.8, 9, 10 Several studies have shown that some patients who have undergone an endometrial ablation could later fully recover the endometrial functional layer, further supporting the presence of endometrial stem cells.11 Padykula et al have later provided the possibility that tissue-resident stem cells exist within the uterine endometrium, although their evidence is indirect.12,13 Since the first two studies revealing the existence of pluripotent tissue-resident stem cell populations in human endometrial tissues in 2004 by Chan et al,14,15 many researchers have extensively investigated biological features, characteristics, significant multilineage differentiation potential, and therapeutic capacities of endometrial stem cells for repairing endometrial injury.16 Currently, endometrial stem cell abnormalities are thought to be associated with the development of various uterine diseases such as female infertility, endometriosis, and endometrial cancers.16 Selectively identifying endometrial stem cells is problematic due to the lack of clearly defined surface markers for investigating their biological features.17 In addition, standardized isolation procedures and culture conditions for endometrial stem cells remain unclear. Further studies are required to optimize their therapeutic effects for clinical application.18
Currently, the exact origin of endometrial stem cells is highly controversial. Interestingly, endometrial stem cells are thought to be originated not only locally from the deeper endometrium basalis, but also from circulating bone marrow-derived stem cells.19 Elucidating the exact origin of endometrial stem cells is a very important research topic not only for identifying causes of various uterine-related diseases, but also for using endometrial stem cells for treating various degenerative diseases. Indeed, endometrial stem cells are promising autologous and allogeneic sources of adult stem cells due to their relatively high differentiation capacity (pluripotency), easy accessibility, abundance (high yield), and ethical (regulatory) aspects.19 In this context, endometrial stem cells can be applied for the repair and regeneration of various tissues outside of the the reproductive organs.20,21 In this review, we summarized a number of recent studies to increase our knowledge and understanding of biological features and characteristics of endometrial stem cells. We also described available information about some surface markers to specifically define endometrial stem cells within uterine tissues and summarized various cell-based therapeutic strategies and several diseases associated with endometrial stem cells.
Dynamic characteristics of uterine endometrium
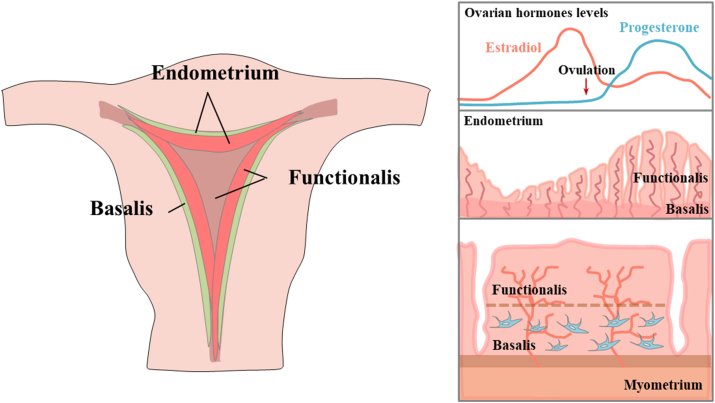
During women's reproductive lifespan, the uterine endometrium (mucosal lining) repeatedly undergoes about 400–500 uterine menstrual cycles of extensive growth, differentiation (decidualization), shedding, and regeneration under the precise control of ovarian steroid hormones, estrogen and progesterone.2 Structurally, the endometrium is a complex tissue consisting of simple columnar epithelium and the endometrial stroma.17 The endometrial epithelium, which extends tubular endometrial glands into the stromal area, is composed of glandular epithelia (GE) and lumina (LE).22 The endometrial stroma is a connective tissue layer that significantly varies in thickness in response to steroid hormonal influences. The endometrium can be divided into two general layers, the functionalis (the upper layer of the endometrium) and the basalis (adjacent to the myometrium) known to experience a significant amount of cell turnover during tissue homeostasis.23,24 The functional layer adjacent to the uterine cavity undergoes dynamic cyclic changes of growth, decidualization, and shedding during each menstrual cycle.25 This layer provides an optimal environment to promote receptivity for implantation, growth, and development of an embryo. The basal layer adjacent to the myometrium and below the functional layer is not shed during the menstrual cycle due to its strong proliferation and repair abilities.3 After the menstrual phase, the functional layer grows from the remaining thin basal layer with a thickness of about 0.5 mm to approximately 8 mm in thickness during the mid-proliferative phase in response to estrogen.26 The proliferation of endometrial epithelial cells is significantly increases (approximately 9-fold) during an early estrous cycle in mice.27 This remarkable regenerative capacity of endometrium is likely mediated by tissue-resident stem cells located in the basal layer, which is not shed during the menstrual cycle14,28 (Fig. 1).
Figure 1.
Schematic diagram of the dynamic endometrial tissue and the location of endometrial stem cells. Endometrium repeatedly undergoes approximately 450 cycles of degeneration and regeneration in ovarian hormones, estrogen and progesterone. The functional endometrial layer is cyclically regenerated from the basalis, which is not shed during menstruation. This cyclic regeneration of endometrium may is mediated by tissue-resident endometrial stem cells located in the basal layer.
Identification of endometrial stem cells
The existence of local pluripotent stem cells within the endometrial tissue due to its amazing regenerative capacity was first suggested by Prianishnikov in 1978.29 Since then, many studies about endometrial stem cells have been reported over the past decade. Clonogenic small human endometrial stem cell populations were identified and characterized for the first time by Chan et al in 2004.14,15 Using purified single-cell populations of two endometrial cell types obtained from women undergoing hysterectomy, they observed that approximately 0.2% of epithelial cell subpopulations and about 1.2% of stromal cell subpopulations generated individual colonies within 15 days after seeding for liquid subculture.14 Their in vitro multilineage differentiation capacities into different cell types have also been assessed. Wolff et al have revealed the existence of pluripotent stromal-like stem cell subpopulation in the endometrium by inducing chondrogenic differentiation in vitro.30 Purifying cell subpopulation with the side population (SP) phenotype is another effective strategy used by many researchers to identify stem-like cell subpopulation from the endometrial tissue. These subsets of cells are characterized by elevated multidrug resistance (MDR)-efflux capability for antimitotic drugs with high levels of ATP-binding cassette transporter protein.31 These human endometrial cell subpopulations with side population phenotype exhibit long-lasting clonogenic potential as well as multilineage differentiation capacity into other endometrial cell types, such as stromal, epithelial, and endothelial cells in vitro.32,33 Currently, it is known that two types of local stem cells exist in human endometrial tissues: epithelial and stromal cell types. Any abnormalities of these tissue-resident stem cells could be the cause of endometrial dysfunction that subsequently leads to infertility.34 However, identifying and isolating epithelial types of human endometrial stem cells are very difficult and challenging due to the lack of certain morphological features and specific surface markers that can be used to definitively isolate and characterize them.35 Endometrial epithelial stem cells are extremely difficult to isolate and culture in vitro for a long time period due to their limited proliferation and functional capacity in traditional 2D culture conditions. Therefore, most of current studies related to endometrial stem cells are focused on stromal types of endometrial stem cells, which are relatively easy to obtain and culture in vitro with traditional 2D culture conditions. For these reasons, most of developed experimental techniques to analyze various characteristics and functions of human endometrial stem cells in vitro are specialized for stromal types of stem cells. In fact, the first endometrial stem cells isolated and characterized by Chan et al in 2004 with specific markers for identification also had the stromal type.14
The origin of endometrial stem cells
Endogenous origin
The remarkable cyclic regenerative capacity of human endometrium during each menstrual cycle supports the hypothesis that endometrial stem cell niche might exist within a specific area of the endometrial tissue that is activated during each menstrual cycle in response to steroid hormones without shedding.29,36 Like any other dynamically regenerating tissues, the endometrium contains a small stem cell population with high clonogenic activity and multilineage differentiation capacity. Recently, many investigators have made efforts to identify tissue-resident stem cells in uterine tissues by analyzing expression patterns of known various adult stem cell markers such as Stro-1,37 CD90,37 CD133,37 CD146,38 and SUSD2.39 Stromal-type endometrial stem cells are found around the luminal and glandular epithelia within both functional and basal endometrial layers, suggesting that although some stem cell subpopulations located in the functional layer are shed during a menstrual period, certain subpopulations located in the basal layer would remain.38, 39, 40 Recently, many researchers have performed experiments to identify and characterize stromal-like endogenous stem cells within the endometrium using multiple genes such as ectonucleoside triphosphate diphosphohydrolase-2 (NTPDase2),41 leucine-rich repeat containing G-protein-coupled receptor 5 (LGR5),42 platelet-derived growth factor receptor beta (PDGFR-ß)/CD146,39 side population (SP),1,43 and sushi domain-containing-2 (SUSD2)44 known to be involved in regulating various biological features and functions of stem cells in other tissues. Although some studies have suggested that epithelial-like endogenous stem cells might have derived from stromal-like endometrial stem cells,45,46 they are known to exist mainly at the base of glands or blood vessels located in the basal endometrial layer.47,48 In addition, epithelial endometrial stem cells have been isolated from epithelial SP fractions generated under hypoxic conditions. They can express undifferentiated cell markers (OCT-4, GDF3, DNMT3B, NANOG, and GABR3) and generate vessel-like tissue structures when transplanted beneath the renal capsule in NOD-SCID mice.1,49 Nguyen et al have also observed subpopulations of immature N-cadherin+/SSEA-1− epithelial endometrial stem cells with high clonogenic potential and differentiation capacity at the basal area of glands adjacent to the myometrium48 (Fig. 2). Schwab et al have observed that clonogenic activities of human endometrial epithelial and stromal cells are not different between proliferative and secretory stages of a menstrual cycle.50 Currently, several growth factors required to effectively increase colony formation of both endometrial stem cell types in vitro have been reported. The combination of epidermal growth factor (EGF), platelet-derived growth factor-BB (PDGF-BB), and transforming growth factor alpha (TGF-α) with fibroblast feeder layers strongly supports the clonogenicity of epithelial-type endometrial stem cells in a serum-free condition,14,50 suggesting the significance of epithelial-stromal cell interactions in clonogenic activities of endometrial stem cells. In addition, EGF, PDGF-BB, and TGF-α with fibroblast growth factor 2 (FGF2)-containing serum-free culture condition is also required to have an effective colony formation of stromal-like endometrial stem cells in vitro.14,50
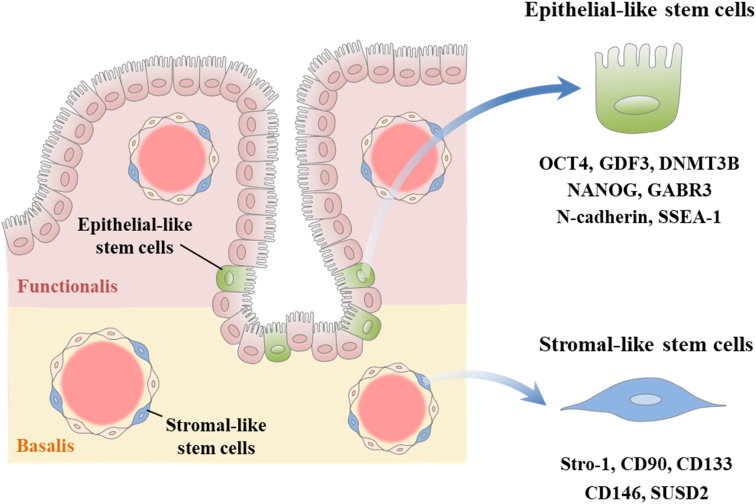
Figure 2.
Schematic diagram of two hypotheses about the endogenous origin of human endometrial stem cells. Stromal-type endometrial stem cells are found around the luminal and glandular epithelia within both functional and basal endometrial layers. Stromal-like stem cells are known to express multiple markers such as CD90, CD133, CD146, LGR5, NTPDase2, Stro-1, and SUSD2. Epithelial-like stem cells are known to exist mainly at the base of glands or blood vessels located in the basal endometrial layer and express various specific genes such as DNMT3B, GDF3, GABR3, NANOG, N-cadherin, OCT-4, and SSEA-1.
Exogenous origin
Interestingly, it has been suggested that circulating bone marrow-derived stem cells, albeit a small number of cells, can be colonized in various tissues.51 In this context, although the vast majority of endometrial stem cells are thought to be endogenously derived from the endometrial tissue itself, it is quite reasonable to assume that some subpopulation might be exogenously derived from circulating bone marrow-derived stem cells. Indeed, bone marrow-derived stem cells have been identified in human endometrial tissues in many studies. Taylor and colleagues have detected donor-derived endometrial cells in human endometrial tissue biopsy samples obtained from all bone marrow stem cells recipients. These cells accounted for up to approximate 52% of endometrial stromal cell populations and 48% of epithelial cell populations, suggesting that bone marrow-derived stem cells can be an exogenous origin of endometrial cells.15 Consistently, several other studies have also observed the existence of bone-marrow derived cells in human endometrium obtained from patients who have received an allogeneic bone marrow stem cell transplant,52, 53, 54 although the number of cells found is quite small. Similarly, Mints et al have found that an average 14% of endometrial endothelial cells within the endometrial vasculature in a bone marrow transplanted patient are exogenously originated from donor-derived bone marrow cells.52 They also detected 6% average of donor-derived endothelial cells at 40 days after bone marrow cell transplant in samples from female mice.52 However, whether endometrial tissue regularly recruits bone marrow-derived stem cells, which can differentiate into various endometrial cell types, under normal physiological conditions or only unregularly occurs under certain disease conditions such endometrial injury is currently unclear. Moreover, it is not yet known whether a local endometrial tissue injury can sufficiently recruit bone marrow-derived stem cells into the damaged site during the menstrual cycle. Various steroid hormones and growth factors have been reported to regulate the translocation of bone-marrow derived stem cells to the endometrial tissue. Indeed, enhanced estrogen secretion is known to stimulate the translocation of circulating endothelial progenitor cells into endometrial blood vessels during an early menstrual cycle.55 Various pathological damage stimuli such as inflammation, ischemia, and reperfusion injury can also stimulate this translocation.56,57 Similarly, estrogen-induced secretions of CXCL12 and its receptor CXCR4 from endometrial stromal cells have been shown to be able to increase migratory capacity of bone marrow-derived cells in vitro,58 while treatment with CXCR4 antagonist, ADM3100, can significantly block bone-marrow derived stem cell recruitment to the injured endometrium in an Asherman rodent model,59 indicating that the CXCL12/CXCR4 signaling cascade plays a key role in the chemotactic migration of bone-marrow derived stem cells to the injured site of the endometrium (Fig. 3). Tal et al have observed that circulating bone marrow-derived cells recruited to the endometrium can subsequently differentiate into stromal decidual cells, which are essential for embryo implantation. They contribute functionally to successful pregnancy in a HOXA11-dependent manner.60 Although multiple biomarkers have been identified to distinguish bone-marrow derived stem cells, there are currently no known genes or biological features that could clearly characterize them from endogenous endometrial stem cells. At least three different types of stem cells (hematopoietic stem cells, mesenchymal stem cells, and endothelial progenitor cells) exist in adult bone marrow. All of them are circulating. Although the hypothesis that the function and shape may differ depending on whether the cells are derived from bone marrow stem cells or cells that are endometrium is convincing enough, but such a hypothesis has not been proven so far. Currently, there are no technical methodology or previous results to distinguish functional and phenotypic characteristics of endometrial stem/progenitor cells according to their origin. Although we do not have enough information on this issue, the hypothesis that the function and morphology of endometrial stem cells may differ according to their origin is convincing enough. In addition, when we analyze endometrial stem cells using recently developed single cell RNA sequencing technique that can differentially analyze the characteristics of certain cell type according to their specific origin, we maybe possibly answer these questions in the future.
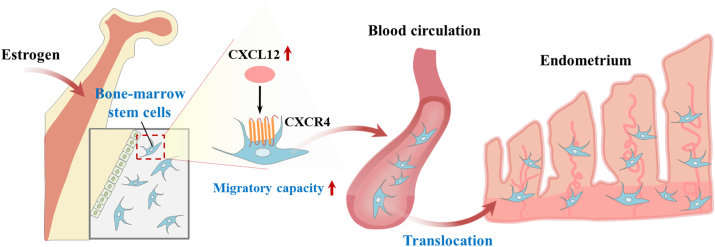
Figure 3.
Schematic diagram of two hypotheses about the exogenous origin (bone marrow-derived stem cells) of human endometrial stem cells. Enhanced estrogen secretion is known to stimulate the translocation of circulating endothelial progenitor cells into endometrial blood vessels through CXCL12/CXCR4 signaling cascade.
Characteristics of endometrial stem cells
Endometrial stem cells have similar biological properties to stromal-like mesenchymal stem cells due to their phenotypes, self-renewing capacity, transdifferentiation potential, and expression patterns of various cell surface antigens.61 Almost all currently available information about characteristics of endometrial stem cells is predominantly obtained from studies on stromal-like endometrial stem cell subpopulations. Although intensive investigations into biological features of endometrial stem cells have been carried out by a number of researchers, their specific surface biomarkers have not yet been completely characterized.20 Currently, studies on biomarkers of endometrial stem cells have been focused on certain genes known to be associated with some critical functions of other stem cell types, such as self-renewal capacity, pluripotency, and multilineage differentiation potential (summarized in Fig. 4). Although endometrial stem cell specific cell surface marker may exclusively distinguish them from other adult stem cells obtained from other tissues such as adipose tissue, umbilical cord blood, or bone marrow, endometrial stem cell specific markers are currently not available. Unfortunately, none of genes or signaling pathways described as endometrial stem cell markers are exclusively specific to endometrial tissue and there is significant overlap with other somatic stem cell types and even with various types of cancer stem cells. In addition, it is not clear how many of the cell surface markers and/or signaling pathways associated with fully differentiated endometrial cells are also present on their corresponding stem cells. For this reason, there is need to find more specific marker or combination of markers to better isolate specific endometrial stem cells from various heterogeneous differentiated tissue constituting cells. We reasoned that a clue to identify potential endometrial stem cell-specific markers can be obtained by extensively investigating certain genes or signaling pathways that are significantly reduced or completely disappeared during their differentiation or cellular aging using large-scale genetic analysis.
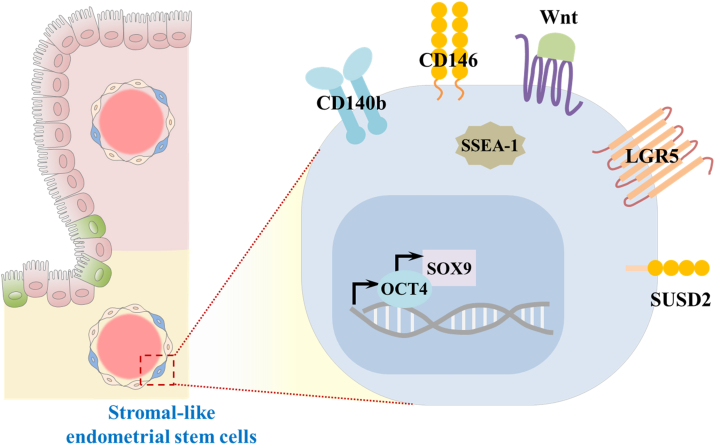
Figure 4.
Schematic diagram of the characteristics of endometrial stem cells. Current studies on biomarkers of endometrial stem cells have been focused on certain genes known to be associated with some critical functions of other stem cell types, such as self-renewal capacity, pluripotency, and multilineage differentiation potential.
140b/CD146 co-expression
140b is a common surface antigen for pericytes and pericyte-like cell subpopulations62 and CD146 is an endothelial and perivascular cell marker originally used to identify and isolate mesenchymal stem cells in bone marrow and dental pulp.63 Crisan et al have used the combination of these surface antigens to identify multipotent stem/progenitor cells in various human tissues.40 This combination can specifically identify the perivascular location of tissue-resident stem cells exhibiting multilineage differentiation capacity in multiple organs.38,40 Schwab et al have found that a small population of MSC-like cells co-expressing these two perivascular cell markers are located perivascularly in human endometrium with stromal cell like phenotypes and that these cells exhibit multilineage differentiation capacities into adipocytes, osteoblasts, and chondrocytes.38 In addition, double positive cell populations of these phenotypic surface markers located near blood vessels exhibit significantly more increased colony-forming capabilities than double negative cell populations.38
N-cadherin
To identify endometrial stem/progenitor cell-specific markers, Nguyen et al compared large scale gene profiling of isolated human endometrial cells from pre-menopausal endometrial tissues and post-menopausal tissues. N-cadherin is a member of the transmembrane-spanning adhesion molecule family which extracellularly mediate calcium-dependent cell–cell adhesion.64 Of the various cell surface molecules changed in post-menopausal endometrial cells, they observed that cell adhesion molecule N-cadherin expression was the most consistently increased.48 Indeed, N-cadherin-positive endometrial cell subpopulations were more clonogenic than the corresponding negative subpopulations, showed greater proliferative capacity and more population doubling levels (PDLs) by serial in vitro passaging.48 Before, N-cadherin expression is known to be significantly correlated with metastasis and progression of endometrioid adenocarcinoma.65 In addition, Poncelet et al also revealed that infertile females with hydrosalpinx showed markedly reduced N-cadherin expression levels in their endometrial tissues compared with healthy control samples.66 Similarly, Liu et al also observed that enhanced expressions of N-cadherin may stimulate the transdifferentiation capacity of endometrial stem cells into glial-like neural cells.67
Musashi-1
The RNA-binding protein Musashi-1 is highly expressed in neural stem/progenitor cells and regulates their various pluripotency-associated functions.68 The number of Musashi-1-positive cells was significantly increased (four-fold) in proliferative endometrium compared to secretory endometrium.69 In addition, Musashi-1 positive cells were mainly located in the stroma region of normal endometrium and the number of Musashi-1 positive cells decreased significantly with increasing gestational age in fetal endometrium.70 Chen et al found that the expression levels of Musashi-1 were significantly higher in endometrial epithelial cells during proliferative phase than the secretory phase of menstrual cycle.71 Consistently, Strauβ et al observed that Musashi-1/2-double-knockdown significantly increased apoptotic cell death and necrosis as well as markedly reduced expressions of various pluripotency-associated genes, self-renewal capacity, and sphere forming ability in both immortalized and primary endometriotic stroma cells.72
LGR5
Leucine-rich repeat containing G-protein-coupled receptor-5 (LGR5, also known as Gpr49) belongs to a family of glycoprotein hormone receptors. It is highly expressed and plays an essential role in multiple types of stem cells, such as hair follicles, small intestine, and stomach by regulating Wnt/β-catenin signaling pathways.73, 74, 75, 76 Lineage tracing experiments have revealed that Lgr5 positive subpopulations mainly consist multipotent stem cells that can differentiate into all cell types in intestinal and colonic epithelia.73 In addition, LGR5 is highly expressed in the female reproductive tract during development. LGR5 positive subpopulations within rodent ovary and the oviduct display long-term stem cell properties ex vivo and in vivo.77,78 LGR5 is also highly expressed in the endometrial epithelium of immature (prepubertal) mouse and in adult endometrial tissues when steroid hormones are deprived.42 However, its expression in endometrium is significantly enhanced in response to ovariectomy but reduced by exogenous administration of steroid hormones estrogen and progesterone.42 Seishima et al have also observed that LGR5 is widely expressed in the müllerian duct during embryonic development and that its expression is enriched in postnatal endometrial glands.79 They also showed that Lgr5 positive endometrial cells were Wnt/β-catenin signaling dependent stem cells that could differentiate into various cell types of endometrial glandular epithelium.79 Xu et al have observed that LGR5 is highly expressed in the perivascular area of the endometrial stromal region where CD140b/CD146 double positive cells are present.80 They also found that both mRNA and protein levels of LGR5 were higher in endometrial stem cells than in unfractionated stromal cells.80
OCT-4
OCT-4 (also known as Oct-3), an undifferentiated phenotype associated antigen, is a homeodomain transcription factor of the Pit-Oct-Unc (POU) family involved in early embryonic development and various cell differentiation.81 Previous studies have revealed that Oct-4 can regulate the self-renewal ability and pluripotency of pluripotent stem cells82 and that it is extensively expressed in embryos at early developmental stages (germ cells83 and embryonic stem cells84) and germ cell tumors.85 At maturity, it is almost exclusively expressed in pluripotent stem cells. Reduced expression of OCT-4 is clearly associated with loss of multilineage differentiation potential of these cells as a master switch during differentiation.86 OCT-4 double-knockout can cause early embryonic lethality in mice due to absence of an inner cell mass of blastocyst.82 Their decreased expression can lead to growth arrest of embryonic stem cells by inhibiting cell-cycle progression in G0/G1 stage.87 Matthai et al have immunohistochemically detected OCT-4 expression mainly in the human endometrial stromal cells from 42% of healthy women and 44% of fibroids uterine or endometriosis patients, although its expression levels are different among samples.88 Salama et al could not find any correlation between the degree of endometriosis and the expression level of OCT-4 in endometrial stem cells.89 Contrary, Shariati et al have observed that mRNA levels of OCT-4 are significantly increased in ectopic endometrial lesions than in normal endometrial tissues.90 Although many investigations have been conducted, the correlation between OCT-4 and endometrial stem cells has not been fully established yet.
Side population (SP)
Side population analysis has been widely used to distinguish undifferentiated stem cell subpopulations from more differentiated cells based on DNA-binding fluorescent dyes (such as Hoechst 33342) efflux properties.91,92 Goodell et al have discovered an SP positive fraction with phenotypic markers of multipotential HSCs having a long-term reconstitution capacity in recipient mice.93,94 These subpopulations can also effectively protect mice from lethal irradiation at low cell doses. They can be categorized into two distinct lineages: myeloid and lymphoid. Kato et al have found SP positive fraction among normal human endometrial cells for the first time. The SP endometrial cell proportion varies widely (0.00–5.11%).32 Most SP cells exhibiting stem cell-like phenotypes show long-term repopulating properties. They could differentiate into gland (CD9+) and stroma (CD13+)-like cells.32 Similarly, SP positive endometrial cell fractions, but not non-SP, exhibit adipogenic and osteogenic differentiation capacities in vitro with reconstruction potential of human endometrial-like tissue in vivo.1
SOX9
Transcription factor SOX9 is known as a marker of stem/progenitor activity in other tissues and a primary downstream target of Wnt/β-catenin signaling pathway. It is best known for its ability to regulate chondrocyte lineage commitment17 and in development of neural crest,95 male gonads,96 and spinal cord.97 Saegusa et al have found that SOX9 expression significantly increased in a stepwise trend according to the grade of endometrial carcinomas.98 They also observed that its expression in normal endometrial tissue was remarkably higher in the proliferative phase than in the secretory phase during a menstrual cycle.98 SOX9 has been detected in the basal endometrial layer, with women having endometriosis showing an increased number of SOX9/SSEA-1 double positive cells in the functional endometrial layer during the secretory stage of the menstrual cycle.99 After purification, these double positive cells can differentiate into large endometriotic lesion-like tissues, suggesting they contribute to ectopic endometriotic lesion formation.99
SSEA-1
SSEA-1 (stage specific embryonic antigen 1), a cell surface antigen defined as Lewis X carbohydrate structure, is highly expressed during early development,100 embryonic stem cells,101 and teratocarcinoma stem cells.102 This antigen is a useful marker for identifying undifferentiated embryonic stem cells as its expression is gradually decreased during stem cell differentiation.103 Valentijn et al have isolated SSEA-1 positive endometrial epithelial cell subpopulations from the basal endometrial layer.47 SSEA-1 positive endometrial cells exhibit significantly enhanced telomerase activity, longer telomere lengths, and a less-differentiated phenotype.47 These cells also generate a significantly greater number of gland-like spheres in 3D culture than SSEA-1 negative epithelial cells, which predominate in the endometrial functional layer.47 More recent work has described that some SSEA1/LGR5 double positive endometrial cells are also present in the luminal epithelium.104 Thus, the location of SSEA1 positive cells is not strictly limited to the endometrial basal layer.
SUSD2
Masuda et al have found that Sushi Domain-containing 2 (SUSD2, also known as W5C5) is a novel biomarker to identify endometrial stem cells. SUSD2 positive subpopulations comprise approximately 4% of total endometrial stromal cells. They are mainly localized in perivascular regions in both functional and basal endometrial layers.39 The self-renewal capacity of SUSD2 positive populations is significantly higher than that of SUSD2 negative cells. SUSD2 positive cells also have multilineage differentiation potential into other cell types such as adipocytes, chondrocytes, endothelial cells, myocytes, and osteocytes.39 They have also revealed that transplanted SUSD2 positive endometrial cells can reconstitute endometrial stromal tissue in vivo.39 Murakami et al have isolated SUSD2 positive and SUSD2 negative subpopulations from mid-luteal endometrial biopsies and then investigated the abundance and clonogenic efficiency of these two cell populations in relation to Body Mass Index. They observed that the clonogenic efficiency of SUSD2 positive populations was significantly lower in obese subjects than in subjects with normal Body Mass Index.17
Notch signaling
The family of Notch proteins are ligand-dependent single-pass transmembrane receptors that play a key roles in many diverse stem cell functions and their fate decisions.105,106 Ables et al observed that significantly fewer neural stem cells and resulted in generation of fewer granule neurons in Notch1 knockdown mice, suggesting the importance roles of Notch1 in maintaining a reservoir of undifferentiated neural stem and progenitor cells.107 Aberrant activation of Notch1 signaling in the endometrium results in complete infertility as a consequence of various endometrium associated developmental and physiological abnormalities, such as the significantly reduced endometrial glands and dysregulation of steroid hormone signaling.108 Notch signaling activities were significantly reduced in endometrial stromal cells isolated from the patients with endometriosis, which was closely related to the impaired decidualization.109 The expression levels of Notch1were significantly higher in endometrial tissues during proliferative phase than the secretory phase of menstrual cycle.110,111 Spitzer et al found that Notch signaling activities were significantly increased in endometrial stem cells compared with differentiated stromal fibroblasts.112 Zhang et al also observed that hypoxic microenvironment during endometrial breakdown and early regeneration activated Notch signaling in endometrial stem cells, leading to markedly increased their self-renewal capacities.113 Notch-1 expression levels in the luminal epithelium was significantly higher in eutopic endometrium of endometriosis patients,114 suggesting the endometrial stem cell hypothesis of endometriosis development.
Wnt/β-catenin signaling
Wnt/β-catenin signaling pathways are widely known to be associated with the regulation of self-renewal capacity and multilineage differentiation potential in multiple types of stem cells.115,116 Indeed, exogenous Wnt ligand Wnt3a exposure significantly increased the self-renewal ability and suppressed apoptotic cell death, but inhibited osteogenic differentiation of mesenchymal stem cells.117 Wnt/β-catenin signaling associated genes (Wnt genes and Fzd receptors) play key roles in endometrial development in response to ovarian steroid hormones. They are also involved in endometrial decidualization and subsequent embryo implantation.118 Kiewisz et al have found that WNT4 is expressed in the basal lamina and glandular/luminal epithelium of porcine endometrial tissues.119 WNT5A is only expressed in the luminal epithelium. WNT7A and β-catenin are also expressed in the glandular and luminal epithelium in response to steroid hormones during the periimplantation period.119 Nguyen et al have observed differential expression patterns of Wnt/β-catenin-associated genes (axin-related protein 2 and β-catenin) between pre- and postmenopausal endometrial cells in both basal and functional layers,120 suggesting that putative endometrial stem cells may reside in the basal layer of premenopausal endometrium and that this signaling pathway is involved in the regulation of these stem cells. Bukowska et al have found that activation of the Wnt/β-catenin signaling is significantly reduced by GSK-3 inhibitor, whereas inhibition of the signaling with XAV939 can enhance levels of various stem cell markers such as CD29, CD73, CD90, and CD105 in endometrial stromal cells.121 They also revealed that Wnt pathway activator BIO could significantly elevate the clonogenicity of endometrial stromal cells.121
Endometrial stem cell associated diseases
Since various aspects of gynaecological pathology are closely associated with abnormal growth of endometrial cells, it is fairly reasonable to postulate that tissue-resident endometrial stem cells may play a regulatory role in the development of gynaecological diseases such as infertility, endometriosis, and endometrial cancer.122,123
Infertility
Recurrent implantation failure (RIF) is defined by pregnancy failure after repeated embryo transfers. Approximately 10% of women undergoing in vitro fertilization (IVF) treatment often experience RIF due to poor endometrial receptivity.124 Although a number of causes for RIF have been suggested, the precise reason has not been clearly elucidated for most patients.125 Various uterine factors associated with endometrial maturation or receptivity might be causes of RIF. Although adequate thickness of the endometrium for a successful pregnancy remains controversial, thin endometrium is one of important causing factors associated with uterine receptivity and subsequent poor reproductive outcome following assisted reproductive technology cycles in which the endometrial functional layer cannot proliferate to be more than 5 mm in thickness.126 Homeobox gene Hoxa 11 homozygous female mutants showing endometrial stem cell dysfunction127 are infertile due to a defective uterine environment. They have an abnormal decidualization of stromal cells. In addition, they lack uterine glandular epithelium, leading to subsequent embryo implantation failure.128 Tewary et al have also observed that reduced number of clonogenic endometrial cell populations at baseline can be associated with the relative severity of RIF.129 Recurrent pregnancy loss (RPL), also referred to as recurrent miscarriage, occurs in up to 3% of reproductive-aged women. It is typically defined as at least two or more consecutive pregnancy losses (miscarriages) before 22 weeks of gestational age.130 RPL is an important reproductive health issue because it affects approximately 1%–2% of couples, although the reproductive health issue of almost half of patients remains unexplained.130 Lucas et al have reported that human endometrial stromal cells derived from RPL patients exhibit reduced levels of CpH (H = A/C/T) methylation which is a hallmark of cellular multipotency and one of key features of pluripotent stem cells, such as induced pluripotent stem cells and embryonic stem cells and131 and decreased clonogenic capacity of endometrial stem cells,132 suggesting that RPL might be closely related to deficiency in clonogenic endometrial cell populations. Similarly, Murakami et al have reported significantly reduced W5C5+ endometrial stem cell subpopulation in patients with obesity who are known to have a high risk for reproductive outcomes,133 indicating that RPL can be caused by endometrial stem cell dysfunction or deficiency.
Endometriosis
Endometriosis is a common inflammatory disease affecting women of reproductive age. It is characterized by the presence of normal uterine endometrial tissue outside the uterine cavity.134 It is commonly associated with intermenstrual bleeding, painful periods, and infertility.135 Endometriosis affects roughly 10%–15% of all women of reproductive age.136 It can affect as high as 35%–50% of patients with pelvic pain or infertility.134 Although the definitive cause for endometriosis remains unknown, currently several hypotheses to explain its cause have been proposed and tested. The retrograde menstruation theory, one of widely accepted mechanisms describing how endometriosis develops, suggests that menstrual endometrial fragments are sloughed via patent fallopian tubes into the peritoneal cavity during a normal menstrual cycle.134 Nisolle et al have revealed that stromal and glandular cells derived from human menstrual endometrial samples have two distinct functions in the development of endometriosis. Stromal cells are mainly involved in the attachment of endometrial fragments. Glandular epithelial cells are mainly involved in their proliferation in the peritoneal cavity.137 Leyendecker et al have also suggested that endometriotic lesions are likely to be derived from shedding endometrial cells or tissue fragments with stem cell properties. Thus, they could reconstitute tissues within the peritoneal cavity in response to steroid hormones.138 Interestingly, growing evidence have shown that menstrual endometrial fragments-derived stem/progenitor cells are involved in the development of endometriosis.9,69,139,140 Kao et al have demonstrated the presence of endometrial stem cells, which can form new blood vessels and invade surrounding tissue, including eutopic and ectopic endometrial tissues. They also compared their characteristics.140 Similarly, Götte has observed an increased expression of adult stem cell marker Musashi-1 controlling maintenance and fate of stem cells in endometriotic tissues with an enhanced expression in proliferative endometrium.69 Schüring et al have found that expression levels of stem cell markers Notch-1 and Numb are significantly increased in endometrial tissues derived from endometriosis patients than in corresponding tissues of healthy women.114 These results suggest a possible direct involvement of endometrial stem cells in endometriosis lesion formation in the peritoneal cavity. However, reflux of endometrial tissue through the fallopian tubes during the menstrual cycle is a common phenomenon. Currently, certain factors or genetic defects that can trigger endometrial stem cells to develop endometriotic lesion within the pelvis have not been fully elucidated yet.
Endometrial cancer
Since stem-like cell subpopulations were first identified in leukemia by Lapidot and colleague in 1994,141 a number of investigators have proposed the existence of cancer stem cells (CSCs) in multiple types of solid tumors, such as stomach,142 breast,143 brain,144 and colon145 cancers. CSCs are functionally defined as a small subset of cancer cells with self-renewal ability, tumor-initiating potential, and ability to give rise to more differentiated cancer cells within the bulk tumor.146,147 It has been reported that levels of ABC multidrug resistance transporters are remarkably enhanced in many different types of CSCs. Thus, they can efficiently pump out intracellular chemotherapeutic drugs or their metabolites. In addition, CSCs, like normal stem cells, reside in a largely dormant state regarding cell-cycle activity, allowing them become resistant to cytotoxic radiation and chemotherapy that target rapidly proliferating cells.148 Several studies have reported the existence of CSCs in endometrial cancers. Gorai et al have reported that precursor (stem) colony-initiating cells can give rise both to mesenchymal and epithelial components during the development of endometrial carcinosarcoma.149 Friel et al have revealed that the proliferation of side population (SP) fraction cells isolated from two human endometrial cancers is significantly slower than the non-SP fraction with enhanced resistance to chemotherapeutic agent paclitaxel.150 Cell cycle analysis showed that the ratio of G0/G1 phase of the cell cycle in these SP cells was higher than that in the non-SP fraction.150 Similarly, Hubbard et al have observed a small population of clonogenic cells derived from 25 endometrial carcinoma samples with high self-renewing ability, differentiation potential, and tumorigenicity, suggesting the existence of endometrial CSC populations.151 Rutella et al have purified CD133 positive subpopulations, ranging from 1.3% to 62.6%, from primary endometrial adenocarcinomas with high resistance to chemotherapeutic agents paclitaxel and cisplatin. These CD133-expressing cells also exhibit stem cell features such as self-renewing capacity and differentiation potential. The can properly recapitulate bulk tumors when they are transplanted into immunodeficient (NOD/SCID) mice.152 Aldehyde dehydrogenase 1 (ALDH1)-expressing cells from endometrioid adenocarcinoma are more tumorigenic, resistant to cytotoxic chemotherapeutic drugs, and more invasive than ALDH1-negative cells, suggesting that ALDH1 can serve as a potential marker for endometrial cancer-initiating cells (CIC).153
Application of endometrial stem cells
Cardiac tissue regeneration
The regenerative capacity of endometrial stem cells for cardiac muscle regeneration was first applied to an animal model of Duchenne muscular dystrophy (DMD), the most common lethal genetic disorder in children, whereby menstrual blood–derived endometrial stem cells that were transplanted into immunodeficient DMD mice and subsequently contributed to muscle repair process by restoring sarcolemmal expression of dystrophin.154 Endometrial stem cells from human menstrual blood also can be used as an easily accessible cellular source to repair damaged heart tissue. Hida et al have engrafted three-dimensional endometrial stem cell sheet manipulation into the region of heart infarct.155 Transplanted endometrial cells differentiated into troponin and α-actinin expressing cardiac muscle cells and significantly decreased the myocardial infarction (MI) area and subsequently restored impaired cardiac function.155 Human endometrium stem cells, compared to human bone marrow MSCs, can significantly restore cardiac functions, decrease infarct size, and increase survival rates after myocardial ischemic injury by stimulating angiogenesis and cardiomyocyte metabolic activity.156 Jiang et al have shown that endometrial stem cell transplantation into the peri-infarct zone of myocardial infarction animals can significantly stimulate endogenous regeneration within the area of ischaemia and improve myocardial function by secreting various cytokines known to activate survival pathways and recruit endogenous c-kit + progenitor cells.157 All these studies suggest that endometrial stem cells with multilineage differentiation potential and angiogenic capacity are excellent cell sources for the regeneration of damaged cardiac muscles.
Endometrial regeneration
Since endometrial stem cells are derived from a dramatically regenerating uterine tissue in response to steroid hormones during each menstrual cycle,158,159 they have remarkable multilineage differentiation capacity. Thus, they are considered as excellent candidates for tissue repair regeneration. Currently, endometrial stem cells have been used in many pre-clinical and small animal studies within a short period of time. Yin et al have isolated SM22α+-derived CD34+/KLF4+ endometrial stem cells from mice uterine tissues and subsequently activated their regeneration capacities by deleting SENP1 expression.160 When these cells were transplanted into the injured endometrial epithelium, they subsequently accelerated endometrial repair with increased cell proliferation and reduced apoptosis by enhancing SENP1-mediated ERα transcriptional activity.160 Injured endometrial epithelium can be significantly regenerated by transplanting bipotent uterine epithelial stem cells that reside in the intersection region between glandular and luminal epithelium.27 Zhang et al have observed that transplantation of menstrual blood-derived endometrial stem cells can remarkably accelerate regeneration of injured endometrial tissue by increasing angiogenesis and endometrial functional layer thickness through AKT and ERK signaling pathways.158 Moreover, reduced pregnancy rates of mice with injured endometrial tissue can be restored by endometrial stem cell transplantation with a higher conception rate.158 Endometrial stem cells can be used for tissue engineering with various natural polymers to repair damaged tissues. Indeed, Park et al have recently developed a 3D stem cell-laden artificial endometrial tissue by incorporating various endometrial cell types including endometrial stem cells and several biodegradable natural polymers (collagen and hyaluronic acid).161 Severe tissue injuries of endometrial ablation mice have been remarkably relieved by transplanting this 3D artificial endometrial tissue.161
Injured skin regeneration
Recent evidence suggests that endometrial stem cells with high tissue regeneration potential might also be applicable to skin wound repair. Eremichev et al have found that endometrial stromal cells undergo transdifferentiation into myofibroblasts162 known to be primary extracellular matrix (ECM)-secreting cells, thus providing mechanical support during wound healing.163 Tavakoli et al firstly revealed evidence about differentiation potential of endometrial stem cells showing high clonogenic ability into keratinocyte and epidermal lineage by evaluating specific markers such as K14, p63, and involucrin.164 Compared to normal keratinocytes from skin tissue samples, differentiated keratinocytes from endometrial stem cells exhibited typical polygonal shape and expressed various keratinocyte specific genes.164 Similarly, Fard et al have successfully differentiated menstrual blood-derived endometrial stem cells into keratinocytes expressing various keratinocytes specific markers in the presence of human foreskin on bilayer amniotic membrane/nano-fibrous fibroin scaffold.165 These studies raised the possibility of endometrial stem cell application for treating skin defects. Indeed, Cuenca et al have observed that endometrial stem cells exhibiting immunomodulatory properties express various wound repair-associated genes such as ANGPT1, ELN MMP3/10, PDGFA, and PDGFB.166 Locally applied cells also show remarkably enhanced wound repair efficiency as well as increased neovascularization activity in a wound splinting animal model.166 Recently, Domnina et al have revealed that transplantation of endometrial stem cell spheroids produced in serum-free media significantly can enhance wound repair efficiency in a rat wound model by secreting various angiogenic and anti-inflammatory factors compared to monolayer cultured endometrial stem cells.167
Liver tissue regeneration
Khademi et al have differentiated endometrial stem cells into functional hepatocyte-like cells in vitro168 and then integrated these cells on an electrospun polyethersulfone (PES) nanofibrous scaffold.169 Yang et al have also developed a multiple-step differentiation protocol of human endometrial stem cells into functional hepatic-like cells in vitro by combining various growth factors such as hepatocyte growth factor, fibroblast growth factor-4, oncostatin M, and trichostatin A.170 These studies raised the possibility of endometrial stem cell application for treating liver disorders. Indeed, transplanted endometrial stem cells remarkably attenuated CCl4-induced liver damage, inhibiting neutrophil infiltration in the liver and reducing pro-inflammatory cytokines (IL-1β, IL-6, and TNF-α) levels.171 They also significantly suppressed CCl4-induced enhancement of aspartate aminotransferase (AST) and alanine aminotransferase (ALT) activities in the serum.171 In addition, the percentage of CD4+CD25+FOXP3+ regulatory T cells in spleen was significantly increased by endometrial stem cells transplantation.171 Kazerooni et al have also differentiated endometrial stem cells into hepatocyte-like cells and then administrated them to CCl4-induced acute liver failure animal models. Several biochemical markers of liver injury (AST, ALT, total bilirubin), pathological changes, and subsequent survival rates were significantly relieved by endometrial stem cell transplantation.172 Human menstrual blood-derived stem cells can also ameliorate liver fibrosis by inhibiting collagen deposition and hepatic stellate cells activity.173 These protective effects might be due to the secretion of various antifibrotic cytokines such as growth-related oncogene, hepatocyte growth factor, interleukin-6, interleukin-8, monocyte chemoattractant protein-1, and osteoprotegerin.173
Lung tissue regeneration
Once injected, endometrial stem cells can be successfully incorporated into damaged regions of lungs. These cells can subsequently ameliorate inflammation (IL-1β and IL-10) in bronchoalveolar lavage fluid and lipopolysaccharide (LPS)-induced acute lung injury.174 Menstrual blood-derived endometrial stem cells can also effectively ameliorate LPS-induced acute lung injury model by secreting anti-inflammatory cytokines (interleukin-10 and keratinocyte growth factor).175 Zhao et al have also observed that menstrual blood-derived endometrial stem cells can significantly attenuate various pulmonary fibrosis symptoms such as collagen deposition, fibrous tissue changes, inflammatory response (IL-1β, TNF-α, TGF-β, and IL-10), and extracellular matrix remodeling by suppressing pro-apoptotic protein Bax, while increasing anti-fibrosis genes HGF and MMP-9 and anti-apoptotic protein Bcl-2.176 In addition, exosomal miRNAs, particularly Let-7, from menstrual blood-derived endometrial stem cells can significantly alleviate alveolar epithelial cell damage by reducing cellular reactive oxygen species (ROS) and mitochondrial DNA damage in bleomycin-(BLM-) induced animal model of pulmonary fibrosis.177 These results suggest that endometrial stem cells might be a promising alternative for treating acute lung injuries.
Neuroprotective potential
Mobarakeh et al have revealed that endometrial stem cells have neural differentiation capacity in vitro in response to various signaling molecules such as bFGF, PDGF, and EGF. Their neural differentiation is characterized by neuron-like morphology and expression patterns of multiple neural lineage specific genes (Nestin, GABA, NF-L, β3-tub, and MAP2).178 Borlongan et al have also observed expression of neuronal differentiation markers (Nestin and MAP2) of endometrial stem cells under appropriate conditioned media.179 They successfully ameliorated neurotoxicant-induced cytotoxicity in primary rat neurons by secreting various neuroprotective factors such as VEGF, BDNF, and NT-3.179 Mohamadi et al have demonstrated that endometrial stem cells seeded in an electrospun poly/collagen/NBG conduits fabricated by electrospinning have therapeutic effects by improving nerve regeneration after a nerve transaction with growth of new blood vessels in a nerve transaction animal model.180 Conditioned medium of human menstrual blood-derived endometrial stem cells can alleviate neurotoxicant-induced inflammatory genes involved in Parkinson's disease such as COX-2, IL-1β, IL-6, iNOS, and TNF-α in vitro.181 This conditioned medium can also promote neuronal survival by reducing cell apoptosis and ROS generation.181 Liu et al have observed that menstrual blood-derived endometrial stem cells can highly express neurotrophic-associated genes such as BDNF, NGF, NT3, and NT4 with differentiation capacity into glial cells under conventional differentiation conditions.67 Intracerebral transplantation of endometrial stem cells can significantly enhance spatial learning and memory of Alzheimer's disease animals by increasing the expression of several Aβ degrading enzymes expression and reducing proinflammatory cytokines.182
Restoration of ovarian function
Lai et al have found that transplantation of human endometrial mesenchymal stem cells from menstrual blood can restore ovarian function by reducing depletion of the germline stem cell pool and cyclicity of chemotherapy-induced infertile mice with premature ovarian failure.183 They also observed that administrated endometrial stem cells could properly infiltrate into chemically-injured ovarian tissue and subsequently differentiate into steroid hormone secreting granulosa cells.183 In addition, administration of green fluorescent protein (GFP)-labeled endometrial stem cells could significantly increase numbers of primordial follicles and ovulated oocytes and serum levels of anti-Müllerian hormone.184 They also observed increases of pregnancy rate and number of delivered pups in mice treated with endometrial stem cells (38.5%) compared to PBS-treated mice (7.7%).184 However, none of these mice delivered pups or oocytes showing incorporated GFP-labeled cells, indicating that there was no infiltration of endometrial stem cells to the germline stem cell pool. Similarly, transplantation of endometrial stem cells can significantly restore ovarian functions (number of follicles, serum sex hormone levels) with premature ovarian failure by secreting FGF2 and decreasing ovarian granulosa cell apoptosis.185 That study also revealed that GFP-labeled endometrial stem cells were located in the ovarian interstitium but not in follicles to repair damaged ovarian tissue rather than directly incorporating into germline cells.185 In addition, Manshadi et al have observed that human menstrual blood-derived endometrial stem cells can improve hormone secretion and differentiate into ovarian-like cells, particularly ovarian granulosa cells, and subsequently improve follicle formation and ovulation in a premature ovarian failure animal model.186 A recent clinical trial has revealed that intraovarian administration of autologous menstrual blood derived-endometrial stem cells to poor ovarian responder women can significantly enhance clinical pregnancy and live birth rates.187 Interestingly, Bu et al have found that endometrial stem cells exert anti-tumor activities by inducing G0/G1 cell cycle arrest and increasing apoptotic cell death through AKT and FoxO3a-mediated signaling pathways. These cells can also disturb mitochondria membrane potential and pro-angiogenic activity of epithelial ovarian cancer cells both in vitro and in vivo.188
Conclusions
Stem cell-based therapy is a rapidly growing field. It has become a promising therapeutic strategy to alleviate numerous degenerative diseases such as autoimmune disorder, fibrosis, Alzheimer's disease, and tissue injury. It is commonly accepted that tissue-resident stem/progenitor cells are present in the basal layer of uterine endometrium. Improved understanding of the biology and unique characteristics of endometrial stem cells provides new insights into the outstanding regenerative capacity of human endometrial tissue during each menstrual cycle. It also provides new insight into endometrial stem cells associated diseases such as infertility, endometriosis, and endometrial cancer, offering new therapeutic strategies targeting endometrial stem cells. In addition, endometrial stem cells can provide unprecedented opportunities for developing powerful treatment options for various degenerative diseases. However, culture protocols for maintaining an undifferentiated state of endometrial stem cells during in vitro expansion and subsequently enhancing their therapeutic effects have not yet been established. In addition, standardized isolation protocols for specific endometrial stem cells within tissues are lacking. Specific markers for identifying endometrial stem cells allow their successful isolation and future therapies of various diseases caused by an inadequate regulation of endometrial stem cells. Moreover, whether endometrial stem cells have full mesenchymal stem cell activity in vivo regarding their multilineage differentiation potential is currently unclear. To overcome current limitations, future studies should also focus on their biological characteristics and underlying molecular action mechanisms in various endometrial stem cell-associated diseases.
Conflict of interests
The authors have no conflict of interests as defined by Genes & Diseases or other interests that might be perceived to influence the results and/or discussion reported in this article.
Funding
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT and Future Planning (No. NRF-2021R1A2C2008424 and NRF-2021M3E5E5094127). This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (No. NRF-2021R1A5A2030333). This work was supported by Korea Environment Industry & Technology Institute (KEITI) through the Project (Technology Development Project for Safety Management of Household Chemical Products), funded by Korea Ministry of Environment (MOE) (No. 1485017593). This research was also supported by a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (No HI21C1847).
Footnotes
Peer review under responsibility of Chongqing Medical University.
References
- 1.Cervelló I., Gil-Sanchis C., Mas A., et al. Human endometrial side population cells exhibit genotypic, phenotypic and functional features of somatic stem cells. PLoS One. 2010;5(6) doi: 10.1371/journal.pone.0010964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jabbour H.N., Kelly R.W., Fraser H.M., et al. Endocrine regulation of menstruation. Endocr Rev. 2006;27(1):17–46. doi: 10.1210/er.2004-0021. [DOI] [PubMed] [Google Scholar]
- 3.Gargett C.E., Nguyen H.P., Ye L. Endometrial regeneration and endometrial stem/progenitor cells. Rev Endocr Metab Disord. 2012;13(4):235–251. doi: 10.1007/s11154-012-9221-9. [DOI] [PubMed] [Google Scholar]
- 4.Timeva T., Shterev A., Kyurkchiev S. Recurrent implantation failure: the role of the endometrium. J Reprod Infertil. 2014;15(4):173–183. [PMC free article] [PubMed] [Google Scholar]
- 5.Bashiri A., Halper K.I., Orvieto R. Recurrent Implantation Failure-update overview on etiology, diagnosis, treatment and future directions. Reprod Biol Endocrinol. 2018;16(1):121. doi: 10.1186/s12958-018-0414-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barker N., Bartfeld S., Clevers H. Tissue-resident adult stem cell populations of rapidly self-renewing organs. Cell Stem Cell. 2010;7(6):656–670. doi: 10.1016/j.stem.2010.11.016. [DOI] [PubMed] [Google Scholar]
- 7.Mimeault M., Batra S.K. Recent progress on tissue-resident adult stem cell biology and their therapeutic implications. Stem Cell Rev. 2008;4(1):27–49. doi: 10.1007/s12015-008-9008-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gargett C.E., Chan R.W., Schwab K.E. Endometrial stem cells. Curr Opin Obstet Gynecol. 2007;19(4):377–383. doi: 10.1097/GCO.0b013e328235a5c6. [DOI] [PubMed] [Google Scholar]
- 9.Sasson I.E., Taylor H.S. Stem cells and the pathogenesis of endometriosis. Ann N Y Acad Sci. 2008;1127:106–115. doi: 10.1196/annals.1434.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gargett C.E., Chan R.W., Schwab K.E. Hormone and growth factor signaling in endometrial renewal: role of stem/progenitor cells. Mol Cell Endocrinol. 2008;288(1–2):22–29. doi: 10.1016/j.mce.2008.02.026. [DOI] [PubMed] [Google Scholar]
- 11.Tresserra F., Grases P., Ubeda A., et al. Morphological changes in hysterectomies after endometrial ablation. Hum Reprod. 1999;14(6):1473–1477. doi: 10.1093/humrep/14.6.1473. [DOI] [PubMed] [Google Scholar]
- 12.Padykula H.A., Coles L.G., McCracken J.A., et al. A zonal pattern of cell proliferation and differentiation in the Rhesus endometrium during the estrogen surge. Biol Reprod. 1984;31(5):1103–1118. doi: 10.1095/biolreprod31.5.1103. [DOI] [PubMed] [Google Scholar]
- 13.Padykula H.A., Coles L.G., Okulicz W.C., et al. The basalis of the primate endometrium: a bifunctional germinal compartment. Biol Reprod. 1989;40(3):681–690. doi: 10.1095/biolreprod40.3.681. [DOI] [PubMed] [Google Scholar]
- 14.Chan R.W., Schwab K.E., Gargett C.E. Clonogenicity of human endometrial epithelial and stromal cells. Biol Reprod. 2004;70(6):1738–1750. doi: 10.1095/biolreprod.103.024109. [DOI] [PubMed] [Google Scholar]
- 15.Taylor H.S. Endometrial cells derived from donor stem cells in bone marrow transplant recipients. JAMA. 2004;292(1):81–85. doi: 10.1001/jama.292.1.81. [DOI] [PubMed] [Google Scholar]
- 16.Zhu X., Péault B., Yan G., et al. Stem cells and endometrial regeneration: from basic research to clinical trial. Curr Stem Cell Res Ther. 2019;14(4):293–304. doi: 10.2174/1574888X14666181205120110. [DOI] [PubMed] [Google Scholar]
- 17.SUBTOTAL hysterectomy for cancer of the cervix and uterus. West J Surg Obstet Gynecol. 1946;54:211. [PubMed] [Google Scholar]
- 18.Gargett C.E., Schwab K.E., Zillwood R.M., et al. Isolation and culture of epithelial progenitors and mesenchymal stem cells from human endometrium. Biol Reprod. 2009;80(6):1136–1145. doi: 10.1095/biolreprod.108.075226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Santamaria X., Mas A., Cervelló I., et al. Uterine stem cells: from basic research to advanced cell therapies. Hum Reprod Update. 2018;24(6):673–693. doi: 10.1093/humupd/dmy028. [DOI] [PubMed] [Google Scholar]
- 20.Gargett C.E., Schwab K.E., Deane J.A. Endometrial stem/progenitor cells: the first 10 years. Hum Reprod Update. 2016;22(2):137–163. doi: 10.1093/humupd/dmv051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mutlu L., Hufnagel D., Taylor H.S. The endometrium as a source of mesenchymal stem cells for regenerative medicine. Biol Reprod. 2015;92(6):138. doi: 10.1095/biolreprod.114.126771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Spencer T.E. Biological roles of uterine glands in pregnancy. Semin Reprod Med. 2014;32(5):346–357. doi: 10.1055/s-0034-1376354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Punyadeera C., Thijssen V.L., Tchaikovski S., et al. Expression and regulation of vascular endothelial growth factor ligands and receptors during menstruation and post-menstrual repair of human endometrium. Mol Hum Reprod. 2006;12(6):367–375. doi: 10.1093/molehr/gal027. [DOI] [PubMed] [Google Scholar]
- 24.Cousins F.L., Pandoy R., Jin S., et al. The elusive endometrial epithelial stem/progenitor cells. Front Cell Dev Biol. 2021;9:640319. doi: 10.3389/fcell.2021.640319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gellersen B., Brosens J.J. Cyclic decidualization of the human endometrium in reproductive health and failure. Endocr Rev. 2014;35(6):851–905. doi: 10.1210/er.2014-1045. [DOI] [PubMed] [Google Scholar]
- 26.McLennan C.E., Rydell A.H. Extent of endometrial shedding during normal menstruation. Obstet Gynecol. 1965;26(5):605–621. [PubMed] [Google Scholar]
- 27.Jin S. Bipotent stem cells support the cyclical regeneration of endometrial epithelium of the murine uterus. Proc Natl Acad Sci U S A. 2019;116(14):6848–6857. doi: 10.1073/pnas.1814597116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gargett C.E. Review article: stem cells in human reproduction. Reprod Sci. 2007;14(5):405–424. doi: 10.1177/1933719107306231. [DOI] [PubMed] [Google Scholar]
- 29.Prianishnikov V.A. On the concept of stem cell and a model of functional-morphological structure of the endometrium. Contraception. 1978;18(3):213–223. doi: 10.1016/s0010-7824(78)80015-8. [DOI] [PubMed] [Google Scholar]
- 30.Wolff E.F., Wolff A.B., Du H., et al. Demonstration of multipotent stem cells in the adult human endometrium by in vitro chondrogenesis. Reprod Sci. 2007;14(6):524–533. doi: 10.1177/1933719107306896. [DOI] [PubMed] [Google Scholar]
- 31.Hirschmann-Jax C., Foster A.E., Wulf G.G., et al. A distinct “side population” of cells with high drug efflux capacity in human tumor cells. Proc Natl Acad Sci U S A. 2004;101(39):14228–14233. doi: 10.1073/pnas.0400067101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kato K., Yoshimoto M., Kato K., et al. Characterization of side-population cells in human normal endometrium. Hum Reprod. 2007;22(5):1214–1223. doi: 10.1093/humrep/del514. [DOI] [PubMed] [Google Scholar]
- 33.Masuda H., Matsuzaki Y., Hiratsu E., et al. Stem cell-like properties of the endometrial side population: implication in endometrial regeneration. PLoS One. 2010;5(4) doi: 10.1371/journal.pone.0010387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.de Miguel-Gómez L., López-Martínez S., Francés-Herrero E., et al. Stem cells and the endometrium: from the discovery of adult stem cells to pre-clinical models. Cells. 2021;10(3):595. doi: 10.3390/cells10030595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim J.Y., Tavaré S., Shibata D. Counting human somatic cell replications: methylation mirrors endometrial stem cell divisions. Proc Natl Acad Sci U S A. 2005;102(49):17739–17744. doi: 10.1073/pnas.0503976102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Teixeira J., Rueda B.R., Pru J.K. StemBook. Harvard Stem Cell Institute; Cambridge,MA: 2008. Uterine stem cells. [PubMed] [Google Scholar]
- 37.Schwab K.E., Hutchinson P., Gargett C.E. Identification of surface markers for prospective isolation of human endometrial stromal colony-forming cells. Hum Reprod. 2008;23(4):934–943. doi: 10.1093/humrep/den051. [DOI] [PubMed] [Google Scholar]
- 38.Schwab K.E., Gargett C.E. Co-expression of two perivascular cell markers isolates mesenchymal stem-like cells from human endometrium. Hum Reprod. 2007;22(11):2903–2911. doi: 10.1093/humrep/dem265. [DOI] [PubMed] [Google Scholar]
- 39.Masuda H., Anwar S.S., Bühring H.J., et al. A novel marker of human endometrial mesenchymal stem-like cells. Cell Transplant. 2012;21(10):2201–2214. doi: 10.3727/096368911X637362. [DOI] [PubMed] [Google Scholar]
- 40.Crisan M., Yap S., Casteilla L., et al. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell. 2008;3(3):301–313. doi: 10.1016/j.stem.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 41.Trapero C., Vidal A., Rodríguez-Martínez A., et al. The ectonucleoside triphosphate diphosphohydrolase-2 (NTPDase2) in human endometrium: a novel marker of basal stroma and mesenchymal stem cells. Purinergic Signal. 2019;15(2):225–236. doi: 10.1007/s11302-019-09656-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sun X., Jackson L., Dey S.K., et al. In pursuit of leucine-rich repeat-containing G protein-coupled receptor-5 regulation and function in the uterus. Endocrinology. 2009;150(11):5065–5073. doi: 10.1210/en.2009-0690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cousins F.L., O D.F., Gargett C.E. Endometrial stem/progenitor cells and their role in the pathogenesis of endometriosis. Best Pract Res Clin Obstet Gynaecol. 2018;50:27–38. doi: 10.1016/j.bpobgyn.2018.01.011. [DOI] [PubMed] [Google Scholar]
- 44.Ulrich D., Tan K.S., Deane J., et al. Mesenchymal stem/stromal cells in post-menopausal endometrium. Hum Reprod. 2014;29(9):1895–1905. doi: 10.1093/humrep/deu159. [DOI] [PubMed] [Google Scholar]
- 45.Huang C.C., Orvis G.D., Wang Y., et al. Stromal-to-epithelial transition during postpartum endometrial regeneration. PLoS One. 2012;7(8) doi: 10.1371/journal.pone.0044285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Garry R., Hart R., Karthigasu K.A., et al. A re-appraisal of the morphological changes within the endometrium during menstruation: a hysteroscopic, histological and scanning electron microscopic study. Hum Reprod. 2009;24(6):1393–1401. doi: 10.1093/humrep/dep036. [DOI] [PubMed] [Google Scholar]
- 47.Valentijn A.J., Palial K., Al-Lamee H., et al. SSEA-1 isolates human endometrial basal glandular epithelial cells: phenotypic and functional characterization and implications in the pathogenesis of endometriosis. Hum Reprod. 2013;28(10):2695–2708. doi: 10.1093/humrep/det285. [DOI] [PubMed] [Google Scholar]
- 48.Nguyen H.P.T., Xiao L., Deane J.A., et al. N-cadherin identifies human endometrial epithelial progenitor cells by in vitro stem cell assays. Hum Reprod. 2017;32(11):2254–2268. doi: 10.1093/humrep/dex289. [DOI] [PubMed] [Google Scholar]
- 49.Cervelló I., Mas A., Gil-Sanchis C., et al. Reconstruction of endometrium from human endometrial side population cell lines. PLoS One. 2011;6(6) doi: 10.1371/journal.pone.0021221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schwab K.E., Chan R.W., Gargett C.E. Putative stem cell activity of human endometrial epithelial and stromal cells during the menstrual cycle. Fertil Steril. 2005;84(Suppl 2):1124–1130. doi: 10.1016/j.fertnstert.2005.02.056. [DOI] [PubMed] [Google Scholar]
- 51.Blau H.M., Brazelton T.R., Weimann J.M. The evolving concept of a stem cell: entity or function? Cell. 2001;105(7):829–841. doi: 10.1016/s0092-8674(01)00409-3. [DOI] [PubMed] [Google Scholar]
- 52.Mints M., Jansson M., Sadeghi B., et al. Endometrial endothelial cells are derived from donor stem cells in a bone marrow transplant recipient. Hum Reprod. 2008;23(1):139–143. doi: 10.1093/humrep/dem342. [DOI] [PubMed] [Google Scholar]
- 53.Ikoma T., Kyo S., Maida Y., et al. Bone marrow-derived cells from male donors can compose endometrial glands in female transplant recipients. Am J Obstet Gynecol. 2009;201(6):608.e1–608.e8. doi: 10.1016/j.ajog.2009.07.026. [DOI] [PubMed] [Google Scholar]
- 54.Cervelló I., Gil-Sanchis C., Mas A., et al. Bone marrow-derived cells from male donors do not contribute to the endometrial side population of the recipient. PLoS One. 2012;7(1) doi: 10.1371/journal.pone.0030260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Masuda H., Kalka C., Takahashi T., et al. Estrogen-mediated endothelial progenitor cell biology and kinetics for physiological postnatal vasculogenesis. Circ Res. 2007;101(6):598–606. doi: 10.1161/CIRCRESAHA.106.144006. [DOI] [PubMed] [Google Scholar]
- 56.Du H., Naqvi H., Taylor H.S. Ischemia/reperfusion injury promotes and granulocyte-colony stimulating factor inhibits migration of bone marrow-derived stem cells to endometrium. Stem Cell Dev. 2012;21(18):3324–3331. doi: 10.1089/scd.2011.0193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Alawadhi F., Du H., Cakmak H., et al. Bone Marrow-Derived Stem Cell (BMDSC) transplantation improves fertility in a murine model of Asherman's syndrome. PLoS One. 2014;9(5) doi: 10.1371/journal.pone.0096662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang X., Mamillapalli R., Mutlu L., et al. Chemoattraction of bone marrow-derived stem cells towards human endometrial stromal cells is mediated by estradiol regulated CXCL12 and CXCR4 expression. Stem Cell Res. 2015;15(1):14–22. doi: 10.1016/j.scr.2015.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sahin Ersoy G., Zolbin M.M., Cosar E., et al. CXCL12 promotes stem cell recruitment and uterine repair after injury in asherman's syndrome. Mol Ther Methods Clin Dev. 2017;4:169–177. doi: 10.1016/j.omtm.2017.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tal R., Shaikh S., Pallavi P., et al. Adult bone marrow progenitors become decidual cells and contribute to embryo implantation and pregnancy. PLoS Biol. 2019;17(9) doi: 10.1371/journal.pbio.3000421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tempest N., MacLean A., Hapangama D.K. Endometrial stem cell markers: current concepts and unresolved questions. Int J Mol Sci. 2018;19(10):3240. doi: 10.3390/ijms19103240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hirschi K.K., D'Amore P.A. Pericytes in the microvasculature. Cardiovasc Res. 1996;32(4):687–698. [PubMed] [Google Scholar]
- 63.Shi S., Gronthos S. Perivascular niche of postnatal mesenchymal stem cells in human bone marrow and dental pulp. J Bone Miner Res. 2003;18(4):696–704. doi: 10.1359/jbmr.2003.18.4.696. [DOI] [PubMed] [Google Scholar]
- 64.Bruner H.C., Derksen P.W.B. Loss of E-cadherin-dependent cell-cell adhesion and the development and progression of cancer. Cold Spring Harbor Perspect Biol. 2018;10(3):a029330. doi: 10.1101/cshperspect.a029330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Xie X., Zheng X., Wang J., et al. Clinical significance of Twist, E-cadherin, and N-cadherin protein expression in endometrioid adenocarcinoma. J Cancer Res Ther. 2017;13(5):817–822. doi: 10.4103/jcrt.JCRT_405_17. [DOI] [PubMed] [Google Scholar]
- 66.Poncelet C., Cornelis F., Tepper M., et al. Expression of E- and N-cadherin and CD44 in endometrium and hydrosalpinges from infertile women. Fertil Steril. 2010;94(7):2909–2912. doi: 10.1016/j.fertnstert.2010.04.055. [DOI] [PubMed] [Google Scholar]
- 67.Liu Y., Yang F., Liang S., et al. N-cadherin upregulation promotes the neurogenic differentiation of menstrual blood-derived endometrial stem cells. Stem Cell Int. 2018;2018:3250379. doi: 10.1155/2018/3250379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Forouzanfar M., Lachinani L., Dormiani K., et al. Intracellular functions of RNA-binding protein, Musashi1, in stem and cancer cells. Stem Cell Res Ther. 2020;11(1):193. doi: 10.1186/s13287-020-01703-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Götte M., Wolf M., Staebler A., et al. Increased expression of the adult stem cell marker Musashi-1 in endometriosis and endometrial carcinoma. J Pathol. 2008;215(3):317–329. doi: 10.1002/path.2364. [DOI] [PubMed] [Google Scholar]
- 70.Lu X., Lin F., Fang H., et al. Expression of a putative stem cell marker Musashi-1 in endometrium. Histol Histopathol. 2011;26(9):1127–1133. doi: 10.14670/HH-26.1127. [DOI] [PubMed] [Google Scholar]
- 71.Chen Y.Z., Wang J.H., Yan J., et al. Increased expression of the adult stem cell marker Musashi-1 in the ectopic endometrium of adenomyosis does not correlate with serum estradiol and progesterone levels. Eur J Obstet Gynecol Reprod Biol. 2014;173:88–93. doi: 10.1016/j.ejogrb.2013.11.025. [DOI] [PubMed] [Google Scholar]
- 72.Strauß T., Greve B., Gabriel M., et al. Impact of musashi-1 and musashi-2 double knockdown on Notch signaling and the pathogenesis of endometriosis. Int J Mol Sci. 2022;23(5):2851. doi: 10.3390/ijms23052851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Barker N., van Es J.H., Kuipers J., et al. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature. 2007;449(7165):1003–1007. doi: 10.1038/nature06196. [DOI] [PubMed] [Google Scholar]
- 74.Jaks V., Barker N., Kasper M., et al. Lgr5 marks cycling, yet long-lived, hair follicle stem cells. Nat Genet. 2008;40(11):1291–1299. doi: 10.1038/ng.239. [DOI] [PubMed] [Google Scholar]
- 75.Barker N., Huch M., Kujala P., et al. Lgr5(+ve) stem cells drive self-renewal in the stomach and build long-lived gastric units in vitro. Cell Stem Cell. 2010;6(1):25–36. doi: 10.1016/j.stem.2009.11.013. [DOI] [PubMed] [Google Scholar]
- 76.Huch M., Dorrell C., Boj S.F., et al. In vitro expansion of single Lgr5+ liver stem cells induced by Wnt-driven regeneration. Nature. 2013;494(7436):247–250. doi: 10.1038/nature11826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Flesken-Nikitin A., Hwang C.I., Cheng C.Y., et al. Ovarian surface epithelium at the junction area contains a cancer-prone stem cell niche. Nature. 2013;495(7440):241–245. doi: 10.1038/nature11979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ng A., Tan S., Singh G., et al. Lgr5 marks stem/progenitor cells in ovary and tubal epithelia. Nat Cell Biol. 2014;16(8):745–757. doi: 10.1038/ncb3000. [DOI] [PubMed] [Google Scholar]
- 79.Seishima R., Leung C., Yada S., et al. Neonatal Wnt-dependent Lgr5 positive stem cells are essential for uterine gland development. Nat Commun. 2019;10(1):5378. doi: 10.1038/s41467-019-13363-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Xu S., Chan R.W.S., Li T., et al. Understanding the regulatory mechanisms of endometrial cells on activities of endometrial mesenchymal stem-like cells during menstruation. Stem Cell Res Ther. 2020;11(1):239. doi: 10.1186/s13287-020-01750-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Schöler H.R., Dressler G.R., Balling R., et al. Oct-4:a germline-specific transcription factor mapping to the mouse t-complex. EMBO J. 1990;9(7):2185–2195. doi: 10.1002/j.1460-2075.1990.tb07388.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Nichols J., Zevnik B., Anastassiadis K., et al. Formation of pluripotent stem cells in the mammalian embryo depends on the POU transcription factor Oct4. Cell. 1998;95(3):379–391. doi: 10.1016/s0092-8674(00)81769-9. [DOI] [PubMed] [Google Scholar]
- 83.Wang H., Wang X., Xu X., et al. Germ cell nuclear factor (GCNF) represses Oct4 expression and globally modulates gene expression in human embryonic stem (hES) cells. J Biol Chem. 2016;291(16):8644–8652. doi: 10.1074/jbc.M115.694208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wu G., Schöler H.R. Role of Oct4 in the early embryo development. Cell Regen. 2014;3(1):7. doi: 10.1186/2045-9769-3-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ben-Shushan E., Sharir H., Pikarsky E., et al. A dynamic balance between ARP-1/COUP-TFII, EAR-3/COUP-TFI, and retinoic acid receptor: retinoid X receptor heterodimers regulates Oct-3/4 expression in embryonal carcinoma cells. Mol Cell Biol. 1995;15(2):1034–1048. doi: 10.1128/mcb.15.2.1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Pan G.J., Chang Z.Y., Schöler H.R., et al. Stem cell pluripotency and transcription factor Oct4. Cell Res. 2002;12(5):321–329. doi: 10.1038/sj.cr.7290134. [DOI] [PubMed] [Google Scholar]
- 87.Lee J., Go Y., Kang I., et al. Oct-4 controls cell-cycle progression of embryonic stem cells. Biochem J. 2010;426(2):171–181. doi: 10.1042/BJ20091439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Matthai C., Horvat R., Noe M., et al. Oct-4 expression in human endometrium. Mol Hum Reprod. 2006;12(1):7–10. doi: 10.1093/molehr/gah254. [DOI] [PubMed] [Google Scholar]
- 89.Salama E., Eldeen G.N., Abdel Rasheed M., et al. Differentially expressed genes: OCT-4, SOX2, STAT3, CDH1 and CDH2, in cultured mesenchymal stem cells challenged with serum of women with endometriosis. J Genet Eng Biotechnol. 2018;16(1):63–69. doi: 10.1016/j.jgeb.2017.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Shariati F., Favaedi R., Ramazanali F., et al. Increased expression of stemness genes REX-1, OCT-4, NANOG, and SOX-2 in women with ovarian endometriosis versus normal endometrium: a case-control study. Int J Reprod Biomed. 2018;16(12) doi: 10.18502/ijrm.v16i12.3684. ijrm.v16i12.3684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Spangrude G.J., Johnson G.R. Resting and activated subsets of mouse multipotent hematopoietic stem cells. Proc Natl Acad Sci U S A. 1990;87(19):7433–7437. doi: 10.1073/pnas.87.19.7433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wolf N.S., Koné A., Priestley G.V., et al. In vivo and in vitro characterization of long-term repopulating primitive hematopoietic cells isolated by sequential Hoechst 33342-rhodamine 123 FACS selection. Exp Hematol. 1993;21(5):614–622. [PubMed] [Google Scholar]
- 93.Goodell M.A., Brose K., Paradis G., et al. Isolation and functional properties of murine hematopoietic stem cells that are replicating in vivo. J Exp Med. 1996;183(4):1797–1806. doi: 10.1084/jem.183.4.1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Matsuzaki Y., Kinjo K., Mulligan R.C., et al. Unexpectedly efficient homing capacity of purified murine hematopoietic stem cells. Immunity. 2004;20(1):87–93. doi: 10.1016/s1074-7613(03)00354-6. [DOI] [PubMed] [Google Scholar]
- 95.Liu J.A., Wu M.H., Yan C.H., et al. Phosphorylation of Sox9 is required for neural crest delamination and is regulated downstream of BMP and canonical Wnt signaling. Proc Natl Acad Sci U S A. 2013;110(8):2882–2887. doi: 10.1073/pnas.1211747110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Gonen N., Lovell-Badge R. The regulation of Sox9 expression in the gonad. Curr Top Dev Biol. 2019;134:223–252. doi: 10.1016/bs.ctdb.2019.01.004. [DOI] [PubMed] [Google Scholar]
- 97.Sun W., Cornwell A., Li J., et al. SOX9 is an astrocyte-specific nuclear marker in the adult brain outside the neurogenic regions. J Neurosci. 2017;37(17):4493–4507. doi: 10.1523/JNEUROSCI.3199-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Saegusa M., Hashimura M., Suzuki E., et al. Transcriptional up-regulation of Sox9 by NF-κB in endometrial carcinoma cells, modulating cell proliferation through alteration in the p14(ARF)/p53/p21(WAF1) pathway. Am J Pathol. 2012;181(2):684–692. doi: 10.1016/j.ajpath.2012.05.008. [DOI] [PubMed] [Google Scholar]
- 99.Hapangama D.K., Drury J., Da Silva L., et al. Abnormally located SSEA1+/SOX9+ endometrial epithelial cells with a basalis-like phenotype in the eutopic functionalis layer may play a role in the pathogenesis of endometriosis. Hum Reprod. 2019;34(1):56–68. doi: 10.1093/humrep/dey336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Cui L., Johkura K., Yue F., et al. Spatial distribution and initial changes of SSEA-1 and other cell adhesion-related molecules on mouse embryonic stem cells before and during differentiation. J Histochem Cytochem. 2004;52(11):1447–1457. doi: 10.1369/jhc.3A6241.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lian Q., Yeo K.S., Que J., et al. Establishing clonal cell lines with endothelial-like potential from CD9(hi), SSEA-1 (-) cells in embryonic stem cell-derived embryoid bodies. PLoS One. 2006;1:e6. doi: 10.1371/journal.pone.0000006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Henderson J.K., Draper J.S., Baillie H.S., et al. Preimplantation human embryos and embryonic stem cells show comparable expression of stage-specific embryonic antigens. Stem Cell. 2002;20(4):329–337. doi: 10.1634/stemcells.20-4-329. [DOI] [PubMed] [Google Scholar]
- 103.Inada E., Saitoh I., Kubota N., et al. Increased expression of cell surface SSEA-1 is closely associated with Naïve-like conversion from human deciduous teeth dental pulp cells-derived iPS cells. Int J Mol Sci. 2019;20(7):1651. doi: 10.3390/ijms20071651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Tempest N., Baker A.M., Wright N.A., et al. Does human endometrial LGR5 gene expression suggest the existence of another hormonally regulated epithelial stem cell niche? Hum Reprod. 2018;33(6):1052–1062. doi: 10.1093/humrep/dey083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Chiba S. Notch signaling in stem cell systems. Stem Cell. 2006;24(11):2437–2447. doi: 10.1634/stemcells.2005-0661. [DOI] [PubMed] [Google Scholar]
- 106.Liang S.J., Li X.G., Wang X.Q. Notch signaling in mammalian intestinal stem cells: determining cell fate and maintaining homeostasis. Curr Stem Cell Res Ther. 2019;14(7):583–590. doi: 10.2174/1574888X14666190429143734. [DOI] [PubMed] [Google Scholar]
- 107.Ables J.L., Decarolis N.A., Johnson M.A., et al. Notch1 is required for maintenance of the reservoir of adult hippocampal stem cells. J Neurosci. 2010;30(31):10484–10492. doi: 10.1523/JNEUROSCI.4721-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Su R.W., Strug M.R., Jeong J.W., et al. Aberrant activation of canonical Notch1 signaling in the mouse uterus decreases progesterone receptor by hypermethylation and leads to infertility. Proc Natl Acad Sci U S A. 2016;113(8):2300–2305. doi: 10.1073/pnas.1520441113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Su R.W., Strug M.R., Joshi N.R., et al. Decreased Notch pathway signaling in the endometrium of women with endometriosis impairs decidualization. J Clin Endocrinol Metab. 2015;100(3):E433–E442. doi: 10.1210/jc.2014-3720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Qi S., Zhao X., Li M., et al. Aberrant expression of Notch1/numb/snail signaling, an epithelial mesenchymal transition related pathway, in adenomyosis. Reprod Biol Endocrinol. 2015;13:96. doi: 10.1186/s12958-015-0084-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Mori M., Miyamoto T., Ohno S., et al. Diagnostic utility of notch-1 immunocytochemistry in endometrial cytology. Acta Cytol. 2012;56(2):166–170. doi: 10.1159/000335485. [DOI] [PubMed] [Google Scholar]
- 112.Spitzer T.L., Rojas A., Zelenko Z., et al. Perivascular human endometrial mesenchymal stem cells express pathways relevant to self-renewal, lineage specification, and functional phenotype. Biol Reprod. 2012;86(2):58. doi: 10.1095/biolreprod.111.095885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Zhang S., Chan R.W.S., Ng E.H.Y., et al. Hypoxia regulates the self-renewal of endometrial mesenchymal stromal/stem-like cells via Notch signaling. Int J Mol Sci. 2022;23(9):4613. doi: 10.3390/ijms23094613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Schüring A.N., Dahlhues B., Korte A., et al. The endometrial stem cell markers notch-1 and numb are associated with endometriosis. Reprod Biomed Online. 2018;36(3):294–301. doi: 10.1016/j.rbmo.2017.11.010. [DOI] [PubMed] [Google Scholar]
- 115.Xu Z., Robitaille A.M., Berndt J.D., et al. Wnt/β-catenin signaling promotes self-renewal and inhibits the primed state transition in naïve human embryonic stem cells. Proc Natl Acad Sci U S A. 2016;113(42):E6382–E6390. doi: 10.1073/pnas.1613849113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Aulicino F., Pedone E., Sottile F., et al. Canonical Wnt pathway controls mESC self-renewal through inhibition of spontaneous differentiation via β-catenin/TCF/LEF functions. Stem Cell Rep. 2020;15(3):646–661. doi: 10.1016/j.stemcr.2020.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Boland G.M., Perkins G., Hall D.J., et al. Wnt 3a promotes proliferation and suppresses osteogenic differentiation of adult human mesenchymal stem cells. J Cell Biochem. 2004;93(6):1210–1230. doi: 10.1002/jcb.20284. [DOI] [PubMed] [Google Scholar]
- 118.Hayashi K., Erikson D.W., Tilford S.A., et al. Wnt genes in the mouse uterus: potential regulation of implantation. Biol Reprod. 2009;80(5):989–1000. doi: 10.1095/biolreprod.108.075416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kiewisz J., Kaczmarek M.M., Andronowska A., et al. Gene expression of WNTs, β-catenin and E-cadherin during the periimplantation period of pregnancy in pigs: involvement of steroid hormones. Theriogenology. 2011;76(4):687–699. doi: 10.1016/j.theriogenology.2011.03.022. [DOI] [PubMed] [Google Scholar]
- 120.Nguyen H.P., Sprung C.N., Gargett C.E. Differential expression of Wnt signaling molecules between pre- and postmenopausal endometrial epithelial cells suggests a population of putative epithelial stem/progenitor cells reside in the basalis layer. Endocrinology. 2012;153(6):2870–2883. doi: 10.1210/en.2011-1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Bukowska J., Ziecik A.J., Laguna J., et al. The importance of the canonical Wnt signaling pathway in the Porcine endometrial stromal stem/progenitor cells: implications for regeneration. Stem Cell Dev. 2015;24(24):2873–2885. doi: 10.1089/scd.2015.0078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Gargett C.E. Stem cells in gynaecology. Aust N Z J Obstet Gynaecol. 2004;44(5):380–386. doi: 10.1111/j.1479-828X.2004.00290.x. [DOI] [PubMed] [Google Scholar]
- 123.Gargett C.E. Identification and characterisation of human endometrial stem/progenitor cells. Aust N Z J Obstet Gynaecol. 2006;46(3):250–253. doi: 10.1111/j.1479-828X.2006.00582.x. [DOI] [PubMed] [Google Scholar]
- 124.Zohni K.M., Gat I., Librach C. Recurrent implantation failure: a comprehensive review. Minerva Ginecol. 2016;68(6):653–667. [PubMed] [Google Scholar]
- 125.Simon A., Laufer N. Repeated implantation failure: clinical approach. Fertil Steril. 2012;97(5):1039–1043. doi: 10.1016/j.fertnstert.2012.03.010. [DOI] [PubMed] [Google Scholar]
- 126.Senturk L.M., Erel C.T. Thin endometrium in assisted reproductive technology. Curr Opin Obstet Gynecol. 2008;20(3):221–228. doi: 10.1097/GCO.0b013e328302143c. [DOI] [PubMed] [Google Scholar]
- 127.Lynch V.J., Brayer K., Gellersen B., et al. HoxA-11 and FOXO1A cooperate to regulate decidual prolactin expression: towards inferring the core transcriptional regulators of decidual genes. PLoS One. 2009;4(9) doi: 10.1371/journal.pone.0006845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Hsieh-Li H.M., Witte D.P., Weinstein M., et al. Hoxa 11 structure, extensive antisense transcription, and function in male and female fertility. Development. 1995;121(5):1373–1385. doi: 10.1242/dev.121.5.1373. [DOI] [PubMed] [Google Scholar]
- 129.Tewary S., Lucas E.S., Fujihara R., et al. Impact of sitagliptin on endometrial mesenchymal stem-like progenitor cells: a randomised, double-blind placebo-controlled feasibility trial. EBioMedicine. 2020;51:102597. doi: 10.1016/j.ebiom.2019.102597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Ford H.B., Schust D.J. Recurrent pregnancy loss: etiology, diagnosis, and therapy. Rev Obstet Gynecol. 2009;2(2):76–83. [PMC free article] [PubMed] [Google Scholar]
- 131.Lister R., Pelizzola M., Kida Y.S., et al. Hotspots of aberrant epigenomic reprogramming in human induced pluripotent stem cells. Nature. 2011;471(7336):68–73. doi: 10.1038/nature09798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Lucas E.S., Dyer N.P., Murakami K., et al. Loss of endometrial plasticity in recurrent pregnancy loss. Stem Cell. 2016;34(2):346–356. doi: 10.1002/stem.2222. [DOI] [PubMed] [Google Scholar]
- 133.Murakami K., Bhandari H., Lucas E.S., et al. Deficiency in clonogenic endometrial mesenchymal stem cells in obese women with reproductive failure–a pilot study. PLoS One. 2013;8(12) doi: 10.1371/journal.pone.0082582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Parasar P., Ozcan P., Terry K.L. Endometriosis: epidemiology, diagnosis and clinical management. Curr Obstet Gynecol Rep. 2017;6(1):34–41. doi: 10.1007/s13669-017-0187-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Malvezzi H., Hernandes C., Piccinato C.A., et al. Interleukin in endometriosis-associated infertility-pelvic pain: systematic review and meta-analysis. Reproduction. 2019;158(1):1–12. doi: 10.1530/REP-18-0618. [DOI] [PubMed] [Google Scholar]
- 136.Giudice L.C., Kao L.C. Endometriosis. Lancet. 2004;364(9447):1789–1799. doi: 10.1016/S0140-6736(04)17403-5. [DOI] [PubMed] [Google Scholar]
- 137.Nisolle M., Casanas-Roux F., Donnez J. Early-stage endometriosis: adhesion and growth of human menstrual endometrium in nude mice. Fertil Steril. 2000;74(2):306–312. doi: 10.1016/s0015-0282(00)00601-4. [DOI] [PubMed] [Google Scholar]
- 138.Leyendecker G., Herbertz M., Kunz G., et al. Endometriosis results from the dislocation of basal endometrium. Hum Reprod. 2002;17(10):2725–2736. doi: 10.1093/humrep/17.10.2725. [DOI] [PubMed] [Google Scholar]
- 139.Figueira P.G.M., Abrão M.S., Krikun G., et al. Stem cells in endometrium and their role in the pathogenesis of endometriosis. Ann N Y Acad Sci. 2011;1221(1):10–17. doi: 10.1111/j.1749-6632.2011.05969.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Kao A.P., Wang K.H., Chang C.C., et al. Comparative study of human eutopic and ectopic endometrial mesenchymal stem cells and the development of an in vivo endometriotic invasion model. Fertil Steril. 2011;95(4):1308–1315.e1. doi: 10.1016/j.fertnstert.2010.09.064. [DOI] [PubMed] [Google Scholar]
- 141.Lapidot T., Sirard C., Vormoor J., et al. A cell initiating human acute myeloid leukaemia after transplantation into SCID mice. Nature. 1994;367(6464):645–648. doi: 10.1038/367645a0. [DOI] [PubMed] [Google Scholar]
- 142.Chen T., Yang K., Yu J., et al. Identification and expansion of cancer stem cells in tumor tissues and peripheral blood derived from gastric adenocarcinoma patients. Cell Res. 2012;22(1):248–258. doi: 10.1038/cr.2011.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Al-Hajj M., Wicha M.S., Benito-Hernandez A., et al. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci U S A. 2003;100(7):3983–3988. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Singh S.K., Clarke I.D., Terasaki M., et al. Identification of a cancer stem cell in human brain tumors. Cancer Res. 2003;63(18):5821–5828. [PubMed] [Google Scholar]
- 145.Tsunekuni K., Konno M., Haraguchi N., et al. CD44/CD133-positive colorectal cancer stem cells are sensitive to trifluridine exposure. Sci Rep. 2019;9(1):14861. doi: 10.1038/s41598-019-50968-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Visvader J.E., Lindeman G.J. Cancer stem cells in solid tumours: accumulating evidence and unresolved questions. Nat Rev Cancer. 2008;8(10):755–768. doi: 10.1038/nrc2499. [DOI] [PubMed] [Google Scholar]
- 147.Reya T., Morrison S.J., Clarke M.F., et al. Stem cells, cancer, and cancer stem cells. Nature. 2001;414(6859):105–111. doi: 10.1038/35102167. [DOI] [PubMed] [Google Scholar]
- 148.Zhou S., Schuetz J.D., Bunting K.D., et al. The ABC transporter Bcrp1/ABCG2 is expressed in a wide variety of stem cells and is a molecular determinant of the side-population phenotype. Nat Med. 2001;7(9):1028–1034. doi: 10.1038/nm0901-1028. [DOI] [PubMed] [Google Scholar]
- 149.Gorai I., Yanagibashi T., Taki A., et al. Uterine carcinosarcoma is derived from a single stem cell: an in vitro study. Int J Cancer. 1997;72(5):821–827. doi: 10.1002/(sici)1097-0215(19970904)72:5<821::aid-ijc19>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 150.Friel A.M., Sergent P.A., Patnaude C., et al. Functional analyses of the cancer stem cell-like properties of human endometrial tumor initiating cells. Cell Cycle. 2008;7(2):242–249. doi: 10.4161/cc.7.2.5207. [DOI] [PubMed] [Google Scholar]
- 151.Hubbard S.A., Friel A.M., Kumar B., et al. Evidence for cancer stem cells in human endometrial carcinoma. Cancer Res. 2009;69(21):8241–8248. doi: 10.1158/0008-5472.CAN-08-4808. [DOI] [PubMed] [Google Scholar]
- 152.Rutella S., Bonanno G., Procoli A., et al. Cells with characteristics of cancer stem/progenitor cells express the CD133 antigen in human endometrial tumors. Clin Cancer Res. 2009;15(13):4299–4311. doi: 10.1158/1078-0432.CCR-08-1883. [DOI] [PubMed] [Google Scholar]
- 153.Rahadiani N., Ikeda J., Mamat S., et al. Expression of aldehyde dehydrogenase 1 (ALDH1) in endometrioid adenocarcinoma and its clinical implications. Cancer Sci. 2011;102(4):903–908. doi: 10.1111/j.1349-7006.2011.01864.x. [DOI] [PubMed] [Google Scholar]
- 154.Cui C.H., Uyama T., Miyado K., et al. Menstrual blood-derived cells confer human dystrophin expression in the murine model of Duchenne muscular dystrophy via cell fusion and myogenic transdifferentiation. Mol Biol Cell. 2007;18(5):1586–1594. doi: 10.1091/mbc.E06-09-0872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Hida N., Nishiyama N., Miyoshi S., et al. Novel cardiac precursor-like cells from human menstrual blood-derived mesenchymal cells. Stem Cell. 2008;26(7):1695–1704. doi: 10.1634/stemcells.2007-0826. [DOI] [PubMed] [Google Scholar]
- 156.Fan X., He S., Song H., et al. Human endometrium-derived stem cell improves cardiac function after myocardial ischemic injury by enhancing angiogenesis and myocardial metabolism. Stem Cell Res Ther. 2021;12(1):344. doi: 10.1186/s13287-021-02423-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Jiang Z., Hu X., Yu H., et al. Human endometrial stem cells confer enhanced myocardial salvage and regeneration by paracrine mechanisms. J Cell Mol Med. 2013;17(10):1247–1260. doi: 10.1111/jcmm.12100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Zhang Y., Lin X., Dai Y., et al. Endometrial stem cells repair injured endometrium and induce angiogenesis via AKT and ERK pathways. Reproduction. 2016;152(5):389–402. doi: 10.1530/REP-16-0286. [DOI] [PubMed] [Google Scholar]
- 159.Li Z., Yan G., Diao Q., et al. Transplantation of human endometrial perivascular cells with elevated CYR61 expression induces angiogenesis and promotes repair of a full-thickness uterine injury in rat. Stem Cell Res Ther. 2019;10(1):179. doi: 10.1186/s13287-019-1272-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Yin M., Zhou H.J., Lin C., et al. CD34 + KLF4 + stromal stem cells contribute to endometrial regeneration and repair. Cell Rep. 2019;27(9):2709–2724. doi: 10.1016/j.celrep.2019.04.088. e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Park S.R., Kim S.R., Im J.B., et al. 3D stem cell-laden artificial endometrium: successful endometrial regeneration and pregnancy. Biofabrication. 2021;13(4) doi: 10.1088/1758-5090/ac165a. [DOI] [PubMed] [Google Scholar]
- 162.Eremichev R., Kulebyakina M., Alexandrushkina N., et al. Scar-free healing of endometrium: tissue-specific program of stromal cells and its induction by soluble factors produced after damage. Front Cell Dev Biol. 2021;9:616893. doi: 10.3389/fcell.2021.616893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Hinz B. Myofibroblasts. Exp Eye Res. 2016;142:56–70. doi: 10.1016/j.exer.2015.07.009. [DOI] [PubMed] [Google Scholar]
- 164.Akhavan-Tavakoli M., Fard M., Khanjani S., et al. In vitro differentiation of menstrual blood stem cells into keratinocytes: a potential approach for management of wound healing. Biologicals. 2017;48:66–73. doi: 10.1016/j.biologicals.2017.05.005. [DOI] [PubMed] [Google Scholar]
- 165.Fard M., Akhavan-Tavakoli M., Khanjani S., et al. Bilayer amniotic membrane/nano-fibrous fibroin scaffold promotes differentiation capability of menstrual blood stem cells into keratinocyte-like cells. Mol Biotechnol. 2018;60(2):100–110. doi: 10.1007/s12033-017-0049-0. [DOI] [PubMed] [Google Scholar]
- 166.Cuenca J., Le-Gatt A., Castillo V., et al. The reparative abilities of menstrual stem cells modulate the wound matrix signals and improve cutaneous regeneration. Front Physiol. 2018;9:464. doi: 10.3389/fphys.2018.00464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Domnina A., Alekseenko L., Kozhukharova I., et al. Generation of therapeutically potent spheroids from human endometrial mesenchymal stem/stromal cells. J Pers Med. 2021;11(6):466. doi: 10.3390/jpm11060466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Khademi F., Soleimani M., Verdi J., et al. Human endometrial stem cells differentiation into functional hepatocyte-like cells. Cell Biol Int. 2014;38(7):825–834. doi: 10.1002/cbin.10278. [DOI] [PubMed] [Google Scholar]
- 169.Khademi F., Ai J., Soleimani M., et al. Improved human endometrial stem cells differentiation into functional hepatocyte-like cells on a glycosaminoglycan/collagen-grafted polyethersulfone nanofibrous scaffold. J Biomed Mater Res B Appl Biomater. 2017;105(8):2516–2529. doi: 10.1002/jbm.b.33758. [DOI] [PubMed] [Google Scholar]
- 170.Yang X.Y., Wang W., Li X. In vitro hepatic differentiation of human endometrial stromal stem cells. In Vitro Cell Dev Biol Anim. 2014;50(2):162–170. doi: 10.1007/s11626-013-9688-z. [DOI] [PubMed] [Google Scholar]
- 171.Lu S., Shi G., Xu X., et al. Human endometrial regenerative cells alleviate carbon tetrachloride-induced acute liver injury in mice. J Transl Med. 2016;14(1):300. doi: 10.1186/s12967-016-1051-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Fathi-Kazerooni M., Tavoosidana G., Taghizadeh-Jahed M., et al. Comparative restoration of acute liver failure by menstrual blood stem cells compared with bone marrow stem cells in mice model. Cytotherapy. 2017;19(12):1474–1490. doi: 10.1016/j.jcyt.2017.08.022. [DOI] [PubMed] [Google Scholar]
- 173.Chen L., Zhang C., Chen L., et al. Human menstrual blood-derived stem cells ameliorate liver fibrosis in mice by targeting hepatic stellate cells via paracrine mediators. Stem Cell Transl Med. 2017;6(1):272–284. doi: 10.5966/sctm.2015-0265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Xiang B., Chen L., Wang X., et al. Transplantation of menstrual blood-derived mesenchymal stem cells promotes the repair of LPS-induced acute lung injury. Int J Mol Sci. 2017;18(4):689. doi: 10.3390/ijms18040689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Ren H., Zhang Q., Wang J., et al. Comparative effects of umbilical cord- and menstrual blood-derived MSCs in repairing acute lung injury. Stem Cell Int. 2018;2018:7873625. doi: 10.1155/2018/7873625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Zhao Y., Lan X., Wang Y., et al. Human endometrial regenerative cells attenuate bleomycin-induced pulmonary fibrosis in mice. Stem Cell Int. 2018;2018:3475137. doi: 10.1155/2018/3475137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Sun L., Zhu M., Feng W., et al. Exosomal miRNA let-7 from menstrual blood-derived endometrial stem cells alleviates pulmonary fibrosis through regulating mitochondrial DNA damage. Oxid Med Cell Longev. 2019;2019:4506303. doi: 10.1155/2019/4506303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Mobarakeh Z.T., Ai J., Yazdani F., et al. Human endometrial stem cells as a new source for programming to neural cells. Cell Biol Int Rep. 2012;19(1) doi: 10.1042/CBR20110009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Borlongan C.V., Kaneko Y., Maki M., et al. Menstrual blood cells display stem cell-like phenotypic markers and exert neuroprotection following transplantation in experimental stroke. Stem Cell Dev. 2010;19(4):439–452. doi: 10.1089/scd.2009.0340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Mohamadi F., Ebrahimi-Barough S., Nourani M.R., et al. Enhanced sciatic nerve regeneration by human endometrial stem cells in an electrospun poly (ε-caprolactone)/collagen/NBG nerve conduit in rat. Artif Cell Nanomed Biotechnol. 2018;46(8):1731–1743. doi: 10.1080/21691401.2017.1391823. [DOI] [PubMed] [Google Scholar]
- 181.Li H., Yahaya B.H., Ng W.H., et al. Conditioned medium of human menstrual blood-derived endometrial stem cells protects against MPP +-induced cytotoxicity in vitro. Front Mol Neurosci. 2019;12:80. doi: 10.3389/fnmol.2019.00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Zhao Y., Chen X., Wu Y., et al. Transplantation of human menstrual blood-derived mesenchymal stem cells alleviates Alzheimer's disease-like pathology in APP/PS1 transgenic mice. Front Mol Neurosci. 2018;11:140. doi: 10.3389/fnmol.2018.00140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Lai D., Wang F., Yao X., et al. Human endometrial mesenchymal stem cells restore ovarian function through improving the renewal of germline stem cells in a mouse model of premature ovarian failure. J Transl Med. 2015;13:155. doi: 10.1186/s12967-015-0516-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Reig A., Mamillapalli R., Coolidge A., et al. Uterine cells improved ovarian function in a murine model of ovarian insufficiency. Reprod Sci. 2019;26(12):1633–1639. doi: 10.1177/1933719119875818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Wang Z., Wang Y., Yang T., et al. Study of the reparative effects of menstrual-derived stem cells on premature ovarian failure in mice. Stem Cell Res Ther. 2017;8(1):11. doi: 10.1186/s13287-016-0458-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Manshadi M.D., Navid S., Hoshino Y., et al. The effects of human menstrual blood stem cells-derived granulosa cells on ovarian follicle formation in a rat model of premature ovarian failure. Microsc Res Tech. 2019;82(6):635–642. doi: 10.1002/jemt.23120. [DOI] [PubMed] [Google Scholar]
- 187.Zafardoust S., Kazemnejad S., Darzi M., et al. Improvement of pregnancy rate and live birth rate in poor ovarian responders by intraovarian administration of autologous menstrual blood derived- mesenchymal stromal cells: phase I/II clinical trial. Stem Cell Rev Rep. 2020;16(4):755–763. doi: 10.1007/s12015-020-09969-6. [DOI] [PubMed] [Google Scholar]
- 188.Bu S., Wang Q., Zhang Q., et al. Human endometrial mesenchymal stem cells exhibit intrinsic anti-tumor properties on human epithelial ovarian cancer cells. Sci Rep. 2016;6:37019. doi: 10.1038/srep37019. [DOI] [PMC free article] [PubMed] [Google Scholar]