Abstract
Objective:
Determine the risk factors and outcomes of white matter brain injury (WMBI) on magnetic resonance imaging (MRI) at term equivalent age in infants with surgical necrotizing enterocolitis (NEC).
Methods:
Retrospective study compared clinical/pathological information between infants with and those without WMBI.
Results:
Out of 69 infants with surgical NEC, 17 (24.6%) had mild WMBI, 13 (18.8%) had moderate WMBI, and six (8.7%) had severe WMBI on the brain MRI. Several clinical factors (gestational age, more RBC transfusions before NEC onset, pneumoperitoneum, earlier NEC onset age, postoperative ileus, AKI by serum creatinine, post-natal steroids, hospital stay) and histopathological findings (necrosis, hemorrhage) had univariate associations with WMBI. Associations with RBC transfusion (OR 23.6 [95%CI: 4.73–117.97]; p=0.0001), age at NEC onset (OR 0.30 [95%CI: 0.11–0.84]; p=0.021), necrosis (OR 0.10 [95%CI: 0.01–0.90]; p =0.040) and bowel hemorrhage (OR 7.79 [95%CI: 2.19–27.72]; p=0.002) persisted in multivariable association with grade 3–4 WMBI. The infants with WMBI had lower mean motor, cognitive, language scores and higher ophthalmic morbidity at two years of age.
Conclusion:
The WMBI was most likely associated with earlier NEC onset, higher RBC transfusions, and less necrosis and greater hemorrhage lesions on intestinal pathology in preterm infants with surgical NEC.
Introduction
Necrotizing enterocolitis (NEC) is a systemic inflammatory disease with multifactorial etiology affecting about 5–10% of premature infants with a birth weight ≤ 1500 grams [1, 2]. NEC remains a leading cause of morbidity and death among preterm infants and leads to increased costs of care and resource utilization [3–9]. One common form of brain injury in the preterm infant is white matter injury, which has been identified to have multiple risk factors, including lower GA at birth, fetal growth restriction, days ventilation and parenteral nutrition, necrotizing enterocolitis, and male sex [10]. Preterm infants with NEC, especially surgical type, have been shown to have higher inflammatory markers in the blood, severe white matter abnormalities on brain imaging, and adverse neurodevelopmental outcomes at two years of age [11–15]. Animal studies have reported systemic inflammation secondary to NEC leading to neuronal injury via microglial activation, inflammatory pathway activation, and brain barrier disruption [16–19].
The clinical course of infants with surgical NEC is affected by the presence or absence of clinical variables, such as degree of acute kidney injury [20, 21], persistent thrombocytopenia, prolonged parenteral nutrition days needing central line usage, cholestasis, prolonged usage of analgesia/sedation and incomplete resection of necrotic bowel lesions by pediatric surgeons after laparotomy, presence or absence of ileocecal valve, or small bowel resection versus combined resection (small and large bowel resection). These factors may influence the nature and extent of white matter injury on brain MRI and neurodevelopmental outcomes in survivors. We have recently reported that infants with some necrosis within the margins of resected bowel had significantly greater mortality (34; 46.6%) and longer hospital stay[22]. A recent study has shown that children with short bowel syndrome with intact ileocecal valves have increased bacterial diversity than patients without ileocecal valves [23], which may modulate the neuroinflammatory axis. Therefore, infants with surgical NEC are at risk of both white and grey matter brain injury that could relate to their pre-, peri-, and post-operative clinical course and intestinal microbiome health.
In the current literature, there is no study combining clinical, postoperative course, and pathology findings in identifying the subgroup of infants with surgical NEC at higher risk of white matter brain injury and poor neurodevelopmental outcomes at two years of corrected age. This study aims to determine the clinical and histopathological risk factors ( Figure 1) for white matter brain injury on MRI brain at term equivalent age in preterm infants with surgical NEC.
Figure 1:
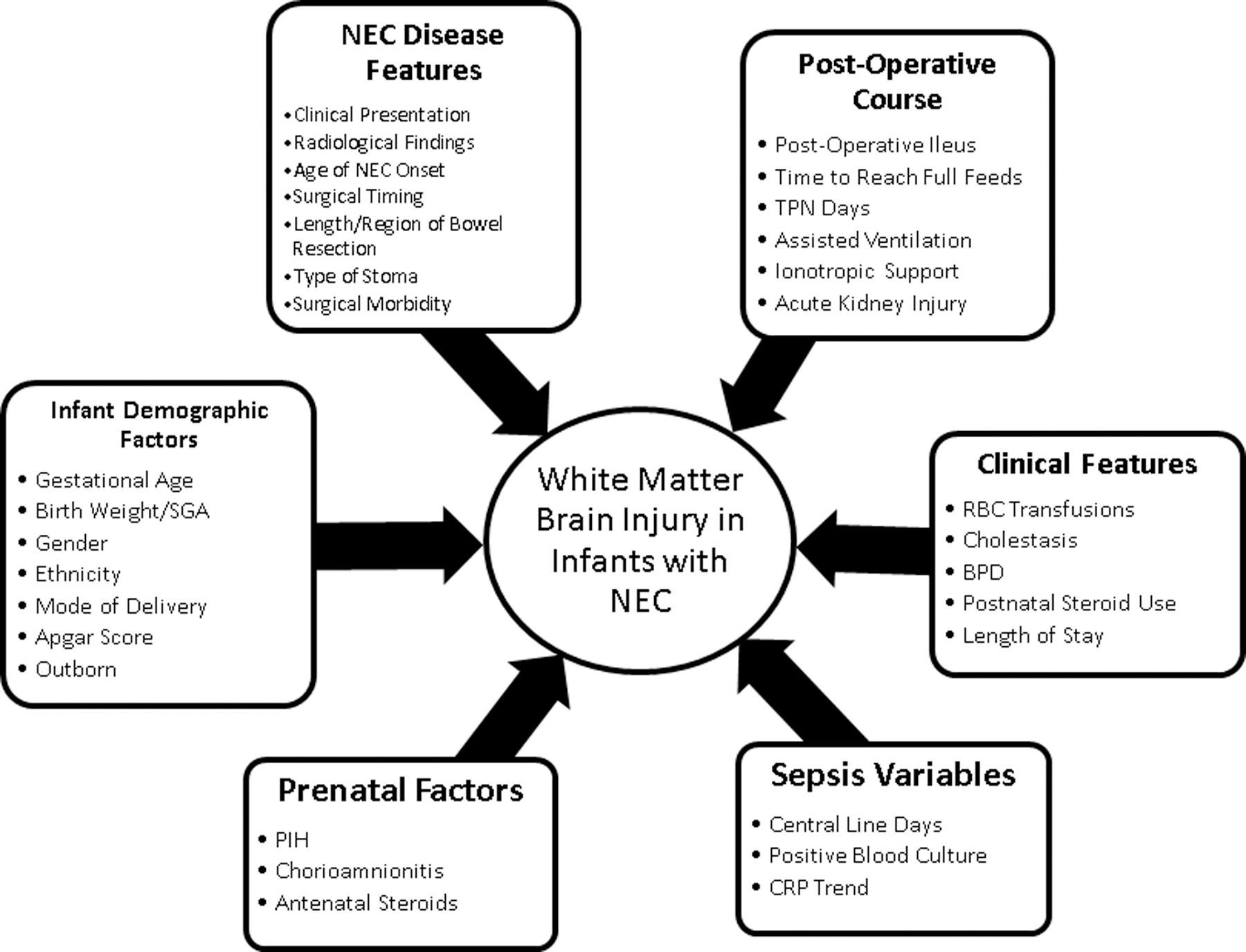
The diagram showing a summary selection of clinical variables in neonates with surgical NEC.
Methods:
This retrospective study was conducted at the University of Mississippi Medical Center (UMMC) at Jackson, Mississippi, after approval by the Institutional Review Board (2017–0127). The protocol was compliant with the Health Insurance Portability and Accountability Act of 1996. The UMMC houses a level 4 neonatal intensive care unit (NICU), which is a regional referral center for neonates with surgical NEC in the entire state. A detailed review of the electronic medical records identified 243 patients with medical and surgical NEC (NEC Bell stage II and above)[24] who underwent NEC management between January 2013 and December 2018. We identified 69 infants with surgical NEC qualifying for the study (see figure 2).
Figure 2:
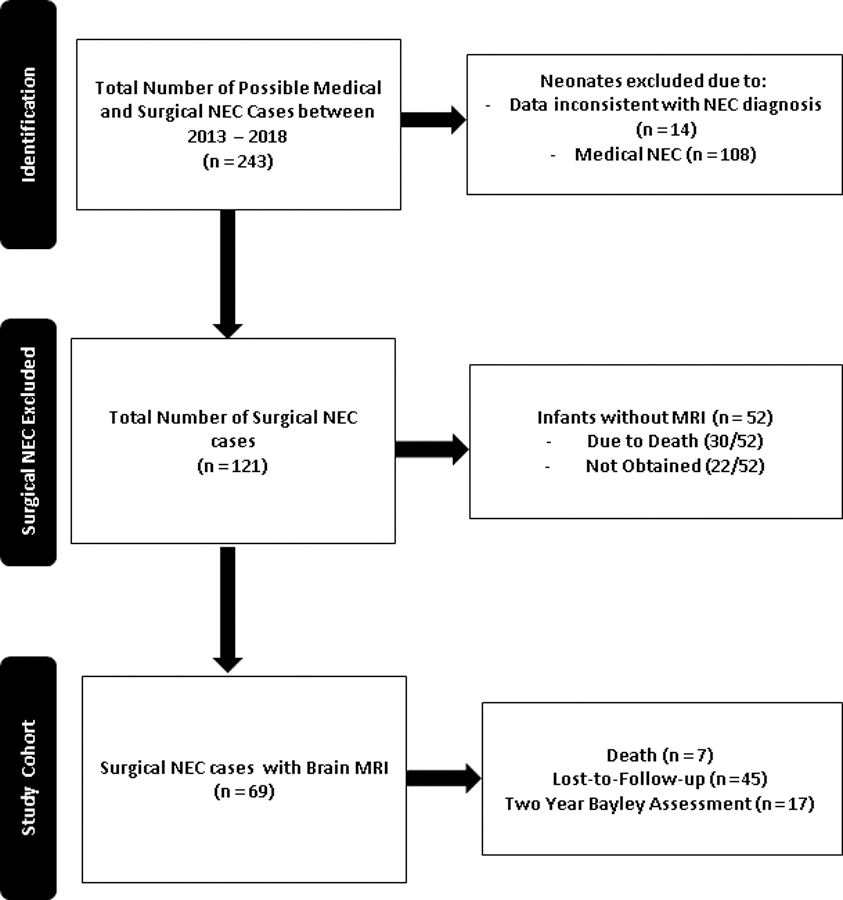
Patient flow algorithm for included, excluded, and enrolled neonates with surgical necrotizing enterocolitis
Clinical information:
We recorded demographic characteristics including birth weight, gestational age, sex, race (African-American, Caucasian, or Latino), and mode of delivery (C-section / Vaginal delivery), APGAR scores at 5 minutes, outborn status, and small for gestational age status. We collected information regarding maternal factors, including pregnancy-induced hypertension, chorioamnionitis, and antenatal steroids.
NEC information:
We noted the NEC features such as the age of onset, pneumatosis, and clinical presentation (abdominal distension, feeding intolerance, and bloody stools). The NEC diagnosis was made by abdominal X-ray by board-certified pediatric radiologists based upon radiological NEC findings such as pneumatosis, pneumoperitoneum, and portal venous gas. We recorded information on Penrose drain, time to laparotomy, length and region of bowel resected, types of stoma creation after NEC surgery. Our surgical team and their approach to the surgical management NEC remained unchanged throughout the study period.
Histopathological Evaluation:
Hematoxylin & eosin-stained surgical resected intestinal tissue sections were evaluated for necrosis, inflammation, hemorrhage, and reparative changes. A score of 0 was assigned when the exam appeared normal, 1 for 1–25% necrosis/ inflammation, 2 when 25–50% area involved, 3 when 50–75% area was affected, and 4 when >75% changes were seen. The two-trained pathologist who was blinded to the clinical course of the infant analyzed the histopathology slides.
Postoperative Morbidity:
To assess postoperative morbidity, we recorded the duration of postoperative ileus, days of parenteral nutrition days, development of short bowel syndrome (SGS), and time to achieve full feeds. Short bowel syndrome was defined as infants requiring TPN at discharge or more than 90 days after NEC onset. Days of parenteral nutrition were defined as the interval between postoperative day 0 until full enteral feedings were achieved (defined as 120 ml/kg/day). Surgical morbidity was classified as surgical site infections (including dehiscence and abscesses), strictures, fistulas, adhesions, and perforations.
We recorded information on the length of stay and mortality. The length of stay was defined as the total hospitalization duration from the day of admission until discharge or death. Mortality was defined as death due to any cause before hospital discharge.
Broncho-pulmonary dysplasia (BPD) data:
We also collected data on bronchopulmonary dysplasia status at 36 weeks and type of steroid hydrocortisone/dexamethasone) used during the clinical course. Bronchopulmonary Dysplasia is classified as mild, moderate, and severe based on the oxygen requirement at the time of assessment[25].
Hematology information:
We recorded complete blood cell count results from the electronic chart before the NEC onset (last available CBC inpatient record before NEC onset), on the day of NEC onset, 24 hours, and 48 hours after onset. We collected data on relative (presented as percentages) as well as on the absolute values. If we had multiple CBC on the same day, we recorded data from what we judged to be the most abnormal. We also collected data on platelet and RBC transfusion before and after the NEC onset
Renal function data:
We captured all serum creatinine (SCr) measurements and daily urine output (UOP) before and five days after NEC onset. After NEC onset, the incidence of AKI was determined using the modified neonatal staging criteria as previously described in the Kidney Disease: Improving Global Outcomes (KDIGO) Clinical Practice Guideline for AKI [26–30].
Neonatal MRI data:
All MRI brain scans (without contrast) were scored independently by two pediatric neuroradiologists unaware of the infants’ clinical course. Our NICU standard of care is to obtain a brain MRI at 36 weeks, corrected age, or before discharge whenever clinically feasible in neonates with a birthweight less than 1500 grams. The most common reason the MRI was not obtained was death or transfer to a quaternary center for bowel transplantation (usually the University of Nebraska Medical Center.) Hence, it is not likely that the missingness of MRI testing is random but is associated with more severe disease and worse outcomes. To further examine this issue, we have compared available baseline features and outcomes of those neonates with and without MRI.
We used a standardized scoring system as used by Woodward et al. and consisting of eight 3-point scales [12]. White-matter brain injury was graded according to five scales, which assessed the nature and extent of white-matter signal abnormality, the loss in the volume of periventricular white matter, and the extent of any cystic abnormalities, ventricular dilatation, or the thinning of the corpus callosum. Gray-matter brain injury was graded according to three scales, which assessed the extent of gray-matter signal abnormality, the quality of gyral maturation, and the subarachnoid space’s size. Composite white-matter scores and composite gray matter scores were created and used to categorize infants according to the extent of their cerebral abnormalities. The categories of white matter brain injury were none (a score of 5 to 6), mild (a score of 7 to 9), moderate (a score of 10 to 12), and severe (a score of 13 to 15). Gray matter was categorized as normal (a score of 3 to 5) or abnormal (a score of 6 to 9). We also assessed cerebellar lesions on MRI.
In our study, the pre-discharge brain MRI was not obtained in 30/121 infants due to death before discharge. Out of 22/52 infants, the MRI was not obtained due to transfer to a quaternary center for bowel transplantation (n=7), and 15 infants were discharged home without an MRI. Given this is a retrospective study, the reasons were not documented in many of these cases (Figure 2).
Cranial Ultrasound:
A trained pediatric neuro-radiologist recorded data on cranial ultrasound (CUS) findings from the patient’s clinical record. The information was collected on the presence and extent of white-matter echolucency or cystic periventricular leukomalacia, ventriculomegaly, and the highest grade of intraventricular hemorrhage before and after the NEC onset.
We selected WMBI on MRI as our primary outcome. We chose MRI because 1) we had a more consistent practice for obtaining MRIs at the time of discharge than CUS, 2) MRI findings are more sensitive and specific and temporally stable than CUS for white matter brain injury, and 3) the timing of CUS both predates and follows NEC onset in our cohort. While we report our CUS findings, we chose not to increase complexity by simultaneously focusing on these as potential outcome measures or covariates.
Neurodevelopment assessment at two years of age:
At our center, infants underwent a comprehensive neurodevelopmental evaluation conducted by child development specialists using Bayley Scales of Infant Development (BSID-III) during the study period who were well aware of the MRI findings and the clinical course. We recorded cognitive and psychomotor development assessment scores. The Mental Development Index (MDI) assesses environmental responsiveness and sensory and perceptual abilities, memory, learning, and early language and communication abilities; the Psychomotor Development Index (PDI) assesses gross and fine motor skills. In our study, 17 infants were evaluated at 2-year neurodevelopmental follow-up. Out of 52 infants who did not follow up at two years of age, seven infants had died, and 45 infants were lost to follow-up, as summarized in Figure 2.
Statistical Methods:
Normally distributed continuous variables are summarized as means and standard deviations (± SD). Comparisons between normally distributed continuous measures for those with and without WMBI were performed using Student’s t-test for equal variance cases and Welch’s unequal variances t-test for unequal variances. For continuous data exhibiting non-normal distributions by Cramér-von Mises test, medians with interquartile range (IQR) [1st quartile; 3rd quartile] are presented, and differences were tested using the Kruskal-Wallis test. Categorical data were summarized as counts with relative frequencies as percentages, and differences in the groups were analyzed using the Chi-squared test (χ² test) or Fisher’s exact test and the Fisher-Freeman-Halton extension for IxJ tables when expected cell counts fail to meet the Chi-squared test criterion.[31]
Univariate logistic regression analyses examined the unadjusted association between each of the risk factors and WMBI. Logistic regression analyses compared clinical and pathological findings among neonates with stage 1–2 WMBI to those with more severe stage 3–4 WMBI. For continuous factors, all odds ratios and their 95% confidence intervals are uniformly expressed per one standard deviation of the factor. Multivariate logistic regression models were used to evaluate the adjusted associations between WMBI and clinical-histological factors, using the absence of white matter brain injury as the reference. Multivariate logistic regressions also were used to assess the relationship between the WMBI grade 3 or 4 and clinical-histological factors with grades 1 and 2 as the reference group. Multivariate models were derived using the forward stepwise approach with a p-value = 0.6 threshold for entry and <0.05 for retention in the models. Ordinal factors were redefined as binary variables in the multivariate models. Four patients missing histopathological data were excluded from all analyses involving histological variables. While missing clinical data was minimal, multiple imputations were performed using the SAS PROC MI regression method, which imputes a subject’s missing data through random value draws from multivariable models predictive of the missing factors conditional on the subject’s known data.[32] While no sufficiently detailed external validation test sample was available, in-sample bootstrap model validation demonstrates good discrimination and model fits assessed by optimism corrected c-statistic (e.g., optimism corrected c-statistic = 0.76 for the final clinical and histopathological multivariable logistic model), calibration plots, and Hosmer-Lemeshow Goodness of Fit tests.[33] All tests were two-sided, and a p-value < 0.05 was considered statistically significant. The statistical analyses were performed in SAS 9.4 statistical software.
Results
Demographic Variables:
Sixty- nine infants were included in the study. The cohort had a mean gestational age of 26.5 ± 2.7 weeks, a mean birth weight of 927 ± 484 grams, was predominantly male (n=46, 66.7%), African-American (n=53, 76.8%), and out-born (n=42, 60.9%). Additional clinical and demographic characteristics are summarized in Table 1.
Table 1 :
Clinical and demographic characteristics of cohort of neonates with surgical NEC from 2013–2018
| N | All | WMBI N=36 |
No WMBI N=33 |
p value | |
|---|---|---|---|---|---|
| Prenatal Information | |||||
| Pregnancy-Induced Hypertension, n (%) | 69 | 18 (26.1) | 9 (25.0) | 9 (27.3) | 0.830 |
| Chronic Hypertension, n (%) | 62 | 11 (17.7) | 4 (12.1) | 7 (24.1) | 0.319 |
| Chorioamnionitis, n (%) | 69 | 8 (11.6) | 3 (8.3) | 5 (15.2) | 0.466 |
| Antenatal Steroids, n (%) | 68 | 53 (77.9) | 30 (83.3) | 23 (71.9) | 0.255 |
| Infant Demographics | |||||
| Gestational Age (weeks); (mean ± SD) | 69 | 26.5 (2.7) | 25.9 (2.1) | 27.1 (3.2) | 0.139 |
| Birth Weight (grams); (mean ± SD) | 69 | 927 (484) | 858 (300) | 1002 (623) | 0.862 |
| Small for Gestational Age, n (%) | 68 | 20 (29.4) | 5 (14.3) | 15 (45.5) | 0.005 |
| Male Gender, n (%) | 69 | 46 (66.7) | 23 (63.9) | 23 (69.7) | 0.609 |
| Race, n (%) | 69 | 0.359 | |||
| Caucasian | 12 (17.4) | 6 (16.7) | 6 (18.2) | ||
| Latino | 2 (2.9) | 0 (0.0) | 2 (6.1) | ||
| African American | 53 (76.8) | 28 (77.8) | 25 (75.8) | ||
| Other | 2 (2.9) | 2 (5.6) | 0 (0.0) | ||
| Mode of Delivery, n (%) | 69 | 0.096 | |||
| C-section | 49 (71.0) | 22 (61.1) | 27 (81.2) | ||
| Vaginal | 20 (29.0) | 14 (38.9) | 6 (18.2) | ||
| Apgar Score <6 at 5 Minutes, n (%) | 69 | 20 (29.0) | 10 (27.8) | 10 (30.3) | 0.817 |
| Outborn, n (%) | 69 | 42 (60.9) | 20 (55.6) | 22 (66.7) | 0.345 |
| Infant Medical Information Prior to NEC | |||||
| Patent Ductus Arteriosus, n (%) | 69 | 44 (63.8) | 25 (69.4) | 19 (57.6) | 0.306 |
| Patent Ductus Arteriosus, Indomethacin, n (%) | 69 | 9 (13.0) | 7 (19.4) | 2 (6.1) | 0.154 |
| Platelet Transfusion Before NEC, n (%) | 69 | 49 (72.0) | 26 (72.2) | 23 (69.7) | 0.817 |
| Red blood Cell Transfusion Before NEC, n (%) | 56 | 16 (28.6) | 14 (41.2) | 2 (9.1) | 0.009 |
| NEC Disease Features | |||||
| Clinical Presentation, n (%) | 69 | 0.615 | |||
| Abdominal Distension | 64 (92.8) | 32 (88.9) | 32 (97.0) | ||
| Bloody Stools | 3 (4.3) | 2 (5.6) | 1 (3.0) | ||
| Feeding Intolerance | 2 (2.9) | 2 (5.6) | 0 (0.0) | ||
| Radiological Findings, n (%) | |||||
| Pneumatosis | 69 | 27 (39.1) | 11 (30.6) | 16 (48.5) | 0.127 |
| Pneumoperitoneum | 69 | 39 (56.5) | 25 (69.4) | 14 (42.4) | 0.024 |
| Portal Venous Gas | 69 | 3 (4.3) | 2 (5.6) | 1 (3.0) | 1.000 |
| Age of NEC Onset (days); (mean ± SD) (median [IQR]) |
69 | 17.4 (15.9) 11 [6–24] |
13.4 (14.8) 8.5 [4.5–14] |
21.6 (16.3) 16 [8–31] |
0.008 |
| Penrose Drain Present, n (%) | 65 | 28 (43.1) | 16 (44.4) | 12 (41.4) | 0.643 |
| Surgery Timing | |||||
| Surgery < 48 Hours, n (%) | 64 | 43 (67.2) | 25 (71.4) | 18 (62.1) | 0.427 |
| Surgery > 48 Hours, n (%) | 64 | 21 (32.8) | 10 (28.6) | 11 (37.9) | 0.427 |
| Length of Bowel Resected (cm; mean ± SD) | 67 | 20.9 (21.4) | 22.0 (21.1) | 19.6 (22.0) | 0.712 |
| Region of Bowel Resected, n (%) | 64 | 0.136 | |||
| Small Bowel Resected | 42 (65.6) | 25 (71.4) | 17 (58.6) | ||
| Large Bowel Resected | 2 (3.1) | 2 (5.7) | 0 (0.0) | ||
| Combined Large and Small Bowel Resected | 20 (31.3) | 8 (22.9) | 12 (41.4) | ||
| Ileocecal Valve Present, n (%) | 68 | 49 (72.1) | 26 (72.2) | 23 (71.9) | 0.673 |
| Type of Stoma | 66 | 0.247 | |||
| Ileostomy, n (%) | 39 (56.5) | 23 (63.9) | 16 (48.5) | ||
| Colostomy, n (%) | 3 (4.3) | 1 (2.8) | 2 (6.1) | ||
| Jejunostomy, n (%) | 21 (30.4) | 9 (25.0) | 12 (36.4) | ||
| Combined Stoma, n (%) | 3 (4.3) | 3 (8.3) | 0 (0.0) | ||
| Short Bowel Syndrome, n (%) | 60 | 34 (56.7) | 19 (59.4) | 15 (53.6) | 0.455 |
| Surgical Morbidity (Infection, Adhesions, Strictures, Dehiscence), n (%) | 69 | 27 (39.1) | 12 (33.3) | 15 (45.5) | 0.302 |
| Post-Operative Intestinal Features | |||||
| Post-Operative Ileus Days (days); (median [IQR]) |
66 | 13 [9–16] | 14 [11–20] | 11 [8–14] | 0.031 |
| Post-Operative Day at Starting Enteral Feedings (days); (median [IQR]) |
65 | 14 [10–18] | 14 [13–20] | 12 [8.5–15.5] | 0.039 |
| Day Attainment of Full Enteral Feedings (120 mL/kg); (median [IQR]) |
61 | 69 [30–89] | 74.5 [30–107] | 61 [27–79] | 0.094 |
| Duration of Parenteral Nutrition (days); (median [IQR]) |
69 | 97 [65–137] | 116 [71.5–159] | 86 [58–117] | 0.071 |
| Post-Operative Systemic Course | |||||
| Assisted Ventilation (intubated), n (%) | 67 | 59 (88.1) | 30 (85.7) | 29 (90.6) | 0.711 |
| 24h Ionotropic Support, n (%) | 68 | 52 (76.5) | 27 (75.0) | 25 (78.1) | 0.762 |
| AKI by Serum Creatinine, n (%) | 61 | 0.027 | |||
| Normal | 29 (47.5) | 15 (48.4) | 14 (46.7) | ||
| Stage 1 | 12 (19.7) | 10 (32.3) | 2 (6.7) | ||
| Stage 2 | 9 (14.8) | 2 (6.5) | 7 (23.3) | ||
| Stage 3 | 11 (18.0) | 4 (12.9) | 7 (23.3) | ||
| AKI by Urine Output, n (%) | 61 | 0.564 | |||
| Normal | 35 (57.4) | 20 (64.5) | 15 (50.0) | ||
| Stage 1 | 2 (3.3) | 1 (3.2) | 1 (3.3) | ||
| Stage 2 | 17 (27.9) | 8 (25.8) | 9 (30.0) | ||
| Stage 3 | 7 (11.5) | 2 (6.5) | 5 (16.7) | ||
| Sepsis Variables | |||||
| Central Line Present (days; mean ± SD) | 66 | 63.5 (41.6) | 67.0 (46.2) | 59.5 (36.0) | 0.812 |
| Positive Blood Culture Sepsis, n (%) | 69 | 24 (34.8) | 13 (36.1) | 11 (33.3) | 0.809 |
| Gram Positive Sepsis, n (%) | 69 | 13 (18.8) | 8 (22.2) | 5 (15.2) | 0.453 |
| Gram Negative Sepsis, n (%) | 69 | 8 (11.6) | 4 (11.1) | 4 (12.1) | 1.000 |
| Positive Blood Culture Sepsis 7 Days after NEC Diagnosis to Discharge, n (%) | 26 | 7 (26.9) | 2 (14.3) | 5 (41.7) | 0.658 |
| CRP on Day of NEC Onset (mean ± SD) | 58 | 7.7 (8.9) | 6.6 (8.3) | 9.0 (9.6) | 0.072 |
| CRP at 1 Week after NEC Onset (mean ± SD) | 47 | 7.9 (9.3) | 8.4 (10.6) | 7.4 (8.4) | 0.822 |
| Cholestasis at NEC Onset, n (%) | 66 | 44 (66.7) | 24 (68.6) | 20 (64.5) | 0.164 |
| BPD, n (%) | 61 | 53 (86.9) | 30 (93.8) | 23 (79.3) | 0.026 |
| Postnatal Use of Steroids, n (%) | 69 | 42 (60.9) | 26 (72.2) | 16 (48.5) | 0.044 |
| Discharge | |||||
| Length of Stay (days); (median [IQR]) |
69 | 161 [109–186] | 173.5 [123–205.5] | 133 [94–171] | 0.038 |
| Death, n (%) | 69 | 6 (8.7) | 4 (11.1) | 2 (6.1) | 0.675 |
Abbreviations: NEC = Necrotizing Enterocolitis; AKI = Acute kidney Injury, CRP = C -reactive protein, WMBI (white matter Brain injury)
Categorical variables are presented as count (column percentage). Continuous variables are presented as mean (standard deviation) and, if not normally distributed, as median with interquartile range. If normality criteria were satisfied, differences in continuous measures’ statistical associations with WMA were tested using a t-test for equal or Welch t-test for unequal variances. When the normality assumption was not satisfied, continuous measures’ statistical associations with WMA were tested with the Kruskal-Wallis Test. Differences in categorical measures’ associations with WMA were tested using the Chi-square test when cell counts were adequate, otherwise Fisher’s Exact and Fisher-Freeman-Halton (FFH) were used with low expected cell counts. All tests were two-sided.
NEC features:
The mean age of NEC onset was 17.4±15.9 days. The abdominal distension was the most common presenting symptom (n=64, 92.8%). The abdominal radiograph showed pneumatosis (n=27, 39.1%), pneumoperitoneum (n=39, 56.5%) and portal venous gas in three (4.3%) cases. The small bowel was resected in 42 (65.6%), and 20 cases (31.3%) had combined (small +large) intestinal resection. Twenty-eight infants (43.1%) had Penrose drain before or post laparotomy. Ileostomy (n=39, 56.5 %) and Jejunostomy (n=21, 30.4%) were the most common stoma after surgery. The additional details have been summarized in Table 1.
Radiology Findings
Brain MRI
Of the 69 infants, 36/69 (47.8%) had normal WMBI, 17/69 (24.6%) had mild WMBI, 13/69 (18.8%) had moderate WMBI, and 6/69 (8.7%) had severe WMBI. The cohort predominantly showed grade one loss of periventricular volume (n=34/68, 49.3%), grade one ventricular dilatation (n=33/68, 47.8%), grade one thinning of corpus callosum (n=35/68, 50.7%), grade one cystic abnormality (n=52/68, 75.4%) on brain MRI. See Table. 2 and Figure 3.
Table 2:
White matter, Grey matter and Cerebellar lesions information from brain MRI and cranial ultrasound
| N | All | WMA | No WMA | p value | |
|---|---|---|---|---|---|
| White Matter Injury (Total Score), n (%) | |||||
| Normal (5–6) | 69 | 33 (47.8) | 33 (47.8) | ||
| Mild (7–9) | 17 (24.6) | 17 (24.6) | |||
| Moderate (10–12) | 13 (18.8) | 13 (18.8) | |||
| Severe (13–15) | 6 (8.7) | 6 (8.7) | |||
| Loss of Periventricular Volume, n (%) | 68 | <0.0001 | |||
| Grade 1 | 34 (49.3) | 2 (2.9) | 32 (47.1) | ||
| Grade 2 | 28 (40.6) | 28 (41.2) | 0 | ||
| Grade 3 | 6 (8.7) | 6 (8.8) | 0 | ||
| Ventricular Dilation, n (%) | <0.0001 | ||||
| Grade 1 | 68 | 33 (47.8) | 3 (4.4) | 30 (44.1) | |
| Grade 2 | 27 (39.1) | 25 (36.8) | 2 (2.9) | ||
| Grade 3 | 8 (11.6) | 8 (11.8) | 0 | ||
| Thinning of Corpus Callosum, n (%) | 68 | <0.0001 | |||
| Grade 1 | 35 (50.7) | 3 (4.4) | 32 (47.1) | ||
| Grade 2 | 26 (37.7) | 26 (38.2) | 0 | ||
| Grade 3 | 7 (10.1) | 7 (10.3) | 0 | ||
| White Matter Signal Abnormality, n (%) | 69 | <0.0001 | |||
| Normal | 41 (59.4) | 8 (11.6) | 33 (47.8) | ||
| Mild | 14 (20.3) | 14 (20.3) | 0 | ||
| Moderate | 14 (20.3) | 14 (20.3) | 0 | ||
| Extent of Cystic Abnormality, n (%) | 68 | 0.0002 | |||
| Grade 1 | 52 (75.4) | 21 (30.9) | 31 (45.6) | ||
| Grade 2 | 11 (15.9) | 10 (14.7) | 1 (1.5) | ||
| Grade 3 | 5 (7.2) | 5 (7.4) | 0 | ||
| Grey Matter Abnormality, n (%) | 68 | 0.012 | |||
| Normal | 61 (88.4) | 29 (42.6) | 32 (47.1) | ||
| Abnormal | 7 (10.1) | 7 (10.3) | 0 | ||
| Loss of Subarachnoid Space, n (%) | 68 | 0.007 | |||
| Grade 1 | 56 (81.2) | 25 (36.8) | 31 (45.6) | ||
| Grade 2 | 10 (14.5) | 9 (13.2) | 1 (1.5) | ||
| Grade 3 | 2 (2.9) | 2 (2.9) | 0 | ||
| Extent of Grey Matter Signal Abnormality, n (%) | 67 | 0.006 | |||
| Grade 1 | 59 (85.5) | 28 (41.8) | 31 (46.3) | ||
| Grade 2 | 6 (8.7) | 6 (9.0) | 0 | ||
| Grade 3 | 2 (2.9) | 2 (5.6) | 0 | ||
| Quality of Gyral Maturation, n (%) | 68 | 0.008 | |||
| Grade 1 | 60 (87.0) | 28 (41.2) | 32 (47.1) | ||
| Grade 2 | 6 (8.7) | 6 (8.8) | 0 | ||
| Grade 3 | 2 (2.9) | 2 (2.9) | 0 | ||
| Cerebellar Injury, n (%) | 65 | <0.0001 | |||
| Normal | 16(24.6) | 28 (43.1) | |||
| Abnormal | 21 (30.4) | 19 (29.2) | 2 (3.1) | ||
| Head US After NEC | |||||
| IVH, n (%) | 58 | 0.019 | |||
| Normal | 29 (42.0) | 14 (24.1) | 15 (25.9) | ||
| Grade 1 | 6 (8.7) | 4 (6.9) | 2 (3.4) | ||
| Grade 2 | 9 (13.0) | 3 (5.2) | 6 (10.3) | ||
| Grade 3 | 6 (8.7) | 5 (8.6) | 1 (1.7) | ||
| Grade 4 | 8 (11.6) | 8 (13.8) | 0 | ||
| Ventriculomegaly, n (%) | 58 | <0.0001 | |||
| Normal | 34 (49.3) | 10 (17.2) | 24 (41.4) | ||
| Grade 1 | 2 (2.9) | 2 (3.4) | 0 | ||
| Grade 2 | 12 (17.4) | 12 (20.7) | 0 | ||
| Grade 3 | 6 (8.7) | 6 (10.3) | 0 | ||
| Grade 4 | 4 (5.8) | 4 (6.9) | 0 | ||
| Periventricular Echogenicity, n (%) | 58 | 0.002 | |||
| Present | 11 (19.0) | 11 (19.0) | 0 (0.0) | ||
| Absent | 47 (81.0) | 23 (39.7) | 24 (41.4) | ||
| Periventricular Cyst, n (%) | 57 | 0.0003 | |||
| Present | 13 (22.8) | 13 (22.8) | 0( 0.0) | ||
| Absent | 44 (77.2) | 20 (35.1) | 24 (42.1) | ||
| Porencephalic Cyst, n (%) | 58 | 0.034 | |||
| Present | 7 (12.1) | 7 (12.1) | 0 (0.0) | ||
| Absent | 51 (87.9) | 27 (46.6) | 24 (41.4) | ||
| Cystic Periventricular Leukomalacia, n (%) | 58 | 0.0003 | |||
| Present | 13 (22.4) | 13 (22.4) | 0 (0.0) | ||
| Absent | 45 (77.6) | 21 (36.2) | 24(41.4) | ||
Abbreviations: NEC = Necrotizing Enterocolitis; WMA =White matter abnormality
Categorical variables are presented as count (percentage). Statistical significance of differences in categorical measures’ associations with WMA were tested using the two-sided Fisher’s Exact test or generalized Fisher-Freeman-Halton (FFH) Exact test.
Figure 3.
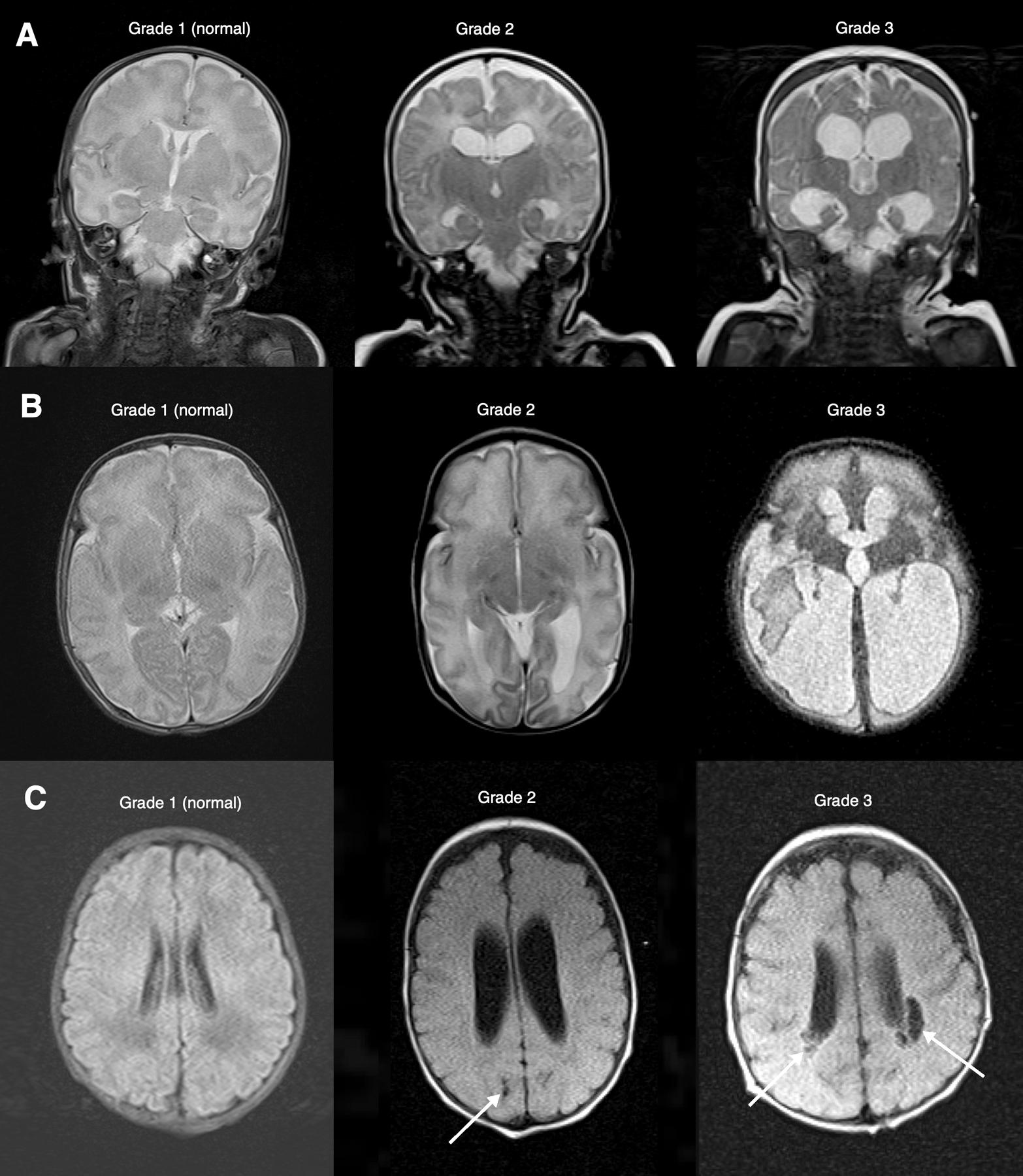
Examples of parameters used for grading white matter abnormalities*. A. Ventricular dilatation. Coronal T2 MRI through the frontal and temporal horns. Grade 1 is normal without ventricular dilatation. Grade 2 shows ventricular enlargement with mild rounding of the frontal horns and minimal enlargement of the temporal horns. Grade 3 shows a significant enlargement of the frontal and temporal horns. B. Periventricular white matter volume loss. Axial T2 MRI at the level of the occipital horns. Grade 1 is normal white matter volume. Grade 2 is mild white matter volume loss with a mild to moderate increase in ventricular size. Grade 3 is a marked reduction in white matter volume, often with severe enlargement of the ventricle. C. Cystic abnormalities. Axial FLAIR MRI at the level of the lateral ventricles. Grade 1 is normal without cystic abnormality. Grade 2 features only a single small (less 2 mm) cyst (arrow). Grade 3 is multiple cysts or a single larger cyst (arrows).
*Adapted from Woodward LJ, Anderson PJ, Austin NC, et al. Neonatal MRI to predict neurodevelopmental outcomes in preterm infants. N Engl J Med 2006;355:685–94.
Seven infants (10.1%) had grey matter abnormality. The included patients mainly showed grade one loss of subarachnoid space (n=56, 81.2%), grade one grey matter signal abnormality (n= 59, 85.5%) and grade one gyral maturation changes (n=60, 87%). Twenty-one infants had a cerebellar injury (30.4%). See Table 2.
Head Sonography
The most frequent head ultrasound lesions after NEC onset were any grade (1–4) intraventricular hemorrhage (n= 29/58, 50%) and grade 1–4 ventriculomegaly (24/58, 41.4%) in the entire cohort. The periventricular echogenicity (n=11/58, 19.0%), periventricular cyst (n=13, 22.8%), and cystic periventricular leukomalacia (n=13, 22.4%) were other lesions noted. The periventricular cyst (13/33, 39.4%) and cystic periventricular leukomalacia lesions (13/34, 38.2%) were significantly more frequent in those with WMBI. The white matter injury, grey matter injury, and head sonography findings have been summarized in Table 2.
Pathology
The cohort had a mean necrosis score of 1.5 ± 1.3, an inflammatory score of 1.5 ± 0.7, and a mean hemorrhage score of 2.2 ± 1.2 on intestine histopathological specimen’s assessment. Table 3 shows the histopathological findings of the resected intestine in infants with surgical necrotizing enterocolitis. Compared to infants with grade 1–2 WMBI, infants with grade 3–4 WMBI had significantly lower necrosis (1.1 ±0.9 vs. 2.0 ±1.4; p= 0.018) and higher mean hemorrhagic scores (3.1 ±1.2 vs. 2.0 ±1.0; p= 0.003).
Table 3:
Histopathological changes in the resected intestine
| All | WMA | No WMA |
P
value |
WMA Grade 3–4 | WMA Grade 1–2 |
P
value |
||
|---|---|---|---|---|---|---|---|---|
| Necrosis, n (%) | 65 | 0.678 | 0.021 | |||||
| 0 | 21 (32.3) | 12 (18.5) | 9 (13.8) | 6 (9.2) | 15 (23.1) | |||
| <25% | 11 (16.9) | 7 (10.8) | 4 (6.2) | 7 (10.8) | 4 (6.2) | |||
| 25–50% | 15 (23.1) | 8 (12.3) | 7 (10.8) | 5 (7.7) | 10 (15.4) | |||
| 50–75% | 16 (24.6) | 8 (12.3) | 8 (12.3) | 1 (1.5) | 15 (23.1) | |||
| >75% | 2 (3.1) | 0 | 2 (3.1) | 0 | 2 (3.1) | |||
|
Necrosis (mean ± SD) (median [IQR]) |
65 | 1.5 (1.3) 2 [0–3] |
1.3 (1.2) 2 [0–3] |
1.7 (1.3) 2 [1–3] |
0.213 |
1.1 (0.9) 1 [0–2] |
2.0 (1.4) 2 [0–3] |
0.018 |
| Inflammation, n (%) | 65 | 0.796 | 0.953 | |||||
| 0 | 4 (6.2) | 2 (3.1) | 2 (3.1) | 1 (1.5) | 3 (4.6) | |||
| <25% | 26 (40.0) | 14 (21.5) | 12 (18.5) | 7 (10.8) | 19 (29.2) | |||
| 25–50% | 33 (50.8) | 19 (29.2) | 14 (21.5) | 11 (16.9) | 22 (33.8) | |||
| 50–75% | 1 (1.5) | 0 | 1 (1.5) | 0 | 1 (1.5) | |||
| >75% | 1 (1.5) | 0 | 1 (1.5) | 0 | 1 (1.5) | |||
|
Inflammation (mean ± SD) (median [IQR]) |
65 | 1.5 (0.7) 2 [1–2] |
1.5 (0.6) 2 [1–2] |
1.6 (0.8) 2 [1–2] |
0.322 |
1.6 (0.6) 2 [1–2] |
1.8 (1.0) 2 [1–2] |
0.611 |
| Hemorrhage, n (%) | 64 | 0.103 | 0.0003 | |||||
| 0 | 4 (6.3) | 1 (1.6) | 3 (4.7) | 0 | 4 (6.3) | |||
| <25% | 14 (21.9) | 9 (14.1) | 5 (7.8) | 5 (7.8) | 9 (14.1) | |||
| 25–50% | 26 (40.6) | 11 (17.2) | 15 (23.4) | 2 (3.1) | 24 (37.5) | |||
| 50–75% | 8 (12.5) | 4 (6.3) | 4 (6.3) | 3 (4.7) | 5 (7.8) | |||
| >75% | 12 (18.8) | 10 (15.6) | 2 (3.1) | 9 (14.1) | 3 (4.7) | |||
|
Hemorrhage (mean ± SD) (median [IQR]) |
64 | 2.2 (1.2) 2 [2–3] |
2.5 (1.3) 2 [2–4] |
2.1 (1.1) 2 [2–3] |
0.242 |
3.1 (1.2) 4 [2–4] |
2.0 (1.0) 2 [1–2] |
0.003 |
| Reparative Change, n (%) | 63 | 25 (39.7) | 15 (23.8) | 10 (15.9) | 0.111 | 9 (14.3) | 16 (25.4) | 0.134 |
Abbreviations: WMA = White Matter Abnormality; Categorical variables are presented as counts (percentage). Continuous variables are presented as mean (standard deviation) if normally distributed and with median (interquartile range) if normality does not hold. If normality criteria were satisfied, differences in continuous measures’ statistical associations with WMA were tested using a t-test for equal or Welch’s t-test for unequal variances. When the normality assumption was not satisfied, continuous measures’ statistical associations with WMA were tested with the Kruskal-Wallis Test. Differences in categorical measures’ associations with WMA were tested using the Chi-square test when cell counts were adequate, otherwise Fisher’s Exact test was used with low expected cell counts. All tests were two-sided.
White Matter Brain Injury
Table 1 compares the baseline demographic, NEC, clinical, and laboratory characteristics between neonates with white matter brain injury (mild, moderate, and severe combined) and without WMBI on MRI brain. On bivariate analysis, compared to those without WMBI, the neonates with WMBI had numerically lower gestational age (25.9 ± 2.1 weeks vs. 27.1 ± 3.2 weeks, p=0.139), significantly higher likelihood of having received red blood cell transfusions before NEC onset [n=14 (41.2%) vs. n=2(9.1%); p=0.009], had more evidence of pneumoperitoneum on abdominal X-ray [n=25 (69.4%) vs n=14(42.4%); p=0.024], had earlier age of NEC onset (13.4±14.8 days vs. 21.6 ± 16.3 days; p=0.008) Figure 4.
Figure 4:
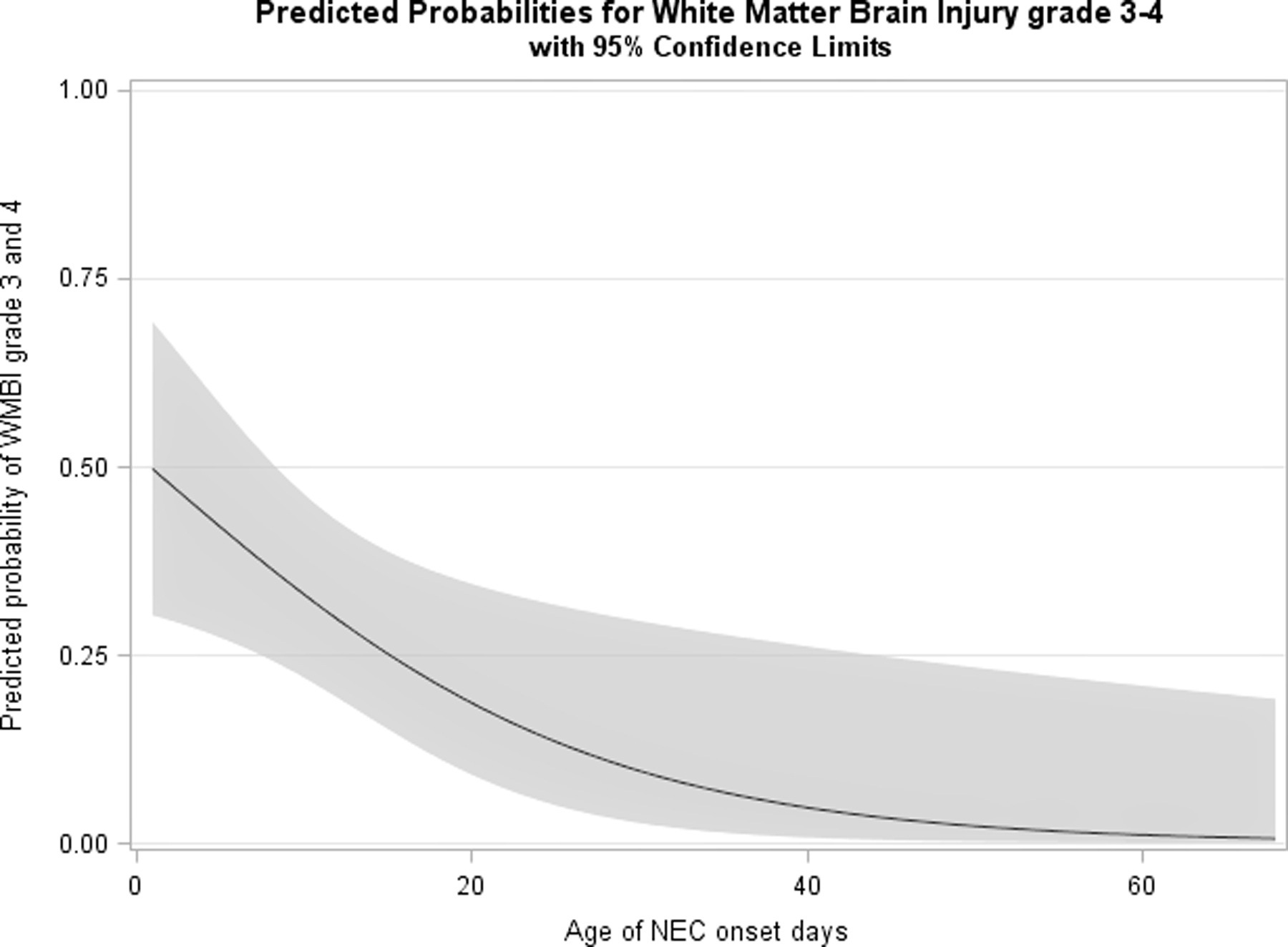
The predictive probability of grade 3–4 WMBI in relation to the age of NEC onset.
Postoperatively, the neonates with WMBI were more likely to have longer-lasting postoperative ileus period (14 days ([IQR: 11–20] vs. 11 days [8–14]; p= 0.031) (Table 1;Figure 5), lower lymphocyte count percentages day 2 after NEC onset [21.8 ±12 vs 30.2 ± 14.3; p=0.014] (Supplemental Table 1), had more acute kidney injury by creatinine (p=0.027), diagnosed more with bronchopulmonary dysplasis at 36 weeks corrected gestational age [n=30 (93.8%) vs. n=23 (79.3%); p=0.026] and received post-natal steroids more frequently [n=26 (72.2%) vs. n=16 (48.5%); p=0.044]. In our cohort, the mean length of stay was 159.3 ± 75.2 days. Compared to those without WMBI, those with WMBI had a longer length of hospitalization (173.5 days [IQR: 123–205.5] vs. 133 days [94–171]; p=0.038). The additional clinical information has been summarized in table 1.
Figure 5:
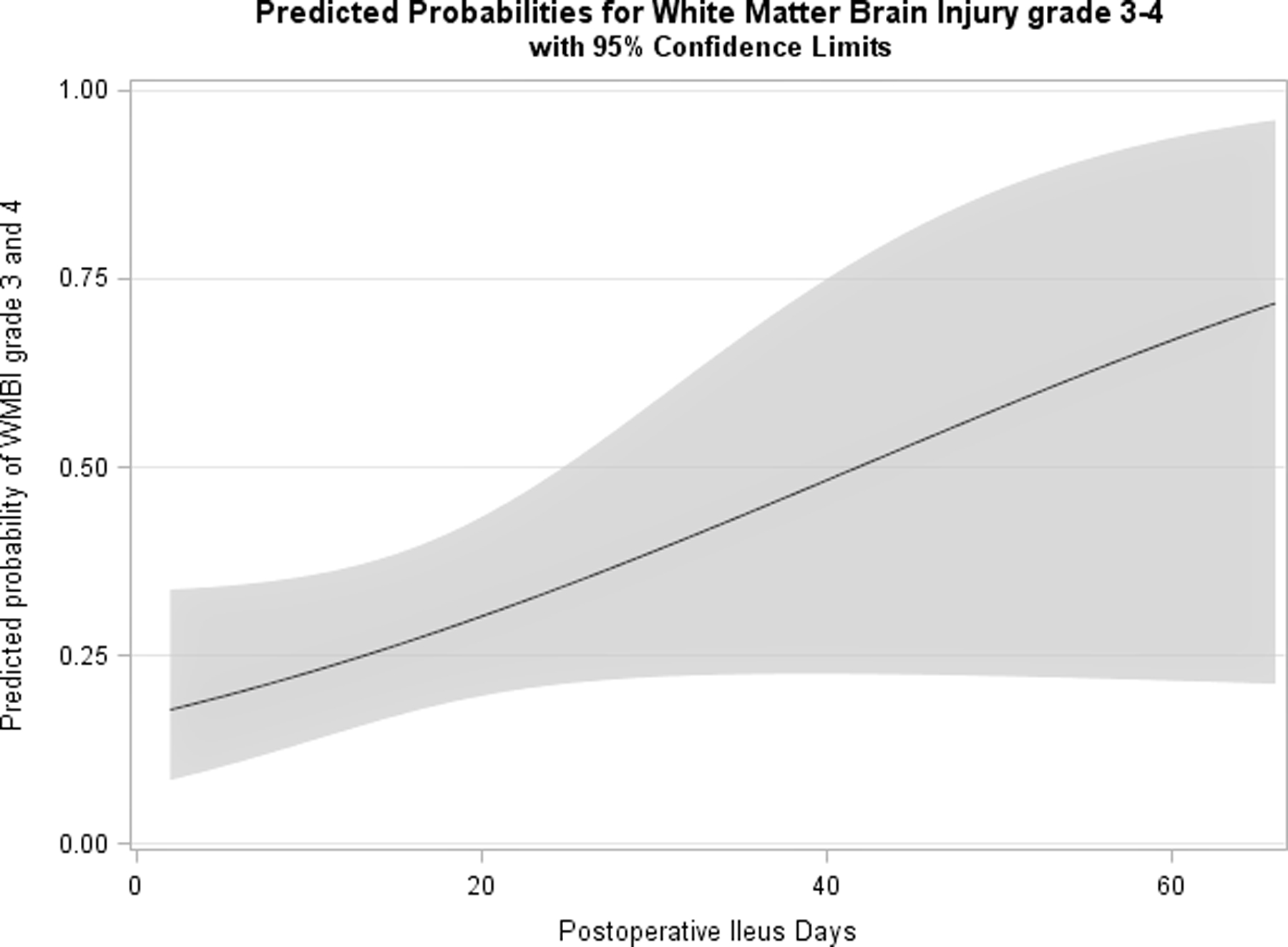
The predictive probability of grade 3–4 WMBI in relation to postoperative ileus days
In our cohort, small for gestational age (OR 0.19 [95%CI: 0.06–0.62]; p=0.006) status at birth and age at NEC onset (OR 0.57 [95%CI: 0.33–0.97]; p=0.040) were significantly associated with lower odds of WMBI. Red blood cell transfusion before NEC onset, pneumoperitoneum on x-ray, and postnatal use of steroids were associated with higher odds of WMBI on univariate logistic regression analysis. On multivariable logistic regression including clinical and histopathological variables in the model, red blood cell transfusion (OR 11.86 [95%CI: 1.89–74.6]; p=0.008) and pneumoperitoneum (OR 4.01 [95%CI: 1.19–13.47]; p=0.025) were independently associated with increased odds of WMBI at term equivalent MRI. Being small for gestational age was independently associated with lower odds (OR 0.10 [95%CI: 0.02–0.45]; p=0.003) of WMBI at term equivalent MRI. The model results have been summarized in Table 4. The clinical factors of red blood cell transfusion before NEC onset (OR 23.6 [95%CI: 4.7–118.0]; p=0.0001) and hemorrhagic lesions on intestinal histopathology (OR 7.79 [95%CI: 2.19–27.72); p=0.002) were associated with higher odds of severe WMBI (grade 3 and grade 4). The preterm infants with later age of NEC onset (OR 0.27 [95%CI: 0.09–0.79]; p=0.016) (Table 4) and lower degree of necrosis (OR 0.57 [95%CI: 0.004–0.82];p =0.036) on the intestinal histopathology had lower odds of grade 3 and 4 WMBI at term equivalent brain MRI. These findings are also summarized in Table 4.
Table 4.
Factors Associated with White Matter Abnormality on Multivariable Regression Analysis
| Factors Associated with any White Matter Abnormality (Present vs. Absent) | Factor Associations with Grade 3 or 4 White Matter Abnormality (Present vs. Absent) | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Clinical Factors | Univariate Associations | Clinical Multivariable Model | Clinical and Histopathologic Model | Univariate Associations | Clinical Multivariable Model | Clinical and Histopathologic Model | ||||||||||||
| Odds Ratio | 95% CI of ORs | p-value | Odds Ratio | 95% CI of ORs | p-value | Odds Ratio | 95% CI of ORs | p-value | Odds Ratio | 95% CI of ORs | p-value | Odds Ratio | 95% CI of ORs | p-value | Odds Ratio | 95% CI of ORs | p- value |
|
| Gestational Age (weeks; SD = 2.7 weeks) | 0.63 | (0.38–1.05) | 0.077 | >0.05 | >0.05 | 0.68 | (0.37–1.25) | 0.218 | >0.05 | >0.05 | ||||||||
| Small for Gestational Age | 0.19 | (0.06–0.62) | 0.006 | 0.10 | (0.02–0.45) | 0.003 | 0.10 | (0.02–0.45) | 0.003 | 0.57 | (0.16–1.98) | 0.374 | >0.05 | >0.05 | ||||
| Mode of Delivery | 2.86 | (0.94–8.69) | 0.063 | >0.05 | >0.05 | 2.30 | (0.75–7.05) | 0.144 | >0.05 | >0.05 | ||||||||
| Patent Ductus Arteriosus, Indomethacin | 3.74 | (0.72–19.50) | 0.117 | >0.05 | >0.05 | 2.40 | (0.57–10.12) | 0.233 | >0.05 | >0.05 | ||||||||
| Red Blood Cell Transfusion Before NEC | 9.86 | (2.03–47.9) | 0.005 | 11.86 | (1.89–74.6) | 0.008 | 11.86 | (1.89–74.6) | 0.008 | 19.71 | (4.94–78.6) | <0.0001 | 23.6 | (4.73–118.0) | 0.0001 | 28.6 | (4.29–190.7) | 0.0005 |
| Pneumatosis | 0.47 | (0.18–1.25) | 0.130 | >0.05 | >0.05 | 0.31 | (0.09–1.08) | 0.065 | >0.05 | >0.05 | ||||||||
| Pneumoperitoneum, (Present vs. Absent) | 3.08 | (1.15–8.30) | 0.026 | 4.01 | (1.19–13.5) | 0.025 | 4.01 | (1.19–13.5) | 0.025 | 2.80 | (0.88–8.95) | 0.082 | >0.05 | >0.05 | ||||
| Age at NEC Onset, (days; SD = 15.9 days) | 0.57 | (0.33–0.97) | 0.040 | >0.05 | >0.05 | 0.29 | (0.11–0.81) | 0.018 | 0.30 | (0.11–0.84) | 0.021 | 0.27 | (0.09–0.79) | 0.016 | ||||
| Region of Bowel Resected, (Large /combined vs. Small) | 0.68 | (0.25–1.83) | 0.442 | >0.05 | >0.05 | 1.55 | (0.52–4.59) | 0.433 | >0.05 | >0.05 | ||||||||
| Post-Operative Ileus Days, (days; SD = 12 days) | 1.18 | (0.72–1.94) | 0.511 | >0.05 | >0.05 | 1.60 | (0.98–2.84) | 0.064 | >0.05 | >0.05 | ||||||||
| Postop Day of Starting Feeds, (days; SD = 14.6 days) | 1.01 | (0.62–1.65) | 0.971 | >0.05 | >0.05 | 1.32 | (0.79–2.22) | 0.295 | >0.05 | >0.05 | ||||||||
| Day to Full Feedings, (days; SD = 42.7 days) | 1.60 | (0.92–2.75) | 0.086 | >0.05 | >0.05 | 1.47 | (0.81–2.53) | 0.198 | >0.05 | >0.05 | ||||||||
| Duration of Parenteral Nutrition, (days; SD = 57.9 days) | 1.50 | (0.89–2.51) | 0.122 | >0.05 | >0.05 | 1.33 | (0.79–2.24) | 0.264 | >0.05 | >0.05 | ||||||||
| AKI by Serum Creatinine, (Stage 2/ 3 vs. Stage 1 or Normal) | 0.85 | (0.33–2.19) | 0.737 | >0.05 | >0.05 | 1.90 | (0.65–5.54) | 0.240 | >0.05 | >0.05 | ||||||||
| BPD, (Grade 3 or 4 vs. Grade 1 or 2) | 2.17 | (0.69–6.86) | 0.185 | >0.05 | >0.05 | 7.71 | (0.94–63.2) | 0.057 | >0.05 | >0.05 | ||||||||
| Postnatal Use of Steroids, (Yes vs. No) | 2.76 | (1.02–7.50) | 0.046 | >0.05 | >0.05 | 1.57 | (0.51–4.80) | 0.430 | >0.05 | >0.05 | ||||||||
| Length of Stay, (days; SD = 75.2 days) | 1.57 | (0.93–2.64) | 0.097 | >0.05 | >0.05 | 1.25 | (0.74–2.11) | 0.393 | >0.05 | >0.05 | ||||||||
| Histopathological Findings from Resected Bowel | Univariate Associations Any WMA | Histopathology Multivariable Model any WMA | Univariate Associations WMA Grade 3 or 4 | Histopathology Multivariable Model WMA Grade 3 or 4 | ||||||||||||||
| Odds Ratio | 95% CI of ORs | p-value | Odds Ratio | 95% CI of ORs | p-value | Odds Ratio | 95% CI of ORs | p-value | Odds Ratio | 95% CI of ORs | p-value | |||||||
| Necrosis, (≥50% vs. <50%) | 0.58 | (0.21–1.64) | 0.308 | 0.08 | (0.01–0.68) | 0.020 | 0.10 | (0.01–0.90) | 0.040 | 0.57 | (0.004–0.82) | 0.036 | ||||||
| Inflammation, (≥50% vs. <50%) | 0.16 | (0.02–1.45) | 0.103 | >0.05 | >0.05 | 0.39 | (0.04–3.43) | 0.393 | >0.05 | >0.05 | ||||||||
| Hemorrhage, (≥50% vs. <50%) | 2.10 | (0.72–6.16) | 0.177 | >0.05 | >0.05 | 9.00 | (2.71–29.9) | 0.0003 | 7.79 | (2.19–27.72) | 0.002 | >0.05 | ||||||
Two-year neurodevelopment assessment:
Out of 69 patients, 17 patients had neurodevelopment assessment at two years of age. Infants with WMBI had significantly lower mean motor (56.3 ±10.7 vs. 81.7 ±5.2; p=0.0004), lower cognitive scores (62.1 ±11.1 vs. 80 ± 5.8; p= 0.004) and numerically lower language score (58.4 ±14.3 vs. 70.6 ±6.4; p = 0.082) on neurodevelopment assessment at 2 years of age. Compared to infants without WMBI, those with WMBI had a higher frequency of abnormal MDI (p=0.007) and PDI scores (p=0.001). Seven infants (10%) had hearing loss in our study population. The infants with WMBI had more retinopathy of prematurity [n=20 (69.0%) vs. n=10 (33.3%); p= 0.003] at hospital discharge and long-term eye complications [n=17 (60.7%) vs. n=9 (29.0%) p=0.014] compared to those without WMBI on brain MRI at term equivalent age. The neurodevelopmental and sensory organ findings have been summarized in Table 5. The Supplemental Table 2 compared available baseline features and outcomes of those neonates with and without two years follow-up and did not significantly differ. We also compared available baseline features and outcomes of those neonates with and without MRI. For the 52 infants without MRIs, we also stratified it by discharge alive. In the 20/52, neonates discharged home alive without MRI, the baseline characteristics such as birth weight and gestational age were not significantly different. The findings are summarized in Supplemental Table 3.
Table 5:
Neurodevelopmental Outcomes at 2 years of corrected age
| N | All | WMBI | No WMBI | p value | |
|---|---|---|---|---|---|
| Motor Scores (mean ± SD) | 17 | 71.2 (15.0) | 56.3 (10.7) | 81.7 (5.2) | 0.0004 |
| Cognitive Scores (mean ± SD) | 17 | 72.7 (12.1) | 62.1 (11.1) | 80.0 (5.8) | 0.004 |
| Language Scores (mean ± SD) | 14 | 66.2 (11.2) | 58.4 (14.3) | 70.6 (6.4) | 0.082 |
| Social/Emotional Scores (mean ± SD) | 14 | 84.3 (17.4) | 78.0 (19.2) | 87.8 (16.4) | 0.311 |
| MDI Cognitive Score, n (%) | 17 | 0.007 | |||
| Normal | 2 (11.8) | 0 (0.0) | 2 (20.0) | ||
| Mild | 8 (47.1) | 1 (14.3) | 7 (70.0) | ||
| Moderate | 6 (35.3) | 5 (71.4) | 1 (10.0) | ||
| Severe | 1 (5.9) | 1 (14.3) | 0 (0.0) | ||
| PDI Psychomotor Development Score, n (%) | 17 | 0.001 | |||
| Normal | 4 (23.5) | 0 (0.0) | 4 (40.0) | ||
| Mild | 7 (41.2) | 1 (14.3) | 6 (60.0) | ||
| Moderate | 3 (17.6) | 3 (42.9) | 0 (0.0) | ||
| Severe | 3 (17.6) | 3 (42.9) | 0 (0.0) | ||
| Hearing Loss, n (%) | 69 | 7 (10.1) | 4 (11.1) | 3 (9.1) | 0.702 |
| ROP, n (%) | 59 | 30 (50.8) | 20 (69.0) | 10 (33.3) | 0.003 |
| Long Term Eye Complications, n (%) | 59 | 26 (44.1) | 17 (60.7) | 9 (29.0) | 0.014 |
Abbreviations: WMBI = White Matter Brain injury; Categorical variables are presented as count (column percentage). Continuous variables are presented as mean (standard deviation) if normally distributed and with median (interquartile range) if normality does not hold. If normality criteria were satisfied, differences in continuous measures’ statistical associations with WMA were tested using a t-test for equal or unequal variances with Satterthwaite degrees of freedom. When the normality assumption was not satisfied, continuous measures’ statistical associations with WMA were tested with the Kruskal-Wallis Test. Differences in categorical measures’ associations with WMA were tested using the Chi-square test when cell counts were adequate, otherwise Fisher’s Exact was used with low expected cell counts. All tests were two-sided.
Bold values indicate statistical significance p<0.05
Discussion:
Our study is the first to report the clinical and histopathological determinants of white matter brain injury in detail in infants with surgical necrotizing enterocolitis (NEC). In our cohort, those with WMBI on term equivalent brain MRI had a unique clinical and histopathological characteristic compared to those without WMBI. Compared to those without WMBI, the infants with WMBI had lower gestational age, lower birth weight, more evidence of pneumoperitoneum, and younger age at NEC onset.
In this cohort, SGA status was significantly associated with lower risk of any WMBI, but SGA was not associated with Grade 3 or 4 WMBI in univariate or multivariable analysis. Our finding for any WMBI is counter to prior studies as well as severe injury within our study. Thus, we think this finding of an association between SGA status and any WMBI in our cohort is due to chance with small numbers. However, in a study by Graz et al., SGA was not a significant, independent predictor of neurodevelopmental outcomes at five years of age[34]. Postoperatively our cohort demonstrated that infants with WMBI had greater rates of AKI, based on the creatinine method. Sarkar et al. reported AKI was independently associated with hypoxic-ischemic lesions on brain MRI in 88 asphyxiated neonates [35]. They reported abnormal MRI in 59% (n=50/88) with an abnormal MRI in 73% (25/34) of the AKI infants compared with 46% (25/54) in the no-AKI. Overall, our infants with surgical NEC demonstrated AKI in 52.5% (n=32/61) by creatinine method and 42.6% (n=26/61) of cases by urine output criteria. The infants with WMBI had predominantly stage 1 AKI by creatinine criteria and stage two AKI by urine out method. These stages of AKI in infants suggest hypovolemia as the pathophysiological mechanism, which could also be hypothesized to adversely influence brain tissue perfusion with an increased risk of ischemic injury. Animal models have shown that AKI appears not to be an isolated event but instead reflects remote multi-organ dysfunction involving the heart, lungs, liver, intestines, and brain through an inflammatory mechanism involving neutrophil migration and cytokine expression increased oxidative stress [36].
In our study, infants with WMBI received more red blood transfusions before NEC onset than the group without WMBI [n=14 (41.2%) vs. n=2(9.1%); p=0.009]. A recent study reported the impact of blood transfusions on neurodevelopmental outcomes in the Preterm Erythropoietin (Epo) Neuroprotection (PENUT) Trial population. Each transfusion was associated with a decrease in mean cognitive score of 0.96 (95% CI [1.34, 0.57]), a decrease in mean motor score of 1.51 [−1.91, −1.12], and a decrease in mean language score of 1.10 [−1.54, −0.66][37]. The exact mechanism of brain injury remains unclear, but possible mechanisms include pro-inflammatory injury, suppression of endogenous erythropoietin, and oxidative stress mediating injury to the pre-oligodendroglia following blood transfusion [38].
The infants with WMBI on brain MRI stayed 30 days longer in the hospital than those without WMBI in our cohort. Length of stay in infants with surgical NEC is affected by the duration of postoperative ileus days, gestational age, birth weight, age of NEC onset, and bowel resected length, as we have demonstrated in our recent study[39]. We have also reported that infants with incomplete resection of necrotic bowel after surgery had a longer length of hospital stay than those with complete resection of necrotic NEC lesions [22]. In our cohort, Infants with WMBI had lower gestational age, lower birth weight, longer postoperative ileus period, took longer time to reach full feeds, lost longer mean bowel length, and needed parenteral nutrition three weeks longer compared to those without WMBI, which in turn was associated with longer length of hospital stay, white matter injury, and adverse neurodevelopmental outcomes.
The infants with WMBI also had lower lymphocyte percentages on day two after NEC onset. A recent study by Zhou et al. has shown that the brains of mice and humans with NEC contained CD4+ T lymphocytes that were required for the development of brain injury [40]. They have also demonstrated that gut-derived IFN-γ-releasing CD4+ T cells may mediate neuroinflammation in neonates with NEC. Low percentages of lymphocytes at day two may reflect sequestration of lymphocytes in the intestine and brain tissue.
In our study, infants with grade 3–4 WMBI had lower necrosis and higher hemorrhagic lesions on pathology examination of the resected bowel. The intestine’s significant blood loss may lead to hypovolemia with associated brain ischemia leading to white or grey matter abnormalities. In the study, infants with WMBI had a higher frequency of laparotomy in less than 48 hours after NEC onset [n=25 (71.4%) vs. n=18 (62.1%); p=0.427] and had a Penrose drain inserted more frequently [n=16 (44.4%) vs. n=12(41.4%); p=0.643]. The urgent need for surgery in less than 48 hours after NEC onset points towards a higher severity of the illness associated with the risk for WMBI.
In our cohort, both groups with and without WMBI had similar rates of any positive blood culture at the time of NEC onset. They did not differ significantly in C-reactive protein levels or central line days. Infants with WMBI had higher gram-positive infection rates and similar rates of gram-negative infections compared to those without WMBI. A prospective study of 192 preterm infants (gestational age <30 weeks) by Shah et al. reported 100 episodes of confirmed sepsis in 68 infants, and nine infants (5%) had confirmed NEC. The sepsis/NEC (n=73/192) group had predominantly gram-positive infection, as noticed in our study cohort, and had significantly more WMBI on the brain MRI than those with no sepsis/ NEC [41]. However, in our cohort, all the infants had surgical NEC and did not include any medical NEC or sepsis patients. Post-natal sepsis due to gram-positive and gram-negative bacteria leading to WMBI may be explained due to release of lipopolysaccharide or peptidoglycan and modulating pro-inflammatory genes in the brain such as Toll-like receptors, nuclear factor-κB, antioxidants, oxidants, and cytokines [42].
Abnormal MRI findings at term equivalent in very preterm infants strongly predict adverse neurodevelopmental outcomes at two years of age [11, 12]. We also noted similar trends in our cohort. MRI imaging studies in infants with surgical NEC have shown severe brain injury compared to infants with medical NEC or spontaneous intestinal perforation [13, 14]. A multi-center clinical study has demonstrated that Infants who developed NEC did not start with high blood levels of inflammatory cytokines, but these rose mainly after the onset of NEC. NEC diagnosis was associated with elevated IL-1β, IL-6, IL-8, IL-10, monocyte chemoattractant protein-1/CC-motif ligand-2, macrophage inflammatory protein-1β/CC-motif ligand-3, and C-reactive protein [15]. Animal studies have shown that systemic inflammation and infection sensitizes the neonatal brain to neuronal injury via various pro-inflammatory pathways [16]. A recent study in piglets with NEC developed systemic inflammation that led to blood-brain barrier disruption, resulting in region-specific (hippocampus) neuronal degeneration [17]. Recently published mouse studies have reported the activation of the intestine’s inflammatory pathway, activating the brain’s microglia, leading to neuronal injury [18, 19].
We acknowledge that our available two-year sample size is very limited. Nonetheless, in the small available sample, we find a highly significant association between WM brain injury and measures of two-year neurodevelopmental outcome consistent with other published studies. Although there are certainly demographic (out-born status) and medical selection (severity of comorbidities, death) biases in those returning for the two-year follow-up, we felt this information was important to convey because it provides evidence of the longer-term prognostic importance of WM brain injury on MRI. WM brain injury on MRI is not just an available and convenient measurable surrogate endpoint or finding but also related to patient-centered definitive longer-term outcomes.
In our cohort, 30.4% (21/65) had cerebellar abnormalities, and these were more commonly associated with WMBI (55.9% vs. 6.5%). Merhar et al. also reported cerebellar hemorrhage on brain MRI in 27% (3/11) of their study (n= 26) infants with NEC [14]. During the most dramatic growth during the preterm period, the cerebellum is vulnerable to large and small hemorrhages, as well as hypoplasia resulting from several potentially modifiable risk factors. These factors include contact with intraventricular blood, crossed cerebrocerebellar diaschisis, postnatal glucocorticoid exposure, pain, opioid exposure, nutrition and somatic growth, cardiorespiratory factors, and socioeconomic status [43, 44].
As noted in Table 1, 77% (53/69) of our cohort is African American which is not highly dissimilar to the overall racial demographic for our medical center (64% African American) and central Mississippi. As also noted in Table 1, WMBI was not significantly associated with race in our cohort. While we cannot comment on this from our data, published studies do suggest African American male pre-term neonates appear to be at higher risk for NEC [45].
This study’s strengths include the only study to identify novel clinical, pathological, and postoperative risk factors for WMBI in neonates with surgical necrotizing enterocolitis. Identification of risk factors associated with WMBI may improve early recognition of at-risk neonates with brain injury and provide useful bedside prognostic information. Limitations of our study include that it is a single-center experience and retrospective. The relatively small sample size may reduce the study’s generalizability and the statistical power to detect other important associations between clinical determinants and white matter injury in a neonate with surgical necrotizing enterocolitis. Secondly, in our cohort, the number of comparisons generates a high probability of type I errors. Again, we view most of our study as a descriptive and exploratory cohort study/experience and, thus, generate a hypothesis. On the other hand, fortunately, surgical NEC is a relatively rare disease with inherently limited numbers, even at relatively high volume centers with a large and uniquely inclusive catchment area such as ours. We did have a few families of pre-specified hypotheses
In conclusion, this study demonstrates that WMBI in infants with surgical NEC occurs in 50% of cases, and 30.4% had a cerebellar injury. In our cohort, the infants with WMBI had lower gestational age, received red blood cell transfusions more frequently before NEC onset, more evidence of pneumoperitoneum, younger age at NEC onset, more prolonged postoperative ileus, acute kidney injury by serum creatinine, hemorrhagic lesions on histopathology, received more postnatal steroids, and had a longer length of hospitalization. In the future, prospective multi-center studies, which allow the inclusion of additional clinical details (e.g., gut perfusion, gut flora) and laboratory predictors such as inflammatory biomarkers, may support earlier recognition of WMBI or identify other risk factors for WMBI after surgical NEC. While some of these exposures are non-modifiable or unavoidable in the setting of an NEC diagnosis, this highlights the importance of assessing clinical and pathological risk factors in infants diagnosed with NEC, given the higher risk for WMBI. Studies that evaluate neuroprotective strategies to prevent white matter, grey matter injury, and consequences are greatly needed to improve neurodevelopmental outcomes in high-risk preterm infants with NEC. Our findings may provide further guidance in targeting experimental neuroprotective or mitigating interventions.
Supplementary Material
Impact.
In preterm infants with surgical NEC, brain magnetic resonance injury (MRI) showed injury in the white matter in 52%, grey matter in 10%, and cerebellar region in 30%.
Preterm infants with severe white matter brain injury (grade 3–4) had less necrosis and greater hemorrhagic lesions on histopathology of the bowel.
Preterm infants with white matter brain injury were more likely to have a more severe postoperative course, AKI, and longer length of hospitalization.
Neuroprotective strategies to prevent brain injury in preterm infants with surgical NEC are needed with the goal of improving the neurodevelopmental outcomes.
Funding:
William Hillegass, MD, Ph.D. is supported by the IDeA grant U54GM115428
Footnotes
Conflicts of interest: The authors disclose no conflicts.
Consent: Patient consent is not required as per IRB
References:
- 1.Neu J and Walker WA, Necrotizing enterocolitis. N Engl J Med, 2011. 364(3): p. 255–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sankaran K, et al. , Variations in incidence of necrotizing enterocolitis in Canadian neonatal intensive care units. J Pediatr Gastroenterol Nutr, 2004. 39(4): p. 366–72. [DOI] [PubMed] [Google Scholar]
- 3.Sjoberg Bexelius T, et al. , Intestinal failure after necrotising enterocolitis: incidence and risk factors in a Swedish population-based longitudinal study. BMJ Paediatr Open, 2018. 2(1): p. e000316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Allin BSR, et al. , One-year outcomes following surgery for necrotising enterocolitis: a UK-wide cohort study . Arch Dis Child Fetal Neonatal Ed, 2018. 103(5): p. F461–f466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Knell J, et al. , Current Status of Necrotizing Enterocolitis. Curr Probl Surg, 2019. 56(1): p. 11–38. [DOI] [PubMed] [Google Scholar]
- 6.Stoll BJ, et al. , Trends in Care Practices, Morbidity, and Mortality of Extremely Preterm Neonates, 1993–2012. Jama, 2015. 314(10): p. 1039–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Santulli TV, et al. , Acute necrotizing enterocolitis in infancy: a review of 64 cases. Pediatrics, 1975. 55(3): p. 376–87. [PubMed] [Google Scholar]
- 8.Mowitz ME, Dukhovny D, and Zupancic JAF, The cost of necrotizing enterocolitis in premature infants. Semin Fetal Neonatal Med, 2018. 23(6): p. 416–419. [DOI] [PubMed] [Google Scholar]
- 9.Ganapathy V, et al. , Long term healthcare costs of infants who survived neonatal necrotizing enterocolitis: a retrospective longitudinal study among infants enrolled in Texas Medicaid. BMC Pediatr, 2013. 13: p. 127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barnett ML, et al. , Exploring the multiple-hit hypothesis of preterm white matter damage using diffusion MRI. Neuroimage Clin, 2018. 17: p. 596–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hintz SR, et al. , Neuroimaging and neurodevelopmental outcome in extremely preterm infants. Pediatrics, 2015. 135(1): p. e32–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Woodward LJ, et al. , Neonatal MRI to predict neurodevelopmental outcomes in preterm infants. N Engl J Med, 2006. 355(7): p. 685–94. [DOI] [PubMed] [Google Scholar]
- 13.Shin SH, et al. , Surgical Necrotizing Enterocolitis versus Spontaneous Intestinal Perforation in White Matter Injury on Brain Magnetic Resonance Imaging. Neonatology, 2016. 110(2): p. 148–54. [DOI] [PubMed] [Google Scholar]
- 14.Merhar SL, et al. , Brain magnetic resonance imaging in infants with surgical necrotizing enterocolitis or spontaneous intestinal perforation versus medical necrotizing enterocolitis. J Pediatr, 2014. 164(2): p. 410–2.e1. [DOI] [PubMed] [Google Scholar]
- 15.Maheshwari A, et al. , Cytokines associated with necrotizing enterocolitis in extremely-low-birth-weight infants. Pediatr Res, 2014. 76(1): p. 100–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Adén U, et al. , Systemic inflammation sensitizes the neonatal brain to excitotoxicity through a pro-/anti-inflammatory imbalance: key role of TNFalpha pathway and protection by etanercept. Brain Behav Immun, 2010. 24(5): p. 747–58. [DOI] [PubMed] [Google Scholar]
- 17.Brunse A, Abbaspour A, and Sangild PT, Brain Barrier Disruption and Region-Specific Neuronal Degeneration during Necrotizing Enterocolitis in Preterm Pigs. Dev Neurosci, 2018. 40(3): p. 198–208. [DOI] [PubMed] [Google Scholar]
- 18.Niño DF, et al. , Cognitive impairments induced by necrotizing enterocolitis can be prevented by inhibiting microglial activation in mouse brain. Sci Transl Med, 2018. 10(471). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Biouss G, et al. , Experimental necrotizing enterocolitis induces neuroinflammation in the neonatal brain. J Neuroinflammation, 2019. 16(1): p. 97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Criss CN, et al. , Acute kidney injury in necrotizing enterocolitis predicts mortality. Pediatr Nephrol, 2018. 33(3): p. 503–510. [DOI] [PubMed] [Google Scholar]
- 21.Garg PM, et al. , Necrotizing enterocolitis in a mouse model leads to widespread renal inflammation, acute kidney injury, and disruption of renal tight junction proteins. Pediatr Res, 2015. 78(5): p. 527–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Garg PM, et al. , Incomplete resection of necrotic bowel may increase mortality in infants with necrotizing enterocolitis. Pediatr Res, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zeichner SL, et al. , The bacterial communities of the small intestine and stool in children with short bowel syndrome. PLoS One, 2019. 14(5): p. e0215351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bell MJ, et al. , Neonatal necrotizing enterocolitis. Therapeutic decisions based upon clinical staging. Ann Surg, 1978. 187(1): p. 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ehrenkranz RA, et al. , Validation of the National Institutes of Health consensus definition of bronchopulmonary dysplasia. Pediatrics, 2005. 116(6): p. 1353–60. [DOI] [PubMed] [Google Scholar]
- 26.Selewski DT, et al. , Neonatal Acute Kidney Injury. Pediatrics, 2015. 136(2): p. e463–73. [DOI] [PubMed] [Google Scholar]
- 27.Jetton JG, et al. , Incidence and outcomes of neonatal acute kidney injury (AWAKEN): a multicentre, multinational, observational cohort study. Lancet Child Adolesc Health, 2017. 1(3): p. 184–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jetton JG, et al. , Assessment of Worldwide Acute Kidney Injury Epidemiology in Neonates: Design of a Retrospective Cohort Study. Front Pediatr, 2016. 4: p. 68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jetton JG and Askenazi DJ, Acute kidney injury in the neonate. Clin Perinatol, 2014. 41(3): p. 487–502. [DOI] [PubMed] [Google Scholar]
- 30.Zappitelli M, et al. , Developing a neonatal acute kidney injury research definition: a report from the NIDDK neonatal AKI workshop. Pediatr Res, 2017. 82(4): p. 569–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mehta C and Patel N, A Network Algorithm for Performing Fisher’s Exact Test in r × c Contingency Tables. Journal of the American Statistical Association, 1983. 78(382): p. 427–434. [Google Scholar]
- 32.SAS Support: Multiple Imputation for Missing Data 2020; Available from: https://support.sas.com/rnd/app/stat/topics/multiple-imputation.html.
- 33.Lankham I and Slaughter M, Simple and Efficient Bootstrap Validation of Predictive Models Using SAS/STAT® Software. Paper 4647-2020. https://www.sas.com/content/dam/SAS/support/en/sas-global-forum-proceedings/2020/4647-2020.pdf. SAS Global Forum; 2020. [Google Scholar]
- 34.Bickle Graz M, Tolsa JF, and Fischer Fumeaux CJ, Being Small for Gestational Age: Does it Matter for the Neurodevelopment of Premature Infants? A Cohort Study. PLoS One, 2015. 10(5): p. e0125769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sarkar S, et al. , Relationship between acute kidney injury and brain MRI findings in asphyxiated newborns after therapeutic hypothermia. Pediatr Res, 2014. 75(3): p. 431–5. [DOI] [PubMed] [Google Scholar]
- 36.Yap SC and Lee HT, Acute kidney injury and extrarenal organ dysfunction: new concepts and experimental evidence. Anesthesiology, 2012. 116(5): p. 1139–48. [DOI] [PubMed] [Google Scholar]
- 37.Vu PT, et al. , Transfusions and neurodevelopmental outcomes in extremely low gestation neonates enrolled in the PENUT Trial: a randomized clinical trial. Pediatr Res, 2021: p. 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dani C, et al. , Red blood cell transfusions can induce proinflammatory cytokines in preterm infants. Transfusion, 2017. 57(5): p. 1304–1310. [DOI] [PubMed] [Google Scholar]
- 39.Garg PM, et al. , Clinical determinants of postoperative outcomes in surgical necrotizing enterocolitis. J Perinatol, 2020. 40(11): p. 1671–1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhou Q, et al. , Necrotizing enterocolitis induces T lymphocyte-mediated injury in the developing mammalian brain. Sci Transl Med, 2021. 13(575). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shah DK, et al. , Adverse neurodevelopment in preterm infants with postnatal sepsis or necrotizing enterocolitis is mediated by white matter abnormalities on magnetic resonance imaging at term. J Pediatr, 2008. 153(2): p. 170–5, 175.e1. [DOI] [PubMed] [Google Scholar]
- 42.Volpe JJ, Postnatal sepsis, necrotizing entercolitis, and the critical role of systemic inflammation in white matter injury in premature infants. J Pediatr, 2008. 153(2): p. 160–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tam EWY, Cerebellar injury in preterm infants. Handb Clin Neurol, 2018. 155: p. 49–59. [DOI] [PubMed] [Google Scholar]
- 44.Limperopoulos C, et al. , Does cerebellar injury in premature infants contribute to the high prevalence of long-term cognitive, learning, and behavioral disability in survivors? Pediatrics, 2007. 120(3): p. 584–93. [DOI] [PubMed] [Google Scholar]
- 45.Jammeh ML, et al. , Racial/ethnic differences in necrotizing enterocolitis incidence and outcomes in premature very low birth weight infants. J Perinatol, 2018. 38(10): p. 1386–1390. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


