Abstract
Objective:
Investigate predictors of post-operative morbidity and mortality in surgical NEC.
Study Design:
We analyzed the clinical outcomes of infants with surgical NEC from the years 2000–2015.
Results:
Ninety infants born at gestation (mean ± standard deviation, SD; standard error of mean, SEM) 27.3±6.6 weeks (SEM ±0.07 weeks) and weighing 1008±456 grams (SEM ±48 grams) developed NEC on 25.2±22.4 days (SEM ±2.4 days). Average bowel resection was 29.2±30.5 centimeters (SEM ±3.2 centimeters). Post-operative Ileus lasted 16.5±12.2 days (SEM ±1.3 days), and was significantly longer in infants with higher gestation and birth weight, age at onset of NEC, length of intestinal resection, maternal chorioamnionitis, and need for pressors. Thirty-eight (42.2%) infants died. Mortality was higher at gestation less than 31 weeks.
Conclusion:
Gestational age, birth weight, age at NEC onset, and length of resected bowel determined post-operative morbidity and mortality in NEC. Length of hospital stay was affected by above factors, and also the duration of post-operative ileus and parenteral nutrition.
Background:
Necrotizing enterocolitis (NEC) is the most common acquired acute gastrointestinal illness of newborn infants, and is seen in 5–10% of those born prior to 28 weeks’ gestation and weighing ≤1500 grams (1). Despite recent reports of reduced incidence (2, 3), NEC remains a life-threatening, unpredictable illness with increased risk of intestinal necrosis/perforation and consequent peritonitis; major abdominal surgery and post-operative morbidity with feeding difficulties, infection, malnutrition, surgical wound-related complications, and developmental delay; intestinal and liver failure, and increased mortality (2, 4–9). The costs of surgical NEC remain high with prolonged hospitalization that may extend to several months (10, 11).
Infants with severe NEC can develop bowel necrosis, severe systemic inflammation, and multi-system organ failure (12). Some of these patients are too unstable and may only tolerate the creation of a decompression stoma at the bedside. Others, who can withstand general anesthesia and surgery, may be able to withstand an exploratory laparotomy and resection of the gangrenous bowel loops. Viable parts of the remaining intestinal segments can be repaired, sometimes with exteriorization and/or anastomosis of the ends (6, 13–15). However, many of these infants remain clinically fragile and very ill for prolonged periods (16).
In this study, we investigated the clinical predictors of post-operative morbidity, length of stay, and mortality in surgical NEC. Specifically, we recorded prenatal factors such as pregnancy-induced hypertension (PIH), chorioamnionitis, and antenatal steroids; demographic data such as gestational age, birth weight, small for gestational age (SGA) status, ethnicity, gender, and outborn status; medical factors preceding NEC such as assisted ventilation, patent ductus arteriosus (PDA), and its medical/surgical treatment. We noted the age at onset of NEC, and indirectly assessed its severity by recoding the length of the resected intestine. Our post-operative markers included hemodynamic instability, duration of paralytic ileus, days on parenteral nutrition, and the time to reach full enteral feedings (120 mL/kg/day). We also recorded less frequent events such as late-onset sepsis, surgical wound dehiscence or infection, strictures, and short bowel syndrome (SBS).
Methods:
This retrospective clinical study was conducted at the University of Mississippi Medical Center (UMMC) at Jackson, Mississippi after approval by the Institutional Review Board. We admit about 900 infants to our neonatal intensive care unit (NICU) every year, and the incidence of surgical NEC remained constant at approximately 2% during the study period January 2000 - December 2015. During this period, we admitted 270 infants with a possible diagnosis of surgical NEC. A careful review of their medical records and available histopathology sections confirmed the diagnosis in 118 infants. Seventeen infants were excluded due to incomplete records, and 11 for confounding conditions (congenital heart disease, intestinal atresia, and spontaneous intestinal perforation in the first 7 days after birth). We finally included 90 patients in our study.
We recorded all patient information listed in the introduction, including the prenatal and demographic data, available information on feeding, medical factors such as PDA, sepsis, and respiratory distress prior to the onset of NEC, age at onset and severity of NEC, post-operative hemodynamic instability, paralytic ileus, parenteral/enteral nutrition, and post-operative complications. Neonatal growth was assessed with the Fenton/World Health Organization growth charts (17). We used indomethacin as the only medication to treat PDA during the study years; ibuprofen was introduced in 2016. Our team of surgeons, and the indications for surgery in NEC remained largely unchanged through the study years. Supplemental figure 1 (A) highlights the relatively consistent gestational age (mean ± standard error) of patients treated for surgical NEC, and panel (B) shows the minimum gestational ages of the operated patients during the study years.
At our center, we typically use post-operative fluid volumes of 100 mL/kg/day, and adjust these infusions based on blood pressures, plasma sodium levels, acid-base balance, urine output, and skin perfusion. Echocardiography may be used selectively to assess right atrial filling in selected patients. To manage post-operative hypotension in infants with longer laparotomy durations, we liberalize total fluid infusion volumes and administer fluid boluses. Pressors such are dopamine infusions are added if the hemodynamic status does not improve with therapeutic measures focused on fluid management.
Descriptive, categorical data were summarized as frequencies (absolute and relative) and tested for differences using a chi-square test. Continuous data, when symmetric, were presented as means ± standard errors, and if skewed, as medians (with quartiles). We used both standard deviations (SD) and standard errors of the mean (SEM) to depict variability in continuous measurements. SD accurately describes study subjects in large clinical studies, but SEM can be useful for conveying variability in disease-specific, small neonatal cohorts as it estimates the precision with which findings from a limited study sample can be extrapolated to the larger universe of preterm infants (18). Two or multiple groups with continuous, parametric data were compared using a Student’s t or analysis of variance (ANOVA), respectively. If the data were non-parametric, we used the Mann-Whitney U or a Kruskal Wallis H test, respectively. If Kruskal-Wallis H test identified significant differences between multiple subgroups of data, secondary comparisons were performed using the Dunn-Bonferroni test. The predictors of various outcome measures (such as post-operative ileus, length of hospital stay, or mortality) were first identified in correlation analyses, and then assessed further in multivariable linear and logistic regression models. Although we started our data analysis with concerns for multicollinearity between gestational age and birth weight, our patients showed a correlation coefficient of 0.394 between these two parameters. The criteria for collinearity, such as the R-squared value in regression analysis (below 0.2), and the variance inflation factors (=1/1-R2; below 0.6) were consistent. Therefore, we tested both gestational age and birth weight in our regression models. Statistical analysis were done with the software programs STATA 15 (Stata Software, College Station, TX) and GraphPad Prism (San Diego, CA). Statistical significance was accepted at p-values below 0.05.
Results
Subjects:
We reviewed the medical records of 90 infants with a confirmed surgical NEC. These infants had a gestational age (mean ± SD) of 27.9±6.6 weeks (±0.7 weeks SEM) and birth weight of 1008±456 grams (±48 grams SEM; Table 1). There were 56 (62.2%) males and 34 (37.8%) females. Fourteen (15.5%) infants were SGA. Eighty-eight (88.8%) were African American, 9 (10%) were Caucasian, and 1 (1.2%) was Latino. Forty (44.4%) infants were outborn and were transferred to our hospital after the onset of NEC. The use of antenatal steroids appeared low when first combined over the 15-year study period. However, in the last 5 years of the study (2010–2015), the rates of steroid administration in intramural cases was higher, at >90%. The diagnosis of PDA was confirmed in all infants by echocardiography. We used only indomethacin for medical treatment of PDA during the study period.
Table 1.
Prenatal and neonatal data prior to NEC
| 90 | |
| Prenatal information | |
| Chorioamnionitis, n (%) | 5 (7%) |
| Antenatal steroids, n (%) | 44 (49%) |
| Maternal Indomethacin, n (%) | 2 (2%) |
| Maternal magnesium sulphate administration, n (%) | 24 (27%) |
| Infant Demographics | |
| Birth weight (grams; mean ± SEM) | 1008±48 |
| Gestational age (weeks; mean ± SEM) | 27.9±0.7 |
| Small for gestational age, n (%) | 14 (16%) |
| Male gender, n (%) | 56 (62%) |
| Ethnicity | |
| African American | 79 (88%) |
| Caucasian | 10 (11%) |
| Latino | 1 (1%) |
| Cesarean section delivery | 48 (54%) |
| Outborn | 45 (53%) |
| Infant medical information prior to NEC | |
| 5 minute Apgar score 0–5, n (%) | 16/90 (18%) |
| Starting of enteral feedings (days, mean ± SEM) | 2±0.49 |
| Enteral feedings | |
| Mother’s own/donor milk, n (%) | 35 (38.4%) |
| Infant formula, n (%) | 55 (61.5%) |
| Patent Ductus Arteriosus, n (%) | 42 (47%) |
| Patent ductus arteriosus, indomethacin treated, n (%) | 16 (19%) |
| Patent ductus arteriosus, surgically ligated, n (%) | 2 (2.2%) |
Clinical presentation of NEC:
The average age at onset of NEC was 25.2±22.4 days (±2.4 days SEM). The corrected gestational age at onset of NEC was 31.4±5.2 weeks (±0.7 weeks SEM). Seventy-four (82.2%) infants were on assisted ventilation prior to surgery. Abdominal distension was the presenting symptom of NEC in 83 (92%), 4 (4.5%) were evaluated for feeding intolerance, and 6 (6.7%) for bloody stools. Some of our patients presented with more than one of these clinical features. These clinical data are summarized in Table 2.
Table 2.
Clinical Characteristics and outcomes.
| Total 90 patients | ||
| Necrotizing enterocolitis disease features | ||
| Age at NEC onset (days; mean ± SEM) | 25.2±2.4 | |
| Gestational age at birth (weeks; mean ± SEM) | 27.9±0.7 | |
| Corrected gestational age at NEC onset (weeks; mean ± SEM) | 31.4±0.7 | |
| Clinical presentation | ||
| Abdominal distension | 83 (92%) | |
| Bloody stools | 6 (6.7%) | |
| Feeding intolerance | 4 (4.5%) | |
| Radiological findings | ||
| Pneumatosis, n (%) | 30 (33.3%) | |
| Pneumoperitoneum, n (%) | 40 (45%) | |
| Portal venous gas, n (%) | 4 (4.4%) | |
| Surgery | ||
| Length of bowel resected (cm; mean ± SEM) | 29.2±3.2 | |
| Bowel resection region | ||
| Small bowel, n (%) | 65 (72%) | |
| Large bowel, n (%) | 1 (1%) | |
| Small bowel + Large bowel, n (%) | 24 (27%) | |
| Intestinal ostomy, n (%) | 73 (81.1%) | |
| Post-operative systemic course | ||
| Duration of antibiotics (days; mean ± SEM) | 11.2±0.08 | |
| Assisted ventilation | ||
| Room air, n (%) | 16 (17.8%) | |
| Assisted ventilation (continuous positive air pressure/intubated), n (%) | 74 (82%) | |
| Positive blood culture for sepsis, n (%) | 9 (10%) | |
| Post-operative intestinal features | ||
| Post-operative ileus [days; n (%)] | 16.49±0.13 | |
| Post-operative day at starting enteral feedings (days; mean ± SEM) | 16.91±0.13 | |
| Abdominal wound infection, n (%) | 15/76 (19.7%) | |
| Intestinal adhesions, n (%) | 12/76 (15.8%) | |
| Intestinal strictures, n (%) | 8/76 (10.5%) | |
| Intestinal fistulas, n (%) | 3/76 (3.9%) | |
| Feedings at start | ||
| Mother’s own/donor milk, n (%) | 35 (38.4%) | |
| Infant formula, n (%) | 55 (61.5%) | |
| Day of attainment of full enteral feedings (120 mL/kg; mean ± SEM) | 75±0.48 | |
| Duration of parenteral nutrition (days; mean ± SEM) | 86.48±0.55 | |
| Short Bowel Syndrome, n (%) | 7 (10%) | |
| Discharge | ||
| Length of stay (days; mean ± SEM) | 132.7±9.1 | |
| Death, n (%) | 38 (42.2%) | |
Well-recorded data on feedings available for the years 2012–2015, since institution of electronic records.
Reliable, well recorded data on post-operative complications available on 76 of our 90 patients
Surgery:
Patients with NEC were taken for surgery if they had clinical worsening due to intestinal perforation (45%), hypotension and metabolic acidosis (61%), increasing ventilatory requirements (82%), or intractable anemia despite repeated RBC transfusions (24%; Table 2). The signs of radiological worsening have also been defined (Table 2). All patients described in this report underwent exploratory laparotomy and surgical treatment at 25.4±22.8 days (±2.8 days SEM). The average length of resected bowel was 29.2±30.5 centimeters (SEM ±3.2 centimeters). The intestine was exteriorized with an ostomy in 73 (81.1%) infants.
Early post-operative outcomes:
The clinical characteristics of these infants are summarized in Table 2. The relationship between patient characteristics and their early post-operative outcomes is summarized in Supplemental table 1. The age of onset of NEC (25.2±22.4 days (SEM ±2.4 days) correlated with the duration of post-operative ileus, days on parenteral nutrition, days to full feedings, and patency of ductus arteriosus. These infants had post-operative ileus after surgery that lasted 16.5±12.2 days (SEM ±1.3 days). Thirty-eight (42.2%) patients died. The hospital stay (average ± SEM) was 132.7±86.2 days (SEM ±9.1 days).
The duration of post-operative ileus correlated with gestational age (r=0.26, p=0.009), birth weight (r=0.31, p=0.002), outborn status (r=0.46, p<0.001), gender (r=0.25, p=0.01), ethnicity (r=0.37, p<0.001), age of onset of NEC (r=0.24, p=0.018), length of resected bowel (r=0.22, p=0.024), day of start of feedings (r=0.96, p<0.001), days on parenteral nutrition (r=0.56, p<0.001), day to reach full feedings (r=0.57, p<0.001), and the length of hospital stay (r=0.31, p<0.001). In multiple regression (R2 = 0.2), the duration of ileus increased only with the duration of antibiotic treatment (t=4.04, p<0.001) and gender (t=2.49, p=0.03).
In the first 24 hours after surgery, 55 (61.1%) patients needed hemodynamic support with intravenous fluids and pressors. These infants had lost more bowel (31.9±28.4 centimeters, SEM ±5.7 centimeters vs. 25±33.6 centimeters, SEM ±3.8 centimeters, p=0.03), had longer paralytic ileus (18.7±14 days, SEM ±1.9 days vs. 13.4±18.4 days, SEM ±1.8 days; p=0.03), and received more antibiotics (12.6 vs. 7.2 days, SEM ±0.9 days, p=0.02). The need for pressors correlated with gender (r=0.22, p=0.016), bowel resection length (r=0.29, p=0.001), days on parenteral nutrition (r=0.2, p=0.046), days of full feedings (r=0.27, p=0.016). Pressors were needed more often in outborn infants (r=0.2, p=0.028) and in those on assisted ventilation (r=0.38, p<0.001).
Late post-operative effects:
The average length of NICU stay was 132.7±86.2 days (SEM ±0.9 days); the survivors stayed for 157.5±86.2 days (SEM ±1.5 days), longer than 97.5±83.7 days (SEM ±2.1 days) in those who died. Infants who survived did not show significant differences in surgical complications such as the need for stoma creation, short bowel syndrome, surgical site infections (dehiscence, abscesses), strictures, fistulas, adhesions, or perforations.
Survival:
Our fifty-two (56.6%) survivors were born at a higher gestational age (29.1±6.6 weeks, SEM ±0.1 weeks) and birth weight (1109.6±456 grams, SEM ±0.6 grams) than the deceased (26.1±3.6 weeks, SEM ±0.5 weeks; p=0.008 and 837.9±402.9 grams, SEM ±8.9 grams; p=0.007; Table 3). The two groups developed NEC at a similar postnatal age (29.1±22.4 days, SEM ±0.2 days in survivors vs. 29.7±14.3 days; SEM ±0.7 days in deceased). After surgery, both groups had begun feedings nearly simultaneously (17.3±12.2 days, SEM ±0.1 days and 16.3±11 days, SEM ±0.3 days, respectively), but survivors reached full feedings later (81.9±43.9 days, SEM ±0.5 days vs. 52.6±34.8 days, SEM ±0.9 days) than those who died, p=0.002). Consequently, survivors needed parenteral nutrition for longer (90.5±50.9 days, SEM ±0.5 days vs. 78.5±54.1 days, SEM ±1.4 days, p=0.03), and had longer hospital stays (157.5±86.2 days, SEM ±1.5 days vs. 97.5±83.6 days, SEM 2.1 days; p<0.001). As expected, multiple regression showed survival to be associated (R2 = 0.42) with birth weight (t=4.47, p<0.001), length of hospital stay (t=3.67, p<0.001), and the age at NEC onset (t=2.44, p=0.018). Interestingly, there was no significant impact of the duration of post-operative ileus.
Table 3.
Comparison of prenatal, neonatal, and NEC data in survivors and those who died.
| All infants (n=90) | Survivors (n=52) | Died (n=38) | p-value |
|---|---|---|---|
| Prenatal information | |||
| Pregnancy-induced hypertension, n (%) | 15 (28.84%) | 10 (26.3%) | 0.07 |
| Chorioamnionitis, n (%) | 2 (3.84%) | 3 (7.89%) | 0.42 |
| Antenatal steroids, n (%) | 28 (53.84%) | 16 (42.1%) | 0.45 |
| Maternal Indomethacin, n (%) | 1 (1.92%) | 1 (2.63%) | 0.82 |
| Infant Demographics | |||
| Birth weight (grams; mean ± SEM) | 1109.58±5.58 | 837.85±8.98 | <0.001 |
| Gestational age (weeks; mean ± SEM) | 29.07±0.09 | 26.12±0.5 | <0.001 |
| Small for gestational age, n (%) | 8 (15.38%) | 6 (15.78%) | 0.34 |
| Male gender, n (%) | 34 (65.38%) | 22 (57.89%) | 0.33 |
| African American ethnicity | 47 (90.4%) | 33 (86.84%) | 0.23 |
| Cesarean section delivery | 28 (53.84%) | 20 (52.63%) | 0.88 |
| Outborn | 30 (57.69%) | 15 (39.47%) | 0.77 |
| Infant medical information prior to necrotizing enterocolitis | |||
| 5 minute Apgar score 0–5, n (%) | 7 (13.46%) | 9 (23.7%) | 0.62 |
| Start enteral feedings (days, mean ± SEM) | 2.3±0.62 | 2.1±0.28 | 0.73 |
| Enteral feedings | |||
| Mother’s own/donor milk, n (%) | 20 (38.46%) | 18 (47.36%) | 0.39 |
| Infant formula, n (%) | 32 (61.53%) | 20 (52.63%) | 0.39 |
| Patent Ductus Arteriosus, n (%) | 40 (53.33%) | 22 (57.9%) | 0.005 |
| Patent ductus arteriosus, medically treated, n (%) | 12 (30%) | 10 (45.45%) | 0.32 |
| Patent ductus arteriosus, surgically ligated, n (%) | 2 (3.84%) | 0 (0%) | 0.22 |
| Necrotizing enterocolitis | |||
| NEC age onset (days; mean ± SEM) | 23.16±0.21 | 29.68±0.72 | 0.46 |
| Length bowel resected (centimeters; mean ± SEM) | 23.10±0.25 | 36.28±0.95 | 0.034 |
| Pressor support 24 hours after NEC onset, n (%) | 32 (61.53%) | 23 (60.5%) | 0.63 |
| Post-operative Ileus (days; mean ± SEM) | 17.1±0.14 | 19.9±0.66 | 0.55 |
| Days of parenteral nutrition (days; mean ± SEM) | 90.52±0.53 | 78.5±1.4 | 0.47 |
| Day of starting feeds (days; mean ± SEM) | 17.31±0.14 | 16.27±0.3 | 0.97 |
| Day to reach full feeds (days; mean ± SEM) | 81.86±0.49 | 52.62±0.87 | 0.06 |
| Antibiotic days (days; mean ± SEM) | 11.67±0.09 | 10.14±0.17 | 0.47 |
| Length of hospital stay (days; mean ± SEM) | 157.53±0.87 | 97.51±2.14 | 0.014 |
SEM = standard error of mean
To evaluate the impact of prematurity on outcomes in surgical NEC, we plotted Kaplan Meier characteristics for mortality vs. gestational age (Figure 1). In our cohort of 90 infants, mortality dropped below 50% beyond 31 weeks, indicating a considerably higher gestational age-determined cut-off for mortality than in infants who do not have NEC (19). The clinical characteristics of patients below and above this 31 weeks’ gestational age threshold are summarized in Table 4.
Figure 1.
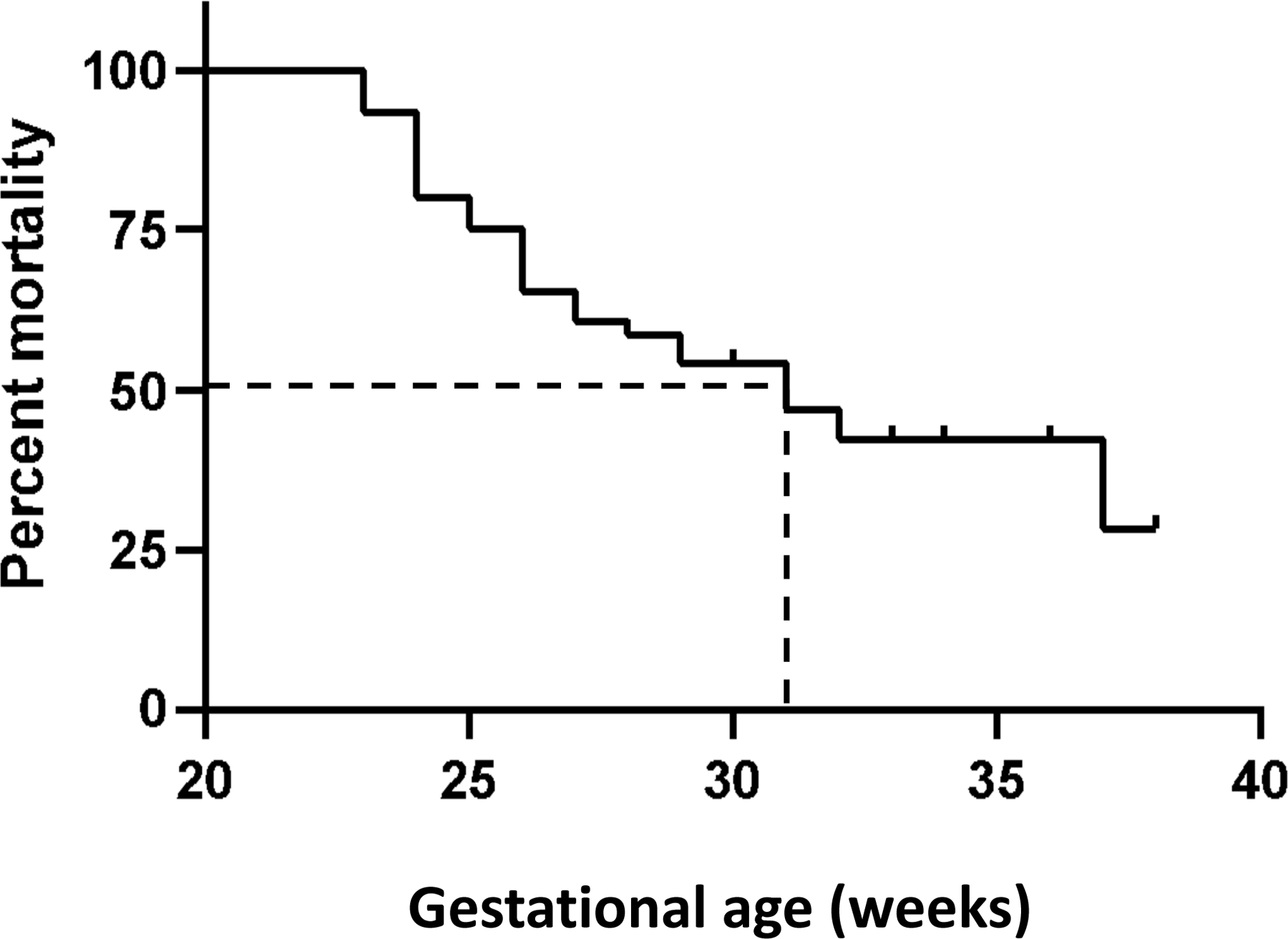
Kaplan -Meier curve shows gestational age (weeks) plotted against mortality (percentage). Dashed lines show the gestational age corresponding to 50% mortality.
Table 4.
Comparison of prenatal, neonatal, and NEC data of infants born at 31 weeks’ gestation
| All infants (n=90) | <31 weeks (n=75) | >31 weeks (n=15) | p-value |
|---|---|---|---|
| Prenatal information | |||
| Pregnancy-induced hypertension, n (%) | 18 (24%) | 7 (46.67%) | 0.07 |
| Chorioamnionitis, n (%) | 5 (6.67%) | 0 (0%) | 0.3 |
| Antenatal steroids, n (%) | 38 (50.67%) | 6/15 (40%) | 0.45 |
| Maternal Indomethacin, n (%) | 2 (2.67%) | 0 (0%) | 0.52 |
| Infant Demographics | |||
| Birth weight (grams; mean ± SEM) | 851.43±29.71 | 1763.6±72.93 | <0.001 |
| Gestational age (weeks; mean ± SEM) | 25.98±0.24 | 33.41±0.36 | <0.001 |
| Small for gestational age, n (%) | 45 (60%) | 7 (46.67%) | 0.34 |
| Male gender, n (%) | 45 (60%) | 11 (73.3%) | 0.33 |
| African American ethnicity | 68 (90.67%) | 12 (80%) | 0.23 |
| C-section delivery | 42 (56%) | 6 (40%) | 0.26 |
| Outborn | 37 (49.33%) | 8 (53.33%) | 0.77 |
| Infant medical information prior to NEC | |||
| 5 min Apgar score 0–5, n (%) | 14 (18.67%) | 2 (13.33%) | 0.62 |
| Start enteral feedings (days, mean ± SEM) | 2±0.49 | 2.1±0.3 | 0.92 |
| Enteral feedings | |||
| Mother’s own/donor milk, n (%) | 35 (47%) | 9 (60%) | 0.34 |
| Infant formula, n (%) | 40 (53%) | 6 (40%) | 0.34 |
| Patent Ductus Arteriosus, n (%) | 40 (53.33%) | 2 (13.33%) | 0.005 |
| Patent Ductus Arteriosus, medically treated, n (%) | 15 (37.5%) | 0 (0%) | 0.39 |
| Patent ductus arteriosus, surgically ligated, n (%) | 2 (2.67%) | 0 (0%) | 0.52 |
| Necrotizing enterocolitis | |||
| NEC age onset (days; mean ± SEM) | 27.35±3.59 | 37.6±7 | 0.023 |
| Length bowel resected (centimeters; mean ± SEM) | 27.51±2.74 | 13.57±1.47 | 0.034 |
| Pressor support 24h after NEC onset, n (%) | 45 (60%) | 10 (66.67%) | 0.63 |
| Post-operative Ileus (days; mean ± SEM) | 16.75±1.45 | 17.64±1.83 | 0.55 |
| Days of PN (days; mean ± SEM) | 89.93±5.47 | 77.64±9.62 | 0.47 |
| Day of starting feeds (days; mean ± SEM) | 16.85±1.42 | 18.08±1.89 | 0.55 |
| Day to reach full feeds (days; mean ± SEM) | 80.89±5.32 | 59±4.45 | 0.2 |
| Antibiotic days (days; mean ± SEM) | 8.28±0.93 | 5.96±0.9 | 0.013 |
| Mortality, n (%) | 34 (37.8%) | 4 (26.7%) | 0.18 |
| Length of hospital stay (days; mean ± SEM) | 143.42±10.12 | 83.27±9.9 | 0.014 |
Discussion:
We reviewed the clinical records of 90 infants with surgical NEC to identify predictors of the length of hospital stay or mortality. These patients had a typical demographic profile and clinical course; an average ± SD (±SEM) gestational age of 27.3±6.6 weeks (SEM ±0.7 weeks), birth weight of 1008±456 grams (SEM ±48 grams), and NEC onset at 25.2±22.4 days (SEM ±2.4 days). Small intestine was resected in 65 (72%), parts of both the small and large bowel in 24 (27%), and some colon in 1. The length of surgical resected small bowel was 29.2±30.5 centimeters (SEM ±3.2 centimeters). Thirty-eight (42%) infants died. The average duration of hospital stay was 132.7±86.2 days (SEM ±0.9 days).
Several studies have focused on outcomes of NEC, but there is considerable variability in the definitions of the outcomes (20–27). Li et al. (22) reported one of the earliest studies. They compared infants with early (<10 days) vs. later NEC onset (<10 days), and noted infants with later onset NEC to have higher mortality (p=0.026), longer time to achieve full feeding (means ± standard deviation 18.1±11.5 vs. 26.3±15.6 days, p=0.008), and a trend towards more infections [29/53 (54.7%) vs. 19/53 (35.8%)]. In another study, Sheng et al. (20) studied the clinical course of 34 preterm infants with NEC. Patients with localized or multifocal disease underwent an enterostomy. Post-operative complications such as sepsis, intestinal stricture, and short bowel syndrome were recorded in 70.5% patients. Several similar studies followed. Hau et al. (23) recently reported a meta-analysis of 58 studies with 4260 patients, focusing on the gastrointestinal sequelae of NEC. Strictures were noted in 24%, recurrent NEC in 8%, intestinal failure in 13%, and adhesion ileus in 6%. Strictures were more frequent after enterostomy than after primary anastomosis (30% vs. 8%), and after enterostomy with no bowel resection than with a concomitant resection. They noted major deficiencies in the analyzed studies; the index of heterogeneity, I2, ranged between 38%−90%. There is an urgent need to define outcomes, standardize study populations, develop ways to identify the risk of bias, and bias-weighted frequencies of sequelae.
Wright et al. (21) described 182 infants with surgical NEC. Fifteen (8%) of their sickest infants could not be transported to the operating room and had to be operated upon at the bedside; 5 had NEC totalis, 4 had multifocal, and 6 had focal disease. Five had an open and close laparotomy, 8 underwent bowel evaluation with a stoma creation, and 2 had bowel resection with primary anastomosis. Ten died at a median of 6.5 (range 2–72) hours after surgery. Interestingly, the Pediatric Index of Mortality-2 scores did not predict outcomes. More recently, Bhatt et al. (27) have tried to address this problem. They developed a receiver operating characteristic curve to predict death or intestinal failure in surgical NEC. They examined a cohort of 147 patients using the American College of Surgeons National Surgical Quality Improvement Program (Pediatric) and reported an area under the curve (AUC) of 0.84 (95% CI 0.77–0.91). Their exciting results were comparable to previously attempts with the Score for Neonatal Acute Physiology Perinatal - Extension II (0.60; 95% CI, 0.48–0.72) and the Vermont Oxford Risk Adjustment Tool (0.74; 95% CI, 0.65–0.83).
The duration of paralytic ileus was determined by birth weight, history of chorioamnionitis, age at NEC onset, need for assisted ventilation, and stoma complications such as wound dehiscence and infection. Many of these patients (61%) needed hemodynamic support in the early post-operative period with extra intravenous fluids and/or pressors to maintain perfusion. This was particularly true for those who lost longer sections of their bowel. However, there was no difference in the duration of ileus, need for parenteral nutrition, or the timing of initiation or attainment of full enteral feedings (120 mL/kg/day). No differences were recorded in short bowel syndrome, surgical site infections, strictures, fistulas, adhesions, or perforations. The impact of NEC on intestinal motility, mucosal immunity, and nutrient absorption is beginning to be understood, but there are important confounders related to maturity and infection.
In the present study, the incidence of NEC was higher in African American infants (89% vs 10%). The impact of ethnic and genetic influences in the pathogenesis of NEC has now been documented in several large studies, but the underlying reasons still remain poorly understood (28, 29). The Pediatrix medical group recently reviewed the medical records of 126,089 infants and identified 8796 (7%) infants to have been treated for NEC (28). NEC was more frequent in African Americans (adjusted odds ratios (AOR) 1.31, 95% confidence interval (CI) [1.24–1.39] and Latinos (1.30 [1.21–1.39]). These infants also had higher mortality than Caucasians (AORs 1.35 [1.15–1.58] and 1.31 [1.09–1.56], respectively). In another study, Janevic et al. (30) reported increased risk of NEC in African American [adjusted relative risk (RR), 1.39; 95% CI, 1.00–1.93] and Latino infants (1.39; 95% CI, 0.98–1.96). Further studies are needed to investigate these health disparities. Several genetic variants have now been associated with NEC, and a detailed study is indicated in larger, multi-ethnic cohorts (31–35).
In our study, the surviving infants had higher gestational ages and birth weights than those who died, by nearly 3 weeks and 250 grams, respectively. Both infants who eventually survived and those who did not had prolonged post-operative periods of ileus and started enteral feedings only at 16–17 days after surgery. However, the survivors took nearly 4 weeks longer to reach full feeding volumes than those who died. The reasons for this delayed maturation of intestinal motility in the survivors, who were gestationally more mature, remain unclear. There were no differences in gender, ethnicity, clinical NEC, surgical complications, or short bowel syndrome. Further studies of gastrointestinal motility, genetics, host-microbial interactions, and/or environmental factors, possibly using advanced, real-time monitoring technology (7), are needed in these critically ill infants (36–40).
In our cohort, the length of resected bowel was an important predictor of clinical outcomes. The survivors lost less bowel than those who died (23.1±30.5 centimeters, SEM ±0.3 centimeter vs. 36.3±32.7 centimeters, ±1 centimeter; p<0.001). These infants had a longer NICU stay for 157.5±86.2 days, SEM ±1.5 days in survivors vs. 97.5±83.6 days, SEM 2.1 days in those who died, but there was no difference in surgical complications such as the need for stoma creation, short bowel syndrome, surgical site infections, strictures, fistulas, adhesions, or perforations. However, these results may also reflect the limitations of a small cohort at our single center; there is an important possibility of our single-center SBS data being skewed due to the death of infants who were most sick and dependent on parenteral nutrition, prior to discharge. Studies in larger groups such as premature infants treated at the research network centers in the Eunice Kennedy Shriver National Institute of Child Health and Human Development, have recorded SBS in 0.7% of 12316 very-low-birth-weight infants (25). Extremely-low-birth-weight infants showed an even higher incidence of SBS (1.1% of 5657 infants). They were likely to remain ill, have feeding difficulties (33%), to have been re-hospitalized (79%), and to have growth delay even at 18 to 22 months (25).
To conclude, our study has generated important data on the clinical status of infants prior to and during NEC and their surgical and post-operative outcomes. However, these associations need further evaluation in larger cohorts. Our study is limited by its retrospective design, relatively small sample size, and single-center format, which increase the risk of bias. The results need validation in a larger, multi-centric cohort in a prospective format, which may also allow the inclusion of additional clinical or laboratory predictors (41–44). There is a need for careful evaluation of the needs and outcomes of infants with surgical NEC.
Supplementary Material
Funding:
National Institutes of Health awards HL124078 and HL133022 to AM
Footnotes
Conflicts of interest: The authors disclose no conflicts.
References:
- 1.Neu J, Modi N, Caplan M. Necrotizing enterocolitis comes in different forms: Historical perspectives and defining the disease. Semin Fetal Neonatal Med 2018;23(6):370–3. [DOI] [PubMed] [Google Scholar]
- 2.Jones IH, Hall NJ. Contemporary Outcomes for Infants with Necrotizing Enterocolitis-A Systematic Review. J Pediatr 2020. [DOI] [PubMed]
- 3.Han SM, Hong CR, Knell J, Edwards EM, Morrow KA, Soll RF, et al. Trends in incidence and outcomes of necrotizing enterocolitis over the last 12 years: A multicenter cohort analysis. J Pediatr Surg 2020. [DOI] [PubMed]
- 4.Sjoberg Bexelius T, Ahle M, Elfvin A, Bjorling O, Ludvigsson JF, Andersson RE. Intestinal failure after necrotising enterocolitis: incidence and risk factors in a Swedish population-based longitudinal study. BMJ paediatrics open 2018;2(1):e000316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Allin BSR, Long AM, Gupta A, Lakhoo K, Knight M. One-year outcomes following surgery for necrotising enterocolitis: a UK-wide cohort study. Archives of disease in childhood Fetal and neonatal edition 2018;103(5):F461–f6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Knell J, Han SM, Jaksic T, Modi BP. Current Status of Necrotizing Enterocolitis. Curr Probl Surg 2019;56(1):11–38. [DOI] [PubMed] [Google Scholar]
- 7.Stoll BJ, Hansen NI, Bell EF, Walsh MC, Carlo WA, Shankaran S, et al. Trends in Care Practices, Morbidity, and Mortality of Extremely Preterm Neonates, 1993–2012. JAMA 2015;314(10):1039–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nair R, Kahlenberg CA, Patel RM, Knesek M, Terry MA. All-Arthroscopic Suprapectoral Biceps Tenodesis. Arthrosc Tech 2015;4(6):e855–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Santulli TV, Schullinger JN, Heird WC, Gongaware RD, Wigger J, Barlow B, et al. Acute necrotizing enterocolitis in infancy: a review of 64 cases. Pediatrics 1975;55(3):376–87. [PubMed] [Google Scholar]
- 10.Mowitz ME, Dukhovny D, Zupancic JAF. The cost of necrotizing enterocolitis in premature infants. Seminars in fetal & neonatal medicine 2018;23(6):416–9. [DOI] [PubMed] [Google Scholar]
- 11.Ganapathy V, Hay JW, Kim JH, Lee ML, Rechtman DJ. Long term healthcare costs of infants who survived neonatal necrotizing enterocolitis: a retrospective longitudinal study among infants enrolled in Texas Medicaid. BMC pediatrics 2013;13:127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kurscheid T, Holschneider AM. Necrotizing enterocolitis (NEC)--mortality and long-term results. Eur J Pediatr Surg 1993;3(3):139–43. [DOI] [PubMed] [Google Scholar]
- 13.Thakkar HS, Lakhoo K. The surgical management of necrotising enterocolitis (NEC). Early human development 2016;97:25–8. [DOI] [PubMed] [Google Scholar]
- 14.Raval MV, Moss RL. Current concepts in the surgical approach to necrotizing enterocolitis. Pathophysiology : the official journal of the International Society for Pathophysiology 2014;21(1):105–10. [DOI] [PubMed] [Google Scholar]
- 15.Rees CM, Hall NJ, Eaton S, Pierro A. Surgical strategies for necrotising enterocolitis: a survey of practice in the United Kingdom. Archives of disease in childhood Fetal and neonatal edition 2005;90(2):F152–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Robinson JR, Rellinger EJ, Hatch LD, Weitkamp JH, Speck KE, Danko M, et al. Surgical necrotizing enterocolitis. Semin Perinatol 2017;41(1):70–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fenton TR, Kim JH. A systematic review and meta-analysis to revise the Fenton growth chart for preterm infants. BMC Pediatr 2013;13:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Davies HT. Describing and estimating: use and abuse of standard deviations and standard errors. Hosp Med 1998;59(4):327–8. [PubMed] [Google Scholar]
- 19.Manuck TA, Rice MM, Bailit JL, Grobman WA, Reddy UM, Wapner RJ, et al. Preterm neonatal morbidity and mortality by gestational age: a contemporary cohort. Am J Obstet Gynecol 2016;215(1):103 e1- e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sheng Q, Lv Z, Xu W, Liu J, Wu Y, Shi J, et al. Short-term surgical outcomes of preterm infants with necrotizing enterocolitis: A single-center experience. Medicine (Baltimore) 2016;95(30):e4379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wright NJ, Thyoka M, Kiely EM, Pierro A, De Coppi P, Cross KM, et al. The outcome of critically ill neonates undergoing laparotomy for necrotising enterocolitis in the neonatal intensive care unit: a 10-year review. J Pediatr Surg 2014;49(8):1210–4. [DOI] [PubMed] [Google Scholar]
- 22.Li X, Li L, Wang Y, Deng C, Guo C. Postoperative characteristics of infants who developed necrotizing enterocolitis with different postnatal ages. Medicine (Baltimore) 2017;96(32):e7774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hau EM, Meyer SC, Berger S, Goutaki M, Kordasz M, Kessler U. Gastrointestinal sequelae after surgery for necrotising enterocolitis: a systematic review and meta-analysis. Arch Dis Child Fetal Neonatal Ed 2019;104(3):F265–F73. [DOI] [PubMed] [Google Scholar]
- 24.Geng Q, Wang Y, Li L, Guo C. Early postoperative outcomes of surgery for intestinal perforation in NEC based on intestinal location of disease. Medicine (Baltimore) 2018;97(39):e12234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cole CR, Hansen NI, Higgins RD, Ziegler TR, Stoll BJ, Eunice Kennedy Shriver NNRN. Very low birth weight preterm infants with surgical short bowel syndrome: incidence, morbidity and mortality, and growth outcomes at 18 to 22 months. Pediatrics 2008;122(3):e573–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Blakely ML, Lally KP, McDonald S, Brown RL, Barnhart DC, Ricketts RR, et al. Postoperative outcomes of extremely low birth-weight infants with necrotizing enterocolitis or isolated intestinal perforation: a prospective cohort study by the NICHD Neonatal Research Network. Ann Surg 2005;241(6):984–9; discussion 9–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bhatt D, Travers C, Patel RM, Shinnick J, Arps K, Keene S, et al. Predicting Mortality or Intestinal Failure in Infants with Surgical Necrotizing Enterocolitis. J Pediatr 2017;191:22–7 e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jammeh ML, Adibe OO, Tracy ET, Rice HE, Clark RH, Smith PB, et al. Racial/ethnic differences in necrotizing enterocolitis incidence and outcomes in premature very low birth weight infants. J Perinatol 2018;38(10):1386–90. [DOI] [PubMed] [Google Scholar]
- 29.Horbar JD, Badger GJ, Carpenter JH, Fanaroff AA, Kilpatrick S, LaCorte M, et al. Trends in mortality and morbidity for very low birth weight infants, 1991–1999. Pediatrics 2002;110(1 Pt 1):143–51. [DOI] [PubMed] [Google Scholar]
- 30.Janevic T, Zeitlin J, Auger N, Egorova NN, Hebert P, Balbierz A, et al. Association of Race/Ethnicity With Very Preterm Neonatal Morbidities. JAMA Pediatr 2018;172(11):1061–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cuna A, George L, Sampath V. Genetic predisposition to necrotizing enterocolitis in premature infants: Current knowledge, challenges, and future directions. Semin Fetal Neonatal Med 2018;23(6):387–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cuna A, Yu W, Menden HL, Feng L, Srinivasan P, Chavez-Bueno S, et al. NEC-like intestinal injury is ameliorated by Lactobacillus rhamnosus GG in parallel with SIGIRR and A20 induction in neonatal mice. Pediatr Res 2020. [DOI] [PMC free article] [PubMed]
- 33.Sampath V, Helbling D, Menden H, Dimmock D, Mulrooney NP, Murray JC, et al. Necrotizing Enterocolitis Is Not Associated With Sequence Variants in Antioxidant Response Genes in Premature Infants. J Pediatr Gastroenterol Nutr 2016;62(3):420–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sampath V, Le M, Lane L, Patel AL, Cohen JD, Simpson PM, et al. The NFKB1 (g.−24519delATTG) variant is associated with necrotizing enterocolitis (NEC) in premature infants. J Surg Res 2011;169(1):e51–7. [DOI] [PubMed] [Google Scholar]
- 35.Sampath V, Menden H, Helbling D, Li K, Gastonguay A, Ramchandran R, et al. SIGIRR genetic variants in premature infants with necrotizing enterocolitis. Pediatrics 2015;135(6):e1530–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Necrotizing enterocolitis in the premature infant. Br Med J 1966;2(5522):1089–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Remon JI, Amin SC, Mehendale SR, Rao R, Luciano AA, Garzon SA, et al. Depth of bacterial invasion in resected intestinal tissue predicts mortality in surgical necrotizing enterocolitis. J Perinatol 2015;35(9):755–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bell MJ, Feigin RD, Ternberg JL, Brotherton T. Evaluation of gastrointestinal microflora in necrotizing enterocolitis. J Pediatr 1978;92(4):589–92. [DOI] [PubMed] [Google Scholar]
- 39.Gewolb IH, Schwalbe RS, Taciak VL, Harrison TS, Panigrahi P. Stool microflora in extremely low birthweight infants. Arch Dis Child Fetal Neonatal Ed 1999;80(3):F167–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Smith B, Bode S, Skov TH, Mirsepasi H, Greisen G, Krogfelt KA. Investigation of the early intestinal microflora in premature infants with/without necrotizing enterocolitis using two different methods. Pediatr Res 2012;71(1):115–20. [DOI] [PubMed] [Google Scholar]
- 41.Andrews WW, Goldenberg RL, Faye-Petersen O, Cliver S, Goepfert AR, Hauth JC. The Alabama Preterm Birth study: polymorphonuclear and mononuclear cell placental infiltrations, other markers of inflammation, and outcomes in 23- to 32-week preterm newborn infants. American journal of obstetrics and gynecology 2006;195(3):803–8. [DOI] [PubMed] [Google Scholar]
- 42.Dorling J, Kempley S, Leaf A. Feeding growth restricted preterm infants with abnormal antenatal Doppler results. Archives of disease in childhood Fetal and neonatal edition 2005;90(5):F359–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Venkatesh KK, Jackson W, Hughes BL, Laughon MM, Thorp JM, Stamilio DM. Association of chorioamnionitis and its duration with neonatal morbidity and mortality. J Perinatol 2019. [DOI] [PubMed]
- 44.Peterslund P, Rasmussen L, Qvist N, Hansen TP, Husby S, Detlefsen S. Frequencies of Immune Cells in the Human Small Bowel During Normal Gestation and in Necrotizing Enterocolitis. Fetal Pediatr Pathol 2019:1–14. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


