Abstract
Background
Adverse mental health conditions including depression, posttraumatic stress disorder (PTSD), and anxiety are prevalent among patients who survive myocardial infarctions (MI) and are associated with adverse outcomes. The mechanisms underlying these associations, however, are not well understood. Inflammatory pathways may mediate the cardiovascular outcomes of patients with mental health disorders. We examined the bidirectional association between PTSD symptoms and inflammatory biomarkers in a young/middle-aged post MI population. We further examined how this association may differ between women and men as well as between Black and non-Black individuals.
Methods
Participants included individuals with early onset MI between the ages 25 and 60. Mental health scores for depression, PTSD, perceived stress, and anxiety as well as inflammatory biomarkers, interleukin-6 (IL-6) and high sensitivity C-reactive protein (hsCRP), were collected at baseline and at six-month follow up. We examined the bidirectional changes in mental health symptoms and inflammatory biomarkers between baseline and follow-up.
Results
Among 244 patients in the study (mean age: 50.8, 48.4% female, 64.3% Black), the geometric means for IL-6 level and hsCRP at rest were 1.7 pg/mL and 2.76 mg/L, respectively. Mental health scores at baseline did not consistently predict changes in inflammatory biomarkers at follow-up. However, baseline levels of both IL-6 and hsCRP were robustly associated with an increase in re-experiencing PTSD symptoms at 6 months: in adjusted linear mixed models, there was a 1.58-point increase in re-experiencing PTSD symptoms per unit of baseline hsCRP (p = 0.01) and 2.59-point increase per unit of baseline IL-6 (p = 0.02). Once the analysis was stratified by race, the association was only noted in Black individuals. Baseline inflammation was not associated with change in any of the other mental health symptom scores.
Conclusion
Markers of inflammation are associated with an increase in post-event PTSD symptoms in younger or middle-aged patients who experienced an MI, especially Black patients. These results suggest a mechanistic link between inflammation and the development of PTSD among individuals with cardiovascular disease.
Keywords: Posttraumatic stress disorder, Myocardial infarction, Inflammation, Longitudinal study
1. Introduction
Cardiovascular disease (CVD) is the leading cause of death globally and has been associated with adverse mental health conditions, especially depression and posttraumatic stress disorder (PTSD) (Scott et al., 2013; Beristianos et al., 2016; Vaccarino et al., 2013). Although lifestyle and traditional cardiovascular risk factors (e.g., diabetes, obesity, hypertension and smoking) are likely to play a role, in most studies these factors did not eliminate the associations between mental health conditions and CVD (Penninx, 2017; Carney and Freedland, 2017; Rozanski et al., 2005).
While the exact mechanisms underlying the association between CVD and mental health disturbances are likely complex and bidirectional, inflammation has been identified as a leading pathway (Carney and Freedland, 2017; Shimbo et al., 2005; Khandaker et al., 2019; Kop et al., 2002). Inflammation is involved in the progression of coronary artery disease and higher levels of inflammation predict future cardiac events (Libby, 2021). Systemic inflammation, as indexed by circulating levels of interleukin 6 (IL-6) and C-reactive protein (CRP) has also been associated with adverse mental health (Penninx, 2017; Kop et al., 2002; Kop and Gottdiener, 2005), and current evidence on depression and inflammation suggests that the association is bidirectional (Huang et al., 2019; Valkanova et al., 2013; Khandaker et al., 2014). Despite some controversy, several studies reported a relationship between PTSD and a number of inflammatory markers, but most were cross-sectional and little is known about the direction of these associations (Passos et al., 2015; Pace and Heim, 2011). Two studies of individuals without prior CVD found that PTSD was associated with increased inflammatory biomarker levels over time (Farr et al., 2015; Sumner et al., 2017). Studies inducing stress challenges found that in people with PTSD, inflammatory markers increase more with stress than in those without PTSD (Bremner et al., 2020; Lima et al., 2019). However, results have been mixed (Jergović et al., 2015; Sumner et al., 2018).
Even fewer data are available on directional pathways linking PTSD and inflammation in populations with pre-existing CVD, a group with high levels of both PTSD and inflammation (Lima et al., 2019; Wolf and Schnurr, 2016). Lima et al. reported minor differences in inflammation in the resting state among patients with a recent myocardial infarction (MI) who developed PTSD compared to those without PTSD; however patients with PTSD, especially those who had greater reexperiencing symptoms, demonstrated an increased inflammatory response during mental stress testing (Lima et al., 2019). This suggests that PTSD symptoms could be implicated in inducing a pro-inflammatory state. On the other hand, in a prospective evaluation of MI survivors, Bielas et al. found that persistently elevated CRP levels after 3 months from admission were associated with the development of post-traumatic stress symptoms (Bielas et al., 2018). This study, however, was small and only included white, primarily male participants. Both the prevalence of mental health conditions and systemic inflammation are higher in women and people of color than other groups (Beydoun et al., 2020; O'Connor et al., 2009). Despite this, there continues to be little research in these populations.
In a sample of men and women recently hospitalized for an MI and followed for six months, we examined the longitudinal relationship between systemic inflammation and PTSD symptoms, and contrasted the results with those for other behavioral outcomes. We focused on two inflammatory biomarkers that have shown established relationships both with CVD and with mental health: high-sensitivity CRP (hsCRP) and IL-6. We further examined the relationship between inflammatory markers and PTSD symptom clusters, given their potentially different association with inflammatory response (Lima et al., 2019), and explored whether those associations differed by race or sex. We hypothesized that the relationship between inflammation and PTSD would be bidirectional and that the reexperiencing symptom cluster would drive most of the associations.
2. Materials and methods
2.1. Study design and sample
The Myocardial Infarction and Mental Stress 2 (MIMS2) study is a longitudinal study of young and middle-aged survivors of a recent MI (Vaccarino et al., 2018). Participants in the MIMS2 study included patients admitted to an Emory-affiliated hospital in Atlanta, Georgia for an acute MI between the ages of 18 and 60 at the time of the event. Patients whose MI hospitalization was within 8 months were recruited for participation in the study. The diagnosis of MI was verified by medical record review based on standard criteria of troponin level increase together with symptoms of ischemia and ECG changes or other evidence of myocardial necrosis. The main exclusion criteria were severe comorbid medical or psychiatric disorders (e.g., cancer, renal failure, sever uncontrolled hypertension, current alcohol/substance abuse, or schizophrenia), pregnancy or breast feeding, or use of immunosuppressant or psychotropic medications other than anti-depressants. MI patients were excluded if they had unstable angina, acute MI or decompensated heart failure within the previous week, and if it was deemed unsafe to withhold anti-ischemic medications for 24 h before testing by study cardiologists. All patients were invited to an initial clinic visit, and a follow-up visit after 6 months.
2.2. Measurements
Demographic information including age, sex, race, marital status, income, and education, was obtained using standardized questionnaires. Study nurses or physicians obtained previous medical history and medication use from the participants and by reviewing medical records.
Inflammatory biomarkers were measured from blood samples collected at rest. In this study we examined levels of hsCRP as our main inflammatory factor of interest given its association with mental health (Kop et al., 2002; Bielas et al., 2018; Empana et al., 2005; An et al., 2015) as well as with cardiovascular risk (Shimbo et al., 2005; Kop et al., 2002; Morrow et al., 1998). However, to establish consistency of results, we also examined IL-6, an established predictor of CVD (Libby, 2021). To quantitate biomarkers, the MesoScale system (Meso Scale Diagnostics, Rockville, Maryland) was employed using the SECTOR Imager 2400 per manufacturer protocols allowing for a lower detection limit of 1.33 pg/mL for hsCRP and 0.06 pg/mL for IL-6. There were no values below the limit of detection for either IL-6 or CRP in this study.
A clinical diagnosis of psychiatric disorders within the past month was obtained using the Structured Clinical Interview for DSM-IV (SCID). In addition to history of psychiatric disorders, various mental health surveys were administered to all participants. Depressive symptoms were assessed using the Beck Depression Inventory (BDI-II), a 21-item self-administered scale (Beck et al., 1996) with acceptable sensitivity and specificity with regards to a clinical diagnosis of depression, and which also provides a continuous measure of depressive symptoms in the past two weeks. It has been used extensively in studies of CVD (Carney et al., 1987; Frasure-Smith et al., 1995). PTSD symptoms were assessed using the civilian version of the PTSD Checklist (PCL-C), a 17-item scale assessing PTSD symptoms in the past month (Blanchard et al., 1996) with good temporal stability, internal consistency, test-retest reliability, and convergent validity (Wilkins et al., 2011). This scale was subdivided into three different DSM-IV PTSD symptom clusters: re-experiencing, avoidance and numbing, and arousal. Anxiety symptoms were evaluated via the State-Trait Anxiety Inventory (STAI), a 40-item self-administered scale assessing current (state) and general (trait) feelings of anxiety. Internal consistency coefficients range from 0.86 to 0.95 and test-retest reliability from 0.65 to 0.75 (Spielberger et al., 1970). Patients returned for a follow-up visit six months after their baseline visit where measures were repeated using identical protocols.
Of 313 CVD patients in the MIMS2, 32 (10%) did not return at the 6-month follow up. Of the 281 participants with information at both baseline and 6-month follow-up, 27 were missing inflammatory marker data at baseline and an additional 10 were missing information on PTSD scores at baseline. Therefore, the analytical sample included 244 participants. This research was approved by the Emory University Institutional Review Board and written informed consent was obtained from all patients. Further details about the study design and sample have been provided elsewhere (Vaccarino et al., 2018).
2.3. Statistical analysis
To assess possible selection bias, we compared the descriptive characteristics of the 281 who participated at both baseline and follow up to the 32 who did not return at 6 months. Next, we calculated descriptive statistics for the overall analytic sample as well as within strata of hsCRP values dichotomized by the median log-transformed value. A median value vs a clinical cut point was used because this was intended as a descriptive analysis and we wanted to achieve groups of approximately equal size. Geometric means were then calculated to express values in regular units.
Differences in biomarkers between follow up and baseline were approximately normally distributed, but actual biomarker levels were not. To examine the direction of the association of PTSD (and other psychological scale scores) with inflammation between baseline and 6 months, we initially analyzed these associations in both directions using ordinary least squares linear regression models to ease interpretation of directionality. We first used the change in psychological scale scores as dependent variables as a function of the natural log of inflammatory biomarkers at baseline, and then examined the opposite direction with the (non-log transformed) change in inflammatory markers as outcomes and baseline psychological scores as predictors. In these models we adjusted for the corresponding baseline levels of the dependent variable.
Since this initial analysis revealed most robust associations in the direction of baseline inflammatory biomarkers predicting worsening PTSD symptoms, in the subsequent stage of the analysis this association became the main focus and we performed multivariable analysis using linear mixed regression models. These models estimated the effects of linear combinations of regression coefficients for the inflammatory marker and time. This approach tends to be more robust in handling longitudinal observational studies with incomplete measurements at follow-up (Molenberghs et al., 1997). In these models we adjusted for potential confounding factors decided a priori based on prior knowledge: 1) demographics (age, race, sex, and years of education), 2) clinical severity (ejection fraction and type of MI, i.e., ST-Elevation Myocardial Infarction (STEMI) or non-STEMI), 3) cardiovascular risk factors (body mass index [BMI], smoking history, history of diabetes, history of hypertension, and a history of MI prior to the index event), and 4) medications (anti-depressants, aspirin, beta-blockers, and statins). Each set of covariates was added to those in the previous model. Because some of these factors could be a consequence of PTSD (such as cardiovascular risk factors and the use of some medications), we used a sequential modeling approach that progressively adjusted for domains of variables to examine the effect of this adjustment on the study estimates.
These analyses were then repeated in sub populations by sex, race (dichotomized as Black and non-Black), and a combination of sex and race. Interaction terms were tested to evaluate whether the measures of association in each stratum were significantly different from one another. The significance level for main effects and interaction effects were set at p < 0.05. All statistical analyses were conducted using SAS version 9.4 (SAS Institute, Cary, NC).
3. Results
3.1. Descriptive characteristics
Baseline characteristics were largely similar comparing patients who participated at both baseline and follow-up visits with those who only participated at baseline, with few exceptions (Supplemental Table 1). Those who did not return at 6-months tended to be younger and had completed fewer years of education compared to those who participated at both baseline and follow-up. Furthermore, those who only attended the baseline visit were less likely to have a history of high cholesterol compared with those who attended both visits, but there were no substantial differences in other cardiovascular risk factors. Notably, both groups had overall similar distributions of psychosocial risk factors, including the Beck Depression Inventory, the State-Trait Anxiety Index and the PTSD Checklist and its subscales.
Table 1 shows the results of descriptive analyses stratified by median hsCRP value. Those with hsCRP levels above the median were, on average, more likely to identify as Black and had a lower income and education compared to those with hsCRP levels below the median. Cardiovascular risk factors were more prevalent in the group with hsCRP above the median than in the group below the median, including a higher BMI, history of hypertension and of high blood cholesterol. However, clinical characteristics of the MI and medication use were overall similar, with exception of aspirin use which was greater in those with hsCRP below the median. The prevalence of major depression and PTSD was higher among those with hsCRP above the median, as were mean symptom scores for depression, PTSD and State-Trait Anxiety Inventory (Table 1).
Table 1.
Baseline characteristics by hsCRP level dichotomized at the median.
| Total N = 244 | Low hsCRP (<median) n = 122 | High hsCRP (≥median) n = 122 | |
|---|---|---|---|
| Demographics | |||
| Age, Years, mean (SD) | 50.8 (6.5) | 50.5 (6.7) | 51.1 (6.4) |
| Women, n (%) | 118 (48.4) | 52 (42.6) | 66 (54.1) |
| Black Race, n (%) | 157 (64.3) | 66 (54.1) | 91 (74.6) |
| Married, n (%) | 101 (41.4) | 58 (47.5) | 43 (35.2) |
| Income <25K, n (%) | 87 (39.0) | 36 (32.1) | 51 (45.9) |
| Education, Years mean (SD) | 13.8 (2.8) | 14.3 (3.0) | 13.3 (2.6) |
| Cardiovascular Risk Factors | |||
| BMI, mean (SD) | 31.5 (7.4) | 28.5 (5.4) | 34.6 (7.9) |
| Ever Smoker, n (%) | 134 (54.9) | 63 (51.6) | 71 (58.2) |
| History of Hypertension, n (%) | 200 (82.0) | 92 (75.4) | 108 (88.5) |
| History of High Cholesterol, n (%) | 198 (81.2) | 92 (75.4) | 106 (86.9) |
| History of Diabetes, n (%) | 80 (32.8) | 33 (27.0) | 47 (38.5) |
| Clinical Characteristics | |||
| Ejection Fraction, mean (SD) | 50.3 (12.1) | 50.9 (11.0) | 49.7 (13.1) |
| Prior MI, n (%) | 47 (19.3) | 23 (18.9) | 24 (19.8) |
| STEMI, n (%) | 67 (27.5) | 33 (27.0) | 34 (27.9) |
| Months since MI, mean (SD) | 5.2 (1.6) | 5.2 (1.7) | 5.3 (1.6) |
| Medications | |||
| Beta Blockers, n (%) | 207 (85.2) | 104 (85.2) | 103 (85.1) |
| Statins, n (%) | 203 (83.5) | 107 (87.7) | 96 (79.3) |
| Aspirin, n (%) | 198 (81.5) | 108 (88.5) | 90 (74.4) |
| ACE Inhibitors, n (%) | 113 (46.5) | 62 (50.8) | 51 (42.1) |
| Antidepressants, n (%) | 39 (16.1) | 16 (13.1) | 23 (19.0) |
| Psychosocial Risk Factors | |||
| Current Major Depression, n (%) | 40 (16.6) | 15 (12.5) | 25 (20.7) |
| Beck Depression Inventory, mean (SD) | 12.3 (10.5) | 11.5 (9.9) | 13.0 (11.0) |
| Current PTSD Diagnosis, n (%) | 31 (12.9) | 14 (11.7) | 17 (14.0) |
| PTSD Score, mean (SD) | 32.4 (15.0) | 31 (14.3) | 33.7 (15.7) |
| PTSD – Reexperiencing Score, mean (SD) | 9.0 (4.8) | 8.5 (4.3) | 9.5 (5.1) |
| PTSD – Hyperarousal Score, mean (SD) | 10.3 (5.1) | 9.8 (4.8) | 10.8 (5.3) |
| PTSD – Avoidance Score, mean (SD) | 13.0 (.64) | 12.7 (6.3) | 13.3 (6.4) |
| State-Trait Anxiety Inventory, mean (SD) | 36.0 (13.0) | 34.3 (11.9) | 37.7 (13.8) |
Median hsCRP levels remained largely similar between baseline and 6-month follow-up (Fig. 1, upper panel). Similarly, median mental health scores for depression, PTSD, and anxiety remained similar, on average, between baseline and follow-up (Fig. 1, lower panel).
Fig. 1.
Upper panel: Comparison of median log-transformed inflammatory markers at baseline and 6-month follow-up (hsCRP p-value = 0.29, IL-6 p-value = 0.51). Lower panel: Comparison of median mental health scores at baseline and 6-month follow-up (BDI p-value = 0.50, PTSD-score p-value = 0.26, STAI p-value = 0.31).
3.2. Initial bidirectional analysis between mental health scores and inflammatory biomarkers
The levels of systemic inflammatory biomarkers (both hsCRP and IL-6) at baseline were most consistently associated with change in the PTSD re-experiencing symptom cluster, as opposed to other symptom clusters or the total PTSD scale score (Table 2a). A one unit increase in the natural log of hsCRP at baseline was associated with a 1.67-point increase in re-experiencing PTSD symptoms between baseline and follow-up. Similarly, a one unit increase in the natural log of IL-6 at baseline was associated with a 2.27-point increase in re-experiencing PTSD symptoms between baseline and follow-up. There was no association between inflammatory biomarker levels at baseline and change in depression or anxiety symptom scores.
Table 2a.
Association between inflammatory biomarkers at baseline and change in mental health scale scores between baseline and 6 months, using linear regression models, after adjusting for the baseline value of the corresponding mental health scale.
| Baseline Inflammation |
Change in Mental Health Scales (Follow-up vs. Baseline) |
||
|---|---|---|---|
| Beta | 95% CI | P | |
| Overall PTSD Score (PCL-C) | |||
| hsCRP (ng/mL) | 1.97 | (0.77,5.10) | 0.156 |
| IL6 (pg/mL) | 7.24 | (0.89,59.15) | 0.065 |
| Re-experiencing PTSD Symptoms Subscale | |||
| hsCRP (ng/mL) | 1.67 | (1.19,2.34) | 0.003 |
| IL6 (pg/mL) | 2.27 | (1.07,4.85) | 0.032 |
| Hyperarousal PTSD Symptoms Subscale | |||
| hsCRP (ng/mL) | 1.25 | (0.90,1.73) | 0.176 |
| IL6 (pg/mL) | 2.46 | (1.20,5.05) | 0.01 |
| Avoidance and Numbing Symptoms Subscale | |||
| hsCRP (ng/mL) | 1.01 | (0.66,1.55) | 0.963 |
| IL6 (pg/mL) | 1.34 | (0.51,3.53) | 0.557 |
| Depression (BDI-II) | |||
| hsCRP (ng/mL) | 1.39 | (0.69,2.80) | 0.359 |
| IL6 (pg/mL) | 0.59 | (0.12,2.83) | 0.507 |
| Anxiety (STAI) | |||
| hsCRP (ng/mL) | 0.82 | (0.31,2.12) | 0.674 |
| IL6 (pg/mL) | 0.43 | (0.05,3.78) | 0.448 |
Abbreviations:
PTSD: Post-Traumatic Stress Disorder.
STAI: State-Trait Anxiety Inventory.
PCL-C: PTSD Checklist – Civilian Version.
CRP: C-Reactive Protein.
BDI-II Beck Depression Inventory II.
IL-6: Interleukin-6.
The natural logs of inflammatory markers at baseline were used as predictors of changes in mental health scores; then, results were transformed back to original units to ease interpretation. The beta coefficients express the unit change in PTSD scale scores between baseline and 6 months per each unit of baseline hsCRP or IL-6. Bolded text indicates significant results at p < 0.05.
For the opposite direction, examining the association between mental health scores at baseline and changes in inflammatory marker levels over time (Table 2b), results were inconsistent, with re-experiencing PTSD symptoms at baseline showing a significant association with an increase in hsCRP levels over time, but not with IL-6. Depression also showed a borderline significant association with an increase in hsCRP over time, but not with IL-6. No significant association continued to be observed for anxiety. Therefore, the rest of the analysis focused on the association between changes in PTSD between baseline and 6 months as the outcome as a function of inflammatory biomarkers at baseline, since this direction provided more robust and consistent results.
Table 2b.
Association between mental health scale scores at baseline and change in inflammatory biomarkers between baseline and 6 months, using linear regression models, after adjusting for the baseline value of the corresponding biomarker.
| Predictors |
Change in Inflammation (Follow-up vs. Baseline) |
||
|---|---|---|---|
| Beta | 95% CI | P | |
| Baseline Mental Health | hsCRP, mg/L | ||
| PTSD Overall PCL-C Score | 0.102 | (-0.02,0.22) | 0.093 |
| Re-Experiencing Subscale | 0.459 | (0.07,0.85) | 0.021 |
| Hyperarousal Subscale | 0.121 | (-0.22.1.35) | 0.483 |
| Avoidance Subscale | 0.250 | (-0.03,0.53) | 0.080 |
| Depression (BDI-II) | 0.167 | (0.001.55.33) | 0.048 |
| Anxiety | 0.046 | (-0.09,0.19) | 0.514 |
| IL-6, pg/L | |||
| PTSD Overall PCL-C Score | 1.377 | (-11.63,14.38) | 0.835 |
| Re-Experiencing Subscale | 9.617 | (-33.32,52.56) | 0.659 |
| Hyperarousal Subscale | 8.189 | (-28.93,45.31) | 0.664 |
| Avoidance Subscale | −2.859 | (-33.69,27.97) | 0.855 |
| Depression (BDI-II) | 9.215 | (-8.68,27.11) | 0.311 |
| Anxiety | 1.508 | (-13.56,16.57) | 0.843 |
Abbreviations:
PTSD: Post-Traumatic Stress Disorder.
CRP: C-Reactive Protein.
BDI-II Beck Depression Inventory II.
IL-6: Interleukin-6.
PTSD scale scores were used as predictors of changes in inflammatory markers (hsCRP or IL-6). The beta coefficients express the unit change in hsCRP or IL-6 between baseline and 6 months per each unit of baseline PTSD scale scores. Bolded text indicates significant results at p < 0.05.
3.3. Associations between baseline inflammatory markers and PTSD symptoms – multivariate mixed models analysis
Table 3 lists the results of progressively adjusted linear mixed regression models examining the association between inflammatory markers at baseline and changes in PTSD overall score and PTSD subscales. Both IL-6 and hsCRP levels measured at baseline remained consistently associated with an increase in the re-experiencing PTSD subscale score in all models, while no associations were noted for the other PTSD symptom subscales.
Table 3.
Multivariable linear mixed model analysis of the relationship between inflammatory biomarker levels at baseline and change in PTSD scores between baseline and 6 months.
| Outcome | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Total PTSD Score |
Re-experiencing Subscale |
Hyperarousal Subscale |
Avoidance Subscale |
|||||||||
| Beta | (95% CI) | P | Beta | (95% CI) | P | Beta | (95% CI) | P | Beta | (95% CI) | P | |
| Model 1: Demographics (age, sex, education, Black race) | ||||||||||||
| CRP | 1.38 | (0.50,3.78) | 0.533 | 1.62 | (1.15,2.29) | 0.007 | 1.00 | (0.68,1.45) | 0.983 | 0.91 | (0.58,1.43) | 0.686 |
| IL-6 | 3.97 | (0.42,37.71) | 0.477 | 2.16 | (0.98,4.71) | 0.056 | 1.67 | (0.73,3.82) | 0.224 | 1.07 | (0.39,2.97) | 0.891 |
| Model 2: Demographics + Clinical Severity (Ejection Fraction, STEMI) | ||||||||||||
| CRP | 1.26 | (0.44,3.53) | 0.666 | 1.57 | (1.09,2.25) | 0.014 | 0.94 | (0.64,1.39) | 0.778 | 0.89 | (0.55,1.42) | 0.606 |
| IL-6 | 7.03 | (0.69,71.52) | 0.099 | 2.51 | (1.01,5.70) | 0.027 | 2.01 | (0.85,4.76) | 0.112 | 1.45 | (0.5,4.14) | 0.492 |
| Model 3: Demographics + Severity + CV Risk Factors (BMI, Ever Smoker, Diabetes, Cholesterol, Hypertension) | ||||||||||||
| CRP | 1.23 | (0.44,3.49) | 0.689 | 1.57 | (1.09,2.25) | 0.014 | 0.94 | (0.64,1.38) | 0.742 | 0.88 | (0.55,1.4) | 0.575 |
| IL-6 | 7.32 | (0.72,73.70) | 0.092 | 2.56 | (1.13,5.75) | 0.025 | 2.03 | (0.86,4.81) | 0.104 | 1.46 | (0.51,4.22) | 0.473 |
| Model 4: Demographics + Severity + Risk Factors + Prescriptions (Statins, Aspirin, Antidepressants) | ||||||||||||
| CRP | 1.26 | (0.44,3.56) | 0.663 | 1.58 | (1.11,2.27) | 0.013 | 0.95 | (0.64,1.39) | 0.784 | 0.88 | (0.55,1.4) | 0.594 |
| IL-6 | 7.69 | (0.75,79.04) | 0.086 | 2.59 | (1.14,5.87) | 0.024 | 2.10 | (0.88,4.95) | 0.094 | 1.49 | (0.52,4.31) | 0.457 |
Abbreviations:
PTSD: Post-Traumatic Stress Disorder.
CRP: C-Reactive Protein.
IL-6: Interleukin-6.
STEMI: ST-Segment Elevation Myocardial Infarction.
CV: Cardiovascular.
BMI: Body Mass Index.
The natural logs of inflammatory markers at baseline were used as predictors of changes in mental health scores; then, results were transformed back to original units to ease interpretation. The beta coefficients refer to the interaction between inflammatory markers and time, therefore expressing the unit change in PTSD scale scores between baseline and 6 months per each unit of baseline hsCRP or IL-6. Bolded text indicates significant results at p < 0.05.
3.4. Stratified analysis
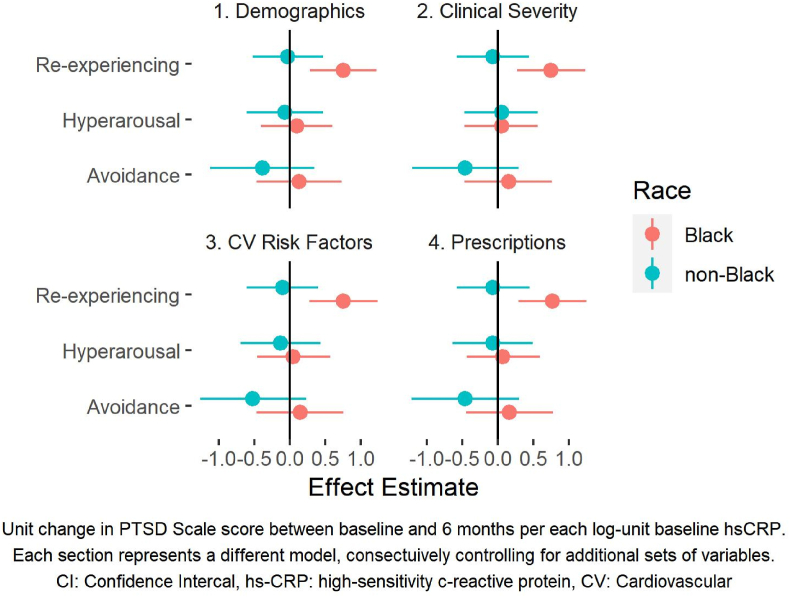
There were 157 Black and 87 non-Black study participants. When stratified by race, baseline hsCRP levels were significantly associated with an increase in both overall PTSD scores and the re-experiencing symptom cluster among Black patients but not among non-Black patients (Table 4a, Fig. 2). The interaction between race and hsCRP measured at baseline on the change in re-experiencing PTSD symptoms was statistically significant in all models. The results for baseline IL-6 were similar (Supplemental Table 2).
Table 4a.
Multivariable linear mixed model analysis of the relationship between hsCRP at baseline and change in PTSD scores between baseline and 6 months, stratified by race.
|
Total PTSD Score |
Re-experiencing Subscale |
|||||||
|---|---|---|---|---|---|---|---|---|
| Beta | (95% CI) | P | Interaction P-value | Beta | (95% CI) | P | Interaction P-value | |
| Model 1: Demographics (age, sex, education) | ||||||||
| Black | 2.56 | (0.66,9.97) | 0.175 | 0.166 | 2.14 | (1.34,3.42) | 0.002 | 0.037 |
| Non-Black | 0.58 | (0.13,2.53) | 0.467 | 0.97 | (0.59,1.6) | 0.915 | ||
| Model 2: Demographics + Clinical Severity (Ejection Fraction, STEMI) | ||||||||
| Black | 2.48 | (0.63,9.97) | 0.194 | 0.163 | 2.12 | (1.31,3.42) | 0.003 | 0.036 |
| Non-Black | 0.52 | (0.11,2.34) | 0.387 | 0.93 | (0.56,1.55) | 0.778 | ||
| Model 3: Demographics + Severity + CV Risk Factors (BMI, Ever Smoker, Diabetes, Cholesterol, Hypertension) | ||||||||
| Black | 2.53 | (0.63,10.07) | 0.188 | 0.144 | 2.14 | (1.32,3.46) | 0.002 | 0.031 |
| Non-Black | 0.47 | (0.1,2.12) | 0.320 | 0.9 | (0.54,1.49) | 0.685 | ||
| Model 4: Demographics + Severity + Risk Factors + Prescriptions (Statins, Aspirin, Antidepressants) | ||||||||
| Black | 2.64 | (0.66,10.59) | 0.167 | 0.137 | 2.16 | (1.34,3.49) | 0.002 | 0.029 |
| Non-Black | 0.53 | (0.12,2.44) | 0.411 | 0.93 | (0.56,1.57) | 0.792 | ||
Abbreviations.
PTSD: Post-Traumatic Stress Disorder.
hsCRP: High Sensitivity C-Reactive Protein.
STEMI: ST-Segment Elevation Myocardial Infarction.
CV: Cardiovascular.
BMI: Body Mass Index.
The natural log of hsCRP at baseline was used as a predictor of change in PTSD scores; then, results were transformed back to original units to ease interpretation. The beta coefficients refer to the interaction between hsCRP and time, therefore expressing the unit change in PTSD scale scores between baseline and 6 months per each unit increase in baseline hsCRP. The interaction p-value is the p-value for the three-way interaction between hsCRP, time, and race. Bolded text indicates significant results at p < 0.05.
Fig. 2.
Beta values and 95% Confidence intervals for the effect of hsCRP on PTSD scores stratified by race. Each panel represents a different model, consecutively controlling for additional sets of variables.
There were 118 women and 126 men in the study. When stratified by sex, hsCRP at baseline was significantly associated with re-experiencing symptoms of PTSD among women but not men (Table 4b), although the interaction was not significant. Results for IL-6 were similar (Supplemental Table 3).
Table 4b.
Multivariable linear mixed model analysis of the relationship between hsCRP at baseline and change in PTSD scores between baseline and 6 months, stratified by sex.
|
Total PTSD Score |
Re-experiencing Subscale |
|||||||
|---|---|---|---|---|---|---|---|---|
| Beta | (95% CI) | P | Interaction P-value | Beta | (95% CI) | P | Interaction P-value | |
| Model 1: Demographics (age, education, Black race) | ||||||||
| Women | 1.82 | (0.45,7.17) | 0.395 | 0.583 | 1.77 | (1.14,2.72) | 0.011 | 0.622 |
| Men | 1.05 | (0.22,4.9) | 0.952 | 1.48 | (0.83,2.64) | 0.181 | ||
| Model 2: Demographics + Clinical Severity (Ejection Fraction, STEMI) | ||||||||
| Women | 1.86 | (0.44,7.69) | 0.394 | 0.483 | 1.75 | (1.12,2.75) | 0.016 | 0.587 |
| Men | 0.9 | (0.18,4.31) | 0.886 | 1.42 | (1.27,2.56) | 0.246 | ||
| Model 3: Demographics + Severity + CV Risk Factors (BMI, Ever Smoker, Diabetes, Cholesterol, Hypertension) | ||||||||
| Women | 1.9 | (0.45,7.85) | 0.379 | 0.414 | 1.77 | (1.13,2.77) | 0.014 | 0.527 |
| Men | 0.9 | (0.19,4.31) | 0.892 | 1.42 | (0.79,2.59) | 0.240 | ||
| Model 4: Demographics + Severity + Risk Factors + Prescriptions (Statins, Aspirin, Antidepressants) | ||||||||
| Women | 1.9 | (0.45,7.92) | 0.381 | 0.522 | 1.77 | (1.12,2.77) | 0.015 | 0.567 |
| Men | 0.95 | (0.2,4.57) | 0.949 | 1.45 | (1.25,2.64) | 0.217 | ||
Abbreviations.
PTSD: Post-Traumatic Stress Disorder.
hsCRP: High Sensitivity C-Reactive Protein.
STEMI: ST-Segment Elevation Myocardial Infarction CV: Cardiovascular.
BMI: Body Mass Index.
The natural log of hsCRP at baseline was used as a predictor of change in PTSD scores; then, results were transformed back to original units to ease interpretation. The beta coefficients refer to the interaction between the natural log of hsCRP level and time, therefore expressing the unit change in PTSD scale scores between baseline and 6 months per each unit increase in baseline hsCRP. The interaction P-value is the p-value for the three-way interaction between hsCRP, time, and sex. Bolded text indicates significant results at p < 0.05.
Black participants were primarily female: there were 87 (55.4%) women among Black participants and 31 (24.6%) among white participants. Stratifying by both sex and race, the associations between hsCRP at baseline and changes in both overall PTSD symptom score and re-experiencing symptoms appeared to be strongest among Black women (Supplemental Table 4). The estimate was elevated but less pronounced and not significant in Black men. In contrast, baseline hsCRP level was not associated with an increase in PTSD symptoms over time in either non-Black men or non-Black women. The results for IL-6 were similar (Supplemental Table 5). This analysis stratified by both sex and race should be considered exploratory given the small number of participants in subgroups of sex and race.
4. Discussion
This study shows that both IL-6 and hsCRP measured at baseline are significantly associated with a longitudinal increase in re-experiencing PTSD symptoms in a population of younger MI patients. This association was found to be significant among Black individuals and especially Black women, but not among non-Black individuals. These findings were independent of clinical and behavioral risk factors associated with inflammation. In contrast, no consistent association was observed for the opposite pathway linking PTSD symptoms at baseline to an increase in inflammation over the follow-up period.
Research has implicated inflammation as an important mechanism to explain the connection of mental health outcomes with increased CHD risk, but most studies have been cross-sectional (Penninx, 2017; Kop et al., 2002; Lima et al., 2019; Empana et al., 2005; Steptoe et al., 2013; Matthews et al., 2010). Furthermore, few studies have specifically examined the longitudinal association between inflammation and PTSD. We recently found that acute mental stress is associated with a larger increase in IL-6 among post-MI patients with elevated re-experiencing symptoms of PTSD; this may suggest that repeated re-experiencing exposures over time in patients with MI and PTSD could lead to higher inflammation (Lima et al., 2019). While we were not able to examine inflammatory responses to stress longitudinally, we found inconsistent evidence for an association between PTSD and increase inflammation longitudinally. However, the reverse direction was true: elevated IL-6 and hsCRP measured at baseline appear to worsen PTSD symptoms over time. This is consistent with Bielas et al.‘s findings, in another post-MI population, showing that persistent elevation of hsCRP from hospital admission to three months predicted PTSD symptoms at follow-up (Bielas et al., 2018).
Our results suggest that inflammation may be implicated in the development or worsening of PTSD, especially of reexperiencing symptoms. Since inflammatory processes are tied with the neuroendocrine system, neuroendocrine dysregulation in PTSD could play a role. Miller and Raison argued that modern humans may have developed an adaptational bias towards inflammation as it enhances the probability of survival and reproduction from an evolutionary perspective (Miller and Raison, 2016). Furthermore, Pace and Heim, based on evidence from prospective cohort studies, suggested that neuroendocrine dysregulation may increase the risk of developing PTSD after a traumatic event (Pace and Heim, 2011). Such dysregulation can facilitate the encoding of traumatic memories, which may explain why reexperiencing symptoms of PTSD are more likely to be expressed. Higher inflammation could signal such neuroendocrine dysregulation.
Studies of the longitudinal associations between depression and inflammation, based on samples from the general population, have suggested that the association is bidirectional (Huang et al., 2019; Valkanova et al., 2013; Khandaker et al., 2014). These findings were also reported in CHD populations; Steptoe et al., for example, reported significant associations between inflammation and somatic symptoms of depression among patients with acute coronary syndromes both at three week and six month follow-up, but no relationship was found for anxiety (Steptoe et al., 2013). In contrast, our study did not find consistent associations between inflammatory biomarkers and changes in depression nor anxiety scores in either direction. Differences in study populations could contribute to differences in results, since our study included a younger post-MI population whereas other studies have largely been conducted in older populations with CHD or in the general population. Furthermore, Steptoe et al. measured inflammation in the acute phase of the cardiac event whereas the present study measured inflammation after complete recovery (Steptoe et al., 2013).
Stratified analyses revealed that inflammatory markers were significantly associated with changes in PTSD symptoms among Black individuals, especially Black women, but not in White Individuals. While the exact reasons for these disparities are unclear, differential exposures to psychosocial stressors could play a role. These groups are at increased risk of psychosocial adversity, such as economic deprivation and discrimination (by gender and race, respectively), which have been associated with both inflammation and adverse mental health (Beydoun et al., 2020; O'Connor et al., 2009; Hodes et al., 2016; Smolderen et al., 2012). Black women, in particular, can face double exposure to sexism and racism. Previous studies have found that women and Black individuals have poorer mental health outcomes and higher levels of inflammation compared to their respective counterparts (Beydoun et al., 2020; O'Connor et al., 2009). Thus, our results could reflect the clustering of psychosocial stressors experienced by these populations which may facilitate the expression of PTSD symptoms (Rozanski et al., 2005; Beydoun et al., 2020).
4.1. Strengths and limitations
This study has some limitations. First, we focused on post-MI patients, thus the results may not be generalizable to non-MI populations. However, this is a high-risk group with elevated rates of both inflammation and PTSD. Second, we conducted many analyses, including the examination of two inflammatory markers and several psychological symptom scores, therefore potentially increasing Type 1 statistical error. However, these multiple analyses were useful to show consistency in results and to contrast PTSD symptoms with other psychological dimensions. Thirty-two individuals did not return at six months; however, a comparison of covariates revealed that those lost to follow up were largely similar to those who remained in the study, suggesting that selection bias is unlikely in our results. Despite these limitations, our study is the first to examine longitudinal trajectories between inflammation and PTSD in both directions in a population of post-MI patients with increased representation of women and Black individuals.
5. Conclusion
In a cohort of younger survivors of a recent MI, inflammation at baseline was associated with an increase in re-experiencing PTSD symptoms between baseline and 6 months. When stratified by race, this association was noted among Black individuals only, especially Black women. These results are consistent with the possibility that inflammation is implicated in the development of PTSD symptoms after an MI, and an increased inflammatory response after the event could be on the pathway between an MI and the development of PTSD, especially among Black individuals and women. Further research is needed to better elucidate these complex relationships. Future studies should also examine specific inflammatory pathways and the clinical utility of specific interventions among diverse populations of post-MI patients. Furthermore, a finer understanding of the cellular and molecular mechanisms linking inflammation, mental health and cardiovascular events may better inform prevention and treatment of PTSD and cardiovascular disease.
Declaration of competing interest
None.
Acknowledgments
This work was supported by the NIH, through the following grants: R01 HL109413, R01 HL109413-02S1, R01 HL125246, R01 HL136205, R01 HL088726, P01 HL101398, KL2 TR000455, K24 HL077506, K24 MH076955, K23 HL127251, K01 HL149982, and T32HL130025.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bbih.2023.100629.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
Data availability
Data will be made available on request.
References
- An K., Salyer J., Kao H.-F.S. Psychological strains, salivary biomarkers, and risks for coronary heart disease among hurricane survivors. Biol. Res. Nurs. 2015;17:311–320. doi: 10.1177/1099800414551164. [DOI] [PubMed] [Google Scholar]
- Beck A., Steer R., Brown G. second ed. 1996. BDI-II Beck Depression Inventory. [Google Scholar]
- Beristianos M.H., Yaffe K., Cohen B., Byers A.L. PTSD and risk of incident cardiovascular disease in aging veterans. Am. J. Geriatr. Psychiatr. 2016;24:192–200. doi: 10.1016/j.jagp.2014.12.003. [DOI] [PubMed] [Google Scholar]
- Beydoun M.A., Obhi H.K., Weiss J., Canas J.A., Beydoun H.A., Evans M.K., Zonderman A.B. Systemic inflammation is associated with depressive symptoms differentially by sex and race: a longitudinal study of urban adults. Mol. Psychiatr. 2020;25:1286–1300. doi: 10.1038/s41380-019-0408-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bielas H., Meister-Langraf R.E., Schmid J.-P., Barth J., Znoj H., Schnyder U., Princip M., von Känel R. C-reactive protein as a predictor of posttraumatic stress induced by acute myocardial infarction. Gen. Hosp. Psychiatr. 2018;53:125–130. doi: 10.1016/j.genhosppsych.2018.03.008. [DOI] [PubMed] [Google Scholar]
- Blanchard E.B., Jones-Alexander J., Buckley T.C., Forneris C.A. Psychometric properties of the PTSD checklist (PCL) Behav. Res. Ther. 1996;34:669–673. doi: 10.1016/0005-7967(96)00033-2. [DOI] [PubMed] [Google Scholar]
- Bremner J.D., Gurel N.Z., Jiao Y., Wittbrodt M.T., Levantsevych O.M., Huang M., Jung H., Shandhi M.H., Beckwith J., Herring I., Rapaport M.H., Murrah N., Driggers E., Ko Y.-A., Alkhalaf M.L., Soudan M., Song J., Ku B.S., Shallenberger L., Hankus A.N., Nye J.A., Park J., Vaccarino V., Shah A.J., Inan O.T., Pearce B.D. Transcutaneous vagal nerve stimulation blocks stress-induced activation of Interleukin-6 and interferon-γ in posttraumatic stress disorder: a double-blind, randomized, sham-controlled trial. Brain Behav. Immun. Health. 2020;9 doi: 10.1016/j.bbih.2020.100138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carney R.M., Freedland K.E. Depression and coronary heart disease. Nat. Rev. Cardiol. 2017;14:145–155. doi: 10.1038/nrcardio.2016.181. [DOI] [PubMed] [Google Scholar]
- Carney R.M., Rich M.W., Tevelde A., Saini J., Clark K., Jaffe A.S. Major depressive disorder in coronary artery disease. Am. J. Cardiol. 1987;60:1273–1275. doi: 10.1016/0002-9149(87)90607-2. [DOI] [PubMed] [Google Scholar]
- Empana J.P., Sykes D.H., Luc G., Juhan-Vague I., Arveiler D., Ferrieres J., Amouyel P., Bingham A., Montaye M., Ruidavets J.B., Haas B., Evans A., Jouven X., Ducimetiere P. Contributions of depressive mood and circulating inflammatory markers to coronary heart disease in healthy European men: the prospective epidemiological study of myocardial infarction (PRIME) Circulation. 2005;111:2299–2305. doi: 10.1161/01.CIR.0000164203.54111.AE. [DOI] [PubMed] [Google Scholar]
- Farr O.M., Ko B.-J., Joung K.E., Zaichenko L., Usher N., Tsoukas M., Thakkar B., Davis C.R., Crowell J.A., Mantzoros C.S. Posttraumatic stress disorder, alone or additively with early life adversity, is associated with obesity and cardiometabolic risk. Nutr. Metabol. Cardiovasc. Dis. 2015;25:479–488. doi: 10.1016/j.numecd.2015.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frasure-Smith N., Lespérance F., Talajic M. Depression and 18-month prognosis after myocardial infarction. Circulation. 1995;91:999–1005. doi: 10.1161/01.cir.91.4.999. [DOI] [PubMed] [Google Scholar]
- Hodes G.E., Ménard C., Russo S.J. Integrating Interleukin-6 into depression diagnosis and treatment. Neurobiol. Stress. 2016;4:15–22. doi: 10.1016/j.ynstr.2016.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang M., Su S., Goldberg J., Miller A.H., Levantsevych O.M., Shallenberger L., Pimple P., Pearce B., Bremner J.D., Vaccarino V. Longitudinal association of inflammation with depressive symptoms: a 7-year cross-lagged twin difference study. Brain Behav. Immun. 2019;75:200–207. doi: 10.1016/j.bbi.2018.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jergović M., Bendelja K., Savić Mlakar A., Vojvoda V., Aberle N., Jovanovic T., Rabatić S., Sabioncello A., Vidović A. Circulating levels of hormones, lipids, and immune mediators in post-traumatic stress disorder – a 3-month follow-up study. Front. Psychiatr. 2015 doi: 10.3389/fpsyt.2015.00049. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4396135/ [Internet] [cited 2021 Jun 28];6. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khandaker G.M., Pearson R.M., Zammit S., Lewis G., Jones P.B. Association of serum interleukin 6 and C-reactive protein in childhood with depression and psychosis in young adult life. JAMA Psychiatr. 2014;71:1121–1128. doi: 10.1001/jamapsychiatry.2014.1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khandaker G.M., Zuber V., Rees J.M.B., Carvalho L., Mason A.M., Foley C.N., Gkatzionis A., Jones P.B., Burgess S. Shared mechanisms between coronary heart disease and depression: findings from a large UK general population-based cohort. Mol. Psychiatr. 2019 doi: 10.1038/s41380-019-0395-3. http://www.nature.com/articles/s41380-019-0395-3 [Internet] [cited 2020 Jun 9];Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kop W.J., Gottdiener J.S. The role of immune system parameters in the relationship between depression and coronary artery disease. Psychosom. Med. 2005;67:S37–S41. doi: 10.1097/01.psy.0000162256.18710.4a. [DOI] [PubMed] [Google Scholar]
- Kop W.J., Gottdiener J.S., Tangen C.M., Fried L.P., McBurnie M.A., Walston J., Newman A., Hirsch C., Tracy R.P. Inflammation and coagulation factors in persons >65 Years of age with symptoms of depression but without evidence of myocardial ischemia. Am. J. Cardiol. 2002;89:419–424. doi: 10.1016/s0002-9149(01)02264-0. [DOI] [PubMed] [Google Scholar]
- Libby P. Targeting inflammatory pathways in cardiovascular disease: the inflammasome, interleukin-1, interleukin-6 and beyond. Cells. 2021;10:951. doi: 10.3390/cells10040951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lima B.B., Hammadah M., Wilmot K., Pearce B.D., Shah A., Levantsevych O., Kaseer B., Obideen M., Gafeer M.M., Kim J.H., Sullivan S., Lewis T.T., Weng L., Elon L., Li L., Bremner J.D., Raggi P., Quyyumi A., Vaccarino V. Posttraumatic stress disorder is associated with enhanced interleukin-6 response to mental stress in subjects with a recent myocardial infarction. Brain Behav. Immun. 2019;75:26–33. doi: 10.1016/j.bbi.2018.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews K.A., Schott L.L., Bromberger J.T., Cyranowski J.M., Everson-Rose S.A., Sowers M. Are there Bi-directional associations between depressive symptoms and C-reactive protein in mid-life women? Brain Behav. Immun. 2010;24:96–101. doi: 10.1016/j.bbi.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller A.H., Raison C.L. The role of inflammation in depression: from evolutionary imperative to modern treatment target. Nat. Rev. Immunol. 2016;16:22–34. doi: 10.1038/nri.2015.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molenberghs G., Bijnens L., Shaw D. In: Linear Mixed Models in Practice: A SAS-Oriented Approach. Verbeke G., Molenberghs G., editors. Springer; New York, NY: 1997. Linear mixed models and missing data. [Internet] [cited 2021 Apr 22]. p. 191–274. [DOI] [Google Scholar]
- Morrow D.A., Rifai N., Antman E.M., Weiner D.L., McCabe C.H., Cannon C.P., Braunwald E. C-reactive protein is a potent predictor of mortality independently of and in combination with troponin T in acute coronary syndromes: a TIMI 11A substudy. Thrombolysis in Myocardial Infarction. J. Am. Coll. Cardiol. 1998;31:1460–1465. doi: 10.1016/s0735-1097(98)00136-3. [DOI] [PubMed] [Google Scholar]
- O'Connor M.-F., Bower J.E., Cho H.J., Creswell J.D., Dimitrov S., Hamby M.E., Hoyt M.A., Martin J.L., Robles T.F., Sloan E.K., Thomas K.S., Irwin M.R. To assess, to control, to exclude: effects of biobehavioral factors on circulating inflammatory markers. Brain Behav. Immun. 2009;23:887–897. doi: 10.1016/j.bbi.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pace T.W.W., Heim C.M. A short review on the psychoneuroimmunology of posttraumatic stress disorder: from risk factors to medical comorbidities. Brain Behav. Immun. 2011;25:6–13. doi: 10.1016/j.bbi.2010.10.003. [DOI] [PubMed] [Google Scholar]
- Passos I.C., Vasconcelos-Moreno M.P., Costa L.G., Kunz M., Brietzke E., Quevedo J., Salum G., Magalhães P.V., Kapczinski F., Kauer-Sant’Anna M. Inflammatory markers in post-traumatic stress disorder: a systematic review, meta-analysis, and meta-regression. Lancet Psychiatr. 2015;2:1002–1012. doi: 10.1016/S2215-0366(15)00309-0. [DOI] [PubMed] [Google Scholar]
- Penninx B.W.J.H. Depression and cardiovascular disease: epidemiological evidence on their linking mechanisms. Neurosci. Biobehav. Rev. 2017;74:277–286. doi: 10.1016/j.neubiorev.2016.07.003. [DOI] [PubMed] [Google Scholar]
- Rozanski A., Blumenthal J.A., Davidson K.W., Saab P.G., Kubzansky L. The epidemiology, pathophysiology, and management of psychosocial risk factors in cardiac practice: the emerging field of behavioral cardiology. J. Am. Coll. Cardiol. 2005;45:637–651. doi: 10.1016/j.jacc.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Scott K.M., de Jonge P., Alonso J., Viana M.C., Liu Z., O'Neill S., Aguilar-Gaxiola S., Bruffaerts R., Caldas-de-Almeida J.M., Stein D.J., de Girolamo G., Florescu S.E., Hu C., Taib N.I., Lépine J.-P., Levinson D., Matschinger H., Medina-Mora M.E., Piazza M., Posada-Villa J.A., Uda H., Wojtyniak B.J., Lim C.C.W., Kessler R.C. Associations between DSM-IV mental disorders and subsequent heart disease onset: beyond depression. Int. J. Cardiol. 2013;168:5293–5299. doi: 10.1016/j.ijcard.2013.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimbo D., Chaplin W., Crossman D., Haas D., Davidson K.W. Role of depression and inflammation in incident coronary heart disease events. Am. J. Cardiol. 2005;96:1016–1021. doi: 10.1016/j.amjcard.2005.05.064. [DOI] [PubMed] [Google Scholar]
- Smolderen K.G., Spertus J.A., Reid K.J., Buchanan D.M., Vaccarino V., Lichtman J.H., Bekelman D.B., Chan P.S. Association of somatic and cognitive depressive symptoms and biomarkers in acute myocardial infarction: insights from the translational research investigating underlying disparities in acute myocardial infarction patients' health status registry. Biol. Psychiatr. 2012;71:22–29. doi: 10.1016/j.biopsych.2011.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielberger C., Gorsuch R., Lushene R. 1970. State-Trait Anxiety (STAI) Manual. [Google Scholar]
- Steptoe A., Wikman A., Molloy G.J., Messerli-Bürgy N., Kaski J.-C. Inflammation and symptoms of depression and anxiety in patients with acute coronary heart disease. Brain Behav. Immun. 2013;31:183–188. doi: 10.1016/j.bbi.2012.09.002. [DOI] [PubMed] [Google Scholar]
- Sumner J.A., Chen Q., Roberts A.L., Winning A., Rimm E.B., Gilsanz P., Glymour M.M., Tworoger S.S., Koenen K.C., Kubzansky L.D. Cross-sectional and longitudinal associations of chronic posttraumatic stress disorder with inflammatory and endothelial function markers in women. Biol. Psychiatr. 2017;82:875–884. doi: 10.1016/j.biopsych.2017.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumner J.A., Chen Q., Roberts A.L., Winning A., Rimm E.B., Gilsanz P., Glymour M.M., Tworoger S.S., Koenen K.C., Kubzansky L.D. Posttraumatic stress disorder onset and inflammatory and endothelial function biomarkers in women. Brain Behav. Immun. 2018;69:203–209. doi: 10.1016/j.bbi.2017.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaccarino V., Goldberg J., Rooks C., Shah A.J., Veledar E., Faber T.L., Votaw J.R., Forsberg C.W., Bremner J.D. Post-traumatic stress disorder and incidence of coronary heart disease: a twin study. J. Am. Coll. Cardiol. 2013;62:970–978. doi: 10.1016/j.jacc.2013.04.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaccarino V., Sullivan S., Hammadah M., Wilmot K., Al Mheid I., Ramadan R., Elon L., Pimple P.M., Garcia E.V., Nye J., Shah A.J., Alkhoder A., Levantsevych O., Gay H., Obideen M., Huang M., Lewis T.T., Bremner J.D., Quyyumi A.A., Raggi P. Mental stress induced-myocardial ischemia in young patients with recent myocardial infarction: sex differences and mechanisms. Circulation. 2018;137:794–805. doi: 10.1161/CIRCULATIONAHA.117.030849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valkanova V., Ebmeier K.P., Allan C.L. CRP, IL-6 and depression: a systematic review and meta-analysis of longitudinal studies. J. Affect. Disord. 2013;150:736–744. doi: 10.1016/j.jad.2013.06.004. [DOI] [PubMed] [Google Scholar]
- Wilkins K.C., Lang A.J., Norman S.B. Synthesis of the psychometric properties of the PTSD checklist (PCL) military, civilian, and specific versions. Depress. Anxiety. 2011;28:596–606. doi: 10.1002/da.20837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf E.J., Schnurr P.P. PTSD-related cardiovascular disease and accelerated cellular aging. Psychiatr. Ann. 2016;46:527–532. doi: 10.3928/00485713-20160729-01. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.