Abstract
Toxoplasma gondii chronic infection is characterized by the establishment of tissue cysts in the brain and increased levels of IFN-γ, which can lead to brain circuitry interference and consequently abnormal behaviour in mice. In this sense, the study presented here sought to investigate the impact of chronic infection by two T. gondii strains in the brain of infection-resistant mice, as a model for studying the involvement of chronic neuroinflammation with the development of behavioural alterations. For that, male BALB/c mice were divided into three groups: non-infected (Ni), infected with T. gondii ME49 clonal strain (ME49), and infected with TgCkBrRN2 atypical strain (CK2). Mice were monitored for 60 days to establish the chronic infection and then submitted to behavioural assessment. The enzyme-linked immunosorbent assay was used for measurement of specific IgG in the blood and levels of inflammatory cytokines and neurotrophic factors in the brain, and the cell's immunophenotype was determined by multiparametric flow cytometry. Mice infected with ME49 clonal strain displayed hyperlocomotor activity and memory deficit, although no signs of depressive- and/or anxiety-like behaviour were detected; on the other hand, chronic infection with CK2 atypical strain induced anxiety- and depressive-like behaviour. During chronic infection by CK2 atypical strain, mice displayed a higher number of T. gondii brain tissue cysts and inflammatory infiltrate, composed mainly of CD3+ T lymphocytes and Ly6Chi inflammatory monocytes, compared to mice infected with the ME49 clonal strain. Infected mice presented a marked decrease of microglia population compared to non-infected group. Chronic infection with CK2 strain produced elevated levels of IFN-γ and TNF-ɑ in the brain, decreased NGF levels in the prefrontal cortex and striatum, and altered levels of fractalkine (CX3CL1) in the prefrontal cortex and hippocampus. The persistent inflammation and the disturbance in the cerebral homeostasis may contribute to altered behaviour in mice, as the levels of IFN-γ were shown to be correlated with the behavioural parameters assessed here. Considering the high incidence and life-long persistence of T. gondii infection, this approach can be considered a suitable model for studying the impact of chronic infections in the brain and how it impacts in behavioural responses.
Keywords: Neuroinflammation, Toxoplasma gondii, Depression, Anxiety, Microglia depletion
1. Introduction
Toxoplasma gondii is an intracellular protozoan, with worldwide distribution and prevalence of human infection varying according to different regions (Dubey et al., 2012; Flegr et al., 2014). Following the parasite distribution, toxoplasmosis outbreaks are reported in many countries, especially in South America (Dubey et al., 2021). The infection is considered a major health problem concerning specific risk groups including pregnant women, fetuses and newborns, and immunocompromised patients (Rostami et al., 2020; Dubey et al., 2021; Graham et al., 2021). In addition, the occurrence of atypical strains, mainly in South America countries (Amouei et al., 2020), represents a major source of infection to humans and act as responsible for the disease outbreak, most of times leading to cases of toxoplasmosis with unusual manifestations (Salvador-Guillouët et al., 2006; Demar et al., 2007; Pardini et al., 2019; Blaizot et al., 2019; Pérez-Grisales et al., 2021). High rates of polymorphisms within the main proteins secreted by T. gondii atypical strains remains as key points in the understanding of the virulence profile and the deleterious impact caused by infection with these strains (Chen et al., 2012; Behnke et al., 2015).
In the past years, studies have been suggesting a possible connection between chronic T. gondii infection identified by positive serology with development of neuropsychiatric conditions (Flegr and Horácek 2020; Pastolache et al., 2021), such as suicidal attempts (Bak et al., 2018; Alvarado-Esquivel et al., 2021a), anxiety (Bay-Richter et al., 2019), depression (Shiadeh et al., 2016; Nasirpour et al., 2020; Alvarado-Esquivel et al., 2021b) and schizophrenia (Al-Hussainy et al., 2015; Esshili et al., 2016; Contopoulos-Ioannidis et al., 2022). However, conflicting studies highlight the absence of significant connections between chronic infection and the occurrence of these behavioural and/or neurological disorders (Gale et al., 2014; Sugden et al., 2016; Chegeni et al., 2019; Bles et al., 2021; Ademe et al., 2022).
In experimental murine models for T. gondii chronic infection, tissue cysts are formed mainly within neurons, which persist throughout the life of the host (Cabral et al., 2016). Chronic infection contributes to the establishment of a constant neuroinflammation, impairing the central nervous system (CNS) homeostasis and functioning, by triggering altered synaptic signals, dysregulation of neurotransmitters, and impairment of perineuronal nets (Torres et al., 2018; Brito et al., 2020; Meurer et al., 2020). The distinct inflammatory patterns and host cerebral modifications in response to the parasite's presence can be explained by the high genetic variability among the T. gondii strains (Robben et al., 2004; Khan et al., 2009; Zhang et al., 2019).
In the establishment of acute and chronic infection by T. gondii, inflammatory monocytes can infiltrate the brain, displaying high expression of NF-kB and promote the activation of signalling pathways responsible for the release of inflammatory cytokines. In addition, microglia can control parasite replication by TNF-ɑ and IFN-γ-dependent production of nitric oxide (Chao et al., 1993, 1994), and act as a key source of the alarmin IL-1ɑ, which stimulates local inflammation and helps control the parasite (Batista et al., 2020). Besides its capacity to control microbial infection, when infected by T. gondii, microglia reactivity is modulated by the release of TGF-β which inhibits the inflammatory pathway and allows parasite dissemination through the tissue (Rozenfeld et al., 2005). Furthermore, the infection can interfere with the balance between microglia and neurons, as the somata of inhibitory neurons was found to be ensheathed by activated microglia and/or myeloid-derived cells and this event may be related to the loss of perisomatic inhibitory synapses found during T. gondii chronic infection (Carrillo et al., 2020).
Disturbances in microglial functioning, combined with persistent neuroinflammation, can play major roles in the development of neuropsychiatric disorders, such as depression and anxiety (Li et al., 2022). Recently, depressive-like behaviour induced by LPS intracerebral injection in C57BL/6 mice was found to be associated with increased microglial activation and enhanced hippocampal inflammation via NLRP3 inflammasome activation and high IL-1β expression (Qiu et al., 2022).
In this sense, considering the parasite's high prevalence rates and the conflicting data regarding the influence of this infection in the establishment of neurological disorders, the understanding of how T. gondii can interfere with and affect the CNS functioning is of great importance to elucidate possible links that may place chronic toxoplasmosis as a risk factor for neuropsychiatric disorders. It is important to highlight that distinct behavioural profile displayed by mice infected with T. gondii reported in the literature reflects the importance of considering not only the parasite strain, but also the mice model applied including their genetic background and even sex. It is already known that BALB/c mice are considered genetically resistant to T. gondii infection, when compared to C57BL/6 mice (Suzuki et al., 1991; Brown et al., 1995; Liesenfeld, 2002; Bergersen et al., 2021); and male mice can also be found to represent a resistant model for the infection (Xiao et al., 2012; Gatkowska et al., 2013; Hegazy et al., 2019).
In this context, the study presented here aimed to investigate the impact of a chronic infection with two strains of T. gondii, focusing on the impact of an atypical strain, as a suitable model for infection-derived brain impairment with microglia depletion and as a trigger for neuroinflammation with repercussions in infection-resistant mice behaviour.
2. Materials and methods
2.1. Maintenance of parasites
Two strains of T. gondii were used to perform this study: a type II clonal strain (ME49) and an atypical strain (TgCkBrRN2, here referred to as CK2). The atypical CK2 strain was first isolated by Clementino-Andrade et al. (2013) from the heart of a chicken obtained from a farm in the state of Rio Grande do Norte, Brazil; being identified as belonging to the #163 genotype registered in ToxoDB.
All parasites were kept frozen in its tachyzoite form in liquid nitrogen. Prior to the experimental infections, the parasites were thawed and inoculated intraperitoneally in Swiss mice. Infected mice were kept for 60 days to establish the chronic stage of infection. After this period, brains were removed, and the cyst load was quantified by light microscopy for further infection of experimental mice as described below.
2.2. Mice and experimental design
This work followed the norms issued by the National Council for the Control of Animal Experimentation (CONCEA) and was approved by the Committee on Ethics in the Use of Animals (CEUA) of the UFMG, under protocol #193/2020.
For the experiments, 8–9 weeks old male BALB/c mice were kept in the animal facility of the Department of Parasitology, ICB/UFMG, under optimal temperature and 12h light/dark cycle and with free access to dry food and water.
For the experimental protocol, mice were organized in three groups, being: 1. Non-infected (Ni); 2. Infected with the clonal strain (ME49); and 3. Infected with the atypical strain (CK2). In the infected groups, mice received 10 brain cysts of each strain via gavage and accompanied for 60 days to establish the chronic infection. For the behavioural assessment, analyses of Ni (N = 10), ME49 (N = 13) and CK2 (N = 10) groups were made during early chronic stage (approximately four weeks post-infection) and chronic stage (eight weeks post-infection). All behavioural evaluations started 14 days before completion of the 4- and 8-weeks post-infection.
2.3. Behavioural assessments
Sixty days after infection, the behavioural tests performed in this study aimed to analyze locomotor activity, memory integrity and existence of anxiety- and depressive-like behaviours in mice chronically infected with T. gondii. For this, the behavioural assessment was performed following the order: the open field test (Horita et al., 2020), object recognition task (Bellozi et al., 2019), elevated plus maze test (Camargos et al., 2020), and forced swim test (Horita et al., 2020).
Each procedure was conducted in a way to minimize the mice's stress, therefore each test happened with a 1–2 days interval to avoid animal exhaustion, in this sense, the behavioural assessments were performed approximately 14 days before the euthanasia date. Mice observation was blindly and randomly performed, recorded by video camera and results were processed using the ANY-MAZE™ software (Soelting Co., IL, USA).
2.3.1. Open field test
For locomotor activity evaluation, mice were individually placed in the center of an open field apparatus for 10 min, and the total distance travelled was measured. For anxiety-like behaviour assessment, the time spent in the center and in the periphery of the apparatus was also analyzed. Tests were recorded and videos were analyzed using ANY-maze software.
2.3.2. Object recognition task
To assess possible cognitive impairment induced by the infections, mice were submitted to the object recognition task (ORT). On day 1, mice were habituated in a box covered with shavings for 5 min. The following 2 days, two identical objects were placed in the apparatus and animals were allowed to freely explore them for 10 and 5 min, respectively. On the test day, one of the familiar objects was replaced by a novel object, and mice were allowed to freely explore it for 5 min. The records were analyzed through ANY-maze software and recognition percentage (RP) was calculated as follow:
For statistical analysis, a one-sample T test was used to compare the group RP to the hypothetical mean of 0.5 (different from random). Mice that did not explore the object were not included in the analysis.
2.3.3. Elevated plus maze
To evaluate anxiety-like behaviour, mice were subjected to the elevated plus maze (EPM) test. The EPM apparatus consists of a plus-shaped maze with two open arms and two closed arms. Each animal was placed in the center of the platform and allowed to freely explore for 5 min. The records were analyzed through ANY-maze software, and the time spent in each arm as well as the number of entries was evaluated.
2.3.4. Forced swim test
To evaluate depressive-like behaviour, mice were subjected to the forced swim test (FST), consisting of an apparatus with 200 mm height and 180 mm diameter, filled with water (25 °C) until 150 mm height. The test lasted 6 min and immobility time was analyzed by EthoVision XT (Noldus, Technology, Leesburg, VA, USA) in the last 4 min. After each session mice were dried and placed in a cage surrounded by a heating pad.
2.4. Euthanasia and organ harvesting
Sixty days post-infection, mice were euthanized, and the blood and brain were collected. Blood was obtained by cardiac puncture, kept for 30 min at room temperature for clot retraction, serum was separated by centrifuging at 600 g for 10 min at 4 °C and stored at −20 °C until use.
After euthanasia and blood collection, all mice received a transcardiac perfusion with 20 mL of phosphate-buffered saline (PBS) at 4 °C to remove any residual blood from the brain tissue. After brain collection, they were transferred to a glass tube containing 1 mL of RPMI and homogenized manually. Forty microliters of the homogenate were used for T. gondii tissue cyst quantification and the supernatant were stored for further cytokine and neurotrophic factors evaluation.
The collection of the brain leukocytes was performed as previously described (Korin et al., 2018) with minor modifications. Briefly, the volume of the brain homogenate was adjusted to 7 mL with RPMI, followed by the addition of 3 mL of stock isotonic percoll (SIP) solution (1:9 PBS and Percoll). The brain homogenate and SIP solution mixture were then carefully transferred to 15 mL falcon tubes containing 2 mL of 30% SIP and centrifuged at 500×g for 30 min at 18 °C without brake for gradient separation. About 4–5 mL of the leukocyte interface was collected, transferred to a new 15 mL tube, and washed three times with PBS 1x at 500×g for 7 min at 4 °C. The pellet was resuspended to 200 μL with wash buffer (NaN3 2 mM, 0.5% BSA and 1x PBS). Cells were counted in the Neubauer chamber and proceeded to immunophenotyping.
2.5. Phenotypic analysis by flow cytometry
Cell staining started by adding the viability dye (LiveDead, AF700, BD Bioscience) followed by an incubation period of 10 min at 4 °C. After incubation, cells were washed twice by centrifugation at 300×g for 8 min at 4 °C with PBS 1x and incubated with a 50 μL mix of surface antibodies (Supplementary Table 1) containing 10% control mice serum for 15 min at 4 °C. Cells were then submitted to two additional rounds of wash with PBS 1x as mentioned above and fixed with 2% paraformaldehyde (PFA) for 20 min at room temperature. After fixation, cells were washed twice with PBS 1x and transferred to FACS tubes. Acquisition was performed in LSR Fortessa (BD Biosciences, USA) and analyzed with the FlowJo software (Tree Star, Ashlan, OR). Data analysis was followed by dimensionality reduction and visualization by t-Distributed Stochastic Neighbor Embedding (tSNE), diffusion map and heatmap using Cytofikit 2 (Chen et al., 2016). Clustering was performed by guided FlowSom. Each experimental group in tSNE analysis represents seven mice with the input of the same number of cells.
2.6. Quantification of systemic anti-Toxoplasma gondii IgG antibodies
The level of systemic anti-T. gondii IgG was detected by an in-house ELISA. Briefly, 96-well plate was incubated with purified T. gondii soluble tachyzoite antigen (STAg) of the RH strain (1μg/100μL/well) for 2 h at 37 °C. The plate was then washed in 0.05% PBS-Tween 20. Three percent of bovine serum albumin in PBS (PBS-BSA) was used to block nonspecific sites for 1 h, at 37 °C, and protected from light. After this step, 50 μL/well of the mice serum (1:100), and negative controls, were added and incubated for 1 h in the same conditions mentioned above, followed by washing as described. Next, it was added 100 μL/well of the peroxidase-conjugated anti-mouse IgG secondary antibody (diluted 1:2000) and incubated for 1 h, at 37 °C and protected from light. After washing, 50 μL/well of O-phenylenediamine dihydrochloride (OPD) were added, followed by 20 min of incubation at 37 °C and protected from light. The reaction was stopped by adding 30 μL/well of stop solution (H2SO4 2 mol/L). The absorbance was measured at 490 nm wavelength and the IgG levels were expressed as optical density (OD).
2.7. Quantification of IFN-γ, CX3CL1, NGF and GDNF in the prefrontal cortex, striatum, and hippocampus
The brains were dissected to isolate the prefrontal cortex, striatum, and hippocampus. In these areas, the levels of IFN-γ, fractalkine (CX3CL1), nerve growth factor (NGF) and glial derived neurotrophic factor (GDNF) were measured by sandwich ELISA (DuoSet® ELISA, R&D Systems) according to the manufacturer's instructions. The levels were quantified based on a standard curve, following the protocol provided by each kit.
2.8. Quantification of nitrite levels in the prefrontal cortex, striatum, and hippocampus
The presence of nitric oxide in the three brain areas was indirectly determined by levels of nitrite measured by the colorimetric method of Griess (Guevara et al., 1998; Sun et al., 2003). Briefly, 100 μL/well of the brain area supernatant were added in a 96-well plate, followed by 100 μL/well of Griess reagent (1% Sulfanilamide in 5% H2PO4 and 0.1% N-1-Naphthyl-ethylenediamine). The plate was incubated for 10 min, at room temperature and protected from light. The absorbance was measured at 540 nm wavelength and the nitrite concentration was determined based on a standard curve.
2.9. Cytokines quantification by Cytometric Beads Array (CBA)
The brain supernatant saved from the flow cytometry was used to determine the levels of local cytokines during T. gondii chronic infection. Levels of IFN-γ, TNF-α, IL-10, IL-2 and IL-6 were measured by CBA, according to the manufacturer's instruction (BD Bioscience, USA). Samples were acquired in BD FACS Callibur flow cytometer (BD Bioscience, USA), and the data processing was performed in the software FlowJo (Tree Star, Ashlan, OR).
2.10. Statistical analysis
The analysis was performed using GraphPad Prism software, version 8.0 (GraphPad, San Diego, CA, USA). The data had their normality analyzed by the Shapiro-Wilk test. Outlier values were identified and excluded based on the ROUT method for outlier identification. To compare data among parametric data groups was performed t-student test or ANOVA test of variance, followed by Tukey post-test. The analysis of non-parametric data was performed by Mann-Whitney or Kruskall-Wallis test, followed by Dunn's post-test. Correlation analysis was made by Pearson's correlation coefficient. The results were considered statistically significant when p < 0.05. The data presented here is representative of two independent experiments.
3. Results
3.1. Chronic infection by atypical strain of Toxoplasma gondii induces anxiety- and depressive-like behaviours in mice
During the experimental protocol, all mice from both infected groups successfully survived. In the acute infection phase, both infected groups displayed similar sickness behaviour, characterized by piloerection and weight loss as the most evident signals. However, mice infected with the CK2 atypical strain exhibited higher and more prolonged weight loss compared to the ME49-infected mice. Additionally, the atypical strain-infected group showed a delay in weight recovery during the establishment of chronic infection, in contrast to the ME49-infected group (data not shown).
Aiming to investigate the impact of brain infection in the development of abnormal behaviours in mice, we evaluated here the locomotor activity, anxiety- and depressive-like behaviour and memory integrity during the establishment of chronic infection by distinct T. gondii strains.
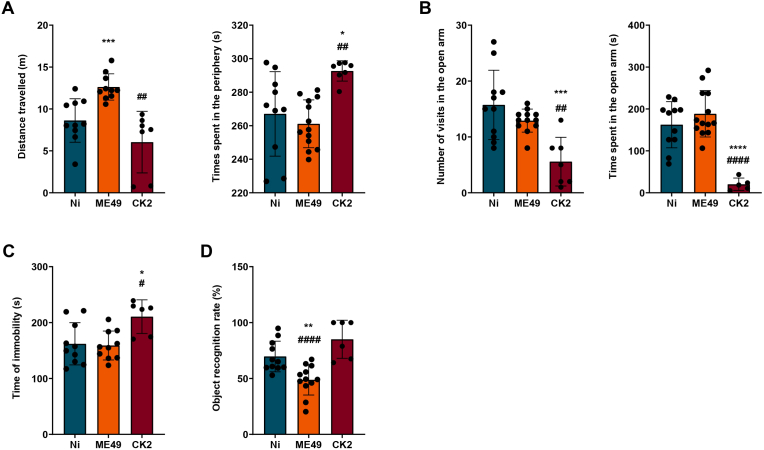
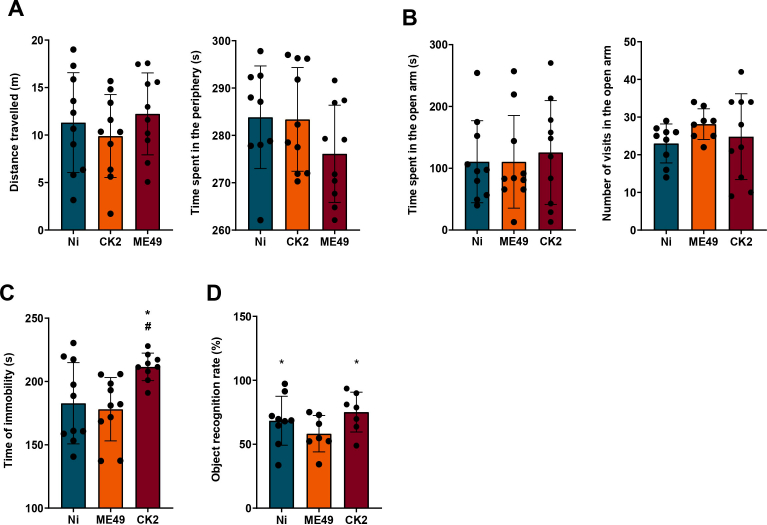
First, to evaluate if the infection could alter mice locomotor activity, mice were submitted to the open field test during the early chronic (4 weeks) and well established chronic (8 weeks) infection. During the early chronic infection, do differences were found in the locomotor activity of both infected groups (Supplementary Fig. 1A). However, in the 8 weeks assessment, we found that chronic infection induced by the ME49 strain, but not by CK2 atypical strain, increased mice's locomotor activity (Fig. 1A, left). In addition, since an open field test can also be used to evaluate anxiety-like behaviour (La-Vu et al., 2020), we also evaluated the time spent in the center and in the periphery of the apparatus. We found that CK2 atypical strain, but not ME49 strain, induced an anxiety-like behaviour in mice, once CK2-infected mice spent significantly more time in the periphery of the apparatus (Fig. 1A, right).
Fig. 1.
Behavioural assessment of mice chronically infected by distinct T. gondii strains. The data represented here is from behavioural analysis for 8 weeks post-infection. (A) data analysis from the open field test, showing the total distance travelled (in meters) by non-infected (blue) and ME49- (orange) and CK2-infected (dark red) mice (left); and time spent in the periphery of the apparatus (right). (B) Data analysis from the elevated plus maze test, showing the number of entries (left) and the time spent (right) in the open arms by non-infected (blue) and ME49- (orange) and CK2-infected (dark red) mice. (C) Data analysis from the forced swim test, showing the time (in seconds) of immobility. (D) Memory analysis performed by the object recognition task. The statistical analysis for the recognition index with 50% cut-off was performed by one sample t-test. * Indicates the statistical difference between non-infected and infected mice groups; # indicates the statistical difference between the infected mice groups. Statistical analysis to find difference among the studied groups was performed using ANOVA test of variance followed by Tukey post-test. Outlier values were removed after ROUT test for outlier identification. Data presented here are expressed as mean ± standard deviation (SD). Non-infected group (n = 10); ME49-infected group (n = 13) and CK2-infected group (n = 10). */#p < 0.05; **/##p < 0.01; ***/###p < 0.001; ****/####p < 0.0001. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
After that, we also evaluated anxiety-like behaviour in the EPM test. Although no differences were found in the assessment during the 4 weeks post-infection (Supplementary Fig. 1 B), in analysis performed during the course of 8 weeks post-infection CK2-infected mice spent less time in the open arm in comparison to control and ME49-infected mice, corroborating the previous data. Moreover, CK2-infected mice had decreased number of entries in the open arm (Fig. 1B).
To evaluate depressive-like behaviour, mice were submitted to FST, which is the most commonly used assay (Petit-Demouliere et al., 2005). We found that CK2 atypical strain induced the development of depressive-like behaviour, which is shown by the increase in immobility time in comparison to the control and ME49 strain. This result was found during both 4 weeks and 8 weeks post-infection behavioural assessment (Supplementary Fig. 1C and 1C).
Finally, to evaluate possible alterations in mice cognition, we used the object recognition task to assess the memory. We found, for both 4- and 8-weeks evaluation, that ME49 strain induced memory impairment once ME49-infected mice couldn't differentiate the familiar object from the novel object (Supplementary Fig. 1D and 1D). However, it is noteworthy to mention that 4 out of 10 CK2-infected mice showed no interest in exploring the objects.
3.2. Chronic infection by atypical strain of Toxoplasma gondii induces higher cerebral inflammation and marked reduction of microglia population
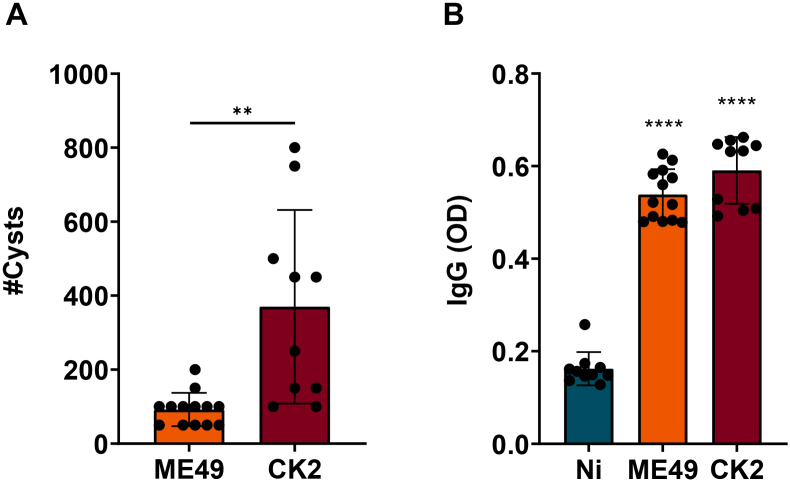
During the experimental procedure, all mice remained infected for 8 weeks to reach chronic infection. After this period, the quantification of the parasite's cyst load in the brain revealed that infection with the atypical strain (CK2) was able to induce a higher number of cysts when compared to mice infected with the clonal strain (ME49) (Fig. 2A). Regarding the levels of specific anti-T. gondii antibodies, both strains were able to induce similar levels of systemic IgG (Fig. 2B).
Fig. 2.
Quantification of the tissue cysts number and systemic anti-T. gondii IgG levels. (A) Tissue cyst burden in the brain homogenate of male BALB/c mice, infected by T. gondii ME49 clonal strain or CK2 atypical strain eight weeks post-infection. (B) Quantification of systemic IgG levels, eight weeks post-infection compared to non-infected group. The statistical analysis was performed by t student test. In “B”, the asterisks represent the statistical difference among infected and non-infected groups. Data presented here are expressed as mean ± standard deviation (SD). OD: optical density. *p < 0.05; ****p < 0.0001.
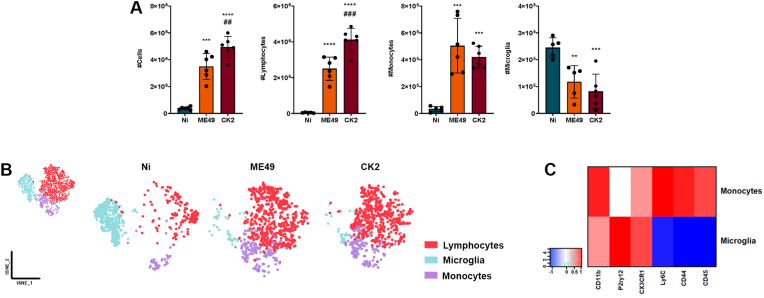
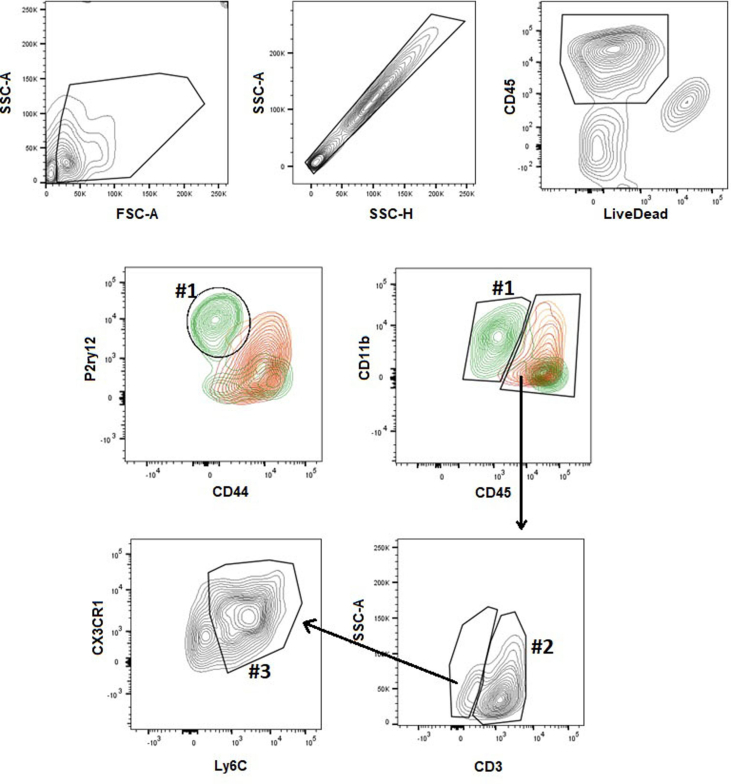
The analysis of the immune cell population in the brain was based on the expression level of the surface markers CD45, CD11b, CD3, CX3CR1, CD44, Ly6C and P2RY12, as evidenced in the analysis strategy used (Supplementary Fig. 2). As expected, non-infected (Ni) mice exhibited mainly the microglia population (Fig. 3A, right), identified based on the P2RY12 expression level. T. gondii-infected mice displayed increased inflammatory infiltrate (Fig. 3A, left), composed mainly by CD3+ T lymphocytes (Fig. 3A, middle-left); however, the atypical strain CK2 induced higher lymphocytic infiltrate when compared to ME49-infected mice.
Fig. 3.
Analysis of the immune cell populations identified in the brain of mice chronically infected with T. gondii. (A) quantification of the total cell number in the brain, followed by the number of CD3+ T lymphocytes, Ly6Chi inflammatory monocytes and P2RY12hi microglia among non-infected mice (N = 6; blue) and mice infected with T. gondii ME49 (N = 6; orange) clonal strain or CK2 atypical strain (N = 6; dark red). (B) tSNE of combined data (upper left side) from non-infected mice and mice after 8 weeks of infection by T. gondii ME49 clonal strain and CK2 atypical strain. This analysis was reached using the group of cells identified as LiveCD45+. The distribution of T lymphocytes (red), microglia (blue) and inflammatory monocytes (purple) were subsequently stratified according to each mice group (Non-infected, Ni; ME49-infected and CK2-infected mice). (C) Heatmap highlighting the expression level of each surface marker, according to the population of microglia and inflammatory monocytes. Data presented here are representative of two independent experiments and expressed as mean ± standard deviation (SD). * Indicates the statistical difference between non-infected and infected mice groups; # indicates the statistical difference between the infected mice groups. Statistical analysis was performed using ANOVA test of variance followed by Tukey post-test. Outlier values were removed after ROUT test for outlier identification. */#p < 0.05; **/##p < 0.01; ***/###p < 0.001; ****/####p < 0.0001. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
Based on the expression level of surface markers, we separated the inflammatory monocytes infiltrated from the periphery (LiveCD45hiCD11bhiCX3CR1+CD44hiSiglecH−Ly6ChiP2RY12-) from the resident microglia population (LiveCD45lowCD11b + CX3CR1hiCD44−SiglecH+Ly6C–P2RY12+). T. gondii chronic infection induced a marked infiltration of inflammatory Ly6Chi monocytes in the brain, which display high expression of CD44 characteristic of periphery-derived infiltrating cells, regardless of the infecting strain (Fig. 3A, middle-right). Concomitantly to the high cell infiltration in the brain, we found a marked reduction of microglia population during the chronic infection, in a strain-independent fashion (Fig. 3A, right).
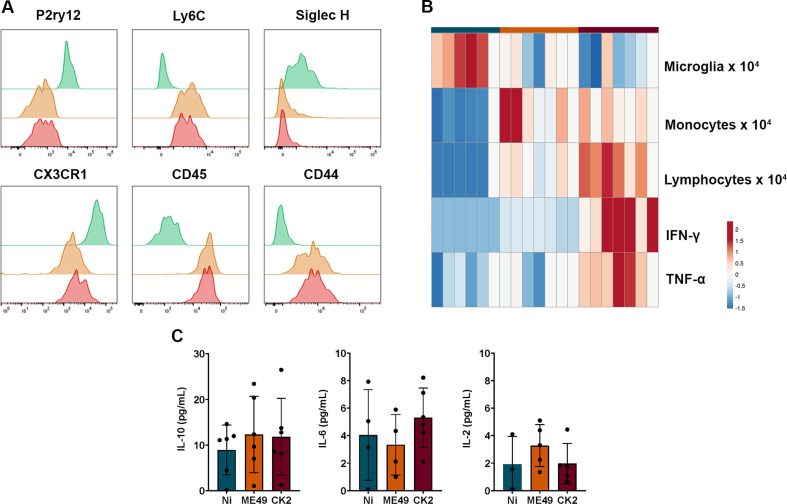
Dimensionality reduction analysis by tSNE identified the three main cell populations, evidencing the CD3+ lymphocytes (red), Ly6Chi monocytes (purple) and P2RY12hi microglia (blue) (Fig. 3B). Analysing each experimental group separately, tSNE analysis revealed the predominance of lymphocytes and inflammatory monocytes in the brain of mice infected with ME49 (Fig. 3B, middle-right) and CK2 (Fig. 3B, right) strains of T. gondii, followed by a significant decrease in the microglia population, when compared to the non-infected mice group (Fig. 3B, middle-left). The differentiation between infiltrated inflammatory monocytes and microglia population was achieved by the differences in the expression level of the surface markers P2RY12, Ly6C, SiglecH, CX3CR1, CD45 and CD44; microglia exhibit high expression levels of P2RY12 and do not express Ly6C, while inflammatory monocytes that infiltrate in the niche do not express P2RY12 and express high levels of Ly6C, CD44 and CD45 (Fig. 3C; Supplementary Fig. 3A).
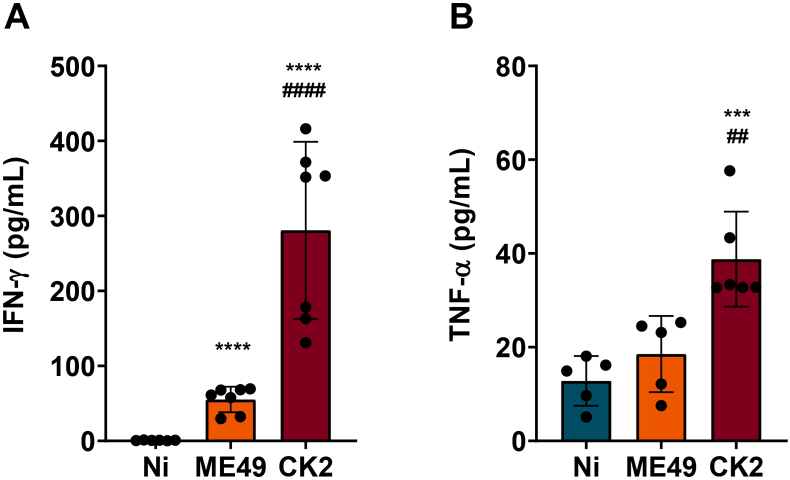
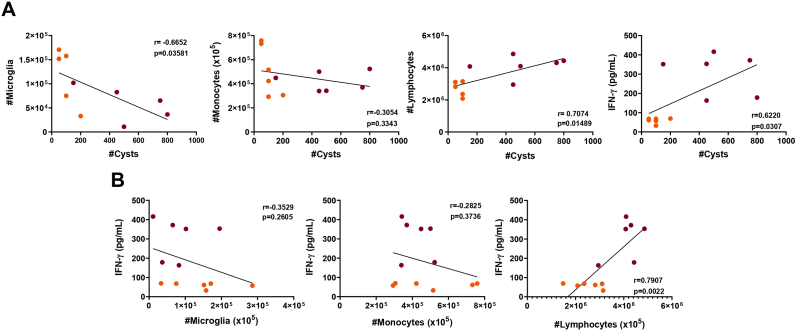
In addition to the inflammatory cells infiltrating, we measured the levels of cytokines in the brain. We found that during chronic infection, besides the marked inflammatory cells infiltrate, mice infected by CK2 strain displayed higher levels of IFN-γ and TNF-α, when compared to ME49-infected and non-infected mice groups (Fig. 4; Supplementary Fig. 3B). No significant differences were observed in the levels of IL-10, IL-6 and IL-2 among the non-infected and infected groups (Supplementary Fig. 3C). In addition, it was found that the T. gondii cyst load in the brain positively correlated to the lymphocyte's infiltration and to the high levels of IFN-γ found during chronic infection; on the other hand, the same cyst load was shown to inversely correlated to the microglia population; thus, as higher is the cyst load in the brain, the lower is the microglia population (Fig. 5A). Furthermore, the levels of IFN-γ were shown to be directly correlated to the number of lymphocytes infiltrated in the brain of infected mice (Fig. 5B), however no correlation was found among the levels of this cytokine and the microglia population or inflammatory monocytes infiltrate (Fig. 5B).
Fig. 4.
Quantification of I IFN-γ and TNF-α levels in the brain of mice eight weeks after T. gondii infection. Chronic infection by atypical strain CK2 (N = 7) lead to significantly higher levels of IFN-γ (A) and TNF-α (B) in the brain of mice, when compared to ME49-infected (N = 7) and non-infected mice (N = 7). Data presented here are expressed as mean ± standard deviation (SD). * Indicates the statistical difference between non-infected and infected mice groups; # indicates the statistical difference between the infected mice groups. Statistical analysis was performed using ANOVA test of variance followed by Tukey post-test. Outlier values were removed after ROUT test for outlier identification. */#p < 0.05; **/##p < 0.01; ***/###p < 0.001; ****/####p < 0.0001.
Fig. 5.
Toxoplasma gondii tissue cyst burden is correlated to the microglia population and to the production of IFN-γ in the brain of infected mice. (A) Correlation analysis between the cell populations of T lymphocytes, inflammatory monocytes, microglia and IFN-γ levels with the T. gondii tissue cyst burden in the brain of infected mice. (B) Correlation analysis of the IFN-γ levels in the brain with the cell populations of microglia, inflammatory monocytes and T lymphocytes in the brain of infected mice. The correlation analysis was performed by the Pearson's coefficient of correlation, followed by linear regression with 95% confidence interval. Orange dots represent mice infected with ME49 clonal strain (N = 6), and purple dots represent mice infected with CK2 atypical strain (N = 6). Outlier values were removed after ROUT test for outlier identification. The results were considered statistically significant when p < 0.05. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
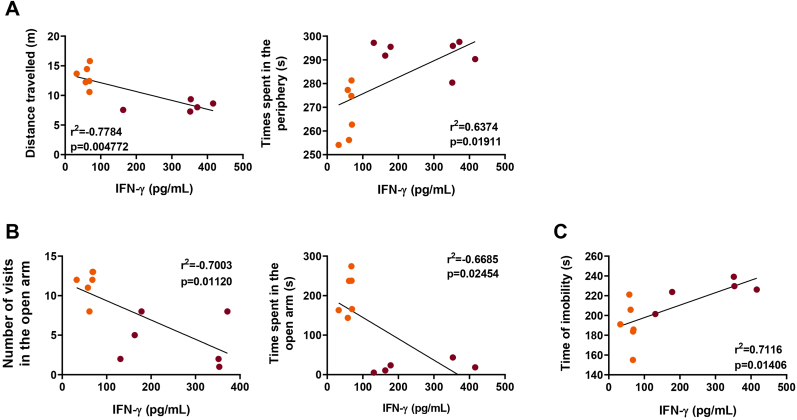
In the search for a possible answer on how T. gondii chronic infection could trigger behavioural changes, the levels of IFN-γ detected in the brain of infected mice were submitted to correlation analysis with the behavioural parameters obtained. Here, we found that, in the open field test, the locomotor activity displayed by the infected mice was inversely correlated with the levels of IFN-γ in the brain (Fig. 6A, left); in addition, the time spent in the periphery was directly correlated with this cytokine concentration in the brain of infected mice (Fig. 6A, right). Correlations between IFN-γ levels and the anxiety-like behaviour identified by the EPM test were also observed (Fig. 6B). Furthermore, the IFN-γ levels were also shown to be directly correlated with the time of immobility of infected mice during chronic infection (Fig. 6C).
Fig. 6.
Total brain levels of IFN-γ are correlated with the behavioural profile displayed by mice chronically infected with T. gondii. The levels of IFN-γ in the brain of infected mice were inversely correlated with the distance travelled, while directly correlated with the time spent in the periphery of the open field apparatus (A). The number of entries and the time spent in the open arms of the elevated plus maze were inversely correlated with the levels of IFN-γ in the brain of infected mice. (C) With the data obtained from the forced swim test, it was found that the time of immobility was directly correlated with the brain levels of IFN-γ in mice chronically infected with T. gondii. The correlation analysis was performed by the Pearson's coefficient of correlation, followed by linear regression with 95% confidence interval. Orange dots represent mice infected with ME49 clonal strain (N = 7), and purple dots represent mice infected with CK2 atypical strain (N = 7). Outlier values were removed after ROUT test for outlier identification. The results were considered statistically significant when p < 0.05. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
3.3. Cerebral inflammation and altered levels of neurotrophic factors are present in distinct brain areas during chronic Toxoplasma gondii infection
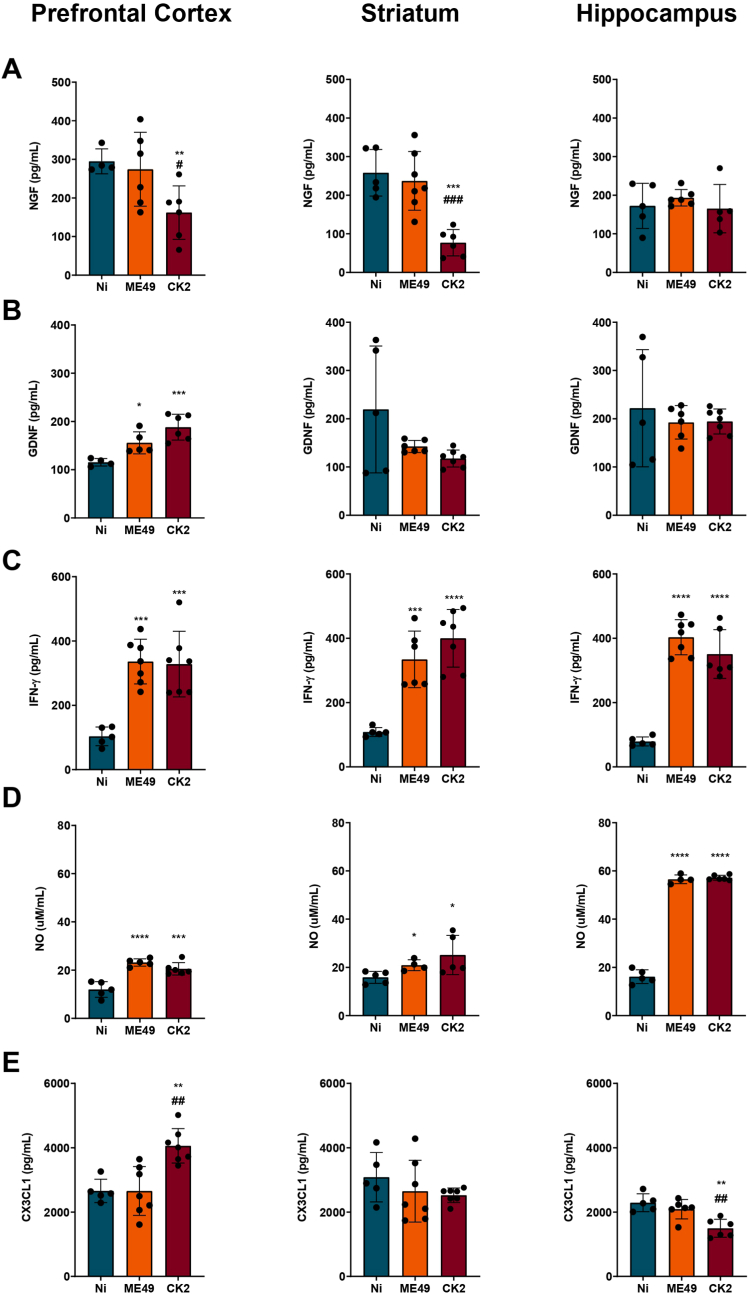
Next, we sought to analyze the chronic T. gondii infection impact over the levels of neurotrophic factors in three different areas of the mice brain: prefrontal cortex, striatum, and hippocampus. The analysis revealed that the infection with T. gondii CK2 atypical strain was capable to interfere in the levels of NGF, leading to the decrease of this factor in the prefrontal cortex (Fig. 7A, left) and striatum (Fig. 7A, middle) when compared to non-infected and ME49-infected mice groups; furthermore, the levels of GDNF in the brain of mice infected with both ME49 and CK2 strains were shown to be significantly higher in the prefrontal cortex (Fig. 7B, left) when compared to naive counterparts.
Fig. 7.
Toxoplasma gondii chronic infection impacts the levels of neurotrophic factors and inflammatory mediators in different brain regions. Quantification of (A) nerve growth factor (NGF) and (B) glia derived neurotrophic factor (GDNF) levels in the prefrontal cortex, striatum, and hippocampus of non-infected (N = 7; blue) and ME49- (N = 7; orange) and CK2-infected (N = 7; dark red) mice. Quantification of IFN-γ (C), nitric oxide (D) and fractalkine – CX3CL1 (E) in the prefrontal cortex, striatum, and hippocampus of non-infected (blue) and ME49- (orange) and CK2-infected (dark red) mice. Data presented here are expressed as mean ± standard deviation (SD). Outlier values were removed after ROUT test for outlier identification. * Indicates the statistical difference between non-infected and infected mice groups; # indicates the statistical difference between the infected mice groups. Statistical analysis was performed using ANOVA test of variance followed by Tukey post-test. NO: nitric oxide. */#p < 0.05; **/##p < 0.01; ***/###p < 0.001; ****/####p < 0.0001. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
After detecting the changes in the neurotrophic factors, we analyzed the levels of inflammatory mediators in the same three brain areas. T. gondii-infected mice displayed high levels of IFN-γ in the three analyzed areas (Fig. 7C); however, no differences on this cytokine levels were found between both infecting strains. The levels of nitrite in the brain, representative of nitric oxide presence due to inflammatory response, were shown to be elevated in all infected mice (Fig. 7D), in a strain-independent fashion. In addition to the inflammatory mediators, we also analyzed the concentration of CX3CL1, which is a chemokine important for the communication between neurons and microglia. During chronic infection by the atypical strain CK2, CX3CL1 levels were significantly higher in the prefrontal cortex (Fig. 7E, left), while reduced levels were found in the hippocampus (Fig. 7E, right); no differences were found in the striatum (Fig. 7E, middle), when compared to non-infected mice.
In summary, our data demonstrated that Toxoplasma chronic infection can lead to behavioural alterations in a strain-dependent fashion and correlated with the IFN-γ levels in the brain. While the ME49 strain induced alterations in mice's locomotor activity and memory, the atypical strain CK2 induced anxiety and depressive-like behaviours. The altered behaviours observed, mainly among mice infected with the atypical strain, were shown to be correlated to the brain IFN-γ levels, supporting the hypothesis that chronic brain infections that lead to persistent neuroinflammation can interfere in the adequate cerebral functioning, which is reflected by abnormal mice behaviour.
4. Discussion
During T. gondii brain infection, neurons are the main target of the parasite, representing 85% of the infected cells (Cabral et al., 2016). It was shown that the chronic infection can induce the retraction of the dendritic tree of neurons in the basolateral amygdala (Mitra et al., 2013), as well as contributing to the degeneration of glutamatergic and GABAergic neurons in different portions of the prefrontal cortex. These neurons under degeneration were found to express high levels of fractalkine (CX3CL1) and to be surrounded by reactive microglia (Li et al., 2019). Fractalkine production by neurons plays a crucial role in modulating the reactive phenotype of microglia, which expresses the CX3CR1 receptor (Harrison et al., 1998) and the CX3CL1-CX3CR1 signalling axis can influence brain homeostasis through the regulation of inflammatory mediators by reactive microglia (Cardona et al., 2006; Limatola and Ransohoff, 2014). Alterations in this pathway can interfere with cerebral functioning, as reduced levels of CX3CL1 were shown to lead to cognitive impairment and long-term memory deficits in mice (Winter et al., 2020). During the chronic infection by the atypical T. gondii strain (CK2), the levels of CX3CL1 were found to be increased and decreased in the prefrontal cortex and hippocampus, respectively. However, only mice infected with the ME49 clonal strain showed memory deficit, despite no changes in CX3CL1 levels. These alterations highlight the impact of chronic infection in the communication pathways between neurons and microglia, as a result of cellular impairment and inflammation generated in the brain due to the presence of T. gondii cysts.
The production and release of neurotrophic factors are crucial for the cerebral function, as they are involved in mechanisms of maintenance, plasticity, and survival of neuronal cells; in addition to being closely involved in the maintenance of cognitive functions (Berry et al., 2012; Budni et al., 2015). In experimental models of brain ischemia, mice harbouring lesions displayed anxiety- and depressive-like behaviours along with high levels of NGF (Fernandes et al., 2020). Conversely, experimental models using rodents were able to induce anxiety- and depressive-like behaviours and displayed reduced levels of NGF in different brain areas (Song et al., 2009; Filho et al., 2015; Hashikawa et al., 2015). Another study found reduced levels of NGF and GDNF in the prefrontal cortex, striatum and hippocampus of rats using an experimental model of bipolar disorder profile and anxiety- and depressive-like behaviours induced by amphetamine (Valvassori et al., 2019). Variations in neurotrophic factors levels and their association with abnormal behaviours might reflect the impact of distinct insults in the CNS, leading to disturbance of brain homeostasis, with the severity being related to the experimental model applied. In our work, during the chronic infection by T. gondii atypical CK2 strain, the availability of NGF was impacted, leading to reduced levels in the prefrontal cortex and striatum when compared to mice infected with ME49 strain and with the non-infected group. The infection led to increased GDNF levels only in the prefrontal cortex. In a previous study, high levels of GDNF were linked to increased locomotor activity in mice (Littrell et al., 2013), however, in our study, such alterations were not observed among mice infected with the atypical strain. In the case of T. gondii infection, factors such as parasite genotype and virulence, widespread tissue cyst load and the cytotoxic stimuli triggered by the neuroinflammation and oxidative environment can contribute to the development of behavioural abnormalities, such as anxiety- and depressive-like behaviours (Kim et al., 2016; Xia et al., 2018; Han et al., 2020).
Herein, the behavioural alterations found in mice infected with T. gondii were shown to be correlated with IFN-γ levels in the brain, offering valuable insights into how chronic neuroinflammation can contribute to behavioural disturbances in mice models. In the context of long-term brain infections, such as T. gondii infection analyzed in this work, behavioural alterations may result from multifactorial parameters, including parasite virulence and burden, the induced neuroinflammation, and unbalanced levels of neurotrophic factors in the brain. In addition, previous studies have shown that T. gondii infection can interfere with the GABAergic and glutamatergic signalling pathways. During infection of C57BL/6 mice with T. gondii ME49 strain, an increased expression of the enzyme GAD67 was observed, while a decrease in the expression of NMDAR was reported (Torres et al., 2018). Another study has reported the production of anti-NMDAR autoantibodies during T. gondii chronic infection in CD-1 mice, being influenced by the tissue cyst load in the brain, and it was also found to be involved in the establishment of behavioural alterations, such as reduction of locomotor and exploratory activities during the chronic infection (Li et al., 2018). These findings can be used to explain the onset of behavioural abnormalities in mice chronically infected by T. gondii.
In a recent study, C57BL/6 mice infected with T. gondii ME49 strain and treated with sulfadiazine and pyrimethamine during the establishment of chronic infection presented an improvement in the behaviour parameters analyzed, with reduced anxiety- and depressive-like behaviours, in addition to the decrease of hyperlocomotor profile. After treatment, which induces the chronic infection, the resolution of behavioural abnormalities was accompanied by reduced tissue cyst load in the brain, with lower levels of neuroinflammation visualized by histological analysis displaying less intense meningitis and reduced mononuclear inflammatory cells infiltrating into the CNS (Castaño et al., 2022). In Castaño et al. (2022) study, the levels of inflammatory cytokines in the serum of treated and untreated mice were measured and a marked reduction in IFN-γ and TNF-ɑ levels was found after treatment. However, measuring cytokine levels directly from the brain tissue could provide a more accurate representation of the neuroinflammatory levels. In our study, higher levels of IFN-γ were found in the brain tissue of mice infected with either strain, indicating that the inflammation induced locally could be involved with the establishment of the abnormal behaviours found. Our experimental model highlights the impact induced by chronic infection even in a resistant host model, leading to an inflamed environment that disrupts cerebral homeostasis. Although, treatment during chronic infection can reduce cerebral damage triggered by the parasite's presence in the brain and its associated neuroinflammation, leading to the remission of behavioural abnormalities as shown by Castaño and colleagues, it is still necessary to evaluate whether treating chronic infection in different hosts, infected with distinct T. gondii strains, can re-establish homeostasis in the brain.
Central nervous system infections have been identified as major players in the disruption of cerebral integrity and impairment of homeostatic brain functioning. Viral infections that reach the CNS are responsible for establishing marked inflammation, leading to encephalitis and triggering damage to brain tissue (Filgueira et al., 2021). Microglia play a key role in regulating brain homeostasis and act as scavenger cells (Tsai et al., 2016) and reduction of 50–60% of microglia population in mice or humans is associated with the loss of myelin integrity (McNamara et al., 2022). The pharmacological depletion of microglia in mice has shown the impact of this cell population disappearance in the control of viral infections, such as West Nile Virus (WNV), Japanese Encephalitis Virus (JEV) and Zika Virus (ZIKV) infections, leading to increased viral load and mice susceptibility with higher mortality rates during these infections (Seitz et al., 2018; Enlow et al., 2021).
In our study of T. gondii chronic infection, it was found a dramatic impact on microglia population in mice infected with either ME49 or CK2 strains, when compared to non-infected mice. A recent study highlighted the ability of T. gondii infection to induce the increase of microglia on the eyes of BALB/c mice after instillation of tachyzoites in the conjunctival sac (Soares et al., 2022). However, the increased microglia population was identified based only in the immunohistochemical staining for Iba-1, which does not allow a real differentiation among microglia and infiltrated inflammatory monocytes (Amici et al., 2017; Jurga et al., 2020). A more accurate differentiation of microglia and inflammatory monocytes can be reached by targeting the receptors Tmem119 and/or P2RY12 (Butovsky et al., 2014; Bennet et al., 2016). Furthermore, the route of infection and virulence profile of the parasite strain must also be considered, as they contribute to the severity of the infection and tissue damage in the host. In this sense, the chronic infection by T. gondii stimulates marked alterations in the resident macrophages, either by leading to cell disappearance or changes in the cell phenotype, and at the same time induces a strong inflammatory infiltrate in the brain. However, we acknowledge that lineage tracing experiments are necessary to definitively determine the cellular origin and dynamics within the neuroinflammatory environment following T. gondii infection. However, the analysis of marker expression conducted in our study provides valuable insights into the profile of the neuroinflammatory response. These markers offer a glimpse into the potential changes occurring in microglia. Nevertheless, to ascertain whether the observed changes are due to microglial disappearance or a drastic shift in their phenotype, lineage tracing experiments would be essential. These experiments would provide a more comprehensive understanding of the cellular changes and clarify the precise contributions of microglia and other immune cells in the context of T. gondii infection.
It is noteworthy to highlight the importance of the host-parasite dynamics displayed by distinct strains of T. gondii isolated from a wide range of intermediate hosts, which directly affect the incidence of human infection in many regions of the world. The identification of T. gondii virulence profile and its ability to induce different levels of neuroinflammation is crucial for understanding the impact on neurocognitive function related to the pathology triggered. The results presented here place T. gondii chronic infection as a suitable model for studying the impact of CNS infections and its relationship with disrupted brain connectivity caused by persistent neuroinflammation, and behavioural disturbances.
Author contributions
RFT, LMDM, LLB, VFAN and RMMB conceptualized the study. RMMB, LMDM, MCMS, JLNS, FVS, CAL, ALB, HBF performed the experiments. RMMB, LMDM, MCMS analyzed the data and wrote the manuscript. RMMB, LMDM, RFT, LLB, VFAN, ACPO and ASM reviewed and edited the manuscript. All authors have reviewed and approved the final version of the manuscript.
Funding
This work was supported by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), the Brazilian National Research Council (CNPq) and Pró-Reitoria de Pesquisa da Universidade Federal de Minas Gerais. RTF, LLB, VFAN, ACPO and ASM are Research Fellows from CNPq (grants RTF #305514/2022-9, LLB #312151/2020-9, VFAN #306036/2019-3, ACPO #310347/2018-1). LMDM is supported by a post-doctoral fellowship from CAPES and RMMB was recipient of the CAPES PhD Scholarship.
Declaration of competing interest
The authors declare that they have no interests to declare.
Acknowledgements
The authors would like to thank Rosálida Estevan Nazar for the technical support.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bbih.2023.100652.
Contributor Information
Ricardo Toshio Fujiwara, Email: rtfujiwara@gmail.com.
Luísa Mourão Dias Magalhães, Email: luisamdmagalhaes@gmail.com.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Supplementary Fig. 1.
Behavioural assessment of mice during early chronic infection by distinct T. gondii strains. The data represented here is from behavioural analysis for 4 weeks post-infection. (A) data analysis from the open field test, showing the total distance travelled (in meters) by non-infected (blue) and ME49- (orange) and CK2-infected (dark red) mice (left); and time spent in the periphery of the apparatus (right). (B) Data analysis from the elevated plus maze test, showing the number of entries (left) and the time spent (right) in the open arms by non-infected (blue) and ME49- (orange) and CK2-infected (dark red) mice. (C) Data analysis from the forced swim test, showing the time (in seconds) of immobility. (D) Memory analysis performed by the object recognition task. The statistical analysis for the recognition index with 50% cut-off was performed by one sample t-test. * Indicates the statistical difference between non-infected and infected mice groups; # indicates the statistical difference between the infected mice groups. Statistical analysis to find difference among the studied groups was performed using ANOVA test of variance followed by Tukey post-test. Outlier values were removed after ROUT test for outlier identification. Non-infected group (n = 10); ME49-infected group (n = 13) and CK2-infected group (n = 10). */#p < 0.05; **/##p < 0.01; ***/###p < 0.001; ****/####p < 0.0001.
Supplementary Fig. 2.
Analysis strategy for the immunophenotyping of the immune cell populations from the brain of studied mice. The cells were initially selected according to the size (FSC-A) and granularity (SSC-A); followed by singlets selection (SSC-A x SSC-H). With the selection of live cells (LiveCD45+), it was possible to identify the microglia population LiveCD45lowCD11b + CX3CR1hiCD44-Ly6C–P2ry12+, in the figure identified as #1); T lymphocytes (LiveCD45hiCD11b-CD44+CD3+, in the figure identified as #2) and inflammatory monocytes (LiveCD45hiCD11b + CX3CR1+CD44hiLy6ChiP2ry12-, in the figure identified as #3).
Supplementary Fig. 3.
(A) Flow cytometry histograms with the frequencies of measured values of surface markers used to differentiate the populations of microglia in the non-infected group (green) and inflammatory monocytes found in the ME49- (orange) and CK2-infected (red) mice. (B) Heatmap evidencing the frequency of microglia, infiltrated inflammatory monocytes, T lymphocytes and cytokines IFN-γ and TNF-a among the non-infected (blue), ME49-infected (orange) and CK2-infected (red) mice. (C) Detected levels of IL-10, IL-6 and IL-2 in the brain of mice from the studied groups; data presented here are expressed by violin plot displaying median and samples distribution pattern.
Data availability
Data will be made available on request.
References
- Ademe M., Kebede T., Teferra S., Alemayehu M., Girma F., Abebe T. Is latente Toxoplasma gondii infection associated with the occurrence of schizophrenia? A case-control study. PLoS One. 2022;17 doi: 10.1371/journal.pone.0270377. 10.137/journal.pone.0270377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Hussainy N.H., Al-saedi A.M., Al-lehaibi J.H., Al-lehaibi Y.A., Al-Sehli Y.M., Afifi M.A. Serological evidences link toxoplasmosis with schizophrenia and major depression disorder. J. Microscopy. Ultrastruc. 2015;3:148–153. doi: 10.1016/j.jmau.2015.03/006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarado-Esquivel C., Estrada-Martinez S., Ramos-Nevarez A., Pérez-Álamos A.R., Beristain-García I., Alvarado-Félix A.O., et al. Association between Toxoplasma gondii exposure and suicidal behavior in patients attending primary health care clinics. Pathogens. 2021;10:677. doi: 10.3390/pathogens10060677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarado-Esquivel C., Mendoza-Larios L.A., Garcia-Dolores F., Sanchez-Anguiano L.F., Antuna-Salcido E.I., Hernández-Tinoco J., et al. Association between Toxoplasma gondii infection in brain and a history of depression in suicide decedents: a cross-sectional study. Pathogens. 2021;10:1313. doi: 10.3390/pathogens10101313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amici S.A., Dong J., Guerau-de-Arellano M. Molecular mechanisms modulating the phenotype of macrophages and microglia. Front. Immunol. 2017;8:1520. doi: 10.3389/fimmu.2017.01520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amouei A., Sarvi S., Sharif M., et al. A systematic review of Toxoplasma gondii genotypes and feline: geographical distribution trends. Transbound. Emerg. Dis. 2020;67:46–64. doi: 10.1111/tbed.13340. [DOI] [PubMed] [Google Scholar]
- Bak J., Shim S.H., Kwon Y.J., Lee H.Y., Kim J.S., Yoon H., Lee Y.J. The association between suicide attempts and Toxoplasma gondii infection. Clin. Psychopharmacol. Neurosci. 2018;16:95–102. doi: 10.9758/cpn.2018.16.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batista S.J., Still K.M., Johanson D., Thompson J.A., O'Brien C.A., Lukens J.R., Harris T.H. Gasdermin-D-dependent IL-1α release from microglia promotes protective immunity during chronic Toxoplasma gondii infection. Nat. Commun. 2020;11:3687. doi: 10.1038/s41467-020-17491-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bay-Richter C., Buttenschon H.N., Mors O., Eskelund A., Budac D., Kaerlev L., Wegener F. Latent toxoplasmosis and psychiatric symptoms – a role of tryptophan metabolism? J. Psychiatr. Res. 2019;110:45–50. doi: 10.1016/j.jpsychires.2018.12.016. [DOI] [PubMed] [Google Scholar]
- Behnke M.S., Khan A., Lauron E.J., et al. Rhoptry proteins ROP5 and ROP18 are major murine virulence factors in genetically divergent South American strains of Toxoplasma gondii. PLoS Genet. 2015;11(8) doi: 10.1371/journal.pgen.1005434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellozi P.M.Q., Gomes G.F., Silva M.C.M., Lima I.V.A., Batista C.R.A., Carneiro-Junior W.O., et al. A positive allosteric modulator of mGluR5 promotes neuroprotective effects in mouse models of Alzheimer's disease. Neuropharmacology. 2019;160 doi: 10.1016/j.neuropharm.2019.107785. [DOI] [PubMed] [Google Scholar]
- Bennet M.L., Bennett F.C., Liddelow S.A., Ajami B., Zamanian J.L., Femhoff N.N., et al. New tools for studying microglia in the mouse and human CNS. Proc. Natl. Acad. Sci. USA. 2016;16:E1739–E1746. doi: 10.1073/pnas.1525528113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergersen K.V., Barnes A., Worth D., David C., Wilson E.H. Targeted transcriptomic analysis of C57BL/6 and BALB/c mice during progressive chronic Toxoplasma gondii infection reveals changes in host and parasite gene expression relating to neuropathology and resolution. Front. Cell. Infect. Microbiol. 2021;11 doi: 10.3389/fcimb.2021.645778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry A., Bindocci E., Alleva E. NGF, brain and behavioral plasticity. Neural Plast. 2012 doi: 10.1155/2012/784040. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaizot R., Nabet C., Blanchet D., et al. Pediatric amazonian toxoplasmosis caused by atypical strains in French guiana, 2002-2017. J. Pediatric Infect. Dis. Soc. 2019;38(3):39–42. doi: 10.1097/INF.0000000000002130. [DOI] [PubMed] [Google Scholar]
- Bles N.J., van der Does J.E.H., Kortbeek L.M., Hofhuis A., van Grootheest G., Vollaard A.M., et al. Toxoplasma gondii seropositivity in patients with depressive and anxiety disorders. Brain, Behavior, & Immunity – Health. 2021;11 doi: 10.1016/j.bbih.2020.100197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brito R.M.M., Meurer Y.S.R., Santos L.S., Marcelino B.M.M., Andrade-Neto V.F. Chronic Toxoplasma gondii infection contributes to decreasing of perineuronal nets surrounding neurons in the corpus striatum of mice. Parasitol. Res. 2020;119:1989–1995. doi: 10.1007/s00436-020-06674-8. [DOI] [PubMed] [Google Scholar]
- Brown C.R., Hunter C.A., Estes R.G., Beckmann E., Forman J., David C., et al. Definitive identification of a gene that confers resistance against Toxoplasma cyst burden and encephalitis. Immunology. 1995;85(3):419–428. [PMC free article] [PubMed] [Google Scholar]
- Budni J., Bellettini-Santos T., Mina F., Garcez M.L., Zugno A.I. The involvement of BDNF, NGF and GDNF in aging and Alzheimer's disease. Aging and Disease. 2015;6:331–341. doi: 10.14336/AD.2015.0825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butovsky O., Jedrychowski M.P., Moore C.S., Cialic R., Lanser A.J., Gabriely G., et al. Identification of a unique TGF-β-dependent molecular and functional signature in microglia. Nat. Neurosci. 2014;17:131–147. doi: 10.1038/nn.3599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabral C.M., Tuladhar S., Dietrich H., Nguyen E., MacDonald W.R., Trivedi T., et al. Neurons are the primary target cell for the brain-tropic intracellular parasite Toxoplasma gondii. PLoS Pathog. 2016;12 doi: 10.1371/journal.ppat.1005447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camargos Q.M., Silva B.C., Silva D.G., Toscano E.C.B., Oliveira B.S., Bellozi P.M.Q., et al. Minocycline treatment prevents depression and anxiety-like behaviors and promotes neuroprotection after experimental ischemic stroke. Brain Res. Bull. 2020;155:1–10. doi: 10.1016/j.brainbull.2019.11.009. [DOI] [PubMed] [Google Scholar]
- Cardona A.E., Pioro E.P., Sasse M.E., Kostenko V., Cardona S.M., Dijkstra I.M., et al. Control of microglial neurotoxicity by the fractalkine receptor. Nat. Neurosci. 2006;9:917–924. doi: 10.1038/nn1715. [DOI] [PubMed] [Google Scholar]
- Carrillo G.L., Ballard V.A., Glausen T., Boone Z., Teamer J., Hinkson C.L., et al. Toxoplasma infection induces microglia-neuron contact and the loss of perisomatic inhibitory synapses. Glia. 2020;68:1968–1986. doi: 10.1002/glia.23816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castaño B.L., Silva A.A., Hernandez-Velasco L.L., Pinheiro A.P.S., Gibaldi D., Mineo J.R., et al. Sulfadiazine plus pyrimethamine therapy reversed multiple behavioral and neurocognitive changes in long-term chronic toxoplasmosis by reducing brain cyst load and inflammation-related alterations. Front. Immunol. 2022;13 doi: 10.3389/fimmu.2022.822567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao C.C., Anderson W.R., Hu S., Gekker G., Martella A., Peterson P.K. Activated microglia inhibit multiplication of Toxoplasma gondii via a nitric oxide mechanism. Clin. Immunol. Immunopathol. 1993;67:178–183. doi: 10.1006/clin.1993.1062. [DOI] [PubMed] [Google Scholar]
- Chao C.C., Gekker G., Peterson P.K. Human microglial cell defense against Toxoplasma gondii. The role of cytokines. J. Immunol. 1994;152:1246–1252. [PubMed] [Google Scholar]
- Chegeni T.N., Sharif M., Sarvi S., Moosazadeh M., Montazeri M., Aghayan S.A., et al. Is there any association between Toxoplasma gondii infection and depression? A systematic review and meta-analysis. PLoS One. 2019;14 doi: 10.1371/journal.pone.0218524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H., Lau M.C., Wong M.T., Newell E.W., Poidinger M., Chen J. Cytofkit: a bioconductor 386 package for an integrated mass cytometry data analysis pipeline. PLoS Comput. Biol. 2016;12 doi: 10.1371/journal.pcbi.1005112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J., Li Z.Y., Zhou D.H., Liu G.H., Zhu X.Q. Genetic diversity among Toxoplasma gondii strains from different hosts and geographical regions revealed by sequence analysis of GRA5 gene. Parasites Vectors. 2012;5(1):279. doi: 10.1186/1756-3305-5-279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clementino-Andrade M.M., Pinheiro B.V., Cunha M.M., Carneiro A.C.A.V., Andrade-Neto V.F., Vitor R.W.A. New genotypes of Toxoplasma gondii obtained from farm animals in Northeast Brazil. Res. Vet. Sci. 2013;94:587–589. doi: 10.1016/j.rvsc.2013.01.006. [DOI] [PubMed] [Google Scholar]
- Contopoulos‐Ioannidis D.G., Giannili M., Truong A.A.N., Montoya J.G. Toxoplasmosis and schizophrenia: a systematic review and meta-analysis of prevalence and associations and future directions. Psychiatr. Res. Clin.l Pract. 2022;4:48–60. doi: 10.1176/appi.prcp.20210041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demar M., Ajzenberg D., Maubon D., et al. Fatal outbreak of human toxoplasmosis along the Maroni River: epidemiological, clinical, and parasitological aspects. Clin. Infect. Dis. 2007;45(7):e88–e95. doi: 10.1086/521246. [DOI] [PubMed] [Google Scholar]
- Dubey J., Lago E.G., Gennari S.M., Su C., Jones J.L. Toxoplasmosis in humans and animals in Brazil: high prevalence, high burden of disease, and epidemiology. Parasitology. 2012;139:1375–1424. doi: 10.1017/S0031182012000765. [DOI] [PubMed] [Google Scholar]
- Dubey J.P., Murata F.H.A., Cerqueira-Cézar C.K., Kwok O.C.H., Villena I. Congenital toxoplasmosis in humans: an update of worldwide rate of congenital infections. Parasitology. 2021;148:1406–1416. doi: 10.1017/S0031182021001013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enlow W., Bordeleau M., Piret J., Ibañez F.G., Uyar O., Venable M.C., et al. Microglia are involved in phagocytosis and extracellular digestion during Zika virus encephalitis in young adult immunodeficient mice. J. Neuroinflammation. 2021;18:178. doi: 10.1186/s12974-021-02221-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esshili A., Thabet S., Jemli A., Trifa F., Mechri A., Zaafrane F., et al. Toxoplasma gondii infection in schizophrenia and associated clinical features. Psychiatric Res. 2016;245:327–332. doi: 10.1016/j.psychres.2016.08.056. [DOI] [PubMed] [Google Scholar]
- Fernandes J.G.A., Victoria E.C.G., Toscano E.C.B., Ferreira R.N., Silva D.G., Oliveira B.S., et al. High levels of NGF during anxiety-like behavior in a murine model of brain ischemic stroke. Neurol. Psychiatr. Brain Res. 2020;38:114–120. doi: 10.1016/j.npbr.2020.10.002. [DOI] [Google Scholar]
- Filgueira L., Larionov A., Lannes N. The influence of virus infection on microglia and accelerated brain aging. Cells. 2021;10:1836. doi: 10.3390/cells10071836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filho C.B., Jesse C.R., Donato F., Giacomeli R., Fabbro L.D., Antunes M.S., et al. Chronic unpredictable mild stress decreases BDNF and NGF levels and Na+, K+-ATPase activity in the hippocampus and prefrontal cortex of mice: antidepressant effect of chrysin. Neuroscience. 2015;289:367–380. doi: 10.1016/j.neuroscience.2014.12.048. [DOI] [PubMed] [Google Scholar]
- Flegr J., Horácek J. Negative effects of latente toxoplasmosis on mental health. Front. Psychiatr. 2020;10:1012. doi: 10.3389/fpsyt.2019.01012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flegr J., Prandota J., Sovickova M., Israili Z.H. Toxoplasmosis – a global threat. Correlation of latent toxoplasmosis with specific disease burden in a set of 88 countries. PLoS One. 2014;24 doi: 10.1371/journal.pone.0090203. journal.pone.0090203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gale S.D., Brown B.L., Berrett A., Erickson L.D., Hedges D.W. Association between latent toxoplasmosis and major depression, generalised anxiety disorder and panic disorder in human adults. Folia Parasitol. 2014;61:285–292. doi: 10.14411/fp.2014.038. [DOI] [PubMed] [Google Scholar]
- Gatkowska J., Wieczorek M., Dziadek B., Dzitko K., Dlugonska H. Sex-dependent neurotransmitter level changes in brain of Toxoplasma gondii infected mice. Exp. Parasitol. 2013;133(1):1–7. doi: 10.1016/j.exppara.2012.10.005. [DOI] [PubMed] [Google Scholar]
- Graham A.K., Fong C., Naqvi A., Lu J.Q. Toxoplasmosis of the central nervous system: manifestations vary with imune responses. J. Neurol. Sci. 2021;420 doi: 10.1016/j.jns.2020.117223. j.jns.2020.117223. [DOI] [PubMed] [Google Scholar]
- Guevara I., Iwanejko J., Dembinska-Kiec A., Pankiewicz J., Wanat A., Anna P., Golabek I., Bartus S., Malczewska-Male c M., Szczudlik A. Determination of nitrite/nitrate in human biological material by the simple Griess Method. Clin. Chim. Acta. 1998;274:177–188. doi: 10.1016/s0009-8981(98)00060-6. [DOI] [PubMed] [Google Scholar]
- Han Y.Y., Jin K., Pan Q.S., Li B., Wu Z.Q., Gan L., et al. Microglial activation in the dorsal striatum participates in anxiety-like behavior in Cyld knockout mice. Brain Behav. Immun. 2020;89:326–338. doi: 10.1016/j.bbi.2020.07.011. [DOI] [PubMed] [Google Scholar]
- Harrison J.K., Jiang Y., Chen S., Xia Y., Maciejewski D., McNamara R.K., et al. Role for neuronally derived fractalkine in mediating interactions between neurons and CX3CR1-expressing microglia. Proc. Natl. Acad. Sci. USA. 1998;95:10896–10901. doi: 10.1073/pnas.95.18.10896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashikawa N., Ogawa T., Sakamoto Y., Ogawa M., Matsuo Y., Zamami Y., Hashikawa-Hobara N. Time course of behavioral alteration and mRNA levels of neurotrophic factor following stress exposure in mouse. Cell. Mol. Neurobiol. 2015;35:807–817. doi: 10.1007/s10571-015-0174-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegazy M.M., Elmehankar M.S., Azab M.S., El-Tantawy N.L., Abdel-Aziz A. Sex dichotomy in the course of experimental latente toxoplasmosis. Exp. Parasitol. 2019;202:15–21. doi: 10.1016/j.exppara.2019.05.003. [DOI] [PubMed] [Google Scholar]
- Horita J.K.H.A., Silva M.C.M., Ferrari C.Z., Vieira E.L.M., Moreira F.A., Oliveira A.C.P., Reis H.J. Evaluation of brain cytokines and the level of brain-derived neurotrophic factor in an inflammatory model of depression. Neuroimmunomodulation. 2020;27:87–96. doi: 10.1159/000511181. [DOI] [PubMed] [Google Scholar]
- Jurga A.M., Paleczna M., Kuter K. Overview of general and discriminating markers of differential microglia phenotypes. Front. Cell. Neurosci. 2020;14:198. doi: 10.3389/fncel.2020.00198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan A., Taylor S., Ajioka J.W., Rosenthal B.M., Sibley L.D. Selection at a single locus leads to widespread expansion of Toxoplasma gondii lineages that are virulent in mice. PLoS Genet. 2009;5 doi: 10.1371/journal.pgen.1000404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y.K., Na K.S., Myint A.M., Leonard B.E. The role of pro-inflammatory cytokines in neuroinflammation, neurogenesis and the neuroendocrine system in major depression. Prog. Neuro Psychopharmacol. Biol. Psychiatr. 2016;64:277–284. doi: 10.1016/j.pnpbp.2015.06.008. [DOI] [PubMed] [Google Scholar]
- Korin B., Dubovik T., Rolls A. Mass cytometry analysis of immune cells in the brain. Nat. Protoc. 2018;13:377–391. doi: 10.1038/nprot.2017.155. [DOI] [PubMed] [Google Scholar]
- La-Vu M., Tobias B.C., Schuette P.J., Adhikari A. To approach or avoid: an introductory overview of the study of anxiety using rodent assays. Front. Behav. Neurosci. 2020;14:145. doi: 10.3389/fnbeh.2020.00145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B., Yang W., Ge T., Wang Y., Cui R. Stress induced microglial activation contributes to depression. Pharmacol. Res. 2022;179 doi: 10.1016/j.phrs.2022.106145. [DOI] [PubMed] [Google Scholar]
- Li Y., Liu Y., Xiu F., Wang J., Cong H., He S., et al. Characterization of exosomes derived from Toxoplasma gondii and their functions in modulating immune responses. Int. J. Nanomed. 2018;13:467–477. doi: 10.2147/IJN.S151110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Severance E.G., Viscidi R.P., Yolken R.H., Xiao J. Persistent Toxoplasma infection of the brain induced neurodegeneration associated with activation of complement and microglia. Infect. Immun. 2019;87 doi: 10.1128/IAI.00139-19. 00139-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liesenfeld O. Oral infection of C57BL/6 mice with Toxoplasma gondii: a new model of inflammatory bowel disease? JID (J. Infect. Dis.) 2002;185:96–101. doi: 10.1086/338006. [DOI] [PubMed] [Google Scholar]
- Limatola C., Ransohoff R.M. Modulating neurotoxicity through CX3CL1/CX3CR1 signalling. Front. Cell. Neurosci. 2014;8:229. doi: 10.3389/fncel.2014.00229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Littrell O.M., Granholm A.C., Gerhardt G.A., Boger H.A. Glial cell-line derived neurotrophic factor (GDNF) replacement attenuates motor impairments and nigrostriatal dopamine deficits in 12-month-old mice with a partial deletion of GDNF. Pharmacol. Biochem. Behav. 2013;104:10–19. doi: 10.1016/j.pbb.2012.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNamara N.B., Munro D.A.D., Bestard-Cuche N., Uyeda A., Bogie J.F.J., Hoffman A., et al. Microglia regulate central nervous system myelin growth and integrity. Nature. 2022;17:1–10. doi: 10.1038/s41586-022-05534-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meurer Y.S.R., Brito R.M.M., Silva V.P., Andrade J.M.A., Linhares S.S.G., Pereira-Junior A., et al. Toxoplasma gondii infection damages the perineuronal nets in a murine model. Memórias do Inst. Oswaldo Cruz. 2020;115 doi: 10.1590/0074-02760200007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitra R., Sapolsky R.M., Vyas A. Toxoplasma gondii infection induces dendritic retraction in basolateral amygdala accompanied by reduced corticosterone secretion. Disease Models and Mechanisms. 2013;6:516–520. doi: 10.1242/dmm.009928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasirpour S., Kheirandish F., Fallahi S. Depression and Toxoplasma gondii infection: assess the possible relationship through a seromolecular case-control study. Arch. Microbiol. 2020;202:2689–2695. doi: 10.1007/s00203-020-01993-x. [DOI] [PubMed] [Google Scholar]
- Pardini L., Bernstein M., Carral L.A., et al. Congenital human toxoplasmosis caused by non-clonal Toxoplasma gondii genotypes in Argentina. Parasitol. Int. 2019;68(1):48–52. doi: 10.1016/j.parint.2018.10.002. [DOI] [PubMed] [Google Scholar]
- Pastolache T.T., Wadhawan A., Rujescu D., Hoisington A.J., Dagdag A., Baca-Garcia E., et al. Toxoplasma gondii, suicidal behavior, and intermediate phenotypes for suicidal behavior. Front. Psychiatr. 2021;12 doi: 10.3389/fpsyt.2021.665682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-Grisales L.J., Cruz-Moncada M., Peláez-Sánchez R., Díaz-Nieto J.F. Toxoplasma gondii infection in Colombia with a review of hosts and their ecogeographic distribution. Zoonoses Public Health. 2021;68(1):38–53. doi: 10.1111/zph.12787. [DOI] [PubMed] [Google Scholar]
- Petit-Demouliere B., Chenu F., Bourin M. Forced swimming test in mice: a review of antidepressant activity. Psychopharmacology. 2005;177:245–255. doi: 10.1007/s00213-004-2048-7. [DOI] [PubMed] [Google Scholar]
- Qiu T., Guo J., Wang L., Shi L., Ai M., Xia Z.X., Peng Z., Kuang L. Dynamic microglial activation is associated with LPS-induced depressive-like behavior in mice: an [18F] DPA-714 PET imaging study. Bosn. J. Basic Med. Sci. 2022;22:649–659. doi: 10.17305/bjbms.2021.6825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robben P.M., Mordue D.G., Truscott S.M., Takeda K., Akira S., Sibley L.D. Production of IL-12 by macrophages infected with Toxoplasma gondii depends on the parasite genotype. J. Immunol. 2004;172:3686–3694. doi: 10.4049/jimmunol.172.6.3686. [DOI] [PubMed] [Google Scholar]
- Rostami A., Riahi S.M., Gamble H.R., Fakhri Y., Shiadeh M.N., Danesh M., et al. Global prevalence of latent toxoplasmosis in pregnant women: a systematic review and meta-analysis. Clin. Microbiol. Infection. 2020;26:673–683. doi: 10.1016/j.cmi.2020.01.008. [DOI] [PubMed] [Google Scholar]
- Rozenfeld C., Martinez R., Seabra S., Sant'Anna C., Gonçalves J.G.R., Bozza M., et al. Toxoplasma gondii prevents neuron degeneration by interferon-y-activated microglia in a mechanism involving inhibition of inducible nitric oxide synthase and transforming growth factor-β1 production by infected microglia. Am. J. Pathol. 2005;167:1021–1031. doi: 10.1016/s0002-9440(10)61191-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvador-Guillouët F., Ajzenberg D., Chaillou-Opitz S., et al. Severe pneumonia during primary infection with an atypical strain of Toxoplasma gondii in an immunocompetent young man. J. Infect. 2006;53(2):e47–e50. doi: 10.1016/j.jinf.2005.10.026. [DOI] [PubMed] [Google Scholar]
- Seitz S., Clarke P., Tyler K.L. Pharmacologic depletion of microglia increases viral load in the brain and enhances mortality in murine models of Flavivirus-induced encephalitis. J. Virol. 2018;92 doi: 10.1128/JVI.00525-18. 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiadeh M.N., Rostami A., Pearce B.D., Gholipourmalekabadi M., Newport D.J., Danesh M., et al. The correlation between Toxoplasma gondii infection and prenatal depression in pregnant women. Eur. J. Clin. Microbiol. Infect. Dis. 2016;35:1829–1865. doi: 10.1007/s10096-016-2734-5. [DOI] [PubMed] [Google Scholar]
- Soares G.L., Leão E.R.L.P., Freitas S.F., Alves R.M.C., Tavares N.P., Costa M.V.N., et al. Behavioral and neuropathological changes after Toxoplasma gondii ocular conjunctival infection in BALB/c mice. Front. Cell. Infect. Microbiol. 2022;12 doi: 10.3389/fcimb.2022.812152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song C., Yang Z.X., Manku M. Neurobiology of disease increased phospholipase a2 activity and inflammatory response but decreased nerve growth factor expression in the olfactory bulbectomized rat model of depression: effects of Chronic Ethyl-Eicosapentaenoate Treatment. J. Neurosci. 2009;29:14–22. doi: 10.1523/JNEUROSCI.3569-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugden K., Moffitt T.E., Pinto L., Poulton R., Williams B.S., Caspi A. Is Toxoplasma gondii infection related to brain and behavior impairments in humans? Evidence from a population-representatitve birth cohort. PLoS One. 2016;11 doi: 10.1371/journal.pone.0148435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J., Zhang X., Broderick M., Fein H. Measurement of nitric oxide production in biological systems by using Griess reaction assay. Sensors. 2003;3:276–284. doi: 10.3390/s60800276. [DOI] [Google Scholar]
- Suzuki Y., Joh K., Orellana M.A., Conley F.K., Remington J.S. A gene(s) within the H-2D region determines the development of toxoplasmic encephalitis in mice. Immunology. 1991;74(4):732–739. [PMC free article] [PubMed] [Google Scholar]
- Torres L., Robinson S.A., Kim D.G., Yan A., Cleland T.A., Bynoe M.S. Toxoplasma gondii alters NMDAR signalling and induces signs of Alzheimer's disease in wild-type, C57BL/6 mice. J. Neuroinflammation. 2018;15:1–19. doi: 10.1186/s12974-018-1086-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai T.T., Chen C.L., Lin Y.S., Chang C.P., Tsai C.C., Cheng Y.L., et al. Microglia retard dengue virus-induced acute viral encephalitis. Sci. Rep. 2016;6 doi: 10.1038/srep27670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valvassori S.S., Mariot E., Varela R.B., Bavaresco D.V., Dal-Pont G.C., Ferreira C.L., et al. The role of neurotrophic factors in manic-, anxious- and depressive-like behaviors induced by amphetamine sensitization: implications to the animal model of bipolar disorder. J. Affect. Disord. 2019;245:1106–1113. doi: 10.1016/j.jad.2018.10.370. [DOI] [PubMed] [Google Scholar]
- Winter A.M., Subbarayan M.S., Grimming B., Weesner J.A., Moss L., Peters M., et al. Two forms of CX3CL1 display differential activity and rescue cognitive deficits in CX3CL1 knockout mice. J. Neuroinflammation. 2020;17:157. doi: 10.1186/s12974-020-01828-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia J., Lu Z., Feng S., Yang J., Ji M. Different effects of immune stimulation on chronic unpredictable mild stress-induced anxiety- and depression-like behaviors depending on timing of stimulation. Int. Immunopharm. 2018;58:48–56. doi: 10.1016/j.intimp.2018.03.010. [DOI] [PubMed] [Google Scholar]
- Xiao J., Kannan G., Jones-Brando L., et al. Sex-specific changes in gene expression and behavior induced by chronic Toxoplasma infection in mice. Neuroscience. 2012;206:39–48. doi: 10.1016/j.neuroscience.2011.12.051. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Lai B.S., Juhas M., Zhang Y. Toxoplasma gondii secretory proteins and their role in invasion and pathogenesis. Microbiol. Res. 2019;227 doi: 10.1016/j.micres.2019.06.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.