Abstract
Phosphorus (P) is often the limiting factor for plant growth because of its low mobility and availability in soils. Phosphate-solubilizing bacteria (PSB) have been shown to increase the availability of soil P fractions, thereby promoting plant growth. We herein investigated the effects of PSB on P availability in two important Chinese soil types: Lateritic red earths (La) and Cinnamon soils (Ci). We initially isolated 5 PSB strains and assessed their effects on soil P fractions. PSB mainly increased moderately labile P in La and labile P in Ci. We then selected the most promising PSB isolate (99% similarity with Enterobacter chuandaensis) and examined its effects on P accumulation in maize seedlings. The results obtained showed that plant P accumulation increased in response to a PSB inoculation in both soil types and the combination of the PSB inoculation and tricalcium phosphate fertilization in La significantly enhanced P accumulation in plant shoots. The present study demonstrated that the PSB isolates tested differed in their ability to mobilize P from distinct P fertilizers and that PSB isolates have potential as a valuable means of sustainably enhancing seedling growth in Chinese agricultural soils.
Keywords: Cinnamon soils, Lateritic red earths, P fractions, P accumulation, Phosphate-solubilizing bacteria (PSB)
Phosphorus (P), a macroelement for plants, is a limiting factor restricting their growth (Aerts and Chapin, 1999; Raghothama, 2005). Although P may be present at high concentrations in soils (Larsen, 1967), very little is directly absorbed by plants. P is mainly present in unavailable forms, namely, insoluble and organic forms. Plant and microbial functions mobilize insoluble P in soils. Since some bacteria were shown to dissolve unavailable natural raw rock phosphate in the early 20th century (Sackett et al., 1908), research on phosphate-solubilizing bacteria (PSB) has been widely pursued (Khan et al., 2009; Ingle and Padole, 2017). The findings obtained have demonstrated that diverse PSB isolates contribute to the mobilization of unavailable P in soils and that they support plant growth. Previous studies examined specific PSB strains and the mechanisms underlying phosphate solubilization (Ding et al., 2021; Amy et al., 2022). Other studies that assessed crop productivities investigated the application of PSB strains to the transformation of unavailable P in soil (Alam et al., 2021; Liu et al., 2021), to the supply of P to crops (Alam et al., 2022; Khan et al., 2022), and even to improvements in soil quality (Pathak et al., 2021; Dasila et al., 2022).
PSB have been shown to accelerate soil P cycling (Hafeez et al., 2019; Liu et al., 2022) and may counteract the antagonistic effects of soil calcification on bioavailable P (Adnan et al., 2017). The PSB population may be closely related to the P fraction (He and Wan, 2022). Moreover, PSB were found to increase available P by reducing soil P retention (Halder et al., 1990; Chen et al., 2006; Ku et al., 2018; Tian et al., 2021), which has been defined as the removal of phosphate from solution by soil (Wild, 1950). The strength of P retention is mainly affected by soil mineralogy, clay content, soil pH, and climate factors, such as temperature and moisture (Batjes, 2011).
P fractions and the retention potential of soil types markedly vary worldwide. Lateritic red earths (La) and Cinnamon soils (Ci) are both important agricultural soils in China. La cover the largest surface area in China, of which approximately 350,000 km2 is cultivated land, accounting for 28% of all agronomically used land in China. La has pH 5.0–5.5 and a clay content of 30–40%. It is rich in iron and aluminum oxides (He et al., 2004), resulting in high P fixation to soil minerals. Moreover, La is mainly distributed in southern China in very high or high P retention potential (PRP) regions (Kochian, 2012). Cultivated lands of Ci cover a smaller area (ca. 20,000 km2) and are mainly planted with corn and wheat. Ci is weakly alkaline, has a clay content of 20–40%, is rich in Ca (Wang, 2003), and is mainly distributed in central China and northeast China, which are moderate or low PRP regions (Kochian, 2012).
PSB exert different effects on P transformation in different soils. Hafeez et al. (2019) found that PSB markedly increased labile P in an alkaline (pH 8.1) sandy loam soil after fertilization with tricalcium phosphate (TCP). Delfim et al. (2020) reported that PSB exerted strong effects on NaOH extractable P in Andisol and Ultisol, which are both acidic soils with similar P fractions. Although these studies are informative, few comparative studies have examined the effects of the same PSB strains on P fractions in La and Ci.
In view of the above research gaps, we isolated 5 PSB strains, examined their P solubilization potential when exposed to various P sources, and assessed their effects on P fractions in unplanted La and Ci. We selected the most promising PSB isolate and investigated its effects on P accumulation in maize seedlings in both soil types.
Materials and Methods
Isolation and identification of PSB
Non-cultivated La and the rhizosphere soil of Chinese cabbage (Brassica rapa L. var. pekinensis rupr) grown in La were used to isolate PSB. One gram of each soil was suspended in 99 mL normal saline (0.85% NaCl sterilized) solution and then gradient diluted. One hundred microliters each of the 10–3, 10–4, and 10–5 dilutions were spread on NBRIP agar (Nautiyal, 1999): glucose, 10 g L–1; MgCl2·6H2O, 5 g L–1; MgSO4·H2O, 0.25 g L–1; KCl, 0.2 g L–1; (NH4)2SO4, 0.1 g L–1; Ca3(PO4)2, 5 g L–1; and Agar, 15 g L–1, followed by an incubation at 28°C for 5 days. Colonies with transparent circles were isolated. We enriched the isolated strains with LB at 28°C for 24 h and then extracted DNA using the TIANamp Bacteria DNA Kit (Tiangen Biotech). Extracted DNA was used as the template for PCR amplification with the following universal bacterial primers targeting the 16S rRNA gene: 27F (Lane, 1991) and 1492R (Turner et al., 1999).
PCR was performed with 1 μL template DNA, 1 μL (10 μM) of each primer, 12.5 μL 2× Taq PCR Mix (KT210; Tiangen Biotech), 9.5 μL double distilled water, and the following steps: initial denaturation (94°C, 3 min), denaturation (94°C, 30 s), annealing (55°C, 30 s), and extension (72°C, 1 min). Final extension (72°C, 5 min) was performed after 30 cycles between denaturation and extension.
PCR products were used for agarose gel electrophoresis. Gels were stained (GeneGreen Nucleic Acid Dye, RT210; Tiangen Biotech) and visualized using the Gel Imaging System (WD-9413B, Beijing Liuyi Biotechnology). PCR products were purified using a purification kit (TIANgel Midi, DP209; Tiangen Biotech). Purified PCR-amplified 16S rDNA fragments were sequenced by AuGCT. The 16S rDNA sequences obtained of the isolated strains were compared and uploaded to apply the NCBI number in the NCBI GenBank.
Identified PSB were enriched in LB at 28°C for 24 h. Bacterial cultures were centrifuged and then washed three times with normal saline to collect bacterial cells. Bacterial cells were suspended with P-free NBRIP and OD600 was adjusted to 0.1. PSB suspensions were used in subsequent experiments.
Test of phosphate release ability
The phosphate release ability of PSB isolates was examined using a shake flask culture. Ca3(PO4)2 (tricalcium phosphate, TCP), FePO4, AlPO4, phytin (inositol hexakisphosphate and Mg and Ca salts), and lecithin (Yuanye) with the same P contents were added to P-free NBRIP. pH was adjusted to 7.0±0.2 with 0.1 M NaOH and HCl. These NBRIP derivatives were then autoclaved at 115°C for 20 min. The PSB suspension was inoculated into sterilized NBRIP derivatives at a ratio of 1% (v/v) of culture medium. PSB was then cultured with agitation at 28°C for 5 days. A rotation speed of 150 rounds per min (rpm) was used with a rotary shaker. Each P treatment had one control without the PSB inoculation; each control and inoculation had three replicates. After the incubation, culture media were centrifuged (12,000×g, 10 min) and the soluble inorganic P contents of the supernatant were measured.
Soil viability test of PSB isolates
To examine whether the PSB isolates obtained were viable in natural soils, 2.5 mL of the PSB suspension and 12.5 mL of sterile distilled water were added to 50 g of sterilized La and Ci and then incubated at 28°C in the dark for 7 days. After the incubation, 0.1 g of soil was suspended in 99 mL of normal saline to obtain dilutions. Fifty microliters of diluted soil suspensions was spread on NBRIP agar medium and cultured at 28°C for 5 days. Strains that formed colonies on NBRIP with transparent circles were considered to be viable in soil.
Soil inoculation experiments
Two soils were used for inoculation experiments: La from Nanning, Guangxi, China (22°50′28.6″N 108°11′25.7″E) and Ci from Fenyang, Shanxi, China (37°17′10.0″N 111°43′11.8″E). Both soils were collected from non-cultivated lands. Soil pH (1:2.5 water), total P, and available P were 5.4, 0.6 g P kg–1 soil, and 0.1 mg P kg–1 soil, respectively, for La and 8.0, 0.7 g P kg–1 soil, and 6.0 mg P kg–1 soil, respectively, for Ci. Soils were air dried after the removal of non-soil components, such as stones and plant roots. Dry soil was crushed and sieved using a 1-mm sieve. In the experiment, soils were autoclaved twice at 121°C for 60 min.
Inoculation experiments included one control and three treatments for each soil: PSB treatment, TCP treatment, and combination treatment. There were 5 experimental groups for both the PSB and combined treatments: A, B, F, G, and H. Each control and treatment (or each experimental group) had three replicates.
Regarding the PSB treatment, 2.5 mL of PSB suspensions and 12.5 mL of sterile distilled water were added to 50 g sterilized soil. In the TCP treatment, 50 g soil was mixed with 1% (w/w) TCP, autoclaved, and then added to 2.5 mL of P-free NBRIP and 12.5 mL of sterile distilled water. The soil of the combination treatment was the same as that of the TCP treatment, except for the addition of 2.5 mL PSB suspensions instead of P-free NBRIP. As a control, 2.5 mL of P-free NBRIP and 12.5 mL of sterile distilled water were added to 50 g sterilized soil. After an incubation at 28°C for 7 days, the dilution of 0.1 g of soil was spread on NBRIP agar using the method described in above to confirm strain survival. The remaining soil was lyophilized to measure P fractions.
Co-culture with maize seedlings
La and Ci were used in the co-culture experiment. As described in above, each soil had one control (no inoculation or fertilizer) and three treatments. One isolate showing an exceptional phosphate-solubilizing capacity was used in this experiment for the PSB and combination treatments. Each control and treatment had three replicates.
Maize (Zea mays L. cv. Guidan 162; Guangxi Zhaohe Seed Industry) seeds were surface sterilized once with 75% (v/v) ethanol and once with 1% (w/v) mercuric chloride for 2 min, respectively, followed by extensive rinsing with sterile distilled water. Germination was conducted at 28°C for 24 h under sterile conditions. Germinated seeds were transferred to culture containers containing 50 g of soil. Five milliliters of P-free Hoagland nutrient solution (Hoagland and Arnon, 1950) and 12.5 mL of sterile distilled water were added to each container. After an incubation at 28°C for 24 h, maize kernels were carefully removed.
In the control and TCP treatments, 2.5 mL P-free NBRIP was added to the soil. In the PSB and combination treatments, 2.5 mL of strain A suspension was added to the soil. Five milliliters of P-free Hoagland nutrient solution was added every other day to support plant growth. All containers were periodically watered with sterile distilled water to maintain the initial weight. After 7 days, maize shoots were harvested, dried, and weighed. After grinding the shoot to a fine powder, 0.05 g of plant material was digested with 1 mL of concentrated H2SO4 and H2O2. The total concentration of P in the digestion solution was assessed using the molybdenum blue method.
The dilution of 0.1 g of soil was spread on NBRIP agar following the procedure described in above to confirm strain survival. The remaining soil was lyophilized after the removal of roots and then used for P fractionation.
Soil P fractionation and measurement of P
The present study adopted Hedley’s sequential P fractionation method (the Hedley method; Hedley et al., 1982), which is widely used to assess soil P fractionation (Yang and Post, 2011; Hou et al., 2018; Xu et al., 2018; Delfim et al., 2020; Liu et al., 2022).
Using the Hedley method, soil P fractions were examined using the following procedure. In the initial step of extraction, 0.5 g soil was placed into a 50-mL centrifuge tube. Extraction was performed using 1) approximately 5 cm2 anion-exchange resin (SelemionTM ion exchangeable resin; AGC Engineering) and 30 mL distilled water, 2) 30 mL 0.5 M NaHCO3, 3) 30 mL 0.1 M NaOH, and 4) 20 mL 1 M HCl in sequence. Each extraction was shaken at 120 rpm at 25°C for 16 h. The resin was set aside and the soil suspension was centrifuged at 6,000 rpm for 20 min to separate the supernatant and soil. Resin was placed into a new tube containing 20 mL 0.5 M HCl and shaken at 120 rpm at 25°C for 2 h to extract resin P. Other extracts were NaHCO3-Pi, NaOH-Pi, and HCl P. Five milliliters of the NaHCO3 extract was mixed with 10 mL 0.9 M H2SO4 and 0.5 g (NH4)2S2O8 was autoclaved at 120°C for 60 min to obtain NaHCO3-PT (total P) (resin P and NaHCO3-PT were identified as labile P, which is available to plants). Five milliliters of the NaOH extract, 10 mL 0.9 M H2SO4, and 0.6 g (NH4)2S2O8 were autoclaved at 120°C for 90 min to obtain NaOH-PT. NaOH-PT has been identified as moderately labile P, which is strongly held by chemisorption to the surfaces of Al and Fe oxides (Hedley et al., 1982; Costa et al., 2016). Po is the difference between PT and the corresponding Pi. The P concentrations of all extracts and the digested solution were quantified using the molybdenum blue method (Murphy and Riley, 1962).
Statistical analysis
All data were analyzed using SPSS (SPSS Statistics, Ver. 21.0.0.0; IBM). Values for phosphate release, the soil P fraction, and P accumulation in maize seedlings are shown as means±SE. Significant differences between means were analyzed with a one-way ANOVA (Tukey’s and Dunnett’s post hoc tests) at a significance level of 5%. Soil P fractions were subjected to a two-way ANOVA with the PSB inoculation and TCP supply. The effects of the soil type, PSB inoculation, and TCP supply on P accumulation in maize seedlings were analyzed by point-biserial correlations.
Results
PSB identification, assessment of phosphate release ability, and viability in soil
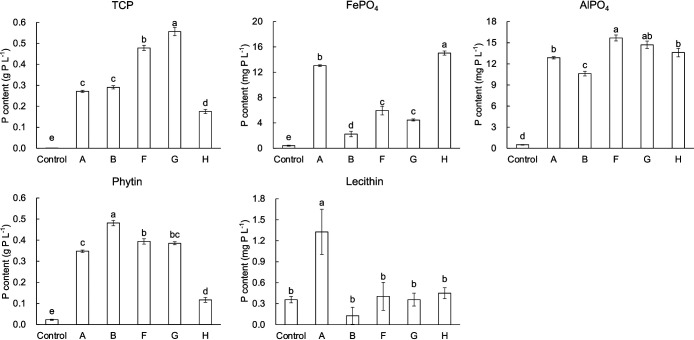
Twenty-eight strains from rhizosphere soil and 7 strains from non-cultivated La were identified as being from 6 genera (data not shown). After the tests on phosphate release ability and viability in soil, 5 strains were selected for subsequent experiments (Table 1): the B strain from the rhizosphere soil of Chinese cabbage released more P from phytin than other strains; the F and G strains released more P from TCP; the A and H strains from non-cultivated La released more P from FePO4 (Fig. 1). These 5 strains showed viability in La and Ci after the viability test (data not shown).
Table 1.
Overview of PSB strains used in experiments
| Strain name | Source | Closest relatives | Similarity (%) | Classification | NCBI number |
|---|---|---|---|---|---|
| A | Lateritic red earths | Enterobacter chuandaensis | 99.44 | Bacteria;Proteobacteria;Gammaproteobacteria;Enterobacterales;Enterobacteriaceae;Enterobacter | ON778739 |
| B | Rhizosphere soil of Chinese cabbage | Pantoea rodasii | 99.72 | Bacteria;Proteobacteria;Gammaproteobacteria;Enterobacterales;Erwiniaceae;Pantoea | ON778745 |
| F | Rhizosphere soil of Chinese cabbage | Klebsiella aerogenes | 92.96 | Bacteria;Proteobacteria;Gammaproteobacteria;Enterobacterales;Enterobacteriaceae;Klebsiella | ON778779.1 |
| G | Rhizosphere soil of Chinese cabbage | Pseudomonas hunanensis | 99.86 | Bacteria;Proteobacteria;Gammaproteobacteria;Pseudomonadales;Pseudomonadaceae;Pseudomonas | ON778780 |
| H | Lateritic red earths | Pseudomonas protegens | 98.38 | Bacteria;Proteobacteria;Gammaproteobacteria;Pseudomonadales;Pseudomonadaceae;Pseudomonas | ON778778 |
Fig. 1.
Soluble inorganic P content in culture solutions containing different P sources after a 5-days incubation with 5 PSB isolates (A, B, F, G, and H) or no bacterial isolate (Control). Different letters indicate significant differences (P<0.05; one-way ANOVA, Tukey, n=3). Error bars=SE.
Soil inoculation experiments
After soil inoculation experiments, the inoculated strains were confirmed to have survived and bacterial growth was not observed in the TCP or control treatment (data not shown). No significant differences were noted in total P in La or Ci between the control and PSB treatments or between the TCP and combination treatments (data not shown).
In the La inoculation experiment (Table 2), all PSB treatment groups had significantly higher NaOH-Pi and lower HCl P than the control group (La-Ctrl). TCP fertilization changed the size of inorganic P fractions (i.e. resin P, NaHCO3-Pi, NaOH-Pi, and HCl P), whereas organic P fractions (NaHCO3-Po, NaOH-Po) remained similar. All combination treatment groups, except for LaFP, had significantly higher NaHCO3-Pi, NaHCO3-Po, and NaOH-Po than the TCP treatment (LaP), while LaAP, LaBP, and LaFP had significantly higher NaOH-Pi. The interaction between the PSB inoculation and TCP supply was significant for all P fractions, except for HCl P.
Table 2.
Soil P fractions (mg P kg–1 soil) in the La inoculation experiment
| Group | Resin P | NaHCO3-Pi | NaHCO3-Po | NaOH-Pi | NaOH-Po | HCl P |
|---|---|---|---|---|---|---|
| La-Ctrl | 0.057±0.003 | 0.005±0.002 | 0.205±0.007 | 14.29±0.24 | 9.41±0.24 | 0.0729±0.0108 |
| LaA | 0.075±0.003 | 0.029±0.004* | 0.210±0.005 | 15.55±0.36* | 9.84±0.28 | 0.0124±0.0032* |
| LaB | 0.084±0.005 | 0.070±0.008* | 0.333±0.009* | 19.96±0.39* | 9.09±0.31 | 0.0007±0.0005* |
| LaF | 0.079±0.003 | 0.043±0.004* | 0.210±0.005 | 15.44±0.25* | 9.56±0.26 | 0.0121±0.0033* |
| LaG | 0.062±0.007 | 0.056±0.005* | 0.210±0.005 | 16.77±0.35* | 9.66±0.35 | 0.0017±0.0008* |
| LaH | 0.069±0.023 | 0.011±0.002 | 0.224±0.006 | 16.62±0.27* | 9.57±0.38 | 0.0210±0.0049* |
| LaP | 0.468±0.016* | 0.263±0.008* | 0.212±0.006 | 34.00±0.38* | 9.43±0.23 | 452±6* |
| LaAP | 1.051±0.022* | 0.605±0.009* | 0.288±0.006* | 43.00±0.32* | 10.78±0.19* | 432±4 |
| LaBP | 0.457±0.012 | 0.490±0.019* | 0.280±0.005* | 40.65±0.22* | 11.39±0.23* | 437±9 |
| LaFP | 0.506±0.016 | 0.266±0.007 | 0.216±0.009 | 35.36±0.29* | 9.56±0.27 | 448±8 |
| LaGP | 0.542±0.009* | 0.335±0.013* | 0.284±0.006* | 34.86±0.30 | 12.68±0.22* | 451±6 |
| LaHP | 0.445±0.009 | 0.456±0.015* | 0.350±0.008* | 34.03±0.33 | 12.71±0.32* | 443±9 |
| PSB | *** | *** | *** | *** | *** | — |
| TCP | *** | *** | *** | *** | *** | *** |
| PSB * TCP | *** | *** | *** | *** | *** | — |
Values represent the mean of three replicates±SE (standard errors).
Significant differences among means were tested with a one-way ANOVA (Dunnett. Use La-Ctrl as the control category for LaA~LaH and LaP; use LaP for LaAP~LaHP).
La-Ctrl: control, La with P-free NBRIP added; LaA~LaH: PSB treatment, La with A~H suspension inoculation; LaP: TCP treatment, La with TCP supply and P-free NBRIP added; LaAP~LaHP: Combination treatment, La with A~H suspension inoculation and TCP supply.
PSB, TCP, and PSB*TCP: Two-way ANOVA for the factors of the PSB inoculation, TCP supply, and the interaction of the PSB inoculation ×TCP supply.
*** P<0.001, ** P<0.01, * P<0.05, — P≥0.05.
Table S1 shows changes in labile P, moderately labile P, and HCl P in the La inoculation experiment. In the La-Ctrl group, moderately labile P (23.71 mg P kg–1 soil) was markedly higher than that of labile P (0.27 mg P kg–1 soil). When TCP was added, the increase in moderately labile P (19.72 mg P kg–1 soil) was greater than that in labile P (0.67 mg P kg–1 soil). Following the PSB inoculation, increases in moderately labile P were also greater than those in labile P in both the PSB and combination treatment groups.
In the Ci inoculation experiment (Table 3), all PSB treatment groups had significantly higher resin P and NaHCO3-Pi than the Ci-Ctrl group. TCP supply had significantly different resin P, NaHCO3-Pi, and HCl P, whereas organic P fractions were unaffected, similar to LaP. All combination treatment groups had significantly higher resin P than the CiP group. The interaction between the PSB inoculation and TCP supply was significant for resin P, NaHCO3-Pi, NaHCO3-Po, and NaOH-Po, whereas NaOH-Pi and HCl P were unaffected.
Table 3.
Soil P fractions (mg P kg–1 soil) in the Ci inoculation experiment
| Group | Resin P | NaHCO3-Pi | NaHCO3-Po | NaOH-Pi | NaOH-Po | HCl P |
|---|---|---|---|---|---|---|
| Ci-Ctrl | 1.76±0.01 | 4.13±0.01 | 0.95±0.01 | 2.38±0.01 | 2.76±0.01 | 307±2 |
| CiA | 3.05±0.01* | 4.49±0.04* | 1.44±0.02* | 2.39±0.01 | 3.41±0.01* | 300±3 |
| CiB | 3.98±0.01* | 4.36±0.05* | 1.54±0.01* | 2.38±0.01 | 2.73±0.02 | 299±2 |
| CiF | 2.39±0.01* | 5.84±0.11* | 1.78±0.05* | 2.28±0.02* | 2.83±0.02* | 300±4 |
| CiG | 1.92±0.01* | 4.97±0.07* | 1.34±0.11* | 2.44±0.02* | 2.74±0.01 | 304±3 |
| CiH | 2.43±0.01* | 4.89±0.05* | 1.12±0.02 | 2.52±0.01* | 2.87±0.01* | 302±3 |
| CiP | 2.02±0.02* | 5.62±0.02* | 0.94±0.03 | 2.38±0.02 | 2.76±0.03 | 1800±9* |
| CiAP | 4.10±0.01* | 6.17±0.02* | 1.43±0.02* | 2.44±0.05 | 3.69±0.01* | 1787±7 |
| CiBP | 4.10±0.01* | 5.82±0.06 | 0.72±0.02* | 2.49±0.04 | 2.80±0.02 | 1790±6 |
| CiFP | 2.82±0.07* | 6.17±0.06* | 1.78±0.06* | 2.36±0.03 | 3.01±0.02* | 1792±9 |
| CiGP | 2.84±0.02* | 5.03±0.03* | 1.36±0.09* | 2.56±0.03* | 3.38±0.04* | 1793±9 |
| CiHP | 2.84±0.01* | 6.05±0.14* | 1.12±0.07 | 2.53±0.04* | 2.99±0.01* | 1793±10 |
| PSB | *** | *** | *** | *** | *** | — |
| TCP | *** | *** | *** | *** | *** | *** |
| PSB * TCP | *** | *** | *** | — | *** | — |
Values represent the mean of three replicates±SE (standard errors).
Significant differences were assessed using a one-way ANOVA (Dunnett. Use Ci-Ctrl as the control category for CiA~CiH and CiP; use CiP for CiAP~CiHP).
Ci-Ctrl: control, Ci with P-free NBRIP added; CiA~CiH: PSB treatment, Ci with A~H suspension inoculation; CiP: TCP treatment, Ci with TCP supply and P-free NBRIP added; CiAP~CiHP: Combination treatment, Ci with A~H suspension inoculation and TCP supply.
PSB, TCP, and PSB×TCP: Two-way ANOVA for the factors of the PSB inoculation, TCP supply, and the interaction of PSB inoculation ×TCP supply.
*** P<0.001, ** P<0.01, * P<0.05, — P≥0.05.
As shown in Table S2, the pool sizes of labile P and moderately labile P in the Ci-Ctrl group were similar: 6.84 and 5.13 mg P kg–1 soil, respectively. TCP supply increased labile P by 1.74 mg kg–1 soil, whereas no increase was noted in moderately labile P. Following the PSB inoculation, increases in labile P were greater than those in moderately labile P in both the PSB and combination treatment groups, except for CiGP.
Pearson’s correlation analysis of the two soils with TCP supply (TCP and combined treatments) and without TCP supply (control and PSB treatments) showed no correlation between labile P and HCl P (data not shown).
Co-culture of PSB with maize seedlings
Strain A was used in the co-culture experiment because it caused higher labile P concentrations than the other strains in both La and Ci under TCP fertilized conditions. After the culture, the strain was confirmed to have survived, and no significant differences were observed in total P in La or Ci between the control and PSB treatments or between the TCP and combination treatments (data not shown).
In the co-culture (Table 4), La treated with PSB isolate A (LaA) showed differences in all fractions, except for NaHCO3-Po, from uninoculated control soil (La-Ctrl), while LaAP (relative to LaP) significantly changed all fractions, except for HCl P; CiA (relative to Ci-Ctrl) significantly changed all fractions, except for NaOH-Po, while CiAP (relative to CiP) significantly changed all fractions, except for NaOH-Po and HCl P. TCP supply significantly increased all fractions, except for NaOH-Po, in LaP, whereas it increased all fractions, except for NaOH-Pi and NaOH-Po, in CiP. The interaction between the PSB inoculation and TCP supply was significant for resin P, NaHCO3-Pi, and NaOH-Pi in La and for labile P (resin P, NaHCO3-Pi, and NaHCO3-Po) in Ci.
Table 4.
Soil P fractions (mg P kg–1 soil) in co-cultured La and Ci
| Group | Resin P | NaHCO3-Pi | NaHCO3-Po | NaOH-Pi | NaOH-Po | HCl P |
|---|---|---|---|---|---|---|
| La-Ctrl | 0.027±0.003d | 0.023±0.002d | 0.212±0.004c | 14.79±0.26d | 9.43±0.23c | 0.065±0.006b |
| LaA | 0.061±0.003c | 0.068±0.004c | 0.226±0.004c | 18.00±0.23c | 10.99±0.20b | 0.007±0.002c |
| LaP | 0.564±0.006b | 0.361±0.006b | 0.256±0.006b | 40.99±0.34b | 10.11±0.23c | 436±3a |
| LaAP | 1.174±0.015a | 0.649±0.021a | 0.292±0.011a | 47.00±0.24a | 12.10±0.30a | 427±2a |
| PSB | *** | *** | *** | *** | *** | * |
| TCP | *** | *** | *** | *** | *** | *** |
| TCP * PSB | *** | *** | — | *** | — | * |
| Ci-Ctrl | 1.86±0.02d | 3.82±0.08d | 1.72±0.05c | 2.05±0.01b | 4.36±0.02b | 306±4b |
| CiA | 2.47±0.03c | 5.11±0.03c | 2.19±0.05b | 2.39±0.01a | 4.39±0.02ab | 291±2c |
| CiP | 3.89±0.09b | 6.09±0.03b | 2.10±0.05b | 2.06±0.04b | 4.30±0.09ab | 1704±8a |
| CiAP | 4.20±0.07a | 6.92±0.08a | 3.32±0.05a | 2.40±0.02a | 4.49±0.04a | 1701±9a |
| PSB | *** | *** | *** | *** | * | — |
| TCP | *** | *** | *** | — | — | *** |
| TCP * PSB | * | *** | *** | — | — | — |
Values represent the mean of three replicates±SE (standard errors).
Significant differences in a column under each group are indicated by different letters (P≤0.05). Significance was analyzed with a one-way ANOVA (Games-Howell).
La-Ctrl: control, La with P-free NBRIP added; LaA: PSB treatment, La with A suspension inoculation; LaP: TCP treatment, La with TCP supply and P-free NBRIP added; LaAP: Combination treatment, La with A suspension inoculation and TCP supply.
Ci-Ctrl: control, Ci with P-free NBRIP added; CiA: PSB treatment, Ci with A suspension inoculation; CiP: TCP treatment, Ci with TCP supply and P-free NBRIP added; CiAP: Combination treatment, Ci with A~H suspension inoculation and TCP supply.
PSB, TCP, and PSB*TCP: Two-way ANOVA for the factors of the PSB inoculation, TCP supply, and the interaction of PSB inoculation ×TCP supply.
*** P<0.001, ** P<0.01, * P<0.05, — P≥0.05.
As shown in Table S3, all treatments increased moderately labile P more than labile P in La, whereas all treatments increased labile P more than moderately labile P in Ci.
In terms of P accumulation in maize seedlings (Fig. 2), LaA and CiA were higher than La-Ctrl and Ci-Ctrl, respectively. LaP and CiP were also higher than La-Ctrl and Ci-Ctrl, respectively, LaAP was higher than La-Ctrl, LaA, and LaP, and CiAP was higher than Ci-Ctrl and CiP. No significant differences were observed between LaA and LaP or between CiA and CiP.
Fig. 2.
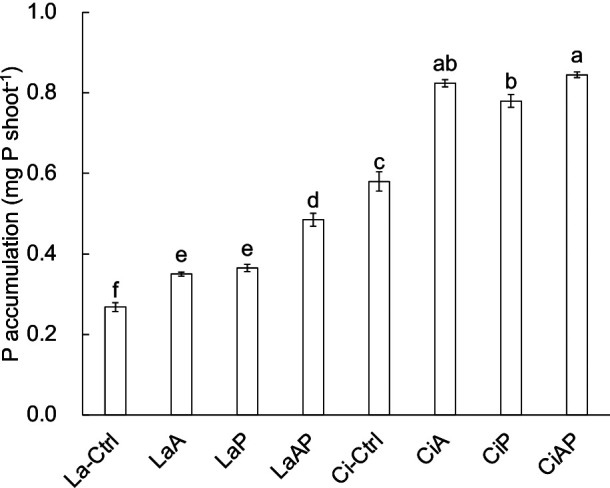
P accumulation in 7-day-old maize seedlings grown on acidic Lateritic red earths (La) or alkaline Cinnamon soils (Ci). Soils were inoculated with PSB strain A (A), fertilized with TCP (P), or treated with both PSB strain A and fertilizer (AP). Different letters indicate significant differences (P<0.05; one-way ANOVA, Tukey, n=3). Error bar=SE.
In La, the PSB inoculation increased P accumulation by 0.082 mg at a rate of approximately 31%; TCP supply increased P accumulation by 0.097 mg at a rate of approximately 36%; and the combination treatment increased P accumulation by 0.217 mg at a rate of approximately 81%. The absolute increase and rate of increase in LaAP were higher than those in LaA. In Ci, the PSB inoculation increased P accumulation by 0.244 mg at a rate of approximately 42%; TCP supply increased P accumulation by 0.200 mg at a rate of approximately 35%; and the combination treatment increased P accumulation by 0.265 mg at a rate of approximately 46%. The absolute increase and rate of increase in CiAP were similar to those in CiA.
Discussion
PSB and their phosphate release ability
In the present study, PSB were isolated with NBRIP, which contains TCP as the sole P source and effectively isolates PSB with high potential to solubilize TCP, but with low potential for mobilization from FePO4 and AlPO4 (Chung et al., 2005; Jiang et al., 2020). However, some of the PSB isolated from unplanted La as well as rhizosphere soil exhibited activity for mobilization not only from TCP, but also from FePO4 and AlPO4, which may be attributed to distinct mechanisms of P release (Zhu et al., 2009; Wang et al., 2014). The 5 PSB isolates used in the present study were closely related to genera that reportedly have the capacity to release P from soils (Lee et al., 2016; Liu et al., 2019; Safirzadeh et al., 2019; Liu et al., 2022). Soil inoculation experiments indicated that the 5 PSB isolated affected phosphate availability from soils with different properties (Tables 2 and 3). Furthermore, the present results showed that the efficiency of these PSB at releasing soil HCl P was inconsistent with their efficiency at releasing P from TCP in shake flasks. For example, F and G released more P from TCP than the other 3 strains (Fig. 1), but did not induce a greater decrease in HCl P than the other strains in the inoculation experiment (Tables S1 and S2). In addition to distinct mechanisms of P release, another reason may be that the environment provided by the culture medium in shake flasks markedly differed from soil. The buffering capacity of soils has been shown to limit the solubilization of soil phosphates by microorganisms (Cabala-Rosand and Wild, 1982; Gyaneshwar et al., 1998); PSB organic acid production was affected by the different nitrogen and carbon conditions of soil (Cuningham and Kuiak, 1992). Previous studies also demonstrated that PSB with high solubility to TCP in the medium did not increase P accumulation in plants (Poonguzhali et al., 2008; Collavino et al., 2010). Therefore, it was considered unreliable to use the solubility of PSB for TCP in media to estimate the release of P from soil and the promotion of plant growth by PSB (Bashan et al., 2013). This study also showed that it is not necessarily reliable to predict the release ability of PSB to soil P by the release ability to TCP.
Relationships between P mobilization by PSB and soil types
Soil inoculation experiments revealed that although different strains may cause different P fraction changes, changes in the same soil were consistent. Regarding La, which is a soil with pH 5.0–5.5 and high PRP, its P fractions are dominated by moderately labile P. The inoculation of PSB resulted in a significant increase in moderately labile P as the main response, regardless of the supply of TCP, while a small amount of released P was transferred to the labile P pool. Delfim et al. (2020) also reported that changes in the P pools of Andisol and Ultisol with pH 5.5 and 5.8 by Bacillus thuringiensis significantly increased NaOH-Pi levels, which is consistent with the present results. In Ci at pH 8.0 with a high HCl P content, PSB mainly increased labile P. Therefore, the changes in P fractions caused by PSB may be dominated by the soil type. In other words, the different phosphate release abilities of PSB led to different changes in P fractions, whereas the changes observed in the same soil were consistently in the same direction.
Effects of the PSB co-culture on maize seedlings
The promotive effects of PSB on plant P uptake and biomass have been widely reported (Biswas et al., 2022; Dasila et al., 2022; Liu et al., 2022; Sabra and Ahmed, 2022). In the present study, strain A also promoted P accumulation in maize seedlings.
The present results indicated that strain A increased P accumulation in maize seedlings in both soils, independent of supplementation with TCP. Furthermore, P accumulation in maize seedlings was higher with strain A combined with TCP supply than with strain A inoculated alone in La, but not in Ci. Overall, the accumulation of and absolute increase in P in maize seedlings were lower in La than in Ci, while those in CiAP and CiA were the highest. In terms of the rate of increase, LaA was lower than CiA, while LaAP was markedly higher than CiAP. Therefore, the combination of PSB and TCP in La significantly optimized planting effects; however, in Ci, the mobilization of soil P using PSB showed promising results.
The results of the point-biserial correlation analysis indicated the significant positive effects of soil types, the PSB inoculation, and TCP supply on P accumulation in maize seedlings (Table S4), and the correlation of soil type was greater than that of PSB inoculation and TCP supply. Nevertheless, the selectivity of the soil type in agricultural production is minimal. Therefore, PSB and P fertilizer may be an effective means to increase crop yield.
Conclusions
The present study examined the effects of PSB isolates on soil P fractions in La and Ci soils and revealed distinct changes in the P fraction caused by PSB in both soil types. Furthermore, the results obtained showed that an inoculation with PSB strain A (cf. Enterobacter chuandaensis) promoted P accumulation in maize seedlings in soil with and without TCP fertilization. The present results suggest that the efficiency of a microbial strain at mobilizing soil P differs with soil types (La and Ci). These differences may be partly attributed to the effects of original soil P fractionation. P was assigned to different fractions during the conversion process. The diversity of global soils may require distinct P fertilization strategies (Mengel, 1997). The results of the present study support this view. La, which is rich in iron and aluminum oxides, is more likely to bind P to the moderately labile P fraction, leading to low labile P conditions.
As reported by Barrow (2022), long-term fertilized soils have already accumulated large amounts of P. PSB are an effective means to mobilize P accumulated in soils. Further studies on P release ability in different soils may contribute to the more efficient use of PSB.
Citation
Long, H., and Wasaki, J. (2023) Effects of Phosphate-solubilizing Bacteria on Soil Phosphorus Fractions and Supply to Maize Seedlings Grown in Lateritic Red Earths and Cinnamon Soils. Microbes Environ 38: ME22075.
https://doi.org/10.1264/jsme2.ME22075
Supplementary Material
References
- Adnan, M., Shah, Z., Fahad, S., Arif, M., Alam, M., Khan, I.A., et al. (2017) Phosphate-solubilizing bacteria nullify the antagonistic effect of soil calcification on bioavailability of phosphorus in alkaline soils. Sci Rep 7: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aerts, R., and Chapin III, F.S. (1999) The mineral nutrition of wild plants revisited: a re-evaluation of processes and patterns. Adv Ecol Res 30: 1–67. [Google Scholar]
- Alam, F., Khan, A., Fahad, S., Nawaz, S., Ahmed, N., Ali, M.A., et al. (2022) Phosphate solubilizing bacteria optimize wheat yield in mineral phosphorus applied alkaline soil. J Saudi Soc Agric Sci 21: 339–348. [Google Scholar]
- Alam, K., Barman, M., Kumar, S., and Ray, P. (2021) Release pattern of soil phosphorus as affected by phosphate solubilizing microorganisms in an Ultisol. Pharma Innov 10: 823–827. [Google Scholar]
- Amy, C., Avice, J.C., Laval, K., and Bressan, M. (2022) Are native phosphate solubilizing bacteria a relevant alternative to mineral fertilizations for crops? Part I. when rhizobacteria meet plant P requirements. Rhizosphere 21: 100476. [Google Scholar]
- Barrow, N.J. (2022) How understanding soil chemistry can lead to better phosphate fertilizer practice: a 68 year journey (so far). Plant Soil 476: 1–15. [Google Scholar]
- Bashan, Y., Kamnev, A.A., and de-Bashan, L.E. (2013) Tricalcium phosphate is inappropriate as a universal selection factor for isolating and testing phosphate-solubilizing bacteria that enhance plant growth: a proposal for an alternative procedure. Biol Fertil Soils 49: 465–479. [Google Scholar]
- Batjes, N.H. (2011) Global distribution of soil phosphorus retention potential. ISRIC-World Soil Information, Wageningen, The Netherlands: ISRIC Report. [Google Scholar]
- Biswas, S.S., Biswas, D.R., Ghosh, A., Sarkar, A., Das, A., and Roy, T. (2022) Phosphate solubilizing bacteria inoculated low-grade rock phosphate can supplement P fertilizer to grow wheat in sub-tropical inceptisol. Rhizosphere 23: 100556. [Google Scholar]
- Cabala-Rosand, P., and Wild, A. (1982) Direct use of low grade phosphate rock from Brazil as fertilizer. Plant Soil 65: 351–362. [Google Scholar]
- Chen, Y.P., Rekha, P.D., Arun, A.B., Shen, F.T., Lai, W.A., and Young, C.C. (2006) Phosphate solubilizing bacteria from subtropical soil and their tricalcium phosphate solubilizing abilities. Appl Soil Ecol 34: 33–41. [Google Scholar]
- Chung, H., Park, M., Madhaiyan, M., Seshadri, S., Song, J., Cho, H., and Sa, T. (2005) Isolation and characterization of phosphate solubilizing bacteria from the rhizosphere of crop plants of Korea. Soil Biol Biochem 37: 1970–1974. [Google Scholar]
- Collavino, M.M., Sansberro, P.A., Mroginski, L.A., and Aguilar, O.M. (2010) Comparison of in vitro solubilization activity of diverse phosphate-solubilizing bacteria native to acid soil and their ability to promote Phaseolus vulgaris growth. Biol Fertil Soils 46: 727–738. [Google Scholar]
- Costa, M.G., Gama-Rodrigues, A.C., Gonçalves, J.L.d.M., Gama-Rodrigues, E.F., Sales, M.V.d.S., and Aleixo, S. (2016) Labile and non-labile fractions of phosphorus and its transformations in soil under eucalyptus plantations, Brazil. Forests 7: 15. [Google Scholar]
- Cuningham, J.E., and Kuiak, C. (1992) Production of citric and oxalic acid and solubilization of calcium phosphate by Penicillium billai. Appl Environ Microbiol 58: 1451–1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dasila, H., Sah, V.K., Jaggi, V., and Sahgal, M. (2022) Phosphate solubilizing bacteria (PSB) a potential tool to enhance soil health and wheat vigor parameters in pot trial experiment. Pharma Innov 11: 1829–1835. [Google Scholar]
- Delfim, J., Gerding, M., and Zagal, E. (2020) Phosphorus fractions in Andisol and Ultisol inoculated with Bacillus thuringiensis and phosphorus uptake by wheat. J Plant Nutr 43: 2728–2739. [Google Scholar]
- Ding, Y., Yi, Z., Fang, Y., He, S., Li, Y., He, K., et al. (2021) Multi-omics reveal the efficient phosphate-solubilizing mechanism of bacteria on rocky soil. Front Microbiol 12: 761972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gyaneshwar, P., Kumar, G.N., and Parekh, L.J. (1998) Effect of buffering on the phosphate-solubilizing ability of microorganisms. World J Microbiol Biotechnol 14: 669–673. [Google Scholar]
- Hafeez, F., Amin, B.A.Z., Akbar, U., Iqbal, A., Faridullah, Bilal, M., and Nazir, R. (2019) Assessment of phosphorus availability in soil by introducing P-solubilizing novel bacterial and fungal strains of Lower Himalaya. Commun Soil Sci Plant Anal 50: 1541–1549. [Google Scholar]
- Halder, A.K., Mishra, A.K., Bhattacharyya, P., and Chakrabartty, P.K. (1990) Solubilization of rock phosphate by Rhizobium and Bradyrhizobium. J Gen Appl Microbiol 36: 81–92. [Google Scholar]
- He, D., and Wan, W. (2022) Distribution of culturable phosphate-solubilizing bacteria in soil aggregates and their potential for phosphorus acquisition. Environ Microbiol 10: e00290-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He, Z., Zhang, M., and Wilson, M.J. (2004) Distribution and classification of red soils in China. In The Red Soils of China. Dordrecht: Springer, pp. 29–33. [Google Scholar]
- Hedley, M.J., White, R.E., and Nye, P.H. (1982) Plant‐induced changes in the rhizosphere of rape (Brassica napus var. Emerald) seedlings: III. Changes in L value, soil phosphate fractions and phosphatase activity. New Phytol 91: 45–56. [Google Scholar]
- Hoagland, D.R., and Arnon, D.I. (1950) The Water-culture Method for Growing Plants Without Soil. Circular 347. Berkeley, CA: University of California, College of Agriculture, Agricultural Experiment Station. [Google Scholar]
- Hou, E., Tan, X., Heenan, M., and Wen, D. (2018) A global dataset of plant available and unavailable phosphorus in natural soils derived by Hedley method. Sci Data 5: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingle, K.P., and Padole, D.A. (2017) Phosphate solubilizing microbes: An overview. Int J Curr Microbiol App Sci 6: 844–852. [Google Scholar]
- Jiang, H., Wang, T., Chi, X., Wang, M., Chen, N., Chen, M., et al. (2020) Isolation and characterization of halotolerant phosphate solubilizing bacteria naturally colonizing the peanut rhizosphere in salt-affected soil. Geomicrobiol J 37: 110–118. [Google Scholar]
- Khan, A.A., Jilani, G., Akhtar, M.S., Naqvi, S.M.S., and Rasheed, M. (2009) Phosphorus solubilizing bacteria: occurrence, mechanisms and their role in crop production. J Agric Biol Sci 1: 48–58. [Google Scholar]
- Khan, H., Akbar, W.A., Shah, Z., Rahim, H.U., Taj, A., and Alatalo, J.M. (2022) Coupling phosphate-solubilizing bacteria (PSB) with inorganic phosphorus fertilizer improves mungbean (Vigna radiata) phosphorus acquisition, nitrogen fixation, and yield in alkaline-calcareous soil. Heliyon 8: e09081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochian, L.V. (2012) Rooting for more phosphorus. Nature 488: 466–467. [DOI] [PubMed] [Google Scholar]
- Ku, Y., Xu, G., Tian, X., Xie, H., Yang, X., and Cao, C. (2018) Root colonization and growth promotion of soybean, wheat and Chinese cabbage by Bacillus cereus YL6. PLoS One 13: e0200181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane, D.J. (1991) 16S/23S rRNA sequencing. In Nucleic Acid Techniques in Bacterial Systematics. Stackebrandt, E., and Goodfellow, M. (eds). Chichester: Jon Wiley & Sons, pp. 115–176. [Google Scholar]
- Larsen, S. (1967) Soil phosphorus. Adv Agron 19: 151–210. [Google Scholar]
- Lee, Y.J., Lee, H.H., Lee, C.J., and Yoon, M.H. (2016) Effect of co-inoculation of two bacteria on phosphate solubilization. Korean J Soil Sci Fert 49: 318–326. [Google Scholar]
- Liu, X., Jiang, X., He, X., Zhao, W., Cao, Y., Guo, T., et al. (2019) Phosphate-solubilizing Pseudomonas sp. strain P34-L promotes wheat growth by colonizing the wheat rhizosphere and improving the wheat root system and soil phosphorus nutritional status. J Plant Growth Regul 38: 1314–1324. [Google Scholar]
- Liu, X., Chen, C., Wang, J., Zou, S., and Long, X. (2021) Phosphorus solubilizing bacteria Bacillus thuringiensis and Pantoea ananatis simultaneously promote soil inorganic phosphate dissolution and soil Pb immobilization. Rhizosphere 20: 100448. [Google Scholar]
- Liu, Y., Hosseini Bai, S., Wang, J., Hu, D., Wu, R., Zhang, W., and Zhang, M. (2022) Strain Klebsiella ZP-2 inoculation activating soil nutrient supply and altering soil phosphorus cycling. J Soils Sediments 22: 2146–2157. [Google Scholar]
- Mengel, K. (1997) Agronomic measures for better utilization of soil and fertilizer phosphates. Eur J Agron 7: 221–233. [Google Scholar]
- Murphy, J.A.M.E.S., and Riley, J.P. (1962) A modified single solution method for the determination of phosphate in natural waters. Anal Chim Acta 27: 31–36. [Google Scholar]
- Nautiyal, C.S. (1999) An efficient microbiological growth medium for screening phosphate solubilizing microorganisms. FEMS Microbiol 170: 265–270. [DOI] [PubMed] [Google Scholar]
- Pathak, A.P., Rathod, M.G., Rakhe, S.A., Bhure, N.U., Borade, R.S., and Kamalapure, P.S. (2021) A step to attain sustainable development in crop production and soil health. In Ecology Research Vol. II. Kolhapur: Bhumi Publishing, pp. 1–16. [Google Scholar]
- Poonguzhali, S., Madhaiyan, M., and Sa, T.M. (2008) Isolation and identification of phosphate solubilizing bacteria from Chinese cabbage and their effect on growth and phosphorus utilization of plants. J Microbiol Biotechnol 18: 773–777. [PubMed] [Google Scholar]
- Raghothama, K.G. (2005) Phosphorus and plant nutrition: an overview. In Phosphorus: Agriculture and the Environment, Vol. 46. Sims J.T., and Sharpley A.N. (eds). Madison, WI: The American Society of Agronomy, Crop Science Society of America, Soil Science Society of America, pp. 353–378. [Google Scholar]
- Sabra, M.A., and Ahmed, A.E. (2022) The influence of the isolated Enterobacter spp and Pantoea sp., on barley’s phosphorus uptake grown in calcareous soil. Egypt Acad J Biolog Sci, G. Microbiolog 14: 47–63. [Google Scholar]
- Sackett, W.G., Patten, A.J., and Brown, C.W. (1908) The solvent action of soil bacteria upon the insoluble phosphates of raw bone meal and natural raw rock phosphate. Centralbl Bakt 20: 688–703. [Google Scholar]
- Safirzadeh, S., Chorom, M., and Enayatizamir, N. (2019) Effect of phosphate solubilising bacteria (Enterobacter cloacae) on phosphorus uptake efficiency in sugarcane (Saccharum officinarum L.). Soil Res 57: 333–341. [Google Scholar]
- Tian, W.H., Ye, J.Y., Cui, M.Q., Chang, J.B., Liu, Y., Li, G.X., et al. (2021) A transcription factor STOP1-centered pathway coordinates ammonium and phosphate acquisition in Arabidopsis. Mol Plant 14: 1554–1568. [DOI] [PubMed] [Google Scholar]
- Turner, S., Pryer, K.M., Miao, V.P., and Palmer, J.D. (1999) Investigating deep phylogenetic relationships among cyanobacteria and plastids by small subunit rRNA sequence analysis. J Eukaryot Microbiol 46: 327–338. [DOI] [PubMed] [Google Scholar]
- Wang, C.M. (2003) Profile characteristics and nutrients of dry Ci soils in dry valley of the upper Minjiang River. Chin J Appl Environ Biol 9: 230–234. [Google Scholar]
- Wang, T., Kong, L., Jiao, J., Liu, M., Hu, F., Sun, B., and Li, H. (2014) Screening of phosphate-solubilizing bacteria in red soil and their acting mechanisms. Acta Petrol Sin 51: 373–380. [Google Scholar]
- Wild, A. (1950) The retention of phosphate by soil. A review. Eur J Soil Sci 1: 221–238. [Google Scholar]
- Xu, G., Shao, H., Zhang, Y., and Junna, S. (2018) Nonadditive effects of biochar amendments on soil phosphorus fractions in two contrasting soils. Land Degrad Dev 29: 2720–2727. [Google Scholar]
- Yang, X., and Post, W.M. (2011) Phosphorus transformations as a function of pedogenesis: A synthesis of soil phosphorus data using Hedley fractionation method. Biogeosciences 8: 2907–2916. [Google Scholar]
- Zhu, Y., Yao, T., Li, Y., and Sun, H. (2009) Screening of phosphate-solubilizing bacteria and their acting mechanisms in the rhizosphere of red clover. Acta Agrestia Sinica 17: 259–263. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.