Abstract
BACKGROUND AND OBJECTIVES:
Heparin-induced thrombocytopenia (HIT) is an antibody-mediated condition that leads to thrombocytopenia and possible thrombosis. Patients with HIT who require cardiac surgery pose a challenge as high doses of heparin or heparin alternatives are required to permit cardiopulmonary bypass (CPB). Intraoperative therapeutic plasma exchange (TPE) is a valuable adjunct in the management of antibody-mediated syndromes including HIT. The clinical impact of TPE on thromboembolic events, bleeding, and mortality after heparin re-exposure is not well established. We hypothesized that TPE with heparin re-exposure will not lead to HIT-related thromboembolic events, bleeding, or increased mortality after cardiac surgery with CPB.
MATERIALS AND METHODS:
We reviewed 330 patients who received perioperative TPE between September 2012 and September 2017.
RESULTS:
24 patients received TPE for HIT before anticipated heparin use for CPB. Most patients were males (79%) scheduled for advanced heart failure therapies. Three patients (12.5%) died within 30 days after surgery but none of the deaths were considered HIT-related. Thromboembolic events (TE) occurred in 3 patients within 7 days of surgery; of those, two were possibly HIT-related.
CONCLUSION:
TPE with heparin re-exposure was not strongly associated with HIT-related thrombosis/death after cardiac surgery with CPB.
Keywords: cardiac surgery, heparin, plasma exchange, platelet factor 4, thrombocytopenia
BACKGROUND
Heparin-induced thrombocytopenia (HIT) is a prothrombotic condition in which antibodies to the antigenic complex of heparin and platelet factor 4 antigen (anti-heparin/PF4) activate platelets to degranulate and aggregate in the microcirculation [1]. These events lead to thrombocytopenia and possible thrombosis [1]. Seroconversion occurs in 8–17% of patients requiring heparin for medical indications, and in approximately 50% of patients exposed to heparin during the course of cardiac surgery. Patients with HIT who require cardiac surgery pose a challenge as high doses of heparin or heparin alternatives are required to permit cardiopulmonary bypass (CPB). The risks of thrombosis associated with early heparin re-exposure during CPB must be balanced against the adverse effects (i.e. bleeding and circuit thrombosis) that are a consequence from the use of irreversible heparin alternatives such as bivalirudin [2–4] for which there are no reversal agents or adequate intraoperative laboratory monitoring strategies to date.
Intraoperative therapeutic plasma exchange (TPE) is a valuable adjunct in the management of antibody-mediated syndromes including HIT. TPE permits heparin use by removing immune complexes and HIT antibodies [5]. Intraoperative TPE allows the use of heparin rather than irreversible heparin alternatives. In a retrospective study of 11 HIT or heparin/PF4 seropositive patients undergoing TPE in preparation for cardiac surgery, a single TPE treatment reduced heparin/PF4 titers by 50–84%, and 7 of 9 patients had normal anti-heparin/PF4 levels after treatment [6]. Following re-exposure to heparin, no serious adverse complications of HIT or to TPE were noted. HIT is associated with postoperative morbidity and mortality. Recent studies have shown that clinical complications of thrombosis and thrombocytopenia are highly correlated with antibody levels [7, 8]. Studies have also shown that TPE effectively removes heparin/PF4 antibodies [6, 9]. The clinical impact of TPE on thromboembolic events, bleeding, and mortality after heparin re-exposure is not well established. We hypothesized that TPE with heparin re-exposure will not lead to HIT-related thromboembolic events, bleeding, or increased mortality after cardiac surgery with CPB.
METHODS
After obtaining IRB approval, we retrospectively reviewed cardiac surgical patients undergoing TPE before CPB with heparin between September 2012 – September 2017. We identified adult patients ≥18 years with a prior history of HIT (decrease in the platelet count of more than 50% from the highest platelet count value after the start of heparin with an onset 5 to 10 days after the start of heparin) or preoperative anti-heparin/PF4 antibody seropositivity (polyclonal ELISA OD > 0.4; GTI PF4 EIA, GTI diagnostics, Waukesha, WI) as their indication for TPE, which was performed as described in a previous study. [6] The American Society for Apheresis (ASFA) designates TPE for pre-CPB in HIT as category III (optimum role of apheresis therapy is not established. Decision making should be individualized).[10] The level of evidence is grade 2C.
TPE was performed using the COBE Spectra® (from 2012 – 2015) or Spectra Optia ® (2014 – 2017) after the induction of general anesthesia. TPE was performed with plasma replacement using a standardized protocol to exchange 1.0 plasma volume (approximately 3500–4500 mL based on the patient’s height, weight, gender, and hematocrit [11].The timing of TPE was dependent on the hemodynamic stability of the patient. Ideally, it was performed before heparinization (400 U/kg as a bolus) but if necessary, heparin was given, CPB initiated to stabilize the patient, and TPE performed during CPB as previously described. [12] Because heparin is removed during TPE, additional heparin was infused during the exchange based on the following estimation. Assuming a hematocrit of 0.25, we replaced heparin lost during TPE as calculated by 4 U/mL of plasma removed administered as a 4000-U bolus after every liter removed with any remainder at the completion of treatment.
Daily minimum platelet counts were abstracted 7 days before to 30 days after TPE. Arterial/venous thromboembolic (TE) events within 7 days of TPE and survival status up to 30 days were determined. Patient demographic and clinical variables were summarized by frequency (%) for categorical and mean (SD) or median [IQR] for continuous variables.
RESULTS
Patient demographic and descriptive data are summarized in Table 1. We reviewed 330 patients who received intraoperative TPE between September 2012 and September 2017 (Figure 1). Of the 164 patients that underwent TPE during cardiac surgery using CPB, 24 patients received TPE for HIT before anticipated heparin use for CPB; the remainder received TPE in order to clear human leukocyte antigen (HLA) specific antibodies prior to heart or lung transplantation. All patients (24) had a history of HIT. Also, 7 patients (29%) had a history of prior VTE, but it was not clear from the records if this was HITT based on timing therefore we did not include this. Of the 24, 12 patients had preoperative anti heparin/PF4 antibody testing at our hospital; we had other patients transferred to us from outside hospitals with a history of HIT and a “positive ELISA” although we did not have these data in our records.
Table 1.
Demographic data
| Variable (n=24 patients) | Mean ± SD Percentage (n) |
|---|---|
| Age (years) | 55.2 ± 9.44 |
| Gender (Male) | 79% (19) |
| Procedure | |
| Heart transplant | 21% (6) |
| VAD insertion/replacement | 54% (13) |
| Other procedures | 22% (5) |
| Race | |
| African American | 37% (9) |
| Caucasian | 54% (13) |
| Other | 4% (2) |
| BMI | 32.0 ± 9.80 |
| Height (m) | 1.75 ± 0.09 |
| Weight (kg) | 98.3 ± 30.2 |
| Preoperative Anti heparin/PF4 Antibody OD value | 1.39 ± 0.89 |
| Nonischemic cardiomyopathy | 62% (15) |
| Ischemic cardiomyopathy | 29% (7) |
| Coronary artery disease | 33% (8) |
| Mod-Sev Aortic stenosis/regurgitation | 50% (12) |
| Atrial fibrillation/flutter | 50% (12) |
| Diabetes | 42% (10) |
| Hypertension | 67% (16) |
| Smoker | 8% (2) |
| Hypercholesterolemia | 62% (15) |
| CKD | 42% (10) |
| ESRD | 4% (1) |
| Previous MI | 25% (6) |
| Previous stroke | 12% (3) |
| Prior VTE | 29% (7) |
| Peripheral Vascular Disease | 8% (2) |
| Mechanical circulatory support | 67% (16) |
| preoperative IABP | 58% (9) |
| preoperative VA ECMO | 8% (5) |
| preoperative VV ECMO | 4% (2) |
| Preoperative LVAD | 25% (6) |
| Redo sternotomy | 29% (7) |
Figure 1.
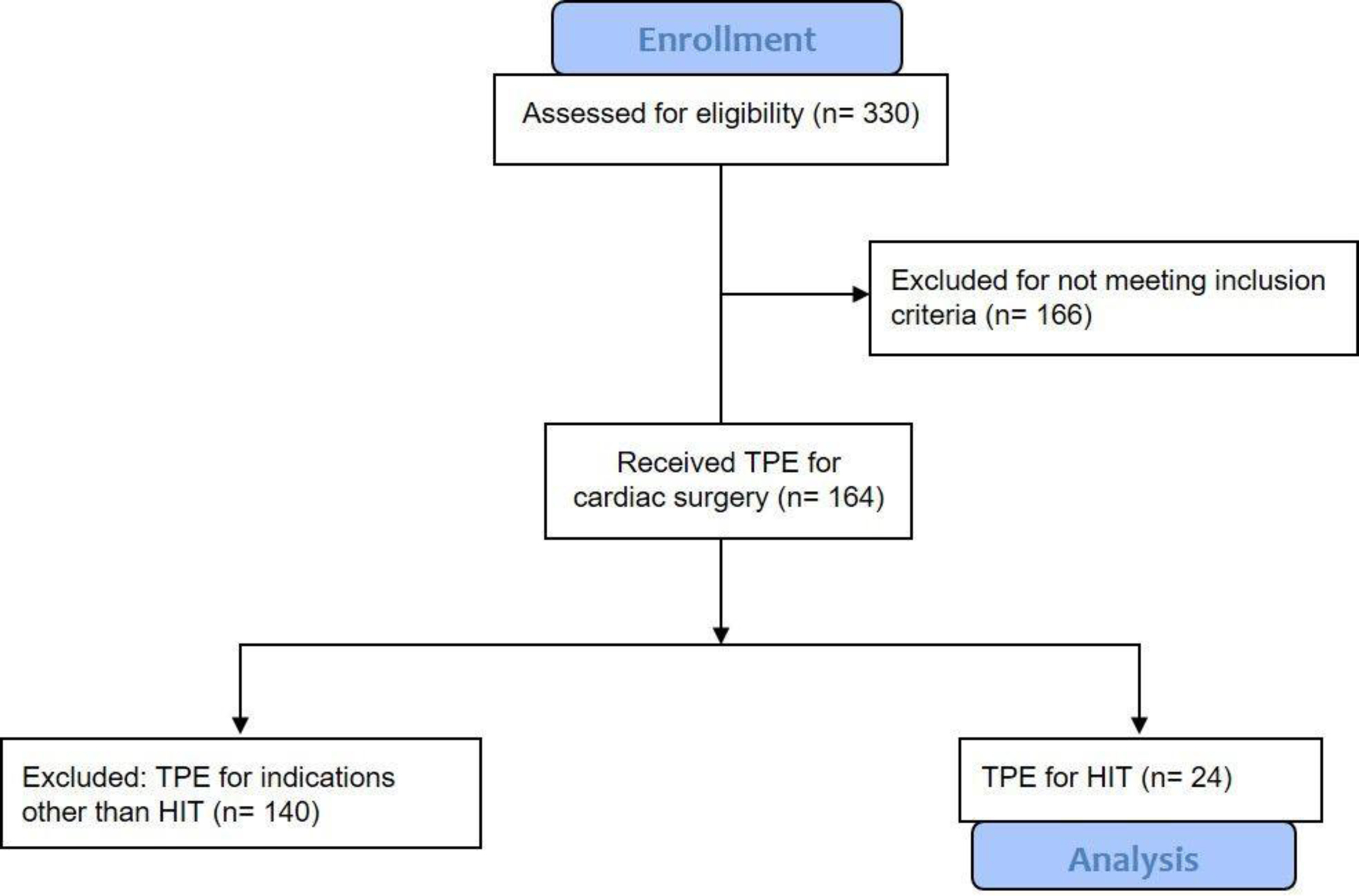
CONSORT 2010 flow diagram
Most patients (79.0%, 18 patients, Table 1) were males scheduled for advanced heart failure therapies (i.e left ventricular assist device insertion or heart transplantation), they all received heparin for CPB. Twenty-one patients were anticoagulated with bivalirudin before surgery, one patient with argatroban, and one patient with coumadin. Postoperative anticoagulation for the LVAD patients used bivaiirudin. The median preoperative HIT ELISA test optical density (OD) of 1.39 [0.67, 2.43]. Two patients with a positive polyclonal heparin/PF4 ELISA, were subsequently negative on the IgG specific assay.
Platelet counts typically reached a nadir on the day of surgery and steadily recovered for most patients during the first ten postoperative days (as shown in Fig. 2). For the 24 patients, in the cohort the preoperative median was 160 ×103/ mm3. The lowest daily median occurred on the day of surgery and had a median of 100 ×103/mm3. Thereafter, the median values monotonically increased through postoperative day 10 to a median of 292 ×103 mm3. By day 16, all platelet counts were above 150 ×103/ mm3.
Figure 2.
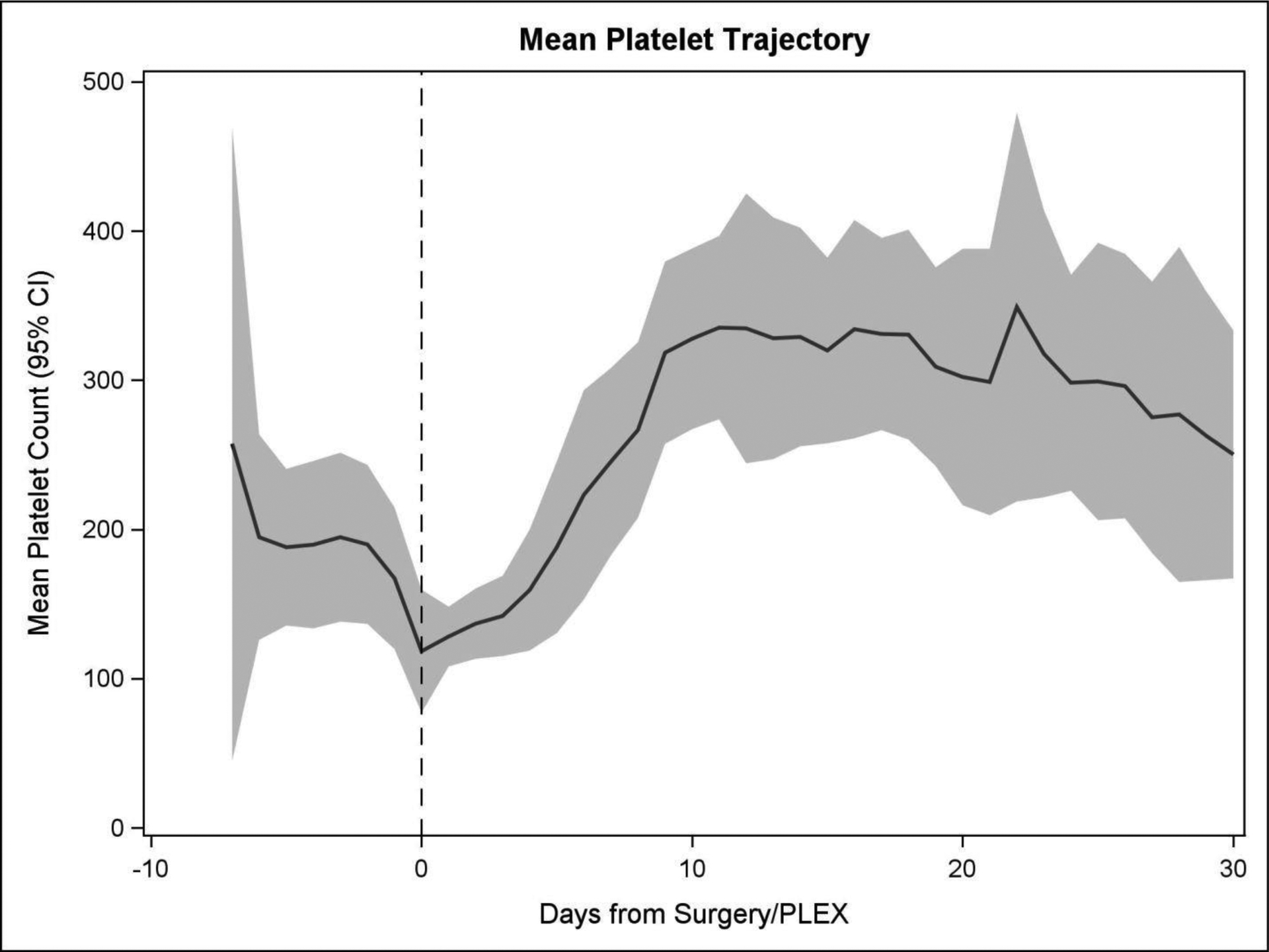
Platelet trajectories for all patients. This graph shows the daily mean platelet count. The band represents the 95% confidence interval.
Only 11 patients had a postoperative HIT ELISA retested. These patients had a preoperative median OD of 1.99 and a single, intraoperative TPE treatment reduced the OD titer to a median of 0.34. One patient had a significantly increased OD titer after TPE (0.9 preoperatively to 1.41 postoperatively), although this was an increase in the polyclonal ELISA only. The IgG specific ELISA was negative. There was no difference between pre and postoperative OD titers in two patients, and for the remaining seven, the OD titers were reduced by 35–85% from the preoperative value. Similarly, seven patients decreased their postoperative OD to <0.4. The median [Q1, Q3] change from pre-to-post surgery is −1.57 [−2.01, 0.01], and the Wilcoxon Signed rank p-value is 0.037, suggesting a significant decrease in OD level in this patient group.
Three patients (12.5%) died within 30 days after surgery. One patient died after a hemorrhagic stroke with subsequent brain death. The platelet counts in the weeks preceding death were normal (Figure 3). The second patient died from respiratory failure secondary to pneumonia. The third patient had multiorgan failure secondary to sepsis; this patient presented with a TE event associated with a drop in his platelet count after an initial recovery, that could have been HIT related. He died later after resolution of thrombocytopenia; the cause of death was unrelated to the prior TE event and was not TE-related. None of the deaths were considered HIT-related, as the platelet counts were normal at the time of death. For these three patients, the preoperative median OD was 2.47 [0.233–2.487]. After TPE, the median OD was 0.467 [0.151–0.933].
Figure 3.
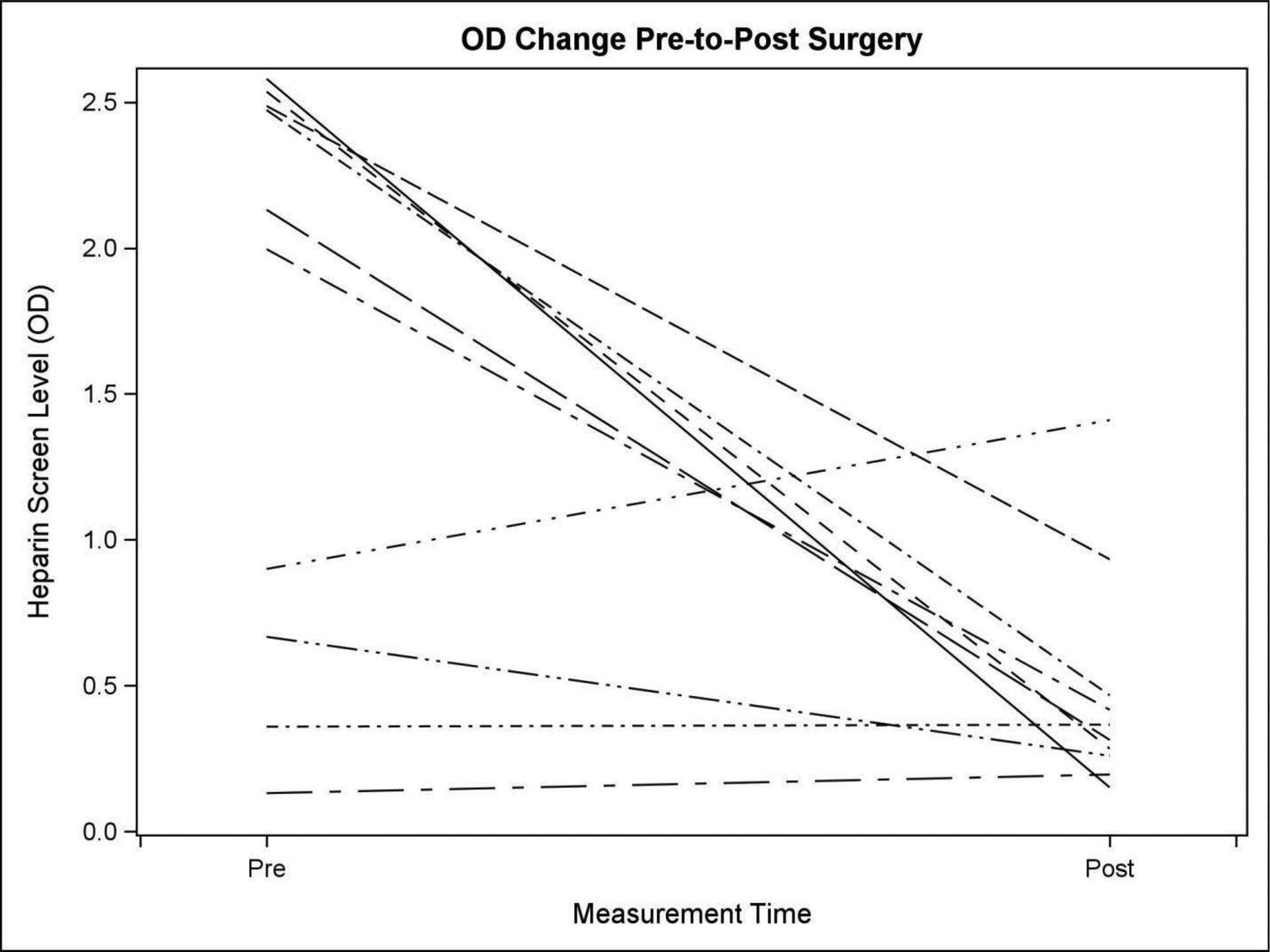
Median [Q1, Q3] change from pre-to-post surgery. the median [Q1, Q3] change from pre-to-post surgery is −1.57 [−2.01, 0.01].The Wilcoxon Signed rank p-value is 0.037.
TE events occurred in 3 patients within 7 days of surgery. Two were possibly HIT-related. One was a stroke with an ELISA OD four days before the TE of 0.47 and a PLT count of 54 × 103.The second event was a deep venous thrombosis with an ELISA OD 1 day before the TE of 0.93 with a PLT count of 106 × 102. A third TE occurred well after platelet count recovery to 330 × 103, and was therefore unlikely HIT-related.
DISCUSSION
We present a series of 24 patients with current or recent HIT that were treated with TPE to remove heparin/PF4 antibodies and allow standard heparin anticoagulation for their complex cardiac surgery. These patients were successfully re-exposed to heparin, without intraoperative incident, to permit cardiopulmonary bypass. In our cohort, platelet counts reached a nadir on the day of surgery. Platelet counts typically decrease during cardiac surgery as a result of blood loss, transfusion, hemodilution, and activation with sequestration in the extracorporeal circuit (primarily the fibers of the oxygenator). It is challenging to differentiate this from HIT, other than it is an expected observation with a typical nadir on the day of surgery, or the day after, with subsequent recovery. The postoperative platelet count profile seems to follow two specific patterns in the setting of HIT. [13] One of the patterns is biphasic and shows an initial recovery of the platelet count post bypass with a subsequent decrease in the count of at least 40% compared to the maximum postoperative value. The second pattern is monophasic with a low platelet count that persists in postoperative days 5–10. The Lillo-Le Loüet score was created to diagnose HIT after CPB and includes the postoperative platelet profile as a variable.[14] Anti heparin/PF4 antibody titers were significantly reduced (by 35–85%) in the majority of the patients with available postoperative antibody testing data. A previous study also reported a reduction of 50%–84% after a single TPE treatment [6]. In our cohort, only two of the three TE events were possibly HIT-related, and none of the deaths were likely HIT-related. Therefore, TPE could be considered a reasonable option for patients with HIT requiring urgent cardiac surgery who were considered clinically unsuitable for heparin alternatives [2], due to complexity of surgery with anticipated long duration and therefore a large expected total bivalirudin dose in the setting of likely renal dysfunction and high bleeding risk.
None of the patients underwent additional TPE treatments aiming to achieve complete antibody removal, provided they had stable platelet counts postoperatively or could tolerate bivalirudin anticoagulation. Repeated postoperative TPE may be unwarranted due to risks of hemodynamic instability and exposure to additional blood products. However, it is unknown whether additional TPE based on repeat anti-heparin/PF4 testing could help further reduce TE complications in this morbid group. Serial PF4-dependent enzyme-immunoassay (EIA) testing for HIT antibodies may be useful especially if the IgG specific ELISA is unavailable. This is a simple, feasible method but is technically challenging as it only detects HIT antibodies indirectly [15]. There is no predetermined value for defining high-titer antibodies, and OD levels correlate poorly with antibody burden. The sensitivity and specificity for PF4/heparin optical density >0.40 is reported as 100% and 26% respectively [16], which means that some patients with positive test results in this assay may never develop HIT [17–19]. Some authors suggest that an OD >1.0–1.4 increases the risk of thrombosis [18, 20]. In our study, of the eleven patients with postoperative OD data, 10 had OD below 1, while one patient had a postoperative OD > 1.4. Interestingly, this patient neither presented with TE nor died in the first 30 days after surgery. The relationship between OD and antibody levels are expressed through a hyperbolic curve function [21]. At high ODs (>2–3), antibody saturation in the ELISA does not accurately reflect the antibody burden or actual titer. Serial dilutions of test samples over a wide range help to quantify the titer of antibody present.
Accuracy in the assessment of titers is particularly relevant for HIT TPE, as one exchange may not be sufficient to remove high titer antibodies [22, 23]. Some HIT patients with high ODs and high titers are likely to need more than one TPE to lower circulating PF4/H antibody burden. Functional assays such as a serotonin release assay (SRA) or heparin-induced platelet activation assay (HIPA), are more specific for immune-mediated HIT but take longer, are more technically demanding, and not widely available [23]. Achieving SRA negativity through multiple sessions of TPE can remove a sufficient amount of HIT antibodies despite persistent positive titers with ELISA [22]. In our study, the patients were not tested with functional assays after TPE, but previous studies have shown that the median time to a negative SRA after a single session is around 50 days [24, 25].To date, TPE is scarcely used for HIT in most institutions, mostly because it is not recognized as an option. In a recent survey [26], only 37% of the surveyed institutions used TPE for HIT for indications such as cardiovascular surgery and HIT-associated thrombosis. There were no major complications from TPE. Hemodynamic instability due to TPE or any plasma reaction was not apparent, although they may have been masked because patients were anesthetized and often already maintained on vasoactive medications. There were no technical issues encountered. Hypocalcemia is expected and was prevented with concomitant intravenous calcium gluconate administration (3–4 grams).
Intravenous immunoglobulin (IVIg) is emerging as a useful approach that can also help to treat patients with HIT. IVIg works by inhibiting HIT antibody-mediated platelet activation, apparently through competitive binding [24]. The inhibition is dependent on the constant domain of IgG (Fc) but not the antigen-binding portion (Fab), and the presence of the HH131 genotype. Other genotypes such as RR131 and HR131 responded favorably to high doses of IVIG, although not as well as the patients with the HH131 genotype. To date, there are no studies relating response to TPE to specific genetic phenotypes. Furthermore, IVIg had no effect on HIT antibody binding in a solid phase PF4-ELISA testing or in the SRA values, which were still strongly positive. In any case, IVIg treatment successfully achieved platelet count recovery [24]. Although laboratory assessments demonstrate antibody persistence after both TPE and IVIg, it is possible that the titer magnitude is not sufficient to trigger a pathological immune and platelet response. Another potential approach involves the concurrent use of cangrelor, a short-acting, intravenous P2Y12 inhibitor, and heparin for cardiopulmonary bypass in patients with HIT but there is limited literature at this time. [27]
This study is a case series without a control group and, consequently, has some limitations. It included a small number of patients, and not all patients had confirmatory tests (e.g SRA). We based the diagnosis on PF4/heparin EIA only, which may be insufficient as only 50% of antibodies causing a positive EIA have clinical relevance. Most of the available SRA results were reported late during the hospital stay, which made them clinically unhelpful. We also did not check for antibody re-emergence which may be a possibility after re-exposure to heparin.
Bivalirudin for complex redo surgery has not been studied, and the bleeding risk in this setting maybe even higher. TPE permits standard anticoagulation for complex procedures and maybe a feasible option for this type of procedures. Genetic assessments may also be useful in future studies to correlate the response to TPE to specific genotypes.
CONCLUSION
Intraoperative TPE is one strategy to facilitate standard heparin anticoagulation during CPB in patients requiring urgent cardiac surgery in the setting of acute HIT. Modifying the protocol to plan for additional TPE based on repeat anti-heparin/PF4 testing could further reduce thromboembolic complications, depending on the results of future studies.
Financial Disclosures:
O. A. Onwuemene: Receives funding from Hemostasis and Thrombosis Research Society, supported by an unrestricted educational grant from Shire, PLC
K. Ghadimi: Receives funding from NIH T32GM008600
I. J. Welsby: Received financial support from Terumo BCT for an Investigator Initiated Trial of Intraoperative Plateletpheresis in 2017
ADDENDUM
I. Moreno-Duarte: This author contributed to data collection and analysis, literature review, manuscript writing and preparation
M. Cooter: This author contributed to data collection and statistical analysis. O. A. Onwuemene: This author contributed to manuscript writing and preparation.
K. Ghadimi: This author contributed to literature review, manuscript writing and preparation
I. J. Welsby: This author contributed to data collection and analysis, literature review, manuscript writing and preparation.
Footnotes
Contributions:
I.Moreno Duarte: Data collection, statistical analysis, data interpretation, manuscript writing.
M. Cooter: Statistical analysis and graphics.
O. A. Onwuemene: Manuscript writing, data interpretation.
K Ghadimi: Study design, Manuscript writing, data interpretation.
I.J Welsby: Study design, Manuscript writing, data interpretation, final approval of the version to be published.
Conflicts of interest: None
REFERENCES
- 1.East JM, Cserti-Gazdewich CM, Granton JT: Heparin-Induced Thrombocytopenia in the Critically Ill Patient. Chest 2018; 154: 678–90. [DOI] [PubMed] [Google Scholar]
- 2.Dyke CM, Smedira NG, Koster A, et al. : A comparison of bivalirudin to heparin with protamine reversal in patients undergoing cardiac surgery with cardiopulmonary bypass: the EVOLUTION-ON study. J Thorac Cardiovasc Surg 2006; 131: 533–9. [DOI] [PubMed] [Google Scholar]
- 3.Koster A, Spiess B, Jurmann M, et al. : Bivalirudin provides rapid, effective, and reliable anticoagulation during off-pump coronary revascularization: results of the “EVOLUTION OFF” trial. Anesth Analg 2006; 103: 540–4. [DOI] [PubMed] [Google Scholar]
- 4.Smedira NG, Dyke CM, Koster A, et al. : Anticoagulation with bivalirudin for off-pump coronary artery bypass grafting: the results of the EVOLUTION-OFF study. J Thorac Cardiovasc Surg 2006; 131: 686–92. [DOI] [PubMed] [Google Scholar]
- 5.Kanellopoulou T, Kostelidou T: Literature review of apheresis procedures performed perioperatively in cardiac surgery for ASFA category indications. J Clin Apher 2018. [DOI] [PubMed] [Google Scholar]
- 6.Welsby IJ, Um J, Milano CA, et al. : Plasmapheresis and heparin reexposure as a management strategy for cardiac surgical patients with heparin-induced thrombocytopenia. Anesth Analg 2010; 110: 30–5. [DOI] [PubMed] [Google Scholar]
- 7.Zwicker JI, Uhl L, Huang WY, et al. : Thrombosis and ELISA optical density values in hospitalized patients with heparin-induced thrombocytopenia. J Thromb Haemost 2004; 2: 2133–7. [DOI] [PubMed] [Google Scholar]
- 8.Chilver-Stainer L, Lammle B, Alberio L: Titre of anti-heparin/PF4-antibodies and extent of in vivo activation of the coagulation and fibrinolytic systems. Thromb Haemost 2004; 91: 276–82. [DOI] [PubMed] [Google Scholar]
- 9.Robinson JA: Apheresis in thoracic organ transplantation. Ther Apher 1999; 3: 34–9. [DOI] [PubMed] [Google Scholar]
- 10.Padmanabhan A, Connelly-Smith L, Aqui N, et al. : Guidelines on the Use of Therapeutic Apheresis in Clinical Practice - Evidence-Based Approach from the Writing Committee of the American Society for Apheresis: The Eighth Special Issue. J Clin Apher 2019; 34: 171–354. [DOI] [PubMed] [Google Scholar]
- 11.Nadler SB, Hidalgo JH, Bloch T: Prediction of blood volume in normal human adults. Surgery 1962; 51: 224–32. [PubMed] [Google Scholar]
- 12.Brady J, Riccio JA, Yumen OH, et al. : Plasmapheresis. A therapeutic option in the management of heparin-associated thrombocytopenia with thrombosis. Am J Clin Pathol 1991; 96: 394–7. [DOI] [PubMed] [Google Scholar]
- 13.Gruel Y, Pouplard C: Post-operative platelet count profile: the most reliable tool for identifying patients with true heparin-induced thrombocypenia after cardiac surgery. J Thromb Haemost 2010; 8: 27–9. [DOI] [PubMed] [Google Scholar]
- 14.Lillo-Le Louet A, Boutouyrie P, Alhenc-Gelas M, et al. : Diagnostic score for heparin-induced thrombocytopenia after cardiopulmonary bypass. J Thromb Haemost 2004; 2: 1882–8. [DOI] [PubMed] [Google Scholar]
- 15.Warkentin TE, Sheppard JI, Moore JC, et al. : Quantitative interpretation of optical density measurements using PF4-dependent enzyme-immunoassays. J Thromb Haemost 2008; 6: 1304–12. [DOI] [PubMed] [Google Scholar]
- 16.Demma LJ, Winkler AM, Levy JH: A diagnosis of heparin-induced thrombocytopenia with combined clinical and laboratory methods in cardiothoracic surgical intensive care unit patients. Anesth Analg 2011; 113: 697–702. [DOI] [PubMed] [Google Scholar]
- 17.Padmanabhan A, Jones CG, Curtis BR, et al. : A Novel PF4-Dependent Platelet Activation Assay Identifies Patients Likely to Have Heparin-Induced Thrombocytopenia/Thrombosis. Chest 2016; 150: 506–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lo GK, Sigouin CS, Warkentin TE: What is the potential for overdiagnosis of heparin-induced thrombocytopenia? Am J Hematol 2007; 82: 1037–43. [DOI] [PubMed] [Google Scholar]
- 19.Rice L: There Is No Such Thing as a “Positive” Antibody Test: Diagnosing Heparin-Induced Thrombocytopenia in 2015. Chest 2015; 148: 1–3. [DOI] [PubMed] [Google Scholar]
- 20.Chan CM, Woods CJ, Warkentin TE, et al. : The Role for Optical Density in Heparin-Induced Thrombocytopenia: A Cohort Study. Chest 2015; 148: 55–61. [DOI] [PubMed] [Google Scholar]
- 21.Engvall E, Perlmann P: Enzyme-linked immunosorbent assay, Elisa. 3. Quantitation of specific antibodies by enzyme-labeled anti-immunoglobulin in antigen-coated tubes. J Immunol 1972; 109: 129–35. [PubMed] [Google Scholar]
- 22.Warkentin TE, Sheppard JA, Chu FV, et al. : Plasma exchange to remove HIT antibodies: dissociation between enzyme-immunoassay and platelet activation test reactivities. Blood 2015; 125: 195–8. [DOI] [PubMed] [Google Scholar]
- 23.Gkalea V, Khaterchi A, Levy P, et al. : Prospective Evaluation of a Rapid Functional Assay for Heparin-Induced Thrombocytopenia Diagnosis in Critically Ill Patients. Crit Care Med 2019; 47: 353–9. [DOI] [PubMed] [Google Scholar]
- 24.Padmanabhan A, Jones CG, Pechauer SM, et al. : IVIg for Treatment of Severe Refractory Heparin-Induced Thrombocytopenia. Chest 2017; 152: 478–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Warkentin TE, Kelton JG: Temporal aspects of heparin-induced thrombocytopenia. N Engl J Med 2001; 344: 1286–92. [DOI] [PubMed] [Google Scholar]
- 26.Onwuemene OA, Zantek ND, Rollins-Raval MA, et al. : Therapeutic plasma exchange for management of heparin-induced thrombocytopenia: Results of an international practice survey. J Clin Apher 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Girgis AM, Golts E, Humber D, et al. : Successful Use of Cangrelor and Heparin for Cardiopulmonary Bypass in a Patient With Heparin-Induced Thrombocytopenia and End-Stage Renal Disease: A Case Report. A A Pract 2019; 13: 10–2. [DOI] [PubMed] [Google Scholar]


