Abstract
Liver fibrosis accompanies the progression of chronic liver diseases independent of etiologies, such as hepatitis viral infection, alcohol consumption, and metabolic-associated fatty liver disease. It is commonly associated with liver injury, inflammation, and cell death. Liver fibrosis is characterized by abnormal accumulation of extracellular matrix components that are expressed by liver myofibroblasts such as collagens and alpha-smooth actin proteins. Activated hepatic stellate cells contribute to the major population of myofibroblasts. Many treatments for liver fibrosis have been investigated in clinical trials, including dietary supplementation (e.g., vitamin C), biological treatment (e.g., simtuzumab), drug (e.g., pegbelfermin and natural herbs), genetic regulation (e.g., non-coding RNAs), and transplantation of stem cells (e.g., hematopoietic stem cells). However, none of these treatments has been approved by Food and Drug Administration. The treatment efficacy can be evaluated by histological staining methods, imaging methods, and serum biomarkers, as well as fibrosis scoring systems, such as fibrosis-4 index, aspartate aminotransferase to platelet ratio, and non-alcoholic fatty liver disease fibrosis score. Furthermore, the reverse of liver fibrosis is slowly and frequently impossible for advanced fibrosis or cirrhosis. To avoid the life-threatening stage of liver fibrosis, anti-fibrotic treatments, especially for combined behavior prevention, biological treatment, drugs or herb medicines, and dietary regulation are needed. This review summarizes the past studies and current and future treatments for liver fibrosis.
Keywords: Liver fibrosis, Molecular mechanism, Therapeutic targets, Treatments, Clinical trials
Core Tip: Liver fibrosis accompanies the progression of chronic liver diseases independent of their etiologies. The initiation and progression of liver fibrosis are mainly driven by liver inflammation, cell death, and metabolic dysregulation, which cause the activation of hepatic stellate cells and excessive accumulation of extracellular matrix proteins. Without effective treatments, liver fibrosis can lead to cirrhosis and primary liver cancer. To date, current therapeutic options for liver fibrosis are limited to prevent the initial causing factors for liver inflammation, hepatocyte cell death, and oxidative stress. However, the reverse of liver fibrosis is slowly and frequently impossible for advanced fibrosis or cirrhosis. To avoid the life-threatening stage of liver fibrosis, anti-fibrotic treatments including biological, medicines, dietary change, and behavior prevention are needed, especially for combined therapy.
INTRODUCTION
Liver fibrosis accompanies the progression of chronic liver diseases independent of etiologies[1], such as hepatitis viral infection, alcohol abuse, and metabolic-associated fatty liver disease (MAFLD). It is commonly associated with liver injury, inflammation, and cell death. Abnormal accumulation of extracellular matrix (ECM) components expressed by liver myofibroblasts, such as collagens and alpha-smooth actin proteins, are the markers of hepatic fibrogenesis[2]. Activated hepatic stellate cells (HSCs) contribute to the major population of myofibroblasts in liver fibrosis[3]. Although many drugs have been investigated in clinical trials, there are no Food and Drug Administration (FDA)-approved treatments for liver fibrosis.
The activation of HSC is a complex pathogenesis in liver fibrosis[4]. Many factors including intrahepatic and extrahepatic factors can drive HSC activation to induce liver fibrosis. A variety of molecular signaling pathways are involved in the regulation of HSC activation[1,5], such as transforming growth factor-β (TGF-β), Toll-like receptors (TLRs), and epigenetic signals (e.g., microRNAs, or miRNAs). The activation of HSCs can be divided into two phases, the initiation and perpetuation phases. RNA sequencing results have shown that fibrogenic transcriptional programs in the initiation phase are also active in the perpetuation phase; therefore, targeting the initial activation of HSC is also critically important for live fibrosis treatment[6].
In this review, the cellular and molecular mechanisms of liver fibrosis are reviewed. Importantly, the currently available treatments for liver fibrosis are summarized and discussed. Some pros and cons of available treatments are discussed. In addition, the future direction for liver fibrosis therapy is predicted.
INITIATION OF LIVER FIBROSIS: CELLULAR AND MOLECULAR MECHANISMS
Hepatic cell death
Liver cell death and inflammation are the initial events in chronic liver disease independent of etiologies. Many factors can cause liver injury and hepatic cell death and inflammation[7,8], including hepatitis viral infection, alcohol consumption, metabolic liver disease, abnormal bile acid products, and genetic factors. These pathogenic factors cause immune cell inflammation, hepatocyte death, mitochondrial dysfunction, and endoplasmic reticulum stress (Figure 1), resulting in HSC activation and differentiation to myofibroblasts to lead to liver fibrosis[5,9].
Figure 1.
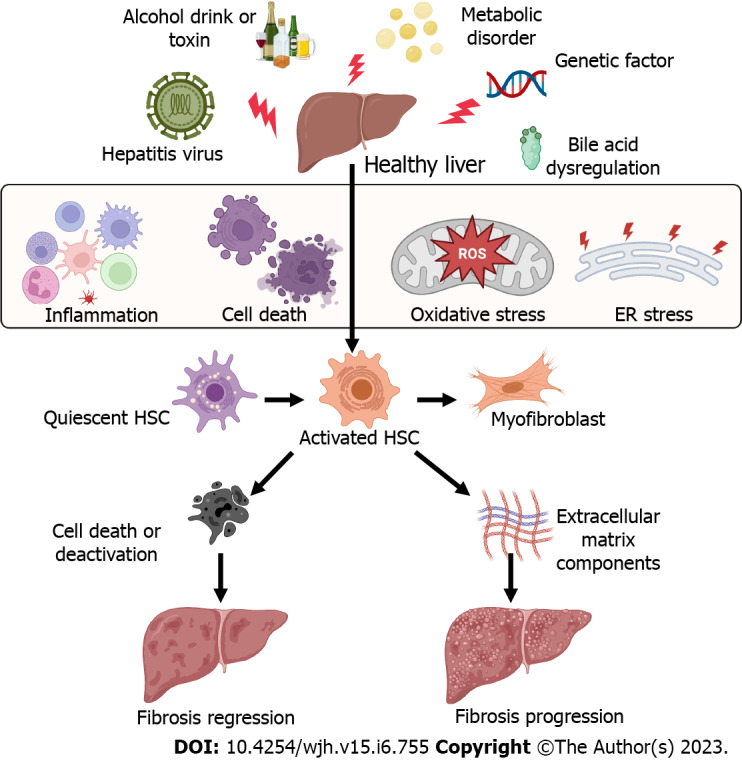
Factors causing the activation of hepatic stellate cells and liver fibrosis. Many factors can cause liver injury and hepatic cell death and inflammation, including hepatitis viral infection, alcohol consumption, metabolic liver disease, abnormal bile acid products, and genetic factors. These pathogenic factors cause immune cell inflammation, hepatocyte death, oxidative stress, and endoplasmic reticulum stress, resulting in hepatic stellate cell activation and differentiation to myofibroblasts to lead to liver fibrosis. All cartoons in this figure were prepared using Biorender (https://biorender.com). ER: Endoplasmic reticulum; HSC: Hepatic stellate cell; ROS: Reactive oxygen species.
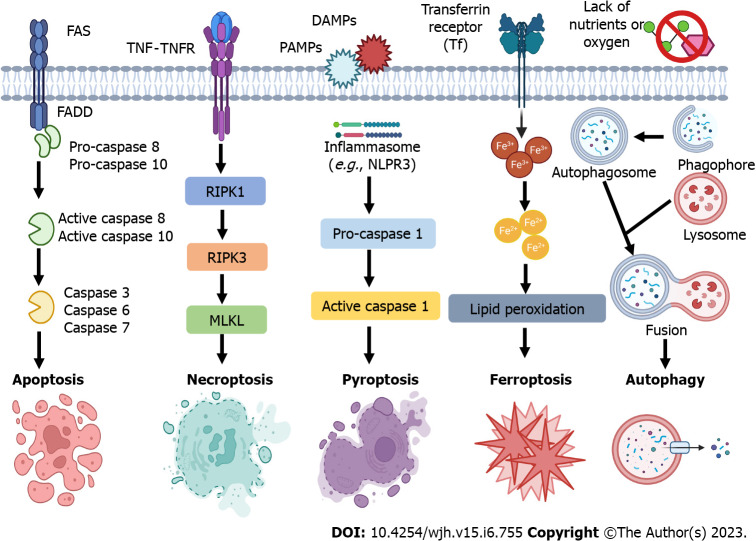
In non-alcoholic steatohepatitis (NASH), hepatocyte death results in the infiltration of monocyte-derived macrophages and upregulation of the expression of inflammatory cytokines[9], such as tumor necrosis factor α (TNF-α), TGF-β1, and interleukin-1β (IL-1β), Hepatocyte death can be classified into programed cell death including pyroptosis, apoptosis, necroptosis, ferroptosis, and autophagy-mediated cell death (Figure 2), as well as non-programed cell death (necrosis). In chronic liver disease, different types of cell death may be associated with the progression of liver fibrosis and end-stage of liver disease, such as hepatocellular carcinoma (HCC). Single-cell RNA sequencing coupled with spatial mapping approaches has been started to dissect the key cellular and molecular functions in liver disease[10].
Figure 2.
Programmed cell death subtypes of hepatic cells, including apoptosis, necroptosis, pyroptosis, ferroptosis, and autophagy-mediated cell death. Apoptosis, necroptosis, pyroptosis, and ferroptosis are programmed forms of cell death, while necrosis is unprogrammed cell death. Autophagy-mediated cell death should be defined when autophagic flux is raised without the involvement of other types of programmed cell death, and pharmacological or genetic inhibition of autophagy blocks cell death. DAMPs: Danger-associated molecular patterns; FADD: Fas-associated protein with a death domain; FAS: Fas cell surface death receptor; MLKL: Mixed lineage kinase domain-like; NLRP3: Nod-like receptor family, pyrin domain containing 3; PAMPs: Pathogen-associated molecular patterns; RIPK1/3: receptor-interacting protein kinase 1/3; TNF: Tumor necrosis factor; TNFR: Tumor necrosis factor receptor. All cartoons in this figure were prepared using Biorender (https://biorender.com).
Pyroptosis is an inflammatory cell death, associated with cell membrane rupture by cleaved gasdermin D[11]. The cleave of gasdermin D is induced by the activation of caspase-1 or caspase-11/4/5[12]. For example, in mice with non-alcoholic fatty liver disease (NAFLD), feeding a high-fat diet can increase the expression of caspase-11 to cause pyroptosis of bone marrow monocyte-derived macrophages by cleave gasdermin D[13]. The expression of pyroptosis-related indicators including gasdermin D, IL-1β, and IL-18 has been shown to be increased in human patients with liver fibrosis and mice with CCl4-induced fibrosis[14]. In addition, S100 calcium-binding protein A8 plays an essential role in macrophage pyroptosis in liver fibrosis, by inducing the expression of nucleotide-binding domain leucine-rich repeat-receptor, pyrin domain-containing-3 (NLRP3) inflammasome, pro-IL-1β, and pro-IL-18 via the activation of TLR4/nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) signaling pathway[14].
Apoptosis is a form of programmed cell death that occurs in all liver cell types. In hepatocytes, kindlin-2 deficiency can increase the apoptosis of hepatocytes, resulting in liver fibrosis and accumulation of ECM components by activating the TNF signaling pathway[15]. In NAFLD, microRNAs such as miR-22 from adipocyte-derived exosomes can cause hepatocyte apoptosis to increase hepatic inflammation, lipid accumulation, and fibrosis by regulating sirtuin 1 expression[16]. In contrast, promoting the apoptosis of HSCs can inhibit and reverse liver fibrosis. For example, treatment with gomisin D can inhibit CCl4-induced HSC proliferation and activation in mice and increase HSC apoptosis to reduce liver fibrosis by regulating the platelet-derived growth factor receptor β signaling pathway[17].
Necroptosis is a regulated cell death that has the features of apoptosis and necrosis. Necroptosis can be induced by TLRs, interferons, and death receptors[18], which is mediated by receptor-interacting protein kinase-3 and its substrate mixed lineage kinase-like[19]. In addition, receptor-interacting serine/threonine-protein kinase 1 has an important role in this process (Figure 2). It contributes to hepatocyte death in NASH. Necroptotic hepatocytes cannot be removed by liver macrophages due to the activation of "don't-eat-me" signaling pathway, the CD47/signal regulatory protein alpha axis[20].
Ferroptosis is an intracellular iron-dependent lytic cell death which is different from apoptosis and necrosis[21,22]. Excessive iron accumulation and the inhibition of glutathione peroxidase 4 trigger ferroptosis, which causes cell plasma membrane rupture[23]. Accumulating research studies have demonstrated that ferroptosis is involved in different liver diseases, including alcoholic liver disease, NASH, cirrhosis, and cancer[24,25]. Several molecular signaling pathways are involved in ferroptosis in liver diseases, such as the nuclear factor erythroid 2-related factor 2[26], heat shock protein family A member 8[27], and nuclear receptor coactivator 4[28]. Pharmacological regulation of HSC ferroptosis is a therapeutic strategy for liver fibrosis[29]. For example, curcumol can induce ferroptosis of activated HSCs to inhibit liver fibrosis by inducing autophagy[28].
Furthermore, some dying cells develop autophagosomes that trigger apoptosis and necroptosis, namely autophagy-mediated cell death (ACD). However, ACD should be defined when some criteria are met[30], including: (1) Cell death happens without the involvement of other types of programmed cell death; (2) autophagic flux is raised; and (3) pharmacological or genetic inhibition of autophagy blocks cell death. Overall, the molecules implicated in the process and signaling pathways of hepatic cell death are potential targets for liver fibrosis.
Hepatic innate and adaptive immunity
Liver resident immune cells and infiltration of myeloid cells or circulating immune cells in the liver during chronic liver injury play key roles in the activation of HSCs. For example, in NASH liver, gut microbiota-diet interplay-resulted metabolite can activate liver macrophages to produce profibrotic factors[31], such as TGF-β1. Treatments such as astaxanthin can suppress the infiltration of monocyte-derived macrophages to suppress HSC activation, liver oxidative stress response, and hepatocyte death by decreasing the expression of proinflammatory cytokines[32], such as TNF-α, TGF-β1, and IL-1β. Another study also during NAFLD progression, mast cells can increase Western diet-induced biliary and liver damage to the development of microvesicular steatosis through microRNA (miR-144-3p)-targeted signaling pathway[33].
Adaptive immune cells including T and B cells have various roles in liver fibrosis. The imbalance of liver regulatory T cells and T helper (Th) cells, such as Th1 cells and Th17 cells plays an essential role in liver fibrosis, cirrhosis, and cancer[34]. The populations of multicytokine-producing CD4 T cells were significantly increased in the livers of patients with NASH compared with patients with NAFLD[35]. The cytokines produced by these CD4 T cells include TNF-α, IFN-γ, IL-17A, and IL-10. The phenotype of T cells is also important for their functions. One study showed that the reduction of tissue-resident memory CD69+CD103−CD8 T cells significantly decreased the resolution of fibrosis in NASH liver. These CD8 T cells can induce FasL/Fas-mediated apoptosis of HSCs[36]. Therefore, targeting immune cells or their secreted inflammatory cytokines is an optional treatment for liver fibrosis[37].
Furthermore, HSC activation is also associated with vascular aging[38], ischemia/reperfusion injury[39], and angiogenesis[40].
SIGNALING PATHWAYS AND MOLECULAR TARGETS FOR LIVER FIBROSIS TREATMENTS
Bile acid receptors
Farnesoid-X-receptor (FXR) and the G protein-coupled bile acid receptors are two widely studied bile acids regulating receptors, which play important roles in lipid and glucose metabolism, inflammation, fibrosis, and immune responses. Many FXR ligands have been investigated in clinical trials for NASH and liver fibrosis treatments, such as EDP-305[41,42], cilofexor[43,44], and MET409[45,46]. Hepatic concentrations of conjugated 12α-hydroxylated bile acids, such as taurodeoxycholate and glycodeoxycholate, were significantly increased in patients with NASH and mouse liver fibrosis models[47]. These bile acids contribute to HSC activation and liver fibrosis by regulating the signaling of G protein-coupled bile acid receptor 1, also known as TGR5.
Caspases
Caspases are involved in liver cell inflammation and hepatocyte cell death. Meanwhile, they play an essential role in liver fibrogenesis. For example, caspase 3-deficient mice on a methionine- and choline-deficient diet had reduced liver collagen production compared to wild-type mice[48]. Lipotoxicity can induce caspase-mediated apoptosis of hepatic cells and liver inflammation and injury in NAFLD. Treatment of caspase inhibitors such as emricasan (IDN-6556, a pan-caspase inhibitor) can reduce liver injury in patients with NAFLD[49].
Chemokine receptors
Chemokine receptors are commonly expressed by immune cells or inflammatory cells, which can be recruited during liver inflammation and fibrosis. For example, C-C chemokine receptor 2 (CCR2) and CCR5 are highly expressed by monocytes and subtypes of liver macrophages, which can be targeted to ameliorate liver fibrosis[50]. A recent study showed that the roles of CCR2 and CCR5 in liver macrophages are different during liver disease progression in mice with a hepatocyte-specific knock-out of NF-κB essential modulator. CCR2 oversees the recruitment of monocytes during liver injury, whereas CCR5 is needed to promote HSC activation[51].
In addition, many other chemokines and their receptors are involved in the pathogenesis of liver fibrosis, including C-X-C motif ligand 12 (CXCL12) /C-X-C receptor 4[52], chemokine (C-X3-C motif) ligand 1/C-X3-C receptor 1 (CX3CR1)[53], CCL19/CCR7[54], and CXCL12/atypical chemokine receptor 3[55].
Fibroblast growth factors
Fibroblast growth factor 15 (FGF15) is an important endocrine regulator for hepatic bile acid and lipid metabolism, which regulates gut-liver crosstalk in mice[56]. A combined treatment using an inhibitor of apical sodium-bile acid transporter (GSK233072) and adeno-associated virus 8-mediated hepatic FGF15 overexpression significantly can improve the therapeutic efficacy against NASH and fibrosis compared to either single treatment[57]. Bile acid nuclear receptor FXR plays an important role in the regulation of the expression of FGF15/19, bile acid homeostasis, and lipid metabolism, which is the target for NASH and liver fibrosis[58]. The expression of hepatic FXR and plasma FGF19 (the ortholog of mouse FGF15) was decreased in children with NASH compared to their expression in healthy subjects[59].
Galectins
Galectins are carbohydrate-binding proteins and play important roles in liver inflammation, immune response, and fibrosis. Galectin-1 (Gal-1) was shown to be highly expressed in the stroma of HCC by cancer-associated fibroblasts. Silencing Gal-1 in these fibroblasts can suppress inflammation and tumor progression[60]. The serum level of Gal-3 was increased in patients with advanced cirrhosis, and liver expression of Gal-3 was also correlated with liver disease severity and inflammation[61].
Lysyl oxidase family members
Lysyl oxidase (LOX) family members are extracellular copper-dependent enzymes, including LOX, lysyl oxidase-like 1 to 4 (LOXL1 to 4) members, which play important roles in the cross-linking of ECM proteins in fibrosis and carcinogenesis. Inhibition of pan-LOX family, LOX, LOXL1, or LOXL2 has been shown to prevent fibrogenesis and accelerate the reversal of liver fibrosis, as well as fibrosis in other organs. However, the roles of LOX family members as therapeutic targets for liver fibrosis need further to be evaluated[62].
NLRP3 inflammasome
Nucleotide-binding oligomerization (NLR) family pyrin domain-containing 3 (NLRP3) plays a pivotal role in liver fibrosis. Activation of NLRP3 can lead to the inflammatory response through the secretion of IL-1β and IL-18 and activation of caspase-1[63], which is involved in liver cell pyroptosis[64]. Activation of NLRP3 inflammasome can induce hepatocyte pyroptosis and liver fibrosis, while inhibiting the activation of NLRP3 inflammasome can inhibit the development of NAFLD and NASH in animal models[65,66]. Activation of NLRP3 inflammasome in pyroptosis is mediated by canonical caspase-1-mediated signaling pathway and noncanonical caspase-11-mediated signaling pathway[67].
Peroxisome proliferator-activated receptors (PPARs)
PPARs, comprised of three subtypes PPARα, β/δ, and γ, play important roles in liver lipid metabolism, inflammation, and fibrosis[68-70]. The expression of liver PPARα was shown to be negatively correlated with NASH severity, visceral fat accumulation, and insulin resistance in human patients[71]. Treatment with PPARα/γ dual agonists decreased the concentrations of total cholesterol, triglyceride (TG), and inflammatory cytokine levels in serum, reduced hepatic steatosis, infiltration of inflammatory cells, and decreased the expression of lipogenic gene and NF-κB protein[72]. Another study showed that the levels of very low-density lipoprotein receptors (VLDLR) were increased in PPARβ/δ-deficient mice. In patients with hepatic steatosis, the mRNA levels of PPARβ/δ were suppressed and associated with an increase in VLDLR levels[73]. A pre-clinical study showed that treatment with pan-PPAR agonist lanifibranor can significantly decrease portal pressure and liver inflammation and induce fibrosis regression[74].
TGF-β/Smad
TGF-β/Smad is the most well-studied signaling pathway in fibrosis. SMAD proteins are essential intracellular effectors of TGF-β and show different roles in liver fibrosis[75], including pro-fibrotic functions (e.g., SMAD3 and SMAD4) and protective functions (e.g., SMAD2 and SMAD7). In addition, many studies have demonstrated that regulating the signaling pathway of TGF-β/Smad can prevent liver fibrosis[76], as well as the protein kinase B (PKB, or AKT)/Forkhead box O3 (FOXO3) signaling pathway.
Wnt/β-catenin
Proteins-derived from human amniotic mesenchymal stem cells, including insulin-like growth factor binding protein-3, Dickkopf-1, and DKK-3, can inhibit HSC activation by suppressing Wnt/β-catenin signaling pathway in vitro[77]. In vivo study also showed that treatment of niclosamide in rats can prevent CCl4-induced liver fibrosis by inhibiting the Wnt/β-catenin pathway and glutaminolysis[78]. Another study also showed that Wnt3a can upregulate the expression of protein regulator of cytokinesis 1 to active β-catenin signaling to promote liver fibrosis[79]. The interaction of β-catenin/transcription factor 4 (TCF4) has been shown to increase during liver fibrosis in mice with bile duct ligation (BDL)[80]. Treatment with ICG-001, an inhibitor of the interaction between cyclic adenosine monophosphate response element binding protein binding protein and β-catenin, together with LF3, a small molecule antagonist that inhibits β-catenin/TCF4 transcriptional activity, can reduce liver fibrosis[80].
Yes-associated protein (YAP)
YAP plays a pivotal role in the sensitivity of HSCs to ferroptosis, apoptosis, and senescence in fibrotic livers. Selective depletion of YAP in myofibroblastic HSCs or activated HSCs can promote their senescence or apoptosis to reduce liver injury and fibrosis[81]. Taurocholic acid can induce the activation of HSCs through the sphingosine-1-phosphate receptor 2/YAP/p38 mitogen-activated protein kinase (p38 MAPK)[82].
CURRENT DIAGNOSIS FOR LIVER FIBROSIS
The golden standard method for liver fibrosis diagnosis is liver biopsy. Histological or histochemical staining can be used to stain the cells or the extracellular matrix proteins to identify liver fibrosis. Common histological staining methods for liver fibrosis evaluation are hematoxylin-eosin staining with Masson's trichrome or Sirius Red staining[83]. Due to the pain and the risk of potential complications of liver biopsy, non-invasive techniques (e.g., elastography scanning) and biomarkers (e.g., aminotransferase to platelet ratio (APRI): The aminotransferase/platelet ratio index) can be applied for diagnosing liver fibrosis[84]. Many available scoring systems can be applied for liver fibrosis diagnosis and evaluation, including fibrosis-4 index (FIB-4), APRI, and NAFLD fibrosis score (NFS)[85].
Imaging methods are commonly applied in the clinic to evaluate the progression of liver fibrosis. For example, ultrasound elastography techniques can be applied to characterize liver fibrosis and its stage in adult patients, such as vibration-controlled transient elastography, the most utilized and validated elastography method[86]. A meta-analysis study also showed that magnetic resonance elastography (MRE) and point-shear wave elastography (pSWE) can be applied for liver fibrosis diagnostic, and MRE is a more accurate imaging technique than pSWE[87]. The pooled sensitivities and specificities for MRE and pSWE were 0.94 (95% confidence level/CI: 0.89-0.97) and 0.95 (95%CI: 0.89-0.98), and 0.86 (95%CI: 0.80-0.90) and 0.88 (95%CI: 0.85-0.91), respectively. Their pooled summary receiver operating characteristic curves showed that the area under the curve (AUC) for MRE was 0.98 (95%CI: 0.96-0.99), whereas the AUC for pSWE was 0.93 (95%CI: 0.90-0.95). Another review paper has updated the conventional and molecular imaging diagnostic methods for liver fibrosis[88]. In addition, artificial intelligence models have been applied for the diagnosis of liver fibrosis[89-91]. For example, the clinical features and imaging data collected from a patient can be analyzed for liver fibrosis diagnosis using a machine learning model.
Recently, studies also have shown that miRNAs, the single-stranded, non-coding RNAs containing 21 to 23 nucleotides, are involved in the pathogenesis of liver fibrosis, which are potential biomarkers for diagnosing liver fibrosis and therapeutic targets for liver fibrosis treatment[92]. The methods for liver fibrosis diagnosis have been reviewed in some recent publications[93-96]. Here, we will not discuss more details and will focus on the treatment options for liver fibrosis.
CURRENT TREATMENT OPTIONS FOR LIVER FIBROSIS
In this section, we review some different treatment options for liver fibrosis, such as biological intervention, anti-fibrotic drugs, and other treatment strategies. These treatments either target causing factors of liver fibrosis to accelerate the recovery of liver injury, or induce the balance of liver metabolism, such as anti-hepatitis viral infection, anti-cell death treatment, and regulators of lipid metabolism.
Biological intervention
Inhibition of LOXL2 in the fibrotic tumor microenvironment can synergistically increase the efficacy of sorafenib and 5-fluorouracil for liver cancer cells[97]. However, some treatments in clinical trials did not show promising results. For example, simtuzumab is a monoclonal antibody against LOXL2. In two phase 2b clinical trials, intravenous infusions of simtuzumab (200 or 700 mg) every other day for 48 wk and 96 wk did not show promising effects to decrease liver fibrosis and the progression of cirrhosis in patients with bridging fibrosis[98]. In a pilot clinical trial, intravenous treatment of simtuzumab (700 mg) every 2 wk for 22 wk did not improve liver biopsy fibrosis score for patients with advanced liver fibrosis[99].
Drug treatment
Aramchol, a partial inhibitor of hepatic stearoyl-CoA desaturase, has been shown to improve NASH and liver fibrosis in rodents and decrease liver triglycerides and fibrosis clinical trials[100].
Anti-hepatitis viral infection drugs: Inhibition of hepatitis viral infection can suppress liver inflammation and hepatocyte death to decrease liver injury, resulting in suppression of liver fibrosis. Drugs such as faldaprevir (also known as BI 201335)[101], ribavirin (HCV treatment)[102], and peginterferon alfa-2a (HBV treatment)[103,104], have been tested in clinical trials for the treatment of liver fibrosis. In addition, many other drugs have been evaluated or are under clinical trial evaluation against hepatitis viral infection[105-107], such as simeprevir, daclatasvir, and sofosbuvir.
Cenicriviroc: C-C chemokine receptors 2 and 5 dual antagonist, has been shown to improve liver fibrosis without worsening NASH compared to the placebo in phase 2 clinical trial (Clinicaltrials.gov, NCT02217475)[108]. A phase 3 clinical trial has been designed to confirm the efficacy and safety of cenicriviroc for liver fibrosis treatment in adults with NASH[109].
Cholangitis treatment: Obeticholic acid and ursodeoxycholic acid are the only two FDA-approved medicines for the treatment of primary biliary cholangitis[110], which have the potential to cholangitis-induced liver fibrosis.
Cyclophilin inhibitors: CRV431, a pan-cyclophilin inhibitor, can decrease liver fibrosis in mice treated with CCl4 for 6 wk and mice with diet-induced NASH[111]. Another cyclophilin inhibitor NV556 also displays an antifibrotic effect in two mouse NASH models, the STAM model (streptozotocin plus a high-fat diet) and methionine- and choline-deficient diet-induced NASH model[112]. In addition, NV556 can also inhibit TGF-β1-induced activation of HSCs in vitro.
FGF regulators or analogues: Treatment of pegbelfermin (BMS-986036, 10 mg or 20 mg daily), a PEGylated human FGF21 analogue, can significantly decrease liver fat accumulation in patients with NASH without treatment-related severe adverse effects, and it can also improve liver fibrosis in patients with obesity and type 2 diabetes[113].
FXR agonists: Treatment of obeticholic acid (INT-747), a potent and orally active FXR agonist, can significantly ameliorate liver fibrosis and the histological and biological markers of NASH in patients with NASH[114].
Gal-3 inhibitors: GB1211, an inhibitor of Gal-3, can inhibit the differentiation of epithelial cells into myofibroblasts and macrophage or myofibroblast-induced fibrosis in the liver[115]. GR-MD-02 (belapectin), a galectin-3 inhibitor, has been shown to inhibit liver fibrosis and portal hypertension in rat fibrosis mode, which is safe and well-tolerated in a phase 1 clinical trial. However, a phase 2b clinical trial showed that treatment of GR-MD-02 did not significantly improve liver fibrosis and reduce portal hypertension (hepatic venous pressure gradient) in patients with NASH[116]. Further studies are required to evaluate these treatments for liver fibrosis.
Glucagon-like peptide-1 (GLP-1) receptor agonist: GLP-1 analogues have been shown to have the effects to reduce liver fat accumulation, liver injury, and insulin resistance in mice with fatty liver disease. Clinical trial (ClinicalTrials.gov, NCT01237119) showed that treatment of GLP-1 analogue liraglutide was well tolerated and suppressed liver fibrosis progression in patients with NASH[117]. Another trial also showed that treatment of liraglutide markedly reduced liver fat content and body weight in patients with uncontrolled type 2 diabetes[118].
Pan-caspase inhibitor: Emricasan (IDN-6556), a pan-caspase inhibitor, can decrease liver cell apoptosis and inflammation and improve portal pressure in rats with CCl4-induced cirrhosis[119]. However, a clinical trial (Clinicaltrials.gov, NCT03205345) did not show the efficacy of emaricasan against liver fibrosis, but it was safe and well-tolerated.
PPAR agonists: In rats with BDL-induced liver fibrosis, treatment of PPAR-γ agonist thiazolidinedione inhibited HSC activation and liver fibrosis by regulating fibrogenic factors, such as TGF-β1, platelet-derived growth factor, and connective tissue growth factor[120]. Farglitazar (GI262570), an agonist of peroxisome proliferator-activated receptor-gamma (PPARγ), can inhibit HSC activation.
Tropifexor: A non-bile acid FXR agonist, can potently inhibit cholestatic liver injury and fibrosis by enhancing the expression of FGF19 in the ileum and the expression of small heterodimer partner in the livers of piglets but inhibit cholesterol 7α-hydroxylase. In addition, tropifexor can increase the abundance of bile acid-biotransforming bacteria and later the amino acid composition in the intestine and decrease intestinal barrier injury in piglets with BLD[121]. Clinical trial (Clinicaltrials.gov, NCT02855164) also showed that treatment of tropifexor (10-90 μg) once daily for 12 weeks was safe and decreased the levels of alanine aminotransferase (ALT) and hepatic fat fraction (HFF) compared to baseline in a dose-dependent manner. The decrease of ALT and HFF can be sustained for up to 48 wk at high doses of tropifexor (140 μg and 200 μg once daily)[122].
Natural products or herbal medicines
Natural products or herbal medicines display diverse roles in the treatment of liver fibrosis. For example, a classical Traditional Chinese Medicine formula Yinchenhao decoction has been shown to ameliorate dimethylnitrosamine-induced liver fibrosis in rats and suppress liver cell apoptosis[123]. Another study showed that Xiaoyaosan decoction significantly reduced CCl4-induced liver fibrosis in rats by regulating both TGF-β1/Smad and AKT/FOXO3 signaling pathways[76]. The major components of these herbal medicines such as Tanshinone IIA extracted from the traditional herbal medicine Salvia miltiorrhiza display broad biological activities, such as anti-inflammatory, antioxidant, antiangiogenic, and anticancer functions[124]. Furthermore, clinical trials also illustrate that these traditional medicine formula such as Fuzheng Huayu display therapeutic effects against hepatitis-B-caused cirrhosis in patients[125].
Dietary regulation or supplementation
Consumption of polyunsaturated fatty acids: The endogenous metabolites of n-3 polyunsaturated fatty acids such as 19,20-epoxy docosapentaenoic acid show a protective effect against liver fibrosis in mouse NASH models[126]. G protein-coupled receptors can be regulated by polyunsaturated fatty acids to reduce liver inflammation and fibrosis[127]. For example, supplementation of docosahexaenoic acid, an omega-3 fatty acid, can reduce liver inflammation and prevent liver fibrosis in diet-induced liver fibrosis model via G protein-coupled receptor 120 (GPR120) signaling, also known as free fatty acid receptor 4[128].
Probiotics: Treatment with probiotic Lactobacillus rhamnosus GG can significantly decrease liver inflammation and fibrosis by reducing the production of hepatic bile acids in mice with BLD[129].
Vitamins: The serum levels of vitamin C have been shown to be negatively associated with the odds of liver fibrosis in patients with NAFLD in United States adults[130]. Another study showed that a decreased serum level of vitamin B12 is associated with an increased risk of liver fibrosis in patients with NAFLD[131]. Treatment of Vitamin D3 can alleviate liver injury and the expression of ECM proteins such as TGF-β and α-SMA in thioacetamide-induced hepatic fibrosis rat model[132]. The data from the National Health and Nutrition Examination Survey (2017-2018) also showed that levels of 25-Hydroxyvitamin D were inversely associated with liver fibrosis during NAFLD development and progression[133].
Antioxidant and anti-inflammatory agents: Supplementation of natural products with antioxidant and anti-inflammatory components can also ameliorate chronic liver disease to improve liver fibrosis and inhibit cancer development[9], such as β-sitosterol and silymarin.
Bariatric surgery
Studies have shown that bariatric surgery (BS) can provide long-term benefits for the resolution of liver fibrosis. The two most common procedures of BS are laparoscopic Roux-en-Y-gastric and laparoscopic sleeve gastrectomy. For example, one study showed that NASH was resolved in 84% of patients (95%CI: 73.1%-92.2%) at year 5 post-BS treatment, while fibrosis was decreased in samples from 70.2% of patients (95%CI: 56.6%-81.6%) compared with baseline and fibrosis was disappeared in samples from 56% of all patients (95%CI: 42.4%-69.3%)[134]. BS has been shown to induce NASH disappearance in nearly 85% (95%CI: 75.8%-92.2%) of patients and to decrease fibrosis in 33.8% of patients (95%CI: 23.6%-45.2%) with NASH at 1 year after surgery[135]. Another clinical study showed that excessive weight loss shown in patients with cirrhosis with 73% (33%–167%), 85% (33%–190%), and 73% (29%–107%) after 1, 2, and 3 years of BS[136], respectively. Among 27 patients with cirrhosis, 3 patients had significant improvement in liver function and did not need liver transplantation, whereas 2 out of 27 patients had deleterious liver function post-BS treatment[136].
Genetic intervention
Gene therapy is a critical tool for disease treatment, including liver fibrosis and cancer. Noncoding RNAs, such as miRNAs and long noncoding RNAs, small interference RNAs, and circular RNAs are important. For example, the treatment of siRNA silencing CCR2 can regulate liver immune to inhibit the infiltration of profibrotic macrophages and neutrophils in murine fibrotic livers[137]. Another study showed that circRNA ASPH regulated liver fibrosis by binding miR-139-5p by regulating neurogenic locus notch homolog protein 1 (Notch 1) expression[138].
Transplantation of stem cells
Transplantation of umbilical cord Wharton's Jelly-derived mesenchymal stem cells to rats with CCl4-induced hepatic fibrosis improved liver function, inflammation, and fibrosis via a paracrine mechanism possibly by targeting TGF-β1 signaling pathway[139]. Another study showed that transplantation of human umbilical cord blood mesenchymal stem cells substantially improved liver fibrosis in histopathological evaluation compared to that in the untreated group[140]. Infusions of hematopoietic stem cells into mice with methionine-choline-deficient diet- or CCl4-induced liver fibrosis can reduce hepatic collagen production and the expression of α-smooth muscle actin[141,142].
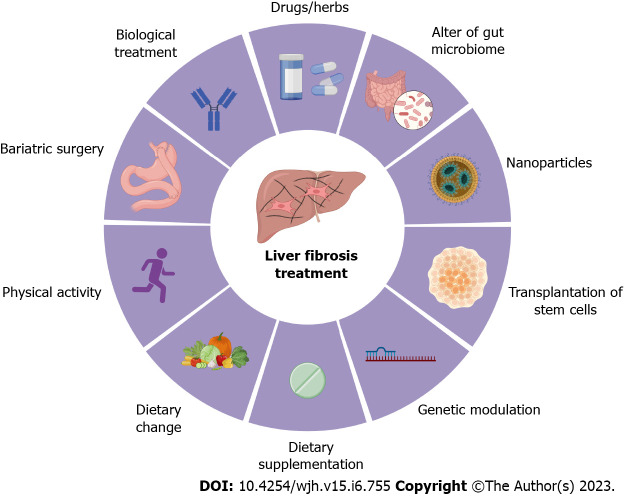
Overall, there are several potent preventive and therapeutic treatments for liver fibrosis, including physical activity (e.g., running), dietary change (e.g., avoid of high-fat and high-sugar diet), dietary supplementation (e.g., vitamin C), biological treatment (e.g., simtuzumab), bariatric surgery (e.g., Roux-en-Y-gastric procedure), drug (e.g., pegbelfermin), change of gut microbiota (e.g., probiotics), nanoparticles (e.g., BMS-986263), genetic regulation (e.g., non-coding RNAs), and transplantation of stem cells (e.g., hematopoietic stem cells) (Figure 3).
Figure 3.
Treatment options for liver fibrosis. Currently, the preventive and therapeutic treatments for liver fibrosis include physical activity (e.g., running), dietary change (e.g., avoid of high-fat and high-sugar diet), dietary supplementation (e.g., vitamin C), biological treatment (e.g., simtuzumab), bariatric surgery (e.g., Roux-en-Y-gastric procedure), drugs and herb medicines (e.g., pegbelfermin), change of gut microbiota (e.g., probiotics), nanoparticles (e.g., BMS-986263), genetic regulation (e.g., non-coding RNAs), and transplantation of stem cells (e.g., hematopoietic stem cells). All cartoons in this figure were prepared using Biorender (https://biorender.com).
CLINICAL TRIALS
In this section, we first review some completed clinical trials (Clinicaltrials.gov, Table 1). These treatments including biological treatment, drugs, dietary supplementation, and infusion of stem cells.
Table 1.
Completed clinical trials (Clinicaltrials.gov, accession date: March 1, 2023)
|
Method
|
Intervention
|
Trial number
|
Phase
|
Title
|
Condition
|
| Biological | Peginterferon alfa-2b (SCH 54031) | NCT00049842 | 3 | Prevention of Disease Progress in Chronic Hepatitis C Patients with Liver Fibrosis (Study P02570AM2) | Chronic HCV; Liver fibrosis |
| PegIntron (peginterferon alfa-2b; SCH 54031) REBETOL (ribavirin; SCH 18908) | NCT00039871 | 3 | PEG-Intron Plus Rebetol Treatment of Chronic Hepatitis C Subjects Who Failed Response to Alpha-Interferon Plus Ribavirin (Study P02370) | Hepatitis; HCV; Fibrosis; Liver cirrhosis | |
| Simtuzumab | NCT01707472 | 2 | Study of Simtuzumab in HIV and/or Hepatitis C- Infected Adults with Liver Fibrosis | Liver fibrosis; HCV infection; HIV | |
| Simtuzumab | NCT01672853 | 2 | Simtuzumab (GS-6624) in the Prevention of Progression of Liver Fibrosis in Adults with PSC | PSC | |
| Dietary regulation | GK#10; Placebo | NCT01598064 | N/A | Probiotics for Liver Cirrhosis with Portal Hypertension | Liver cirrhosis; Portal hypertension |
| Drug | Aramchol | NCT02279524 | 2 | A Clinical Trial to Evaluate the Efficacy and Safety of Two Aramchol Doses Versus Placebo in Patients With NASH | Fatty liver; NASH; Liver fibrosis |
| BI 201335 | NCT01909778 | 1 | Open Label Single Dose Phase I Trial of BI 201335 to Study Pharmacokinetics and Safety in Patients with Compensated Liver Cirrhosis | HCV; Liver cirrhosis | |
| BMS-986036; Placebo | NCT03486912 | 2 | A Study of Experimental Medication BMS-986036 in Adults with NASH and Liver Cirrhosis | Hepatic cirrhosis; Liver fibrosis; NAFLD; NASH | |
| BMS-98603; Other: Placebo | NCT03486899 | 2 | A Study of Experimental Medication BMS-986036 in Adults with NASH and Stage 3 Liver Fibrosis | Liver fibrosis; NAFLD; NASH | |
| BMS-986263 placebo | NCT03420768 | 2 | A Study of Experimental Medication BMS-986263 in Adults with Advanced Hepatic Fibrosis After Cure of Hepatitis C | Hepatic cirrhosis; Liver fibrosis | |
| Cenicriviroc placebo | NCT02217475 | 2 | Efficacy and Safety Study of Cenicriviroc for the Treatment of NASH in Adult Participants with Liver Fibrosis | NASH | |
| CRV431 placebo | NCT04480710 | 2 | A Study of CRV431 Dosed Once Daily in NASH-induced F2 and F3 Subjects | NASH; Fibrosis; NAFLD | |
| Fuzheng Huayu placebo | NCT00854087 | 2 | Assess the Antifibrotic Activity of Fuzheng Huayu in Chronic Hepatitis C Patients with Hepatic Fibrosis | Chronic HCV | |
| GI262570 placebo | NCT00244751 | 2 | Antifibrotic Activity of GI262570 In Chronic Hepatitis C Subjects | Cirrhosis, liver | |
| GR-MD-02 placebo | NCT02462967 | 2 | Clinical Trial to Evaluation the Safety and Efficacy of GR-MD-02 for the Treatment of Liver Fibrosis and Resultant Portal Hypertension in Patients with Nash Cirrhosis | Hypertension, portal | |
| GR-MD-02; Placebo | NCT02421094 | 2 | Clinical Trial to Evaluate Efficacy of GR-MD-02 for Treatment of Liver Fibrosis in Patients with NASH With Advanced Fibrosis | NASH | |
| IDN-6556; Placebo | NCT02230670 | 2 | A Study of IDN-6556 in Subjects with Liver Cirrhosis | Liver cirrhosis; Hepatic cirrhosis | |
| IDN-6556; Placebo | NCT02138253 | 2 | A Trial of IDN-6556 in Post Orthotopic Liver Transplant for Chronic HCV | Liver fibrosis; Liver cirrhosis | |
| INT-747; Ursodeoxycholic acid; Placebo | NCT00550862 | 2 | Study of INT 747 in Combination with URSO in Patients with PBC | Liver cirrhosis; Biliary injury | |
| Nitazoxanide; BID | NCT03656068 | 2 | An Evaluation of the Safety and Efficacy of Nitazoxanide on Collagen Turnover in NASH Patients with Fibrosis | NASH; Fatty liver; Fibrosis; Compensated cirrhosis | |
| Placebo obeticholic acid | NCT00570765 | 2 | Study of INT-747 as Monotherapy in Participants with PBC | Liver cirrhosis, biliary injury | |
| SEL; Simtuzumab | NCT02466516 | 2 | Safety, Tolerability, and Efficacy of GS-4997 Alone or in Combination with Simtuzumab in Adults with NASH and Fibrosis Stages F2-F3 | NASH | |
| Simeprevir; Daclatasvir; Sofosbuvir | NCT02349048 | 2 | Study to Assess Efficacy, Safety, Tolerability and Pharmacokinetics of Simeprevir, Daclatasvir and Sofosbuvir in Treatment-naive Participants with Chronic HCV Genotype 1 Infection | HCV | |
| Tropifexor (LJN452) CVC | NCT03517540 | 2 | Study of Safety, Tolerability, and Efficacy of a Combination Treatment of LJN452 and CVC in Adult Patients with NASH and Liver Fibrosis | NASH | |
| Peginterferon alfa-2a + Ribavirin; Peginterferon alfa-2a | NCT00006164 | 3 | Long Term Interferon for Patients Who Did Not Clear HCV with Standard Treatment | Chronic HCV; Cirrhosis; Fibrosis; Hepatic cirrhosis | |
| OMACOR placebo oral capsule | NCT00760513 | 4 | Treatment of non-Alcoholic Fatty Liver Disease With n-3 Fatty Acids | NAFLD | |
| Ceftriaxone normal saline | NCT04218695 | 4 | Prophylactic Antibiotics in Admitted Cirrhotics | Cirrhosis, LIVER | |
| Proton pump inhibitors placebo | NCT03175731 | 4 | PPIs and Gastroesophageal Varices in Liver Cirrhosis (PPIs: Proton pump inhibitors) | Liver cirrhosis; Hypertension, portal | |
| Other | Human fetal liver cell transplantation | NCT01013194 | 1 or 2 | Human Fetal Liver Cell Transplantation in Chronic Liver Failure | Liver cirrhosis |
| G-colony stimulating factor and infusion of the mobilized monocyte cells | NCT01503749 | 1 | Safety and Efficacy Study of Peripheral Blood Mononucleated Cells for Treatment of Liver Cirrhosis | Liver cirrhosis | |
| Leukapheresis; Infusion of stem cells via image-guided scan | NCT00147043 | N/A | Adult Stem Cell Therapy in Liver Insufficiency | Liver cirrhosis |
N/A: Not applicable; CVC: Cenicriviroc; HCV: Hepatitis C virus; HIV: Human immunodeficiency virus; NAFLD: non-alcoholic fatty liver disease; NASH: Nonalcoholic steatohepatitis; PBC: Primary biliary cirrhosis; PSC: Primary sclerosing cholangitis.
Future treatments
There is an unmet need for treatments for liver fibrosis due to the efficacy of available treatments. Some drugs with potent anti-fibrotic effects in pre-clinical models are now waiting to be further evaluated in clinical trials (Table 2). The promising preventive and therapeutic treatments for liver fibrosis, including treatment of hepatitis viral infection (e.g., Peginterferon Alfa 2a), transplantation of mesenchymal stem cells, bariatric surgery for patients with obesity and NAFLD, dietary modification (e.g., Mediterranean diet or Calorie-restricted diet).
Table 2.
Recruiting and active clinical trials (Clinicaltrials.gov, accession date: March 1, 2023)
|
Method
|
Interventions
|
NCT number
|
Phases
|
Title
|
Conditions
|
| Drug | ZED1227; Placebo | NCT05305599 | 2 | Different Doses of ZED1227 vs Placebo in NAFLD | NAFLD; Fibrosis |
| Tropifexor; Licogliflozin; Placebo | NCT04065841 | 2 | Efficacy, Safety, and Tolerability of the Combination of Tropifexor & Licogliflozin and Each Monotherapy, Compared with Placebo in Adult Patients with NASH and Liver Fibrosis | NASH; Liver fibrosis | |
| Tenofovir disoproxil Fumarate; PEG-Interferon alfa 2a | NCT03957629 | N/A | Optimized Treatment of Peginterferon Alfa 2a in Treatment Experienced Patients with HBV-Related Liver Fibrosis | Hepatitis B; Fibrosis | |
| Sildenafil | NCT04908657 | 4 | Sildenafil for Liver Fibrosis in Adolescents and Adults After Fontan Operation | Fibrosis | |
| Saroglitazar magnesium | NCT05011305 | 2 | Saroglitazar Magnesium for the Treatment of NASH with Fibrosis | NASH; Fibrosis | |
| Saroglitazar magnesium | NCT05045482 | 1 | Hepatic Impairment with Cirrhosis Due to Cholestatic Liver Disease | Hepatic impairment; Cirrhosis | |
| Rivaroxaban apixaban | NCT04874428 | 1 | Direct Oral Anticoagulants (Rivaroxaban and Apixaban) in Patients with Liver Cirrhosis | Cirrhosis | |
| Resmetirom; Placebo | NCT05500222 | 3 | A Phase 3 Study to Evaluate the Effect of Resmetirom on Clinical Outcomes in Patients with Well-compensated NASH Cirrhosis (MAESTRO-NASH-OUTCOMES) | NASH; Cirrhosis | |
| Rencofilstat; Placebo | NCT05402371 | 2 | A Study to Evaluate the Efficacy and Safety of Rencofilstat in Subjects with NASH and Advanced Liver Fibrosis | NASH; Fibrosis, Liver NAFLD | |
| Placebo; Esomeprazole | NCT04448028 | 4 | Stop of Proton-pump Inhibitor Treatment in Patients with Liver Cirrhosis - a Double-blind, Placebo-controlled Trial | Liver cirrhosis | |
| PHIN-214 | NCT05490888 | 1 | Single Dose Escalation of PHIN-214 in Child-Pugh A and B Liver Cirrhotics | Cirrhosis; Fibrosis; Hepatic ascites | |
| Placebo zibotentan + dapagliflozin | NCT05516498 | 2 | Zibotentan and Dapagliflozin Combination, EvAluated in Liver Cirrhosis (ZEAL Study) | Cirrhosis | |
| L-ornithine; L-aspartate | NCT05737030 | 4 | Effect of L-ornithine-L-aspertate (LOLA) on the Gut Microbiome | Cirrhosis | |
| Hydronidone capsules; The placebo capsules | NCT05115942 | 3 | Hydronidone for the Treatment of Liver Fibrosis Associated with Chronic Viral Hepatitis B Phase 3 Trial | Liver fibrosis | |
| Growth hormone | NCT05253287 | 2/3 | Growth Hormone in Decompensated Liver Cirrhosis | Cirrhosis; Fibrosis | |
| Empagliflozin 10 MG; Placebo pills | NCT05147090 | 4 | Effects of Empagliflozin on Fibrosis and Cirrhosis in Chronic Hepatitis B | NAFLD; Cirrhosis; Fibrosis | |
| Cotadutide; Placebo | NCT05364931 | 2/3 | A Study to Evaluate the Safety and Efficacy of Cotadutide Given by Subcutaneous Injection in Adult Participants with Non-cirrhotic Non-alcoholic Steatohepatitis with Fibrosis | Non-cirrhotic NASH with Fibrosis | |
| Candesartan; Ramipril | NCT03770936 | 3 | Effect of Some Drugs on Liver Fibrosis | Liver fibrosis | |
| Branched-chain amino acid; Placebo | NCT03633279 | 4 | Treatment of Sarcopenia Improves the Muscle Mass and Muscle Strength of Patients with Liver Cirrhosis-Child C | Liver cirrhosis | |
| BMS-986263; Placebo | NCT04267393 | 2 | Safety and Effectiveness of BMS-986263 in Adults with Compensated Cirrhosis (Liver Disease) From Nonalcoholic Steatohepatitis (NASH) | NASH | |
| AZD4831; Placebo | NCT05638737 | 2 | A Study in Participants with Non-cirrhotic NASH With Fibrosis | Non-cirrhotic NASH with fibrosis | |
| Atorvastatin; Placebo | NCT05028829 | 2 | Safety and Efficacy of Atorvastatin v. Placebo on HCC Risk | Liver fibrosis; Cirrhosis | |
| Dietary supplement | Leucine enriched essential amino acid; Balanced amino acid supplement (BAA) | NCT03208868 | N/A | Leucine-Enriched Essential Amino Acid Mixture to Reverse Muscle Loss in Cirrhosis | Cirrhosis |
| Hydroxy methyl butyrate; Balanced Amino Acids | NCT05166499 | N/A | HMB Enriched Amino Acids to Reverse Muscle Loss in Cirrhosis | Cirrhosis | |
| Biological | Umbilical cord-derived mesenchymal stem cell comprehensive treatment | NCT03945487 | 2 | Mesenchymal Stem Cells Treatment for Decompensated Liver Cirrhosis | Decompensated liver cirrhosis |
| Mesenchymal stem cell | NCT03254758 | 1/2 | A Study of ADR-001 in Patients with Liver Cirrhosis | Decompensated liver cirrhosis | |
| Human umbilical cord-derived mesenchymal stem cells | NCT05227846 | 1 | Human Umbilical Cord-derived Mesenchymal Stem Cells for Decompensated Cirrhosis (MSC-DLC-1) | Decompensated cirrhosis | |
| Human umbilical cord-derived mesenchymal stem cell infusion | NCT05331872 | 1 | Umbilical Cord-derived Mesenchymal Stem Cell Infusion in the Management of Adult Liver Cirrhosis | Liver cirrhosis | |
| Fecal microbiota transplantation; Placebo | NCT04932577 | 2/3 | Fecal Microbiota Transplantation for Liver Cirrhosis | Cirrhosis | |
| Cellgram-LC | NCT04689152 | 3 | Clinical Trial to Evaluate the Efficacy and Safety of Cellgram-LC Administration in Patients with Alcoholic Cirrhosis | Alcoholic cirrhosis | |
| Autologous BM MSC | NCT03626090 | 1/2 | Mesenchymal Stem Cell Therapy for Liver Cirrhosis | Cirrhosis | |
| Allogeneic umbilical cord mesenchymal stem cell | NCT04357600 | 1/2 | Umbilical Cord Mesenchymal Stem Cell for Liver Cirrhosis Patient Caused by Hepatitis B | Cirrhosis | |
| Other | Weight loss | NCT05104541 | N/A | Impact of Weight Loss in Cirrhosis with Obesity and MAFLD | Liver cirrhosis |
| Lifestyle therapy bariatric surgery | NCT03472157 | N/A | A Randomized Controlled Study Evaluating Bariatric Surgery as a Treatment for Severe NASH With Advanced Liver Fibrosis in Non-severe Obese Patients | Surgery; Obesity; NASH; Cirrhosis | |
| Indo mediterranean diet calorie restricted diet | NCT05073588 | N/A | Effect of Indo-Mediterranean Diet on Hepatic Steatosis and Fibrosis in NAFLD Children | NAFLD |
N/A: Not applicable; HCC: Hepatocellular carcinoma; NAFLD: Non-alcoholic fatty liver disease; NASH: Nonalcoholic steatohepatitis.
Furthermore, deliver system can be applied to increase the efficiency of anti-fibrotic treatments. For example, BMS-986263, a lipid nanoparticle, has been applied to deliver small interfering RNA to degrade mRNA of heat shock protein 47, a key collagen chaperone involved in the pathogenesis of fibrosis. Treatment of MS-986263 in patients with HCV infection and sustained virologic response improved the Ishak score, the histology activity index score for levels of liver fibrosis[143]. Many other types of nanoparticles have been applied to treat liver fibrosis or its causing chronic liver disease, such as Fibroblast growth factor 2 conjugated superparamagnetic iron oxide nanoparticles[144], cerium oxide nanoparticles[145], and silymarin-conjugated gold nanoparticles[146].
CONCLUSION
Liver fibrosis accompanies the progression of chronic liver diseases independent of their etiologies. The initiation and progression of liver fibrosis are mainly driven by liver inflammation and hepatocyte or cholangiocyte injury and damage, resulting in the activation of HSCs and their differentiation into ECM protein-producing myofibroblasts. Thus, current therapeutic options for liver fibrosis are to prevent the initial causing factors for liver inflammation, hepatocyte cell death and oxidative stress. Unfortunately, the reverse of liver fibrosis is slowly and frequently impossible for advanced fibrosis or cirrhosis. Liver transplantation is the only therapeutic option for the late stage of liver cirrhosis and cancer. To avoid the life-threatening stage of advanced liver fibrosis and cirrhosis, anti-fibrotic treatments including biological, medicines, dietary change, and behavior prevention are needed. Currently, promising treatments for liver fibrosis are still the preventive strategies, such as treatment of hepatitis viral infection (e.g., Peginterferon Alfa 2a), inhibition of the progression of MAFLD and obesity (e.g., bariatric surgery), dietary modification (e.g., Mediterranean diet or Calorie-restricted diet). In addition, nano-delivery systems have been applied to improve the treatment efficacy and specifically deliver the treatments. Pre-clinical and clinical evaluations for new treatments of liver fibrosis are required while we still lack currently effective strategies for liver fibrosis treatment. The treatment efficacy can be evaluated by histological staining methods, imaging methods, and serum biomarkers, as well as fibrosis scoring systems, such as FIB-4, APRI, and NFS. Although many anti-fibrotic candidate agents have shown robust effects in experimental animal models, their anti-fibrotic effects in clinical trials are less clear. The development of patient-derived organoid models for liver fibrosis may advance the development of compounds with anti-fibrotic properties in the future. In addition, new delivery systems can improve the efficacy of potent treatments and reduce the side effects of therapy. Meanwhile, additional clinical studies are required to confirm the efficacy and safety of treatments.
Footnotes
Conflict-of-interest statement: The authors declare no conflicts of interest.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Peer-review started: March 12, 2023
First decision: March 23, 2023
Article in press: April 18, 2023
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Boteon YL, Brazil; Su SB, China S-Editor: Ma YJ L-Editor: A P-Editor: Yuan YY
Contributor Information
Chun-Ye Zhang, Bond Life Sciences Center, University of Missouri, Columbia, MO 65211, United States.
Shuai Liu, Department of Radiology,The First Affiliated Hospital, Zhejiang University, Hangzhou 310006, Zhejiang Province, China.
Ming Yang, Department of Surgery, University of Missouri, Columbia, MO 65211, United States. yangmin@health.missouri.edu.
References
- 1.Higashi T, Friedman SL, Hoshida Y. Hepatic stellate cells as key target in liver fibrosis. Adv Drug Deliv Rev. 2017;121:27–42. doi: 10.1016/j.addr.2017.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kisseleva T, Brenner D. Molecular and cellular mechanisms of liver fibrosis and its regression. Nat Rev Gastroenterol Hepatol. 2021;18:151–166. doi: 10.1038/s41575-020-00372-7. [DOI] [PubMed] [Google Scholar]
- 3.Mederacke I, Hsu CC, Troeger JS, Huebener P, Mu X, Dapito DH, Pradere JP, Schwabe RF. Fate tracing reveals hepatic stellate cells as dominant contributors to liver fibrosis independent of its aetiology. Nat Commun. 2013;4:2823. doi: 10.1038/ncomms3823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tsuchida T, Friedman SL. Mechanisms of hepatic stellate cell activation. Nat Rev Gastroenterol Hepatol. 2017;14:397–411. doi: 10.1038/nrgastro.2017.38. [DOI] [PubMed] [Google Scholar]
- 5.Hou W, Syn WK. Role of Metabolism in Hepatic Stellate Cell Activation and Fibrogenesis. Front Cell Dev Biol. 2018;6:150. doi: 10.3389/fcell.2018.00150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.De Smet V, Eysackers N, Merens V, Kazemzadeh Dastjerd M, Halder G, Verhulst S, Mannaerts I, van Grunsven LA. Initiation of hepatic stellate cell activation extends into chronic liver disease. Cell Death Dis. 2021;12:1110. doi: 10.1038/s41419-021-04377-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Koyama Y, Brenner DA. Liver inflammation and fibrosis. J Clin Invest. 2017;127:55–64. doi: 10.1172/JCI88881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Del Campo JA, Gallego P, Grande L. Role of inflammatory response in liver diseases: Therapeutic strategies. World J Hepatol. 2018;10:1–7. doi: 10.4254/wjh.v10.i1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang CY, Liu S, Yang M. Antioxidant and anti-inflammatory agents in chronic liver diseases: Molecular mechanisms and therapy. World J Hepatol. 2023;15:180–200. doi: 10.4254/wjh.v15.i2.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ramachandran P, Matchett KP, Dobie R, Wilson-Kanamori JR, Henderson NC. Single-cell technologies in hepatology: new insights into liver biology and disease pathogenesis. Nat Rev Gastroenterol Hepatol. 2020;17:457–472. doi: 10.1038/s41575-020-0304-x. [DOI] [PubMed] [Google Scholar]
- 11.Yu P, Zhang X, Liu N, Tang L, Peng C, Chen X. Pyroptosis: mechanisms and diseases. Signal Transduct Target Ther. 2021;6:128. doi: 10.1038/s41392-021-00507-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kovacs SB, Miao EA. Gasdermins: Effectors of Pyroptosis. Trends Cell Biol. 2017;27:673–684. doi: 10.1016/j.tcb.2017.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Drummer C 4th, Saaoud F, Jhala NC, Cueto R, Sun Y, Xu K, Shao Y, Lu Y, Shen H, Yang L, Zhou Y, Yu J, Wu S, Snyder NW, Hu W, Zhuo J', Zhong Y, Jiang X, Wang H, Yang X. Caspase-11 promotes high-fat diet-induced NAFLD by increasing glycolysis, OXPHOS, and pyroptosis in macrophages. Front Immunol. 2023;14:1113883. doi: 10.3389/fimmu.2023.1113883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu Y, Kong X, You Y, Xiang L, Zhang Y, Wu R, Zhou L, Duan L. S100A8-Mediated NLRP3 Inflammasome-Dependent Pyroptosis in Macrophages Facilitates Liver Fibrosis Progression. Cells. 2022;11 doi: 10.3390/cells11223579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gao H, Zhong Y, Zhou L, Lin S, Hou X, Ding Z, Li Y, Yao Q, Cao H, Zou X, Chen D, Bai X, Xiao G. Kindlin-2 inhibits TNF/NF-κB-Caspase 8 pathway in hepatocytes to maintain liver development and function. Elife. 2023;12 doi: 10.7554/eLife.81792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen K, Lin T, Yao W, Chen X, Xiong X, Huang Z. Adipocytes-derived exosomal miR-122 promotes non-alcoholic fat liver disease progression via targeting Sirt1. Gastroenterol Hepatol. 2022 doi: 10.1016/j.gastrohep.2022.12.003. [DOI] [PubMed] [Google Scholar]
- 17.Wang R, Liu F, Chen P, Li S, Gu Y, Wang L, Chen C, Yuan Y. Gomisin D alleviates liver fibrosis through targeting PDGFRβ in hepatic stellate cells. Int J Biol Macromol. 2023;235:123639. doi: 10.1016/j.ijbiomac.2023.123639. [DOI] [PubMed] [Google Scholar]
- 18.Dhuriya YK, Sharma D. Necroptosis: a regulated inflammatory mode of cell death. J Neuroinflammation. 2018;15:199. doi: 10.1186/s12974-018-1235-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pasparakis M, Vandenabeele P. Necroptosis and its role in inflammation. Nature. 2015;517:311–320. doi: 10.1038/nature14191. [DOI] [PubMed] [Google Scholar]
- 20.Shi H, Wang X, Li F, Gerlach BD, Yurdagul A Jr, Moore MP, Zeldin S, Zhang H, Cai B, Zheng Z, Valenti L, Tabas I. CD47-SIRPα axis blockade in NASH promotes necroptotic hepatocyte clearance by liver macrophages and decreases hepatic fibrosis. Sci Transl Med. 2022;14:eabp8309. doi: 10.1126/scitranslmed.abp8309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu J, Kang R, Tang D. Signaling pathways and defense mechanisms of ferroptosis. FEBS J. 2022;289:7038–7050. doi: 10.1111/febs.16059. [DOI] [PubMed] [Google Scholar]
- 22.Zhang C, Liu X, Jin S, Chen Y, Guo R. Ferroptosis in cancer therapy: a novel approach to reversing drug resistance. Mol Cancer. 2022;21:47. doi: 10.1186/s12943-022-01530-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen X, Kang R, Kroemer G, Tang D. Ferroptosis in infection, inflammation, and immunity. J Exp Med. 2021;218 doi: 10.1084/jem.20210518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhu L, Luo S, Zhu Y, Tang S, Li C, Jin X, Wu F, Jiang H, Wu L, Xu Y. The Emerging Role of Ferroptosis in Various Chronic Liver Diseases: Opportunity or Challenge. J Inflamm Res. 2023;16:381–389. doi: 10.2147/JIR.S385977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Capelletti MM, Manceau H, Puy H, Peoc'h K. Ferroptosis in Liver Diseases: An Overview. Int J Mol Sci. 2020;21 doi: 10.3390/ijms21144908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Abu-Halaka D, Shpaizer A, Zeigerman H, Kanner J, Tirosh O. DMF-Activated Nrf2 Ameliorates Palmitic Acid Toxicity While Potentiates Ferroptosis Mediated Cell Death: Protective Role of the NO-Donor S-Nitroso-N-Acetylcysteine. Antioxidants (Basel) 2023;12 doi: 10.3390/antiox12020512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang Y, Zhao M, Zhao L, Geng Y, Li G, Chen L, Yu J, Yuan H, Zhang H, Yun H, Yuan Y, Wang G, Feng J, Xu L, Wang S, Hou C, Yang G, Zhang N, Lu W, Zhang X. HBx-Induced HSPA8 Stimulates HBV Replication and Suppresses Ferroptosis to Support Liver Cancer Progression. Cancer Res. 2023;83:1048–1061. doi: 10.1158/0008-5472.CAN-22-3169. [DOI] [PubMed] [Google Scholar]
- 28.Zheng Y, Zhao T, Wang J, Jiang R, Huang J, Li W. Curcumol alleviates liver fibrosis through inducing autophagy and ferroptosis in hepatic stellate cells. FASEB J. 2022;36:e22665. doi: 10.1096/fj.202200933RR. [DOI] [PubMed] [Google Scholar]
- 29.Li L, Zhu Z. Pharmacological modulation of ferroptosis as a therapeutic target for liver fibrosis. Front Pharmacol. 2022;13:1071844. doi: 10.3389/fphar.2022.1071844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jung S, Jeong H, Yu SW. Autophagy as a decisive process for cell death. Exp Mol Med. 2020;52:921–930. doi: 10.1038/s12276-020-0455-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang M, Qi X, Li N, Kaifi JT, Chen S, Wheeler AA, Kimchi ET, Ericsson AC, Scott Rector R, Staveley-O'Carroll KF, Li G. Western diet contributes to the pathogenesis of non-alcoholic steatohepatitis in male mice via remodeling gut microbiota and increasing production of 2-oleoylglycerol. Nat Commun. 2023;14:228. doi: 10.1038/s41467-023-35861-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang M, Kimchi ET, Staveley-O'Carroll KF, Li G. Astaxanthin Prevents Diet-Induced NASH Progression by Shaping Intrahepatic Immunity. Int J Mol Sci. 2021;22 doi: 10.3390/ijms222011037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kennedy L, Meadows V, Sybenga A, Demieville J, Chen L, Hargrove L, Ekser B, Dar W, Ceci L, Kundu D, Kyritsi K, Pham L, Zhou T, Glaser S, Meng F, Alpini G, Francis H. Mast Cells Promote Nonalcoholic Fatty Liver Disease Phenotypes and Microvesicular Steatosis in Mice Fed a Western Diet. Hepatology. 2021;74:164–182. doi: 10.1002/hep.31713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang CY, Liu S, Yang M. Regulatory T cells and their associated factors in hepatocellular carcinoma development and therapy. World J Gastroenterol. 2022;28:3346–3358. doi: 10.3748/wjg.v28.i27.3346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Woestemeier A, Scognamiglio P, Zhao Y, Wagner J, Muscate F, Casar C, Siracusa F, Cortesi F, Agalioti T, Müller S, Sagebiel A, Konczalla L, Wahib R, Karstens KF, Giannou AD, Duprée A, Wolter S, Wong MN, Mühlig AK, Bielecka AA, Bansal V, Zhang T, Mann O, Puelles VG, Huber TB, Lohse AW, Izbicki JR, Palm NW, Bonn S, Huber S, Gagliani N. Multicytokine-producing CD4+ T cells characterize the livers of patients with NASH. JCI Insight. 2023;8 doi: 10.1172/jci.insight.153831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Koda Y, Teratani T, Chu PS, Hagihara Y, Mikami Y, Harada Y, Tsujikawa H, Miyamoto K, Suzuki T, Taniki N, Sujino T, Sakamoto M, Kanai T, Nakamoto N. CD8(+) tissue-resident memory T cells promote liver fibrosis resolution by inducing apoptosis of hepatic stellate cells. Nat Commun. 2021;12:4474. doi: 10.1038/s41467-021-24734-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.He Y, Hwang S, Ahmed YA, Feng D, Li N, Ribeiro M, Lafdil F, Kisseleva T, Szabo G, Gao B. Immunopathobiology and therapeutic targets related to cytokines in liver diseases. Cell Mol Immunol. 2021;18:18–37. doi: 10.1038/s41423-020-00580-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wan Y, Li X, Slevin E, Harrison K, Li T, Zhang Y, Klaunig JE, Wu C, Shetty AK, Dong XC, Meng F. Endothelial dysfunction in pathological processes of chronic liver disease during aging. FASEB J. 2022;36:e22125. doi: 10.1096/fj.202101426R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cheng F, Li Y, Feng L, Li S. Hepatic stellate cell activation and hepatic fibrosis induced by ischemia/reperfusion injury. Transplant Proc. 2008;40:2167–2170. doi: 10.1016/j.transproceed.2008.06.052. [DOI] [PubMed] [Google Scholar]
- 40.Elpek GÖ. Angiogenesis and liver fibrosis. World J Hepatol. 2015;7:377–391. doi: 10.4254/wjh.v7.i3.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ratziu V, Rinella ME, Neuschwander-Tetri BA, Lawitz E, Denham D, Kayali Z, Sheikh A, Kowdley KV, Desta T, Elkhashab M, DeGrauw J, Goodwin B, Ahmad A, Adda N. EDP-305 in patients with NASH: A phase II double-blind placebo-controlled dose-ranging study. J Hepatol. 2022;76:506–517. doi: 10.1016/j.jhep.2021.10.018. [DOI] [PubMed] [Google Scholar]
- 42.Ahmad A, Adda N. Assessment of drug-drug interaction potential with EDP-305, a farnesoid X receptor agonist, in healthy subjects. Clin Transl Sci. 2022;15:2146–2158. doi: 10.1111/cts.13348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Patel K, Harrison SA, Elkhashab M, Trotter JF, Herring R, Rojter SE, Kayali Z, Wong VW, Greenbloom S, Jayakumar S, Shiffman ML, Freilich B, Lawitz EJ, Gane EJ, Harting E, Xu J, Billin AN, Chung C, Djedjos CS, Subramanian GM, Myers RP, Middleton MS, Rinella M, Noureddin M. Cilofexor, a Nonsteroidal FXR Agonist, in Patients With Noncirrhotic NASH: A Phase 2 Randomized Controlled Trial. Hepatology. 2020;72:58–71. doi: 10.1002/hep.31205. [DOI] [PubMed] [Google Scholar]
- 44.Trauner M, Bowlus CL, Gulamhusein A, Hameed B, Caldwell SH, Shiffman ML, Landis C, Muir AJ, Billin A, Xu J, Liu X, Lu X, Chung C, Myers RP, Kowdley KV. Safety and sustained efficacy of the farnesoid X receptor (FXR) agonist cilofexor over a 96-week open-label extension in patients with PSC. Clin Gastroenterol Hepatol. 2022 doi: 10.1016/j.cgh.2022.07.024. [DOI] [PubMed] [Google Scholar]
- 45.Harrison SA, Bashir MR, Lee KJ, Shim-Lopez J, Lee J, Wagner B, Smith ND, Chen HC, Lawitz EJ. A structurally optimized FXR agonist, MET409, reduced liver fat content over 12 weeks in patients with non-alcoholic steatohepatitis. J Hepatol. 2021;75:25–33. doi: 10.1016/j.jhep.2021.01.047. [DOI] [PubMed] [Google Scholar]
- 46.Jiang H, Chen HC, Lafata KJ, Bashir MR. Week 4 Liver Fat Reduction on MRI as an Early Predictor of Treatment Response in Participants with Nonalcoholic Steatohepatitis. Radiology. 2021;300:361–368. doi: 10.1148/radiol.2021204325. [DOI] [PubMed] [Google Scholar]
- 47.Xie G, Jiang R, Wang X, Liu P, Zhao A, Wu Y, Huang F, Liu Z, Rajani C, Zheng X, Qiu J, Zhang X, Zhao S, Bian H, Gao X, Sun B, Jia W. Conjugated secondary 12α-hydroxylated bile acids promote liver fibrogenesis. EBioMedicine. 2021;66:103290. doi: 10.1016/j.ebiom.2021.103290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Thapaliya S, Wree A, Povero D, Inzaugarat ME, Berk M, Dixon L, Papouchado BG, Feldstein AE. Caspase 3 inactivation protects against hepatic cell death and ameliorates fibrogenesis in a diet-induced NASH model. Dig Dis Sci. 2014;59:1197–1206. doi: 10.1007/s10620-014-3167-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shiffman M, Freilich B, Vuppalanchi R, Watt K, Chan JL, Spada A, Hagerty DT, Schiff E. Randomised clinical trial: emricasan versus placebo significantly decreases ALT and caspase 3/7 activation in subjects with non-alcoholic fatty liver disease. Aliment Pharmacol Ther. 2019;49:64–73. doi: 10.1111/apt.15030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Puengel T, Lefere S, Hundertmark J, Kohlhepp M, Penners C, Van de Velde F, Lapauw B, Hoorens A, Devisscher L, Geerts A, Boehm S, Zhao Q, Krupinski J, Charles ED, Zinker B, Tacke F. Combined Therapy with a CCR2/CCR5 Antagonist and FGF21 Analogue Synergizes in Ameliorating Steatohepatitis and Fibrosis. Int J Mol Sci. 2022;23 doi: 10.3390/ijms23126696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bartneck M, Koppe C, Fech V, Warzecha KT, Kohlhepp M, Huss S, Weiskirchen R, Trautwein C, Luedde T, Tacke F. Roles of CCR2 and CCR5 for Hepatic Macrophage Polarization in Mice With Liver Parenchymal Cell-Specific NEMO Deletion. Cell Mol Gastroenterol Hepatol. 2021;11:327–347. doi: 10.1016/j.jcmgh.2020.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wu X, Qian L, Zhao H, Lei W, Liu Y, Xu X, Li J, Yang Z, Wang D, Zhang Y, Tang R, Yang Y, Tian Y. CXCL12/CXCR4: An amazing challenge and opportunity in the fight against fibrosis. Ageing Res Rev. 2023;83:101809. doi: 10.1016/j.arr.2022.101809. [DOI] [PubMed] [Google Scholar]
- 53.Ni Y, Zhuge F, Ni L, Nagata N, Yamashita T, Mukaida N, Kaneko S, Ota T, Nagashimada M. CX3CL1/CX3CR1 interaction protects against lipotoxicity-induced nonalcoholic steatohepatitis by regulating macrophage migration and M1/M2 status. Metabolism. 2022;136:155272. doi: 10.1016/j.metabol.2022.155272. [DOI] [PubMed] [Google Scholar]
- 54.Liu XB, Liu H, Liu J, Cheung AKL, Zheng MZ, Cheng JL, Liu QS, Lo CM, Chen ZW, Man K. Cytomegalovirus Latency Exacerbated Small-for-size Liver Graft Injury Through Activation of CCL19/CCR7 in Hepatic Stellate Cells. Transplantation. 2022;106:519–530. doi: 10.1097/TP.0000000000003846. [DOI] [PubMed] [Google Scholar]
- 55.Van Loy T, De Jonghe S, Castermans K, Dheedene W, Stoop R, Verschuren L, Versele M, Chaltin P, Luttun A, Schols D. Stimulation of the atypical chemokine receptor 3 (ACKR3) by a small-molecule agonist attenuates fibrosis in a preclinical liver but not lung injury model. Cell Mol Life Sci. 2022;79:293. doi: 10.1007/s00018-022-04317-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bhat N, Esteghamat F, Chaube BK, Gunawardhana K, Mani M, Thames C, Jain D, Ginsberg HN, Fernandes-Hernando C, Mani A. TCF7L2 transcriptionally regulates Fgf15 to maintain bile acid and lipid homeostasis through gut-liver crosstalk. FASEB J. 2022;36:e22185. doi: 10.1096/fj.202101607R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Matye DJ, Wang H, Luo W, Sharp RR, Chen C, Gu L, Jones KL, Ding WX, Friedman JE, Li T. Combined ASBT Inhibitor and FGF15 Treatment Improves Therapeutic Efficacy in Experimental Nonalcoholic Steatohepatitis. Cell Mol Gastroenterol Hepatol. 2021;12:1001–1019. doi: 10.1016/j.jcmgh.2021.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schumacher JD, Guo GL. Pharmacologic Modulation of Bile Acid-FXR-FGF15/FGF19 Pathway for the Treatment of Nonalcoholic Steatohepatitis. Handb Exp Pharmacol. 2019;256:325–357. doi: 10.1007/164_2019_228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nobili V, Alisi A, Mosca A, Della Corte C, Veraldi S, De Vito R, De Stefanis C, D'Oria V, Jahnel J, Zohrer E, Scorletti E, Byrne CD. Hepatic farnesoid X receptor protein level and circulating fibroblast growth factor 19 concentration in children with NAFLD. Liver Int. 2018;38:342–349. doi: 10.1111/liv.13531. [DOI] [PubMed] [Google Scholar]
- 60.Tsai YT, Li CY, Huang YH, Chang TS, Lin CY, Chuang CH, Wang CY, Anuraga G, Chang TH, Shih TC, Lin ZY, Chen YL, Chung I, Lee KH, Chang CC, Sung SY, Yang KH, Tsui WL, Yap CV, Wu MH. Galectin-1 orchestrates an inflammatory tumor-stroma crosstalk in hepatoma by enhancing TNFR1 protein stability and signaling in carcinoma-associated fibroblasts. Oncogene. 2022;41:3011–3023. doi: 10.1038/s41388-022-02309-7. [DOI] [PubMed] [Google Scholar]
- 61.Cervantes-Alvarez E, Limon-de la Rosa N, Vilatoba M, Pérez-Monter C, Hurtado-Gomez S, Martinez-Cabrera C, Argemi J, Alatorre-Arenas E, Yarza-Regalado S, Tejeda-Dominguez F, Lizardo-Thiebaud MJ, Mendez-Guerrero O, Gamboa-Dominguez A, Aguilar-Salinas CA, Huang CA, Kershenobich D, Bataller R, Torre A, Navarro-Alvarez N. Galectin-3 is overexpressed in advanced cirrhosis and predicts post-liver transplant infectious complications. Liver Int. 2022;42:2260–2273. doi: 10.1111/liv.15326. [DOI] [PubMed] [Google Scholar]
- 62.Chen W, Yang A, Jia J, Popov YV, Schuppan D, You H. Lysyl Oxidase (LOX) Family Members: Rationale and Their Potential as Therapeutic Targets for Liver Fibrosis. Hepatology. 2020;72:729–741. doi: 10.1002/hep.31236. [DOI] [PubMed] [Google Scholar]
- 63.Jiang S, Zhang Y, Zheng JH, Li X, Yao YL, Wu YL, Song SZ, Sun P, Nan JX, Lian LH. Potentiation of hepatic stellate cell activation by extracellular ATP is dependent on P2X7R-mediated NLRP3 inflammasome activation. Pharmacol Res. 2017;117:82–93. doi: 10.1016/j.phrs.2016.11.040. [DOI] [PubMed] [Google Scholar]
- 64.Gan C, Cai Q, Tang C, Gao J. Inflammasomes and Pyroptosis of Liver Cells in Liver Fibrosis. Front Immunol. 2022;13:896473. doi: 10.3389/fimmu.2022.896473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wree A, Eguchi A, McGeough MD, Pena CA, Johnson CD, Canbay A, Hoffman HM, Feldstein AE. NLRP3 inflammasome activation results in hepatocyte pyroptosis, liver inflammation, and fibrosis in mice. Hepatology. 2014;59:898–910. doi: 10.1002/hep.26592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gaul S, Leszczynska A, Alegre F, Kaufmann B, Johnson CD, Adams LA, Wree A, Damm G, Seehofer D, Calvente CJ, Povero D, Kisseleva T, Eguchi A, McGeough MD, Hoffman HM, Pelegrin P, Laufs U, Feldstein AE. Hepatocyte pyroptosis and release of inflammasome particles induce stellate cell activation and liver fibrosis. J Hepatol. 2021;74:156–167. doi: 10.1016/j.jhep.2020.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhu Y, Zhao H, Lu J, Lin K, Ni J, Wu G, Tang H. Caspase-11-Mediated Hepatocytic Pyroptosis Promotes the Progression of Nonalcoholic Steatohepatitis. Cell Mol Gastroenterol Hepatol. 2021;12:653–664. doi: 10.1016/j.jcmgh.2021.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.de Souza Basso B, Haute GV, Ortega-Ribera M, Luft C, Antunes GL, Bastos MS, Carlessi LP, Levorse VG, Cassel E, Donadio MVF, Santarém ER, Gracia-Sancho J, Rodrigues de Oliveira J. Methoxyeugenol deactivates hepatic stellate cells and attenuates liver fibrosis and inflammation through a PPAR-ɣ and NF-kB mechanism. J Ethnopharmacol. 2021;280:114433. doi: 10.1016/j.jep.2021.114433. [DOI] [PubMed] [Google Scholar]
- 69.Pawlak M, Lefebvre P, Staels B. Molecular mechanism of PPARα action and its impact on lipid metabolism, inflammation and fibrosis in non-alcoholic fatty liver disease. J Hepatol. 2015;62:720–733. doi: 10.1016/j.jhep.2014.10.039. [DOI] [PubMed] [Google Scholar]
- 70.Chen J, Montagner A, Tan NS, Wahli W. Insights into the Role of PPARβ/δ in NAFLD. Int J Mol Sci. 2018;19 doi: 10.3390/ijms19071893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Francque S, Verrijken A, Caron S, Prawitt J, Paumelle R, Derudas B, Lefebvre P, Taskinen MR, Van Hul W, Mertens I, Hubens G, Van Marck E, Michielsen P, Van Gaal L, Staels B. PPARα gene expression correlates with severity and histological treatment response in patients with non-alcoholic steatohepatitis. J Hepatol. 2015;63:164–173. doi: 10.1016/j.jhep.2015.02.019. [DOI] [PubMed] [Google Scholar]
- 72.Zhang Y, Cui Y, Wang XL, Shang X, Qi ZG, Xue J, Zhao X, Deng M, Xie ML. PPARα/γ agonists and antagonists differently affect hepatic lipid metabolism, oxidative stress and inflammatory cytokine production in steatohepatitic rats. Cytokine. 2015;75:127–135. doi: 10.1016/j.cyto.2015.05.031. [DOI] [PubMed] [Google Scholar]
- 73.Zarei M, Barroso E, Palomer X, Dai J, Rada P, Quesada-López T, Escolà-Gil JC, Cedó L, Zali MR, Molaei M, Dabiri R, Vázquez S, Pujol E, Valverde ÁM, Villarroya F, Liu Y, Wahli W, Vázquez-Carrera M. Hepatic regulation of VLDL receptor by PPARβ/δ and FGF21 modulates non-alcoholic fatty liver disease. Mol Metab. 2018;8:117–131. doi: 10.1016/j.molmet.2017.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Boyer-Diaz Z, Aristu-Zabalza P, Andrés-Rozas M, Robert C, Ortega-Ribera M, Fernández-Iglesias A, Broqua P, Junien JL, Wettstein G, Bosch J, Gracia-Sancho J. Pan-PPAR agonist lanifibranor improves portal hypertension and hepatic fibrosis in experimental advanced chronic liver disease. J Hepatol. 2021;74:1188–1199. doi: 10.1016/j.jhep.2020.11.045. [DOI] [PubMed] [Google Scholar]
- 75.Xu F, Liu C, Zhou D, Zhang L. TGF-β/SMAD Pathway and Its Regulation in Hepatic Fibrosis. J Histochem Cytochem. 2016;64:157–167. doi: 10.1369/0022155415627681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhou Y, Wu R, Cai FF, Zhou WJ, Lu YY, Zhang H, Chen QL, Su SB. Xiaoyaosan decoction alleviated rat liver fibrosis via the TGFβ/Smad and Akt/FoxO3 signaling pathways based on network pharmacology analysis. J Ethnopharmacol. 2021;264:113021. doi: 10.1016/j.jep.2020.113021. [DOI] [PubMed] [Google Scholar]
- 77.Liu QW, Ying YM, Zhou JX, Zhang WJ, Liu ZX, Jia BB, Gu HC, Zhao CY, Guan XH, Deng KY, Xin HB. Human amniotic mesenchymal stem cells-derived IGFBP-3, DKK-3, and DKK-1 attenuate liver fibrosis through inhibiting hepatic stellate cell activation by blocking Wnt/β-catenin signaling pathway in mice. Stem Cell Res Ther. 2022;13:224. doi: 10.1186/s13287-022-02906-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.El-Ashmawy NE, Al-Ashmawy GM, Fakher HE, Khedr NF. The role of WNT/β-catenin signaling pathway and glutamine metabolism in the pathogenesis of CCl(4)-induced liver fibrosis: Repositioning of niclosamide and concerns about lithium. Cytokine. 2020;136:155250. doi: 10.1016/j.cyto.2020.155250. [DOI] [PubMed] [Google Scholar]
- 79.Rao S, Xiang J, Huang J, Zhang S, Zhang M, Sun H, Li J. PRC1 promotes GLI1-dependent osteopontin expression in association with the Wnt/β-catenin signaling pathway and aggravates liver fibrosis. Cell Biosci. 2019;9:100. doi: 10.1186/s13578-019-0363-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gao Y, Fan S, Zhao P, Li H, Cai C, Li X, Zhou Y, Huang M, Bi H. β-catenin/TCF4 inhibitors ICG-001 and LF3 alleviate BDL-induced liver fibrosis by suppressing LECT2 signaling. Chem Biol Interact. 2023;371:110350. doi: 10.1016/j.cbi.2023.110350. [DOI] [PubMed] [Google Scholar]
- 81.Du K, Maeso-Díaz R, Oh SH, Wang E, Chen T, Pan C, Xiang K, Dutta RK, Wang XF, Chi JT, Diehl AM. Targeting YAP-mediated hepatic stellate cell death susceptibility and senescence for treatment of liver fibrosis. Hepatology. 2023 doi: 10.1097/HEP.0000000000000326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yang J, Tang X, Liang Z, Chen M, Sun L. Taurocholic Acid Promotes Hepatic Stellate Cell Activation via S1PR2/p38 MAPK/YAP Signaling under Cholestatic Conditions. Clin Mol Hepatol. 2023 doi: 10.3350/cmh.2022.0327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lo RC, Kim H. Histopathological evaluation of liver fibrosis and cirrhosis regression. Clin Mol Hepatol. 2017;23:302–307. doi: 10.3350/cmh.2017.0078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lurie Y, Webb M, Cytter-Kuint R, Shteingart S, Lederkremer GZ. Non-invasive diagnosis of liver fibrosis and cirrhosis. World J Gastroenterol. 2015;21:11567–11583. doi: 10.3748/wjg.v21.i41.11567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kumar R, Teo EK, How CH, Wong TY, Ang TL. A practical clinical approach to liver fibrosis. Singapore Med J. 2018;59:628–633. doi: 10.11622/smedj.2018145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Taru MG, Neamti L, Taru V, Procopciuc LM, Procopet B, Lupsor-Platon M. How to Identify Advanced Fibrosis in Adult Patients with Non-Alcoholic Fatty Liver Disease (NAFLD) and Non-Alcoholic Steatohepatitis (NASH) Using Ultrasound Elastography-A Review of the Literature and Proposed Multistep Approach. Diagnostics (Basel) 2023;13 doi: 10.3390/diagnostics13040788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Schambeck JPL, Forte GC, Gonçalves LM, Stuker G, Kotlinski JBF, Tramontin G, Altmayer S, Watte G, Hochhegger B. Diagnostic accuracy of magnetic resonance elastography and point-shear wave elastography for significant hepatic fibrosis screening: Systematic review and meta-analysis. PLoS One. 2023;18:e0271572. doi: 10.1371/journal.pone.0271572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Li S, Sun X, Chen M, Ying Z, Wan Y, Pi L, Ren B, Cao Q. Liver Fibrosis Conventional and Molecular Imaging Diagnosis Update. J Liver. 2019;8 [PMC free article] [PubMed] [Google Scholar]
- 89.Decharatanachart P, Chaiteerakij R, Tiyarattanachai T, Treeprasertsuk S. Application of artificial intelligence in non-alcoholic fatty liver disease and liver fibrosis: a systematic review and meta-analysis. Therap Adv Gastroenterol. 2021;14:17562848211062807. doi: 10.1177/17562848211062807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ahmed Y, Hussein RS, Basha TA, Khalifa AM, Ibrahim AS, Abdelmoaty AS, Abdella HM, Fahmy AS. Detecting liver fibrosis using a machine learning-based approach to the quantification of the heart-induced deformation in tagged MR images. NMR Biomed. 2020;33:e4215. doi: 10.1002/nbm.4215. [DOI] [PubMed] [Google Scholar]
- 91.He L, Li H, Dudley JA, Maloney TC, Brady SL, Somasundaram E, Trout AT, Dillman JR. Machine Learning Prediction of Liver Stiffness Using Clinical and T2-Weighted MRI Radiomic Data. AJR Am J Roentgenol. 2019;213:592–601. doi: 10.2214/AJR.19.21082. [DOI] [PubMed] [Google Scholar]
- 92.Tadokoro T, Morishita A, Masaki T. Diagnosis and Therapeutic Management of Liver Fibrosis by MicroRNA. Int J Mol Sci. 2021;22 doi: 10.3390/ijms22158139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kaya A, Barutcu S, Gulsen MT. Evaluation of fibrosis with noninvasive biochemical tests in chronic viral hepatitis B. Hepatol Forum. 2023;4:25–29. doi: 10.14744/hf.2022.2022.0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wang J, Qin T, Sun J, Li S, Cao L, Lu X. Non-invasive methods to evaluate liver fibrosis in patients with non-alcoholic fatty liver disease. Front Physiol. 2022;13:1046497. doi: 10.3389/fphys.2022.1046497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Qi RB, Wu ZH. Association between COVID-19 and chronic liver disease: Mechanism, diagnosis, damage, and treatment. World J Virol. 2023;12:22–29. doi: 10.5501/wjv.v12.i1.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sang C, Yan H, Chan WK, Zhu X, Sun T, Chang X, Xia M, Sun X, Hu X, Gao X, Jia W, Bian H, Chen T, Xie G. Diagnosis of Fibrosis Using Blood Markers and Logistic Regression in Southeast Asian Patients With Non-alcoholic Fatty Liver Disease. Front Med (Lausanne) 2021;8:637652. doi: 10.3389/fmed.2021.637652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Gong L, Zhang Y, Yang Y, Yan Q, Ren J, Luo J, Tiu YC, Fang X, Liu B, Lam RHW, Lam KO, Lee AW, Guan XY. Inhibition of lysyl oxidase-like 2 overcomes adhesion-dependent drug resistance in the collagen-enriched liver cancer microenvironment. Hepatol Commun. 2022;6:3194–3211. doi: 10.1002/hep4.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Harrison SA, Abdelmalek MF, Caldwell S, Shiffman ML, Diehl AM, Ghalib R, Lawitz EJ, Rockey DC, Schall RA, Jia C, McColgan BJ, McHutchison JG, Subramanian GM, Myers RP, Younossi Z, Ratziu V, Muir AJ, Afdhal NH, Goodman Z, Bosch J, Sanyal AJ GS-US-321-0105 and GS-US-321-0106 Investigators. Simtuzumab Is Ineffective for Patients With Bridging Fibrosis or Compensated Cirrhosis Caused by Nonalcoholic Steatohepatitis. Gastroenterology. 2018;155:1140–1153. doi: 10.1053/j.gastro.2018.07.006. [DOI] [PubMed] [Google Scholar]
- 99.Meissner EG, McLaughlin M, Matthews L, Gharib AM, Wood BJ, Levy E, Sinkus R, Virtaneva K, Sturdevant D, Martens C, Porcella SF, Goodman ZD, Kanwar B, Myers RP, Subramanian M, Hadigan C, Masur H, Kleiner DE, Heller T, Kottilil S, Kovacs JA, Morse CG. Simtuzumab treatment of advanced liver fibrosis in HIV and HCV-infected adults: results of a 6-month open-label safety trial. Liver Int. 2016;36:1783–1792. doi: 10.1111/liv.13177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ratziu V, de Guevara L, Safadi R, Poordad F, Fuster F, Flores-Figueroa J, Arrese M, Fracanzani AL, Ben Bashat D, Lackner K, Gorfine T, Kadosh S, Oren R, Halperin M, Hayardeny L, Loomba R, Friedman S ARREST investigator study group, Sanyal AJ. Aramchol in patients with nonalcoholic steatohepatitis: a randomized, double-blind, placebo-controlled phase 2b trial. Nat Med. 2021;27:1825–1835. doi: 10.1038/s41591-021-01495-3. [DOI] [PubMed] [Google Scholar]
- 101.Wu J, Gießmann T, Lang B, Elgadi M, Huang F. Investigation of the effect of food and omeprazole on the relative bioavailability of a single oral dose of 240 mg faldaprevir, a selective inhibitor of HCV NS3/4 protease, in an open-label, randomized, three-way cross-over trial in healthy participants. J Pharm Pharmacol. 2016;68:459–466. doi: 10.1111/jphp.12538. [DOI] [PubMed] [Google Scholar]
- 102.Ahmed S, Ullah N, Parveen S, Javed I, Jalil NAC, Murtey MD, Sheikh IS, Khan S, Ojha SC, Chen K. Effect of Silymarin as an Adjunct Therapy in Combination with Sofosbuvir and Ribavirin in Hepatitis C Patients: A Miniature Clinical Trial. Oxid Med Cell Longev. 2022;2022:9199190. doi: 10.1155/2022/9199190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Chen S, Zhou J, Wu X, Meng T, Wang B, Liu H, Wang T, Zhao X, Kong Y, Wu S, Ou X, Jia J, Sun Y, You H. Comparison of fibrosis regression of entecavir alone or combined with pegylated interferon alpha2a in patients with chronic hepatitis B. Hepatol Int. 2021;15:611–620. doi: 10.1007/s12072-021-10162-1. [DOI] [PubMed] [Google Scholar]
- 104.Mieli-Vergani G, Bansal S, Daniel JF, Kansu A, Kelly D, Martin C, Tizzard S, Wirth S, Zhou J, Vergani D. Peginterferon Alfa-2a (40KD) Plus Lamivudine or Entecavir in Children With Immune-Tolerant Chronic Hepatitis B. J Pediatr Gastroenterol Nutr. 2021;73:156–160. doi: 10.1097/MPG.0000000000003118. [DOI] [PubMed] [Google Scholar]
- 105.Said EM, Abdulaziz BA, El Kassas M, El Attar IH, Emadeldeen M, Abd-Elsalam SM. High success rates for the use of sofosbuvir/ombitasvir/paritaprevir/ritonavir + ribavirin and sofosbuvir/simeprevir/daclatasvir + ribavirin in retreatment of chronic hepatitis C infection after unsuccessful sofosbuvir/daclatasvir therapy: a real-life experience. Arch Virol. 2020;165:1633–1639. doi: 10.1007/s00705-020-04639-x. [DOI] [PubMed] [Google Scholar]
- 106.Gupta N, Manirambona L, Shumbusho F, Kabihizi J, Murangwa A, Serumondo J, Makuza JD, Nsanzimana S, Muvunyi CM, Mukabatsinda C, Musabeyezu E, Camus G, Grant PM, Kateera F. Safety and efficacy of sofosbuvir-velpatasvir-voxilaprevir for re-treatment of chronic hepatitis C virus infection in patients with previous direct-acting antiviral treatment failure in Rwanda (SHARED-3): a single-arm trial. Lancet Gastroenterol Hepatol. 2022;7:542–551. doi: 10.1016/S2468-1253(21)00399-X. [DOI] [PubMed] [Google Scholar]
- 107.Ueno T, Osawa M, Shiozaki T, Green M, Garimella T. Exposure-Response Analysis for Efficacy of Daclatasvir, Asunaprevir, and Beclabuvir Combinations in HCV-Infected Patients. Clin Pharmacol Drug Dev. 2019;8:903–913. doi: 10.1002/cpdd.646. [DOI] [PubMed] [Google Scholar]
- 108.Ratziu V, Sanyal A, Harrison SA, Wong VW, Francque S, Goodman Z, Aithal GP, Kowdley KV, Seyedkazemi S, Fischer L, Loomba R, Abdelmalek MF, Tacke F. Cenicriviroc Treatment for Adults With Nonalcoholic Steatohepatitis and Fibrosis: Final Analysis of the Phase 2b CENTAUR Study. Hepatology. 2020;72:892–905. doi: 10.1002/hep.31108. [DOI] [PubMed] [Google Scholar]
- 109.Anstee QM, Neuschwander-Tetri BA, Wong VW, Abdelmalek MF, Younossi ZM, Yuan J, Pecoraro ML, Seyedkazemi S, Fischer L, Bedossa P, Goodman Z, Alkhouri N, Tacke F, Sanyal A. Cenicriviroc for the treatment of liver fibrosis in adults with nonalcoholic steatohepatitis: AURORA Phase 3 study design. Contemp Clin Trials. 2020;89:105922. doi: 10.1016/j.cct.2019.105922. [DOI] [PubMed] [Google Scholar]
- 110.Barba Bernal R, Ferrigno B, Medina Morales E, Castro CM, Goyes D, Trivedi H, Patwardhan VR, Bonder A. Management of Primary Biliary Cholangitis: Current Treatment and Future Perspectives. Turk J Gastroenterol. 2023;34:89–100. doi: 10.5152/tjg.2023.22239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kuo J, Bobardt M, Chatterji U, Mayo PR, Trepanier DJ, Foster RT, Gallay P, Ure DR. A Pan-Cyclophilin Inhibitor, CRV431, Decreases Fibrosis and Tumor Development in Chronic Liver Disease Models. J Pharmacol Exp Ther. 2019;371:231–241. doi: 10.1124/jpet.119.261099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Simón Serrano S, Grönberg A, Longato L, Rombouts K, Kuo J, Gregory M, Moss S, Elmér E, Mazza G, Gallay P, Pinzani M, Hansson MJ, Massoumi R. Evaluation of NV556, a Novel Cyclophilin Inhibitor, as a Potential Antifibrotic Compound for Liver Fibrosis. Cells. 2019;8 doi: 10.3390/cells8111409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Sanyal A, Charles ED, Neuschwander-Tetri BA, Loomba R, Harrison SA, Abdelmalek MF, Lawitz EJ, Halegoua-DeMarzio D, Kundu S, Noviello S, Luo Y, Christian R. Pegbelfermin (BMS-986036), a PEGylated fibroblast growth factor 21 analogue, in patients with non-alcoholic steatohepatitis: a randomised, double-blind, placebo-controlled, phase 2a trial. Lancet. 2019;392:2705–2717. doi: 10.1016/S0140-6736(18)31785-9. [DOI] [PubMed] [Google Scholar]
- 114.Younossi ZM, Ratziu V, Loomba R, Rinella M, Anstee QM, Goodman Z, Bedossa P, Geier A, Beckebaum S, Newsome PN, Sheridan D, Sheikh MY, Trotter J, Knapple W, Lawitz E, Abdelmalek MF, Kowdley KV, Montano-Loza AJ, Boursier J, Mathurin P, Bugianesi E, Mazzella G, Olveira A, Cortez-Pinto H, Graupera I, Orr D, Gluud LL, Dufour JF, Shapiro D, Campagna J, Zaru L, MacConell L, Shringarpure R, Harrison S, Sanyal AJ REGENERATE Study Investigators. Obeticholic acid for the treatment of non-alcoholic steatohepatitis: interim analysis from a multicentre, randomised, placebo-controlled phase 3 trial. Lancet. 2019;394:2184–2196. doi: 10.1016/S0140-6736(19)33041-7. [DOI] [PubMed] [Google Scholar]
- 115.Zetterberg FR, MacKinnon A, Brimert T, Gravelle L, Johnsson RE, Kahl-Knutson B, Leffler H, Nilsson UJ, Pedersen A, Peterson K, Roper JA, Schambye H, Slack RJ, Tantawi S. Discovery and Optimization of the First Highly Effective and Orally Available Galectin-3 Inhibitors for Treatment of Fibrotic Disease. J Med Chem. 2022;65:12626–12638. doi: 10.1021/acs.jmedchem.2c00660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Chalasani N, Abdelmalek MF, Garcia-Tsao G, Vuppalanchi R, Alkhouri N, Rinella M, Noureddin M, Pyko M, Shiffman M, Sanyal A, Allgood A, Shlevin H, Horton R, Zomer E, Irish W, Goodman Z, Harrison SA, Traber PG Belapectin (GR-MD-02) Study Investigators. Effects of Belapectin, an Inhibitor of Galectin-3, in Patients With Nonalcoholic Steatohepatitis With Cirrhosis and Portal Hypertension. Gastroenterology. 2020;158:1334–1345.e5. doi: 10.1053/j.gastro.2019.11.296. [DOI] [PubMed] [Google Scholar]
- 117.Armstrong MJ, Gaunt P, Aithal GP, Barton D, Hull D, Parker R, Hazlehurst JM, Guo K LEAN trial team, Abouda G, Aldersley MA, Stocken D, Gough SC, Tomlinson JW, Brown RM, Hübscher SG, Newsome PN. Liraglutide safety and efficacy in patients with non-alcoholic steatohepatitis (LEAN): a multicentre, double-blind, randomised, placebo-controlled phase 2 study. Lancet. 2016;387:679–690. doi: 10.1016/S0140-6736(15)00803-X. [DOI] [PubMed] [Google Scholar]
- 118.Petit JM, Cercueil JP, Loffroy R, Denimal D, Bouillet B, Fourmont C, Chevallier O, Duvillard L, Vergès B. Effect of Liraglutide Therapy on Liver Fat Content in Patients With Inadequately Controlled Type 2 Diabetes: The Lira-NAFLD Study. J Clin Endocrinol Metab. 2017;102:407–415. doi: 10.1210/jc.2016-2775. [DOI] [PubMed] [Google Scholar]
- 119.Frenette C, Kayali Z, Mena E, Mantry PS, Lucas KJ, Neff G, Rodriguez M, Thuluvath PJ, Weinberg E, Bhandari BR, Robinson J, Wedick N, Chan JL, Hagerty DT, Kowdley KV IDN-6556-17 Study Investigators. Emricasan to prevent new decompensation in patients with NASH-related decompensated cirrhosis. J Hepatol. 2021;74:274–282. doi: 10.1016/j.jhep.2020.09.029. [DOI] [PubMed] [Google Scholar]
- 120.Alatas FS, Matsuura T, Pudjiadi AH, Wijaya S, Taguchi T. Peroxisome Proliferator-Activated Receptor Gamma Agonist Attenuates Liver Fibrosis by Several Fibrogenic Pathways in an Animal Model of Cholestatic Fibrosis. Pediatr Gastroenterol Hepatol Nutr. 2020;23:346–355. doi: 10.5223/pghn.2020.23.4.346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Xiao Y, Wang Y, Liu Y, Wang W, Tian X, Chen S, Lu Y, Du J, Cai W. A nonbile acid farnesoid X receptor agonist tropifexor potently inhibits cholestatic liver injury and fibrosis by modulating the gut-liver axis. Liver Int. 2021;41:2117–2131. doi: 10.1111/liv.14906. [DOI] [PubMed] [Google Scholar]
- 122.Sanyal AJ, Lopez P, Lawitz EJ, Lucas KJ, Loeffler J, Kim W, Goh GBB, Huang JF, Serra C, Andreone P, Chen YC, Hsia SH, Ratziu V, Aizenberg D, Tobita H, Sheikh AM, Vierling JM, Kim YJ, Hyogo H, Tai D, Goodman Z, Schaefer F, Carbarns IRI, Lamle S, Martic M, Naoumov NV, Brass CA. Tropifexor for nonalcoholic steatohepatitis: an adaptive, randomized, placebo-controlled phase 2a/b trial. Nat Med. 2023;29:392–400. doi: 10.1038/s41591-022-02200-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Cai FF, Bian YQ, Wu R, Sun Y, Chen XL, Yang MD, Zhang QR, Hu Y, Sun MY, Su SB. Yinchenhao decoction suppresses rat liver fibrosis involved in an apoptosis regulation mechanism based on network pharmacology and transcriptomic analysis. Biomed Pharmacother. 2019;114:108863. doi: 10.1016/j.biopha.2019.108863. [DOI] [PubMed] [Google Scholar]
- 124.Shi MJ, Dong BS, Yang WN, Su SB, Zhang H. Preventive and therapeutic role of Tanshinone ⅡA in hepatology. Biomed Pharmacother. 2019;112:108676. doi: 10.1016/j.biopha.2019.108676. [DOI] [PubMed] [Google Scholar]
- 125.Song YN, Sun JJ, Lu YY, Xu LM, Gao YQ, Zhang W, Wang XS, Xue DY, Zheng QS, Su SB. Therapeutic efficacy of fuzheng-huayu tablet based traditional chinese medicine syndrome differentiation on hepatitis-B-caused cirrhosis: a multicenter double-blind randomized controlled trail. Evid Based Complement Alternat Med. 2013;2013:709305. doi: 10.1155/2013/709305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Aoki H, Isobe Y, Yoshida M, Kang JX, Maekawa M, Arita M. Enzymatically-epoxidized docosahexaenoic acid, 19,20-EpDPE, suppresses hepatic crown-like structure formation and nonalcoholic steatohepatitis fibrosis through GPR120. Biochim Biophys Acta Mol Cell Biol Lipids. 2023;1868:159275. doi: 10.1016/j.bbalip.2022.159275. [DOI] [PubMed] [Google Scholar]
- 127.Yang M, Zhang CY. G protein-coupled receptors as potential targets for nonalcoholic fatty liver disease treatment. World J Gastroenterol. 2021;27:677–691. doi: 10.3748/wjg.v27.i8.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Nakamoto K, Shimada K, Harada S, Morimoto Y, Hirasawa A, Tokuyama S. DHA supplementation prevent the progression of NASH via GPR120 signaling. Eur J Pharmacol. 2018;820:31–38. doi: 10.1016/j.ejphar.2017.11.046. [DOI] [PubMed] [Google Scholar]
- 129.Liu Y, Chen K, Li F, Gu Z, Liu Q, He L, Shao T, Song Q, Zhu F, Zhang L, Jiang M, Zhou Y, Barve S, Zhang X, McClain CJ, Feng W. Probiotic Lactobacillus rhamnosus GG Prevents Liver Fibrosis Through Inhibiting Hepatic Bile Acid Synthesis and Enhancing Bile Acid Excretion in Mice. Hepatology. 2020;71:2050–2066. doi: 10.1002/hep.30975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Zhao Y, Li H. Association of serum vitamin C with liver fibrosis in adults with nonalcoholic fatty liver disease. Scand J Gastroenterol. 2022;57:872–877. doi: 10.1080/00365521.2022.2041085. [DOI] [PubMed] [Google Scholar]
- 131.Li L, Huang Q, Yang L, Zhang R, Gao L, Han X, Ji L, Zou X. The Association between Non-Alcoholic Fatty Liver Disease (NAFLD) and Advanced Fibrosis with Serological Vitamin B12 Markers: Results from the NHANES 1999-2004. Nutrients. 2022;14 doi: 10.3390/nu14061224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Megahed A, Gadalla H, Abdelhamid FM, Almehmadi SJ, Khan AA, Albukhari TA, Risha EF. Vitamin D Ameliorates the Hepatic Oxidative Damage and Fibrotic Effect Caused by Thioacetamide in Rats. Biomedicines. 2023;11 doi: 10.3390/biomedicines11020424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Ciardullo S, Muraca E, Cannistraci R, Perra S, Lattuada G, Perseghin G. Low 25 (OH) vitamin D levels are associated with increased prevalence of nonalcoholic fatty liver disease and significant liver fibrosis. Diabetes Metab Res Rev. 2023:e3628. doi: 10.1002/dmrr.3628. [DOI] [PubMed] [Google Scholar]
- 134.Lassailly G, Caiazzo R, Ntandja-Wandji LC, Gnemmi V, Baud G, Verkindt H, Ningarhari M, Louvet A, Leteurtre E, Raverdy V, Dharancy S, Pattou F, Mathurin P. Bariatric Surgery Provides Long-term Resolution of Nonalcoholic Steatohepatitis and Regression of Fibrosis. Gastroenterology. 2020;159:1290–1301.e5. doi: 10.1053/j.gastro.2020.06.006. [DOI] [PubMed] [Google Scholar]
- 135.Lassailly G, Caiazzo R, Buob D, Pigeyre M, Verkindt H, Labreuche J, Raverdy V, Leteurtre E, Dharancy S, Louvet A, Romon M, Duhamel A, Pattou F, Mathurin P. Bariatric Surgery Reduces Features of Nonalcoholic Steatohepatitis in Morbidly Obese Patients. Gastroenterology. 2015;149:379–88; quiz e15. doi: 10.1053/j.gastro.2015.04.014. [DOI] [PubMed] [Google Scholar]
- 136.Mehdorn AS, Moulla Y, Mehdorn M, Dietrich A, Schönfels W, Becker T, Braun F, Beckmann JH, Linecker M. Bariatric surgery in liver cirrhosis. Front Surg. 2022;9:986297. doi: 10.3389/fsurg.2022.986297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Tian T, Zhao C, Li S, Huang Z, Guo Y, Dai W, Bai R, Tang C, Lin Y, Gao J. Liver-Targeted Delivery of Small Interfering RNA of C-C Chemokine Receptor 2 with Tetrahedral Framework Nucleic Acid Attenuates Liver Cirrhosis. ACS Appl Mater Interfaces. 2023;15:10492–10505. doi: 10.1021/acsami.2c22579. [DOI] [PubMed] [Google Scholar]
- 138.Meng H, Jiang L, Jia P, Niu R, Bu F, Zhu Y, Pan X, Li J, Liu J, Zhang Y, Huang C, Lv X. Inhibition of circular RNA ASPH reduces the proliferation and promotes the apoptosis of hepatic stellate cells in hepatic fibrosis. Biochem Pharmacol. 2023;210:115451. doi: 10.1016/j.bcp.2023.115451. [DOI] [PubMed] [Google Scholar]
- 139.Li M, Yan L, Yuan X, Deng M, Xi Y, Zhu D. Umbilical Cord Wharton's Jelly-Derived Mesenchymal Stem Cells Inhibit the TGF-β1 Pathway in Hepatic Fibrosis Rats Through a Paracrine Regulation Process. Clin Lab. 2023;69 doi: 10.7754/Clin.Lab.2022.220357. [DOI] [PubMed] [Google Scholar]
- 140.Abou Rayia DM, Ashour DS, Abo Safia HS, Abdel Ghafar MT, Amer RS, Saad AE. Human umbilical cord blood mesenchymal stem cells as a potential therapy for schistosomal hepatic fibrosis: an experimental study. Pathog Glob Health. 2023;117:190–202. doi: 10.1080/20477724.2022.2064795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.King A, Houlihan DD, Kavanagh D, Haldar D, Luu N, Owen A, Suresh S, Than NN, Reynolds G, Penny J, Sumption H, Ramachandran P, Henderson NC, Kalia N, Frampton J, Adams DH, Newsome PN. Sphingosine-1-Phosphate Prevents Egress of Hematopoietic Stem Cells From Liver to Reduce Fibrosis. Gastroenterology. 2017;153:233–248.e16. doi: 10.1053/j.gastro.2017.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Berardis S, Dwisthi Sattwika P, Najimi M, Sokal EM. Use of mesenchymal stem cells to treat liver fibrosis: current situation and future prospects. World J Gastroenterol. 2015;21:742–758. doi: 10.3748/wjg.v21.i3.742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Lawitz EJ, Shevell DE, Tirucherai GS, Du S, Chen W, Kavita U, Coste A, Poordad F, Karsdal M, Nielsen M, Goodman Z, Charles ED. BMS-986263 in patients with advanced hepatic fibrosis: 36-week results from a randomized, placebo-controlled phase 2 trial. Hepatology. 2022;75:912–923. doi: 10.1002/hep.32181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Kurniawan DW, Booijink R, Pater L, Wols I, Vrynas A, Storm G, Prakash J, Bansal R. Fibroblast growth factor 2 conjugated superparamagnetic iron oxide nanoparticles (FGF2-SPIONs) ameliorate hepatic stellate cells activation in vitro and acute liver injury in vivo. J Control Release. 2020;328:640–652. doi: 10.1016/j.jconrel.2020.09.041. [DOI] [PubMed] [Google Scholar]
- 145.Oró D, Yudina T, Fernández-Varo G, Casals E, Reichenbach V, Casals G, González de la Presa B, Sandalinas S, Carvajal S, Puntes V, Jiménez W. Cerium oxide nanoparticles reduce steatosis, portal hypertension and display anti-inflammatory properties in rats with liver fibrosis. J Hepatol. 2016;64:691–698. doi: 10.1016/j.jhep.2015.10.020. [DOI] [PubMed] [Google Scholar]
- 146.Abdullah AS, El Sayed IET, El-Torgoman AMA, Alghamdi NA, Ullah S, Wageh S, Kamel MA. Preparation and Characterization of Silymarin-Conjugated Gold Nanoparticles with Enhanced Anti-Fibrotic Therapeutic Effects against Hepatic Fibrosis in Rats: Role of MicroRNAs as Molecular Targets. Biomedicines. 2021;9 doi: 10.3390/biomedicines9121767. [DOI] [PMC free article] [PubMed] [Google Scholar]