This cohort study characterizes the clinical phenotype of systemic sclerosis sine scleroderma vs limited cutaneous and diffuse cutaneous systemic sclerosis within the EUSTAR database.
Key Points
Question
What are the main clinical features of systemic sclerosis (SSc) sine scleroderma (ssSSc) compared with limited cutaneous SSc (lcSSc) and diffuse cutaneous SSc (dcSSc) within the EUSTAR database?
Findings
In this cohort study of 4263 patients, 376 patients (8.8%) were classified as having ssSSc, and survival was higher in patients with ssSSc compared with lcSSc and dcSSc. The only independent factor for the onset of skin fibrosis in ssSSc was anti–Scl-70 antibody positivity; patients with ssSSc had a lower prevalence of previous or current digital ulcers than patients with lcSSc and dcSSc, and skin telangiectasias were associated with diastolic dysfunction in patients with ssSSc.
Meaning
Systemic sclerosis sine scleroderma accounted for nearly 10% of patients with SSc; cutaneous findings in this subgroup may be associated with internal organ dysfunction, and in particular, skin telangiectasias in ssSSc were associated with diastolic heart dysfunction.
Abstract
Importance
Systemic sclerosis (SSc) sine scleroderma (ssSSc) is a subset of SSc defined by the absence of skin fibrosis. Little is known about the natural history and skin manifestations among patients with ssSSc.
Objective
To characterize the clinical phenotype of patients with ssSSc compared with patients with limited cutaneous SSc (lcSSc) and diffuse cutaneous SSc (dcSSc) within the EUSTAR database.
Design, Setting, and Participants
This longitudinal observational cohort study based on the international EUSTAR database included all patients fulfilling the classification criteria for SSc assessed by the modified Rodnan Skin score (mRSS) at inclusion and with at least 1 follow-up visit; ssSSc was defined by the absence of skin fibrosis (mRSS = 0 and no sclerodactyly) at all available visits. Data extraction was performed in November 2020, and data analysis was performed from April 2021 to April 2023.
Main Outcomes and Measures
Main outcomes were survival and skin manifestations (onset of skin fibrosis, digital ulcers, telangiectasias, puffy fingers).
Results
Among the 4263 patients fulfilling the inclusion criteria, 376 (8.8%) were classified as having ssSSc (mean [SD] age, 55.3 [13.9] years; 345 [91.8%] were female). At last available visit, in comparison with 708 patients with lcSSc and 708 patients with dcSSc with the same disease duration, patients with ssSSc had a lower prevalence of previous or current digital ulcers (28.2% vs 53.1% in lcSSc; P < .001; and 68.3% in dcSSc; P < .001) and puffy fingers (63.8% vs 82.4% in lcSSc; P < .001; and 87.6% in dcSSc; P < .001). By contrast, the prevalence of interstitial lung disease was similar in ssSSc and lcSSc (49.8% and 57.1%; P = .03) but significantly higher in dcSSc (75.0%; P < .001). Skin telangiectasias were associated with diastolic dysfunction in patients with ssSSc (odds ratio, 4.778; 95% CI, 2.060-11.081; P < .001). The only independent factor for the onset of skin fibrosis in ssSSc was the positivity for anti–Scl-70 antibodies (odds ratio, 3.078; 95% CI, 1.227-7.725; P = .02). Survival rate was higher in patients with ssSSc (92.4%) compared with lcSSc (69.4%; P = .06) and dcSSc (55.5%; P < .001) after up to 15 years of follow-up.
Conclusions and Relevance
Systemic sclerosis sine scleroderma should not be neglected considering the high prevalence of interstitial lung disease (>40%) and SSc renal crisis (almost 3%). Patients with ssSSc had a higher survival than other subsets. Dermatologists should be aware that cutaneous findings in this subgroup may be associated with internal organ dysfunction. In particular, skin telangiectasias in ssSSc were associated with diastolic heart dysfunction.
Introduction
Systemic sclerosis (SSc) is a rare connective tissue disease characterized by the association of autoimmune features with vascular manifestations and, in the majority of patients, fibrosis of the skin and internal organs, predominantly heart, lungs, and kidneys.1 There is a high heterogeneity among patients with SSc regarding the presence and severity of skin and visceral involvement.2,3,4,5 The LeRoy classification defines 2 main subsets of SSc based on the extent of skin fibrosis6: limited cutaneous SSc (lcSSc) with skin thickening sparing the trunk and distal to the elbow and knees, and diffuse cutaneous SSc (dcSSc) with proximal and distal skin thickening. These subsets notably differ in terms of survival and frequency of visceral involvement, with dcSSc being less prevalent but having a higher mortality rate with more frequent visceral manifestations.7,8 Systemic sclerosis sine scleroderma (ssSSc) is a third subset, initially described by Rodnan and Fennell9 and characterized by the absence of skin fibrosis (ie, without [sine in Latin] scleroderma) but with SSc-associated visceral manifestations.9,10,11,12
In many observational studies and clinical trials, no distinction is made between lcSSc and ssSSc. There is a current emphasis in improving SSc patient selection for clinical trials, based on stratification strategies combining the extent of skin involvement with autoantibody status.13,14 There is also a growing interest in precision medicine in SSc to foster individual management. To that extent, ssSSc may constitute a subset with a distinct clinical trajectory differing from lcSSc or dcSSc. Although therapeutic research was mainly focused on dcSSc initially, there is rising interest on other subsets of the disease.15,16,17,18,19 Patients with lcSSc or ssSSc may experience higher morbidity than expected, justifying dedicated clinical trials and validation of relevant outcome measures for these subsets.20,21,22 Dermatologists play a key role in the diagnosis and management of patients with SSc, as early features of the disease include important skin manifestations such as Raynaud phenomenon (RP), digital ulcers, and puffy fingers. There is a recent emphasis on nonfibrotic skin manifestations of SSc, including puffy fingers as part of the diagnostic strategy or telangiectasias as surrogate markers of the severity of SSc-related vasculopathy.4,23 Thus, dermatologists should be especially aware of such nonfibrotic manifestations that are crucial for the diagnosis of ssSSc and included in the American College of Rheumatology 2013 classification criteria of the disease. Patients with ssSSc may also secondarily develop skin fibrosis, although this question is still to be explored.
To date, little is known on the natural history of skin involvement and on skin manifestations (digital ulcers, telangiectasias, or puffy fingers) in patients with ssSSc. Previous studies exploring this subset had limited statistical power due to small sample size, precluding relevant evaluations of skin outcomes.10,11,12 These studies were mainly based on single-center or nationwide cohorts, and data on patients with ssSSc from multicentric international cohorts are still missing. To our knowledge, there are no international studies exploring risk factors of skin fibrosis onset in patients with ssSSc. The present study aimed to characterize the main clinical features, with a specific focus on cutaneous manifestations, of patients with ssSSc in comparison with lcSSc and dcSSc within the international EUSTAR (European Scleroderma Trials and Research) database.
Methods
EUSTAR Cohort
The EUSTAR database prospectively collects data from participating centers using a predetermined data set. The structure of the database, the collected data set, and definitions of clinical variables have been previously published in detail.21,24 All included patients provided written consent with institutional review board authorization from each center. The EUSTAR database and this study comply with the recommendations of the Declaration of Helsinki. For this study, data extraction was performed in November 2020 (n = 19 115 patients entered in EUSTAR).
Patient Population and Definitions of SSc Sine Scleroderma
All patients from the EUSTAR database (1) fulfilling the American College of Rheumatology 2013 or 1980 classification criteria for SSc and with available date of first non-RP symptom, (2) assessed by the modified Rodnan Skin score (mRSS) at inclusion, (3) with at least 1 follow-up visit and available disease duration based on the first non-RP symptom were eligible for the study (n = 4263).25,26 The definition of ssSSc was derived from Diab et al10 and included all patients without skin fibrosis (mRSS = 0 and no sclerodactyly) at all available visits (including baseline and all follow-up visits). Survival and clinical characteristics of patients with ssSSc were compared with those of patients with dcSSc and lcSSc, matched by disease duration (±1 year) at last available visit. The pairing ratio was 2 patients with lcSSc and 2 patients with dcSSc for 1 patient with ssSSc. Interstitial lung disease (ILD) was attested by the presence of signs of ILD on high-resolution computed tomography (HRCT) and/or radiography, or when a date for a diagnosis of ILD was notified by the evaluator any time during the study. Current digital ulcers (DUs) were recorded by physicians. The history of DUs was based on medical record and patients’ reporting.
Mortality
All-cause mortality was assessed in patients with ssSSc and in paired patients with dcSSc and lcSSc until last available visit. Systemic sclerosis–related cause of death was not available in the EUSTAR database and was thus not explored.
Statistical Analysis
Statistical methods are detailed in eMethods in Supplement 1. Comparison between groups were assessed using t test for quantitative variables with Gaussian distribution, Wilcoxon rank sum test for quantitative variables with non-Gaussian distribution, and χ2 or Fisher exact test as appropriate for qualitative variables. We performed all tests with a significance level of P < .05 (2-tailed). Analyses were conducted in SAS, version 9.4 (SAS Institute), and figures were plotted via R package “survival” and “survminer” (R, version 4.0.2; R Foundation for Statistical Computing).
Results
Clinical Characteristics of Patients With ssSSc at Inclusion and Risk Factors for Onset of Skin Fibrosis
Among the 4263 eligible patients from EUSTAR based on availability of mRSS at baseline and during follow-up, 376 (8.8%) (mean [SD] age, 55.3 [13.9] years; 345 [91.8%] were female) were classified as having constant ssSSc according to the adapted 2014 definition of Diab et al10 (eFigure in Supplement 1). In terms of phenotype at inclusion visit, the majority of patients with constant ssSSc had RP (97.2%) (Table 1). Key dermatological features at inclusion visit included telangiectasias in 47.8%, puffy fingers in 40.3%, current or previous DUs in 4.7% and 19.0%, respectively, and pitting scars in 11.5% of patients with ssSSc. The most frequent visceral manifestations at inclusion visit were esophageal symptoms (57%) and ILD (39.4%) (Table 1). Presence of anticentromere antibodies was reported in 61% of patients with ssSSc, followed by antitopoisomerase antibodies (anti–Scl-70) in 15.1% of the patients.
Table 1. Baseline Characteristics (Inclusion Visit) of Patients With Constant ssSSc (ie Without Skin Fibrosis Ever, at Baseline, or During Follow-up).
| Characteristics at inclusion visit | Data available, No. (n = 376) | ssSSc at inclusion visit, No. (%) (n = 376) |
|---|---|---|
| Demographics | ||
| Age, mean (SD), y | 376 | 55.3 (13.9) |
| Sex | ||
| Female | 376 | 345 (91.8) |
| Male | 376 | 31 (8.2) |
| Disease duration since first non-RP symptom, mean (SD), y | 376 | 8.3 (9.4) |
| Disease duration since RP onset, mean (SD), y | 338 | 11.9 (11.7) |
| Definition of ssSSc (adapted from Diab et al,10 2014) | 376 | 376 (100) |
| Composite definition of ssSSc (adapted from Poormoghim et al,12 2000) | 376 | 323 (85.9) |
| Disease characteristics | ||
| Skin manifestations | ||
| RP | 353 | 343 (97.2) |
| Telangiectasia | 276 | 132 (47.8) |
| Current DUs | 274 | 13 (4.7) |
| Previous DUs | 274 | 52 (19.0) |
| Current pitting scars | 269 | 31 (11.5) |
| Previous pitting scars | 269 | 31 (11.5) |
| Current puffy fingers | 365 | 147 (40.3) |
| Previous puffy fingers | 365 | 26 (7.1) |
| Other manifestations | ||
| Joint synovitis | 373 | 28 (7.5) |
| Tendon friction rubs | 369 | 5 (1.4) |
| Muscle weakness | 376 | 48 (12.8) |
| CK elevation | 280 | 16 (5.7) |
| Esophageal symptoms | 374 | 213 (57.0) |
| Stomach symptoms | 369 | 61 (16.5) |
| Intestinal symptoms | 373 | 101 (27.1) |
| History of scleroderma renal crisis | 375 | 6 (1.6) |
| Proteinuria | 313 | 16 (5.1) |
| Lung fibrosis on radiography or HRCT or presence of ILD | 327 | 129 (39.4) |
| DLCO (%pred), mean (SD) | 315 | 73.6 (21.1) |
| FVC (%pred), mean (SD) | 328 | 102.6 (21.5) |
| TLC (%pred), mean (SD) | 246 | 99.6 (20.9) |
| sPAP >40 mm Hg (echocardiography) | 259 | 20 (7.7) |
| Left ventricular ejection fraction (%), mean (SD) | 301 | 61.9 (6.2) |
| Diastolic heart dysfunction | 301 | 80 (26.6) |
| Conduction block | 274 | 25 (9.1) |
| Disease activity at baseline | ||
| EScSG disease activity index (2001), mean (SD) | 354 | 0.8 (1.0) |
| EScSG disease activity index (2016), mean (SD) | 376 | 0.4 (0.8) |
| Immunological findings | ||
| ANA+ | 373 | 360 (96.5) |
| ACA+ | 359 | 219 (61.0) |
| ATA+ | 357 | 54 (15.1) |
| RNA pol III+ | 252 | 7 (2.8) |
| PmScl+ | 236 | 12 (5.1) |
| U1RNP+ | 281 | 12 (4.3) |
| CRP >5 mg/L | 250 | 5 (2.0) |
Abbreviations: ACA, anticentromere antibodies; ANA, antinuclear antibodies; ATA, antitopoisomerase I antibodies; CK, creatine kinase; CRP, C-reactive protein; DLCO, diffusion capacities of carbon monoxide; DUs, digital ulcers; EScSG, European Systemic Sclerosis research group; FVC, forced vital capacity; HRCT, high-resolution computed tomography; ILD, interstitial lung disease; %pred, percent predicted; RNA pol III, anti–RNA polymerase III antibodies; RP, Raynaud phenomenon; sPAP, systolic pulmonary arterial pressure; ssSSc, systemic sclerosis sine scleroderma; TLC, total lung capacities; U1RNP, anti–U1 ribonuclease protein antibodies.
Among the 4263 eligible patients, in addition to the 376 patients with constant ssSSc, 184 patients (4.3%) had no skin fibrosis at inclusion visit but showed skin fibrosis onset during follow-up (eFigure in Supplement 1), with 171 of them subsequently fulfilling the definition of lcSSc and 13 the definition of dcSSc. In multivariable analysis, the only independent risk factor for the onset of skin fibrosis (ie, progression from ssSSc to cutaneous SSc, either lcSSc or dcSSc) in these baseline patients with ssSSc was the positivity for antitopoisomerase antibody (anti–Scl-70) (odds ratio, 3.078; 95% CI, 1.227-7.725; P = .02) (Table 2). The presence of puffy fingers at baseline was not associated with the onset of skin fibrosis in ssSSc.
Table 2. Risk Factors for Onset of Skin Fibrosis During Follow-up in ssSSc (ie, Risk Factors of Progression to lcSSc or dcSSc During Follow-up).
| Characteristics of patients at inclusion visit | Parameters at inclusion visit associated with onset of skin fibrosis in patients with ssSSc during follow-up | |||
|---|---|---|---|---|
| Univariate analysis | Multivariate analysis | |||
| Odds ratio (95% CI) | P value | Odds ratio (95% CI) | P value | |
| Age, y | 0.987 (0.975-1.000) | .04 | 1.016 (0.989-1.044) | .24 |
| Sex (reference = female) | 1.511 (0.848-2.692) | .16 | 0.926 (0.281-3.048) | .90 |
| Disease duration since first non-RP symptom | 0.989 (0.970-1.009) | .27 | NA | NA |
| Disease duration since RP onset | 0.985 (0.969-1.003) | .09 | 1.005 (0.971-1.041) | .77 |
| RP (reference = no) | 1.651 (0.449-6.077) | .45 | NA | NA |
| Telangiectasia (reference = no) | 1.045 (0.651-1.675) | .86 | NA | NA |
| DUs (reference = never) | 1.582 (0.948-2.641) | .08 | 1.745 (0.627-4.859) | .29 |
| Pitting scars (reference = never) | 1.533 (0.902-2.603) | .11 | 2.348 (0.837-6.582) | .10 |
| Puffy fingers (reference = never) | 0.890 (0.619-1.280) | .53 | NA | NA |
| Joint synovitis (reference = no) | 1.771 (0.989-3.172) | .05 | 1.078 (0.348-3.333) | .90 |
| Tendon friction rubs (reference = no) | 1.220 (0.288-5.163) | .79 | NA | NA |
| Muscle weakness (reference = no) | 1.376 (0.838-2.258) | .21 | NA | NA |
| CK elevation (reference = no) | 1.820 (0.873-3.792) | .11 | 1.282 (0.336-4.889) | .72 |
| Esophageal symptoms (reference = no) | 0.911 (0.638-1.300) | .61 | NA | NA |
| Stomach symptoms (reference = no) | 1.237 (0.783-1.952) | .36 | NA | NA |
| Intestinal symptoms (reference = no) | 1.012 (0.680-1.506) | .95 | NA | NA |
| History of scleroderma renal crisis (reference = no) | 0.347 (0.042-2.908) | .33 | NA | NA |
| Proteinuria (reference = no) | 0.748 (0.287-1.950) | .55 | NA | NA |
| Lung fibrosis on radiography or HRCT or presence of ILD (reference = no) | 0.625 (0.415-0.940) | .02 | 0.851 (0.369-1.961) | .70 |
| Significant dyspnea (reference = no) | 0.481 (0.178-1.300) | .15 | <0.001 (<0.001->999.999) | .98 |
| DLCO (%pred) | 1.001 (0.992-1.011) | .75 | NA | NA |
| FVC (%pred) | 1.000 (0.991-1.009) | .98 | NA | NA |
| TLC (%pred) | 0.999 (0.988-1.011) | .91 | NA | NA |
| sPAP >40 mm Hg (reference = no) | 1.124 (0.521-2.422) | .77 | NA | NA |
| Left ventricular ejection fraction | 0.996 (0.964-1.030) | .83 | NA | NA |
| Diastolic heart dysfunction (reference = no) | 0.810 (0.511-1.282) | .37 | NA | NA |
| Conduction block (reference = no) | 0.772 (0.360-1.657) | .51 | NA | NA |
| EScSG disease activity index (2001) | 1.080 (0.907-1.286) | .39 | NA | NA |
| EScSG disease activity index (2016) | 0.815 (0.638-1.041) | .10 | 1.070 (0.738-1.551) | .72 |
| ANA+ (reference = negative) | 0.887 (0.348-2.264) | .80 | NA | NA |
| ACA+ (reference = negative) | 0.849 (0.586-1.230) | .39 | NA | NA |
| ATA+ (reference = negative) | 1.930 (1.229-3.032) | .004 | 3.078 (1.227-7.725) | .02 |
| RNA pol III+ (reference = negative) | 1.843 (0.571-5.948) | .31 | NA | NA |
| PmScl+ (reference = negative) | 0.839 (0.264-2.671) | .77 | NA | NA |
| U1RNP+ (reference = negative) | 0.966 (0.333-2.805) | .95 | NA | NA |
| CRP >5 mg/L (reference = no) | 1.181 (0.225-6.201) | .84 | NA | NA |
Abbreviations: ACA, anticentromere antibodies; ANA, antinuclear antibodies; ATA, antitopoisomerase I antibodies; CK, creatine kinase; CRP, C-reactive protein; DLCO, diffusion capacities of carbon monoxide; dcSSc, diffuse cutaneous systemic sclerosis; DUs, digital ulcers; EScSG, European Systemic Sclerosis research group; FVC, forced vital capacity; HRCT, high-resolution computed tomography; ILD, interstitial lung disease; lcSSc, limited cutaneous systemic sclerosis; NA, not applicable; %pred, percent predicted; RNA pol III, anti–RNA polymerase III antibodies; RP, Raynaud phenomenon; sPAP, systolic pulmonary arterial pressure; ssSSc, systemic sclerosis sine scleroderma; TLC, total lung capacities; U1RNP, anti–U1 ribonuclease protein antibodies.
Clinical Phenotype of ssSSc Depending on Presence of Key Skin Manifestations
In comparison with patients with ssSSc with no DUs ever, patients with ssSSc who experienced DUs (history of DUs or DUs during follow-up) tended to be younger, with more frequent esophageal manifestations and higher prevalence of creatine kinase elevation at last available visit in univariate and multivariate analysis (Table 3). No antibody subtype was associated with DUs at entry or during follow-up in ssSSc. Digital ulcers were associated with the presence of digital pitting scars but not with puffy fingers.
Table 3. Clinical Characteristics Associated With DUs Ever (ie, History of DUs at Inclusion and/or DUs During Follow-up) in Patients With ssSSc.
| Characteristics of patients with ssSSc at last visit | Univariate modeling for DUs (ever) vs never | Multivariable modeling for DUs (ever) vs never | ||
|---|---|---|---|---|
| Odds ratio (95% CI) | P value | Odds ratio (95% CI) | P value | |
| Age at last visit, y | 0.984 (0.968-1.001) | .06 | 0.971 (0.949-0.994) | .01 |
| Sex (reference = female) | 1.071 (0.453-2.532) | .88 | NA | NA |
| Disease duration since RP onset (last visit), y | 0.993 (0.972-1.013) | .48 | NA | NA |
| RP (reference = never) | >999.999 (<0.001->999.999) | .99 | NA | NA |
| Telangiectasia (reference = never) | 0.737 (0.455-1.191) | .21 | NA | NA |
| Pitting scars (reference = never) | 15.334 (8.733-26.924) | <.001 | NA | NA |
| Puffy fingers (reference = never) | 1.085 (0.661-1.780) | .75 | NA | NA |
| Joint synovitis (reference = never) | 0.695 (0.364-1.327) | .27 | NA | NA |
| Tendon friction rubs (reference = never) | 1.962 (0.868-4.433) | .10 | 2.416 (0.888-6.574) | .08 |
| CK elevation (reference = never) | 2.008 (0.988-4.084) | .05 | 3.268 (1.388-7.694) | .007 |
| Esophageal symptoms (reference = never) | 2.491 (1.331-4.663) | .004 | 4.797 (1.806-12.741) | .002 |
| Stomach symptoms (reference = never) | 1.308 (0.809-2.115) | .27 | NA | NA |
| Intestinal symptoms (reference = never) | 0.947 (0.596-1.505) | .82 | NA | NA |
| History of scleroderma renal crisis (reference = never) | 0.717 (0.146-3.513) | .68 | NA | NA |
| Proteinuria (reference = never) | 0.784 (0.367-1.673) | .53 | NA | NA |
| Lung fibrosis on radiography or HRCT or presence of ILD (reference = never) | 0.813 (0.505-1.309) | .39 | NA | NA |
| Significant dyspnea (reference = never) | 1.088 (0.564-2.100) | .80 | NA | NA |
| DLCO (%pred) (last visit) | 0.992 (0.977-1.007) | .31 | NA | NA |
| FVC (%pred) (last visit) | 0.999 (0.986-1.011) | .82 | NA | NA |
| TLC (%pred) (last visit) | 1.001 (0.985-1.017) | .89 | NA | NA |
| sPAP >40 mm Hg (reference = never) | 1.170 (0.546-2.505) | .69 | NA | NA |
| Pulmonary hypertension (reference = never) | 0.996 (0.508-1.955) | .99 | NA | NA |
| Left ventricular ejection fraction (last visit) | 0.959 (0.907-1.014) | .14 | NA | NA |
| Diastolic heart dysfunction (reference = never) | 0.970 (0.598-1.573) | .90 | NA | NA |
| Conduction block (reference = never) | 0.501 (0.240-1.046) | .07 | 0.401 (0.160-1.005) | .05 |
| EScSG disease activity index (2001) (last visit) | 0.651 (0.423-1.004) | .05 | NA | NA |
| EScSG disease activity index (2016) (last visit) | 0.658 (0.377-1.148) | .14 | 0.433 (0.179-1.052) | .06 |
| ANA+ (reference = negative) | 2.788 (0.339-22.958) | .34 | NA | NA |
| ACA+ (reference = negative) | 0.982 (0.607-1.589) | .94 | NA | NA |
| ATA+ (reference = negative) | 1.010 (0.545-1.873) | .97 | NA | NA |
| RNA pol III+ (reference = negative) | 0.451 (0.098-2.079) | .31 | NA | NA |
| PmScl+ (reference = negative) | 0.923 (0.285-2.986) | .89 | NA | NA |
| U1RNP+ (reference = negative) | 2.666 (0.969-7.330) | .06 | 2.323 (0.702-7.685) | .17 |
| CRP >5 mg/L (reference = never) | 0.553 (0.203-1.506) | .25 | NA | NA |
Abbreviations: ACA, anticentromere antibodies; ANA, antinuclear antibodies; ATA, antitopoisomerase I antibodies; CK, creatine kinase; CRP, C-reactive protein; DLCO, diffusion capacities of carbon monoxide; DUs, digital ulcers; EScSG, European Systemic Sclerosis research group; FVC, forced vital capacity; HRCT, high-resolution computed tomography; ILD, interstitial lung disease; NA, not applicable; %pred, percent predicted; RNA pol III, anti–RNA polymerase III antibodies; RP, Raynaud phenomenon; sPAP, systolic pulmonary arterial pressure; ssSSc, systemic sclerosis sine scleroderma; TLC, total lung capacities; U1RNP, anti–U1 ribonuclease protein antibodies.
Patients with sine scleroderma with skin telangiectasias had longer disease duration in multivariate analysis, more frequent intestinal symptoms, and more frequent diastolic dysfunction, suggesting an association between skin telangiectasias and visceral microangiopathy in patients with ssSSc (Table 4). In univariate analysis, patients with ssSSc with skin telangiectasias also had a higher prevalence of history of or currently elevated systolic pulmonary arterial pressure (>40 mm Hg), although this result was not significant in multivariate analysis (Table 4).
Table 4. Clinical Characteristics Associated With Telangiectasia (Ever) in Patients With ssSSc.
| Characteristics of patients with ssSSc at last visit | Univariate modeling for telangiectasia (ever vs never) | Multivariable modeling for telangiectasia (ever vs never) | ||
|---|---|---|---|---|
| Odds ratio (95% CI) | P value | Odds ratio (95% CI) | P value | |
| Age at last visit, y | 1.013 (0.997-1.030) | .10 | 0.991 (0.964-1.019) | .51 |
| Sex (reference = female) | 0.721 (0.323-1.606) | .42 | NA | NA |
| Disease duration since RP onset (last visit), y | 1.031 (1.009-1.053) | .006 | 1.057 (1.015-1.101) | .007 |
| RP (reference = never) | <0.001 (<0.001->999.999) | .99 | NA | NA |
| Puffy fingers (reference = never) | 1.013 (0.635-1.616) | .96 | NA | NA |
| Pitting scars (reference = never) | 1.093 (0.680-1.757) | .71 | NA | NA |
| DUs (reference = never) | 0.737 (0.455-1.191) | .21 | NA | NA |
| Joint synovitis (reference = never) | 2.732 (1.366-5.464) | .004 | 2.583 (0.911-7.324) | .07 |
| Tendon friction rubs (reference = never) | 1.759 (0.687-4.504) | .24 | NA | NA |
| CK elevation (reference = never) | 0.743 (0.365-1.512) | .41 | NA | NA |
| Esophageal symptoms (reference = never) | 0.978 (0.586-1.633) | .93 | NA | NA |
| Stomach symptoms (reference = never) | 1.448 (0.904-2.319) | .12 | 0.509 (0.237-1.096) | .08 |
| Intestinal symptoms (reference = never) | 1.872 (1.200-2.921) | .006 | 2.479 (1.185-5.187) | .02 |
| History of scleroderma renal crisis (reference = never) | 1.017 (0.250-4.141) | .98 | NA | NA |
| Proteinuria (reference = never) | 1.050 (0.521-2.116) | .89 | NA | NA |
| Lung fibrosis on radiography or HRCT or presence of ILD (reference = never) | 0.975 (0.618-1.536) | .91 | NA | NA |
| Significant dyspnea (reference = never) | 1.670 (0.851-3.275) | .14 | 1.328 (0.438-4.025) | .62 |
| DLCO (%pred) (last visit) | 0.988 (0.973-1.003) | .11 | NA | NA |
| FVC (%pred) (last visit) | 0.997 (0.985-1.009) | .61 | NA | NA |
| TLC (%pred) (last visit) | 1.009 (0.994-1.024) | .25 | NA | NA |
| sPAP >40 mm Hg (reference = never) | 2.714 (1.091-6.753) | .03 | 2.204 (0.553-8.793) | .26 |
| Pulmonary hypertension (reference = never) | 0.938 (0.500-1.760) | .84 | NA | NA |
| Left ventricular ejection fraction (last visit) | 0.997 (0.944-1.052) | .90 | NA | NA |
| Diastolic heart dysfunction (reference = never) | 4.986 (2.891-8.601) | <.001 | 4.778 (2.060-11.081) | <.001 |
| Conduction block (reference = never) | 1.800 (0.902-3.593) | .10 | 0.634 (0.243-1.656) | .35 |
| EScSG disease activity index (2001) (last visit) | 1.707 (1.131-2.576) | .01 | NA | NA |
| EScSG disease activity index (2016) (last visit) | 1.155 (0.755-1.767) | .51 | NA | NA |
| ANA+ (reference = negative) | 0.647 (0.129-3.255) | .60 | NA | NA |
| ACA+ (reference = negative) | 1.294 (0.822-2.038) | .27 | NA | NA |
| ATA+ (reference = negative) | 0.843 (0.477-1.492) | .56 | NA | NA |
| RNA pol III+ (reference = negative) | 3.036 (0.661-13.954) | .15 | 1.673 (0.312-8.961) | .55 |
| PmScl+ (reference = negative) | 2.247 (0.619-8.152) | .22 | NA | NA |
| U1RNP+ (reference = negative) | 2.653 (0.746-9.431) | .13 | 1.621 (0.303-8.684) | .57 |
| CRP >5 mg/L (reference = never) | 1.464 (0.599-3.575) | .40 | NA | NA |
Abbreviations: ACA, anticentromere antibodies; ANA, antinuclear antibodies; ATA, antitopoisomerase I antibodies; CK, creatine kinase; CRP, C-reactive protein; DLCO, diffusion capacities of carbon monoxide; DUs, digital ulcers; EScSG, European Systemic Sclerosis research group; FVC, forced vital capacity; HRCT, high-resolution computed tomography; ILD, interstitial lung disease; NA, not applicable; %pred, percent predicted; RNA pol III, anti–RNA polymerase III antibodies; RP, Raynaud phenomenon; sPAP, systolic pulmonary arterial pressure; ssSSc, systemic sclerosis sine scleroderma; TLC, total lung capacities; U1RNP, anti–U1 ribonuclease protein antibodies.
There were no relevant clinical characteristics differentiating patients with ssSSc with or without puffy fingers, notably in terms of disease duration (eTable 1 in Supplement 1). Regarding visceral involvement in ssSSc, the presence of ILD and/or lung fibrosis on HRCT or lung radiography was associated with a higher prevalence of dyspnea and altered pulmonary function test parameters (eTables 3 and 4 in Supplement 1).
Comparison With lcSSc and dcSSc at Last Available Visit
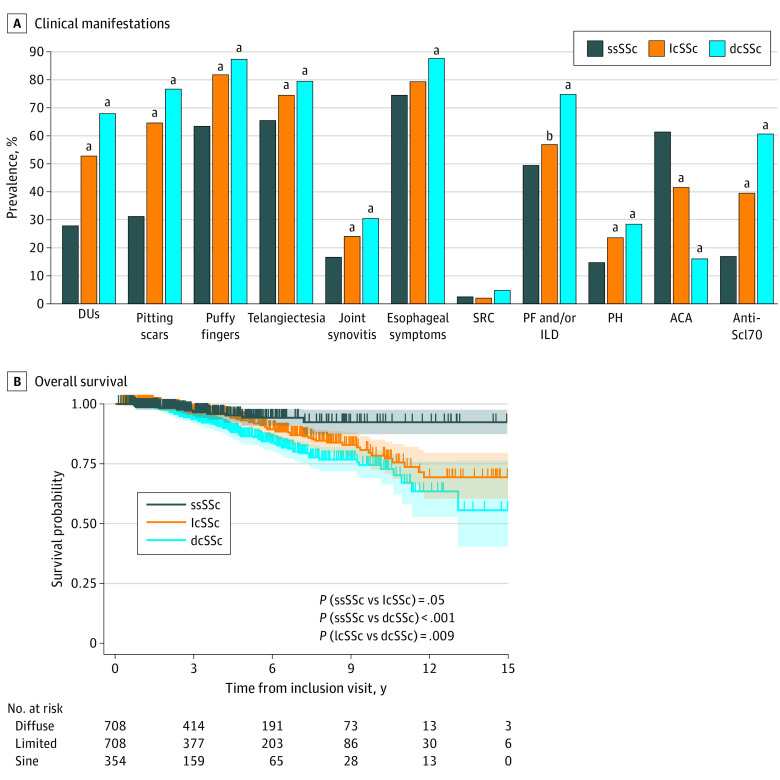
Based on disease duration since the presence of the first non-RP symptom at last available visit, 354 patients with ssSSc were paired to 708 patients with lcSSc and 708 patients with dcSSc (eFigure in Supplement 1). Patients with ssSSc were less likely to be men (8.8%) in comparison with both lcSSc (15.7% of men; P = .002) and dcSSc (25.7% of men; P < .001). Patients with ssSSc had a lower prevalence of previous or current DUs (28.2% vs 53.1% in lcSSc; P < .001; and 68.3% in dcSSc; P < .001) despite similar prevalence for RP (Figure, A; eTable 2 in Supplement 1). Patients with ssSSc also had a lower prevalence of puffy fingers (ever) (63.8% vs 82.4% in lcSSc; P < .001; and 87.6% in dcSSc; P < .001). Skin telangiectasias were also less frequent in patients with ssSSc (65.8%) compared with patients with lcSSc (74.7%; P < .001) or with dcSSc (79.7%; P < .001) (Figure, A; eTable 2 in Supplement 1).
Figure. Comparison of Clinical Presentation and Survival of Patients With ssSSc vs Cutaneous Subsets (lcSSc and dcSSc).
A, Prevalence of main SSc-related clinical manifestations in the 3 SSc subsets (χ2 test or Fisher test as appropriate; P value for comparisons of ssSSc with lcSSc and ssSSc with dcSSc, detailed results and comparisons for other manifestations are provided in eTable 2 in Supplement 1). B, Comparison of overall survival in patients with ssSSc, lcSSc, and dcSSc (censored at after 15 years of follow-up). Shaded areas represent 95% CIs. ACA indicates anticentromere antibodies; dcSSc, diffuse cutaneous systemic sclerosis; DUs, digital ulcers; ILD, interstitial lung disease; lcSSc, limited cutaneous systemic sclerosis; PF, pulmonary fibrosis; PH, pulmonary hypertension; SRC, history of scleroderma renal crisis; SSc, systemic sclerosis; ssSSc, systemic sclerosis sine scleroderma.
aP < .001.
bP = .03.
Pulmonary hypertension or conduction blocks were more frequent in patients with lcSSc and dcSSc in comparison with ssSSc. Prevalence of diastolic dysfunction was similar in all subsets (eTable 2 in Supplement 1). The prevalence of ILD was similar in ssSSc and lcSSc (49.8% and 57.1%; P = .03) and significantly higher in dcSSc (75.0%; P < .001). There were no significant differences in the prevalence of scleroderma renal crisis among the 3 subsets (Figure, A; eTable 2 in Supplement 1).
Regarding therapeutics, although almost half of patients with ssSSc (49.4%) had received immunomodulatory therapies at some point during their disease course; these treatments were less frequently prescribed in ssSSc than in lcSSc (64.2%; P < .001) and dcSSc (75.0%; P < .001) (eTable 2 in Supplement 1). Calcium channel inhibitors and sildenafil were equally prescribed among all disease subsets, while DU-related therapies (including bosentan or iloprost) were less frequently prescribed for patients with ssSSc compared with patients with lcSSc and dcSSc (eTable 2 in Supplement 1).
In survival analyses, median (IQR) follow-up duration was 3.3 (1.6-6.1) years. Overall survival tended to be higher in patients with ssSSc compared with lcSSc (P = .06) and was significantly higher compared with dcSSc (P < .001), all matched for disease duration at last available visit (supporting data in Figure, B). Overall survival was also significantly lower in dcSSc compared with lcSSc (P = .009; Figure, B).
Discussion
This cohort study based on the EUSTAR longitudinal database, including more than 350 patients with ssSSc, provides unique insight on this specific SSc subtype and the associated skin manifestations. The numbers of patients with ssSSc in previous studies exploring this subset were 48 in the Pittsburgh cohort,12 57 in the Canadian registry,10 79 in the Brazilian cohort,11 22 in the German registry,27 and 118 in the Spanish registry.28 Of note, the Spanish and German studies did not focus on patients with ssSSc, but only mentioned the clinical characteristics of this subset among others.27,28 To our knowledge, it is also the first international multicenter study specifically exploring patients with ssSSc. Our results suggest that ssSSc is not a rare subset, as it accounted for almost 10% of patients with SSc in the EUSTAR registry. Although lcSSc and ssSSc have so far been mostly considered as similar nosological entities, the present study highlights key differences in terms of clinical phenotype and survival, further supporting the need to separate ssSSc from lcSSc for future investigations.10,12
In our study, in patients that could be classified as having ssSSc at the inclusion visit but whose disease then progressed to lcSSc or dcSSc, the only independent risk factor for progression, ie, risk factor for the onset of skin fibrosis, was the positivity for anti–Scl-70 antibodies. This population of patients with ssSSc with positivity for anti–Scl-70 antibodies might be a new population to be considered for therapeutic trials with early SSc aiming at reducing the progression of skin fibrosis, as the inclusion of these patients at an early stage may show that active therapy could prevent the onset of skin fibrosis in this specific Scl-70–positive population of patients with ssSSc.29,30,31 Puffy fingers were not associated with the onset of skin fibrosis in patients with ssSSc experiencing skin fibrosis during follow-up, and disease duration as well as the prevalence of anti–Scl-70 antibodies between ssSSc with or without puffy fingers were similar, suggesting that the hypothesis regarding a potential continuum between puffy fingers and sclerodactyly may not be accurate in all patients with SSc.32,33 Histological characterization of puffy fingers may help to understand if such manifestations are the result of early inflammatory infiltrate and/or subsequent to vascular leakage.31,33,34,35
Considering the large sample size of patients with ssSSc in our study, we were able to explore parameters associated with other skin manifestations, including DUs and skin telangiectasias in this population. Our study revealed that patients with ssSSc with DU tended to have a higher prevalence of anti-U1RNP antibodies, an antibody classically associated with mixed connective tissue disease (MCTD or Sharp syndrome). The association of DUs with creatine kinase elevation in patients with ssSSc also suggested some phenotypic similarities between patients with ssSSc with digital ischemia and patients with MCTD.36 In comparison with patients with lcSSc, patients with ssSSc had a lower prevalence of DUs. This higher prevalence of DUs in lcSSc compared with ssSSc is consistent with data from the Pittsburgh cohort, the Canadian registry, and the Brazilian registry.10,11,12 Our results confirm that lcSSc have more severe peripheral skin manifestations and suggest that skin fibrosis of the finger pulp (ie, sclerodactyly), the main dermatological feature differentiating lcSSc and ssSSc, directly participates in the pathogenesis of DUs in these patients.
Skin telangiectasias in patients with ssSSc were associated with important visceral manifestations, such as elevated systolic pulmonary arterial pressure on echocardiography in univariate analysis or intestinal symptoms and diastolic dysfunction in univariate and multivariate analysis, independently from age or disease duration.37 This result is consistent with previous results in SSc and strengthens the need for a careful assessment of all skin manifestations of SSc, as nonfibrotic skin manifestations of the disease may also inform on the risk of visceral manifestations.4 This association of skin telangiectasias with some cardiac manifestations and digestive involvement in ssSSc also strengthens the hypothesis that SSc-related vasculopathy is involved in the pathogenesis of these visceral manifestations.34,35,38
The ssSSc subtype should not be neglected considering the prevalence of severe visceral manifestations. Our study reveals a high prevalence of ILD in patients with ssSSc, as ILD and/or lung fibrosis was reported in almost 40% of included patients. This important finding supports that even patients with SSc without skin fibrosis should be investigated at baseline by HRCT.39,40,41,42 Although ILD was less severe in patients with ssSSc than in other cutaneous subsets, the high prevalence of ILD in ssSSc also suggests that the pathogenesis driving skin fibrosis and lung fibrosis may differ.43 Almost 3% of patients with ssSSc had scleroderma renal crisis, and this prevalence was not different in patients with dcSSc (5%; P > .99). These results confirm the systemic nature of SSc with widespread visceral involvement in the sine scleroderma subset as well. From a nosological viewpoint, our data strengthen the need to abandon the naming scleroderma and to systematically prefer using systemic sclerosis to designate the disease and its related visceral manifestations, such as SSc-ILD or SSc renal crisis, since these manifestations are not uncommon in patients with sine scleroderma, ie, patients without scleroderma.2,44 As recently proposed for the taxonomy of morphea (ie, localized scleroderma) in JAMA Dermatology,44,45 our data suggest that the nosological frame of SSc should be revised, and the term SSc should definitively replace scleroderma when designating this systemic autoimmune disease. Beyond these considerations on naming, there is a current initiative for a revision of SSc subsets, from the 1988 LeRoy classification based on skin involvement (ie, lcSSc vs dcSSc) to a more refined classification including autoantibodies and gene expression patterns to predict clinical trajectories in patients with SSc.2,3,46,47,48,49,50 Our study strengthens the relevance of autoantibody subtypes to predict clinical trajectories, as the presence of anti–Scl-70 antibodies (antitopoisomerase I antibodies) was an independent risk factor of the onset of skin fibrosis in patients with ssSSc. Moreover, in univariate analyses, anti–Scl-70 antibodies were associated with ILD, and anti-U1RNP antibodies were associated with DUs in patients with ssSSc, strengthening the relevance of using autoantibody subtypes to define specific phenotypes within the subsets defined by the extent of skin fibrosis. The specific gene signatures in the skin or blood are still to be further explored in patients with ssSSc but may participate in implementing personalized medicine in SSc by refining the current subsets.5,51
Limitations and Strengths
Our study comes with limitations, including our selection strategy of patients with ssSSc in EUSTAR: only considering patients with mRSS of 0 at all visits may have led to a selection bias and to underestimation of the prevalence of ssSSc; isolated puffy fingers with no skin fibrosis can be rated as Rodnan 2 by some experts, and patients with puffy fingers and Rodnan skin score 1 or 2 (1 for fingers on 1 or both hands) may therefore either match the definition of ssSSc or lcSSc. We thus decided to exclude these patients from our selection strategy of patients with ssSSc to ensure that no patients with lcSSc were wrongly classified as having ssSSc in our study. The prevalence of ssSSc could then be even higher than 10%, supporting the importance of further considering and better characterizing the ssSSc subset. Despite the large sample size allowed by the EUSTAR cohort (n = 19 115 patients with SSc), the number of patients with dcSSc to be paired with patients with ssSSc with similar disease duration was limited, and only 354 patients with ssSSc were included in the comparison analysis to preserve the 1 patient with ssSSc for 2 patients with dcSSc ratio. The EUSTAR database does not include data on itching, specific cause of death, or overlap syndromes; thus, we could not further explore these questions. The number of missing data on Nailfold capillaroscopy and the lack of systematic screening for calcinosis in EUSTAR precluded relevant analyses regarding these parameters.
The strengths of this study include its large ssSSc sample size. To our knowledge, it is the largest study ever conducted on this specific subtype, allowing unprecedented subgroup analyses with statistical power to explore skin manifestations and survival differences with lcSSc and dcSSc. To our knowledge, this study is the first international multicenter study conducted on patients with ssSSc, offering greater generalizability than previous studies.
Conclusions
In this cohort study, ssSSc accounted for almost 10% of all patients with SSc. The positivity for anti–Scl-70 was the only independent parameters associated with the onset of skin fibrosis in ssSSc, strengthening the relevance of antibody subtypes to predict the trajectory of skin involvement in patients with SSc. Systemic sclerosis sine scleroderma should not be neglected, considering the high prevalence of ILD (>40%) and of scleroderma renal crisis (almost 3%). Dermatologists should be aware of the prevalence of these visceral associations in ssSSc and their associations with cutaneous findings. Even in patients without skin fibrosis, the assessment of other dermatological features, such as skin telangiectasias, is of utmost importance, as such nonfibrotic manifestations were also associated with visceral manifestations, such as diastolic dysfunction. Acknowledging the specific prognosis and phenotype of ssSSc is among the necessary steps toward precision medicine and updated classification for SSc, and the term systemic sclerosis should be systematically preferred to scleroderma when designating this systemic autoimmune disease to reflect the risk of organ involvement even in patients without skin fibrosis, ie, sine (without) scleroderma.2,5,13,47,52
eMethods
eFigure: Flow Chart and selection strategy
eTable 1: Clinical characteristics associated with Puffy fingers (ever) in sine scleroderma patients (ssSSc)
eTable 2: Comparison of the clinical characteristics of ssSSc with lcSSc and dcSSc with same disease duration at last available visit
eTable 3: Comparison of the clinical characteristics of ssSSc (with Lung fibrosis/ILD) with lcSSc with Lung fibrosis/ILD) and dcSSc with Lung fibrosis/ILD)
eTable 4: Clinical characteristics associated with ILD and/or Lung Fibrosis ever (i.e. history of ILD and/or Lung Fibrosis at inclusion and/or ILD and/or Lung Fibrosis during follow-up) in sine scleroderma patients (ssSSc)
EUSTAR collaborators
Data Sharing Statement
References
- 1.Denton CP, Khanna D. Systemic sclerosis. Lancet. 2017;390(10103):1685-1699. doi: 10.1016/S0140-6736(17)30933-9 [DOI] [PubMed] [Google Scholar]
- 2.Lescoat A, Cavalin C, Ehrlich R, et al. The nosology of systemic sclerosis: how lessons from the past offer new challenges in reframing an idiopathic rheumatological disorder. Lancet Rheumatol. 2019;1(4):e257-e264. doi: 10.1016/S2665-9913(19)30038-4 [DOI] [PubMed] [Google Scholar]
- 3.Sobanski V, Lescoat A, Launay D. Novel classifications for systemic sclerosis: challenging historical subsets to unlock new doors. Curr Opin Rheumatol. 2020;32(6):463-471. doi: 10.1097/BOR.0000000000000747 [DOI] [PubMed] [Google Scholar]
- 4.Jouvray M, Launay D, Dubucquoi S, et al. Whole-body distribution and clinical association of telangiectases in systemic sclerosis. JAMA Dermatol. 2018;154(7):796-805. doi: 10.1001/jamadermatol.2018.0916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lescoat A, Varga J, Matucci-Cerinic M, Khanna D. New promising drugs for the treatment of systemic sclerosis: pathogenic considerations, enhanced classifications, and personalized medicine. Expert Opin Investig Drugs. 2021;30(6):635-652. doi: 10.1080/13543784.2021.1923693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.LeRoy EC, Black C, Fleischmajer R, et al. Scleroderma (systemic sclerosis): classification, subsets and pathogenesis. J Rheumatol. 1988;15(2):202-205. [PubMed] [Google Scholar]
- 7.Medsger TA Jr, Benedek TG. History of skin thickness assessment and the Rodnan skin thickness scoring method in systemic sclerosis. J Scleroderma Relat Disord. 2019;4(2):83-88. doi: 10.1177/2397198318823122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ioannidis JPA, Vlachoyiannopoulos PG, Haidich AB, et al. Mortality in systemic sclerosis: an international meta-analysis of individual patient data. Am J Med. 2005;118(1):2-10. doi: 10.1016/j.amjmed.2004.04.031 [DOI] [PubMed] [Google Scholar]
- 9.Rodnan GP, Fennell RH Jr. Progressive systemic sclerosis sine scleroderma. JAMA. 1962;180:665-670. doi: 10.1001/jama.1962.03050210027006 [DOI] [PubMed] [Google Scholar]
- 10.Diab S, Dostrovsky N, Hudson M, et al. ; Canadian Scleroderma Research Group . Systemic sclerosis sine scleroderma: a multicenter study of 1417 subjects. J Rheumatol. 2014;41(11):2179-2185. doi: 10.3899/jrheum.140236 [DOI] [PubMed] [Google Scholar]
- 11.Marangoni RG, Rocha LF, Del Rio APT, Yoshinari NH, Marques-Neto JF, Sampaio-Barros PD. Systemic sclerosis sine scleroderma: distinct features in a large Brazilian cohort. Rheumatology (Oxford). 2013;52(8):1520-1524. doi: 10.1093/rheumatology/ket163 [DOI] [PubMed] [Google Scholar]
- 12.Poormoghim H, Lucas M, Fertig N, Medsger TA Jr. Systemic sclerosis sine scleroderma: demographic, clinical, and serologic features and survival in forty-eight patients. Arthritis Rheum. 2000;43(2):444-451. doi: [DOI] [PubMed] [Google Scholar]
- 13.Nihtyanova SI, Sari A, Harvey JC, et al. Using autoantibodies and cutaneous subset to develop outcome-based disease classification in systemic sclerosis. Arthritis Rheumatol. 2020;72(3):465-476. doi: 10.1002/art.41153 [DOI] [PubMed] [Google Scholar]
- 14.Johnson SR, Soowamber ML, Fransen J, et al. There is a need for new systemic sclerosis subset criteria: a content analytic approach. Scand J Rheumatol. 2018;47(1):62-70. doi: 10.1080/03009742.2017.1299793 [DOI] [PubMed] [Google Scholar]
- 15.Allanore Y. Limited cutaneous systemic sclerosis: the unfairly neglected subset. J Scleroderma Relat Disord. 2016;1:241-246. doi: 10.5301/jsrd.5000216 [DOI] [Google Scholar]
- 16.Lescoat A, Roofeh D, Townsend W, et al. Domains and outcome measures for the assessment of limited cutaneous systemic sclerosis: a scoping review protocol. BMJ Open. 2021;11(3):e044765. doi: 10.1136/bmjopen-2020-044765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lescoat A, Murphy SL, Chen YT, et al. Symptom experience of limited cutaneous systemic sclerosis from the patients’ perspective: a qualitative study. Semin Arthritis Rheum. 2022;52:151926. doi: 10.1016/j.semarthrit.2021.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lescoat A, Sandler RD, Zimmermann F, et al. Domains and outcome measures for the assessment of limited cutaneous systemic sclerosis: an international collaborative scoping review. Rheumatology (Oxford). 2022;61(8):3132-3148. doi: 10.1093/rheumatology/keac049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zanatta E, Huscher D, Ortolan A, et al. ; EUSTAR collaborators . Phenotype of limited cutaneous systemic sclerosis patients with positive anti-topoisomerase I antibodies: data from the EUSTAR cohort. Rheumatology (Oxford). 2022;61(12):4786-4796. doi: 10.1093/rheumatology/keac188 [DOI] [PubMed] [Google Scholar]
- 20.Karanth R, Abignano G, Kakkar V, Ross R, Denton C, Del Galdo F. Serum IFN score predicts long term outcome in limited cutaneous SSc. ACR Meeting Abstract No. 1856. Accessed November 7, 2021. https://acrabstracts.org/abstract/serum-ifn-score-predicts-long-term-outcome-in-limited-cutaneous-ssc/
- 21.Frantz C, Huscher D, Avouac J, et al. ; EUSTAR co-authors . Outcomes of limited cutaneous systemic sclerosis patients: Results on more than 12,000 patients from the EUSTAR database. Autoimmun Rev. 2020;19(2):102452. doi: 10.1016/j.autrev.2019.102452 [DOI] [PubMed] [Google Scholar]
- 22.Lescoat A, Murphy SL, Roofeh D, et al. Considerations for a combined index for limited cutaneous systemic sclerosis to support drug development and improve outcomes. J Scleroderma Relat Disord. 2021;6(1):66-76. doi: 10.1177/2397198320961967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bellando-Randone S, Galdo FD, Lepri G, et al. Progression of patients with Raynaud’s phenomenon to systemic sclerosis: a five-year analysis of the European Scleroderma Trial and Research group multicentre, longitudinal registry study for Very Early Diagnosis of Systemic Sclerosis (VEDOSS). Lancet Rheumatol. 2021;3(12):e834-e843. doi: 10.1016/S2665-9913(21)00244-7 [DOI] [PubMed] [Google Scholar]
- 24.Meier FMP, Frommer KW, Dinser R, et al. ; EUSTAR Co-authors . Update on the profile of the EUSTAR cohort: an analysis of the EULAR Scleroderma Trials and Research group database. Ann Rheum Dis. 2012;71(8):1355-1360. doi: 10.1136/annrheumdis-2011-200742 [DOI] [PubMed] [Google Scholar]
- 25.Preliminary criteria for the classification of systemic sclerosis (scleroderma). Subcommittee for scleroderma criteria of the American Rheumatism Association Diagnostic and Therapeutic Criteria Committee. Arthritis Rheum. 1980;23(5):581-590. doi: 10.1002/art.1780230510 [DOI] [PubMed] [Google Scholar]
- 26.van den Hoogen F, Khanna D, Fransen J, et al. 2013 Classification criteria for systemic sclerosis: an American College of Rheumatology/European League against Rheumatism collaborative initiative. Arthritis Rheum. 2013;65(11):2737-2747. doi: 10.1002/art.38098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hunzelmann N, Genth E, Krieg T, et al. ; Registry of the German Network for Systemic Scleroderma . The registry of the German Network for Systemic Scleroderma: frequency of disease subsets and patterns of organ involvement. Rheumatology (Oxford). 2008;47(8):1185-1192. doi: 10.1093/rheumatology/ken179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tolosa-Vilella C, Morera-Morales ML, Simeón-Aznar CP, et al. ; RESCLE Investigators, Autoimmune Diseases Study Group (GEAS) . Digital ulcers and cutaneous subsets of systemic sclerosis: clinical, immunological, nailfold capillaroscopy, and survival differences in the Spanish RESCLE Registry. Semin Arthritis Rheum. 2016;46(2):200-208. doi: 10.1016/j.semarthrit.2016.04.007 [DOI] [PubMed] [Google Scholar]
- 29.Kuzumi A, Ebata S, Fukasawa T, et al. Long-term z-up of the DESIRES Trial With a Focus on Serum Immunoglobulin Levels. JAMA Dermatol. 2023;159(4):374-383. doi: 10.1001/jamadermatol.2022.6340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ebata S, Yoshizaki A, Oba K, et al. Safety and efficacy of rituximab in systemic sclerosis (DESIRES): a double-blind, investigator-initiated, randomised, placebo-controlled trial. Lancet Rheumatol. 2021;3(7):e489-e497. doi: 10.1016/S2665-9913(21)00107-7 [DOI] [PubMed] [Google Scholar]
- 31.Jaafar S, Lescoat A, Huang S, et al. Clinical characteristics, visceral involvement, and mortality in at-risk or early diffuse systemic sclerosis: a longitudinal analysis of an observational prospective multicenter US cohort. Arthritis Res Ther. 2021;23(1):170. doi: 10.1186/s13075-021-02548-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lescoat A. Very early diagnosis of systemic sclerosis: deciphering the heterogeneity of systemic sclerosis in the very early stages of the disease. J Scleroderma Relat Disord. 2023;8(1):3-6. doi: 10.1177/23971983221129211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Naredo E, Pascau J, Damjanov N, et al. Performance of ultra-high-frequency ultrasound in the evaluation of skin involvement in systemic sclerosis: a preliminary report. Rheumatology (Oxford). 2020;59(7):1671-1678. doi: 10.1093/rheumatology/kez439 [DOI] [PubMed] [Google Scholar]
- 34.Matucci-Cerinic M, Kahaleh B, Wigley FM. Review: evidence that systemic sclerosis is a vascular disease. Arthritis Rheum. 2013;65(8):1953-1962. doi: 10.1002/art.37988 [DOI] [PubMed] [Google Scholar]
- 35.Bruni C, Frech T, Manetti M, et al. Vascular leaking, a pivotal and early pathogenetic event in systemic sclerosis: should the door be closed? Front Immunol. 2018;9:2045. doi: 10.3389/fimmu.2018.02045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pope JE. Other manifestations of mixed connective tissue disease. Rheum Dis Clin North Am. 2005;31(3):519-533, vii. doi: 10.1016/j.rdc.2005.04.011 [DOI] [PubMed] [Google Scholar]
- 37.Tennøe AH, Murbræch K, Andreassen JC, et al. Left ventricular diastolic dysfunction predicts mortality in patients with systemic sclerosis. J Am Coll Cardiol. 2018;72(15):1804-1813. doi: 10.1016/j.jacc.2018.07.068 [DOI] [PubMed] [Google Scholar]
- 38.Allanore Y, Distler O, Matucci-Cerinic M, Denton CP. Review: defining a unified vascular phenotype in systemic sclerosis. Arthritis Rheumatol. 2018;70(2):162-170. doi: 10.1002/art.40377 [DOI] [PubMed] [Google Scholar]
- 39.Hoffmann-Vold AM, Maher TM, Philpot EE, et al. The identification and management of interstitial lung disease in systemic sclerosis: evidence-based European consensus statements. Lancet Rheumatol. 2020;2:e71-e83. doi: 10.1016/S2665-9913(19)30144-4 [DOI] [PubMed] [Google Scholar]
- 40.Khanna D, Lescoat A, Roofeh D, et al. Systemic sclerosis-associated interstitial lung disease: how to incorporate two Food and Drug Administration-approved therapies in clinical practice. Arthritis Rheumatol. 2022;74(1):13-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Roofeh D, Jaafar S, Vummidi D, Khanna D. Management of systemic sclerosis-associated interstitial lung disease. Curr Opin Rheumatol. 2019;31(3):241-249. doi: 10.1097/BOR.0000000000000592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Roofeh D, Lescoat A, Khanna D. Treatment for systemic sclerosis-associated interstitial lung disease. Curr Opin Rheumatol. 2021;33(3):240-248. doi: 10.1097/BOR.0000000000000795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lescoat A, Roofeh D, Kuwana M, Lafyatis R, Allanore Y, Khanna D. Therapeutic approaches to systemic sclerosis: recent approvals and future candidate therapies. Clin Rev Allergy Immunol. 2021. doi: 10.1007/s12016-021-08891-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Prasad S, Zhu JL, Schollaert-Fitch K, Torok KS, Jacobe HT. An evaluation of the performance of current morphea subtype classifications. JAMA Dermatol. 2021;157(4):1-8. doi: 10.1001/jamadermatol.2020.5809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fett NM. Morphea (localized scleroderma). JAMA Dermatol. 2013;149(9):1124-1124. doi: 10.1001/jamadermatol.2013.5079 [DOI] [PubMed] [Google Scholar]
- 46.Varga J, Hinchcliff M. Connective tissue diseases: systemic sclerosis: beyond limited and diffuse subsets? Nat Rev Rheumatol. 2014;10(4):200-202. doi: 10.1038/nrrheum.2014.22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hinchcliff M, Mahoney JM. Towards a new classification of systemic sclerosis. Nat Rev Rheumatol. 2019;15(8):456-457. doi: 10.1038/s41584-019-0257-z [DOI] [PubMed] [Google Scholar]
- 48.Hinchcliff M, Huang CC, Wood TA, et al. Molecular signatures in skin associated with clinical improvement during mycophenolate treatment in systemic sclerosis. J Invest Dermatol. 2013;133(8):1979-1989. doi: 10.1038/jid.2013.130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Skaug B, Khanna D, Swindell WR, et al. Global skin gene expression analysis of early diffuse cutaneous systemic sclerosis shows a prominent innate and adaptive inflammatory profile. Ann Rheum Dis. 2020;79(3):379-386. doi: 10.1136/annrheumdis-2019-215894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sobanski V, Giovannelli J, Allanore Y, et al. ; EUSTAR Collaborators . Phenotypes determined by cluster analysis and their survival in the prospective European scleroderma trials and research cohort of patients with systemic sclerosis. Arthritis Rheumatol. 2019;71(9):1553-1570. doi: 10.1002/art.40906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Khanna D, Spino C, Johnson S, et al. Abatacept in early diffuse cutaneous systemic sclerosis: results of a phase II investigator-initiated, multicenter, double-blind, randomized, placebo-controlled trial. Arthritis Rheumatol. 2020;72(1):125-136. doi: 10.1002/art.41055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Elhai M, Sritharan N, Boubaya M, et al. Stratification in systemic sclerosis according to autoantibody status versus skin involvement: a study of the prospective EUSTAR cohort. Lancet Rheumatol. 2022;4(11):e785-e794. doi: 10.1016/S2665-9913(22)00217-X [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods
eFigure: Flow Chart and selection strategy
eTable 1: Clinical characteristics associated with Puffy fingers (ever) in sine scleroderma patients (ssSSc)
eTable 2: Comparison of the clinical characteristics of ssSSc with lcSSc and dcSSc with same disease duration at last available visit
eTable 3: Comparison of the clinical characteristics of ssSSc (with Lung fibrosis/ILD) with lcSSc with Lung fibrosis/ILD) and dcSSc with Lung fibrosis/ILD)
eTable 4: Clinical characteristics associated with ILD and/or Lung Fibrosis ever (i.e. history of ILD and/or Lung Fibrosis at inclusion and/or ILD and/or Lung Fibrosis during follow-up) in sine scleroderma patients (ssSSc)
EUSTAR collaborators
Data Sharing Statement