This cohort study evaluates whether coronary atherosclerotic plaque activity as assessed by noninvasive imaging is associated with coronary events in patients with myocardial infarction.
Key Points
Question
Is coronary atherosclerotic plaque activity (assessed by 18F-sodium fluoride positron emission tomography) associated with coronary events in patients with myocardial infarction?
Findings
In this cohort study of 704 patients with myocardial infarction, coronary atherosclerotic plaque activity was not associated with the primary composite end point of cardiac death, nonfatal myocardial infarction, or revascularization. In a secondary analysis, elevated plaque activity was associated with the composite end point of cardiac death or nonfatal myocardial infarction.
Meaning
The findings suggest that coronary atherosclerotic plaque activity is not associated with cardiac death, nonfatal myocardial infarction, or revascularization.
Abstract
Importance
Recurrent coronary events in patients with recent myocardial infarction remain a major clinical problem. Noninvasive measures of coronary atherosclerotic disease activity have the potential to identify individuals at greatest risk.
Objective
To assess whether coronary atherosclerotic plaque activity as assessed by noninvasive imaging is associated with recurrent coronary events in patients with myocardial infarction.
Design, Setting, and Participants
This prospective, longitudinal, international multicenter cohort study recruited participants aged 50 years or older with multivessel coronary artery disease and recent (within 21 days) myocardial infarction between September 2015 and February 2020, with a minimum 2 years’ follow-up.
Intervention
Coronary 18F-sodium fluoride positron emission tomography and coronary computed tomography angiography.
Main Outcomes and Measures
Total coronary atherosclerotic plaque activity was assessed by 18F-sodium fluoride uptake. The primary end point was cardiac death or nonfatal myocardial infarction but was expanded during study conduct to include unscheduled coronary revascularization due to lower than anticipated primary event rates.
Results
Among 2684 patients screened, 995 were eligible, 712 attended for imaging, and 704 completed an interpretable scan and comprised the study population. The mean (SD) age of participants was 63.8 (8.2) years, and most were male (601 [85%]). Total coronary atherosclerotic plaque activity was identified in 421 participants (60%). After a median follow-up of 4 years (IQR, 3-5 years), 141 participants (20%) experienced the primary end point: 9 had cardiac death, 49 had nonfatal myocardial infarction, and 83 had unscheduled coronary revascularizations. Increased coronary plaque activity was not associated with the primary end point (hazard ratio [HR], 1.25; 95% CI, 0.89-1.76; P = .20) or unscheduled revascularization (HR, 0.98; 95% CI, 0.64-1.49; P = .91) but was associated with the secondary end point of cardiac death or nonfatal myocardial infarction (47 of 421 patients with high plaque activity [11.2%] vs 19 of 283 with low plaque activity [6.7%]; HR, 1.82; 95% CI, 1.07-3.10; P = .03) and all-cause mortality (30 of 421 patients with high plaque activity [7.1%] vs 9 of 283 with low plaque activity [3.2%]; HR, 2.43; 95% CI, 1.15-5.12; P = .02). After adjustment for differences in baseline clinical characteristics, coronary angiography findings, and Global Registry of Acute Coronary Events score, high coronary plaque activity was associated with cardiac death or nonfatal myocardial infarction (HR, 1.76; 95% CI, 1.00-3.10; P = .05) but not with all-cause mortality (HR, 2.01; 95% CI, 0.90-4.49; P = .09).
Conclusions and Relevance
In this cohort study of patients with recent myocardial infarction, coronary atherosclerotic plaque activity was not associated with the primary composite end point. The findings suggest that risk of cardiovascular death or myocardial infarction in patients with elevated plaque activity warrants further research to explore its incremental prognostic implications.
Introduction
Recurrent coronary events are common following acute myocardial infarction (MI) but are challenging to predict. Clinical risk scores, such as the Global Registry of Acute Coronary Events (GRACE) score,1 estimate risk of early events but have limitations and lack precision.2,3 The presence of obstructive coronary artery disease has also been seen as a major factor associated with future risk, leading to strategies of coronary revascularization to reduce subsequent events.4,5 However, most index MIs arise from nonobstructive coronary plaques, and recurrent events commonly occur at sites remote from the culprit plaque.5,6,7 This has led to attempts to detect high-risk coronary artery plaques that drive downstream events and, thereby, identify patients at risk of future coronary events.8 Previous studies have assessed coronary plaque characteristics using invasive imaging approaches, including intravascular ultrasonography either alone6 or in combination with near-infrared spectroscopy.7 Coronary plaques associated with high-risk features, such as thin-cap fibroatheroma or lipid-rich plaque, are associated with future coronary events, especially those associated with subsequent coronary revascularization.6,7 However, these techniques are impractical for widespread application because of the requirement for direct instrumentation of the coronary arteries with its attendant risks.
Advances in noninvasive imaging have enabled the assessment of coronary anatomic and biologic features without the need to instrument the coronary arteries. Coronary computed tomography (CT) angiography has comparable accuracy to invasive coronary angiography9 and is more sensitive at detecting coronary atheroma.10 When complemented by positron emission tomography (PET), the anatomic and biologic features of coronary artery plaque can be assessed simultaneously to identify coronary atherosclerotic plaque activity.11,12 We and others have previously shown that combined 18F-sodium fluoride PET and coronary CT angiography can identify high-risk and active coronary atherosclerotic plaque in patients with recent MI.11,13,14 Coronary artery 18F-sodium fluoride uptake is a marker of active calcification driven by the lipid-rich necrotic core of the atheromatous plaque15,16,17,18,19 and is associated with progression of coronary calcification.20,21 In retrospective post hoc pooled analyses of patients with cardiovascular disease,22,23 increased coronary 18F-sodium fluoride uptake was associated with an increased risk of fatal and nonfatal MI. We therefore wished to establish whether this technique was generalizable and sufficiently robust for clinical application. In a regulated, international, multicenter, prospective cohort study, we aimed to assess whether combined 18F-sodium fluoride PET and coronary CT angiography would be associated with future risk of coronary events in patients with recent MI.
Methods
Study Design
This was an international, multicenter, prospective longitudinal cohort study conducted in 9 centers across 4 countries (eTable 1 in Supplement 1) between September 2015 and February 2020 and was overseen by the Edinburgh Clinical Trials Unit and an independent trial steering committee. The study was performed under a clinical trial authorization from the Medicines and Healthcare products Regulatory Agency, with approval from the South East Scotland Research Ethics Committee in accordance with the Declaration of Helsinki24 and with the written informed consent of each participant. The study has been reported in line with the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline.
Study Population
The study population consisted of patients aged 50 years or older with a recent (within 21 days) type 1 MI and multivessel coronary artery disease shown on invasive coronary angiography, defined as at least 2 major epicardial vessels with either more than 50% luminal stenosis or previous coronary revascularization (percutaneous coronary intervention or coronary artery bypass graft surgery). Exclusion criteria included the inability or unwillingness to give informed consent; pregnancy, breastfeeding, or child-bearing potential; major intercurrent illness with life expectancy less than 2 years; kidney dysfunction (estimated glomerular filtration rate ≤30 mL/min/1.73 m2); atrial fibrillation; or contraindication to iodinated contrast media, PET, or CT.
Image Acquisition
Study participants were administered a target dose of 250 MBq 18F-sodium fluoride intravenously and rested in a quiet environment for 60 minutes. Participants underwent an attenuation-correction CT scan followed by a dual cardiac- and respiratory-gated PET scan of the thorax in list mode for 30 minutes.11,13,22,23 Thereafter, electrocardiography-gated coronary CT angiography was undertaken in held expiration either on the same hybrid scanner or using an alternative CT scanner optimized for coronary angiography (eTable 2 in Supplement 1).25 When required, patients received an oral or intravenous β-blockade, such as metoprolol, 5 to 100 mg, to slow the heart rate below 65 beats per minute to maximize image quality and facilitate prospective gating to reduce radiation exposure. A glyceryl trinitrate spray or tablet was administered sublingually (200-400 μg) to induce coronary vasodilatation to enhance image quality of the coronary angiogram. Injected activity and CT dose-length product were recorded. Effective radiation dose was calculated using a conversion factor of 0.024 mSv per MBq for 18F-sodium fluoride and 0.014 mSv per Gy cm for CT.26,27
Image Analysis
All data were anonymized before transfer to the core laboratory for analysis. Coronary CT angiography findings were analyzed according to the Society of Cardiovascular Computed Tomography guidelines using the 2022 Coronary Artery Disease–Reporting and Data System (CAD-RADS 2.0) score.28 The list mode data sets of the PET scans were reconstructed into 10 electrocardiography-gated bins using a standard ordered expectation maximization algorithm with time of flight and the point spread function correction.29,30 Coronary PET image analysis was performed using dedicated software (FusionQuant, Cedars-Sinai Medical Center) as described previously.31,32,33,34 In brief, we extracted whole-vessel tubular and 3-dimensional volumes of interest (4-mm radius) from the CT angiogram and used these to measure the coronary microcalcification activity (CMA) on the PET scan. This finding represents the overall coronary atherosclerotic plaque activity based on both the volume and intensity of 18F-sodium fluoride uptake, analogous to the Agatston score used for coronary artery calcium scoring (eFigure 1 in Supplement 1). All investigator site staff and study participants were blinded to the CMA findings.
Clinical Follow-up and Outcomes
Participants were followed up by site investigators until the last recruited patient had completed their 2-year follow-up visit. Because of concealment of the CMA findings, clinical outcomes were reported by site investigators according to a standardized clinical proforma.35,36 The primary clinical outcomes of interest were cardiac death or nonfatal MI, but this was expanded during study conduct to include unscheduled coronary revascularization due to lower than anticipated event rates. The latter was defined as any coronary revascularization that occurred beyond 6 weeks from the screening visit to exclude planned staged revascularization procedures.
Sample Size
At study inception, the primary end point was cardiac death or recurrent nonfatal MI. Given the inclusion criteria of patients with multivessel disease, we anticipated that approximately one-third of participants would have low coronary atherosclerotic plaque activity (CMA = 0)22,23 and an event rate of 20% and two-thirds would have increased coronary atherosclerotic plaque activity (CMA>0) and an event rate of 30%. For 80% power and 2-sided P < .05, we estimated a sample size of 692. As the time-to-first event analysis would require approximately 10% fewer patients, this would allow for 10% missing data. During study conduct, review of the total study population demonstrated a lower than anticipated event rate. The trial steering committee recommended an extended follow-up and the inclusion of unscheduled coronary revascularization into the primary end point on the basis that increased coronary atherosclerotic plaque activity may be associated with disease progression and coronary revascularization.6,7
Statistical Analysis
Categorical data are presented as number (percentage) and continuous variables as mean (SD) or median (IQR). The primary end point was defined as the composite of cardiac death, nonfatal recurrent MI, or unscheduled coronary revascularization. Secondary analyses were performed for all-cause death, the original primary end point of cardiac death or MI, and each of the components of the primary end point. The association of active coronary atherosclerotic plaque (CMA = 0 vs CMA>0) with the time to first event was assessed using cumulative incidence plots and a log-rank test as well as hazard ratios (HRs) with 95% CIs using Cox proportional hazards regression analysis. Requested post hoc analyses included comparisons of baseline characteristics of participants’ clinical profile and coronary CT angiography findings as well as further Cox proportional hazards regression models to explore adjustments for clinical characteristics (in which P < .10 between participants with [CMA>0] or without [CMA = 0] plaque activity), CAD-RADS 2.0 score, GRACE score, and the severity of obstructive coronary artery disease. Statistical significance was taken as a 2-sided P < .05. For post hoc analyses, P values should be considered indicative only. Statistical analyses were performed using SAS, version 9.4 (SAS Institute Inc).
Results
Study Population
Among 2684 patients screened, 995 were eligible, and a total of 712 participants were recruited and attended for baseline 18F-sodium fluoride PET and CT scans. Of these, 6 participants received the radiotracer but were unable to complete the scan, and 2 patients were scanned but image reconstruction could not be completed (eFigure 2 in Supplement 1). The study population comprised 704 patients (mean [SD] age, 63.8 [8.2] years; 103 [15] female; 601 [85%] male) with a high prevalence of cardiovascular risk factors receiving guideline-directed medical therapy, in whom 671 (95%) underwent index coronary revascularization (Table 1). Identifiable coronary atherosclerotic plaque activity (CMA>0) was seen in 421 participants (60%), who had clinical profiles, CAD-RADS 2.0 scores, mean GRACE scores, and severity of coronary artery disease broadly similar to those of 283 participants (40%) without demonstrable activity (CMA = 0) (Table 1).
Table 1. Baseline Characteristics of the Study Population.
| Characteristic | Participantsa | P valued | ||
|---|---|---|---|---|
| Total (N = 704) | Low coronary atherosclerotic plaque activity (n = 283)b | High coronary atherosclerotic plaque activity (n = 421)c | ||
| Age, mean (SD), y | 63.8 (8.2) | 61.8 (7.4) | 65.1 (8.4) | <.001 |
| Sex | ||||
| Female | 103 (15) | 61 (22) | 42 (10) | <.001 |
| Male | 601 (85) | 222 (78) | 379 (90) | |
| Body mass index, mean (SD)e | 28.3 (4.4) | 28.6 (4.7) | 28.1 (4.2) | .11 |
| Cardiovascular risk factors | ||||
| Smoking status | ||||
| Current | 193 (27) | 90 (32) | 103 (24) | .06 |
| Former | 225 (32) | 91 (32) | 134 (32) | |
| Never | 286 (41) | 102 (36) | 184 (44) | |
| Hypertension | 351 (50) | 119 (42) | 232 (55) | <.001 |
| Hypercholesterolemia | 398 (57) | 162 (58) | 236 (56) | .62 |
| Diabetes | 118 (17) | 40 (14) | 78 (19) | .15 |
| Prior cardiovascular disease | ||||
| Coronary artery disease | 139 (20) | 41 (14) | 98 (23) | .01 |
| Myocardial infarction | 102 (14) | 36 (13) | 66 (16) | .33 |
| Percutaneous coronary intervention | 100 (14) | 28 (10) | 72 (17) | .01 |
| Coronary artery bypass graft surgery | 31 (4) | 12 (4) | 19 (5) | >.99 |
| Peripheral vascular disease | 21 (3) | 12 (4) | 9 (2) | .17 |
| Cerebrovascular disease | 33 (5) | 10 (4) | 23 (5) | .31 |
| Electrocardiography findingsf | ||||
| ST-segment elevation myocardial infarction | 463 (66) | 189 (67) | 274 (65) | .76 |
| Non–ST-segment elevation myocardial infarction | 239 (34) | 94 (33) | 145 (35) | |
| GRACE score, mean (SD) | 118 (25) | 113 (22) | 121 (26) | <.001 |
| Severity of obstructive coronary artery diseaseg | ||||
| 1 Vessel | 28 (4) | 12 (4) | 16 (4) | .64 |
| 2 Vessels | 387 (55) | 163 (58) | 224 (53) | |
| 3 Vessels | 239 (34) | 90 (32) | 149 (35) | |
| Left main stem disease | 50 (7) | 18 (6) | 32 (8) | |
| Percutaneous coronary intervention | 671 (95) | 267 (94) | 404 (96) | .42 |
| CT coronary angiography, CAD-RADS 2.0 scoreh | ||||
| 0 | 31 (4) | 21 (7) | 10 (2) | <.001 |
| 1 or 2 | ||||
| P1/2 | 108 (15) | 52 (18) | 56 (13) | |
| P3/4 | 59 (8) | 18 (6) | 41 (10) | |
| 3 | ||||
| P1/2 | 64 (9) | 31 (11) | 33 (8) | |
| P3/4 | 119 (17) | 46 (16) | 73 (17) | |
| 4 or 5 | ||||
| P1/2 | 51 (7) | 27 (10) | 24 (6) | |
| P3/4 | 272 (39) | 88 (31) | 184 (44) | |
| Medication | ||||
| Aspirin | 673 (96) | 268 (95) | 405 (96) | .45 |
| P2Y12 receptor antagonist | 688 (98) | 279 (99) | 409 (97) | .32 |
| Anticoagulant therapy | 42 (6) | 17 (6) | 25 (6) | >.99 |
| Statin | 653 (93) | 260 (92) | 393 (93) | .55 |
| ACE inhibitor or ARB | 623 (88) | 250 (88) | 373 (89) | >.99 |
| β-Adrenergic receptor antagonist | 573 (82) | 233 (82) | 340 (81) | .67 |
| Calcium-channel antagonist | 64 (9) | 19 (7) | 45 (11) | .10 |
| Nitrate | 384 (55) | 158 (56) | 226 (54) | .63 |
| Other antianginal therapy | 22 (3) | 8 (3) | 14 (3) | .88 |
| Mineralocorticoid receptor antagonist | 42 (6) | 21 (7) | 21 (5) | .24 |
| Other diuretic therapy | 54 (8) | 22 (8) | 32 (8) | >.99 |
Abbreviations: ACE, angiotensin-converting enzyme; ARB, angiotensin receptor blocker; CAD-RADS 2.0, 2022 Coronary Artery Disease–Reporting and Data System28; CMA, coronary microcalcification activity; CT, computed tomography; GRACE, Global Registry of Acute Coronary Events.1
Data are reported as number (percentage) of participants unless otherwise indicated.
Low coronary atherosclerotic plaque activity was defined as a CMA of 0.
High coronary atherosclerotic plaque activity was defined as a CMA greater than 0.
P value for comparison between low and high coronary atherosclerotic plaque activity (CMA = 0 vs CMA >0) using a 2-sample t test for continuous variables and a χ2 test for categorical variables. This was a post hoc analysis and should be taken as indicative values.
Calculated as weight in kilograms divided by height in meters squared.
Two missing data points.
From index-invasive coronary angiography.
For participants with a residual CAD-RADS 2.0 score of 0, 6 had 2 or more stented vessels and 20 had limited CT coronary angiogram quality.
Clinical Outcomes
Clinical follow-up was available for all study participants. At study completion, follow-up was available for 693 participants (98.4%). Over a median of 4 years (IQR, 3-5 years), there were 2582 patient-years of follow-up, and 141 participants (20%) experienced the composite primary end point; the first event was cardiac death in 9 participants, nonfatal MI in 49, and unscheduled coronary revascularization in 83. There was no difference in the primary end point or its components between those who did and did not have increased coronary atherosclerotic plaque activity (Figure 1 and Table 2). Increased coronary plaque activity was not associated with the primary end point (HR, 1.25; 95% CI, 0.89-1.76; P = .20) or unscheduled revascularization (HR, 0.98; 95% CI, 0.64-1.49; P = .91). In contrast, higher rates of the original primary end point of cardiac death or recurrent nonfatal MI (47 of 421 patients with high plaque activity [11.2%] vs 19 of 283 with low plaque activity [6.7%]) and all-cause death (30 of 421 patients with high plaque activity [7.1%] vs 9 of 283 with low plaque activity [3.2%]) were observed in participants with increased coronary atherosclerotic activity (Figure 2 and Table 2), and increased coronary atherosclerotic activity was associated with these end points (cardiac death or nonfatal myocardial infarction: HR, 1.82 [95% CI, 1.07-3.10; P = .03]; all-cause mortality: HR, 2.43 [95% CI, 1.15-5.12; P = .02]). After adjustment for clinical characteristics, CAD-RADS 2.0 score, GRACE score, or the severity of obstructive coronary artery disease, high coronary plaque activity was associated with cardiac death or nonfatal myocardial infarction (HR, 1.76; 95% CI, 1.00-3.10; P = .05) but not with all-cause mortality (HR, 2.01; 95% CI, 0.90-4.49; P = .09) (Table 3). Findings were similar across quartiles of increased CMA (eTable 3 in Supplement 1).
Figure 1. Cumulative Incidence Plots of the Primary End Points.
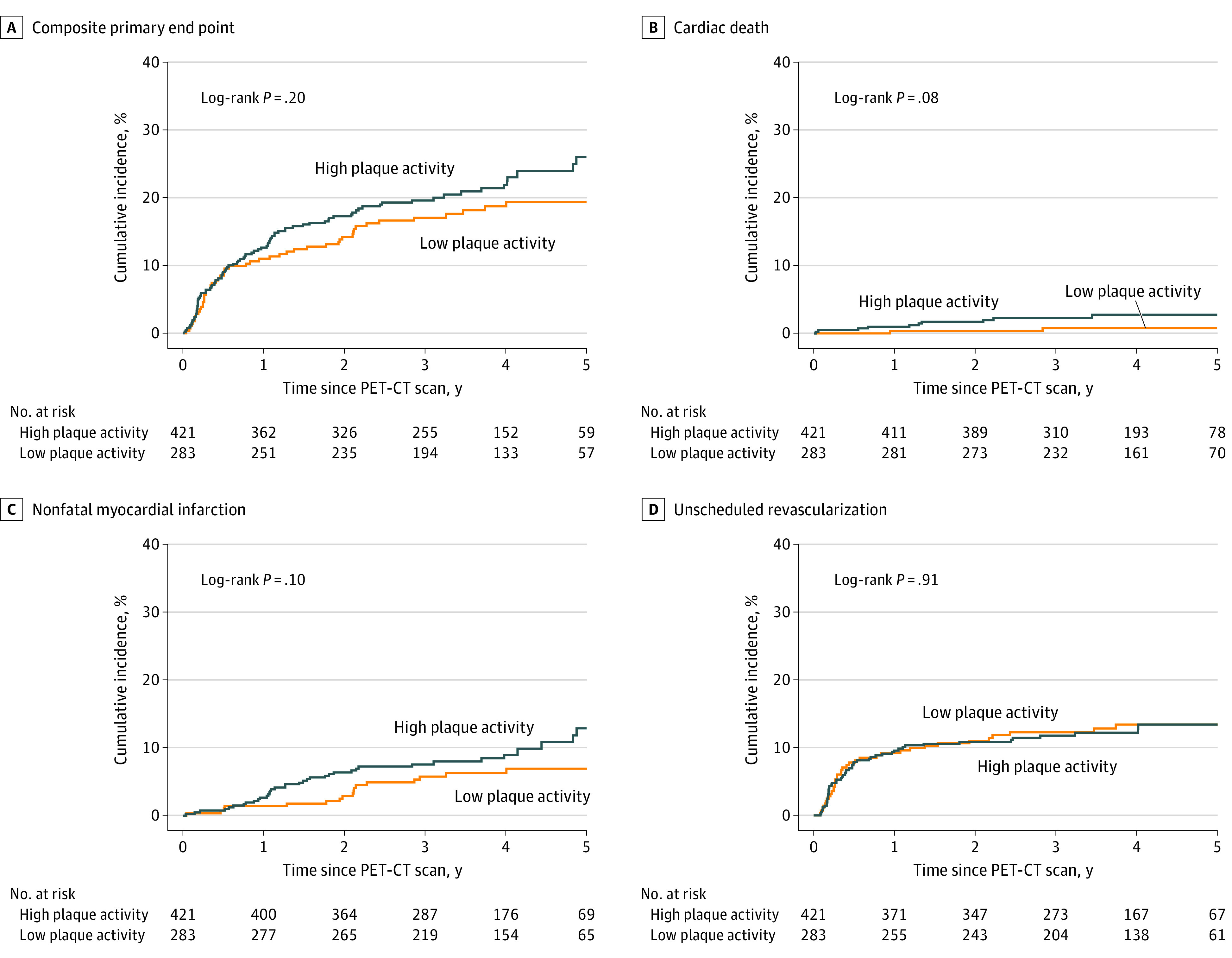
Low coronary atherosclerotic plaque activity was defined as coronary microcalcification activity [CMA] of 0; high coronary atherosclerotic plaque activity was defined as a CMA greater than 0. PET-CT indicates positron emission tomography–computed tomography.
Table 2. Clinical Outcomes in the Study Population.
| Outcome | Participants, No. (%) | Hazard ratio (95% CI) | P value | ||
|---|---|---|---|---|---|
| Total (N = 704) | Low coronary atherosclerotic plaque activity (n = 283)a | High coronary atherosclerotic plaque activity (n = 421)b | |||
| Primary end point | 141 (20.0) | 51 (18.0) | 90 (21.4) | 1.25 (0.89-1.76) | .20 |
| All-cause death | 39 (5.5) | 9 (3.2) | 30 (7.1) | 2.43 (1.15-5.12) | .02 |
| Components of the primary end point | |||||
| Cardiac death | 12 (1.7) | 2 (0.7) | 10 (2.4) | 3.51 (0.77-16.04) | .10 |
| Nonfatal myocardial infarction | 54 (7.7) | 17 (6.0) | 37 (8.8) | 1.61 (0.91-2.86) | .10 |
| Unscheduled coronary revascularization | 87 (12.4) | 36 (12.7) | 51 (12.1) | 0.98 (0.64-1.49) | .91 |
| Cardiac death or nonfatal myocardial infarction | 66 (9.4) | 19 (6.7) | 47 (11.2) | 1.82 (1.07-3.10) | .03 |
Abbreviation: CMA, coronary microcalcification activity.
Low coronary atherosclerotic plaque activity was defined as a CMA of 0.
High coronary atherosclerotic plaque activity was defined as a CMA greater than 0.
Figure 2. Cumulative Incidence Plots of the Secondary End Points.
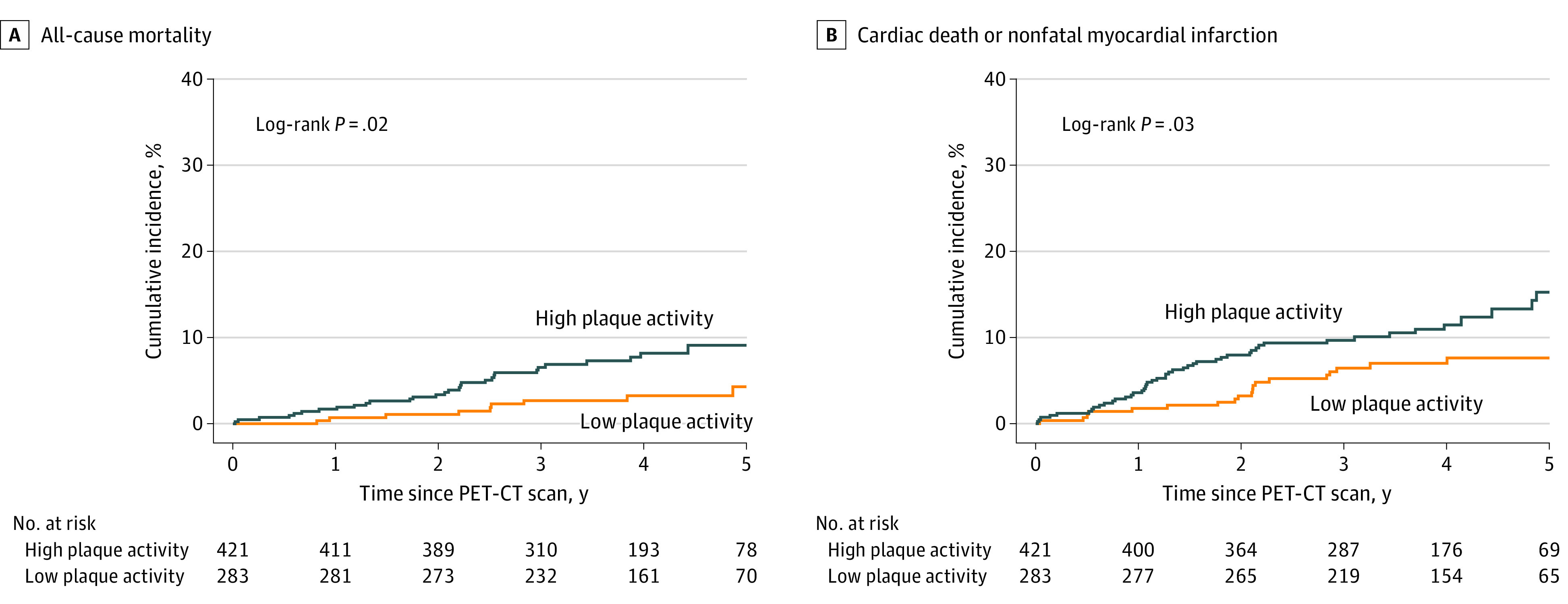
Low coronary atherosclerotic plaque activity was defined as coronary microcalcification activity [CMA] of 0; high coronary atherosclerotic plaque activity was defined as a CMA greater than 0. PET-CT indicates positron emission tomography–computed tomography.
Table 3. Adjusted Analyses for Clinical Outcomesa.
| Adjusted outcome | Adjusted hazard ratio (95% CI) | P valueb |
|---|---|---|
| Cardiac death or nonfatal myocardial infarction | ||
| Age, sex, smoking habit, hypertension, history of coronary artery disease, and prior percutaneous coronary intervention | 1.76 (1.02-3.04) | .04 |
| CAD-RADS 2.0 scorec | 1.78 (1.03-3.06) | .04 |
| GRACE scored | 1.73 (1.01-2.97) | .05 |
| Severity of obstructive coronary artery diseasee | 1.76 (1.03-3.00) | .04 |
| Age, sex, smoking habit, hypertension, history of coronary artery disease, prior percutaneous coronary intervention, CAD-RADS 2.0 score, GRACE score, and severity of obstructive coronary artery diseasec,d,e | 1.76 (1.00-3.10) | .05 |
| All-cause death | ||
| Age, sex, smoking habit, hypertension, history of coronary artery disease, and prior percutaneous coronary intervention | 2.12 (0.98-4.55) | .06 |
| CAD-RADS 2.0 scorec | 2.32 (1.09-4.95) | .03 |
| GRACE scoref | 1.80 (0.84-3.86) | .13 |
| Severity of obstructive coronary artery diseasee | 2.25 (1.06-4.74) | .03 |
| Age, sex, smoking habit, hypertension, history of coronary artery disease, prior percutaneous coronary intervention, CAD-RADS 2.0 score, GRACE score, and severity of obstructive coronary artery diseasec,e,f | 2.01 (0.90-4.49) | .09 |
Abbreviations: CAD-RADS 2.0, 2022 Coronary Artery Disease-Reporting and Data System28; CMA, coronary microcalcification activity; GRACE, Global Registry of Acute Coronary Events.1
Adjusted outcomes are shown for low (CMA = 0) vs high (CMA >0) coronary atherosclerotic plaque activity. A post hoc analysis assessed the association between CMA and cardiac death or nonfatal recurrent myocardial infarction and all-cause death in Cox proportional hazards regression models adjusting for clinical characteristics, CAD-RADS 2.0 score, GRACE score, and invasive angiographic severity of obstructive coronary artery disease.
This was a post hoc analysis, and P values should be taken as indicative values.
The segment involvement score of the CAD-RADS 2.0 represented overall coronary plaque burden.
GRACE risk score for prediction of death or myocardial infarction at 6 months after discharge.
Severity of obstructive coronary artery disease by invasive coronary angiography was categorized into 4 groups: (1) 1 vessel, (2) 2 vessels, (3) 3 vessels, and (4) left main stem disease.
GRACE risk score for prediction of death at 6 months after discharge.
Safety End Point
The safety population was composed of all 712 participants who received the 18F-sodium fluoride radiotracer. The mean (SD) radiation exposure attributable to the radiotracer was 6.0 (0.3) mSv (mean [SD] injected activity, 248 [13] MBq), and the mean (SD) total radiation exposure for the CT scanning protocol was 4.9 (3.0) mSv (mean [SD] dose-length product, 348 [215] Gy cm). Performance of PET and coronary CT angiography was associated with 15 adverse events, which were predominantly iodinated contrast reactions. Two events were graded as serious: palpitation and β-blocker–induced bradycardia (eTable 4 in Supplement 1).
Discussion
The prediction of recurrent coronary events in patients with MI is imprecise and currently relies on clinical risk scores and the presence of obstructive coronary artery disease. In this cohort study, we tested the hypothesis that coronary atherosclerotic plaque activity would identify patients at risk of future coronary events and would be associated with future coronary events. We did not demonstrate that increased coronary atherosclerotic plaque activity was associated with the primary composite end point of cardiac death, nonfatal MI, or unscheduled coronary revascularization. However, it was associated with the secondary end point of cardiac death or nonfatal MI as well as all-cause mortality. These findings are consistent with the importance of coronary atherosclerotic plaque biologic features and activity in the association with spontaneous atherothrombotic events. The findings suggesting risk of cardiovascular death or MI in patients with elevated plaque activity warrant further research to explore its incremental prognostic implications.
Human coronary atherosclerosis is a slow and progressive condition that evolves over years with a central role for the insudation of toxic and inflammatory oxidized lipids into the arterial intima. This leads to a procalcific reaction that attempts to contain and constrain the lipid-rich necrotic plaque and thereby prevent plaque rupture. The early stages of developing microcalcification are markers of high-risk plaques that have the potential to rupture, causing acute coronary occlusion and MI before macrocalcification can contain and stabilize the atherosclerotic plaque.37 This underlies the theoretical basis of 18F-sodium fluoride uptake within coronary atherosclerotic plaques, identifying an active and potentially unstable phase of the disease that appears associated with clinical atherothrombotic events.15,16,17 Uptake of 18F-sodium fluoride is also associated with high-risk plaque features on intravascular ultrasonography and optical coherence tomography,11,14,38,39 and in a retrospective case series of 293 patients with predominantly stable coronary artery disease,22 CMA was associated with future risk of fatal or nonfatal MI. In our prospective cohort study, we found that this noninvasive measure of coronary atherosclerotic plaque activity was associated with the secondary outcome of cardiac death or nonfatal MI. In post hoc analyses, this association was independent of clinical profile, GRACE score, or the severity of obstructive coronary artery disease and underscored the importance of coronary plaque biologic features in the risk of fatal and nonfatal MI.
We found no association between unscheduled coronary revascularization and coronary atherosclerotic plaque activity, and our revised hypothesis that such activity would be associated with unscheduled coronary revascularization was not established. The participant profile and the frequency of revascularization events within our study are consistent with those of the Providing Regional Observations to Study Predictors of Events in the Coronary Tree (PROSPECT) study.6 In that intravascular ultrasonography study, rates of recurrent coronary revascularization were 17%, representing the largest component of the primary end point. This dominance of coronary revascularization events was in keeping with the study’s main findings that plaque burden over 70% and a small luminal area were the key predictors of outcome. However, our findings suggest that such coronary revascularization events are not associated with coronary atherosclerotic plaque activity. Moreover, as with the PROSPECT study,6 we observed that most of the coronary revascularization events occurred within the first year of follow-up. Such a time course would suggest that the predominant factors associated with these revascularization events were the characteristics of the index presentation, coronary anatomic features, and interventional procedures rather than the underlying atherosclerotic plaque activity throughout the coronary circulation. Thus, coronary 18F-sodium fluoride uptake was not associated with coronary revascularization, and as a marker of active calcification that attempts to constrain the atherosclerotic plaque, this was perhaps unsurprising.
We observed an association between coronary atherosclerotic plaque activity and all-cause mortality with a 2-fold to 3-fold increase in risk of death, although there was no association after multivariable adjustment. We also found that coronary atherosclerotic plaque activity was associated with spontaneous coronary events. Although we found lower numbers of events than anticipated, we observed twice the number of cardiac death or nonfatal MI events than prior studies,6,7 likely reflecting our inclusion of patients with multivessel disease and the longer follow-up period. These factors enabled us to explore whether coronary atherosclerotic plaque activity was associated with spontaneous atherothrombotic coronary events rather than relying on surrogates of plaque volumes and coronary revascularization events. We demonstrated the central importance of coronary atherosclerotic plaque activity for these fatal and nonfatal events and that it was independent of the severity of obstructive coronary artery disease. This finding suggests that identification of coronary atherosclerotic plaque activity is associated with the likelihood of recurrent spontaneous coronary events and provides a potential basis for intensification of preventive therapeutic interventions, such as more intensive antiplatelet, lipid-lowering, or anti-inflammatory therapies.
Positron emission tomography is not a straightforward technique, and some may question whether this approach is applicable to widespread clinical practice. However, PET is routinely used in modern oncological practice, and 18F-sodium fluoride is a simple, inexpensive, and readily available radiotracer. Combined with the widespread use of coronary CT angiography in routine cardiological practice, the delivery of such a technique is likely to become readily achievable, particularly as coronary 18F-sodium fluoride PET assessments can be combined with previously acquired coronary CT angiograms.25
Although the severity of coronary artery disease was similar, there were some differences in the patient characteristics between those with and without increased coronary atherosclerotic plaque activity. Those with increased activity were, on average, 3 years older and more likely to be male and to have a higher frequency of hypertension and prior diagnosis of coronary artery disease. These overall differences were not surprising given their known association with coronary artery disease and their potential role in promoting atherosclerotic plaque activity. Moreover, these differences are consistent with contemporary, prospective registry data of over 3000 patients with recent MI.40 Patients with recurrent coronary events were also older, more likely to be male, and had a higher frequency of hypertension and prior coronary artery disease. It may therefore have been unexpected and incongruous if coronary atherosclerotic plaque activity had not corresponded with these characteristics. The current standard of care uses the GRACE score for risk prediction, which in large meta-analyses may have the best predictive performance and incorporates factors such as age.1 It is also predictive of not only short-term outcomes but also 5-year outcomes.41 We found that coronary atherosclerotic plaque activity was associated with the secondary end point of cardiac death or nonfatal MI despite adjustment for a range of covariates, including baseline clinical characteristics, coronary CT angiography findings, GRACE score, and the extent of obstructive disease on invasive coronary angiography. Coronary microcalcification activity would therefore appear to provide added prognostic value for spontaneous atherothrombotic coronary events.
Limitations
There are several study limitations. We had a lower than anticipated event rate in the study population despite recruiting patients with MI and multivessel disease. This may, in part, reflect our inclusion criteria for multivessel disease: at least 2 major epicardial vessels with either more than 50% luminal stenosis or previous coronary revascularization. The low event rate also led us to change our primary end point during the conduct of the study. The inclusion of unscheduled coronary revascularization was misplaced, and the occurrence of this event did not appear to be associated with plaque activity, as determined by 18F-sodium fluoride uptake. Our study was a longitudinal cohort study, and we could only assess associations rather than causality. We had low inclusion of women in our study, which may reflect the lower proportion of women who present with ST-segment elevation MI and multivessel disease and was comparable with rates reported in prior studies and prospective registries.5,6,40 We intentionally did not undertake end point adjudication because there was strict blinding of the study imaging findings, and there was no opportunity for the site investigators to be influenced by the results of the PET scan. In such circumstances, systematic reviews35,36 have found no differences in outcomes whether events have been assessed by site investigators or by independent clinical end point adjudication committees.
Conclusions
This cohort study found that coronary atherosclerotic plaque activity was not associated with the primary composite end point of cardiac death, nonfatal MI, or unplanned revascularization. In a secondary analysis, plaque activity appeared to be associated with combined cardiac death and MI, warranting further prospective study to explore the incremental prognostic implications of these findings.
eTable 1. Participant Recruitment per Site
eTable 2. Scanner Type by Center
eTable 3. Clinical Outcomes in Quartiles of Increased Coronary Microcalcification Activity Compared With No Coronary Microcalcification Activity
eTable 4. Site Investigator Reported Adverse Events
eFigure 1. Measurement of Coronary Microcalcification Activity With 18F-Sodium Fluoride Uptake
eFigure 2. CONSORT Diagram
PRE18FFIR Investigators
Data Sharing Statement
References
- 1.D’Ascenzo F, Biondi-Zoccai G, Moretti C, et al. TIMI, GRACE and alternative risk scores in acute coronary syndromes: a meta-analysis of 40 derivation studies on 216,552 patients and of 42 validation studies on 31,625 patients. Contemp Clin Trials. 2012;33(3):507-514. doi: 10.1016/j.cct.2012.01.001 [DOI] [PubMed] [Google Scholar]
- 2.Granger CB, Goldberg RJ, Dabbous O, et al. ; Global Registry of Acute Coronary Events Investigators . Predictors of hospital mortality in the global registry of acute coronary events. Arch Intern Med. 2003;163(19):2345-2353. doi: 10.1001/archinte.163.19.2345 [DOI] [PubMed] [Google Scholar]
- 3.van der Sangen NMR, Azzahhafi J, Chan Pin Yin DRPP, et al. External validation of the GRACE risk score and the risk-treatment paradox in patients with acute coronary syndrome. Open Heart. 2022;9(1):e001984. doi: 10.1136/openhrt-2022-001984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Collet JP, Thiele H, Barbato E, et al. ; ESC Scientific Document Group . 2020 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur Heart J. 2021;42(14):1289-1367. doi: 10.1093/eurheartj/ehaa575 [DOI] [PubMed] [Google Scholar]
- 5.Mehta SR, Wood DA, Storey RF, et al. ; COMPLETE Trial Steering Committee and Investigators . Complete revascularization with multivessel PCI for myocardial infarction. N Engl J Med. 2019;381(15):1411-1421. doi: 10.1056/NEJMoa1907775 [DOI] [PubMed] [Google Scholar]
- 6.Stone GW, Maehara A, Lansky AJ, et al. ; PROSPECT Investigators . A prospective natural-history study of coronary atherosclerosis. N Engl J Med. 2011;364(3):226-235. doi: 10.1056/NEJMoa1002358 [DOI] [PubMed] [Google Scholar]
- 7.Waksman R, Di Mario C, Torguson R, et al. ; LRP Investigators . Identification of patients and plaques vulnerable to future coronary events with near-infrared spectroscopy intravascular ultrasound imaging: a prospective, cohort study. Lancet. 2019;394(10209):1629-1637. doi: 10.1016/S0140-6736(19)31794-5 [DOI] [PubMed] [Google Scholar]
- 8.Arbab-Zadeh A, Fuster V. From detecting the vulnerable plaque to managing the vulnerable patient. J Am Coll Cardiol. 2019;74(12):1582-1593. doi: 10.1016/j.jacc.2019.07.062 [DOI] [PubMed] [Google Scholar]
- 9.Haase R, Schlattmann P, Gueret P, et al. ; COME-CCT Consortium . Diagnosis of obstructive coronary artery disease using computed tomography angiography in patients with stable chest pain depending on clinical probability and in clinically important subgroups: meta-analysis of individual patient data. BMJ. 2019;365:l1945. doi: 10.1136/bmj.l1945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maurovich-Horvat P, Bosserdt M, Kofoed KF, et al. ; DISCHARGE Trial Group . CT or invasive coronary angiography in stable chest pain. N Engl J Med. 2022;386(17):1591-1602. doi: 10.1056/NEJMoa2200963 [DOI] [PubMed] [Google Scholar]
- 11.Joshi NV, Vesey AT, Williams MC, et al. 18F-fluoride positron emission tomography identifies ruptured and high-risk coronary atherosclerotic plaques. Lancet. 2014; 383(9918):705-713. doi: 10.1016/S0140-6736(13)61754-7 [DOI] [PubMed] [Google Scholar]
- 12.Tarkin JM, Joshi FR, Evans NR, et al. Detection of atherosclerotic inflammation by 68Ga-DOTATATE PET compared to [18F]FDG PET imaging. J Am Coll Cardiol. 2017;69(14):1774-1791. doi: 10.1016/j.jacc.2017.01.060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dweck MR, Chow MWL, Joshi N, et al. Coronary arterial 18F-NaF uptake: a novel marker of cardiovascular risk. J Am Coll Cardiol. 2012; 59(17):1539-1548. doi: 10.1016/j.jacc.2011.12.037 [DOI] [PubMed] [Google Scholar]
- 14.Majeed K, Bellinge JW, Butcher SC, et al. Coronary 18F-sodium fluoride PET detects high-risk plaque features on optical coherence tomography and CT-angiography in patients with acute coronary syndrome. Atherosclerosis. 2021;319:142-148. doi: 10.1016/j.atherosclerosis.2020.12.010 [DOI] [PubMed] [Google Scholar]
- 15.Irkle A, Vesey AT, Lewis DY, et al. Identifying active vascular microcalcification by (18)F-sodium fluoride positron emission tomography. Nat Commun. 2015;6:7495. doi: 10.1038/ncomms8495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Creager MD, Hohl T, Hutcheson JD, et al. 18F-fluoride signal amplification identifies microcalcifications associated with atherosclerotic plaque instability in PET-CT images. Circ Cardiovasc Imaging. 2019; 12(1):e007835. doi: 10.1161/CIRCIMAGING.118.007835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moss AJ, Sim AM, Adamson PD, et al. Ex vivo 18F-fluoride uptake and hydroxyapatite deposition in human coronary atherosclerosis. Sci Rep. 2020;10(1):20172. doi: 10.1038/s41598-020-77391-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Youn T, Al’Aref SJ, Narula N, et al. 18F-sodium fluoride positron emission tomography/computed tomography in ex vivo human coronary arteries with histological correlation. Arterioscler Thromb Vasc Biol. 2020;40(2):404-411. doi: 10.1161/ATVBAHA.119.312737 [DOI] [PubMed] [Google Scholar]
- 19.Wen W, Gao M, Yun M, et al. In vivo coronary 18F-sodium fluoride activity. correlations with coronary plaque histological vulnerability and physiological environment. JACC Cardiovasc Imaging. 2023;16(4):508-520. doi: 10.1016/j.jcmg.2022.03.018 [DOI] [PubMed] [Google Scholar]
- 20.Bellinge JW, Francis RJ, Lee SC, et al. 18F-sodium fluoride positron emission tomography activity predicts the development of new coronary artery calcifications. Arterioscler Thromb Vasc Biol. 2021;41(1):534-541. doi: 10.1161/ATVBAHA.120.315364 [DOI] [PubMed] [Google Scholar]
- 21.Doris MK, Meah MN, Moss AJ, et al. Coronary 18F-fluoride uptake and progression of coronary artery calcification. Circ Cardiovasc Imaging. 2020;13(12):e011438. doi: 10.1161/CIRCIMAGING.120.011438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kwiecinski J, Tzolos E, Adamson PD, et al. 18F-sodium fluoride coronary uptake predicts outcome in patients with coronary artery disease. J Am Coll Cardiol. 2020;75(24):3061-3074. doi: 10.1016/j.jacc.2020.04.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fletcher AJ, Tew YY, Tzolos E, et al. Thoracic aortic 18F-sodium fluoride activity and ischemic stroke in patients with established cardiovascular disease. JACC Cardiovasc Imaging. 2022;15(7):1274-1288. doi: 10.1016/j.jcmg.2021.12.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.World Medical Association . World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191-2194. doi: 10.1001/jama.2013.281053 [DOI] [PubMed] [Google Scholar]
- 25.Kwiecinski J, Adamson PD, Lassen ML, et al. Feasibility of coronary 18F-sodium fluoride PET assessment with the utilization of previously acquired CT angiography. Circ Cardiovasc Imaging. 2018; 11(12):e008325. doi: 10.1161/CIRCIMAGING.118.008325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Halliburton SS, Abbara S, Chen MY, et al. ; Society of Cardiovascular Computed Tomography . SCCT guidelines on radiation dose and dose-optimization strategies in cardiovascular CT. J Cardiovasc Comput Tomogr. 2011;5(4):198-224. doi: 10.1016/j.jcct.2011.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Segall G, Delbeke D, Stabin MG, et al. ; SNM . SNM practice guideline for sodium 18F-fluoride PET/CT bone scans 1.0. J Nucl Med. 2010;51(11):1813-1820. doi: 10.2967/jnumed.110.082263 [DOI] [PubMed] [Google Scholar]
- 28.Cury RC, Leipsic J, Abbara S, et al. CAD-RADS™ 2.0—2022 Coronary Artery Disease-Reporting and Data System: an expert consensus document of the Society of Cardiovascular Computed Tomography (SCCT), the American College of Cardiology (ACC), the American College of Radiology (ACR), and the North America Society of Cardiovascular Imaging (NASCI). J Cardiovasc Comput Tomogr. 2022;16(6):536-557. doi: 10.1016/j.jcct.2022.07.002 [DOI] [PubMed] [Google Scholar]
- 29.Doris MK, Otaki Y, Krishnan SK, et al. Optimization of reconstruction and quantification of motion-corrected coronary PET-CT. J Nucl Cardiol. 2020;27(2):494-504. doi: 10.1007/s12350-018-1317-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rubeaux M, Joshi NV, Dweck MR, et al. Motion correction of 18F-sodium fluoride PET for imaging coronary atherosclerotic plaques. J Nucl Med. 2016;57(1):54-59. doi: 10.2967/jnumed.115.162990 [DOI] [PubMed] [Google Scholar]
- 31.Kwiecinski J, Cadet S, Daghem M, et al. Whole-vessel coronary 18F-sodium fluoride PET for assessment of the global coronary microcalcification burden. Eur J Nucl Med Mol Imaging. 2020;47(7):1736-1745. doi: 10.1007/s00259-019-04667-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kwiecinski J, Dey D, Cadet S, et al. Predictors of 18F-sodium fluoride uptake in patients with stable coronary artery disease and adverse plaque features on computed tomography angiography. Eur Heart J Cardiovasc Imaging. 2020;21(1):58-66. doi: 10.1093/ehjci/jez152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tzolos E, Kwiecinski J, Lassen ML, et al. Observer repeatability and interscan reproducibility of 18F-sodium fluoride coronary microcalcification activity. J Nucl Cardiol. 2022;29(1):126-135. doi: 10.1007/s12350-020-02221-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tzolos E, Lassen ML, Pan T, et al. Respiration-averaged CT versus standard CT attenuation map for correction of 18F-sodium fluoride uptake in coronary atherosclerotic lesions on hybrid PET/CT. J Nucl Cardiol. 2022;29(2):430-439. doi: 10.1007/s12350-020-02245-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Meah MN, Denvir MA, Mills NL, Norrie J, Newby DE. Clinical endpoint adjudication. Lancet. 2020;395(10240):1878-1882. doi: 10.1016/S0140-6736(20)30635-8 [DOI] [PubMed] [Google Scholar]
- 36.Ndounga Diakou LA, Trinquart L, Hróbjartsson A, et al. Comparison of central adjudication of outcomes and onsite outcome assessment on treatment effect estimates. Cochrane Database Syst Rev. 2016;3(3):MR000043. doi: 10.1002/14651858.MR000043.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Motoyama S, Sarai M, Harigaya H, et al. Computed tomographic angiography characteristics of atherosclerotic plaques subsequently resulting in acute coronary syndrome. J Am Coll Cardiol. 2009;54(1):49-57. doi: 10.1016/j.jacc.2009.02.068 [DOI] [PubMed] [Google Scholar]
- 38.Lee JM, Bang JI, Koo BK, et al. Clinical relevance of 18F-sodium fluoride positron-emission tomography in non-invasive identification of high-risk plaque in patients with coronary artery disease. Circ Cardiovasc Imaging. 2017;10(11):e006704. doi: 10.1161/CIRCIMAGING.117.006704 [DOI] [PubMed] [Google Scholar]
- 39.Wurster TH, Landmesser U, Abdelwahed YS, et al. Simultaneous [18F]fluoride and gadobutrol enhanced coronary positron emission tomography/magnetic resonance imaging for in vivo plaque characterization. Eur Heart J Cardiovasc Imaging. 2022;23(10):1391-1398. doi: 10.1093/ehjci/jeab276 [DOI] [PubMed] [Google Scholar]
- 40.Song J, Murugiah K, Hu S, et al. ; China PEACE Collaborative Group . Incidence, predictors, and prognostic impact of recurrent acute myocardial infarction in China. Heart. 2020;107(4):313-318. doi: 10.1136/heartjnl-2020-317165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fox KA, Carruthers KF, Dunbar DR, et al. Underestimated and under-recognized: the late consequences of acute coronary syndrome (GRACE UK-Belgian Study). Eur Heart J. 2010;31(22):2755-2764. doi: 10.1093/eurheartj/ehq326 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Participant Recruitment per Site
eTable 2. Scanner Type by Center
eTable 3. Clinical Outcomes in Quartiles of Increased Coronary Microcalcification Activity Compared With No Coronary Microcalcification Activity
eTable 4. Site Investigator Reported Adverse Events
eFigure 1. Measurement of Coronary Microcalcification Activity With 18F-Sodium Fluoride Uptake
eFigure 2. CONSORT Diagram
PRE18FFIR Investigators
Data Sharing Statement


