Key Points
Question
What are the lengths of the pooled learning curves for different parameters of minimally invasive distal pancreatectomy (MIDP) in experienced pancreatic centers?
Findings
This cohort study of 2041 MIDP procedures in 26 centers estimated a learning curve length at 40 procedures based on conversion rate and 56 and 71 procedures based on operation time and blood loss, respectively. Ultimately, 85 procedures were needed to complete the learning curve for a textbook outcome.
Meaning
These findings suggest that although learning curves for conversion rate, operation time, and intraoperative blood loss are completed earlier, extensive experience may be needed to master the learning curve of MIDP.
This cohort study evaluates the length of pooled learning curves for minimally invasive distal pancreatectomy in experienced European centers.
Abstract
Importance
Understanding the learning curve of a new complex surgical technique helps to reduce potential patient harm. Current series on the learning curve of minimally invasive distal pancreatectomy (MIDP) are mostly small, single-center series, thus providing limited data.
Objective
To evaluate the length of pooled learning curves of MIDP in experienced centers.
Design, Setting, and Participants
This international, multicenter, retrospective cohort study included MIDP procedures performed from January 1, 2006, through June 30, 2019, in 26 European centers from 8 countries that each performed more than 15 distal pancreatectomies annually, with an overall experience exceeding 50 MIDP procedures. Consecutive patients who underwent elective laparoscopic or robotic distal pancreatectomy for all indications were included. Data were analyzed between September 1, 2021, and May 1, 2022.
Exposures
The learning curve for MIDP was estimated by pooling data from all centers.
Main Outcomes and Measures
The learning curve was assessed for the primary textbook outcome (TBO), which is a composite measure that reflects optimal outcome, and for surgical mastery. Generalized additive models and a 2-piece linear model with a break point were used to estimate the learning curve length of MIDP. Case mix–expected probabilities were plotted and compared with observed outcomes to assess the association of changing case mix with outcomes. The learning curve also was assessed for the secondary outcomes of operation time, intraoperative blood loss, conversion to open rate, and postoperative pancreatic fistula grade B/C.
Results
From a total of 2610 MIDP procedures, the learning curve analysis was conducted on 2041 procedures (mean [SD] patient age, 58 [15.3] years; among 2040 with reported sex, 1249 were female [61.2%] and 791 male [38.8%]). The 2-piece model showed an increase and eventually a break point for TBO at 85 procedures (95% CI, 13-157 procedures), with a plateau TBO rate at 70%. The learning-associated loss of TBO rate was estimated at 3.3%. For conversion, a break point was estimated at 40 procedures (95% CI, 11-68 procedures); for operation time, at 56 procedures (95% CI, 35-77 procedures); and for intraoperative blood loss, at 71 procedures (95% CI, 28-114 procedures). For postoperative pancreatic fistula, no break point could be estimated.
Conclusion and Relevance
In experienced international centers, the learning curve length of MIDP for TBO was considerable with 85 procedures. These findings suggest that although learning curves for conversion, operation time, and intraoperative blood loss are completed earlier, extensive experience may be needed to master the learning curve of MIDP.
Introduction
Minimally invasive distal pancreatectomy (MIDP) is increasingly considered a valuable alternative for open distal pancreatectomy (ODP).1 Two recent randomized trials showed clear benefits of the minimally invasive approach over ODP with respect to time to functional recovery, hospital stay, and intraoperative blood loss, with similar overall complication rates.2,3 These findings are in line with promising results of cohort studies and systematic reviews.4,5,6,7 However, implementation of MIDP has been rather slow due to perceived technical difficulty and lack of specific training.8,9,10
The development of a new surgical technique requires careful stepwise progression according to the Idea, Development, Exploration, Assessment, and Long-term follow-up (IDEAL) model in order to protect patients as much as possible from potential harm.11 Assessment of the learning curve is typically done after implementation in multiple centers (IDEAL stage 2b) and is important for understanding the possible difficulties when implementing a new technique. A previous systematic review evaluating the learning curve of bariatric surgery suggested several phases of a learning curve, namely competency, proficiency, and mastery.12 Herein, competency reflects improvement of operative parameters, such as operative time while focusing on patient safety; proficiency reflects further reduction of postoperative complications and stabilization of operative time; and mastery reflects when operation time and complication rates plateau, even with more complicated procedures.
Most studies on the learning curve for MIDP were single-center series that focused on single intraoperative parameters, such as conversion rate or mean operative time.13,14,15 However, recent studies have suggested that a single marker may not be representative for the quality of care received by a patient and may not truly reflect the mastering of a surgical procedure.16,17 Therefore, assessing the implementation of a novel technique should ideally be assessed using a composite measure representing the most optimal outcome for a patient. Textbook outcome (TBO) is a composite measure that reflects optimal outcome and an uneventful course. Therefore, TBO is an excellent outcome representative for mastering a procedure.18 This study among experienced European pancreatic centers evaluates the length of the learning curve of MIDP for the clinically most relevant parameter TBO.
Methods
Setting and Population
In this retrospective cohort study, all centers participating in the European Consortium on Minimally Invasive Pancreatic Surgery were invited to participate, and those that performed at least 15 distal pancreatectomies annually and with a minimum experience of 50 MIDPs were eligible. All consecutive patients aged 18 years or older who underwent either elective laparoscopic or robotic distal pancreatectomy for both malignant and benign disease of the distal pancreas were included. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline was followed for this study.19 The medical ethics review committee of the Amsterdam UMC approved this study and waived the need for informed consent owing to the retrospective observational study design.
Data Collection
Data were obtained from local databases in 26 participating centers using a predefined selection of required parameters from January 1, 2006, through June 30, 2019. All centers had ongoing databases completed by local collaborators using predefined definitions of required parameters.
Outcome Measures
The learning curve of MIDP was evaluated using TBO as the optimal outcome for patients, hereby reflecting surgical mastery. The definition of TBO was previously developed by an international group of experts in pancreatic surgery as a composite measure reflecting an optimal outcome and uneventful course in a patient.18 A TBO is achieved when the following criteria are met: no postoperative pancreatic fistula (POPF) (grade B/C according to International Study Group of Pancreatic Surgery [ISGPS] definitions),20 no postpancreatectomy hemorrhage (grade B/C ISGPS),21 no major morbidity (Clavien-Dindo grade III or higher),22 no readmission, and no in-hospital or 30-day mortality. Two researchers (S.L. and L.C.) classified an operation as TBO when these criteria were met. Secondary outcomes were operative team performance, namely operation time, intraoperative blood loss, conversion to open rate, and POPF grade B/C ISGPS.
Statistical Analysis
Among the 26 participating centers, there was a wide range in number of procedures of their series. To accommodate this range, only the first 100 consecutive procedures per center were pooled, ensuring that the plotted learning curves were at all times based on more than 10 centers. To fit the pooled incidence of both the primary and secondary outcomes, generalized additive models were used.23 In addition, a 2-piece model was used to describe the learning curve.24 The first piece represents the learning phase and is the linear descending or ascending section of the graph. The second piece is the plateau phase, which indicates a stable outcome and starts when the data are best fitted to a horizontal line. The procedure number at the transition from the first piece (the linear ascending or descending line) to the second piece (the horizontal line) is the break point. The break point represents the length of the learning curve when improvement was seen in the first piece of the 2-piece model. An improvement in the first piece indicates a learning curve. If there was no improvement in the outcomes, no learning curve was assumed. When improvement was seen and, consequently, a break point was found, learning-associated morbidity was calculated. The learning-associated morbidity is the closed triangular area under the descending part and above the horizontal line of the 2-piece model when prolonged under the descending part. For TBO, this closed triangular area is above the ascending parts and below the horizontal line of the 2-piece model when prolonged above the ascending part and represents the percent less TBO compared with when the break point was estimated.
The following case-mix parameters were used to assess to what extent outcomes were expected based on case mix: age, sex, body mass index, American Society of Anesthesiologists classification, tumor histology, splenectomy, vascular and multivisceral resection, duct diameter, and tumor size. Data on race and ethnicity were not collected, as this is not common practice in Europe. Missing data are presented. Missing data were assumed to be missing at random and were estimated using multiple imputation with the creation of 10 data sets (eMethods in Supplement 1). Per imputed data set, a prediction model was calculated, and the regression coefficients of the created data sets were pooled according to the Rubin rule.25 This pooled prediction model was used to calculate the expected outcome per consecutive case number. The expected outcomes based on case mix per center were pooled. To assess to what extent outcomes were expected based on case mix, these outcomes were plotted and visually compared with the observed outcome parameters. Data were analyzed between September 1, 2021, and May 1, 2022, using SPSS Statistics for Windows, version 26.0 (IBM Corporation) and R, version 3.6.1 (R Foundation for Statistical Computing) statistical software.
Results
Study Population
A total of 2610 patients from 26 European centers in 8 countries were included, varying from 50 to 280 patients per center. Only the first 100 consecutive procedures per center (2041 procedures in total) were used in the learning curve analyses (15 centers with ≤100 procedures, 11 centers with >100 procedures). Among those 2041 patients, the mean (SD) age was 58 (15.3) years; of 2040 with recorded sex, 1249 [61.2%] were female and 791 (38.8%) were male. In total 2041 procedures, were used in the learning curve analyses . In total, 1692 patients (82.9%) underwent laparoscopic MIDP and 349 (17.1%) robotic MIDP. Patient and surgical characteristics are presented in the Table.
Table. Patient and Surgical Characteristics and Outcomes for 2041 Patients.
| Patient characteristics | No. (%) | Missing, No. (%) |
|---|---|---|
| Age, y, mean (SD) | 58 (15.3) | 1 (<0.1) |
| Sex | ||
| Female | 1249 (61.2) | 1 (<0.1) |
| Male | 791 (38.8) | |
| Body mass index, mean (SD)a | 25.9 (4.9) | 275 (13.5) |
| American Society of Anesthesiologists classification | ||
| I | 335 (18.0) | 181 (8.9) |
| II | 1061 (57.0) | |
| III | 459 (24.7) | |
| IV | 5 (0.3) | |
| Comorbidities | 1214 (65.5) | 188 (9.2) |
| Neoadjuvant therapy | 39 (2.1) | 222 (10.9) |
| Tumor pathology | ||
| Neuroendocrine tumors (including insulinoma) | 565 (27.6) | 2 (<0.1) |
| Pancreatic ductal adenocarcinoma | 362 (17.8) | |
| Mucinous cystic neoplasm | 290 (14.2) | |
| Intraductal papillary mucinous neoplasm | 276 (13.5) | |
| Serous cystadenoma | 190 (9.3) | |
| Chronic pancreatitis or pseudocyst | 98 (4.8) | |
| Solid pseudopapillary tumor | 68 (3.3) | |
| Metastases | 50 (2.4) | |
| Undetermined or other cyst | 39 (1.9) | |
| No pathology found | 25 (1.2) | |
| Other malignant tumor | 23 (1.1) | |
| Undetermined mass | 2 (0.1) | |
| Other | 51 (2.5) | |
| Surgical characteristics and outcome | ||
| Approach | ||
| Laparoscopic | 1692 (82.9) | 0 |
| Robotic | 349 (17.1) | |
| Converted to open | 264 (12.9) | 1 (<0.1) |
| Splenectomy | 1329 (65.1) | 1 (<0.1) |
| Vascular resection | 17 (0.9) | 165 (8.1) |
| Multivisceral resection | 168 (8.7) | 113 (5.5) |
| Operation time, min, mean (SD) | 243 (89.9) | 62 (3.0) |
| Blood loss, mL, median (IQR) | 120 (70-250) | 383 (18.8) |
| Postoperative pancreatic fistula grade B/C | 469 (22.9) | 3 (<0.1) |
| Textbook outcome | 1286 (65.8) | 88 (4.3) |
Data were collected from the first 100 consecutive patients per center, for a total of 2041 patients. Some characteristics were not available for all patients; the numbers of missing patients are shown in the far right column
As measured by weight in kilograms divided by height in meters squared.
Learning Curve
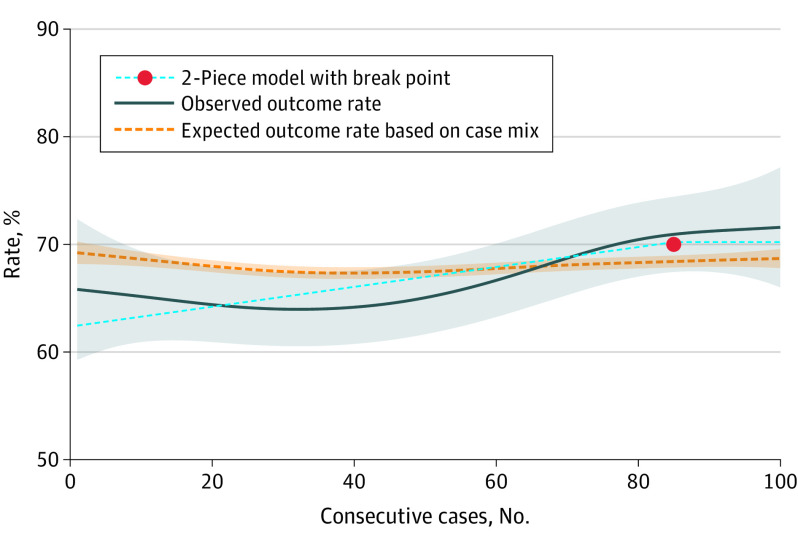
The primary outcome, TBO, was achieved for 1286 of 2041 patients (65.8%). At the beginning of the learning curve, a lower TBO rate (63%) was observed than expected based on case mix; however, at the end, the observed outcome was higher than the expected outcome based on case mix (Figure 1) and reached a plateau at 70% (95% CI, 65%-75%). The break point was estimated at 85 procedures (95% CI, 13-157 procedures). Learning-associated loss of TBO was estimated at 3.3%.
Figure 1. Learning Curve of Minimally Invasive Distal Pancreatectomy for Textbook Outcome.
The solid blue line represents the generalized additive model (pooled incidence) with a 95% CI (shaded area). The dotted orange line represents the predicted outcomes based on case mix (shaded area indicates 95% CI). The light blue dashed line is the 2-piece model. The first phase is the linear ascending section, the second phase is the plateau phase, which starts after the break point. The break point represents the length of the learning curve.
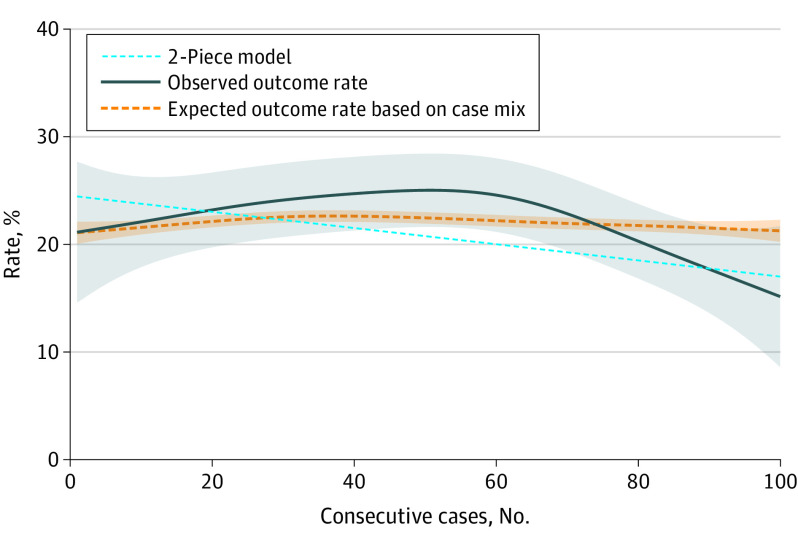
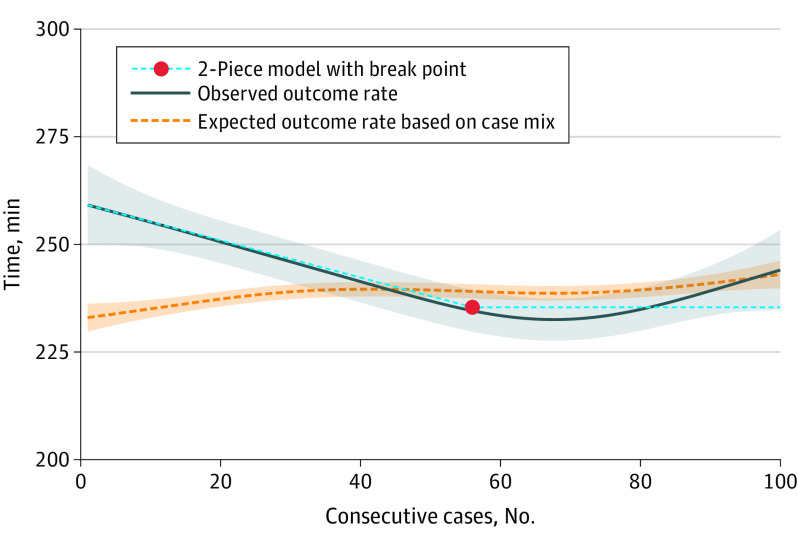
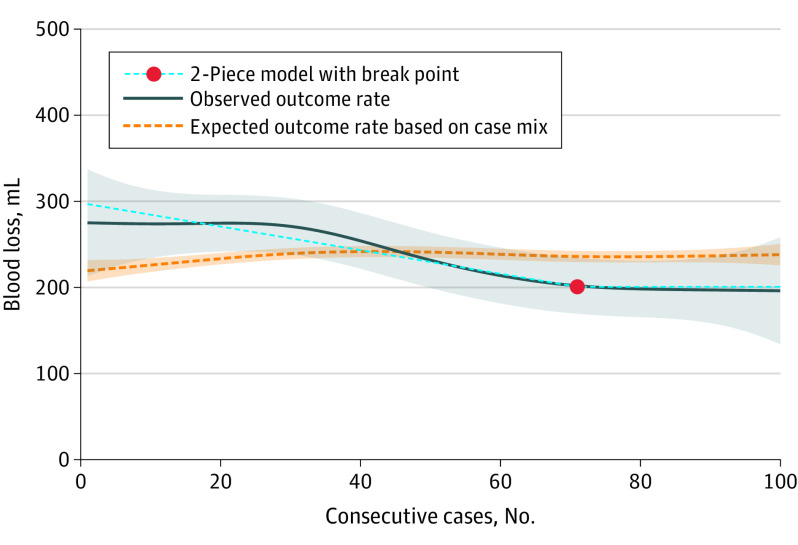
The length of the learning curve was also assessed for the secondary outcomes. The overall POPF rate was 22.9% (469 patients). For POPF, the first piece of the 2-piece model continued to descend without reaching a plateau, indicating a decrease in POPF rate during the study period without reaching a stable outcome. At the end of the study period, the descending part of the 2-piece model reached a 15% pancreatic fistula rate, which is below the expected outcome based on case mix (Figure 2). Operation time descended, indicating a decrease in operation time, to a plateau at 235 minutes (95% CI, 231-240 minutes), with a break point estimated at 56 procedures (95% CI, 35-77 procedures). At the beginning of the study period, the observed operation time was longer than expected based on case mix; however, at the end, the observed and expected lines were more congruous (Figure 3). Intraoperative blood loss decreased, indicating an improvement to a plateau at 201 mL (95% CI, 165-236 mL) with a break point estimated at 71 procedures (95% CI, 28-114 procedures) (Figure 4). The conversion to open rate descended to a plateau at 11% (95% CI, 9%-13%), with a break point estimated at 40 procedures (95% CI, 11-68 procedures) (eFigure in Supplement 1). At the end of the study period, the observed outcomes were below the outcomes expected based on case mix, indicating an improvement above expectation.
Figure 2. Learning Curve of Minimally Invasive Distal Pancreatectomy for Postoperative Pancreatic Fistula Grade B/C.
The solid blue line represents the generalized additive model (pooled incidence) with a 95% CI (shaded area). The dotted orange line represents the predicted outcomes based on case mix (shaded area indicates 95% CI). The light blue dashed line is the 2-piece model. The first phase is the linear descending section; there is no second phase since a plateau was not reached within 100 procedures.
Figure 3. Learning Curve of Minimally Invasive Distal Pancreatectomy for Operation Time.
The solid blue line represents the generalized additive model (pooled incidence) with a 95% CI (shaded area). The dotted orange line represents the predicted outcomes based on case mix (shaded area indicates 95% CI). The light blue dashed line is the 2-piece model. The first phase is the linear descending section, and the second phase is the plateau phase, which starts after the break point. The break point represents the length of the learning curve.
Figure 4. Learning Curve of Minimally Invasive Distal Pancreatectomy for Intraoperative Blood Loss.
The solid blue line represents the generalized additive model (pooled incidence) with a 95% CI (shaded area). The dotted orange line represents the predicted outcomes based on case mix (shaded area indicates 95% CI). The light blue dashed line is the 2-piece model. The first phase is the linear descending section. The second phase is the plateau phase, which starts after the break point. The break point represents the length of the learning curve.
Discussion
Based on this European multicenter cohort study among 2610 patients after MIDP in 26 centers from 8 countries, the length of the mastery learning curve was estimated at 85 procedures, after which a stable plateau in TBO rate of 70% was reached. For operation time, intraoperative blood loss, and conversion to open rate, the length of the learning curve varied between 40 and 71 procedures.
This international, multicenter study is the first in our knowledge to focus on the mastery of learning MIDP as reflected by TBO. Previous studies that assessed the learning curve of MIDP used risk-adjusted cumulative sum, analysis of variance, or time intervals as statistical methods. These small case series focused mainly on conversion rate, operation time, and blood loss as primary outcomes and reported learning curve lengths between 10 and 40 procedures.5,13,14,15,16,26,27 These lower cutoffs may be explained by the different methods to establish the length of the learning curve. Most studies divided patients into consecutive subgroups or periods and compared the rates of the variables of interest between the groups.5,13,14,16,27 Other studies, however, used a cumulative sum or receiver operating characteristic curve analyses to assess the length of the learning curve for mostly operation time.15,26 Although dividing patients into consecutive subgroups or assessing through a cumulative sum and receiver operating characteristic analysis may help in determining the length of the learning curve, there was no correction for case mix (ie, differences in patient or tumor selection for MIDP), and with these methods, the CI of the plateau found is questionable. Interestingly, no data exist regarding the length of the learning curve for ODP.28 A comparison between the length of the learning curves between ODP and MIDP is, therefore, not feasible.
The extensive length of the learning curve in our study of the patients’ clinically most relevant parameter, TBO, was not unexpected. This composite outcome parameter includes not only complications but also readmission rates and is associated with many different factors. According to a previous study, several factors, such as female sex or the absence of neoadjuvant treatment, were associated with improved TBO rates and may not be attributable to surgeon experience.18 Still, our study findings show an improvement with increasing experience. While in the early experience, TBO was achieved in 63% of patients, improving to 70% when the plateau stabilized. This rate is comparable to the rate in the Netherlands, where TBO was achieved in 67.4% of ODP and MIDP cases,18 and higher than for US Medicare patients, where TBO was achieved in 47.8% cases.29 This difference might be explained by inclusion of low-volume hospitals and prolonged length of hospital stay in the TBO definition in the latter study.29
A limitation of the definition used for TBO is that it includes only short-term outcomes of MIDP. For patients with cancer undergoing surgery, other oncologic-associated parameters may be more important than those in the current definition. However, in this study, a substantial number of procedures were performed because of benign disease. The TBO was used because it is a representative end point for operations performed for both malignant and benign disease.
An interesting finding is that the length of learning curve for the POPF rate could not be estimated for the first 100 consecutive procedures, which is in contrast to pancreatoduodenectomy, for which an association between surgeons’ performance and the occurrence of POPF was found.30 In contrast to pancreatoduodenectomy, a distal pancreatectomy mostly does not require an anastomosis. Although some studies on the MIDP learning curve found a decreasing rate of POPF with increasing experience,5,27 these studies did not adjust for risk factors associated with POPF. The POPF rate following MIDP may be associated with patient characteristics (ie, body mass index), pancreatic characteristics (ie, pancreatic thickness or texture), and closure methods rather than surgical performance.31,32 Information regarding pancreatic texture or closure methods was not available for this study.
For the other secondary outcome parameters, clear improvement was seen with a plateau reached after 40 to 71 MIDP procedures. Although MIDP is seen as a valuable alternative to ODP,2 also in experienced European centers, this procedure is often performed fewer than 15 times per year. Therefore, a stable outcome, indicated by a plateau, may be reached only after several years of performing MIDP. It remains uncertain whether centers performing fewer than 15 distal pancreatectomy procedures annually are able to reach a plateau for the studied learning curve parameters. Furthermore, our study concerns centers’ level, not individual surgeons. Additional studies should focus on the learnability of the procedure by individual surgeons and its association with the length of the learning curve. Research should also determine which other factors are associated with the length of learning curves and to what extent the learning curve can be reduced, for instance, through a training program with hands-on courses or applying technology, such as simulations, virtual reality, or artificial intelligence, and proctoring programs prior to implementation. Such a program could increase efficient learning and may thus decrease patient harm after implementing MIDP.33 Potentially, surgeons may benefit from learning a new procedure in centers with a high annual volume before introducing the procedure to centers with lower annual volumes. However, the difference in TBO rate with increasing experience was small with limited learning-associated morbidity.
A systematic review evaluating the learning curve of bariatric procedures proposes a standardization of phases of the learning curve, namely, competency, proficiency, and mastery.12 The first phase, competency, correlates with the number of procedures necessary to achieve improvement in 1 or more operative parameters. The second phase, proficiency, correlates with stabilization (reaching a plateau) of operative parameters and reduction of postoperative complications. The third phase, mastery, is achieved when operative parameters and complication rates plateau, even in more complicated cases. These phases correspond with the learning curves found in this study. First, the conversion rate stabilized, which can be considered as achieving competency. Second, the other operative parameters, operation time and intraoperative blood loss, stabilized or continued to decrease, such as for the POPF rate (ie, achieving proficiency). Third, TBO reached a plateau, (ie, achieving mastery). Although our data did not show an increase in case complexity, TBO reflects the complete outcome in a patient and, therefore, may be representative of achieving mastery.
Strengths and Limitations
A major strength of this study is the international, multicenter design to evaluate the different learning curves for MIDP. The analysis was based on not only single intraoperative parameters but also clinically relevant parameters for patients and parameters representative of team performance. The calculated 2-piece model did not differ substantially from the observed outcomes as seen in the figures, since it largely overlapped the generalized additive model of the observed outcomes. Furthermore, the learning curve of MIDP was evaluated at the center level. Although evaluating the learning curve of the surgeons individually would be interesting, patient outcomes were determined not only by the individual performances of the operating surgeon but also by the quality of care given by the complete team.
This study also has several limitations. First, the retrospective design comes with inherent limitations. For instance, data on pancreatic transection and stump closure methods were not available. Second, the inclusion of both malignant and benign diseases may have created some heterogeneity. Some variations in surgical technique, extent of the resection (eg, splenopancreatectomy, spleen-preserving distal pancreatectomy, radical antegrade modular pancreatosplenectomy) or different postoperative policies might be associated with the learning curve. However, the impact of this heterogeneity was minimized by using a case-mix model and pooling data from 26 different centers. Third, learning curve length was estimated; however, the 95% CI was broad, which may have diminished the certainty of the results. Since it is difficult to calculate an exact learning curve length because the learning curve is subject to many unaccountable variables, these results should be considered as an indication for the length of the learning curve and associated morbidity, not as an exact number. Fourth, given the hierarchical structure of the data, a multilevel analysis would have been the most accurate method to analyze these data. However, as a possible consequence of attempting to fit models that were too complex to be properly supported by the data, the models did not converge. Therefore, models on pooled data were used, ignoring the clustering of patients within institutions. Fifth, both robotic and laparoscopic distal pancreatectomy techniques were included in the learning curve analysis. Although both techniques show comparable outcomes in terms of major morbidity and POPF rates, robotic distal pancreatectomy is associated with improved conversion rates.34 In addition, the length of the learning curve of robotic distal pancreatectomy is potentially shorter.26,35 However, the low number of robotic procedures did not allow for a separate analysis. Future research assessing the learning curves of both approaches or for different indications (ie, pancreatic ductal adenocarcinoma or spleen preservation) would be interesting.
Conclusions
This international, multicenter cohort study found that within a center’s first 100 MIDP procedures, the length of the learning curve for parameters indicating team performance varied between 40 and 71 procedures. The length of the learning curve of the clinically most relevant parameter, TBO rate, stabilized at 70% after 85 procedures. Although MIDP is considered a valuable alternative to ODP, our findings suggest that it may take extensive experience to master the learning curve.
eMethods. Missing Data and Multiple Imputation
eReferences.
eFigure. Learning Curve for MIDP of Conversion to Open Rate
Nonauthor Collaborators
Data Sharing Statement
References
- 1.Asbun HJ, Moekotte AL, Vissers FL, et al. ; International Study Group on Minimally Invasive Pancreas Surgery (I-MIPS) . The Miami international evidence-based guidelines on minimally invasive pancreas resection. Ann Surg. 2020;271(1):1-14. doi: 10.1097/SLA.0000000000003590 [DOI] [PubMed] [Google Scholar]
- 2.de Rooij T, van Hilst J, van Santvoort H, et al. ; Dutch Pancreatic Cancer Group . Minimally invasive versus open distal pancreatectomy (LEOPARD): a multicenter patient-blinded randomized controlled trial. Ann Surg. 2019;269(1):2-9. doi: 10.1097/SLA.0000000000002979 [DOI] [PubMed] [Google Scholar]
- 3.Björnsson B, Larsson AL, Hjalmarsson C, Gasslander T, Sandström P. Comparison of the duration of hospital stay after laparoscopic or open distal pancreatectomy: randomized controlled trial. Br J Surg. 2020;107(10):1281-1288. doi: 10.1002/bjs.11554 [DOI] [PubMed] [Google Scholar]
- 4.de Rooij T, Jilesen AP, Boerma D, et al. A nationwide comparison of laparoscopic and open distal pancreatectomy for benign and malignant disease. J Am Coll Surg. 2015;220(3):263-270.e1. [DOI] [PubMed] [Google Scholar]
- 5.Lof S, Moekotte AL, Al-Sarireh B, et al. ; Minimally Invasive Liver and Pancreatic Surgery Study Group - UK (MI-LAPS UK) . Multicentre observational cohort study of implementation and outcomes of laparoscopic distal pancreatectomy. Br J Surg. 2019;106(12):1657-1665. doi: 10.1002/bjs.11292 [DOI] [PubMed] [Google Scholar]
- 6.Mehrabi A, Hafezi M, Arvin J, et al. A systematic review and meta-analysis of laparoscopic versus open distal pancreatectomy for benign and malignant lesions of the pancreas: it’s time to randomize. Surgery. 2015;157(1):45-55. doi: 10.1016/j.surg.2014.06.081 [DOI] [PubMed] [Google Scholar]
- 7.Riviere D, Gurusamy KS, Kooby DA, et al. Laparoscopic versus open distal pancreatectomy for pancreatic cancer. Cochrane Database Syst Rev. 2016;4(4):CD011391. doi: 10.1002/14651858.CD011391.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abu Hilal M, Takhar AS. Laparoscopic left pancreatectomy: current concepts. Pancreatology. 2013;13(4):443-448. doi: 10.1016/j.pan.2013.04.196 [DOI] [PubMed] [Google Scholar]
- 9.van Hilst J, de Rooij T, Abu Hilal M, et al. Worldwide survey on opinions and use of minimally invasive pancreatic resection. HPB (Oxford). 2017;19(3):190-204. doi: 10.1016/j.hpb.2017.01.011 [DOI] [PubMed] [Google Scholar]
- 10.Tran Cao HS, Lopez N, Chang DC, et al. Improved perioperative outcomes with minimally invasive distal pancreatectomy: results from a population-based analysis. JAMA Surg. 2014;149(3):237-243. doi: 10.1001/jamasurg.2013.3202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McCulloch P, Altman DG, Campbell WB, et al. ; Balliol Collaboration . No surgical innovation without evaluation: the IDEAL recommendations. Lancet. 2009;374(9695):1105-1112. doi: 10.1016/S0140-6736(09)61116-8 [DOI] [PubMed] [Google Scholar]
- 12.Wehrtmann FS, de la Garza JR, Kowalewski KF, et al. Learning curves of laparoscopic Roux-en-Y gastric bypass and sleeve gastrectomy in bariatric surgery: a systematic review and introduction of a standardization. Obes Surg. 2020;30(2):640-656. doi: 10.1007/s11695-019-04230-7 [DOI] [PubMed] [Google Scholar]
- 13.Braga M, Ridolfi C, Balzano G, Castoldi R, Pecorelli N, Di Carlo V. Learning curve for laparoscopic distal pancreatectomy in a high-volume hospital. Updates Surg. 2012;64(3):179-183. doi: 10.1007/s13304-012-0163-2 [DOI] [PubMed] [Google Scholar]
- 14.Nachmany I, Pencovich N, Ben-Yehuda A, et al. Laparoscopic distal pancreatectomy: learning curve and experience in a tertiary center. J Laparoendosc Adv Surg Tech A. 2016;26(6):470-474. doi: 10.1089/lap.2016.0098 [DOI] [PubMed] [Google Scholar]
- 15.Ricci C, Casadei R, Buscemi S, et al. Laparoscopic distal pancreatectomy: what factors are related to the learning curve? Surg Today. 2015;45(1):50-56. doi: 10.1007/s00595-014-0872-x [DOI] [PubMed] [Google Scholar]
- 16.de Rooij T, Cipriani F, Rawashdeh M, et al. Single-surgeon learning curve in 111 laparoscopic distal pancreatectomies: does operative time tell the whole story? J Am Coll Surg. 2017;224(5):826-832.e1. doi: 10.1016/j.jamcollsurg.2017.01.023 [DOI] [PubMed] [Google Scholar]
- 17.Busweiler LAD, Schouwenburg MG, van Berge Henegouwen MI, et al. ; Dutch Upper Gastrointestinal Cancer Audit (DUCA) group . Textbook outcome as a composite measure in oesophagogastric cancer surgery. Br J Surg. 2017;104(6):742-750. doi: 10.1002/bjs.10486 [DOI] [PubMed] [Google Scholar]
- 18.van Roessel S, Mackay TM, van Dieren S, et al. ; Dutch Pancreatic Cancer Group . Textbook outcome: nationwide analysis of a novel quality measure in pancreatic surgery. Ann Surg. 2020;271(1):155-162. doi: 10.1097/SLA.0000000000003451 [DOI] [PubMed] [Google Scholar]
- 19.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP; STROBE Initiative . The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370(9596):1453-1457. doi: 10.1016/S0140-6736(07)61602-X [DOI] [PubMed] [Google Scholar]
- 20.Bassi C, Marchegiani G, Dervenis C, et al. ; International Study Group on Pancreatic Surgery (ISGPS) . The 2016 update of the International Study Group (ISGPS) definition and grading of postoperative pancreatic fistula: 11 years after. Surgery. 2017;161(3):584-591. doi: 10.1016/j.surg.2016.11.014 [DOI] [PubMed] [Google Scholar]
- 21.Wente MN, Veit JA, Bassi C, et al. Postpancreatectomy hemorrhage (PPH): an International Study Group of Pancreatic Surgery (ISGPS) definition. Surgery. 2007;142(1):20-25. doi: 10.1016/j.surg.2007.02.001 [DOI] [PubMed] [Google Scholar]
- 22.Clavien PA, Barkun J, de Oliveira ML, et al. The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg. 2009;250(2):187-196. doi: 10.1097/SLA.0b013e3181b13ca2 [DOI] [PubMed] [Google Scholar]
- 23.Hastie T, Tibshirani R. Generalized additive models for medical research. Stat Methods Med Res. 1995;4(3):187-196. doi: 10.1177/096228029500400302 [DOI] [PubMed] [Google Scholar]
- 24.Papachristofi O, Jenkins D, Sharples LD. Assessment of learning curves in complex surgical interventions: a consecutive case-series study. Trials. 2016;17(1):266. doi: 10.1186/s13063-016-1383-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rubin D. Multiple Imputation for Nonresponse in Surveys. Wiley; 1987. [Google Scholar]
- 26.Napoli N, Kauffmann EF, Perrone VG, Miccoli M, Brozzetti S, Boggi U. The learning curve in robotic distal pancreatectomy. Updates Surg. 2015;67(3):257-264. doi: 10.1007/s13304-015-0299-y [DOI] [PubMed] [Google Scholar]
- 27.Shakir M, Boone BA, Polanco PM, et al. The learning curve for robotic distal pancreatectomy: an analysis of outcomes of the first 100 consecutive cases at a high-volume pancreatic centre. HPB (Oxford). 2015;17(7):580-586. doi: 10.1111/hpb.12412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Müller PC, Kuemmerli C, Cizmic A, et al. Learning curves in open, laparoscopic, and robotic pancreatic surgery. Ann Surg Open. 2022;3(1):e111. doi: 10.1097/AS9.0000000000000111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Merath K, Chen Q, Bagante F, et al. Textbook outcomes among Medicare patients undergoing hepatopancreatic surgery. Ann Surg. 2020;271(6):1116-1123. doi: 10.1097/SLA.0000000000003105 [DOI] [PubMed] [Google Scholar]
- 30.Hogg ME, Zenati M, Novak S, et al. Grading of surgeon technical performance predicts postoperative pancreatic fistula for pancreaticoduodenectomy independent of patient-related variables. Ann Surg. 2016;264(3):482-491. doi: 10.1097/SLA.0000000000001862 [DOI] [PubMed] [Google Scholar]
- 31.Ratnayake CBB, Wells C, Hammond J, French JJ, Windsor JA, Pandanaboyana S. Network meta-analysis comparing techniques and outcomes of stump closure after distal pancreatectomy. Br J Surg. 2019;106(12):1580-1589. doi: 10.1002/bjs.11291 [DOI] [PubMed] [Google Scholar]
- 32.De Pastena M, van Bodegraven EA, Mungroop TH, et al. Distal Pancreatectomy Fistula Risk Score (D-FRS). Ann Surg. 2023;277(5):e1099-e1105. doi: 10.1097/SLA.0000000000005497 [DOI] [PubMed] [Google Scholar]
- 33.de Rooij T, van Hilst J, Boerma D, et al. ; Dutch Pancreatic Cancer Group . Impact of a nationwide training program in minimally invasive distal pancreatectomy (LAELAPS). Ann Surg. 2016;264(5):754-762. doi: 10.1097/SLA.0000000000001888 [DOI] [PubMed] [Google Scholar]
- 34.Lof S, van der Heijde N, Abuawwad M, et al. ; European Consortium on Minimally Invasive Pancreatic Surgery (E-MIPS) . Robotic versus laparoscopic distal pancreatectomy: multicentre analysis. Br J Surg. 2021;108(2):188-195. doi: 10.1093/bjs/znaa039 [DOI] [PubMed] [Google Scholar]
- 35.Shyr B-U, Chen S-C, Shyr Y-M, Wang S-E. Learning curves for robotic pancreatic surgery-from distal pancreatectomy to pancreaticoduodenectomy. Medicine (Baltimore). 2018;97(45):e13000. doi: 10.1097/MD.0000000000013000 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods. Missing Data and Multiple Imputation
eReferences.
eFigure. Learning Curve for MIDP of Conversion to Open Rate
Nonauthor Collaborators
Data Sharing Statement