Abstract
Sepsis represents a deranged and exaggerated systemic inflammatory response to infection and is associated with vascular and metabolic abnormalities that trigger systemic organic dysfunction. Mitochondrial function has been shown to be severely impaired during the early phase of critical illness, with a reduction in biogenesis, increased generation of reactive oxygen species and a decrease in adenosine triphosphate synthesis of up to 50%. Mitochondrial dysfunction can be assessed using mitochondrial DNA concentration and respirometry assays, particularly in peripheral mononuclear cells. Isolation of monocytes and lymphocytes seems to be the most promising strategy for measuring mitochondrial activity in clinical settings because of the ease of collection, sample processing, and clinical relevance of the association between metabolic alterations and deficient immune responses in mononuclear cells. Studies have reported alterations in these variables in patients with sepsis compared with healthy controls and non-septic patients. However, few studies have explored the association between mitochondrial dysfunction in immune mononuclear cells and unfavorable clinical outcomes. An improvement in mitochondrial parameters in sepsis could theoretically serve as a biomarker of clinical recovery and response to oxygen and vasopressor therapies as well as reveal unexplored pathophysiological mechanistic targets. These features highlight the need for further studies on mitochondrial metabolism in immune cells as a feasible tool to evaluate patients in intensive care settings. The evaluation of mitochondrial metabolism is a promising tool for the evaluation and management of critically ill patients, especially those with sepsis. In this article, we explore the pathophysiological aspects, main methods of measurement, and the main studies in this field.
Keywords: Sepsis, Mitochondria, Mitochondrial dysfunction, Oxidative phosphorylation, Inflammation, Respirometry
Core Tip: The evaluation of mitochondrial metabolism is a promising tool for the evaluation and management of critically ill patients, particularly those with sepsis. In this article, we explore the pathophysiological aspects, main methods of measurement, and main studies in this field.
INTRODUCTION
Sepsis is a major health problem worldwide and can be characterized by a dysregulated host response to infection[1-3]. Particularly, an imbalanced systemic inflammatory response to infection contribute to the clinical progress to multi-organ dysfunction[2,4]. There is a great effort in the recognition and prompt treatment of sepsis, especially with regard to early antibiotic administration, hemodynamic resuscitation, and evacuation of septic foci[1]. The ultimate cause of death in patients with sepsis, however, remains unclear. Infections usually are eradicated through an intense, but localized, inflammatory response. Nonetheless, fatal infections are characterized by an inability to resolve the inflammatory response because cytokines released into the systemic circulation, activates inflammatory cells in remote locations[5]. This response leads to organ injury and dysfunction, which cannot be completely explained by a decrease in tissue oxygenation due to an impairment of blood flow[2,4,5] but likely to problems in oxygen utilization.
Mitochondria is a highly specialized organelle that is considered the power plant of cells thus supporting energy in the form of adenosine triphosphate (ATP) according to functional demands[6]. In the blood, except for erythrocytes, all cell types possess mitochondria which imply they are dependent on oxidative metabolism. Remarkably, mitochondria tightly connect ATP biosynthesis with oxygen consumption, and even minor changes in mitochondrial functional integrity affect many aspects of cellular homeostasis. Although, the main proposed function of mitochondria is to generate ATP via oxidative phosphorylation (OXPHOS) of adenosine diphosphate (ADP), additional functions include generation and detoxification of reactive oxygen species (ROS), calcium homeostasis, involvement in apoptosis, synthesis and catabolism of metabolites, and transport of organelles within the cell[6,7]. Any alteration in one of these processes can be defined as mitochondrial dysfunction[8]. Actually, we know that impaired mitochondrial metabolism is an important mechanism that leads to organ dysfunction[2,5]. In acute diseases, such as sepsis, the measurement of mitochondrial metabolism, through cellular respiration, has the potential to identify those patients at risk of progressing in their organ failures. In addition, it can potentially help in monitoring the therapeutic response of these patients[6,9].
Considering that mitochondria interact with various other pathways involved in inflammation, Ca2+ balance, redox signaling, and apoptosis, it can be assumed that mitochondria are fundamental in cell survival and death[10]. Mitochondrial metabolism has been shown to be severely impaired during the early phase of an acute disease, with a reduction in biogenesis, increased generation of ROS, and a decrease in ATP synthesis[11,12]. The presence of an impaired mitochondrial metabolism is associated with the presence of a multiple organ failure syndrome, highlighting mitochondrial components as potential targets for therapeutic strategies[4,11,13]. There has been an increased interest in this field, with new studies exploring mitochondrial impairment and organ dysfunction and their relationship with prognosis in sepsis[14-17].
The main objective of this review is analyze the current state of the art in this field, explore potential methods for assessing mitochondrial metabolism in intensive care settings, and explore future perspectives on mitochondrial metabolism as a biomarker of clinical outcomes in sepsis.
PHYSIOPATHOLOGY
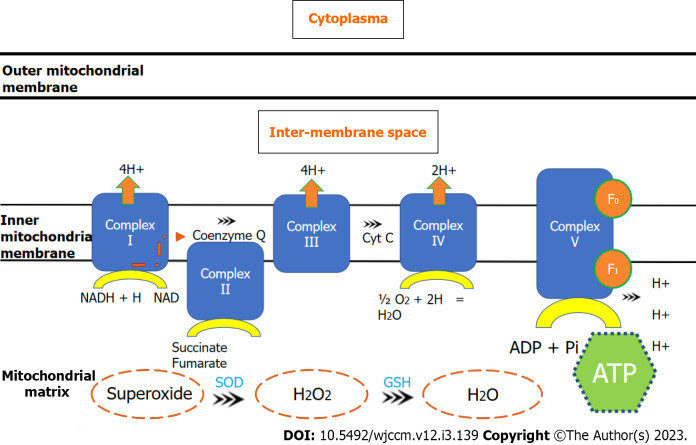
ATP is produced by mitochondria through OXPHOS by F1Fo-ATP synthase. ATP is generated to a greater extent by the oxidation of metabolic substrates in the tricarboxylic acid cycle leading to reduction of the electron acceptors NAD+ and FAD to nicotinamide adenine dinucleotide (NADH) and 1,5-dihydroflavin adenine dinucleotide (FADH2)[18]. Both NADH and FADH2 are subsequently oxidized in the electron transport system of mitochondria. The electron transport chain is composed by enzyme complexes I to IV and the transporters ubiquinone and cytochrome c. With the electrons movement across the respiratory chain, protons are pumped across the inner mitochondrial membrane, generating an electrical potential. This proton-motive force provides the energy for F1Fo-ATP synthase, as known as Complex V, to phosphorylate ADP to ATP (Figure 1). This mechanism is favored by the electrochemical gradient produced by the proton motive force. Oxygen is the terminal electron acceptor of the chain in Complex IV and is reduced to water[19]. An incomplete reduction of oxygen increases superoxide radical production in Complex III and at Complex I. As part of this process, ROS are generated as by-products of the incomplete four-electron reduction of molecular oxygen to water[20,21].
Figure 1.
Electron transport chain through mitocondrial complexes and oxidative phosphorylation. ADP: Adenosine diphosphtate; ATP: Adenosine triphosphate; Cyt c: Cytochrome c; GSH: Gluthatione synthetase; NAD: Nicotinamide adenine dinucleotide; NADH: Nicotinamide adenine dinucleotide (reduced); SOD: Superoxide dismuthase.
Under physiological conditions, mitochondria consume approximately 90% of the cellular O2; however, 1%-4% of the respiratory chain reactions lead to a leak of electrons that directly react with O2 to form O2(*-), which can oxidize lipids, DNA, or proteins[22]. To avoid self-damage, mitochondria have intrinsic defense mechanisms that protect against ROS-induced damage through a large array of antioxidants[12]. In sepsis, not only ROS are generated, but also reactive nitrogen species (RNS), nitric oxide, and peroxynitrite[7,20,21]. Enzymatic defenses, such as the superoxide dismutase 2 (SOD2), convert O2(*-) into hydrogen peroxide (H2O2), which can then be detoxified to water by catalase or selenium-containing glutathione peroxidase[23,24]. SOD2 expression is higher in survivors of critical illness[22]. Glutathione and Coenzyme Q10 (CoQ10) also have an important mitochondrial antioxidant function. CoQ10 Levels are lower in sepsis, suggesting a potential role in mitochondrial dysfunction[25].
Mitochondria are also essential for other cellular functions, such as calcium homeostasis, apoptosis, autophagy, and cellular signaling[6,9,12]. Mitochondrial DNA (mtDNA) is susceptible to mutations and deletions due to ROS increased levels and requires a set of self-regulated repair mechanisms[6]. mtDNA damage resulting from this phenomenon is associated with reduced mitochondrial respiratory capacity, which are potentially irreversible, depending on the intensity of oxidant “attack”[23]. Mitochondrial function is maintained by an equilibrium between fission, fusion, biogenesis, and autophagy[26]. In the case of an impairment in mitochondrial metabolism, various signaling routes allow an interaction between the mitochondria and nucleus, triggering mitochondrial biogenesis[10]. Defective mitochondria can become toxic by excessive ROS production, that can lead to apoptosis. Autophagy compensates for nutrient depletion or copes with cellular stress by recycling cellular components, to produce amino acids and fatty acids that can be metabolized and used in OXPHOS[27]. Despite being important for critical illness recovery, excessive induction of autophagy can trigger apoptosis[28]. ROS is one of some signaling pathways that regulate autophagy, and, since mitochondria are the primary source of ROS, mitochondria themselves play a key role in regulating autophagy[10]. Disturbances in mitochondrial function leading to impaired ATP biosynthesis, increased ROS production, and oxidative stress are associated with skeletal muscle damage, which correlates with septic shock severity and is associated with impaired clinical outcomes[18].
Many inflammatory mediators are linked to the altered mitochondrial metabolism. Tumoral necrosis factor-alpha (TNF-α) is a major interleukin that participates in the host response to sepsis and is capable of causing mitochondrial impairment[29]. TNF-α binds to several TNF receptors, ultimately promoting the intracellular release of ceramides and production of ROS, which may lead to mitochondrial dysfunction. TNF receptor activation promotes pro-inflammatory responses in polymorphonuclear leukocytes and monocytes, that induce ROS formation, leading to mtDNA damage and inhibition of mitochondrial metabolism in these cells[30]. Inhibition of mitochondrial complexes causes deviation of electrons, also producing even more ROS. These phenomena can lead to an imbalance between ROS production and mitochondrial antioxidant capacity, through manganese superoxide dismutase and glutathione reductase. This imbalance can trigger mitochondrial uncoupling related to the opening of mitochondrial permeability transition pores (PTP)[31]. The resulting mitochondrial permeability transition leads to dissolution of the electrochemical gradient required to form ATP, and these dysfunctional mitochondria are targeted for removal via autophagy. The induction of mitochondrial PTP win this context can promote apoptosis. Therefore, the pro-inflammatory activity in sepsis, especially in its initial phase, can lead to both a reduction in mitochondrial mass and a decrease in its function.
MECHANISMS OF MITOCHONDRIAL DYSFUNCTION
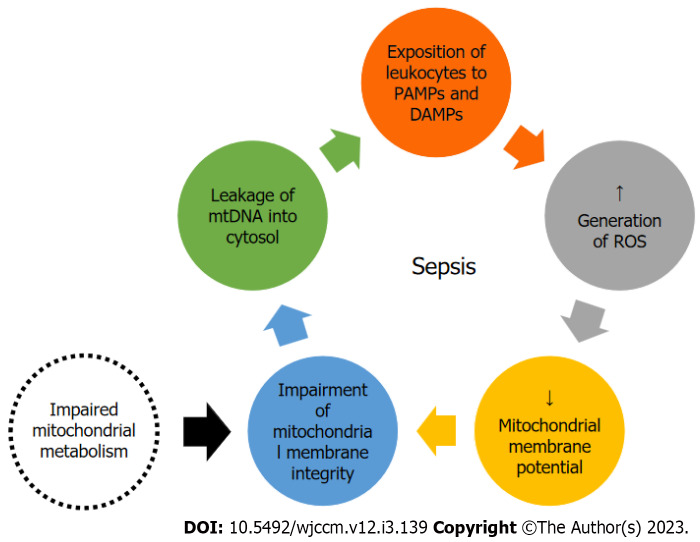
Hypoxia has been assumed to be the main causative agent of mitochondrial dysfunction[32]. However, it was later shown that tissue oxygen levels are normal or even elevated in sepsis[33]. Instead of a lower availability of oxygen, there is a lower use of it in septic patients[24]. Thus, OXPHOS dysfunction explain the inability to maintain ATP levels in this context, leading to increased glycolysis and increased lactate levels[6]. This imbalance between ROS production and antioxidant capacity leads to an oxidative stress that damage the electron transport chain and mtDNA, creating a vicious circle of mitochondrial damage and ROS production[20]. ROS and calcium overload cause an increased membrane permeability, and mitochondrial products such as mtDNA leak into the circulation, acting as danger-associated molecular patterns and contributing to multiorganic failure[13] in a vicious cycle (Figure 2). This cascade can trigger cell apoptosis[7], a component of tissue damage and organ failure.
Figure 2.
Mitochondrial DNA damage as a potential trigger for the inflammatory response. DAMP: Damage associated molecular patterns; mtDNA: Mitochondrial deoxyribonucleic acid; PAMP: Pathogen associated molecular patterns; ROS: Reactive oxygen species.
Changes in mitochondrial form and function in critical illnesses suggest that mitochondria try to rescue mechanisms and adapt to harmful environments. Mitochondrial fission and fusion are upregulated during critical illness, although it appears to be insufficient for restoring mitochondrial function[34]. Mitochondrial biogenesis was observed in skeletal muscle taken on days 1 to 2 in intensive care unit (ICU) survivors, but not in non-survivors[35]. During extreme conditions, such as refractory shock, mitochondrial damage is disseminated, causing the induction of mitochondrial PTP in many mitochondria and a great decrease in ATP production[36]. In contrast, a reduction in mitochondrial density was observed after the onset of sepsis, suggesting that although upregulated, biogenesis may be insufficient to maintain homeostasis[10].
Mitochondrial metabolism and inflammatory activity in sepsis-what is the relationship?
The hallmark of sepsis inflammatory response is an imbalance between a systemic inflammatory response and compensatory anti-inflammatory response[37,38]. The imbalance of pro- and anti-inflammatory responses often results in immunoparalysis among critically ill patients, making them more vulnerable to additional infections, and is linked to higher mortality rates[39]. Actually, the cause of immune dysfunction is matter of debate[38], and immune response is dependent of metabolic pathways[40], that is known as “immunometabolism”. Mitochondria are a hub of the immune system, playing a crucial role in regulating the function of immune cells and shaping and modulating the response of the immune system to infection[7]. Evidence suggests that leukocytes from critically ill patients display mitochondrial dysfunction, which is thought to be the root cause of immunoparalysis and could be responsible for the onset of organ dysfunction[41,42]. Moreover, the recovery of mitochondrial function is associated with improved recovery in critically ill patients[14]. These changes are mostly detected in lymphocytes, monocytes, and macrophages[43].
Lymphocytes respond to cytokine stimuli induced by monocyte-macrophages, dendritic cells, and neutrophils, which are responsible for the innate immune response. Activated phagocytic cells, such as monocytes and macrophages, release the proinflammatory cytokines interleukin-1 (IL-1) and IL-6[44]. This phenomenon has been associated with energy deprivation[45]. Lymphocytes also attenuate the potentially harmful effects of the proinflammatory response, and this modulation of the immune response has a major impact on prognosis in septic patients[46]. On the other hand, IL-10 plays a major role in modulating the immune system by inhibiting monocyte-macrophage activation and suppressing the production of TNF-α, IL-1, and interferon-gamma (IFN-γ) from lymphocytes acting at the level of accessory cells[47]. In addition to cytokines, Krebs cycle intermediates, such as citrate, succinate, and itaconate, can activate pro-inflammatory gene expression[48]. Metabolites from the Krebs cycle impact the reprogramming of macrophages from the M1 phenotype (pro-inflammatory) to the M2 phenotype (anti-inflammatory). M1 macrophages have impaired OXPHOS, and M2 macrophages have an intact Krebs cycle, with OXPHOS as the main source of ATP generation[43].
Two major pathways are implicated in this interaction between mitochondrial function and inflammatory responses. The mammalian target of rapamycin (mTOR) pathway plays a pivotal role in metabolic regulation by modulating glycolysis. Furthermore, metabolic reprogramming and a transition to glycolysis for energy production in CD4+ and CD8+ T cells are also induced by the activation of mTOR and OXPHOS[30]. The nuclear factor kappabeta (NF-κβ) is a stress-induced pathway (i.e., tissue damage, cytokine, and PAMPs release) that promotes the expression of target genes involved in the immune response. Upon activation of the NF-κβ pathway and subsequent induction of cytokine expression, macrophages undergo differentiation into either M1 or M2 subtypes, contingent on the local cytokine milieu present at the site of infection. IFN-γ typically drives the differentiation of M1 macrophages, which in turn produce pro-inflammatory cytokines. Conversely, M2 cells refer to macrophages exposed to immune complexes, IL-4, IL-13, and IL-10[30].
MEASUREMENT OF MITOCHONDRIAL DYSFUNCTION
Regrettably, it is currently impractical to perform a thorough and accurate real-time analysis of the modified cells and tissues within malfunctioning organs of living human patients in a hospital setting. Therefore, mechanisms must be inferred from tissue specimens obtained from nonvital organs (e.g., blood, skeletal muscle) or from postmortem examinations. In postmortem studies, septic patients exhibit mild to moderate mitochondrial swelling and autophagocytosis, with minimal cell death or indications of permanent damage, such as tissue fibrosis[49].
Mitochondrial biogenesis
The process of mitochondrial biogenesis encompasses the synthesis of mitochondrial proteins encoded by nuclear DNA, which are subsequently imported and integrated into the mitochondria. Additionally, biogenesis can also occur through mitochondrial DNA, which encodes 13 proteins primarily located within the OXPHOS pathway. In this way, biogenesis serves to replace damaged proteins and enhances the ability to generate energy if energy demand increases over time[4]. Increased mitochondrial biogenesis is detected in postmortem studies in critically ill patients, with increased expression of transcription factors[34]. A decrease in mitochondrial content has also been reported in the muscles of critically ill patients with sepsis-induced multiple organ failure[50]. Taken together, these data suggest that biogenesis activation may have a role in the recovery phase of critical illness[22], time when there was also an increase in RNA expression, participating in the restorative process[16]. These data point to compromised mitochondrial biogenesis in critically ill patients; and an activation of the biogenesis pathway may represent a key prognostic factor in critically ill patients, associated with recovering of the initial injury[22].
From this point of view, it seems reasonable to imagine that the dynamic processes of mitochondrial fusion and fission must change during acute inflammatory injury observed in sepsis. This activity can be measured by assessing the concentrations of mitochondrial fusion proteins (mitofusins 1 and 2, optic atrophy 1 protein) and mitochondrial fission proteins (dynamin-related protein 1 and fission 1 protein)[22]. Currently, adequate characterization in relation to the pro-fusion or mitochondrial fission profile in sepsis is merely speculative, lacking clinical studies that may show some signs regarding its in vivo effects.
Mitochondrial DNA
Mitochondria experience various morphological changes during fusion and fission events, which help to sustain a healthy mitochondrial population by facilitating mitochondrial DNA exchange, preserving mitochondrial DNA integrity, and regulating the size, quantity, distribution, and upkeep of OXPHOS capacity[22]. These morphological changes also play a crucial role in cell division and proliferation, as well as in the selective elimination of damaged or excess mitochondria through a process referred to as mitophagy[4]. Proteins that facilitate fusion events (such as mitofusin-2) and fission events (such as dynamin related protein-1) have been linked to changes in mitochondrial membrane potentials and diminished oxygen consumption[51]. Fission and fusion processes become more prevalent under stressful conditions and play a crucial role in eliminating damaged mitochondria and enhancing repair mechanisms. Currently, there is insufficient data regarding these mitochondrial dynamics in septic patients, and the data may vary based on the tissue type. From a hypothetical perspective, the balance of mitochondrial dynamics in septic patients may shift in favor of mitochondrial fusion, which could represent a cellular response aimed at improving mitochondrial function and decreasing oxidative stress[52].
However, mitochondrial DNA levels in the serum should be interpreted as a potential damage-associated molecular pattern, propagating an inflammatory response through interactions with the immune system[12,53]. Thus, mtDNA damage can lead to a pathological cycle, resulting in metabolic dysfunction, especially in white blood cells[54]. A reduction in mtDNA content in the peripheral blood, observed in the acute phase of sepsis, could be due to an increased concentration of neutrophils in the peripheral blood[55]. Therefore, it remains uncertain whether there is an interaction between DNA concentration and changes in mitochondrial function, especially in immune cells. Mitochondria contain their own DNA, and the depletion of mtDNA in an injury process may theoretically cause a respiratory chain defect and compromise ATP synthesis[23,55].
Qualitative measurements of mitochondrial metabolism
In addition to changes in mitochondrial mass, the quality of mitochondrial function and a shift towards glycolytic pathways are regulated by several hormones, enzymes, and regulatory pathways within cells. Reactive derivatives of nitric oxide and superoxide anion (such as peroxynitrite), which cause oxidative stress, promote glycolysis by activating the rate-limiting step of the pentose pathway, glucose-6-phosphate dehydrogenase. The pentose pathway results in the formation of NADPH relative to NADH. While NADH is the substrate for mitochondrial OXPHOS of high-energy phosphates, NADPH is crucial for the formation and repair of proteins, DNA, and lipids. Thus, by diverting glycolytic intermediates from the Krebs cycle to suppress aerobic mitochondrial respiration, cells and tissues transition to a state of decreased oxygen consumption and ATP production. This phenomenon is commonly referred to as the “Warburg effect”, particularly in the context of cancer[41]. In this context, cells are less dependent on oxidative metabolism, thus reducing oxidative stress and promoting the formation of reducing equivalents (e.g., lactic acid and NADPH) that induce cell repair[56]. The Warburg effect and related mediators, such as HIF-1α, are induced under conditions that model sepsis, confer cytoprotection to vital organs, and inhibit inflammation under conditions of acute cell stress[57]. However, the HIF-1α activation in immune cells could perpetuate the activation of the pro-inflammatory pathway[58].
The proton pumps of the electron transport chain, in conjunction with F1Fo-ATP synthase, establish a proton gradient across the inner membrane, generating both an electrochemical potential (proton motive force, pmf, in mV) and a flux of protons (proton current in nmol of protons/min). The mitochondrial membrane potential is a critical component of healthy mitochondrial metabolism and contributes to determining the pmf[8].
Reductions in both the expression and activity of complexes I, II, III, and IV have been reported in critically ill patients[14,22]. However, it is still doubtful whether these alterations are determinants of the patient's prognosis, or if they are just epiphenomena in the acute context of critical illness. However, they are useful and commonly used measurements to assess mitochondrial activity[35,59-61], especially when normalized by enzymatic activity, protein, or DNA concentration.
Respirometry
Measurement of mitochondrial respiration is a cost-effective and time-efficient method compared to traditional methods of assessing mitochondrial function in biopsies, making it readily available for use. Advanced instruments equipped with highly sensitive micro-cathode oxygen electrodes enable high-resolution measurements of mitochondrial respiration and can be utilized in acute care settings. Mitochondrial respiration can be quantified by performing substrate-uncoupler-inhibitor titrations, commonly known as the SUIT protocol. This protocol involves titration with various combinations of substrates, uncouplers, and inhibitors to assess mitochondrial respiratory function[9]. This protocol allows the study of complex interactions of coupling and substrate control in a single assay, measuring multiple aspects of mitochondrial physiology[62].
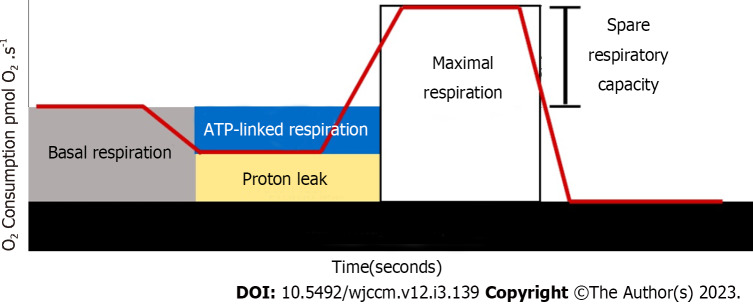
Respirometry enables the real-time measurement of mitochondrial respiration, with key parameters obtainable through the use of established inhibitors and uncouplers that act as sensitive indicators of response to mitochondrial stress. Figure 3 depicts the mitochondrial respiration trace derived from the SUIT protocol, which was employed in the following procedures[9,62]: (1) Routine respiration: Routine respiration also known as basal respiration, measures the oxygen consumption resulting from ATP production and proton leak. This represents energy demand under steady-state conditions. Changes in routine respiration in patients with disease compared to controls may indicate altered mitochondrial function and should be interpreted in the context of the following mitochondrial parameters; (2) proton leak: After measuring routine respiration, cells are exposed to oligomycin, an inhibitor of complex V. The remaining mitochondrial respiration after the addition of oligomycin is attributable to proton leak. While some proton leaks are expected under physiological conditions, significant proton leak may indicate damage to the mitochondrial membrane and/or complex damage. The use of oligomycin also allows for the estimation of oxygen consumption secondary to ATP production, often referred to as ATP-linked respiration; (3) maximal respiration: The addition of a mitochondrial uncoupler, such as dinitrophenol or carbonyl cyanide-4-(trifluoromethoxy) phenylhydrazone, stimulates maximal respiration by mimicking the physiological energy demand, leading to an increase in oxygen consumption. The difference between maximal respiration and routine respiration represents the spare respiratory capacity (SRC) of the cell. SRC indicates the ability of the cell to respond to energetic stress and is a measure of a cell’s fitness. A decrease in SRC may limit the cell's ability to handle stressors, resulting in mitochondrial dysfunction; and (4) residual oxygen consumption: The addition of mitochondrial inhibitors, such as the combination of rotenone (complex I) and antimycin (complex III), completely inhibits electron transport system. The remaining oxygen is consumed by non-mitochondrial respiration in the form of oxidases and other cellular enzymes that use oxygen. Residual oxygen consumption may increase in the presence of a stress response.
Figure 3.
Example of a respirometry assay. ATP: Adenosine triphosphate.
Under normal conditions with excess ADP and oxygen, mitochondrial respiration occurs rapidly, known as state 3 respiration. Conversely, when ADP is fully consumed, state 4 respiration occurs, which is significantly slower. This state 4 respiration can be induced by “uncoupling” oxygen consumption from OXPHOS, leading to proton leakage back into the mitochondrial matrix without the production of cellular energy. One of the most promising indicators of mitochondrial function is the biochemical coupling efficiency (BCE), which is calculated as the quotient between OXPHOS and proton leak. BCE reflects the true effectiveness of mitochondria in utilizing oxygen for ATP production[62]. It is a useful way to gain more insight into the site of the dysfunction, namely, respiratory control decreases because of dysfunction in localized sites of substrate oxidation, ATP synthesis, proton conductance, or F1Fo-ATP synthase[8].
Although we understand that small clinical centers may have limited access to equipment for measuring real-time mitochondrial respiratory rates, this limitation could be easily overcome using simpler biochemical colorimetric methods that still maintain a reasonable level of sensitivity, the time to assay is usually short, and easy to implement in the laboratory hospital routine. For instance, the measurements of enzymatic activity of succinate dehydrogenase: Complex II (succinate: DCIP-oxidoreductase), complex, and complex V are routinely performed in research laboratories, and at the current state require standardization as a step forward to reach clinical settings[63,64]. However, the aforementioned colorimetric methods restrict the evaluation of metabolic activity to one complex each time, whereas in mitochondrial respirometry assays the metabolic activity of complexes can be assayed both, individually or more than one at the same time.
Which cells are ideal for measuring the mitochondrial activity? And what is the most suitable method?
Although it is logical that dysfunction in mitochondrial metabolism leads to a certain degree of organ failure, its measurement is often not feasible, because the ethical questions, costs, and viability of obtaining adequate mitochondrial samples from vital organs in critically ill patients[61]. Therefore, it is feasible to assess mitochondrial dysfunction in cells that are easy to collect, especially those that may reflect the “systemic” effect of sepsis on the body. Peripheral blood cells have been used to access bioenergetic functions in translational research. Peripheral blood mononuclear cells (PBMCs) are mitochondria-rich with high rates of respiration[16]; therefore, they are prime candidates in circulating blood to provide reliable estimation of global oxidative metabolism, particularly the metabolism linked to immune response. Lymphocytes comprise the majority of PBMCs and are traditionally used to measure defects in mitochondrial OXPHOS[30]. Exhaustion of lymphocytes, especially T cells, leads to an increased risk of secondary infections, which is correlated with mortality[65]. Circulating immune cells play an important role in the pathophysiology of sepsis because their activation may remotely induce inflammation in non-infected organs[66]. The measurement of mitochondrial respiration in PBMCs of septic patients has the potential to identify those at risk of negative outcomes, and also can monitor the clinical course and response to treatment, making it a useful marker for acute care settings[9]. Thus, it is a candidate biomarker in sepsis.
In addition, this approach allows us to unravel mechanist readouts associated with impaired metabolism that follow this syndrome and also challenges how or whether this could be improved by therapeutic intervention. These primary observations collected from the literature are encouraging, and more clinical studies will help to advance methodological issues, clinical validation, and the level of reproducibility and sensitivity (Table 1).
Table 1.
Different methods of mensuration of mitochondrial damage and recovery
| Item | Description |
| Mitochondrial biogenesis | PPAR-γ coactivator 1-α, nuclear respiratory factor 1, mitochondrial transcription factor A. Material obtained from tissue biopsy or from peripheral PBMC |
| Mitochondrial content | Mitochondrial mass, concentration and area |
| Mitochondrial DNA content | PGC-1α, NRF-1, absolute DNA number of copies |
| Mitochondrial fusion | Mitofusins 1 and 2, optic atrophy 1 protein |
| Mitochondrial fission | Dynamin-related protein 1 and fission 1 protein |
PPAR-γ: Peroxisome proliferator-activated receptor gamma; PGC-1α: Peroxisome proliferator-activated receptor gamma coactivator-1 alpha; NRF-1: Nuclear respiratory factor-1; PBMC: Peripheral blood mononuclear cells.
SEPSIS AND MULTI-ORGANIC FAILURE
Various systemic inflammatory processes can exert different effects on mitochondria. In the early stages of sepsis, reduced perfusion resulting from intrinsic and extrinsic fluid losses, decreased intake, myocardial depression, microcirculatory redistribution of blood flow, and loss of vascular tone, can lead to tissue hypoxia. This condition, characterized by insufficient oxygen levels at the mitochondrial level, impedes the ability of mitochondria to carry out OXPHOS, leading to a deficit in ATP production[12]. Although Complex IV exhibits distinctive enzyme properties that facilitate its functionality under hypoxic conditions, severely diminished oxygen concentrations may compromise ATP generation and activate cell death pathways, thereby adversely impacting cellular homeostasis[12,67,68]. Hormonal alterations in sepsis also affect mitochondrial function and efficiency. For example, thyroid hormones are believed to work predominantly through the modulation of mitochondrial activity[4,33]. Thirdly, genes that transcribe mitochondrial proteins are downregulated early in the inflammatory response. This was first recognized in human volunteers receiving endotoxins[69] and subsequently described in critically ill patients[35].
Impairment of mitochondrial metabolism in sepsis
Different studies, in different contexts, have evaluated mitochondrial activity in septic compared to nonseptic or control patients. Muscular cells are prone to impaired mitochondrial metabolism during critical illness[70] and are an important research field. Carré et al[35] used muscle tissue biopsies from critically ill patients, comparing them to those of controls subjected to hip surgery. They found a decrease in mitochondrial density in critically ill patients, without a decrease in Complex I and Complex IV activity. In a study evaluating patients with ICU-acquired weakness, comparing ATP synthesis in this population with metabolically healthy controls, ICU patients had an approximately 50% reduction in the ability of skeletal muscle to synthesize ATP in mitochondria, with a depletion of complex III and IV concentrations[71]. A similar loss of mitochondrial activity was detected in a previous study in a population with sepsis and multiorgan failure[72]. Complex I and complex IV activity was reduced in the intercostal and leg muscles, respectively, compared to controls.
Belikova demonstrated a higher baseline PBMC oxygen consumption and attenuated response to ADP stimulation in patients with sepsis than in healthy volunteers[66]. In blood mononuclear cells, Kraft et al[16] demonstrated an early sepsis-mediated disruption of mitochondrial quality control in septic patients, with a later activation of mitochondrial biogenesis in this population. These patients also showed increased mitochondrial damage (measured by mtDNA levels) during the early phase of sepsis management. In a cohort of septic and non-septic ICU patients with measurement of mitochondrial function in isolated lymphocytes, critically ill patients had increased mitochondrial oxygen consumption but no significant difference in mitochondrial membrane potential[17]. Jang et al[15] also found a lower routine, uncoupled Complex I, and maximal respiration in septic patients, when compared to controls in the early sepsis management. A respirometric study in PBMC developed by Japiassú et al[73] reported reduced F1Fo-ATP synthase activity, thereby reducing ATP production. This may contribute to the energetic failure reported in these cells during the course of septic insult. In addition, septic shock PBMC have reduced O2 consumption, ADP-induced state 3 respiration, and respiratory control ratio compared to control PBMC. Inhibition of complexes I, III, and IV in PBMCs from septic patients compared with controls was also detected in another study[74]. In a pediatric population, Weiss et al[75] detected a decrease in spare respiratory capacity on days 1-2 of sepsis compared with controls. Spare respiratory capacity normalized on days 5-7. Patients with sepsis also had a higher ratio of leak to maximal respiration than controls, with normalization in the later phase of sepsis. Patients with sepsis did not show differences in basal or ATP-linked oxygen consumption or membrane potential.
In patients admitted to the emergency department with and without sepsis, Puskarich[76] did not find differences in plasma levels of cytochrome B, NADH, and Cox-III mtDNA between groups. Pyle et al[55], in a protocol that evaluated mononuclear cell mtDNA content, found that these levels were lower in patients with sepsis, with depletion of monocyte and lymphocyte mtDNA. Platelet studies have also evaluated mitochondrial metabolism in patients with sepsis. Sjövall et al[61] found an increase in state 3 and a decrease in RCR in patients with sepsis compared to controls during sequential evaluations in the first week of sepsis diagnosis. Additionally, patients with sepsis had increased rates of complex I and complex II respiration compared to controls. However, the mtDNA concentration did not differ between the platelets of patients with sepsis and controls.
Are mitochondrial metabolisms associated with mortality?
A criticism can be made of the differences in mitochondrial measurements between survivors and non-survivors observed in studies. Whether they are pathological or just another measure of disease severity requires further investigation[77]. In human subjects, a significant constraint of this research approach is the uncertainty surrounding whether mitochondria sourced from PBMCs, platelets, or muscle cells can accurately serve as proxies for the mitochondria present in essential organs like the liver, kidneys, and heart[77]. The establishment of a workable, all-encompassing strategy to investigate the complete energy production pathway in human beings would signify a more significant achievement, as it would facilitate a deeper comprehension of the origin of lactate in distinct patients and across time, thereby enabling more targeted clinical trials for novel treatments for bioenergetic dysfunction.
Defects in leukocyte energy metabolism[78], particularly in T lymphocyte cells[79], are intrinsically associated with the state of immunoparalysis in sepsis. Metabolic events in the mitochondria of macrophages, dendritic cells, and T-lymphocytes have profound effects on immunity. When exposed to infectious injury, OXPHOS levels decrease, with a concomitant increase in glycolysis. An outcome of the reduced ATP production via OXPHOS is the redirection of mitochondria towards generating mitochondrial ROS, which function as signaling molecules essential for eliciting an appropriate immune response[54]. Therefore, mitochondrial respiration is essential for the functioning of these cells.
Despite the fact that the current knowledge suggests a potential role of mitochondrial metabolism impairment in septic patients (when compared with controls) with a potential impact on prognosis, the literature is quite heterogeneous with regard to the findings of mitochondrial dysfunction (Table 2). It is still necessary to define the most practical way of measuring, with the greatest clinical applicability, the greatest prognostic impact, and, above all, the most accurate in predicting the clinical course of the disease.
Table 2.
Studies that explored association of mitochondrial dysfunction and prognosis in sepsis
|
Ref.
|
n of critically ill septic patients
|
Mitochondrial measurement
|
Main findings (survivors vs nonsurvivors)
|
| Belikova et al[66], 2007 | |||
| Brealey et al[18], 2003 | 28 | ATP concentration, Complex I, II, and IV activities in biopsied muscle cells | Sepsis survivors had an increased level of ATP and Complex I and IV activity |
| Carré et al[35], 2010 | 16 | Mitochondrial morphology (surface density and volume), RT-PCR of mitochondrial biogenesis factors and concentration of Complex I and Complex IV mitochondrial proteins and OXPHOS transcripts in muscle biopsy specimens | Nonsurvivors had an increased decline in mitochondrial surface density, with a similar mitochondrial volume in these groups; OXPHOS transcripts were more abundant in survivors; increased ATP content in survivors; no difference between groups in Complex I and Complex IV activity |
| Kraft et al[16], 2019 | 37 | qRT-PCR for genes that regulate mitochondrial biogenesis in PBMCs | Increased genetic activation of mitochondrial biogenesis in day 3 compared with day 1; decrease in mtDNA in septic patients compared with controls, with a recovery on day 5; early activation of mitochondrial biogenesis by day 1 associated with ICU discharge; increased mRNA levels in survivors |
| Japiassú et al[73], 2011 | 20 | Respirometry of PBMC evaluating state 3, 4 and respiratory control ratio | No difference in ADP-stimulated respiration in nonsurvivors, when compared with survivors |
| Nedel et al[14], 2021 | 90 patients | Respirometry of permeabilized lymphocytes | Improvement in Complex I, Complex II, basal and in BCE in day 3, compared with day 1, were associated with lower mortality. In multivariate analysis, BCE improvement was associated with lower 6-mo mortality |
| Puskarich et al[77], 2015 | 28 patients | Respirometry of platelets | Routine and state 3 respiration were significantly higher in non-survivors compared to survivors; state 4 respiration had a non-significant increase in non-survivors |
| Pyle et al[55], 2010 | Not reported (147 patients, including septic) | mtDNA content from mononuclear cells | No relationship between mtDNA content and survival outcome at 180 d |
| Sjövall et al[61], 2010 | 18 patients | Respirometry of isolated platelets evaluating Complex I, state 3, 4 and respiratory control ratio | Non-survivors had an increased Complex I, Complex II, state 3 respiration and an increased respiratory control ratio at day 6-7 of sepsis when compared with survivors |
| Sjövall et al[82], 2013 | 20 patients | Respirometry of permeabilized peripheral blood immune cells | Survivors and non-survivors at 90 d after sepsis did not have difference in Complex I plus Complex II respiration normalized to citrate synthase, mtDNA, and cytochrome c |
ATP: Adenosine triphosphate; BCE: Biochemical coupling efficiency; ICU: Intensive care unit; qRT-PCR: Quantitative reverse transcriptase-polymerase chain reaction; mtDNA: Mitochondrion desoxyribonucleic acid; OXPHOS: Oxidative phosphorylation; PBMC: Peripheral blood mononuclear cells; ADP: Adenosine diphosphtate.
Are mitochondrial metabolisms associated with recovery?
Therefore, mitochondrial biogenesis is critical for recovery, and the recovery from organ dysfunction is preceded by an increased mitochondrial biogenesis[33]. In our study of patients with multi-organ failure in intensive care, we found that those who ultimately survived had higher levels of PGC-1α and better-preserved levels of Complex protein, along with a more robust antioxidant response (specifically, manganese superoxide) in the early stages of their disease progression[35]. The ability to clear damaged mitochondria is another important phenomenon[80]. Mitophagy (autophagic degradation) and mitoptosis (programmed destruction) are the processes by which cells deal with impaired mitochondria[12]. The efficiency of these processes may be an important contributing factor to the pathogenesis of various states of the disease. The process of mitophagy entails the targeted sequestration and subsequent degradation of damaged mitochondria, which occurs prior to their ability to activate cell death pathways and potentially jeopardize the viability of the entire cell. Thus, mitophagy operates as an initial protective response. Conversely, heightened levels of oxidative stress and apoptotic proteases can impede the function of mitophagy and stimulate additional inflammatory responses[81].
CONCLUSION
Alterations caused by acute inflammatory conditions, such as sepsis, is associated with impaired function of mitochondrial components including protein content, mtDNA concentration, oxidative complexes activity, and F1Fo-ATP synthase. Often, these alterations are associated with clinical outcomes. Given these features mitochondria deserve to be better explored regarding its role as potential biomarker in prognosis, sepsis rehabilitation, and its association with different spectrum of organ failure.
Footnotes
Conflict-of-interest statement: The authors state that there was no conflict of interest.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Peer-review started: January 20, 2023
First decision: February 20, 2023
Article in press: April 14, 2023
Specialty type: Critical care medicine
Country/Territory of origin: Brazil
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Liu LP, China; Zhong GQ, China S-Editor: Chen YL L-Editor: A P-Editor: Xu ZH
Contributor Information
Wagner Nedel, Intensive Care Unit, Grupo Hospitalar Conceição, Porto Alegre 91350200, Brazil; Laboratory of Neurotrauma and Biomarkers, Departamento de Bioquímica, ICBS, Universidade Federal do Rio Grande do Sul, Porto Alegre 90035-003, Brazil; Brazilian Research in Intensive Care Network-BRICNet, São Paulo 04039-002, Brazil. wagnernedel@gmail.com.
Caroline Deutschendorf, Infection Control Committee, Hospital de Clínicas de Porto Alegre, Porto Alegre 90410-000, Brazil.
Luis Valmor Cruz Portela, Laboratory of Neurotrauma and Biomarkers, Departamento de Bioquímica, ICBS, Universidade Federal do Rio Grande do Sul, Porto Alegre 90035-003, Brazil.
References
- 1.Evans L, Rhodes A, Alhazzani W, Antonelli M, Coopersmith CM, French C, Machado FR, Mcintyre L, Ostermann M, Prescott HC, Schorr C, Simpson S, Wiersinga WJ, Alshamsi F, Angus DC, Arabi Y, Azevedo L, Beale R, Beilman G, Belley-Cote E, Burry L, Cecconi M, Centofanti J, Coz Yataco A, De Waele J, Dellinger RP, Doi K, Du B, Estenssoro E, Ferrer R, Gomersall C, Hodgson C, Hylander Møller M, Iwashyna T, Jacob S, Kleinpell R, Klompas M, Koh Y, Kumar A, Kwizera A, Lobo S, Masur H, McGloughlin S, Mehta S, Mehta Y, Mer M, Nunnally M, Oczkowski S, Osborn T, Papathanassoglou E, Perner A, Puskarich M, Roberts J, Schweickert W, Seckel M, Sevransky J, Sprung CL, Welte T, Zimmerman J, Levy M. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock 2021. Crit Care Med. 2021;49:e1063–e1143. doi: 10.1097/CCM.0000000000005337. [DOI] [PubMed] [Google Scholar]
- 2.Cecconi M, Evans L, Levy M, Rhodes A. Sepsis and septic shock. Lancet. 2018;392:75–87. doi: 10.1016/S0140-6736(18)30696-2. [DOI] [PubMed] [Google Scholar]
- 3.Angus DC, van der Poll T. Severe sepsis and septic shock. N Engl J Med. 2013;369:840–851. doi: 10.1056/NEJMra1208623. [DOI] [PubMed] [Google Scholar]
- 4.Singer M. The role of mitochondrial dysfunction in sepsis-induced multi-organ failure. Virulence. 2014;5:66–72. doi: 10.4161/viru.26907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Leite HP, de Lima LF. Metabolic resuscitation in sepsis: a necessary step beyond the hemodynamic? J Thorac Dis. 2016;8:E552–E557. doi: 10.21037/jtd.2016.05.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Supinski GS, Schroder EA, Callahan LA. Mitochondria and Critical Illness. Chest. 2020;157:310–322. doi: 10.1016/j.chest.2019.08.2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arulkumaran N, Deutschman CS, Pinsky MR, Zuckerbraun B, Schumacker PT, Gomez H, Gomez A, Murray P, Kellum JA ADQI XIV Workgroup. MITOCHONDRIAL FUNCTION IN SEPSIS. Shock. 2016;45:271–281. doi: 10.1097/SHK.0000000000000463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brand MD, Nicholls DG. Assessing mitochondrial dysfunction in cells. Biochem J. 2011;435:297–312. doi: 10.1042/BJ20110162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jang DH, Greenwood JC, Spyres MB, Eckmann DM. Measurement of Mitochondrial Respiration and Motility in Acute Care: Sepsis, Trauma, and Poisoning. J Intensive Care Med. 2017;32:86–94. doi: 10.1177/0885066616658449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moonen HPFX, Van Zanten ARH. Mitochondrial dysfunction in critical illness during acute metabolic stress and convalescence: consequences for nutrition therapy. Curr Opin Crit Care. 2020;26:346–354. doi: 10.1097/MCC.0000000000000741. [DOI] [PubMed] [Google Scholar]
- 11.Maestraggi Q, Lebas B, Clere-Jehl R, Ludes PO, Chamaraux-Tran TN, Schneider F, Diemunsch P, Geny B, Pottecher J. Skeletal Muscle and Lymphocyte Mitochondrial Dysfunctions in Septic Shock Trigger ICU-Acquired Weakness and Sepsis-Induced Immunoparalysis. Biomed Res Int. 2017;2017:7897325. doi: 10.1155/2017/7897325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kozlov AV, Bahrami S, Calzia E, Dungel P, Gille L, Kuznetsov AV, Troppmair J. Mitochondrial dysfunction and biogenesis: do ICU patients die from mitochondrial failure? Ann Intensive Care. 2011;1:41. doi: 10.1186/2110-5820-1-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McClave SA, Wischmeyer PE, Miller KR, van Zanten ARH. Mitochondrial Dysfunction in Critical Illness: Implications for Nutritional Therapy. Curr Nutr Rep. 2019;8:363–373. doi: 10.1007/s13668-019-00296-y. [DOI] [PubMed] [Google Scholar]
- 14.Nedel WL, Kopczynski A, Rodolphi MS, Strogulski NR, De Bastiani M, Montes THM, Abruzzi J Jr, Galina A, Horvath TL, Portela LV. Mortality of septic shock patients is associated with impaired mitochondrial oxidative coupling efficiency in lymphocytes: a prospective cohort study. Intensive Care Med Exp. 2021;9:39. doi: 10.1186/s40635-021-00404-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jang DH, Orloski CJ, Owiredu S, Shofer FS, Greenwood JC, Eckmann DM. Alterations in Mitochondrial Function in Blood Cells Obtained From Patients With Sepsis Presenting to an Emergency Department. Shock. 2019;51:580–584. doi: 10.1097/SHK.0000000000001208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kraft BD, Chen L, Suliman HB, Piantadosi CA, Welty-Wolf KE. Peripheral Blood Mononuclear Cells Demonstrate Mitochondrial Damage Clearance During Sepsis. Crit Care Med. 2019;47:651–658. doi: 10.1097/CCM.0000000000003681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ayala JC, Grismaldo A, Aristizabal-Pachon AF, Mikhaylenko EV, Nikolenko VN, Mikhaleva LM, Somasundaram SG, Kirkland CE, Aliev G, Morales L. Mitochondrial Dysfunction in Intensive Care Unit Patients. Curr Pharm Des. 2021;27:3074–3081. doi: 10.2174/1381612826666201207112931. [DOI] [PubMed] [Google Scholar]
- 18.Brealey D, Singer M. Mitochondrial Dysfunction in Sepsis. Curr Infect Dis Rep. 2003;5:365–371. doi: 10.1007/s11908-003-0015-9. [DOI] [PubMed] [Google Scholar]
- 19.Hsiao CP, Hoppel C. Analyzing mitochondrial function in human peripheral blood mononuclear cells. Anal Biochem. 2018;549:12–20. doi: 10.1016/j.ab.2018.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Galley HF. Oxidative stress and mitochondrial dysfunction in sepsis. Br J Anaesth. 2011;107:57–64. doi: 10.1093/bja/aer093. [DOI] [PubMed] [Google Scholar]
- 21.Mantzarlis K, Tsolaki V, Zakynthinos E. Role of Oxidative Stress and Mitochondrial Dysfunction in Sepsis and Potential Therapies. Oxid Med Cell Longev. 2017;2017:5985209. doi: 10.1155/2017/5985209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Preau S, Vodovar D, Jung B, Lancel S, Zafrani L, Flatres A, Oualha M, Voiriot G, Jouan Y, Joffre J, Uhel F, De Prost N, Silva S, Azabou E, Radermacher P. Energetic dysfunction in sepsis: a narrative review. Ann Intensive Care. 2021;11:104. doi: 10.1186/s13613-021-00893-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Crouser ED. Mitochondrial dysfunction in septic shock and multiple organ dysfunction syndrome. Mitochondrion. 2004;4:729–741. doi: 10.1016/j.mito.2004.07.023. [DOI] [PubMed] [Google Scholar]
- 24.Nagar H, Piao S, Kim CS. Role of Mitochondrial Oxidative Stress in Sepsis. Acute Crit Care. 2018;33:65–72. doi: 10.4266/acc.2018.00157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vassiliou AG, Mastora Z, Jahaj E, Keskinidou C, Pratikaki ME, Kampisiouli E, Orfanos SE, Kotanidou A, Dimopoulou I. Serum Coenzyme Q10 Levels are Decreased in Critically-Ill Septic Patients: Results From a Preliminary Study. Biol Res Nurs. 2021;23:198–207. doi: 10.1177/1099800420944489. [DOI] [PubMed] [Google Scholar]
- 26.Zamponi N, Zamponi E, Cannas SA, Billoni OV, Helguera PR, Chialvo DR. Mitochondrial network complexity emerges from fission/fusion dynamics. Sci Rep. 2018;8:363. doi: 10.1038/s41598-017-18351-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roca-Agujetas V, de Dios C, Lestón L, Marí M, Morales A, Colell A. Recent Insights into the Mitochondrial Role in Autophagy and Its Regulation by Oxidative Stress. Oxid Med Cell Longev. 2019;2019:3809308. doi: 10.1155/2019/3809308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gunst J, Derese I, Aertgeerts A, Ververs EJ, Wauters A, Van den Berghe G, Vanhorebeek I. Insufficient autophagy contributes to mitochondrial dysfunction, organ failure, and adverse outcome in an animal model of critical illness. Crit Care Med. 2013;41:182–194. doi: 10.1097/CCM.0b013e3182676657. [DOI] [PubMed] [Google Scholar]
- 29.Pool R, Gomez H, Kellum JA. Mechanisms of Organ Dysfunction in Sepsis. Crit Care Clin. 2018;34:63–80. doi: 10.1016/j.ccc.2017.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Koutroulis I, Batabyal R, McNamara B, Ledda M, Hoptay C, Freishtat RJ. Sepsis Immunometabolism: From Defining Sepsis to Understanding How Energy Production Affects Immune Response. Crit Care Explor. 2019;1:e0061. doi: 10.1097/CCE.0000000000000061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Exline MC, Crouser ED. Mitochondrial dysfunction during sepsis: still more questions than answers. Crit Care Med. 2011;39:1216–1217. doi: 10.1097/CCM.0b013e31821487cb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wesselink E, Koekkoek WAC, Grefte S, Witkamp RF, van Zanten ARH. Feeding mitochondria: Potential role of nutritional components to improve critical illness convalescence. Clin Nutr. 2019;38:982–995. doi: 10.1016/j.clnu.2018.08.032. [DOI] [PubMed] [Google Scholar]
- 33.Singer M. Critical illness and flat batteries. Crit Care. 2017;21:309. doi: 10.1186/s13054-017-1913-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vanhorebeek I, Gunst J, Derde S, Derese I, Boussemaere M, D'Hoore A, Wouters PJ, Van den Berghe G. Mitochondrial fusion, fission, and biogenesis in prolonged critically ill patients. J Clin Endocrinol Metab. 2012;97:E59–E64. doi: 10.1210/jc.2011-1760. [DOI] [PubMed] [Google Scholar]
- 35.Carré JE, Orban JC, Re L, Felsmann K, Iffert W, Bauer M, Suliman HB, Piantadosi CA, Mayhew TM, Breen P, Stotz M, Singer M. Survival in critical illness is associated with early activation of mitochondrial biogenesis. Am J Respir Crit Care Med. 2010;182:745–751. doi: 10.1164/rccm.201003-0326OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Exline MC, Crouser ED. Mitochondrial mechanisms of sepsis-induced organ failure. Front Biosci. 2008;13:5030–5041. doi: 10.2741/3061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Duggal NA, Snelson C, Shaheen U, Pearce V, Lord JM. Innate and adaptive immune dysregulation in critically ill ICU patients. Sci Rep. 2018;8:10186. doi: 10.1038/s41598-018-28409-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Surbatovic M, Vojvodic D, Khan W. Immune Response in Critically Ill Patients. Mediators Inflamm. 2018;2018:9524315. doi: 10.1155/2018/9524315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Frazier WJ, Hall MW. Immunoparalysis and adverse outcomes from critical illness. Pediatr Clin North Am. 2008;55:647–668, xi. doi: 10.1016/j.pcl.2008.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Faas MM, de Vos P. Mitochondrial function in immune cells in health and disease. Biochim Biophys Acta Mol Basis Dis. 2020;1866:165845. doi: 10.1016/j.bbadis.2020.165845. [DOI] [PubMed] [Google Scholar]
- 41.McBride MA, Owen AM, Stothers CL, Hernandez A, Luan L, Burelbach KR, Patil TK, Bohannon JK, Sherwood ER, Patil NK. The Metabolic Basis of Immune Dysfunction Following Sepsis and Trauma. Front Immunol. 2020;11:1043. doi: 10.3389/fimmu.2020.01043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Angajala A, Lim S, Phillips JB, Kim JH, Yates C, You Z, Tan M. Diverse Roles of Mitochondria in Immune Responses: Novel Insights Into Immuno-Metabolism. Front Immunol. 2018;9:1605. doi: 10.3389/fimmu.2018.01605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Iwasaki Y, Takeshima Y, Fujio K. Basic mechanism of immune system activation by mitochondria. Immunol Med. 2020;43:142–147. doi: 10.1080/25785826.2020.1756609. [DOI] [PubMed] [Google Scholar]
- 44.de Pablo R, Monserrat J, Prieto A, Alvarez-Mon M. Role of circulating lymphocytes in patients with sepsis. Biomed Res Int. 2014;2014:671087. doi: 10.1155/2014/671087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kelly B, O'Neill LA. Metabolic reprogramming in macrophages and dendritic cells in innate immunity. Cell Res. 2015;25:771–784. doi: 10.1038/cr.2015.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.de Pablo R, Monserrat J, Reyes E, Diaz-Martin D, Rodriguez Zapata M, Carballo F, de la Hera A, Prieto A, Alvarez-Mon M. Mortality in patients with septic shock correlates with anti-inflammatory but not proinflammatory immunomodulatory molecules. J Intensive Care Med. 2011;26:125–132. doi: 10.1177/0885066610384465. [DOI] [PubMed] [Google Scholar]
- 47.Andrea AD, Aste-Amezaga M, Valiante NM, Ma X, Kubin M, Trinchieri G. Interleukin 10 (IL-10) Inhibits Human Lymphocyte Interferon 3,-Production by Suppressing Natural Killer Cell Stimulatory Factor/IL-12 Synthesis in Accessory Cells. J Exp Med. 1993;178:1041–1048. doi: 10.1084/jem.178.3.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Murphy MP, O'Neill LAJ. Krebs Cycle Reimagined: The Emerging Roles of Succinate and Itaconate as Signal Transducers. Cell. 2018;174:780–784. doi: 10.1016/j.cell.2018.07.030. [DOI] [PubMed] [Google Scholar]
- 49.Takasu O, Gaut JP, Watanabe E, To K, Fagley RE, Sato B, Jarman S, Efimov IR, Janks DL, Srivastava A, Bhayani SB, Drewry A, Swanson PE, Hotchkiss RS. Mechanisms of cardiac and renal dysfunction in patients dying of sepsis. Am J Respir Crit Care Med. 2013;187:509–517. doi: 10.1164/rccm.201211-1983OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fredriksson K, Tjäder I, Keller P, Petrovic N, Ahlman B, Schéele C, Wernerman J, Timmons JA, Rooyackers O. Dysregulation of mitochondrial dynamics and the muscle transcriptome in ICU patients suffering from sepsis induced multiple organ failure. PLoS One. 2008;3:e3686. doi: 10.1371/journal.pone.0003686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liesa M, Palacín M, Zorzano A. Mitochondrial dynamics in mammalian health and disease. Physiol Rev. 2009;89:799–845. doi: 10.1152/physrev.00030.2008. [DOI] [PubMed] [Google Scholar]
- 52.Willems PH, Rossignol R, Dieteren CE, Murphy MP, Koopman WJ. Redox Homeostasis and Mitochondrial Dynamics. Cell Metab. 2015;22:207–218. doi: 10.1016/j.cmet.2015.06.006. [DOI] [PubMed] [Google Scholar]
- 53.Harrington JS, Choi AMK, Nakahira K. Mitochondrial DNA in Sepsis. Curr Opin Crit Care. 2017;23:284–290. doi: 10.1097/MCC.0000000000000427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mills EL, Kelly B, O'Neill LAJ. Mitochondria are the powerhouses of immunity. Nat Immunol. 2017;18:488–498. doi: 10.1038/ni.3704. [DOI] [PubMed] [Google Scholar]
- 55.Pyle A, Burn DJ, Gordon C, Swan C, Chinnery PF, Baudouin SV. Fall in circulating mononuclear cell mitochondrial DNA content in human sepsis. Intensive Care Med. 2010;36:956–962. doi: 10.1007/s00134-010-1823-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Meloche J, Pflieger A, Vaillancourt M, Paulin R, Potus F, Zervopoulos S, Graydon C, Courboulin A, Breuils-Bonnet S, Tremblay E, Couture C, Michelakis ED, Provencher S, Bonnet S. Role for DNA damage signaling in pulmonary arterial hypertension. Circulation. 2014;129:786–797. doi: 10.1161/CIRCULATIONAHA.113.006167. [DOI] [PubMed] [Google Scholar]
- 57.Cicchillitti L, Di Stefano V, Isaia E, Crimaldi L, Fasanaro P, Ambrosino V, Antonini A, Capogrossi MC, Gaetano C, Piaggio G, Martelli F. Hypoxia-inducible factor 1-α induces miR-210 in normoxic differentiating myoblasts. J Biol Chem. 2012;287:44761–44771. doi: 10.1074/jbc.M112.421255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tannahill GM, Curtis AM, Adamik J, Palsson-McDermott EM, McGettrick AF, Goel G, Frezza C, Bernard NJ, Kelly B, Foley NH, Zheng L, Gardet A, Tong Z, Jany SS, Corr SC, Haneklaus M, Caffrey BE, Pierce K, Walmsley S, Beasley FC, Cummins E, Nizet V, Whyte M, Taylor CT, Lin H, Masters SL, Gottlieb E, Kelly VP, Clish C, Auron PE, Xavier RJ, O'Neill LA. Succinate is an inflammatory signal that induces IL-1β through HIF-1α. Nature. 2013;496:238–242. doi: 10.1038/nature11986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Svistunenko DA, Davies N, Brealey D, Singer M, Cooper CE. Mitochondrial dysfunction in patients with severe sepsis: an EPR interrogation of individual respiratory chain components. Biochim Biophys Acta. 2006;1757:262–272. doi: 10.1016/j.bbabio.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 60.Brealey D, Brand M, Hargreaves I, Heales S, Land J, Smolenski R, Davies NA, Cooper CE, Singer M. Association between mitochondrial dysfunction and severity and outcome of septic shock. Lancet. 2002;360:219–223. doi: 10.1016/S0140-6736(02)09459-X. [DOI] [PubMed] [Google Scholar]
- 61.Sjövall F, Morota S, Hansson MJ, Friberg H, Gnaiger E, Elmér E. Temporal increase of platelet mitochondrial respiration is negatively associated with clinical outcome in patients with sepsis. Crit Care. 2010;14:R214. doi: 10.1186/cc9337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Makrecka-Kuka M, Krumschnabel G, Gnaiger E. High-Resolution Respirometry for Simultaneous Measurement of Oxygen and Hydrogen Peroxide Fluxes in Permeabilized Cells, Tissue Homogenate and Isolated Mitochondria. Biomolecules. 2015;5:1319–1338. doi: 10.3390/biom5031319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rustin P, Chretien D, Bourgeron T, Gérard B, Rötig A, Saudubray JM, Munnich A. Biochemical and molecular investigations in respiratory chain deficiencies. Clin Chim Acta. 1994;228:35–51. doi: 10.1016/0009-8981(94)90055-8. [DOI] [PubMed] [Google Scholar]
- 64.Fischer JC, Ruitenbeek W, Berden JA, Trijbels JM, Veerkamp JH, Stadhouders AM, Sengers RC, Janssen AJ. Differential investigation of the capacity of succinate oxidation in human skeletal muscle. Clin Chim Acta. 1985;153:23–36. doi: 10.1016/0009-8981(85)90135-4. [DOI] [PubMed] [Google Scholar]
- 65.Brady J, Horie S, Laffey JG. Role of the adaptive immune response in sepsis. Intensive Care Med Exp. 2020;8:20. doi: 10.1186/s40635-020-00309-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Belikova I, Lukaszewicz AC, Faivre V, Damoisel C, Singer M, Payen D. Oxygen consumption of human peripheral blood mononuclear cells in severe human sepsis. Crit Care Med. 2007;35:2702–2708. doi: 10.1097/01.ccm.0000295593.25106.c4. [DOI] [PubMed] [Google Scholar]
- 67.Srinivasan S, Avadhani NG. Cytochrome c oxidase dysfunction in oxidative stress. Free Radic Biol Med. 2012;53:1252–1263. doi: 10.1016/j.freeradbiomed.2012.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Szabó C, Módis K. Pathophysiological roles of peroxynitrite in circulatory shock. Shock. 2010;34 Suppl 1:4–14. doi: 10.1097/SHK.0b013e3181e7e9ba. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Calvano SE, Xiao W, Richards DR, Felciano RM, Baker HV, Cho RJ, Chen RO, Brownstein BH, Cobb JP, Tschoeke SK, Miller-Graziano C, Moldawer LL, Mindrinos MN, Davis RW, Tompkins RG, Lowry SF Inflamm and Host Response to Injury Large Scale Collab. Res. Program. A network-based analysis of systemic inflammation in humans. Nature. 2005;437:1032–1037. doi: 10.1038/nature03985. [DOI] [PubMed] [Google Scholar]
- 70.De Jonghe B, Sharshar T, Lefaucheur JP, Authier FJ, Durand-Zaleski I, Boussarsar M, Cerf C, Renaud E, Mesrati F, Carlet J, Raphaël JC, Outin H, Bastuji-Garin S Groupe de Réflexion et d'Etude des Neuromyopathies en Réanimation. Paresis acquired in the intensive care unit: a prospective multicenter study. JAMA. 2002;288:2859–2867. doi: 10.1001/jama.288.22.2859. [DOI] [PubMed] [Google Scholar]
- 71.Jiroutková K, Krajčová A, Ziak J, Fric M, Waldauf P, Džupa V, Gojda J, Němcova-Fürstová V, Kovář J, Elkalaf M, Trnka J, Duška F. Mitochondrial function in skeletal muscle of patients with protracted critical illness and ICU-acquired weakness. Crit Care. 2015;19:448. doi: 10.1186/s13054-015-1160-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fredriksson K, Hammarqvist F, Strigård K, Hultenby K, Ljungqvist O, Wernerman J, Rooyackers O. Derangements in mitochondrial metabolism in intercostal and leg muscle of critically ill patients with sepsis-induced multiple organ failure. Am J Physiol Endocrinol Metab. 2006;291:E1044–E1050. doi: 10.1152/ajpendo.00218.2006. [DOI] [PubMed] [Google Scholar]
- 73.Japiassú AM, Santiago AP, d'Avila JC, Garcia-Souza LF, Galina A, Castro Faria-Neto HC, Bozza FA, Oliveira MF. Bioenergetic failure of human peripheral blood monocytes in patients with septic shock is mediated by reduced F1Fo adenosine-5'-triphosphate synthase activity. Crit Care Med. 2011;39:1056–1063. doi: 10.1097/CCM.0b013e31820eda5c. [DOI] [PubMed] [Google Scholar]
- 74.Garrabou G, Morén C, López S, Tobías E, Cardellach F, Miró O, Casademont J. The effects of sepsis on mitochondria. J Infect Dis. 2012;205:392–400. doi: 10.1093/infdis/jir764. [DOI] [PubMed] [Google Scholar]
- 75.Weiss SL, Selak MA, Tuluc F, Perales Villarroel J, Nadkarni VM, Deutschman CS, Becker LB. Mitochondrial dysfunction in peripheral blood mononuclear cells in pediatric septic shock. Pediatr Crit Care Med. 2015;16:e4–e12. doi: 10.1097/PCC.0000000000000277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Puskarich MA, Shapiro NI, Trzeciak S, Kline JA, Jones AE. Plasma levels of mitochondrial DNA in patients presenting to the emergency department with sepsis. Shock. 2012;38:337–340. doi: 10.1097/SHK.0b013e318266a169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Puskarich MA. The Challenge and the Promise of Studying Mitochondrial Dysfunction in Humans with Sepsis. Ann Am Thorac Soc. 2015;12:1595–1596. doi: 10.1513/AnnalsATS.201509-592ED. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Cheng SC, Scicluna BP, Arts RJ, Gresnigt MS, Lachmandas E, Giamarellos-Bourboulis EJ, Kox M, Manjeri GR, Wagenaars JA, Cremer OL, Leentjens J, van der Meer AJ, van de Veerdonk FL, Bonten MJ, Schultz MJ, Willems PH, Pickkers P, Joosten LA, van der Poll T, Netea MG. Broad defects in the energy metabolism of leukocytes underlie immunoparalysis in sepsis. Nat Immunol. 2016;17:406–413. doi: 10.1038/ni.3398. [DOI] [PubMed] [Google Scholar]
- 79.Luperto M, Zafrani L. T cell dysregulation in inflammatory diseases in ICU. Intensive Care Med Exp. 2022;10:43. doi: 10.1186/s40635-022-00471-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kubli DA, Gustafsson ÅB. Mitochondria and mitophagy: the yin and yang of cell death control. Circ Res. 2012;111:1208–1221. doi: 10.1161/CIRCRESAHA.112.265819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zhou R, Yazdi AS, Menu P, Tschopp J. A role for mitochondria in NLRP3 inflammasome activation. Nature. 2011;469:221–225. doi: 10.1038/nature09663. [DOI] [PubMed] [Google Scholar]
- 82.Sjövall F, Morota S, Persson J, Hansson MJ, Elmér E. Patients with sepsis exhibit increased mitochondrial respiratory capacity in peripheral blood immune cells. Crit Care. 2013;17:R152. doi: 10.1186/cc12831. [DOI] [PMC free article] [PubMed] [Google Scholar]