Abstract
Fuel properties of oil-bearing kukui (Aleurites moluccana) nuts, a commonly found crop in Hawaii and tropical Pacific regions, were comprehensively studied to evaluate their potential for bioenergy production. Proximate and ultimate analyses, heating value, and elemental composition of the seed, shell, and de-oiled seed cake were determined across five sampling locations in Hawaii. The aged and freshly harvested kukui seeds were found to have similar oil contents, ranging from 61 to 64%wt. Aged seeds, however, have 2 orders of magnitude greater free fatty acids than those freshly harvested (50% vs 0.4%). The nitrogen content of the de-oiled kukui seed cake was found to be comparable to that of the soybean cake. Aging of kukui seeds can decrease the flashpoint temperature and increase the liquid–solid phase transition temperatures of kukui oil obtained. Mg and Ca are the major ash-forming elements present in the kukui shells, >80%wt of all metal elements detected, which may reduce deposition problems for thermochemical conversion in comparison with hazelnut, walnut, and almond shells. The study also revealed that kukui oil has similar characteristics to canola, indicating that it is well-suited for biofuel production.
1. Introduction
In 2021, the Intergovernmental Panel on Climate Change vividly illustrates the necessity of limiting global warming to 1.5 °C to avoid severe climate impacts; achieving this target will require reaching global net-zero greenhouse gases emissions by 2050 or soon after.1 Biomass is recognized as a key component to decarbonizing the energy sector and improving energy security, and biomass energy production accounts for over 50% of the total world renewable energy supply in 2021.2 Despite being the most petroleum-dependent state in the US with >80% of primary energy consumption from petroleum,3 Hawaii possesses significant biomass resources that can be harnessed for energy production throughout its islands.4
Kukui (candlenut, Aleurites moluccana), the Hawaii state tree, is widespread in Hawaii’s islands, India, the Philippines, Malaysia, Indonesia, and Australia.5−7 Based on an assessment of historical imagery, the current coverage of naturally occurring kukui trees in Hawaii is ∼4170 ha7 with an estimated annual seed production of ∼80 kg/tree6 and a productivity of 3200 kg/ha oil.5 Although the kukui seed is marketed as a natural weight-loss supplement, many adverse symptoms were reported, which may be associated with the presence of phorbol esters.8 Currently, the primary application is oil for the cosmetic industry in Hawaii and biodiesel production in Malaysia and Indonesia.5,9−17 With the high oil content of the seeds,5 the potentially valuable caffeoyl alcohol monolignols or C-lignin-containing shells,18,19 and the high-grade bioadsorbents derived from the shells,20,21 kukui nuts have the potential to serve as a feedstock for sustainable aviation fuel (SAF) and high-value chemicals. A comprehensive understanding of its fuel characteristics can remove one limitation to the use of kukui nuts for SAF and chemical production.6 Production of kukui nuts at scale and its biocultural significance in Hawaii and other tropical regions remain to be addressed.
The objective of this study is to conduct a comprehensive characterization of kukui nuts to evaluate their potential for bioenergy production. Nuts were collected from trees in five locations in Honolulu, Hawaii. Essential fuel and biomass properties of seeds, oils, de-oiled seed cakes, and shells were determined and compared with other oil seeds. Impacts of storage on these properties were determined by separately analyzing nuts collected a few days after the fruits fell from the trees (fresh) and those that were aged under ambient conditions (aged).
2. Materials and Methods
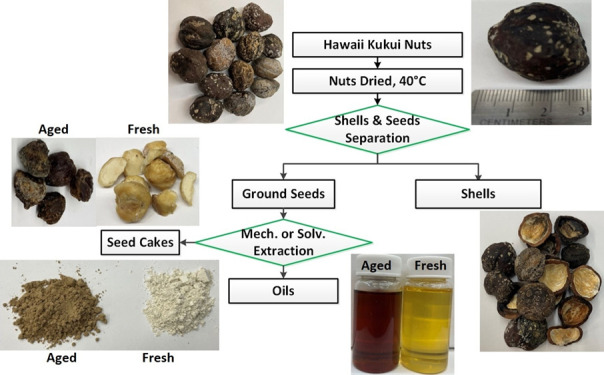
2.1. Test Materials
Kukui nuts were collected in 2021 from five locations in Honolulu, HI. Four locations, i.e., near Hale Aloha-Ilima Tower (HA), Bilger Hall (BH), former National Oceanic and Atmospheric Administration site (NOAA), and Hawaii Institute of Geophysics (HIG), were on the University of Hawaii Manoa (UHM) campus (21°17′48″N, 158°49′01″W) shown in Figure S1; the fifth location was on Saint Louis Heights (SLH) (21°18’09″N, 158°48′21″W). The fallen nuts were picked from the ground near the kukui trees and then oven-dried at 40 ± 1 °C for 7 days. The seeds were separated through mechanical shelling, and the oil was extracted from the seeds by solvent extraction (SE)22 and mechanical extraction (ME). Process details are described in the Supplementary Information (SI).
2.2. Oil Fatty Acid Profile
The oil samples were converted to their corresponding fatty acid methyl esters by KOH-catalyzed transesterification.23,24 This sample was analyzed using a Bruker 436-GC gas chromatograph (GC) and a SCION-MS select, single quadrupole mass spectrometer (Bruker Corp., Billerica, MA, USA). The GC was equipped with a 60 m Agilent DB1701 capillary column [low/mid polarity (14%-cyanopropyl-phenyl)-methylpolysiloxane] with a 15 m guard column before the backflush valve. American Oil Chemists’ Society (AOCS) Animal & Vegetable Reference Set and customized standards were purchased from AccuStandard (New Haven, CT) for chemical identification and quantification, respectively.
2.3. Characterization
The physicochemical properties of the seeds, cakes, shells, and oils were determined according to the methods and procedures listed in Table 1. Details on the proximate, ultimate, thermogravimetric, element content, and phase transition analyses are provided in the Supplementary Information.
Table 1. Properties of Samples Determined Based on Methods and Procedures.
| analysis | standard | instrument model (manufacturer) |
|---|---|---|
| ultimate | ISO16948 | CHN628 Elemental Analyzer (LECO Corp., St. Joseph, MI, USA) |
| proximate | ASTM D1756, D872, D1755 | TGA801 macro thermogravimetric analyzer (LECO Corporation, St. Joseph, MI USA) |
| higher heating value (HHV) | ASTM D4809 | 6200 Isoperibol Calorimeter (Parr Instrument Company, Moline, IL, USA) |
| elemental (solids) | S8 TIGER XRF spectrometer (Bruker Corp., Billerica, MA, USA) | |
| thermogravimetric | TGA 5500 thermogravimetric analyzer (TA Instruments, New Castle, DE, USA) | |
| phase transition | Q2000 differential scanning calorimetry (DSC) with an RCS90 temperature control (TA Instruments, New Castle, DE, USA) | |
| viscosity and density | ASTM D7042 | SVM3000 Stabinger Viscometer (Anton Paar USA Inc., Ashland, VA, USA) |
| flash point | ASTM D3828 | Setaflash 82000-2 U closed cup flash point analyzer (Stanhope-Seta, London, UK) |
| free fatty acid (FFA) | AOCS Ca 5a-40 | chemicals required were purchased from Fisher Scientific, Hampton, NH, USA |
| iodine value (IV) | AOCS Cd 1d-92 | chemicals required were purchased from Fisher Scientific, Hampton, NH, USA |
3. Results and Discussion
3.1. Kukui Nuts
The kukui nuts collected were generally elliptical-shaped and ∼2.5–3.5 cm in diameter with hard, rough, and brown-to-black shells. Nut weights (dry basis) were ∼8–12 g, and the mass ratio of the fleshy seed to the shell was ∼2:1, consistent with the values reported in the literature.6,11 Although the sampling locations were all near the UHM campus, the kukui trees at the NOAA location were located close to the Manoa stream, and the tree at SLH was at a higher elevation. The aged nuts were separated from the fresh nuts based on seed color, i.e., the aged and fresh seeds are waxy brown and light tan, respectively. The age of the nuts was not determined. The aged seeds varied from a brown surface color only to a completely dark brown color on the surface and throughout the interior of the whole seed. Note that the aged seeds collected from the ground could be one or multiple seasons old.
Reported oil extraction from the candlenut seeds includes three techniques, solvent (SE),15,25,26 mechanical (ME),10,11,13,27 and supercritical carbon dioxide (scCO2)28−30 processes, summarized in Table 2. Fresh and aged seeds in this study were processed via SE, and values were averaged across sampling locations. Fresh and aged seed oil contents were not significantly different. The reported oil contents of the seeds obtained by SE ranged from 20 to 56%wt,15,25,26 slightly lower than the values obtained in this study. ME yields less oil, 20–43%wt of the seed.10,11,27 Oil equal to ∼13% of the initial seed mass was retained in the ME seed cake.11 This resulted in a larger HHV of the ME cake11 in comparison with the SE cake (Table S1). Although the green scCO2 technology can yield a comparably higher fraction of oil, high equipment and operating costs may hinder its application for biofuel production. In general, the kukui oil content is within the upper range of other nuts that include hazelnut, almond, walnut, and macadamia.31
Table 2. Oil Content of Kukui Seeds Extracted Using Various Technologies.
| reference | oil content %wt | method |
|---|---|---|
| fresh seeds (this study, n = 5) | 63.8 ± 3.4 | solvent |
| aged seeds (this study, n = 4) | 61.2 ± 3.8 | solvent |
| Kibazohi and Sangwan25 | 20.11 | solvent |
| Cabral et al.26 | 42 | solvent |
| Villarante et al.15 | 56 | solvent |
| Sulistyo et al.10 | 30 | mechanical |
| Martín et al.11 | 43.2 | mechanical |
| Budianto et al.27 | 39 | mechanicala |
| Pham et al.13 | 20–30 | mechanical |
| Martín et al.11 | 56.3 | mechanical + solventb |
| Nik Norulaini et al.28 | 52.6 | supercritical CO2 |
| Siddique et al.29 | 70.12–77.27 | supercritical CO2c |
| Subroto et al.30 | 61.4 | supercritical CO2 |
The oil content was reported as 425 mL oil/kg seed, and this value was calculated based on oil density = 0.92 g cm–3.
Solvent extraction was performed for the oil cake obtained from mechanical extraction, and the resulting oil yield was 13.1% of the initial seed mass.
The values are the maximum oil yield under the optimized extraction conditions.
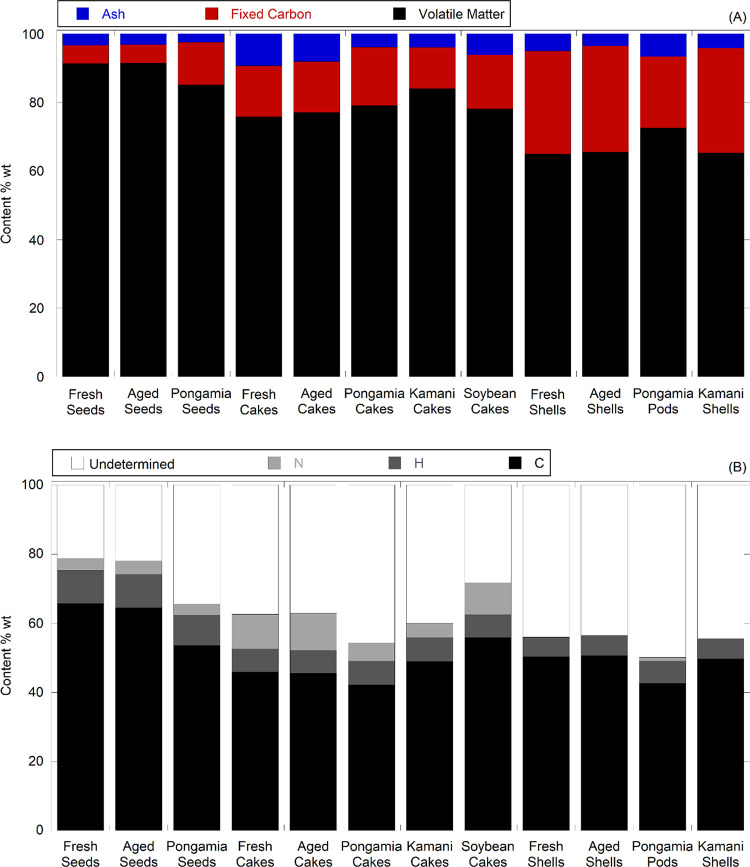
Figure 1A,B compares the proximate and ultimate analysis results of kukui seeds, seed cakes, and shells with those of pongamia (Millettia pinnata),22 kamani (Calophyllum inophyllum), and soybean (Glycine max),32 respectively; data, error estimates, and literature values are presented in Table S1. Note that Table S1 summarizes Tables S2–S4 as averages, as no significant statistical differences were observed across the sampling locations. The proximate and ultimate results and energy content of fresh and aged kukui seeds, seed cakes, and shells were similar. The volatile matter and carbon content of the kukui seeds are 5–6%wt higher than those of pongamia seeds owing to the higher oil content of the kukui seeds, >60%wt (SE) vs 20–30% wt of pongamia seeds (SE).22 Although the ash content of kukui seeds is ∼1%wt higher than that of pongamia seeds (2.49%wt),22 the ash content of kukui cakes is the highest among the four cakes in Figure 1A, i.e., over 2-fold higher than that of pongamia and kamani cakes (3.9 and 4.0%wt, respectively) and ∼50% higher than that of soybean cakes (6.14%wt).32 The nitrogen content of the kukui cakes, 10.04 and 10.63%wt for fresh and aged cakes, was slightly higher than the literature values for soybean cakes, 9.29%wt,32 and ∼100% higher than that of pongamia cakes, 5.30%wt,22 indicating the potential to serve as a protein source.
Figure 1.
Comparison of proximate (A) and ultimate (B) analysis results of kukui seeds, de-oiled cakes, and shells with those of kamani, pongamia,22 and soybean.32 Note: the data utilized for plotting are derived from the values presented in Tables S1, S2, and S6 of ref (22) and Table 1 of ref (32).
Kukui shells have a lower nitrogen content, <0.5%wt, than shells of widely produced almonds, hazelnut, and walnut, 1.1–1.6%wt.33 The ash content of kukui shells, 3.58 and 5.01%wt for fresh and aged shells, respectively, is slightly higher than shells from these three commercial nuts, 1.4–3.3%wt,33 and that of macadamia shells, 0.82%wt.34 The kukui shells reportedly contain C-lignin and could be a potential sustainable feedstock for catechol production.18,19
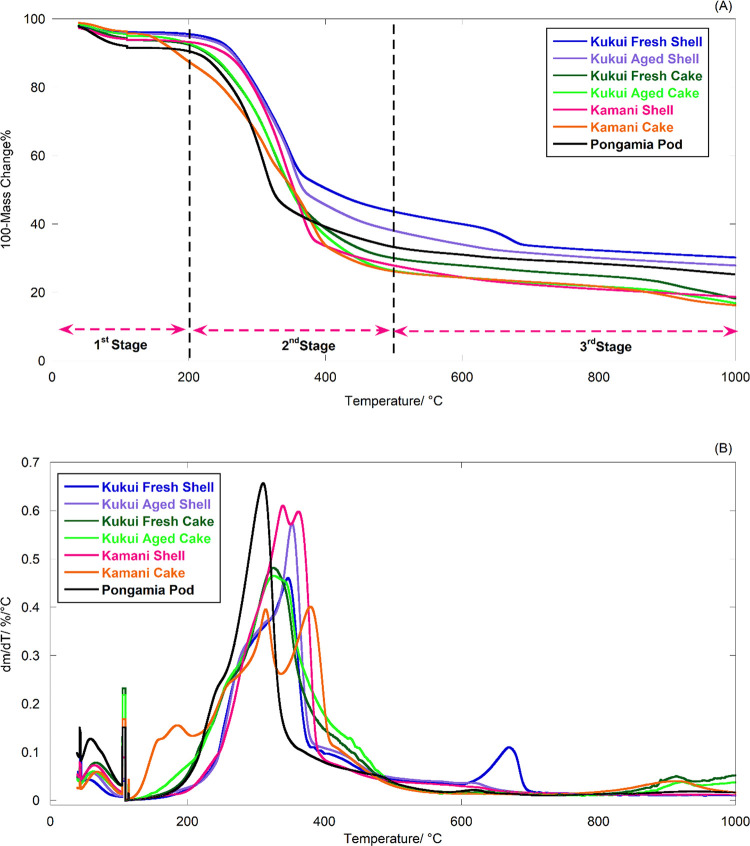
Thermogravimetric (TG) and derivative thermogravimetric (DTG) analysis results for kukui SE cakes and shells, kamani, and pongamia are compared in Figure 2 (data for individual locations are shown in Figure S2). A significant loss of mass occurs for all materials in a range of 200–500 °C due to the decomposition of hemicellulose, cellulose, and lignin. At T > 500 °C, only a limited weight loss (10–15%wt) associated with the degradation of other heavy components (remaining lignin) was observed.35,36 The mass loss at temperatures <200 °C includes water and the release of some light volatile species. The temperature corresponding to the rate of maximum mass loss (peak temperature in Figure 2B) of kukui shells is >30 °C higher than that of pongamia pods. The potassium concentration of kukui shells is an order of magnitude lower than that of pongamia pods (Figure 3), and K catalytically decreases pyrolysis reactions’ energy barriers.37,38 Correspondingly, the kukui cakes’ maximum mass loss occurs at ∼20 °C lower than that of kukui shells. The temperature for kamani cakes is similar to that of pongamia pods owing to the synergetic catalytic impacts of K and P.37−39
Figure 2.
TG analysis of kukui, kamani, and pongamia: (A) TG curve; (B) DTG curve.
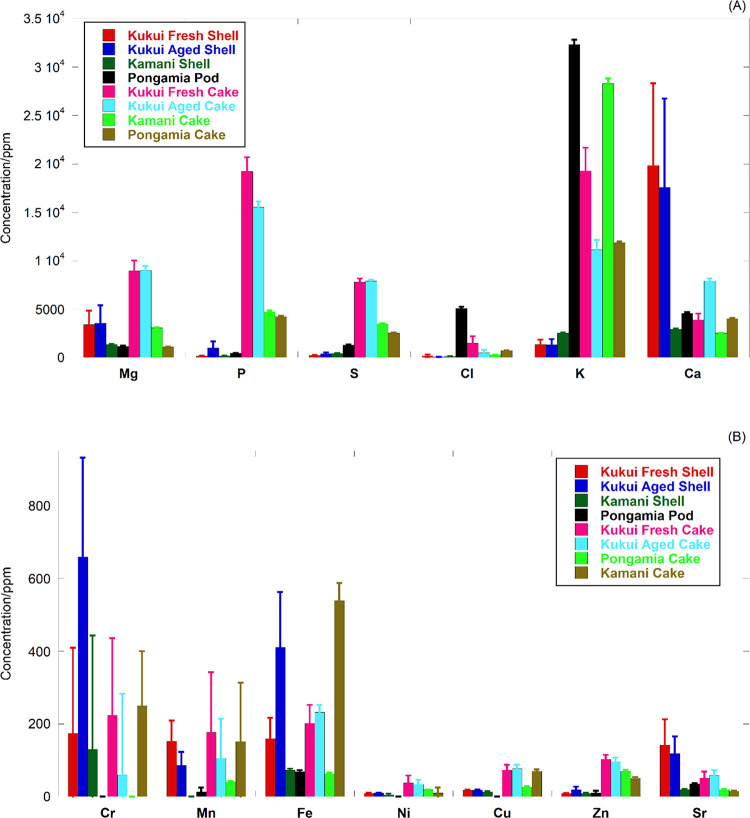
Figure 3.
XRF elemental analysis results: (A) major elements; (B) minor elements.
Figure 3 presents the X-ray fluorescence (XRF) results of the kukui cakes and shells (data and error estimates in Tables S5–S8 for each location). XRF analysis was not conducted on seeds because their high oil content prevented the formation of stable sample pellets. The higher total mineral content of the kukui cakes compared to the shells is consistent with the ash content results from the proximate analysis. For the major elements shown in Figure 3A, concentrations are higher in kukui cakes than in shells, with the exception of Ca. Greater than 80% of the total inorganic elements detected in kukui shells are alkaline earth metals, i.e., Mg and Ca, and the maximum concentrations of Cl and K are 337 and 2117 ppm, respectively, suggesting a reduced risk of ash deposition during thermochemical conversion.40 The two primary ash components in almond, hazelnut, and walnut shells, K2O (30–49%wt) and SiO2 (10–27%wt), are significantly higher than those in kukui shells (<10 and <3%wt of K2O and SiO2, respectively).33 An elevated level of Cr and Fe was detected in aged shells.
Required plant micronutrients, Mn, Fe, Ni, Cu, and Zn, were detected in the kukui shells and cakes at concentrations of 10–400 ppm and 30–230 ppm, respectively. Sr originates from basalt lava, Asian dust, and rainfall (ocean derived) in Hawaii41 and was detected in both kukui cakes and shells. The P and S contents of kukui cakes were higher than those of kamani and pongamia cakes. Note that Na was not included in Figure 3A, as its concentration was not consistently above the lower limit of quantification. The aged cakes, however, were found to have elevated levels of Na (Table S6).
Overall, kukui nuts possess several valuable properties. The high oil yield of the seeds, ∼60%wt, indicates that they have the potential to serve as a viable source of oil for biofuel production. The high nitrogen content of the de-oiled seed cakes could make them a useful protein source, potentially for animal feed or as an ingredient in food products. Additionally, the shells are abundant in alkaline earth metals, particularly Mg and Ca, which suggests a reduced risk of ash deposition and fouling during thermochemical conversion. However, more research is necessary to determine the cost-effective and efficient ways to convert de-oiled seed cakes into animal feed and shells into value-added chemicals and bioenergy.
3.2. Kukui Oil
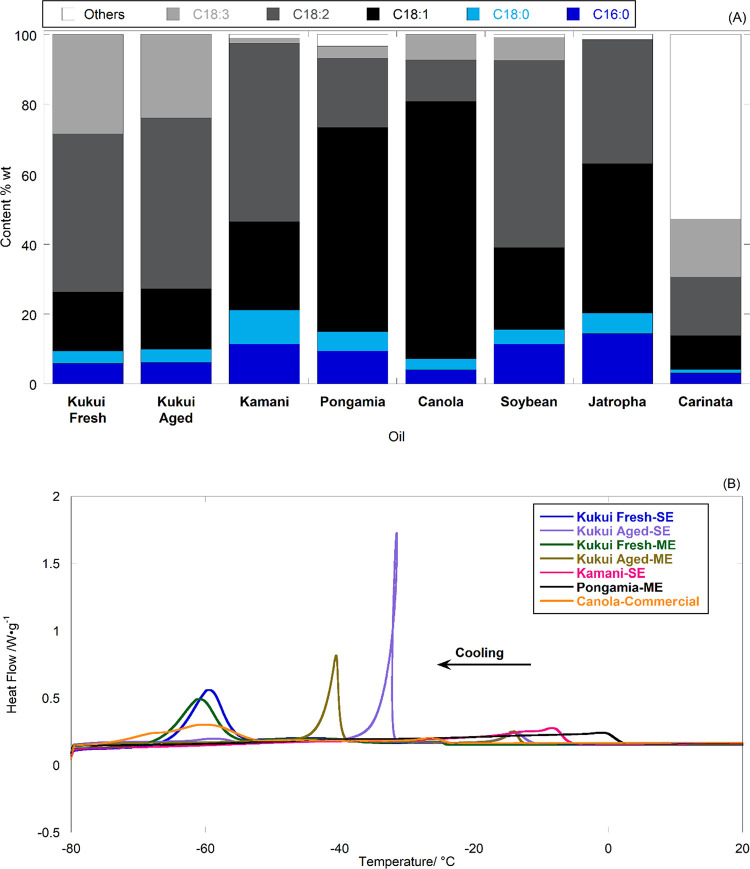
The physicochemical properties of kukui oils obtained from SE and ME are listed in Table 3 (data and error estimates in Table S9 for SE oils from each location). The FFA of aged seed oils (ASO) was found to be 2 orders of magnitude higher than that of the fresh seed oil (FSO) and 5–7-fold higher than the literature-reported values.13,42,43 In general, up to 5% FFA may be found in crude vegetable oil,44 and the high FFA of ASO may be associated with cell damage and the gradual decomposition of organic components, which is also reflected by the color of the aged seeds. The density, viscosity, and HHV of the FSO and ASO from both SE and ME are similar and close to the literature-reported range for kukui oils, 0.92 g cm–3 at 15 °C,13,14,30 24.89–26.91 mm2 s–1 at 40 °C,13,14 and 37.61 MJ/kg,43 respectively. Figure 4A shows the fatty acid profile (FAP) of the SE kukui oils (data and error estimates and comparison with the literature values in Tables S10 and S11) along with that of kamani, canola,22 pongamia,22 soybean,45 jatropha,45 and carinata oils.46 Although a significant difference was found between the FFA contents of ASO and FSO, the FAPs of ASO and FSO are almost identical, consistent with the results obtained from IV analysis, i.e., 161.4 and 157.9, respectively. The total unsaturated fatty acids account for ∼90%wt of the kukui oils with linoleic acid (C18:2) as the primary acid, in good agreement with the literature results summarized in Table S11. Although the total unsaturated fraction of the kukui oil is somewhat similar to that of canola oils, ∼92%wt, the 114 iodine value of kukui oil is ∼40% higher22 owing to the higher fractions of linoleic (C18:2) and linolenic (C18:3) acids. Consequently, kukui oil would require more hydrogen for complete saturation, leading to increased cost and energy consumption for hydrotreated renewable fuel production. Additionally, when processed through transesterification, the oil may produce less stable biodiesel.
Table 3. Properties of Kukui Oil Obtained.
| properties | SE-fresh | SE-aged | ME-fresh | ME-aged | literature |
|---|---|---|---|---|---|
| FFA % | 0.39 ± 0.26 | 52.51 ± 10.79 | 0.19 ± 0.03 | 39.11 ± 0.43 | 6.9–7.8a |
| iodine value | 161.4 ± 5.0 | 157.9 ± 6.5 | 173.3 ± 4.0 | 156.9 ± 8.2 | 131–137a |
| ν at 40 °C/mm2 s–1 | 23.11 ± 3.52 | 20.45 ± 1.19 | 26.69 ± 0.00 | 26.41 ± 0.01 | 24.89–26.91b |
| ρ at 15 °C/g cm–3 | 0.92 ± 0.01 | 0.90 ± 0.00 | 0.93 ± 0.00 | 0.93 ± 0.00 | 0.92c |
| Tonset/°C (L-S) | –23.07 ± 1.42 | –10.28 ± 2.01 | –24.03 | –12.85 | –11.08d |
| Tpeak/°C (L-S) | –60.81 ± 1.01 | –29.75 ± 4.24 | –61.16 | –40.15 | –65d |
| Tonset/°C (S-L) | –21.99 ± 4.06 | –16.71 ± 1.99 | –22.34 | –22.01 | |
| Tpeak/°C (S-L) | –31.86 ± 0.95 | –22.40 ± 5.23 | –30.96 | –26.55 | |
| HHV MJ kg–1 | 38.94 ± 0.26 | 38.81 ± 0.17 | 39.19 ± 0.11 | 38.73 ± 0.05 | 37.61e |
| flash point/°C | 206 ± 2 | 184 ± 2 | >284f |
Figure 4.
Oil analysis: (A) FAP of kukui oils in comparison with that of kamani, pongamia,22 canola, soybean,45 jatropha,45 and carinata oils.46 (B) DSC cooling curves of oils. Note: the data utilized for plotting are derived from the values presented in Table S5 of ref (22), Table 1 of ref (45), and Table 5 of ref (46).
The degree of unsaturation in oils is also reflected in the DSC crystallization curves. This is because the crystallization curve is affected only by the chemical composition of the oils, rather than the initial crystalline state, making it more reproducible and simpler than the DSC melting curve.44 The information obtained, therefore, can be utilized to monitor the oil quality and to track changes in oil properties over time, such as during storage or processing.47Figure 4B compares the DSC crystallization curve of kukui oil with kamani, pongamia, and canola oils from DSC analysis. FSO has an onset liquid-to-solid phase transition temperature (Tonset(L-S)) over 60 °C lower than that of pongamia and kamani oils due to their higher level of unsaturation (∼90%) compared to ∼81 and 77% in pongamia and kamani oils, respectively. It is worth noting that the FSO exhibits similar low-temperature characteristics to commercial canola oil, despite the significant difference in the fraction of unsaturated fatty acids, i.e., oleic acid (C18:1) and C18:2 + C18:3 account for ∼80%wt of the total unsaturated fatty acids in canola and FSO, respectively. Additionally, the DSC analysis revealed the deterioration of oil quality during long-term storage, despite the similarities in FAP and viscosity between ASO and FSO. The liquid-to-solid phase transition (L-S) temperatures of ASO, i.e., Tonset (L-S) and Tpeak (L-S), are about 10 and 30 °C higher than those of FSO, indicating the presence of higher melting temperature compounds in ASO. In contrast, the flash point of ASO, 184 °C, is lower than the FSO, 206 °C, indicating the formation of more volatile compounds in the aging process.
In summary, the physicochemical properties of FSO and ASO were analyzed, with the FFA of ASO found to be significantly higher than that of FSO and literature-reported values. The density, viscosity, and HHV of both FSO and ASO were similar and in line with the literature values. The FAPs of ASO and FSO were nearly identical, with the total unsaturated fatty acids accounting for ∼90% wt. However, the high degree of unsaturation in kukui oil, particularly with linoleic (C18:2) and linolenic (C18:3) acids, makes it less stable and requires increased cost and energy consumption for hydrotreated renewable fuel production. The DSC crystallization curves of the oils revealed the impacts of storage, with ASO showing the formation of higher melting temperature as well as more volatile compounds compared to FSO. Future research is needed to explore methods for maintaining the oil quality and to advance technologies for SAF production.
4. Conclusions
The fuel properties of kukui nuts from Hawaii were comprehensively characterized. The physicochemical properties of the kukui seeds, shells, oils, and de-oiled seed cakes were determined and compared with those of other tropical biomass and oil plants. The following conclusions were drawn:
The kukui seeds yield >60%wt oil, at the upper range of common commercial nuts.
The de-oiled seed cakes have a nitrogen content of ∼10%wt, slightly higher than that of soybeans, and have the potential to be utilized as a protein source.
Alkaline earth metals, Mg and Ca, were found to be the primary ash-forming elements in the shells, accounting for >80% of the total inorganic element mass, favorable characteristics for thermochemical conversion.
The kukui oil contains >90% unsaturated C18 fatty acids and has physicochemical properties most similar to canola oil in a group that includes kamani, pongamia, soybean, jatropha, and carinata oils.
The ASO has abnormally high levels of FFA and poorer low-temperature properties in comparison with FSO, which would affect oil stability.
Acknowledgments
This research was funded in part by the U.S. Federal Aviation Administration Office of Environment and Energy through ASCENT, the FAA Center of Excellence for Alternative Jet Fuels and the Environment, and Project 001 through FAA Award Number 13-C-AJFE-UH under the supervision of James Hileman and Nathan Brown. Funding was also provided by Hawaii’s Environmental Response, Energy, and Food Security Tax (HRS Section 243-3.5 “Barrel Tax”) through the Hawaii Natural Energy Institute’s Energy Systems Development Special Fund and the Office of Naval Research, Asia Pacific Research Initiative for Sustainable Energy Systems Program (Award No. N00014-18-1-2127). Any opinions, findings, conclusions, or recommendations expressed in this material are those of the authors and do not necessarily reflect the views of the FAA.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.3c00860.
Details on the oil extraction methods and proximate, ultimate, thermogravimetric, element content, and phase transition analyses (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- IPCC . Climate Change 2021: The Physical Science Basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK, 2021. [Google Scholar]
- International Energy Agency World Energy Outlook 2022, 2022.
- Administration, U. S. D. o. E. E. I . Hawaii End-use energy consumption 2019, estimates.
- Turn S. Q.; Keffer V.; Staackmann M., Biomass and bioenergy resource assessment state of Hawaii; Hawaii Natural Energy Institute, Honolulu, HI: 2002. [Google Scholar]
- Shaah M. A.; Allafi F.; Hossain M. S.; Alsaedi A.; Ismail N.; Ab Kadir M. O.; Ahmad M. I. Candlenut oil: review on oil properties and future liquid biofuel prospects. Int. J. Energy Res. 2021, 45, 17057–17079. 10.1002/er.6446. [DOI] [Google Scholar]
- Elevitch C. R.; Abbott I. A.; Leakey R. R. B., Traditional trees of Pacific Islands: their culture, environment, and use. 1st ed. ed.; Permanent Agriculture Resources: Ho̅lualoa, Hawai‘i, 2006. [Google Scholar]
- Lincoln N.; Zhang Q.; Chen Q. State of the State Tree: Historical and Modern Ecology of Kukui (Candlenut, Aleurites Moluccanus) in Hawai’i. Pac. Sci. 2020, 74, 419–434. 10.2984/74.4.9. [DOI] [Google Scholar]
- Koons A. L.; Laubach L. T.; Katz K. D.; Beauchamp G. A. Mobitz Type II Atrioventricular Heart Block After Candlenut Ingestion. J. Am. Osteopath. Assoc. 2020, 120, 839–843. 10.7556/jaoa.2020.136. [DOI] [PubMed] [Google Scholar]
- Sulistyo H.; Suardjaja I.; Rahayu S. In Transesterification of candlenut oil with ethanol to biodiesel, Proceeding on Regional Symposium on Chemical Engineering; 2006; 4–6.
- Sulistyo H.; Rahayu S. S.; Winoto G.; Suardjaja I. Biodiesel production from high iodine number candlenut oil. Int. J. Chem. Mater. Biomol. Sci. 2008, 2, 485–488. 10.5281/zenodo.1332938. [DOI] [Google Scholar]
- Martín C.; Moure A.; Martin G.; Carrillo E.; Dominguez H.; Parajo J. C. Fractional characterisation of jatropha, neem, moringa, trisperma, castor and candlenut seeds as potential feedstocks for biodiesel production in Cuba. Biomass Bioenergy 2010, 34, 533–538. 10.1016/j.biombioe.2009.12.019. [DOI] [Google Scholar]
- Imdadul H. K.; Zulkifli N. W. M.; Masjuki H. H.; Kalam M. A.; Kamruzzaman M.; Rashed M. M.; Rashedul H. K.; Alwi A. Experimental assessment of non-edible candlenut biodiesel and its blend characteristics as diesel engine fuel. Environ. Sci. Pollut. Res. Int. 2017, 24, 2350–2363. 10.1007/s11356-016-7847-y. [DOI] [PubMed] [Google Scholar]
- Pham L. N.; Luu B. V.; Phuoc H. D.; Le H. N. T.; Truong H. T.; Luu P. D.; Furuta M.; Imamura K.; Maeda Y. Production of Biodiesel from Candlenut Oil Using a Two-step Co-solvent Method and Evaluation of Its Gaseous Emissions. J. Oleo Sci. 2018, 67, 617–626. 10.5650/jos.ess17220. [DOI] [PubMed] [Google Scholar]
- Atabani A. E.; Mekaoussi M.; Uguz G.; Arpa O.; Ayanoglu A.; Shobana S. Evaluation, characterization, and engine performance of complementary fuel blends of butanol-biodiesel-diesel fromAleurites moluccanusas potential alternative fuels for CI engines. Energy Environ. 2020, 31, 755–784. 10.1177/0958305X18790953. [DOI] [Google Scholar]
- Villarante N. R.; Ibarrientos C. H. Physicochemical Characterization of Candlenut (Aleurites moluccana)-derived Biodiesel Purified with Deed Eutectic Solvents. J. Oleo Sci. 2021, 70, 113–123. 10.5650/jos.ess20152. [DOI] [PubMed] [Google Scholar]
- Syimir Fizal A. N.; Hossain M. S.; Zulkifli M.; Khalil N. A.; Abd Hamid H.; Ahmad Yahaya A. N. Implementation of the supercritical CO2 technology for the extraction of candlenut oil as a promising feedstock for biodiesel production: potential and limitations. Int. J. Green Energy 2022, 19, 72–83. 10.1080/15435075.2021.1930007. [DOI] [Google Scholar]
- Shaah M. A.; Hossain M. S.; Allafi F.; Ab Kadir M. O.; Ahmad M. I. Biodiesel production from candlenut oil using a non-catalytic supercritical methanol transesterification process: optimization, kinetics, and thermodynamic studies. RSC Adv. 2022, 12, 9845–9861. 10.1039/D2RA00571A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su S.; Wang S.; Song G. Disassembling catechyl and guaiacyl/syringyl lignins coexisting in Euphorbiaceae seed coats. Green Chem. 2021, 23, 7235–7242. 10.1039/D1GC02131A. [DOI] [Google Scholar]
- Tobimatsu Y.; Chen F.; Nakashima J.; Escamilla-Treviño L. L.; Jackson L.; Dixon R. A.; Ralph J. Coexistence but Independent Biosynthesis of Catechyl and Guaiacyl/Syringyl Lignin Polymers in Seed Coats. Plant Cell 2013, 25, 2587–2600. 10.1105/tpc.113.113142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariana M.; Mistar E. M.; Syabriyana M.; Zulkipli A. S.; Aswita D.; Alfatah T. Properties and adsorptive performance of candlenut shell and its porous charcoals for aqueous mercury(II) removal. Bioresour. Technol. Rep. 2022, 19, 101182 10.1016/j.biteb.2022.101182. [DOI] [Google Scholar]
- Muliani S.; Zakir M.; Fauziah S. Surface Modification of Activated Carbon from Candlenut Shell (Aleurites moluccana) with HNO3 and its Application as an Adsorbent Methyl Orange Dyes. Egypt. J. Chem. 2022, 10.21608/ejchem.2022.149651.6476. [DOI] [Google Scholar]
- Fu J.; Summers S.; Morgan T. J.; Turn S. Q.; Kusch W. Fuel Properties of Pongamia (Milletia pinnata) Seeds and Pods Grown in Hawaii. ACS Omega 2021, 6, 9222–9233. 10.1021/acsomega.1c00635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu K.-S. Preparation of fatty acid methyl esters for gas-chromatographic analysis of lipids in biological materials. J. Am. Oil Chem. Soc. 1994, 71, 1179–1187. 10.1007/BF02540534. [DOI] [Google Scholar]
- Zhong J.; Wang Y.; Yang R.; Liu X.; Yang Q.; Qin X. The application of ultrasound and microwave to increase oil extraction from Moringa oleifera seeds. Ind. Crops Prod. 2018, 120, 1–10. 10.1016/j.indcrop.2018.04.028. [DOI] [Google Scholar]
- Kibazohi O.; Sangwan R. S. Vegetable oil production potential from Jatropha curcas, Croton megalocarpus, Aleurites moluccana, Moringa oleifera and Pachira glabra: Assessment of renewable energy resources for bio-energy production in Africa. Biomass Bioenergy 2011, 35, 1352–1356. 10.1016/j.biombioe.2010.12.048. [DOI] [Google Scholar]
- Cabral M. R. P.; dos Santos S. A. L.; Stropa J. M.; da Silva R. C. d. L.; Cardoso C. A. L.; de Oliveira L. C. S.; Scharf D. R.; Simionatto E. L.; Santiago E. F.; Simionatto E. Chemical composition and thermal properties of methyl and ethyl esters prepared from Aleurites moluccanus (L.) Willd (Euphorbiaceae) nut oil. Ind. Crops Prod. 2016, 85, 109–116. 10.1016/j.indcrop.2016.02.058. [DOI] [Google Scholar]
- Budianto A.; Prajitno D. H.; Budhikarjono K. Biofuel production from candlenut oil using catalytic cracking process with Zn/HZSM-5 catalyst. ARPN J. Eng. Appl. Sci. 2014, 9, 2121–2124. [Google Scholar]
- Nik Norulaini N. A.; Rahmad Setia B.; Anuar O.; Md Zaidul L. S.; Mohd Omar A. K. Major chemical constituents of candle nut oil extract using supercritical carbon dioxide. Malays. J. Pharm. Sci. 2004, 2, 61–72. [Google Scholar]
- Siddique B. M.; Ahmad A.; Alkarkhi A. F.; Ibrahim M. H.; Mohd Omar K. Chemical composition and antioxidant properties of candlenut oil extracted by supercritical CO2. J. Food Sci. 2011, 76, C535–C542. 10.1111/j.1750-3841.2011.02146.x. [DOI] [PubMed] [Google Scholar]
- Subroto E.; Widjojokusumo E.; Veriansyah B.; Tjandrawinata R. R. Supercritical CO2 extraction of candlenut oil: process optimization using Taguchi orthogonal array and physicochemical properties of the oil. J. Food Sci. Technol. 2017, 54, 1286–1292. 10.1007/s13197-017-2542-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maestri D.; Cittadini M. C.; Bodoira R.; Martínez M. Tree Nut Oils: Chemical Profiles, Extraction, Stability, and Quality Concerns. Eur. J. Lipid Sci. Technol. 2020, 122, 1900450 10.1002/ejlt.201900450. [DOI] [Google Scholar]
- Uzun B. B.; Pütün A. E.; Pütün E. Fast pyrolysis of soybean cake: Product yields and compositions. Bioresour. Technol. 2006, 97, 569–576. 10.1016/j.biortech.2005.03.026. [DOI] [PubMed] [Google Scholar]
- Demirbaş A. Fuel Characteristics of Olive Husk and Walnut, Hazelnut, Sunflower, and Almond Shells. Energy Sources 2002, 24, 215–221. 10.1080/009083102317243601. [DOI] [Google Scholar]
- Turn S. Q.; Keffer V.; Staackmann M., Analysis of Hawaii biomass energy resources for distributed energy applications; Hawaii Natural Energy Institute, University of Hawaii: Honolulu, 2002, 21. [Google Scholar]
- Yang H.; Yan R.; Chen H.; Lee D. H.; Zheng C. Characteristics of hemicellulose, cellulose and lignin pyrolysis. Fuel 2007, 86, 1781–1788. 10.1016/j.fuel.2006.12.013. [DOI] [Google Scholar]
- Chen W.-H.; Kuo P.-C. A study on torrefaction of various biomass materials and its impact on lignocellulosic structure simulated by a thermogravimetry. Energy 2010, 35, 2580–2586. 10.1016/j.energy.2010.02.054. [DOI] [Google Scholar]
- Mayer Z. A.; Apfelbacher A.; Hornung A. A comparative study on the pyrolysis of metal- and ash-enriched wood and the combustion properties of the gained char. J. Anal. Appl. Pyrolysis 2012, 96, 196–202. 10.1016/j.jaap.2012.04.007. [DOI] [Google Scholar]
- Nowakowski D. J.; Jones J. M.; Brydson R. M. D.; Ross A. B. Potassium catalysis in the pyrolysis behaviour of short rotation willow coppice. Fuel 2007, 86, 2389–2402. 10.1016/j.fuel.2007.01.026. [DOI] [Google Scholar]
- Nowakowski D. J.; Woodbridge C. R.; Jones J. M. Phosphorus catalysis in the pyrolysis behaviour of biomass. J. Anal. Appl. Pyrolysis 2008, 83, 197–204. 10.1016/j.jaap.2008.08.003. [DOI] [Google Scholar]
- Niu Y.; Tan H.; Hui S. Ash-related issues during biomass combustion: Alkali-induced slagging, silicate melt-induced slagging (ash fusion), agglomeration, corrosion, ash utilization, and related countermeasures. Prog. Energy Combust. Sci. 2016, 52, 1–61. 10.1016/j.pecs.2015.09.003. [DOI] [Google Scholar]
- Chadwick O. A.; Derry L. A.; Bern C. R.; Vitousek P. M. Changing sources of strontium to soils and ecosystems across the Hawaiian Islands. Chem. Geol. 2009, 267, 64–76. 10.1016/j.chemgeo.2009.01.009. [DOI] [Google Scholar]
- Sulistyo H.; Rahayu S. S.; Suardjaja I. M.; Setiadi U. H., Crude Candlenut Oil Ethanolysis to Produce Renewable Energy at Ambient Condition; Proc. World Congr. Eng. Comput. Sci: San Francisco, USA, 2009. [Google Scholar]
- Setiawan D. I.; Irawadi T. T.; Mas’ud Z. A. Hydrotreating of Sunan Candlenut (Reutealis trisperma Airy Shaw) Oil by Using NiMo-gamma Al2O3 as Renewable Energy. Indones. J. Chem. 2019, 19, 78–88. 10.22146/ijc.27274. [DOI] [Google Scholar]
- Hammond E. W., VEGETABLE OILS | Composition and Analysis. In Encyclopedia of Food Sciences and Nutrition (Second Edition), Caballero B., Ed.; Academic Press: Oxford, 2003, 5916–5921. [Google Scholar]
- Dwivedi G.; Sharma M. P. Potential and limitation of straight vegetable oils as engine fuel – An Indian perspective. Renew. Sustainable Energy Rev. 2014, 33, 316–322. 10.1016/j.rser.2014.02.004. [DOI] [Google Scholar]
- Cardone M.; Mazzoncini M.; Menini S.; Rocco V.; Senatore A.; Seggiani M.; Vitolo S. Brassica carinata as an alternative oil crop for the production of biodiesel in Italy: agronomic evaluation, fuel production by transesterification and characterization. Biomass Bioenergy 2003, 25, 623–636. 10.1016/S0961-9534(03)00058-8. [DOI] [Google Scholar]
- Tan C. P.; Che Man Y. B. Differential scanning calorimetric analysis of edible oils: Comparison of thermal properties and chemical composition. J. Am. Oil Chem. Soc. 2000, 77, 143–155. 10.1007/s11746-000-0024-6. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.