Abstract
Introduction:
Diurnal variations in physical performance can affect athletes’ success in competitive sports depending on whether the time of peak performance concurs with the time of competition. The purpose of this systematic review was to investigate the diurnal variation in maximum endurance and strength performance.
Methods:
The databases PubMed, EMBASE, and Web-of-Science were searched from inception to November 2020. The search string was externally reviewed according to PRESS guidelines, the review was conducted in accordance to PRISMA guidelines, and registered beforehand on PROSPERO. Eligibility criteria were that [1] the studies included humans, [2] any kind of maximum endurance or maximum strength test was performed at [3] a minimum of three different times of the day. There were no restrictions regarding study design, participants’ sex, age or fitness levels.
Results:
From 10,460 screened articles 63 articles met all three inclusion criteria. Meta-analysis on the harmonizable 29 studies provided evidence for diurnal variations in physical performance. In detail, the overall effect sizes (95% confidence intervals) were 0.23 (0.05; 0.40), 0.73 (0.37; 1.09), 0.39 (0.18; 0.60), and 0.79 (0.28, 1.30) for endurance exercise tests, maximum power output in Wingate-test, handgrip strength, and jump height all in favor of higher performance in the evening. The overall risk of bias in individual studies was moderately high.
Conclusions:
There is strong evidence that anaerobic power as well as jump height are maximal between 13:00 and 19:00. There is some evidence that handgrip strength peaks between 13:00 and 21:00, but only little evidence that there is a time of peak performance in maximum endurance.
Keywords: Circadian, diurnal, performance, endurance, strength
INTRODUCTION
The global sports market size is hundreds of billion US-dollars per year. In competitive sports small values can make a difference between victory and defeat, which is accompanied with big financial consequences through price money and sponsoring. Thus, it is not surprising that decades of research have focused on factors influencing physical performance including investigating the time of peak performance (1–8). In professional sports, differences in the time of peak performance can cause two dilemmas. First, if competitions are scheduled at the time of peak performance it will increase the chance of reaching new world records, but this time may not match with prime time on television which brings the biggest advertising revenue. Second, individual differences in the time of peak performance result in disadvantages for those athletes in whom the time of peak performance does not match with the time of competition.
For recreationally active people, exercising closer to the time of peak performance enables them to exercise with higher intensities which, as compared to exercising closer to their nadir, could in the long-term lead to improved physical adaptions such as higher maximum oxygen uptake or muscular strength. Researchers performing exercise intervention studies and assessing physical fitness pre- and post-intervention as an outcome would need to consider the test time, because the effects of the intervention might be overestimated or underestimated if pre-and post-assessments do not take place at the same time of the day. In a clinical setting, maximum strength (9) or cardiorespiratory fitness (10) are valid and independent risk predictors for morbidity and mortality. Hence, large variations across a day could affect risk estimation. Notably, performing exercise tests always at the same time of the day is often not possible in research studies or clinical routine. Therefore, it is necessary to at least know the magnitude of this influence.
That peak performance varies over the course of a day and that the time of the peak can differ between individuals may be due to myriad factors, (11) including time relative to prior physical activity, habitual exercise time, food intake, sleep, environmental factors, and the endogenous circadian system. Alterations that are induced by the endogenous circadian timing system include many physiological functions that can directly influence performance such as core body temperature, (12) respiratory control (13) or subjective alertness (14). Thus, it has been frequently hypothesized that such a complex task as performing maximum physical performance is likely to show diurnal rhythms too. In total, at least eight reviews have already summarized the results of studies investigating diurnal variation in physical performance (1–8). However, six of these eight are narrative reviews and outdated. Of the two most recent articles one investigated maximal strength, which is a predictor of mortality and has clear implications for several populations, but the other review exclusively focused on short-duration maximal exercise performance which is only relevant to athletic young adults (4, 5). More importantly, none of the eight reviews conducted a meta-analysis or assessed the risk of bias in individual studies. This is of high relevance though, because a meta-analysis could provide statistical evidence for diurnal variations and assessing the risk of bias is critical for interpreting the results and to provide recommendations for future study design to prevent or limit bias. Hence, the purpose of this work was to address these knowledge gaps by conducting a systematic review and meta-analysis including a risk of bias assessment, screening all relevant studies investigating diurnal variations in any maximum endurance and any maximum strength performance measures. The aim of this meta-analysis was to test the hypothesis that a higher maximum physical performance is achieved at a certain time of the day as compared to any other time of the day.
MATERIALS AND METHODS
This systematic review was conducted in accordance to “Preferred Reporting Items for Systematic reviews and meta-Analyses” (PRISMA)(15). The review was registered in October, 2018 and updated in January 2019 and January 2021 on PROSPERO (CRD42018109068; http://www.crd.york.ac.uk/PROSPERO/display_record.php?ID=CRD42018109068). No IRB approval was required for this review.
Search and study selection
The search was performed on November 17, 2020 in the databases EMBASE, Pubmed and Web of Science (see Figure 1). Each of the three search strings was reviewed by an external data specialist in compliance to the guideline statement for “Peer Review of Electronic Search Strategies” (16). The search strings are available in the supplement (see Appendix, Supplemental Digital Content, pages 1 – 3). Two independent reviewers conducted the literature search and screened all titles, abstracts, and full texts, in that order, for inclusion and exclusion. Disagreements between reviewers were resolved by mutual consensus and by involvement of a third expert. To reduce the risk of screening fatigue, the researches screened the studies in opposite order. We were able to obtain the full text articles for all studies that were assessed for eligibility. Eligibility criteria were that: [1] the studies included humans, [2] any kind of maximum endurance or maximum strength test was performed, and [3] these tests were performed at a minimum of three different times of the day. Studies investigating just two different times of the day were excluded, because these usually only measure morning and evening performance, which is likely to miss the true peak and/or nadir in performance, thus susceptible to underestimation of the true magnitude of the diurnal variation. There were no restrictions regarding date of publication, study design, or participants’ sex, age or fitness level. Letters to the editor, conference abstracts, and literature reviews were excluded.
Figure 1:
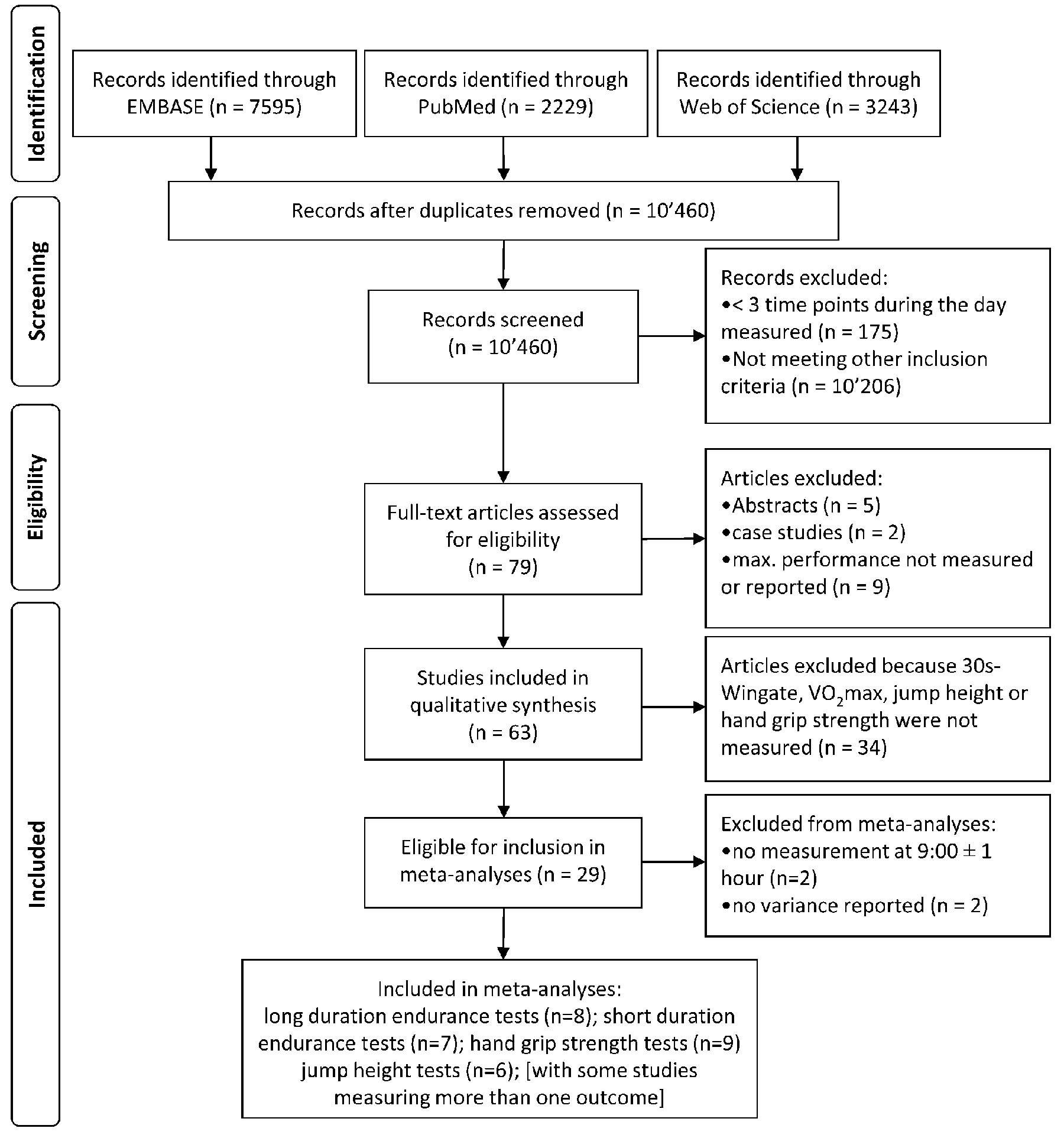
PRISMA Flow Diagram of the literature screening.
Data collection process and data items
Data extraction were independently performed by two researchers using a data abstraction sheet including participant characteristics, test times, kind of exercise test, outcomes, and times of peak/nadir performance. Mean values, standard deviations and other statistics were extracted for all outcomes. Where data was not provided in tables or text the online tool web plot digitizer (https://automeris.io/WebPlotDigitizer/) was used to estimate the data from graphs.
Risk of bias assessment
To assess the methodological quality of the studies, a slightly modified version of the “Joanna Briggs Institute Critical Appraisal tools for use in systematic reviews” was used (17). The assessment was done by two independent reviewers. In case of discrepancies, a third researcher was consulted. Due to the nature of the study designs, it was unlikely that the study participants and investigators were blinded; therefore, blinding was not included as a criterion. The criteria to define low risk or high risk were determined before the data extraction process started. Risk of bias was assessed by checking if participant characteristics were reported (i.e. selection bias); if test conditions were standardized and the gold standard method was used (i.e. detection bias); if all relevant data was presented with absolute values, effect sizes and confidence intervals (i.e. reporting bias); and if the authors controlled for confounding factors, such as sufficient regeneration time between exercise test, randomization of sequence, or use of familiarization trials (see Table S1, Appendix, Supplemental Digital Content, for more details and examples). Risk of bias across studies (i.e. publication bias) was assessed with funnel plots (see Figure S1, Appendix, Supplemental Digital Content, page 21). To create the funnel plot, the same assumptions were used as for performing the meta-analyses (see Figure 3).
Figure 3:
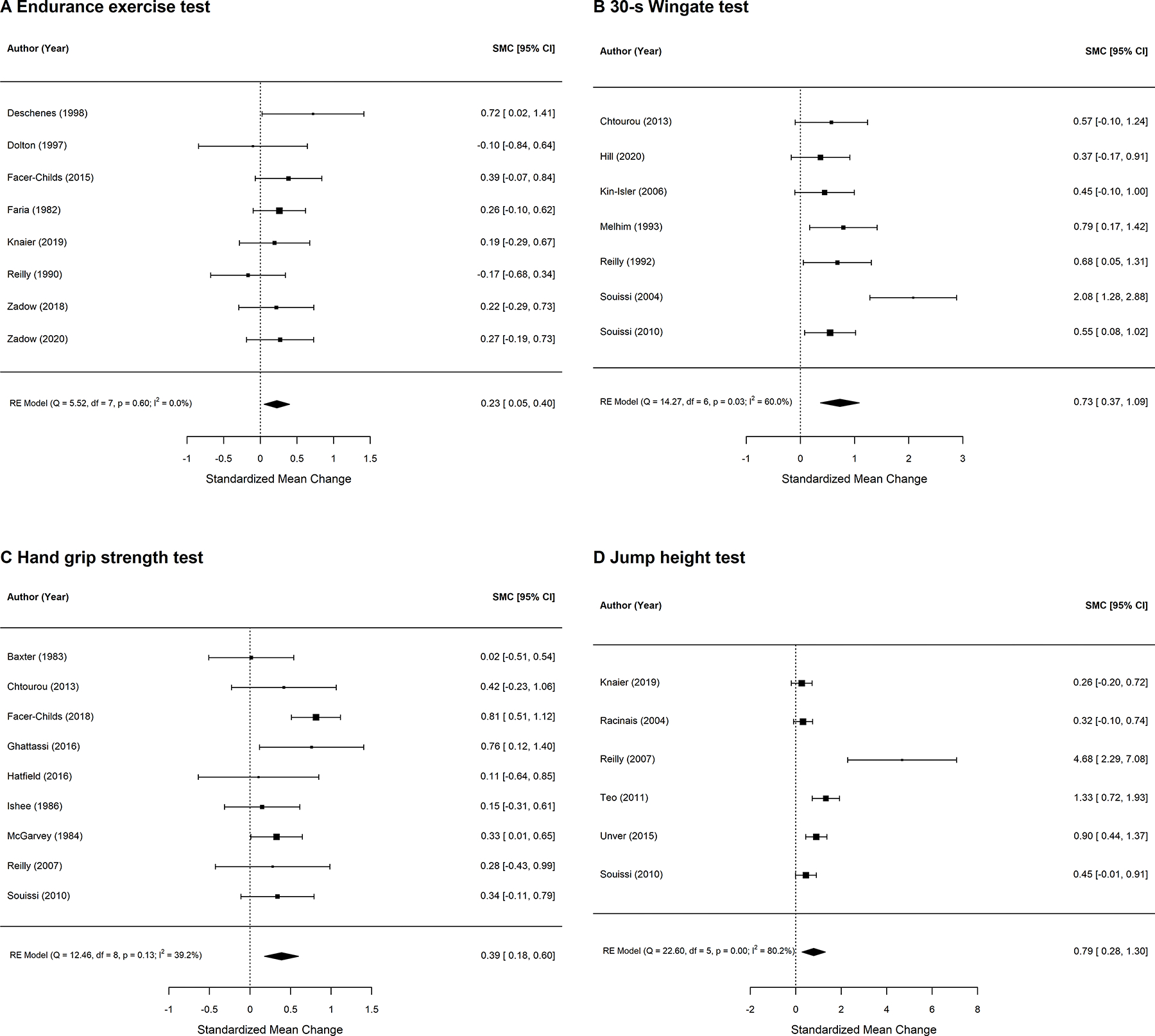
Forest plot for standardized mean change for A: Endurance exercise tests; B: 30-s Wingate test; C: handgrip strength test; D: jump height. Effects are calculated on an assumed correlation between within-participant measurements of r=0.8.
Synthesis of results
The detailed results of individual studies are displayed in Table S2 in the supplementary digital content (see Appendix, Supplemental Digital Content, pages 8 – 20). In addition, the most frequent outcomes for each of the four main categories are displayed graphically (see Figure 2). Our initially planned meta-analysis could not be performed due to insufficient reporting of results in the original studies (see Appendix, Supplemental Digital Content, pages 4 – 5). Therefore, we adapted our meta-analysis. Given that data on time-of-day specific variations within each participant were not available for most of the included studies, we computed Cohen’s d to estimate effect size for within-participant designs (18, 19). As Cohen’s d is biased, we applied Hedge’s correction factor (20). Considering that the included studies assessed the outcomes at different time intervals (2–6 hours) across varying time spans (7–24 hours), we used the differences between the peak measurement and the measurement between 8:00 – 10:00 am to generate a comparable effect size among the studies (see Appendix, Supplemental Digital Content, pages 5 – 6 for details). Positive effect sizes indicate a higher performance in the evening, while negative effect sizes indicate higher performance in the morning. However, it is worth pointing out that some degree of underestimation is unavoidable, because the observed highest/lowest measurements are likely to miss the true peak/nadir with the infrequent sampling rate and the short testing window. A random effects model was applied to estimate overall Cohen’s d using calculated Cohen’s d from individual studies. We used restricted maximum likelihood to estimate the between-study variance (21). Forest plots were used to display and compare estimates across studies. Heterogeneity among studies was estimated by the Cochran Q test and quantified by the I2 statistic (22). To receive a quantifiable measure for the time of peak performance, the mean and standard deviation was calculated for each category based on the reported times of peak performance.
Figure 2:
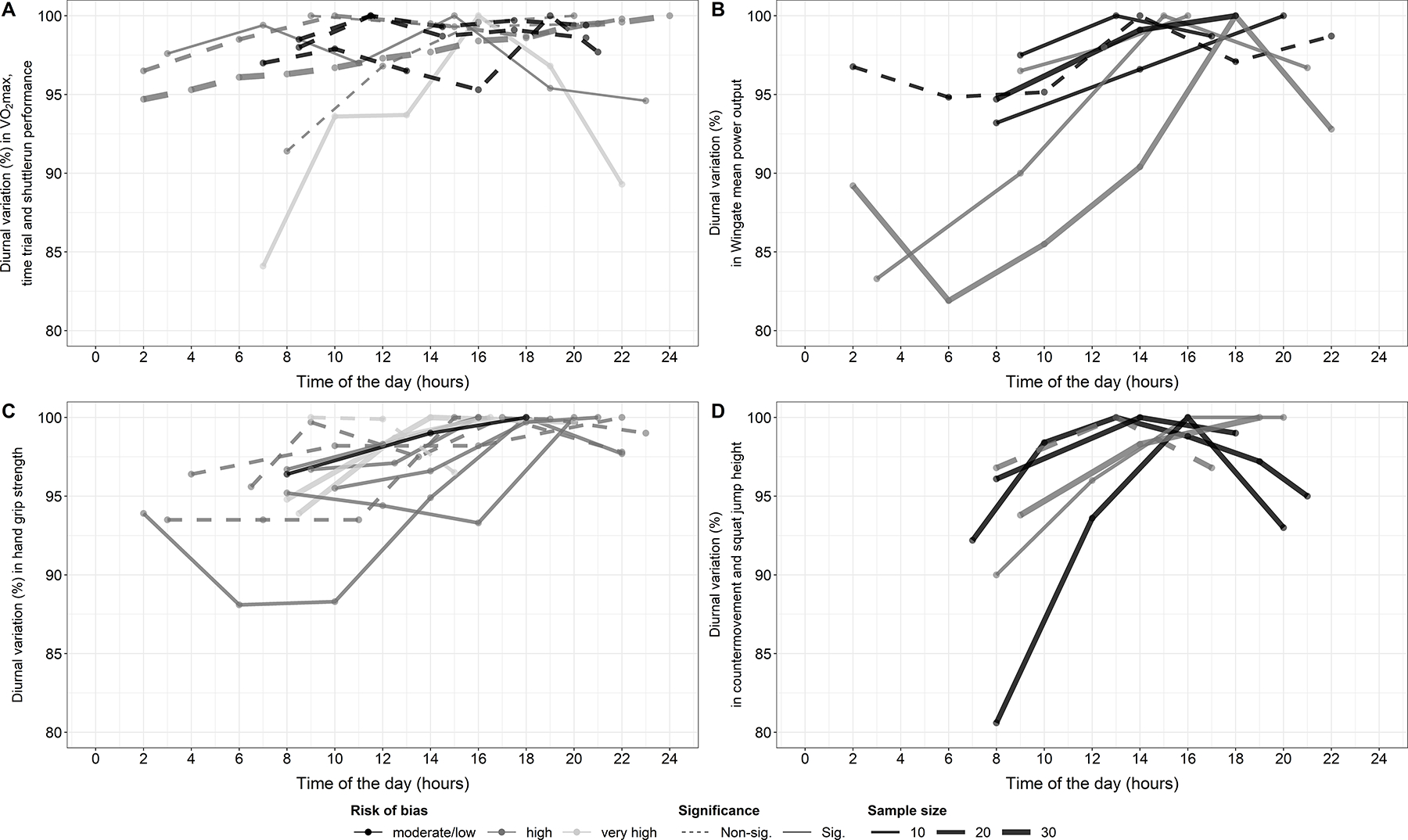
Diurnal variations in physical performance reported in the individual studies.
RESULTS
Study selection and characteristics
An overview of the study selection and the final 63 studies (23–85) included in this review is presented in Figure 1. In total 841 participants (78% male, 19% female, 3% sex not reported) were included. The fitness levels of the participants were “active” in 14 and “trained” in 28 studies, and 21 studies not reporting the fitness level. The mean age reported in 57 studies ranged between 18 and 33 years. Five studies investigated adults with a mean age above 38 years and one study tested adolescents.
Risk of bias within and across studies
The risk of bias within studies is presented in Table 1 (23–85). The overall risk of bias was moderate to high. Many studies failed to report basic participant characteristics, did not perform the tests in standardized conditions, and/or showed insufficient data reporting. Furthermore, the risk of bias remains unclear for several factors due to insufficient reporting. However, it is likely that if for example a familiarization trial had been performed the authors would have reported this. Therefore, the risk of bias is likely to be even higher than seen in Table 1. Finally, a sample size calculation was missing in almost every study. There is only little evidence for a publication bias (see Figure S1, Appendix, Supplemental Digital Content, page 21).
Table 1:
Risk of bias for individual studies.
| Selection bias | Detection bias | Confounding | Reporting bias | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||
| First-author (Year) | N | Participant characteristics reported | Gold standard used | Standardized conditions | Regeneration between tests | Familiarization performed | Sequence randomized | Controlled for sleep / PA | Effect size/ 95% CI | All absolute data | All times of day |
|
| |||||||||||
| Araujo (2011) [23] | 8 | yes | yes | yes | yes | yes | unclear | yes | yes | yes | yes |
| Atkinson (1993) [24] | 14 | yes | yes | unclear | yes | unclear | unclear | yes | no | yes | no |
| Baxter (1983) [25] | 14 | no | no | yes | yes | unclear | unclear | unclear | no | yes | yes |
| Bernard (1998) [26] | 23 | yes | no | yes | yes | yes | yes | unclear | no | yes | yes |
| Bowdle (2016) [27] | 25 | yes | yes | yes | yes | yes | yes | yes | no | no | yes |
| Buckner (2016) [28] | 7 | yes | yes | unclear | no | yes | unclear | yes | no | yes | yes |
| Callard (2000a) [29] | 6 | yes | yes | yes | no | unclear | unclear | unclear | no | no | yes |
| Callard (2000b) [30] | 7 | yes | yes | unclear | no | unclear | no | unclear | no | no | yes |
| Chtourou (2013) [31] | 10 | yes | no | no | unclear | yes | unclear | unclear | no | yes | yes |
| Coldwells (1994) [32] | 4 | no | yes | unclear | yes | yes | unclear | unclear | no | yes | yes |
| Deschenes (1998) [33] | 10 | yes | yes | yes | yes | yes | yes | unclear | no | yes | yes |
| Deschenes (1998b) [34] | 10 | yes | yes | yes | yes | unclear | yes | unclear | no | yes | yes |
| Deschenes (2002) [35] | 10 | yes | yes | yes | yes | yes | yes | yes | no | no | no |
| Deschodt (2004) [36] | 11 | yes | no | yes | yes | yes | yes | unclear | no | yes | yes |
| Dolton (1997) [37] | 7 | yes | no | yes | yes | unclear | yes | unclear | no | yes | yes |
| Facer-Childs (2015) [38] | 20 | no | no | unclear | unclear | unclear | unclear | unclear | no | no | yes |
| Facer-Childs (2018) [39] | 56 | no | yes | unclear | yes | unclear | unclear | no | no | no | yes |
| Falgairette (2003) [40] | 9 | yes | no | yes | yes | yes | yes | yes | no | yes | yes |
| Faria (1982) [41] | 31 | no | yes | yes | yes | yes | no | unclear | no | yes | yes |
| Freivalds (1983) [42] | 3 | no | no | no | no | unclear | unclear | unclear | no | yes | yes |
| Gauthier (1996) [43] | 13 | yes | yes | unclear | no | unclear | yes | yes | no | no | yes |
| Gauthier (1997) [44] | 14 | yes | yes | unclear | no | unclear | yes | unclear | no | no | no |
| Gauthier (2001) [45] | 8 | no | yes | unclear | yes | unclear | unclear | unclear | no | no | no |
| Ghattassi (2016) [46] | 12 | yes | no | yes | yes | yes | yes | unclear | no | yes | yes |
| Giacomoni (2005) [47] | 20 | yes | yes | yes | yes | yes | yes | unclear | no | no | no |
| Guette (2005) [48] | 10 | yes | yes | unclear | yes | yes | yes | unclear | no | no | yes |
| Hatfield (2016) [49] | 7 | yes | no | yes | yes | yes | yes | unclear | no | no | yes |
| Hill (1991) [50] | 6 | yes | no | unclear | yes | unclear | yes | unclear | no | no | no |
| Hill (2020) [51] | 14 | yes | no | yes | yes | yes | yes | yes | no | yes | yes |
| Ilmarinen (1975) [52] | 6 | yes | yes | unclear | unclear | unclear | unclear | unclear | no | yes | yes |
| Ilmarinen (1980) [53] | 4 | yes | no | unclear | yes | unclear | no | unclear | no | yes | yes |
| Ishee (1986) [54] | 18 | no | no | unclear | yes | no | yes | unclear | no | yes | no |
| Jasper (2009) [55] | 10 | no | yes | no | yes | yes | unclear | unclear | no | yes | yes |
| Javierre (1996) [56] | 8 | yes | no | yes | no | no | yes | no | no | yes | yes |
| Kin-Isler (2006) [57] | 14 | yes | no | yes | yes | yes | yes | yes | no | yes | yes |
| Kline (2007) [58] | 25 | yes | no | yes | yes | unclear | yes | yes | no | no | yes |
| Knaier (2019a) [59] | 17 | yes | yes | yes | yes | no | yes | yes | yes | yes | yes |
| Knaier (2019b) [60] | 19 | yes | yes | yes | yes | yes | yes | yes | yes | yes | yes |
| Lyddan (1971) [61] | 10 | no | yes | no | yes | yes | no | unclear | no | yes | no |
| McGarvey (1984) [62] | 40 | no | yes | no | no | unclear | unclear | unclear | no | yes | yes |
| Melhim (1993) [63] | 13 | yes | no | no | yes | no | unclear | yes | no | yes | yes |
| Pereira (2011) [64] | 30 | no | yes | yes | yes | unclear | yes | unclear | no | yes | yes |
| Petit (2013) [65] | 13 | yes | no | yes | no | unclear | yes | unclear | no | yes | yes |
| Racinais (2004) [66] | 23 | yes | no | yes | yes | unclear | yes | unclear | no | yes | yes |
| Reilly (1984) [67] | 10 | yes | no | yes | yes | unclear | no | yes | no | yes | yes |
| Reilly (1990) [68] | 15 | yes | yes | unclear | yes | unclear | unclear | unclear | no | yes | yes |
| Reilly (1992) [69] | 12 | yes | yes | yes | yes | yes | yes | yes | no | yes | yes |
| Reilly (2007) [70] | 8 | yes | no | unclear | yes | yes | unclear | unclear | no | yes | yes |
| Sargent (2010) [71] | 11 | no | no | no | yes | yes | unclear | yes | no | no | yes |
| Sedliak (2007) [72] | 11 | yes | yes | yes | no | yes | unclear | yes | no | yes | yes |
| Sedliak (2008) [73] | 32 | yes | no | yes | no | yes | no | yes | no | yes | yes |
| Sedliak (2011) [74] | 17 | yes | yes | yes | no | yes | yes | yes | no | no | yes |
| Sinclair (2013) [75] | 24 | no | yes | yes | yes | yes | unclear | unclear | no | yes | yes |
| Souissi (2004) [76] | 19 | yes | no | unclear | yes | yes | yes | yes | no | yes | yes |
| Souissi (2010) [77] | 20 | yes | yes | yes | yes | yes | yes | yes | no | yes | yes |
| Souissi (2019) [78] | 15 | yes | no | yes | yes | yes | yes | yes | yes | yes | yes |
| Strutton (2003) [79] | 6 | no | no | unclear | yes | unclear | unclear | unclear | no | yes | yes |
| Tamm (2009) [80] | 18 | no | yes | unclear | no | yes | unclear | yes | no | no | yes |
| Teo (2011) [81] | 20 | yes | no | yes | yes | yes | yes | unclear | no | yes | yes |
| Unver (2015) [82] | 25 | yes | no | unclear | yes | unclear | yes | unclear | no | yes | yes |
| Wyse (1994) [83] | 9 | yes | yes | yes | no | yes | unclear | unclear | no | yes | yes |
| Zadow (2018) [84] | 15 | yes | yes | yes | yes | yes | yes | yes | yes | yes | yes |
| Zadow (2020) [85] | 19 | yes | yes | yes | yes | yes | yes | yes | no | yes | yes |
Abbreviations: PA, physical activity; 95% CI, 95% confidence interval.
Results of individual studies
The detailed results of individual studies are displayed in Table S2 (see Appendix, Supplemental Digital Content, pages 8 – 20). There was high homogeneity in the study population (i.e. young male adults), but high heterogeneity regarding the performance tests used and at what times of day the tests were performed. Endurance exercise tests used in the studies were shuttle-run, incremental, step, and time to exhaustion test as well as time trials. Further, the test protocols differed in increment, duration, and exercise intensity. Further tests that were used were short swim tests, force velocity tests on cycle ergometers and sprint tests, with all of them having different durations or distances. To investigate diurnal variations in strength a total 13 different muscle groups were tested. In addition, for isokinetic testing different velocities and ranges of motion were used adding a further layer of complexity. Jump performance was measured by countermovement jump, unload squat jump, loaded squat jump, multi-jump, broad jump and long-jump. In addition, even the same test could often not be compared directly. For example, countermovement jump results were reported as flight duration (s), jump height (cm), and/or peak power (W/kg).
Qualitative synthesis of results
Figure 2A shows that the nine studies investigating diurnal variations in endurance exercise provide little evidence for consistent diurnal variations. In contrast, the studies investigating 30-s Wingate test performance (Figure 2B) and jump height (Figure 2D) show a significant diurnal variation for both displaying a peak between 13:00 and 19:00 in individual studies. Notably, in most studies jump height decreased in the late evening again after reaching its peak. Figure 2C shows that eight out of twelve studies investigating maximum handgrip strength showed a significant diurnal variation, with similar times of peak strength.
Quantitative synthesis of results
Figure 3 shows the results of the meta-analyses for the four main categories: endurance exercise tests (Figure 3A) and 30-s Wingate test (Figure 3B), maximum handgrip strength (Figure 3C), and jump height (Figure 3D). The positive effect sizes indicate a higher performance in the evening. When assumed that the within-subject correlation is r=0.8, all four categories show small to large overall effect sizes for diurnal variations and thus provide evidence for a significant diurnal variation in endurance and strength performance, as their peak performance is significantly higher than that at 8:00 – 10:00. In detail, the overall effect sizes (95% CI) are 0.23 (0.05; 0.40), 0.73 (0.37; 1.09), 0.39 (0.18; 0.60), and 0.79 (0.28, 1.30) for the four categories A, B, C, and D, respectively. Notably, with a more conservative assumption of the correlation with r=0.5 the overall effect sizes are still between small and medium for B, C, and D, but become negligible for A as the effect size for A is 0.15 (−0.02; 0.33) (see Figure S2, Appendix, Supplemental Digital Content, page 22). The mean and standard deviation for the clock hour of peak performance when considering all studies, regardless of reporting significant diurnal variation or not, were 15:06 ± 5:30, 16:18 ± 2:30, 17:00 ± 3:42, and 15:12 ± 2:18 for endurance exercise, 30-s Wingate test, handgrip strength and jump height, respectively. Notably, in endurance exercise only one study showed a significant difference with a higher performance in the evening as compared to the morning.
DISCUSSION
This is the first review investigating diurnal variation in endurance and strength performance to perform a meta-analysis. Hence, for the first time substantial statistical evidence is provided for the existence of diurnal variation in maximum physical performance in humans. In detail, we provide evidence for a significantly higher performance in the late afternoon and early evening in 30-s Wingate test, handgrip strength, and jump height as compared to the morning. The highest effect sizes were observed for jump height and 30-s Wingate test with effect sizes of 0.79 and 0.73, respectively. In endurance exercise tests, the peak performance was observed at different time of day across studies and often was not significant different from the nadir in performance.
Although the methodological limitations of this review per se are low its generalizability and the conclusions that can be drawn are limited in some respects. First, the conclusions are not generalizable, because only one fifth of the participants were female and most studies included only young adults. Second, the overall risk of bias in individual studies is rather high, thereby limiting the confidence in some results. Forth, it is unclear to which extend the magnitude of diurnal variation in performance is affected by participants’ level of effort, because this factor was not assessed in most studies. However, those studies that assessed objective or subjective measures in effort (e.g. 34, 41, 52, 59, 60, 68, 84, 85), suggest that diurnal variations in performance were not explained by different levels of effort or exhaustion.
Diurnal variations in endurance exercise tests
While performing a Wingate test, handgrip strength test, or a countermovement jump is a straightforward process, assessing aerobic endurance capacity is more complex. First, as shown in Table S2 (see Appendix, Supplemental Digital Content, pages 8 – 20), many different test protocols can be used resulting in a large heterogeneity between the studies. Second, the required time and cost, is higher for investigators due to longer test durations as well as the need to calibrate the devices, perform extensive data analysis, and to fulfill participant safety requirements such as performing medical examinations. Further, participants need to maintain their effort over a longer duration when performing endurance exercise tests. Thus, it is remarkable that the studies investigating endurance exercise tests were among those testing the most time points during the day and having relatively larger sample sizes. The reported magnitudes in diurnal variations in most studies were very similar and ranging from 3% to 5% (37, 41, 52, 59, 68, 84, 85). These relatively small diurnal variations were not expected from just a mathematical point of view, because a high number of measurement points bares a low risk to miss the actual peak and nadir and is therefore likely to increase the measured magnitude in diurnal variation. Interestingly, the only two studies reporting significant results were Ilmarinen et al. (52) which tested only six subjects and Facer-Childs et al. (38) which had a rather high risk of bias based on our pre-defined criteria (see Table S1, Appendix, Supplemental Digital Content, page 7). Facer-Childs et al. (2015) reported variations of 16% on average and up to 26% for the sub-group of late chronotypes. These magnitudes are about three and five times as high as those reported by other studies with moderate or low risk of bias, while the sample sizes were similar in all studies.
Diurnal variations in 30-s Wingate test
There was general agreement across studies that mean power output from 30-s Wingate test peaks in the late afternoon and early evening. In detail, all seven studies consistently showed higher mean power output in the late afternoon and early evening as compared to the morning, with six of the seven showing significant diurnal variations (see Figure 2B) (31, 51, 57, 62, 76, 77). Four studies tested participants at three time points ranging from 8:00 and 21:00 and showed the magnitudes of diurnal variations ranging from 2.5% to 7% (31, 51, 57, 77). However, the two studies testing participants at four (62) and six (76) different times of the day in the time window between 02:00 and 22:00 showed larger diurnal variations with 17% and 18%, respectively. Thus, those studies testing participants in a narrow time window might have captured the peak but most likely missed the nadir in performance during the day.
Diurnal variations in maximum handgrip strength
Half of the studies investigating maximum handgrip strength (31, 39, 46, 49, 54, 61, 77) had only three measurement points during the day and just one of the twelve studies showed a low risk of bias. However, the sample size of those studies investigating this outcome were higher as compared to that for the other outcomes. While a handgrip strength test is easy to carry out and to standardize, it is debatable if it is a good marker for general muscular strength in young adults, due to the relatively small number of muscle groups recruited. However, synthesizing other strength measures was not possible due to the wide range of different muscle groups tested and test protocols used. Thus, a strength test that can be carried out in almost every lab by every group would enable a comparison across studies in future meta-analysis. Isometric testing like and isometric squat or the isometric mid-thigh-pull used by Teo et al. (81) is simple to perform, standardize, and analyze. Further, it represents a large muscle group and requires only a force plate and a power rack but no isokinetic dynamometer. Thus, we recommend that all future studies include this strength test into their protocols to provide data for future meta-analyses.
Diurnal variations in jump height
Studies investigating jump height had the highest proportion of studies with low risk of bias and five (60, 70, 77, 81, 82) out of six studies reported a significant diurnal variation. The time window for peak performance was between 13:00 and 19:00 in all five studies with significant results. In addition, the diurnal pattern of countermovement jump performance is identical to that of the squat jump displaying an increase in performance in the morning, peaking in the afternoon, and a decrease in the late evening. The one study showing no significant results differed in several aspects. First, the tests were conducted in a tropical environment (temperature 28°C and 63% relative humidity). Second, it is the only study including females. Third, the mean jump height was 62 ± 10 cm (66) and therefore much higher as in the other studies. However, it is still unclear if subjects with higher fitness levels show smaller diurnal variations or not, since only one study tested and proved this hypothesis so far (24). Notably, the largest effect size in Figure 3D was seen in a study with just eight participants (70). This is explained by the large difference in jump height during the day with 5 cm in relation to the observed small standard deviations of 1.5 and 0.9 cm.
Inter-individual differences
When looking at Figure 2A one might conclude that there are no diurnal variations in endurance exercise test. Nonetheless, we want to point out that the absence of substantial diurnal variation on a group level does not necessarily mean that on an individual level there are no changes in performance over the course of a day. The reason is that individuals might reach their peak and nadir at the different times of the day. For example, if half of the subjects achieve a 10% higher performance in the morning and the other half of subjects achieves a 10% higher performance in the evening the estimated difference on a group level would be zero. Hence, individuals showing large diurnal variations could still be classified incorrectly if they are tested at a disadvantages time of the day. Therefore, future studies should ideally not only report the diurnal variation on a group level as done before, but additionally report the maximum differences in performance between any two time points for each participant individually. Two previous studies demonstrated that the time of peak performance as well as the magnitude of diurnal variations can vary strongly between subjects for endurance (59) and strength performance as well as for jump height (60). Though, a clear limitation of the latter two studies is, as in almost every study included in this review, that each subject was only tested once at each time of the day. Future studies should ideally test each subject twice at each time of the day. First, it could be investigated if the observed individual patterns in diurnal variations are reproducible. Second, this would help to assess the day-to-day variation too, which helps to put the diurnal variation into perspective.
An individual’s chronotype might is a subject characteristic that relates to inter-individual differences in diurnal variations in physical performance. Facer-Childs et al. (2015) were one of the first who investigated this innovative question. Their results suggest that the time of peak performance significantly differs between chronotypes for endurance (38) and handgrip strength performance (39). In detail, in early and intermediate chronotypes the peak in performance was reported to be about 5.5 to 6.5 hours after the habitual wakening time, while in late chronotypes it took more than 11 hours (38). However, a physiological explanation for the large difference between the wake-up and time of peak performance requires further study. The chronotype-dependent results of Facer-Childs et al. (2015) are supported by further studies investigating plantar flexion (80) and swimming time trials (86). However, there is conflicting evidence and several studies reported no different times of peak performance between chronotypes for VO2max (87), mean or peak power output in Wingate test (51), muscular strength (60), countermovement jump (60), or in a self-paced walking test (88). Notably, one study even reported a tendency for endurance performance being higher in the morning in late chronotypes (59). For a recent review on the impact of chronotype on maximum performance and other physiological functions please see Vitale et al. (89).
Possible underlying mechanisms
While this systematic review did not focus on mechanistic studies, we will briefly discuss potential mechanisms that may underlie diurnal variations in maximum endurance and strength. For a more detailed review of possible factors contributing to diurnal variations in maximum performance, please see Kusumoto et al. (11) Core body temperature has repeatedly been suggested as a main underlying mechanism. In support, it has been shown that lowering core body temperature in the evening to morning levels by cold water baths (16 – 17°C) decreases muscular strength (90) and repeated sprint performance (91). However, long baths in cold water might decrease subjects’ motivation. It has been shown that raising core body temperature in the morning to evening levels through an active and/or passive warm-up does elevate isokinetic or isometric strength (92), cycling time trial (93), or repeated sprint test performance (94) indicating that core body temperature is a contributing factor. However, the performance is not elevated to the same level as evening performance indicating that it is not the only factor. Increasing core body temperature through active warm-up for example drives chemical and metabolic rates (95). Notably, in long duration exercise pre-cooling has shown to improve performance by decreasing heat stress (96). In conclusion, core body temperature might play a role in diurnal variations in performance but can definitely not explain the variation entirely. Apart from core body temperature, other possible underlying mechanisms may include the central clock influences, muscle clocks, autonomic nervous system output to the muscle, rhythms in cardiopulmonary function, environmental temperature, nutritional state or sleep homeostasis. However, to date studies investigating the underlying mechanism are rare.
Methodological strengths and limitations of the review
The review was done in accordance to PRISMA and PRESS guidelines and it was registered beforehand on PROSPERO. The review did not exclude any studies by publication dates, and it included studies in English (n=60), German (n=1) and French (n=2) language which were translated by native speakers. Furthermore, full texts were made available for all eligible studies and it is the first review on this topic that includes a risk of bias assessment to describe the quality of the individual studies. One limitation of this review is that despite our efforts to receive the required data from the authors, we were not successful in retrieving the individual-level data to perform the four different priori meta-analyses. However, we did design a post hoc meta-analysis based on available study-level data. Notably, the times during the day that were investigated were discrete and differed between individual studies, which limits the estimation of the time of peak performance. The second limitation of this review is that the risk of bias assessment was performed based on those categories and criteria that we anticipated to produce the most severe bias (see Table S1, Appendix, Supplemental Digital Content, page 7). Although this was done to the best of our abilities and the criteria were defined before extracting the data from individual studies, we are aware that other groups might have focused on other kinds of bias and that other criteria could be used and would lead to different assessments. For example, the study by Facer-Childs et al. (2015) stated the sex for the participants screened but not for the participants included in the study and was subsequently rated as participants’ characterization missing.
Recommendations and perspectives for future studies
It is unfortunate, that despite the fact that many studies have been conducted on this topic, we could not perform our initially intended meta-analysis (see Appendix, Supplemental Digital Content, pages 4 – 5). Several studies seem to have been conducted with rigor, but reported the results insufficiently. Although we acknowledge that there has been a clear decrease in risk of bias in the past years, we provide detailed recommendations for future studies in Table 2 to facilitate a solid meta-analysis on this topic in the near future.Further, we identified three important knowledge gaps that should be addressed in the future.
Table 2:
Recommendations for future research.
| Essential | Additional | |
|---|---|---|
|
| ||
| Participants | ||
| Sex/Age/Race | Include male and female subjects as well as subjects with different race and ethnicity. | Investigate adolescents and older subjects, because they are underrepresented in the current literature. |
| Inclusion criteria | Use pre-specified inclusion criteria based on age, sex, and level of fitness. | Depending on the research question consider including further criteria, such as training history, habitual exercise time or chronotype. |
| Blinding | Ensure that subjects are unaware of the performance they achieved at a certain time of the day until the end of the study. | Blind the investigator analyzing the data. |
| Sample size | A priori sample size needs to be performed at least for the primary outcome of the study. | If subgroup analyses are planned (e.g. for different chronotypes) the sample size needs to be adequate for these analyses too. |
|
| ||
| Measurements | ||
| Time points | Measure at least five time points during the day. | Previous studies mainly focused on the time windows from 07:00 to 20:00. Consider investigating broader time windows. |
| Gold standard | Use gold standard methods for each outcome. | Ensure that the test-retest reliability is sufficient to enable showing the difference anticipated in the sample size calculation. |
| Outcomes | If two or more outcomes are measured pre-specify the primary one. | For all studies investigating strength performance we recommend to include hand grip strength, countermovement jump and isometric mid-thigh-pull-up to the protocol. |
| Familiarization | Perform at least one familiarization trial. | Time trials for example might require more than one familiarization trial. |
| Calibration | Ensure that the devices are calibrated in sufficient intervals according to the recommendation of the manufacturer. | |
| External factors | Ensure equal preparation (i.e. warm-up, preparation before test) and equal external factors (i.e. room temperature, humidity). | |
| Randomization | Randomize the sequence of time points measured. | |
| Verification | Implement methods to ensure that subjects actually reached their maximum (e.g. secondary VO2max criteria). | Measure each time of the day twice to ensure that the pattern of diurnal variation seen is repetitive and to assess the coefficient of variation at each time of the day. |
| Regeneration | Ensure sufficient regeneration time between tests. The required duration will depend on the task and can be short for tasks like hand grip strength test but may be long for long duration time trials. | Include physiological markers (e.g. blood) to ensure the regeneration. |
|
| ||
| Monitoring during study period | ||
| Sleep | Give instructions to maintain a constant sleeping routine. | Use diaries, questionnaires or ideally objective methods to monitor sleep. |
| Physical activity | Give instructions how long to refrain from exercise before each test. | Use diaries, questionnaires or ideally objective methods to monitor activity. |
| Nutrition | Give instructions such as allowed diets, required fasting durations before the tests, or time windows subjects are allowed to eat. | Use diaries, questionnaires or ideally less subjective methods such as recording nutrition and meal timing by taking pictures with a reference sized measure. |
|
| ||
| Reporting | ||
| Study | Report everything that is recommended by CONSORT. | |
| Results | Report effect sizes or mean differences including 95% confidence intervals | |
| Real data | Report data as it is measured (e.g. L/min, N, s, cm – ideally SI-units) and not only as percentage values. | Report absolute and relative data (e.g. L/min and mL/kg/min or N and N/kg) and if possible report different units for the same outcome (e.g. jump height in cm, flight time in s, and peak power N/kg for the countermovement jump). |
| Times of day | Report all times of the day tested and not only the peak and nadir. | |
| Deviations | Report if the actual test time deviated from intended test time. Report for which outcomes and time points data is missing. |
|
| Outcomes | Report all outcomes measured or provide an explanation if some outcomes are not reported. | |
(1) There is a clear need to identify the underlying mechanisms causing diurnal variations in performance, since this will create methods to shift the time of peak performance or to reduce the magnitude of the diurnal decline. Especially, athletes competing at disadvantageous times of the day would highly benefit from such methods. However, investigating the causality between for example changes in core body temperature and diurnal variations in maximum performance may be challenging. Increasing body temperature too much may trigger counter regulatory mechanisms that try to maintain temperature within set-range boundaries. These counter regulatory mechanisms by themselves cost energy which then might not be available for performance. Thus, experimentally testing the effect of endogenously-driven temperature changes compared to exogenously imposed temperature changes is challenging.
(2) Diurnal variations in maximum performance have been broadly investigated to date, but endogenous circadian rhythms have not been. Hence, highly controlled in-laboratory studies using specific protocols (97) such as forced desynchronization are required in upcoming studies to separate the homeostatic process, environmental/behavioural cycles from the circadian timing process (71). Understanding the relative contribution of the circadian system to the diurnal variations in maximum performance may help to evaluate the possibility to implement chronobiology-based approach to shift timing of peak performance (e.g., light treatment (98)).
(3) The long-term effects of exercising at a certain time of the day have been investigated to a much smaller extent than diurnal variations in maximum performance. Some studies addressed this research question for endurance and strength performance (99), but only few investigated the effects on other health related outcomes. Furthermore, previous research mainly focused on healthy participants and athletes, but not on vulnerable participants (100).
CONCLUSIONS
There is strong evidence that 30-s Wingate test performance as well as jump height are maximal between 13:00 and 19:00. There is some evidence that handgrip strength peaks between 13:00 and 21:00, but only little evidence that there is a time of peak performance in endurance exercises. The effect sizes for jump height and 30-s Wingate test are medium to large at 0.79 and 0.73, while the effect sizes for handgrip strength and long endurance performance are small to medium at 0.39 and 0.23, respectively.
Supplementary Material
Acknowledgments
We thank Hannah Ewald, PhD, MPH Information Specialist, University Medical Library, University of Basel, Switzerland for performing the review of the search strategy according to the guidelines for Peer Review of Electronic Search Strategies. We also thank Justin Carrard and Gilles Neve for translating the four articles that were published in French.
Funding:
R.K. was funded by the Swiss National Science Foundation (Grant P2BSP3_191755). J.Q. was supported in part by the NIH (Grant K99HL148500 and R01DK102696). F.A.J.L.S. was supported in part by NIH grants R01DK102696, R01DK105072 and R01HL140574.
Footnotes
Conflict of interest statement:
None of the authors involved in the present study have any conflict of interest, financial, personal, or otherwise, which would influence this research, and the results do not constitute endorsement by the American College of Sports Medicine. The authors declare that the results of the study are presented clearly, honestly, and without fabrication, falsification, or inappropriate data manipulation.
REFERENCES
- 1.Atkinson G, Reilly T. Circadian variation in sports performance. Sports Med. 1996;21(4):292–312. [DOI] [PubMed] [Google Scholar]
- 2.Chtourou H, Souissi N. The effect of training at a specific time of day: a review. J Strength Cond Res. 2012;26(7):1984–2005. [DOI] [PubMed] [Google Scholar]
- 3.Drust B, Waterhouse J, Atkinson G, Edwards B, Reilly T. Circadian rhythms in sports performance—an update. Chronobiol Int. 2005;22(1):21–44. [DOI] [PubMed] [Google Scholar]
- 4.Mirizio GG, Nunes RSM, Vargas DA, Foster C, Vieira E. Time-of-day effects on short-duration maximal exercise performance. Sci Rep. 2020;10(1):9485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pullinger SA, Cocking S, Robertson CM, et al. Time-of-day variation on performance measures in repeated-sprint tests: a systematic review. Chronobiol Int. 2020;37(4):451–68. [DOI] [PubMed] [Google Scholar]
- 6.Reilly T, Waterhouse J. Sports performance: is there evidence that the body clock plays a role? Eur J Appl Physiol. 2009;106(3):321–32. [DOI] [PubMed] [Google Scholar]
- 7.Thun E, Bjorvatn B, Flo E, Harris A, Pallesen S. Sleep, circadian rhythms, and athletic performance. Sleep Med Rev. 2015;23:1–9. [DOI] [PubMed] [Google Scholar]
- 8.Winget CM, DeRoshia CW, Holley DC. Circadian rhythms and athletic performance. Med Sci Sports Exerc. 1985;17(5):498–516. [PubMed] [Google Scholar]
- 9.Metter EJ, Talbot LA, Schrager M, Conwit R. Skeletal muscle strength as a predictor of all-cause mortality in healthy men. J Gerontol A Biol Sci Med Sci. 2002;57(10):B359–365. [DOI] [PubMed] [Google Scholar]
- 10.Kodama S, Saito K, Tanaka S, et al. Cardiorespiratory fitness as a quantitative predictor of all-cause mortality and cardiovascular events in healthy men and women: a meta-analysis. JAMA. 2009;301(19):2024–35. [DOI] [PubMed] [Google Scholar]
- 11.Kusumoto H, Ta C, Brown SM, Mulcahey MK. Factors contributing to diurnal variation in athletic performance and methods to reduce within-day performance variation: a systematic review. J Strength Cond Res. 2020; online ahead of print [DOI] [PubMed] [Google Scholar]
- 12.Hiddinga AE, Beersma DG, Van den Hoofdakker RH. Endogenous and exogenous components in the circadian variation of core body temperature in humans. J Sleep Res. 1997;6(3):156–63. [DOI] [PubMed] [Google Scholar]
- 13.Spengler CM, Czeisler CA, Shea SA. An endogenous circadian rhythm of respiratory control in humans. J Physiol. 2000;526.3:683–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dijk DJ, Duffy JF, Czeisler CA. Circadian and sleep/wake dependent aspects of subjective alertness and cognitive performance. J Sleep Res. 1992;1(2):112–7. [DOI] [PubMed] [Google Scholar]
- 15.Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McGowan J, Sampson M, Salzwedel DM, Cogo E, Foerster V, Lefebvre C. PRESS Peer Review of Electronic Search Strategies: 2015 Guideline Statement. J Clin Epidemiol. 2016;75:40–6. [DOI] [PubMed] [Google Scholar]
- 17.Moola S, Munn Z, Tufanaru C. Systematic reviews of etiology and risk. Joanna Briggs Institute Reviewer’S Manual, The Joanna Briggs Institute, Adelaide, Australia. 2017. [Google Scholar]
- 18.Cohen J Statistical Power Analysis for the Behavioral Sciences. Routledge Academic Press; 2013. 459 p. [Google Scholar]
- 19.Lakens D. Calculating and reporting effect sizes to facilitate cumulative science: a practical primer for t-tests and ANOVAs. Front Psychol. 2013;4:863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hedges L, Olkin I. Statistical methods in meta-analysis. Stat Med. 1985;20:191–200. [Google Scholar]
- 21.Langan D, Higgins JPT, Jackson D, et al. A comparison of heterogeneity variance estimators in simulated random-effects meta-analyses. Res Synth Methods. 2019;10(1):83–98. [DOI] [PubMed] [Google Scholar]
- 22.Borenstein M, Hedges L, Higgins J, Rothenstein H. Introduction to Meta-Analysis. John Wiley and Sons; 2011. [Google Scholar]
- 23.Araujo LG, Waterhouse JM, Edwards B, Santos EHR, Tufik S, Tlio De Mello M. Twenty-four-hour rhythms of muscle strength with a consideration of some methodological problems. Biol Rhythm Res. 2011;42(6):473–90. [Google Scholar]
- 24.Atkinson G, Coldwells A, Reilly T, Waterhouse J. A comparison of circadian rhythms in work performance between physically active and inactive subjects. Ergonomics. 1993;36(1–3):273–81. [DOI] [PubMed] [Google Scholar]
- 25.Baxter C, Reilly T. Influence of time of day on all-out swimming. Br J Sports Med. 1983;17(2):122–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bernard T, Giacomoni M, Gavarry O, Seymat M, Falgairette G. Time-of-day effects in maximal anaerobic leg exercise. Eur J Appl Physiol. 1998;77(1–2):133–8. [DOI] [PubMed] [Google Scholar]
- 27.Bowdle RHW, Warren BL, Kim J. Time of day effect on isokinetic peak torque during knee flexion and extension. Isokinet Exerc Sci. 2016;24(4):285–93. [Google Scholar]
- 28.Buckner SL, Dankel SJ, Counts BR, et al. Do rhythms exist in elbow flexor torque, oral temperature and muscle thickness during normal waking hours? Physiol Behav. 2016;160:12–7. [DOI] [PubMed] [Google Scholar]
- 29.Callard D, Davenne D, Gauthier A, Lagarde D, Van Hoecke J. Circadian rhythms in human muscular efficiency: Continuous physical exercise versus continuous rest. A crossover study. Chronobiol Int. 2000;17(5):693–704. [DOI] [PubMed] [Google Scholar]
- 30.Callard D, Gauthier A, Maffiuletti N, Davenne D, Van Hoecke J Circadian fluctuations in the muscular efficiency of athletes: with sleep versus sleep deprivation. J Société Biol. 2000;194(3–4):165–9. [PubMed] [Google Scholar]
- 31.Chtourou H, Aloui A, Hammouda O, Chaouachi A, Chamari K, Souissi N. The effect of time-of-day and judo match on short-term maximal performances in judokas. Biol Rhythm Res. 2013;44(5):797–806. [DOI] [PubMed] [Google Scholar]
- 32.Coldwells A, Atkinson G, Reilly T. Sources of variation in back and leg dynamometry. Ergonomics. 1994;37(1):79–86. [DOI] [PubMed] [Google Scholar]
- 33.Deschenes MR, Kraemer WJ, Bush JA, et al. Biorhythmic influences on functional capacity of human muscle and physiological responses. Med Sci Sports Exerc. 1998;30(9):1399–407. [DOI] [PubMed] [Google Scholar]
- 34.Deschenes MR, Sharma JV, Brittingham KT, Casa DJ, Armstrong L.E., Maresh C.M. Chronobiological effects on exercise performance and selected physiological responses. Eur J Appl Physiol. 1998;77(3):249–56. [DOI] [PubMed] [Google Scholar]
- 35.Deschenes MR, Bronson LL, Cadorette MP, Powers JE, Weinlein JC. Aged men display blunted biorhythmic variation of muscle performance and physiological responses. J Appl Physiol. 2002;92(6):2319–25. [DOI] [PubMed] [Google Scholar]
- 36.Deschodt VJ, Arsac LM. Morning vs. evening maximal cycle power and technical swimming ability. J Strength Cond Res. 2004;18(1):149–54. [DOI] [PubMed] [Google Scholar]
- 37.Dolton B, McNaughton L, Davoren B. Circadian rhythms have no effect on cycling performance. Int J Sports Med. 1997;18(7):538–42. [DOI] [PubMed] [Google Scholar]
- 38.Facer-Childs E, Brandstaetter R. The impact of circadian phenotype and time since awakening on diurnal performance in athletes. Curr Biol. 2015;25(4):518–22. [DOI] [PubMed] [Google Scholar]
- 39.Facer-Childs ER, Boiling S, Balanos GM. The effects of time of day and chronotype on cognitive and physical performance in healthy volunteers. Sports Med - Open. 2018;4(1):47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Falgairette G, Billaut F, Ramdani S Effects of recovery duration and time of day on sprint performance. Can J Appl Physiol. 2003;28(2):213–24. [DOI] [PubMed] [Google Scholar]
- 41.Faria IE, Drummond BJ. Circadian changes in testing heart rate and body temperature, maximal oxygen consumption and perceived exertion. Ergonomics. 1982;25(5):381–6. [DOI] [PubMed] [Google Scholar]
- 42.Freivalds A, Chaffin DB, Langolf GD. Quantification of human performance circadian rhythms. Am Ind Hyg Assoc J. 1983;44(9):643–8. [DOI] [PubMed] [Google Scholar]
- 43.Gauthier A, Davenne D, Martin A, Cometti G, Van Hoecke J Diurnal rhythm of the muscular performance of elbow flexors during isometric contractions. Chronobiol Int. 1996;13(2):135–46. [DOI] [PubMed] [Google Scholar]
- 44.Gauthier A, Davenne D, Gentil C, Van Hoecke J Circadian rhythm in the torque developed by elbow flexors during isometric contraction. Effect of sampling schedules. Chronobiol Int. 1997;14(3):287–94. [DOI] [PubMed] [Google Scholar]
- 45.Gauthier A, Davenne D, Martin A, Van Hoecke J Time of day effects on isometric and isokinetic torque developed during elbow flexion in humans. Eur J Appl Physiol. 2001;84(3):249–52. [DOI] [PubMed] [Google Scholar]
- 46.Ghattassi K, Hammouda O, Graja A, et al. Morning melatonin ingestion and diurnal variation of short-term maximal performances in soccer players. Acta Physiol Hung. 2016;103(1):94–104. [DOI] [PubMed] [Google Scholar]
- 47.Giacomoni M, Edwards B, Bambaeichi E Gender differences in the circadian variations in muscle strength assessed with and without superimposed electrical twitches. Ergonomics. 2005;48(11–14):1473–87. [DOI] [PubMed] [Google Scholar]
- 48.Guette M, Gondin J, Martin A Time-of-day effect on the torque and neuromuscular properties of dominant and non-dominant quadriceps femoris. Chronobiol Int. 2005;22(3):541–58. [DOI] [PubMed] [Google Scholar]
- 49.Hatfield DL, Nicoll JX, Kraemer WJ Effects of circadian rhythm on power, force, and hormonal response in young men. J Strength Cond Res. 2016;30(3):725–32. [DOI] [PubMed] [Google Scholar]
- 50.Hill DW, Smith JC Effect of time of day on the relationship between mood state, anaerobic power, and capacity. Percept Mot Skills. 1991;72(1):83–7. [DOI] [PubMed] [Google Scholar]
- 51.Hill DW, Chtourou H. The effect of time of day and chronotype on the relationships between mood state and performance in a Wingate test. Chronobiol Int. 2020;37(11):1599–610. [DOI] [PubMed] [Google Scholar]
- 52.Ilmarinen J, Rutenfranz J, Kylian H, Klimt F Study of the circadian variation of different circulatory and respiratory functions at submaximal and maximal ergometer work. Eur J Appl Physiol. 1975;34(4):255–67. [DOI] [PubMed] [Google Scholar]
- 53.Ilmarinen J, Ilmarinen R, Korhonen C, Nurminen M Circadian variation of physiological functions related to physical work capacity. Scand J Work Environ Health. 1980;6(2):112–22. [DOI] [PubMed] [Google Scholar]
- 54.Ishee J, Titlow L. Diurnal-variations in physical performance. Percept Mot Skills. 1986;63(2):835–8. [Google Scholar]
- 55.Jasper I, Häussler A, Baur B, Marquardt C, Hermsdörfer J Circadian variations in the kinematics of handwriting and grip strength. Chronobiol Int. 2009;26(3):576–94. [DOI] [PubMed] [Google Scholar]
- 56.Javierre C, Calvo M, Díez A, Garrido E, Segura R, Ventura JL. Influence of sleep and meal schedules on performance peaks in competitive sprinters. Int J Sports Med. 1996;17(06):404–8. [DOI] [PubMed] [Google Scholar]
- 57.Kin-Isler A Time-of-day effects in maximal anaerobic performance and blood lactate concentration during and after a supramaximal exercise. Isokinet Exerc Sci. 2006;14(4):335–40. [Google Scholar]
- 58.Kline CE, Durstine JL, Davis JM, et al. Circadian variation in swim performance. J Appl Physiol. 2007;102(2):641–9. [DOI] [PubMed] [Google Scholar]
- 59.Knaier R, Infanger D, Niemeyer M, Cajochen C, Schmidt-Trucksäss A. In athletes, the diurnal variations in maximum oxygen uptake are more than twice as large as they day-to-day variations. Front Physiol. 2019;10:219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Knaier R, Infanger D, Cajochen C, Schmidt-Trucksaess A, Faude O, Roth R. Diurnal and day-to-day variations in isometric and isokinetic strength. Chronobiol Int. 2019;36(11):1537–49. [DOI] [PubMed] [Google Scholar]
- 61.Lyddan MJ, Caldwell LS, Alluisi EA. Measurements of muscular strength, endurance, and recovery over fifteen successive days. J Mot Behav. 1971;3(3):213–23. [DOI] [PubMed] [Google Scholar]
- 62.McGarvey SR, Morrey BF, Askew LJ, An KN Reliability of isometric strength testing. Temporal factors and strength variation. Clin Orthop. 1984;185:301–5. [PubMed] [Google Scholar]
- 63.Melhim A Investigation of circadian rhythms in peak power and mean power of female physical education students. Int J Sports Med. 1993;14(6):303–6. [DOI] [PubMed] [Google Scholar]
- 64.Pereira R, Machado M, Ribeiro W, Russo AK, de Paula A, Lazo-Osorio RA. Variation of explosive force at different times of day. Biol Sport. 2011;28(1):3–9. [Google Scholar]
- 65.Petit E, Bourdin H, Mougin F, Tio G, Haffen E. Time-of-day effects on psychomotor and physical performances in highly trained cyclists. Percept Mot Skills. 2013;117(2):376–88. [DOI] [PubMed] [Google Scholar]
- 66.Racinais S, Hue O, Hertogh C, Damiani M, Blonc S Time-of-day effects in maximal anaerobic leg exercise in tropical environment: A first approach. Int J Sports Med. 2004;25(3):186–90. [DOI] [PubMed] [Google Scholar]
- 67.Reilly T, Robinson G, Minors DS. Some circulatory responses to exercise at different times of day. Med Sci Sports Exerc. 1984;16(5):477–82. [DOI] [PubMed] [Google Scholar]
- 68.Reilly T, Brooks GA. Selective persistence of circadian rhythms in physiological responses to exercise. Chronobiol Int. 1990;7(1):59–67. [DOI] [PubMed] [Google Scholar]
- 69.Reilly T, Down A. Investigation of circadian rhythms in anaerobic power and capacity of the legs. J Sports Med Phys Fitness. 1992;32(4):343–7. [PubMed] [Google Scholar]
- 70.Reilly T, Atkinson G, Edwards B, Waterhouse J, Farrelly K, Fairhurst E Diurnal variation in temperature, mental and physical performance, and tasks specifically related to football (soccer). Chronobiol Int. 2007;24(3):507–19. [DOI] [PubMed] [Google Scholar]
- 71.Sargent C, Ferguson SA, Darwent D, Kennaway DJ, Roach GD The influence of circadian phase and prior wake on neuromuscular function. Chronobiol Int. 2010;27(5):911–21. [DOI] [PubMed] [Google Scholar]
- 72.Sedliak M, Finni T, Cheng S, Kraemer WJ, Häkkinen K Effect of time-of-day-specific strength training on serum hormone concentrations and isometric strength in men. Chronobiol Int. 2007;24(6):1159–77. [DOI] [PubMed] [Google Scholar]
- 73.Sedliak M, Finni T, Cheng S, Haikarainen T, Häkkinen K Diurnal variation in maximal and submaximal strength, power and neural activation of leg extensors in men: Multiple sampling across two consecutive days. Int J Sports Med. 2008;29(3):217–24. [DOI] [PubMed] [Google Scholar]
- 74.Sedliak M, Haverinen M, Häkkinen K Muscle strength, resting muscle tone and EMG activation in untrained men: Interaction effect of time of day and test order-related confounding factors. J Sports Med Phys Fitness. 2011;51(4):560–70. [PubMed] [Google Scholar]
- 75.Sinclair J, Wright J, Hurst HT, Taylor PJ, Atkins S The influence of circadian rhythms on peak isokinetic force of quadriceps and hamstring muscles. Isokinet Exerc Sci. 2013;21(4):279–84. [Google Scholar]
- 76.Souissi N, Gauthier A, Sesboüé B, Larue J, Davenne D Circadian rhythms in two types of anaerobic cycle leg exercise: force-velocity and 30-s Wingate tests. Int J Sports Med. 2004;25(1):14–9. [DOI] [PubMed] [Google Scholar]
- 77.Souissi H, Chaouachi A, Chamari K, Dogui M, Amri M, Souissi N. Time-of-day effects on short-term exercise performances in 10- to 11-year-old boys. Pediatr Exerc Sci. 2010;22(4):613–23. [DOI] [PubMed] [Google Scholar]
- 78.Souissi Y, Souissi M, Chtourou H. Effects of caffeine ingestion on the diurnal variation of cognitive and repeated high-intensity performances. Pharmacol Biochem Behav. 2019;177:69–74. [DOI] [PubMed] [Google Scholar]
- 79.Strutton PH, Catley M, Davey NJ Stability of corticospinal excitability and grip force in intrinsic hand muscles in man over a 24-h period. Physiol Behav. 2003;79(4–5):679–82. [DOI] [PubMed] [Google Scholar]
- 80.Tamm AS, Lagerquist O, Ley AL, Collins DF Chronotype influences diurnal variations in the excitability of the human motor cortex and the ability to generate torque during a maximum voluntary contraction. J Biol Rhythms. 2009;24(3):211–24. [DOI] [PubMed] [Google Scholar]
- 81.Teo W, McGuigan MR, Newton MJ The effects of circadian rhythmicity of salivary cortisol and testosterone on maximal isometric force, maximal dynamic force, and power output. J Strength Cond Res. 2011;25(6):1538–45. [DOI] [PubMed] [Google Scholar]
- 82.Unver S, Atan T. Investigation of the changes in performance measurements based on circadian rhythm. Anthropologist. 2015;19(2):423–30. [Google Scholar]
- 83.Wyse JP, Mercer TH, Gleeson NP. Time-of-day dependence of isokinetic leg strength and associated interday variability. Br J Sports Med. 1994;28(3):167–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zadow EK, Kitic CM, Wu SSX, Fell JW, Adams MJ Time of day and short-duration high-intensity exercise influences on coagulation and fibrinolysis. Eur J Sport Sci. 2018;18(3):367–75. [DOI] [PubMed] [Google Scholar]
- 85.Zadow EK, Fell JW, Kitic CM, Han J, Wu SSX. Effects of time of day on pacing in a 4-km time trial in trained cyclists. Int J Sports Physiol Perform. 2020;15(10):1455–1459. [DOI] [PubMed] [Google Scholar]
- 86.Rae DE, Stephenson KJ, Roden LC Factors to consider when assessing diurnal variation in sports performance: the influence of chronotype and habitual training time-of-day. Eur J Appl Physiol. 2015;115(6):1339–49. [DOI] [PubMed] [Google Scholar]
- 87.Sugawara J, Hamada Y, Nishijima T, Matsuda M. Diurnal variations of post-exercise parasympathetic nervous reactivation in different chronotypes. Jpn Heart J. 2001;42(2):163–71. [DOI] [PubMed] [Google Scholar]
- 88.Rossi A, Formenti D, Vitale JA, Calogiuri G, Weydahl A. The effect of chronotype on psychophysiological responses during aerobic self-paced exercises. Percept Mot Skills. 2015;121(3):840–55. [DOI] [PubMed] [Google Scholar]
- 89.Vitale JA, Weydahl A. Chronotype, physical activity, and sport performance: a systematic review. Sports Med. 2017;47(9):1859–68. [DOI] [PubMed] [Google Scholar]
- 90.Robinson WR, Pullinger SA, Kerry JW, et al. Does lowering evening rectal temperature to morning levels offset the diurnal variation in muscle force production? Chronobiol Int. 2013;30(8):998–1010. [DOI] [PubMed] [Google Scholar]
- 91.Pullinger SA, Oksa J, Brocklehurst EL, et al. Controlling rectal and muscle temperatures: Can we offset diurnal variation in repeated sprint performance? Chronobiol Int. 2018;35(7):959–68. [DOI] [PubMed] [Google Scholar]
- 92.Edwards BJ, Pullinger SA, Kerry JW, et al. Does raising morning rectal temperature to evening levels offset the diurnal variation in muscle force production? Chronobiol Int. 2013;30(4):486–501. [DOI] [PubMed] [Google Scholar]
- 93.Atkinson G, Todd C, Reilly T, Waterhouse J. Diurnal variation in cycling performance: influence of warm-up. J Sports Sci. 2005;23(3):321–9. [DOI] [PubMed] [Google Scholar]
- 94.Pullinger SA, Oksa J, Clark LF, et al. Diurnal variation in repeated sprint performance cannot be offset when rectal and muscle temperatures are at optimal levels (38.5°C). Chronobiol Int. 2018;35(8):1054–65. [DOI] [PubMed] [Google Scholar]
- 95.McGowan CJ, Pyne DB, Thompson KG, Rattray B. Warm-Up strategies for sport and exercise: mechanisms and applications. Sports Med. 2015;45(11):1523–46. [DOI] [PubMed] [Google Scholar]
- 96.Bongers CCWG, Hopman MTE, Eijsvogels TMH. Cooling interventions for athletes: An overview of effectiveness, physiological mechanisms, and practical considerations. Temp Austin. 2017;4(1):60–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Qian J, Scheer FAJL. Circadian System and Glucose Metabolism: Implications for physiology and disease. Trends Endocrinol Metab. 2016;27(5):282–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Knaier R, Schäfer J, Rossmeissl A, et al. Prime time light exposures do not seem to improve maximal physical performance in male elite athletes, but enhance end-spurt performance. Front Physiol. 2017;8:264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Küüsmaa-Schildt M, Liukkonen J, Vuong MK, Nyman K, Häkkinen K, Häkkinen A. Effects of morning vs. evening combined strength and endurance training on physical performance, sleep and well-being. Chronobiol Int. 2019;36(6):811–25. [DOI] [PubMed] [Google Scholar]
- 100.Qian J, Walkup MP, Chen S-H, et al. Association of objectively measured timing of physical activity bouts with cardiovascular health in type 2 diabetes. Diabetes Care. 2021;44(4):1046–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


