Abstract
N 6-methyladenosine (m6A), one of the most abundant RNA modifications, is involved in the progression of many diseases, but its role and related molecular mechanisms in endometriosis remain unknown. To address these issues, we detected m6A levels in normal, eutopic, and ectopic endometrium and found the m6A levels decreased in eutopic and ectopic endometrium compared with normal endometrium. In addition, we proved that methyltransferase-like 3 (METTL3) downregulation accounted for m6A reduction in endometriosis. Furthermore, we observed that METTL3 knockdown facilitated the migration and invasion of human endometrial stromal cells (HESCs), whereas METTL3 overexpression exerted opposite effects, suggesting that METTL3 downregulation might contribute to endometriosis development by enhancing cellular migration and invasion. Mechanistically, METTL3-dependent m6A was involved in the DGCR8-mediated maturation of primary microRNA126 (miR126 and pri-miR126). Moreover, miR126 inhibitor significantly enhanced the migration and invasion of METTL3-overexpressing HESCs, whereas miR126 mimics attenuated the migration and invasion of METTL3-silenced HESCs. Our study revealed the METTL3/m6A/miR126 pathway, whose inhibition might contribute to endometriosis development by enhancing cellular migration and invasion. It also showed that METTL3 might be a novel diagnostic biomarker and therapeutic target for endometriosis.
Keywords: endometriosis, methyltransferase-like 3 (METTL3), N6-methyladenosine (m6A), primary microRNA126 (pri-miR126), microRNA126 (miR126)
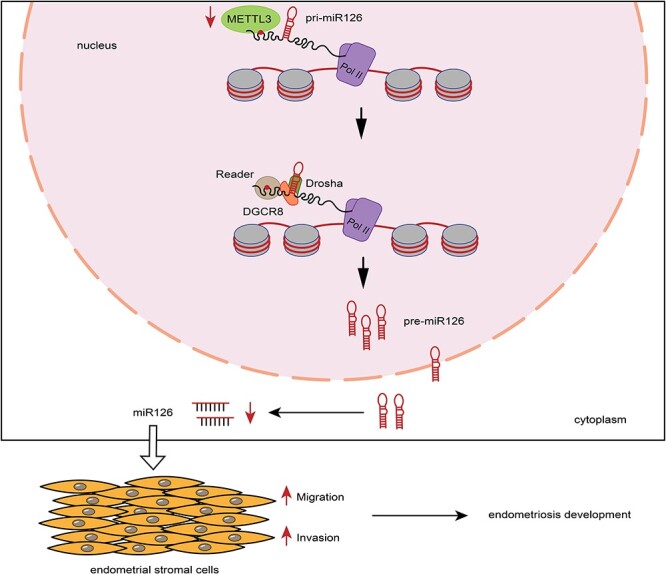
Suppressive METTL3 enhances cell migration and invasion by attenuating DGCR8-mediated maturation of pri-miR126 in an m6A-dependent manner, thus contributing to endometriosis development.
Graphical Abstract
Graphical Abstract.
Introduction
Endometriosis, defined as the presence of endometrial-like tissue within extra-uterine sites [1], is associated with chronic pelvic pain and female infertility and causes negative quality of life of patients, sexual dysfunction, and unsatisfactory personal relationships [2–4]. It affects ~200 million women worldwide, with >10% of women of reproductive age [5]. It is a benign disease but similar to malignancy in certain aspects, such as migration and invasion [6], thus contributing to endometriosis development and resulting in the postsurgical recurrence of patients. Despite multifactorial etiologies in endometriosis in the last few decades [7–12], the pathogenesis of endometriosis remains unclear.
N 6-methyladenosine (m6A) is one of the most abundant RNA modifications [13], with a dynamic and reversible regulatory feature, similar to DNA methylation. Modification of m6A is catalyzed by a methyltransferase complex composed of two subcomplexes, methyltransferase-like 3 (METTL3) and methyltransferase-like 14 (METTL14), and other components, such as WTAP, RBM15, VIRMA, ZC3H13, and HAKAI [14]. As for demethylases, two molecules, fat mass and obesity-associated protein (FTO; [15]) and alkB homolog 5 (ALKBH5; [16]), are required for demethylation. The changes in balance among m6A methyltransferases and demethylases are involved in the dynamic regulation of m6A. Increasing evidence shows that m6A plays a crucial role in developing diseases, especially in tumor metastasis. METTL3 functions as an oncogene to promote cell growth, invasion, and survival in lung cancer by increasing the expression of epidermal growth factor receptor (EGFR) and transcriptional co-activator with PDZ-binding motif (TAZ) [17]. METTL14 downregulation contributes to the metastasis of hepatocellular carcinoma by regulating m6A-dependent primary microRNA (pri-miRNA) processing [18]. However, the status of m6A and the m6A-associated mechanisms involved in endometriosis development remain unknown and need to be further studied.
This study aimed to clarify whether m6A levels differed among normal control, eutopic, and ectopic endometrium and to elucidate the catalytic mediator of m6A and underlying molecular mechanisms involved in endometriosis development. The study revealed the importance of METTL3/m6A in endometriosis development and suggested that METTL3 might be a novel target in the diagnosis and therapy with respect to endometriosis.
Materials and methods
Patients and tissue collection
The local ethics committee of Union Hospital, Tongji Medical College, Huazhong University of Science and Technology approved the study. Human tissues were collected with the written informed consent of patients, following the guidelines of the Declaration of Helsinki.
A total of 50 cases of normal control endometrium (NC) were curetted from patients without endometriosis, who visited for tubal infertility. Also, 25 cases of eutopic endometrium (EU) and 23 cases of ectopic endometriosis (EC) were obtained from patients with ovarian endometriosis in stages III and IV [19]. No strict match was found between eutopic and ectopic endometrium. All tissues were obtained during the proliferative phase of the menstrual cycle, and the menstrual cycle phases were established based on their menstrual history and endometrial histology by an independent pathologist. All patients were premenopausal and had regular menstrual cycles. None of the patients had received any hormonal treatments for at least 3 months.
RNA m6A quantitative assays
The m6A levels in total RNAs were detected using an EpiQuik m6A RNA Methylation Quantification Kit (Epigentek, NY, USA). Briefly, RNAs were added to the strip wells with RNA high binding solution. Capture antibody solution and detection antibody solution were then added to the wells separately in a suitable diluted concentration. The m6A levels were then quantified colorimetrically by reading the absorbance of each well at a wavelength of 450 nm. The data were calculated using relative quantification.
RNA extraction and quantitative real-time polymerase chain reaction
Total RNAs, including mRNAs, microRNA (miRNAs), and pri-miRNAs, were extracted from tissues and cells with TRIzol (TaKaRa, Tokyo, Japan). The cDNAs were synthesized from mRNAs and pri-miRNAs using PrimeScript RT Master Mix (Takara, Tokyo, Japan) and quantitative real-time polymerase chain reaction (qRT-PCR) was conducted with SyberGreen qPCR master mix (DBI, Ludwigshafen, Germany). Besides, the cDNA of miRNAs was synthesized and quantified using an miDETECT A Track miRNA qRT-PCR Starter Kit (Ribobio, Guangzhou, China). All data were analyzed by 2−ΔΔCt methods. Supplemental Table S1 presents the primer sequences.
Western blot analysis
The protein was routinely extracted using radio-immunoprecipitation assay buffer (Beyotime, Shanghai, China). Then the protein concentration was quantified using a BCA protein assay kit (Beyotime, Shanghai, China). Equal amounts of protein (40 μg) were resolved by 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis and transferred onto polyvinyl difluoride membranes (Millipore, MA, USA). After blocking with 5% skimmed milk in Tris-buffered saline containing 0.1% Tween-20 for 1 h, the membranes were incubated with primary antibodies against METTL3 (0.4 μg/mL, Proteintech, Wuhan, China), METTL14 (3.51 μg/mL, Abclonal, Wuhan, China), FTO (1.13 μg/mL, Abclonal, Wuhan, China), ALKBH5 (1.7625 μg/mL, Abclonal, Wuhan, China), and GAPDH (0.25 μg/mL, Proteintech, Wuhan, China) overnight at 4°C. After washing, the membranes were incubated with anti-rabbit antibody (0.25 μg/mL, Affinity Biosciences, OH, USA) or anti-mouse antibody (0.25 μg/mL, Affinity Biosciences, OH, USA) at 25°C for 1 h. After washing, the membranes were visualized using the detection system following incubation with enhanced ECL detection reagent (Millipore, MA, USA). Then the gray value of protein bands was analyzed using ImageJ software.
Immunohistochemistry
All tissues were immediately placed into 4% buffered formalin for immunohistochemistry (IHC). Servicebio Biotechnology Co., Ltd (Wuhan, China) was employed for paraffin embedding, paraffin sectioning, and IHC staining. IHC staining was performed with primary antibody against METTL3 (1.5 μg/mL, Proteintech, Wuhan, China) on paraffin sections. Finally, Image Pro Plus software was used to analyze the data.
Cell culture and transfection
Human endometrial stromal cells (HESCs; ATCC, VA, USA) were cultured in Dulbecco modified Eagle medium (DMEM)/F12 medium (Gibco, CA, USA) with 10% fetal bovine serum (FBS, Gibco) and maintained in a humidified 5% CO2 incubator at 37°C.
Qijing Company (Wuhan, China) completed the construction and design of small interfering RNAs (siRNA), microRNA-126 (miR126) mimics, miR126 inhibitor, and miR-NC. Hanbio Company (Shanghai, China) completed the construction and design of METTL3-overexpressing plasmid and negative control. They were transfected into HESCs using Lipofectamine 3000 (Invitrogen, CA, USA) following the manufacturer’s protocol. The sequences are presented in Supplemental Table S2 and Material S3.
RNA m6A dot blot assays
VAHTS mRNA Capture Beads (Vazyme, Nanjing, China) were used to collect the poly(A)+ RNAs from the isolated total RNAs. The diluted poly(A)+ RNAs were spotted onto N+ nylon membranes (GE Healthcare, MD, USA). After ultraviolet cross-linking, the membranes were blocked with 5% fat-free milk in Tris-buffered saline containing 0.1% Tween-20 for 1 h, and then incubated with an anti-m6A antibody (3.09 μg/mL, Abclonal, Wuhan, China) overnight at 4°C. After washing, the membranes were incubated with an anti-rabbit antibody (0.25 μg/mL, Affinity Biosciences, OH, USA) for 1 h at 25°C. After further washing, the membranes were incubated with enhanced ECL detection reagent (Millipore, MA, USA) and visualized using the detection system. Equal amounts of poly(A)+ RNAs were spotted on the other membranes, stained with 0.02% methylene blue, and washed with ribonuclease (RNase)-free water.
Transwell assays
Transwell chambers (Corning, NY, USA) were used to perform these experiments. For cell invasion, Matrigel (Becton, Dickinson and Company, NJ, USA) was diluted with FBS-free DMEM/F12 medium in a ratio of 1:3 and then loaded into the upper chamber. However, Matrigel was not required for cell migration. Further, 8 × 104 cells were resuspended in 200 μL of serum-free DMEM/F12 medium and then plated into the upper chamber, and 600 μL of 20% FBS-containing medium was added to the lower chamber. After 24 h, the cells on the underside of the membrane were fixed, stained, and subsequently counted under a microscope in five random high-power fields.
Co-immunoprecipitation assays
HESCs were washed with pre-chilled phosphate-buffered saline and then lysed with NP-40 lysis buffer (Boster, Wuhan, China). After centrifugation, the supernatant was collected. Subsequently, protein A/G beads were added into the supernatant and rotated for 10 min at 4°C. After beads were separated magnetically, the protein content of the supernatant was quantified with a BCA protein assay kit (Beyotime, Shanghai, China). Afterward, an anti-METTL3 antibody (Proteintech, Wuhan, China) was used to perform immunoprecipitation. Protein A/G beads were added to capture the anti-METTL3 antibody. After washing, RNase was added to the immunoprecipitated complex for 15 min at 37°C. Subsequently, beads were separated magnetically and boiled in the loading buffer for 10 min at 95°C. Finally, a primary antibody against DiGeorge critical region 8 (DGCR8; 0.7 μg/mL, Proteintech, Wuhan, China) was used to perform western blot analysis.
RNA immunoprecipitation-PCR
HESCs transfected with the negative control and METTL3-overexpressing vector were ultraviolet-irradiated and lysed with lysis buffer (Bersinbio RIP Kit, Guangzhou, China). Immunoprecipitation of endogenous DGCR8 was performed using an anti-DGCR8 antibody (Proteintech, Wuhan, China) overnight at 4°C. Then protein A/G beads were added to capture the anti-DGCR8 antibody. After washing, proteinase K was added to the immunoprecipitated complex to remove the excess proteins. Then RNAs were extracted using TRIzol and quantified by qRT-PCR using primers for primary microRNA-126 (pri-miR126) and normalizing to input.
Methylated RNA immunoprecipitation-PCR
Similar to RNA immunoprecipitation (RIP), the RNAs of HESCs transfected with negative control and METTL3-overexpressing vector were isolated and fragmented by ultrasonication for 1.5 min. An anti-m6A antibody (Abclonal, Wuhan, China) was used to perform immunoprecipitations. Protein A/G beads were then added to capture the anti-m6A antibody. After washing, proteinase K was added to the immunoprecipitated complex to remove the excess proteins. Then RNAs were extracted using TRIzol and quantified by qRT-PCR using primers for pri-miR126 and normalizing to input.
Statistical analysis
GraphPad Prism 7 was used for statistical analysis. All data were presented as mean ± SEM. All experiments were repeated in triplicate or quadruplicate. For data variables with normal distribution, the Student t-test was used to analyze differences between the two groups and one-way analysis of variance was performed to analyze differences between multiple groups. For non-normally distributed data, the Mann–Whitney test was used for two groups, whereas the Kruskal–Wallis test was used for multiple groups. Statistical significance was defined as P < 0.05.
Results
Downregulation of METTL3-mediated m6A modification in endometriosis
To investigate the role of m6A modification in endometriosis, we first measured the m6A levels in NC (n = 50), EU (n = 25), and EC (n = 23) by a colorimetric method. The results showed that the m6A levels were downregulated in EU and EC compared with NC. No significant difference was found between EU and EC in m6A levels (Figure 1A). METTL3 and METTL14 are the core components of the m6A methyltransferase complex, involved in the formation of m6A [14]. The FTO [15] and ALKBH5 [16], as demethylases, are involved in demethylation. Therefore, we measured the expression of these m6A-associated genes in NC, EU, and EC by qRT-PCR and western blot analysis to determine the key molecule that resulted in m6A downregulation in endometriosis. The results showed that the mRNA and protein levels of METTL3 were both downregulated in EU and EC compared with NC, with no difference between EU and EC (Figure 1B and C). These findings were completely consistent with the changes in m6A, suggesting a potential role of METTL3 for m6A formation in endometriosis. Besides, we observed that METTL14 had no difference in mRNA and protein expression among these tissues (Figure 1B and D). Both the mRNA and protein levels of ALKBH5 were upregulated in EC compared with NC and EU (Figure 1B and E). Contrary to ALKBH5, the mRNA and protein expression of FTO was downregulated in EC compared with NC and EU (Figure 1B and F). Neither ALKBH5 nor FTO had a significant difference in mRNA and protein levels between NC and EU. Overall, METTL3, which exhibited a positive and completely consistent correlation with m6A, was selected for in-depth studies to explore the function of m6A in endometriosis. For further confirmation, we also detected METTL3 expression in NC, EU, and EC by IHC. The results showed that METTL3 levels were also downregulated in EU and EC compared with NC (Figure 1G), similar to the results of qRT-PCR and western blot analysis. In addition, we observed that METTL3 was expressed in the nuclei of epithelial and stromal cells (Figure 1G).
Figure 1.
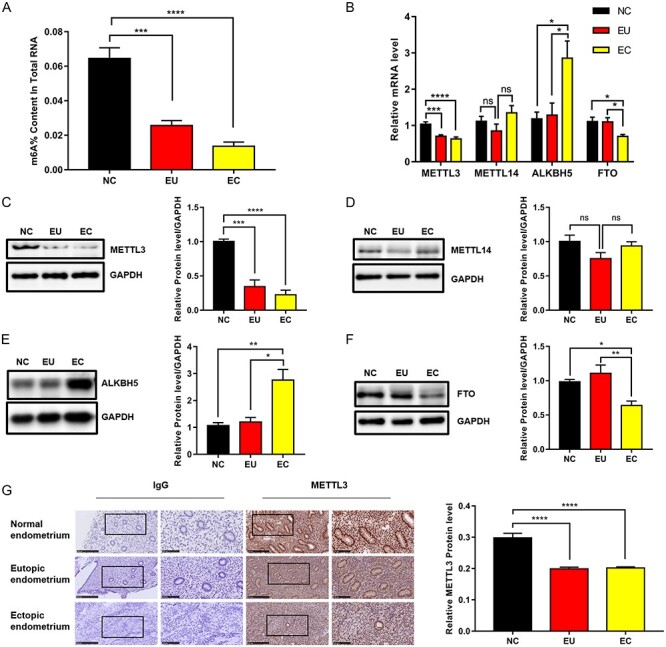
M6A levels were reduced in endometriosis and downregulated METTL3 accounted for aberrant m6A modification. (A) M6A levels in normal control endometrium (NC; n = 50), EU (n = 25), and ectopic endometrium (EC; n = 23). (B) mRNA levels of m6A-associated genes in the tissues detected by qRT-PCR. NC (n = 16), EU (n = 11), and EC (n = 11). (C–F) Protein levels of m6A-associated genes in the tissues detected by western blot analysis. NC (n = 8), EU (n = 8), and EC (n = 8). (G) Protein levels of METTL3 in the tissues detected by immunohistochemical analysis. NC (n = 7), EU (n = 7), and EC (n = 7). All experiments were repeated in triplicate or quadruplicate. Data with error bars are presented to indicate the mean ± SEM values. *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001.
To further confirm the correlation between METTL3 and m6A in endometriosis, we carried out m6A dot blot assays on the poly(A)+ RNAs, which were extracted from HESCs transfected with siRNAs of METTL3 and METTL3-overexpressing vector, respectively. The results showed that METTL3 was effectively overexpressed (Figure 2A and C) and silenced (Figure 2B and D) in mRNA and protein levels. After assessing the knockdown efficacy of siRNAs targeting METTL3, si-METTL3-3 was chosen for subsequent studies as it exhibited the best knockdown efficacy to METTL3 (Figure 2B). As expected, METTL3 overexpression increased the m6A levels compared with negative control, whereas METTL3 knockdown decreased the m6A levels (Figure 2E), suggesting that METTL3 truly accounted for m6A downregulation in endometriosis.
Figure 2.
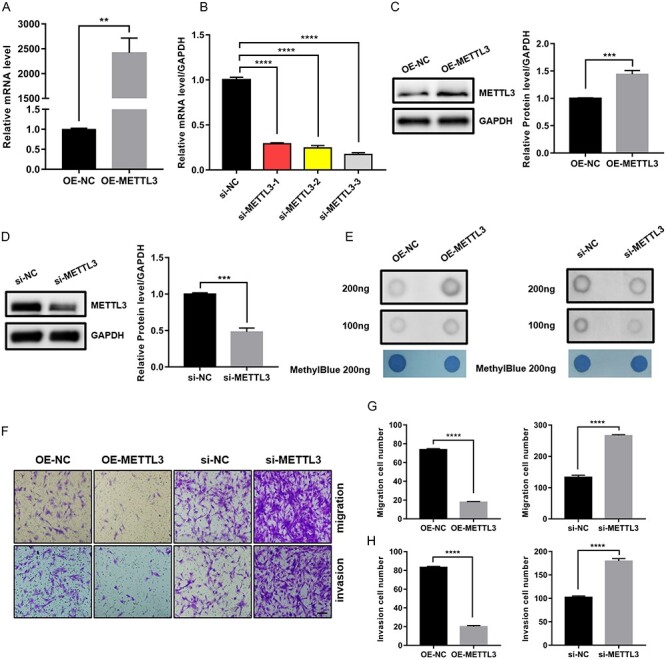
METTL3 regulated the migration and invasion of HESCs. (A and B) Overexpression and knockdown efficacy of METTL3 at mRNA levels in HESCs (si-METTL3-3 was chosen to perform in subsequent studies as it exhibited the best knockdown efficacy to METTL3). (C and D) Overexpression and knockdown efficacy of METTL3 at protein levels in HESCs. (E) Dot blot assays were used to detect the m6A changes upon METTL3 overexpression or knockdown in HESCs. Methylene blue stain was performed as control. (F–H) Transwell assays were used to evaluate the migrating and invasive abilities of HESCs upon METTL3 overexpression or knockdown (scale bar = 100 μm). The number of migrating and invasive cells is presented on the right. All experiments were repeated in triplicate or quadruplicate. Data with error bars are presented to indicate the mean ± SEM values. **P < 0.01, ***P < 0.001, and ****P < 0.0001.
METTL3-regulated migration and invasion of HESCs
Next, given the reports that METTL3 was involved in regulating cell migration and invasion [20–22], we wondered about the effect of METTL3 on the migration and invasion of endometrial stromal cells. Therefore, we examined the migrating and invasive abilities of HESCs on METTL3 overexpression and knockdown using Transwell assays. The results showed that METTL3 overexpression inhibited the migration and invasion of HESCs, whereas METTL3 knockdown promoted the migration and invasion of HESCs (Figure 2F–H), suggesting that METTL3 was involved in regulating cell migration and invasion.
DGCR8-mediated maturation of pri-miR126 by METTL3 modulation in an m6A-dependent manner
We planned to determine the potential mechanisms by which METTL3 regulated cellular migration and invasion. Various studies have pointed out the vital role of miRNAs in regulating the migration and invasion of endometrial stromal cells [23–25]. Moreover, studies demonstrated that METTL3 could label pri-miRNAs with m6A tags and then promoted the maturation of pri-miRNAs by regulating the combination of DGCR8, a microprocessor for RNA processing, with pri-miRNAs in an m6A-dependent manner [26, 27]. Thus, we proposed a hypothesis that METTL3 might regulate the DGCR8-mediated maturation of pri-miRNAs to miRNAs in an m6A-dependent manner, thus affecting cell migration and invasion. To test this hypothesis, our first step was to perform co-immunoprecipitation in HESCs using the anti-METTL3 antibody. The results showed that METTL3 could co-precipitate with DGCR8. Furthermore, we treated the immunoprecipitated complex with RNase. We found that the interrelationship between METTL3 and DGCR8 could be attenuated by RNase, suggesting that their interrelationship might be partly mediated by RNAs (Figure 3A). This finding implied that METTL3 might participate in the DGCR8-mediated maturation of pri-miRNAs in HESCs.
Figure 3.
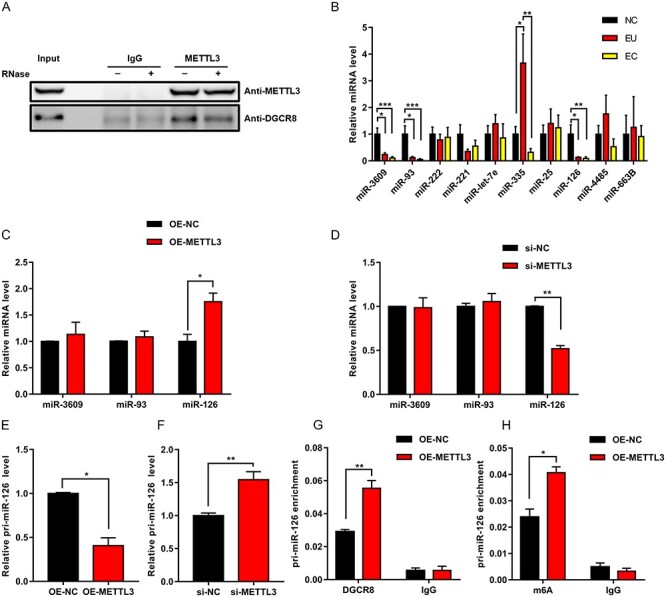
METTL3 regulated DGCR8-mediated maturation of pri-miR126 in an m6A-dependent manner. (A) Interaction between METTL3 and DGCR8 was evaluated by co-immunoprecipitation in HESCs, and immunoglobulin G (IgG) was used as control. Ribonuclease (RNase) was used as indicated. (B) Levels of miRNAs with m6A tags in the tissues. NC (n = 10), EU (n = 9), and EC (n = 9). (C and D) Levels of miRNAs were quantified by qRT-PCR in METTL3-overexpressing or METTL3-silenced HESCs. (E–F) Level of pri-miR126 was quantified by qRT-PCR in METTL3-overexpressing or METTL3-silenced HESCs. (G) RNA immunoprecipitation-PCR assays showed obvious enrichment of pri-miR126 bound to DGCR8 in METTL3-overexpressing HESCs. (H) Methylated RIP-PCR assays showed obvious enrichment of pri-miR126 with m6A in METTL3-overexpressing HESCs. All experiments were repeated in triplicate or quadruplicate. Data with error bars are presented to indicate the mean ± SEM values. *P < 0.05, **P < 0.01, and ***P < 0.001.
A previous study reported that a series of miRNAs with m6A tags in their pri-miRNAs were governed by METTL3 in an m6A-dependent manner, including miR3609, miR221/222, miR126, miR93, miR25, miR-let-7e, miR335, miR4485, and miR663B [26]. We wondered whether these miRNAs were differentially expressed in endometriosis and regulated by METTL3 in endometrial stromal cells. Thus, we first examined the expression levels of these miRNAs in NC, EU, and EC. The results showed that miR3609, miR93, and miR126 were downregulated in EU and EC compared with NC, with no difference in the expression levels of these miRNAs between EU and EC. In addition, miR335 was upregulated in EU compared with NC but downregulated in EC compared with EU. MiR335 had no difference in the expression level between NC and EC (Figure 3B). As we know, Alarcón et al. [26] demonstrated that METTL3 played a positive role in m6A-dependent processing of pri-miRNAs to miRNAs. However, our previous results showed that METTL3 was downregulated in EU and EC compared with NC. Therefore, we concluded that downregulated METTL3 would arrest the maturation of pri-miRNAs. Thus, candidate miRNAs should be miRNAs downregulated in EU and EC compared with NC. Given the aforementioned conclusion, we chose miR3609, miR93, and miR126 as the candidate miRNAs. Subsequently, we examined the expression levels of these three miRNAs by qRT-PCR on METTL3 overexpression or knockdown in HESCs to validate whether these three miRNAs were governed by METTL3 in endometrial stromal cells. The results showed that only miR126 expression significantly increased on METTL3 overexpression (Figure 3C), whereas it decreased on METTL3 knockdown (Figure 3D). Furthermore, we examined the pri-miR126 expression level on METTL3 overexpression and knockdown in HESCs. Then, we observed a decrease in pri-miR126 in METTL3-overexpressing cells (Figure 3E) and an accumulation of pri-miR126 in METTL3-silenced cells (Figure 3F), suggesting that METTL3 exerted a positive function in regulating the maturation of pri-miR126 in HESCs. RIP-PCR using the anti-DGCR8 antibody and MeRIP-PCR using the anti-m6A antibody were performed in HESCs to further test METTL3 regulation on pri-miR126. We observed that the level of pri-miR126 bound to DGCR8 and the level of pri-miR126 modified by m6A were both elevated in METTL3-overexpressing cells (Figure 3G and H), suggesting that METTL3-mediated m6A facilitated the recognition and combination of pri-miR126 by DGCR8, which subsequently promoted the processing of pri-miR126 to miR126. Taken together, we concluded that METTL3 modulated DGCR8-mediated maturation of pri-miR126 in an m6A-dependent manner.
Involvement of miR-126 in METTL3-mediated cell migration and invasion
Finally, we assessed the effects of miR126 on METTL3-mediated migration and invasion of HESCs. We transfected miR126 inhibitor into METTL3-overexpressing cells and transfected miR126 mimics into METTL3-silenced cells. As expected, miR126 inhibitor significantly restored the migrating and invasive abilities of METTL3-overexpressing cells, whereas miR126 mimics attenuated the migrating and invasive abilities of METTL3-silenced cells (Figure 4A and B).
Figure 4.
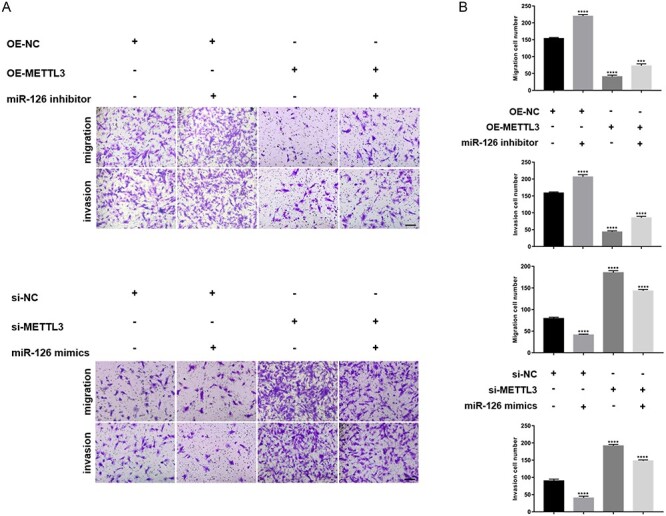
MiR-126 was involved in METTL3-mediated cell migration and invasion. (A) Migrating and invasive abilities of HESCs transfected with the negative control and METTL3-overexpressing vector were evaluated in the absence or presence of miR126 inhibitor (the panel above, scale bar = 100 μm). The migrating and invasive abilities of HESCs transfected with the negative control and siRNAs targeting METTL3 were evaluated in the absence or presence of miR126 mimics (the panel below, scale bar = 100 μm). (B) Number of migrating and invasive cells was presented. All experiments were repeated in triplicate or quadruplicate. Data with error bars are presented to indicate the mean ± SEM values. ***P < 0.001, and ****P < 0.0001.
Based on the results, we concluded that METTL3 downregulation reduced the m6A level of pri-miR126, thus attenuating DGCR8-mediated maturation of pri-miR126 to miR126, which enhanced the migration and invasion of endometrial stromal cells, thus contributing to endometriosis development (Figure 5).
Figure 5.
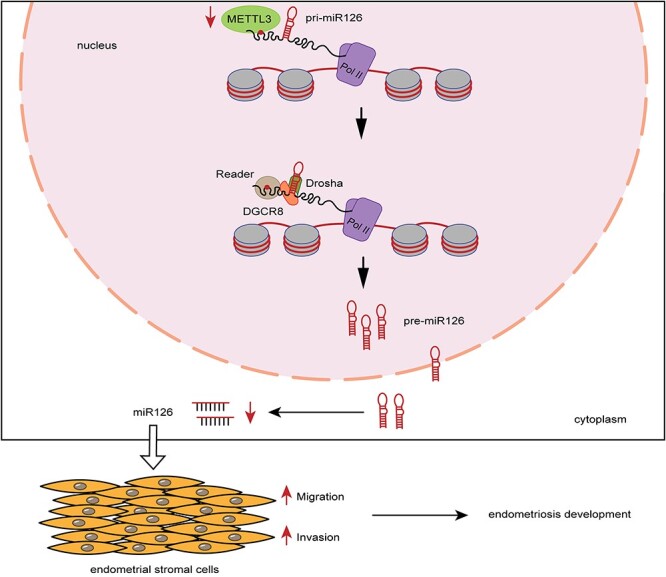
Model of a pattern of METTL3 in regulating endometriosis. METTL3 downregulation reduced the m6A level of pri-miR126, thus attenuating the maturation of pri-miR126, which enhanced the migration and invasion of endometrial stromal cells, thus contributing to endometriosis development.
Discussion
The present study demonstrated that the inhibition of METTL3/m6A/miR126 promoted the migration and invasion of endometrial stromal cells in endometriosis. The major findings were as follows: (i) m6A levels in endometriosis were downregulated, and METTL3 was the key cause of m6A downregulation in endometriosis; (ii) METTL3 regulated the migration and invasion of HESCs; (iii) METTL3 modulated DGCR8-mediated maturation of pri-miR126 in an m6A-dependent manner; and (iv) MiR-126 was involved in METTL3-mediated cell migration and invasion.
M6A modification, one of the most abundant RNA modifications [13], is involved in many cellular processes, such as RNA transcription [28], splicing [29–31], RNA stability [32–34], protein translation [17, 35, 36], and RNA processing [26, 27], all of which, if abnormal, can cause diseases. Despite the crucial role of m6A in developing diseases, little is known regarding m6A-associated cellular and molecular events involved in endometriosis development.
As we all know, the most well-accepted pathophysiological hypothesis for endometriosis is based on retrograde menstruation. However, Sampson hypothesis is a plausible mechanism for most endometriotic lesions; however, it does not explain why endometriosis only develops in some, but not in all, women who have reflux menstruation into the peritoneal cavity [37]. To further answer the question, many new hypotheses have been put forward over the past decades. Among these hypotheses, we noticed the importance of EU in endometriosis. Studies have confirmed that EU in women with endometriosis exhibits multiple cellular and molecular abnormalities [1, 38–42]. The pre-existing endometrial abnormalities enhance the implantation and growth of these pathological tissues upon attachment to the peritoneum or the ovary [43–45]. According to this hypothesis, we examined the expression of m6A and m6A-associated genes in eutopic and ectopic endometrium of ovarian endometriosis as well as normal endometrium from the control group. Then, we found m6A downregulation in eutopic and ectopic tissues compared with the normal group. Meanwhile, METTL3 was identified as the key cause for m6A downregulation. METTL3, as the core active component of the multicomponent m6A methyltransferase complex, exerts multiple functions in various types of cancers, including regulating the pluripotency of cancer stem cells, cancer proliferation, cancer metastasis, and tumor immunity [46, 47]. Here, in our study, we used HESCs to investigate the function of METTL3 in cellular migration and invasion. We observed that the overexpression and knockdown of METTL3 in endometrial stromal cells originating from normal endometrium exerted a negative effect on cellular migration and invasion. Therefore, we suggested that METTL3 downregulation in EU rendered endometrial stromal cells stronger migrating and invasive abilities, which enhanced their implantation and growth upon attachment to the ovary, thus contributing to endometriosis development.
Of note was the finding that METTL14 had no significant difference in expression among these tissues. Initially, METTL14 was regarded as the second methyltransferase enzyme that could mediate the formation of different m6A sites in the transcriptome [48]. However, three separate crystallization studies overturned this concept [49–51]. They suggested that METTL14 was catalytically inactive but played a stabilization role for METTL3 and enhanced its enzymatic activity by forming a complex with METTL3; this complex finally provided a platform for the recognition and binding of substrate RNA [52, 53]. Thus, METTL14 might function as a pseudomethyltransferase required for METTL3 activity in endometriosis. In addition, ALKBH5 and FTO are the only two known m6A demethylases, belonging to the AlkB family of Fe(II)/a-ketoglutarate-dependent dioxygenases [15, 16], sharing a similar catalytic core but exerting different substrate preferences [54, 55]. Our findings showed a partly consistent trend of ALKBH5 with m6A, suggesting that ALKBH5 was possibly involved in dynamically regulating m6A in endometriosis. Meanwhile, FTO showed an opposite trend to m6A in ectopic lesions compared with normal tissues, suggesting that FTO might exhibit other functions in endometriosis, rather than regulating m6A demethylation. For example, a previous study reported that FTO preferentially demethylated N6,2′-O-dimethyladenosine (m6Am) rather than m6A, and reduced the stability of m6Am mRNAs [56]. As mentioned earlier, further studies are needed to clarify the role of other m6A-associated molecules in endometriosis.
Furthermore, we investigated the potential mechanism of METTL3 regulating cell migration and invasion. Then, we first identified miR126 as the target of METTL3. Many studies showed the importance of miR126 in endometriosis [57, 58]. For example, a previous study pointed miR126 downregulation in endometriosis and that miR126 might play an initial role in the development and progression of endometriosis [59]. Subsequently, we demonstrated that the METTL3/m6A/miR126 axis, in which METTL3-mediated m6A was involved in DGCR8-mediated maturation of pri-miR126 to miR126, was inhibited and thus promoted the migration and invasion of endometrial stromal cells. Of note were the studies showing that the METTL3-m6A-miRNA model was established in other diseases similarly [22, 60]. For example, METTL3 played an oncogenic role in bladder cancer through modulating the m6A-dependent pri-miR221/222 process [60]. In addition, we noticed that some downstream targets, mainly the target genes of miRNAs, were added to this model in tumors [22, 61]. For example, upregulated METTL3-mediated miR126 promoted ovarian cancer progression via the PTEN-mediated PI3K/AKT/ mTOR pathway [61]. Thus, we were curious about some new factors regulated by the METTL3/m6A/miR126 axis in endometriosis. A previous study reported that miR-126 negatively regulated the expression of BCAR3 and its effect on the migration and invasion of endometrial stromal cells [57]. In addition, long noncoding RNA (lncRNAs) MALAT1-mediated miR126 inhibited the apoptosis of endometrial stromal cells via activating the CREB1-mediated PI3K/AKT pathway [62]. Therefore, we proposed a hypothesis that functional downstream genes or pathways of the METTL3/m6A/miR126 axis might exist and hence more studies needed to be performed to test the hypothesis in the future. Moreover, m6A exists in many other pri-miRNAs, but we merely identified pri-miR126 as a target. Thus, it will be intriguing and essential for us to identify other pri-miRNAs in the future. Similarly, m6A also exists in lncRNAs [63] and circular RNAs (circRNAs) [64], suggesting that not only miRNAs but also lncRNAs and circRNAs might be regulated by m6A in endometriosis. Thus, further studies are needed to identify the possible lncRNAs and circRNAs regulated by m6A in endometriosis.
Although we only explored the effect of METTL3 on the endometrial stromal cells, examining the effect of METTL3 on endometrial epithelial cells remains very important. Until now, various studies have demonstrated that endometrial epithelial cells derived from patients with endometriosis exhibit some abnormal biological functions, including decreased apoptosis [65, 66], increased proliferation [67–69], and so on. Interestingly, METTL3 was proved to be involved in regulating these biological functions [70, 71], suggesting that METTL3 might contribute to endometriosis development via affecting these functions of endometrial epithelial cells. Moreover, our group previously demonstrated that the epithelial-mesenchymal transition (EMT) played a crucial role in endometriosis development [72, 73]. The EMT is characterized by the transformation from a polarized and epithelial phenotype to a highly motile fibroblastoid or mesenchymal phenotype [74]. Recently, accumulating evidence shows that METTL3 is associated with EMT. In lung cancer, METTL3 is indispensable for TGF-β-induced EMT of lung cancer cells through the regulation of JUNB [75]. In gastric cancer, METTL3-mediated m6A modification promotes the EMT process and metastasis via the METTL3/ZMYM1/E-cadherin axis [76]. Thus, we hypothesized that METTL3 might be a core molecule affecting the EMT of endometrial epithelial cells in endometriosis, thus contributing to disease progression. Consequently, investigating the effect of METTL3 on endometrial epithelial cells is urgently needed in the future.
As we only focused on ovarian endometriosis in this study, we did not include the roles of METTL3 in other types of endometriosis. In addition, we quantified the m6A levels using only a single strategy; therefore, it might be better to add more strategies to quantify the m6A levels in the future. All in all, further studies are needed: (i) to use more approaches to quantify the m6A levels of tissues, such as mass spectrometry; (ii) to identify the target miRNAs (or lncRNAs or circRNAs) of METTL3/m6A through high-throughput analysis and further study the downstream target genes and related regulatory pathways of the METTL3/m6A/miR126 axis; (iii) to clear the possible role and mechanism of ALKBH5 and FTO in endometriosis; (iv) to further study the potential role of METTL3 in endometrial epithelial cells; and (v) to expand the types of tissues and conclude the function of m6A in other types of endometriosis.
In summary, our research delineated an METTL3-m6A model, whose downregulation enhanced the migration and invasion of endometrial stromal cells by attenuating DGCR8-mediated maturation of pri-miR126, thus contributing to endometriosis development. Our current findings provided a novel sight that METTL3 might be a possible biomarker for noninvasive diagnosis and a therapeutic target to suppress endometriosis development.
Data availability
The data underlying this article will be shared on reasonable request to the corresponding author.
Authors’ contributions
Y. L., L. Z., and X. L. (Xiaoou Li) designed the study and X. L. (Xiaoou Li) performed the experiments, analyzed the data, and drafted the manuscript. W. X., X. L., (Xuefeng Long), X. D., Y. P., and Y. X. collected tissue samples. Z. Z., L. Z., and Y. L. assisted in revision of the manuscript. All authors have approved the final version of the manuscript.
Supplementary Material
Acknowledgements
The authors thank all the patients who agreed to participate in this study.
Conflict of Interest: The authors declare no conflicts of interest.
Footnotes
† Grant Support: This study was financially supported by the National Natural Science Foundation of China (grant numbers: 82071722, 81701417, 81974242, and 81901460).
Contributor Information
Xiaoou Li, Department of Obstetrics and Gynecology, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, Hubei, China.
Wenqian Xiong, Department of Obstetrics and Gynecology, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, Hubei, China.
Xuefeng Long, Department of Obstetrics and Gynecology, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, Hubei, China.
Xin Dai, Department of Obstetrics and Gynecology, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, Hubei, China.
Yuan Peng, Department of Obstetrics and Gynecology, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, Hubei, China.
Ying Xu, Department of Obstetrics and Gynecology, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, Hubei, China.
Zhibing Zhang, Department of Physiology, Wayne State University, Detroit, MI, USA.
Ling Zhang, Department of Obstetrics and Gynecology, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, Hubei, China.
Yi Liu, Department of Obstetrics and Gynecology, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, Hubei, China.
References
- 1. Giudice LC, Kao LC. Endometriosis. Lancet 2004; 364:1789–1799. [DOI] [PubMed] [Google Scholar]
- 2. Nnoaham KE, Hummelshoj L, Webster P, d'Hooghe T, de Cicco NF, de Cicco NC, Jenkinson C, Kennedy SH, Zondervan KT. Impact of endometriosis on quality of life and work productivity: a multicenter study across ten countries. Fertil Steril 2011; 96:366–373.e368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Pluchino N, Wenger JM, Petignat P, Tal R, Bolmont M, Taylor HS, Bianchi-Demicheli F. Sexual function in endometriosis patients and their partners: effect of the disease and consequences of treatment. Hum Reprod Update 2016; 22:762–774. [DOI] [PubMed] [Google Scholar]
- 4. Simoens S, Dunselman G, Dirksen C, Hummelshoj L, Bokor A, Brandes I, Brodszky V, Canis M, Colombo GL, DeLeire T, Falcone T, Graham B et al. The burden of endometriosis: costs and quality of life of women with endometriosis and treated in referral centres. Hum Reprod 2012; 27:1292–1299. [DOI] [PubMed] [Google Scholar]
- 5. As-Sanie S, Black R, Giudice LC, Gray Valbrun T, Gupta J, Jones B, Laufer MR, Milspaw AT, Missmer SA, Norman A, Taylor RN, Wallace K et al. Assessing research gaps and unmet needs in endometriosis. Am J Obstet Gynecol 2019; 221:86–94. [DOI] [PubMed] [Google Scholar]
- 6. Swiersz LM. Role of endometriosis in cancer and tumor development. Ann N Y Acad Sci 2002; 955:281–292 discussion 293-285, 396-406. [DOI] [PubMed] [Google Scholar]
- 7. Sampson JA. Metastatic or embolic endometriosis, due to the menstrual dissemination of endometrial tissue into the venous circulation. Am J Pathol 1927; 3:93–110.143. [PMC free article] [PubMed] [Google Scholar]
- 8. Batt RE, Smith RA, Buck Louis GM, Martin DC, Chapron C, Koninckx PR, Yeh J. Müllerianosis. Histol Histopathol 2007; 22:1161–1166. [DOI] [PubMed] [Google Scholar]
- 9. Batt RE, Yeh J. Müllerianosis: four developmental (embryonic) mullerian diseases. Reprod Sci 2013; 20:1030–1037. [DOI] [PubMed] [Google Scholar]
- 10. Hufnagel D, Li F, Cosar E, Krikun G, Taylor HS. The role of stem cells in the etiology and pathophysiology of endometriosis. Semin Reprod Med 2015; 33:333–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jerman LF, Hey-Cunningham AJ. The role of the lymphatic system in endometriosis: a comprehensive review of the literature. Biol Reprod 2015; 92:64. [DOI] [PubMed] [Google Scholar]
- 12. Vercellini P, Viganò P, Somigliana E, Fedele L. Endometriosis: pathogenesis and treatment. Nat Rev Endocrinol 2014; 10:261–275. [DOI] [PubMed] [Google Scholar]
- 13. Rottman F, Shatkin AJ, Perry RP. Sequences containing methylated nucleotides at the 5′ termini of messenger RNAs: possible implications for processing. Cell 1974; 3:197–199. [DOI] [PubMed] [Google Scholar]
- 14. Knuckles P, Lence T, Haussmann IU, Jacob D, Kreim N, Carl SH, Masiello I, Hares T, Villaseñor R, Hess D, Andrade-Navarro MA, Biggiogera M et al. Zc3h13/Flacc is required for adenosine methylation by bridging the mRNA-binding factor Rbm15/Spenito to the m(6)A machinery component Wtap/Fl(2)d. Genes Dev 2018; 32:415–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jia G, Fu Y, Zhao X, Dai Q, Zheng G, Yang Y, Yi C, Lindahl T, Pan T, Yang YG, He C. N6-methyladenosine in nuclear RNA is a major substrate of the obesity-associated FTO. Nat Chem Biol 2011; 7:885–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zheng G, Dahl JA, Niu Y, Fedorcsak P, Huang CM, Li CJ, Vågbø CB, Shi Y, Wang WL, Song SH, Lu Z, Bosmans RP et al. ALKBH5 is a mammalian RNA demethylase that impacts RNA metabolism and mouse fertility. Mol Cell 2013; 49:18–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lin S, Choe J, Du P, Triboulet R, Gregory RI. The m(6)A methyltransferase METTL3 promotes translation in human cancer cells. Mol Cell 2016; 62:335–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ma JZ, Yang F, Zhou CC, Liu F, Yuan JH, Wang F, Wang TT, Xu QG, Zhou WP, Sun SH. METTL14 suppresses the metastatic potential of hepatocellular carcinoma by modulating N(6) -methyladenosine-dependent primary MicroRNA processing. Hepatology 2017; 65:529–543. [DOI] [PubMed] [Google Scholar]
- 19. Revised American Society for Reproductive Medicine classification of endometriosis: 1996. Fertil Steril 1997; 67:817–821. [DOI] [PubMed] [Google Scholar]
- 20. Deng R, Cheng Y, Ye S, Zhang J, Huang R, Li P, Liu H, Deng Q, Wu X, Lan P, Deng Y. m(6)A methyltransferase METTL3 suppresses colorectal cancer proliferation and migration through p38/ERK pathways. Onco Targets Ther 2019; 12:4391–4402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Liu T, Yang S, Sui J, Xu SY, Cheng YP, Shen B, Zhang Y, Zhang XM, Yin LH, Pu YP, Liang GY. Dysregulated N6-methyladenosine methylation writer METTL3 contributes to the proliferation and migration of gastric cancer. J Cell Physiol 2020; 235:548–562. [DOI] [PubMed] [Google Scholar]
- 22. Peng W, Li J, Chen R, Gu Q, Yang P, Qian W, Ji D, Wang Q, Zhang Z, Tang J, Sun Y. Upregulated METTL3 promotes metastasis of colorectal Cancer via miR-1246/SPRED2/MAPK signaling pathway. J Exp Clin Cancer Res 2019; 38:393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cho S, Mutlu L, Zhou Y, Taylor HS. Aromatase inhibitor regulates let-7 expression and let-7f-induced cell migration in endometrial cells from women with endometriosis. Fertil Steril 2016; 106:673–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lv X, Chen P, Liu W. Down regulation of MiR-93 contributes to endometriosis through targeting MMP3 and VEGFA. Am J Cancer Res 2015; 5:1706–1717. [PMC free article] [PubMed] [Google Scholar]
- 25. Wang X, Ren R, Shao M, Lan J. MicroRNA16 inhibits endometrial stromal cell migration and invasion through suppression of the inhibitor of nuclear factorkappaB kinase subunit beta/nuclear factorkappaB pathway. Int J Mol Med 2020; 46:740–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Alarcón CR, Lee H, Goodarzi H, Halberg N, Tavazoie SF. N6-methyladenosine marks primary microRNAs for processing. Nature 2015; 519:482–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Alarcon CR, Goodarzi H, Lee H, Liu X, Tavazoie S, Tavazoie SF. HNRNPA2B1 is a mediator of m(6)A-dependent nuclear RNA processing events. Cell 2015; 162:1299–1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Barbieri I, Tzelepis K, Pandolfini L, Shi J, Millan-Zambrano G, Robson SC, Aspris D, Migliori V, Bannister AJ, Han N, De Braekeleer E, Ponstingl H et al. Promoter-bound METTL3 maintains myeloid leukaemia by m(6)A-dependent translation control. Nature 2017; 552:126–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Dominissini D, Moshitch-Moshkovitz S, Schwartz S, Salmon-Divon M, Ungar L, Osenberg S, Cesarkas K, Jacob-Hirsch J, Amariglio N, Kupiec M, Sorek R, Rechavi G. Topology of the human and mouse m6A RNA methylomes revealed by m6A-seq. Nature 2012; 485:201–206. [DOI] [PubMed] [Google Scholar]
- 30. Taketo K, Konno M, Asai A, Koseki J, Toratani M, Satoh T, Doki Y, Mori M, Ishii H, Ogawa K. The epitranscriptome m6A writer METTL3 promotes chemo- and radioresistance in pancreatic cancer cells. Int J Oncol 2018; 52:621–629. [DOI] [PubMed] [Google Scholar]
- 31. Horiuchi K, Kawamura T, Iwanari H, Ohashi R, Naito M, Kodama T, TJJoBC H. Identification of Wilms' tumor 1-associating protein complex and its role in alternative splicing and the cell cycle. J Biol Chem 2013; 288:33292–33302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Du H, Zhao Y, He J, Zhang Y, Xi H, Liu M, Ma J, LJNc W. YTHDF2 destabilizes m 6 A-containing RNA through direct recruitment of the CCR4–NOT deadenylase complex. Nat Commun 2016; 7:12626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Chen M, Wei L, Law CT, Tsang FH, Shen J, Cheng CL, Tsang LH, Ho DW, Chiu DK, Lee JM, Wong CC, Ng IO et al. RNA N6-methyladenosine methyltransferase-like 3 promotes liver cancer progression through YTHDF2-dependent posttranscriptional silencing of SOCS2. Hepatology 2018; 67:2254–2270. [DOI] [PubMed] [Google Scholar]
- 34. Shi HL, Wang X, Lu ZK, Zhao BXS, Ma HH, Hsu PJ, Liu C, He C. YTHDF3 facilitates translation and decay of N-6-methyladenosine-modified RNA. Cell Research 2017; 27:315–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Coots RA, Liu XM, Mao Y, Dong L, Zhou J, Wan J, Zhang X, Qian SB. m(6)A facilitates eIF4F-independent mRNA translation. Mol Cell 2017; 68:504–514 e507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wang X, Zhao BS, Roundtree IA, Lu Z, Han D, Ma H, Weng X, Chen K, Shi H, He CJC. N6-methyladenosine modulates messenger RNA translation efficiency. Cell 2015; 161:1388–1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Bulun SE. Endometriosis. N Engl J Med 2009; 360:268–279. [DOI] [PubMed] [Google Scholar]
- 38. Wu Y, Kajdacsy-Balla A, Strawn E, Basir Z, Halverson G, Jailwala P, Wang Y, Wang X, Ghosh S, Guo SW. Transcriptional characterizations of differences between eutopic and ectopic endometrium. Endocrinology 2006; 147:232–246. [DOI] [PubMed] [Google Scholar]
- 39. Hornung D, Ryan IP, Chao VA, Vigne JL, Schriock ED, Taylor RN. Immunolocalization and regulation of the chemokine RANTES in human endometrial and endometriosis tissues and cells. J Clin Endocrinol Metab 1997; 82:1621–1628. [DOI] [PubMed] [Google Scholar]
- 40. Osteen KG, Bruner KL, Sharpe-Timms KL. Steroid and growth factor regulation of matrix metalloproteinase expression and endometriosis. Semin Reprod Endocrinol 1996; 14:247–255. [DOI] [PubMed] [Google Scholar]
- 41. Kao LC, Germeyer A, Tulac S, Lobo S, Yang JP, Taylor RN, Osteen K, Lessey BA, Giudice LC. Expression profiling of endometrium from women with endometriosis reveals candidate genes for disease-based implantation failure and infertility. Endocrinology 2003; 144:2870–2881. [DOI] [PubMed] [Google Scholar]
- 42. Tseng JF, Ryan IP, Milam TD, Murai JT, Schriock ED, Landers DV, Taylor RN. Interleukin-6 secretion in vitro is up-regulated in ectopic and eutopic endometrial stromal cells from women with endometriosis. J Clin Endocrinol Metab 1996; 81:1118–1122. [DOI] [PubMed] [Google Scholar]
- 43. Nair AS, Nair HB, Lucidi RS, Kirchner AJ, Schenken RS, Tekmal RR, Witz CA. Modeling the early endometriotic lesion: mesothelium-endometrial cell co-culture increases endometrial invasion and alters mesothelial and endometrial gene transcription. Fertil Steril 2008; 90:1487–1495. [DOI] [PubMed] [Google Scholar]
- 44. Chapron C, Marcellin L, Borghese B, Santulli P. Rethinking mechanisms, diagnosis and management of endometriosis. Nat Rev Endocrinol 2019; 15:666–682. [DOI] [PubMed] [Google Scholar]
- 45. Bulun SE, Yilmaz BD, Sison C, Miyazaki K, Bernardi L, Liu S, Kohlmeier A, Yin P, Milad M, Wei J. Endometriosis. Endocr Rev 2019; 40:1048–1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Pan Y, Ma P, Liu Y, Li W, Shu Y. Multiple functions of m(6)A RNA methylation in cancer. J Hematol Oncol 2018; 11:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Chen XY, Zhang J, Zhu JS. The role of m(6)A RNA methylation in human cancer. Mol Cancer 2019; 18:103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Liu J, Yue Y, Han D, Wang X, Fu Y, Zhang L, Jia G, Yu M, Lu Z, Deng X, Dai Q, Chen W et al. A METTL3-METTL14 complex mediates mammalian nuclear RNA N6-adenosine methylation. Nat Chem Biol 2014; 10:93–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Sledz P, Jinek M. Structural insights into the molecular mechanism of the m(6)A writer complex. Elife 2016; 5:e18434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Wang P, Doxtader KA, Nam Y. Structural basis for cooperative function of Mettl3 and Mettl14 methyltransferases. Mol Cell 2016; 63:306–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Wang X, Feng J, Xue Y, Guan Z, Zhang D, Liu Z, Gong Z, Wang Q, Huang J, Tang C, Zou T, Yin P. Structural basis of N(6)-adenosine methylation by the METTL3-METTL14 complex. Nature 2016; 534:575–578. [DOI] [PubMed] [Google Scholar]
- 52. Wu B, Li L, Huang Y, Ma J, Min J. Readers, writers and erasers of N(6)-methylated adenosine modification. Curr Opin Struct Biol 2017; 47:67–76. [DOI] [PubMed] [Google Scholar]
- 53. Wang X, Huang J, Zou T, Yin P. Human m(6)A writers: Two subunits, 2 roles. RNA Biol 2017; 14:300–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. AA E, He C, Klungland A. ALKBHs-facilitated RNA modifications and de-modifications. DNA Repair (Amst) 2016; 44:87–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Yi C, Yang CG, He C. A non-heme iron-mediated chemical demethylation in DNA and RNA. Acc Chem Res 2009; 42:519–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Mauer J, Luo X, Blanjoie A, Jiao X, Grozhik AV, Patil DP, Linder B, Pickering BF, Vasseur JJ, Chen Q, Gross SS, Elemento O et al. Reversible methylation of m(6)Am in the 5′ cap controls mRNA stability. Nature 2017; 541:371–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Meng X, Liu J, Wang H, Chen P, Wang D. MicroRNA-126-5p downregulates BCAR3 expression to promote cell migration and invasion in endometriosis. Mol Cell Endocrinol 2019; 494:110486. [DOI] [PubMed] [Google Scholar]
- 58. Sepahi N, Kohan L, Jahromi AR, Daneshbod Y, Hoveidi EN. mir-126 rs4636297 and TGFbetaRI rs334348 functional gene variants are associated with susceptibility to endometriosis and its severity. Gynecol Endocrinol 2017; 33:429–432. [DOI] [PubMed] [Google Scholar]
- 59. Liu S, Gao S, Wang XY, Wang DB. Expression of miR-126 and Crk in endometriosis: miR-126 may affect the progression of endometriosis by regulating Crk expression. Arch Gynecol Obstet 2012; 285:1065–1072. [DOI] [PubMed] [Google Scholar]
- 60. Han J, Wang JZ, Yang X, Yu H, Zhou R, Lu HC, Yuan WB, Lu JC, Zhou ZJ, Lu Q, Wei JF, Yang H. METTL3 promote tumor proliferation of bladder cancer by accelerating pri-miR221/222 maturation in m6A-dependent manner. Mol Cancer 2019; 18:110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Bi X, Lv X, Liu D, Guo H, Yao G, Wang L, Liang X, Yang Y. METTL3-mediated maturation of miR-126-5p promotes ovarian cancer progression via PTEN-mediated PI3K/Akt/mTOR pathway. Cancer Gene Ther 2021; 28:335–349. [DOI] [PubMed] [Google Scholar]
- 62. Feng Y, Tan BZ. LncRNA MALAT1 inhibits apoptosis of endometrial stromal cells through miR-126-5p-CREB1 axis by activating PI3K-AKT pathway. Mol Cell Biochem 2020; 475:185–194. [DOI] [PubMed] [Google Scholar]
- 63. Yang D, Qiao J, Wang G, Lan Y, Li G, Guo X, Xi J, Ye D, Zhu S, Chen W, Jia W, Leng Y et al. N6-Methyladenosine modification of lincRNA 1281 is critically required for mESC differentiation potential. Nucleic Acids Res 2018; 46:3906–3920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Yang Y, Fan X, Mao M, Song X, Wu P, Zhang Y, Jin Y, Yang Y, Chen LL, Wang Y, Wong CC, Xiao X et al. Extensive translation of circular RNAs driven by N(6)-methyladenosine. Cell Res 2017; 27:626–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Meresman GF, Vighi S, Buquet RA, Contreras-Ortiz O, Tesone M, Rumi LS. Apoptosis and expression of Bcl-2 and Bax in eutopic endometrium from women with endometriosis. Fertil Steril 2000; 74:760–766. [DOI] [PubMed] [Google Scholar]
- 66. Dmowski WP, Ding J, Shen J, Rana N, Fernandez BB, Braun DP. Apoptosis in endometrial glandular and stromal cells in women with and without endometriosis. Hum Reprod 2001; 16:1802–1808. [DOI] [PubMed] [Google Scholar]
- 67. Ruiz A, Ruiz L, Colon-Caraballo M, Torres-Collazo BJ, Monteiro JB, Bayona M, Fazleabas AT, Flores I. Pharmacological blockage of the CXCR4-CXCL12 axis in endometriosis leads to contrasting effects in proliferation, migration, and invasion. Biol Reprod 2018; 98:4–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Meresman GF, Auge L, Baranao RI, Lombardi E, Tesone M, Sueldo C. Oral contraceptives suppress cell proliferation and enhance apoptosis of eutopic endometrial tissue from patients with endometriosis. Fertil Steril 2002; 77:1141–1147. [DOI] [PubMed] [Google Scholar]
- 69. Oh HK, Choi YS, Yang YI, Kim JH, Leung PC, Choi JH. Leptin receptor is induced in endometriosis and leptin stimulates the growth of endometriotic epithelial cells through the JAK2/STAT3 and ERK pathways. Mol Hum Reprod 2013; 19:160–168. [DOI] [PubMed] [Google Scholar]
- 70. Yang J, Liu J, Zhao S, Tian F. N(6)-Methyladenosine METTL3 Modulates the Proliferation and Apoptosis of Lens Epithelial Cells in Diabetic Cataract. Mol Ther Nucleic Acids 2020; 20:111–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Cai X, Wang X, Cao C, Gao Y, Zhang S, Yang Z, Liu Y, Zhang X, Zhang W, Ye L. HBXIP-elevated methyltransferase METTL3 promotes the progression of breast cancer via inhibiting tumor suppressor let-7g. Cancer Lett 2018; 415:11–19. [DOI] [PubMed] [Google Scholar]
- 72. Xiong Y, Liu Y, Xiong W, Zhang L, Liu H, Du Y, Li N. Hypoxia-inducible factor 1alpha-induced epithelial-mesenchymal transition of endometrial epithelial cells may contribute to the development of endometriosis. Hum Reprod 2016; 31:1327–1338. [DOI] [PubMed] [Google Scholar]
- 73. Du Y, Zhang Z, Xiong W, Li N, Liu H, He H, Li Q, Liu Y, Zhang L. Estradiol promotes EMT in endometriosis via MALAT1/miR200s sponge function. Reproduction 2019; 157:179–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Huber MA, Kraut N, Beug H. Molecular requirements for epithelial-mesenchymal transition during tumor progression. Curr Opin Cell Biol 2005; 17:548–558. [DOI] [PubMed] [Google Scholar]
- 75. Wanna-Udom S, Terashima M, Lyu H, Ishimura A, Takino T, Sakari M, Tsukahara T, Suzuki T. The m6A methyltransferase METTL3 contributes to Transforming Growth Factor-beta-induced epithelial-mesenchymal transition of lung cancer cells through the regulation of JUNB. Biochem Biophys Res Commun 2020; 524:150–155. [DOI] [PubMed] [Google Scholar]
- 76. Yue B, Song C, Yang L, Cui R, Cheng X, Zhang Z, Zhao G. METTL3-mediated N6-methyladenosine modification is critical for epithelial-mesenchymal transition and metastasis of gastric cancer. Mol Cancer 2019; 18:142. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.


