Abstract

Cetyltrimethylammonium bromide (CTAB) surfactant was proven to be a reliable emulsifier for creating stable emulsions used for drilling, well stimulation, and EOR. The presence of acids like HCl during such operations may lead to the formation of acidic emulsions. No previous comprehensive investigations have been done to study the performance of CTAB-based acidic emulsions. This paper, therefore, presents experimental investigations of the stability, rheological behavior, and pH responsiveness of a CTAB/HCl-based acidic emulsion. The effects of temperature, pH, and CTAB concentration on the emulsion stability and rheology have been investigated using a bottle test and a TA Instrument DHR1 rheometer. Viscosity and flow sweep were analyzed for the steady state at a shear range of 2.5–250 s–1. For the dynamic tests, the storage modulus (G′) and loss modulus (G″) were observed by applying the oscillation test at the range of shear frequency from 0.1 to 100 rad/s. The results revealed that the emulsion exhibits steady rheological behaviors ranging from Newtonian to shear-dependent (psedosteady), depending on the temperature and CTAB concentration. The tendency of the emulsion to exhibit a solid-like behavior is also dependent on CTAB concentration, temperature, and pH. However, the pH responsiveness of the emulsion is more significantly observed within the acidic range of the pH.
1. Introduction
Surfactants are widely employed as emulsified agents for the purpose of producing tight emulsions and microemulsions that are useful in allied applications of petroleum engineering. These applications comprise of drilling engineering, enhanced oil recovery (EOR), and well stimulations.1−7 Additionally, surfactants are utilized in the good gravel packing process and solubilization of crude oil.8,9 Other utilities of surfactants include reducing the frictional loss in turbulent pipe flow, enhancing oil recovery rates via modifying capillary forces, and altering the wettability, and as shale-swelling inhibitors for averting unstable wellbores.10−15 Among the latest surfactants verified is cetyltrimethylammonium bromide (CTAB), which has been established as a dependable emulsifier for petroleum engineering applications. CTAB is a cationic quaternary ammonium surfactant applicable as an emulsifying agent because of its micelle-forming capability in an aqueous solution. In water, a micelle forms an aggregate with the hydrophilic head sections that contact the solvent surrounding it to restructure the single-tail hydrophobic section at the micelle’s center. CTAB can emulsify two immiscible liquids due to the interaction of the hydrophilic head and hydrophobic tail with the two liquids. Surfactants like the CTAB also are widely known as amphiphilic compounds that lower the surface and interfacial tension between two phases. CTAB also is well recognized for having properties of dispersion, suspension, solubilization, and transportation. These properties make CTAB a unique compound to be mixed or interacted with other solutions or materials to form the emulsion. Of late, petroleum industry applications for CTAB have been extensive, for example, as an additive for drilling fluid and in assisting enhanced oil recovery.16 A key function of employing CTAB in drilling fluids is the conservation of good stability by means of shale inhibition and enhancing the rheology of drilling fluids. New research has shown that the addition of CTAB with graphene formulation into drilling fluid produces high shale dispersion recovery and low clay swelling degrees in comparison to drilling fluids that had no such formulations added.16 Additionally, introducing CTAB additives into drilling fluids helps diminish formation damage affected by mud invasion, but then it could also potentially lead to the separation of the clay to produce a thick mud cake around the borehole.17 A recent study by Moslemizadeh et al. examined the swelling inhibitive behavior of CTAB on clay.18 The shale’s wellbore stability was enhanced by means of CTAB and its wettability alteration.19 It was stated by Yue et al.19 that the CTAB addition into the water-based drilling fluid causes an increase in the contact angle with the shale and improves the stability of the wellbore. The study by Bi et al.20 revealed that an ionic attraction causes the inhibition of CTAB, as it is positively charged and attracts negative charges on the shale surface.
In the area of enhanced oil recovery, a number of research studies have verified the utility of CTAB surfactant as a singular agent, or when mixed with other materials, in reducing interfacial tension (IFT) and augmenting the mobility ratio.21 Furthermore, CTAB has been effectively used in the creation of tight and dependable viscoelastic surfactants (VES) that are applied for the hydraulic fracturing of shale gas and in constricted reservoirs.22 Additionally, the recent papers by Li et al.,23 Ashtari Larki et al.,24 and Rajabi et al.25 demonstrate the practicality of nanosilica and CTAB in enhancing the efficiency of oil recovery. Moreover, CTAB combined with silica nanoparticles has been utilized for increasing the ultimate oil recovery as well.26 Another study by Hassan et al.27 demonstrated the effectiveness of CTAB with MgCl2 in ultimate oil recovery enhancement, mobility control of the gas, and improvement of displacement efficiency. Earlier, Golombok and Wijst28 had investigated the utility of CTAB with NaSal (sodium salicylate) in decelerating the water flow rate in high-permeability zones. Both CTAB and dodecyltrimethylammonium bromide (DTAB) surfactants enhance wettability and reduce interfacial tension (IFT) as well as surface tension. Therefore, both CTAB and DTAB may well augment EOR in comparison to BTAB surfactants.29,30 Later, Bera et al.31 applied CTAB for improving EOR by conducting investigations on their effects on wettability. A similar study showed that CTAB combined with the nanosilica can improve the emulsion stability and increase the emulsion’s superficial viscosity.32 According to Yekeen et al.,33 CTAB was identified as the most stable surfactant-stabilized emulsion. Also, CTAB exhibited better outcomes in inhibiting asphaltene precipitation.34
Acidic emulsions are frequently formed, intentionally or unintentionally, during different petroleum engineering-related operations. Emulsified acids, for instance, are regularly used as a means of matrix acidizing in carbonate and sandstone reservoirs, by which formation damage is removed and/or formation permeability is increased. An emulsified acid is created by emulsifying an oil phase (e.g., diesel oil) in water-diluted acid by means of a surfactant carefully chosen as an emulsifier agent. The core purpose of emulsified acids is to slow down the acid reaction with the rock in order to maximize the penetration of acid within the formation. The emulsions produced by the surfactants depend on the rheological properties of the emulsion.35 During the past two years, we have conducted rigorous experimental probes to research the performance of emulsified acids produced via two common types of nonionic surfactant, namely, tweet 80 and Span 80. The performance evaluations were completed by way of experimental study of stability, rheological properties, and corrosivity.2,36 Hence, in this work, we are willing to investigate the performance of the oil in water acidic emulsion formed by the cationic surfactant cetyltrimethylammonium bromide (CTAB). The behavior of the emulsion is studied to observe the rheological properties and stability of the emulsion in different conditions and concentrations of CTAB. Stability and rheological properties are important parameters that need to be observed in the CTAB-based emulsion. They are crucial parameters to ensure that the emulsion is valid in EOR, fracturing, or as drilling fluid additives. In this paper, therefore, the stability, rheological properties, and pH responsiveness are experimentally investigated using a rheometer and bottle test. The CTAB-based acidic emulsion used in this study has been investigated at acidity and alkalinity values of pH. The acidic emulsion was prepared by mixing the emulsion with hydrochloric acid (HCl) to reduce its pH value.
2. Methodology
2.1. Materials
The cetyltrimethylammonium bromide (CTAB) surfactant of >97% purity was procured from CSI LabShop Malaysia (Ipoh-Perak, Malaysia). The surfactant was used to form the oil-in-water emulsion. The oil type used is a diesel that contains 75% saturated hydrocarbon and 25% aromatic hydrocarbon. We deemed CTAB as appropriate for formulating these kinds of emulsions on account of its moderate hydrophilic–lipophilic (HLB) value of 10.37
This formulated emulsion was blended by magnetic stirring. Then, the emulsion was stored in a bottle that was sealed with aluminum foil to prevent evaporation. The CTAB-based emulsion utilized in this investigation has been evaluated at acidity and alkalinity pH values. The acidic emulsion preparation involved combining the water/diesel emulsion with hydrochloric acid (HCl) to lower its pH value. By itself, the emulsion at this acidic condition can characterize the standard emulsified acids utilized during carbonate reservoir stimulation.
2.2. Bottle Test (Stability Study)
A bottle test was conducted to assess the ability of CTAB-based acidic emulsion. The emulsion stability was evaluated by monitoring the time required for the emulsion to break up into its separate phases. The emulsion sample was formulated in a test tube and placed in a static condition under visual observation till a well-defined separation of the two phases was noticed. Straight away, the time taken was noted as a marker of the stability of the emulsion, wherein a shorter time denoted a weaker emulsion. Various factors were controlled for numerous samples with the purpose of studying the stability and analyzing the effect of these factors, viz., pH values, temperature, and surfactant concentration.
2.3. Measurement of Rheological Properties
The rheological properties were examined at steady and dynamic states at various conditions, such as different pH values, temperatures, and surfactant concentrations. A TA Instrument DHR1 Rheometer model at Universiti Teknologi PETRONAS (UTP), Seri Iskandar-Perak, Malaysia was employed for conducting rotational (steady rheology) and oscillation (dynamic rheology) tests for the samples of CTAB-based emulsions. Viscosity was studied by means of a rotational test on a rheometer with a shear range of 0.1–100 s–1. Alternatively, for studying the dynamic rheology, we observed the storage modulus (G′) and loss modulus (G″) by employing an oscillation test that also used a rheometer with a shear frequency of 0.1 to 100 rad/s.
The emulsion pH value was controlled by adding NaOH and HCl to achieve two pH values of the emulsion, viz., 1 (strong acidity) and 10 (strong alkalinity) to assess the pH responsiveness of the emulsion. The rheological properties were studied at 60 and 100 °C to assess the effect of temperature.
The results obtained from the bottle test and rheological measurements have been utilized in combination to optimize the dosage of CTAB. From the bottle test, the optimum CTAB concentration is the concentration at which the longest time elapsed before the emulsion phases are separated from each other. The results obtained from the bottle test were further confirmed by the rheological investigation, where the stability is indirectly represented by rheology (i.e., higher viscosity indicates higher stability).
3. Results and Discussion
3.1. Emulsion Stability Results
3.1.1. Effect of CTAB Concentration on the Emulsion Stability (Visual Observation)
Visual investigations were carried out to evaluate the stability of the emulsion formed by mixing 30 mL of water and 10 mL of diesel oil at three different values CTAB concentration of 0.5, 1.0, and 1.5 g. The function of the visual investigation is to observe the phase behavior of the emulsion at different elapsed standing times in order to identify the concentration among the three values, which can produce the emulsion with the highest stability. In Figure 1, the pictorial image of the emulsion at various standing times is shown, which begins from 1 min (at the most left) to 1 day (at the most right) for each of the three CTAB concentration values, viz., 05 g (top), 1 g (middle), and 1.5 g (bottom). The results reveal that the stability of the emulsion is proportionate to the CTAB concentration, such that, higher concentrations of CTAB produces more stable emulsions. It can be observed that increasing the concentration of CTAB from 0.5 to 1 g is considerably more effectual than increasing the CTAB concentration from 1 to 1.5 g. Thus, the CTAB concentration of 1 g may be regarded as the optimum CTAB quantity required for formulating a stable emulsion in the experimental conditions, i.e., ambient conditions. Even though the emulsion at all three CTAB concentration values exhibited a complete separation after 1 h, the transformation of the emulsion from entirely transparent at 0.5 g to cloudy at 1 and 1.5 g can signify the alteration of stability with an increase in CTAB concentration. The cloudier phase behavior at 1 g may indicate incomplete separation and hence a more stable emulsion than that at 0.5 and 1.5 g. However, the phase behavior presented in the figure cannot be taken as strong evidence that the emulsion exhibits the highest stability at 1 g as compared to 0.5 and 1.5 g. Therefore, this result will be substantiated later through additional quantitative results attained from the subsequent rheological measurements (as shown in Figure 4). Nevertheless, this tendency might be modified by differing from the ambient temperature. In addition, the influence of heating on the stability of emulsion shall be examined in the subsequent sections as well through the investigations of the rheological properties at 60 and 100 °C.
Figure 1.
Visual observation results of 30 mL of water and 10 mL of diesel emulsion with 0.5, 1, and 1.5 g CTAB concentrations after 1 min to 1 day.
Figure 4.
Change of viscosity with CTAB concentration and different shear rates.
3.1.2. Effect of pH on the Stability of CTAB-Based Emulsion
Two CTAB-based emulsions were formulated with the purpose of studying the effect of pH (i.e., acidity) on the stability of CTAB-based emulsions. Hydrochloric acid or sodium hydroxide was added to modify the pH value and produce an acidic (pH = 1) or alkaline (pH = 10) emulsion. As demonstrated in Figure 2, the water and diesel oil are nearly separated from each other entirely after one day for both pH values. This shows that the CTAB-based emulsion in the acidic condition has virtually identical stability as the CTAB-based emulsion in the neutral condition. Nevertheless, in the case of pH 1, the emulsion begins to separate soon after 1 min, signifying low emulsion stability under acidic conditions. This outcome coincides accurately with our formerly published outcomes regarding the effect of pH on the phase behavior of CTAB-based viscoelastic surfactants. The imperative explanation of the effect of pH on phase behavior and stability of the emulsions is associated with the electrostatic interaction forces of a double layer repulsion and van der Waals attraction and the hydrogen bonds between the cationic surfactant and hydrochloric acid, via altering their molecular state distributions before.22 Ordinarily, no distinct tendency exists that defines the effect of pH on emulsion stability, and certain emulsions might even display a pH-irresponsive behavior. Also, the effect is extensively dependent on temperature, where the tendency that ensues at a low temperature might not be identical to that at a high temperature. Furthermore, it has been previously observed that certain CTAB-based viscoelastic surfactants can show pH-responsive behavior at acidic conditions, but then show no such behavior at alkaline conditions.22
Figure 2.
Effect of acidity on the stability of CTAB-based emulsion for 1 g of CTAB.
3.2. Results of the Rheological Properties of the Emulsion
This segment describes the results of the rheological experimentations on the emulsion at various CTAB concentrations, pH values, and temperatures. This shall consist of both the results from steady and dynamic rheological measurements.
3.2.1. Steady Rheological Measurements
The steady rheological measurements were taken with the aid of a rotational rheometer that gauged the sample viscosity related to the shear rate, which is controlled by the rotation of the rheometer’s spindle. This can define the rheological behavior of the emulsion and illustrate the shear dependence of the emulsion, that is, in what manner viscosity responds to the shear rate changes. For studying the effect of CTAB concentration on the rheology of the emulsion, the viscosity of the emulsion at a temperature of 60 °C was quantified for each of the three CTAB concentration samples of 0.5, 1.0, and 1.5 g at the shear rate values ranging from 2.5 to 250 s–1. Figure 3a–c illustrates the flow curves of the emulsion at the three CTAB amounts. The linear relationship between shear stress and shear rate at 0.5 and 1.5 g CTAB concentrations specifies that the emulsion at these two concentrations shows Newtonian flow behavior, in which the viscosity is independent of the shear rate. Then again, shear dependence at low shear rates (less than 50 s–1) is observed, at which a sharp viscosity decrease occurs with the increase in the shear rate. When CTAB concentration is 1 g, an obvious pseudoplastic shear-dependent non-Newtonian flow behavior is exhibited by the emulsion, in which a steady decrease in viscosity occurs with the increase in the shear rate. Figure 3a–c similarly shows that the viscosity increases steadily with higher CTAB concentrations. Nonetheless, an optimum amount of CTAB exists, beyond which the viscosity begins to decline with the increase in CTAB concentrations. Based on Figure 4, we can deduce that this optimum CTAB concentration value exists between the amounts of 1 and 1.5 g of CTAB. We can consider 1 g as a recommended optimum CTAB amount for this emulsion sample in order to prevent viscosity loss. Also, we can observe that the effect of CTAB concentration turns out to be insignificant at a high shear rate. That is, at a shear rate of 250 s–1, the dependence of viscosity on the concentration of CTAB becomes minor. The pseudoplastic rheological behavior of the emulsion observed at the optimum CTAB amount (i.e., 1 g) may be due to the “micelle solubilization” as explained before by Zhang et al.38 At an amount less than the optimum value, the stability (which is directly related to the rheological behavior) increases steadily due to the increase in the surfactant molecules adsorbed to the interface between the two phases forming the emulsion. However, at a critical surfactant amount (1 g in this case), the surfactant may form micelles that have a solubilization tendency. The solubilization of micelles may become higher than the molecule adsorption at the interface, leading to a decline in the stability and disrupting the pseudoplastic rheological behavior developed at the optimum surfactant amount. The emulsion can then return to its low stability low viscosity Newtonian behavior.
Figure 3.
Emulsion flow curves at different CTAB concentrations.
In Figure 5, further details about the rheological behavior of the emulsions at the three CTAB concentrations are illustrated. The viscosity variations with shear rate signify that all three samples display shear-thinning capacities; however, this behavior is more perceptible at 1 g of CTAB. This might corroborate our previous conclusion, in which we had considered 1 g of CTAB as the optimum concentration of CTAB. At 1 g of CTAB, the emulsion has greater viscosity and rheological complex and therefore is more stable than the emulsion at 1.5 g of CTAB. At low concentrations of CTAB, the surfactant molecules tend to form micelles, which are small aggregates of surfactant molecules that can help thicken the liquid. This is because the micelles create a sort of network within the liquid that impedes the flow of the liquid, resulting in higher viscosity. As the concentration of CTAB is increased beyond the optimum amount, the micelles become more closely packed together and begin to overlap, which can actually reduce the viscosity of the liquid.39 Shear thinning, which is identified as a decrease of viscosity at high shear rates, can be related to the transient structure of the internal network structure of the solution.40 At low CTAB concentrations, the surfactant molecules tend to form smaller and less stable micelles in the liquid. These micelles can break apart more easily under high shear rates, such as those induced by stirring or pouring, leading to a decrease in viscosity. This is why shear-thinning behavior is more perceptible at low CTAB concentrations, as the surfactant molecules are more likely to break apart and reduce the viscosity of the liquid. At higher CTAB concentrations, the surfactant molecules tend to form larger and more stable micelles or bilayers, which are more resistant to shear forces. This can make shear-thinning behavior less perceptible at high CTAB concentrations, as the surfactant molecules are less likely to break apart and reduce the viscosity of the liquid.
Figure 5.
Viscosity and shear stress vs. shear rate of the emulsion at three CTAB amounts (0.5, 1.0, and 1.5 g).
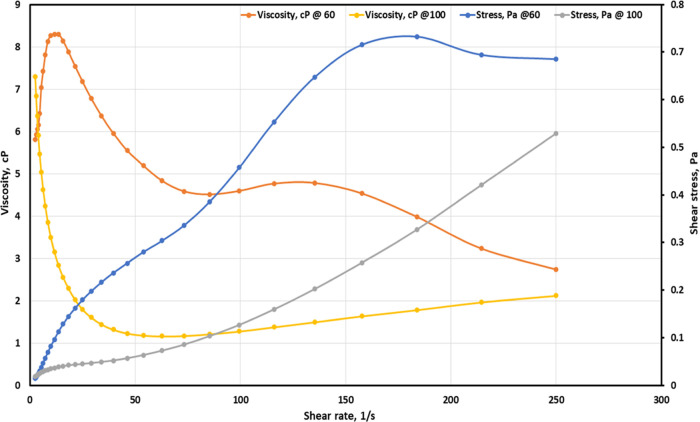
To measure the rheological properties of the emulsion in the acidic or alkaline environments, the pH was changed to the values of 1 and 10, respectively, by means of the method discussed previously. Afterward, the emulsion’s rheological behavior was evaluated at 60 °C temperature and within the same shear rate range as before (i.e., 2.5–2.5 × 102 s–1) with the aid of a DHR1 rheometer. In Figure 6, we see the viscosity dependence of the emulsion on shear stress in both acidic (i.e., pH = 1) and alkaline (i.e., pH = 10) conditions. In both these conditions, the emulsion displays a degree of pseudoplasticity, which exhibits shear dependency. Nevertheless, when the shear rate is low, the emulsion displays this additional pseudoplastic behavior under acidic conditions rather than in alkaline conditions. This outcome conflicts with the result of phase behavior, as shown in Figure 6, in which the emulsion at pH 1 separates more quickly than the emulsion at pH 10. On the other hand, this effect only occurs at the very start of shearing (i.e., below 10 s–1). Beyond the shearing rate of 10 s–1, the emulsion’s viscosity in the alkaline conditions surpasses the emulsion’s viscosity in acidic conditions up until the shear rate of approximately 30 s–1; then, at that point, the two lines display similar viscosity values. Therefore, we can deduce that the emulsion is pH responsive at static and low values of shear rate, and the viscosity dependence of pH turns is reduced at a high shear rate.
Figure 6.
Viscosity–shear relation of the emulsion in acidic and alkaline conditions.
In Figure 7, we specify that the emulsion shows non-Newtonian, shear-thinning behavior at both pH values. Nevertheless, when the shear rate is low, the emulsion viscosity in acidic conditions of pH 1 is higher than the emulsion viscosity in alkaline conditions of pH 10.
Figure 7.
Flow sweep result of the emulsion in acidic and alkaline conditions.
The succeeding study was performed in order to evaluate the effect of temperature on rheology at steady conditions. In Figure 8, the viscosity and temperature are indicated as having an inverse relationship, which is valid for all liquids. At both 60 and 100 °C temperatures, the viscosity steadily declines with the shear rate. Nevertheless, the shear dependence of the viscosity at a high temperature is lesser than the shear dependence at a low temperature. A noteworthy observation from the figure is that at a low temperature, a visible shear thickening occurs at low shear rate values, i.e., viscosity increases with the increase in shear rate. Likewise, the tendency of shear thickening trailed by shear thinning has been noted by Wolf et al.41 as well in particle-stabilized emulsions and was validated by the emulsion being shown as suspension.
Figure 8.
Viscosity variation of CTAB-based emulsion at temperatures of 60 and 100 °C.
Figure 9 illustrates the flow sweep curves of the emulsion at temperatures of 60 and 100 °C. The flow curves (shear rate–shear stress) specify that at 100 °C, the emulsion is more prone to show Newtonian flow behavior, although its viscosity–shear rate curve displays a shear dependence behavior at a low shear rate value.
Figure 9.
Flow sweep curves for the CTAB-based emulsion at temperatures of 60 and 100 °C.
3.2.2. Dynamic Rheological Measurements
The dynamic rheological measurement (oscillatory test) is performed with the objective of investigating the viscoelastic properties of the samples. The measurements of storage modulus (G′) and loss modulus (G″) are taken at various angular frequencies applied in the range of 0.1–100 rad/s with the aim of observing the sample’s elastic and viscous behaviors. In Figure 10, we illustrate the variation of storage modulus (G′) and loss modulus (G″) with the frequency of the three emulsion samples with 0.5, 1, and 1.5 g of CTAB, designated a, b, and c, respectively, at 60 °C. The emulsion having 0.5 g of CTAB displays G′ and G″ modulus curves of mild slopes with a couple of cross points. When the frequency is low, the sample displays a solid-like behavior, which is specified by the storage modulus being larger than the loss modulus. Following the first cross point, the emulsion begins to show a liquid-like behavior (G″ > G′), even though the gap between the two moduli is not very apparent. What this means is that in the case of this sample, the emulsion shows viscoelastic properties most often. As the CTAB concentration is increased to 1.0 g, the emulsion shows elastic behavior from frequency ranges of 0.1 until 80 rad/s, which is specified via the marginally higher values of the storage modulus in comparison to the loss modulus. In the case of the emulsion with 1.5 g of CTAB, the sample shows viscous behavior at a low frequency up until around 15 rad/s. At that moment, the curve displays G′ moduli higher than G″ moduli, which specifies a conversion from liquid-like behavior to solid-like behavior as a result of the presence of wormlike micelles.
Figure 10.
Frequency sweep graph for CTAB-based emulsion at different concentrations: (a) 0.5 g, (b) 1.0 g, and (c) 1.5 g.
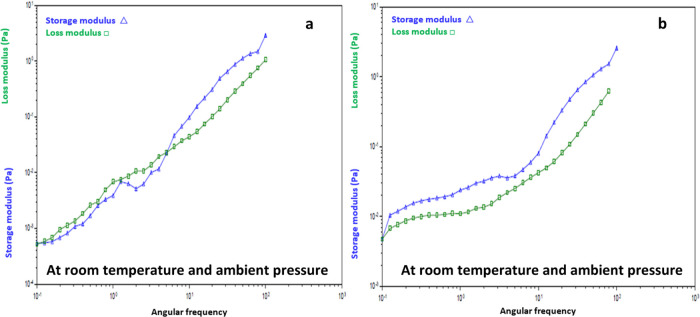
To evaluate the effect of pH, the frequency sweep of the emulsion at pH 1 and 10 is shown in Figure 11a,b, respectively. In acidic conditions of pH 1, the emulsion exhibits a viscous behavior (G″ > G′) till a frequency of 5 rad/s is reached and subsequently begins to exhibit a viscoelastic fluid characteristic, as shown in Figure 11a. In contrast, when alkaline conditions are prevalent, the sample displays viscoelastic behavior (G′ > G″) from the beginning till the end of the experiment, as shown in Figure 11b. Hence, it may be understood that the variation of pH value can alter the structure of the micelle, which thereby changes the CTAB-based emulsion’s viscoelastic properties.
Figure 11.
Frequency sweep graph for CTAB-based emulsion at (a) acidic and (b) alkaline conditions.
In order to evaluate the effect of temperature on the dynamic rheology, the frequency sweep of 1 g of CTAB-based emulsion at 60 and 100 °C is shown in Figure 12a,b. Figure 12a illustrates the CTAB-based emulsion at a low temperature of 60 °C, exhibiting a solid-like behavior at 0.1–50 rad/s and a liquid-like behavior at 50–10 rad/s. At a high temperature of 100 °C, the CTAB-based emulsion shows a liquid-like behavior as the angular frequency increases. At a low frequency, no perceptible difference is observed, and the curves exhibit similar behavior up to the frequency of 10 rad/s. Subsequently, beyond 10 rad/s up until 100 rad/s, the storage modulus becomes more substantial than the loss modulus, revealing that the sample displays solid-like behavior at a higher frequency, as Figure 12b represents.
Figure 12.
Frequency sweep graph for CTAB-based emulsion at (a) low and (b) high temperatures.
4. Conclusions
This paper describes the experimental study of the stability, rheological properties, and pH responsiveness of an oil-in-water CTAB-based emulsion. The emulsion preparation involved emulsifying the water in diesel oil by utilizing a CTAB surfactant as an emulsified agent at different concentrations. From the findings of the experimental investigation, the resulting conclusions are drafted as follows:
The stability of the CTAB-based emulsion is proportionate to the CTAB concentration, such that, higher CTAB concentrations can produce emulsions that are more stable. Even so, increasing the CTAB concentration from 0.5 to 1 g is more effective than increasing the CTAB concentration from 1 to 1.5 g.
An optimum amount of CTAB concentration exists, beyond which the emulsion stability might decline. At ambient conditions, the optimum CTAB amount was found to be 1 g.
The CTAB-based emulsion was discovered to be pH-responsive at static conditions as well as at low shearing rate values, at which it displays greater stability in an alkaline environment than in an acidic environment. Nevertheless, when the shear rate is high, the emulsion is irresponsive to pH, and at high temperatures, the acidic environment could generate more stable emulsions at extremely low shear rate values.
At high temperatures, the shear dependence of the emulsion viscosity is lesser than that at low temperatures. There is an observable shear thickening as the viscosity increases with the increase in the shear rate at a low temperature and shear rate values. At high temperatures, the emulsion has a greater tendency to display a Newtonian behavior except for a small degree of shear dependency.
The dynamic rheological examination unveiled that the emulsion will possibly show both viscous and viscoelastic behaviors contingent on the CTAB concentration, pH, and temperature. A frequency point always exists, exceeding which the emulsion transforms from solid-like to liquid-like behavior, and this frequency point is contingent on CTAB concentration, pH, and temperature. For instance, at a low concentration of CTAB, the emulsion exhibits a liquid-like behavior for the entire frequency range except for the low frequency.
Acknowledgments
The authors would like to express their profound gratitude to the Universiti Teknologi PETRONAS (UTP) for supporting this study under YUTP-Grant cost center 15LC0-428 and 15LC0-226.
Glossary
Nomenclature
- CTAB
cetyltrimethylammonium bromide
- EOR
enhanced oil recovery
- G′
storage modulus
- G″
loss modulus
- IFT
interfacial tension
- VES
viscoelastic surfactants
- MgCl2
magnesium chloride
- NaSal
sodium salicylate
- DTAB
dodecyltrimethylammonium bromide
- g
gram
The authors declare no competing financial interest.
References
- Mohyaldinn M. E.; Hassan A. M.; Ayoub M. A.. Application of Emulsions and Microemulsions in Enhanced Oil Recovery and Well Stimulation. In Microemulsion—A Chemical Nanoreactor, Mejut J. C., Ed.; IntechOpen, 2019. [Google Scholar]
- Yousufi M. M.; Elhaj M. E. M.; Moniruzzaman M.; Ayoub M. A.; Nazri A. B. M.; binti Husin H.; bin Mohd Saaid I. Synthesis and Evaluation of Jatropha Oil-Based Emulsified Acids for Matrix Acidizing of Carbonate Rocks. J. Pet. Explor. Prod. Technol. 2019, 9, 1119–1133. 10.1007/s13202-018-0530-8. [DOI] [Google Scholar]
- Hosseini-Kaldozakh S. A.; Khamehchi E.; Dabir B.; Alizadeh A.; Mansoori Z. Experimental Investigation of Water Based Colloidal Gas Aphron Fluid Stability. Colloids Interfaces 2019, 3, 31. 10.3390/colloids3010031. [DOI] [Google Scholar]
- Siddig O.; Al-Afnan S.; Elkatatny S.; Bahgat M. Novel Cake Washer for Removing Oil-Based Calcium Carbonate Filter Cake in Horizontal Wells. Sustainability 2020, 12, 3427. 10.3390/su12083427. [DOI] [Google Scholar]
- Chatterjee P.; Sowiak G. A.; Underhill P. T. Effect of Phase Change on the Rheology and Stability of Paraffin Wax-in-Water Pickering Emulsions. Rheol. Acta 2017, 56, 601–613. 10.1007/s00397-017-1021-4. [DOI] [Google Scholar]
- Zhang W.; Mao J.; Yang X.; Zhang H.; Zhang Z.; Yang B.; Zhang Y.; Zhao J. Study of a Novel Gemini Viscoelastic Surfactant with High Performance in Clean Fracturing Fluid Application. Polymers 2018, 10, 1215. 10.3390/polym10111215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Supee A.; Idris A. K. Effects of Surfactant-Polymer Formulation and Salinities Variation towards Oil Recovery. Arab. J. Sci. Eng. 2014, 39, 4251–4260. 10.1007/s13369-014-1025-7. [DOI] [Google Scholar]
- Zhao J.; Fan J.; Mao J.; Yang X.; Zhang H.; Zhang W. High Performance Clean Fracturing Fluid Using a New Tri-Cationic Surfactant. Polymers 2018, 10, 535. 10.3390/polym10050535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahal S.; Khalladi R.; Moulai-Mostefa N. Solubilization of Crude Oil by Extended and Other Anionic Surfactants. Arab. J. Sci. Eng. 2016, 41, 111–117. 10.1007/s13369-015-1618-9. [DOI] [Google Scholar]
- Alvarez J. O.; Schechter D. S. Wettability Alteration and Spontaneous Imbibition in Unconventional Liquid Reservoirs by Surfactant Additives. SPE Reserv. Eval. Eng. 2017, 20, 107–117. 10.2118/177057-PA. [DOI] [Google Scholar]
- Quintero L. An Overview of Surfactant Applications in Drilling Fluids for the Petroleum Industry. J. Dispers. Sci. Technol. 2002, 23, 393–404. 10.1080/01932690208984212. [DOI] [Google Scholar]
- Chen S.; Shi Y.; Yang X.; Xie K.; Cai J. Design and Evaluation of a Surfactant–Mixed Metal Hydroxide-Based Drilling Fluid for Maintaining Wellbore Stability in Coal Measure Strata. Energies 2019, 12, 1862. 10.3390/en12101862. [DOI] [Google Scholar]
- Sousa R. P. F. d.; Braga G. S.; Silva R. R.; da Leal G. L. R.; Freitas J. C. O.; Madera V. S.; Garnica A. I. C.; Curbelo F. D. S. Formulation and Study of an Environmentally Friendly Microemulsion-Based Drilling Fluid (O/W) with Pine Oil. Energies 2021, 14, 7981. 10.3390/en14237981. [DOI] [Google Scholar]
- Ohlendorf D.; Interthal W.; Hoffmann H. Surfactant Systems for Drag Reduction: Physico-Chemical Properties and Rheological Behaviour. Rheol. Acta 1986, 25, 468–486. 10.1007/BF01774397. [DOI] [Google Scholar]
- Khan M. N.; Wan Sulaiman W. R.; Abbas A. H. Study of Sulfosuccinate and Extended Sulfated Sodium Surfactants on the Malaysian Crude/Water Properties for ASP Application in Limestone. Arab. J. Sci. Eng. 2021, 46, 6915–6924. 10.1007/s13369-020-05252-5. [DOI] [Google Scholar]
- Rana A.; Arfaj M. K.; Yami A. S.; Saleh T. A. Cetyltrimethylammonium Modified Graphene as a Clean Swelling Inhibitor in Water-Based Oil-Well Drilling Mud. J. Environ. Chem. Eng. 2020, 8, 103802. 10.1016/j.jece.2020.103802. [DOI] [Google Scholar]
- Liew C. X.; Gholami R.; Safari M.; Raza A.; Rabiei M.; Fakhari N.; Rasouli V.; Vettaparambil J. V. A New Mud Design to Reduce Formation Damage in Sandstone Reservoirs. J. Pet. Sci. Eng. 2019, 181, 106221 10.1016/j.petrol.2019.106221. [DOI] [Google Scholar]
- Moslemizadeh A.; Aghdam S. K.; Shahbazi K.; Aghdam H. K.; Alboghobeish F. Assessment of Swelling Inhibitive Effect of CTAB Adsorption on Montmorillonite in Aqueous Phase. Appl. Clay Sci. 2016, 127–128, 111–122. 10.1016/j.clay.2016.04.014. [DOI] [Google Scholar]
- Yue Y.; Chen S.; Wang Z.; Yang X.; Peng Y.; Cai J.; Nasr-El-Din H. A. Improving Wellbore Stability of Shale by Adjusting Its Wettability. J. Pet. Sci. Eng. 2018, 161, 692–702. 10.1016/j.petrol.2017.12.023. [DOI] [Google Scholar]
- Bi Z.; Zhang Z.; Xu F.; Qian Y.; Yu J. Wettability, Oil Recovery, and Interfacial Tension with an SDBS–Dodecane–Kaolin System. J. Colloid Interface Sci. 1999, 214, 368–372. 10.1006/jcis.1999.6208. [DOI] [PubMed] [Google Scholar]
- Bhui U. K.; Sanyal S.; Saha R.; Rakshit S.; Pal S. K. Steady-State and Time-Resolved Fluorescence Spectroscopic Study of Petroleum Crudes in Aqueous-Surfactant Solutions: Its Implications for Enhanced Oil Recovery (EOR) during Surfactant Flooding. Fuel 2018, 234, 1081–1088. 10.1016/j.fuel.2018.08.006. [DOI] [Google Scholar]
- Chieng Z. H.; Mohyaldinn M. E.; Hassan A. M.; Bruining H. Experimental Investigation and Performance Evaluation of Modified Viscoelastic Surfactant (VES) as a New Thickening Fracturing Fluid. Polymers 2020, 12, 1470. 10.3390/polym12071470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S.; Qiao C.; Li Z.; Wanambwa S. Properties of Carbon Dioxide Foam Stabilized by Hydrophilic Nanoparticles and Hexadecyltrimethylammonium Bromide. Energy Fuels 2017, 31, 1478–1488. 10.1021/acs.energyfuels.6b03130. [DOI] [Google Scholar]
- Ashtari Larki S.; Banashooshtari H.; Shokrollahzadeh Behbahani H.; Najafi-Marghmaleki A. Effect of Acid Number of Crude Oil on Oil Recovery of Smart Water Coupled with Silica Nanoparticles. Pet. Sci. Technol. 2018, 36, 343–349. 10.1080/10916466.2018.1425716. [DOI] [Google Scholar]
- Rajabi M. S.; Moradi R.; Mehrizadeh M. Experimental Investigation of Chemical Solutions Effects on Wettability Alteration and Interfacial Tension Reduction Using Nano-Alkaline–Surfactant Fluid: An EOR Application in Carbonate Reservoirs. J. Pet. Explor. Prod. 2021, 11, 1925–1941. 10.1007/s13202-021-01155-9. [DOI] [Google Scholar]
- Rezaei A.; Derikvand Z.; Parsaei R.; Imanivarnosfaderani M. Surfactant-Silica Nanoparticle Stabilized N2-Foam Flooding: A Mechanistic Study on the Effect of Surfactant Type and Temperature. J. Mol. Liq. 2021, 325, 115091 10.1016/j.molliq.2020.115091. [DOI] [Google Scholar]
- Hassan A.; Ayoub M.; Eissa M.; Bruining H.; Al-Mansour A.; Al-Quraishi A. In A New Hybrid Improved and Enhanced Oil Recovery IOR/EOR Process Using Smart Water Assisted Foam SWAF Flooding in Carbonate Rocks; A Laboratory Study Approach, International Petroleum Technology Conference, OnePetro, 2021.
- Golombok M.; van der Wijst R. Permeability Thickening Fluids for Improved Secondary Oil Recovery. J. Pet. Sci. Eng. 2013, 110, 22–26. 10.1016/j.petrol.2013.08.040. [DOI] [Google Scholar]
- Kumar S.; Panigrahi P.; Saw R. K.; Mandal A. Interfacial Interaction of Cationic Surfactants and Its Effect on Wettability Alteration of Oil-Wet Carbonate Rock. Energy Fuels 2016, 30, 2846–2857. 10.1021/acs.energyfuels.6b00152. [DOI] [Google Scholar]
- Dordzie G.; Dejam M. Enhanced Oil Recovery from Fractured Carbonate Reservoirs Using Nanoparticles with Low Salinity Water and Surfactant: A Review on Experimental and Simulation Studies. Adv. Colloid Interface Sci. 2021, 293, 102449 10.1016/j.cis.2021.102449. [DOI] [PubMed] [Google Scholar]
- Bera A.; Mandal A.; Kumar T. The Effect of Rock-Crude Oil-Fluid Interactions on Wettability Alteration of Oil-Wet Sandstone in the Presence of Surfactants. Pet. Sci. Technol. 2015, 33, 542–549. 10.1080/10916466.2014.998768. [DOI] [Google Scholar]
- Pei H.; Shu Z.; Zhang G.; Ge J.; Jiang P.; Qin Y.; Cao X. Experimental Study of Nanoparticle and Surfactant Stabilized Emulsion Flooding to Enhance Heavy Oil Recovery. J. Pet. Sci. Eng. 2018, 163, 476–483. 10.1016/j.petrol.2018.01.025. [DOI] [Google Scholar]
- Yekeen N.; Padmanabhan E.; Syed A. H.; Sevoo T.; Kanesen K. Synergistic Influence of Nanoparticles and Surfactants on Interfacial Tension Reduction, Wettability Alteration and Stabilization of Oil-in-Water Emulsion. J. Pet. Sci. Eng. 2020, 186, 106779 10.1016/j.petrol.2019.106779. [DOI] [Google Scholar]
- Mohamed R. S.; Loh W.; Ramos A. C. S.; Delgado C. C.; Almeida V. R. Reversibility and Inhibition of Asphaltene Precipitation in Brazilian Crude Oils. Pet. Sci. Technol. 1999, 17, 877–896. 10.1080/10916469908949754. [DOI] [Google Scholar]
- Haq B. Green Enhanced Oil Recovery for Carbonate Reservoirs. Polymers 2021, 13, 3269. 10.3390/polym13193269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yousufi M. M.; Elhaj M. E. M.; Moniruzzaman M. Comparative Analysis of Corrosion Inhibition: Between Jatrophacurcas, Palm and Diesel Oil Based Emulsified Acids for Acid Stimulation Operations. IOP Conf. Ser. Earth Environ. Sci. 2018, 164, 012006. 10.1088/1755-1315/164/1/012006. [DOI] [Google Scholar]
- Barut K. D.; Coşkun Ari F. F.; Öner F. Development and Characterization of a Cationic Emulsion Formulation as a Potential PDNA Carrier System. Turkish J. Chem. 2005, 29, 27–40. [Google Scholar]
- Zhang J.; Ge D.; Wang X.; Wang W.; Cui D.; Yuan G.; Wang K.; Zhang W. Influence of Surfactant and Weak-Alkali Concentrations on the Stability of O/W Emulsion in an Alkali-Surfactant-Polymer Compound System. ACS Omega 2021, 6, 5001–5008. 10.1021/acsomega.0c06142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Z.; Cai J. J.; Scriven L. E.; Davis H. T. Spherical-to-Wormlike Micelle Transition in CTAB Solutions. J. Phys. Chem. A 1994, 98, 5984–5993. 10.1021/j100074a027. [DOI] [Google Scholar]
- Dorosti A. H.; Ghatee M.; Norouzi M. Preparation and Characterization of Water-Based Magnetorheological Fluid Using Wormlike Surfactant Micelles. J. Magn. Magn. Mater. 2020, 498, 166193 10.1016/j.jmmm.2019.166193. [DOI] [Google Scholar]
- Wolf B.; Lam S.; Kirkland M.; Frith W. J. Shear Thickening of an Emulsion Stabilized with Hydrophilic Silica Particles. J. Rheol. 2007, 51, 465–478. 10.1122/1.2714642. [DOI] [Google Scholar]