Abstract
Novel barium heteroleptic complexes were synthesized through the substitution of the bis(trimethylsilyl)amide of Ba(btsa)2·2DME with aminoalkoxide and β-diketonate ligands. Compounds [Ba(ddemap)(tmhd)]2 (1) and [Ba(ddemmp)(tmhd)]2 (2) were obtained and analyzed through Fourier transform infrared spectroscopy, nuclear magnetic resonance, thermogravimetric analysis, and elemental analysis (ddemapH = 1-(dimethylamino)-5-((2-(dimethylamino)ethyl) (methyl)amino)pentan-3-ol and ddemmpH = 1-(dimethylamino)-5-((2-(dimethylamino)ethyl) (methyl)amino)-3-methylpentan-3-ol). In single-crystal X-ray crystallography, complex 1 exhibited a dimeric structure with μ2-O bonds of the ddemap ligand. All complexes exhibited high volatility and could be sublimed under reduced pressure (0.5 Torr) at 160 °C, indicating that these complexes are promising candidates as atomic layer deposition or chemical vapor deposition precursors for the growth of barium-containing thin films.
Introduction
Heavier alkaline-earth metal compounds containing calcium, strontium, and barium are key components of magnetoresistive, ferroelectric, and piezoelectric materials for dynamic random access memory, a precursor for thin-film growth, organic synthesis, and electrically tunable microwave devices.1−15 In particular, electroceramics containing barium oxide (BaO) and transition-metal oxides such as BaTiO3, BaSrTiO3, and Y-doped BaZrO3 exhibit high dielectric constants and high proton conductivity.16−21
Therefore, they are commonly used in low-temperature superconductors, solar cells, high-k capacitors, and fuel cells. For the aforementioned applications, the fabrication of thin films containing these structures is required, which can be deposited using chemical vapor-phase methods such as metal–organic chemical vapor deposition (MOCVD) and atomic layer deposition (ALD). During these processes, it is essential to use precursor compounds with appropriate volatility and thermal stability.
However, the synthesis of barium complexes as CVD/ALD precursors is often limited by poor volatility and thermal stability owing to the formation of oligomeric structures as a result of their large atomic radius, high coordination sphere, and low oxidation number. To prevent the formation of oligomeric compounds, sterically bulky ligands or neural ligands such as tetraethylene glycol dimethyl ethereal (tetraglyme), tetramethylethylenediamine, β-diketonates, and cyclopentadienyl groups have been used to synthesize volatile Ba precursors with saturated metal centers. Precursors such as Ba(β-diketonate)2(glyme), Ba(thd)2, Ba(Me5C5)2, Ba(nPrMe4C5)2, and Ba(tBu3C5H2)2 have been reported to be volatile.22−29
However, there have been challenges such as the instability of the β-diketonate precursors at their evaporation temperatures and the extreme sensitivity of cyclopentadienyl complexes to air (oxygen) and moisture.30 To address these challenges, we synthesized heteroleptic precursors, where the central atom is bonded to different types of ligands such as multidentate aminoalkoxy ligands and β-diketonate ligands, which have a distinct advantage of having a different dissociation pattern for each ligand and might be useful in the development of thin films. Recently, we reported the synthesis of heteroleptic Sr complexes, which exhibited a saturated metal center and improved volatility and thermal stability.9,31 Among these complexes, a heteroleptic strontium complex with amino alkoxide ligand ([Sr(demamp)(tmhd)]2) was used as the ALD precursor to fabricate thin films. Based on the results of our previous study, we conducted further research on heteroleptic Ba precursors using aminoalkoxides and β-diketone ligands. Complexes [Ba(ddemmp)(tmhd))2 and [Ba(ddemap)(tmhd)]2 were prepared through the in situ reaction of barium bis(trimethylsilyl)amides [Ba(btsa)2·2DME] (Scheme 1). All compounds were characterized through Fourier transform infrared spectroscopy (FT-IR), elemental analysis (EA), thermogravimetric analysis (TGA), and nuclear magnetic resonance (NMR) spectroscopy. Additionally, the molecular structure of complex 1 was characterized via X-ray crystallography.
Scheme 1. Synthesis of Complexes 1 and 2.
Experimental Section
General Remarks
NMR spectra were recorded using a Bruker 500 MHz spectrometer (1H) and a Bruker 500 MHz spectrometer (13C) with C6D6 as the solvent and reference, respectively. Infrared (IR) spectra were obtained using a Nicolet Nexus FT-IR spectrophotometer. EA was performed using a Thermo Scientific OEA Flash 2000 analyzer. TGAs were conducted using a SETARAM 92–18 TG-DTA instrument with a constant flow of nitrogen (500 mL/min) throughout the experiment under inert conditions.
Ba[N(SiMe3)]2·2DME was prepared according to previously reported method.32 All reactions were performed under inert dry conditions in an argon-filled glovebox. Hexane was purified using an innovative technology PS-MD-4 solvent-purification system. All other chemicals were purchased from Aldrich and Alfa Aesar without further purification. The melting point was measured using a Stuart SMP40 automatic melting point apparatus.
ddemapH: dimethyl amine solution [2.0 M in tetrahydrofuran (THF)/50 mL, 0.1 mol] was added dropwise at 0 °C with constant stirring to 40% ethanol solution of epichlorohydrin (9.2 g, 0.1 mol) in a three-necked flask fitted with a reflux condenser. After the addition, the reaction mixture was warmed to room temperature and stirring was continued for another 6 h. Then, trimethylethane-1,2-diamine (10.2 g, 0.1 mol) was added to the reaction mixture, which was then refluxed for 12 h. After the volatiles and solvent (ethanol and THF) were removed, the product was extracted with methylene chloride three times. The solvent was removed, and the crude product was distilled (110 °C/0.5 Torr) to obtain a colorless liquid (10 g, 50%).1H NMR (500 MHz, C6D6): δ 2.05 (s, 6H, (CH3)2NCH2) 2.20–2.45 (m, br, 8H, NCH2CH, CHCH2N, NCH2CH2,CH2CH2N), 2.13 (s, 6H, (CH3)2NCH2), 2.15 (s, 3H, CH3NCH2), 3.84 (m, 1H, CH2CHCH2). 13C NMR (125 MHz, C6D6): δ 44.0 ((CH3)2NCH2), 45.4 (CH3NCH2), 46.3 (CH3)2NCH2), 56.0 (NCH2CH2), 57.8 (NCH2CH), 62.1 (CH2CHCH2), 64.3 (CH2CHCH2), and 67.1 (CH2CHCH2) ppm.
ddemmpH: dimethyl amine solution (2.0 M in THF/50 mL, 0.1 mol) was added dropwise at 0 °C with constant stirring to 40% ethanol solution of 2-(chloromethyl)-2-methyloxirane (10 g, 0.1 mol) in a three-necked flask fitted with a reflux condenser. After the addition, the reaction mixture was warmed to room temperature and stirring was continued for another 6 h. Then, trimethylethane-1,2-diamine (10.2 g, 0.1 mol) was added to the reaction mixture, which was then refluxed for 12 h. After the volatiles and solvent (ethanol and THF) were removed, the product was extracted with methylene chloride three times. The solvent was removed, and the crude product was distilled (110 °C/0.5 Torr) to obtain a colorless liquid (12 g, 60%).1H NMR (500 MHz, C6D6): δ = 1.24 (s, 3H, CH3CCH2), 2.02 (s, 6H, (CH3)2NCH2) 2.08–2.62 (m, br, 8H, NCH2CH, CHCH2N, NCH2CH2,CH2CH2N), 2.27 (s, 3H, CH3NCH2), 2.30 (s, 6H, (CH3)2NCH2). 13C NMR (125 MHz, C6D6): δ 26.0 (CH3CCH2), 45.1 ((CH3)2NCH2), 46.9 (CH3NCH), 48.2 (CH3)2NCH2), 57.7 (NCH2CH2), 58.1 (NCH2CH), 65.6 (CH2CHCH2), 68.3 (CH2CHCH2), and 73.0 (CH2CHCH2) ppm.
General Procedure of the Synthesis of [Ba(ddemap)(tmhd)]2 and [Ba(ddemmp)(tmhd)]2
A hexane solution (10 mL) containing aminoalcohol ligands, ddemapH or ddemmpH, was added dropwise to a solution of Ba[(btsa)]2·2DME in hexane (20 mL) at room temperature with constant stirring. After stirring for 20 min at room temperature, 2,2,6,6-tetramethyl-3,5-heptadione (tmhdH) was added to the reaction mixture and stirred for another 15 h at room temperature. Subsequently, the mixture was filtered and the volatiles were removed in vacuo to obtain the product as a white solid. X-ray-quality crystals were grown from a saturated solution in toluene at −30 °C.
[Ba(ddemmp)(tmhd)]2 (1). Ba(btsa)2·2DME (0.64 g, 1.0 mmol), ddemapH (0.20 g, 1.0 mmol), and tmhdH (0.18, 1.0 mmol) were used. Yield: 0.38 g (82%). mp > 200 °C. 1H NMR (500 MHz, C6D6): δ 1.35 (s, 18H, (CH3)3CCH), 1.85–2.66 (m, br, 8H, NCH2CH, CHCH2N, NCH2CH2,CH2CH2N), 2.13 (s, 3H, CH3NCH2), 2.17 (s, 6H, (CH3)2NCH2), 2.33 (s, 6H, CH2N(CH3)2), 4.34 (s, 1H, CH2CHCH2), and 5.81 (s, 1H, CCHC) ppm. 13C NMR (125 MHz, C6D6): δ 29.3 ((CH3)3CCO), 41.1 ((CH3)3CCO), 41.3 ((CH3)2NCH2), 44.7 (CH3NCH2), 57.4 (CH3)2NCH2), 67.4 (NCH2CH2), 68.9 (OCCH2), 69.3 (NCH2CH), 72.7 (CHCH2N), 87.1 (CH2CHCH2), and 197.0 (COCHCO) ppm. FT-IR (KBr, cm–1) = 2949 (s), 2898 (m), 2820(s), 2790(s), 1602 (s), 1576 (m), 1473 (m), 1455 (s), 1419 (s), 1356 (m), 1182 (m), 1144 (m), 1039 (w), and 863 (w). C42H86N6O6Ba2 (1045.82) Calcd: C, 48.23; H, 8.29; N, 8.04. Found: C, 47.78; H, 8.59; N, 7.99.
[Ba(ddemmp)(tmhd)]2 (2). Ba(btsa)2·2DME (0.64 g, 1.0 mmol), ddemmpH (0.22 g, 1.0 mmol), and tmhdH (0.18, 1.0 mmol) were used. Yield: 0.41 g (85%). mp > 200 °C. 1H NMR (500 MHz, C6D6): δ 1.35 (s, 18H, (CH3)3CCH), 1.40 (s, 3H, CH3CCH2) 2.11–2.43 (m, br, 8H, NCH2CH, CHCH2N, NCH2CH2,CH2CH2N), 2.19 (s, br, 3H, CH3NCH2), 2.26 (s, br, 6H, (CH3)2NCH2), 2.28 (s, 6H, CH2N(CH3)2) and 5.81 (s, 1H, CCHC) ppm. 13C NMR (125 MHz, C6D6): δ 29.3 ((CH3)3CCO), 41.2 ((CH3)3CCO), 45.4 (CH3CCH2), 46.3 ((CH3)2NCH2), 48.4 (CH3NCH2), 58.1 (CH2NCH3), 71.6 (OCCH3), 74.2 (CH2CCH2), 75.6 (CH2CCH2), 87.1 (CH2CHCH2), and 197.0 (COCHCO) ppm. FT-IR (KBr, cm–1) = 2949 (s), 2898 (m), 2861 (m), 2820 (m), 2790 (m), 1602 (s), 1576 (m), 1505 (m), 1473 (m), 1455 (s), 1419 (s), 1356 (m), 1182 (m), 1144 (m), 1039 (w), 960 (w), and 863 (w). C43H88N6O6Ba2 (1059.85) Calcd: C, 48.73; H, 8.37; N, 7.93. Found: C, 47.95; H, 8.60; N, 7.99.
Crystallography
A single crystal of 1 was grown through slow evaporation from a saturated solution of hexane in a glovebox at room temperature. Specimens of suitable size and quality were coated with paratone oil and mounted onto a glass capillary. Reflection data were collected using a Bruker Apex II-CCD area detector diffractometer with graphite-monochromatized Mo-Kα radiation (λ = 0.71073 Å). The cell parameters were determined and refined using the SMART program, and data reduction was performed using SAINT software. The data were corrected for Lorentz and polarization effects, and an empirical absorption correction was applied using the SADABS program. The structures were determined using direct methods, and all nonhydrogen atoms were subjected to anisotropic refinement by full-matrix least-squares on F2 using the SHELXTL/PC package. The hydrogen atoms were placed at their geometrically calculated positions and refined based on the corresponding carbon atoms with isotropic thermal parameters. The supplementary crystallographic data used in this study can be found at the Cambridge Crystallographic Data Centre (CCDC):2258733 (complex 1). These data can be obtained free of charge from the CCDC.
Results and Discussion
We have previously reported that the heteroleptic strontium complex [Sr(tmtad)(tmhd)]2 with tmtad and tmhd as the coordinating bulky ligands saturates the metal center30 (tmtadH = 2,5,9,12-tetramethyl-2,5,9,12-tetraazatridecan-7-ol). This complex exhibited a dimeric structure with a nonbonding amine group in its crystal structure and volatile properties. Based on these results, we conducted further research to prepare barium precursors with penta- and tetradentate ligands, where only barium compounds using tetradentate ligands were synthesized as the desired products.
The simple preparation of [Ba(ddemap)(tmhd)]2 and [Ba(ddemmp)(tmhd)]2 complexes was conducted by a controlled in situ reaction using barium bis(trimethylsilyl)amide [Ba(btsa)2·2DME] and appropriate ligands, such as ddemapH, ddemmpH, and tmhdH, to yield the desired products, [Ba(ddemap)(tmhd)]2 (1) and [Ba(ddemmp)(tmhd)]2 (2) (Scheme 1). All complexes were obtained in moderate yields of 80–90% as a white powder and purified through recrystallization from saturated hexane solution at −30 °C or sublimation (160 °C/0.5 Torr). In single X-ray crystallography, complex 1 exhibited a fully saturated dimeric structure without a nonbonding amine group. The 1H NMR spectra of complexes 1 and 2 in the benzene-d6 solution exhibited a downfield shift of the two amino groups [−N(CH3)2] compared to the free ligands [CCH2N(CH3)2] and the absence of the btsa peak (Figures S1–S4), where 1H NMR spectra of complex 2 exhibited broad peaks. The FT-IR spectra of all complexes indicated the absence of Si–CH3 rocking vibration and −NH peaks from the btsa group; however, they exhibited peaks for C=O stretching in coordinated β-diketonate at 1602 and 1602 cm–1, respectively, indicating that the reaction proceeded successfully (Figures S5 and S6).
Crystallography
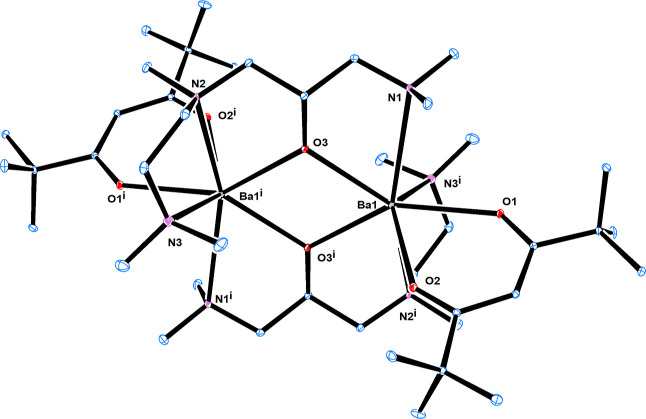
The single-crystal X-ray study revealed that complex 1 crystallizes in the triclinic space group and exists as a dimer with an alkoxy oxygen bridging the two barium atoms in the complex through μ2-O bonding. In complex 1, each of the metal centers is fully saturated by four oxygen atoms and three nitrogen atoms from the ddemap and one tmhd ligand, exhibiting a distorted capped trigonal prismatic structure, which is similar to the result of a previous report [Ba(tmtad)(tmhd)]2 (Figure 1).30 The distance between the two barium atoms in complex 1 is 4.112(8) Å, which is shorter than that of [Ba(demamp)(btsa)]2 [4.134(2) Å].9 The reason for the reduced intermetal distance may be attributed to the replacement of the sterically bulky btsa group with a more compact tmhd group and ddemap ligand that includes an additional amine group. On average, the distance between the metal centers and the μ2-bridging alkoxide oxygen atoms is 2.587 Å, which is slightly longer than the corresponding distance in [Ba(demamp)(btsa)]2 (2.558 Å) (Table 1). The average bond lengths of Ba–N and Ba–O were 2.951 and 2.625 Å, respectively, and the bridging angle of Ba–O–Ba was 105.59 (7)°.
Figure 1.
Oak Ridge Thermal Ellipsoid Plot drawing of the crystal structure of [Ba(ddemmp)(tmhd)]2 (1). The ellipsoids represent a probability of 30%.
Table 1. Selected Bond Lengths (Å) and Bond Angles (°) for 1.
| bond lengths (Å) | 1 | bond angles (°) | 1 |
|---|---|---|---|
| Ba1–O3 | 2.588(2) | O3i–Ba1–O3 | 74.41(7) |
| Ba1–O3i | 2.585(2) | O3i–Ba1–N2i | 61.63(7) |
| Ba1–O1 | 2.638(2) | N2i–Ba1–N3i | 61.78(8) |
| Ba1–O2 | 2.603(2) | O3–Ba1–N1 | 60.98(7) |
| Ba1–N2i | 2.928(3) | O1–Ba1–O2 | 65.82(7) |
| Ba1–N3i | 2.959(3) | Ba1–O3–Ba1i | 105.59(7) |
| Ba1–N1 | 2.969(3) | ||
| Ba1–Ba1i | 4.112(8) |
Thermogravimetric Analysis
TGA of complexes 1 and 2 was conducted from room temperature to 500 °C (Figure 2) Prior to this, sampling was conducted in an argon-filled glovebox, and data were collected under a constant flow of nitrogen to avoid any air contact. Complexes 1 and 2 exhibited 2% weight loss before reaching 250 °C. This mass loss can be explained by the evaporation of hexane from the sample. All compounds displayed clean one-step curves with 87% and 81% mass losses in the temperature range of 250–365 °C, where residual masses of 11% and 17% were observed, respectively. The residual masses of complexes 1 and 2 are comparable to those of BaO and BaCO3 (calcd. 14% and 18%). All compounds exhibited high volatility and a low amount of nonvolatile residues. The sublimation tests of complexes 1 and 2 were performed under 0.5 Torr (160 °C), where the yields of sublimed products were in the range of 80–90%.
Figure 2.
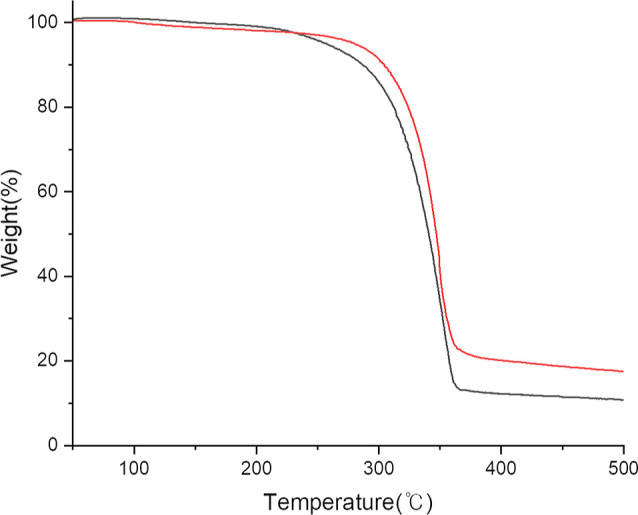
TGA plot of complexes 1 (black) and 2 (red).
Conclusions
In this study, we successfully synthesized and characterized novel heteroleptic barium complexes; [Ba(ddemmp)(tmhd))2(1) and [Ba(ddemap)(tmhd)]2(2). All complexes were obtained as white crystalline powders, where complex 1 exhibited a dimeric structure with a μ2-bridging alkoxide oxygen and distorted capped trigonal prismatic geometry in single-crystal X-ray crystallography. All the complexes are promising candidates for thin-film applications, as evidenced by their volatility and TGA results. These compounds exhibited a clean single-step TG curve and low nonvolatile residue and were sublimed at 0.5 Torr (160 °C). These results indicate the advantage of designing suitable heteroleptic complexes with fully saturated metal centers..
Acknowledgments
This work was supported by the Materials and Components Technology Development Program (20010275, Development of ALD precursors for high k thin film in logic and DRAM Flash memory devices) funded by the Ministry of Trade, Industry & Energy (MOTIE, Republic of Korea), and the Development of smart chemical materials for IoT devices Project through the Korea Research Institute of Chemical Technology (KRICT) of Republic of Korea (KS2321-10).
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.3c01697.
The authors declare no competing financial interest.
Supplementary Material
References
- Žurauskienė N.; Balevičius S.; Stankevič V.; Paršeliu̅nas J.; Keršulis S.; Abrutis A.; Plaušinaitienė V. Influence of Sr Content on CMR effect in polycrystalline La1-xSrxMnO3 thin films. Acta Phys. Pol., A 2009, 115, 1136–1138. 10.12693/aphyspola.115.1136. [DOI] [Google Scholar]
- Pignard S.; Yu-Zhang K.; Leprince-Wang Y.; Han K.; Vincent H.; Sénateur J. P. Correlation between Agnetoresistive properties and growth morphology of La1-xMnO3-δ thin films deposited on SrTiO3, LaAlO3 and MgO. Thin Solid Films 2001, 391, 21–27. 10.1016/s0040-6090(01)00970-1. [DOI] [Google Scholar]
- Gorbenko O. Y.; Bosak A. A.; Kaul A. R.; Babushkina N. A.; Belova L. M. Colossal magnetoresistive thin films of (La1-xPrx)0.7Ca0.3MnO3 Prepared by Aerosol MOCVD. Mater. Res. Soc. Symp. Proc. 1997, 495, 333–338. 10.1557/proc-495-333. [DOI] [Google Scholar]
- Sakabe Y.; Takeshima Y.; Tanaka K. Multilayer ceramic capacitors with thin (Ba,Sr)TiO3 layers by MOCVD. J. Electroceram. 1999, 3, 115–121. 10.1023/a:1009986825169. [DOI] [Google Scholar]
- Regnery S.; Ehrhart P.; Fitsilis F.; Waser R.; Ding Y.; Jia C. L.; Schumacher M.; Schienle F.; Juergensen H. (Ba, Sr) TiO3 thin film growth in a batch processing MOCVD reactor. J. Eur. Ceram. Soc. 2004, 24, 271–276. 10.1016/s0955-2219(03)00235-8. [DOI] [Google Scholar]
- Matthews J. S.; Rees W. S. Group 2 element precursors for the chemical vapor deposition of electronic materials. Adv. Inorg. Chem. 2000, 50, 173–192. 10.1016/s0898-8838(00)50004-x. [DOI] [Google Scholar]
- Wojtczak W. A.; Fleig P. F.; Hampden-Smith M. J. A review of Group 2 (Ca, Sr, Ba) metal-organic compounds as precursors for chemical vapor deposition. Adv. Organomet. Chem. 1996, 40, 215–340. [Google Scholar]
- Marks T. J. Coordination chemistry routes to films for superconducting electronics. Pure Appl. Chem. 1995, 67, 313–318. 10.1351/pac199567020313. [DOI] [Google Scholar]
- George S. M.; Park B. K.; Kim C. G.; Chung T.-M. Heteroleptic Group 2 metal precursors for metal oxide thin films. Eur. J. Inorg. Chem. 2014, 2014, 2002–2010. 10.1002/ejic.201301296. [DOI] [Google Scholar]
- Buch F.; Harder S. A study on chiral organocalcium complexes: attempts in enantioselective catalytic hydrosilylation and intramolecular hydroamination of alkenes. Z. Naturforsch. 2008, 63, 169–177. 10.1515/znb-2008-0209. [DOI] [Google Scholar]
- Spielmann J.; Harder S. Reduction of Ketones with Hydrocarbon-Soluble Calcium Hydride: Stoichiometric Reactions and Catalytic Hydrosilylation. Eur. J. Inorg. Chem. 2008, 2008, 1480–1486. 10.1002/ejic.200701255. [DOI] [Google Scholar]
- Crimmin M. R.; Barrett A. G. M.; Hill M. S.; Hitchcock P. B.; Procopiou P. A. Calcium-Catalyzed Intermolecular Hydrophosphination. Organometallics 2007, 26, 2953–2956. 10.1021/om070200k. [DOI] [Google Scholar]
- Spielmann J.; Harder S. Hydrocarbon-soluble calcium hydride: A “Worker-Bee” in calcium chemistry. Chemistry 2007, 13, 8928–8938. 10.1002/chem.200701028. [DOI] [PubMed] [Google Scholar]
- Ruspic C.; Harder S. Big Ligands for Stabilization of Small Functionalities in Calcium Chemistry. Inorg. Chem. 2007, 46, 10426–10433. 10.1021/ic701479r. [DOI] [PubMed] [Google Scholar]
- Barrett A. G. M.; Crimmin M. R.; Hill M. S.; Hitchcock P. B.; Procopiou P. A. Trifluoromethyl Coordination and C-F Bond Activation at Calcium. Angew. Chem. 2007, 46, 6339–6342. 10.1002/anie.200701945. [DOI] [PubMed] [Google Scholar]
- Malghe Y. S.; Gurjar A. V.; Dharwadkar S. R. Synthesis of BaTiO3 powder from barium titanyl oxalate (BTO) precursor employing microwave heating technique. Bull. Mater. Sci. 2004, 27, 217–220. 10.1007/bf02708508. [DOI] [Google Scholar]
- Win T.; Naing K.; Tun K. M. Synthesis of barium titanate from titanyl acylate precursor by sol-precipitate method. J. Myanmar Acad. Arts Sci. 2008, 6, 61–70. [Google Scholar]
- Hatanpää T.; Vehkamäki M.; Mutikainen I.; Kansikas J.; Ritala M.; Leskelä M. Synthesis and characterisation of cyclopentadienyl complexes of barium: precursors for atomic layer deposition of BaTiO3. Dalton Trans. 2004, 8, 1181–1188. 10.1039/b400235k. [DOI] [PubMed] [Google Scholar]
- Acharya S.; Torgersen J.; Kim Y.; Park J.; Schindler P.; Dadlani A. L.; Winterkorn M.; Xu S.; Walch S. P.; Usui T.; Schildknecht C.; Prinz F. B. Self-limiting atomic layer deposition of barium oxide and barium titanate thin films using a novel pyrrole based precursor. J. Mater. Chem. C 2016, 4, 1945–1952. 10.1039/c5tc03561a. [DOI] [Google Scholar]
- Daly S. R.; Bellott B. J.; Nesbit M. A.; Girolami G. S. Synthesis and structural diversity of barium (N,N-dimethylamino)diboranates. Inorg. Chem. 2012, 51, 6449–6459. 10.1021/ic2016879. [DOI] [PubMed] [Google Scholar]
- Oh S. H.; Ko J. H.; Lee H. Y.; Lazar I.; Roleder K. Precursor phenomena of barium titanate single crystals grown using a solid-state single crystal growth method studied with inelastic Brillouin light scattering and birefringence measurements. Molecules 2018, 23, 3171–3184. 10.3390/molecules23123171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumayer D. A.; Studebaker D. B.; Hinds B. J.; Stern C. L.; Marks T. J. New precursors for barium metal-organic chemical vapor deposition. In situ growth of epitaxial barium titanate films using a liquid barium precursor. Chem. Mater. 1994, 6, 878–880. 10.1021/cm00043a002. [DOI] [Google Scholar]
- Tammenmaa M.; Antson H.; Asplund M.; Hiltunen L.; Leskelä M.; Niinistö L.; Ristolainen E. Alkaline earth sulfide thin films grown by atomic layer epitaxy. J. Cryst. Growth 1987, 84, 151–154. 10.1016/0022-0248(87)90122-9. [DOI] [Google Scholar]
- Saanila V.; Ihanus J.; Ritala M.; Leskelä M. Atomic layer epitaxy growth of BaS and BaS:Ce thin Filmsfrom in situ synthesized Ba(thd)2. Chem. Vap. Deposition 1998, 04, 227–233. . [DOI] [Google Scholar]
- Ihanus J.; Hänninen T.; Hatanpää T.; Aaltonen T.; Mutikainen I.; Sajavaara T.; Keinonen J.; Ritala M.; Leskelä M. Atomic layer deposition of SrS and BaS thin films using cyclopentadienyl precursors. Chem. Mater. 2002, 14, 1937–1944. 10.1021/cm0111130. [DOI] [Google Scholar]
- Ihanus J.; Hänninen T.; Hatanpää T.; Ritala M.; Leskelä M. Electroluminescent SrS and BaS thin films deposited by ALD using cyclopentadienyl precursors. J. Electrochem. Soc. 2004, 151, 221–225. 10.1149/1.1787633. [DOI] [Google Scholar]
- Shim J. H.; Park J. S.; An J.; Gür T. M.; Kang S.; Prinz F. B. Intermediate-temperature ceramic fuel cells with thin film yttrium-doped barium zirconate electrolytes. Chem. Mater. 2009, 21, 3290–3296. 10.1021/cm900820p. [DOI] [Google Scholar]
- Vehkamäki M.; Hatanpää T.; Ritala M.; Leskelä M.; Väyrynen S.; Rauhala E. Atomic layer deposition of BaTiO3 thin films – Effect of barium hydroxide formation. Chem. Vap. Deposition 2007, 13, 239–246. 10.1002/cvde.200606538. [DOI] [Google Scholar]
- Condorelli G. G.; Malandrino G.; Fragalà I. L. Engineering of molecular architectures of β-diketonate precursors toward new advanced materials. Coord. Chem. Rev. 2007, 251, 1931–1950. 10.1016/j.ccr.2007.04.016. [DOI] [Google Scholar]
- Hatanpää T.; Ritala M.; Leskelä M. Precursors as enablers of ALD technology: contributions from University of Helsinki. Coord. Chem. Rev. 2013, 257, 3297–3322. 10.1016/j.ccr.2013.07.002. [DOI] [Google Scholar]
- Park C.; Cho C.; Park B. K.; Hong C. S.; Chung T.-M. Synthesis and characterization of new strontium complexes with multidentate ligands. ChemistrySelect 2022, 7, e202104606 10.1002/slct.202104606. [DOI] [Google Scholar]
- Westerhausen M. Synthesis and spectroscopic properties of bis(trimethylsilyl)amides of the alkaline-earth metals magnesium, calcium, strontium, and barium. Inorg. Chem. 1991, 30, 96–101. 10.1021/ic00001a018. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.