Abstract
Background: The effect of europinidin on alcoholic liver damage in rats was examined in this research. Methods: A total of 24 Wistar rats were grouped in the same way into four groups: normal control (normal), ethanol control (EtOH), europinidin low dose (10 mg/kg), and europinidin higher dose (20 mg/kg). The test group rats were orally treated with europinidin-10 and europinidin-20 for 4 weeks, whereas 5 mL/kg distilled water was administered to control rats. In addition, 1 h after the last dose of the above-mentioned oral treatment, 5 mL/kg (i.p.) EtOH was injected to induce liver injury. After 5 h of EtOH treatment, samples of blood were withdrawn for biochemical estimations. Results: Administration of europinidin at both doses restored all of the estimated serum, i.e., liver function tests (ALT, AST, ALP), biochemical test (Creatinine, albumin, BUN, direct bilirubin, and LDH), lipid assessment (TC and TG), endogenous antioxidants (GSH-Px, SOD, and CAT), malondialdehyde (MDA), nitric oxide (NO), cytokines (TGF-β, TNF-α, IL-1β, IL-6, IFN-γ, and IL-12), caspase-3, and nuclear factor kappa B (NF-κB) associated with the EtOH group. Conclusion: The results of the investigation showed that europinidin had favorable effects in rats given EtOH and may have hepatoprotective potential property.
1. Introduction
Alcohol is a popularly consumed beverage worldwide.1 Consumption of excessive alcohol for a prolonged time can lead to a variety of sociomedical and public health issues around the world1 and can contribute to hepatic damage.2 Alcohol-prompted hepatic damage causes steatosis, necrosis, and decreased liver cell regeneration, which eventually leads to cirrhosis and liver fibrosis.3 Most population die worldwide every year as a consequence of alcohol-producing hepatic damage.4 Hence, alcohol-producing hepatic disease prevention and treatment are becoming more important public health concerns around the world.4−6
Alcohol processed into acetaldehyde in the liver and high levels together can contribute to oxidative pressure and apoptosis in hepatocytes, which can result in tissue inflammation and fibrosis.3 Alcohol-evoked hepatic toxicity is largely influenced by oxidative pressure, lipid peroxidation, tenderness, and the creation of dangerous byproducts, according to an earlier study.4−6 The liver’s cellular immune system can be stimulated by ethanol (EtOH) and acetaldehyde, which can lead to the generation of inflammatory markers like tumor necrosis factor-(TNF-α),7 extracellular medium buildup, and nuclear factor-κB (NF-κB) secretion.3 Alcohol-prompted hepatic damage is heightened by TNF-α and NF-κB, which trigger an inflammatory response and increase oxidative stress.3 The development of novel therapeutic techniques for alcohol-induced liver disease patients remains a significant necessity.
The most often utilized animal models for alcohol-induced liver disease research are rodents (rats). Animal experiments with EtOH can imitate some symptoms of human illness and support identifying the process behind the growth of alcoholic liver disease.2
Flavonoids are phytochemical substances found in many herbs that have therapeutic applications.8,9 Anthocyanidins are sugar-free anthocyanins, which are common plant colors found in fruits, leaves, and flowers.10 Anthocyanins derived from black raspberry have been shown in network analysis and docking studies to protect against alcoholic liver impairment.11 The anthocyanin isolated from Lonicera caerulea L. was reported to have protective action in alcoholic hepatosteatosis in rodents.12
Europinidin is a compound, found in the plants Plumbago Europea and Ceratostigma plumbaginoides, that belongs to the Plumbaginaceae family, and it is an o-methylated derivative of delphinidin.13,14 Potent anti-inflammatory and antioxidant effects have been demonstrated in Europium, a naturally occurring metal.15 The flavonoid europinidin, which is generated from the metal europium, has been demonstrated to have positive effects on liver health.16,17
Previously, europinidin published rotenone-activated Parkinson’s disease by inhibited inflammation and lipid peroxidation,18 effectiveness in preventing streptozotocin-induced memory impairment,19 and ulcer protective activity in rats.20 The cause of the current appraisal was to analyze the consequences of europinidin on EtOH-induced liver damage in rats.
2. Methods
2.1. Animals
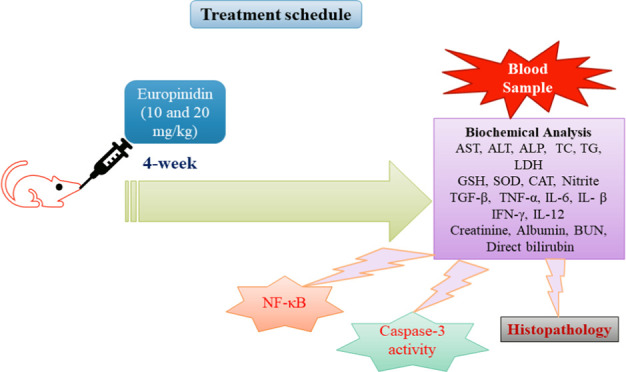
Male Wistar rats (10–12 weeks) weighing 180 ± 20 g were housed in a standard research setup with pellet food, unrestricted access to water, and natural daylight hours (cycle: dark and light). Before being used in research, all of the rats were adjusted to the laboratory surroundings for a week. The study protocol was approved by the Institutional animal ethics committee (CPCSEA-IAEC/TRS/PT/023/028) and conducted with ARRIVE (Animal Research: Reporting of In Vivo Experiments) guidelines.21
2.2. Drugs and Solutions
Europinidin was obtained as a gift sample from SKL in Maharashtra, India. Analytical kits, i.e., TNF-α, transforming growth factor β (TGF-β), interleukins (IL-1β, IL-6, and IL-12), interferon-γ (IFN-γ), NF-κB, and caspase-3 were analyzed using rat enzyme-linked immunosorbent (ELISA), Sigma-Aldrich (St. Louis, MO) assay kits.
2.3. Experimental Design
A total of 24 Wistar rats were divided into four groups with six rats each:
Group I: Normal (normal control)
Group II: EtOH control
Group III: Europinidin-10 mg/kg (EtOH+ Europinidin 10 mg/kg)
Group IV: Europinidin-20 mg/kg (EtOH+ Europinidin 20 mg/kg)18−20
Over the course of four weeks, each of these treatments was given orally every day via an intragastric tube. In addition, 1 h after the last above-mentioned oral dose rats from the normal group received saline (5 mL/kg), rats of groups EtOH, europinidin-10 mg/kg, and europinidin-20 mg/kg were injected with 5 mL/kg EtOH (56%, i.p.).4,22 After 5 h of EtOH treatment, samples of blood were withdrawn through the retro-orbital plexus and serum was separated and evaluated for biochemical tests. Then, rats from all groups were excised liver tissue for the following biochemical analysis.
2.4. Biochemical Analysis
2.4.1. Serum Biochemical Analysis
Biochemical analysis was conducted as per the protocol given by the kit manufacturer (Modern Lab, M.S. India). Alanine transaminase (ALT), aspartate transaminase (AST), alkaline phosphatase (ALP), lactate dehydrogenase (LDH), total cholesterol (TC), and triglycerides (TG) activity were elaborated as per the process given on the kit.
2.4.2. Preparation of Tissue Homogenate
Liver tissue was washed with isotonic saline and homogenized buffer in ice-cold conditions with phosphate-buffered saline. The homogenate was centrifuged at 3000 rpm at 4 °C for 10 min, and then the supernatants were collected for biochemical analysis by using commercially available kits.
2.5. Endogenous Antioxidants and Malondialdehyde
The expression of oxidative stress-related indexes malondialdehyde (MDA), catalase (CAT), glutathione peroxidase (GSH-Px), and superoxide dismutase (SOD) was examined in this study.23,24 To evaluate the quantity of GSH-Px, the Ellman method was utilized.25 1.0 mL of centrifuged Ellman reagent and 10% trichloroacetic acid were contorted to the homogenate. The absorbance of the reactive assortment was monitored at 412 nm, and U/mL tissue demonstrated GSH-Px activity. The Misra and Frodvich method was employed to analyze SOD.26 To start the reaction, 0.2 mL of brain homogenate supernatant was combined with 0.8 mL of glycine buffer (50 mM, pH 10.4) and 0.02 mL of epinephrine. The mixture was then left undisturbed for 5 min. Every minute, the difference in optical density at 480 nm was calculated and normalized to a blank reagent. SOD activity was demonstrated as a unit of measurement (U/mL). CAT activity was determined by the H2O2 decomposition method.27 0.1 mL of tissue buoyant and 1.9 mL of phosphate buffer were added to the cuvette (50 mM, pH 7.0). To start the reaction, 1.0 mL of newly made H2O2 (30 mM) was assorted in the cuvette. The absorbance at 240 nm wavelength was examined every 10 s for 1 min. The number of enzymes required to mold 1 mmol of peroxide every minute at 25 °C with pH 7.0 was determined to be 1 min of CAT. The Wills technique was used to analyze MDA. Thiobarbituric acid (TBA, Sigma-Aldrich)-sensitive substances combine with thiobarbituric acid to form the metabolite TBA-MDA. Peak absorbance was determined spectrophotometrically at 532 nm, and activity was signified as nmol/mL tissue.23
2.6. Cytokines and Nitric Oxide
The quantity of nitric oxide (NO), TGF-β, TNF-α, IL-1β, IL-6, IFN-γ, and IL-12 was estimated using an analytical kit as per the manufacturer’s guidelines in compliance with that recommendation.28 For the NO determination, the Griess reagent [0.1% N-(1-naphthyl) ethylenediamine dihydrochloride, 1% sulfanilamide, and 2.5% H3PO4] was combined with 100 mL of supernatants from various groups. The sample was first homogenized or sonicated to ensure uniformity and then centrifuged to remove any solid debris or cellular material. The resulting supernatant contains the soluble components of the sample. Total nitrites were quantified spectrophotometrically at 540 nm after being nurtured for 10 min at ambient temperature in the dark. Using a NaNO2 standard curve, the concentration of nitrite in the specimen was calculated.29
2.7. Creatinine, Albumin, Blood Urea Nitrogen (BUN), and Direct Bilirubin
Using a commercial kit, the Henry approach was used to quantify serum creatinine, total bilirubin, and BUN using a Coulter STKS Counter model SPlus.30 Serum albumin was analyzed and measured using an automatic analyzer.31,32
2.8. Inflammatory Biomarker (NF-κB) and Caspase-3
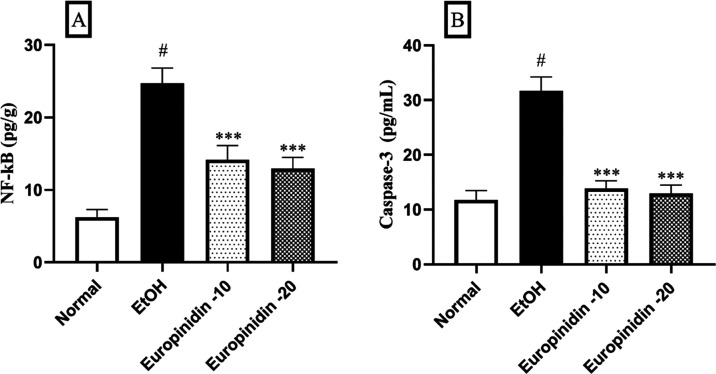
The intensities of NF-κB and caspase-3 were determined with an ELISA kit by carrying out the assay with the protocol. Concentrations of inflammatory biomarkers NF-κB were shown in pg/g, and caspase-3 concentrations were displayed in pg/mL.
2.9. Histopathology
The liver samples were swiftly taken from rats to investigate pathological changes. They were preserved in a 10% buffered formalin solution for 24 h, then embedded in paraffin, and sliced into 5 mm pieces. The samples were stained with hematoxylin and eosin (H&E) to visualize the alterations. Histopathological analysis was done using a light microscope (Olympus Corporation, Tokyo, Japan).33,34
2.10. Statistics
The values of results were analyzed using GraphPad Prism (version 8.0.1) Software, Inc., and represented as mean ± SEM. The data were examined by one-way analysis of variance (ANOVA) followed by Tukey’s test. P < 0.05 was measured as statistically substantial.
3. Results
3.1. Liver Parameters
The AST, ALT, and ALP levels were elevated considerably (P < 0.001) in the EtOH group than the normal group. Europinidin-10 and -20 mg/kg decreased with the rise of AST [F (3, 20) = 12.77 (P < 0.0001)], ALT [F (3, 20) = 58.69 (P < 0.001)] and ALP [F (3, 20) = 14.49 (P < 0.001)] levels in EtOH-treated animals. The outcomes were found to be significant vs the EtOH group. The outcomes are graphically represented in Figure 1A–C.
Figure 1.
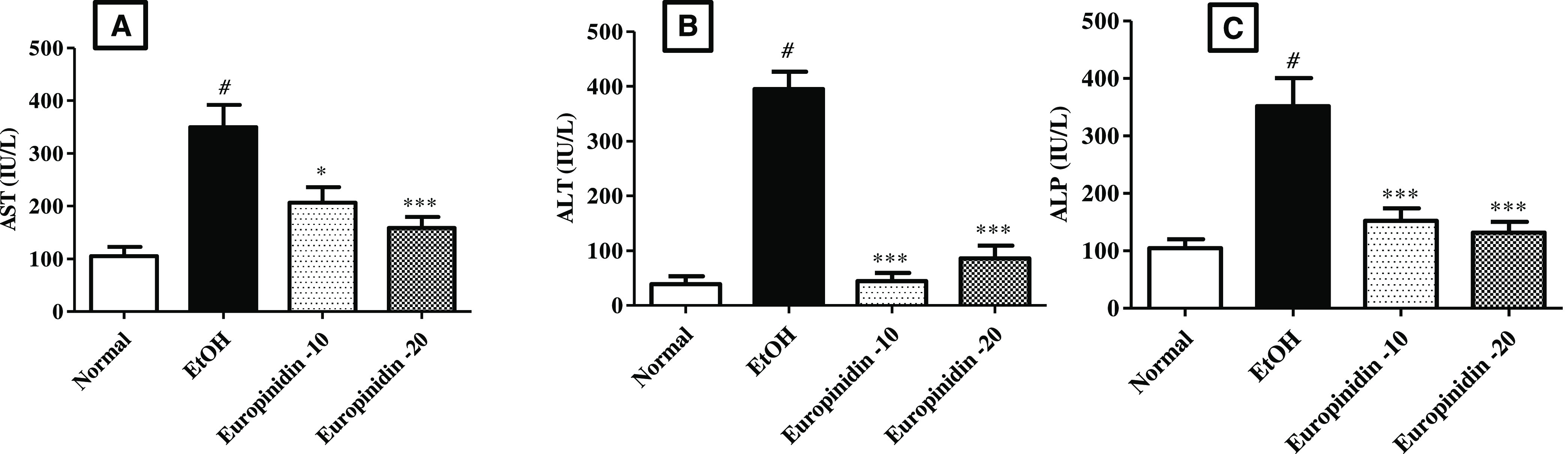
(A–C) Effect of europinidin on (A) AST, (B) ALT, and (C) ALP in alcoholic liver-diseased rats. #P < 0.001 vs normal and *P < 0.05 and ***P < 0.0001 vs EtOH”.
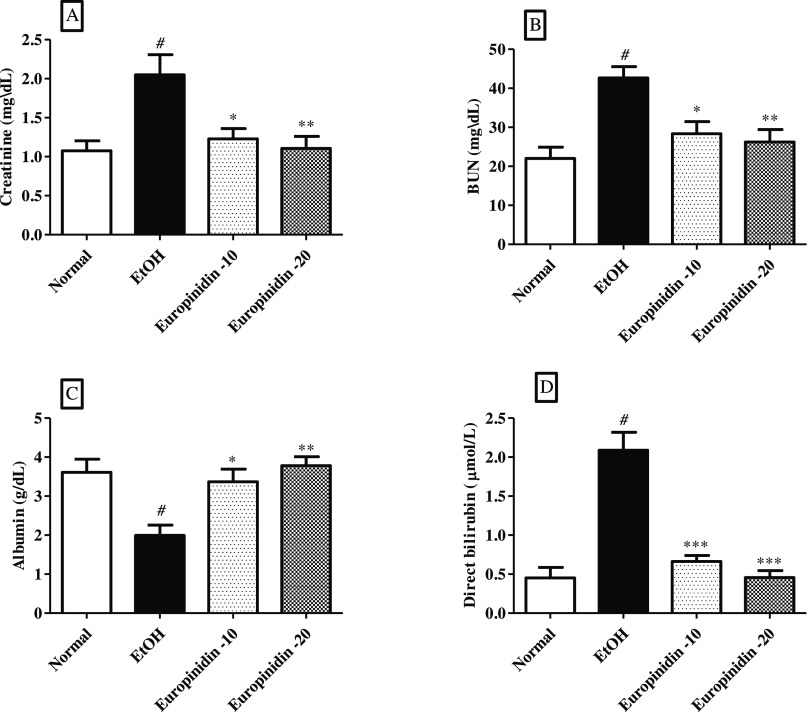
3.2. Creatinine, Albumin, BUN, and Direct Bilirubin
Creatinine, BUN, and bilirubin quantity shoot up in EtOH-induced group in comparison to normal control group, whereas albumin levels reduced in EtOH-control group as related to the normal group. The results of creatinine [F (3,20) = 6.833 (P = 0.0024)], BUN [F (3, 20) = 8.742 (P = 0.0007)], bilirubin [F (3, 20) = 29.66 (P < 0.0001)], and albumin [F (3, 20) = 7.864 (P = 0.0012)] in the europinidin-treated animals were found to be statistically significant. The outcomes are graphically represented in Figure 2A–D.
Figure 2.
(A–D) Effect of europinidin on (A) Creatinine, (B) BUN, (C) albumin, and (D) direct bilirubin in alcoholic liver-diseased rats. #P < 0.001 vs normal and *P < 0.05, **P < 0.001, and ***P < 0.0001 vs EtOH”.
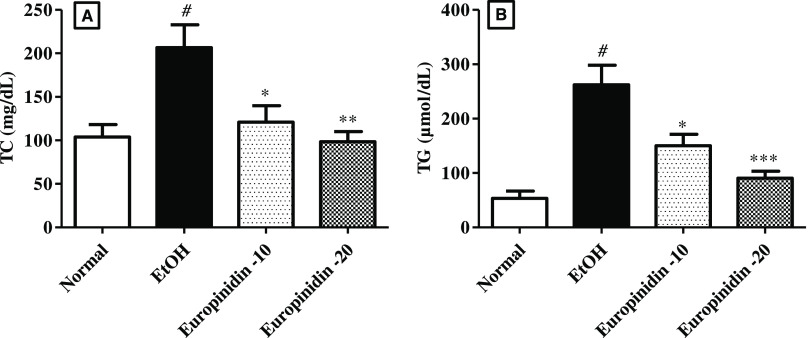
3.3. Serum Lipid Profile
The effect of europinidin on TC and TG levels in EtOH-treated animals is represented in Figure 3A,B. TC [F (3, 20) = 7.314 (P = 0.0017)] and TG [F (3, 20) = 16.00 (P < 0.0001)] were drastically evaluated in EtOH-treated animals than normal. Administration of europinidin (10 and 20 mg/kg) to EtOH-given rats lessened the levels of TC [F (3, 20) = 7.314 (P < 0.0001)] and TG [F (3, 20) = 16.00 (P < 0.0001)] toward normal vs EtOH-control group.
Figure 3.
(A, B) Effect of europinidin on (A) TC and (B) TG in alcoholic liver-diseased rats. #P < 0.001 vs normal and *P < 0.05, **P < 0.001, and ***P < 0.0001 vs EtOH”.
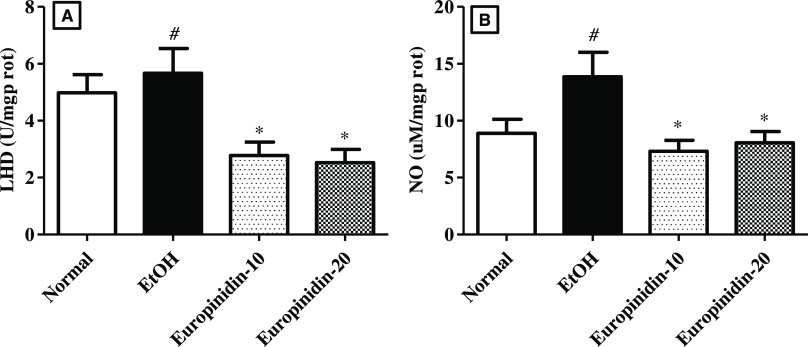
3.4. LDH
The circulating LDH intensity was elevated considerably (P < 0.001) in animals of the EtOH group vs normal animals. Europinidin attenuated the quantity of LDH in the rats compared to the EtOH group animals [F (3, 20) = 6.156 (P = 0.0039)]. The results consequence of europinidin on LDH in EtOH-treated animals are shown in Figure 4A.
Figure 4.
(A, B) Effect of europinidin on (A) LDH and (B) NO in alcoholic liver-diseased rats. #P < 0.001 vs normal and *P < 0.05 vs EtOH”.
3.5. Nitric Oxide
The production of NO (P < 0.05) was drastically upraised in EtOH-treated rats vs normal. Dosing of europinidin (10 and 20 mg/kg) attenuated the intensities of NO [F (3, 20) = 4.385 (P < 0.0001)] vs EtOH group. The results are depicted in Figure 4B
3.6. Endogenous Antioxidants and MDA
Figure 5A–C represents the influence of europinidin on hepatic tissue endogenous antioxidant levels in EtOH-treated animals. The quantity of GSH-Px, SOD, and CAT level decreased in the EtOH group (P < 0.001). Europinidin (10 and 20 mg/kg) reinstated the amounts of GSH [F (3, 20) = 10.17 (P = 0.0003)], SOD [F (3, 20) = 16.33 (P < 0.0001)] and catalase [F (3, 20) = 6.799 (P = 0.0024)] in EtOH-treated animals. The outcomes were significant (P < 0.05) vs the EtOH group.
Figure 5.
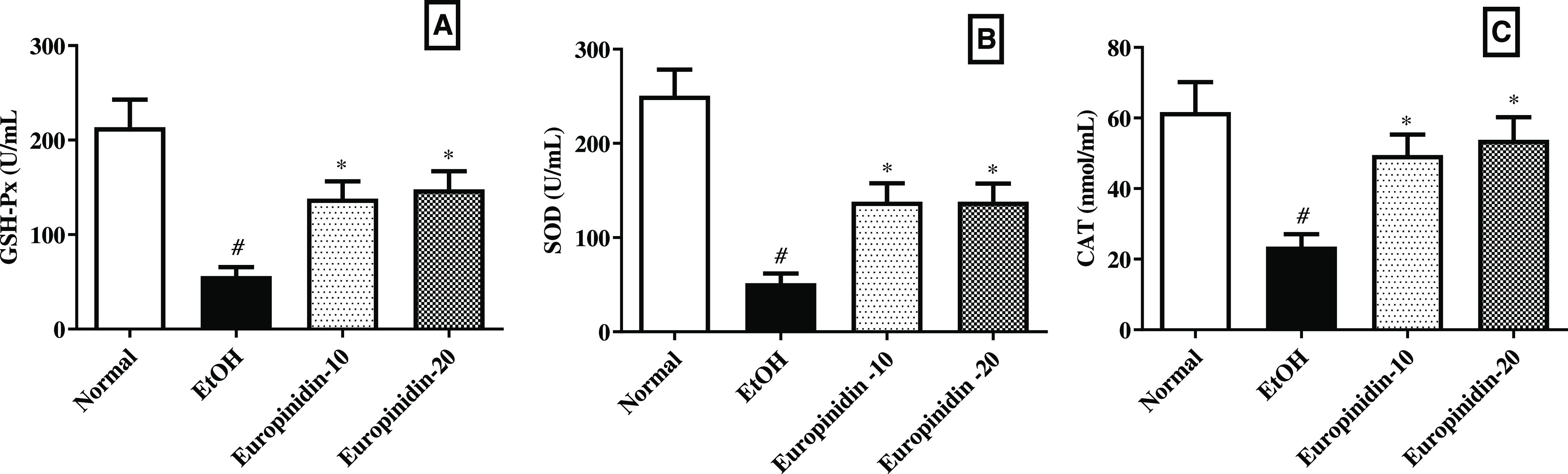
(A–C) Effect of europinidin on (A) GSH, (B) SOD, and (C) CAT in alcoholic liver-diseased rats. #P < 0.001 vs normal and *P < 0.05 vs EtOH”.
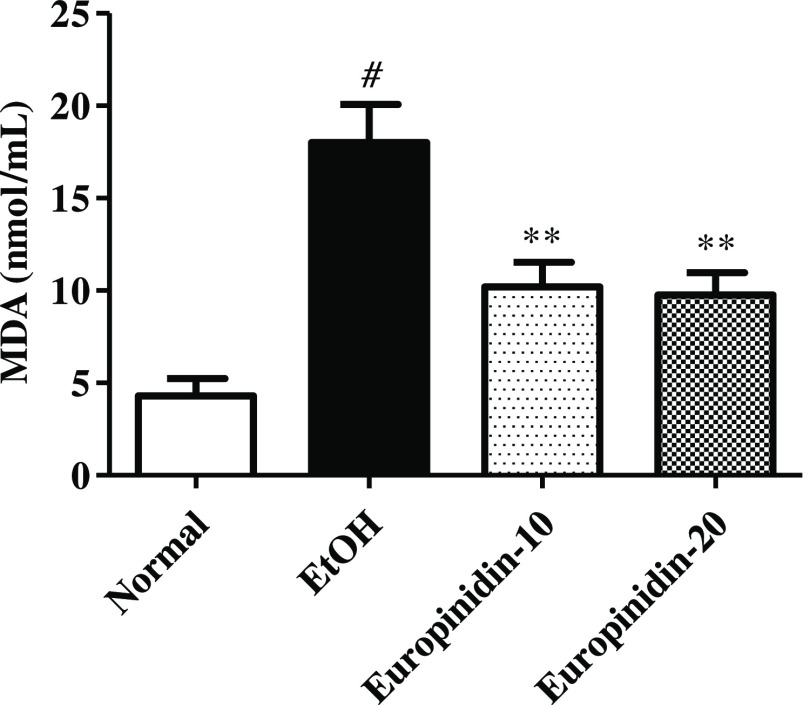
The concentration of MDA was improved drastically in the EtOH group vs normal. Dosing of europinidin (10 and 20 mg/kg) lessened the intensities of MDA [F (3, 20) =15.21 (P < 0.0001)] vs EtOH group. The outcome of europinidin on MDA levels in EtOH-treated animals is displayed in Figure 6.
Figure 6.
Effect of europinidin on MDA levels in alcoholic liver-diseased rats. #P < 0.001 vs normal and **P < 0.001 vs EtOH”.
3.7. Cytokines
TNF-α, TGF-β, IL-1β, IFN-Y, IL-12, and IL-6 intensities increased in EtOH-given animals. The results of TNF-α, TGF-β, IL-1β, IFN-γ, IL-12, and IL-6 levels in EtOH-control animals were shown to be statistically notable vs normal animals. Treatment of europinidin (10 and 20 mg/kg) diminished the increased IL-6 [F (3, 20) = 11.46 (P = 0.0001)], TNF-α [F (3, 20) = 6.436 (P = 0.0032)], TGF-β [F (3, 20) (P = 0.0004)], IL-β [F (3, 20) = 13.53 (P < 0.0001)], IL-12 [F (3, 20) = 33.43 (P < 0.0001)], and IFN-γ [F (3, 20) = 12.77 (P < 0.0001)] when associated with the EtOH group. Effects of europinidin on IL-6, TNF-α, IL-1β, IL-12, IFN-γ, and TGF-β levels in the EtOH group are shown in Figure 7A–E.
Figure 7.
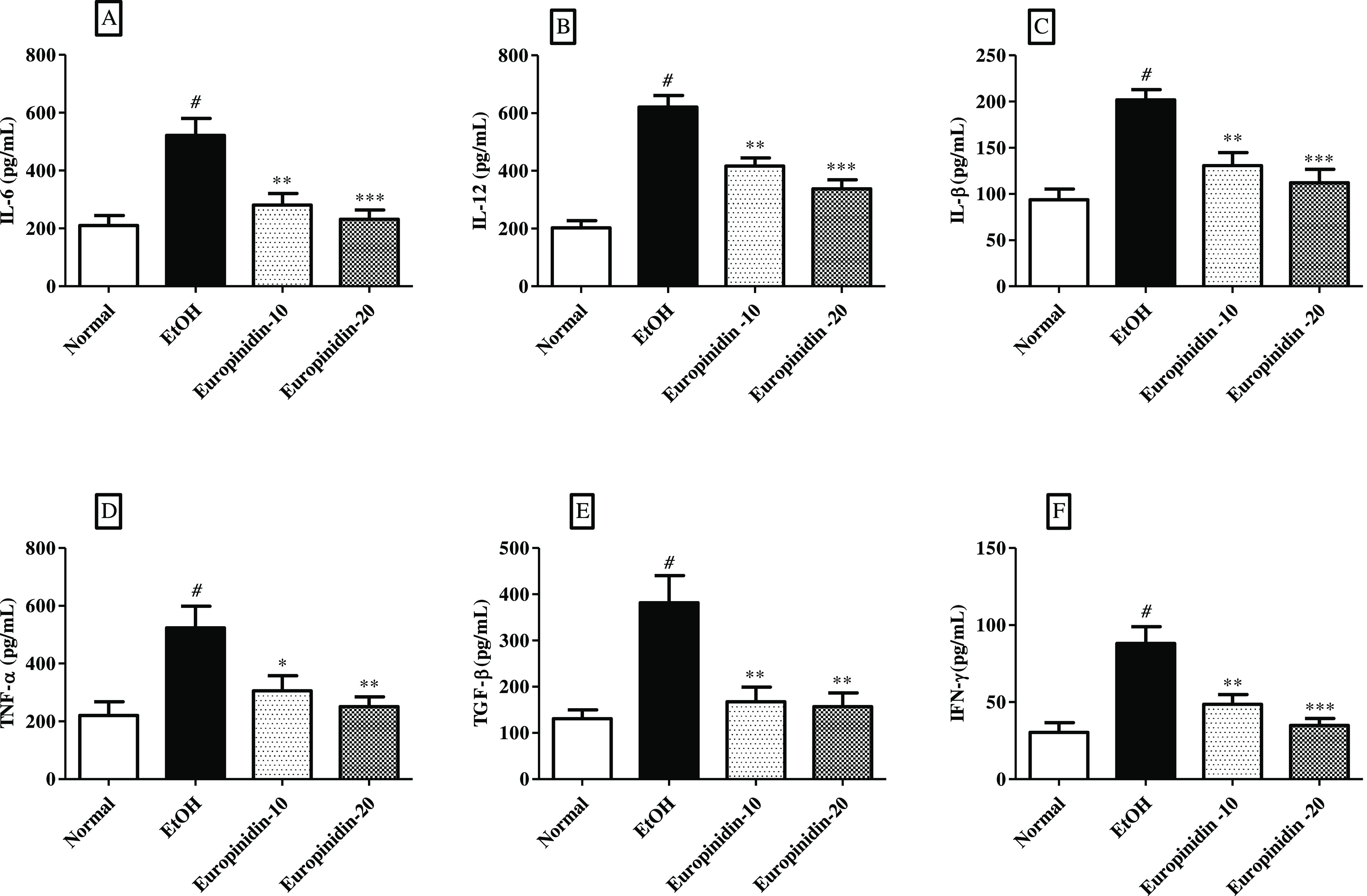
(A–F) Effect of europinidin on (A) IL-6, (B) IL-12, (C) IL-1β, (D) TNF-α, (E) TGF-β, and (F) IFN- γ in alcoholic liver-diseased rats. #P < 0.001 vs normal and *P < 0.05, **P < 0.001, and ***P < 0.0001 vs EtOH”.
3.8. NF-κB and Caspase-3
EtOH group displayed a significant increase of NF-κB and Caspase-3 levels (P < 0.05). Europinidin (10 and 20 mg/kg) inhibited the pathway of NF-κB [F (3, 20) = 118.7 (P < 0.0001)] and Caspase-3 [F (3, 20) = 155.3 (P < 0.0001)] in EtOH-treated animals. The outcomes were significant (P < 0.001) vs the EtOH group (Figure 8A,B).
Figure 8.
(A, B) Effect of europinidin on (A) NF-κB and (B) caspase-3 in alcoholic liver-diseased rats. #P < 0.001 vs normal and ***P < 0.0001 vs EtOH”.
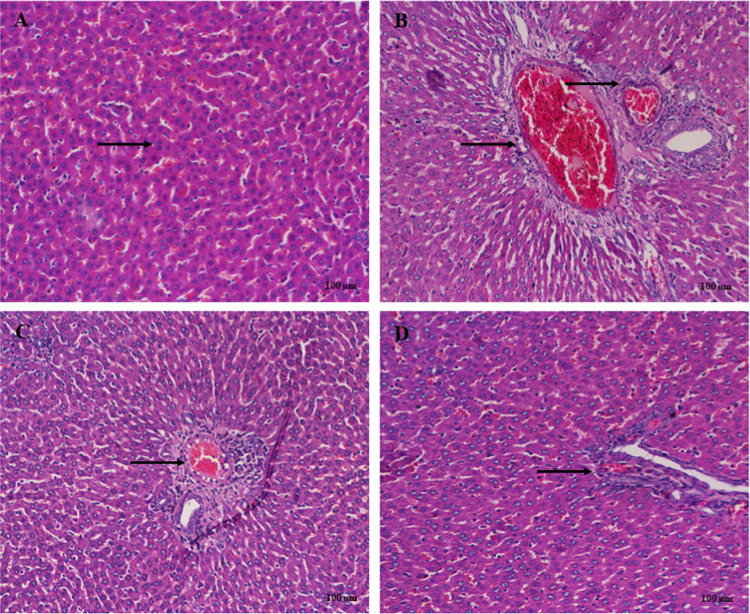
3.9. Histopathology
Liver histology showed normal liver structure in the normal group (Figure 9A). Rats treated with EtOH displayed extensive necrosis, fatty changes, and inflammation (Figure 9B). Europinidin-10 mg/kg showed moderate necrosis or degeneration, and inflammation (Figure 9C). This reduction was better than that seen in the europinidin-20 mg/kg group with progress in the pathological characteristics shown in Figure 9D.
Figure 9.
(A–D) Histological changes in liver rats stained with H&E (A) Normal: normal arrangement of hepatocytes. (B) EtOH control: section of liver tissue of ethanol-treated group showing extensive necrosis, fatty changes, and inflammation. (C) EtOH + Europinidin 10 mg/kg: indicated moderate tissue necrosis and inflammation. (D) EtOH + Europinidin 20 mg/kg: showed few tissue necrosis and mild inflammation indicated by arrows.
4. Discussion
Alcohol-related liver disease (ALD) encompasses a range of liver conditions that are caused by excessive and prolonged alcohol consumption.35 In the current study, europinidin was evaluated for hepaprotective activity against EtOH-induced liver damage in rats. Liver function tests, liver antioxidant enzymes, lipid peroxidation, cytokines levels, and histological studies were done to assess hepatoprotective.
The hepatic microsomal EtOH-oxidizing system’s major enzyme, CYP2E1, is crucial for the metabolism of EtOH.36,37 Alcohol consumption promotes CYP2E1 activity, increasing the generation of ROS.38 Europinidin considerably enhanced GSH-Px, SOD, and CAT and reduced MDA levels in the liver.
ALD is also associated with increased TNF-α and IL-6, as well as NO levels, all of which contribute to hepatocyte dysfunction.39,40 In addition, all of the systemic signs of alcoholic hepatitis are assumed to be caused by cytokine production.41 In our investigation, TNF-α, IL-6, IFN-γ, IL-1β, and IL-12 levels in the liver of EtOH-given rats were lowered by europinidin. These results imply that europinidin may cushion animals from EtOH-prompted liver trauma by decreasing the inflammatory cascade caused by EtOH. These results suggested that europinidin may exert a protective effect by reducing inflammatory mediator production.28,32 Pro-fibrotic cytokine TGF-β is prevalent in ALD, and it plays a main role in several chronic liver disorders.42 Alcohol administration significantly raised TGF-β levels in liver tissue in the current investigation, whereas treatment with europinidin attenuated TGF-β in alcohol-treated rats, which indicates its protective action in liver fibrosis in the animals.43
The liver enzyme such as AST, ALT, and ALP are the most sensitive indicators of the hepatocytes; when the liver is injured, ALT and AST are released from the hepatocytes.44 It is reported that ALD elevates AST, ALT, and ALP and AST/ALT ratio.35,41 However, europinidin administered to ALD-induced rats significantly attenuated AST, ALT, and ALP toward normal. From the results, europinidin may be a protective consequence against hepatic injury. LDH is an anaerobic metabolism enzyme that reversibly transforms pyruvate to lactate utilizing NADH. A literature survey revealed that LDH synthesis in hepatocytes increases early in acute liver failure.45 Creatinine, BUN, and total bilirubin levels accelerated liver damage, which was significantly reduced by europinidin.46,47 Albumin, a critical component of plasma proteins that acts in transporting and binding proteins, lipids, medicines, and chemicals, is produced by the liver. While alcohol consumption can cause a decrease in albumin levels, it is worth mentioning that rats treated with europinidin had higher amounts of this important protein.48
Liver disease can affect hepatic lipid metabolism, causing changes in circulating lipid levels and contributing to dyslipidemia.49 Europinidin reduced the elevated TG and TC in EtOH-treated rats implying that europinidin may have a protective function against EtOH-induced irregularities in hepatic lipid breakdown in rats.
The NF-kB pathway has a significant impact on various cellular processes such as inflammation, oxidative damage, and apoptosis.50 NF-kB is a vital part of the expansion and progression of hepatotoxicity.51 By activating in response to pro-inflammatory stimuli such as oxidative stress and cytokines, NF-kB triggers the expression of pro-inflammatory genes and the creation of pro-inflammatory cytokines, resulting in inflammation and fibrosis of the liver.51 NF-kB is a transcription factor that can regulate both pro- and anti-apoptotic genes, thereby controlling liver apoptosis as well.52 Europinidin can diminish the appearance of pro-inflammatory genes and the generation of pro-inflammatory cytokines by declining NF-kB initiation. This reduces liver fibrosis and inflammation.53
Caspase-3 is a key effector protease in the procedure of apoptosis, or planned cell death. In the context of hepatic injury, caspase-3 has been demonstrated to be essential for the beginning and completion of liver cell apoptosis, promoting the development of fibrosis and liver damage.54 The effects of europinidin on caspase-3, an essential effector protease in the process of apoptosis or programmed cell death, may also contribute to its protective effects on rat’s liver injury.55
In the presence of hepatic damage, europinidin can minimize liver cell death and enhance liver function by blocking caspase-3 activation. Europinidin has been shown to have beneficial effects against hepatic injury in rats, potentially through its inhibitory effects on both caspase-3 and the NF-kB pathway.
Histopathological alterations in the liver on account of alcohol consumption result in pathological damage, characterized by disruptions in normal architecture, apoptosis, and cellular permeation. In that aspect, Reddy et al. (2010) also found that a certain EtOH damages the liver, resulting in alterations to its regular histology, improved absorptivity, and hepatocyte necrosis.56 Additionally, all of the positive outcomes of europinidin on the liver are seen in histopathology examinations as well as our biochemical results.57 Europinidin reduces oxidative stress and lipid peroxidation in the hepatic and increases endogenous antioxidants, suggesting that it can protect the organ from EtOH-induced injury. The research also sheds light on the mechanisms underlying this protective effect, demonstrating that europinidin inhibits the TNF-α, TGF-β, IFN-γ, NF-kB, and caspase-3 signaling pathway, which is known to be involved in the development of liver damage in response to alcohol intake. These findings suggest that europinidin could be a potential therapeutic target for the prevention and treatment of ALD. It is imperative to confirm the mechanism with further studies because the current study only tested a small number of animals for a short time frame. An in-depth understanding of the mechanism will require different genetic models, immunochemical analysis, and more histology analysis with Masson’s trichrome and Sirius red staining to confirm collagen deposits in liver sections and western blotting.
5. Conclusions
Our study revealed that europinidin can protect the livers of rats from damage caused by alcohol consumption. This protective effect is likely due to the ability of europinidin to restore liver function tests, liver antioxidant enzymes, lipid peroxidation, cytokines levels, NF-kB, and caspase-3 triggered by alcohol in the rat liver. These findings are encouraging and suggest that further research is necessary to determine the potential use of europinidin as a treatment option for ALD damage.
Acknowledgments
The authors are thankful to the Researchers Supporting Project number (RSP2023R516) at King Saud University, Riyadh, Saudi Arabia.
Author Contributions
Conceptualization: I.K.; Methodology and the first draft of the manuscript: N.S. and W.A.M.; Critical revision of the manuscript: S.A.A., A.M.A., S.S.I., S.A., M.A.A., B.M.H., and F.A.A. All authors read and approved the current version of the manuscript.
The authors declare no competing financial interest.
References
- Nowak A. J.; Relja B. The Impact of Acute or Chronic Alcohol Intake on the NF-κB Signaling Pathway in Alcohol-Related Liver Disease. Int J. Mol. Sci. 2020, 21, 9407. 10.3390/ijms21249407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh Dastidar S.; Warner J. B.; Warner D. R.; McClain C. J.; Kirpich I. A. Rodent Models of Alcoholic Liver Disease: Role of Binge Ethanol Administration. Biomolecules 2018, 8, 3. 10.3390/biom8010003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun L.; Wen S.; Li Q.; Lai X.; Chen R.; Zhang Z.; Li D.; Sun S. L-theanine relieves acute alcoholic liver injury by regulating the TNF-α/NF-κB signaling pathway in C57BL/6J mice. J. Funct. Foods 2021, 86, 104699 10.1016/j.jff.2021.104699. [DOI] [Google Scholar]
- Chen L.; Liu L.; Abdulla R.; Tursun X.; Xin X.; Aisa H. A.; Du C. Chemical Components and Hepatoprotective Mechanism of Xwak Granule in Mice Treated with Acute Alcohol. J. Evidence-Based Complementary Altern. Med. 2020, 2020, 8538474 10.1155/2020/8538474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Askgaard G.; Kjær M. S.; Tolstrup J. S. Opportunities to Prevent Alcoholic Liver Cirrhosis in High-Risk Populations: A Systematic Review With Meta-Analysis. Am. J. Gastroenterol. 2019, 114, 221–232. 10.1038/s41395-018-0282-6. [DOI] [PubMed] [Google Scholar]
- Rodriguez W. E.; Wahlang B.; Wang Y.; Zhang J.; Vadhanam M. V.; Joshi-Barve S.; Bauer P.; Cannon R.; Ahmadi A. R.; Sun Z.; Cameron A.; Barve S.; Maldonado C.; McClain C.; Gobejishvili L. Phosphodiesterase 4 Inhibition as a Therapeutic Target for Alcoholic Liver Disease: From Bedside to Bench. Hepatology 2019, 70, 1958–1971. 10.1002/hep.30761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J. H.; Lee D. H.; Park M. S.; Jung Y. S.; Hong J. T. C-C chemokine receptor type 5 deficiency exacerbates alcoholic fatty liver disease through pro-inflammatory cytokines and chemokines-induced hepatic inflammation. J. Gastroenterol. Hepatol. 2017, 32, 1258–1264. 10.1111/jgh.13657. [DOI] [PubMed] [Google Scholar]
- Ullah A.; Munir S.; Badshah S. L.; Khan N.; Ghani L.; Poulson B. G.; Emwas A.-H.; Jaremko M. Important Flavonoids and Their Role as a Therapeutic Agent. Molecules 2020, 25, 5243. 10.3390/molecules25225243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monteiro A. F. M.; Viana J. D. O.; Nayarisseri A.; Zondegoumba E. N.; Mendonça Junior F. J. B.; Scotti M. T.; Scotti L. Computational studies applied to flavonoids against Alzheimer’s and Parkinson’s diseases. Oxid. Med. Cell. Longevity 2018, 2018, 1–21. 10.1155/2018/7912765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao Y.; Bao J.. 8 - Rice phenolics and other natural products. In Rice, 4th ed.; Bao J., Ed.; AACC International Press, 2019; pp 221–271. [Google Scholar]
- Xiao T.; Luo Z.; Guo Z.; Wang X.; Ding M.; Wang W.; Shen X.; Zhao Y. Multiple Roles of Black Raspberry Anthocyanins Protecting against Alcoholic Liver Disease. Molecules 2021, 26, 2313. 10.3390/molecules26082313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo A.; Wang S.; Liu L.; Yao Y.; Guo J. Understanding the effect of anthocyanin extracted from Lonicera caerulea L. on alcoholic hepatosteatosis. Biomed. Pharmacother. 2019, 117, 109087 10.1016/j.biopha.2019.109087. [DOI] [PubMed] [Google Scholar]
- Harborne J. B. Comparative biochemistry of the flavonoids-IV.: correlations between chemistry, pollen morphology and systematics in the family plumbaginaceae. Phytochemistry 1967, 6, 1415–1428. 10.1016/S0031-9422(00)82884-8. [DOI] [Google Scholar]
- Altharawi A.; Alharthy K. M.; Althurwi H. N.; Albaqami F. F.; Alzarea S. I.; Al-Abbasi F. A.; Nadeem M. S.; Kazmi I. Europinidin Inhibits Rotenone-Activated Parkinson’s Disease in Rodents by Decreasing Lipid Peroxidation and Inflammatory Cytokines Pathways. Molecules 2022, 27, 7159. 10.3390/molecules27217159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdel-Lateef M. A.; Albalawi M. A.; Al-Ghamdi S. N.; Mahdi W. A.; Alshehri S.; El Hamd M. A. Determination of metanil yellow dye in turmeric powder using a unique fluorescence Europium doped carbon dots. Spectrochim. Acta, Part A 2023, 287, 122124 10.1016/j.saa.2022.122124. [DOI] [PubMed] [Google Scholar]
- Lauro G. J.; Francis J.. Natural Food Colorants: Science and Technology; CRC Press, 2000. [Google Scholar]
- Delgado-Vargas F.; Paredes-Lopez O.. Natural Colorants for Food and Nutraceutical Uses; CRC Press, 2002. [Google Scholar]
- Altharawi A.; Alharthy K. M.; Althurwi H. N.; Albaqami F. F.; Alzarea S. I.; Al-Abbasi F. A.; Nadeem M. S.; Kazmi I. Europinidin Inhibits Rotenone-Activated Parkinson’s Disease in Rodents by Decreasing Lipid Peroxidation and Inflammatory Cytokines Pathways. Molecules 2022, 27, 7159. 10.3390/molecules27217159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmad A. Neuroprotective Efficacy of Europinidin in Streptozotocin-Induced Memory Impairment by Modulation of Oxidative Stress, Inflammatory Mediators, and Cholinesterase Activity in Rats. Oxid. Med. Cell. Longevity 2023, 2023, 5248127 10.1155/2023/5248127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Afzal M.; Alharbi K. S.; Alenezi S. K.; Alshammari M. S.; Alomar F. A.; Kazmi I. Europinidin Enhances Healing through Modulating Antioxidant Processes in Experimentally Induced-Stomach Ulcer Condition. Int. J. Pharmacol. 2022, 18, 1509–1520. 10.3923/ijp.2022.1509.1520. [DOI] [Google Scholar]
- Percie du Sert N.; Hurst V.; Ahluwalia A.; Alam S.; Avey M. T.; Baker M.; Browne W. J.; Clark A.; Cuthill I. C.; Dirnagl U.; Emerson M.; Garner P.; Holgate S. T.; Howells D. W.; Karp N. A.; Lazic S. E.; Lidster K.; MacCallum C. J.; Macleod M.; Pearl E. J.; Petersen O. H.; Rawle F.; Reynolds P.; Rooney K.; Sena E. S.; Silberberg S. D.; Steckler T.; Würbel H. The ARRIVE guidelines 2.0: Updated guidelines for reporting animal research. J. Cereb. Blood Flow Metab. 2020, 40, 1769–1777. 10.1177/0271678X20943823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S.; Sui S.; Liu Z.; Peng C.; Liu J.; Luo D.; Fan X.; Liu C.; Lu W. Y. Protective roles of hepatic gamma-aminobutyric acid signaling in acute ethanol exposure-induced liver injury. J. Appl. Toxicol. 2018, 38, 341–350. 10.1002/jat.3544. [DOI] [PubMed] [Google Scholar]
- Shi X.; Zhu W.; Chen T.; Cui W.; Li X.; Xu S. Paraquat induces apoptosis, programmed necrosis, and immune dysfunction in CIK cells via the PTEN/PI3K/AKT axis. Fish Shellfish Immunol. 2022, 130, 309–316. 10.1016/j.fsi.2022.09.024. [DOI] [PubMed] [Google Scholar]
- Shi X.; Xu T.; Li X.; Sun X.; Zhang W.; Liu X.; Wang Y.; Zhang Y.; Xu S. ROS mediated pyroptosis-M1 polarization crosstalk participates in inflammation of chicken liver induced by bisphenol A and selenium deficiency. Environ. Pollut. 2023, 324, 121392 10.1016/j.envpol.2023.121392. [DOI] [PubMed] [Google Scholar]
- Ellman G. L. Tissue sulfhydryl groups. Arch. Biochem. Biophys. 1959, 82, 70–77. 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- Misra H. P.; Fridovich I. The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J. Biol. Chem. 1972, 247, 3170–3175. 10.1016/S0021-9258(19)45228-9. [DOI] [PubMed] [Google Scholar]
- Aebi H.; Wyss S. R.; Scherz B.; Skvaril F. Heterogeneity of erythrocyte catalase II. Isolation and characterization of normal and variant erythrocyte catalase and their subunits. Eur. J. Biochem. 1974, 48, 137–145. 10.1111/j.1432-1033.1974.tb03751.x. [DOI] [PubMed] [Google Scholar]
- Wang L.-W.; Wang L.-K.; Chen H.; Fan C.; Li X.; He C.-M.; Gong Z.-J. Ethyl pyruvate protects against experimental acute-on-chronic liver failure in rats. World J. Gastroenterol 2012, 18, 5709. 10.3748/wjg.v18.i40.5709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green L. C.; Wagner D. A.; Glogowski J.; Skipper P. L.; Wishnok J. S.; Tannenbaum S. R. Analysis of nitrate, nitrite, and [15N] nitrate in biological fluids. Anal. Biochem. 1982, 126, 131–138. 10.1016/0003-2697(82)90118-X. [DOI] [PubMed] [Google Scholar]
- Liu J. Ethanol and liver: recent insights into the mechanisms of ethanol-induced fatty liver. World J. Gastroenterol. 2014, 20, 14672. 10.3748/wjg.v20.i40.14672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bujanda L.; Hijona E.; Larzabal M.; et al. Resveratrol inhibits nonalcoholic fatty liver disease in rats. BMC gastroenterology 2008, 8 (1), 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusuma R. A.; Noer E. R.; Purnomo H. D.; Pramono A.; Mahati E.; Nissa C. Hepatogomax Improves Serum Albumin and Transaminase Enzyme Activity Levels in Sprague Dawley Rats Liver Cirrhosis. J. Biomed. Transl. Res. 2022, 8 (3), 112–118. 10.14710/jbtr.v8i3.15078. [DOI] [Google Scholar]
- Li X.-X.; Jiang Z.-H.; Zhou B.; Chen C.; Zhang X.-Y. Hepatoprotective effect of gastrodin against alcohol-induced liver injury in mice. J. Physiol. Biochem. 2019, 75, 29–37. 10.1007/s13105-018-0647-8. [DOI] [PubMed] [Google Scholar]
- Shi X.; Xu T.; Cui W.; Qi X.; Xu S. Combined negative effects of microplastics and plasticizer DEHP: The increased release of Nets delays wound healing in mice. Sci. Total Environ. 2023, 862, 160861 10.1016/j.scitotenv.2022.160861. [DOI] [PubMed] [Google Scholar]
- Torruellas C.; French S. W.; Medici V. Diagnosis of alcoholic liver disease. World J. Gastroenterol 2014, 20, 11684–11699. 10.3748/wjg.v20.i33.11684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X.; Wang Y.; Wu D.; Li S.; Wang C.; Han Z.; Wang J.; Wang K.; Yang Z.; Wei Z. Magnolol Prevents Acute Alcoholic Liver Damage by Activating PI3K/Nrf2/PPARγ and Inhibiting NLRP3 Signaling Pathway. Front. Pharmacol. 2019, 10, 1459. 10.3389/fphar.2019.01459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galicia-Moreno M.; Gutiérrez-Reyes G. The role of oxidative stress in the development of alcoholic liver disease. Rev. Gastroenterol. Mex. 2014, 79, 135–144. 10.1016/j.rgmxen.2014.06.007. [DOI] [PubMed] [Google Scholar]
- Zeng T.; Zhang C.-L.; Zhao N.; Guan M.-J.; Xiao M.; Yang R.; Zhao X.-L.; Yu L.-H.; Zhu Z.-P.; Xie K.-Q. Impairment of Akt activity by CYP2E1 mediated oxidative stress is involved in chronic ethanol-induced fatty liver. Redox Biol. 2018, 14, 295–304. 10.1016/j.redox.2017.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han J. H.; Ju J. H.; Lee Y. S.; Park J. H.; Yeo I. J.; Park M. H.; Roh Y. S.; Han S. B.; Hong J. T. Astaxanthin alleviated ethanol-induced liver injury by inhibition of oxidative stress and inflammatory responses via blocking of STAT3 activity. Sci. Rep. 2018, 8, 14090 10.1038/s41598-018-32497-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X.; Dong K.; Ma Y.; Jin Q.; Yin S.; Wang S. Hepatoprotective effects of chamazulene against alcohol-induced liver damage by alleviation of oxidative stress in rat models. Open Life Sci. 2020, 15, 251–258. 10.1515/biol-2020-0026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramaniyan V.; Chakravarthi S.; Jegasothy R.; Seng W. Y.; Fuloria N. K.; Fuloria S.; Hazarika I.; Das A. Alcohol-associated liver disease: A review on its pathophysiology, diagnosis and drug therapy. Toxicol. Rep. 2021, 8, 376–385. 10.1016/j.toxrep.2021.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaitantzi H.; Meyer C.; Rakoczy P.; Thomas M.; Wahl K.; Wandrer F.; Bantel H.; Alborzinia H.; Wölfl S.; Ehnert S.; Nüssler A.; Bergheim I.; Ciuclan L.; Ebert M.; Breitkopf-Heinlein K.; Dooley S. Ethanol sensitizes hepatocytes for TGF-β-triggered apoptosis. Cell Death Dis. 2018, 9, 51. 10.1038/s41419-017-0071-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S.; Chen L.; Shi X.; Wang Y.; Xu S. Polystyrene microplastics-induced macrophage extracellular traps contributes to liver fibrotic injury by activating ROS/TGF-β/Smad2/3 signaling axis. Environ. Pollut. 2023, 324, 121388 10.1016/j.envpol.2023.121388. [DOI] [PubMed] [Google Scholar]
- Dasgupta A.Liver Enzymes as Alcohol Biomarkers. In Alcohol and its Biomarkers, Dasgupta A., Ed.; Elsevier: San Diego, 2015; pp 121–137, Chapter 5. [Google Scholar]
- Kotoh K.; Kato M.; Kohjima M.; Tanaka M.; Miyazaki M.; Nakamura K.; Enjoji M.; Nakamuta M.; Takayanagi R. Lactate dehydrogenase production in hepatocytes is increased at an early stage of acute liver failure. Exp. Ther. Med. 2011, 2, 195–199. 10.3892/etm.2011.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F.; Liu Y.; Peng Q.; Wang G.; Tan Q.; Ou Z.; Xu Q.; Liu C.; Zuo D.; Zhao J. Creatinine accelerates APAP-induced liver damage by increasing oxidative stress through ROS/JNK signaling pathway. Front. Pharmacol. 2022, 13, 3339. 10.3389/fphar.2022.959497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palipoch S.; Punsawad C. Biochemical and histological study of rat liver and kidney injury induced by Cisplatin. J. Toxicol. Pathol. 2013, 26, 293–299. 10.1293/tox.26.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolankaya D.; Selmanoğlu G.; Sorkun K.; Salih B. Protective effects of Turkish propolis on alcohol-induced serum lipid changes and liver injury in male rats. Food Chem. 2002, 78, 213–217. 10.1016/S0308-8146(01)00400-9. [DOI] [Google Scholar]
- Heeren J.; Scheja L. Metabolic-associated fatty liver disease and lipoprotein metabolism. Mol. Metab. 2021, 50, 101238 10.1016/j.molmet.2021.101238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lingappan K. NF-κB in oxidative stress. Curr. Opin. Toxicol. 2018, 7, 81–86. 10.1016/j.cotox.2017.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papa S.; Bubici C.; Zazzeroni F.; Franzoso G. Mechanisms of liver disease: cross-talk between the NF-κB and JNK pathways. bchm 2009, 390, 965. 10.1515/BC.2009.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arab-Nozari M.; Ahangar N.; Mohammadi E.; Lorigooini Z.; Shokrzadeh M.; Amiri F. T.; Shaki F. Ginkgo biloba attenuated hepatotoxicity induced by combined exposure to cadmium and fluoride via modulating the redox imbalance, Bax/Bcl-2 and NF-kB signaling pathways in male rats. Mol. Biol. Rep. 2020, 47, 6961–6972. 10.1007/s11033-020-05755-2. [DOI] [PubMed] [Google Scholar]
- Calis Z.; Mogulkoc R.; Baltaci A. K. The roles of flavonols/flavonoids in neurodegeneration and neuroinflammation. Mini-Rev. Med. Chem. 2020, 20, 1475–1488. 10.2174/1389557519666190617150051. [DOI] [PubMed] [Google Scholar]
- Jaeschke H.; Ramachandran A. Acetaminophen-induced apoptosis: Facts versus fiction. J. Clin. Transl. Res. 2020, 6, 36. [PMC free article] [PubMed] [Google Scholar]
- Hagar H.; Husain S.; Fadda L. M.; Attia N. M.; Attia M. M.; Ali H. M. Inhibition of NF-κB and the oxidative stress-dependent caspase-3 apoptotic pathway by betaine supplementation attenuates hepatic injury mediated by cisplatin in rats. Pharmacol. Rep. 2019, 71, 1025–1033. 10.1016/j.pharep.2019.06.003. [DOI] [PubMed] [Google Scholar]
- Damodara Reddy V.; Padmavathi P.; Gopi S.; Paramahamsa M.; Varadacharyulu N. C. Protective effect of Emblica officinalis against alcohol-induced hepatic injury by ameliorating oxidative stress in rats. Indian J. Clin. Biochem. 2010, 25, 419. 10.1007/s12291-010-0058-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senthilkumar R.; Sengottuvelan M.; Nalini N. Protective effect of glycine supplementation on the levels of lipid peroxidation and antioxidant enzymes in the erythrocyte of rats with alcohol-induced liver injury. Cell Biochem. Funct. 2004, 22, 123–128. 10.1002/cbf.1062. [DOI] [PubMed] [Google Scholar]