Abstract
Introduction
Restoration of podocyte autophagy is considered as a feasible strategy for the treatment of diabetic kidney disease (DKD). This study aimed at investigating the protective effect and potential mechanism of vitamin D on podocyte injury of DKD.
Methods
Type 2 diabetic db/db mice received intraperitoneal injections of vitamin D analog paricalcitol 400 ng/kg per day for 16 weeks. Immortalized mouse podocytes were cultured in high glucose (HG) medium with active vitamin D3 calcitriol or autophagy inhibitor 3-methyladenine. Renal function and urine albumin creatinine ratio were assessed at week 24. HE, PAS staining, and electron microscopy were used to evaluate renal histopathology and morphological changes. Immunohistochemistry, immunofluorescence, and Western blot were used to evaluate protein expression of nephrin and podocin in kidney tissue and podocytes. The expression of autophagy-related proteins (LC3, Beclin-1, Vps34) and apoptosis-related proteins (cleaved caspase-3, Bax) was determined by Western blotting. Podocyte apoptosis was further evaluated by using flow cytometer.
Results
Albuminuria in a db/db mouse model was markedly attenuated after treatment with paricalcitol. This was accompanied by alleviation of mesangial matrix expansion and podocyte injury. Besides, the impaired autophagy in podocytes under diabetic conditions was also markedly enhanced after paricalcitol or calcitriol treatment, accompanied by restored decreased podocyte slit diaphragm proteins podocin and nephrin. Furthermore, the protective effect of calcitriol against HG-induced podocyte apoptosis could be abated by autophagy inhibitor 3-methyladenine.
Conclusion
Vitamin D ameliorates podocyte injury of DKD by enhancing podocyte autophagy activity, which may become a potential candidate autophagy activator for the therapeutic interventions for DKD.
Keywords: Diabetic kidney disease, Vitamin D, Autophagy, Podocyte
Introduction
Diabetic kidney disease (DKD) is a microvascular complication of diabetes and the leading cause of end-stage renal disease [1, 2]. DKD with massive proteinuria is still a difficult problem in clinical treatment. Podocytes are an essential part for the formation of the glomerular filtration barrier. There is compelling evidence identifying injury and loss of podocytes as the key mediators in the pathogenesis of DKD [3–5]. Therefore, identification of potential novel therapeutic strategy for protecting podocyte may provide clinical benefits for DKD.
Autophagy and apoptosis are two important pathophysiological processes. Autophagy is a highly conserved catabolic pathway that degrades proteins and organelles through the lysosome. The autophagy process involves the regulation of autophagy genes and signal transduction pathways, among which Beclin-1 and vacuolar sorting protein 34 (Vps34) complex formation is a key signal for early initiation of autophagy [6, 7]. The basic level of autophagy is essential to maintain the homeostasis of podocytes [4]. Currently, a growing body of evidence indicates that defective autophagy mediated the podocyte lesion [5, 8]. The capacity for autophagy in podocytes is markedly impaired in type 2 diabetes, and this deficiency contributes to the intensity of renal injury [9, 10]. These evidences show that upregulating autophagy activity may be a potential novel therapeutic strategy for DKD.
Vitamin D, a steroid hormone, has been widely utilized for the treatment of calcium homeostasis and bone metabolism. Our previous studies found that vitamin D exerts renoprotective effects by improving podocyte injury in rats with puromycin nephropathy [11]. Recently emerging evidence suggests that vitamin D has a protective effect on diabetes and DKD [12–14]. In addition to reducing proteinuria, anti-inflammatory and anti-fibrosis effects in various animal models and clinic trials, it also can prevent the loss of podocytes to a certain extent [15]. A recent study indicated that vitamin D-vitamin D receptor regulates defective autophagy in renal tubular epithelial cells in streptozotocin-induced diabetic mice via the AMPK pathway [16]. However, the molecular mechanism of vitamin D on podocyte protection remains not fully clarified. Here, we investigated the effects and potential mechanism of vitamin D on podocyte injury of DKD.
Materials and Methods
Animals and Treatment
8-week-old male db/db mice and db/m mice were purchased from Nanjing Biomedical Research Institute, Nanjing University. The mice were kept in an animal house with a bright/dark cycle of 12 h, a constant temperature of 22 ± 2°C, and a humidity of 5 ± 10%. The mice were free to assess clean drinking and common feed. The experiment was carried out after feeding for a week. The mice were divided into three groups (8 db/m mice as control group, 8 db/db mice as DM group, 8 db/db mice as DM + P group). The DM + P group was given paricalcitol by intraperitoneal injection at a dose of 400 ng/kg/day for 16 weeks. The db/db and db/m groups were given an equivalent amount of saline by oral gavage for the same period. Body weight and fasting blood glucose was measured every 2 weeks or every 4 weeks in all animals. Before the mice were sacrificed, the urine samples were collected for the urinary albumin-to-creatinine ratio (UACR). Blood samples were collected for biochemical assays, and the kidneys were harvested for histological experiment.
Plasma and Urine Biochemical Analysis
Plasma samples were acquired by centrifugation of the blood samples at 3,000 × g and 4°C, and blood urea nitrogen (BUN) was measured using a Beckman Coulter automated biochemical analyzer (DXC800, Beckman Coulter Inc., Pasadena, CA). Urinary albumin or urinary and serum creatinine (Scr) levels were, respectively, measured using mouse albumin-specific enzyme-linked immunosorbent assay kits (Bethyl Laboratories Inc., Montgomery, TX, USA) and creatinine kits (Cayman Chemical, Ann Arbor, MI, USA) according to the manufacturer’s instructions.
Kidney Histology and Immunohistochemistry
Paraffin-embedded tissues were cut into 4-μm sections and stained with periodic acid-Schiff for light microscopic examination. The sections were deparaffinized in xylene, rehydrated in decreasing percentage of ethanol solutions, and incubated in 3% H2O2 for 20 min at 37°C. They were then washed three times with phosphate-buffered saline for 5 min. Antigen retrieval was performed in a microwave oven, and the sections were then blocked in 5% bovine serum albumin. The sections were incubated overnight with an anti-nephrin primary antibody or anti-podocin primary antibody at 4°C and then incubated with a biotinylated secondary antibody (Maixin, Fuzhou, China). Finally, diaminobenzidine tetrachlorohydrochloride substrates were added for reaction. Histological evaluation was performed in a blinded manner using an Olympus Microscopy System (cx41, Olympus, Japan). The degree of glomerular mesangial dilatation was assessed using the mesangial fractional volume density, defined as the percentage of the cross-sectional area of the glomerular tuft made up by mesangium [17]. The glomerular mesangial expansion index was scored from 0 to 3 points: 0–25%, 26–50%, 51–75%, and >75%. Semi-quantitative analysis is carried out through Image-Pro Plus 6.0 software (Media Cybernetics, MD).
Cell Culture and Treatment
The conditionally immortalized mouse podocyte cell line, kindly provided by Professor J. Reiser of Rush University (Chicago, IL, USA), was cultured as previously reported [18, 19]. Briefly, podocytes were cultured at 33°C with 100% relative humidity and 5% CO2 in RPMI 1640 medium (Gibco BRL, Gaithersburg, MD, USA) containing 10% fetal bovine serum (Gibco®; Thermo Fisher Scientific, Waltham, MA, USA), recombinant IFN-γ (CYT-358, ProSpec, Tany TechnoGene Ltd., Ness Ziona, Israel), and 100 U/mL penicillin/streptomycin. To induce differentiation, podocytes were transferred into cell culture dishes which were coated with type I collagen (BD Bioscience, Bedford, MA, USA), cultured in IFN-γ-free RPMI 1640 medium containing 5% fetal bovine serum at 37°C for 10–14 days. Differentiated and mature podocytes were maintained in serum-free medium overnight. Cultured podocytes were divided into six groups according to various treatments: normal glucose (NG, 5.6 mm glucose), mannitol control group (MA, 5.6 mm glucose plus 24.4 mm D-mannitol), high glucose (HG, 30 mm glucose), high glucose plus 1 nm 1,25(OH)2D3 (HG + 1VD), high glucose plus 10 nm 1,25(OH)2D3 (HG + 10VD), high glucose plus 100 nm 1,25(OH)2 D3 (HG + 100VD). Cells were treated with the above intervention for 48 h.
Real-Time Quantitative PCR
Total RNA from cultured podocytes, prepared using Trizol Reagent (Invitrogen), was reverse transcribed into complementary DNAs with a Prime Script RT Reagent Kit (Takara Biotechnology, Dalian, China) according to the manufacturer’s instructions. The RT-qPCR assays were performed in an Applied Biosystems 7500 Real-Time PCR System (Applied Biosystems, Foster, CA, USA) using Power SYBR Green PCR Master Mix (Takara Biotechnology, Dalian, China). The sequences of the specific primer are as follows: Beclin-1: forward: 5′-ATGGAGGGGTCTAAGGCGT C-3′; reverse: 5′-TGGGCTGTGGTAAGTAATGGA-3′; Vps34: forward: 5′-GGGCTAT ACCAAGAGACATGC-3′; reverse: 5′-CGCCTTGTAGGATGTTCTGACT-3′; GAPDH: forward: 5′-AGGTCGGTGTGAACGGATTTG-3′; reverse: 5′-TGTAGACCATGTAG TTGAGGTCA-3′. The relative expression ratio of messenger RNA was calculated by2−ΔΔCT method using GAPDH as endogenous control.
Western Blot
Total proteins were extracted from cells of each group using RIPA lysis buffer with protease inhibitor phenyl methanesulfonyl fluoride and quantified using Pierce BCA Protein Assay Kit (NCI3225CH, Thermo Scientific, Milford, MA) according to the manufacturer’s instructions. After being boiled at 99°C for 10 min, the denatured protein was electrophoresed on 10.5% sodium dodecyl sulfate polyacrylamide gel electrophoresis and then transferred onto polyvinylidene fluoride membranes (Millipore, Billerica, MA, USA) for 2 h at 200 mA. Membranes were blocked in 5% non-fat dry milk in TBS-T (137 mm NaCl, 20 mm Tris, pH 7.6, with 0.1% Tween 20) for 1 h at room temperature and then incubated overnight at 4°C with the following primary antibodies: rabbit anti-nephrin (1:3,000, Abcam, Cambridge, UK), rabbit anti-podocin (1:3,000, Abcam, Cambridge, UK), rabbit anti-LC3A/B (1:1,000, Cell Signaling Technology, Boston, USA), rabbit anti-Beclin-1 (1:1,000, Cell Signaling Technology, Boston, USA), rabbit anti-Vps34 (1:1,000, Cell Signaling Technology, Boston, USA), rabbit anti-Bax, rabbit anti-capase-3 (1:1,000, Cell Signaling Technology, Boston, USA), rabbit anti-GAPDH (1:5,000, Cell Signaling Technology, Boston, USA), rabbit anti-β-actin (1:5,000, Abcam, Cambridge, UK). Subsequently, the membranes were incubated with the horseradish peroxidase-conjugated goat anti-rabbit IgG (1:3,000, Cell Signaling Technology, Boston, USA) for 1 h at room temperature. Finally, membranes treated with ECL Western Blotting Detection Reagents (Advansta, MenioPark, CA, USA) were imaged on Kodak X-ray film (Eastman Kodak, Rochester, NY, USA). Scanned images were quantified densitometrically using ImageJ software (National Institutes of Health, Bethesda, MD, USA).
Immunofluorescence
Cells cultured on six-well plates containing coverslips were fixed in cold methanol at −20°C for 20 min and permeabilized with 0.5% Triton X-100 (Sigma-Aldrich) in PBS for 10 min at room temperature. After washing three times with PBS, the cells were blocked with 5% BSA for 1 h at room temperature and then incubated with rabbit anti-LC3 (1:200, Cell Signaling Technology, Boston USA), goat anti-synaptopodin (1:50, Santa Cruz, CA, USA) overnight at 4°C. The cells were washed with PBS and stained with the goat anti-rabbit Alexa Fluor 555, donkey anti-goat IgG 488 (1:250, Cell Signaling Technology, Boston USA) for 1 h at room temperature in the dark. Finally, the cells were stained with 4′,6-diamidino-2-phenylindole (Guangzhou Whiga Technology Co., Ltd.) for 5 min. The images were captured using a laser scanning confocal microscopy (LCSM, Zeiss KS 400, Postfach, Germany).
Transmission Electron Microscopy
Renal cortex tissues and immortalized mouse podocytes under different conditions were fixed in 2.5% glutaraldehyde buffered and washed with cacodylate (Sigma-Aldrich, St. Louis, MO, USA). After being fixed with 1% osmium tetroxide (Sigma-Aldrich, St. Louis, MO, USA), the cells were embedded into epoxy resin and then sections were cut ultrathin (90 nm), placed on copper mesh. Images were examined by transmission electron miscroscopy (TEM) using a JEM-100CX transmission electron microscope (Jeol, Tokyo, Japan).
Flow Cytometric Analysis
The apoptotic rate of podocytes was detected by the Annexin V-FITC Apoptosis Detection Kits (KeyGen, Nanjing, China) according to the manufacturer's instructions. After various treatments, podocytes were trypsinized, washed twice with PBS, and collected by centrifugation at 1,000 g for 4 min. Cells were resuspended in 500 μL 1 × binding buffer and then 5 μL of Annexin V-FITC and 5 μL propidium iodide were added before incubating at room temperature for 5 min in the dark. A total of 20,000 cells were analyzed using a flow cytometer (FACSARIA II; BD Biosciences, San Jose, CA, USA).
Statistical Analysis
All data were expressed as means ± SD. Multiple comparisons between groups were assessed by one-way ANOVA with least significant difference test or Tamhane’s test using SPSS 22.0 software (SPSS, Inc., Chicago, IL, USA). p < 0.05 was considered as statistically significant.
Results
Vitamin D Improved the Metabolic Characteristics of db/db Mice
To investigate the potential impact of vitamin D in DKD, we investigated the effect of vitamin D analog paricalcitol in db/db mice, a typical type 2 diabetes animal model, and followed the development of DKD. As shown in Figure 1a, b, the body weight and kidney-to-body weight ratio were significantly increased in diabetic db/db mice compared with db/m mice. The db/db mice exhibited hyperglycemia associated with obesity throughout the experimental periods (8–24 weeks of age) (Fig. 1c). Moreover, higher levels of UACR in db/db mice were observed compared with db/m mice (Fig. 1d). However, BUN and Scr did not differ between the db/db mice and db/m mice (Fig. 1e, f). In contrast, 16 weeks of treatment with paricalcitol significantly decreased the body weight, kidney-to-body weight ratio, and blood glucose in db/db mice. Meanwhile, treatment with paricalcitol significantly reduced UACR levels compared to the untreated DKD group. However, no statistical difference was noticed in the Scr and BUN. These above results demonstrate that paricalcitol may improve metabolic characteristics of db/db mice.
Fig. 1.
a Vitamin D improved the metabolic characteristics of db/db mice. Body weight levels at 8, 10, 12, 16, 20, and 24 weeks. b The ratio of kidney weight to body weight was detected at 24 weeks. c Blood glucose levels were detected at 8, 10, 12, 16, 20, and 24 weeks in the db/m mice, diabetic db/db mice, and diabetic db/db mice treated with paricalcitol (400 ng/kg/d) groups. d The urinary albumin-to-creatinine ratio before the experiment and at the time of sacrifice. e, f BUN and Scr levels were determined at 24 weeks. All the data are expressed as mean ± SD. *p < 0.05, versus db/m mice group. #p < 0.05, versus db/db mice group.
Vitamin D Alleviated the Histopathological Changes of the Kidneys in db/db Mice
The histopathological alterations were investigated in renal tissues via PAS staining. As shown with PAS-stained kidney tissues (Fig. 2a, b), compared with the db/m group, the untreated db/db group exhibited kidney damage, including glomerular hypertrophy, mesangial matrix expansion. In contrast, these kidney damages were significantly reduced by paricalcitol treatment. The kidney ultrastructure was further examined by electron microscopy. As shown in Figure 2c, normal morphology of the glomerular filtration barrier including GBM and podocyte foot process was seen in non-diabetic BKS. As expected, chronic diabetes caused GBM thickening and foot process effacement (Fig. 2c). The impairment of the glomerular filtration barrier is consistent with the more severe albuminuria observed in db/db mice. Interestingly, the extent of foot process effacement and GBM thickening was markedly alleviated in the db/db mice treated with paricalcitol compared to diabetic db/db mice (Fig. 2c).
Fig. 2.
Treatment with paricalcitol ameliorated glomerular histological injury in db/db mice. a Representative photomicrographs of PAS staining in the db/m mice, db/db mice, and db/db +P (400 ng/kg/d) groups at 24 weeks (magnification, ×400). b The mesangial expansion index of glomerulus. Data are the results of experiments in each group (n = 8). c Electron microscopy of ultrathin kidney sections shows morphological changes in the foot process and the glomerular basement membrane in each group (magnification, ×7,000). *p < 0.01 versus db/m mice group, #p < 0.01 versus db/db mice group.
Vitamin D Increased Nephrin and Podocin Expression in Renal Tissues of db/db Mice
Podocytes are essential for maintaining the filtration barrier of the glomerulus. The integrity of this filtration barrier depends on the podocyte slit diaphragm proteins nephrin and podocin. To evaluate the role of vitamin D in podocyte injury in vivo, we sought to determine the protein expression of nephrin and podocin in renal tissues of db/db mice by using immunohistochemical staining and immunofluorescence. As shown in Figure 3a, immunohistochemical staining indicated that the protein expressions of nephrin and podocin were significantly decreased in db/db mice, compared to db/m group; in contrast, they were markedly upregulated after paricalcitol treatment (Fig. 3a, b). Similar results are also obtained by immunofluorescence assay (Fig. 3c, d). These results indicate that paricalcitol prevents the decreased nephrin and podocin expression of podocytes in renal tissues from db/db mice.
Fig. 3.
Treatment with paricalcitol increased nephrin and podocin expression in renal tissues of db/db mice. Representative photomicrographs of the immunohistochemistry (IHC) and immunofluorescence (IF) for nephrin and podocin of the db/m mice, db/db mice, and db/db +P (paricalcitol 400 ng/kg/d) groups at 24 weeks (magnification, ×400). a, b Analysis of the expression of nephrin and podocin detected by IHC. c, d Analysis of the expression of nephrin and podocin detected by IF. All the data are expressed as mean ± SD. *p < 0.01 versus db/m mice group, #p < 0.01 versus db/db mice group.
Vitamin D Restored the Impaired Autophagy in db/db Mice
To further clarify whether autophagy is involved in the renal protection of vitamin D in db/db mice, the expressions of autophagic protein LC3-II, Beclin-1, and Vps34 were analyzed by Western blotting. Immunoblotting results showed that the expressions of LC3-II, Beclin-1, and Vps34 were lower in db/db mice than in the db/m mice, while treatment with paricalcitol restored these protein levels in db/db mice (Fig. 4a, b). Furthermore, autophagic activity was also examined by counting the number of autophagosomes with TEM, a gold standard to detect autophagy. We found the increasement of autophagosomes in podocytes of db/db mice after treatment of paricalcitol (Fig. 4c). These results indicate podocyte autophagy impairment under the diabetic state, whereas paricalcitol treatment restored podocyte autophagy activities.
Fig. 4.
Treatment with paricalcitol restored the impaired autophagy in db/db mice. a–b Western blot analysis revealed the expression of Beclin-1, Vps34, and LC3-II in the db/m mice, db/db mice, and db/db mice+P (paricalcitol 400 ng/kg/d) groups at 24 weeks. c Representative electron micrographs showing autolysosomes in podocytes of mice at 24 weeks. The red arrow indicates autolysosomes (×20,000). All the data are expressed as mean ± SD. *p < 0.05, versus db/m mice group, #p < 0.05 versus db/db mice group.
Vitamin D Attenuates HG-Induced Apoptosis in Podocytes
Hyperglycemia promotes podocyte apoptosis and plays a key role in the pathogenesis of DKD. To examine the role of vitamin D in podocyte apoptosis, the cells were treated with various concentrations of active vitamin D analog calcitriol (1, 10, 100 nm) and high glucose (HG) for 48 h. Increases in the protein levels of cleaved caspase-3 and Bax were observed in the HG-treated group compared with the control group. By contrast, the expressions of cleaved caspase-3 and Bax were significantly decreased in a dose-dependent manner after treatment with calcitriol (Fig. 5a, b). Meanwhile, the apoptosis-inducing effect of HG was abolished after treatment with calcitriol (Fig. 5c, d). These results suggest that vitamin D may potentially play a proapoptotic role in HG-induced podocyte apoptosis.
Fig. 5.
Vitamin D attenuates HG-induced apoptosis in podocytes. Podocytes were treated with different concentrations of calcitriol (1, 10 and 100 nm) followed by HG treatment for 48 h. a, b The expressions of cleaved caspase-3 and Bax in podocytes were determined by Western blotting. Data are mean ± SD of three experiments. *p < 0.05 versus NG, #p < 0.05 versus HG. c, d The apoptosis rate of cells was examined by flow cytometry. All the data are expressed as mean ± SD. *p < 0.05 versus NG, #p < 0.05 versus HG. NG, normal glucose (5.6 mm); HG, high glucose (30 mm); HG + 1VD, high glucose+calcitriol (1 nm); HG + 10VD, high glucose calcitriol (10 nm); HG + 100VD, high glucose+calcitriol (100 nm).
Vitamin D Enhances Autophagy in HG-Cultured Podocytes
As shown in Figure 6a, the messenger RNA expressions of Vps34 and Beclin-1 were significantly increased in podocytes after treatment with vitamin D analog calcitriol in a dose-dependent manner when compared with the HG group. Similar results are also obtained by immunoblotting (Fig. 6b, c). LC3-II was also examined by immunofluorescence assay. The number and distribution of LC3-II punctate staining were decreased in HG group compared with the control group, which were reversed after treatment with calcitriol (Fig. 6d). Furthermore, autophagic activity was examined by counting the number of autophagosomes with TEM. As shown in Figure 6e, the number of autophagosomes was gradually increased in vitamin D groups in a dose-dependent manner.
Fig. 6.
Vitamin D enhances autophagy in HG-cultured podocytes. a The mRNA levels of Beclin-1 and Vps34 were examined by real-time quantitative PCR. b LC3 exhibits red fluorescence, synaptopodin exhibits green fluorescence, and DAPI-stained nuclei exhibit blue fluorescence (magnification, ×400). c, d Cells were treated with different concentrations of calcitriol (1, 10, and 100 nmol/L) followed by HG treatment for 48 h. The expressions of LC3-II, Beclin-1, and Vps34 in podocytes were determined by Western blotting. e Electron microscopic evaluation of autophagy in podocytes. The red arrows represent autophagosomes (magnification, ×6,000 or ×20,000). Data are mean ± SD of three experiments. *p < 0.05 versus NG, #p < 0.05 versus HG. NG, normal glucose (5.6 mm); HG, high glucose (30 mm); HG + 1VD, high glucose calcitriol (1 nm); HG + 10VD, high glucose calcitriol (10 nm); HG + 100VD, high glucose + calcitriol (100 nm). mRNA, messenger RNA.
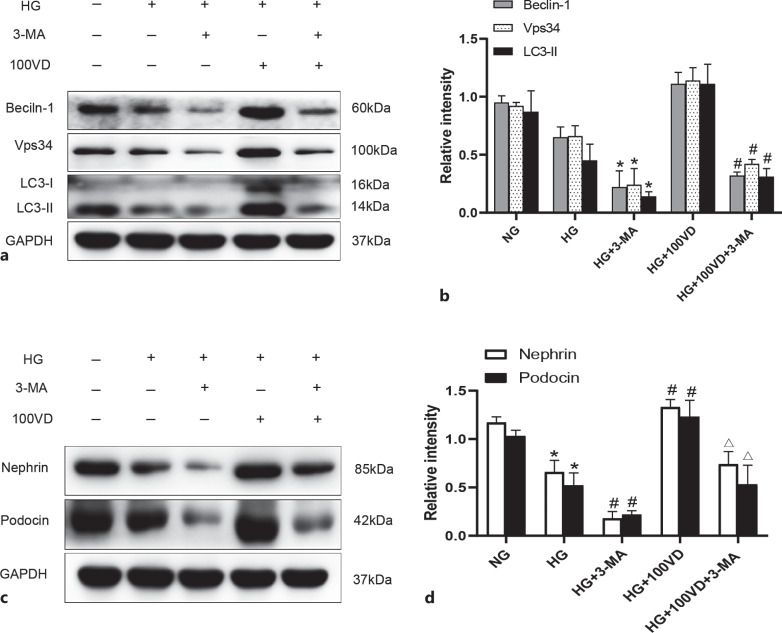
3-Methyladenine Abolished the Enhanced Autophagy and Increased Podocin and Nephrin Protein in HG-Cultured Podocytes Treated with Vitamin D
The role of vitamin D in autophagy and podocyte injury was further confirmed in HG-cultured podocytes by using autophagy inhibitor 3-methyladenine (3-MA). As shown in Figure 7a, b, the increased protein expressions of Beclin-1, Vps34, and LC3-II in HG-cultured podocytes treated with calcitriol were significantly inhibited after addition of 3-MA. Meanwhile, we also observed that the increased protein expressions of podocin and nephrin in HG-cultured podocytes treated with calcitriol were significantly decreased after addition of 3-MA. These findings further indicate that vitamin D enhances autophagy and protects podocytes under HG condition.
Fig. 7.
3-MA abolished the enhanced autophagy and increased podocin and nephrin protein in HG-cultured podocytes treated with vitamin D. a, b Podocytes were cultured in NG (5.6 mm), HG (30 mm), HG + 3-MA (10 mm), or HG + 100VD (100 nm) for 48 h. All the data are expressed as mean ± SD. Western blot analysis of Beclin-1, Vps34, LC3, and GAPDH protein expression in each group. Quantitative analysis for Beclin-1, Vps34, LC3-II protein expression. All data are expressed as mean ± SD. *p < 0.05 versus HG, #p < 0.05 versus HG + 100VD. c, d Protein expression of nephrin and podocin in podocytes was determined by Western blot. All the data are expressed as mean ± SD. *p < 0.05 versus NG, #p < 0.05 versus HG, △p < 0.05 vs. 3-MA. NG, normal glucose; HG, high glucose; MA, 3-methyladenine; VD, calcitriol.
Discussion
In the present study, we explored the protective effect and potential mechanism of vitamin D in DKD. Our results suggested that vitamin D supplementation attenuated podocyte damage by the restoration of autophagic activity in db/db mice and HG-cultured podocytes. Vitamin D plays a role renoprotection and enhances autophagy activity in podocytes, providing a novel therapeutic strategy in DKD.
DKD is the most common cause of CKD and ESRD and has become a serious public health problem worldwide. Despite current therapies, there is large residual risk of DKD onset and progression. Therefore, effective therapeutic options are urgently needed to improve the prognosis of DKD. Albuminuria results from the damage of the glomerular filtration barrier, which leads to the excessive filtration of plasma protein and other macromolecular substances and exceeds the reabsorption ability of renal tubules. Studies of both clinical and experimental DKD implicate podocyte injury and dysfunction as resulting in albuminuria and glomerulosclerosis [20–22]. Thus, exploration of the drugs which target the improvement of the damaged podocyte would be conducive to improving albuminuria and delaying the progression of DKD.
Vitamin D is well known for its role in the regulation of bone metabolism and calcium-phosphorus homeostasis and has been studied in recent years for its extensive noncalcemic functions. Numerous pieces of evidence suggest that vitamin D and its analogs have antiproteinuric effects and slow the progression of chronic kidney disease [23–25]. In DKD, vitamin D was also shown to ameliorate renal injury and prevent the loss of podocytes to a certain extent [25]. However, the underlying mechanism is not fully understood.
Autophagy and apoptosis of podocytes are considered as the two main reasons for loss of podocytes in DKD. Autophagy is a highly conserved lysosomal degradation pathway that removes protein aggregates and damaged organelles to maintain cellular homeostasis. Autophagy has been shown to be essential to the maintenance of cellular homeostasis in podocytes [26, 27]. Emerging evidence has suggested that dysregulated autophagy may contribute to both glomerular and tubulointerstitial pathologies in the kidneys of DKD [17, 28, 29]. Recent studies have also revealed that vitamin D-vitamin D receptor regulates defective autophagy in renal tubular epithelial cells and podocytes [13, 16]. In the current study, we explored whether vitamin D can be used as autophagy regulator to play a protective role against podocyte injury under diabetic state. We found that levels of autophagy-related proteins LC3-II, Beclin-1, Vps34 were lower in renal tissues of db/db mice than those in the control group, while treatment with activated vitamin D restored these autophagy protein levels in db/db mice (Fig. 4). Similarly, TEM results also further demonstrated the increasement of autophagosomes in podocytes of db/db mice treated with activated vitamin D (Fig. 4e). These results indicate that the impaired autophagy in podocytes of db/db mice was also markedly enhanced after activated vitamin D treatment. Meanwhile, this was accompanied by alleviation of albuminuria, podocyte injury, and mesangial matrix expansion. Besides, decreased podocyte slit diaphragm proteins podocin and nephrin was also markedly restored in db/db mice after activated vitamin D treatment.
In the in vitro context, HG exposure in murine podocytes altered several key proteins, including the downregulation of nephrin, podocin, and autophagy-related proteins (LC3-II, Beclin-1, and Vps34), as well as upregulation of apoptosis-related proteins (Bax, cleaved caspase-3), and podocyte apoptosis was accompanied by reductions in autophagy. However, the addition of exogenous activated vitamin D increased the expression of nephrin, podocin, and autophagy-related proteins, while the expressions of apoptosis-related proteins Bax and cleaved caspase-3 decreased. Furthermore, the protective effect of activated vitamin D against HG decreased expression of podocin and nephrin proteins and induced podocyte apoptosis could be abated by autophagy inhibitor 3-methyladenine. These results above suggest that activated vitamin D can prevent podocyte injury in DKD through modulating autophagy. However, more prospective studies are needed to validate our hypothesis, and results on long-term outcomes are also needed.
Conclusion
This study demonstrated that podocyte autophagy was impaired in the context of DKD. Notably, activated vitamin D treatment alleviated podocyte injury through enhancing autophagic activity, thus preventing the progression of DKD.
Statement of Ethics
This study protocol was reviewed and approved by the Institutional Animal Care and Use Committee of Jinan University (20180910-11).
Conflict of Interest Statement
The authors declared that they have no conflict of interest.
Funding Sources
This study was supported by the National Natural Science Foundation (81470950, 81970625), the Natural Science Foundation of Guangdong Province (2022A15150 12282), and GDPH Supporting Fund for Talent Program (DFJH201916).
Author Contributions
Houqin Xiao conceived and designed the study as well as critically revised the manuscript. Xiaoyi Zhang performed the experiments, analyzed the data, and drafted the manuscript. Li Zhang participated in the study design and manuscript revision. Yingzhen Wen and Mengxian Zhang performed the experiments and data collection. Shuangxin Liu participated in the study design. All authors critically reviewed the manuscript and approved the final draft.
Funding Statement
This study was supported by the National Natural Science Foundation (81470950, 81970625), the Natural Science Foundation of Guangdong Province (2022A15150 12282), and GDPH Supporting Fund for Talent Program (DFJH201916).
Data Availability Statement
All data generated or analyzed during the study are included in this article. Further inquiries can be directed to the corresponding author.
References
- 1. Saeedi P, Petersohn I, Salpea P, Malanda B, Karuranga S, Unwin N, et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: results from the international diabetes federation diabetes atlas, 9th edition. Diabetes Res Clin Pract. 2019;157:107843. 10.1016/j.diabres.2019.107843. [DOI] [PubMed] [Google Scholar]
- 2. Tervaert TW, Mooyaart AL, Amann K, Cohen AH, Cook HT, Drachenberg CB, et al. Pathologic classification of diabetic nephropathy. J Am Soc Nephrol. 2010;21(4):556–63. 10.1681/ASN.2010010010. [DOI] [PubMed] [Google Scholar]
- 3. Reiser J, Altintas MM. Podocytes. F1000Res. 2016;5:F1000. Faculty Rev-114. [Google Scholar]
- 4. Hartleben B, Godel M, Meyer-Schwesinger C, Liu S, Ulrich T, Köbler S, et al. Autophagy influences glomerular disease susceptibility and maintains podocyte homeostasis in aging mice. J Clin Invest. 2010;120(4):1084–96. 10.1172/JCI39492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cao H, Fang L, Zhou Y, Wen P, Jiang L, He W, et al. Autophagy attenuates diabetic glomerular damage through protection of hyperglycemia-induced podocyte injury. PLoS One. 2013;8(4):e60546. 10.1371/journal.pone.0060546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mauthe M, Jacob A, Freiberger S, Hentschel K, Stierhof YD, Codogno P, et al. Resveratrol-mediated autophagy requires WIPI-1-regulated LC3 lipidation in the absence of induced phagophore formation. Autophagy. 2011;7(12):1448–61. 10.4161/auto.7.12.17802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Funderburk SF, Wang QJ, Yue Z. The Beclin 1-VPS34 complex--at the crossroads of autophagy and beyond. Trends Cell Biol. 2010;20(6):355–62. 10.1016/j.tcb.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yang D, Livingston MJ, Liu Z, Dong G, Zhang M, Chen JK, et al. Autophagy in diabetic kidney disease: regulation, pathological role and therapeutic potential. Cell Mol Life Sci. 2018;75(4):669–88. 10.1007/s00018-017-2639-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Koch EAT, Nakhoul R, Nakhoul F, Nakhoul N. Autophagy in diabetic nephropathy: a review. Int Urol Nephrol. 2020;52(9):1705–12. 10.1007/s11255-020-02545-4. [DOI] [PubMed] [Google Scholar]
- 10. Yasuda-Yamahara M, Kume S, Tagawa A, Maegawa H, Uzu T. Emerging role of podocyte autophagy in the progression of diabetic nephropathy. Autophagy. 2015;11(12):2385–6. 10.1080/15548627.2015.1115173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Xiao HQ, Shi W, Liu SX, Zhang B, Xu LX, Liang XL, et al. Podocyte injury is suppressed by 1,25-dihydroxyvitamin D via modulation of transforming growth factor-beta 1/bone morphogenetic protein-7 signalling in puromycin aminonucleoside nephropathy rats. Clin Exp Pharmacol Physiol. 2009;36(7):682–9. 10.1111/j.1440-1681.2008.05133.x. [DOI] [PubMed] [Google Scholar]
- 12. Maddaloni E, Cavallari I, Napoli N, Conte C. Vitamin D and diabetes mellitus. Front Horm Res. 2018;50:161–76. 10.1159/000486083. [DOI] [PubMed] [Google Scholar]
- 13. Shi L, Xiao C, Zhang Y, Xia Y, Zha H, Zhu J, et al. Vitamin D/vitamin D receptor/Atg16L1 axis maintains podocyte autophagy and survival in diabetic kidney disease. Ren Fail. 2022;44(1):694–705. 10.1080/0886022X.2022.2063744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lei M, Liu Z, Guo J. The emerging role of vitamin D and vitamin D receptor in diabetic nephropathy. BioMed Res Int. 2020;2020:4137268. 10.1155/2020/4137268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kuhlmann A, Haas CS, Gross ML, Reulbach U, Holzinger M, Schwarz U, et al. 1,25-Dihydroxyvitamin D3 decreases podocyte loss and podocyte hypertrophy in the subtotally nephrectomized rat. Am J Physiol Renal Physiol. 2004;286(3):F526–33. 10.1152/ajprenal.00316.2003. [DOI] [PubMed] [Google Scholar]
- 16. Li A, Yi B, Han H, Yang S, Hu Z, Zheng L, et al. Vitamin D-VDR (vitamin D receptor) regulates defective autophagy in renal tubular epithelial cell in streptozotocin-induced diabetic mice via the AMPK pathway. Autophagy. 2022;18(4):877–90. 10.1080/15548627.2021.1962681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fogo AB, Haas M, de Heer E, Cohen AH, Cook HT, Drachenberg CB, et al. Renal pathology society. Pathologic classification of diabetic nephropathy. J Am Soc Nephrol. 2010;21(4):556–63. [DOI] [PubMed] [Google Scholar]
- 18. Xiao H, Shi W, Liu S, Wang W, Zhang B, Zhang Y, et al. 1,25-Dihydroxyvitamin D(3) prevents puromycin aminonucleoside-induced apoptosis of glomerular podocytes by activating the phosphatidylinositol 3-kinase/Akt-signaling pathway. Am J Nephrol. 2009;30(1):34–43. 10.1159/000200769. [DOI] [PubMed] [Google Scholar]
- 19. Li R, Zhang L, Shi W, Zhang B, Liang X, Liu S, et al. NFAT2 mediates high glucose-induced glomerular podocyte apoptosis through increased Bax expression. Exp Cell Res. 2013;319(7):992–1000. 10.1016/j.yexcr.2013.01.007. [DOI] [PubMed] [Google Scholar]
- 20. Pagtalunan ME, Miller PL, Jumping-Eagle S, Nelson RG, Myers BD, Rennke HG, et al. Podocyte loss and progressive glomerular injury in type II diabetes. J Clin Invest. 1997;99(2):342–8. 10.1172/JCI119163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Meyer TW, Bennett PH, Nelson RG. Podocyte number predicts long-term urinary albumin excretion in Pima Indians with Type II diabetes and microalbuminuria. Diabetologia. 1999;42(11):1341–4. 10.1007/s001250051447. [DOI] [PubMed] [Google Scholar]
- 22. Pavenstädt H, Kriz W, Kretzler M. Cell biology of the glomerular podocyte. Physiol Rev. 2003;83(1):253–307. 10.1152/physrev.00020.2002. [DOI] [PubMed] [Google Scholar]
- 23. de Borst MH, Hajhosseiny R, Tamez H, Wenger J, Thadhani R, Goldsmith DJ. Active vitamin D treatment for reduction of residual proteinuria: a systematic review. J Am Soc Nephrol. 2013;24(11):1863–71. 10.1681/ASN.2013030203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Haussler MR, Whitfield GK, Haussler CA, Sabir MS, Khan Z, Sandoval R, et al. 1,25-Dihydroxyvitamin D and klotho: a tale of two renal hormones coming of age. Vitam Horm. 2016;100:165–230. 10.1016/bs.vh.2015.11.005. [DOI] [PubMed] [Google Scholar]
- 25. Wang Y, Deb DK, Zhang Z, Sun T, Liu W, Yoon D, et al. Vitamin D receptor signaling in podocytes protects against diabetic nephropathy. J Am Soc Nephrol. 2012;23(12):1977–86. 10.1681/ASN.2012040383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bork T, Liang W, Yamahara K, Lee P, Tian Z, Liu S, et al. Podocytes maintain high basal levels of autophagy independent of mtor signaling. Autophagy. 2020;16(11):1932–48. 10.1080/15548627.2019.1705007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lin Q, Banu K, Ni Z, Leventhal JS, Menon MC. Podocyte autophagy in homeostasis and disease. J Clin Med. 2021;10(6):1184. 10.3390/jcm10061184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wang B, Qian JY, Tang TT, Lin LL, Yu N, Guo HL, et al. VDR/Atg3 Axis regulates slit diaphragm to tight junction transition via p62-mediated autophagy pathway in diabetic nephropathy. Diabetes. 2021;70(11):2639–51. 10.2337/db21-0205. [DOI] [PubMed] [Google Scholar]
- 29. Liu N, Xu L, Shi Y, Zhuang S. Podocyte autophagy: a potential therapeutic target to prevent the progression of diabetic nephropathy. J Diabetes Res. 2017;2017:3560238. 10.1155/2017/3560238. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during the study are included in this article. Further inquiries can be directed to the corresponding author.