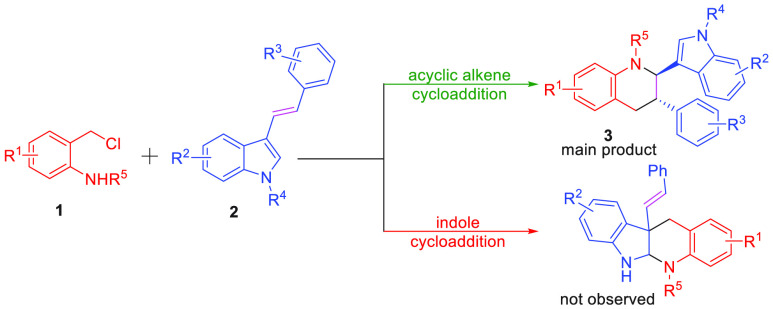
Abstract
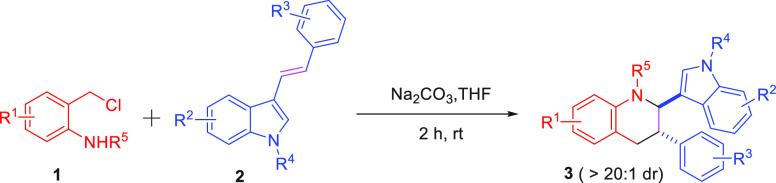
The chemoselective annulation of aza-ortho-quinone methide generated by in situ o-chloromethyl sulfonamide has been achieved with bifunctional acyclic olefin. This efficient approach provides access to the diastereoselective synthesis of functionalized tetrahydroquinoline derivatives containing indole scaffolds through the inverse-electron-demand aza-Diels–Alder reaction under mild reaction conditions with excellent results (up to 93% yield, > 20:1 dr). Moreover, this article realized the cyclization of α-halogeno hydrazone with electron-deficient alkene affording the tetrahydropyridazine derivatives, which had never been reported.
Introduction
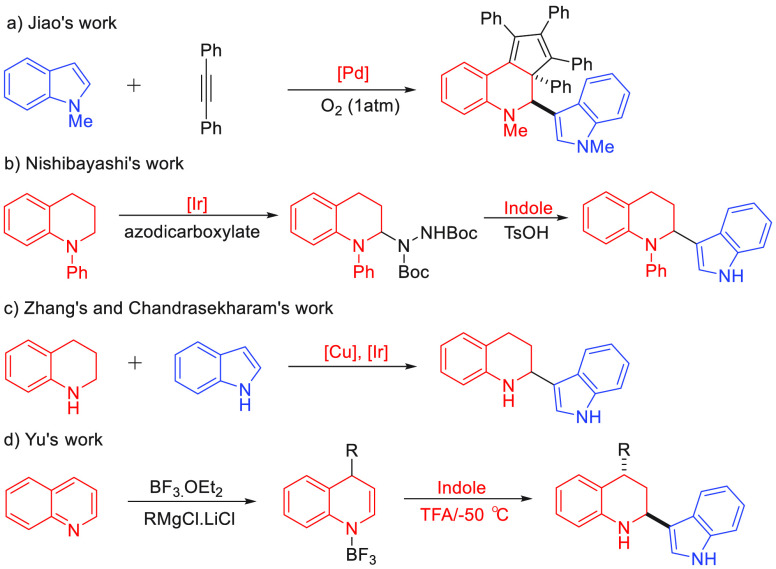
Recent research indicated N-biheteroarenes play an important role in dyes and pharmaceuticals, such as antibacterial agent 2-(1H-indol-3-yl)tetrahydroquinoline.1 Tetrahydroquinoline and indole skeleton widely exist in the core structure of the natural product and exhibits a broad spectrum of biological activities, respectively.2 Therefore, it is of great value to construct the tetrahydroquinoline-indole linked heterobiarene framework for the discovery of functional and pharmaceutically active molecules. To date, these methods for the synthesis of tetrahydroquinoline containing indole scaffold are very limited. An early example, Jiao’s group developed a selective ring-expansion reaction mediated by the Pd(OAc)2 providing the polysubstituted tetrahydroquinoline-indole scaffold (Scheme 1a).3 In 2012, the C–H amination of tetrahydroquinoline was contributed by Nishibayashi and co-workers affording biheteroarenes with the assistance of a visible-light-photoredox catalyst (Scheme 1b).4 Later, Zhang and Chandrasekharam’s group disclosed a Cu/Ir-catalyzed direct α-functionalization strategy through the dehydrogenative cross C(sp3)-C(sp2) coupling of tetrahydroquinolines and indoles (Scheme 1c).5 Recently, the dearomative double nucleophilic addition to quinolines accessing tetrahydroquinoline was described by Yu’s group (Scheme 1d).6 Although some efficient strategies have been established, it limited their application using a metal catalyst, oxidation, or harsh reaction condition. Thus, it is still highly desirable to exploit a mild, metal-free, and easy-to-operate method for constructing the tetrahydroquinoline skeleton bearing indole.
Scheme 1. Synthesis of Tetrahydroquinoline.
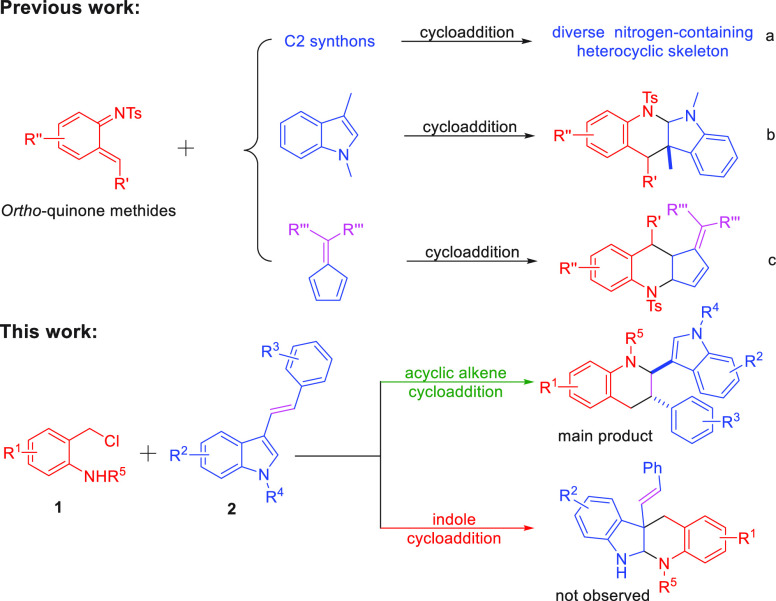
Aza-ortho-quinone methides (aza-o-QMs) generated in situ via the o-chloromethyl sulfonamide were widely employed as the four atoms building blocks for the construction of N-containing heterocyclic compounds through [4 + n] annulation reaction.7−10 Especially, the [4 + 2] cycloaddition reaction attracted extensive attention since the Diels–Alder reaction of aza-o-QMs with C2 synthons has been disclosed by Corey’s group.11 In 2019, Liu’s group discovered the cycloaddition reaction between 1,3,5-triazinane and o-chloromethyl sulfonamide could form various tetrahydroquinazoline derivatives with the assistance of the base.12 Besides, much effort has been devoted to developing the cycloaddition of aza-o-QMs with a cyclic alkene, such as furan, azlactone, bicyclic alkene oxabenzonorbornene, and [60] fullerene affording diverse quinoline scaffold through hetero-Diels–Alder reaction (Scheme 2a).13−15 Moreover, You’s group reported a concise synthesis of tetrahydro-5H-indolo[2,3-b]quinoline using o-chloromethyl sulfonamide and 1,3-dimethyl-1H-indole as the substances (Scheme 2b).16 It is worth noting that the cycloaddition product of acyclic olefin was not detected, while the substrate contains acyclic olefin and cyclic olefin functional groups (Scheme 2c).17 Furthermore, because indole has excellent reactivity,16,18 there is no example of the chemoselective intermolecular [4 + 2] annulation of aza-ortho-quinone methide with 1,2 disubstituted acyclic olefin in the presence of acyclic olefin and indole. Achievement of such a transformation is particularly challenging, because of (1) the potential competing dimerization of the aza-o-QMs and self-nucleophilic addition reaction;14b,19 (2) it may suppress the occurrence of this transformation that indole could react with aza-o-QMs;16,18 (3) compared with acyclic olefin, cyclic olefins have priority reactivity.17,18 In view of our continued interest in the annulation reaction of aza-ortho-quinone methides,7c,9e,19 we envisioned the chemoselective annulation of aza-ortho-quinone methide 1 with 3-vinylindoles 2(20) would occur, which could provide a mild and metal-free method to form the functionalized tetrahydroquinoline containing indole framework.
Scheme 2. [4 + 2] Annulation of Aza-ortho-quinone Methides.
Results and Discussion
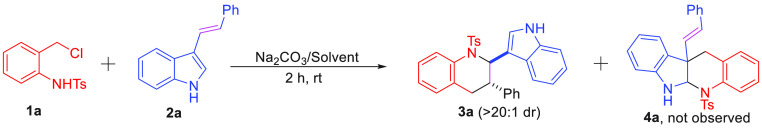
With these considerations in mind, we began our investigation using o-chloromethyl sulfonamide 1a as the four-atom building blocks and bifunctional acyclic olefin 2a as the C2 synthon under the basic conditions to screen effective parameters. The initial experiment was performed in the presence of KOH (0.2 mmol), 1a (0.15 mmol), and 2a (0.1 mmol) in dichloromethane (DCM) at room temperature. The corresponding [4 + 2] annulation product 3a was obtained in 48% isolated yield (Table 1, entry 1). The structure of 3a was identified through NMR analysis and confirmed by X-ray crystallographic analysis.21 Interestingly, the cycloaddition product 4a between indole and aza-o-QMs was not obtained, which indicated the reaction has excellent selectivity. As shown in Table 1, screening of various bases was conducted at room temperature (Table 1, entries 1–10). These results indicated: (1) the type of base has a significant impact on this transformation (Table 1, entries 1–3 vs entries 4–6); (2) organic base 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU), triethylamine (TEA), and 1,4-diazabicyclo[2.2.2]octane (TEDA) could not effectively promote the annulation proceeding (Table 1, entries 4–6); and (3) inorganic bases presented a better performance in yield (Table 1, entries 7–10), and Na2CO3 was proven to be the appropriate base for this cycloaddition reaction with 83% yield (Table 1, entry 8). To find the optimal condition, various solvents were screened with Na2CO3 as the base. Moderate yields (42%–86%) were provided in dichloroethane (DCE), trichloromethane (CHCl3), and acetonitrile (MeCN) (Table 1, entries 11–13). The best yield (90%) was afforded using THF (Table 1, entry 14). In addition, raising and decreasing the temperature could not further improve the yield of 3a (Table 1, entries 15–16).
Table 1. Screening of Optimal Reaction Conditionsa.
| entry | base | solvent | yield of 3a (%)b |
|---|---|---|---|
| 1 | KOH | DCM | 48 |
| 2 | NaOH | DCM | 50 |
| 3 | KOtBu | DCM | 19 |
| 4 | DBU | DCM | <10 |
| 5 | TEA | DCM | <5 |
| 6 | TEDA | DCM | trace |
| 7 | K2CO3 | DCM | 71 |
| 8 | Na2CO3 | DCM | 83 |
| 9 | Cs2CO3 | DCM | 67 |
| 10 | KHCO3 | DCM | 79 |
| 11 | Na2CO3 | DCE | 42 |
| 12 | Na2CO3 | CHCl3 | 74 |
| 13 | Na2CO3 | MeCN | 86 |
| 14 | Na2CO3 | THF | 90 |
| 15c | Na2CO3 | THF | 76c |
| 16d | Na2CO3 | THF | 84d |
Reaction conditions: 1a (0.15 mmol), 2a (0.1 mmol), and base (0.2 mmol) were reacted for 2 h at room temperature. The dr value was determined by the crude 1HNMR analysis. 4a was not detected in this transformation.
Isolated yield based on 2a.
Reaction was performed at 0 °C.
Reaction was performed at 66 °C.
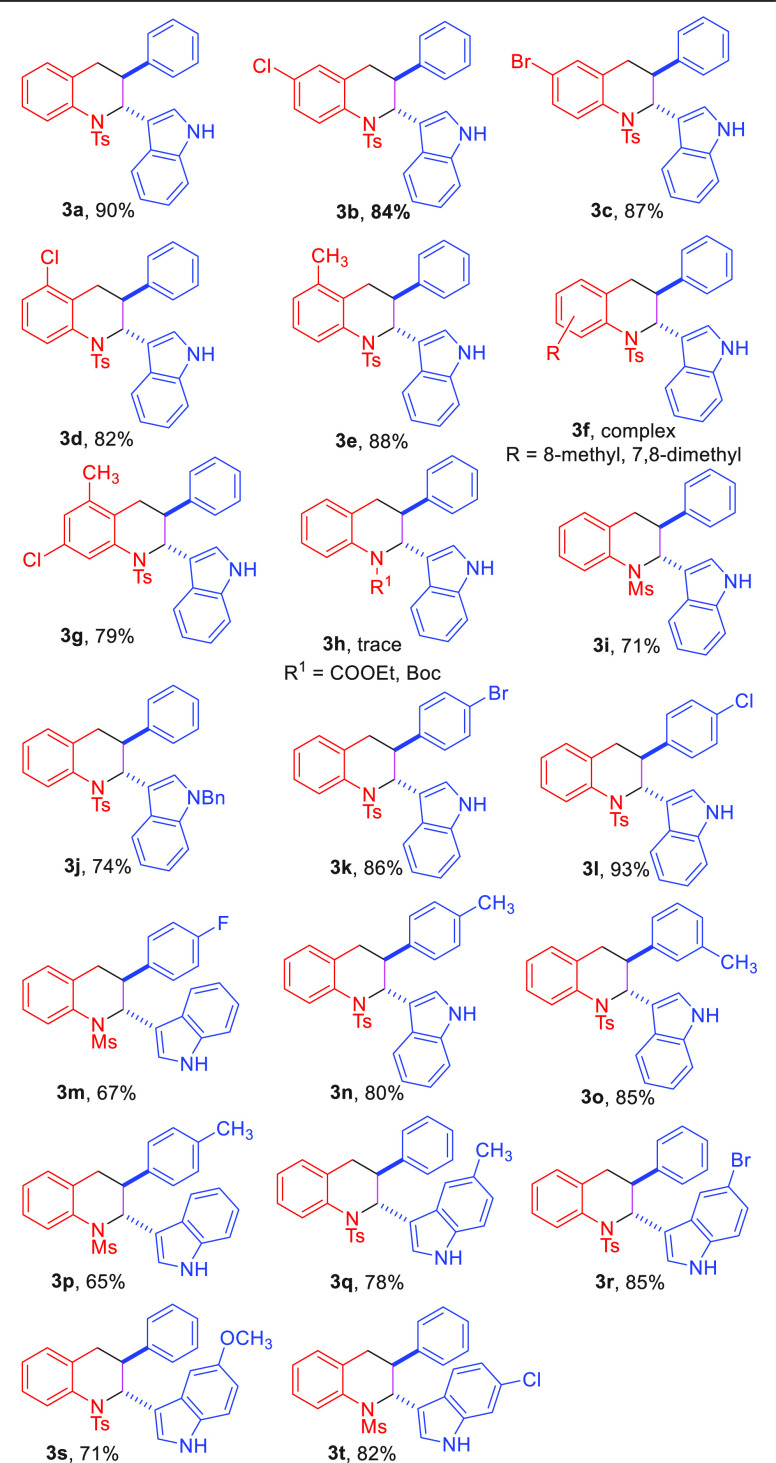
With the aforementioned optimal reaction conditions established, the scope of o-chloromethyl sulfonamides 1 and bifunctional acyclic olefin 2 with various substituted groups was examined. As shown in Table 2, the first part of the substrate scope was screened using (E)-3-styryl-1H-indole 2a and the aza-ortho-quinone methides 1 bearing various substituted phenyl rings as the starting material. A series of o-chloromethyl sulfonamides 1 bearing different electron-withdrawing substituents (−Cl, −Br) on the 3- or 4- position of aromatic ring underwent smoothly, offering the [4 + 2] annulation product in good to excellent yield (3b, 3c, and 3d). Besides, the tetrahydroquinoline derivative 3e featuring electron-donating groups on the aromatic group was formed in 88% yield under standard conditions. However, when the methyl group was at the 7- and 8-positions on the aromatic ring of 1, the reaction mixture was so complicated that the expected product could not be obtained. Significantly, starting material 1 containing different electrical properties of the group on the aromatic ring was tolerated in this reaction providing the desired product 3g in 79% yield. Subsequently, the effect of substrates possessing different protecting groups on this reaction was studied. Due to the strong electron-withdrawing group (−COOEt) and large sterically hindered group (−Boc) could block the cyclization process, 3h could not be accessed. To our delight, N-(2-(chloromethyl) phenyl) methanesulfonamide was compatible with the [4 + 2] cycloaddition reaction affording 3i in 71% yield with >20:1 dr. Encouraged by these promising results, 3-vinylindole 2 containing different substituents was synthesized to further evaluate the generality of this transformation. First, the substituted group on the nitrogen atom of indole was tested. When switching the hydrogen atom to the benzyl group, the desired compound 3j could be obtained in 74%. Furthermore, this protocol was amenable to the 3-vinylindole substrates with electron-withdrawing groups, such as −F, −Cl, −Br groups and delivered the corresponding product 3k–3m in good to excellent yields with high diastereoselectivities (67%–93% yields, >20:1 dr). Moreover, when the methyl group is on the 3- or 4- position of the benzene ring, tetrahydroquinoline skeletons bearing indole were achieved as a single diastereoisomer in 80% and 85% yields, respectively (3n, 3o). Compared with 3i, 3m, and 3p, it was found that the [4 + 2] annulation between the mesyloxy-protected o-chloromethyl sulfonamide 1 and bifunctional acyclic olefin 2 containing an electron-withdrawing or electron-donating group could proceed smoothly, giving the desired product with excellent diastereoselectivity. In addition, bifunctional olefin 2 with a methyl or Br group at the five positions on the indole ring could also work well under the optimal reaction conditions, providing the cycloaddition products 3q–3r in moderate yields (78%, 85%, >20:1 dr). In addition, the ether functional group was also compatible in this process, giving the cycloaddition product 3s in 71%. Furthermore, 3t could be delivered in 82% yield via this conversion using the Cl substituted group at the six positions on the indole ring and aza-o-QMs 1a as the starting materials.
Table 2. Substrate Scope of Reactiona.
Reaction conditions: 1 (0.15 mmol), 2 (0.1 mmol), and Na2CO3 (0.2 mmol) were reacted at room temperature. The dr value was determined by the crude 1HNMR analysis;
Isolated yield based on 2.
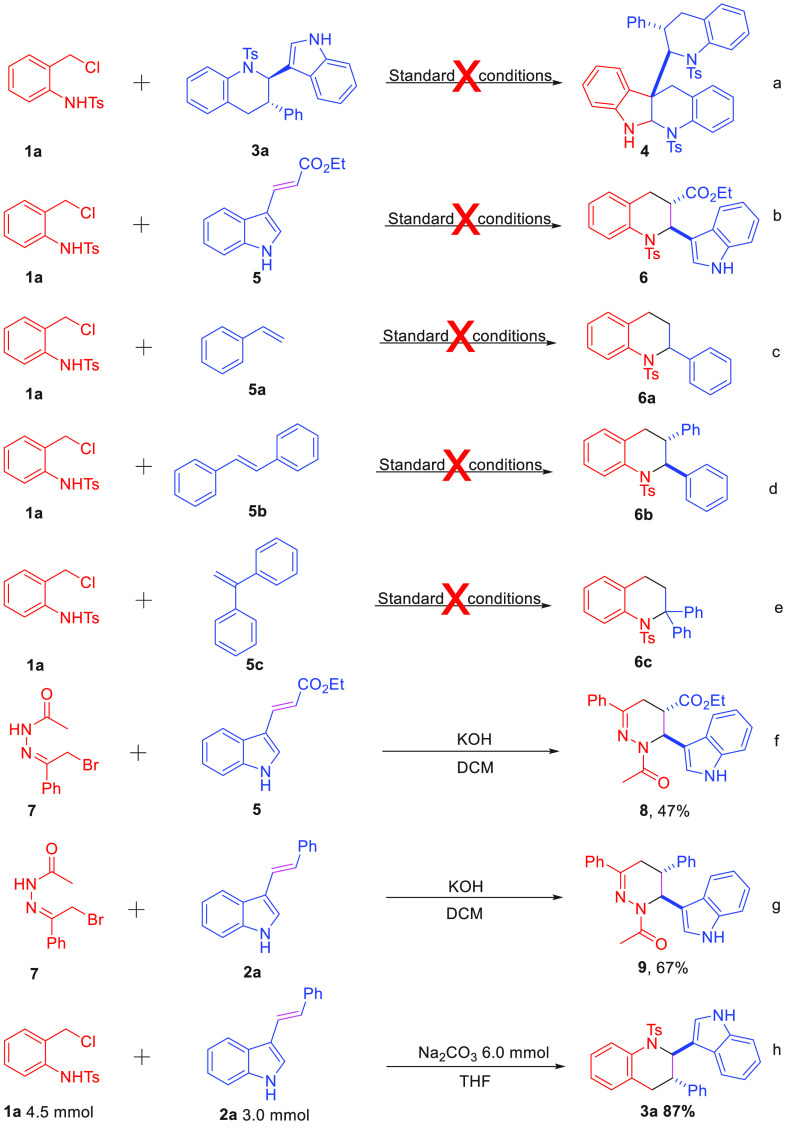
Encouraged by these excellent results, the cyclization of o-chloromethyl sulfonamide 1a with tetrahydroquinoline scaffold bearing indole 3a was performed. To our disappointment, the desired product 4 could not be afforded (Scheme 3a). Although various bifunctional olefins, such as ethyl (E)-3-(1H-indol-3-yl) acrylate 5, styrene 5a, (E)-1,2-diphenylethene 5b, and ethene-1,1-diyldibenzene 5c, were applied in this process, the [4 + 2] cycloaddition product could not be furnished, which indicated bifunctional acyclic olefins containing indole is necessary for this transformation (Scheme 3b–e). Our previous work showed the reactivity of α-halogeno hydrazone 7 is similar to 1a,9e and the [4 + 2] annulation reaction between α-halogeno hydrazone 7 and the electron-deficient bifunctional acyclic olefin 5 has been studied, utilizing DCM as the solvent in the presence of KOH. This process proceeded smoothly affording the tetrahydropyridazine 8 in 47% yield (>20:1 dr) (Scheme 3f), which had never been reported. Moreover, α-halogeno hydrazone was a good C4 building block for the synthesis of 1-(6-(1H-indol-3-yl)-3,5-diphenyl-5,6-dihydropyridazin-1(4H)-yl)ethan-1-one 9 (Scheme 3g). To gain insight into the utility of this chemoselective cyclization of aza-ortho-quinone methide precursor, a gram-scale experiment was performed under standard conditions. Expected cycloaddition product 3a was generated in 87% yield with excellent dr (>20:1) (Scheme 3h).
Scheme 3. Gram-Scale Reactions and Transformations.
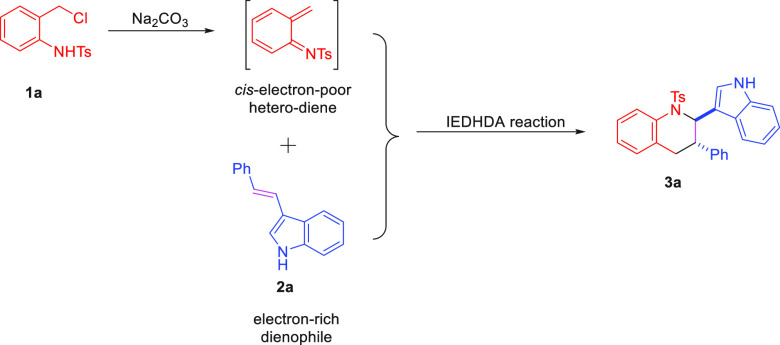
A plausible mechanism was proposed as shown in Scheme 4. The cis-electron-poor heterodiene intermediate was generated by 1a, in situ, under basic conditions. Meanwhile, 3-vinylindole 2a was employed as the electron-rich dienophile. Then, the inverse-electron-demand hetero-Diels–Alder reaction between 2a and cis-electron-poor heterodiene would occur delivering 3a.
Scheme 4. Proposed Mechanism for Forming Product 3.
Conclusions
In conclusion, we herein developed an inverse-electron-demand hetero-Diels–Alder reaction between aza-ortho-quinone methide precursor and bifunctional acyclic olefin mediated by an inorganic base. This strategy provided a convenient method to produce the highly functionalized tetrahydroquinoline derivative containing indole scaffold under mild reaction conditions with excellent results (63–91% yields, >20:1 dr). Furthermore, our approach realized the cyclization of α-halogeno hydrazone with electron-deficient alkene which had never been reported.
Experimental Section
General Information
The 1H and 13C NMR spectra were recorded on a 400 MHz spectrometer with chloroform-d and dimethyl sulfoxide-d6 as the solvent. High-resolution mass spectra (HRMS) were recorded on an FT-ICR MS spectrometer. Column chromatography was performed on silica gel 200–300 mesh. azoalkene precursors 1 and bifunctional acyclic olefin 2 were synthesized according to literature methods.9b,20b,22
General Procedure for the Preparation of Dihydropyrazole 3
To a stirred solution of the aza-ortho-quinone methide precursor 1 (0.15 mmol) and Na2CO3 (0.2 mmol) in THF (2 mL) at room temperature, bifunctional acyclic olefin 2 (0.1 mmol) was added. After 2 h, bifunctional acyclic olefin 2 disappeared, as indicated by the TLC. The mixture was concentrated in vacuo, and the crude product was purified by flash chromatography eluting with (petroleum ether/ethyl acetate 10:1) to afford the products 3.
2-(1H-Indol-3-yl)-3-phenyl-1-tosyl-1,2,3,4-tetrahydroquinoline (3a)
Ethyl acetate/petroleum ether = 1:10 as an eluent, yellow solid (43 mg, 90%). MP: 164.4–175.9 °C. 1H NMR (400 MHz, DMSO-d6) δ 10.89 (d, J = 2.6 Hz, 1H), 7.55–7.50 (m, 1H), 7.45–7.29 (m, 6H), 7.28–7.17 (m, 5H), 7.06–6.91 (m, 5H), 6.81 (ddd, J = 8.0, 7.0, 1.0 Hz, 1H), 5.54 (d, J = 9.3 Hz, 1H), 3.31 (ddd, J = 12.4, 9.3, 3.4 Hz, 1H), 2.54–2.50 (m, 1H), 2.37 (s, 3H), 1.92 (dd, J = 14.2, 12.0 Hz, 1H). 13C NMR (100 MHz, DMSO-d6) δ 143.80, 142.19, 136.51, 136.16, 135.78, 135.35, 129.71, 128.57, 127.74, 127.39, 127.27, 126.89, 126.21, 124.85, 123.83, 120.99, 119.01, 118.56, 115.63, 111.76, 61.41, 50.08, 33.36, 21.07. HRMS (ESI): m/z calcd for C30H26N2NaO2S [M + Na]+: 501.1607, found 501.1601.
6-Chloro-2-(1H-indol-3-yl)-3-phenyl-1-tosyl-1,2,3,4-tetrahydroquinoline (3b)
Ethyl acetate/petroleum ether = 1:10 as an eluent, yellow solid (43 mg, 84%). MP: 109.1–121.2 °C. 1H NMR (400 MHz, Chloroform-d): δ 8.01 (s, 1H), 7.63 (d, J = 8.6 Hz, 1H), 7.44–7.35 (m, 2H), 7.30 (dd, J = 8.8, 2.0 Hz, 2H), 7.23–7.09 (m, 7H), 7.01 (d, J = 8.0 Hz, 1H), 6.93 (ddd, J = 8.0, 6.9, 1.1 Hz, 1H), 6.87–6.80 (m, 2H), 6.78 (d, J = 2.3 Hz, 1H), 5.50 (d, J = 9.1 Hz, 1H), 3.39 (ddd, J = 12.2, 9.1, 3.6 Hz, 1H), 2.50 (dd, J = 14.5, 3.5 Hz, 1H), 2.42 (s, 3H), 2.10 (dd, J = 14.5, 11.5 Hz, 1H). 13C NMR (100 MHz, Chloroform-d): δ 143.95, 142.03, 136.78, 136.60, 136.29, 135.47, 131.27, 129.68, 128.68, 127.78, 127.68, 127.64, 127.62, 127.28, 127.20, 124.96, 123.43, 122.01, 119.74, 119.58, 116.61, 111.58, 62.44, 49.45, 33.12, 21.75. HRMS (ESI): m/z calcd for C30H25ClN2NaO2S [M + Na]+: 535.1217, found 535.1217.
6-Bromo-2-(1H-indol-3-yl)-3-phenyl-1-tosyl-1,2,3,4-tetrahydroquinoline (3c)
Ethyl acetate/petroleum ether = 1:10 as an eluent, yellow solid (48 mg, 87%). MP: 138.5–142.4 °C. 1H NMR (400 MHz, Chloroform-d) δ 7.97 (d, J = 2.4 Hz, 1H), 7.57 (d, J = 8.6 Hz, 1H), 7.44 (dd, J = 8.6, 2.3 Hz, 1H), 7.39–7.35 (m, 2H), 7.31 (d, J = 8.3 Hz, 1H), 7.27 (d, J = 2.3 Hz, 1H), 7.22–7.16 (m, 5H), 7.13 (ddd, J = 8.1, 6.9, 1.2 Hz, 1H), 7.03 (d, J = 8.0 Hz, 1H), 6.95 (ddd, J = 8.0, 6.9, 1.0 Hz, 1H), 6.88–6.83 (m, 2H), 6.81 (d, J = 2.5 Hz, 1H), 5.52 (d, J = 9.0 Hz, 1H), 3.44–3.38 (m, 1H), 2.51 (dd, J = 14.7, 3.7 Hz, 1H), 2.42 (s, 3H), 2.14 (dd, J = 14.6, 11.3 Hz, 1H). 13C NMR (100 Hz, Chloroform-d) δ 143.94, 142.03, 136.78, 136.71, 136.31, 136.05, 130.62, 130.59, 129.68, 128.70, 127.90, 127.64, 127.32, 127.22, 124.97, 123.39, 122.08, 119.65, 119.15, 116.70, 111.55, 62.34, 49.27, 32.96, 21.76. HRMS (ESI): m/z calcd for C30H25BrN2NaO2S [M + Na]+: 579.0712, found 579.0718.
5-Chloro-2-(1H-indol-3-yl)-3-phenyl-1-tosyl-1,2,3,4-tetrahydroquinoline (3d)
Ethyl acetate/petroleum ether = 1:10 as an eluent, yellow solid (42 mg, 82%). MP: 186.8–191.6 °C. 1H NMR (400 MHz, Chloroform-d) δ 8.00 (s, 1H), 7.59 (d, J = 7.4 Hz, 1H), 7.33–7.10 (m, 12H), 6.99–6.87 (m, 3H), 6.79 (d, J = 2.5 Hz, 1H), 5.64 (d, J = 8.4 Hz, 1H), 3.47 (ddd, J = 10.3, 8.4, 4.0 Hz, 1H), 2.97 (dd, J = 15.4, 4.1 Hz, 1H), 2.40 (s, 3H), 2.18 (dd, J = 15.4, 10.4 Hz, 1H). 13C NMR (100 MHz, Chloroform-d) δ 143.95, 142.18, 138.42, 136.76, 136.44, 132.59, 131.93, 129.64, 128.72, 127.70, 127.66, 127.38, 127.20, 126.32, 125.01, 124.25, 123.38, 122.13, 119.73, 119.65, 116.65, 111.55, 61.91, 48.19, 29.55, 21.75. HRMS (ESI): m/z calcd for C30H25ClN2NaO2S [M + Na]+: 535.1217, found 535.1214.
2-(1H-Indol-3-yl)-5-methyl-3-phenyl-1-tosyl-1,2,3,4-tetrahydroquinoline (3e)
Ethyl acetate/petroleum ether = 1:10 as an eluent, yellow solid (44 mg, 88%). MP: 118.9–124.6 °C. 1H NMR (400 MHz, DMSO-d6) δ 10.88 (d, J = 2.7 Hz, 1H), 7.36–7.19 (m, 10H), 7.12–6.97 (m, 5H), 6.91 (d, J = 2.6 Hz, 1H), 6.87–6.81 (m, 1H), 5.61 (d, J = 8.8 Hz, 1H), 3.34 (ddt, J = 11.0, 8.7, 3.8 Hz, 1H), 2.58 (dd, J = 14.8, 3.8 Hz, 1H), 2.35 (s, 3H), 2.17 (s, 3H), 1.85 (dd, J = 14.9, 11.0 Hz, 1H). 13C NMR (100 MHz, DMSO-d6) δ 143.72, 142.53, 136.47, 136.14, 135.96, 135.00, 133.15, 129.59, 128.58, 127.46, 127.39, 126.99, 126.88, 126.32, 124.89, 123.73, 123.46, 121.04, 119.06, 118.59, 115.68, 111.74, 60.75, 49.00, 29.37, 21.07, 19.05. HRMS (ESI): m/z calcd for C31H28N2NaO2S [M + Na]+: 515.1764, found 515.1763.
7-Chloro-2-(1H-indol-3-yl)-5-methyl-3-phenyl-1-tosyl-1,2,3,4-tetrahydroquinoline (3g)
Ethyl acetate/petroleum ether = 1:10 as an eluent, yellow solid (42 mg, 79%). MP: 108.7–119.1 °C. 1H NMR (400 MHz, Chloroform-d) δ 7.92 (s, 1H), 7.63 (d, J = 8.3 Hz, 2H), 7.34 (d, J = 8.0 Hz, 2H), 7.32–7.27 (m, 2H), 7.17–7.09 (m, 4H), 7.01 (d, J = 2.4 Hz, 1H), 6.89 (ddd, J = 8.1, 7.0, 1.0 Hz, 1H), 6.77–6.67 (m, 4H), 5.26 (d, J = 9.8 Hz, 1H), 3.21 (ddd, J = 13.2, 9.7, 3.2 Hz, 1H), 2.49 (s, 3H), 2.31 (dd, J = 14.1, 3.3 Hz, 1H), 2.25 (s, 3H), 2.01 (t, J = 13.7 Hz, 1H). 13C NMR (100 Hz, Chloroform-d) δ 144.21, 142.10, 141.53, 141.25, 136.88, 136.58, 134.39, 132.66, 130.08, 129.82, 128.53, 128.10, 127.47, 127.08, 125.09, 125.03, 123.28, 122.08, 119.73, 119.68, 116.50, 111.42, 63.06, 51.23, 34.44, 21.85, 19.63. HRMS (ESI): m/z calcd for C31H27ClN2NaO2S [M + Na]+: 5491374, found 5491374.
2-(1H-Indol-3-yl)-1-(methylsulfonyl)-3-phenyl-1,2,3,4-tetrahydroqui-noline (3i)
Ethyl acetate/petroleum ether = 1:10 as an eluent, yellow solid (28 mg, 71%). MP: 129.8–134.7 °C. 1H NMR (400 MHz, Chloroform-d) δ 7.99 (s, 1H), 7.61 (d, J = 8.2 Hz, 1H), 7.36–7.15 (m, 11H), 7.02 (ddd, J = 8.1, 7.1, 1.0 Hz, 1H), 6.82 (d, J = 2.5 Hz, 1H), 5.82 (d, J = 7.6 Hz, 1H), 3.62 (td, J = 8.2, 4.3 Hz, 1H), 3.18 (dd, J = 15.2, 8.7 Hz, 1H), 2.95 (dd, J = 15.2, 4.4 Hz, 1H), 2.42 (s, 3H). 13C NMR (100 MHz, Chloroform-d) δ 142.17, 137.36, 136.74, 131.03, 128.80, 128.64, 127.98, 127.59, 127.20, 124.79, 124.54, 123.68, 122.39, 121.68, 119.92, 119.59, 115.78, 111.64, 61.27, 46.81, 40.28, 32.33. HRMS (ESI): m/z calcd for C24H22N2NaO2S [M + Na]+: 425.1294, found 425.1299.
2-(1-Benzyl-1H-indol-3-yl)-3-phenyl-1-tosyl-1,2,3,4-tetrahydroquinoline (3j)
Ethyl acetate/petroleum ether = 1:10 as an eluent, yellow solid (42 mg, 74%). MP: 164.1–168.7 °C. 1H NMR (400 MHz, Chloroform-d) δ 7.69 (dd, J = 8.0, 1.1 Hz, 1H), 7.42–7.38 (m, 2H), 7.34 (td, J = 7.8, 1.6 Hz, 1H), 7.25–7.05 (m, 12H), 7.00 (d, J = 8.0 Hz, 1H), 6.90 (dd, J = 6.9, 1.4 Hz, 3H), 6.81 (dd, J = 6.6, 2.9 Hz, 2H), 6.77 (s, 1H), 5.41 (d, J = 9.6 Hz, 1H), 5.22–5.09 (m, 2H), 3.35 (ddd, J = 12.6, 9.6, 3.3 Hz, 1H), 2.51 (dd, J = 14.2, 3.4 Hz, 1H), 2.40 (s, 3H), 2.15–2.07 (m, 1H). 13C NMR (100 MHz, Chloroform-d) δ 143.57, 142.53, 137.62, 137.02, 136.87, 136.66, 135.76, 129.49, 128.71, 128.49, 127.69, 127.61, 127.58, 127.56, 127.48, 127.28, 127.22, 127.03, 126.53, 126.24, 125.93, 121.69, 120.31, 119.32, 116.03, 110.06, 62.73, 50.59, 49.89, 33.55, 21.73. HRMS (ESI): m/z calcd for C37H32N2NaO2S [M + Na]+: 591.2077, found 591.2070.
3-(4-Bromophenyl)-2-(1H-indol-3-yl)-1-tosyl-1,2,3,4-tetrahydroquinoline (3k)
Ethyl acetate/petroleum ether = 1:10 as an eluent, yellow solid (48 mg, 86%). MP: 168.5–173.2 °C. 1H NMR (400 MHz, Chloroform-d) δ 8.02 (s, 1H), 7.61 (dd, J = 7.9, 1.4 Hz, 1H), 7.38–7.02 (m, 12H), 6.98 (ddd, J = 8.0, 6.9, 1.0 Hz, 1H), 6.82–6.74 (m, 3H), 5.59 (d, J = 8.2 Hz, 1H), 3.47 (ddd, J = 10.0, 8.2, 4.2 Hz, 1H), 2.94 (dd, J = 15.5, 4.2 Hz, 1H), 2.41 (s, 3H), 2.19 (dd, J = 15.5, 10.0 Hz, 1H). 13C NMR (100 MHz, Chloroform-d) δ143.93, 141.07, 138.25, 136.65, 136.27, 132.56, 131.65, 131.05, 129.49, 129.25, 127.66, 127.18, 126.18, 124.71, 123.85, 123.35, 122.12, 120.92, 119.63, 119.42, 116.05, 111.51, 61.51, 47.17, 31.92, 21.61. HRMS (ESI): m/z calcd for C30H25BrN2NaO2S [M + Na]+: 579.0712, found 579.0719.
3-(4-Chlorophenyl)-2-(1H-indol-3-yl)-1-tosyl-1,2,3,4-tetrahydroquinoline (3l)
Ethyl acetate/petroleum ether = 1:10 as an eluent, yellow solid (48 mg, 93%). MP: 114.0–123.7 °C. 1H NMR (400 MHz, Chloroform-d) δ 7.99 (s, 1H), 7.61 (dd, J = 7.9, 1.4 Hz, 1H), 7.36–7.26 (m, 4H), 7.26–7.06 (m, 8H), 6.98 (ddd, J = 8.0, 7.0, 1.0 Hz, 1H), 6.86–6.80 (m, 3H), 5.59 (d, J = 8.3 Hz, 1H), 3.48 (ddd, J = 10.0, 8.2, 4.1 Hz, 1H), 2.95 (dd, J = 15.5, 4.2 Hz, 1H), 2.41 (s, 3H), 2.19 (dd, J = 15.5, 10.1 Hz, 1H). 13C NMR (100 MHz, Chloroform-d) δ 144.06, 140.70, 138.42, 136.79, 136.46, 132.99, 132.70, 131.30, 129.63, 129.04, 128.85, 127.80, 127.33, 126.35, 124.90, 124.05, 123.45, 122.29, 119.80, 119.60, 116.29, 111.63, 61.72, 47.33, 29.35, 21.76. HRMS (ESI): m/z calcd for C30H25ClN2NaO2S [M + Na]+: 535.1217, found 535.1213.
3-(4-Fluorophenyl)-2-(1H-indol-3-yl)-1-(methylsulfonyl)-1,2,3,4-tetrahydroquinoline (3m)
Ethyl acetate/petroleum ether = 1:10 as an eluent, yellow solid (28 mg, 67%). MP: 242.3–244.6 °C. 1H NMR (400 MHz, Chloroform-d) δ 8.14 (s, 1H), 7.53 (dd, J = 8.1, 1.1 Hz, 1H), 7.38–7.31 (m, 2H), 7.26–7.16 (m, 6H), 7.07–6.99 (m, 3H), 6.82 (d, J = 2.5 Hz, 1H), 5.68 (d, J = 8.2 Hz, 1H), 3.54 (dt, J = 8.6, 4.4 Hz, 1H), 3.14 (dd, J = 14.9, 9.6 Hz, 1H), 2.92 (dd, J = 15.0, 4.1 Hz, 1H), 2.51 (s, 3H). 13C NMR (100 MHz, Chloroform-d) δ 140.57, 137.19, 136.62, 132.78, 131.18, 129.14, 128.60, 128.49, 128.20, 127.58, 124.57, 123.68, 122.34, 121.87, 119.89, 119.39, 115.15, 111.62, 61.13, 47.11, 40.69, 32.67. HRMS (ESI): m/z calcd for C24H21FN2NaO2S [M + Na]+: 443.1200, found 443.1209.
2-(1H-Indol-3-yl)-3-(p-tolyl)-1-tosyl-1,2,3,4-tetrahydroquinoline (3n)
Ethyl acetate/petroleum ether = 1:10 as an eluent, yellow solid (39 mg, 80%). MP: 110.8–114.9 °C. 1H NMR (400 MHz, Chloroform-d) δ 8.00 (d, J = 2.5 Hz, 1H), 7.59 (dd, J = 7.9, 1.3 Hz, 1H), 7.35–7.08 (m, 10H), 7.01 (d, J = 7.8 Hz, 2H), 6.95 (ddd, J = 8.0, 7.0, 1.0 Hz, 1H), 6.81–6.76 (m, 3H), 5.60 (d, J = 8.5 Hz, 1H), 3.43 (ddd, J = 10.5, 8.5, 4.0 Hz, 1H), 2.94 (dd, J = 15.3, 4.0 Hz, 1H), 2.40 (s, 3H), 2.30 (s, 3H), 2.13 (dd, J = 15.4, 10.6 Hz, 1H). 13C NMR (100 MHz, Chloroform-d) δ 143.91, 139.17, 138.42, 136.78, 136.76, 136.46, 132.53, 132.21, 129.59, 129.35, 127.61, 127.52, 127.38, 126.35, 125.02, 124.40, 123.46, 122.05, 119.77, 119.57, 116.63, 111.56, 62.00, 47.90, 29.75, 21.74, 21.19. HRMS (ESI): m/z calcd for C31H28N2NaO2S [M + Na]+: 515.1764, found 515.1763.
(1H-indol-3-yl)-3-(m-tolyl)-1-tosyl-1,2,3,4-tetrahydroquinoline (3o)
Ethyl acetate/petroleum ether = 1:10 as an eluent, yellow solid (41 mg, 85%). MP: 109.9–118.6 °C. 1H NMR (400 MHz, Chloroform-d) δ 8.05 (s, 1H), 7.60 (dd, J = 8.0, 1.2 Hz, 1H), 7.39–7.35 (m, 2H), 7.33–6.91 (m, 11H), 6.79 (d, J = 2.5 Hz, 1H), 6.72 (d, J = 1.8 Hz, 1H), 6.69 (dt, J = 7.4, 1.6 Hz, 1H), 5.64 (d, J = 8.5 Hz, 1H), 3.46–3.40 (m, 1H), 2.97 (dd, J = 15.3, 4.1 Hz, 1H), 2.41 (s, 3H), 2.25 (s, 3H), 2.15 (dd, J = 15.3, 10.7 Hz, 1H). 13C NMR (100 MHz, Chloroform-d) δ 143.95, 142.19, 138.44, 138.26, 136.77, 136.51, 132.49, 132.23, 129.64, 128.54, 128.32, 127.92, 127.63, 127.40, 126.41, 124.99, 124.78, 124.51, 123.43, 121.99, 119.72, 119.51, 116.61, 111.56, 61.89, 48.48, 29.87, 21.72, 21.57. HRMS (ESI): m/z calcd for C31H28N2NaO2S [M + Na]+: 515.1764, found 515.1763.
2-(1H-indol-3-yl)-1-(methylsulfonyl)-3-(p-tolyl)-1,2,3,4-tetrahydroquinoline (3p)
Ethyl acetate/petroleum ether = 1:10 as an eluent, yellow solid (27 mg, 65%). MP: 196.4–201.1 °C. 1H NMR (400 MHz, Chloroform-d) δ 8.11 (d, J = 2.5 Hz, 1H), 7.58 (d, J = 7.7 Hz, 1H), 7.31–7.22 (m, 4H), 7.17–7.12 (m, 2H), 7.07–6.93 (m, 5H), 6.75 (d, J = 2.5 Hz, 1H), 5.77 (d, J = 7.7 Hz, 1H), 3.55 (ddd, J = 8.8, 7.7, 4.3 Hz, 1H), 3.12 (dd, J = 15.1, 8.9 Hz, 1H), 2.90 (dd, J = 15.2, 4.3 Hz, 1H), 2.41 (s, 3H), 2.27 (s, 3H). 13C NMR (100 MHz, Chloroform-d) δ 139.14, 137.33, 136.75, 136.72, 131.33, 129.26, 128.73, 127.78, 127.50, 124.77, 124.53, 123.75, 122.24, 121.76, 119.77, 119.54, 115.62, 111.71, 61.35, 46.62, 40.26, 32.59, 21.14. HRMS (ESI): m/z calcd for C25H24N2NaO2S [M + Na]+: 439.1451, found 439.1456.
2-(5-Methyl-1H-indol-3-yl)-3-phenyl-1-tosyl-1,2,3,4-tetrahydroquinoline (3q)
Ethyl acetate/petroleum ether = 1:10 as an eluent, yellow solid (38 mg, 78%). MP: 176.3–183.2 °C. 1H NMR (400 MHz, Chloroform-d) δ 7.84 (s, 1H), 7.60 (dd, J = 7.9, 1.3 Hz, 1H), 7.35–7.08 (m, 11H), 6.99–6.92 (m, 3H), 6.82 (d, J = 1.7 Hz, 1H), 6.78 (d, J = 2.5 Hz, 1H), 5.67 (d, J = 8.1 Hz, 1H), 3.47 (ddd, J = 10.0, 8.0, 4.2 Hz, 1H), 2.96 (dd, J = 15.5, 4.2 Hz, 1H), 2.40 (s, 3H), 2.30 (s, 3H), 2.24 (dd, J = 15.5, 10.1 Hz, 1H). 13C NMR (100 MHz, Chloroform-d) δ143.88, 142.33, 138.58, 136.61, 135.06, 132.69, 131.70, 129.62, 128.72, 127.79, 127.51, 127.43, 127.18, 126.18, 125.25, 124.07, 123.74, 123.29, 119.56, 116.40, 111.10, 61.80, 48.09, 29.46, 21.74, 21.68. HRMS (ESI): m/z calcd for C31H28N2NaO2S [M + Na]+: 515.1764, found 515.1769.
2-(5-Bromo-1H-indol-3-yl)-3-phenyl-1-tosyl-1,2,3,4-tetrahydroquinoline (3r)
Ethyl acetate/petroleum ether = 1:10 as an eluent, yellow solid (47 mg, 85%). MP: 145.5–153.6 °C. 1H NMR (400 MHz, Chloroform-d) δ 8.16 (s, 1H), 7.66 (dd, J = 7.4, 1.9 Hz, 1H), 7.35–7.05 (m, 13H), 6.90–6.86 (m, 2H), 6.75 (d, J = 1.9 Hz, 1H), 5.52 (d, J = 8.8 Hz, 1H), 3.27 (ddd, J = 10.9, 8.7, 3.8 Hz, 1H), 2.95 (dd, J = 15.2, 3.8 Hz, 1H), 2.42 (s, 3H), 2.09 (dd, J = 15.3, 11.0 Hz, 1H). 13C NMR (100 MHz, Chloroform-d) δ 144.22, 141.79, 138.12, 135.96, 135.25, 132.56, 132.39, 129.73, 128.79, 127.80, 127.72, 127.40, 127.34, 126.70, 126.55, 124.84, 124.67, 124.27, 122.20, 116.64, 112.99, 112.83, 61.95, 49.21, 29.75, 21.76. HRMS (ESI): m/z calcd for C30H25BrN2NaO2S [M + Na]+: 579.0712, found 579.0711.
2-(5-Methoxy-1H-indol-3-yl)-3-phenyl-1-tosyl-1,2,3,4-tetrahydroquinoline (3s)
Ethyl acetate/petroleum ether = 1:10 as an eluent, yellow solid (36 mg, 71%). MP: 122.4–127.8 °C. 1H NMR (400 MHz, Chloroform-d) δ 7.84 (s, 1H), 7.73 (dd, J = 8.0, 1.1 Hz, 1H), 7.46–7.41 (m, 2H), 7.37–7.32 (m, 1H), 7.26–7.09 (m, 8H), 6.84 (ddt, J = 5.8, 2.6, 1.3 Hz, 2H), 6.79–6.72 (m, 2H), 6.31 (d, J = 2.4 Hz, 1H), 5.43 (d, J = 9.4 Hz, 1H), 3.48 (s, 3H), 3.35–3.28 (m, 1H), 2.50 (dd, J = 14.1, 3.5 Hz, 1H), 2.42 (s, 3H), 2.10 (dd, J = 14.0, 12.6 Hz, 1H). 13C NMR (100 MHz, Chloroform-d) δ 153.73, 143.65, 142.66, 137.12, 136.83, 135.99, 131.88, 129.55, 128.59, 127.74, 127.60, 127.58, 127.38, 127.25, 127.09, 126.37, 125.42, 123.77, 117.12, 112.53, 112.12, 101.55, 62.62, 55.52, 50.81, 33.90, 21.74. HRMS (ESI): m/z calcd for C31H28N2NaO3S [M + Na]+: 531.1713, found 515.1710.
2-(6-Chloro-1H-indol-3-yl)-3-phenyl-1-tosyl-1,2,3,4-tetrahydroquinoline (3t)
Ethyl acetate/petroleum ether = 1:10 as an eluent, yellow solid (36 mg, 82%). MP: 125.4–130.8 °C. 1H NMR (400 MHz, Chloroform-d) δ 8.00 (s, 1H), 7.63–7.58 (m, 1H), 7.34–7.11 (m, 10H), 6.98 (dd, J = 8.6, 1.9 Hz, 1H), 6.82 (d, J = 2.5 Hz, 1H), 5.76 (d, J = 7.9 Hz, 1H), 3.52 (td, J = 8.5, 4.2 Hz, 1H), 3.17 (dd, J = 15.1, 9.1 Hz, 1H), 2.93 (dd, J = 15.1, 4.2 Hz, 1H), 2.46 (s, 3H). 13C NMR (100 MHz, Chloroform-d) δ 141.94, 137.16, 137.09, 131.36, 128.78, 128.72, 128.41, 127.93, 127.74, 127.33, 124.84, 124.23, 123.44, 122.06, 120.74, 120.45, 116.32, 111.59, 61.16, 47.49, 40.15, 32.58. HRMS (ESI): m/z calcd for C24H21ClN2NaO2S [M + Na]+: 459.0904, found 459.0908.
General Procedure for the Preparation of 8
To a stirred solution of ethyl (E)-3-(1H-indol-3-yl)acrylate 5 (0.1 mmol) in DCM (2 mL) at room temperature in the presence of KOH (2 mmol), α-halogeno hydrazone 7 (0.15 mmol) was added. After 8 h, 5 disappeared, as indicated by TLC. The mixture was concentrated in vacuo, and the crude product was purified by flash chromatography eluting with (petroleum ether/ethyl acetate 10:1) to afford the product 8 in 47%.
Ethyl 2-Acetyl-3-(1H-indol-3-yl)-6-phenyl-2,3,4,5-tetrahydropyridazine-4-carboxylate (8)
Ethyl acetate/petroleum ether = 1:8 as an eluent, white solid (18 mg, 47%). MP: 191.4–198.3 °C. 1H NMR (400 MHz, Chloroform-d) δ 8.19 (s, 1H), 7.82–7.71 (m, 2H), 7.65 (d, J = 7.7 Hz, 1H), 7.45–7.26 (m, 4H), 7.15 (dtd, J = 18.1, 7.2, 1.2 Hz, 2H), 6.70 (d, J = 2.8 Hz, 1H), 6.61 (s, 1H), 4.29–4.07 (m, 2H), 3.55–3.47 (m, 1H), 3.02 (dt, J = 17.9, 1.7 Hz, 1H), 2.50 (s, 3H), 2.35 (dd, J = 17.8, 6.7 Hz, 1H), 1.31–1.23 (m, 3H). 13C NMR (100 MHz, Chloroform-d) δ 172.19, 171.78, 145.80, 137.31, 136.93, 129.50, 128.57, 125.59, 124.55, 122.58, 121.83, 119.97, 118.70, 113.81, 111.70, 61.59, 47.11, 39.31, 29.84, 21.68, 14.34. HRMS (ESI): m/z calcd for C23H23N3NaO3 [M + Na]+: 412.1632, found 412.1637.
General Procedure for the Preparation of 9
To a stirred solution of 2a (0.1 mmol) in DCM (2 mL) at room temperature in the presence of KOH (2 mmol), α-halogeno hydrazone 7 (0.15 mmol) was added. After 8 h, 2a disappeared, as indicated by the TLC. The mixture was concentrated in vacuo, and the crude product was purified by flash chromatography eluting with (petroleum ether/ethyl acetate 10:1) to afford the product 9 in 67%.
1-(6-(1H-Indol-3-yl)-3,5-diphenyl-5,6-dihydropyridazin-1(4H)-yl)ethan-1-one (9)
Ethyl acetate/petroleum ether = 1:10 as an eluent, yellow solid (26 mg, 67%). MP: 221.5–227.6 °C. 1H NMR (400 MHz, Chloroform-d) δ 8.27 (s, 1H), 7.85–7.78 (m, 2H), 7.77–7.69 (m, 1H), 7.41 (dd, J = 5.4, 1.9 Hz, 3H), 7.32–7.19 (m, 6H), 7.19–7.11 (m, 2H), 6.70–6.65 (m, 1H), 6.27 (s, 1H), 3.88–3.82 (m, 1H), 2.85 (d, J = 18.1 Hz, 1H), 2.69 (dd, J = 18.3, 7.1 Hz, 1H), 2.48 (s, 3H). 13C NMR (100 MHz, Chloroform-d) δ 172.62, 146.43, 142.39, 137.38, 136.95, 129.57, 128.95, 128.66, 127.17, 126.94, 125.55, 124.83, 122.38, 121.58, 119.80, 118.78, 115.20, 111.74, 51.03, 38.32, 24.79, 21.78. HRMS (ESI): m/z calcd for C26H23N3NaO [M + Na]+: 416.1733, found 416.1742.
Acknowledgments
We are grateful for financial support from the Youth Science and Technology Personal Growth Project of the Educational Department of Guizhou Province (KY [2021] 227), the Guizhou Province Science and Technology plan program of China (QKHPTRC [2019]-034) and CK1187-029.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.2c07036.
1H, 13C{1H} NMR spectra for all of the products (PDF)
Accession Codes
CCDC 2205697 contains the supplementary crystallographic data for compound 3e. These data can be obtained free of charge from the Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
The authors declare no competing financial interest.
Supplementary Material
References
- a Goebel G. L.; Hohnen L.; Borgelt L.; Hommen P.; Qiu X. Q.; Lightfoot H.; Wu P. Small molecules with tetrahydroquinoline-containing Povarov scaffolds as inhibitors disrupting the Protein–RNA interaction of LIN28–let-7. Eur. J. Med. Chem. 2022, 228, 114014. 10.1016/j.ejmech.2021.114014. [DOI] [PubMed] [Google Scholar]; b Shen S. D.; Picci C.; Ustinova K.; Benoy V.; Kutil Z.; Zhang G. P.; Tavares M. T.; Pavlíček J.; Zimprich C. A.; Robers M. B.; BosCh L. V.; Bařinka C.; Langley B.; Kozikowski A. P. Tetrahydroquinoline-capped histone deacetylase 6 inhibitor SW-101 ameliorates pathological phenotypes in a Charcot–Marie–Tooth type 2A mouse model. J. Med. Chem. 2021, 64 (8), 4810. 10.1021/acs.jmedchem.0c02210. [DOI] [PubMed] [Google Scholar]; c Yadav P.; Kumar A.; Althagafi I.; Nemaysh V.; Rai R.; Pratap R. The recent development of tetrahydro-quinoline/isoquinoline based compounds as anticancer agents. Curr. Top. Med. Chem. 2021, 21 (17), 1587. 10.2174/1568026621666210526164208. [DOI] [PubMed] [Google Scholar]
- a Hoemann M. Z.; Xie R. L.; Rossi R. F.; Meyer S.; Sidhu A.; Cuny G. D.; Hauske J. R. Potent in vitro methicillin-resistant staphylococcus aureus activity of 2-(1H-indol-3-yl) tetrahydroquinoline derivatives. Bioorg. Med. Chem. Lett. 2002, 12, 129. 10.1016/S0960-894X(01)00714-4. [DOI] [PubMed] [Google Scholar]; b Zhou M. X.; Gu L. B.; Li W.; Wu Z. Z.. WO 2021050721, 2021.; c Cuny G. D.; Hauske J. R.; Heefner D. L.; Hoemann M. Z.; Kumaravel G.; Melikian-Badalian A.; Rossi R. F.; Xie R. L.. WO 2000034265 A2, 2000.; d Zeng H. H.; Cao R.; Zhang H. B. Combined 3D-QSAR modeling and molecular docking study on quinoline derivatives as inhibitors of P-selectin. Chem. Biol. Drug. Des. 2009, 74, 596. 10.1111/j.1747-0285.2009.00893.x. [DOI] [PubMed] [Google Scholar]; e Hassan J.; Sévignon M.; Gozzi C.; Schulz E.; Lemaire M. Aryl–aryl bond formation one century after the discovery of the Ullmann reaction. Chem. Rev. 2002, 102 (5), 1359. 10.1021/cr000664r. [DOI] [PubMed] [Google Scholar]; f Li C. J. Cross-dehydrogenative coupling (CDC): exploring C–C bond formations beyond functional group transformations. Acc. Chem. Res. 2009, 42 (2), 335. 10.1021/ar800164n. [DOI] [PubMed] [Google Scholar]
- Shi Z. Z.; Zhang B.; Cui Y. X.; Jiao N. Palladium-catalyzed ring-expansion reaction of indoles with alkynes: from indoles to tetrahydroquinoline derivatives under mild reaction conditions. Angew. Chem., Int. Ed. 2010, 49, 4036. 10.1002/anie.201001237. [DOI] [PubMed] [Google Scholar]
- Miyake Y.; Nakajima K.; Nishibayashi Y. Direct sp3 C-H amination of nitrogen-containing benzoheterocycles mediated by visible-light-photoredox catalysts. Chem.—Eur. J. 2012, 18, 16473. 10.1002/chem.201203066. [DOI] [PubMed] [Google Scholar]
- a Chen X. W.; Zhao H.; Chen C.; Jiang H. F.; Zhang M. Iridium-catalyzed dehydrogenative α-functionalization of (hetero)aryl-fused cyclic secondary amines with indoles. Org. Lett. 2018, 20, 1171. 10.1021/acs.orglett.8b00096. [DOI] [PubMed] [Google Scholar]; b Ramana D. V.; Chandrasekharam M. Copper-catalyzed direct oxidative α-functionalization of tetrahydroquinoline in water under mild conditions. Adv. Synth. Catal. 2018, 360 (21), 4080. 10.1002/adsc.201800684. [DOI] [Google Scholar]
- Wang D.; Wang Z. T.; Liu Z. L.; Huang M. D.; Hu J. Y.; Yu P. Strategic C–C bond-forming dearomatization of pyridines and quinolines. Org. Lett. 2019, 21, 4459. 10.1021/acs.orglett.9b01247. [DOI] [PubMed] [Google Scholar]
- For some selected examples about [4 + 3] annulation, see:; a Wang L.; Li S.; Blumel M.; Philipps A. R.; Wang A.; Puttreddy R.; Rissanen K.; Enders D. Asymmetric synthesis of spirobenzazepinones with atroposelectivity and spiro-1,2-diazepinones by NHC-catalyzed [3 + 4] annulation reactions. Angew. Chem., Int. Ed. 2016, 55, 11110. 10.1002/anie.201604819. [DOI] [PubMed] [Google Scholar]; b Mei G. J.; Zhu Z. Q.; Zhao J. J.; Bian C. Y.; Chen J.; Chen R. W.; Shi F. Brønsted acid-catalyzed stereoselective [4 + 3] cycloadditions of ortho-hydroxybenzyl alcohols with N,N-cyclic azomethine imines. Chem. Commun. 2017, 53, 2768. 10.1039/C6CC09775H. [DOI] [PubMed] [Google Scholar]; c Zhang X.; Pan Y.; Liang P.; Pang L.; Ma X.; Jiao W.; Shao H. Oxadiazepine synthesis by formal [4 + 3] cycloaddition of o-chloromethyl arylsulfonamides with nitrones promoted by NaHCO3. Adv. Synth. Catal. 2018, 360, 3015. 10.1002/adsc.201800663. [DOI] [Google Scholar]; d Wang X. Y.; Li Z. F.; Feng C.; Zhen Q.; Guo M. Z.; Yao Y. N.; Zou X. Y.; Wang P. F.; Hou Y. L.; Gong P. A [4 + 3] Cycloaddition Reaction of Aza-ortho-quinone Methides with C,N-Cyclic Azomethine Imines for Synthesis of 1,2,4-Triazepines. Synlett. 2021, 32, 2090. 10.1055/a-1585-4490. [DOI] [Google Scholar]; e Meng Z. R.; Yang W. R.; Zheng J. [4 + 3]-Cycloaddition of aza-o-quinone methides and azomethine imines to make 1,2,4-triazepines. Tetrahedron Lett. 2019, 60, 1758. 10.1016/j.tetlet.2019.05.038. [DOI] [Google Scholar]; f Long W. Y.; Chen S. Q.; Zhang X. H.; Fang L.; Wang Z. Y. Diversity-oriented synthesis of 1,2,3,5-tetrahydrobenzo[e][1,2,4]oxadiazepines and 2,3-dihydro-1H-benzo[e][1,2,4] triazepines by base-induced [4 + 3] annulation reactions. Tetrahedron. 2018, 74 (42), 6155. 10.1016/j.tet.2018.09.004. [DOI] [Google Scholar]; g Guo Z. Y.; Jia H.; Liu H. L.; Wang Q. J.; Huang J. X.; Guo H. C. A [4 + 3] Annulation Reaction of aza-o-Quinone Methides with Arylcarbohydrazonoyl Chlorides for Synthesis of 2,3-Dihydro-1H-benzo[e][1,2,4]triazepines. Org. Lett. 2018, 20 (10), 2939. 10.1021/acs.orglett.8b00990. [DOI] [PubMed] [Google Scholar]; h Zheng Y. S.; Tu L.; Gao L. M.; Huang R.; Feng T.; Sun H.; Wang W. X.; Li Z. H.; Liu J. K. Accessing benzooxadiazepines via formal [4 + 3] cycloadditions of aza-o-quinone methides with nitrones. Org. Biomol Chem. 2018, 16 (15), 2639. 10.1039/C8OB00201K. [DOI] [PubMed] [Google Scholar]; i Zhi Y.; Zhao K.; Shu T.; Enders D. Synthesis of Benzotriazepine Derivatives via [4 + 3] Cycloaddition of Aza-o-quinone Methide Intermediates and Azomethine Imines. Synthesis. 2016, 48 (02), 238. 10.1055/s-0035-1560809. [DOI] [Google Scholar]; j Chen L.; Yang G. M.; Wang J.; Jia Q. F.; Wei J.; Du Z. Y. An efficient [4 + 3] cycloaddition reaction of aza-o-quinodimethanes with C,N-cyclic azomethine imines: stereoselective synthesis of 1,2,4-triazepines. RSC Adv. 2015, 5, 76696. 10.1039/C5RA15903B. [DOI] [Google Scholar]
- For some selected examples about [4 + 2] annulation, see:; a Li L. Z.; Wang C. S.; Guo W. F.; Mei G. X.; Shi F. Catalytic asymmetric [4 + 2] cycloaddition of in Situ generated o-quinone methide Imines with o-hydroxystyrenes: diastereo- and enantioselective construction of tetrahydroquinoline frameworks. J. Org. Chem. 2018, 83 (2), 614. 10.1021/acs.joc.7b02533. [DOI] [PubMed] [Google Scholar]; b Boal B. W.; Schammel A. W.; Garg N. K. An interrupted fischer indolization approach toward fused indoline-containing natural products. Org. Lett. 2009, 11, 3458. 10.1021/ol901383j. [DOI] [PubMed] [Google Scholar]; c Liao H. H.; Hsiao C. C.; Atodiresei I.; Rueping M. Multiple hydrogen-bond activation in asymmetric brønsted acid catalysis. Chem.—Eur. J. 2018, 24, 7718. 10.1002/chem.201800677. [DOI] [PubMed] [Google Scholar]; d Alden-Danforth E.; Scerba M. T.; Lectka T. Asymmetric cycloadditions of o-quinone methides employing chiral ammonium fluoride precatalysts. Org. Lett. 2008, 10 (21), 4951. 10.1021/ol802029e. [DOI] [PMC free article] [PubMed] [Google Scholar]; e Lee A.; Younai A.; Price C. K.; Izquierdo J.; Mishra R. K.; Scheidt K. A. Enantioselective annulations for dihydroquinolones by in situ generation of azolium enolates. J. Am. Chem. Soc. 2014, 136 (30), 10589. 10.1021/ja505880r. [DOI] [PMC free article] [PubMed] [Google Scholar]; f Mukhina O. A.; Kuznetsov D. M.; Cowger T. M.; Kutateladze A. G. Amino azaxylylenes photogenerated from o-amido imines: photo assisted access to complex spiro-poly-heterocycles. Angew. Chem., Int. Ed. 2015, 54, 11516. 10.1002/anie.201504455. [DOI] [PMC free article] [PubMed] [Google Scholar]; g Kretzschmar M.; Hodik T.; Schneider C. Brønsted acid catalyzed addition of enamides to ortho-quinone methide imines—an efficient and highly enantioselective synthesis of chiral tetrahydroacridines. Angew. Chem., Int. Ed. 2016, 55, 9788. 10.1002/anie.201604201. [DOI] [PubMed] [Google Scholar]; h Ji H. J.; He C. L.; Gao H. J.; Fu W. J.; Xu J. F. DBU-Promoted Formal [4 + 2] Annulation Reactions of o-Chloromethyl Anilines with Azlactones. Synthesis. 2021, 53, 1349. 10.1055/s-0040-1706549. [DOI] [Google Scholar]; i Zheng Y. S.; Tu L.; Li N.; Huang R.; Feng T.; Sun H.; Li Z. H.; Liu J. K. Inverse-Electron-Demand [4 + 2]-Cycloaddition of 1,3,5-triazinanes: Facile Approaches to Tetrahydroquinazolines. Adv. Synth. Catal. 2019, 361 (j), 44. 10.1002/adsc.201801063. [DOI] [Google Scholar]; i Han S.; Vogt F.; May J. A.; Krishnan S.; Gatti M.; Virgil S. C.; Stoltz B. M. Evolution of a Unified, Stereodivergent Approach to the Synthesis of Communesin F and Perophoramidine. J. Org. Chem. 2015, 80, 528. 10.1021/jo502534g. [DOI] [PMC free article] [PubMed] [Google Scholar]; k Schammel A. W.; Chiou G.; Garg N. K. Interrupted Fischer Indolization Approach toward the Communesin Alkaloids and Perophoramidine. Org. Lett. 2012, 14 (17), 4556. 10.1021/ol302023q. [DOI] [PMC free article] [PubMed] [Google Scholar]; l Schammel A. W.; Boal B. W.; Zu L. S.; Mesganaw T.; Garg N. K. Exploration of the interrupted Fischer indolization reaction. Tetrahedron. 2010, 66 (26), 4687. 10.1016/j.tet.2010.02.050. [DOI] [PMC free article] [PubMed] [Google Scholar]; m Rahul P.; Veena S.; Jubi J. Inverse Electron Demand Diels Alder Reaction of Aza-o-Quinone Methides and Enaminones: Accessing 3-Aroyl Quinolines and Indeno[1,2-b]quinolinones. J. Org. Chem. 2022, 87 (21), 13708. 10.1021/acs.joc.2c01361. [DOI] [PubMed] [Google Scholar]; n May J. A.; Stoltz B. The structural and synthetic implications of the biosynthesis of the calycanthaceous alkaloids, the communesins, and nomofungin. Tetrahedron. 2006, 62 (22), 5262. 10.1016/j.tet.2006.01.105. [DOI] [Google Scholar]; n May J. A.; Zeidan R. K.; Stoltz B. M. Biomimetic approach to communesin B (a.k.a. nomofungin). Tetrahedron. Lett. 2003, 44 (6), 1203. 10.1016/S0040-4039(02)02790-9. [DOI] [Google Scholar]
- For some selected examples about [4 + 1] annulation, see:; a Sharma H. A.; Hovey M. T.; Scheidt K. A. Azaindole synthesis through dual activation catalysis with N-heterocyclic carbenes. Chem. Commun. 2016, 52, 9283. 10.1039/C6CC04735A. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Yang Q. Q.; Xiao C.; Lu L. Q.; An J.; Tan F.; Li B. J.; Xiao W. J. Synthesis of indoles through highly efficient cascade reactions of sulfur ylides and N-(ortho-chloromethyl) aryl amides. Angew. Chem., Int. Ed. 2012, 51, 9137. 10.1002/anie.201203657. [DOI] [PubMed] [Google Scholar]; c Chen M. W.; Cao L. L.; Ye Z. S.; Jiang G. F.; Zhou Y. G. A mild method for generation of o-quinone methides under basic conditions. The facile synthesis of trans-2,3-dihydrobenzofurans. Chem. Commun. 2013, 49, 1660. 10.1039/c3cc37800d. [DOI] [PubMed] [Google Scholar]; d Huang H.; Yang Y.; Zhang X. Y.; Zeng W. L.; Liang Y. Transition-metal-free approach to synthesis of indolines from N-(ortho-chloromethyl) aryl amides and iodonium ylides. Tetrahedron. Lett. 2013, 54, 6049. 10.1016/j.tetlet.2013.08.096. [DOI] [Google Scholar]; e Zhang X. K.; Wang H. B.; Li Z. W.; Shu Y.; Gan S.; Zhang X. F.; Shao H. W.; Wang C. Y. Chemodivergent synthesis of aza-Heterocycles with a quarternary carbon center via [4 + 1] annulation between azoalkenes and α-bromo carbonyl compounds. ACS. Omega. 2022, 7, 40963. 10.1021/acsomega.2c04127. [DOI] [PMC free article] [PubMed] [Google Scholar]; f Yang Q. Q.; Wang Q.; An J.; Chen J. R.; Lu L. Q.; Xiao W. J. Construction of optically active indolines by formal [4 + 1] annulation of sulfur ylides and N-(ortho-chloromethyl) aryl amides. Chem.—Eur. J. 2013, 19, 8401. 10.1002/chem.201300988. [DOI] [PubMed] [Google Scholar]; g Zhang Y.; Liu T. D.; Liu L.; Guo H. Y.; Zeng H. Y.; Bi W.; Qiu G. Y. S.; Gao W.; Ran X.; Yang L.; Du G. B.; Zhang L. P. Palladium-Catalyzed Preparation of N-Substituted Benz[c, d]indol-2-imines and N-Substituted Amino-1-naphthylamides. J. Org. Chem. 2022, 87 (13), 8515. 10.1021/acs.joc.2c00620. [DOI] [PubMed] [Google Scholar]; h Li H.; Yu Z.; Sun H.; Liu B.; Wang X.; Shao Z.; Wang M.; Xie W.; Yao X.; Yao Q.; Zhi Y. Efficient Synthesis of 2,3′-Spirobi (Indolin)-2’-Ones and Preliminary Evaluation of Their Damage to Mitochondria in HeLa Cells. Front. Pharmacol. 2022, 12, 821518. 10.3389/fphar.2021.821518. [DOI] [PMC free article] [PubMed] [Google Scholar]; i Hua T. B.; Chao F.; Wang L.; Yan C. Y.; Xiao C.; Yang Q. Q.; Xiao W. J. Tandem Phospha-Michael Addition/N-Acylation/ Intramolecular Wittig Reaction of aza-o-Quinone Methides: Approaches to 2,3-Disubstituted Indoles. Adv. Synth. Catal. 2020, 362 (13), 2615. 10.1002/adsc.202000343. [DOI] [Google Scholar]; j Gui H. Z.; Wu X. Y.; Wei Y.; Shi M. A Formal Condensation and [4 + 1] Annulation Reaction of 3-Isothiocyanato Oxindoles with Aza-o-Quinone Methides. Adv. Synth. Catal. 2019, 361 (23), 5466. 10.1002/adsc.201901124. [DOI] [Google Scholar]; k Jong J. A. W.; Bao X.; Wang Q.; Zhu J. P. Formal [4 + 1] cycloaddition of o-aminobenzyl chlorides with isocyanides: synthesis of 2-amino-3-substituted indoles. Helv. Chim. Acta 2019, 102 (3), e1900002 10.1002/hlca.201900002. [DOI] [Google Scholar]
- For some selected examples about the generation of ortho-quinone methide, see:; a Walden D. M.; Jaworski A. A.; Johnston R. C.; Hovey M. T.; Baker H. V.; Meyer M. P.; Scheidt K. A.; Cheong P. H. Y. Formation of aza-ortho-quinone methides under room temperature conditions: Cs2CO3 effect. J. Org. Chem. 2017, 82 (14), 7183. 10.1021/acs.joc.7b00697. [DOI] [PubMed] [Google Scholar]; b Liao H. H.; Miñoza S.; Lee S. C.; Rueping M. Aza-ortho-quinone methides as reactive intermediates: generation and utility in contemporary asymmetric synthesis. Chem.—Eur. J. 2022, 28, e202201112 10.1002/chem.202201112. [DOI] [PubMed] [Google Scholar]; c Lewis R. S.; Garza C. J.; Dang A. T.; Pedro T. K. A.; Chain W. J. Michael additions of highly basic enolates to ortho-quinone methides. Org. Lett. 2015, 17 (9), 2278. 10.1021/acs.orglett.5b00972. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Pathak T. P.; Sigman M. S. A. Applications of ortho-quinone methide intermediates in catalysis and asymmetric synthesis. J. Org. Chem. 2011, 76, 9210. 10.1021/jo201789k. [DOI] [PMC free article] [PubMed] [Google Scholar]; e Liu X. J.; Wang K.; Guo W. G.; Liu Y.; Li C. An organic-base catalyzed asymmetric 1,4-addition of tritylthiol to in situ generated aza-o-quinone methides at the H2O/DCM interface. Chem. Commun. 2019, 55, 2668. 10.1039/C8CC09382B. [DOI] [PubMed] [Google Scholar]
- Steinhagen H.; Corey E. J. A convenient and versatile route to hydroquinolines by inter- and intramolecular aza-Diels–Alder pathways. Angew. Chem., Int. Ed. 1999, 38, 1928.. [DOI] [PubMed] [Google Scholar]
- Zheng Y. S.; Tu L.; Li N.; Huang R.; Feng T.; Sun H.; Li Z. H.; Liu J. K. Inverse-electron-demand [4 + 2]-cycloaddition of 1,3,5-triazinanes: facile approaches to tetrahydroquinazolines. Adv. Synth. Catal. 2019, 361, 44. 10.1002/adsc.201801063. [DOI] [Google Scholar]
- Wang H. Q.; Ma W. J.; Sun A.; Sun X. Y.; Jiang C.; Zhang Y. C.; Shi F. (4 + 2) Cyclization of aza-o-quinone methides with azlactones: construction of biologically important dihydroquinolinone frameworks. Org. Biomol. Chem. 2021, 19, 1334. 10.1039/D0OB02388D. [DOI] [PubMed] [Google Scholar]
- a Lei L.; Liang Y. F.; Liang C.; Qin J. K.; Pan C. X.; Su G. F.; Mo D. L. Copper (i)-catalyzed [4+ 2] cycloaddition of aza-ortho-quinone methides with bicyclic alkenes. Org. Biomol. Chem. 2021, 19, 3379. 10.1039/D1OB00319D. [DOI] [PubMed] [Google Scholar]; b Lei L.; Yao Y.-Y.; Jiang L.-J.; Lu X.; Liang C.; Mo D.-L. Synthesis of furo [3,2-b] quinolines and furo[2,3-b:4,5-b] diquinolines through [4 + 2] cycloaddition of aza-o-quinone methides and furans. J. Org. Chem. 2020, 85, 3059. 10.1021/acs.joc.9b02953. [DOI] [PubMed] [Google Scholar]
- Jiang S. P.; Lu W. Q.; Liu Z.; Wang G. W. Synthesis of fullerotetrahydroquinolines via [4 + 2] cycloaddition reaction of [60] fullerene with in situ generated aza-o-quinone methides. J. Org. Chem. 2018, 83, 1959. 10.1021/acs.joc.7b02897. [DOI] [PubMed] [Google Scholar]
- Shao W.; Xu-Xu Q. F.; You S. L. Highly diastereoselective synthesis of polycyclic indolines through formal [4 + 2] propargylic cycloaddition of indoles with ethynyl benzoxazinanones. Chem.—Asian J. 2020, 15 (16), 2462. 10.1002/asia.202000640. [DOI] [PubMed] [Google Scholar]
- Cheng H.; Yan D. C.; Wang G.; He Z. L. [4+ 2]-Cycloaddition reactions of aza-o-quinone methides with fulvenes: construction of tetrahydroquinoline derivatives. Synlett. 2022, 33 (08), 795. 10.1055/a-1796-7444. [DOI] [Google Scholar]
- a Dandia A.; Sachdeva H.; Ahmed N.; Joshi K. One pot Synthesis of fluorine containing diastereoisomeric spiro [3H-indol-3,2’-oxiran]-2(1H)-ones and their conversion to 5a, 10b-dihydrdro-5H,6H-indole[2,3-b] quinoline-11-ones. Heterocycl. Commun. 2000, 6 (2), 181. 10.1515/HC.2000.6.2.181. [DOI] [Google Scholar]; b Wu H. X.; Xue F.; Xiao X.; Qin Y. Total synthesis of (+)-perophoramidine and determination of the absolute configuration. Angew. Chem., Int. Ed. 2010, 132 (40), 14052. 10.1021/ja1070043. [DOI] [PubMed] [Google Scholar]; c Robertson F. J.; Kenimer B. D.; Wu J. Direct annulation and alkylation of indoles with 2-aminobenzyl alcohols catalyzed by TFA. Tetrahedron. 2011, 67, 4327. 10.1016/j.tet.2011.02.067. [DOI] [Google Scholar]; d Luo M.; Chen J. X.; Yu L. Q.; Wei W. G. Concise Synthesis of Polycyclic Indoline Scaffolds through an InIII-Catalyzed Formal [4 + 2] Annulation of 2,3-Disubstituted Indoles with o-Aminobenzyl Alcohols. Eur. J. Org. Chem. 2017, 18, 2652. 10.1002/ejoc.201700432. [DOI] [Google Scholar]
- Zhang X. K.; Pan Y.; Liang P.; Ma X. F.; Jiao W.; Shao H. W. An effective method for the synthesis of 1,3-dihydro-2H-indazoles via N-N bond formation. Adv. Synth. Catal. 2019, 361, 5552. 10.1002/adsc.201901331. [DOI] [Google Scholar]
- a Tu M. S.; Chen K. W.; Wu P.; Zhang Y. C.; Liu X. Q.; Shi F. Advances in organocatalytic asymmetric reactions of vinylindoles: powerful access to enantioenriched indole derivatives. Org. Chem. Front. 2021, 8, 2643. 10.1039/D0QO01643H. [DOI] [Google Scholar]; b Guan X. K.; Liu G. F.; An D.; Zhang H.; Zhang S. Q. Chiral imidodiphosphoric acid-catalyzed highly diastereo- and enantioselective synthesis of poly-substituted 3,4-dihydro-2H-pyrans: [4 + 2] cycloadditions of β, γ-unsaturated α-ketoesters and 3-vinylindoles. Org. Lett. 2019, 21, 5438. 10.1021/acs.orglett.9b01675. [DOI] [PubMed] [Google Scholar]; c Zhu Z. Q.; Shen Y.; Sun X. X.; Tao J. Y.; Liu J. X.; Shi F. Catalytic asymmetric [3 + 2] cycloadditions of C-3 unsubstituted 2-indolylmethanols: regio-, diastereo- and enantioselective construction of the cyclopenta[b]indole framework. Adv. Synth. Catal. 2016, 358, 3797. 10.1002/adsc.201600931. [DOI] [Google Scholar]; d Tan B.; Hernández-Torres G.; Barbas C. F. Highly efficient hydrogen-bonding catalysis of the Diels–Alder reaction of 3-vinylindoles and methyleneindolinones provides carbazolespirooxindole skeletons. J. Am. Chem. Soc. 2011, 133 (32), 12354. 10.1021/ja203812h. [DOI] [PubMed] [Google Scholar]
- CCDC 2205697 contains the supplementary crystallographic data for compound 3a.
- Wagner A. M.; knezevic C. E. K.; Wall J. L.; Sun V. L.; Buss J. A.; Allen L. T.; Wenzel A. G. Green synthesis of novel chalcone and coumarin derivatives via Suzuki coupling reaction. Tetrahedron. Lett. 2012, 53, 833. 10.1016/j.tetlet.2011.12.017. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.