Abstract

Organ transplantation is understood as a technique where an organ from a donor patient is transferred to a recipient patient. This practice gained strength in the 20th century and ensured advances in areas of knowledge such as immunology and tissue engineering. The main problems that comprise the practice of transplants involve the demand for viable organs and immunological aspects related to organ rejection. In this review, we address advances in tissue engineering for reversing the current challenges of transplants, focusing on the possible use of decellularized tissues in tissue engineering. We address the interaction of acellular tissues with immune cells, especially macrophages and stem cells, due to their potential use in regenerative medicine. Our goal is to exhibit data that demonstrate the use of decellularized tissues as alternative biomaterials that can be applied clinically as partial or complete organ substitutes.
1. Introduction
Organ transplantation is a surgical procedure aimed at substituting nonfunctional organs for functional ones. The technique consists, basically, of transferring a healthy tissue or organ from a donor to a recipient.1 The transplant can be performed from one individual to another (allografts), from one region to another in the same person (autograft), or using a functional organ or tissue from an animal (xenograft).2 Transplantation methodology has stimulated advances in numerous areas of knowledge, for example: (i) development of immunosuppressive agents, (ii) improvement of tissue preservation methods, (iii) better understanding of the major histocompatibility complex (MCH), and (iv) innovation in surgical techniques.3,4 Despite these advances, a major problem after organ transplantation is rejection due to an immune response against the new organ, which stems from genetic disparities involving the human leukocyte antigen (HLA) between the donor and recipient.5
In the 20th century, the practice of transplantation gained strength, which allowed a great search to unravel problems such as rejection, which was defined in 1944 by Medawar as a host-versus-graft response (HVG), characterized by an immunological event (sensitization, memory, and tolerance).6 Years after Medawar’s publication, in 1954, the first successful human organ transplantation occurred. The process was performed on two twin brothers, where Ronald donated a kidney to Richard Herrick, who died 8 years after the surgical process due to recurrence of kidney disease, while Ronald died in 2010 after complications from a heart surgery.7
Organ transplant rejection occurs by immune responses mainly mediated by mononuclear cells, B and T lymphocytes.8 The drug regimens are used to suppress the immunity of recipient patients before and after the transplant procedure in order to prevent tissue rejection. However, at the same time, the drugs should be dosed to allow the immune system to maintain sufficient functionality to fight, e.g., infections.9,10 Currently, immunosuppression involves the use of 3 types of drugs: a calcineurin inhibitor, an antiproliferative agent, and corticosteroids.11,12 In addition, it is possible to use monoclonal or polyclonal antibody therapy, which aims to prevent cases of acute rejection of the transplanted organ through T lymphocyte depletion.13,14
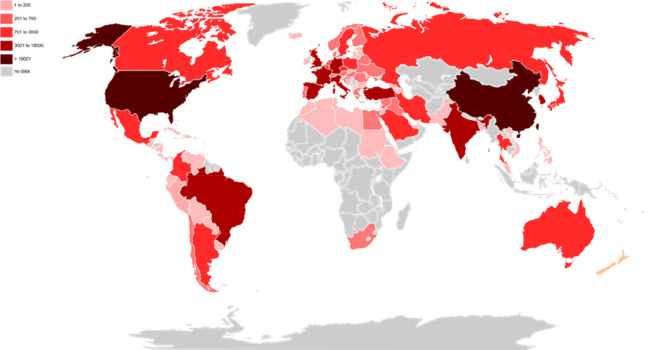
Despite these difficulties, the main factor that limits the practice of transplants is the scarcity of suitable organs, from either the lack of donors or the low quality of available organs (virus-positive donors and recipients; blood group incompatibility; structural condition of the organ).3,4,15,16 In 2020 over 132.193 transplants were performed worldwide (Figure 1); however, in the United States alone there are over 106.088 individuals waiting to receive a new organ, which demonstrates the great inequality between supply and demand for organs.17
Figure 1.
Distribution of transplants performed worldwide in 2020. Reported data of transplants performed in the year 2020 by 85 countries, listed by IRODaT - International Registry in Organ Donation and Transplantation.
The number of deaths of patients with late-stage organ failure is dependent on technological innovations, such as the development of artificial tissues. To achieve this, tissue engineering and regenerative medicine strategies for the replacement of nonfunctional organs have been studied over the last years.18−22
Tissue engineering uses the knowledge of a natural extracellular matrix (ECM) as a template for the synthesis of biomaterials and scaffolds, aiming to mimic the structure and composition of the original organ.23 Among the biomaterials, the use of decellularized tissues shows great promise in regenerative medicine. The decellularized organs preserve native ECM composition and structure, allowing recellularization with, e.g., recipient compatible cells, reducing the host immune response.24−27
Considering the two main problems of organ transplantation, low organ availability and rejection, our objective in this review is to show some advances in tissue engineering in an attempt to overcome these difficulties. Among the approaches, we will mainly focus on the use of decellularized tissues and how they can be used in therapies. We will also address how these acellular tissues interact with cells of the immune system, specifically macrophages, and with stem cells, both of them with great potential for use in regenerative medicine. Our objective is to demonstrate that the use of decellularized tissues can be an alternative for the generation of scaffolds with potential application for partial or complete organ replacement.
2. Tissue Engineering Strategies
Tissue engineering (TE) was first defined, in 1993, as “an interdisciplinary field that applies engineering principles to the life sciences in order to develop biological substitutes capable of restoring, maintaining, or improving tissue function”.28 This is a growing area of interest that aims to develop biological substitutes to be used in vivo transplant, in order to help solve the problems of high demand and low availability of organs.29 This strategy involves the use of scaffolds produced from biological and nonbiological materials, which can provide mechanical support in 3D form for cell development and mimic tissue structure30,31 (Table 1). Studies corroborate that scaffolds favor growth, migration, proliferation, and differentiation and are essential in providing a structure capable of housing human mesenchymal stem cells.32 It is possible to use scaffolds in transplantation therapy, as in the process of meniscal allograft, and in this process the meniscus can be extracted in its totality or partially, with collagen- or polyurethane-based scaffolds being used in partial extraction.33 Another study points out the scaffold composed of gelatin and copper capillary alginate, a 3D structure that provides favorable conditions for viable multipotent astrocytic stem cells (neural stem cells) under in vitro conditions.34
Table 1. Current Strategies for Tissue Engineering.
| STRATEGIES | DESCRIPTION | REFERENCES |
|---|---|---|
| Hydrogels | Hydrophilic materials capable of generating 3D structures similar to the extracellular matrix. Their properties include: porous structure, flexibility in the synthesis of the biomaterial, physical properties similar to natural tissues, biocompatibility, and the ability to store nanoparticles and therapeutic biomolecules. | (35) |
| 3D Bioprinting | Allows the deposit with spatial precision of bioinks, such as hydrogels, cells, and growth factors, mimicking in vitro the structure of native tissue and cellular and vascular composition from computational projections or from digitalization of magnetic resonance or computed tomography images. | (36, 37) |
| Decellularization | The use of dECM in regenerative medicine aims to minimize adverse reactions to the host, taking advantage of the unique characteristics of this biomaterial: maintaining the biological, mechanical, and structural properties of ECM which ensures the biocompatibility of the graft and the extraction of cells, and cellular debris provide a nonimmunogenic environment and in turn low cytotoxicity. | (38) |
There are many types of biomaterials that can be used to produce scaffolds for tissue engineering. The most commonly used definition of biomaterial was conceived during the 1991 Chester Consensus Conference, described as a substance or combination of substances of natural or synthetic origin used for indeterminate periods of time that can partially or totally replace a tissue, maintaining its function, with the aim of generating quality of life for the individual.39 Biomaterials are classified as natural or synthetic. In general, biomaterials of natural origin are composed of polypeptides, polysaccharides, nucleic acids, and hydroxyapatites and presented advantages over synthetic biomaterials, such as (i) selective cell adhesion, (ii) similarities in the mechanical properties of tissues in vivo, and (iii) biodegrability. Synthetic biomaterials are mostly polymers that are relatively inexpensive and with evident elastic characteristics, contributing to the increase of biocompatibility and the control of degradation of these structures.40
Several techniques are used to obtain biomaterials: one of them is the use of hydrogels. The 3D structures generated by hydrogels, which mimic the ECM, must meet the basic requirements of biocompatibility and mechanical and physical characteristics appropriate for each tissue, which provide a suitable environment for cell adhesion, which contributes to tissue regeneration.41 Hydrogels are used in the therapy of many diseases, mostly in regenerative transport of molecules and cells, and these applications are better addressed elsewhere.42−44
3D bioprinting, which consists of printing biomaterials consisting of living cells or active biomolecules, is a method that involves the deposition of layers of bioink, resulting in 3D structures.45 The technique has been a breakthrough in tissue engineering and regenerative medicine, generating supports capable of maintaining cells in appropriate microenvironmental conditions.46,47 Macro-architectural properties are the main characteristics of 3D bioprinting, which allow cell distribution within the constructs; however, these structures have little control over the microarchitecture of the tissue, and it is difficult to control cell orientation.48
So, the main objective of TE is to create a scaffold that mimics the composition and structure of the tissue or organ of interest, is biocompatible, has low immunogenictiy, and is capable of maintaining cells or stimulating host cells to repopulate the scaffold, making it a functional tissue. Synthetic scaffolds have some limitations, such as the difficulty in creating the complex vascular network needed to keep cells viable and in reproducing the mechanical properties of the native organ, and because the synthetic matrix components are discrepant from the natural matrix, these materials become potentially immunogenic.49−51 Therefore, considering the complexity of the organs, in terms of both structure and composition, an interesting alternative would be to use the organ itself as a scaffold. This can be achieved through decellularization.
2.1. Decellularization
Decellularization is a method that aims to remove all cellular and nuclear material of tissue while maintaining the ECM with preserved composition, biological activity, and mechanical integrity.52 A tissue is considered decellularized when (i) it possesses double-stranded DNA in a concentration less than 50 ng/mg of dry weight tissue, (ii) it possesses fragments of DNA only less than 200 base pairs, and (iii) in the absence of visible nuclear material in the stains of DAPI and hematoxilin and eosin. The use of nucleases assists in the removal of residual genetic material, which results in complete decellularization, avoiding immune reactions in the host.53,54
Decellularization aims to keep the 3D structure and vasculature of the entire organ intact for future applications, including recellularization of the tissue for use in transplants.55 Vascularized decellularized tissues are a breakthrough in tissue engineering because they present unique characteristics. These tissues are biocompatible and have a high capacity of endothelialization, which ensures better tissue cell repopulation, and the conformational structure of the tissue is respected. The biomaterial is composed of components of the natural extracellular matrix (collagen, elastin, glycosaminoglycans, and macromolecules) that generate a microenvironment suitable for cellular activity. Collagen and elastin fibers are responsible for providing elasticity to the tissue, ensuring resistance to tissue damage by pulsating blood flow. The use of dECM aims in regenerative medicine to minimize adverse reactions to the recipient, by maintaining the biological, mechanical, and structural properties of the ECM, and the extraction of cells and cellular debris provides a nonimmunogenic environment of low cytotoxicity, ensuring the biocompatibility of the graft.56
Decellularization can be performed with detergents, enzymes, chelating agents, physical agents, and a combination of physical and chemical agents.57−60 The substances most commonly used in the decellularization process are ionic detergents since these molecules are efficient in breaking cell membranes and lipids.61 The main techniques and reagents for obtaining decellularized tissue are shown in Figure 2, and the other ways of obtaining a decellularized extracellular matrix (dECM) are laid out in the review by Zhang et al.62
Figure 2.
Overview of decellularization strategies. There are numerous techniques for obtaining decellularized tissue. The final objective of the process is to destroy the cells and extract the cellular debris generated, keeping the ECM intact.
After decellularization, a cell-free scaffold is obtained, with the structure and composition of the native ECM of the organ or tissue of interest. This decellularized scaffold can be used as a whole or, for example, in the form of hydrogels, which lose 3D conformation but maintain the composition of the ECM.63,64
Studies have advanced in obtaining decellularized tissues capable of replacing those already damaged,65 focused on the replacement of muscle tissues of the upper limbs. The use of decellularized tissues that aid in the differentiation of pluripotent stem cells into functional cells is also performed.66
It is expected that this cell-free scaffold will generate a lower immune response if applied to patients. In addition, it allows the inclusion of cells compatible with the receptor, contributing to a reduction in the immune response and, consequently, in the rejection of the new tissue. Considering the complexity of the interaction of immune system cells and other cell types used for recellularization of dECM, we will address this in more depth, with more specific examples, in the following topics.
3. Interaction of a Decellularized Extracellular Matrix and Cells
The ECM is a complex structure composed of different types of collagens, glycosaminoglycans, proteoglycans, elastin, fibronectin, and laminin.67 The ECM, in addition to providing support to cells, is capable of stimulating cell proliferation, migration, differentiation, and other processes, not only due to its composition but also, e.g., due to its three-dimensional structure and rigidity.68
The ECM has also fundamental participation in immune events, such as monocyte chemoattraction and polarization.69 Biochemically, some studies suggest that ECM molecules, for example, type I collagen, seem to influence macrophage activation, differentiation, and secretion of metalloproteinase 9 in vitro. Another experiment shows that some molecules like vitronectin, laminin, and matrigel, as well as the structural geometry of macrophages, increase the expression of arginase-1 (Arg1) in vitro, a marker of anti-inflammatory macrophages. Thus, ECM composition directly influences macrophage phenotyping.70,71 Similarly, molecules such as heparan-sulfate are responsible for binding to cytokines, e.g. IL-2, allowing them to induce an innate response.72 In addition, Gvaramia et al.73 showed that the dECM can bind IL-4 in vitro, stimulating the polarization of human monocytes to an anti-inflammatory profile and consequently decreasing the inflammatory response. Besides interacting with molecules in the intercellular space, some products of the ECM that show partial proteolysis, the matrikines, can also regulate the cellular behavior.74 The following subsections will show examples of how immune system cells, more specifically macrophages, and stem cells interact with the dECM.
3.1. Macrophage Profiles and Transplant Rejection
Macrophage polarization is one key event in the rejection of transplanted organs. Macrophage polarization refers to changes in gene expression, surface markers, and factors secreted by these cells upon stimulation by different cytokines and microenvironment-related factors. The polarization of monocytes recruited from the circulation results in different profiles of macrophages in the tissues, with a greater pro-inflammatory or pro-resolving tendency.75
It is common to consider a binary distinction between populations of these cells: the M1, also referred to as classically activated macrophages, acting to promote inflammatory processes, and the M2, also called alternatively activated, with an anti-inflammatory or pro-resolving character.75 Commonly, these different macrophage phenotypes are classified based on the molecules involved in polarization induction, the classically polarized ones being obtainable in vitro through stimulation with lipopolysaccharide (LPS) and interferon γ (IFNγ), a situation that seeks to mimic a microenvironment related to Th1 lymphocytes in vivo. The alternatively polarized ones are related to stimulation by IL-4, referring to a microenvironment with a greater influence on Th2 lymphocytes.75,76 Regarding the action of these cells, pro-inflammatory ones demonstrate greater activation of signaling pathways involving STAT1 and NF-kB and the production of molecules such as induced nitric oxide synthase (iNOS). Anti-inflammatory macrophages, on the other hand, would be more active in the context of inflammation resolution, tissue repair, and fibrosis involving PPAR receptor pathways and the STAT6 factor and higher expression of arginase and IL-10, for example.75
However, this dichotomy does not seem to faithfully reflect macrophage diversity and plasticity. First, the so-called classically and alternatively activated macrophages in vitro do not seem to correspond exactly to the cells analyzed in vivo.76 Moreover, different subtypes of macrophages related to the M2 phenotype have already been described, such as M2a, M2b, and M2c,77 as well as another macrophage polarization phenotype, M4.78 Because of that, the idea of a spectrum of macrophage activation has been gaining strength, in which solely the subtype M1 and M2 phenotypes would be simplifications.75,79 Despite that, the simplistic division between pro- and anti-inflammatory macrophages is still widely used in the literature and will be adopted in the following sections.
Considering the transplant environment and the mechanisms surrounding transplant tolerance, there are several molecules that can polarize macrophages. In early graft tolerance, a lack of IL-33 could be responsible for increased iNOS+ macrophages and early graft rejection.80 Considering long-term rejection, analysis of kidney biopsies described that anti-inflammatory macrophage infiltration correlates with a progressively reduced glomerular filtration rate.81 Furthermore, a cytokine environment related to pro-inflammatory macrophages is related to the context of acute graft rejection, while anti-inflammatory macrophages appear to play an important role in the process of fibrosis and chronic rejection.82 These and other results point to macrophages, their polarization possibilities, and interactions with the ECM as key elements in the context of transplant tolerance and rejection (Figure 3), requiring further detailed studies to optimize the techniques of this procedure.
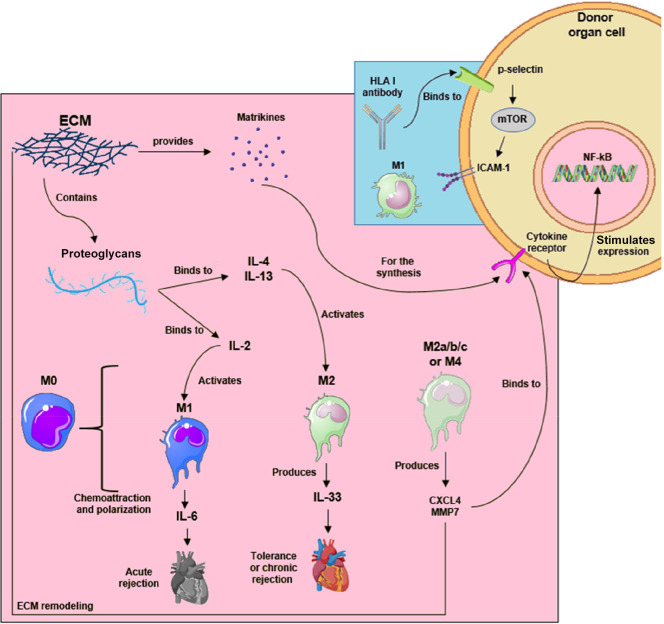
Figure 3.
Innate and adaptive responses to transplantation. ECM participation on macrophage activation in transplantation outcomes. Recognition of HLA antibodies in adaptive transplant rejection. The images were obtained from Servier Medical Art (http://smart.servier.com/), Creative Commons Attribution 3.0 Unported License (https://creativecommons.org/licenses/by/3.0/).
It was described that the use of porcine brain-derived dECM, in a murine spinal cord injury model, could modify macrophage polarization, which was commonly pro-inflammatory to macrophages toward an anti-inflammatory macrophage profile.83 Similarly, using a tooth-tissue-derived dECM combined with a drug, rosiglitazone, to improve teeth root regeneration demonstrated a downregulation in the expression of the NOS2+ pro-inflammatory macrophage by activating the PPAR-γ-NF-κB pathway.84 On the other hand, Chakraborty et al.85 demonstrated that decellularized goat corneas implanted in rabbits were able to evoke an immune response with a predominance of M1 macrophages due to exposure of epitopes of ECM structural molecules during the decellularization process. The main epitope pointed out as the cause of immune response was the α-1,3-galactosyltransferase (α-gal). This epitope is also observed in porcine decellularized tissues and is of great importance since humans may develop antibodies against it.86
The use of cross-linking of the dECM with chondroitin sulfate—a substance naturally found in cartilage—was able to reverse the inflammatory response caused by the dECM.85 All these findings point out that, despite being promising, the xenotransplantation of the dECM can lead to different immune responses and that the dECM association with drugs and other natural substances could attenuate a possible inflammatory response caused by the dECM and therefore promote an effective dECM treatment.
Given emphasis on the use of dECMs as scaffolds in the context of organ bioengineering, Petrosyan et al.87 showed that the renal dECM from healthy mice as well as from animals with Alport syndrome—in which there is progressive renal fibrosis—stimulates macrophage polarization to an anti-inflammatory macrophage in in vitro experiments, whereas a synthetic ECM tends to stimulate the polarization of these cells to a pro-inflammatory macrophage profile.87
Not only does the dECM contribute to an anti-inflammatory environment but also an anti-inflammatory environment facilitates the formation of the ECM. In this regard, Witherel et al.88 demonstrated that in in vitro experiments a “mixed” pro-inflammatory/anti-inflammatory macrophage phenotype, originating from stimulation with factors related to both phenotypes, was related to the deposition of an ECM with lower fiber alignment when compared to the “pure” M2 phenotype, suggesting a lower pro-fibrotic character. Furthermore, in a murine model, subcutaneous implantation of hydrogels containing IL-4 and IL-13 stimulated macrophage polarization toward M2 compared to hydrogels without cytokines, which elicited predominantly M1 response. The presence of M2 was associated with greater production of glycosaminoglycan-rich ECM and less fiber alignment, consistent with a less fibrotic ECM.88
In another point of view, we could highlight how macrophages influence the ECM. Thus, Sapudom et al.89 suggest that the most commonly activated anti-inflammatory M2a macrophages90 act on the differentiation of fibroblasts involving the TGF-β1 pathway and promoting a period with increased cell migration and remodeling of the ECM, while anti-inflammatory M2c macrophages stimulate the process of dedifferentiation of myofibroblasts into fibroblasts, a process related to the termination of the resolution process. The results also imply that the presence of a predominantly M2a environment for a long period may be related to the fibrotic process and that IL-10 is related to the defining stage of the resolution process.89 The anti-inflammatory macrophage (M2a) undergoes activation in the presence of IL-4/IL-13. In particular, IL-4 contributes to the increased expression of CD36, responsible for binding to oxidized low density lipoproteins, and the increased presence of these receptor proteins is involved in the elimination of debris, helping to end the inflammatory response. The anti-inflammatory macrophages (M2c) are activated by glucocorticoids, IL-10, and TGF-β. Glucocorticoids are involved in macrophage down adhesion, dissemination, phagocytosis, and apoptosis. IL-10 is a Th1 cell inhibitor, which binds to the IL-10 receptor; when the receptor is autophosphorylated, there is activation of the transcription factor STAT3, responsible for regulating the expression of pro-inflammatory cytokines.90
3.2. Stem Cell–dECM Interaction
Tissue engineering could involve, in addition to different types of biomaterials, the association with cell types that may further favor the regeneration of injured tissue. One of the most researched cell types in this area is the stem cell. Stem cells were first described in 1961, by Drs. James A. Till and Ernest A. McCulloch at the University of Toronto in Canada, based on the study and observation that rare cells present in the bone marrow of mice were capable of differentiating into other cell types.91 Stem cells are an unspecialized cell type with the ability to self-renew and differentiate into various cell types.92
Regarding the use of stem cells in clinical studies, the most used cell types so far are mesenchymal stem/stromal cells (MSCs). These cells can be isolated from different adult tissues (allowing autologous transplantation) such as bone marrow, adipose tissue, umbilical cord cells, menstrual blood, and others;93,94 that is, they can be virtually present in all tissues of the adult organism (Figure 4a). These cells could self-renew and can generate cells of mesodermal origin, such as adipocytes, osteocytes, and chondrocytes. In normal conditions, the MSC and other adult stem cells present in tissues are present in specific niches.95 They help maintain tissue homeostasis and regenerate small lesions94 (Figure 4b).
Figure 4.
Mesenchymal stem/stromal cells and their niche. (a) Mesenchymal stem/stromal cells can be isolated from different tissues and have the potential to differentiate into adipocytes, osteoblasts, and chondroblasts. (b) Stem cell niche composition. The images were obtained from Servier Medical Art (http://smart.servier.com/), Creative Commons Attribution 3.0 Unported License (https://creativecommons.org/licenses/by/3.0/).
MSCs have already been used to treat different types of diseases and injuries, including infarct, spinal cord injury, diabetes, and others, demonstrating beneficial effects.96,41 For example, mice that received an injection of MSCs showed preservation of some cardiac functions, due to a reduction of scar stiffness, attenuation of postinfarction remodeling, and improved cardiac muscle compliance, when compared to animals that received a vehicle.97 However, over the years of research, it was observed that the potential of MSCs went beyond differentiating into tissue cells and that their effects were caused much more by the paracrine factors secreted by them. These factors can reduce apoptosis, induce proliferation, promote angiogenesis,98−100 and regulate the immune response, e.g., suppressing the activity of B lymphocytes, natural killer (NK) cells, and dendritic cells.101−103
Currently, about 650 clinical trials are involved in the use of the regenerative potential of MSCs, all phase 2 for diseases such as osteoarthritis, neuropathies, lung disorders, spinal cord injury, heart disease, Crohn’s disease, and diabetes mellitus.104 In a study related to osteogenesis imperfecta, infants underwent transplantation of stem cells extracted from bone marrow; these cells acted to increase bone mineral density; and a reduction in the number of bone fractures was observed.105
Another type of stem cell that has potential use in tissue engineering is pluripotent stem cells (PSCs): embryonic stem cells (ESCs) and induced pluripotent stem cells (iPSCs). These cells have a greater capacity for self-renewal than MSCs, as they can differentiate into all cell types of the adult organism. Currently, different protocols allow the differentiation of these cells into cardiac cells,106,107 hepatocytes,108,109 neurons,110,111 and retinal epithelial cells,112 etc. Considering the ethical issues and rejection problems of ESCs, human iPSCs have gained prominence since they are obtained from the reprogramming of adult cells, allowing autologous transplantation and reducing ethical issues. All these associated characteristics reveal the potential use of stem cells in regenerative medicine and tissue engineering.
It is known that the ECM can influence the behavior of stem cells, and this relationship is being extensively studied.113,114,95,115 So, specific combinations of different ECMs could lead to specific cellular behaviors. In this context, the use of decellularized matrices of specific organs and tissues may contain the set of factors and molecules of ECM that favor the achievement of specific phenotypes. As it is a field of great interest, the dECM has been used for the treatment of several diseases and as a way to improve the differentiation or phenotype of cells in vitro. The relationship between various cell types, including stem cells, with different decellularized matrices from different organs and by different methodologies was recently reviewed by Cramer and Badylak,116 Agmon and Christman,117 and Hillebrandt et al.118 Here we will show some recent examples of the interaction of different dECMs with stem cells, attempts at dECM recellularization, and some therapeutic possibilities.
Regarding cardiac regeneration, many studies have developed association strategies between dECsM and different stem cell types.119 Cardiac-tissue-derived dECM has already been used in 3D cultures to increase the maturation of cardiomyocytes generated from iPSC,120 to generate bioinks for 3D printing of patches associated with cardiac progenitor stem cells and MSCs, and to improve regeneration after cardiac injury.121 Cardiac-tissue-derived dECM was also associated with MSC for muscle regeneration in cases of volumetric muscle loss,122 by promoting the differentiation of iPSC cells and cardiopoietic human amniotic fluid cells123 among others. A relevant point is choosing the best ECM region to be decellularized, if not the whole organ, as the ECM derived from different regions of the heart influenced, e.g., adipose-derived MSCs and human pulmonary microvascular endothelial cells in specific ways.124 Furthermore, there have been several attempts to recellularize the whole organ, with undifferentiated PSCs, partially spontaneously committed PSCs, and PSCs differentiated to cardiac progenitors or cardiomyocytes.125−130
One critical point in recellularization is the vascularization of the organ. Ciampi et al.131 showed that human iPSC-derived endothelial cells were efficient in recellularizing the vessels of decellularized rat kidney scaffolds, being able to cover more than 80% of the vasculature (glomerular and peritubular capillaries and small vessels).131
Recently, the formation of iPSC-derived islet organoids coated with a decellularized rat pancreatic ECM was demonstrated. The results showed that the organoids were able to respond to insulin and glucagon, in addition to being composed of endocrine cell types, including α, β, δ, and pancreatic polypeptide cells.132 Complementary analyses showed that one of the main ECM components related to the effects was type 4 collagen.132 It was also recently demonstrated that the decellularized retina of mice and pigs repopulated with hiPSC-derived retinal pigment epithelial cells or ocular progenitor cells was able to guide these cells to a specific organization, indicating the specificity of the ECM.133
Another possible use of the dECM is in liver regeneration. Some studies have shown that pretreatment of dECMs with a conditioned medium derived from a liver cell line (HepG2) contributes to better recellularization with iPSC-derived MSCs, liver cells (HepG2), and endothelial cells.134 Furthermore, it is not necessarily only hepatic ECMs that can have beneficial effects on hepatocyte culture. Kehtari et al.135 showed that decellularized Wharton’s jelly was able to promote hiPSC differentiation to hepatocytes.135 A new strategy aimed at tissue engineering application for liver regeneration was to generate a bioink from the liver ECM associated with hiPSC-derived hepatocytes. These cells remained viable, in addition to presenting greater area and better functionality.136
Applications of the dECM have also been described for neural and adipose tissue engineering. A recent strategy used for spinal cord injury regeneration was using the decellularized optic nerve loaded with a neurotrophin-3-overexpressing oligodendrocyte precursor cell, mimicking the white matter-like tissue and which, when applied to a white matter defect model, showed improvement in the animal’s condition.137 For adipose tissue engineering, for example, an injectable hydrogel-associating muscle-adhesive protein with poly(N-isopropyl acrylamide) and decellularized adipose tissue powder was described. This hydrogel with rat-adipose-derived stem cells was able to induce adipogenic differentiation.138
Other approaches involved the use of ECMs derived from cell cultures rather than specific tissues or organs. For example, in 2014, it was reported that dECM obtained from cultures of MSC, MSC-derived osteoblasts, and two types of smooth muscle cells had different effects on uninduced MSCs. While the first maintained the stemness and improved proliferation and motility, the others promoted the commitment to osteoblast and different smooth muscle cells, respectively.139 More recently, another work used the dECM from MSC and showed that it was nonimmunogenic and able to improve the proliferation and trilineage differentiation (adipocytes, osteoblasts, and chondrocytes) of MSCs.113
Briefly, we note that the use of the dECM helps to mimic the microenvironment of the tissue of interest, contributing to the differentiation and maturation of stem cells in vitro, which can be used not only for future therapies but also in drug testing assays, for example. In addition, the studies showed that scaffolds, at least those of smaller proportions or in hydrogel form, can be recellularized with different stem cells and, when implanted, assist in the regeneration of damaged tissue. In other words, the examples showed here demonstrate the potential of dECM, not only for in vivo applications, alone or associated with cells, but for also in vitro assays.
4. Concluding Remarks
The present review made explicit the search of tissue engineering for new biomaterials for partial or complete replacement of “diseased” tissues, with the intention of decreasing the high demand for organs for transplantation. Besides the search for organs, keeping them healthy is another concern because in most synthetic tissues biomaterials are responsible for causing immunogenicity, characterized by the imbalance between pro- and anti-inflammatory immune cells, requiring the use of antirejection drugs to prevent tissue loss.
In the current landscape, tissue engineering is looking for a biomaterial that can work on both fronts, and one option may be the use of decellularized tissues. These biomaterials are pointed out by studies as tissues that do not generate immunogenicity because their composition is practically identical among human beings, besides using the advantages of the extracellular matrix (recruitment and fixation of cells). Another important aspect is the use of stem cells from the patient himself, as mesenchymal stem cells, which end up in the tissue being differentiated by differentiation factors secreted by the matrix, besides the secretion of factors with immunomodulatory properties (reduction in immunogenicity) and stimulation of local cells to differentiate themselves. The use of more advanced technologies, such as the 3D printer, is being used to obtain synthetic structures and organs. Studies indicate that the use of extracellular matrix and human cells for the synthesis of bioprinting have resulted in the printing of entire organs.
Currently the commercialization of decellularized tissues is already a reality;51 however, even with the numerous advances and benefits that have been exposed in relation to the use of a decellularized extracellular matrix, there are still many challenges in whole organ bioengineering. For this technology to become viable in clinical transplant practice, some conditions are necessary, such as standardization of decellularization protocols for different organs and large-scale production of different cell types since the number of cells for recellularization of more complex organs can be high, in addition to being necessary to ensure that the entire tissue is completely recellularized, with all the cell types required for the correct functioning of the organ.49,50
Similarly, ethical aspects should be taken into consideration since the biomaterials are mostly obtained by financing from large private biotechnology companies, which can make the practice controversial since the donated organs would generate for-profit products.140 Finally, it is also necessary to define the best model for evaluating the interaction between the recipient and the transplanted organ, ensuring patient safety.50
Acknowledgments
We thank Capes for the support.
The authors declare no competing financial interest.
References
- Bezinover D.; Saner F. Organ Transplantation in the Modern Era. BMC Anesthesiol 2019, 19 (1), 1–4. 10.1186/s12871-019-0704-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cozzi E.; Schneeberger S.; Bellini M. I.; Berglund E.; Böhmig G.; Fowler K.; Hoogduijn M.; Jochmans I.; Marckmann G.; Marson L.; Neuberger J.; Oberbauer R.; Pierson R. N.; Reichart B.; Scobie L.; White C.; Naesens M. Organ Transplants of the Future: Planning for Innovations Including Xenotransplantation. Transpl. Int. 2021, 34 (11), 2006–2018. 10.1111/tri.14031. [DOI] [PubMed] [Google Scholar]
- Calne R. Clinical Transplantation: Current Problems, Possible Solutions. Philos. Trans. R. Soc. B Biol. Sci. 2005, 360 (1461), 1797–1801. 10.1098/rstb.2005.1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linden P. K. History of Solid Organ Transplantation and Organ Donation. Crit. Care Clin. 2009, 25 (1), 165–184. 10.1016/j.ccc.2008.12.001. [DOI] [PubMed] [Google Scholar]
- Wood-Trageser M. A.; Xu Q.; Zeevi A.; Randhawa P.; Lesniak D.; Demetris A. J. Precision Transplant Pathology. Curr. Opin. Organ Transplant 2020, 25 (4), 412–419. 10.1097/MOT.0000000000000772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MEDAWAR P. B. The behaviour and fate of skin autografts and skin homografts in rabbits: A report to the War Wounds Committee of the Medical Research Council. J. Anat. 1944, 78, 176–99. [PMC free article] [PubMed] [Google Scholar]; https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1272490/
- Timsit M. O.; Kleinclauss F.; Thuret R. Histoire Chirurgicale de La Transplantation Rénale. Prog. en Urol. 2016, 26 (15), 874–881. 10.1016/j.purol.2016.08.003. [DOI] [PubMed] [Google Scholar]
- Lu T.; Yang B.; Wang R.; Qin C. Xenotransplantation: Current Status in Preclinical Research. Front. Immunol. 2020, 10.3389/fimmu.2019.03060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allison T. L. Immunosuppressive Therapy in Transplantation. Nurs. Clin. North Am. 2016, 51 (1), 107–120. 10.1016/j.cnur.2015.10.008. [DOI] [PubMed] [Google Scholar]
- Liu Y.; Zhou P.; Han M.; Xue C. B.; Hu X. P.; Li C. Basiliximab or Antithymocyte Globulin for Induction Therapy in Kidney Transplantation: A Meta-Analysis. Transplant. Proc. 2010, 42 (5), 1667–1670. 10.1016/j.transproceed.2010.02.088. [DOI] [PubMed] [Google Scholar]
- Mancini M. C.; Cush E. M.; Launius B. K.; Brown P. A. The Management of Immunosuppression: The Art and the Science. Crit. Care Nurs. Q. 2004, 27 (1), 61–64. 10.1097/00002727-200401000-00005. [DOI] [PubMed] [Google Scholar]
- Pham M. X.; Yee J.. Cardiac transplant rejection. In Genomic and personalized medicine; Ginsberg G. H., Willard H. S., Eds.; Elsevier: Tokyo, 2013; pp 557–71. [Google Scholar]
- Gabardi S.; Tichy E. M.. Overview of Immunosuppressive Therapies in Renal Transplantation; Springer, 2012. 10.1007/978-1-4614-0008-0_6. [DOI] [Google Scholar]
- Yeung M. Y.; Gabardi S.; Sayegh M. H. Use of Polyclonal/Monoclonal Antibody Therapies in Transplantation. Expert Opin. Biol. Ther. 2017, 17 (3), 339–352. 10.1080/14712598.2017.1283400. [DOI] [PubMed] [Google Scholar]
- Lewis A.; Koukoura A.; Tsianos G. I.; Gargavanis A. A.; Nielsen A. A.; Vassiliadis E. Organ Donation in the US and Europe: The Supply vs Demand Imbalance. Transplant. Rev. 2021, 35 (2), 100585. 10.1016/j.trre.2020.100585. [DOI] [PubMed] [Google Scholar]
- Branger P.; Samuel U.. Annual Report 2018; 2018. https://www.eurotransplant.org/wp-content/uploads/2019/12/032675-_ET_Jaarverslag_2018_v7-1.pdf.
- National Foundation for Transplants , 2022. Visualized in https://transplants.org/.
- Mehrotra R. Advancing American Kidney Health an Introduction. Clin. J. Am. Soc. Nephrol. 2019, 14 (12), 1788. 10.2215/CJN.11840919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Himmelfarb J.; Vanholder R.; Mehrotra R.; Tonelli M. The Current and Future Landscape of Dialysis. Nat. Rev. Nephrol. 2020, 16 (10), 573–585. 10.1038/s41581-020-0315-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leuning D. G.; Witjas F. M. R.; Maanaoui M.; de Graaf A. M. A.; Lievers E.; Geuens T.; Avramut C. M.; Wiersma L. E.; van den Berg C. W.; Sol W. M. P. J.; de Boer H.; Wang G.; LaPointe V. L. S.; van der Vlag J.; van Kooten C.; van den Berg B. M.; Little M. H.; Engelse M. A.; Rabelink T. J. Vascular Bioengineering of Scaffolds Derived from Human Discarded Transplant Kidneys Using Human Pluripotent Stem Cell–Derived Endothelium. Am. J. Transplant. 2019, 19 (5), 1328–1343. 10.1111/ajt.15200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homan K. A.; Gupta N.; Kroll K. T.; Kolesky D. B.; Skylar-Scott M.; Miyoshi T.; Mau D.; Valerius M. T.; Ferrante T.; Bonventre J. V.; Lewis J. A.; Morizane R. Flow-Enhanced Vascularization and Maturation of Kidney Organoids in Vitro. Nat. Methods 2019, 16 (3), 255–262. 10.1038/s41592-019-0325-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoogduijn M. J.; Montserrat N.; van der Laan L. J. W.; Dazzi F.; Perico N.; Kastrup J.; Gilbo N.; Ploeg R. J.; Roobrouck V.; Casiraghi F.; Johnson C. L.; Franquesa M.; Dahlke M. H.; Massey E.; Hosgood S.; Reinders M. E. J. The Emergence of Regenerative Medicine in Organ Transplantation: 1st European Cell Therapy and Organ Regeneration Section Meeting. Transpl. Int. 2020, 33 (8), 833–840. 10.1111/tri.13608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nam S. Y.; Park S. H. ECM Based Bioink for Tissue Mimetic 3D Bioprinting. Adv. Exp. Med. Biol. 2018, 1064, 335–353. 10.1007/978-981-13-0445-3_20. [DOI] [PubMed] [Google Scholar]
- Ozbek S.; Balasubramanian P. G.; Chiquet-Ehrismann R.; Tucker R. P.; Adams J. C. The Evolution of Extracellular Matrix. Mol. Biol. Cell 2010, 21 (24), 4300–4305. 10.1091/mbc.e10-03-0251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussey G. S.; Dziki J. L.; Badylak S. F. Extracellular Matrix-Based Materials for Regenerative Medicine. Nat. Rev. Mater. 2018, 3 (7), 159–173. 10.1038/s41578-018-0023-x. [DOI] [Google Scholar]
- Kaukonen R.; Jacquemet G.; Hamidi H.; Ivaska J. Cell-Derived Matrices for Studying Cell Proliferation and Directional Migration in a Complex 3D Microenvironment. Nat. Protoc. 2017, 12 (11), 2376–2390. 10.1038/nprot.2017.107. [DOI] [PubMed] [Google Scholar]
- Goldstein S.; Clarke D. R.; Walsh S. P.; Black K. S.; O’brien M. F. Transpecies Heart Valve Transplant: Advanced Studies of a Bioengineered Xeno-Autograft. Ann. Thorac. Surg. 2000, 70 (6), 1962–1969. 10.1016/S0003-4975(00)01812-9. [DOI] [PubMed] [Google Scholar]
- Langer R.; Vacanti J. Advances in Tissue Engineering. J. Pediatr. Surg. 2016, 51 (1), 8–12. 10.1016/j.jpedsurg.2015.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu W.; Ma X.; Gou M.; Mei D.; Zhang K.; Chen S. 3D Printing of Functional Biomaterials for Tissue Engineering. Curr. Opin. Biotechnol. 2016, 40, 103–112. 10.1016/j.copbio.2016.03.014. [DOI] [PubMed] [Google Scholar]
- Bajaj P.; Schweller R. M.; Khademhosseini A.; West J. L.; Bashir R. 3D Biofabrication Strategies for Tissue Engineering and Regenerative Medicine. Annu. Rev. Biomed. Eng. 2014, 16 (May), 247–276. 10.1146/annurev-bioeng-071813-105155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akbari M.; Tamayol A.; Bagherifard S.; Serex L.; Mostafalu P.; Faramarzi N.; Mohammadi M. H.; Khademhosseini A. Textile Technologies and Tissue Engineering: A Path Toward Organ Weaving. Adv. Healthc. Mater. 2016, 5 (7), 751–766. 10.1002/adhm.201500517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamalpoor Z.; Taromi N.; Soleimani M.; Koudehi M. F.; Asgari A. In Vitro Interaction of Human Wharton’s Jelly Mesenchymal Stem Cells with Biomimetic 3D Scaffold. J. Biomed. Mater. Res. - Part A 2019, 107 (6), 1166–1175. 10.1002/jbm.a.36608. [DOI] [PubMed] [Google Scholar]
- Pereira H.; Fatih Cengiz I.; Gomes S.; Espregueira-Mendes J.; Ripoll P. L.; Monllau J. C.; Reis R. L.; Oliveira J. M. Meniscal Allograft Transplants and New Scaffolding Techniques. EFORT Open Rev. 2019, 4 (6), 279–295. 10.1302/2058-5241.4.180103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willenberg B. J.; Zheng T.; Meng F. W.; Meneses J. C.; Rossignol C.; Batich C. D.; Terada N.; Steindler D. A.; Weiss M. D. Gelatinized Copper-Capillary Alginate Gel Functions as an Injectable Tissue Scaffolding System for Stem Cell Transplants. J. Biomater. Sci. Polym. Ed. 2011, 22 (12), 1621–1637. 10.1163/092050610X519453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pourshahrestani S.; Zeimaran E.; Kadri N. A.; Mutlu N.; Boccaccini A. R. Polymeric Hydrogel Systems as Emerging Biomaterial Platforms to Enable Hemostasis and Wound Healing. Adv. Healthc. Mater. 2020, 9 (20), 2000905. 10.1002/adhm.202000905. [DOI] [PubMed] [Google Scholar]
- Liu N.; Ye X.; Yao B.; Zhao M.; Wu P.; Liu G.; Zhuang D.; Jiang H.; Chen X.; He Y.; Huang S.; Zhu P. Advances in 3D Bioprinting Technology for Cardiac Tissue Engineering and Regeneration. Bioact. Mater. 2021, 6 (5), 1388–1401. 10.1016/j.bioactmat.2020.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma X.; Liu J.; Zhu W.; Tang M.; Lawrence N.; Yu C.; Gou M.; Chen S. 3D Bioprinting of Functional Tissue Models for Personalized Drug Screening and in Vitro Disease Modeling. Adv. Drug Delivery Rev. 2018, 132, 235–251. 10.1016/j.addr.2018.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X.; Chen X.; Hong H.; Hu R.; Liu J.; Liu C. Decellularized extracellular matrix scaffolds: Recent trends and emerging strategies in tissue engineering. Bioact Mater. 2022, 10, 15–31. 10.1016/j.bioactmat.2021.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marin E.; Boschetto F.; Pezzotti G. Biomaterials and Biocompatibility: An Historical Overview. J. Biomed. Mater. Res. - Part A 2020, 108 (8), 1617–1633. 10.1002/jbm.a.36930. [DOI] [PubMed] [Google Scholar]
- Bharadwaz A.; Jayasuriya A. C. Recent Trends in the Application of Widely Used Natural and Synthetic Polymer Nanocomposites in Bone Tissue Regeneration. Mater. Sci. Eng., C 2020, 110, 110698. 10.1016/j.msec.2020.110698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavakoli S.; Klar A. S. Advanced Hydrogels as Wound Dressings. Biomolecules 2020, 10 (8), 1–20. 10.3390/biom10081169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmadian E.; Dizaj S. M.; Eftekhari A.; Dalir E.; Vahedi P.; Hasanzadeh A.; Samiei M. The Potential Applications of Hyaluronic Acid Hydrogels in Biomedicine. Drug Res. (Stuttg). 2020, 70 (1), 6–11. 10.1055/a-0991-7585. [DOI] [PubMed] [Google Scholar]
- Wu J.; Chen Q.; Deng C.; Xu B.; Zhang Z.; Yang Y.; Lu T. Exquisite Design of Injectable Hydrogels in Cartilage Repair. Theranostics 2020, 10 (21), 9843–9864. 10.7150/thno.46450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang G.; Yang X.; Wang Q.; Zhang A.; Tang B. Hydrogels-Based Ophthalmic Drug Delivery Systems for Treatment of Ocular Diseases. Mater. Sci. Eng., C 2021, 127 (May), 112212. 10.1016/j.msec.2021.112212. [DOI] [PubMed] [Google Scholar]
- Dey M.; Ozbolat I. T. 3D Bioprinting of Cells, Tissues and Organs. Sci. Rep. 2020, 10 (1), 10–12. 10.1038/s41598-020-70086-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy S. V.; Atala A. 3D Bioprinting of Tissues and Organs. Nat. Biotechnol. 2014, 32 (8), 773–785. 10.1038/nbt.2958. [DOI] [PubMed] [Google Scholar]
- Patel D. K.; Sakhaei A. H.; Layani M.; Zhang B.; Ge Q.; Magdassi S. Highly Stretchable and UV Curable Elastomers for Digital Light Processing Based 3D Printing. Adv. Mater. 2017, 29 (15), 1606000. 10.1002/adma.201606000. [DOI] [PubMed] [Google Scholar]
- Chansoria P.; Shirwaiker R. Characterizing the Process Physics of Ultrasound-Assisted Bioprinting. Sci. Rep. 2019, 9 (1), 1–17. 10.1038/s41598-019-50449-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peloso A.; Dhal A.; Zambon J. P.; Li P.; Orlando G.; Atala A.; Soker S. Current Achievements and Future Perspectives in Whole-Organ Bioengineering Rocky Tuan; Timothy O’Brien. Stem Cell Res. Ther. 2015, 10.1186/s13287-015-0089-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajab T. K.; O’Malley T. J.; Tchantchaleishvili V. Decellularized Scaffolds for Tissue Engineering: Current Status and Future Perspective. Artif. Organs 2020, 44 (10), 1031–1043. 10.1111/aor.13701. [DOI] [PubMed] [Google Scholar]
- Nakamura N.; Kimura T.; Kishida A. Overview of the Development, Applications, and Future Perspectives of Decellularized Tissues and Organs. ACS Biomater. Sci. Eng. 2017, 3 (7), 1236–1244. 10.1021/acsbiomaterials.6b00506. [DOI] [PubMed] [Google Scholar]
- Nouri Barkestani M.; Naserian S.; Uzan G.; Shamdani S. Post-Decellularization Techniques Ameliorate Cartilage Decellularization Process for Tissue Engineering Applications. J. Tissue Eng. 2021, 12, 12–14. 10.1177/2041731420983562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M. Y.; Fang J. J.; Lee J. N.; Periasamy S.; Yen K. C.; Wang H. C.; Hsieh D. J. Supercritical Carbon Dioxide Decellularized Xenograft-3D CAD/CAM Carved Bone Matrix Personalized for Human Bone Defect Repair. Genes (Basel) 2022, 13 (5), 755. 10.3390/genes13050755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crapo P. M.; Gilbert T. W.; Badylak S. F. An Overview of Tissue and Whole Organ Decellularization Processes. Biomaterials 2011, 32 (12), 3233–3243. 10.1016/j.biomaterials.2011.01.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choudhury D.; Yee M.; Sheng Z. L. J.; Amirul A.; Naing M. W. Decellularization Systems and Devices: State-of-the-Art. Acta Biomater 2020, 115, 51–59. 10.1016/j.actbio.2020.07.060. [DOI] [PubMed] [Google Scholar]
- Hsia K.; Lin C. H.; Lee H. Y.; Chen W. M.; Yao C. L.; Chen C. C.; Ma H.; Wang S. J.; Lu J. H. Sphingosine-1-Phosphate in Endothelial Cell Recellularization Improves Patency and Endothelialization of Decellularized Vascular Grafts in Vivo. Int. J. Mol. Sci. 2019, 20 (7), 1641. 10.3390/ijms20071641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods T.; Gratzer P. F. Effectiveness of Three Extraction Techniques in the Development of a Decellularized Bone-Anterior Cruciate Ligament-Bone Graft. Biomaterials 2005, 26 (35), 7339–7349. 10.1016/j.biomaterials.2005.05.066. [DOI] [PubMed] [Google Scholar]
- Faulk D. M.; Carruthers C. A.; Warner H. J.; Kramer C. R.; Reing J. E.; Zhang L.; D’Amore A.; Badylak S. F. The Effect of Detergents on the Basement Membrane Complex of a Biologic Scaffold Material. Acta Biomater. 2014, 10 (1), 183–193. 10.1016/j.actbio.2013.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levorson E. J.; Hu O.; Mountziaris P. M.; Kasper F. K.; Mikos A. G. Cell-Derived Polymer/Extracellular Matrix Composite Scaffolds for Cartilage Regeneration, Part 2: Construct Devitalization and Determination of Chondroinductive Capacity. Tissue Eng Parct C Methods 2014, 10.1089/ten.tec.2013.0288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilpin A.; Yang Y. Decellularization Strategies for Regenerative Medicine: From Processing Techniques to Applications. Biomed Res. Int. 2017, 2017, 1. 10.1155/2017/9831534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keane T. J.; Swinehart I. T.; Badylak S. F. Methods of Tissue Decellularization Used for Preparation of Biologic Scaffolds and in Vivo Relevance. Methods 2015, 84 (March), 25–34. 10.1016/j.ymeth.2015.03.005. [DOI] [PubMed] [Google Scholar]
- Zhang X.; Chen X.; Hong H.; Hu R.; Liu J.; Liu C. Decellularized Extracellular Matrix Scaffolds: Recent Trends and Emerging Strategies in Tissue Engineering. Bioact. Mater. 2022, 10, 15–31. 10.1016/j.bioactmat.2021.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massaro M. S.; Pálek R.; Rosendorf J.; Červenková L.; Liška V.; Moulisová V. Decellularized Xenogeneic Scaffolds in Transplantation and Tissue Engineering: Immunogenicity versus Positive Cell Stimulation. Mater. Sci. Eng., C 2021, 127, 112203. 10.1016/j.msec.2021.112203. [DOI] [PubMed] [Google Scholar]
- Daviran M.; Schultz K. M. Characterizing the Dynamic Rheology in the Pericellular Region by Human Mesenchymal Stem Cell Re-Engineering in PEG-Peptide Hydrogel Scaffolds. Rheol. Acta 2019, 58 (8), 421–437. 10.1007/s00397-019-01142-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naik A.; Griffin M.; Szarko M.; Butler P. E. Optimizing the Decellularization Process of an Upper Limb Skeletal Muscle; Implications for Muscle Tissue Engineering. Artif. Organs 2020, 44 (2), 178–183. 10.1111/aor.13575. [DOI] [PubMed] [Google Scholar]
- Abazari M. F.; Soleimanifar F.; Enderami S. E.; Nasiri N.; Nejati F.; Mousavi S. A.; Soleimani M.; Kiani J.; Ghoraeian P.; Kehtari M. Decellularized Amniotic Membrane Scaffolds Improve Differentiation of IPSCs to Functional Hepatocyte-like Cells. J. Cell. Biochem. 2020, 121 (2), 1169–1181. 10.1002/jcb.29351. [DOI] [PubMed] [Google Scholar]
- Theocharis A. D.; Skandalis S. S.; Gialeli C.; Karamanos N. K. Extracellular Matrix Structure. Adv. Drug Delivery Rev. 2016, 97, 4–27. 10.1016/j.addr.2015.11.001. [DOI] [PubMed] [Google Scholar]
- Karamanos N. K.; Theocharis A. D.; Piperigkou Z.; Manou D.; Passi A.; Skandalis S. S.; Vynios D. H.; Orian-Rousseau V.; Ricard-Blum S.; Schmelzer C. E. H.; Duca L.; Durbeej M.; Afratis N. A.; Troeberg L.; Franchi M.; Masola V.; Onisto M. A Guide to the Composition and Functions of the Extracellular Matrix. FEBS J. 2021, 288 (24), 6850–6912. 10.1111/febs.15776. [DOI] [PubMed] [Google Scholar]
- Chakraborty J.; Roy S.; Ghosh S. Regulation of Decellularized Matrix Mediated Immune Response. Biomater. Sci. 2020, 8 (5), 1194–1215. 10.1039/C9BM01780A. [DOI] [PubMed] [Google Scholar]
- Wesley R. B.; Meng X.; Godin D.; Galis Z. S. Extracellular Matrix Modulates Macrophage Functions Characteristic to Atheroma: Collagen Type I Enhances Acquisition of Resident Macrophage Traits by Human Peripheral Blood Monocytes in Vitro. Arterioscler. Thromb. Vasc. Biol. 1998, 18 (3), 432–440. 10.1161/01.ATV.18.3.432. [DOI] [PubMed] [Google Scholar]
- Luu T. U.; Liu W. F. Regulation of Macrophages by Extracellular Matrix Composition and Adhesion Geometry. Regen. Eng. Transl. Med. 2018, 4 (4), 238–246. 10.1007/s40883-018-0065-z. [DOI] [Google Scholar]
- Wrenshall L. Role of the Microenvironment in Immune Responses to Transplantation. Springer Semin. Immunopathol. 2003, 25 (2), 199–213. 10.1007/s00281-003-0138-y. [DOI] [PubMed] [Google Scholar]
- Gvaramia D.; Kern J.; Jakob Y.; Tritschler H.; Brenner R. E.; Breiter R.; Kzhyshkowska J.; Rotter N. Modulation of the inflammatory response to decellularized collagen matrix for cartilage regeneration. Journal Of Biomedical Materials Research Part A, [S.L.] 2022, 110, 1021. 10.1002/jbm.a.37349. [DOI] [PubMed] [Google Scholar]
- Maquart F. X.; Pasco S.; Ramont L.; Hornebeck W.; Monboisse J. C. An Introduction to Matrikines: Extracellular Matrix-Derived Peptides Which Regulate Cell Activity - Implication in Tumor Invasion. Crit. Rev. Oncol. Hematol. 2004, 49 (3), 199–202. 10.1016/j.critrevonc.2003.06.007. [DOI] [PubMed] [Google Scholar]
- Murray P. J. Macrophage Polarization. Annu. Rev. Physiol. 2017, 79, 541–566. 10.1146/annurev-physiol-022516-034339. [DOI] [PubMed] [Google Scholar]
- Orecchioni M.; Ghosheh Y.; Pramod A. B.; Ley K. Macrophage Polarization: Different Gene Signatures in M1(Lps+) vs. Classically and M2(LPS-) vs. Alternatively Activated Macrophages. Front. Immunol. 2019, 10 (MAY), 1–14. 10.3389/fimmu.2019.01084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iqbal S.; Kumar A. Characterization of In Vitro Generated Human Polarized Macrophages. J. Clin. Cell. Immunol. 2015, 10.4172/2155-9899.1000380. [DOI] [Google Scholar]
- Erbel C.; Tyka M.; Helmes C. M.; Akhavanpoor M.; Rupp G.; Domschke G.; Linden F.; Wolf A.; Doesch A.; Lasitschka F.; Katus H. A.; Gleissner C. A. CXCL4-Induced Plaque Macrophages Can Be Specifically Identified by Co-Expression of MMP7+S100A8+ in Vitro and in Vivo. Innate Immun 2015, 21 (3), 255–265. 10.1177/1753425914526461. [DOI] [PubMed] [Google Scholar]
- Martinez F. O.; Gordon S. The M1 and M2 Paradigm of Macrophage Activation: Time for Reassessment. F1000Prime Rep 2014, 6 (March), 1–13. 10.12703/P6-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T.; Zhang Z.; Bartolacci J. G.; Dwyer G. K.; Liu Q.; Mathews L. R.; Velayutham M.; Roessing A. S.; Lee Y. C.; Dai H.; Shiva S.; Oberbarnscheidt M. H.; Dziki J. L.; Mullet S. J.; Wendell S. G.; Wilkinson J. D.; Webber S. A.; Wood-Trageser M.; Watkins S. C.; Demetris A. J.; Hussey G. S.; Badylak S. F.; Turnquist H. R. Graft IL-33 regulates infiltrating macrophages to protect against chronic rejection. J. Clin Invest 2020, 130 (10), 5397–5412. 10.1172/JCI133008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toki D.; Zhang W.; Hor K. L. M.; Liuwantara D.; Alexander S. I.; Yi Z.; Sharma R.; Chapman J. R.; Nankivell B. J.; Murphy B.; O’Connell P. J. The Role of Macrophages in the Development of Human Renal Allograft Fibrosis in the First Year after Transplantation. Am. J. Transplant. 2014, 14 (9), 2126–2136. 10.1111/ajt.12803. [DOI] [PubMed] [Google Scholar]
- Li J.; Li C.; Zhuang Q.; Peng B.; Zhu Y.; Ye Q.; Ming Y. The Evolving Roles of Macrophages in Organ Transplantation. J. Immunol. Res. 2019, 2019, 1. 10.1155/2019/5763430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong J. Y.; Seo Y.; Davaa G.; Kim H. W.; Kim S. H.; Hyun J. K. Decellularized Brain Matrix Enhances Macrophage Polarization and Functional Improvements in Rat Spinal Cord Injury. Acta Biomater 2020, 101, 357–371. 10.1016/j.actbio.2019.11.012. [DOI] [PubMed] [Google Scholar]
- Lan T.; Chen J.; Zhang J.; Huo F.; Han X.; Zhang Z.; Xu Y.; Huang Y.; Liao L.; Xie L.; Tian W.; Guo W. Xenoextracellular Matrix-Rosiglitazone Complex-Mediated Immune Evasion Promotes Xenogenic Bioengineered Root Regeneration by Altering M1/M2Macrophage Polarization. Biomaterials 2021, 276, 121066. 10.1016/j.biomaterials.2021.121066. [DOI] [PubMed] [Google Scholar]
- Chakraborty J.; Roy S.; Murab S.; Ravani R.; Kaur K.; Devi S.; Singh Di.; Sharma S.; Mohanty S.; Dinda A. K.; Tandon R.; Ghosh S. Modulation of Macrophage Phenotype, Maturation, and Graft Integration through Chondroitin Sulfate Cross-Linking to Decellularized Cornea. ACS Biomater. Sci. Eng. 2019, 5 (1), 165–179. 10.1021/acsbiomaterials.8b00251. [DOI] [PubMed] [Google Scholar]
- Liang R.; Fisher M.; Yang G.; Hall C.; Woo S. L. Y. Alpha1,3-Galactosyltransferase Knockout Does Not Alter the Properties of Porcine Extracellular Matrix Bioscaffolds. Acta Biomater 2011, 7 (4), 1719–1727. 10.1016/j.actbio.2011.01.001. [DOI] [PubMed] [Google Scholar]
- Petrosyan A.; Da Sacco S.; Tripuraneni N.; Kreuser U.; Lavarreda-Pearce M.; Tamburrini R.; De Filippo R. E.; Orlando G.; Cravedi P.; Perin L. A Step towards Clinical Application of Acellular Matrix: A Clue from Macrophage Polarization. Matrix Biol. 2017, 57–58, 334–346. 10.1016/j.matbio.2016.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witherel C. E.; Sao K.; Brisson B. K.; Han B.; Volk S. W.; Petrie R. J.; Han L.; Spiller K. L. Regulation of Extracellular Matrix Assembly and Structure by Hybrid M1/M2Macrophages. Biomaterials 2021, 269, 120667. 10.1016/j.biomaterials.2021.120667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapudom J.; Karaman S.; Mohamed W. K. E.; Garcia-Sabate A.; Quartey B. C.; Teo J. C. M. 3D in vitro M2 macrophage model to mimic modulation of tissue repair. npj Regen Med. 2021, 10.1038/s41536-021-00193-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X.; Li Y.; Fu M.; Xin H.-B. Polarizing Macrophages In Vitro. Methods Mol. Biol. 2018, 1784, 119–126. 10.1007/978-1-4939-7837-3_12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Till J. E.; Mcculloch E. A. A Direct Measurement of the Radiation Sensitivity of Normal Mouse Bone Marrow Cells’. Radiat. Res. 1961, 14, 213–222. 10.2307/3570892. [DOI] [PubMed] [Google Scholar]
- Zakrzewski W.; Dobrzynski M.; Szymonowicz M.; Rybak Z. Stem Cells: Past, Present, and Future. Stem Cell Res. Ther. 2019, 10 (5), 329–332. 10.1186/s13287-019-1165-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macrin D.; Joseph J. P.; Pillai A. A.; Devi A. Eminent Sources of Adult Mesenchymal Stem Cells and Their Therapeutic Imminence. Stem Cell Rev. Reports 2017, 13 (6), 741–756. 10.1007/s12015-017-9759-8. [DOI] [PubMed] [Google Scholar]
- Gurusamy N.; Alsayari A.; Rajasingh S.; Rajasingh J.. Adult Stem Cells for Regenerative Therapy, 1st ed.; Elsevier Inc., 2018; Vol. 160. 10.1016/bs.pmbts.2018.07.009. [DOI] [PubMed] [Google Scholar]
- Gattazzo F.; Urciuolo A.; Bonaldo P. Extracellular Matrix: A Dynamic Microenvironment for Stem Cell Niche. Biochim. Biophys. Acta - Gen. Subj. 2014, 1840 (8), 2506–2519. 10.1016/j.bbagen.2014.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown C.; McKee C.; Bakshi S.; Walker K.; Hakman E.; Halassy S.; Svinarich D.; Dodds R.; Govind C. K.; Chaudhry G. R. Mesenchymal Stem Cells: Cell Therapy and Regeneration Potential. J. Tissue Eng. Regen. Med. 2019, 13 (9), 1738–1755. 10.1002/term.2914. [DOI] [PubMed] [Google Scholar]
- Berry M. F.; Engler A. J.; Woo Y. J.; Pirolli T. J.; Bish L. T.; Jayasankar V.; Morine K. J.; Gardner T. J.; Discher D. E.; Sweeney H. L. Mesenchymal Stem Cell Injection after Myocardial Infarction Improves Myocardial Compliance. Am. J. Physiol. - Hear. Circ. Physiol. 2006, 290 (6), H2196. 10.1152/ajpheart.01017.2005. [DOI] [PubMed] [Google Scholar]
- Gnecchi M.; Zhang Z.; Ni A.; Dzau V. J. Paracrine Mechanisms in Adult Stem Cell Signaling and Therapy. Circ. Res. 2008, 103 (11), 1204–1219. 10.1161/CIRCRESAHA.108.176826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S. K.; Jacobus S. J.; Cohen A. D.; Weiss M.; Callander N.; Singh A. K.; Parker T. L.; Menter A.; Yang X.; Parsons B.; Kumar P.; Kapoor P.; Rosenberg A.; Zonder J. A.; Faber E.; Lonial S.; Anderson K. C.; Richardson P. G.; Orlowski R. Z.; Wagner L. I.; Rajkumar S. V. Carfilzomib or Bortezomib in Combination with Lenalidomide and Dexamethasone for Patients with Newly Diagnosed Multiple Myeloma without Intention for Immediate Autologous Stem-Cell Transplantation (ENDURANCE): A Multicentre, Open-Label, Phase 3, Randomise. Lancet Oncol 2020, 21 (10), 1317–1330. 10.1016/S1470-2045(20)30452-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan D.; Zeng M.; Xia Q.; Wu S.; Ye S.; Rao J.; Lin D.; Zhang H.; Ma H.; Han Z.; Guo X.; Liu Z. Efficacy and Safety of Umbilical Cord Mesenchymal Stem Cells in Treatment of Cesarean Section Skin Scars: A Randomized Clinical Trial. Stem Cell Res. Ther 2020, 11 (1), 244. 10.1186/s13287-020-01695-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang X. X.; Zhang Y.; Liu B.; Zhang S. X.; Wu Y.; Yu X. D.; Mao N. Human Mesenchymal Stem Cells Inhibit Differentiation and Function of Monocyte-Derived Dendritic Cells. Blood 2005, 105 (10), 4120–4126. 10.1182/blood-2004-02-0586. [DOI] [PubMed] [Google Scholar]
- Sotiropoulou P. A.; Perez S. A.; Salagianni M.; Baxevanis C. N.; Papamichail M. Cell Culture Medium Composition and Translational Adult Bone Marrow-Derived Stem Cell Research. Stem Cells 2006, 24 (5), 1409–1410. 10.1634/stemcells.2005-0654. [DOI] [PubMed] [Google Scholar]
- Luz-Crawford P.; Ipseiz N.; Espinosa-Carrasco G.; Caicedo A.; Tejedor G.; Toupet K.; Loriau J.; Scholtysek C.; Stoll C.; Khoury M.; Noël D.; Jorgensen C.; Krönke G.; Djouad F. PPARβ/δ Directs the Therapeutic Potential of Mesenchymal Stem Cells in Arthritis. Ann. Rheum. Dis. 2016, 75 (12), 2166–2174. 10.1136/annrheumdis-2015-208696. [DOI] [PubMed] [Google Scholar]
- Samsonraj R. M.; Raghunath M.; Nurcombe V.; Hui J. H.; van Wijnen A. J.; Cool S. M. Concise Review: Multifaceted Characterization of Human Mesenchymal Stem Cells for Use in Regenerative Medicine. Stem Cells Transl. Med. 2017, 6 (12), 2173–2185. 10.1002/sctm.17-0129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz E. M.; Prockop D. J.; Fitzpatrick L. A.; Koo W. W. K.; Gordon P. L.; Neel M.; Sussman M.; Orchard P.; Marx J. C.; Pyeritz R. E.; Brenner M. K. Transplantability and Therapeutic Effects of Bone Marrow-Derived Mesenchymal Cells in Children with Osteogenesis Imperfecta. Nat. Med. 1999, 5 (3), 309–313. 10.1038/6529. [DOI] [PubMed] [Google Scholar]
- Kattman S. J.; Witty A. D.; Gagliardi M.; Dubois N. C.; Niapour M.; Hotta A.; Ellis J.; Keller G. Stage-Specific Optimization of Activin/Nodal and BMP Signaling Promotes Cardiac Differentiation of Mouse and Human Pluripotent Stem Cell Lines. Cell Stem Cell 2011, 8 (2), 228–240. 10.1016/j.stem.2010.12.008. [DOI] [PubMed] [Google Scholar]
- Lian X.; Hsiao C.; Wilson G.; Zhu K.; Hazeltine L. B.; Azarin S. M.; Raval K. K.; Zhang J.; Kamp T. J.; Palecek S. P. Robust Cardiomyocyte Differentiation from Human Pluripotent Stem Cells via Temporal Modulation of Canonical Wnt Signaling. Proc. Natl. Acad. Sci. U. S. A. 2012, 10.1073/pnas.1200250109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siller R.; Greenhough S.; Naumovska E.; Sullivan G. J. Small-Molecule-Driven Hepatocyte Differentiation of Human Pluripotent Stem Cells. Stem Cell Reports 2015, 4 (5), 939–952. 10.1016/j.stemcr.2015.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Y.-m.; Zhuansun Y.-x.; Chen R.; Lin L.; Lin Y.; Li J.-g. Mesenchymal Stem Cell Exosomes Promote Immunosuppression of Regulatory T Cells in Asthma. Exp. Cell Res. 2018, 363 (1), 114–120. 10.1016/j.yexcr.2017.12.021. [DOI] [PubMed] [Google Scholar]
- Hong Y. J.; Do J. T. Neural Lineage Differentiation From Pluripotent Stem Cells to Mimic Human Brain Tissues. Front. Bioeng. Biotechnol. 2019, 7, 1–17. 10.3389/fbioe.2019.00400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galiakberova A. A.; Dashinimaev E. B. Neural Stem Cells and Methods for Their Generation From Induced Pluripotent Stem Cells in Vitro. Front. Cell Dev. Biol. 2020, 10.3389/fcell.2020.00815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruotti J.; Sripathi S. R.; Bharti K.; Fuller J.; Wahlin K. J.; Ranganathan V.; Sluch V. M.; Berlinicke C. A.; Davis J.; Kim C.; Zhao L.; Wan J.; Qian J.; Corneo B.; Temple S.; Dubey R.; Olenyuk B. Z.; Bhutto I.; Lutty G. A.; Zack D. J. Small-Molecule-Directed, Efficient Generation of Retinal Pigment Epithelium from Human Pluripotent Stem Cells. Proc. Natl. Acad. Sci. U. S. A. 2015, 112 (35), 10950–10955. 10.1073/pnas.1422818112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novoseletskaya E.; Grigorieva O.; Nimiritsky P.; Basalova N.; Eremichev R.; Milovskaya I.; Kulebyakin K.; Kulebyakina M.; Rodionov S.; Omelyanenko N.; Efimenko A. Mesenchymal Stromal Cell-Produced Components of Extracellular Matrix Potentiate Multipotent Stem Cell Response to Differentiation Stimuli. Front. Cell Dev. Biol. 2020, 10.3389/fcell.2020.555378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han F.; Bi J.; Qiao L.; Arancio O. Stem Cell Therapy for Alzheimer’s Disease. Adv. Exp. Med. Biol. 2020, 1266, 39–55. 10.1007/978-981-15-4370-8_4. [DOI] [PubMed] [Google Scholar]
- Ahmed M.; ffrench-Constant C. Extracellular Matrix Regulation of Stem Cell Behavior. Curr. Stem Cell Reports 2016, 2 (3), 197–206. 10.1007/s40778-016-0056-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cramer M. C.; Badylak S. F. Extracellular Matrix-Based Biomaterials and Their Influence Upon Cell Behavior. Ann. Biomed. Eng. 2020, 48 (7), 2132–2153. 10.1007/s10439-019-02408-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agmon G.; Christman K. L. Controlling Stem Cell Behavior with Decellularized Extracellular Matrix Scaffolds. Curr. Opin. Solid State Mater. Sci. 2016, 20 (4), 193–201. 10.1016/j.cossms.2016.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillebrandt K. H.; Everwien H.; Haep N.; Keshi E.; Pratschke J.; Sauer I. M. Strategies Based on Organ Decellularization and Recellularization. Transpl. Int. 2019, 32 (6), 571–585. 10.1111/tri.13462. [DOI] [PubMed] [Google Scholar]
- Bejleri D.; Davis M. E. Decellularized Extracellular Matrix Materials for Cardiac Repair and Regeneration. Adv. Healthc. Mater. 2019, 8 (5), 1801217. 10.1002/adhm.201801217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida A.; Lira R.; Oliveira M.; Martins M.; Azevedo Y.; Silva K. R.; Carvalho S.; Cortez E.; Stumbo A. C.; Carvalho L.; Thole A. Bone marrow-derived mesenchymal stem cells transplantation ameliorates renal injury through anti-fibrotic and anti-inflammatory effects in chronic experimental renovascular disease. Biomed J. 2022, 45 (4), 629–641. 10.1016/j.bj.2021.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang K. W.; Tu T. W.; Nagle M. E.; Lewis B. K.; Burks S. R.; Frank J. A. Molecular and Histological Effects of MR-Guided Pulsed Focused Ultrasound to the Rat Heart. J. Transl. Med. 2017, 15 (1), 1–12. 10.1186/s12967-017-1361-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu G.; Zheng G.; Ge M.; Wang J.; Huang R.; Shu Q.; Xu J. Mesenchymal Stem Cell-Derived Extracellular Vesicles Affect Disease Outcomes via Transfer of MicroRNAs. Stem Cell Res. Ther. 2018, 9 (1), 1–9. 10.1186/s13287-018-1069-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaggi G.; Di Credico A.; Izzicupo P.; Antonucci I.; Crescioli C.; Di Giacomo V.; Di Ruscio A.; Amabile G.; Alviano F.; Di Baldassarre A.; Ghinassi B. Epigenetic Features of Human Perinatal Stem Cells Redefine Their Stemness Potential. Cells 2020, 9 (5), 1304. 10.3390/cells9051304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liguori G. R.; Liguori T. T. A.; de Moraes S. R.; Sinkunas V.; Terlizzi V.; van Dongen J. A.; Sharma P. K.; Moreira L. F. P.; Harmsen M. C. Molecular and Biomechanical Clues From Cardiac Tissue Decellularized Extracellular Matrix Drive Stromal Cell Plasticity. Front. Bioeng. Biotechnol. 2020, 10.3389/fbioe.2020.00520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu T. Y.; Lin B.; Kim J.; Sullivan M.; Tobita K.; Salama G.; Yang L. Repopulation of Decellularized Mouse Heart with Human Induced Pluripotent Stem Cell-Derived Cardiovascular Progenitor Cells. Nat. Commun. 2013, 4, 1–11. 10.1038/ncomms3307. [DOI] [PubMed] [Google Scholar]
- Chamberland C.; Martinez-Fernandez A.; Beraldi R.; Nelson T. J. Embryonic Decellularized Cardiac Scaffold Supports Embryonic Stem Cell Differentiation to Produce Beating Cardiac Tissue. ISRN Stem Cells 2014, 2014, 1–10. 10.1155/2014/625164. [DOI] [Google Scholar]
- Nguyen Q. H.; Pervolarakis N.; Blake K.; Ma D.; Davis R. T.; James N.; Phung A. T.; Willey E.; Kumar R.; Jabart E.; Driver I.; Rock J.; Goga A.; Khan S. A.; Lawson D. A.; Werb Z.; Kessenbrock K. Profiling Human Breast Epithelial Cells Using Single Cell RNA Sequencing Identifies Cell Diversity. Nat. Commun. 2018, 9 (1), 1–12. 10.1038/s41467-018-04334-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyette J. P.; Charest J. M.; Mills R. W.; Jank B. J.; Moser P. T.; Gilpin S. E.; Gershlak J. R.; Okamoto T.; Gonzalez G.; Milan D. J.; Gaudette G. R.; Ott H. C. Bioengineering Human Myocardium on Native Extracellular Matrix. Circ. Res. 2016, 118 (1), 56–72. 10.1161/CIRCRESAHA.115.306874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng S. L. J.; Narayanan K.; Gao S.; Wan A. C. A. Lineage Restricted Progenitors for the Repopulation of Decellularized Heart. Biomaterials 2011, 32 (30), 7571–7580. 10.1016/j.biomaterials.2011.06.065. [DOI] [PubMed] [Google Scholar]
- Hochman-mendez C.; Mesquita F. C. P.; Morrissey J.; Da Costa E. C.; Hulsmann J.; Tang-Quan K.; Xi Y.; Lee P. F.; Sampaio L. C.; Taylor D. A. Restoring anatomical complexity of a left ventricle wall as a step toward bioengineering a human heart with human induced pluripotent stem cell-derived cardiac cells. Acta Biomater 2022, 141, 48–58. 10.1016/j.actbio.2021.12.016. [DOI] [PubMed] [Google Scholar]
- Ciampi O.; Bonandrini B.; Derosas M.; Conti S.; Rizzo P.; Benedetti V.; Figliuzzi M.; Remuzzi A.; Benigni A.; Remuzzi G.; Tomasoni S. Engineering the Vasculature of Decellularized Rat Kidney Scaffolds Using Human Induced Pluripotent Stem Cell-Derived Endothelial Cells. Sci. Rep. 2019, 9 (1), 1–10. 10.1038/s41598-019-44393-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi H.; Karanth S. S.; Ye K.; Stein R.; Jin S. Decellularized Tissue Matrix Enhances Self-Assembly of Islet Organoids from Pluripotent Stem Cell Differentiation. ACS Biomater. Sci. Eng. 2020, 6 (7), 4155–4165. 10.1021/acsbiomaterials.0c00088. [DOI] [PubMed] [Google Scholar]
- Maqueda M.; Mosquera J. L.; García-Arumí J.; Veiga A.; Duarri A. Repopulation of Decellularized Retinas with HiPSC-Derived Retinal Pigment Epithelial and Ocular Progenitor Cells Shows Cell Engraftment, Organization and Differentiation. Biomaterials 2021, 276, 121049. 10.1016/j.biomaterials.2021.121049. [DOI] [PubMed] [Google Scholar]
- Caires-Júnior L. C.; Goulart E.; Telles-Silva K. A.; Araujo B. H. S.; Musso C. M.; Kobayashi G.; Oliveira D.; Assoni A.; Carvalho V. M.; Ribeiro-Jr A. F.; Ishiba R.; Braga K. A. O.; Nepomuceno N.; Caldini E.; Rangel T.; Raia S.; Lelkes P. I.; Zatz M. Pre-Coating Decellularized Liver with HepG2-Conditioned Medium Improves Hepatic Recellularization. Mater. Sci. Eng., C 2021, 121, 111862. 10.1016/j.msec.2020.111862. [DOI] [PubMed] [Google Scholar]
- Kehtari M.; Zeynali B.; Soleimani M.; Kabiri M.; Seyedjafari E. Fabrication of a Co-Culture Micro-Bioreactor Device for Efficient Hepatic Differentiation of Human Induced Pluripotent Stem Cells (HiPSCs). Artif. Cells, Nanomedicine Biotechnol 2018, 46 (sup2), 161–170. 10.1080/21691401.2018.1452753. [DOI] [PubMed] [Google Scholar]
- Mao Q.; Wang Y.; Li Y.; Juengpanich S.; Li W.; Chen M.; Yin J.; Fu J.; Cai X. Fabrication of Liver Microtissue with Liver Decellularized Extracellular Matrix (DECM) Bioink by Digital Light Processing (DLP) Bioprinting. Mater. Sci. Eng., C 2020, 109, 110625. 10.1016/j.msec.2020.110625. [DOI] [PubMed] [Google Scholar]
- Lai Y. F.; Lin T. Y.; Ho P. K.; Chen Y. H.; Huang Y. C.; Lu D. W. Erythropoietin in Optic Neuropathies: Current Future Strategies for Optic Nerve Protection and Repair. Int. J. Mol. Sci. 2022, 23 (13), 7143. 10.3390/ijms23137143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon O.; Kim T. H.; Alsberg E. Reversible Dynamic Mechanics of Hydrogels for Regulation of Cellular Behavior. Acta Biomater 2021, 136, 88–98. 10.1016/j.actbio.2021.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattabhi S. R.; Lehaf A. M.; Schlenoff J. B.; Keller T. C. S. Human Mesenchymal Stem Cell Osteoblast Differentiation, ECM Deposition, and Biomineralization on PAH/PAA Polyelectrolyte Multilayers. J. Biomed. Mater. Res. - Part A 2015, 103 (5), 1818–1827. 10.1002/jbm.a.35322. [DOI] [PubMed] [Google Scholar]
- Lee E.; Milan A.; Urbani L.; De Coppi P.; Lowdell M. W. Decellularized Material as Scaffolds for Tissue Engineering Studies in Long Gap Esophageal Atresia. Expert Opin. Biol. Ther. 2017, 17 (5), 573–584. 10.1080/14712598.2017.1308482. [DOI] [PubMed] [Google Scholar]