Abstract
Objectives:
To use a novel, validated bioassay to monitor serum concentrations of a breakdown product of collagen X in a prospective longitudinal study of patients sustaining isolated tibial plateau fractures. Collagen X is the hallmark extracellular matrix protein present during conversion of soft, cartilaginous callus to bone during endochondral repair. Previous preclinical and clinical studies demonstrated a distinct peak in collagen X biomarker (CXM) bioassay levels after long bone fractures.
Setting:
Level 1 academic trauma facility.
Patients/Participants:
Thirty-six patients; isolated tibial plateau fractures.
Intervention:
(3) Closed treatment, ex-fix (temporizing/definitive), and open reduction internal fixation.
Main Outcome Measurements:
Collagen X serum biomarker levels (CXM bioassay).
Results:
Twenty-two men and 14 women (average age: 46.3 y; 22.6–73.4, SD 13.3) enrolled (16 unicondylar and 20 bicondylar fractures). Twenty-five patients (72.2%) were treated operatively, including 12 (33.3%) provisionally or definitively treated by exfix. No difference was found in peak CXM values between sexes or age. Patients demonstrated peak expression near 1000 pg/mL (average: male—986.5 pg/mL, SD 369; female—953.2 pg/mL, SD 576). There was no difference in peak CXM by treatment protocol, external fixator use, or fracture severity (Schatzker). Patients treated with external fixation (P = 0.05) or staged open reduction internal fixation (P = 0.046) critically demonstrated delayed peaks.
Conclusions:
Pilot analysis demonstrates a strong CXM peak after fractures commensurate with previous preclinical and clinical studies, which was delayed with staged fixation. This may represent the consequence of delayed construct loading. Further validation requires larger cohorts and long-term follow-up. Collagen X may provide an opportunity to support prospective interventional studies testing novel orthobiologics or fixation techniques.
Keywords: biomarkers, fracture healing, collagen X, tibial plateau
INTRODUCTION
All primary clinical methods currently used to monitor fracture healing are qualitative in nature. Fracture healing is typically observed through serial radiographs that, in the case of long bone fractures, can be scored using the modified Radiographic Union Score for Tibial Fractures (mRUST) method.1–3 Radiographs are complimented with subjective physical examinations, clinical experience, and visual comparisons between serial radiographs to determine whether fracture healing matches clinical expectations. In tibial plateau fractures, radiographs can be a poor indicator of healing, and the mRUST scoring method is not validated for use in metaphyseal injuries.4 A further limitation of the mRUST method is that the peak signal occurs late in the healing process, limiting its utility for early predictions. With progressively complex periarticular reconstructions, the entirety of the osteosynthesis construct frequently obscures the metaphysis and interval healing is not visualizable.5 Given that there are no validated quantitative methods to monitor tibial plateau fracture healing, there is an unmet clinical need to find novel technologies that can complement standard radiographs.
The introduction of such a method to measure fracture healing activity at a biological level would be a substantial advancement and could allow clinicians to personalize postfracture care and identify delayed healing or nonunion at early time points in care. In addition, a quantitative method could give clarity to interventional questions, such as the appropriate time to weight-bearing or the consequence of medications such as non-steroidal antiinflammatories (NSAIDs). Biomarkers used to date in fracture healing have primarily measured bone turnover markers (BTM) and are therefore considered later indicators of healing consistent with radiographic evidence of bone mineralization.6,7 Importantly, no studies to date have investigated BTMs after tibial plateau fractures. The ability of established BTMs to monitor fracture healing in diaphyseal fractures remains uncertain.8,9
In this study, we aimed to test the novel collagen X (ColX) biomarker (CXM) associated with the transient cartilaginous callus tissue present in fracture healing by endochondral ossification. The use of this biomarker is prompted by studies demonstrating that fractures heal through a mixture of endochondral (indirect) and intramembranous (direct) bone formation.10–13 The relative contribution of these 2 repair processes is likely modulated by the strain experienced within the fracture field. We have previously validated the biomarker through preclinical work, demonstrating that the marker is measurable in serum during long bone healing.14,15 During endochondral ossification, before conversion to bone tissue, chondrocytes within the soft callus undergo hypertrophic maturation and synthesize a provisional collagen X–rich extracellular matrix. This provisional matrix catalyzes bone formation by promoting matrix mineralization and angiogenesis such that the cartilage callus plays a critical role in vascularized bone regeneration.16–20 The hypertrophic cartilaginous phase in fracture healing is transient, and the breakdown product of the homotrimeric ColX protein is detectable in serum using a sandwich ELISA-based assay (CXM) reactive to the intact trimeric noncollagenous 1 domain of ColX. The CXM assay was developed and validated as a measurement of skeletal growth velocities in children because growth plate activity requires expansion of the cartilaginous region.15 This first publication included a 3-patient case series demonstrating that the CXM bioassay also displayed distinctive peaks during the endochondral phase of long bone fracture repair.15 More recently, we have rigorously studied the CXM biomarker in a murine animal model of long bone fracture healing, validating that serum levels of the CXM biomarker followed expected patterns of col10 gene and protein expression, consistently tracking with quantitative histomorphometric advancement of healing.21
Importantly, ColX is not normally expressed outside of the processes of limb growth and fracture healing. Baseline adult measurements are minimal.15 Tibial plateau fractures are generally fixed with both absolute and relative stability concepts. Although the articular surface is treated with absolute stability goals and perhaps therefore intramembranous healing, we hypothesize that most plateau fractures heal in the metaphysis through large contributions from endochondral ossification. There are a wide range of treatments for plateau fractures, including nonoperative management, external fixation, primary open reduction internal fixation (ORIF), and staged fixation. Unicondylar and bicondylar fractures are addressed with different protocols resulting in differential durations of weight-bearing restrictions based on the injury pattern, surgeon preference, and patient adherence.22 This produces an opportunity for detection and observation of differential CXM expression in a clinical cohort across varied strain microenvironments.
Therefore, the purpose of this study was to prospectively measure the CXM expression profile present in a cohort of patients sustaining tibial plateau fractures. We sought to understand the magnitude and timing of the CXM spike and to investigate fundamental differences in CXM expression as a function of age, sex, or basic fixation protocol during tibial plateau fracture healing. We hypothesized that age and sex would not demonstrate differences in expression within comparable treatment paradigms. The literature provides clear evidence that strain modulation affects fracture healing and callus formation.23,24 As such, we further hypothesized that the addition of external or internal fixation would change the strain environment and CXM response.
MATERIALS AND METHODS
Definitions
Collagen X: Spoken “Collagen type ten.” Refers to the endogenous matrix protein. Abbreviated “ColX.”
CXM: Spoken “C-X-M.” Refers to the results of the ELISA-based biomarker test, quantifying a breakdown product in serum of collagen X.
Patient Care and Exclusion
Institutional review board’s review and approval was obtained at all sites. All patients (2 Level 1 academic trauma centers, Level 3 trauma hospital, 2019–2021) sustaining isolated tibial plateau fractures presenting within 20 days of injury were offered inclusion. Standard of care was delivered by treatment teams independent of the research effort. Treatment strategies included (1) nonoperative management (“non-op”), (2) immediate open reduction internal fixation (“immediate ORIF”), and (3) staged open reduction internal fixation (“staged ORIF”). Patients were excluded for other acute concomitant fractures, prior fractures or major orthopaedic surgery within 12 months, pathologic fracture, genetic diseases of cartilage development/growth/metabolism, and known benign or malignant chondroid processes.
Collagen X Sampling, ELISA, and CXM
Patients gave blood samples at first presentation and at standard follow-ups (3, 6, and 12 weeks postoperatively and all further visits). Blood was captured using Whatman 903 Protein saver cards, creating dried blood spots (DBS) by lancet finger pricks. Protein cards were stored in a −20°C freezer until assay. Human DBS were sampled (3.1 mm punch in duplicate, 250 μL of sample diluent, and extracted overnight at 4°C), before assay per previously published procedure,21 resulting in measurements in pg/mL. Biomarker performance metrics were measured including each patient’s peak CXM value, the time-to-peak CXM, and “ΔCXM,” which was defined as the peak CXM subtracted from their initial “injury” CXM value.
Patient and Injury Details
Demographic information and clinical details (mechanism, injury and surgery dates, operative notes, and radiographs) were collected. Injury mechanism was classified by previous methodology25,26 into bins for suspected high-energy and low-energy groups.
Statistical Analysis
Summary statistics and frequencies were calculated (Stata) for demographics and each experimental outcome. All data were plotted in Prism GraphPad v8.2.1 with each individual data point representing an enrolled patient. Statistical calculations were performed for subgroup analysis using paired and unpaired t tests with Welch correction and Pearson correlations (P < 0.05). One-way Brown–Forsythe analyses of variance with Welch corrections were performed for 3 or more group comparisons. Linear regressions or a cubic spline curve (5 knots, smoothing fit) were computed for all scatter plots.
RESULTS
Thirty-six patients with tibial plateau fractures and longitudinal collagen X biomarker measurements were enrolled (patient demographics: Table 1). Twenty-two patients (61.1%) were male and 14 (38.9%) were female, with an average age of 46.3 years (range 22.6–73.4; SD 13.3). Twenty-six patients (72.2%) were treated operatively, including 12 (33.3%) provisionally or definitely treated with external fixation. Fracture severity was grouped using the Schatzker classification (16 unicondylar and 20 bicondylar fractures). An XY scatter of all individual CXM values in time shows highest values tend to fall between 20 and 60 days (Fig. 1A), with median time-to-peak CXM at 30.5 days (Fig. 1B).
TABLE 1.
Patient Demographics and Injury Patterns
| Unicondylar (n = 16) |
Bicondylar (n = 20) |
Total (n = 36) |
|
|---|---|---|---|
| N (%) | N (%) | N (%) | |
|
| |||
| Male | 10 (62.5) | 12 (60.0) | 22 (61.1) |
| Female | 6 (37.5) | 8 (40.0) | 14 (38.9) |
| Average age (range) | 46.1 (23.8–72.1) | 48.2 (22.6–73.4) | 46.3 (22.6–73.4) |
| BMI (range) | 28.8 (21.3–40.4) | 30.9 (16.1–48.3) | 29.9 (16.1–48.3) |
| Schatzker classification | |||
| I | 4 | ||
| II | 6 | ||
| III | 1 | ||
| IV | 6 | ||
| VI | 19 | ||
| Management | |||
| Operative | 8 (50) | 18 (84.2) | 26 (72.2) |
| Nonoperative | 8 (50) | 2 (15.8) | 10 (27.8) |
FIGURE 1.
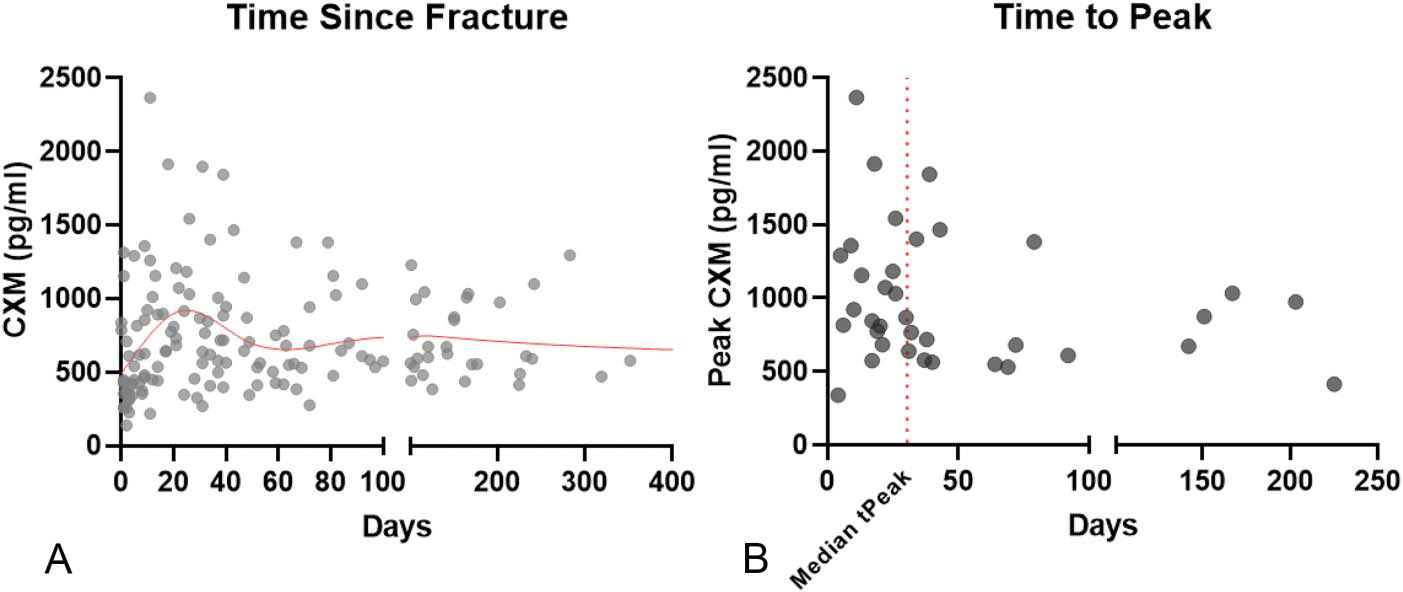
Overall CXM values suggest the median time to peak at 31-day postinjury. A, Individual CXM levels (pg/mL) with a cubic spline curve fit (red line) over time suggest a peak followed by a resolution back toward the baseline. Each dot represents an individual patient value. B, Time-to-peak CXM value for each patient. Median time to peak (tpeak) = 30.5 days (red line).
CXM values were segregated by sex. Both men and women demonstrated peak expression near 1000 pg/mL (average peak: male—986.5 pg/mL, SD 369; female—953.2 pg/mL, SD 576; Fig. 2A, P = 0.849). Neither time-to-peak CXM (Fig. 2E, P = 0.726) nor ΔCXM (P = 0.867) values were different by sex. Smoking and diabetes were tested using unpaired t tests with the Welch correction. There was no difference in peak CXM, regardless of smoking (Fig. 2B, P = 0.886) or diabetic status (Fig. 2C, P = 0.655). Time-to-peak CXM by smoking (Fig. 2F, P = 0.056) approached significance, whereas time-to-peak CXM by diabetes did not (Fig. 2G, P = 0.376). CXM values were tested against age. No correlation was demonstrated between age and CXM behavior, including peak CXM (Fig. 2D, P = 0.652), time-to-peak CXM (Fig. 2H, P = 0.560), or ΔCXM (not shown, P = 0.564).
FIGURE 2.
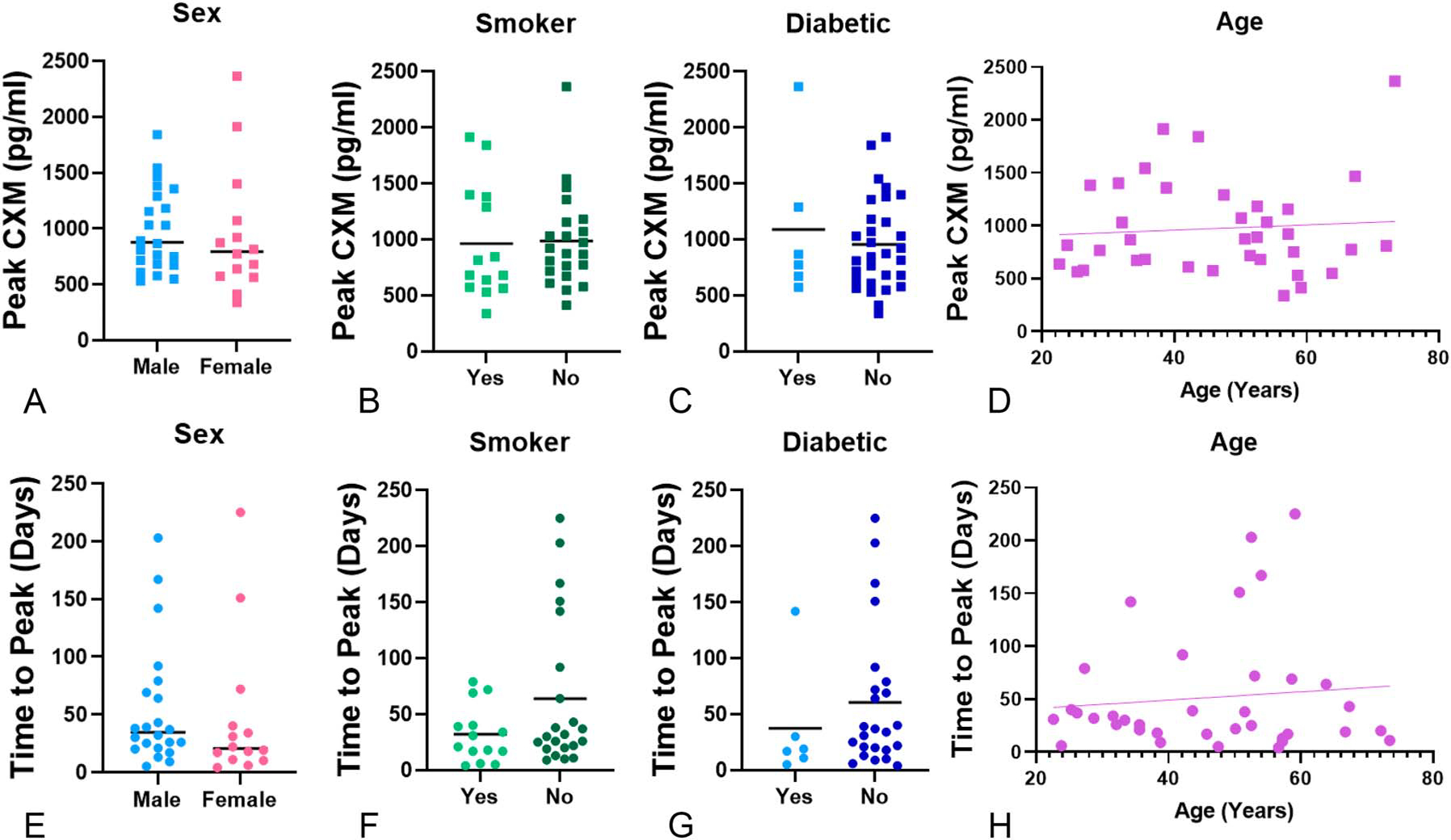
CXM values do not segregate by patient demographics. There was no difference based on sex and (A) peak CXM (P = 0.849) or (E) time-to-peak CXM (P = 0.726). Graphs represent individual CXM values for men (N = 22) versus women (N = 14). There was no difference between smoking status and (B) peak CXM (P = 0.886); however, (F) time-to-peak CXM (P = 0.056) neared significance. Diabetic status (C), peak CXM (P = 0.655), and (G) time-to-peak CXM (P = 0.376) showed no significance. Similarly, there was no correlation between age and (D) peak CXM value (P = 0.652) or (H) time-to-peak CXM (P = 0.560).
There was no difference in peak CXM (Fig. 3A, P = 0.271) or time-to-peak CXM (Fig. 3B, P = 0.404) by fracture severity (unicondylar vs. bicondylar). Mechanism of injury (low vs. high) demonstrated no difference in peak CXM (Fig. 3C, P = 0.217) or time-to-peak CXM (Fig. 3D, P = 0.527).
FIGURE 3.
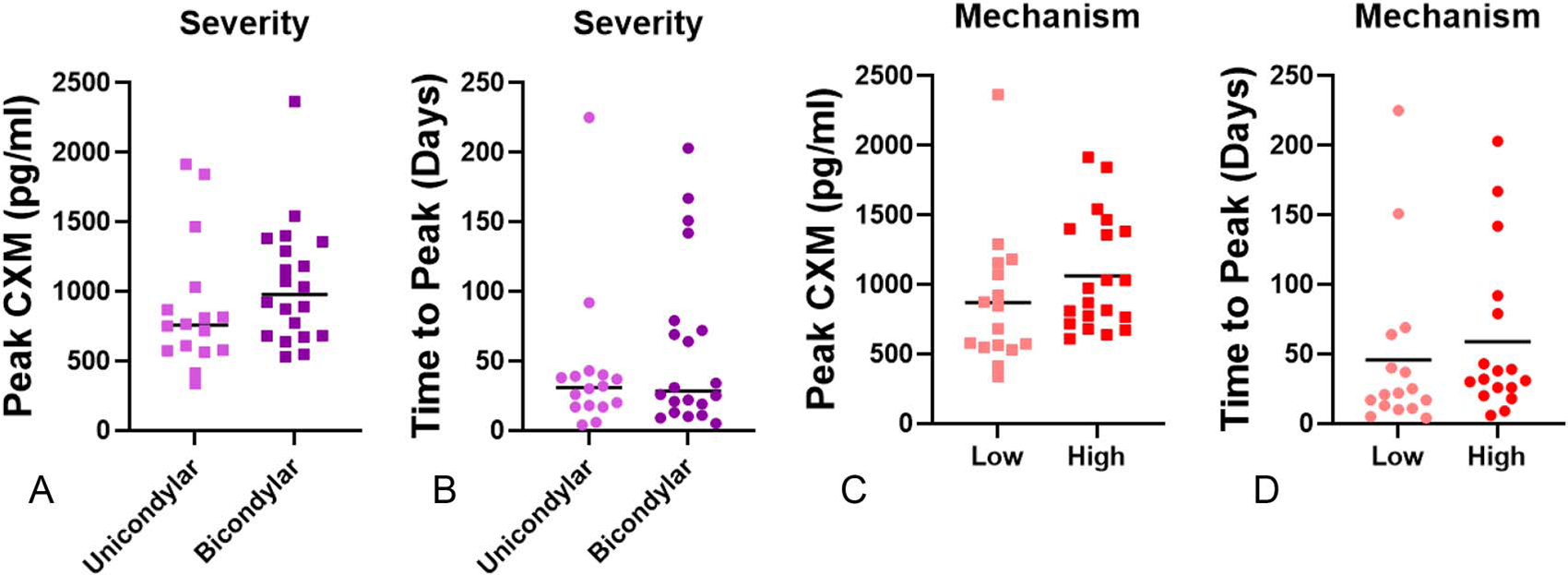
Injury severity and mechanism have no effect on CXM peak or time-to-peak CXM. Unicondylar and bicondylar injuries demonstrated no difference in (A) peak CXM (P = 0.271) or (B) time-to-peak CXM (P = 0.404). Mechanism of the injury demonstrated no significant difference for (C) peak CXM (P = 0.217) or (D) time-to-peak CXM (P = 0.527).
The use of external fixation yielded no significant difference in peak CXM (Fig. 4A, P = 0.843), but time-to-peak CXM was significantly delayed with the use of external fixators (Fig. 4E; 37 days without and 90 days with, P = 0.050). No differences were found in peak CXM values and treatment protocol (Fig. 4B, P = 0.844). However, the time-to-peak CXM was significantly delayed in patients receiving staged ORIF (32 days with nonoperative care, 28 days with immediate ORIF, and 90 days with staged ORIF; Fig. 4F; F2,19 = 3.66, P = 0.0458). There was no correlation between the time to definitive surgery and either peak CXM (Fig. 4C, P = 0.855) or time-to-peak CXM (Fig. 4G, P = 0.305). Time to weight-bearing correlated with peak CXM for all patients although not significant (Fig. 4D, P = 0.134). Time to weight-bearing was strongly correlated with time-to-peak CXM (Fig. 4H, P = 0.0089), demonstrating later peaks with later clearance for weight-bearing. Zero patients experienced a loss of reduction or failure of hardware during the clinical follow-up.
FIGURE 4.

Staged ORIF delays time to CXM peak. The use of external fixators did not affect (A) the peak CXM value (P = 0.843) but did delay (E) time to peak (P = 0.050). Surgical treatment (nonoperational, immediate ORIF, and staged ORIF) had no difference between groups for (B) peak CXM (P = 0.844), although it did show significance between groups for (F) time-to-peak CXM (P = 0.0458). Of those who received surgical intervention, time to definitive surgery showed no correlation between (C) peak CXM (P = 0.855) and (G) time-to-peak CXM (P = 0.305). Similarly, there was no correlation in time to weight-bearing (D) peak CXM (P = 0.134), but there was a significant correlation between time to weight-bearing and (H) time to peak (P = 0.0089).
DISCUSSION
This study describes the prospective clinical observation of a circulating collagen X biomarker, analyzed longitudinally in dried blood spots and quantified using a validated CXM protein ELISA,15 in a cohort of patients sustaining tibial plateau fractures. CXM differs from other BTMs used in fracture healing because CXM measures the serum concentration of a collagen X breakdown product created during the intermediate cartilaginous phase in fracture healing. To date, clinical validation of the CXM biomarker has been performed primarily in children, proving equivalence to height growth velocity based on collagen X breakdown activity in growth plates. In a preclinical murine tibia fracture model, we have characterized the temporal expression pattern of collagen X and the CXM biomarker during normal fracture repair. We found that col10a gene expression spikes early postfracture and is followed by a commensurate rise in collagen X protein presence in the matrix of the fracture callus. A rise in CXM serum concentration follows, which peaks at 14 days before the process of bone remodeling finishes.21 Similar peaks in CXM have been shown in a pilot human cohort.15,27 However, the heterogenous and nuanced nature of fracture care will likely require investigation at each anatomical fracture site to make comparisons between CXM assays of relevant patients.
The most critical findings from this cohort of tibial plateau patients are that there were no differences in CXM expression based on patient demographics (sex and age). These are fundamental findings which imply, but do not confirm at this early stage, that there may be no quantifiable difference in tibial plateau fracture healing between men and women. Similarly, we observed no correlation in the peak CXM value between young and old across a well-distributed age span from 22 to 73 years. These findings need to be validated in a larger cohort of metaphyseal tibial plateau fractures but suggest the potential power of this biomarker to answer basic questions on the influence of demographics in rate of fracture repair that remain unclear.
The literature is unclear regarding whether sex-based differences in fracture healing exist. Fundamentally different hormonal environments and body sizes could give rise to differential phenotypic healing rates.28–30 Preclinical studies to date have not provided definitive clarity with conflicting results. There are studies demonstrating more rapid bone generation in male rats and mice,31,32 but other studies refute this.33 Deng et al32 found that male mice formed larger bone calluses than female mice during tibial fracture healing because of increased IGF-1 expression, stronger activation of Wnt/β-catenin signaling pathway, and more osteoblasts during callus formation. More recently, Haffner-Luntzer et al34 showed that male mice demonstrated differences in fracture healing, starting with a more prominent cartilaginous callus and ending with higher tissue mineral density and bending stiffness at day 21, attributed mechanistically to greater activation of Wnt/β-catenin signaling. The authors hypothesized that the differences found in fracture healing associated with sex could be attributable to differences in mouse size alone. In our preclinical validation of the CXM biomarker, we did not find significant differences in temporal biomarker expression according to sex but did not quantify functional bone repair using CT or biomechanics.21 The lack of difference by sex of CXM levels in this study would support the idea that differences in endochondral ossification as a function of sex may be small or insignificant. An important difference between clinical and preclinical studies to consider is that it is standard in human treatment to have a period of 6–8 weeks without weight-bearing, whereas small animal preclinical studies allow immediate ambulation of the animals and thus may amplify differences in loading. Sex differences in humans may not be relevant in fractures initially treated with protected modes of weight-bearing. Finally, the preclinical studies investigating sex differences were all based on long bone fractures, which may not match metaphyseal behavior.
The effect of age on fracture healing has a strongly supported mechanistic foundation at the preclinical level, with multiple studies demonstrating delayed healing with increased age associated with increased systemic inflammation and reduced vascularization.35 Lu et al36 demonstrated differences in healing between juvenile (4 weeks old), middle-aged (6 months old), and elderly (18 months old) mice. They concluded that there was a significant change in fracture healing between juvenile and middle-aged animals, with a much smaller decrease in healing from the middle-aged to the elderly mice. This seemed to be due to slower chondrocyte maturation and decreased vascular invasion leading to delayed endochondral repair in older mice. Later, the team investigated vascularization, demonstrating a higher density of blood vessels and greater expression of proangiogenesic factors (hypoxia-inducible factor-1 alpha protein and transcripts of vascular endothelial growth factor) in juvenile mice compared with older-aged animals.37 More recent murine studies build on these initial mechanistic data to show senescent periosteal progenitors and decreased proliferation within fracture callus, and chronic inflammation contribute to delayed fracture healing in aged animals.38–43 Taken together, these data suggest that the elderly may exhibit a baseline level of inflammation (inflamm-aging44) that interferes with the catabolic stages of fracture healing. One of the major challenges, however, of interpreting preclinical research on aging is mapping the mouse to human life span. Our data suggest that older patients trend toward a longer time to peak and difference in CXM (DCXM), but a larger patient cohort will be needed to clarify the impact of age on tibial plateau healing.
Important secondary findings of our research include the delay to peak CXM in patients who received staged ORIF of their plateau fractures relative to nonoperative or immediate ORIF and the delay to peak CXM associated with delayed time to weight-bearing. We hypothesize that the delayed definitive surgery and a prolonged period of non–weight-bearing (up to 8 weeks after definitive fixation) associated with this protocol may limit early strain at the fracture and delay endochondral ossification. This has interesting implications regarding the differential rate of healing between operative and nonoperative fractures, as well as the potential pros and cons of the protocols such as early total care45,46 versus staged periarticular fixation.47,48 Finding appropriately matched control patients with similar fracture patterns may prove difficult, but these data justify additional work to increase the power of this observation in a larger cohort.
Research quantifying fracture healing in human patients is limited fundamentally; no methods exist for direct quantification of biological healing. The excellent multicenter work that yielded first the RUST3 followed by the mRUST scoring system1,2,49,50 is the current standard for fracture healing and is a qualitative scoring system. The mRUST has been adapted into multiple other systems for other anatomic locations (hip51 and humerus52,53 fractures), but this system has not been used successfully or validated for any metaphyseal anatomic location to date. Wojahn et al50 performed an elegant study that characterized the spread of tibial healing performance across the mRUST spectrum, demonstrating remarkably large ranges for time(s) to score(s). A simple interpretation of this study is that tibias demonstrate a wide range of phenotypic healing. A fair criticism of all literature on fracture healing scoring is that none of the analyses have been segmented to consider the effect of age or sex. In many ways, this is remarkable because application to large-scale grant funding is not possible without consideration of these factors, yet the orthopaedic literature remains exclusively focused on parameters such as fracture geometry and size and construct factors. These are all important but are perhaps drowned out in effect size by central and nonmodifiable patient factors. Our current study found no differences in CXM levels as a function of age or sex in patients with tibial plateau fractures.
The strength of our study is the exploration of a unique diagnostic opportunity through quantifying a novel serum biomarker in patients with isolated tibial plateau fractures. No previous investigation exists linking biomarkers to endochondral ossification in a clinical cohort of patients with bone fractures. Weaknesses of the research presented here center around the exploratory and novel nature of the collagen X bioassay. In addition, there is no validated scoring system for metaphyseal healing, and we have no qualitative scores to compare CXM values with tibial plateau fractures. Finally, this work faces a common limitation of biomarker studies in that the resolution of sampling can greatly affect the conclusions. Collagen X may prove to be a superior biomarker based on ease of capture alone; DBS sampling is superior in this regard to serum collection in that it only requires a finger prick rather than a phlebotomist for blood acquisition and has potential applicability for austere environments.
This study represents a first clinical cohort of fractures observed for behavior of CXM levels after an isolated fracture and demonstrates that in vivo CXM levels spike initially and subsequently resolve, which is related to resorption of the cartilage callus and consolidation of the bone matrix. The CXM spike magnitude was not affected by patient age or sex or fracture type. Differences were found within the treatment protocols because staged treatment and delayed weight-bearing resulted in a delayed peak CXM. In the future, we hope to expand on these findings by investigating larger cohorts of other fracture types to uncover granular associations between patient and injury factors and CXM expression.
Acknowledgments
This research was funded by grants from AO Trauma North America, Orthopaedic Research and Education Foundation, the Gerlinger Foundation, the UCSF New Orthopaedic Vision Award, and a philanthropic gift from Donna Giordano.
Footnotes
C. S Bahney discloses an unpaid position on the Board of Directors for Orthopaedic Research Society (ORS), Tissue Engineering and Regenerative Medicine International Society (TERMIS), and the International Section of Fracture Repair (ISFR). Furthermore, C. S. Bahney is a paid employee of the nonprofit Steadman Philippon Research Institute (SPRI). SPRI exercises special care to identify any financial interests or relationships related to research conducted here. During the past calendar year, SPRI has received grant funding or in-kind donations from Arthrex, DJO, MLB, Ossur, Siemens, Smith & Nephew, XTRE, and philanthropy. These funding sources provided no support for the work presented in this manuscript. T. Miclau discloses board or committee positions for the AO Foundation, Inman Abbott Society, International Combined Orthopaedic Research Societies, International Orthopaedic Trauma Association, Orthopaedic Research Society, Orthopaedic Trauma Association, Osteosynthesis and Trauma Care Foundation, and San Francisco General Hospital Foundation. He has received research support from Baxter and is a paid consultant for Arquos, Bone Therapeutics, NXTSENS, Surrozen, and Synthes with stock or stock options at Arquos. None of the paid positions are related to the work presented in this manuscript. R. Coughlan reports patent rights to the CXM biomarker assay technology and a portion of royalties generated through its licensure. He is a paid consultant to Therachon AG and BioMarin Pharmaceutical. None of the paid positions are related to the work presented in this manuscript. The other authors report no conflict of interest.
Level of Evidence: Level II, prospective clinical observational study.
REFERENCES
- 1.Cooke ME, Hussein AI, Lybrand KE, et al. Correlation between RUST assessments of fracture healing to structural and biomechanical properties: RUST correlation to callus properties. J Orthop Res. 2018;36:943–945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Litrenta J, III PT, Mehta S, et al. Determination of radiographic healing: an assessment of consistency using RUST and modified RUST in metadiaphyseal fractures. J Orthop Trauma. 2015;29:512–516. [DOI] [PubMed] [Google Scholar]
- 3.Whelan DB, Bhandari M, Stephen D, et al. Development of the radiographic union score for tibial fractures for the assessment of tibial fracture healing after intramedullary fixation. J Trauma. 2010;68:629–632. [DOI] [PubMed] [Google Scholar]
- 4.Ramoutar DN, Lefaivre K, Broekhuyse H, et al. Mapping recovery in simple and complex tibial plateau fracture fixation. Bone Joint J. 2019;101-B:1009–1014. [DOI] [PubMed] [Google Scholar]
- 5.Barei DP. Functional outcomes of severe bicondylar tibial plateau fractures treated with dual incisions and medial and lateral plates. J Bone Joint Surg Am. 2006;88:1713–1721. [DOI] [PubMed] [Google Scholar]
- 6.Cox G, Einhorn TA, Tzioupis C, et al. Bone-turnover markers in fracture healing. J Bone Joint Surg Br. 2010;92-B:329–334. [DOI] [PubMed] [Google Scholar]
- 7.Sousa CP, Dias IR, Lopez-peña M, et al. Bone turnover markers for early detection of fracture healing disturbances: a review of the scientific literature. An Acad Bras Ciênc. 2015;87:1049–1061. [DOI] [PubMed] [Google Scholar]
- 8.Kumar M, Shelke D, Shah S. Prognostic potential of markers of bone turnover in delayed-healing tibial diaphyseal fractures. Eur J Trauma Emerg Surg. 2019;45:31–38. [DOI] [PubMed] [Google Scholar]
- 9.Sousa CP, Lopez-Peña M, Guzón FM, et al. Evaluation of bone turnover markers and serum minerals variations for predicting fracture healing versus non-union processes in adult sheep as a model for orthopedic research. Injury. 2017;48:1768–1775. [DOI] [PubMed] [Google Scholar]
- 10.Hellwinkel JE, Miclau T, Provencher MT, et al. The life of a fracture: biologic progression, healing gone awry, and evaluation of union. JBJS Rev. 2020;8:e1900221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bahney CS, Zondervan RL, Allison P, et al. Cellular biology of fracture healing. J Orthop Res. 2019;37:35–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Le AX, Miclau T, Hu D, et al. Molecular aspects of healing in stabilized and non-stabilized fractures. J Orthop Res. 2001;19:78–84. [DOI] [PubMed] [Google Scholar]
- 13.Marsell R, Einhorn TA. The biology of fracture healing. Injury. 2011;42:551–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bahney CS, Hu DP, Taylor AJ, et al. Stem cell-derived endochondral cartilage stimulates bone healing by tissue transformation: cartilage transformation stimulates bone healing. J Bone Miner Res. 2014;29:1269–1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Coghlan RF, Oberdorf JA, Sienko S, et al. A degradation fragment of type X collagen is a real-time marker for bone growth velocity. Sci Transl Med. 2017;9:eaan4669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Houben A, Kostanova-Poliakova D, Weissenböck M, et al. β-catenin activity in late hypertrophic chondrocytes locally orchestrates osteoblastogenesis and osteoclastogenesis. Development. 2016;143:3826–3838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gerber HP, Vu TH, Ryan AM, et al. VEGF couples hypertrophic cartilage remodeling, ossification and angiogenesis during endochondral bone formation. Nat Med. 1999;5:623–628. [DOI] [PubMed] [Google Scholar]
- 18.Gerstenfeld LC, Shapiro FD. Expression of bone-specific genes by hypertrophic chondrocytes: implication of the complex functions of the hypertrophic chondrocyte during endochondral bone development. J Cel Biochem. 1996;62:1–9. [DOI] [PubMed] [Google Scholar]
- 19.Kuhlman RE, McNamee MJ. The biochemical importance of the hypertrophic cartilage cell area to enchondral bone formation. J Bone Joint Surg Am. 1970;52:1025–1032. [PubMed] [Google Scholar]
- 20.Lian JB, McKee MD, Todd AM, et al. Induction of bone-related proteins, osteocalcin and osteopontin, and their matrix ultrastructural localization with development of chondrocyte hypertrophy in vitro. J Cel Biochem. 1993;52:206–219. [DOI] [PubMed] [Google Scholar]
- 21.Working ZM, Morris ER, Chang JC, et al. A quantitative serum biomarker of circulating collagen X effectively correlates with endochondral fracture healing. J Orthop Res. 2021;39:53–62. [DOI] [PubMed] [Google Scholar]
- 22.Haller JM, Potter MQ, Kubiak EN. Weight bearing after a periarticular fracture. Orthop Clin North Am. 2013;44:509–519. [DOI] [PubMed] [Google Scholar]
- 23.Glatt V, Samchukov M, Cherkashin A, et al. Reverse dynamization accelerates bone-healing in a large-animal osteotomy model. J Bone Joint Surg Am. 2021;103:257–263. [DOI] [PubMed] [Google Scholar]
- 24.Litrenta J, Tornetta P, Vallier H, et al. Dynamizations and exchanges: success rates and indications. J Orthop Trauma. 2015;29:569–573. [DOI] [PubMed] [Google Scholar]
- 25.Working ZM, Elliott I, Marchand LS, et al. Predictors of amputation in high-energy forefoot and midfoot injuries. Injury. 2017;48:536–541. [DOI] [PubMed] [Google Scholar]
- 26.Marchand LS, Working ZM, Rane AA, et al. Unstable pelvic ring injuries: how soon can patients safely bear weight? J Orthop Trauma. 2019;33:71–77. [DOI] [PubMed] [Google Scholar]
- 27.Working ZM. Collagen X biomarker indicates early healing trajectory in a longitudinal fracture cohort. Presented at: Orthopaedic Research Society annual meeting; February 13, 2021; virtual. [Google Scholar]
- 28.Beil FT, Barvencik F, Gebauer M, et al. Effects of estrogen on fracture healing in mice. J Trauma. 2010;69:1259–1265. [DOI] [PubMed] [Google Scholar]
- 29.Haffner-Luntzer M, Fischer V, Prystaz K, et al. The inflammatory phase of fracture healing is influenced by oestrogen status in mice. Eur J Med Res. 2017;22:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fischer V, Kalbitz M, Müller-Graf F, et al. Influence of menopause on inflammatory cytokines during murine and human bone fracture healing. Int J Mol Sci. 2018;19:2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mehta M, Schell H, Schwarz C, et al. A 5-mm femoral defect in female but not in male rats leads to a reproducible atrophic non-union. Arch Orthop Trauma Surg. 2011;131:121–129. [DOI] [PubMed] [Google Scholar]
- 32.Deng Z, Gao X, Sun X, et al. Gender differences in tibial fractures healing in normal and muscular dystrophic mice. Am J Transl Res. 2020;12:2640–2651. [PMC free article] [PubMed] [Google Scholar]
- 33.Collier CD, Hausman BS, Zulqadar SH, et al. Characterization of a reproducible model of fracture healing in mice using an open femoral osteotomy. Bone Rep. 2020;12:100250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Haffner-Luntzer M, Fischer V, Ignatius A. Differences in fracture healing between female and male C57BL/6J Mice. Front Physiol. 2021;12:712494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Clark D, Nakamura M, Miclau T, et al. Effects of aging on fracture healing. Curr Osteoporos Rep. 2017;15:601–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lu C, Miclau T, Hu D, et al. Cellular basis for age-related changes in fracture repair. J Orthop Res. 2005;23:1300–1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lu C, Hansen E, Sapozhnikova A, et al. Effect of age on vascularization during fracture repair. J Orthop Res. 2008;26:1384–1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Clark D, Brazina S, Yang F, et al. Age-related changes to macrophages are detrimental to fracture healing in mice. Aging Cell. 2020;19:e13112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xing Z, Lu C, Hu D, et al. Rejuvenation of the inflammatory system stimulates fracture repair in aged mice. J Orthop Res. 2010;28:1000–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xing Z, Lu C, Hu D, et al. Multiple roles for CCR2 during fracture healing. Dis Model Mech. 2010;3:451–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Baht GS, Silkstone D, Vi L, et al. Exposure to a youthful circulaton rejuvenates bone repair through modulation of β-catenin. Nat Commun.2015;6:7131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Josephson AM, Bradaschia-Correa V, Lee S, et al. Age-related inflammation triggers skeletal stem/progenitor cell dysfunction. Proc Natl Acad Sci USA. 2019;116:6995–7004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hebb JH, Ashley JW, McDaniel L, et al. Bone healing in an aged murine fracture model is characterized by sustained callus inflammation and decreased cell proliferation. J Orthop Res. 2018;36:149–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Franceschi C, Bonafè M, Valensin S, et al. Inflamm-aging: an evolutionary perspective on immunosenescence. Ann N Y Acad Sci. 2006;908:244–254. [DOI] [PubMed] [Google Scholar]
- 45.Pape HC, Tornetta P, Tarkin I, et al. Timing of fracture fixation in multi-trauma patients: the role of early total care and damage control surgery. J Am Acad Orthop Surg. 2009;17:541–549. [DOI] [PubMed] [Google Scholar]
- 46.Unno F, Lefaivre KA, Osterhoff G, et al. Is early definitive fixation of bicondylar tibial plateau fractures safe? An observational cohort study. J Orthop Trauma. 2017;31:151–157. [DOI] [PubMed] [Google Scholar]
- 47.Tscherne H, Lobenhoffer P. Tibial plateau fractures. Management and expected results. Clin Orthop Relat Res. 1993;292:87–100. [PubMed] [Google Scholar]
- 48.Egol KA, Tejwani NC, Capla EL, et al. Staged management of high-energy proximal tibia fractures (OTA types 41): the results of a prospective, standardized protocol. J Orthop Trauma. 2005;19:448–455; discussion 456. [DOI] [PubMed] [Google Scholar]
- 49.Mitchell SL, Obremskey WT, Luly J, et al. Inter-rater reliability of the modified Radiographic Union Score for Diaphyseal Tibial (mRUST) fractures with bone defects. J Orthop Trauma. 2019;33:301–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wojahn RD, Bechtold D, Abraamyan T, et al. Progression of tibia fracture healing using RUST: are early radiographs helpful? J Orthop Trauma. 2021. [epub ahead of print]. doi: 10.1097/BOT.0000000000002146. [DOI] [PubMed] [Google Scholar]
- 51.Frank T, Osterhoff G, Sprague S, et al. The Radiographic Union Score for Hip (RUSH) identifies radiographic nonunion of femoral neck fractures. Clin Orthop Relat Res. 2016;474:1396–1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Christiano AV, Goch AM, Burke CJ, et al. Radiographic Humerus Union Measurement (RHUM) demonstrates high inter- and intraobserver reliability in assessing humeral shaft fracture healing. HSS J. 2020;16:216–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schneble CA, Li DT, Kahan J, et al. Reliability of radiographic union scoring in humeral shaft fractures. J Orthop Trauma. 2020;34:e437–e441. [DOI] [PubMed] [Google Scholar]


