Abstract
Neural mechanisms of perceptual decision making have been extensively studied in experimental settings that mimic stable environments with repeating stimuli, fixed rules and payoffs. In contrast, we live in an ever-changing environment and have varying goals and behavioral demands. To accommodate variability, our brain flexibly adjusts decision-making processes depending on context. Here, we review a growing body of research that explores the neural mechanisms underlying this flexibility. We highlight diverse forms of context dependency in decision making implemented through a variety of neural computations. Context-dependent neural activity is observed in a distributed network of brain structures, including posterior parietal, sensory, motor, and subcortical regions, as well as the prefrontal areas classically implicated in cognitive control. We propose that investigating the distributed network underlying flexible decisions is key to advancing our understanding and discuss a path forward for experimental and theoretical investigations.
Keywords: sensory-guided behavior, flexible decision-making, task switch, stimulus-action mapping, decision policy, distributed neural networks
1. Introduction
A hallmark of our intelligence is the ability to make appropriate decisions under different circumstances. We gather information from the external world, combine it with internal knowledge, and commit to mental propositions and plans of action — decisions that shape our lives. Among the experimental paradigms developed to uncover the neural mechanisms of decision making, sensory psychophysics has played a key role in shaping our current understanding (1). A common finding has been that many sensory discriminations (e.g., judging the direction of moving dots) are based on the accumulation of sensory evidence toward a decision bound (1, 2, 3). Models built on this key computation have been remarkably successful, offering a unified explanation for various aspects of behavior, including choice, response time, and confidence (4).
However, in the experiments that gave rise to these models, subjects often repeated the same decision-making process in a stationary task environment with stable stimulus-action associations and rules. This stationarity removed the need to determine appropriate stimulus features, actions, and rules for each decision, substantially reducing the complexity of the decision-making process. However, such stationarity rarely happens in our daily lives, where consecutive decisions often differ from each other. Even for the same sensory inputs, our actions can greatly vary depending on context. For example, when we run into a colleague in a hallway, we may stop to chat, but not during a fire drill. Decision making in a non-stationary environment often has a hierarchical structure, where we must first infer the relevant inputs, actions, payoffs, task solutions, and decision policies before acting on the inputs. We can implement these context-dependent hierarchical decisions rapidly and effortlessly, and thus there should be neural mechanisms that flexibly alter behavior without “re-wiring” the decision-making neural circuits. How can decision-making models be extended to account for such context dependency?
A growing body of research has now begun to explore flexible decision-making, but empirical and theoretical results are diverse and often hard to connect. Many studies have examined how decision-making processes unfold under different task rules. Task rules could be modified in a number of ways. For example, one can change relevant sensory features, the association between stimuli and actions, the reward associated with different choices, and so on. Adjustments of behavior to any of these manipulations are attributed to “context-dependent” computations, but the computations could be vastly different and they may not share the same neural underpinnings.
The first section of this review introduces perceptual decisions, where the rich history of past studies and the maturity of existing empirical and theoretical frameworks provide an ideal testbed for investigating context-dependent decisions. After introducing the neural mechanisms of perceptual decisions, we explain the diversity of flexible behavior associated with these decisions. We suggest that past studies tapped into different forms of flexibility, shedding light on the diverse brain regions and neural responses observed in those studies. The next section of the paper focuses on a key observation that, even for a single task, a network of brain regions is engaged during decision making. We contrast these observations to a currently dominant doctrine that the prefrontal cortex operates as a central hub that controls flexible behavior (5, 6). In the last section, we briefly discuss how network models have been designed to implement flexibility.
We end this introduction with two notes to clarify the scope of this review. First, although our review focuses on perceptual decision making, the line is blurred between perceptual decisions and other kinds. For example, experiments on value-based decision making or reinforcement learning typically involve sensory components (e.g., options with different values are presented as visual cues) and use similar task structures (e.g., binary decisions). Furthermore, much of the existing literature on cognitive control uses perceptual tasks such as the Wisconsin Card Sorting Test to examine subjects’ capacity to flexibly switch behavior. We believe our core takeaways apply to those tasks, too, although we do not deeply explore them here. Second, our main interest is in flexible mechanisms that allow rapid behavioral adjustment, but context-dependent behavior can also be studied across blocks, sessions, subjects, or different training levels. By summarizing these broad studies, we will demonstrate the diversity of flexible computations and the distributedness of the neural activity associated with them.
2. Perceptual decision making in a stationary context
Substantial progress has been made in understanding the computational and neural mechanisms of perceptual decision making. For thorough reviews of this progress, see refs. 2, 3, 4. Here we summarize the key findings, focusing on those most relevant to context-dependent decision making.
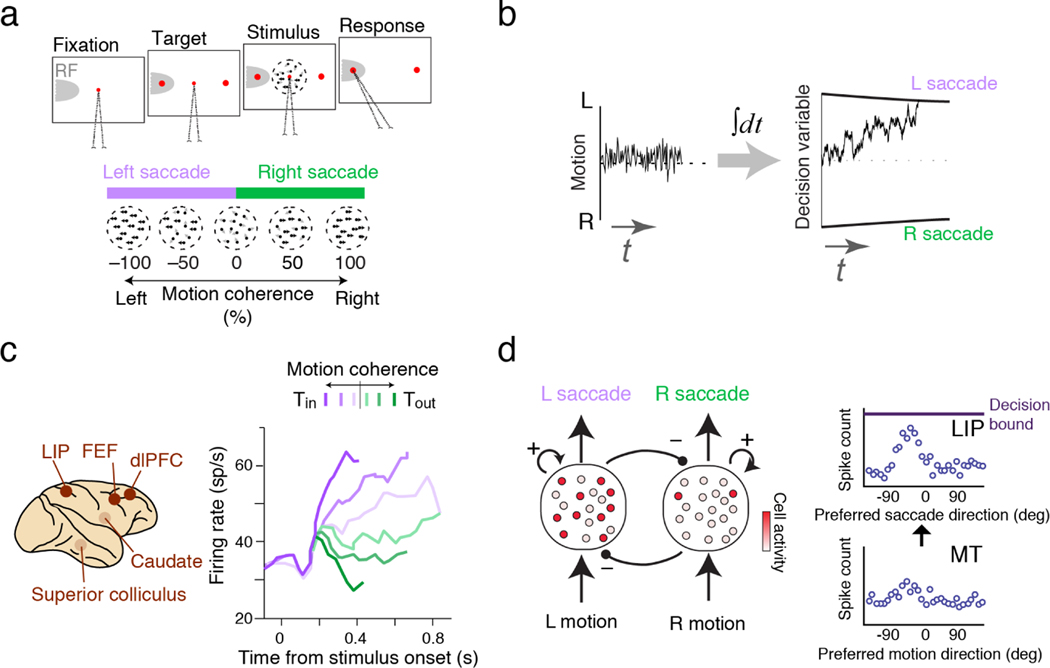
In typical perceptual decision-making tasks, subjects discriminate sensory stimuli (e.g., motion toward right or left), reporting their choice with specific motor actions (e.g., reaching right or left). The stimuli vary across trials, usually along a parametrically controlled dimension, which enables precise manipulation of task difficulty. For example, in the motion direction discrimination task with random dots (Figure 1a), the percentage of dots moving coherently together (motion coherence) determines the difficulty of the trial (7, 8). Motion direction and coherence vary randomly across trials, but subjects typically perform hundreds of similar discriminations consecutively, applying the same computations and task rules on every trial.
Figure 1.
Perceptual decision making in a stationary context. (a) Design of a typical visual discrimination task. Subjects report the net direction of random-dots motion (left or right) by making a saccadic eye movement. The percentage of coherently moving dots varies across trials. Neural recording is typically made from neurons whose response field (RF, gray shading) overlaps with one of the targets. (b) A bounded accumulation model accounts for the behavior. The model accumulates noisy sensory evidence to form a decision variable (DV) and commits to a choice when the DV reaches a bound. (c) Neural activity similar to the DV can be found in diverse brain regions involved in oculomotor control. For example, neurons in LIP increase their firing rates when the stimulus supports a saccade to the target in their RFs. Tin, target in neuron RF; Tout, target outside RF. Right panel, adapted with permission from Reference 8; copyright 2002 Society for Neuroscience. (d) Circuit models. In bistable attractor dynamics models (left; 19, 20), two pools of neurons, each supporting one of the choices, compete until one pool dominates. In probabilistic population codes, networks that integrate neural activity can perform optimal evidence accumulation (right; adapted with permission from Reference 21; copyright 2008 Elsevier). dlPFC, dorsolateral prefrontal cortex; FEF, frontal eye field; LIP, lateral intraparietal area; MT, middle temporal area.
This stationary setting provides ample data to address a key question: how are decisions made in the face of uncertainty about stimulus difficulty and noise in sensory inputs and sensory neural responses? An optimal solution would be to integrate all available sensory information, that is, to accumulate independent pieces of evidence over time for temporally extended stimuli (Figure 1b) and to integrate evidence across space for spatially extended stimuli (e.g., for multi-feature stimuli) (9, 10). When there is cost associated with gathering information (e.g., maintaining fixation or sustaining attention), the reward rate is maximized by continuing the evidence accumulation until a time-dependent decision criterion or bound is reached (11, 12, 13; Figure 1b). This accumulation to bound process can be further adjusted to accommodate factors such as prior probabilities of the stimuli and expected rewards of different choices to make reward-maximizing decisions (14, 15, 16, 17, 18).
Empirical results indicate a close match between the bounded accumulation model and the neural computations that underlie behavior. The model accurately fits and can even predict the distribution of choice, reaction time, and confidence (22, 23, 24, 25). Moreover, dynamics of neural responses in multiple brain regions resemble the evidence accumulation process (8, 26, 27, 28, 29, 30, 31, 32). Shadlen and Newsome were the first to identify neurons in the monkey lateral intraparietal area (LIP) that gradually increase their firing rates when the motion stimulus supports the target in the response field of neurons (33, 34; Figure 1c). Critically, these neural responses reach a common level immediately before the decision, offering a neural signature for committing to a choice through reaching the decision bound (8). Since those first studies, similar neural signatures have been identified in a variety of motor planning regions, including frontal eye fields (FEF) (28), pre-arcuate gyrus (15, 35), dorsolateral prefrontal cortex (dlPFC) (29, 36), caudate (27), superior colliculus (26), and motor cortex (37, 38, 39) (Figure 1c). Further support for the involvement of motor planning regions in bounded accumulation comes from visual search tasks, where subjects find a target in the midst of distractors (40), and from stop signal tasks, where subjects are instructed to generate a motor movement but later cued to withhold the movement in a random portion of trials (41).
Motivated by these empirical results, neural circuit models have been proposed for the implementation of the bounded accumulation process (19, 20, 21, 40, 42, 43, 44, 45). These models typically comprise neural pools that accumulate evidence for their preferred choice through a mixture of self-excitation, mutual inhibition, or feedforward inhibition (Figure 1d). Sensory inputs bias the competition until one pool wins, signifying a commitment to a choice and triggering a behavioral response. Bounded accumulation and its circuit models have also been adopted beyond perceptual tasks, especially for value-based decisions (46, 47) and memory-guided decisions (48, 49).
3. Diversity of flexible decisions and their neural correlates
Making perceptual decisions in realistic, non-stationary environments requires processes beyond those studied in stationary task settings (Figure 2a). Before gathering sensory evidence and using it to form action plans, decision makers must first determine the context and “set up” the decision making process accordingly. They should “decide” what are the relevant sensory information, relevant actions, expected payoffs, fruitful solutions (e.g., integrate or differentiate), and decision policies (e.g., how much evidence to accumulate). This hierarchical decision making structure —making decisions about how to make a decision—enables flexible adjustment of behavior in a manner appropriate for different task contexts. In this section, we summarize recent studies that examined these adjustment mechanisms through comparing behavior and neural responses across multiple tasks.
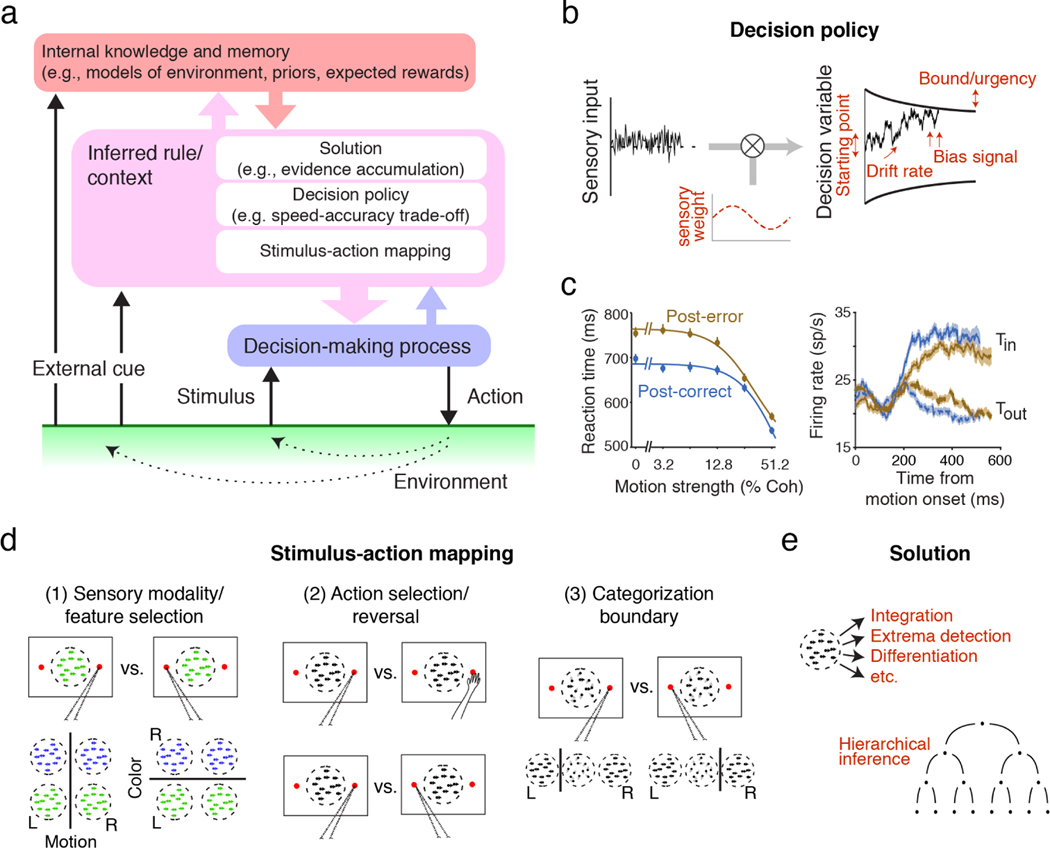
Figure 2.
Diverse forms of context dependency in perceptual decision making. (a) Decision making is hierarchical in nature. Even for the simplest decision, the brain first chooses relevant stimuli, actions, solutions, and policies. (b) Adjustments in decision policy are often explained as changes in the parameters of decision-making models. Adjustable parameters of the bounded accumulation model are shown in red. (c) Post-error slowing is an example of policy adjustment. Slower reaction times after error trials (left panel) are explained by reduced sensory sensitivity and lower urgency. Correspondingly, LIP buildup activity decreases after error trials (right panel). Adapted with permission from Reference 50; copyright 2016 Elsevier. (d) Common task designs that test flexibility in stimulus-action mapping: (1) Change in relevant sensory modality or feature. (2) Change in effectors (e.g., saccade and reach; top) or reversal of stimulus-action mapping (bottom). (3) Change in categorization boundary. (e) Flexibility to adopt different solutions. For example, the same stimulus could be integrated, differentiated, matched to a template, and so on (top). More complex tasks that involve hierarchical inference, offer a rich ground for studying flexibility of strategy (bottom; adapted with permission from Reference 51; copyright 2021 Ariel Zylberberg).
A key challenge in summarizing these studies is the numerous ways that a decision maker can adjust decision-making processes. Different forms of flexibility possibly engage different neural mechanisms, but any change in behavior or neural activity across tasks is labeled as “flexible” or “context-dependent” in the literature. To highlight this diversity, we classify flexibility into three types (Figure 2). At the highest level, a decision maker could be flexible in their solutions for the task. For example, they can choose to integrate, differentiate, or even ignore sensory inputs (“flexibility in task solution”). Second, for a given solution, a decision maker could flexibly adjust the decision policy for a given solution. This includes, for example, how much evidence to accumulate, or whether to favor one option over others (“flexibility in decision policy”). Finally, a decision maker can flexibly associate different sensory inputs to different actions (“flexibility in stimulus-action mapping”).
3.1. Flexibility in decision policy
We first discuss adjustments in decision policy (Figure 2b) as successful demonstrations of how flexible choice behavior could be described mechanistically using the existing theoretical framework for perceptual decision making.
In experimental settings, changes in decision policy can be induced through a variety of task manipulations, including changes in prior probabilities of stimuli (16, 52), costs and rewards associated with each choice (18, 53, 54, 55), variability in stimulus (56), spatiotemporal effects of sensory signals on decisions (57), urgency to respond (58), or even parameters as subtle as the duration of inter-trial intervals (59) and timing of reward delivery (60). Further, the history of choices and feedback influences subsequent decision policies (15, 17, 50, 61). These manipulations—which can happen at various time scales ranging from trial-to-trial to session-to-session—impact different aspects of decisions, including choice biases, accuracy, reaction time, and decision confidence.
Consider the adjustment of decision policy upon receipt of feedback in previous trials. Post-error slowing (PES) is a commonly observed effect in which subjects elongate their reaction times following an error feedback (Figure 2c left). Purcell et al. (50) used evidence accumulation models to identify the neural underpinnings of these behavioral changes. PES in the direction discrimination task (Figure 1a) arises from reduced sensitivity (lower drift rate) and increased decision bound (decreased urgency). Increased bound compensates for reduced sensitivity to maintain the overall accuracy but at the expense of longer reaction times. Consistent with these modeling insights, LIP neural activity following an error shows reduced dependence on stimulus strength, manifested as decreased buildup rates (Figure 2c right) as predicted by lower sensitivity. Further, urgency signals calculated from LIP responses are reduced, indicating an effective increase in decision bound by an amount matching the model predictions. Similarly successful applications of decision-making models can be found in studies of speed-accuracy trade-off (60, 62), prior probability effects (16, 52), and reward bias (53, 54). Thus, the modeling framework developed for a stationary setting naturally extends to certain forms of flexible computations.
The key strength of this approach is a mechanistic understanding through quantitative comparison between models and data in multiple task conditions. For behavioral data, these models explain changes in choice, reaction time, confidence, and psychophysical kernels (63). Remarkably, model parameters could be fit to one aspect of behavior (e.g., reaction time) and then used to generate accurate predictions about the other aspects (e.g., choice) (22, 24, 25, 44). Neural data could also be examined in the same framework. For example, Hanks et al. (60) used neural data to estimate changes in model parameters between two speed-accuracy regimes and then confirmed that the neurally-inferred parameters account for the observed behavioral changes.
Despite the success of this approach, there remain key unanswered questions. Most notable, we do not know what neural mechanisms control the necessity, type and magnitude of policy adjustments. Multiple brain regions have been suggested to play a role in setting the decision bound, most prominently the basal ganglia (58, 64, 54) and possibly the superior colliculus (65). When human subjects are urged to respond quickly, striatum activity is enhanced compared to the condition where accuracy is prioritized (58). Deep brain stimulation of the subthalamic nucleus reduces decision threshold and triggers faster responses (64). Choice biases induced by reward imbalance are also dependent on the striatum. Doi et al. (54) found that the activity of caudate neurons is modulated based on the association between the target in the neuron RF and reward such that the microstimulation of caudate could enhance the monkey’s choice bias. In the cortex, the posterior parietal cortex (PPC) (61) and PFC (15) encode past stimuli and feedback, shaping history-dependent behavioral biases. Network models that consider subcortical structures may give us clues for how decision policy is adjusted in the evidence accumulation circuits (66).
3.2. Flexibility in stimulus-action mapping
At the core of perceptual tasks is the mapping of sensory stimuli onto actions (Figure 2d). However, the flexible stimulus-action mapping is rarely a focus of the existing decision making models, as many of them presume a fixed stimulus-action association and instead focus on the integration of evidence (Figure 1b, d). Is the selection of appropriate mapping for a particular decision independent of the decision formation itself? Empirical findings summarized below suggest that they are rather tightly intermingled in the brain.
3.2.1. Selection of relevant sensory features.
To perform a perceptual task, the decision maker must first decide what sensory information to base the decision on. A common experimental design to understand this process involves comparing neural responses when subjects generate the same set of actions in response to different sensory stimuli (31, 67, 68, 69). For example, Raposo et al. (70) trained rats to choose one of the two nose ports based on either the frequency of visual flashes or the frequency of auditory clicks. Another task variant uses a multi-feature stimulus space and instructs subjects to discriminate the same stimuli based on different features (36, 69, 71, 72, 73, 74, 75, 76) (Figure 2d(1)). For example, Mante et al. (36) trained monkeys to report either the dominant color (red vs. green) or dominant motion (left vs. right) of colorful random dots kinematograms. Because the two sensory features could support opposing choices, subjects should ideally base their decisions only on the relevant stimulus feature in each task.
One important question is whether the selection of sensory features happens independently of the evidence accumulation process. If so, we would expect modality general integration signals that reflect evidence accumulation regardless of the source of information. Such a task-invariant implementation permits identical readouts of the decision variable across tasks. In support of modality-general mechanisms, human electroencephalography (EEG) studies have found neural signals reflecting decision formation across sensory modalities (31, 77, 78). For example, “centroparietal positivity” reflects accumulated sensory evidence for motion discrimination, contrast detection, and auditory detection tasks (31, 77). Further, functional magnetic resonance imaging (fMRI) studies suggest that some regions in the prefrontal and posterior parietal cortex may reflect the decision variable regardless of sensory stimuli (79).
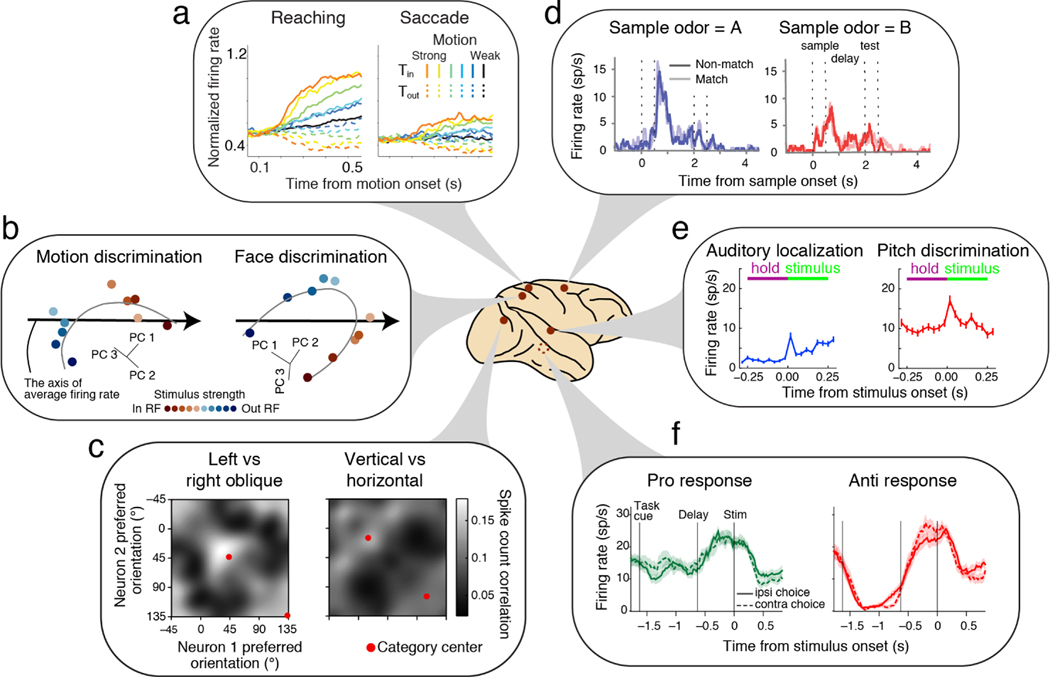
By contrast, electrophysiological studies of spiking activity have found that the encoding of the decision variable is task specific. Okazawa et al. (69) report a qualitative difference in the firing rates of LIP neurons during motion discrimination and face discrimination tasks. During face discrimination, LIP firing rates are lower for the strongest stimuli supporting saccade to the target in the RF, the opposite of the motion discrimination task. The LIP population represents the accumulation of evidence in both tasks. However, neural responses form a curved manifold that rotates and shifts depending on tasks (Figure 3b). The rotation of the manifold occurs even when monkeys switch between categorization of the identity or expression of the same face. An earlier study by Stoet et al. (72) also found that LIP neurons discharged differently for the same reach choice between color and orientation categorizations of the same stimuli.
Figure 3.
Diverse brain regions are implicated in context-dependent decision making. Dark red circles on the brain indicate the site recorded in the study highlighted in each panel. We depicted a monkey brain for illustrative purposes, but several results are from rodents. (a) Medial intraparietal (MIP) neurons encode the decision variable with different strengths when monkeys report their decisions through reaching or eye movements. Adapted with permission from Reference 85; copyright 2015 Society for Neuroscience. (b) Lateral intraparietal (LIP) neurons encode the decision variable for their preferred saccade targets along curved manifolds in population state space that are distinct for motion and face discrimination tasks. Adapted with permission from Reference 69; copyright 2021 Elsevier. (c) Monkey V1 neurons show distinct patterns of noise correlations depending on the category boundary in an orientation discrimination task. Adapted with permission from Reference 106; copyright 2018 Springer Nature. (d) When mice report if two sequentially presented odors (sample and test stimuli) match, premotor (PM) neurons encode sample odor during the delay period before any motor plan can be made. Adapted with permission from Reference 102; copyright 2020 Elsevier. (e) A1 neurons encode task rules before stimulus presentation when rats report either the location or the pitch of the same auditory stimulus. Adapted with permission from Reference 75; copyright 2014 Elsevier. (f) Superior colliculus (SC) neurons encode task rules before stimulus presentation when rats switch between pro (orienting toward a stimulus) and anti (orienting away) stimulus-action associations. Adapted with permission from Reference 97; copyright 2021 Springer Nature.
Mechanistic accounts of such task-specific encoding of the decision variable remain elusive. These variable representations imply that optimal readout of the decision variable has to be task dependent. Curved manifolds appear to be ubiquitous across brain regions including the medial and lateral frontal cortices (69) and likely the basal ganglia (27) and the superior colliculus (26), but task dependence of the manifold should yet be investigated in all these regions. One possibility is that some of these regions, unlike LIP, include task invariant integrator mechanisms. Alternatively, all regions may include task-dependent representations and collectively coordinate decision making through task-dependent readouts. Examination of single-cell activity is needed because EEG and fMRI, which record aggregated activity of brain tissue, may lack enough resolution to separate distinct neural responses within a circuit.
What is the mechanism for the selection of relevant sensory information? It has been long considered that the prefrontal cortex (PFC) is central to flexible sensory selection. For example, Wisconsin Card Sorting Test (WCST) has been used to demonstrate behavioral inflexibility in patients with prefrontal damage (80). Lesion studies in monkeys trained on WCST-analog tasks suggest differential contributions of PFC subregions to the update and maintenance of task rules or evaluation of outcomes (80, 81).
Focusing on selective sensory routing, Mante et al. (36) attempted to provide a mechanistic model. During color and motion discrimination, they found that the same neural population in FEF encodes both color and motion information but changes their response dynamics such that only the task-relevant information is integrated along the choice axis. These response patterns resemble the activity of a recurrent neural network trained to perform the same task. While this is an appealing idea, the selection process may not entirely depend on a local circuitry within the prefrontal cortex. Using a task similar to Mante’s, Siegel et al. (74) showed that task context information is represented not only in PFC but also in LIP and then propagates to visual areas. Kamigaki et al. (82) also showed that neurons in the posterior parietal cortex modulate their firing rates when monkeys switch task rules in a similar experiment. As mentioned above, the encoding of decision variable in motor-planning areas depends on relevant sensory dimensions (69), implying that neural mechanisms for sensory selection and evidence accumulation for action are intermingled.
There is also an important, long-standing question of whether a part of the selection process happens in sensory circuits (75, 83, 84). Attention modulates responses of sensory neurons selective for different locations or features (84). However, the role of attention in gating relevant features in decision making remains debated. Sasaki et al. (83) found virtually no change in MT neural responses when monkeys discriminated the direction or disparity of random dots stimuli. By contrast, Rodgers et al. (75) found large differences in A1 activity when rats switched between auditory localization and pitch discrimination tasks (Figure 3e). Furthermore, selection between visual and auditory signals could be happening at the level of the thalamus (68). Wimmer et al. (68) trained mice to switch between using visual or auditory features of a bi-modal stimulus. Neural activity of the visual thalamic reticular nucleus reflected task contexts, and disruption of activity affected decision making based on the relevant modality. To what extent differences of results across these experiments depend on species or tasks should be determined by future experiments.
In summary, several key questions remain unanswered or only partially answered. While many studies implicate the prefrontal cortex for the selection of sensory features, other cortical and subcortical regions also appear to engage in the process. Moreover, task-specific encoding of the decision variable suggests that sensory selection and accumulation of evidence may not be independent processes. Addressing the outstanding questions would be aided by expanding the existing decision-making models to incorporate flexible sensory selection, which provides a quantitative framework for hypothesis testing in future experiments.
3.2.2. Selection of appropriate motor outputs.
Besides sensory selection, the decision maker should also choose appropriate motor actions. As in sensory selection, a core question is whether the brain uses the same accumulators regardless of motor actions. This question can be answered with tasks in which subjects report the outcome of the same sensory discrimination through different motor actions (78, 79, 85, 86, 87). For example, de Lafuente et al. (85) trained monkeys to report the direction of random dots either with a saccadic eye movement or by reaching to one of the two targets (Figure 2d(2) top).
Comparison of neural responses across different actions has demonstrated specificity for action modality. As explained earlier, the discovery of evidence accumulation signals in motor planning brain areas (Figure 1c) supports an “intentional framework” in which potential motor intentions compete to form a decision (88, 89, 90). Consistent with this framework, when monkeys reported their choices with reaching movements, the medial intraparietal (MIP) area —a reach-related region of the parietal cortex— strongly reflects decision formation, whereas in the saccade task, MIP neural responses attenuate (85; Figure 3a). Overall, the neural populations reflecting decision formation and motor planning are not fully aligned in the neural population state space (69), but the same neural population encodes both the decision formation and action plans.
What if the decision maker is not informed of available action options prior to decision making? The intentional framework predicts that in such cases decisions are formed at a more abstract level beyond motor circuits (88). Multiple tasks have been developed to test this with monkeys, but experimental control is challenging and outcomes remain controversial. One task design is to obscure target locations and reveal them only after the stimulus offset (91). Alternatively, subjects could be instructed to select a target based on color (e.g., left/right motion corresponds to red/green target) but the target colors are revealed only after the decision period (92, 93, 94). As expected, the inability to form an actionable motor plan alters neural responses in motor planning regions (92, 93). However, the interpretation of results is complicated by the possibility that subjects might still form provisional action plans (88) during the stimulus-viewing period and then adjust those plans in response to the appearance of the targets. Recently, Shushruth et al. (95) tackled this issue with a similar task but trained animals to avoid the formation of provisional action plans. Curiously, they found that LIP encoded decision formation only after the target positions were revealed, effectively representing the accumulation of sensory memory rather than ongoing sensory evidence.
Perhaps the best controlled task design for studying stimulus-action mapping is to simply reverse the associations between the stimuli and actions (96, 97, 98, 99, 100, 101). For example, the left and right directions in a motion discrimination task could be associated with left and right saccades, respectively, in one task (pro-saccade task) and with right and left saccade in another (anti-saccade task) (96; Figure 2d(2) bottom). Match-to-sample tasks have a similar task structure as subjects should switch responses to the same test stimulus depending on the match with the sample stimulus (102).
As in the selection of sensory information, classical cognitive control theories attribute the role of such behavioral switching to the prefrontal cortex (PFC). Reversal learning tasks have been extensively used to study cognitive flexibility, including in clinical patients and lesioned animal models (103). Human patients with prefrontal lesions exhibit perseverative behavior and thus fail to reverse their behavior in response to rule changes (103). Similarly, rodents and monkeys with orbitofrontal cortex lesions show reversal learning deficits (104, 103). Asaad et al. (98) recorded from PFC while monkeys switched associations between object stimuli and saccade targets, finding that PFC neurons encode flexible transformation from stimuli to actions.
However, responses in motor-planning areas suggest a more intricate scenario. If PFC is fully responsible for routing sensory evidence to appropriate motor plans, neurons in motor areas would merely reflect signals supporting their preferred actions. However, experimental observations refute this prediction. For example, Wu et al. (102) examined the premotor cortex of mice performing odor match-to-sample, finding that premotor neurons encode the sample odor before the presentation of the test stimulus, when no action plan is supposed to be formed (Figure 3d). Duan et al. (97) trained rats to switch the association between light direction and nose pokes based on task cues. Some neurons in the deep layer of the superior colliculus (SC) maintained task context regardless of the light direction or nose poke direction (Figure 3f). Muhammad et al. (105) showed that, when monkeys were cued to respond to either a matching or a non-matching stimulus in a match-to-sample task, the premotor cortex encoded the task rule even earlier than PFC does.
Overall, we see multiplex neural signals reflecting decision formation, selection of actions, and maintenance of rules in diverse brain regions. These findings suggest that, as for the sensory selection, the selection of motor outputs is a process inextricably tied to decision formation.
3.2.3. Changes in readout along the same sensory dimension.
Decision makers could also alter stimulus-action mapping by shifting their criterion for categorizing stimuli into action plans (106, 107, 108, 109, 110, 111) (Figure 2d(3)). For example, Liu et al. (107) trained mice to report if a tone frequency exceeded a criterion level, with the level being 10 kHz in some blocks and 20 kHz in others. Therefore, successful performance depended on adjusting the decision criterion rather than gating different sensory features or modalities.
The behavioral outcome of this criterion shift seems similar to those when subjects adjust their decision policy to favor one option over others. For example, by changing the probability of choices (16, 52, 109) or reward magnitudes associated with each choice (54, 53), the subjects’ decision criterion for the same sensory judgment can shift. These changes can be accounted for by parameters in decision-making models such as a dynamic bias in drift rate (16) or starting point of the accumulation process (52). In theory, even when subjects are explicitly instructed to shift decision boundary through supervised or reinforcement feedback, a similar decision-making mechanism can operate.
However, empirical findings suggest that the effect is not limited to decision-making circuits. Rather, sensory neural responses also reflect criterion shifts. For example, between the high and low tone boundaries in auditory tone discrimination, neural selectivity of the mouse auditory cortex shifts toward the discriminating boundary (112, but see 111). Also, noise correlations in sensory areas are influenced by category boundaries. Bondy et al. (106) showed that, in orientation categorization with two different category boundaries, monkey V1 neurons whose preferred orientations match the stimuli in the same category have higher noise correlations (Figure 3c). These task-dependent modulations likely arise from interactions between sensory and decision-making brain areas (42, 106, 113, 114, 115). Thus, in circuit models, the interaction between sensory and decision-making processes should be taken into account to implement the criterion shift.
3.3. Flexible adoption of task solutions
Not only can decision makers adjust the parameters of a computation (e.g., evidence accumulation) for decision making (decision policy changes, section 3.1), but also they can change the computation itself. While evidence accumulation can be an optimal solution for discrimination or categorization of stable sensory information (116, 117), different solutions could become appropriate in other task conditions (Figure 2e top). For example, when the task is to compare the magnitudes of two stimuli, then an appropriate solution is to subtract inputs rather than integrate them (118, 119). Similarly, an optimal solution for a detection task against a stable background is differentiation, not integration. How can the brain flexibly adopt different task solutions?
Responses in higher cortical areas likely reflect the chosen solutions, but we are still far from understanding the principles underlying the process. One key fact is that the brain uses common resources to perform different computations and thus likely employs coding schemes that generalize over different tasks (120, 121, 122, 123). The population neural code of the prefrontal cortex and hippocampus has geometries suitable for task generalizations (120). Recurrent neural networks trained to perform multiple tasks show compositional codes that reflect computations shared across tasks (121). Also, circuit models could be designed such that they perform different computations such as maintenance and comparison of sensory inputs through small changes in parameters (119). These could be the ingredients for implementing multiple task solutions.
At the same time, differences in task solutions can also shape how sensory neurons respond or contribute to behavior (124, 125, 126). For example, Koida et al. (124) trained monkeys to report the category of a color patch (e.g., red or green) in one task and to report the match of the color with a reference color in another task —two tasks with distinct solutions. They found color selective neurons in the IT cortex strongly modulate their activity in a task-dependent manner, with some neurons almost abolishing their responses in one task (124). In another study, Chowdhury et al. (125) showed that the involvement of MT neurons in coarse disparity discrimination depends on whether monkeys were trained to perform fine disparity discrimination beforehand. These results suggest that the brain may dynamically modulate or select sensory activity for a given task solution. But the nature of such interaction between sensory activity and task solutions remains largely unexplored.
In richer task designs, subjects may employ multiple solutions for the same task context. It can happen when high task complexity requires subjects to decompose the task into multiple sub-tasks, for example, a tree-like (hierarchical) task structure, where subjects should make multiple binary decisions to reach a goal (51, 127, 128, 129; Figure 2e bottom). In human neuroimaging literature, it has been suggested that the prefrontal cortex forms a hierarchical structure along the rostrocaudal axis, where more rostral regions are involved in making higher decisions in a decision tree (130). Relatedly, there is a gradient for plasticity in the lateral prefrontal cortex of monkeys, with higher plasticity in the more anterior regions (131).
Another scenario where subjects may employ multiple solutions is less constrained experimental designs with multiple task goals. For example, Yang et al. (132) trained monkeys to perform a complex computer game where multiple items are associated with reward and punishment and each requires different actions. They showed that monkeys flexibly switch across distinct behavioral strategies. Modeling studies suggest that rodent behavior could be decomposed into distinct behavioral states even during simple perceptual tasks (133). But models with increased complexity can be overparameterized, requiring careful handling (1, 134). Quantitative and yet parsimonious decision-making models —such as those developed for stationary settings (Figure 1)— are much needed to provide a significant advance in these topics.
3.4. Mechanisms of deciding to switch task rules
So far we have mainly asked how perceptual decisions are formed under different task rules and how these rules are implemented in the brain. However, there is an equally important question common to any form of flexibility: how does the brain “decide” to switch task rules? In the real world, we are usually not instructed to follow predefined task rules but rather choose them ourselves based on internal knowledge or external cues, e.g., outcomes of past decisions (Figure 2a). As briefly mentioned earlier, tasks like WCST or reversal learning have been extensively used to study how subjects switch rules based on feedback (80, 81, 135, 136), but they are rarely associated with the existing frameworks of perceptual decision making. A unique challenge for perceptual decisions is that a decision maker should adopt correct rules and perform correct perceptual discrimination at the same time. Failure in either leads to error, hence there is the ambiguity of whether errors arise from a wrong solution or erroneous perceptual discrimination.
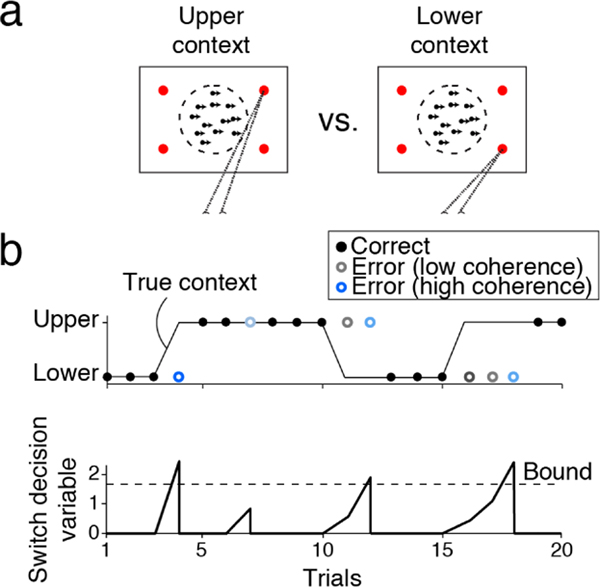
Multiple recent studies have approached this question and started to form mechanistic models of rule switching (137, 138). Purcell et al. (137) designed two task contexts in a motion direction discrimination task, where subjects should select either upper or lower direction targets depending on the hidden context (Figure 4a). To perform the task, subjects correctly infer both the current context and the motion direction. Negative feedback on a trial with higher certainty about a perceptual decision is evidence for a context change. Subjects accumulate their perceptual certainty and feedback over trials and switch their context choice when the accumulated switch evidence reaches a bound (Figure 4b). Using a similar task structure, Sarafyazd et al. (138) revealed neural correlates of such an inference process in the anterior cingulate cortex. These findings not only reveal close interactions across high- and low-levels of hierarchical decision-making processes but also indicate an intriguing possibility that common computational motifs such as evidence accumulation account for multiple levels of decision making.
Figure 4.
Mechanisms of deciding to switch task rules proposed in Purcell et al. (137). (a) Subjects report motion direction using either the upper or lower pair of direction targets depending on the context. (b) The context is not cued and must be inferred from feedback. Subjects switch context after errors (open circles; color indicates motion coherence) depending on the history of feedback and motion coherence on the previous trials (top). Behavior could be modeled as the accumulation of switch evidence to a bound (bottom). Switch evidence is formed by combining feedback and the certainty of the previous trial. Adapted with permission from Reference 137; the authors hold the copyright.
4. Distributed circuits for flexible decision making
In the previous section, we highlighted context-dependent signals in multiple brain regions (Figure 3). Here we discuss the implication of these findings. While the dominant perspective in the field is that a centralized module (i.e., the prefrontal cortex or a frontoparietal network) enables flexible behavior (Figure 5a), empirical evidence suggests an alternative: a distributed network as the neural substrate for flexible behavior (Figure 5b).
Figure 5.
Schematic of network architectures proposed to explain flexible perceptual decision-making. (a) A prevalent theory is that the prefrontal cortex or a frontoparietal network operates as a central hub that gates relevant sensory information and generates appropriate motor plans. Its internal circuits flexibly adjust computations applied to sensory inputs, sending final decisions to appropriate motor regions for execution. (b) A distributed architecture consistent with recent experimental findings. There are no distinct central controls, but multiple brain regions have the capacity to maintain task contexts and flexibly modulate behavior. Dynamics of activity in these brain regions flexibly form decisions. There are gradients within the network such that regions deeper in the sensorimotor hierarchy play more prominent roles in creating flexible behavior.
4.1. Centralized or distributed mechanisms?
The prefrontal cortex (PFC) has long been thought of as a central region that enables cognitive flexibility. PFC receives inputs from various sensory modalities and limbic regions and in turn projects to these regions as well as motor-planning areas. These connections make PFC an ideal hub for flexible stimulus-action mapping. As recent human neuroimaging studies have shown close coordination between PFC and posterior parietal cortex (PPC) (139, 140), the special status of PFC has also been extended to PPC. The resulting frontoparietal network, which can change its functional connectivity with other brain areas during the adoption of different task rules, is theorized to underlie cognitive flexibility and control (6).
By contrast, studies on perceptual decision making have long suggested a distributed architecture for implementation of neural computations (90, 4). As explained earlier, sensory and motor areas reflect decision formation beyond momentary sensory and motor signals (85, 88, 106, 90, 141, 142, 143; Figure 3a, c). While this does not deny domain-general mechanisms for decision making (31, 77, 78, 144), the prevalence of neural signals encoding the decision variable (Figure 1c) indicates that many brain structures form an interconnected network for decision making.
How do we compare the centralized and distributed perspectives in the study of flexible perceptual decision making? Empirical findings summarized thus far indicate that a variety of brain regions change their activity depending on task rules. Centralized theories would claim that such modulations are driven by the central unit and meant to facilitate efficient routing of information through the central unit. However, patterns of neural responses observed in sensory or motor brain regions are difficult to interpret based on this idea.
First, sensory and motor areas directly encode task contexts (72, 75, 97, 101, 105, 124; Figure 3e, f) as opposed to mere modulation of sensory/motor responses by context. These contextual signals cannot be explained as attention-like enhancement of task-relevant information. For example, neurons encode task rules even outside the epoch where relevant sensory or motor events are happening (75, 97). While such contextual signals could be maintained through inputs from PFC, it is unclear why such inputs should be received when not needed. Perhaps, these signals modulate computations within sensory and motor areas in a context-dependent manner, counter to the core idea of a centralized module for implementing flexibility.
Second, these areas also reflect signals that do not directly pertain to their roles in sensory and motor processing (69, 70, 102; Figure 3b, d). For example, the encoding of decision variable in an oculomotor area is modulated by what sensory features are distinguished (69). Or a premotor area reflects sample stimuli during a match-to-sample task (102). These findings indicate that motor-planning areas do not merely receive abstract decision variables from an amodal central module capable of flexible decision making. Rather, they could be part of the network that flexibly transforms sensory signals into action plans (Figure 5b; 143, 145, 146).
In this distributed network, there is no clear parcellation of brain regions into those responsible for flexible control and others (147, 145). However, there are gradients across brain structures, where some areas have more capacity to maintain task rules, flexibly adjust computations, and exert influence over other regions. Therefore, higher-order sensory and motor-planning cortical regions and subcortical areas that have access to both sensory and motor information could play significant roles in flexible behavior, and even early sensory areas can reflect context-dependent behavior, albeit to a lesser extent. PFC lies at the top of this distributed network as the most flexible circuitry but it does not play a unique role as a control area (147).
Multiple brain structures could be responsible for different forms of flexibility in the distributed network (section 3), while the extent of their involvement varies depending on the task. This perspective might reconcile diverse theories of perceptual decision making such as those advocating active inference by sensory neurons (148, 115) or the intentional framework that emphasizes selection at motor stages (88, 90). Distributed architecture is also sensible from an evolutionary perspective (145) as the development of PFC is phylogenetically recent (149) whereas flexibility in behavior is a common requirement for many organisms (150).
4.2. How to study distributed networks?
How can we study computations distributed in a brain-wide network? To study such processes, it would be more fruitful to focus on common computational principles across the network rather than trying to pinpoint brain regions responsible for individual operations.
Studies that aim to infer the role of individual areas in the network through causal manipulations are strongly challenged (151, 152). If there were a central hub for flexible decision making, inactivating this hub region would have disrupted all forms of flexible behavior. But when multiple brain regions interact in a distributed network to make decisions, perturbing a single region may yield a wide range of results, including no effect, transient behavioral deficits, and lasting deficits (143, 152, 153). Indeed, recent studies have found that inactivating saccade-selective LIP neurons has limited effects on perceptual decision making (154, 155). More precisely, choice biases appear transiently after inactivation and disappear within a session (156). There are also mixed results on the inactivation effects of rodent PPC on perceptual decision making (157, 158, 159). The inactivation of a more peripheral structure, the superior colliculus, shows stronger effects (160). It is possible that more peripheral brain regions tend to be bottlenecks while higher areas form more robust networks. Therefore, the strength of perturbation effects alone is inadequate to determine the extent of involvement of the perturbed brain region in the studied behavior. More meaningful conclusions could be made by multi-pronged studies that combine subtle perturbations with electrophysiological recordings from other nodes of the network during an array of tasks, each carefully tailored to investigate an aspect of flexible behavior.
Understanding a distributed network is greatly aided by adopting population-level analyses within and across the network. In distributed networks, even neurons in “modalityspecific” areas may show mixed selectivity for sensory, motor, and contextual information (161, 162, 163). These complex representations would be difficult to probe through conventional analysis methods that do not appreciate the diversity of neural responses (e.g., averaging activity across the population) (69, 70). Emerging analysis techniques such as targeted or unsupervised dimensionality reduction (164, 165) and geometric data visualization (166, 167) may provide better insights into the representations afforded by each brain area (69). Furthermore, understanding the distributed network would be impossible without discovering the principles of communications across brain areas. Using the language of population neural activity and communication subspace offers a practical path forward (168).
Finally, we would like to emphasize the importance of recognizing the properties and requirements of different task designs (Figure 2). Besides identifying brain regions responsible for flexible behavior in a specific task design, it would become important to examine what forms of flexibility are related to each brain region and what forms are not. Furthermore, specific details of behavioral tasks —such as how animals are trained, how often tasks switch, how task contexts are cued— can be critical for interpreting behavioral and neural data. An advance in the field relies not only on novel experimental techniques but also on better understanding and designing of behavioral tasks (1, 169).
5. Implementing flexibility in network models
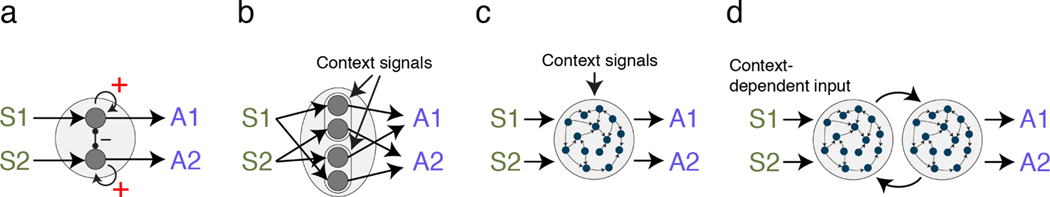
In this last section, we briefly summarize circuit motifs used in existing computational models of perceptual decision making and flexible control. As mentioned in section 2, existing circuit models formulate decision making as competition across neural modules encoding different actions. These models readily account for some flexible aspects of decision policy (Figure 2b). For example, tweaking the strength of self-excitation of action-selective modules alters the speed-accuracy trade-off of decisions (20, 170; Figure 6a). However, they do not address flexible adjustment in stimulus-action mapping.
Figure 6.
Example circuit motifs proposed to model decision-making and its flexible adjustments. S1 and S2 are inputs from sensory neurons selective to the discriminated stimuli (e.g., left and right motion directions). A1 and A2 are drives for the two actions (e.g., leftward and rightward saccade). (a) When a decision is made through competition of choice-selective neural modules, changes in self-excitation (red plus signs) alter speed-accuracy trade-off. (b) To achieve flexible stimulus-action mapping, a control module could switch the routing of sensory information depending on contextual signals. (c) More recent models implement similar computations by training recurrent neural networks. (d) Embracing the distributed nature of neural processing and interactions in the actual brain can yield mechanistic models that better explain the neural responses. Multi-module RNNs are a fruitful step in that direction.
Traditionally, flexible sensory-action mapping are addressed by models of cognitive control (171, 172, 173, 174, 175). These models include control modules that influence sensory and motor modules (Figure 6b). Often, different control modules are responsible for different tasks. Through external contextual signals and mutual inhibition, one control module becomes active, gating information flow from sensory to motor modules. The gating could be implemented through a range of mechanisms (171, 176, 177, 178) such as a thresholding operation that triggers outputs only when inputs from both the contextual and sensory signals are present (171). Also, models could be designed to learn novel sensory-action associations through rapid synaptic plasticity (174, 173). Overall, these models are powerful but usually operate within the narrow scope they are designed for and depend on careful handcrafting by model designers to properly associate task inputs with outputs.
Recent developments in artificial neural networks (ANNs) have introduced an alternative route to engineer circuit models for cognition (36, 121). Without handcrafting the detailed circuit architecture and computations, a fully connected recurrent neural network (RNN) could be trained to solve diverse tasks similar to those used in human or animal studies as long as proper objective functions and learning rules are implemented (36, 121, 179, 180, 181; Figure 6c). For example, to switch between color and motion discrimination (Figure 2d(1)), an RNN that receives color, motion, and contextual signals can be trained to generate correct binary choices for each task (36). With a similar approach, Yang et al. (121) successfully trained an RNN to perform 20 perceptual tasks including context-dependent decisions. The computations happening within RNNs could be partly inferred through analyzing their neural response dynamics (182, 183). The geometry of population activity provides another fruitful method for understanding RNNs and their connection with neural responses recorded from the brain (36, 180, 181), with the caveat that similar geometric responses may also arise from different network architectures (184).
RNNs appear to be particularly well suited for describing centralized mechanisms (Figure 5a). There are several architectural properties worth discussing. First, many existing RNNs assume a uniform network architecture composed of randomly connected units with similar physiological properties and thus lack the concept of neuron type diversity and hierarchical structures. Second, sensory inputs and motor outputs of RNNs are highly stylized. For example, the context-dependent RNN (Figure 6c) only has to deal with one-dimensional inputs of color and motion information and generate a binary output. This already circumvents the challenge of decoding meaningful information from high-dimensional sensory representations (185) and generating dynamic motor control signals inextricably tied to decision formation in the real brain (186). Third, many RNNs receive contextual signals, but such signals presume knowledge about the number and type of contexts. Such knowledge is unlikely to be readily available to the real brain operating in complex environments. Finally, typical RNNs are unencumbered with biophysical constraints of the real brain that might affect population response properties. For example, one-dimensional signals may be better encoded on a curved manifold than on a linear manifold in a network if the network needs to reduce the number of spikes due to metabolic costs (187, 69).
Incorporating the distributed neural architecture of the brain in ANNs could lead to a better understanding of brain-like neural computations (42, 143, 188, 189). Such models would have a hierarchical architecture comprising multiple interconnected neural modules (Figure 5b). For example, Pinto et al. (143) modeled RNNs composed of input and output modules to account for task-dependent effects of perturbation they observed experimentally (Figure 6d). Multi-module circuits also explain immunity to the perturbation of individual circuits (153, 152). Further, modeling a large-scale cortical network accounts for the emergence of a hierarchical structure, where higher areas have longer timescales and stronger control over network connectivity (190). We expect that further expansion of theories that embrace the distributed brain architecture provides more insights into interpreting diverse experimental observations highlighted in this review.
6. Concluding remarks
Advances in theoretical and experimental studies of perceptual decision making have brought an understanding of cognitive flexibility within our grasp. In particular, existing behavioral and neural models can successfully account for various forms of flexible adjustments in decision policy (Figure 2b–c). However, it is much less understood how stimulus-action mapping (Figure 2d) and task solutions (Figure 2e) are implemented in a context-dependent manner. Empirical results suggest that the same neurons that encode decision formation often show activity reflecting changes in these task rules (Figure 3). This implies a potential link between evidence accumulation and flexible control mechanisms. We hope to see future efforts to integrate context-dependent computations into decision-making models.
A notable neurophysiological observation is the distributed nature of context-dependent neural responses (Figure 3). Neural activity reflecting maintenance, change, or implementation of task rules abounds outside PFC, including PPC, sensory, motor, and subcortical brain areas. While mixed selectivity for diverse behavioral variables is particularly highlighted in recent rodent literature (161, 162, 163), past primate studies have also repeatedly reported context-dependent signals across brain regions as summarized above. These observations invite us to focus on computational principles governing the whole network rather than testing roles for individual brain regions. Apart from perceptual decision making, context-dependent computations are essential in sensory, motor, memory, and many other brain functions. We expect that advances on this topic would open a window for understanding cognition and intelligence in general (4).
ACKNOWLEDGMENTS
This work was supported by the Simons Collaboration on the Global Brain (542997 and 988247), McKnight Scholar Award, Pew Scholarship in the Biomedical Sciences, and National Institute of Mental Health (R01 MH109180 and R01 MH127375). G.O. was supported by post-doctoral fellowships from the Charles H. Revson Foundation and the Japan Society for the Promotion of Science.
Footnotes
DISCLOSURE STATEMENT
The authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
LITERATURE CITED
- 1.Waskom ML, Okazawa G, Kiani R. 2019. Designing and interpreting psychophysical investigations of cognition. Neuron 104(1):100–112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.O’Connell RG, Kelly SP. 2021. Neurophysiology of human perceptual decision-making. Annu Rev Neurosci 44:495–516 [DOI] [PubMed] [Google Scholar]
- 3.Hanks TD, Summerfield C. 2017. Perceptual decision making in rodents, monkeys, and humans. Neuron 93(1):15–31 [DOI] [PubMed] [Google Scholar]
- 4.Shadlen MN, Kiani R. 2013. Decision making as a window on cognition. Neuron 80(3):791–806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Miller EK, Cohen JD. 2001. An integrative theory of prefrontal cortex function. Annu Rev Neurosci 24:167–202 [DOI] [PubMed] [Google Scholar]
- 6.Cole MW, Reynolds JR, Power JD, Repovs G, Anticevic A, Braver TS. 2013. Multi-task connectivity reveals flexible hubs for adaptive task control. Nat Neurosci 16(9):1348–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Britten KH, Shadlen MN, Newsome WT, Movshon JA. 1992. The analysis of visual motion: a comparison of neuronal and psychophysical performance. J Neurosci 12(12):4745–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roitman JD, Shadlen MN. 2002. Response of neurons in the lateral intraparietal area during a combined visual discrimination reaction time task. J Neurosci 22(21):9475–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Drugowitsch J, DeAngelis GC, Klier EM, Angelaki DE, Pouget A. 2014. Optimal multisensory decision-making in a reaction-time task. Elife 3:e03005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Okazawa G, Sha L, Kiani R. 2021. Linear integration of sensory evidence over space and time underlies face categorization. J Neurosci 41(37):7876–7893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Drugowitsch J, Moreno-Bote R, Churchland AK, Shadlen MN, Pouget A. 2012. The cost of accumulating evidence in perceptual decision making. J Neurosci 32(11):3612–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Deneve S. 2012. Making decisions with unknown sensory reliability. Front Neurosci 6:75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Khalvati K, Kiani R, Rao RPN. 2021. Bayesian inference with incomplete knowledge explains perceptual confidence and its deviations from accuracy. Nat Commun 12(1):5704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gold JI, Shadlen MN. 2007. The neural basis of decision making. Annu Rev Neurosci 30:535–74 [DOI] [PubMed] [Google Scholar]
- 15.Mochol G, Kiani R, Moreno-Bote R. 2021. Prefrontal cortex represents heuristics that shape choice bias and its integration into future behavior. Curr Biol 31(6):1234–1244 e6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hanks TD, Mazurek ME, Kiani R, Hopp E, Shadlen MN. 2011. Elapsed decision time affects the weighting of prior probability in a perceptual decision task. J Neurosci 31(17):6339–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Urai AE, de Gee JW, Tsetsos K, Donner TH. 2019. Choice history biases subsequent evidence accumulation. Elife 8:e46331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Noorbaloochi S, Sharon D, McClelland JL. 2015. Payoff information biases a fast guess process in perceptual decision making under deadline pressure: Evidence from behavior, evoked potentials, and quantitative model comparison. J Neurosci 35(31):10989–1011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang XJ. 2002. Probabilistic decision making by slow reverberation in cortical circuits. Neuron 36(5):955–68 [DOI] [PubMed] [Google Scholar]
- 20.Wong KF, Wang XJ. 2006. A recurrent network mechanism of time integration in perceptual decisions. J Neurosci 26(4):1314–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Beck JM, Ma WJ, Kiani R, Hanks T, Churchland AK, et al. 2008. Probabilistic population codes for Bayesian decision making. Neuron 60(6):1142–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Palmer J, Huk AC, Shadlen MN. 2005. The effect of stimulus strength on the speed and accuracy of a perceptual decision. J Vis 5(5):376–404 [DOI] [PubMed] [Google Scholar]
- 23.Smith PL, Vickers D. 1988. The accumulator model of two-choice discrimination. J Mathematical Psychol 32(2):135–168 [Google Scholar]
- 24.Kiani R, Shadlen MN. 2009. Representation of confidence associated with a decision by neurons in the parietal cortex. Science 324(5928):759–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kiani R, Corthell L, Shadlen MN. 2014. Choice certainty is informed by both evidence and decision time. Neuron 84(6):1329–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Horwitz GD, Newsome WT. 1999. Separate signals for target selection and movement specification in the superior colliculus. Science 284(5417):1158–61 [DOI] [PubMed] [Google Scholar]
- 27.Ding L, Gold JI. 2010. Caudate encodes multiple computations for perceptual decisions. J Neurosci 30(47):15747–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ding L, Gold JI. 2012. Neural correlates of perceptual decision making before, during, and after decision commitment in monkey frontal eye field. Cereb Cortex 22(5):1052–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim B, Basso MA. 2008. Saccade target selection in the superior colliculus: a signal detection theory approach. J Neurosci 28(12):2991–3007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Donner TH, Siegel M, Fries P, Engel AK. 2009. Buildup of choice-predictive activity in human motor cortex during perceptual decision making. Curr Biol 19(18):1581–5 [DOI] [PubMed] [Google Scholar]
- 31.O’Connell RG, Dockree PM, Kelly SP. 2012. A supramodal accumulation-to-bound signal that determines perceptual decisions in humans. Nat Neurosci 15(12):1729–35 [DOI] [PubMed] [Google Scholar]
- 32.Philiastides MG, Heekeren HR, Sajda P. 2014. Human scalp potentials reflect a mixture of decision-related signals during perceptual choices. J Neurosci 34(50):16877–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shadlen MN, Newsome WT. 2001. Neural basis of a perceptual decision in the parietal cortex (area LIP) of the rhesus monkey. J Neurophysiol 86(4):1916–36 [DOI] [PubMed] [Google Scholar]
- 34.Shadlen MN, Newsome WT. 1996. Motion perception: seeing and deciding. Proc Natl Acad Sci U S A 93(2):628–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kiani R, Cueva CJ, Reppas JB, Newsome WT. 2014. Dynamics of neural population responses in prefrontal cortex indicate changes of mind on single trials. Curr Biol 24(13):1542–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mante V, Sussillo D, Shenoy KV, Newsome WT. 2013. Context-dependent computation by recurrent dynamics in prefrontal cortex. Nature 503(7474):78–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cisek P, Kalaska JF. 2005. Neural correlates of reaching decisions in dorsal premotor cortex: specification of multiple direction choices and final selection of action. Neuron 45(5):801–14 [DOI] [PubMed] [Google Scholar]
- 38.Peixoto D, Verhein JR, Kiani R, Kao JC, Nuyujukian P, et al. 2021. Decoding and perturbing decision states in real time. Nature 591(7851):604–609 [DOI] [PubMed] [Google Scholar]
- 39.Chandrasekaran C, Peixoto D, Newsome WT, Shenoy KV. 2017. Laminar differences in decision-related neural activity in dorsal premotor cortex. Nat Commun 8(1):614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Purcell BA, Heitz RP, Cohen JY, Schall JD, Logan GD, Palmeri TJ. 2010. Neurally constrained modeling of perceptual decision making. Psychol Rev 117(4):1113–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hanes DP, Schall JD. 1996. Neural control of voluntary movement initiation. Science 274(5286):427–30 [DOI] [PubMed] [Google Scholar]
- 42.Wimmer K, Compte A, Roxin A, Peixoto D, Renart A, de la Rocha J. 2015. Sensory integration dynamics in a hierarchical network explains choice probabilities in cortical area MT. Nat Commun 6:6177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Deco G, Rolls ET, Albantakis L, Romo R. 2013. Brain mechanisms for perceptual and reward-related decision-making. Prog Neurobiol 103:194–213 [DOI] [PubMed] [Google Scholar]
- 44.Ditterich J. 2006. Stochastic models of decisions about motion direction: behavior and physiology. Neural Netw 19(8):981–1012 [DOI] [PubMed] [Google Scholar]
- 45.Usher M, McClelland JL. 2001. The time course of perceptual choice: the leaky, competing accumulator model. Psychol Rev 108(3):550–92 [DOI] [PubMed] [Google Scholar]
- 46.Tajima S, Drugowitsch J, Pouget A. 2016. Optimal policy for value-based decision-making. Nat Commun 7:12400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Louie K, LoFaro T, Webb R, Glimcher PW. 2014. Dynamic divisive normalization predicts time-varying value coding in decision-related circuits. J Neurosci 34(48):16046–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ratcliff R, Starns JJ. 2013. Modeling confidence judgments, response times, and multiple choices in decision making: recognition memory and motion discrimination. Psychol Rev 120(3):697–719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shadlen MN, Shohamy D. 2016. Decision making and sequential sampling from memory. Neuron 90(5):927–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Purcell BA, Kiani R. 2016. Neural mechanisms of post-error adjustments of decision policy in parietal cortex. Neuron 89(3):658–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zylberberg A. 2021. Decision prioritization and causal reasoning in decision hierarchies. PLoS Comput Biol 17(12):e1009688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rao V, DeAngelis GC, Snyder LH. 2012. Neural correlates of prior expectations of motion in the lateral intraparietal and middle temporal areas. J Neurosci 32(29):10063–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fan Y, Gold JI, Ding L. 2020. Frontal eye field and caudate neurons make different contributions to reward-biased perceptual decisions. Elife 9:e60535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Doi T, Fan Y, Gold JI, Ding L. 2020. The caudate nucleus contributes causally to decisions that balance reward and uncertain visual information. Elife 9:e56694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hagura N, Haggard P, Diedrichsen J. 2017. Perceptual decisions are biased by the cost to act. Elife 6:e18422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zylberberg A, Fetsch CR, Shadlen MN. 2016. The influence of evidence volatility on choice, reaction time and confidence in a perceptual decision. Elife 5:e17688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Levi AJ, Yates JL, Huk AC, Katz LN. 2018. Strategic and dynamic temporal weighting for perceptual decisions in humans and macaques. eNeuro 5(5):ENEURO.0169–18.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Forstmann BU, Dutilh G, Brown S, Neumann J, von Cramon DY, et al. 2008. Striatum and presma facilitate decision-making under time pressure. Proc Natl Acad Sci U S A 105(45):17538–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Simen P, Contreras D, Buck C, Hu P, Holmes P, Cohen JD. 2009. Reward rate optimization in two-alternative decision making: empirical tests of theoretical predictions. J Exp Psychol Hum Percept Perform 35(6):1865–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hanks T, Kiani R, Shadlen MN. 2014. A neural mechanism of speed-accuracy tradeoff in macaque area LIP. Elife 3:e02260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Akrami A, Kopec CD, Diamond ME, Brody CD. 2018. Posterior parietal cortex represents sensory history and mediates its effects on behaviour. Nature 554(7692):368–372 [DOI] [PubMed] [Google Scholar]
- 62.Heitz RP, Schall JD. 2012. Neural mechanisms of speed-accuracy tradeoff. Neuron 76(3):616–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Okazawa G, Sha L, Purcell BA, Kiani R. 2018. Psychophysical reverse correlation reflects both sensory and decision-making processes. Nat Commun 9(1):3479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cavanagh JF, Wiecki TV, Cohen MX, Figueroa CM, Samanta J, et al. 2011. Subthalamic nucleus stimulation reverses mediofrontal influence over decision threshold. Nat Neurosci 14(11):1462–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Stine GM, Trautmann EM, Jeurissen D, Shadlen MN. 2022. A neural mechanism for terminating decisions. bioRxiv :2022.05.02.490327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lo CC, Wang XJ. 2006. Cortico-basal ganglia circuit mechanism for a decision threshold in reaction time tasks. Nat Neurosci 9(7):956–63 [DOI] [PubMed] [Google Scholar]
- 67.Yeung N, Nystrom LE, Aronson JA, Cohen JD. 2006. Between-task competition and cognitive control in task switching. J Neurosci 26(5):1429–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wimmer RD, Schmitt LI, Davidson TJ, Nakajima M, Deisseroth K, Halassa MM. 2015. Thalamic control of sensory selection in divided attention. Nature 526(7575):705–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Okazawa G, Hatch CE, Mancoo A, Machens CK, Kiani R. 2021. Representational geometry of perceptual decisions in the monkey parietal cortex. Cell 184(14):3748–3761 e18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Raposo D, Kaufman MT, Churchland AK. 2014. A category-free neural population supports evolving demands during decision-making. Nat Neurosci 17(12):1784–1792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kumano H, Suda Y, Uka T. 2016. Context-dependent accumulation of sensory evidence in the parietal cortex underlies flexible task switching. J Neurosci 36(48):12192–12202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Stoet G, Snyder LH. 2004. Single neurons in posterior parietal cortex of monkeys encode cognitive set. Neuron 42(6):1003–12 [DOI] [PubMed] [Google Scholar]
- 73.Roy JE, Riesenhuber M, Poggio T, Miller EK. 2010. Prefrontal cortex activity during flexible categorization. J Neurosci 30(25):8519–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Siegel M, Buschman TJ, Miller EK. 2015. Cortical information flow during flexible sensorimotor decisions. Science 348(6241):1352–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rodgers CC, DeWeese MR. 2014. Neural correlates of task switching in prefrontal cortex and primary auditory cortex in a novel stimulus selection task for rodents. Neuron 82(5):1157–70 [DOI] [PubMed] [Google Scholar]
- 76.Flesch T, Juechems K, Dumbalska T, Saxe A, Summerfield C. 2022. Orthogonal representations for robust context-dependent task performance in brains and neural networks. Neuron 110(7):1258–1270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kelly SP, O’Connell RG. 2013. Internal and external influences on the rate of sensory evidence accumulation in the human brain. J Neurosci 33(50):19434–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tosoni A, Galati G, Romani GL, Corbetta M. 2008. Sensory-motor mechanisms in human parietal cortex underlie arbitrary visual decisions. Nat Neurosci 11(12):1446–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Heekeren HR, Marrett S, Ruff DA, Bandettini PA, Ungerleider LG. 2006. Involvement of human left dorsolateral prefrontal cortex in perceptual decision making is independent of response modality. Proc Natl Acad Sci U S A 103(26):10023–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mansouri FA, Freedman DJ, Buckley MJ. 2020. Emergence of abstract rules in the primate brain. Nat Rev Neurosci 21(11):595–610 [DOI] [PubMed] [Google Scholar]
- 81.Buckley MJ, Mansouri FA, Hoda H, Mahboubi M, Browning PG, et al. 2009. Dissociable components of rule-guided behavior depend on distinct medial and prefrontal regions. Science 325(5936):52–8 [DOI] [PubMed] [Google Scholar]
- 82.Kamigaki T, Fukushima T, Miyashita Y. 2009. Cognitive set reconfiguration signaled by macaque posterior parietal neurons. Neuron 61(6):941–51 [DOI] [PubMed] [Google Scholar]
- 83.Sasaki R, Uka T. 2009. Dynamic readout of behaviorally relevant signals from area MT during task switching. Neuron 62(1):147–57 [DOI] [PubMed] [Google Scholar]
- 84.Katzner S, Busse L, Treue S. 2009. Attention to the color of a moving stimulus modulates motion-signal processing in macaque area MT: Evidence for a unified attentional system. Front Syst Neurosci 3:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.de Lafuente V, Jazayeri M, Shadlen MN. 2015. Representation of accumulating evidence for a decision in two parietal areas. J Neurosci 35(10):4306–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ho TC, Brown S, Serences JT. 2009. Domain general mechanisms of perceptual decision making in human cortex. J Neurosci 29(27):8675–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Liu T, Pleskac TJ. 2011. Neural correlates of evidence accumulation in a perceptual decision task. J Neurophysiol 106(5):2383–98 [DOI] [PubMed] [Google Scholar]
- 88.Shadlen MN, Kiani R, Hanks TD, Churchland AK. 2008. Neurobiology of decision making: An intentional framework, book section 4. MIT Press, 71–101 [Google Scholar]
- 89.Snyder LH, Batista AP, Andersen RA. 2000. Intention-related activity in the posterior parietal cortex: a review. Vision Res 40(10–12):1433–41 [DOI] [PubMed] [Google Scholar]
- 90.Cisek P. 2012. Making decisions through a distributed consensus. Curr Opin Neurobiol 22(6):927–36 [DOI] [PubMed] [Google Scholar]
- 91.Salzman CD, Newsome WT. 1994. Neural mechanisms for forming a perceptual decision. Science 264(5156):231–7 [DOI] [PubMed] [Google Scholar]
- 92.Bennur S, Gold JI. 2011. Distinct representations of a perceptual decision and the associated oculomotor plan in the monkey lateral intraparietal area. J Neurosci 31(3):913–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wang M, Montanede C, Chandrasekaran C, Peixoto D, Shenoy KV, Kalaska JF. 2019. Macaque dorsal premotor cortex exhibits decision-related activity only when specific stimulus-response associations are known. Nat Commun 10(1):1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Twomey DM, Kelly SP, O’Connell RG. 2016. Abstract and effector-selective decision signals exhibit qualitatively distinct dynamics before delayed perceptual reports. J Neurosci 36(28):7346–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Shushruth S, Zylberberg A, Shadlen MN. 2022. Sequential sampling from memory underlies action selection during abstract decision-making. Curr Biol 32(9):1949–1960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Gold JI, Shadlen MN. 2003. The influence of behavioral context on the representation of a perceptual decision in developing oculomotor commands. J Neurosci 23(2):632–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Duan CA, Pagan M, Piet AT, Kopec CD, Akrami A, et al. 2021. Collicular circuits for flexible sensorimotor routing. Nat Neurosci 24(8):1110–1120 [DOI] [PubMed] [Google Scholar]
- 98.Asaad WF, Rainer G, Miller EK. 1998. Neural activity in the primate prefrontal cortex during associative learning. Neuron 21(6):1399–407 [DOI] [PubMed] [Google Scholar]
- 99.Johnston K, Levin HM, Koval MJ, Everling S. 2007. Top-down control-signal dynamics in anterior cingulate and prefrontal cortex neurons following task switching. Neuron 53(3):453–62 [DOI] [PubMed] [Google Scholar]
- 100.Stokes MG, Kusunoki M, Sigala N, Nili H, Gaffan D, Duncan J. 2013. Dynamic coding for cognitive control in prefrontal cortex. Neuron 78(2):364–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.van den Brink RL, Hagena K, Wilming N, Murphy PR, Buechel C, Donner TH. 2022. Largescale circuit configuration for flexible sensory-motor decisions. bioRxiv 2022.03.10.483758 [Google Scholar]
- 102.Wu Z, Litwin-Kumar A, Shamash P, Taylor A, Axel R, Shadlen MN. 2020. Context-dependent decision making in a premotor circuit. Neuron 106(2):316–328 e6 [DOI] [PubMed] [Google Scholar]
- 103.Izquierdo A, Brigman JL, Radke AK, Rudebeck PH, Holmes A. 2017. The neural basis of reversal learning: An updated perspective. Neuroscience 345:12–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Rudebeck PH, Murray EA. 2014. The orbitofrontal oracle: cortical mechanisms for the prediction and evaluation of specific behavioral outcomes. Neuron 84(6):1143–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Muhammad R, Wallis JD, Miller EK. 2006. A comparison of abstract rules in the prefrontal cortex, premotor cortex, inferior temporal cortex, and striatum. J Cogn Neurosci 18(6):974–89 [DOI] [PubMed] [Google Scholar]
- 106.Bondy AG, Haefner RM, Cumming BG. 2018. Feedback determines the structure of correlated variability in primary visual cortex. Nat Neurosci 21(4):598–606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Liu Y, Xin Y, Xu NL. 2021. A cortical circuit mechanism for structural knowledge-based flexible sensorimotor decision-making. Neuron 109(12):2009–2024 e6 [DOI] [PubMed] [Google Scholar]
- 108.Wang TY, Liu J, Yao H. 2020. Control of adaptive action selection by secondary motor cortex during flexible visual categorization. Elife 9:e54474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Crapse TB, Lau H, Basso MA. 2018. A role for the superior colliculus in decision criteria.Neuron 97(1):181–194 e6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ferrera VP, Yanike M, Cassanello C. 2009. Frontal eye field neurons signal changes in decision criteria. Nat Neurosci 12(11):1458–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Jaramillo S, Borges K, Zador AM. 2014. Auditory thalamus and auditory cortex are equally modulated by context during flexible categorization of sounds. J Neurosci 34(15):5291–301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Xin Y, Zhong L, Zhang Y, Zhou T, Pan J, Xu NL. 2019. Sensory-to-category transformation via dynamic reorganization of ensemble structures in mouse auditory cortex. Neuron 103(5):909–921 e6 [DOI] [PubMed] [Google Scholar]
- 113.Quinn KR, Seillier L, Butts DA, Nienborg H. 2021. Decision-related feedback in visual cortex lacks spatial selectivity. Nat Commun 12(1):4473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Zhao Y, Yates JL, Levi AJ, Huk AC, Park IM. 2020. Stimulus-choice (mis)alignment in primate area MT. PLoS Comput Biol 16(5):e1007614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Lange RD, Chattoraj A, Beck JM, Yates JL, Haefner RM. 2021. A confirmation bias in perceptual decision-making due to hierarchical approximate inference. PLoS Comput Biol 17(11):e1009517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Waskom ML, Kiani R. 2018. Decision making through integration of sensory evidence at prolonged timescales. Curr Biol 28(23):3850–3856 e9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Stine GM, Zylberberg A, Ditterich J, Shadlen MN. 2020. Differentiating between integration and non-integration strategies in perceptual decision making. Elife 9:e55365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Romo R, de Lafuente V. 2013. Conversion of sensory signals into perceptual decisions. Prog Neurobiol 103:41–75 [DOI] [PubMed] [Google Scholar]
- 119.Machens CK, Romo R, Brody CD. 2005. Flexible control of mutual inhibition: a neural model of two-interval discrimination. Science 307(5712):1121–4 [DOI] [PubMed] [Google Scholar]
- 120.Bernardi S, Benna MK, Rigotti M, Munuera J, Fusi S, Salzman CD. 2020. The geometry of abstraction in the hippocampus and prefrontal cortex. Cell 183(4):954–967 e21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Yang GR, Joglekar MR, Song HF, Newsome WT, Wang XJ. 2019. Task representations in neural networks trained to perform many cognitive tasks. Nat Neurosci 22(2):297–306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Mohan K, Zhu O, Freedman DJ. 2021. Interaction between neuronal encoding and population dynamics during categorization task switching in parietal cortex. Neuron 109(4):700–712 e4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Cole MW, Etzel JA, Zacks JM, Schneider W, Braver TS. 2011. Rapid transfer of abstract rules to novel contexts in human lateral prefrontal cortex. Front Hum Neurosci 5:142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Koida K, Komatsu H. 2007. Effects of task demands on the responses of color-selective neurons in the inferior temporal cortex. Nat Neurosci 10(1):108–16 [DOI] [PubMed] [Google Scholar]
- 125.Chowdhury SA, DeAngelis GC. 2008. Fine discrimination training alters the causal contribution of macaque area MT to depth perception. Neuron 60(2):367–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Koldaeva A, Schaefer AT, Fukunaga I. 2019. Rapid task-dependent tuning of the mouse olfactory bulb. Elife 8:e43558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Solway A, Botvinick MM. 2015. Evidence integration in model-based tree search. Proc Natl Acad Sci U S A 112(37):11708–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Huys QJ, Lally N, Faulkner P, Eshel N, Seifritz E, et al. 2015. Interplay of approximate planning strategies. Proc Natl Acad Sci U S A 112(10):3098–103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Bv Opheusden, Ma WJ. 2019. Tasks for aligning human and machine planning. Current Opinion in Behavioral Sciences 29:127–133 [Google Scholar]
- 130.Badre D, Nee DE. 2018. Frontal cortex and the hierarchical control of behavior. Trends Cogn Sci 22(2):170–188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Riley MR, Qi XL, Zhou X, Constantinidis C. 2018. Anterior-posterior gradient of plasticity in primate prefrontal cortex. Nat Commun 9(1):3790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Yang Q, Lin Z, Zhang W, Li J, Chen X, et al. 2022. Monkey plays pac-man with compositional strategies and hierarchical decision-making. eLife 11:e74500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Ashwood ZC, Roy NA, Stone IR, International Brain L, Urai AE, et al. 2022. Mice alternate between discrete strategies during perceptual decision-making. Nat Neurosci 25(2):201–212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Churchland AK, Kiani R. 2016. Three challenges for connecting model to mechanism in decision-making. Curr Opin Behav Sci 11:74–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Bartolo R, Averbeck BB. 2020. Prefrontal cortex predicts state switches during reversal learning. Neuron 106(6):1044–1054 e4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Murray EA, Rudebeck PH. 2018. Specializations for reward-guided decision-making in the primate ventral prefrontal cortex. Nat Rev Neurosci 19(7):404–417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Purcell BA, Kiani R. 2016. Hierarchical decision processes that operate over distinct timescales underlie choice and changes in strategy. Proc Natl Acad Sci U S A 113(31):E4531–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Sarafyazd M, Jazayeri M. 2019. Hierarchical reasoning by neural circuits in the frontal cortex. Science 364(6441):eaav8911 [DOI] [PubMed] [Google Scholar]
- 139.Chiu YC, Yantis S. 2009. A domain-independent source of cognitive control for task sets: shifting spatial attention and switching categorization rules. J Neurosci 29(12):3930–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Xu Y. 2018. The posterior parietal cortex in adaptive visual processing. Trends Neurosci 41(11):806–822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Tajima S, Koida K, Tajima CI, Suzuki H, Aihara K, Komatsu H. 2017. Task-dependent recurrent dynamics in visual cortex. Elife 6:e26868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Akrami A, Liu Y, Treves A, Jagadeesh B. 2009. Converging neuronal activity in inferior temporal cortex during the classification of morphed stimuli. Cereb Cortex 19(4):760–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Pinto L, Rajan K, DePasquale B, Thiberge SY, Tank DW, Brody CD. 2019. Task-dependent changes in the large-scale dynamics and necessity of cortical regions. Neuron 104(4):810–824 e9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Heekeren HR, Marrett S, Bandettini PA, Ungerleider LG. 2004. A general mechanism for perceptual decision-making in the human brain. Nature 431(7010):859–62 [DOI] [PubMed] [Google Scholar]
- 145.Cisek P. 2022. Evolution of behavioural control from chordates to primates. Philos Trans R Soc Lond B Biol Sci 377(1844):20200522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Hunt LT, Hayden BY. 2017. A distributed, hierarchical and recurrent framework for reward-based choice. Nat Rev Neurosci 18(3):172–182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Fine JM, Hayden BY. 2022. The whole prefrontal cortex is premotor cortex. Philos Trans R Soc Lond B Biol Sci 377(1844):20200524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Lee TS, Mumford D. 2003. Hierarchical bayesian inference in the visual cortex. J Opt Soc Am A Opt Image Sci Vis 20(7):1434–48 [DOI] [PubMed] [Google Scholar]
- 149.Preuss TM, Wise SP. 2022. Evolution of prefrontal cortex. Neuropsychopharmacology 47(1):3–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Calhoun AJ, Pillow JW, Murthy M. 2019. Unsupervised identification of the internal states that shape natural behavior. Nat Neurosci 22(12):2040–2049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Wolff SB, Olveczky BP. 2018. The promise and perils of causal circuit manipulations. Curr Opin Neurobiol 49:84–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Bredenberg C, Savin C, Kiani R. 2021. Recurrent neural circuits overcome partial inactivation by compensation and re-learning. bioRxiv 2021.11.12.468273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Li N, Daie K, Svoboda K, Druckmann S. 2016. Robust neuronal dynamics in premotor cortex during motor planning. Nature 532(7600):459–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Katz LN, Yates JL, Pillow JW, Huk AC. 2016. Dissociated functional significance of decision-related activity in the primate dorsal stream. Nature 535(7611):285–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Zhou Y, Freedman DJ. 2019. Posterior parietal cortex plays a causal role in perceptual and categorical decisions. Science 365(6449):180–185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Jeurissen D, Shushruth S, El-Shamayleh Y, Horwitz GD, Shadlen MN. 2022. Deficits in decision-making induced by parietal cortex inactivation are compensated at two timescales. Neuron 110(12):1924–1931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Yao JD, Gimoto J, Constantinople CM, Sanes DH. 2020. Parietal cortex is required for the integration of acoustic evidence. Curr Biol 30(17):3293–3303 e4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Zhong L, Zhang Y, Duan CA, Deng J, Pan J, Xu NL. 2019. Causal contributions of parietal cortex to perceptual decision-making during stimulus categorization. Nat Neurosci 22(6):963–973 [DOI] [PubMed] [Google Scholar]
- 159.Erlich JC, Brunton BW, Duan CA, Hanks TD, Brody CD. 2015. Distinct effects of prefrontal and parietal cortex inactivations on an accumulation of evidence task in the rat. Elife 4:e05457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Jun EJ, Bautista AR, Nunez MD, Allen DC, Tak JH, et al. 2021. Causal role for the primate superior colliculus in the computation of evidence for perceptual decisions. Nat Neurosci 24(8):1121–1131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Koay SA, Thiberge SY, Brody CD, Tank DW. 2019. Neural correlates of cognition in primary visual versus neighboring posterior cortices during visual evidence-accumulation-based navigation. bioRxiv 568766 [Google Scholar]
- 162.Musall S, Kaufman MT, Juavinett AL, Gluf S, Churchland AK. 2019. Single-trial neural dynamics are dominated by richly varied movements. Nat Neurosci 22(10):1677–1686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Stringer C, Pachitariu M, Steinmetz N, Reddy CB, Carandini M, Harris KD. 2019. Spontaneous behaviors drive multidimensional, brainwide activity. Science 364(6437):255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Kobak D, Brendel W, Constantinidis C, Feierstein CE, Kepecs A, et al. 2016. Demixed principal component analysis of neural population data. Elife 5:e10989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Williams AH, Kim TH, Wang F, Vyas S, Ryu SI, et al. 2018. Unsupervised discovery of demixed, low-dimensional neural dynamics across multiple timescales through tensor component analysis. Neuron 98(6):1099–1115 e8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Kriegeskorte N, Wei XX. 2021. Neural tuning and representational geometry. Nat Rev Neurosci 22(11):703–718 [DOI] [PubMed] [Google Scholar]
- 167.Ebitz RB, Hayden BY. 2021. The population doctrine in cognitive neuroscience. Neuron 109(19):3055–3068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Kohn A, Jasper AI, Semedo JD, Gokcen E, Machens CK, Yu BM. 2020. Principles of corticocortical communication: Proposed schemes and design considerations. Trends Neurosci 43(9):725–737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Fetsch CR. 2016. The importance of task design and behavioral control for understanding the neural basis of cognitive functions. Curr Opin Neurobiol 37:16–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Roxin A, Ledberg A. 2008. Neurobiological models of two-choice decision making can be reduced to a one-dimensional nonlinear diffusion equation. PLoS Comput Biol 4(3):e1000046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Cohen JD, Dunbar K, McClelland JL. 1990. On the control of automatic processes: a parallel distributed processing account of the stroop effect. Psychol Rev 97(3):332–61 [DOI] [PubMed] [Google Scholar]
- 172.Salinas E. 2004. Context-dependent selection of visuomotor maps. BMC Neurosci 5:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Loh M, Deco G. 2005. Cognitive flexibility and decision-making in a model of conditional visuomotor associations. Eur J Neurosci 22(11):2927–36 [DOI] [PubMed] [Google Scholar]
- 174.Fusi S, Asaad WF, Miller EK, Wang XJ. 2007. A neural circuit model of flexible sensorimotor mapping: learning and forgetting on multiple timescales. Neuron 54(2):319–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Zylberberg A, Fernandez Slezak D, Roelfsema PR, Dehaene S, Sigman M. 2010. The brain’s router: a cortical network model of serial processing in the primate brain. PLoS Comput Biol 6(4):e1000765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Yang GR, Murray JD, Wang XJ. 2016. A dendritic disinhibitory circuit mechanism for pathway-specific gating. Nat Commun 7:12815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Akam T, Kullmann DM. 2010. Oscillations and filtering networks support flexible routing of information. Neuron 67(2):308–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Kremkow J, Aertsen A, Kumar A. 2010. Gating of signal propagation in spiking neural networks by balanced and correlated excitation and inhibition. J Neurosci 30(47):15760–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Song HF, Yang GR, Wang XJ. 2017. Reward-based training of recurrent neural networks for cognitive and value-based tasks. Elife 6:e21492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Chaisangmongkon W, Swaminathan SK, Freedman DJ, Wang XJ. 2017. Computing by robust transience: How the fronto-parietal network performs sequential, category-based decisions. Neuron 93(6):1504–1517 e4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Wang J, Narain D, Hosseini EA, Jazayeri M. 2018. Flexible timing by temporal scaling of cortical responses. Nat Neurosci 21(1):102–110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Sussillo D, Barak O. 2013. Opening the black box: low-dimensional dynamics in high-dimensional recurrent neural networks. Neural Comput 25(3):626–49 [DOI] [PubMed] [Google Scholar]
- 183.Zhang X, Liu S, Chen ZS. 2021. A geometric framework for understanding dynamic information integration in context-dependent computation. iScience 24(8):102919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Maheswaranathan N, Williams AH, Golub MD, Ganguli S, Sussillo D. 2019. Universality and individuality in neural dynamics across large populations of recurrent networks. Adv Neural Inf Process Syst 2019:15629–15641 [PMC free article] [PubMed] [Google Scholar]
- 185.Kohn A, Coen-Cagli R, Kanitscheider I, Pouget A. 2016. Correlations and neuronal population information. Annu Rev Neurosci 39:237–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Kaufman MT, Churchland MM, Ryu SI, Shenoy KV. 2015. Vacillation, indecision and hesitation in moment-by-moment decoding of monkey motor cortex. Elife 4:e04677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Keemink SW, Machens CK. 2019. Decoding and encoding (de)mixed population responses. Curr Opin Neurobiol 58:112–121 [DOI] [PubMed] [Google Scholar]
- 188.Perich MG, Rajan K. 2020. Rethinking brain-wide interactions through multi-region ‘network of networks’ models. Curr Opin Neurobiol 65:146–151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Kleinman M, Chandrasekaran C, Kao JC. 2020. Recurrent neural network models of multi-area computation underlying decision-making. bioRxiv 798553 [Google Scholar]
- 190.. Chaudhuri R, Knoblauch K, Gariel MA, Kennedy H, Wang XJ. 2015. A large-scale circuit mechanism for hierarchical dynamical processing in the primate cortex. Neuron 88(2):419–31 [DOI] [PMC free article] [PubMed] [Google Scholar]