Abstract
Background
Women with gestational diabetes mellitus (GDM) are at greater risk of abnormal birth weight. Since the level of biochemical indicators could often affect the intrauterine growth and development of the fetus, it is of great practical significance to understand the changes of biochemical levels across pregnancy in women with GDM and to find out the indicators that play an important role in predicting birth weight.
Methods
The data source of this study was from the Xi'an Longitudinal Mother–Child Cohort study (XAMC), in which women with GDM with normal and high pre-pregnancy body mass index (BMI) and their newborns between January 1st and March 31st in 2018 were included. The data of ferritin, serum lipid profile and fasting plasma glucose (FPG) of mothers in the three trimesters of pregnancy, as well as birth weight of newborns were all collected from medical records. Multiple linear regression and multivariate logistic regression analyses were used to explore the association of the biochemical indexes and birth weight. A P value < 0.05 was considered statistically significant.
Results
A total of 782 mother-infant pairs were finally included and divided into normal weight group (NG) (n = 530, 67.8%) and overweight/obesity group (OG) (n = 252, 32.2%) according to maternal pre-pregnancy BMI. The level of ferritin in both NG and OG decreased during pregnancy (P for trend < 0.001 for all), whereas the levels of total cholesterol (TC), high density cholesterol (HDL-C), low density cholesterol (LDL-C) and triglycerides (TG) all showed an upward trend (P for trend < 0.05 for all). The levels of FPG in the two groups remained in a relatively stable during the whole pregnancy even though it was higher in OG during the 2nd and 3rd trimesters, whilst HbAlc levels in NG women increased (P for trend = 0.043) during pregnancy. Meanwhile, the risk of macrosomia and large-for-gestational-age (LGA) increased with the increase of FPG level (P for trend < 0.05). Multivariate logistic regression analyses results showed that only FPG level in the 3rd trimester was correlated with birth weight, with birth weight increased by 44.9 g for each SD increase in FPG level.
Conclusion
Maternal FPG in the 3rd trimester is an independent predictor of newborn birth weight, and a higher level of that is associated with an increased risk of macrosomia and LGA.
Keywords: Gestational diabetes mellitus, Ferritin, Lipid profile, Fasting plasma glucose, Birth weight
Background
Gestational diabetes mellitus (GDM) is defined as "diabetes diagnosed in the second or third trimester of pregnancy that is not clearly overt diabetes prior to gestation" [1], is one of the most common metabolic diseases during pregnancy. The incidence has been on the rise in recent decades, although there are significant differences globally [2]. GDM could lead to adverse maternal and neonatal outcomes including shoulder dystocia, stillbirth, metabolic diseases in childhood and adolescence, etc [3–5]. The effect of GDM on birth weight is a matter of concern as it could increase the risk of abnormal birth weight [6]. Hence, there is an urgent need for effective measures for prenatal intervention.
The level of maternal biochemical indicators could often reflect the health of the mother during pregnancy and their offspring. It is well known that birth weight is associated with maternal metabolic disturbances, such as hyperglycemia, dyslipidemia, and other health problems like high iron content which is suggested as the level of ferritin. Hence, it is helpful to predict and monitor the birth weight through the detection of those clinical biochemical indicator, such as ferritin, serum lipid profile, plasma glucose and glycosylated hemoglobin (HbAlc), and some of them were well documented in normal pregnant women [7–9]. Since the level of maternal biochemical indexes change as pregnancy progresses [10], their levels in different stages of pregnancy may have various predictive effects on birth weight of newborns. Hence, it is not enough to only focused on the level of biochemical index in a certain stage of pregnancy [11–13]. Until now, the occurrence of altered serum ferritin, lipid profile and metrics of sugar metabolism have not been fully elucidated in women with GDM. Therefore, the present investigation was carried out to assess the changes of some important biochemical indexes, such as ferritin, serum lipid profile and glucose level in the different trimesters of pregnancy in women with GDM and their relationship with birth weight.
Methods
Data source and study population
The data source of this study was from the Xi'an longitudinal mother–child cohort (XAMC), which is an ongoing large-scale longitudinal mother–child cohort study to investigate the role of external and internal factors on the short-term and long-term outcomes of mothers and children [14]. All pregnant women delivered at Northwest Women's and Children's Hospital between January 1st and March 31st in 2018 were included. From the medical records, we excluded subjects with type 1 or 2 diabetes mellitus before pregnancy as well as those who were not diagnosed with GDM. Patients with multiple birth, incomplete or incorrect data, and stillbirth, or women with a history of severe systemic disease such as cancer, chronic renal failure, severe anemia, or immune disorders and other endocrinopathies were also excluded. Only women with GDM with normal and high pre-pregnancy BMI who had more than one of biochemical index records were selected.
The data of maternal age, parity, gestational age, admission/discharge diagnosis, sociodemographic information, anthropometric characteristics of mothers and newborns, maternal biochemical parameters including ferritin, lipid profile, such as total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), triglyceride (TG), as well as fasting plasma glucose (FPG) and HbAlc were all extracted from the electronic information system of hospital. Generally, the biochemical parameters were measured in the first (gestational age < 13 weeks), middle (13–28 gestational weeks) and third (> 28 weeks) trimesters of pregnancy.
The protocol was approved by the ethical committee of Xi'an Jiaotong University (XJTU 2016–053) and the Northwest Women's and Children's Hospital (NWCH 2012–013). All patients provided written informed consent. The principles of the Helsinki Declaration were followed throughout the study.
GDM diagnosis
All women underwent a 75-g oral glucose tolerance test (OGTT) during 24–28 gestational weeks. The diagnosis was carried out by obstetricians based on the recommendations of the International Association of Diabetes and Pregnancy Study Groups Consensus Panel [15]: fasting glucose 5.1 mmol/L, 1-h glucose 10.0 mmol/L, or 2-h glucose 8.5 mmol/L. The plasma glucose greater than or equal to any of these thresholds was diagnosed as GDM.
Classification and definition
Pre-pregnancy BMI (weight (kg)/ height (m)2) was calculated by maternal height and pre-pregnancy weight. Mothers were divided into two groups according to pre-pregnancy BMI: Normal weight group (NG, BMI:18.5–23.9 kg/m2) and Overweight/Obese group (OG, BMI ≥ 24.0 kg/m2) [16]. Low birth weight (LBW) was defined as birth weight less than 2500 g whilst macrosomia was defined as over 4000 g after 37 weeks' gestation. Infants whose fetal growth less than the 10th percentile at each completed week of gestation were small for gestational age (SGA), and greater than 90th percentile were large for gestational age (LGA) [17]. For educational level, "senior high and below", "undergraduate", and "bachelor above" were regarded as "low", "middle", and "high", respectively.
Statistical analysis
Continuous measures were expressed as mean and standard deviation (SD), while discrete variables were expressed as number and percentage. For continuous variables with normal distribution, Independent-Samples T Test was used for intergroup comparison, whilst Kruskal–Wallis H test was used for those with non-normal distribution. The Chi-square test (Fisher exact test) was used for categorical variables. Trend tests were used to evaluate the changing trends of the data. In multiple linear regression, the biochemical variables were standardized by Z-score method which was implemented by SPSS, and the linear relationships between biochemical indexes and birth weight were analyzed and presented as β (95% CI). Moreover, the relationships between the level of biochemical indexes and the risk of macrosomia and LGA were analyzed by logistic regression analyses and presented as OR (95% CI). All the analyses were performed by the statistical package SPSS (version 23.0, Chicago, IL), and GraphPad Prism 8.0.1 was used for drawing. P-value less than 0.05 was considered statistically significant.
Results
Characteristics of mother-infant pairs
A total of 782 mother-infant pairs were included and 67.8% (n = 530) of them had normal pre-pregnancy BMI (Table 1). The average age of mothers at birth was 31.9 ± 4.2 years old, and 71.1% of mothers had medium education level. More than sixty percent of mothers were uniparas, and 1/10 of mothers had a family history of diabetes. The gestational weight gain in OG women was lower than that in NG (10.6 ± 4.5 kg vs 13.2 ± 4.7 kg, P < 0.001). Compared with NG, the proportion of preterm delivery and cesarean section in OG was much higher (7.5% vs 3.6%, P < 0.05; 61.1% vs 44.9%, P < 0.001). For newborns, the average birth weight was 3405.6 ± 460.7 g, and that is higher in OG although it was just marginally significant (3382.6 ± 429.6 g vs 3454.1 ± 517.6 g, P = 0.058). Moreover, both the ratios of LBW and LGA in OG were higher than those in NG (5.6% vs 1.9%; 17.1% vs 10.9%, P < 0.05 for all).
Table 1.
Baseline characteristics and neonatal outcomes of women with gestational diabetes mellitus
| Total (n = 782) |
NG (n = 530) |
OG (n = 252) |
P | |
|---|---|---|---|---|
| Maternal characteristics | ||||
| Age (years) | 31.9 ± 4.2 | 31.8 ± 4.2 | 32.3 ± 4.1 | 0.097 |
| Educational level (%) | 0.279 | |||
| Low | 113(14.5) | 72(13.6) | 41(16.3) | |
| Middle | 556(71.1) | 375(70.7) | 181(71.8) | |
| High | 113(14.4) | 83(15.7) | 30(11.9) | |
| Preterm (%) | 38(4.9) | 19(3.6) | 19(7.5) | 0.016 |
| Gestational weight gain (kg) | 12.4 ± 4.8 | 13.2 ± 4.7 | 10.6 ± 4.5 | < 0.001 |
| Parity (%) | 0.294 | |||
| 0 | 480(61.4) | 332(62.6) | 148(58.7) | |
| ≥ 1 | 302(38.6) | 198(37.4) | 104(41.3) | |
| Birth delivery (%) | < 0.001 | |||
| Vaginal delivery | 390(49.9) | 292(55.1) | 98(38.9) | |
| Cesarean section | 392(50.1) | 238(44.9) | 154(61.1) | |
| Family history of diabetes (%) | 79(10.1) | 51(9.6) | 28(11.1) | 0.519 |
| Neonatal outcomes | ||||
| Birth weight (g) | 3405.6 ± 460.7 | 3382.6 ± 429.6 | 3454.1 ± 517.6 | 0.058 |
| LBW (%) | 24(3.1) | 10(1.9) | 14(5.6) | 0.005 |
| SGA (%) | 22(2.8) | 19(3.6) | 3(1.2) | 0.058 |
| Macrosomia (%) | 75(9.6) | 44(8.3) | 31(12.3) | 0.076 |
| LGA (%) | 101(12.9) | 58(10.9) | 43(17.1) | 0.017 |
| Sex (%) | 0.918 | |||
| Male | 421(53.8) | 286(54.0) | 135(53.6) | |
| Female | 361(46.2) | 244(46.0) | 117(46.4) | |
NG Normal Group, OG Overweight/obese Group
LBW Low Birth Weight (birth weight < 2500 g); Macrosomia (birth weight ≥ 4000 g), SGA Small for Gestational Age (birth weight less than the 10th percentile at each completed week of gestation), LGA Large for Gestational Age (birth weight greater than 90th percentileat each completed week of gestation)
For educational level, "senior high and below", "undergraduate", and "bachelor above" were regarded as "low", "middle", and "high", respectively
P < 0.05 means there was a statistical difference between the two groups
Trends of biochemical indexes’ levels during pregnancy
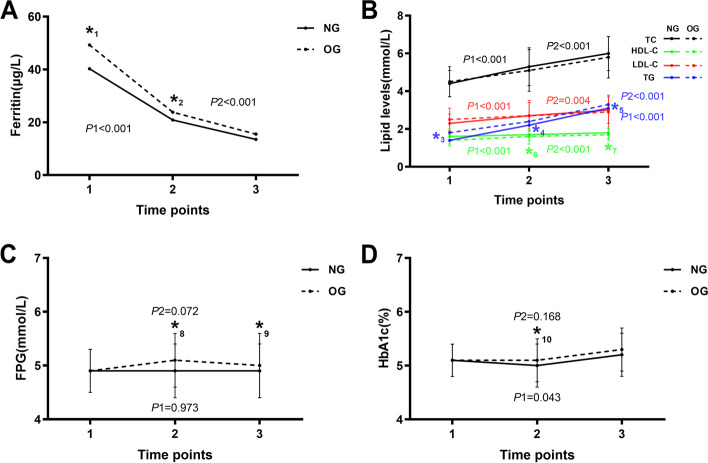
We analyzed the changing trend of the average level of biochemical indexes in different trimesters in NG and OG, respectively (Fig. 1). The results showed statistically significant reduction in ferritin concentration in both groups (P for trend < 0.001 for all). Nevertheless, compared with NG, the level of ferritin in OG was remarkably higher in the 1st and 2nd trimesters [49.3 (32.7, 70.3) μg/L vs 40.3 (23.2, 65.5) μg/L, P < 0.05; 23.8 (13.5, 47.7) μg/L vs 20.9 (11.6, 36.7) μg/L, P < 0.05]. Contrarily, lipid profile data showed pronounced elevations of TC, HDL-C, LDL-C and TG during the whole pregnancy (P for trend < 0.05 for all). Moreover, in the 2nd and 3rd trimester, the HDL-C level of NG was higher than that of OG [(1.7 ± 0.3) mmol/L vs (1.6 ± 0.4) mmol/L; (1.8 ± 0.3) mmol/L vs (1.7 ± 0.3) mmol/L, P < 0.05 for all], while the TG level of OG was significantly higher than that of NG during the whole pregnancy [1.8 (1.3, 2.6) mmol/L vs 1.4 (1.2, 1.8) mmol/L; 2.4 (1.8, 3.3) mmol/L vs 2.2 (1.7, 2.9) mmol/L; 3.3 (2.6, 4.5) mmol/L vs 3.1 (2.4, 3.9) mmol/L]. However, the levels of FPG and HbAlc in two groups were relatively comparable during the pregnancy. Even so, comparisons between the groups showed that the FPG level of OG was higher than that of NG in the 2nd [(5.1 ± 0.5) mmol/L vs (4.9 ± 0.5) mmol/L, P < 0.001] and 3rd trimester [(5.0 ± 0.6) mmol/L vs (4.9 ± 0.5) mmol/L, P < 0.05], while HbAlc level was only higher than that of NG in the 2nd trimester [(5.1 ± 0.4) % vs (5.0 ± 0.4) %, P < 0.001].
Fig. 1.
Level changes of a ferritin, b lipid, c FPG and d HbAlc across pregnancy in women with GDM. NG: Normal Group; OG: Overweight/obese Group; TC: total cholesterol; HDL-C: high-density lipoprotein cholesterol; LDL-C: low-density lipoprotein cholesterol; TG: triglyceride; FPG: fasting plasma glucose. 1: The 1st trimester; 2: The 2nd trimester; 3: The 3rd trimester. *: There was a statistical difference between the two groups at this time point. P values: *1: 0.013; *2:0.042; *3:0.021; *4:0.047; *5: 0.035; *6: 0.030; *7: 0.034; *8: 0.019; *9: 0.044; *10: 0.038. P1: P for trend in NG. P2: P for trend in OG
Relationship between biochemical indexes and birth weight
A simple linear regression analysis between each Z-score standardized index and birth weight was made (Table 2). We found that TG and FPG levels in the 1st trimester and FPG level in the 3rd trimester were positively correlated with birth weight. For each SD increase in TG and FPG level in the 1st trimester and FPG level in the 3rd trimester, birth weight increased by 89.0 g, 61.9 g and 58.9 g, respectively. However, there was a negative correlation between maternal ferritin level and birth weight in the 3rd trimester, and birth weight decreased by 51.1 g for each SD increase in ferritin level. Then, the indexes with P < 0.2 in Table 2 were chosen for further analysis (Table 3). After adjusting for maternal age, educational level, pre-pregnancy BMI, gestational age, gestational weight gain, parity and newborn sex, only the FPG level in the 3rd trimester was positively correlated with birth weight, with birth weight increased by 44.9 g (14.0–75.7 g) for each SD increase in FPG level.
Table 2.
Simple linear regression analyses between each Z-score standardized indexes and neonatal birth weight
| The 1st trimester | The 2nd trimester | The 3rd trimester | |||||||
|---|---|---|---|---|---|---|---|---|---|
| N | β per SD (95% CI) | P | N | β per SD (95% CI) | P | N | β per SD (95% CI) | P | |
| Ferritin (μg/L) | 334 |
5.9 (-44.3, 56.1) |
0.817 | 483 |
6.2 (-34.9, 47.4) |
0.766 | 357 |
-51.1 (-100.3, -1.8) |
0.042 |
| TC (mmol/L) | 164 |
14.4 (-66.1, 94.9) |
0.724 | 255 |
-20.2 (-75.7, 35.4) |
0.475 | 405 |
-12.3 (-57.9, 33.2) |
0.595 |
| HDL-C (mmol/L) |
-17.5 (-98.0, 63.0) |
0.668 |
-20.2 (-75.7, 35.3) |
0.474 |
-31.8 (-77.2, 13.7) |
0.170 | |||
| LDL-C (mmol/L) |
26.5 (-54.4, 107.4) |
0.519 |
-11.5 (-67.1, 44.1) |
0.684 |
-17.2 (-62.9, 28.5) |
0.460 | |||
| TG (mmol/L) |
89.0 (9.7, 168.4) |
0.028 |
30.4 (-25.1, 85.8) |
0.282 |
34.1 (-11.3, 79.5) |
0.141 | |||
| FPG (mmol/L) | 287 |
61.9 (8.6, 115.3) |
0.023 | 293 |
32.0 (-19.2, 83.2) |
0.219 | 597 |
58.9 (22.0, 95.7) |
0.002 |
| HbAlc (%) | 88 |
42.1 (-51.6, 135.9) |
0.374 | 637 |
8.5 (-27.3, 44.2) |
0.643 | 239 |
38.2 (-19.6, 96.0) |
0.194 |
TC Total cholesterol, HDL-C High-density lipoprotein cholesterol, LDL-C Low-density lipoprotein cholesterol, TG Triglyceride, FPG Fasting plasma glucose
The biochemical variables were standardized by Z-score
Table 3.
Beta coefficients (95% CI) for maternal ferritin, serum lipid and glucose level across pregnancy (per SD), in association with neonatal birth weight
| N | Crude- β per SD | P | Adjusted-βa per SD | P | |
|---|---|---|---|---|---|
| The 1st trimester | |||||
| TG (mmol/L) | 164 | 89.0 (9.7, 168.4) | 0.028 | 55.4 (-10.2, 121.1) | 0.097 |
| FPG (mmol/L) | 287 | 61.9 (8.6, 115.3) | 0.023 | 35.8 (-7.3, 78.9) | 0.103 |
| The 3rd trimester | |||||
| Ferritin (μg/L) | 357 | -51.1 (-100.3, -1.8) | 0.042 | -12.1 (-53.5, 29.3) | 0.564 |
| HDL-C (mmol/L) | 405 | -31.8 (-77.2, 13.7) | 0.170 | -21.2 (-59.9, 17.5) | 0.282 |
| TG (mmol/L) | 405 | 34.1 (-11.3, 79.5) | 0.141 | 30.1 (-8.4, 68.5) | 0.125 |
| FPG (mmol/L) | 597 | 58.9 (22.0, 95.7) | 0.002 | 44.9 (14.0, 75.7) | 0.004 |
a: Adjusted for maternal age, educational level, pre-pregnancy BMI, gestational age, gestational weight gain, parity and newborn sex
TG triglyceride, FPG Fasting plasma glucose, HDL-C High-density lipoprotein cholesterol
The biochemical variables were standardized by Z-score
Relationship between FPG level in the 3rd trimester and the risk of high birth weight
Next, we divided the FPG level in the 3rd trimester into tertiles and calculated the proportions of macrosomia and LGA in each category (Fig. 2). As expected, the prevalence of macrosomia and LGA increased with the increase of FPG levels (P for trend < 0.05), with 12.1% and 23.8% of marcrosomia and LGA, respectively in the 3rd tertile. Logistic regression analyses results (Table 4) showed that the risk of macrosomia and LGA increased with the level of FPG in the 3rd trimester (P for trend < 0.05). Even in Model 3 after adjusting for all confounders mentioned above, the risk of macrosomia and LGA increased by 1.44 and 1.50 times in the 3rd tertile of FPG level compared to the 1st tertile.
Fig. 2.
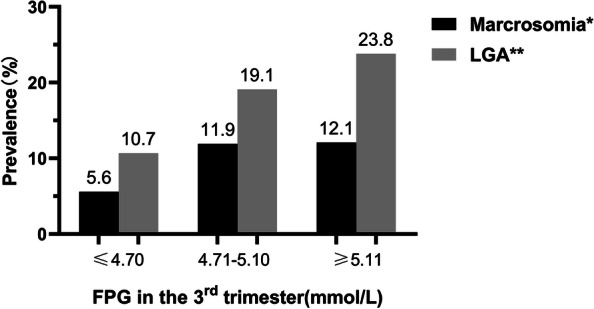
Prevalence of macrosomia and LGA newborns according to tertiles of FPG level in the 3.rd trimester. FPG: fasting plasma glucose; LGA: large-for-gestational-age; *: P for trend = 0.004 < 0.05; **: P for trend < 0.001
Table 4.
Odds Ratios and 95% Confidence Interval for macrosomia and LGA according to tertiles of FPG level in the three trimesters
|
1st tertile (≤ 4.70 mmol/L) |
2nd tertile (4.71–5.10 mmol/L) |
3rd tertile (≥ 5.11 mmol/L) |
P for trend | |
|---|---|---|---|---|
| Macrosomia | ||||
| Model 1 | 1.00 (Ref) | 2.27 (1.08–4.81) | 2.34 (1.12–4.89) | 0.030 |
| Model 2 | 1.00 (Ref) | 2.42 (1.13–5.20) | 2.33 (1.09–4.95) | 0.038 |
| Model 3 | 1.00 (Ref) | 2.52 (1.14–5.57) | 2.44 (1.12–5.33) | 0.035 |
| LGA | ||||
| Model 1 | 1.00 (Ref) | 1.98 (1.11–3.52) | 2.62 (1.50–4.56) | 0.001 |
| Model 2 | 1.00 (Ref) | 2.05 (1.14–3.69) | 2.58 (1.46–4.56) | 0.001 |
| Model 3 | 1.00 (Ref) | 2.06 (1.13–3.76) | 2.50 (1.40–4.47) | 0.002 |
Model 1: unadjusted; Model 2: adjusted by maternal age, pre-pregnancy BMI, educational level, family history of diabetes and parity; Model 3: adjusted by maternal age, pre-pregnancy BMI, educational level, family history of diabetes and parity plus gestational age, gestational weight gain and newborn sex
LGA Large-for-gestational-age
Discussion
In this study, we explored the trend changes of ferritin, serum lipid profile, plasma glucose and HbAlc levels in different pregnant trimesters in women with GDM with normal and high pre-pregnancy BMI and analyzed their effect on newborns’ birth weight. We found that regardless of pre-pregnancy BMI, the level of ferritin showed a downward trend during pregnancy whereas the levels of lipid profiles all showed upward trends while the levels of FPG remained relatively stable, although the average levels were generally higher in women who were overweight/obese before pregnancy. However, only FPG in the third trimester was positively associated with birth weight and the risk of macrosomia and LGA.
A large number of studies have shown that ferritin is closely related to the risk of GDM [8, 18–22]. The mechanism may be that the increase of serum ferritin induces the inflammatory process, which leads to the decrease of insulin secretion, the increase of insulin resistance and the disorder of liver function, and finally the decrease of muscle glucose uptake and the increase of gluconeogenesis, leading to the development of GDM [23, 24]. So we firstly analyzed the changes of ferritin levels in women with GDM during the whole pregnancy, and found that ferritin levels decreased in both women with GDM with normal and high pre-pregnancy BMI, which was consistent with the results of a randomized, double-blinded study of black women [25]. In addition, we also found that the ferritin levels of women with GDM who were overweight/obese before pregnancy were higher than those with normal pre-pregnancy BMI in the first and second trimester of pregnancy (Fig. 1). Previous studies have shown that obese and overweight adolescents have higher ferritin level than adolescents of normal weight [26, 27], possibly because inflammatory factors were activated during obesity and, accordingly, the iron regulating protein hepcidin was released as a defense mechanism, caused an increase in ferritin level [28]. Moreover, we did not find any effect of ferritin levels at all stages of pregnancy on birth weight after adjustment, which was similar to the opinions from previous studies [24].
Serum lipids are important indicators to reflect the health of the mother and fetus during pregnancy and dyslipidemia during pregnancy could increase the risk of adverse pregnancy outcomes such as GDM, preeclampsia and preterm delivery [7, 29]. The change of maternal lipid metabolism is a normal part of pregnancy. Fat accumulates in maternal tissue in the early stage of pregnancy and hyperlipidemia occurs in the third trimester of pregnancy [10]. But how is the situation in women with GDM? Ryckman et al. [30] once found that TG levels continued to increase throughout pregnancy in GDM patients. A previous study further revealed that changes in TG and TC levels were mainly caused by pre-pregnancy BMI [31]. In this study, we also found that the level of TC、HDL-C、LDL-C、TG increased during pregnancy in all women with GDM. And, the TG level of women with GDM with high pre-pregnancy BMI was higher than that of people with normal pre-pregnancy BMI. Since hypertriglyceridemia was considered to be one of the key driving factors of macrosomia [32], women with GDM with high BMI before pregnancy should be more vigilant against the occurrence of high birth weight. However, after adjusting for possible confounders, we found lipid levels at all stages of pregnancy had no effect on birth weight. This was inconsistent with the results of a multi-center Korean study in which a positive association between serum TG levels and birth weight in both the second and third trimester was found [33]. The main reason for the discrepancy may be that the population we studied was only women with GDM, which may affect the relationship between TG and birth weight.
Glucose metabolism is of particular concern throughout pregnancy and often played crucial role in the development of GDM. Previous studies showed that the mean HbA1c level decreased from the 1st trimester until the 2nd trimester, and increased towards the 3rd trimester in women with pre-gestational diabetes [34], and there was a slight change in FPG level in the cohort of healthy pregnant women [35]. In this study, the levels of FPG and HbAlc of women with GDM remained in a relatively stable range during the whole pregnancy, because the glucose of women with GDM was controlled more strictly than that of normal women. Even so, further comparisons between the groups showed that the FPG level of OG was higher than that of NG in the 2nd and 3rd trimester, while HbAlc level was higher than that of NG in the 2nd trimester. This is consistent with the finding that the values of OGTT in obese women were on the high side in the second trimester [36]. In addition, a study from Norway also suggested that women who were overweight/obese had significantly higher FPG in the 3rd trimester than women with normal weight [35]. The results of multiple linear regression analysis showed that there was only a correlation between FPG in the 3rd trimester and birth weight (Table 3). And the results of further exploration showed that high levels of FPG in the 3rd trimester could increase the risk of macrosomia and LGA. The risk of macrosomia and LGA in GDM patients with FPG > 4.71 mmol/L in late pregnancy is more than twice as high as that in women with FPG ≤ 4.70 mmol/L based on the data of our study (Table 4), which means that women with GDM with normal and high pre-pregnancy BMI should pay special attention to FPG in the 3rd trimester. However, a large cohort study of healthy pregnant women found that FPG in both 1st and 3rd trimester of pregnancy were associated with birth weight, and there was a negative correlation between HDL-C and birth weight in the 3rd trimester [35], which is slightly different from the findings of this study. The reason may be the difference in study population or sample size and confounding factors, which still needs further study.
Strengths and limitations
One of the major strengths of our study was that, to the best of our knowledge, this was the first time the associations of key clinical maternal biochemical indexes of three trimesters with neonatal birth weight were examined. Previous studies indicated that the level of maternal biochemical indexes, such as ferritin, serum lipid, plasma glucose and so on may have a predictive effect on neonatal birth weight [11–13]. However, majority of those studies only focused on a certain time point of pregnancy and it was hard to reveal the real effect on birth weight. Secondly, we did all analyses based on different pre-pregnancy BMI levels of subjects since the BMI before pregnancy has an important influence on the pregnancy outcome of women with GDM, including neonatal birth weight [37, 38]. However, some limitations of our study have to be considered. Firstly, this was a cross-sectional study needs to be further validated in conjunction with cohort studies. Based on our XAMC cohort, an ongoing observation study will prospectively provide more useful hints. Secondly, since women with GDM who were underweight before pregnancy were not included because of very small proportion, whether the conclusion can be applied to women with GDM with lower BMI before pregnancy is unknown.
Conclusions
In women with GDM regardless of pre-pregnancy BMI, the level of ferritin decreased whist the levels of lipid profiles increased, and the levels of FPG remained relatively stable during the whole pregnancy. However, only FPG in the 3rd trimester was an independent predictor of newborn birth weight, and that is associated with an increased risk of macrosomia and LGA. These observations highlight the importance of controlling the levels of FPG levels of women with GDM during pregnancy clinically.
Acknowledgements
Thanks to the staff of Northwest Women's and Children's Hospital for their support of this study.
Abbreviations
- GDM
Gestational diabetes mellitus
- BMI
Body mass index
- XAMC
Xi’an Longitudinal Mother–Child Cohort study
- NG
Normal group
- OG
Overweight/obesity group
- FPG
Fasting plasma glucose
- TG
Triglycerides
- TC
Total cholesterol
- HDL-C
High density cholesterol
- LDL-C
Low density cholesterol
- HbA1c
Glycosylated hemoglobin
- OGTT
Oral glucose tolerance test
- LGA
Large for gestational age
- SGA
Small for gestational age
- LBW
Low birth weight
- OR
Odds ratio
- CI
Confidence interval
Authors’ contributions
XQL designed the study. JJ and PW wrote the manuscript. JJ, PW, GHL, ZYH and SSW collected the data. JJ, PW, GHL, SSW, and WLY were involved in data cleaning and statistics. YM and CL contributed to the interpretation of the results and critical revision of the manuscript for important intellectual content. All authors gave the final approval of the version to be published.
Funding
This research was funded by the National Natural Science Foundation of China (No. 81874263).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
The protocol was approved by the ethical committee of Xi’an Jiaotong University (XJTU 2016–053) and the Northwest Women and Children's Hospital (NWCH 2012–013). All women provided gave written informed consent. The principles of the Helsinki Declaration were followed throughout the study.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Jing Ji and Pei Wu contributed equally to this work.
References
- 1.Classification and Diagnosis of Diabetes Standards of medical care in diabetes-2022. Diabetes Care. 2022;45(Suppl 1):S17–s38. doi: 10.2337/dc22-S002. [DOI] [PubMed] [Google Scholar]
- 2.Zhu Y, Zhang C. Prevalence of gestational diabetes and risk of progression to type 2 diabetes: a global perspective. Curr DiabRep. 2016;16(1):7. doi: 10.1007/s11892-015-0699-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Billionnet C, Mitanchez D, Weill A, et al. Gestational diabetes and adverse perinatal outcomes from 716,152 births in France in 2012. Diabetologia. 2017;60(4):636–644. doi: 10.1007/s00125-017-4206-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lemieux P, Benham JL, Donovan LE, Moledina N, Pylypjuk C, Yamamoto JM. The association between gestational diabetes and stillbirth: a systematic review and meta-analysis.Diabetologia. 2021. 10.1007/s00125-021-05579-0. [DOI] [PubMed]
- 5.Perng W, Ringham BM, Smith HA, Michelotti G, Kechris KM, Dabelea D. A prospective study of associations between in utero exposure to gestational diabetes mellitus and metabolomic profiles during late childhood and adolescence. Diabetologia. 2020;63(2):296–312. doi: 10.1007/s00125-019-05036-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shang M, Wen Z. Increased placental IGF-1/mTOR activity in macrosomia born to women with gestational diabetes. Diabetes Res Clin Pract. 2018;146:211–219. doi: 10.1016/j.diabres.2018.10.017. [DOI] [PubMed] [Google Scholar]
- 7.Adank MC, Benschop L, Peterbroers KR, et al. Is maternal lipid profile in early pregnancy associated with pregnancy complications and blood pressure in pregnancy and long term postpartum? Am J Obstet Gynecol. 2019;221(2):150.e151–150.e113. doi: 10.1016/j.ajog.2019.03.025. [DOI] [PubMed] [Google Scholar]
- 8.Chen X, Scholl TO, Stein TP. Association of elevated serum ferritin levels and the risk of gestational diabetes mellitus in pregnant women: the Camden study. Diabetes Care. 2006;29(5):1077–1082. doi: 10.2337/diacare.2951077. [DOI] [PubMed] [Google Scholar]
- 9.Yamamoto JM, Kellett JE, Balsells M, et al. Gestational diabetes mellitus and diet: a systematic review and meta-analysis of randomized controlled trials examining the impact of modified dietary interventions on maternal glucose control and neonatal birth weight. Diabetes Care. 2018;41(7):1346–1361. doi: 10.2337/dc18-0102. [DOI] [PubMed] [Google Scholar]
- 10.Alvarez JJ, Montelongo A, Iglesias A, Lasunción MA, Herrera E. Longitudinal study on lipoprotein profile, high density lipoprotein subclass, and postheparin lipases during gestation in women. J Lipid Res. 1996;37(2):299–308. doi: 10.1016/S0022-2275(20)37617-3. [DOI] [PubMed] [Google Scholar]
- 11.Judistiani RTD, Madjid TH, Irianti S, et al. Association of first trimester maternal vitamin D, ferritin and hemoglobin level with third trimester fetal biometry: result from cohort study on vitamin D status and its impact during pregnancy and childhood in Indonesia. BMC Pregnancy Childbirth. 2019;19(1):112. doi: 10.1186/s12884-019-2263-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Spencer JA, Chang TC, Crook D, et al. Third trimester fetal growth and measures of carbohydrate and lipid metabolism in umbilical venous blood at term. Arch Dis Child Fetal Neonatal Ed. 1997;76(1):F21–25. doi: 10.1136/fn.76.1.f21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yachi Y, Tanaka Y, Nishibata I, et al. Second trimester postload glucose level as an important predictor of low birth weight infants: Tanaka Women's Clinic Study. Diabetes Res Clin Pract. 2014;105(3):e16–19. doi: 10.1016/j.diabres.2014.02.006. [DOI] [PubMed] [Google Scholar]
- 14.Ji J, He Z, Qu P, et al. The Xi'an longitudinal mother-child cohort study: design, study population and methods. Eur J Epidemiol. 2021;36(2):223–232. doi: 10.1007/s10654-020-00704-6. [DOI] [PubMed] [Google Scholar]
- 15.Metzger BE, Gabbe SG, Persson B, et al. International association of diabetes and pregnancy study groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care. 2010;33(3):676–682. doi: 10.2337/dc09-1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet (London, England). 2004;363(9403):157–163. doi:10.1016/s0140-6736(03)15268-3. [DOI] [PubMed]
- 17.Dai L, Deng C, Li Y, et al. Birth weight reference percentiles for Chinese. PloS one. 2014;9(8):e104779. doi: 10.1371/journal.pone.0104779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bowers KA, Olsen SF, Bao W, Halldorsson TI, Strøm M, Zhang C. Plasma concentrations of ferritin in early pregnancy are associated with risk of gestational diabetes mellitus in women in the Danish national birth cohort. J Nutr. 2016;146(9):1756–1761. doi: 10.3945/jn.115.227793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang X, Wu M, Zhong C, et al. Association between maternal plasma ferritin concentration, iron supplement use, and the risk of gestational diabetes: a prospective cohort study. Am J Clin Nutr. 2021;114(3):1100–1106. doi: 10.1093/ajcn/nqab162. [DOI] [PubMed] [Google Scholar]
- 20.Behboudi-Gandevani S, Safary K, Moghaddam-Banaem L, Lamyian M, Goshtasebi A, Alian-Moghaddam N. The relationship between maternal serum iron and zinc levels and their nutritional intakes in early pregnancy with gestational diabetes. Biol Trace Elem Res. 2013;154(1):7–13. doi: 10.1007/s12011-013-9703-y. [DOI] [PubMed] [Google Scholar]
- 21.Cheng Y, Li T, He M, et al. The association of elevated serum ferritin concentration in early pregnancy with gestational diabetes mellitus: a prospective observational study. Eur J Clin Nutr. 2020;74(5):741–748. doi: 10.1038/s41430-019-0542-6. [DOI] [PubMed] [Google Scholar]
- 22.Soubasi V, Petridou S, Sarafidis K, et al. Association of increased maternal ferritin levels with gestational diabetes and intra-uterine growth retardation. Diabetes Metab. 2010;36(1):58–63. doi: 10.1016/j.diabet.2009.06.010. [DOI] [PubMed] [Google Scholar]
- 23.Swaminathan S, Fonseca VA, Alam MG, Shah SV. The role of iron in diabetes and its complications. Diabetes Care. 2007;30(7):1926–1933. doi: 10.2337/dc06-2625. [DOI] [PubMed] [Google Scholar]
- 24.Barbieri M, Ragno E, Benvenuti E, et al. New aspects of the insulin resistance syndrome: impact on haematological parameters. Diabetologia. 2001;44(10):1232–1237. doi: 10.1007/s001250100634. [DOI] [PubMed] [Google Scholar]
- 25.Goldenberg RL, Tamura T, DuBard M, Johnston KE, Copper RL, Neggers Y. Plasma ferritin and pregnancy outcome. Am J Obstet Gynecol. 1996;175(5):1356–1359. doi: 10.1016/s0002-9378(96)70054-6. [DOI] [PubMed] [Google Scholar]
- 26.Huang YF, Tok TS, Lu CL, Ko HC, Chen MY, Chen SC. Relationship Between being Overweight and Iron Deficiency in Adolescents. Pediatr Neonatol. 2015;56(6):386–392. doi: 10.1016/j.pedneo.2015.02.003. [DOI] [PubMed] [Google Scholar]
- 27.Shattnawi KK, Alomari MA, Al-Sheyab N, Bani SA. The relationship between plasma ferritin levels and body mass index among adolescents. Sci Rep. 2018;8(1):15307. doi: 10.1038/s41598-018-33534-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Eftekhari M, Mozaffari-Khosravi H, Shidfar F. The relationship between BMI and iron status in iron-deficient adolescent Iranian girls. Public Health Nutr. 2009;12(12):2377–2381. doi: 10.1017/s1368980009005187. [DOI] [PubMed] [Google Scholar]
- 29.Jin WY, Lin SL, Hou RL, et al. Associations between maternal lipid profile and pregnancy complications and perinatal outcomes: a population-based study from China. BMC Pregnancy Childbirth. 2016;16:60. doi: 10.1186/s12884-016-0852-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ryckman KK, Spracklen CN, Smith CJ, Robinson JG, Saftlas AF. Maternal lipid levels during pregnancy and gestational diabetes: a systematic review and meta-analysis. BJOG. 2015;122(5):643–651. doi: 10.1111/1471-0528.13261. [DOI] [PubMed] [Google Scholar]
- 31.Schreuder YJ, Hutten BA, van Eijsden M, et al. Ethnic differences in maternal total cholesterol and triglyceride levels during pregnancy: the contribution of demographics, behavioural factors and clinical characteristics. Eur J Clin Nutr. 2011;65(5):580–589. doi: 10.1038/ejcn.2010.282. [DOI] [PubMed] [Google Scholar]
- 32.Kitajima M, Oka S, Yasuhi I, Fukuda M, Rii Y, Ishimaru T. Maternal serum triglyceride at 24–32 weeks' gestation and newborn weight in nondiabetic women with positive diabetic screens. Obstet Gynecol. 2001;97(5 Pt 1):776–780. doi: 10.1016/s0029-7844(01)01328-x. [DOI] [PubMed] [Google Scholar]
- 33.Hwang JY, Choi HI, Kim H, et al. Relationship of maternal grain intake and serum triglyceride levels with infant birth weight: Mothers and Children's Environmental Health (MOCEH) study. Eur J Clin Nutr. 2015;69(6):676–680. doi: 10.1038/ejcn.2014.271. [DOI] [PubMed] [Google Scholar]
- 34.Toft JH, Dalen I, Skadberg Ø, Gøransson LG, Økland I, Bleskestad IH. Glycated albumin and continuous glucose monitoring metrics across pregnancy in women with pre-gestational diabetes. Endocrinol Diabetes Metab. 2022;5(6):e376. doi: 10.1002/edm2.376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Roland MCP, Lekva T, Godang K, Bollerslev J, Henriksen T. Changes in maternal blood glucose and lipid concentrations during pregnancy differ by maternal body mass index and are related to birthweight: A prospective, longitudinal study of healthy pregnancies. PloS one. 2020;15(6):e0232749. doi: 10.1371/journal.pone.0232749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Farah N, McGoldrick A, Fattah C, O'Connor N, Kennelly MM, Turner MJ. Body Mass Index (BMI) and Glucose Intolerance during Pregnancy in White European Women. J Reprod Infertil. 2012;13(2):95–99. [PMC free article] [PubMed] [Google Scholar]
- 37.Byström M, Liu A, Quinton AE, et al. Gestational Diabetes Independently Increases Birth Length and Augments the Effects of Maternal BMI on Birth Weight: A Retrospective Cohort Study. Front Pediatr. 2014;2:112. doi: 10.3389/fped.2014.00112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Silva S, Saunders C, Zajdenverg L, et al. Predictive factors for birth weight of newborns of mothers with gestational diabetes mellitus. Diabetes Res Clin Pract. 2018;138:262–270. doi: 10.1016/j.diabres.2018.01.032. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.