ABSTRACT
Facultative marine bacterial pathogens sense environmental signals so that the expression of virulence factors is upregulated on entry into hosts and downregulated during the free-living lifestyle in the environment. In this study, we utilized transcriptome sequencing to compare the transcriptional profiles of Photobacterium damselae subsp. damselae, a generalist pathogen that causes disease in diverse marine animals and fatal infections in humans at NaCl concentrations that mimic the free-living lifestyle or host internal milieu, respectively. We here show that NaCl concentration constitutes a major regulatory signal that shapes the transcriptome and uncover 1,808 differentially expressed genes (888 upregulated and 920 downregulated in response to low-salt conditions). Growth at 3% NaCl, a salinity that mimics the free-living lifestyle, upregulated genes involved in energy production, nitrogen metabolism, transport of compatible solutes, utilization of trehalose and fructose, and carbohydrate and amino acid metabolism with strong upregulation of the arginine deiminase system (ADS). In addition, we observed a marked increase in resistance to antibiotics at 3% NaCl. On the contrary, the low salinity conditions (1% NaCl) that mimic those encountered in the host triggered a virulence gene expression profile that maximized the production of the type 2 secretion system (T2SS)-dependent cytotoxins damselysin, phobalysin P, and a putative PirAB-like toxin, observations that were corroborated by the analysis of the secretome. Low salinity also upregulated the expression of iron-acquisition systems, efflux pumps, and other functions related to stress response and virulence. The results of this study greatly expand our knowledge of the salinity-responsive adaptations of a generalist and versatile marine pathogen.
IMPORTANCE
Pathogenic Vibrionaceae species experience continuous shifts of NaCl concentration in their life cycles. However, the impact of salinity changes in gene regulation has been studied in a small number of Vibrio species. In this study, we analyzed the transcriptional response of Photobacterium damselae subsp. damselae (Pdd), a generalist and facultative pathogen, to changes in salinity, and demonstrate that growth at 1% NaCl in comparison to 3% NaCl triggers a virulence program of gene expression, with a major impact in the T2SS-dependent secretome. The decrease in NaCl concentration encountered by bacteria on entry into a host is proposed to constitute a regulatory signal that upregulates a genetic program involved in host invasion and tissue damage, nutrient scavenging (notably iron), and stress responses. This study will surely inspire new research on Pdd pathobiology, as well as on other important pathogens of the family Vibrionaceae and related taxa whose salinity regulons still await investigation.
KEYWORDS: Photobacterium damselae, salinity, NaCl, transcriptome, secretome, virulence, hemolysin, arginine deiminase, damselysin
INTRODUCTION
Bacterial pathogens need to orchestrate the expression of virulence factors in a cell economy-driven fashion that guarantees the production of these factors only when and where they are actually needed. This is of special importance in generalist and facultative marine pathogens that thrive in a free-living lifestyle and also are capable of colonizing and infecting animals (1). In marine ecosystems, one of the main environmental signals that is highly informative of the transition from the free-living phase in seawater bodies to an infective stage is the abrupt drop of NaCl concentration encountered on entry into the internal milieu of an animal host.
The family Vibrionaceae comprises a genetically and metabolically diverse group of marine bacteria, and many species include animal and human pathogenic strains (2, 3). Most species enjoy a free-living existence in ocean water, attached to organic particles, and colonizing biotic and abiotic surfaces (4, 5). Thus, the association of pathogenic Vibrios with animals can be considered an optional part of their life cycle, and only a very reduced number of species might be viewed as obligate pathogens. Though pathogenic Vibrionaceae species experience continuous shifts of NaCl concentration in their life cycles, very few species of this family have been the subject of global transcriptomic studies aimed at investigating the role of salinity in gene expression modulation. These include Vibrio cholerae (6, 7), Vibrio parahaemolyticus (8 - 10), Vibrio brasiliensis (11), and Vibrio fujianensis (12). However, some of these studies focused on the study of salt stress and employed NaCl conditions that do not mimic those encountered in nature (12). Other studies compared conditions that differed in NaCl concentrations but also in additional variables, as is the case of a study with Vibrio vulnificus grown in human serum versus seawater (13).
Photobacterium damselae subsp. damselae (hereafter Pdd), formerly known as Vibrio damsela, is a member of the Vibrionaceae that has been isolated in marine ecosystems from diverse sources such as coastal ocean waters (14), sediment (15), shellfish (16), marine bird droppings (17), digestive tract of whales (18), fish and shrimp intestines (19, 20), and internal organs of apparently healthy fish (21, 22). It is also a strong histamine-producing bacterium commonly isolated from the retailed fish (23). In addition, Pdd is a highly versatile and virulent generalist pathogen of increasing financial concern that causes primary disease in crustaceans and in countless species of fish, wild as well as cultivated in aquaculture facilities in different parts of the globe (24, 25). Of note, Pdd is also an opportunistic human pathogen that can cause wound-associated infections on contact with the marine milieu and fatal necrotizing fasciitis (26).
The highly virulent strains of Pdd cause massive tissue damage in the infected hosts due to the plasmid (pPHDD1)-encoded cytotoxins damselysin (Dly) and phobalysin P (PhlyP), and the chromosome-encoded phobalysin C (PhlyC), which are secreted in very high amounts by the type 2 secretion system (T2SS) (27). Dly is a phospholipase D active against sphingomyelin, whereas PhlyP and PhlyC are pore-forming toxins. Additional demonstrated virulence factors of Pdd include a polysaccharide capsule that contributes to Pdd resistance to host defense mechanisms (28).
The biological and environmental signals that regulate gene expression in Pdd and, specifically, virulence factors are scarcely understood. Expression of the cytotoxins and biogenesis of the polysaccharide capsule are positively regulated by the two-component system RstAB (27 - 29), homologous to the V. cholerae CarSR (30), but the specific signal that triggers the activation of the RstAB system remains unknown. Previous studies have reported that iron limitation upregulates the expression of genes encoding Dly, PhlyP, and PhlyC cytotoxins (31, 32). Also, NaCl levels were found to modulate the transcriptional activity of the promoters of the three aforementioned cytotoxins (31), as well as phospholipase and hemolytic activities (33), but no further insights have been taken to study the role of salinity in Pdd gene regulation. In this study, we use RNA sequencing (RNA-seq) to analyze the transcriptional profiles of the highly virulent Pdd strain RM-71 grown at average seawater salinity (3% NaCl) and at the salinity of the internal milieu of vertebrate hosts (1% NaCl), respectively. Our results reveal that a shift from high to low salinity triggers a virulence gene expression profile with a strong impact in the T2SS-dependent secretome, including the upregulation of cytotoxins, iron-acquisition systems, and other functions potentially involved in pathogenicity.
MATERIALS AND METHODS
Standard culture conditions
Pdd strains were routinely grown at 25°C on tryptic soy agar (TSA) or tryptic soy broth (TSB) and in M9 minimal medium (34) supplemented with 0.2% casamino acids (Difco) (CM9) and 0.5% glucose. NaCl was adjusted to final concentrations of 1% (TSA-1, TSB-1, CM9-1) or 3% (TSA-3, TSB-3, CM9-3), as necessary. When necessary, antibiotics were supplied at the following final concentrations: kanamycin (Km) at 50 µg/mL and rifampicin (Rif) at 20 µg/mL.
RNA extraction and RNA-seq
The highly virulent Pdd strain RM-71 selected for the present study carries the virulence plasmid pPHDD1 and was isolated from a diseased turbot in 1988 during an outbreak in Galicia (northwest Spain) (35). For RNA-seq, three independent precultures of RM-71 for each salinity condition (1% NaCl and 3% NaCl) in TSB were grown until they reached an optical density at 600 (OD600) of 0.3. Then, each preculture was diluted (1:100) and grown in 10-mL TSB until an OD600 of 0.55. At this point, cultures were instantly treated with RNAprotect Bacteria Reagent (Qiagen) for RNA stabilization. Pelleted cells were resuspended in TE buffer (30 mM Tris-HCl, 1 mM EDTA, pH 8.0) with 10-µL lysozyme (15 mg/mL) (Sigma-Aldrich) and 15-µL Proteinase K (20 mg/mL) (Qiagen). The RNeasy Mini Kit (Qiagen) was used for RNA extraction and on-column digestion of DNA with DNase I was performed using the RNase-free DNase kit (Qiagen). The integrity and the amount of the total RNA were evaluated using a Bioanalyzer 2100 (RNA 6000 Nano chip assay) and a Qubit 3.0 (Quant-It dsRNA BR Assay). rRNA depletion was performed with the Ribo-Zero rRNA Removal Kit (Gram-negative bacteria) (Illumina), and cDNA libraries were generated using the TruSeq RNA kit in accordance with Illumina’s instructions. First, rRNA-depleted RNA was chemically fragmented before being subjected to reverse transcription for cDNA synthesis. A reparative process was performed by adding a single “A” base to the 3′ end of cDNA fragments following adapters’ ligation. Finally, cleaned products were enriched by PCR to generate the double-stranded cDNA library that was sequenced on an Illumina HiSeq 2500 sequencer.
RNA-seq data analysis
RNA-seq raw reads were analyzed with FastQC program as previously described (36) and mapped against the genome of RM-71 (RefSeq assembly accession no.: GCF_001708035.2) using the Bowtie2 v2.2.6 algorithm (37). Poor-quality readings were eliminated using PicardTools (http://picard.sourceforge.net). Several quality steps were performed to evaluate sequencing and mapping processes. Distribution of GC content and duplicate proportion of mappable readings were evaluated. Genetic quantification was performed by the HTSeq software (0.6.1 version) (38). A correlation and distance study between samples from the same condition was carried out to evaluate them as biological replicates. For this, the transcriptome normalized by the size of the library was analyzed with statistics program R. The analysis of the differential expression was performed using DESeq2 method (1.18.1 version) (39), and a differential negative binomial distribution was applied to determine statistical significance. A Python script developed at Sistemas Genómicos (Valencia, Spain) was employed to generate a data matrix with the counts obtained for each sample (each of the three replicates at each of the two salinities). Genes were considered as differentially expressed when fold change (FC) values were lower than −1.5 or higher than 1.5 and a P-value adjusted by false discovery rate (FDR) ≤ 0.05. Differentially expressed genes (DEGs) were mapped against Uniprot, COG (Cluster of Orthologous Groups), GO (Gene Ontology) and KEGG (Kyoto Encyclopaedia of Genes and Genomes) databases and analyzed using the hyper-geometric test. An FDR-adjusted P-value of 0.05 was used to determine a functional category as statistically significant or over-represented (40).
Growth experiments
For growth assays, an inoculum of Pdd RM-71 strain adjusted to an OD600 of 0.3 was diluted (1:100) in 100 µL of the tested media in a 96-well plate. Bacterial cultures were incubated at 25°C under shaking conditions, and growth was monitored for 24–48 hours using the spectrophotometer Epoch2 microplate reader (BioTek). The experiments were independently performed twice, and at least two replicates per condition were included. Growth media were prepared according to the aim of the experiments as detailed below.
Growth in seawater microcosms. To evaluate Pdd growth in marine-like microcosms, coastal seawater samples were collected at Lodeiro beach, Rianxo, Galicia (northwest Spain). One sample consisting of plain seawater without macroscopically visible particulate material was sterilized in an autoclave and named as NSW (natural seawater). A second sample of 100-mL seawater was supplemented with 30 g (wet weight) of macroalgae (Ulva lactuca) occurring in the surroundings of the same collection site, and the mix was mechanically ground and then boiled at 95°C for 1 minute and labeled as ESW (eutrophicated seawater). The two types of samples were supplemented with 0.2% casamino acids (Difco) when necessary. For growth experiments, three replicates per condition were prepared using an inoculum of Pdd RM-71 adjusted to an OD600 of 0.3 and diluted (1:100) in 100 µL of NSW or ESW in a 96-well plate. Bacterial growth was monitored for 24 or 48 hours using the spectrophotometer Epoch2 microplate reader (BioTek) under shaking conditions.
Carbohydrate-supplemented minimal medium. Pdd RM-71 strain was tested for the ability to ferment the carbohydrates fructose and trehalose under different NaCl concentrations. For this purpose, OF (Oxidation/Fermentation) basal medium (Difco) was supplemented with agar (15 g/L) and NaCl to a final concentration of 1% or 3%. Fructose and trehalose (Sigma-Aldrich) were added individually to a final concentration of 0.5%, and plates were streaked with RM-71 strain from a freshly grown TSA-1 plate. Plates were incubated at 25°C and monitored for 3 days. The ability to ferment the sugars was indicated by a color change from green to acidic yellow. For growth experiments, trehalose-minimal medium and fructose-minimal medium were prepared by supplementing CM9 minimal medium with 0.5% of each carbohydrate. NaCl concentrations were adjusted to final concentrations of 1% and 3%, as necessary. Growth in CM9-minimal medium with 0.5% glucose at both NaCl concentrations was included in the experiment as a control.
Growth with antibiotics under different NaCl concentrations. To assess the impact of NaCl concentration on growth in the presence of antibiotics, Pdd RM-71 was grown in TSB-1 and TSB-3 supplemented with kanamycin (50 µg/mL), ampicillin (100 µg/mL), and vancomycin (25 µg/mL). After 40 hours, cells grown in TSB-3 were passaged (1:100) to fresh antibiotic-supplemented TSB medium at both NaCl conditions in a 96-well plate and growth was monitored for 40 hours.
Antimicrobial activity of piscidin peptides against Pdd. Piscidin peptide 1 (PP1) was designed and produced as described by Barroso et al. (41). PP1 at 15 µM (previously determined minimum inhibitory concentration) was serially diluted (1:2) up to a concentration of 0.12 µM and incubated with Pdd RM-71 (108 CFU/mL) on TSB-1 and TSB-3 in a final volume of 100 µL in 96-well plates. Wells without bacteria and wells with no added peptide were used as blanks and controls, respectively.
Hemolysis and phospholipase assays
Hemolysis and phospholipase activities were evaluated on TSA plates supplemented with 5% sheep blood agar (Thermo Scientific) and 3% egg yolk extract (Oxoid), respectively. To determine the impact of NaCl concentration in the phenotypical observation of these two activities, NaCl was added to a final concentration of 1% or 3% during plate preparation. RM-71 cultures were grown in TSB-1 and TSB-3 up to an OD600 of 1.2, and 2 µL of the suspension were spotted on each assay plate and incubated at 25°C for 24–48 hours. The hemolytic and phospholipase activities were calculated by dividing the halo diameter by the colony diameter. A Student’s t-test was used to determine statistical significance.
Motility assays
Motility was determined using the swim-migration assay (42). For this, 4 µL of cultures grown on TSB-1 or TSB-3 up to an OD600 of 0.2 were vertically inoculated into a semisolid TSA plate, containing 0.25% agar, 10 mM of L-arginine (Sigma-Aldrich), and either 1% or 3% NaCl. Plates were incubated at 25°C under aerobic and anaerobic conditions for 24 hours and motility haloes were measured. An AnaeroJar containing AnaeroGen sachets (Thermo Fisher Scientific) was used to generate the anaerobic conditions, and resazurin strips (Thermo Fisher Scientific) were used to confirm the absence of oxygen during incubation.
Arginine decarboxylase/dihydrolase test
Arginine Moeller’s medium was used to test arginine decarboxylase/dihydrolase activity by dissolving 10 g/L of Decarboxylase Base Moeller (Difco), 10 g/L of L-arginine (Sigma-Aldrich), and supplemented with 1% or 3% NaCl. Tubes were inoculated with bacteria precultured in TSB-1 or TSB-3 medium to an OD600 of 1.4 and covered with 1 mL of sterile mineral oil. A non-inoculated tube was included as a negative control. Tubes were incubated at 25°C for 48 hours and monitored every 12 hours for changes.
Acid survival assays under different NaCl conditions
To investigate the effect of salinity on Pdd sensitivity to acid, growth kinetics at various pH levels and survival assays were determined under different NaCl concentrations. For growth assays, three replicates of RM-71 adjusted to an OD600 of 0.3 were inoculated (1:100) in 100 µL of TSB-1 and TSB-3 adjusted to pH 7, 6, 5, and 4. Growth was monitored for 48 hours using the spectrophotometer Epoch2 microplate reader (BioTek) under constant stirring. To determine bacterial viability on acid exposure, overnight cultures of RM-71 and ∆arcA strains in 5-mL of TSB-1 (pH 7) were diluted in fresh TSB-1 and TSB-3 and grown to an OD600 of 0.3. Bacterial cells were harvested (4,000 × g for 5 minutes) and suspended in 1 mL of acidified TSB-1 and TSB-3 (pH 4). Then, 1:100 dilutions were inoculated in TSB-1 and TSB-3 (pH 4.0), with and without supplemental L-arginine (10 mM) (Sigma-Aldrich), in 96-well plates. For colony counts, 5-µL aliquots of 10-fold dilutions at 0 and 16 hours were drop-plated in TSA-1. Plates were incubated at 25°C for 24 hours, and the number of viable cells (CFU/mL) was determined for each time point.
Scanning and transmission electron microscopy (SEM and TEM)
For SEM, exponentially growing cultures of Pdd RM-71 in TSB-1 and TSB-3 (OD600 of 0.55) were pelleted down by centrifugation (4,000 × g, 5 minutes, 4°C). Cells were fixed as previously described (27), sputter-coated with iridium, and imaged using an Ultra Plus ZEISS scanning electron microscope. For polysaccharide capsule visualization, sample processing and TEM analyses were conducted as previously described (28). Images were digitally recorded using a CCD digital camera Orius 1100 W (Gatan). Cell length, cell width, and capsule thickness at both salinities were determined by measuring 30 cells with the Fiji software (ImageJ version 1.51n) (43). Capsule thickness for each cell was calculated as the average of six measurements at different points. An unpaired t-test was used to determine statistical significance.
Allelic exchange mutagenesis
Non-polar deletion mutants for δ-endotoxin gene pirB (A0J47_RS13275) and arginine deiminase arcA (A0J47_RS14010) were constructed by allelic exchange mutagenesis. Primers used for the construction and screening of mutants are listed in Table S3. In brief, 2-kb DNA fragments upstream and downstream of each gene were obtained by amplification with Hi-Fidelity Kapa Taq (Kapa) and subsequently ligated, resulting in an in-frame deletion of >90% of the coding sequence for each target gene. For allelic exchange, the suicide vector pNidKan (KmR), bearing the sucrose sensitivity gene sacB and the pir-dependent R6K ori, was used as previously described (28). To select the first event of recombination, 100-µL serial dilutions of transconjugants were seeded on thiosulfate-citrate-bile salts-sucrose (TCBS) agar supplemented with kanamycin. Colonies that were resistant to kanamycin were seeded on TSA-1 plates supplemented with sucrose (15% [wt/vol]) to select for a second recombination event. As a control to study whether PirAB toxin has an impact on the virulence of Pdd for fish, we needed to construct a Pdd RM-71 mutant defective for four previously characterized toxins Dly, PhlyP, PhlyC, and PlpV (31, 44). Such a tetra-mutant (dubbed here AVL442) lacks these four toxins but maintains intact pirA and pirB genes. This tetra-mutant was constructed by deleting plpV as previously described (44) on the genetic background of the previously constructed triple (Dly, PhlyP, PhlyC) mutant AR89 (31). All gene deletions were confirmed by PCR, and the DNA regions involved in recombination were sequenced to ensure that no point mutations were generated during the process.
SDS-PAGE analysis of culture supernatants
To analyze the effect of NaCl changes on the abundance of secreted proteins, the extracellular products (ECPs) from three replicates of Pdd RM-71 cultures grown in TSB-1 or TSB-3 were collected. In a first attempt, to be able to correlate the protein profiles with the transcriptomic results, we obtained ECPs from cultures obtained in the same conditions used for RNA-seq analysis (OD600 of 0.55). However, due to the low protein abundance in these early exponential supernatants, for the analysis of the secretome we used cultures grown to stationary phase (OD600: 1.7). Bacterial suspensions were centrifuged (13,000 × g, 5 minutes, 4°C), and supernatants were filtered through 0.22-µm-pore size filters (Schleicher & Schuell, Dassel, Germany). Protein precipitation of cell-free supernatants was performed as previously described (27), and precipitated proteins were subjected to SDS-PAGE in 14% polyacrylamide gels using the Laemmli discontinuous buffer system (45). Coomassie Brilliant Blue R250 was used for protein staining.
Protein quantification by densitometry
SDS-PAGE gel images acquired in a GS-900 calibrated densitometer (Bio-Rad) were analyzed using the Image Lab Software version 6.0.1 (Bio-Rad). Bands were automatically detected by the software by selecting the high-sensitivity setting. Rolling disk values were set for the lane with less intensity, applied to all lanes, and adjusted to determine the background-subtracted intensity for each band. Normalization between lanes was determined using the sialidase band as a housekeeping secreted protein (HKP) given its stable expression under the experimental conditions tested. For this, the lane normalization factor (LNF) was calculated for each gel by dividing the HPK signal of each lane by the HPK value with the highest intensity. Then, normalized band intensity was determined for each target protein by dividing its signal value by the corresponding LNF. Mean of normalized band intensities of three replicates from independent cultures for each condition (1% NaCl and 3% NaCl) over three gels was calculated for each protein. An unpaired, two-tailed Student’s t-test was used for the statistical analysis of densitometric data using the GraphPad Prism 9 Software.
Fish virulence assays
For experimental infection assays on turbot, groups of 10 fish (6 ± 1.2 g) were acclimated at 18°C for 1 week before the infection assay. Virulence challenges were conducted by intraperitoneal injection of 0.1 mL of bacterial suspensions in 0.85% NaCl solution at a dose of 4 × 105 CFU/fish per all the tested strains. A negative control group of 10 fish was inoculated with 0.1 mL of sterile 0.85% NaCl. Fish mortality was recorded for 7 days. Reisolation on TSA-1 and TCBS and identification of bacteria from the kidney of dead fish was performed by PCR with ureD primers (46). The protocols of animal experimentation used in this study have been reviewed and approved by the Animal Ethics Committee of the Universidade de Santiago de Compostela.
RESULTS AND DISCUSSION
General overview of the transcriptional profile of Pdd RM-71 under different conditions of salinity
Pdd requires the addition of NaCl for growth and is capable of growing at 1%, 3%, 5%, and 6% NaCl (47). To assess the impact of NaCl concentration on Pdd growth dynamics, precultures of the highly virulent strain RM-71 prepared at 1% NaCl in TSB medium were used as starters (1:100 dilution) of new cultures at two different salinities, 1% or 3% NaCl. Pdd achieved a slightly higher optical density at 3% NaCl at the end of the exponential phase (Fig. 1A).
Fig 1.
(A) Growth of Pdd RM-71 cultured in TSB medium at 1% and 3% NaCl. Data are presented as mean ± SD from three biological replicates and three independent experiments. (B) Volcano plot showing statistical significance of fold change (FC) values. Genes with FDR adjusted P-values ≤ 0.05 and Log2 FC values ≥ 0.59 and ≤ –0.59 were considered to be upregulated DEGs (orange dots) or downregulated DEGs (blue dots) at 1% NaCl. (C) Distribution of the number of upregulated (orange), downregulated (blue), and unchanged (gray) genes within the genome of strain RM-71 at 1% NaCl versus 3% NaCl. Distribution of DEGs is balanced in both chromosomes as well as in the virulence plasmid pPHDD1. (D) Functional classification of upregulated (orange) and downregulated (blue) DEGs at 1% NaCl versus 3% NaCl based on COG (Cluster of Orthologous Groups) database.
To investigate the transcriptional response of Pdd to NaCl changes, triplicates of early exponential RM-71 cultures grown in TSB at 3% NaCl and 1% NaCl were used to purify RNA and to generate six cDNA libraries that were subjected to Illumina sequencing. An average of 54.27 million raw reads were generated and mapped against the complete genome of Pdd RM-71. Principal component analysis demonstrated a clear distinction between the two sample groups (1% NaCl and 3% NaCl) (Fig. S1). Detailed information on the RNA-seq data for each replicate is described in Table S1. To visualize the changes in gene expression that might be elicited in Pdd on entry into an animal host, growth at 3% NaCl was established as the control condition in comparison to growth at 1% NaCl. The comparative analysis of the transcriptomic profiles identified 1,808 DEGs: 888 upregulated (FC > 1.5) and 920 downregulated (FC < −1.5) at 1% NaCl compared to 3% NaCl (Fig. 1B). The complete list of DEGs at each salinity condition is summarized in Table S2. The number of upregulated and downregulated genes was balanced in the two chromosomes, as well as in the virulence plasmid pPHDD1 (Fig. 1C).
The proteins encoded by the DEGs were mapped to COG, KEGG, and GO databases. Interestingly, translation (J) was the most abundant functional category assigned by the COG database within the low salt-upregulated profile (Fig. 1D). In addition, genes upregulated at 1% NaCl were mostly assigned to functional categories involved in inorganic ion metabolism and transport (P), membrane biogenesis (M), cell motility (N), and intracellular secretion and trafficking (U). Conversely, categories related to general metabolism, such as energy production and conversion (C), amino acid and carbohydrate metabolism and transport (E and G, respectively) were the most represented among genes upregulated at 3% NaCl. Figures showing the top 15 enriched KEGG pathways and GO terms involved in biological processes are presented in Fig. S2.
As a general overview, the comparative study of the transcriptomes revealed the upregulation of genes involved in energy metabolism, biosynthesis of amino acids, ADS, and uptake of compatible solutes at 3% NaCl (Table 1), whereas growth at 1% NaCl upregulated genes encoding cytotoxins and other proteins secreted via the T2SS, iron uptake systems, and other virulence factors (Table 2). Therefore, we propose that growth at 3% NaCl upregulates an “environmental profile” in Pdd, whereas a shift to 1% NaCl upregulates a “virulence profile” (Fig. 2). Study of DEG distribution along the two chromosomes of the Pdd RM-71 genome revealed that some of the top DEGs mapped to multigene operons (Fig. 3). The most relevant results obtained from the detailed study of the transcriptomes are discussed below.
TABLE 1.
List of selected DEGs significantlyater samples were collected at L upregulated at 3% NaCl
| Locus_tag | Product/function | FC | Log2FC | P-value | Location a |
|---|---|---|---|---|---|
| Energy metabolism | |||||
| A0J47_RS18510 | Hypothetical protein | –19.83 | –4.31 | 4.52E-66 | ChrII |
| A0J47_RS18515 | F0F1 ATP synthase subunit a | –16.75 | –4.07 | 1.31E-93 | ChrII |
| A0J47_RS18520 | F0F1 ATPase subunit c | –17.46 | –4.13 | 7.67E-31 | ChrII |
| A0J47_RS18525 | F0F1 ATPase subunit b | –12.28 | –3.62 | 1.12E-65 | ChrII |
| A0J47_RS18540 | F0F1 ATPase subunit gamma | –10.65 | –3.41 | 4.22E-82 | ChrII |
| A0J47_RS00375 | Fumarate reductase flavoprotein subunit FrdA | –3.95 | –1.98 | 4.53E-76 | ChrI |
| A0J47_RS16570 | Cytochrome c-type subunit TorY | –62.18 | –5.96 | 1.56E-17 | ChrII |
| A0J47_RS16575 | Putative molybdoenzyme reductase TorZ | –16.80 | –4.07 | 1.86E-23 | ChrII |
| A0J47_RS08095 | Anaerobic sulfite reductase subunit AsrA | –2.20 | –1.14 | 2.52E-07 | ChrI |
| Nitrogen metabolism and nitrosative stress response | |||||
| A0J47_RS06275 | Hydroxylamine reductase | –58.34 | –5.87 | 0 | ChrI |
| A0J47_RS17605 | Nitrite reductase (NADH) small subunit | –6.58 | –2.72 | 1.61E-09 | ChrII |
| A0J47_RS06260 | Nitrous oxide-stimulated promoter family protein | –18.74 | –4.23 | 2.83E-162 | ChrI |
| A0J47_RS00170 | Nitric oxide-sensing transcriptional repressor NsrR | –1.60 | –0.68 | 5.44E-30 | ChrI |
| Carbohydrate metabolism | |||||
| Pyruvate metabolism and acetate production | |||||
| A0J47_RS00915 | Phosphoenolpyruvate carboxykinase PckA | –2.13 | –1.09 | 2.41E-61 | ChrI |
| A0J47_RS06270 | Pyruvate-ferredoxin/flavodoxin oxidoreductase NifJ | –6.59 | –2.72 | 4.31E-37 | ChrI |
| A0J47_RS07640 | Alcohol dehydrogenase | –2.69 | –1.43 | 1.88E-41 | ChrI |
| A0J47_RS13735 | Pyruvate-formate lyase GrcA | –27.94 | –4.80 | 0 | ChrI |
| A0J47_RS05575 | Formate C-acetyltransferase PflB | –6.60 | –2.72 | 3.94E-287 | ChrI |
| A0J47_RS05580 | Formate efflux transporter FocA | –6.10 | –2.71 | 6.74E-196 | ChrI |
| A0J47_RS18485 | Acetate kinase AckA | –3.80 | –1.93 | 3.18E-84 | ChrII |
| Fructose and mannose metabolism | |||||
| A0J47_RS05660 | Mannose-6-phosphate isomerase ManA | –1.58 | –0.66 | 2.77E-41 | ChrI |
| A0J47_RS05670 | PTS system, fructose-specific IIA component | –4.47 | –2.16 | 9.64E-21 | ChrI |
| A0J47_RS08255 | Fructose operon transcriptional repressor FruR | –3.74 | –1.90 | 1.58E-67 | ChrI |
| A0J47_RS08260 | PTS system, fructose-specific IIA component FruB | –8.93 | –3.16 | 1.91E-20 | ChrI |
| A0J47_RS08265 | 1-phosphofructokinase FruK | –6.91 | –2.79 | 1.44E-12 | ChrI |
| A0J47_RS08270 | PTS system, fructose-specific IIB/C component FruA | –4.48 | –2.16 | 2.19E-77 | ChrI |
| Starch and glucose metabolism | |||||
| A0J47_RS15340 | Glycogen phosphorylase | –3.14 | –1.65 | 2.10E-67 | ChrII |
| A0J47_RS18420 | Trehalose operon repressor TreR | –2.13 | –1.09 | 3.69E-51 | ChrII |
| A0J47_RS18430 | PTS system, trehalose-specific IIBC component PTS system TreB | –14.92 | –3.90 | 0 | ChrII |
| A0J47_RS18435 | Trehalose-6-phosphate hydrolase TreC | –7.00 | –2.81 | 1.83E-30 | ChrII |
| Amino sugar and nucleotide metabolism | |||||
| A0J47_RS17085 | Anaerobic ribonucleoside-triphosphate reductase activating protein NrdG | –3.39 | –1.76 | 8.82E-58 | ChrII |
| A0J47_RS17090 | Anaerobic Ribonucleoside-triphosphate reductase (thioredoxin) NrdD | –15.70 | –3.97 | 0 | ChrII |
| A0J47_RS06860 | Uridine phosphorylase Udp | –4.12 | –2.04 | 4.58E-195 | ChrI |
| A0J47_RS11155 | N-acetylneuraminate lyase NanA | –4.65 | –2.22 | 1.38E-75 | ChrI |
| Amino acid metabolism | |||||
| A0J47_RS14010 | Arginine deiminase ArcA | –39.70 | –5.31 | 6.85E-147 | ChrI |
| A0J47_RS14015 | Carbamate kinase ArcC | –35.99 | –5.17 | 7.93E-172 | ChrI |
| A0J47_RS14020 | Ornithine carbamoyltransferase ArcB | –15.11 | –3.92 | 3.70E-302 | ChrI |
| A0J47_RS14005 | Arginine/ornithine antiporter ArcD | –2.20 | –3.12 | 0 | ChrI |
| A0J47_RS02700 | Arginine N-succinyltransferase AstA | –2.28 | –1.19 | 1.46E-60 | ChrI |
| A0J47_RS02705 | Succinylglutamic semialdehyde dehydrogenase AstD | –2.35 | –1.23 | 4.96E-40 | ChrI |
| A0J47_RS01975 | Arginine decarboxylase AdiA | –2.20 | –1.14 | 8.73E-29 | ChrI |
| A0J47_RS00430 | Aspartate ammonia-lyase AspA | –4.51 | –2.17 | 1.73E-111 | ChrI |
| A0J47_RS04485 | Glutaminase GlsA | –2.99 | –1.58 | 4.10E-17 | ChrI |
| A0J47_RS16645 | Glycine cleavage system aminomethyltransferase GcvT | –4.54 | –2.18 | 1.08E-156 | ChrII |
| A0J47_RS10545 | Acetolactate synthase I/II/III large subunit AlsS | –3.84 | –1.94 | 5.49E-72 | ChrI |
| A0J47_RS14750 | Hippurate hydrolase HipO | –5.31 | –2.41 | 6.95E-76 | ChrII |
| Compatible solute uptake systems | |||||
| A0J47_RS18900 | Glycine betaine/proline transport system substrate-binding protein | –10.69 | –3.42 | 1.02E-165 | ChrII |
| A0J47_RS18905 | Glycine betaine/proline transport system permease protein | –5.00 | –2.32 | 3.06E-31 | ChrII |
| A0J47_RS18910 | Glycine betaine/proline transport system ATP-binding protein | –3.36 | –1.75 | 1.76E-51 | ChrII |
| A0J47_RS08670 | Betaine/Carnitine/Choline Transporter (BCCT) family transporter | –11.85 | –3.57 | 5.68E-288 | ChrI |
| A0J47_RS07020 | Betaine/Carnitine/Choline Transporter (BCCT) family transporter | –4.80 | –2.26 | 2.82E-198 | ChrI |
| Histamine production | |||||
| A0J47_RS13250 | Histidine-histamine antiporter HdcT | –3.98 | –1.99 | 3.24E-28 | ChrI |
| A0J47_RS13255 | Histidine decarboxylase HdcA | –2.76 | –1.46 | 1.66E-33 | ChrI |
| A0J47_RS13260 | Histidine-tRNA ligase HisRS | –1.59 | –0.67 | 8.24E-13 | ChrI |
| Peptidases | |||||
| A0J47_RS07600 | M20 family metallopeptidase | –26.89 | –4.75 | 0 | ChrI |
| A0J47_RS13435 | U32 family peptidase | –8.41 | –3.07 | 1.55E-119 | ChrI |
| A0J47_RS19220 | C69 family dipeptidase | –5.32 | –2.41 | 1.27E-78 | ChrII |
| Porins, permeases, and transporters | |||||
| A0J47_RS05530 | Outer Membrane Protein C | –12.49 | –3.64 | 0 | ChrI |
| A0J47_RS14790 | Maltoporin LamB | –11.89 | –3.57 | 9.44E-172 | ChrII |
| A0J47_RS06290 | Uncharacterized membrane protein YjiH | –12.44 | –3.64 | 1.67E-24 | ChrI |
| A0J47_RS08595 | C4-dicarboxylate transporter DcuC | –9.03 | –3.17 | 2.11E-111 | ChrI |
| Hypothetical and uncharacterized proteins | |||||
| A0J47_RS17280 | Hypothetical protein | –139.01 | –7.12 | 0 | ChrII |
| A0J47_RS17285 | Hypothetical protein | –84.29 | –6.40 | 0 | ChrII |
| A0J47_RS12415 | Helix-turn-helix domain-containing protein | –97.17 | –6.60 | 1.31E-288 | ChrI |
| A0J47_RS10160 | Hypothetical protein | –24.70 | –4.63 | 0 | ChrI |
| A0J47_RS04560 | Hypothetical protein | –24.69 | –4.63 | 1.10E-94 | ChrI |
ChrI, chromosome I; ChrII, chromosome II.
TABLE 2.
List of selected DEGs significantly upregulated at 1% NaCl
| Locus_tag | Product/function | FC | Log2FC | P-value | Location a |
|---|---|---|---|---|---|
| Virulence and antimicrobial resistance | |||||
| A0J47_RS13280 | PirA-like | 348.13 | 8.44 | 0 | ChrI |
| A0J47_RS13275 | PirB-like | 83.44 | 6.38 | 0 | ChrI |
| A0J47_RS20355 | Pore-forming toxin PhlyP | 11.13 | 3.48 | 0 | pPHDD1 |
| A0J47_RS10995 | Pore-forming toxin PhlyC | 2.32 | 1.22 | 1.29E-59 | ChrI |
| A0J47_RS20350 | Damselysin | 11.01 | 3.46 | 0 | pPHDD1 |
| A0J47_RS20585 | Outer membrane protein OmpU | 3.70 | 1.89 | 9.76E-100 | pPHDD1 |
| A0J47_RS20130 | RNAase toxin Ntox44 | 4.77 | 2.25 | 1.14E-89 | pPHDD1 |
| A0J47_RS20325 | AcrB/MacB-like ABC transporter ATP-binding protein | 3.64 | 1.86 | 1.78E-153 | pPHDD1 |
| A0J47_RS20340 | TolC family protein | 3.60 | 1.85 | 6.53E-189 | pPHDD1 |
| A0J47_RS20345 | AcrA/MacA-like membrane fusion protein | 3.72 | 1.90 | 9.20E-173 | pPHDD1 |
| A0J47_RS20135 | PAAR domain-containing protein | 3.55 | 1.83 | 7.77E-79 | pPHDD1 |
| A0J47_RS11245 | Putative lipoprotein | 9.43 | 3.24 | 0 | ChrI |
| A0J47_RS20555 | TonB-dependent transferrin receptor Vep20-like | 9.50 | 3.25 | 1.08E-220 | pPHDD1 |
| A0J47_RS20550 | Serum resistance protein Vep07-like | 4.69 | 2.23 | 2.21E-150 | pPHDD1 |
| A0J47_RS03660 | Fe3+ ABC transporter substrate-binding protein FbpA | 18.74 | 4.23 | 0 | ChrI |
| A0J47_RS11430 | Fe2+ transporter permease subunit FeoB | 7.40 | 2.89 | 4.37E-197 | ChrII |
| A0J47_RS11435 | Fe2+ transport protein FeoA | 5.98 | 2.58 | 6.13E-73 | ChrII |
| A0J47_RS14665 | TonB-dependent siderophore receptor FhuE | 7.05 | 2.82 | 0 | ChrII |
| Protein export and secretion | |||||
| A0J47_RS19585 | Preprotein translocase subunit SecD | 8.10 | 3.02 | 0 | ChrII |
| A0J47_RS00865 | Type II secretion system protein EpsJ | 2.88 | 1.52 | 2.55E-50 | ChrI |
| A0J47_RS00870 | Type II secretion system protein EpsI | 3.21 | 1.68 | 1.14E-34 | ChrI |
| A0J47_RS00875 | Type II secretion system protein EpsH | 4.66 | 2.22 | 1.06E-91 | ChrI |
| A0J47_RS00880 | Type II secretion system protein EpsG | 4.54 | 2.18 | 2.82E-198 | ChrI |
| A0J47_RS00885 | Type II secretion system protein EpsF | 3.55 | 1.83 | 2.28E-88 | ChrI |
| Flagellar motility and chemotaxis | |||||
| A0J47_RS12245 | Chemotaxis protein CheV | 1.69 | 0.75 | 3.38E-53 | ChrII |
| A0J47_RS12040 | Chemotaxis response regulator CheY | 2.69 | 1.43 | 3.47E-80 | ChrI |
| A0J47_RS05380 | Methyl-accepting chemotaxis protein | 2.58 | 1.37 | 8.54E-152 | ChrI |
| A0J47_RS12030 | Chemotaxis protein histidine kinase CheA | 2.42 | 1.28 | 4.93E-56 | ChrI |
| A0J47_RS12035 | Chemotaxis regulator CheZ | 2.42 | 1.28 | 2.59E-51 | ChrI |
| A0J47_RS12075 | Flagellar biosynthetic protein FliQ | 2.31 | 1.21 | 3.72E-07 | ChrI |
| A0J47_RS12200 | Flagellar P-ring protein precursor FlgI | 1.98 | 0.98 | 4.34E-26 | ChrI |
| A0J47_RS12210 | Flagellar basal-body rod protein FlgG | 1.84 | 0.88 | 2.08E-20 | ChrI |
| Translation, ribosomal structure, and biogenesis | |||||
| A0J47_RS00405 | 30S ribosomal protein S6-L-glutamate ligase RimK | 52.24 | 5.71 | 0 | ChrI |
| A0J47_RS16560 | 2OG-Fe(II) oxygenase | 11.78 | 3.56 | 3.93E-161 | ChrII |
| A0J47_RS00505 | tRNA-dihydrouridine synthase B DusB | 6.43 | 2.69 | 1.13E-196 | |
| A0J47_RS06710 | 50S ribosomal protein L32 RpmF | 4.73 | 2.24 | 1.70E-29 | ChrI |
| A0J47_RS07485 | Small subunit ribosomal protein S1 RpsA | 4.29 | 2.10 | 3.66E-195 | ChrI |
| A0J47_RS02550 | 50s ribosomal protein L31 RpmE | 3.85 | 1.94 | 2.64E-102 | ChrI |
| A0J47_RS09500 | 50S ribosomal protein L25 RplY | 4.71 | 2.24 | 0 | ChrI |
| Metabolism and transport | |||||
| A0J47_RS17020 | Glutathione synthase GshB | 38.42 | 5.26 | 0 | ChrII |
| A0J47_RS15265 | Class I SAM-dependent methyltransferase | 29.87 | 4.90 | 1.43E-302 | ChrII |
| A0J47_RS12810 | Myo-inositol-1(or 4)-monophosphatase ShuB | 26.88 | 4.75 | 0 | ChrI |
| A0J47_RS08575 | Agmatinase SpeB | 11.13 | 3.48 | 0 | ChrI |
| A0J47_RS17735 | Gamma-glutamylputrescine oxidase | 9.63 | 3.27 | 1.30E-244 | ChrII |
| A0J47_RS01525 | Glutamine-fructose-6-phosphate transaminase GlmS | 7.50 | 2.91 | 9.23E-41 | ChrI |
| A0J47_RS17685 | Cysteine desulfurase | 7.34 | 2.87 | 3.41E-147 | ChrII |
| A0J47_RS17170 | Carboxynorspermidine decarboxylase | 4.47 | 2.16 | 1.99E-118 | ChrII |
| A0J47_RS17175 | Carboxynorspermidine synthase | 5.42 | 2.44 | 0 | ChrII |
| Transporters | |||||
| A0J47_RS09135 | Spermidine/putrescine binding protein PotD2 | 9.02 | 3.17 | 2.40E-96 | ChrI |
| A0J47_RS10120 | Energy-coupling factor ATP-binding protein EcfA | 8.28 | 3.05 | 4.99E-268 | ChrI |
| A0J47_RS01035 | 7-cyano-7-deazaguanine/7-aminomethyl-7-deazaguanine transporter YhhQ | 22.65 | 4.50 | 0 | ChrI |
| A0J47_RS11695 | TolC family protein CusC | 9.73 | 3.28 | 8.73E-73 | ChrI |
| A0J47_RS07645 | GPR1/FUN34/yaaH putative acetate transporter | 23.91 | 4.58 | 0 | ChrI |
| A0J47_RS19570 | NupC/NupG family nucleoside CNT transporter | 9.46 | 3.24 | 7.88E-273 | ChrII |
| A0J47_RS13535 | Magnesium transporter MgtE | 5.35 | 2.42 | 3.37E-182 | ChrI |
| A0J47_RS06610 | Na+/H+-dicarboxylate symporter | 7.76 | 2.96 | 1.58E-55 | ChrI |
| A0J47_RS10440 | Na+-driven multidrug efflux pump (MATE) | 6.13 | 2.62 | 1.92E-152 | ChrI |
| A0J47_RS15280 | MFS transporter | 32.63 | 5.03 | 0 | ChrII |
| A0J47_RS08650 | MFS transporter | 17.63 | 4.14 | 0 | ChrI |
| A0J47_RS15440 | MFS transporter | 6.17 | 2.62 | 3.73E-150 | ChrII |
| Peptidases | |||||
| A0J47_RS12990 | Putative protease YegQ | 11.93 | 3.58 | 0 | ChrI |
| A0J47_RS08645 | Peptidoglycan DD-metalloendopeptidase | 9.97 | 3.32 | 0 | ChrI |
| A0J47_RS18555 | Trypsin-like serine protease | 5.67 | 2.50 | 5.84E-262 | ChrII |
| A0J47_RS03380 | Do family serine endopeptidase | 4.26 | 2.09 | 9.46E-17 | ChrI |
| Transcriptional regulators | |||||
| A0J47_RS00510 | DNA-binding transcriptional regulator Fis | 5.81 | 2.54 | 5.61E-172 | ChrI |
| A0J47_RS12800 | Fe-S cluster assembly transcriptional regulator IscR | 5.61 | 2.49 | 1.82E-256 | ChrI |
| A0J47_RS01530 | DeoR family transcriptional regulator | 5.51 | 2.46 | 1.99E-21 | ChrI |
| A0J47_RS08475 | LysR family transcriptional regulator | 2.78 | 1.47 | 2.60E-51 | ChrI |
| Histone acetylation and tRNA binding/modification | |||||
| A0J47_RS19900 | Histone acetyltransferase HPA2 | 17.18 | 4.10 | 1.21E-208 | ChrII |
| A0J47_RS09065 | tRNA 2-thiocytidine(32) synthetase TtcA | 6.59 | 2.72 | 0 | ChrI |
| A0J47_RS00505 | tRNA dihydrouridine synthase DusB | 6.43 | 2.69 | 1.13E-196 | ChrI |
| A0J47_RS11195 | tRNA 5-methoxyuridine(34)/uridine 5-oxyacetic acid(34)synthase CmoB | 5.17 | 2.37 | 1.27E-218 | ChrI |
| Hypothetical and uncharacterized proteins | |||||
| A0J47_RS17025 | Flavohemoglobin expression-modulating QEGLA motif protein | 27.51 | 4.78 | 0 | ChrII |
| A0J47_RS17005 | Hypothetical protein | 18.64 | 4.22 | 0 | ChrII |
| A0J47_RS10190 | Hypothetical protein | 13.27 | 3.73 | 1.35E-08 | ChrI |
| A0J47_RS10185 | Hypothetical protein | 11.90 | 3.57 | 1.16E-55 | ChrI |
| A0J47_RS19195 | Uncharacterized membrane protein | 9.61 | 3.27 | 3.45E-68 | ChrII |
| A0J47_RS19225 | DUF3316 domain-containing protein | 9.59 | 3.26 | 5.71E-162 | ChrII |
| A0J47_RS20565 | Uncharacterized protein | 4.78 | 2.26 | 9.38E-172 | pPHDD1 |
ChrI, chromosome I; ChrII, chromosome II; pPHDD1, virulence plasmid.
Fig 2.
Heat map view showing the general transcriptional response of Pdd at 1% NaCl (upregulation of a virulence profile) compared to 3% NaCl (upregulation of an environmental profile). Differential expression values are given as Log2FC-based color scale. Orange segments represent induced expression (positive FC), whereas blue segments represent downregulation (negative FC).
Fig 3.
Mapping of differentially expressed genes (DEGs) in response to NaCl changes within chromosome I and chromosome II of Pdd RM-71 genome. Expression values are depicted in a color-based scale as Log2fold change (FC). In the detailed view, DEGs are shown in orange (upregulated at 1% NaCl), blue (upregulated at 3% NaCl), and gray (unchanged) and annotated with the corresponding National Center for Biotechnology Information (NCBI) Locus Tag of RM-71 genome (GCF_001708035.2). Operon-predicted functions are shown.
Salinity changes slightly impact cell morphology and capsule production in Pdd RM-71
Comparative analysis of the transcriptomes revealed that some genes involved in cell shape regulation were upregulated at low salt (Table S2), including the rod shape-determining protein RodA (A0J47_RS14950) and the peptidoglycan (PG) glycosyltransferase MrdB (A0J47_RS05095), both part of the shape, elongation, division, and sporulation (SEDS) family (48). In line with these findings, the V. cholerae gene (pbpB, VCA0870) homologous to Pdd RM-71 D-alanyl-D-alanine endopeptidase PbpG (A0J47_RS09265) was induced in the absence of NaCl and suggested to be involved in PG modulation under no salt (6, 49).
Cell morphology of RM-71 was assessed by SEM and TEM. SEM images revealed that cells grown at low salt exhibited a significantly more elongated shape and were narrower than cells at 3% NaCl (Fig. 4A and B). Polysaccharide capsule constitutes a virulence factor in Pdd RM-71, playing a role in protection against fish serum, and a gene cluster participating in capsule biogenesis has been functionally characterized (28). In the present study, we found that capsule biogenesis genes are slightly upregulated at 1% NaCl (Fig. 3, chromosome I panel; Table S2), with FC values ranging from 1.7 to 3.57. However, TEM analysis revealed a significant, albeit moderate increase in capsule thickness at 3% NaCl (average thickness of 65.55 and 69.43 nm for 1% and 3% NaCl, respectively) (Fig. 4C). These apparently contradictory observations suggest the participation of additional, yet-unknown regulatory mechanisms in Pdd capsule production. The presence of fully capsulated cells at either salinity condition indicates that Pdd expresses the polysaccharide capsule during its environmental lifestyle as well as during an infection. While the protective role of the capsule during animal infection has been previously established in Pdd (28), the function of the capsule during the free-living lifestyle is unknown. Previous studies have shown that Pdd can be predated by Bdellovibrio bacteriovorus (50) and by heterotrophic marine flagellates and microzooplankton (51, 52). It can be speculated that the capsule may play a protective role against predation in the marine environment. It is pertinent to note that not all Pdd strains are capsulated (A. V. Barca, A. do Vale, and C. R. Osorio, unpublished data), emphasizing the need for in-depth studies to clarify the role of capsule in predation scenarios.
Fig 4.
(A) Cell morphology and polysaccharide capsule of Pdd RM-71 grown at 1% NaCl and 3% NaCl, assessed by SEM (top panel) and TEM (bottom panel), respectively. (B) Box plot showing cell length and width of Pdd RM-71 cells at two assayed salinities. Cells were significantly longer and narrower at 1% NaCl. (C) Capsule thickness (in nanometer) of Pdd RM-71 at both salinities. Capsule was more compact and significantly thicker at 3% NaCl. Statistical difference was assessed by Student’s t-test: ****P < 0.0001, **P < 0.01.
Energy production and carbohydrate metabolism are upregulated at 3% NaCl
Pdd is a highly heterogeneous subspecies, and the populations causing disease in marine animals are considered to be of multiclonal nature (25, 53). Nevertheless, there are conserved biochemical features that define the subspecies. Pdd is heterotrophic, non-nitrogen fixing, facultative anaerobic capable of reducing nitrate to nitrite, ferments glucose with the production of gas, produces arginine deiminase, and most strains produce urease (47). The comparative study of the Pdd transcriptomic profiles revealed the upregulation, at 3% NaCl, of genes involved in energy production and carbohydrate and amino acid metabolism (Fig. 2; Table 1), being notable that the genes encoding F0F1 ATP synthase subunits were among the top-20 upregulated genes. This upregulation of the ATP synthesis machinery might account, at least in part, for the increased growth observed at 3% NaCl (Fig. 1A). Enzymes involved in aerobic and anaerobic respiration were markedly upregulated at 3% NaCl including the frdABCD fumarate reductase operon, the asrABC sulfite reductase operon, and nitrite reductase subunits (Table 1). The enhanced expression of aerobic and anaerobic respiration enzymes under high salt has also been reported in Shewanella sp. and Escherichia coli (54, 55).
We observed a strong upregulation of genes involved in response to reactive nitrogen species. Noteworthy, the gene encoding a hydroxylamine reductase is upregulated 58-fold at 3% NaCl. This enzyme, homologous to hybrid cluster protein Hcp of E. coli, catalyzes the reduction of hydroxylamine to NH3 and H2O and is predicted to have a role in protection against nitrosative stress (56). In addition, it was observed the upregulation of a nitrous oxide stimulated promoter family protein homologous to E. coli YgbA. These two genes are under the control of the regulatory protein NsrR, a nitric oxide–sensitive regulator of transcription, characterized in E. coli (57) and whose homolog in Pdd identified in the present study (A0J47_RS00170) is also upregulated at 3% NaCl (Table 1). These findings clearly suggest that seawater salinity boosts nitrogen metabolism in Pdd, leading to the potential activation of nitrosative stress response mechanisms.
The cytochrome c-type subunit TorY (56% amino acid identity to E. coli TorY/Yeck) and a molybdoenzyme reductase (68% amino acid identity to E. coli TorZ/BisZ) are 62- and 16-fold upregulated at seawater salinity, respectively (Table 1). This system was first described in E. coli as an additional anaerobic respiration system that uses trimethylamine N-oxide (TMAO) and biotin sulfoxide as alternative electron acceptors (58). Of note, it has been reported in a previous study that Pdd is capable of reducing TMAO to TMA (trimethylamine) (59). Additionally, genes involved in glucolysis/gluconeogenesis, pyruvate and acetate metabolism were upregulated at 3% NaCl (Table 1). So are genes encoding an acetate kinase and an alcohol dehydrogenase, suggesting that acetate production is enhanced at seawater salinity in Pdd. The upregulation of genes involved in acetate production has been reported in V. vulnificus as an antipredation strategy against protozoans (60).
Considering all the functions upregulated at 3% NaCl, energy production systems including aerobic and anaerobic respiration systems as well as carbon metabolism might be of high priority when Pdd grows in a free-living state. The enhanced expression of these gene categories was also observed in P. damselae subsp. piscicida when shifting from low to high salt (61) and in E. coli in response to growth in seawater (55).
Growth at 3% NaCl upregulates genes involved in the uptake of compatible solutes and in the use of trehalose and fructose as carbon sources
To thrive in high-osmolarity environments, bacterial cells accumulate compatible solutes (osmolytes) in the cytoplasm, via either uptake or biosynthesis (62, 63). Osmolytes can be sugars (as trehalose), amino acids (proline and glutamine), and quaternary amines (glycine betaine, choline). Acquisition of compatible solutes from the environment is preferable in terms of cell economy, since the biosynthesis of osmolytes is energetically costly. In line with this, we observed that the growth of Pdd at 3% NaCl enhanced the expression of uptake systems for compatible solutes (Fig. 2; Table 1), namely the chromosome II-encoded ProU system for the uptake of glycine betaine and L-proline. The enhanced expression of proXVW genes under hyper-osmotic conditions has been reported in V. parahaemolyticus (8) and V. vulnificus (64).
Albeit the type strain of Pdd (ATCC33539) was reported to be unable to use trehalose (47), we here found three genes of the trehalose-specific PTS (phosphotransferase system) (treB, treC, and treR) in the genome of RM-71 (genes absent in the type strain) that were 2- to 15-fold upregulated at high salt. This observation suggests that Pdd RM-71 may use trehalose as a compatible solute (osmolyte) similar to what has been described in other members of the family Vibrionaceae (63), and trehalose may also be used as a carbon source (65). When we streaked Pdd RM-71 on plates of O/F (oxidation–fermentation) medium supplemented with trehalose, we observed a visibly higher production of acid at 3% NaCl than at 1% (Fig. 5A). To quantitatively measure the possible preference of trehalose versus glucose at 3% NaCl by Pdd, we monitored the growth of RM-71 at each salinity condition in the presence of either glucose or trehalose, using CM9 (minimal medium M9 supplemented with casamino acids). Interestingly, at 3% NaCl trehalose conferred higher growth levels than glucose, whereas at 1% NaCl glucose was the carbon source that allowed for higher growth rates (Fig. 5A).
Fig 5.
Acid production of Pdd RM-71 (left panels) by fermentation of trehalose (A) and fructose (B) under 1% NaCl and 3% NaCl in oxidation–fermentation (O/F) medium. Growth curves of RM-71 in CM9 medium (right panels) supplemented with 0.5% glucose compared with the growth in CM9 medium with 0.5% trehalose (A) or 0.5% fructose (B) at 1% NaCl and 3% NaCl. Data are presented as mean ± SD from three biological replicates and two independent experiments.
An early study reported that the type strain of Pdd encodes a putative PTS system for fructose utilization (66). Our RNA-seq data also revealed that 3% NaCl causes the upregulation of genes of a PTS system for fructose (Table 1). Similar to trehalose, we observed that the growth of Pdd RM-71 at 3% NaCl in presence of fructose is higher than in presence of glucose, whereas at 1% NaCl glucose is the preferred carbon source and allows higher growth rates (Fig. 5B). All these observations clearly indicate that at seawater salinity, the use of trehalose and fructose is enhanced in comparison to glucose. This poses a notable biological significance, considering that trehalose and fructose are highly abundant sugars in marine environments (67 - 69). Trehalose constitutes the main sugar in some species of macroalgae (70) and is also a major oligosaccharide in the hemolymph of some crustaceans (71). Fructose availability would come mainly from the hydrolysis of sucrose, which is abundant in seagrass meadows (69, 72) and is produced by green algae and cyanobacteria (73).
Growth at 3% NaCl enhances tolerance to kanamycin, vancomycin, and ampicillin
We found that Pdd RM-71 grown at standard conditions of 1% NaCl exhibits intrinsic resistance to ampicillin and vancomycin, similar to other species of the family Vibrionaceae (74, 75) while it is sensitive to kanamycin (Fig. 6). Surprisingly, Pdd RM-71 showed increased tolerance to kanamycin, ampicillin, and vancomycin at 3% NaCl compared to 1% NaCl (Fig. 6). The most drastic increase was observed with kanamycin, as RM-71 at 3% NaCl grows up to an OD600: 0.8 with antibiotic concentrations (50 µg/mL) that resulted inhibitory at 1% NaCl. To demonstrate that the NaCl-promoted antibiotic tolerance constitutes a salinity-dependent response of Pdd cells and is not a consequence of the selection of stable mutations conferring antibiotic resistance, late stationary-phase cells grown at 3% NaCl in the presence of each antimicrobial were passaged to fresh antibiotic-supplemented media at the two NaCl concentrations, and the growth was monitored. As shown in Fig. 6, the phenotype of increased tolerance at 3% NaCl with respect to 1% NaCl is maintained for the three antibiotics tested, suggesting that the NaCl-induced antibiotic tolerance is due to a regulatory, adaptive mechanism in Pdd.
Fig 6.
NaCl changes impact antibiotic resistance in Pdd. Growth curves of RM-71 in TSB-1% NaCl and 3% NaCl supplemented with kanamycin (50 µg/mL) (A), ampicillin (100 µg/mL) (B), and vancomycin (25 µg/mL). After 40 hours, RM-71 cells grown at 3% NaCl in each antibiotic were passaged to fresh antibiotic-supplemented TSB medium at both NaCl conditions (a, b, and c). Data are presented as mean ± SD (n = 2).
The induced tolerance to antimicrobials under high salt has been reported in Vibrios (76, 77), but the underlying molecular mechanisms are poorly understood. Several mechanisms have been described for beta-lactam resistance in Vibrios (78, 79). Contradictorily, most genes categorized in beta-lactam resistance (ko01501 pathway in KEGG), such as penicillin-binding proteins (PBPs), multidrug efflux pumps (A0J47_RS15280, A0J47_RS08650), and outer membrane (OM) proteins were upregulated at low salt (Table 2; Table S2). However, it is pertinent to clarify that the ligands of these putative multidrug pumps remain completely undeciphered in Pdd, and they may serve functions different from antibiotic resistance.
We identified genes whose upregulation at 3% NaCl could account for the increased resistance to ampicillin. These include a gene for a D-alanyl-D-alanine carboxypeptidase (A0J47_RS09080) (Pbp5) (FC: −1.94) (Table S2) whose deletion in E. coli led to an increase in ampicillin susceptibility (80). In addition, previous research reported that some PG peptidases from V. cholerae, particularly ShyA, are critical to maintain cell viability on treatment with beta-lactams (79). Supporting our data on growth in the presence of ampicillin at 3% salt, a PG endopeptidase (A0J47_RS18260) homolog to V. cholerae ShyA (52% ID, VCA0079) was induced under high salt in Pdd (FC: −3.01).
Mechanisms of aminoglycoside resistance include the acquisition of resistance plasmids, changes in membrane permeability, and/or active efflux of antibiotics (81). We observed that the expression of a Na+-driven multidrug efflux pump (A0J47_RS00420) is upregulated at 3% NaCl in Pdd (FC: −1.82). This protein is homologous to V. cholerae VcmA (VC1540; 27% identity, 65% coverage), a protein responsible for increased resistance to kanamycin (82).
Regulation of bacterial metabolism has been identified as an additional mechanism that supports antibiotic resistance in Vibrio and other species (83 - 85). Indeed, a recent work has demonstrated that NaCl negatively regulates proton motive force (PMF) in Vibrio alginolyticus, leading to a reduction in aminoglycoside uptake and a subsequent increase in antibiotic resistance at high salt (86). Nevertheless, our transcriptomic data did not reveal a clear contribution of NaCl to PMF changes and its possible impact on aminoglycoside uptake.
Gram-negative OM is an extraordinary barrier to high-molecular-weight antibiotics, such as vancomycin (87). A previous research identified the inner membrane protein VigA conferring vancomycin resistance in V. cholerae (74), but no homologs of VigA were identified in the genome of Pdd RM-71. Vancomycin exerts its action by binding to D-Ala-D-Ala residues from the bacterial PG, affecting normal cell growth. The enzyme D-ala-D-ala ligase (Ddl) catalyzes the ligation of these residues in the assembly of the bacterial PG, but alternative Ddl ligases (Van ligases) produce other PG precursors with lower affinity for the antibiotic (88). Interestingly, we found a gene (A0J47_RS04780) upregulated (FC: −1.73) at high salt in Pdd (Table S2), whose protein shares 33% identity (BlastP searches in CARD: The Comprehensive Antibiotic Resistance Database) with VanL of Enterococcus faecalis (ABX54687.1), a ligase that synthesizes the alternative D-Ala-D-Ser substrate with less affinity for vancomycin (89). The contribution of this gene in the enhanced vancomycin resistance observed at high salt has not been previously reported in Pdd and would surely deserve future investigations.
Pdd is sensitive to antimicrobial peptides produced by marine fish and arthropoda, such as hepcidin (90), pleurocidin-amide and tachyplesin (91), and piscidin (41). We here tested the possible effect of salinity on the sensitivity of Pdd RM-71 to piscidin but did not find differences at 3% NaCl versus 1% NaCl (Fig. S3). Considering that piscidin is believed to act at the level of the cell membrane (92), our results suggest that salinity does not cause appreciable differential regulation of cell functions that might be involved in susceptibility to this fish-derived, antimicrobial peptide.
Salt-mediated regulation of amino acid metabolism highlights major changes in arginine catabolism
One of the best-studied traits of the amino acid metabolism in Pdd is its high histamine-producing ability (>1,000 ppm), being able to release toxic levels of histamine in fish even under refrigeration temperatures (4°C) and hence posing a considerable risk for human health (93). Notably, the transcriptomics analysis revealed that at 3% NaCl Pdd upregulates the genes hdcT, hdcA, and hisRS involved in histamine production (Table 1), a finding consistent with histamine production taking place in decomposing fish in the marine environment and, hence, at salinities close to 3% NaCl. To better understand which amino acid pathways undergo significant changes in response to NaCl, salt-regulated pathways were identified by mapping DEGs to KEGG database. We observed a general upregulation of genes involved in amino acid pathways at 3% NaCl (Fig. S4) suggesting that amino acid metabolism is key to support bacterial growth and survival in a free-living lifestyle. The modulation of amino acid metabolism in response to NaCl has also been reported in V. parahaemolyticus (9) and E. coli (94). Though Pdd survives in seawater microcosms at 14°C and 22°C for longer than 1 year (95), we observed that natural coastal seawater does not support detectable growth of Pdd RM-71 (measured as an increase in optical density) after 40 hours, even when using ESW (Fig. 7A). Interestingly, the addition of casamino acids substantially improved growth but only in eutrophicated water (Fig. 7A). We also observed that Pdd RM-71 is capable of growing in minimal medium with glucose in presence of NH4 + ions as sole nitrogen source at 1% NaCl but not at 3% NaCl (Fig. 7B). This result suggests that inorganic nitrogen is not routinely used by Pdd under environmental conditions in seawater, but it is used under low-salt stress. All these observations suggest that Pdd thrives in organic matter–rich microenvironments but not so much in pelagic, nutrient-scarce seawater, and amino acid availability may constitute a limiting factor for the build up of Pdd populations in the environment. In a study of coastal vibrioplankton, it was found that the presence of Pdd was minor in comparison with, for instance, the Vibrio splendidus group (96). In fact, albeit Pdd is occasionally isolated from coastal seawater, it is more prevalent in nutrient-rich ecological niches and surfaces, such as gastrointestinal tracts of sharks and teleost fish (97, 98), fish gill microbiota (99), decomposing fish (100), and, especially, in the internal milieu of animal hosts suffering a Pdd infection, where it can constitute the major pathogen isolated, in many instances as pure culture (101).
Fig 7.
(A) Growth curves of Pdd RM-71 in plain coastal seawater (NSW) and eutrophicated seawater (ESW) with or without casamino acids (CasAA). (B) Growth of Pdd RM-71 in minimal medium M9 with 1% NaCl or 3% NaCl supplemented (CM9) or not (M9) with CasAA. The OD600 was monitored for 24 hours. Data are presented as mean ± SD from three biological replicates and two independent experiments.
Pdd is one of the few vibrios that produces arginine deiminase (also known as arginine dihydrolase), a key phenotypical test employed in taxonomy and identification of vibrios (102). In fact, Pdd (which was formerly known as V. damsela) was classically included in the “group F vibrios” or “group EF6” together with Vibrio furnissii and Vibrio fluvialis (103), positive for arginine deiminase. Through the arginine deiminase (ADI) pathway, L-arginine is converted to L-ornithine, yielding ATP, carbon dioxide, and ammonia through three metabolic steps (Fig. 8A). Intriguingly, our RNA-seq analysis revealed that the arcACBD operon encoding the ADS, responsible for L-arginine internalization and catabolism, is listed among the top upregulated genes at 3% NaCl, being arcA and arcC genes ca. 40-fold upregulated (Table 1; Fig. 8B). Additionally, genes of another L-arginine catabolic pathway, the arginine succinyltransferase system, were also upregulated at high salinity (Table 1).
Fig 8.
(A) Metabolic map showing differentially expressed genes (DEGs) related to arginine catabolism. DEGs are placed in the corresponding metabolic reaction with a color-based scale. Expression values are depicted in the heat map as Log2FC. Genes most upregulated at 3% NaCl (blue) correspond to the arginine deiminase pathway (arcABCD). Genes involved in the arginine succinyltransferase system (AST) were also upregulated at high salinity. (B) Gene map of the arginine deiminase system in RM-71 genome. The NCBI locus tag and the corresponding fold change (FC) value are displayed for each gene. (C) Moeller’s test results for arginine decarboxylation/deamination of ∆arcA, RM-71wt (wild type), and non-inoculated control (C-). The light violet color observed in ∆arcA strain suggests the activity of other arginine-based alkalinizing enzymes. (D) Graph showing RM-71wt and ∆arcA viability after 16 hours of incubation at pH 4 in TSB-1 and TSB-3 and supplemental L-arginine (10 mM). A Student’s t-test was used to assess statistical significance (***P < 0.001, **P < 0.01; ns, not significant). (E) Graph showing swimming motility diameters of RM-71 and ∆arcA in TSB-1 and TSB-3 supplemented with 10-mM L-arginine (L-arg) under aerobic and anaerobic conditions. ∆arcA motility is significantly reduced in the presence of L-arg at 1% NaCl. Data represent the mean ± SD from three biological replicates and two independent experiments.
The fitness advantages to Pdd in upregulating ADI pathway under marine-like salinity or, to put it the other way, to downregulate it under low salinity, remain unknown. To gain an insight into this, we deleted arcA gene in Pdd RM-71 by allelic exchange. Deletion of arcA caused a visible phenotypical change in the classical test for arginine deiminase, which detects alkalinization of the medium indicated by a purple coloration (Fig. 8C). This medium detects alkalinization caused by more than one enzymatic activity, including both arginine deiminase (also known as arginine dihydrolase) and arginine decarboxylase, and this may explain why the arcA mutant still produces residual purple color in this test. The arcA mutant did not show impairment for growth either in TSB medium at 1% NaCl and 3% NaCl (Fig. S5A) or in the presence of supplemented L-arginine (Fig. S5B) with respect to the parental strain.
The ADI pathway has a role in bacterial acid stress survival in many species of bacteria (104 - 106). We, therefore, aimed at investigating whether the mutation of arcA exerted any effect on resistance to acidic pH in Pdd. We first analyzed the growth of RM-71 in TSB-1 and TSB-3 at various pH levels (pH 7, pH 6, pH 5, and pH 4) and observed that pH 4 did not allow Pdd replication either at 1% NaCl or at 3% NaCl (data not shown). We next evaluated the viability of RM-71 and ∆arcA on acid stress (pH 4) in TSB-1 and TSB-3 supplemented or not with L-arginine (10 mM). Interestingly, the survival of RM-71 and ∆arcA strains after 16-hour exposure to acid conditions was ca. 3 orders of magnitude higher at 3% NaCl than at 1% NaCl (Fig. 8D), thus demonstrating that salinity has a major impact on Pdd survival at pH 4. In line with these findings, previous studies have demonstrated that high salinity increased V. parahaemolyticus survival against acid stress (107, 108). In addition, it was observed that the ∆arcA strain exhibited a slight, but significant, impairment for acid resistance in comparison to RM-71, suggesting a role of the ADI pathway on Pdd resistance to acidic conditions (Fig. 8D). This impairment was detected in TSB-3 with no additional L-arginine supplement but was not detected in presence of a surplus of L-arginine. This last observation suggests that an excess of arginine might allow other arginine-degrading pathways (e.g., the arginine decarboxylase system) to contribute to acid resistance and thus compensate for the absence of a functional ADI pathway.
Pseudomonas aeruginosa uses ADI activity to fermentatively generate ATP (109) and to maintain motility under anaerobiosis (110). We noted that the arcA mutant was not affected in swimming motility in anaerobiosis at either salinity condition but exhibited significant impairment under aerobiosis in the presence of supplemented L-arginine at 1% NaCl (Fig. 8E), a seemingly contradictory observation (ADI pathway genes were here found to be upregulated at 3% NaCl) that indicates that the involvement of the ADI pathway in Pdd motility needs additional investigation. Nevertheless, the observation that arginine deiminase is also upregulated in Pdd under iron-excess conditions (32) supports the hypothesis of a preferential role of this enzymatic activity during the free-living lifestyle versus the pathogenic lifestyle. It has been shown that uptake and respiration of L-arginine are maintained under nutrient starvation in Vibrio sp. (111). This strategy might be important for Pdd in its survival for long periods in low-nutrient seawater (95).
Low salt triggers a virulence profile with a major impact on the upregulation of the T2SS-dependent secretome
Entry into the internal milieu of a vertebrate host leads to an abrupt drop in salinity. We found that 1% NaCl upregulates many gene functions potentially related to virulence. These functions include motility and chemotaxis, synthesis and uptake of polyamines, stress response mechanisms and efflux systems, and, notably, iron acquisition mechanisms and cytotoxins (Fig. 2; Table 2). The glutathione synthetase GshB was within the top upregulated functions at low salt, with an FC value of 38.4. Mutation of this gene has been associated with a strong impairment in colonization ability in V. cholerae (112). Previous studies reported the presence of polyamines putrescine, cadaverine, norspermidine, and spermidine in Pdd cells (93, 113, 114). We found that low salinity upregulates genes for the production of putrescine and norspermidine synthesis and transport (Table 2). The implication of spermidine and putrescine in the virulence of bacterial pathogens has been reported in several studies (115 - 117). However, the role of polyamines in Pdd pathobiology remains unknown.
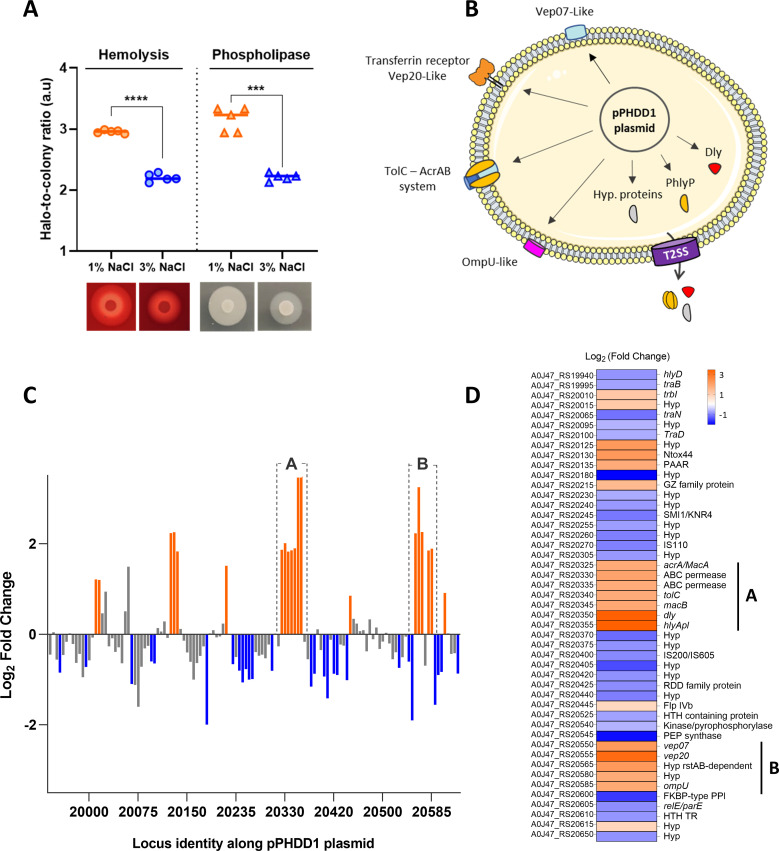
The hallmarks of Pdd pathobiology in infected animals include the extensive hemorrhages and tissue damage caused by the secretion, in very high amounts, of the T2SS-dependent phospholipase D Dly and the two pore-forming toxins PhlyP and PhlyC (24). A pioneering study on Pdd toxin production reported that cytolytic activity was maximal when the bacteria were grown at 1% NaCl (118). In agreement with these findings, the plasmid-borne genes encoding Dly (dly, A0J47_RS20350) and PhlyP (hlyApl , A0J47_RS20355) were 11-fold upregulated at low salt, whereas the hlyAch gene encoding the chromosomal hemolysin PhlyC (A0J47_RS10995) was 2-fold upregulated (Table 2).
Unexpectedly, the list of top-upregulated genes at 1% NaCl was dominated by two genes encoding a small, 11-kDa protein (A0J47_RS13280) (PirA) and a putative δ-endotoxin (A0J47_RS13275) (PirB), respectively (Table 2). These two proteins are candidates to constitute a PirAB-like binary toxin for its similarity to the PirAB toxin produced by the strains of V. parahaemolyticus causing acute hepatopancreatic necrosis disease in crustaceans (119). However, the role of this putative PirAB-like toxin in Pdd remains unknown. The 11-kDa and δ-endotoxin proteins were previously identified in a study that characterized the T2SS-dependent secretome of Pdd RM-71 (27) and were subsequently confirmed as genes of the RstAB regulon, a two-component system that is a master positive regulator of virulence in Pdd (28). The genes for 11-kDa (PirA) and δ-endotoxin (PirB) are not ubiquitous in the subspecies. They occur in some Pdd isolates and are located within a highly variable region of the Pdd chromosome II, suggesting their acquisition by horizontal gene transfer (53).
This strong upregulation of the 11-kDa and δ-endotoxin genes under salinity conditions that mimic the hosts’ milieu prompted us to construct a knockout mutant of the larger protein (δ-endotoxin or PirB) and assess its impact in virulence for turbot, the host of Pdd RM-71. We found that deletion of the δ-endotoxin gene did not cause a detectable impairment in virulence (Fig. S6A). The δ-endotoxin mutant produced hemolytic activity at levels identical to the parental strain (Fig. S6B) and exhibited normal growth (Fig. S6C). As a comparison, a quadruple mutant of Pdd RM-71 defective in Dly, PhlyP, PhlyC, and PlpV cytotoxins, but with intact δ-endotoxin gene, was non-virulent under the conditions tested (Fig. S6A). These results suggest that the δ-endotoxin gene is expendable for maximal virulence of Pdd RM-71 in a turbot fish model. Notwithstanding, considering the broad host range of Pdd, it would be interesting to conduct future studies to assess the role of this putative PirAB-like toxin for other animal hosts, including fish and crustaceans.
Changes in expression at the transcriptional level may not necessarily reflect the final changes in actual protein amounts since other regulatory mechanisms may tune ultimate protein abundance (120). To gain an insight into the effect of salinity in the secretome of Pdd, RM-71 culture supernatants obtained at 3% NaCl and 1% NaCl were examined by SDS-PAGE, and protein bands were subjected to quantitative analysis. As shown in Fig. 9A, the majority of the secreted proteins were significantly more abundant at 1% NaCl than at 3% NaCl. Notably, cytotoxins Dly, PhlyP, and PhlyC and proteins of the putative PirAB-like toxin were more abundant in the profile at low salt. The most abundant protein bands corresponded to Dly and the PirA-like 11-kDa protein (A0J47_RS13280), which were 25 and 13 times more abundant, respectively, at 1% than at 3% NaCl (Fig. 9B). Altogether, these results clearly confirm that a shift from high to low salinity triggers a virulence profile in Pdd. In concordance with the results of the analysis of secreted proteins, the phenotypical plate tests for the detection of hemolysis (a phenotype attributable to synergistic effects of Dly with PhlyP and PhlyC) and phospholipase (a phenotype mainly attributable to Dly) showed increased activities at 1% compared to 3% NaCl (Fig. 10A). These observations are in the same line as previous reports documenting the induction of cytotoxin expression in Vibrio species in response to low salt, as seen with V. vulnificus vvhA hemolysin (121). Similarly, cytotoxicity of V. parahaemolyticus has been shown to be higher when grown in 1% NaCl when compared to 3% NaCl (107).
Fig 9.
Salinity modulates the abundance of secreted proteins in Pdd. (A) Comparative analysis of SDS-PAGE profiles of culture supernatants from RM-71wt at 1% NaCl and 3% NaCl. Previously identified protein bands (27) are numbered from 1 to 12. Bands with higher abundance at low salt are marked with an asterisk (*). Identification and tagging of the proteins is shown in the adjacent table. (B) Protein quantification results were obtained by densitometric analysis using ImageLab Software (Bio-Rad). Sialidase band (2, HKP) was used as a normalization control. Data are presented as mean ± SD of normalized protein intensity (expressed as a.u, arbitrary units) from three replicates over three independent experiments. The corresponding band on the SDS-PAGE gel shown in (A) is given in parentheses. nd, not detected. Statistical difference was assessed by Student’s t-test: ****P < 0.0001, ***P < 0.001, **P < 0.01, *P < 0.05.
Fig 10.
Differential gene expression patterns in the virulence plasmid pPHDD1 in response to changes in NaCl concentration. (A) Box plot showing hemolytic and phospholipase activities (expressed as a.u, arbitrary units, of the halo-to-colony ratio) of RM-71 at two NaCl conditions. Statistical significance was determined using Student’s t-test: ****P < 0.0001, ***P < 0.001. (B) Cell illustration depicting pPHDD1-encoded genes that are part of the low-salt stimulon of Pdd RM-71. Cellular membrane was used from Servier Medical Art templates, licensed under a Creative Commons Attribution 3.0 Unported License. (C) Graph showing the distribution of upregulated (orange), downregulated (blue), and unchanged genes (gray) along pPHDD1 virulence plasmid of RM-71 at 1% NaCl versus 3% NaCl. All NCBI locus tags in x-axis must be preceded by “A0J47_RS.” “A” and “B” denote the groups where most of the upregulated genes are concentrated. (D) Heat map depicting upregulated and downregulated DEGs in RM-71 pPHDD1 plasmid at 1% NaCl versus 3% NaCl. Expression values are represented as Log2 (Fold Change); hypothetical proteins are denoted as “Hyp.”
Additional secreted proteins with increased abundance at low salt were a lipoprotein (A0J47_RS11245) and an RstAB-dependent hypothetical protein (A0J47_RS20565). In accordance with these results, their respective genes were also upregulated at 1% NaCl in the transcriptomic assay. However, the trypsin-like serine protease (band 8 in Fig. 9A), which was 5.67-fold upregulated at 1% NaCl in the RNA-seq data, exhibited more protein abundance at 3% NaCl. It has to be noted that, due to the low amount of protein obtained from cultures grown to the exponential phase (OD600 of 0.55), the comparative analysis of the secretome at the two NaCl conditions was performed using cultures grown to the stationary phase (OD600 of 1.7). Therefore, it might be expected that the relative levels of some proteins in the Pdd secretome when comparing the two salinity conditions do not exactly mimic their relative levels in the transcriptomic data.
Altogether, our data showing that secreted proteins are much more abundantly produced at low salinity (Fig. 9) indicate that Pdd cells allocate valuable resources for the production of high amounts of proteins. Consistent with these observations, several genes involved in protein synthesis and regulation were transcriptionally induced at low salt (Fig. 2 and Table 2), and as many as 52 genes encoding ribosomal proteins were upregulated at 1% NaCl (Table S2). Additionally, the genes encoding ribosomal RpsF modification protein RimK (A0J47_RS00405) as well as YegQ (A0J47_RS12990), involved in tRNA hydroxylation, were listed among the 20 top DEGs under this condition.
Iron acquisition systems are upregulated at 1% NaCl
Iron (Fe) is an essential element for most microorganisms, but its availability within the host is strongly limited, as it is chelated by proteins (122). In a vertebrate host, most Fe is complexed to heme, and hemoglobin is contained within erythrocytes. Previous studies reported that Pdd can use ferric citrate, ferrous iron, transferrin, and heme as iron sources (123, 124). Being low salinity an informative signal of entry into a host, it would be expected that iron uptake systems be upregulated at 1% in Pdd. Confirming this hypothesis, RNA-seq unveiled the upregulation, at low salt, of Fbp and Feo systems, involved in ferric and ferrous iron transport, respectively (Table 2). fbpA gene was the most upregulated with an FC of 18.74 (Table 2), but the upregulation was also observed for the gene encoding the TonB-dependent siderophore receptor FhuE and for some genes of the cluster encoding the heme iron utilization system (Fig. 3, chromosome I panel). Furthermore, the upregulation of erythrocyte-lysing hemolysins Dly and PhlyP at low salt is expected to contribute to the release of heme iron. Also of note, low salt conditions upregulated (9.5-fold) a plasmid (pPHDD1)-encoded TonB-dependent receptor (A0J47_RS20555) homologous (60% amino acid identity) to Vep20, a transferrin receptor that is a key element for V. vulnificus virulence in eels (125).
Additional candidate virulence factors encoded within pPHDD1 plasmid are upregulated at low salt
Previous studies have demonstrated that the deletion of the pPHDD1-encoded cytotoxin genes dly and hlyApl in Pdd RM-71 is not sufficient to mimic the low-virulence profile of a naturally plasmidless strain of Pdd (44), emphasizing the potential implications of yet uncharacterized virulence factors encoded within pPHDD1 plasmid. As said above, the hemolytic and phospholipase activities are upregulated at low salinity and are mainly attributable to the cytotoxins encoded within pPHDD1 (Fig. 10A). By studying the comparative transcriptional landscape of pPHDD1 plasmid in response to low salt, we detected the upregulation of additional, potential virulence factors (Fig. 10B), whose genes are distributed in specific regions within the plasmid, suggesting that the genes within a cluster may be co-transcribed and co-regulated (Fig. 10C and D). A cluster A of five upregulated genes includes the AcrAB proteins, TolC, and two additional ABC permeases, where AcrAB-TolC constitutes a putative multidrug efflux transporter. Cluster B includes two interesting candidate virulence genes that have not been studied in Pdd so far. The first gene encodes an OM lipoprotein (A0J47_RS20550) homologous to Vep07 involved in resistance to eel serum in V. vulnificus biotype 2, and mutations in vep07 were correlated with loss of virulence for eels (126). The second gene encodes the Vep20-like TonB-dependent transferrin receptor mentioned above (A0J47_RS20555). It is so far unknown whether these two genes encoding the Vep07- and the Vep20-like proteins play a role in Pdd pathobiology, but they certainly will deserve special attention in future studies. In addition, A0J47_RS20585, encoding an OM protein OmpU, is also part of the low salt-induced profile (FC 3.7). Several studies have disclosed the crucial role of OmpU in proliferation, colonization, and adhesion in different Vibrios (127). In accordance with our results, high salt also induced the upregulation of OmpU in V. parahaemolyticus (8). Finally, two uncharacterized pPHDD1-encoded proteins are also part of the low-salt stimulon of Pdd and correspond to gene A0J47_RS20580, induced 3.6-fold at 1% NaCl, and the T2SS-secreted RstAB-dependent protein (A0J47_RS20565; FC: 4.7) (27).
Concluding remarks
Here, we propose that the decrease in NaCl concentration experienced by Pdd on entry into a host (from 3% to 1% NaCl) triggers a virulence program by strongly upregulating cytotoxin abundance in the secretome fraction, protein translation, expression of iron acquisition systems, and additional virulence-related functions. On the contrary, growth at marine salinity upregulates compatible solute uptake and enhances energy production mechanisms, utilization of trehalose and fructose as carbon sources, nitrogen metabolism, and global amino acid metabolism, with emphasis on enzymes of the ADS that might play a role in acid resistance. It is noticeable that the salinity regulon does not overlap with the previously reported RstAB regulon in Pdd, suggesting that this two-component system may not be primarily responsible for transducing information on changes in NaCl concentration. As an example, while mutation of RstAB system caused a 1,300-fold downregulation of capsule biogenesis genes (28), the impact of NaCl changes in the transcription of capsule genes in the present study was very discrete (with FCs ranging from 1.6 to 3.57). Among the species of the Vibrionaceae, few examples are known about the regulatory systems that transduce information on NaCl concentrations, but it is evident that transcriptome responses to salinity changes are transduced via several mechanisms in Vibrios, rather than via a single global regulator. In V. cholerae, OscR (6), CosR (7), and EnvZ/OmpR (128) constitute osmolarity-responsive regulators, and new regulatory genes have recently been described that mediate salinity-responsive modulation of gene expression in V. parahaemolyticus (129). The strong induction of a virulence genetic program in Pdd on exposure to low salinity, and the conspicuous changes elicited in the T2SS-dependent secretome, will surely boost future studies aimed at identifying novel osmolarity-sensitive systems in this generalist and highly versatile marine bacterium and will inspire studies in other Vibrionaceae species.
ACKNOWLEDGMENTS
This work was supported by the grant PID2019-110558RB-I00 funded by Spanish MCIN/AEI/10.13039/501100011033; “ERDF A way of making Europe”; the European Union; and Xunta de Galicia (Spain) (grant nos ED431C 2018/18 and ED431C 2022/23). Alba V. Barca thanks the IACOBUS program (also supported by the INTERREG VI A España-Portugal (POPTEC) 2021-2027) for a predoctoral mobility grant for a 3 month research period at IBMC, Porto, Portugal. Ana do Vale was funded by Portuguese national funds through the FCT–Fundação para a Ciência e a Tecnologia, I.P. and, when eligible, by COMPETE 2020 FEDER funds, under the Scientific Employment Stimulus-Individual Call 2021.02251.CEECIND/CP1663/CT0016.
We are grateful to Insuiña S.L. (Oia, Galicia, Spain) for their valuable support in providing fish for the experiments.
The authors declare no conflict of interest.
Contributor Information
Carlos R. Osorio, Email: cr.osorio@usc.es.
E. Maggie Sogin, University of California Merced, Merced, California, USA
DATA AVAILABILITY
A list of all differentially expressed genes (DEGs) is provided in Table S2. The complete genome of Pdd RM-71 strain is available under the accession number GCF_001708035.2.
SUPPLEMENTAL MATERIAL
The following material is available online at https://doi.org/10.1128/msystems.01253-22.
ASM does not own the copyrights to Supplemental Material that may be linked to, or accessed through, an article. The authors have granted ASM a non-exclusive, world-wide license to publish the Supplemental Material files. Please contact the corresponding author directly for reuse.
REFERENCES
- 1. Choi G, Choi SH. 2022. Complex regulatory networks of virulence factors in Vibrio vulnificus. Trends Microbiol 30:1205–1216. doi: 10.1016/j.tim.2022.05.009 [DOI] [PubMed] [Google Scholar]
- 2. Thompson FL, Iida T, Swings J. 2004. Biodiversity of vibrios. Microbiol Mol Biol Rev 68:403–431, doi: 10.1128/MMBR.68.3.403-431.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Baker-Austin C, Oliver JD, Alam M, Ali A, Waldor MK, Qadri F, Martínez J. 2018. Vibrio spp. infections. Nat Rev Dis Primers 4:7. doi: 10.1038/s41572-018-0010-y [DOI] [PubMed] [Google Scholar]
- 4. Lyons MM, Lau Y-T, Carden WE, Ward JE, Roberts SB, Smolowitz R, Vallino J, Allam B. 2007. Characteristics of marine aggregates in shallow-water ecosystems: implications for disease ecology. EcoHealth 4:406–420. doi: 10.1007/s10393-007-0134-0 [DOI] [Google Scholar]
- 5. Takemura AF, Chien DM, Polz MF. 2014. Associations and dynamics of Vibrionaceae in the environment, from the genus to the population level. Front Microbiol 5:38. doi: 10.3389/fmicb.2014.00038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Shikuma NJ, Yildiz FH. 2009. Identification and characterization of OscR, a transcriptional regulator involved in osmolarity adaptation in Vibrio cholerae. J Bacteriol 191:4082–4096. doi: 10.1128/JB.01540-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Shikuma NJ, Davis KR, Fong JNC, Yildiz FH. 2013. The transcriptional regulator, CosR, controls compatible solute biosynthesis and transport, motility and biofilm formation in Vibrio cholerae. Environ Microbiol 15:1387–1399. doi: 10.1111/j.1462-2920.2012.02805.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yang L, Zhan L, Han H, Gao H, Guo Z, Qin C, Yang R, Liu X, Zhou D. 2010. The low-salt stimulon in Vibrio parahaemolyticus. Int J Food Microbiol 137:49–54. doi: 10.1016/j.ijfoodmicro.2009.11.006 [DOI] [PubMed] [Google Scholar]
- 9. Ma R, Wang Y, Huang L, Zhao S, Li L, Yin M, Fang W. 2021. Effects of different salinity on the transcriptome and antibiotic resistance of two Vibrio parahaemolyticus strains isolated from Penaeus vannamei cultured in seawater and freshwater ponds. J Fish Dis 44:2055–2066. doi: 10.1111/jfd.13520 [DOI] [PubMed] [Google Scholar]
- 10. García K, Yáñez C, Plaza N, Peña F, Sepúlveda P, Pérez-Reytor D, Espejo RT. 2017. Gene expression of Vibrio parahaemolyticus growing in laboratory isolation conditions compared to those common in its natural ocean environment. BMC Microbiol 17:118. doi: 10.1186/s12866-017-1030-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hu S, Li Y, Wang B, Yin L, Jia X. 2022. Effects of Nacl concentration on the behavior of Vibrio Brasiliensis and Transcriptome analysis. Foods 11:840. doi: 10.3390/foods11060840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Huang Z, Yu K, Fang Y, Dai H, Cai H, Li Z, Kan B, Wei Q, Wang D. 2020. Comparative Genomics and Transcriptomics analyses reveal a unique environmental adaptability of Vibrio Fujianensis. Microorganisms 8:555. doi: 10.3390/microorganisms8040555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Williams TC, Blackman ER, Morrison SS, Gibas CJ, Oliver JD. 2014. Transcriptome sequencing reveals the virulence and environmental genetic programs of Vibrio Vulnificus exposed to host and Estuarine conditions. PLoS One 9:e114376. doi: 10.1371/journal.pone.0114376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ortigosa M, Esteve C, Pujalte MJ. 1989. Vibrio species in seawater and mussels: abundance and numerical taxonomy. Syst Appl Microbiol 12:316–325. doi: 10.1016/S0723-2020(89)80080-3 [DOI] [Google Scholar]
- 15. Cortés G, Antillón F. 1990. Aislamiento de vibrios enteropatógenos de bivalvos y cieno del golfo de Nicoya, Costa Rica. Rev Biol Trop 38:437–440. [PubMed] [Google Scholar]
- 16. Kelly MT, Stroh EM. 1988. Occurrence of Vibrionaceae in natural and cultivated oyster populations in the Pacific Northwest. Diagn Microbiol Infect Dis 9:1–5. doi: 10.1016/0732-8893(88)90054-5 [DOI] [PubMed] [Google Scholar]
- 17. Buck JD. 1990. Potentially pathogenic marine Vibrio species in seawater and marine animals in the Sarasota, Florida, area. J Coast Res 6:943–948. [Google Scholar]
- 18. Herwig RP, Staley JT. 1986. Anaerobic bacteria from the digestive tract of North Atlantic fin whales (Balaenoptera physalus) . FEMS Microbiol Ecol 38:361–371. doi: 10.1111/j.1574-6968.1986.tb01749.x [DOI] [Google Scholar]
- 19. Yu J, Kang MJ, Kim YJ, Park M-J, Lim JK, Noh CH, Kang SG, Lee HS, Lee J-H, Kwon KK. 2021. Comparison of intestine microbiota between wild and farmed Korean rockfish, Sebastes schlegelii. Ocean Sci J 56:297–306. doi: 10.1007/s12601-021-00022-2 [DOI] [Google Scholar]
- 20. Wang C, Lin G, Yan T, Zheng Z, Chen B, Sun F. 2014. The cellular community in the intestine of the shrimp Penaeus penicillatus and its culture environments. Fish Sci 80:1001–1007. doi: 10.1007/s12562-014-0765-3 [DOI] [Google Scholar]
- 21. Pujalte MJ, Sitjà-Bobadilla A, Alvarez-Pellitero P, Garay E. 2003. Carriage of potentially fish-pathogenic bacteria in Sparus aurata cultured in Mediterranean fish farms. Dis Aquat Organ 54:119–126. doi: 10.3354/dao054119 [DOI] [PubMed] [Google Scholar]
- 22. Raharjo HM, Budiyansah H, Mursalim MF, Chokmangmeepisarn P, Sakulworakan R, Madyod S, Sewaka M, Sonthi M, Debnath PP, Elayaraja S, Rung-Ruangkijkrai T, Dong HT, Rodkhum C. 2022. Distribution of Vibrionaceae in farmed Asian sea bass, Lates calcarifer in Thailand and their high prevalence of antimicrobial resistance. J Fish Dis 45:1355–1371. doi: 10.1111/jfd.13667 [DOI] [PubMed] [Google Scholar]
- 23. Torido Y, Ohshima C, Takahashi H, Miya S, Iwakawa A, Kuda T, Kimura B. 2014. Distribution of psychrophilic and mesophilic histamine-producing bacteria in retailed fish in Japan. Food Control 46:338–342. doi: 10.1016/j.foodcont.2014.05.045 [DOI] [Google Scholar]
- 24. Osorio CR, Vences A, Matanza XM, Terceti MS. 2018. Photobacterium damselae subsp. damselae, a generalist pathogen with unique virulence factors and high genetic diversity. J Bacteriol 200:e00002–18. doi: 10.1128/JB.00002-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Terceti MS, Ogut H, Osorio CR. 2016. Photobacterium damselae subsp. damselae, an emerging fish pathogen in the Black Sea: evidence of a multiclonal origin. Appl Environ Microbiol 82:3736–3745. doi: 10.1128/AEM.00781-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Martin DP, Barron OA, Wu CH. 2022. Photobacterium damsela necrotizing fasciitis of the arm. J Hand Surg Am 47:905. doi: 10.1016/j.jhsa.2021.07.033 [DOI] [PubMed] [Google Scholar]
- 27. Terceti MS, Vences A, Matanza XM, Barca AV, Noia M, Lisboa J, Dos Santos NMS, do Vale A, Osorio CR. 2019. The Rstab system impacts virulence, motility, cell morphology, penicillin tolerance and production of type II secretion system-dependent factors in the fish and human pathogen Photobacterium Damselae Subsp. Damselae. Front Microbiol 10:897. doi: 10.3389/fmicb.2019.00897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Matanza XM, López-Suárez L, do Vale A, Osorio CR. 2021. The two-component system RstAB regulates production of a polysaccharide capsule with a role in virulence in the marine pathogen Photobacterium damselae subsp. damselae. Environ Microbiol 23:4859–4880. doi: 10.1111/1462-2920.15731 [DOI] [PubMed] [Google Scholar]
- 29. Terceti MS, Rivas AJ, Alvarez L, Noia M, Cava F, Osorio CR. 2017. RstB regulates expression of the Photobacterium damselae subsp. damselae major virulence factors damselysin, phobalysin P and phobalysin C. Front Microbiol 08:582. doi: 10.3389/fmicb.2017.00582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bilecen K, Yildiz FH. 2009. Identification of a calcium-controlled negative regulatory system affecting Vibrio cholerae biofilm formation. Environ Microbiol 11:2015–2029. doi: 10.1111/j.1462-2920.2009.01923.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Rivas AJ, Balado M, Lemos ML, Osorio CR. 2013. Synergistic and additive effects of chromosomal and plasmid-encoded hemolysins contribute to hemolysis and virulence in Photobacterium damselae subsp. damselae. Infect Immun 81:3287–3299. doi: 10.1128/IAI.00155-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Puentes B, Balado M, Bermúdez-Crespo J, Osorio CR, Lemos ML. 2017. A proteomic analysis of the iron response of Photobacterium damselae subsp. damselae reveals metabolic adaptations to iron levels changes and novel potential virulence factors. Vet Microbiol 201:257–264. doi: 10.1016/j.vetmic.2017.01.040 [DOI] [PubMed] [Google Scholar]
- 33. von Hoven G, Neukirch C, Meyenburg M, Schmidt S, Vences A, Osorio CR, Husmann M, Rivas AJ. 2018. Cytotoxin- and chemotaxis-genes cooperate to promote adhesion of Photobacterium damselae subsp. damselae. Front Microbiol 9:2996. doi: 10.3389/fmicb.2018.02996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Miller JH. 1972. Experiments in molecular genetics. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. [Google Scholar]
- 35. Fouz B, Larsen JL, Nielsen B, Barja JL, Toranzo AE. 1992. Characterization of Vibrio damsela strains isolated from turbot Scophthalmus maximus in Spain. Dis Aquat Org 12:155–166. doi: 10.3354/dao012155 [DOI] [Google Scholar]
- 36. Matanza XM, Osorio CR. 2018. Transcriptome changes in response to temperature in the fish pathogen Photobacterium damselae subsp. damselae: clues to understand the emergence of disease outbreaks at increased seawater temperatures. PLoS One 13: e0210118. doi: 10.1371/journal.pone.0210118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Langmead B, Salzberg SL. 2012. Fast gapped-read alignment with Bowtie 2. Nat Methods 9:357–359. doi: 10.1038/nmeth.1923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Anders S, Pyl PT, Huber W. 2015. HTSeq -- a Python framework to work with high-throughput sequencing data. Bioinformatics 31:166–169. doi: 10.1093/bioinformatics/btu638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Love MI, Huber W, Anders S. 2014. Moderated estimation of fold change and dispersion for RNA-Seq data with DESeq2. Genome Biol 15:550. doi: 10.1186/s13059-014-0550-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Benjamini Y, Hochberg Y. 1995. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Series B Stat Methodol 57:289–300. doi: 10.1111/j.2517-6161.1995.tb02031.x [DOI] [Google Scholar]
- 41. Barroso C, Carvalho P, Carvalho C, Santarém N, Gonçalves JFM, Rodrigues PNS, Neves JV. 2020. The diverse Piscidin repertoire of the European sea Bass (Dicentrarchus Labrax): Molecular characterization and antimicrobial activities. Int J Mol Sci 21:4613. doi: 10.3390/ijms21134613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Adler J, Hazelbauer GL, Dahl MM. 1973. Chemotaxis toward sugars in Escherichia coli. J Bacteriol 115:824–847. doi: 10.1128/jb.115.3.824-847.1973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S, Schmid B, Tinevez J-Y, White DJ, Hartenstein V, Eliceiri K, Tomancak P, Cardona A. 2012. Fiji: an open-source platform for biological-image analysis. Nat Methods 9:676–682. doi: 10.1038/nmeth.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Vences A, Rivas AJ, Lemos ML, Husmann M, Osorio CR. 2017. Chromosome-encoded Hemolysin, Phospholipase, and collagenase in Plasmidless isolates of Photobacterium damselae subsp. damselae contribute to virulence for fish. Appl Environ Microbiol 83:e00401-17. doi: 10.1128/AEM.00401-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Laemmli UK. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685. doi: 10.1038/227680a0 [DOI] [PubMed] [Google Scholar]
- 46. Osorio CR, Toranzo AE, Romalde JL, Barja JL. 2000. Multiplex PCR assay for ureC and 16S rRNA genes clearly discriminates between both subspecies of Photobacterium damselae. Dis Aquat Organ 40:177–183. doi: 10.3354/dao040177 [DOI] [PubMed] [Google Scholar]
- 47. Love M, Teebken-Fisher D, Hose JE, Farmer JJ, Hickman FW, Fanning GR. 1981. Vibrio damsela, a marine bacterium, causes skin ulcers on the damselfish Chromis punctipinnis. Science 214:1139–1140. doi: 10.1126/science.214.4525.1139 [DOI] [PubMed] [Google Scholar]
- 48. Meeske AJ, Riley EP, Robins WP, Uehara T, Mekalanos JJ, Kahne D, Walker S, Kruse AC, Bernhardt TG, Rudner DZ. 2016. SEDS proteins are a widespread family of bacterial cell wall polymerases. Nature 537:634–638. doi: 10.1038/nature19331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Möll A, Dörr T, Alvarez L, Davis BM, Cava F, Waldor MK. 2015. A D, D-carboxypeptidase is required for Vibrio cholerae halotolerance. Environ Microbiol 17:527–540. doi: 10.1111/1462-2920.12779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Schoeffield AJ, Williams HN. 1990. Efficiencies of recovery of bdellovibrios from brackish-water environments by using various bacterial species as prey. Appl Environ Microbiol 56:230–236. doi: 10.1128/aem.56.1.230-236.1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Nagata T, Kirchman DL. 1992. Release of macromolecular organic complexes by heterotrophic marine flagellates. Mar Ecol Prog Ser 83:233–240. doi: 10.3354/meps083233 [DOI] [Google Scholar]
- 52. Landry MR, Kirshtein J, Constantinou J. 1995. A refined dilution technique for measuring the community grazing impact of microzooplankton, with experimental tests in the central equatorial Pacific. Mar Ecol Prog Ser 120:53–63. doi: 10.3354/meps120053 [DOI] [Google Scholar]
- 53. Terceti MS, Vences A, Matanza XM, Dalsgaard I, Pedersen K, Osorio CR. 2018. Molecular epidemiology of Photobacterium damselae subsp. damselae outbreaks in Marine Rainbow Trout farms reveals extensive horizontal Gene transfer and high genetic diversity. Front Microbiol 9:2155. doi: 10.3389/fmicb.2018.02155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Liu Y, Gao W, Wang Y, Wu L, Liu X, Yan T, Alm E, Arkin A, Thompson DK, Fields MW, Zhou J. 2005. Transcriptome analysis of Shewanella Oneidensis MR-1 in response to elevated salt conditions. J Bacteriol 187:2501–2507. doi: 10.1128/JB.187.7.2501-2507.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Rozen Y, Larossa RA, Templeton LJ, Smulski DR, Belkin S. 2002. Gene expression analysis of the response by Escherichia coli to seawater. Antonie Van Leeuwenhoek 81:15–25. doi: 10.1023/a:1020500821856 [DOI] [PubMed] [Google Scholar]
- 56. Wang J, Vine CE, Balasiny BK, Rizk J, Bradley CL, Tinajero-Trejo M, Poole RK, Bergaust LL, Bakken LR, Cole JA. 2016. The roles of the hybrid cluster protein, HCP and its reductase, HCR, in high affinity nitric oxide reduction that protects anaerobic cultures of Escherichia coli against nitrosative stress. Mol Microbiol 100:877–892. doi: 10.1111/mmi.13356 [DOI] [PubMed] [Google Scholar]
- 57. Filenko N, Spiro S, Browning DF, Squire D, Overton TW, Cole J, Constantinidou C. 2007. The NsrR regulon of Escherichia coli K-12 includes genes encoding the hybrid cluster protein and the periplasmic, respiratory nitrite reductase. J Bacteriol 189:4410–4417. doi: 10.1128/JB.00080-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Gon S, Patte JC, Méjean V, Iobbi-Nivol C. 2000. The TorYZ (yeck bisz) operon encodes a third respiratory trimethylamine N-oxide reductase in Escherichia coli. J Bacteriol 182:5779–5786. doi: 10.1128/JB.182.20.5779-5786.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Malle P, Valle M, Eb P, Tailliez R. 1998. Optimization of culture conditions for enumeration of H2S bacteria in the flesh of seafish. J Rapid Methods Auto Microbiol 6:129–141. doi: 10.1111/j.1745-4581.1998.tb00193.x [DOI] [Google Scholar]
- 60. Rasheedkhan Regina V, Noorian P, Sim CBW, Constancias F, Kaliyamoorthy E, Booth SC, Espinoza-Vergara G, Rice SA, McDougald D. 2022. Loss of the acetate switch in Vibrio Vulnificus enhances Predation defense against Tetrahymena Pyriformis. Appl Environ Microbiol 88:e0166521. doi: 10.1128/AEM.01665-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Chuang MY, Tsai WC, Kuo TY, Chen HM, Chen WJ. 2016. Comparative proteome analysis reveals proteins involved in salt adaptation in Photobacterium damselae subsp. piscicida. J Basic Microbiol 56:1234–1243. doi: 10.1002/jobm.201600091 [DOI] [PubMed] [Google Scholar]
- 62. Csonka LN. 1989. Physiological and genetic responses of bacteria to osmotic stress. Microbiol Rev 53:121–147. doi: 10.1128/mr.53.1.121-147.1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Gregory GJ, Dutta A, Parashar V, Boyd EF. 2020. Investigations of dimethylglycine, glycine betaine, and ectoine uptake by a betaine-carnitine-choline transporter family transporter with diverse substrate specificity in Vibrio species. J Bacteriol 202: e00314-20. doi: 10.1128/JB.00314-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Rao NV, Shashidhar R, Bandekar JR. 2013. Comparative analysis of induction of osmotic-stress-dependent genes in Vibrio vulnificus exposed to hyper- and hypo-osmotic stress. Can J Microbiol 59:333–338. doi: 10.1139/cjm-2012-0749 [DOI] [PubMed] [Google Scholar]
- 65. Amaral GRS, Dias GM, Wellington-Oguri M, Chimetto L, Campeão ME, Thompson FL, Thompson CC. 2014. Genotype to phenotype: identification of diagnostic Vibrio phenotypes using whole genome sequences. Int J Syst Evol Microbiol 64:357–365. doi: 10.1099/ijs.0.057927-0 [DOI] [PubMed] [Google Scholar]
- 66. Meadow ND, Revuelta R, Chen VN, Colwell RR, Roseman S. 1987. Phosphoenolpyruvate:glycose phosphotransferase system in species of Vibrio, a widely distributed marine bacterial genus. J Bacteriol 169:4893–4900. doi: 10.1128/jb.169.11.4893-4900.1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Mopper K, Dawson R, Liebezeit G, Ittekkot V. 1980. The monosaccharide spectra of natural waters. Marine Chemistry 10:55–66. doi: 10.1016/0304-4203(80)90058-4 [DOI] [Google Scholar]
- 68. Sogin EM, Puskás E, Dubilier N, Liebeke M. 2019. Marine metabolomics: a method for nontargeted measurement of metabolites in seawater by gas chromatography-mass spectrometry. mSystems 4: e00638-19. doi: 10.1128/mSystems.00638-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Sogin EM, Michellod D, Gruber-Vodicka HR, Bourceau P, Geier B, Meier DV, Seidel M, Ahmerkamp S, Schorn S, D’Angelo G, Procaccini G, Dubilier N, Liebeke M. 2022. Sugars dominate the seagrass rhizosphere. Nat Ecol Evol 6:866–877. doi: 10.1038/s41559-022-01740-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Leyton A, Flores L, Mäki-Arvela P, Lienqueo ME, Shene C. 2019. Macrocystis pyrifera source of nutrients for the production of carotenoids by a marine yeast Rhodotorula mucilaginosa. J Appl Microbiol 127:1069–1079. doi: 10.1111/jam.14362 [DOI] [PubMed] [Google Scholar]
- 71. Johnston MA, Davies PS. 1972. Carbohydrates of the hepatopancreas and blood tissues of Carcinus. Comp Biochem Physiol B Biochem 41:433–443. doi: 10.1016/0305-0491(72)90046-6 [DOI] [Google Scholar]
- 72. Martínez-Crego B, Vizzini S, Califano G, Massa-Gallucci A, Andolina C, Gambi MC, Santos R. 2020. Resistance of seagrass habitats to ocean acidification via altered interactions in a tri-trophic chain. Sci Rep 10: 5103. doi: 10.1038/s41598-020-61753-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Lunn JE. 2002. Evolution of sucrose synthesis. Plant Physiol 128:1490–1500. doi: 10.1104/pp.010898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Dörr T, Delgado F, Umans BD, Gerding MA, Davis BM, Waldor MK. 2016. A transposon screen identifies genetic determinants of Vibrio cholerae resistance to high-molecular-weight antibiotics. Antimicrob Agents Chemother 60:4757–4763. doi: 10.1128/AAC.00576-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Kapetanović D, Vardić Smrzlić I, Kazazić S, Omanović D, Cukrov N, Cindrić A-M, Rapljenović A, Perić L, Orlić K, Mijošek T, Redžović Z, Gavrilović A, Radočaj T, Filipović Marijić V. 2023. A preliminary study of the cultivable microbiota on the plastic litter collected by commercial fishing trawlers in the south-eastern Adriatic Sea, with emphasis on Vibrio isolates and their antibiotic resistance. Mar Pollut Bull 187:114592. doi: 10.1016/j.marpolbul.2023.114592 [DOI] [PubMed] [Google Scholar]
- 76. Coronado MJ, Vargas C, Kunte HJ, Galinski EA, Ventosa A, Nieto JJ. 1995. Influence of salt concentration on the susceptibility of moderately halophilic bacteria to antimicrobials and its potential use for genetic transfer studies. Curr Microbiol 31:365–371. doi: 10.1007/BF00294701 [DOI] [PubMed] [Google Scholar]
- 77. Ottaviani D, Bacchiocchi I, Masini L, Leoni F, Carraturo A, Giammarioli M, Sbaraglia G. 2001. Antimicrobial susceptibility of potentially pathogenic halophilic vibrios isolated from seafood. Int J Antimicrob Agents 18:135–140. doi: 10.1016/s0924-8579(01)00358-2 [DOI] [PubMed] [Google Scholar]
- 78. Weaver AI, Murphy SG, Umans BD, Tallavajhala S, Onyekwere I, Wittels S, Shin JH, VanNieuwenhze M, Waldor MK, Dörr T. 2018. Genetic determinants of penicillin tolerance in Vibrio Cholerae. Antimicrob Agents Chemother 62:e01326-18. doi: 10.1128/AAC.01326-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Dörr T, Davis BM, Waldor MK. 2015. Endopeptidase-mediated beta lactam tolerance. PLoS Pathog 11: e1004850. doi: 10.1371/journal.ppat.1004850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Sarkar SK, Chowdhury C, Ghosh AS. 2010. Deletion of penicillin-binding protein 5 (PBP5) sensitises Escherichia coli cells to beta-lactam agents. Int J Antimicrob Agents 35:244–249. doi: 10.1016/j.ijantimicag.2009.11.004 [DOI] [PubMed] [Google Scholar]
- 81. Garneau-Tsodikova S, Labby KJ. 2016. Mechanisms of resistance to aminoglycoside antibiotics: overview and perspectives. Medchemcomm 7:11–27. doi: 10.1039/C5MD00344J [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Huda MN, Morita Y, Kuroda T, Mizushima T, Tsuchiya T. 2001. Na+-driven multidrug efflux pump VcmA from Vibrio cholerae non-O1, a non-halophilic bacterium. FEMS Microbiol Lett 203:235–239. doi: 10.1111/j.1574-6968.2001.tb10847.x [DOI] [PubMed] [Google Scholar]
- 83. Su Y, Peng B, Han Y, Li H, Peng X. 2015. Fructose restores susceptibility of multidrug-resistant Edwardsiella tarda to kanamycin. J Proteome Res 14:1612–1620. doi: 10.1021/pr501285f [DOI] [PubMed] [Google Scholar]
- 84. Su Y-B, Peng B, Li H, Cheng Z-X, Zhang T-T, Zhu J-X, Li D, Li M-Y, Ye J-Z, Du C-C, Zhang S, Zhao X-L, Yang M-J, Peng X-X. 2018. Pyruvate cycle increases aminoglycoside efficacy and provides respiratory energy in bacteria. Proc Natl Acad Sci U S A 115:E1578–E1587. doi: 10.1073/pnas.1714645115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Keller M, Han X, Dörr T. 2023. Disrupting central carbon metabolism increases Β-lactam antibiotic susceptibility in Vibrio Cholerae. J Bacteriol 205:e0047622. doi: 10.1128/jb.00476-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Yang J, Zeng Z-H, Yang M-J, Cheng Z-X, Peng X-X, Li H. 2018. NaCl promotes antibiotic resistance by reducing redox states in Vibrio alginolyticus. Environ Microbiol 20:4022–4036. doi: 10.1111/1462-2920.14443 [DOI] [PubMed] [Google Scholar]
- 87. Stokes JM, French S, Ovchinnikova OG, Bouwman C, Whitfield C, Brown ED. 2016. Cold stress makes Escherichia coli susceptible to glycopeptide antibiotics by altering outer membrane integrity. Cell Chem Biol 23:267–277. doi: 10.1016/j.chembiol.2015.12.011 [DOI] [PubMed] [Google Scholar]
- 88. Stogios PJ, Savchenko A. 2020. Molecular mechanisms of vancomycin resistance. Protein Sci 29:654–669. doi: 10.1002/pro.3819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Boyd DA, Willey BM, Fawcett D, Gillani N, Mulvey MR. 2008. Molecular characterization of Enterococcus faecalis N06-0364 with low-level vancomycin resistance harboring a novel D-Ala-D-Ser gene cluster, vanL. Antimicrob Agents Chemother 52:2667–2672. doi: 10.1128/AAC.01516-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Huang PH, Chen JY, Kuo CM. 2007. Three different hepcidins from tilapia, Oreochromis mossambicus: analysis of their expressions and biological functions. Mol Immunol 44:1922–1934. doi: 10.1016/j.molimm.2006.09.031 [DOI] [PubMed] [Google Scholar]
- 91. Dorrington T, Gomez-Chiarri M. 2008. Antimicrobial peptides for use in oyster aquaculture: effect on pathogens, commensals, and eukaryotic expression systems. J Shellfish Res 27:365–373. doi: 10.2983/0730-8000(2008)27[365:APFUIO]2.0.CO;2 [DOI] [Google Scholar]
- 92. Park NG, Silphaduang U, Moon HS, Seo JK, Corrales J, Noga EJ. 2011. Structure-activity relationships of piscidin 4, a piscine antimicrobial peptide. Biochemistry 50:3288–3299. doi: 10.1021/bi101395j [DOI] [PubMed] [Google Scholar]
- 93. Bjornsdottir-Butler K, Abraham A, Harper A, Dunlap PV, Benner RA Jr. 2018. Biogenic amine production by and phylogenetic analysis of 23 Photobacterium species. J Food Prot 81:1264–1274. doi: 10.4315/0362-028X.JFP-18-022 [DOI] [PubMed] [Google Scholar]
- 94. Li F, Xiong X-S, Yang Y-Y, Wang J-J, Wang M-M, Tang J-W, Liu Q-H, Wang L, Gu B. 2021. Effects of Nacl concentrations on growth patterns, phenotypes associated with virulence, and energy metabolism in Escherichia Coli Bw25113. Front Microbiol 12:705326. doi: 10.3389/fmicb.2021.705326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Fouz B, Toranzo AE, Marco-Noales E, Amaro C. 1998. Survival of fish-virulent strains of Photobacterium damselae subsp. damselae in seawater under starvation conditions. FEMS Microbiol Lett 168:181–186. doi: 10.1111/j.1574-6968.1998.tb13271.x [DOI] [PubMed] [Google Scholar]
- 96. Thompson JR, Pacocha S, Pharino C, Klepac-Ceraj V, Hunt DE, Benoit J, Sarma-Rupavtarm R, Distel DL, Polz MF. 2005. Genotypic diversity within a natural coastal bacterioplankton population. Science 307:1311–1313. doi: 10.1126/science.1106028 [DOI] [PubMed] [Google Scholar]
- 97. Juste-Poinapen NMS, Yang L, Ferreira M, Poinapen J, Rico C. 2019. Community profiling of the intestinal microbial community of juvenile Hammerhead sharks (Sphyrna Lewini) from the Rewa Delta, Fiji. Sci Rep 9:7182. doi: 10.1038/s41598-019-43522-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Pacheco-Sandoval A, Lago-Lestón A, Abadía-Cardoso A, Solana-Arellano E, Schramm Y. 2022. Age as a primary driver of the gut microbial composition and function in wild harbor seals. Sci Rep 12: 14641. doi: 10.1038/s41598-022-18565-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Itay P, Shemesh E, Ofek-Lalzar M, Davidovich N, Kroin Y, Zrihan S, Stern N, Diamant A, Wosnick N, Meron D, Tchernov D, Morick D. 2022. An insight into gill microbiome of eastern Mediterranean wild fish by applying next generation sequencing. Front. Mar. Sci 9:1008103. doi: 10.3389/fmars.2022.1008103 [DOI] [Google Scholar]
- 100. Peters L, Chikweto A, McKIBBEN J, Gibson K. 2021. Potential for scombroid poisoning from ingestion of Selar crumenophthalmus due to increased histamine levels in Grenada, West Indies. J Food Prot 84:368–371. doi: 10.4315/JFP-20-255 [DOI] [PubMed] [Google Scholar]
- 101. Pedersen K, Dalsgaard I, Larsen JL. 1997. Vibrio damsela associated with diseased fish in Denmark. Appl Environ Microbiol 63:3711–3715. doi: 10.1128/aem.63.9.3711-3715.1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Holt JG, Krieg NR, Sneath PHA, Staley JT, Williams ST. 1994. Bergey’s manual of determinative bacteriology. 9th ed. Lippincott Williams & Wilkins, Philadelphia, Pa. [Google Scholar]
- 103. Seidler RJ, Allen DA, Colwell RR, Joseph SW, Daily OP. 1980. Biochemical characteristics and virulence of environmental group F bacteria isolated in the United States. Appl Environ Microbiol 40:715–720. doi: 10.1128/aem.40.4.715-720.1980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Casiano-Colón A, Marquis RE. 1988. Role of the arginine deiminase system in protecting oral bacteria and an enzymatic basis for acid tolerance. Appl Environ Microbiol 54:1318–1324. doi: 10.1128/aem.54.6.1318-1324.1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Ryan S, Begley M, Gahan CGM, Hill C. 2009. Molecular characterization of the arginine deiminase system in Listeria monocytogenes: regulation and role in acid tolerance. Environ Microbiol 11:432–445. doi: 10.1111/j.1462-2920.2008.01782.x [DOI] [PubMed] [Google Scholar]
- 106. Xiong L, Teng JLL, Watt RM, Kan B, Lau SKP, Woo PCY. 2014. Arginine Deiminase pathway is far more important than Urease for acid resistance and intracellular survival in Laribacter hongkongensis: a possible result of arc Gene cassette duplication. BMC Microbiol 14:42. doi: 10.1186/1471-2180-14-42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Whitaker WB, Parent MA, Naughton LM, Richards GP, Blumerman SL, Boyd EF. 2010. Modulation of responses of Vibrio parahaemolyticus O3:K6 to pH and temperature stresses by growth at different salt concentrations. Appl Environ Microbiol 76:4720–4729. doi: 10.1128/AEM.00474-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Kalburge SS, Whitaker WB, Boyd EF. 2014. High-salt preadaptation of Vibrio parahaemolyticus enhances survival in response to lethal environmental stresses. J Food Prot 77:246–253. doi: 10.4315/0362-028X.JFP-13-241 [DOI] [PubMed] [Google Scholar]
- 109. Vander Wauven C, Piérard A, Kley-Raymann M, Haas D. 1984. Pseudomonas aeruginosa mutants affected in anaerobic growth on arginine: evidence for a four-gene cluster encoding the arginine deiminase pathway. J Bacteriol 160:928–934. doi: 10.1128/jb.160.3.928-934.1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Shoesmith JH, Sherris JC. 1960. Studies on the mechanism of arginine-activated motility in a Pseudomonas strain. J Gen Microbiol 22:10–24. doi: 10.1099/00221287-22-1-10 [DOI] [PubMed] [Google Scholar]
- 111. Faquin WC, Oliver JD. 1984. Arginine uptake by a psychrophilic marine Vibrio sp. during starvation-induced morphogenesis. Microbiology 130:1331–1335. doi: 10.1099/00221287-130-6-1331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Merrell DS, Hava DL, Camilli A. 2002. Identification of novel factors involved in colonization and acid tolerance of Vibrio cholerae. Mol Microbiol 43:1471–1491. doi: 10.1046/j.1365-2958.2002.02857.x [DOI] [PubMed] [Google Scholar]
- 113. Yamamoto S, Chowdhury MA, Kuroda M, Nakano T, Koumoto Y, Shinoda S. 1991. Further study on polyamine compositions in Vibrionaceae. Can J Microbiol 37:148–153. doi: 10.1139/m91-022 [DOI] [PubMed] [Google Scholar]
- 114. Hamama K, Okada M, Saito T, Nogi Y. 2000. Polyamine distribution profiles among some members of the gamma subclass of the class Proteobacteria. Microbiol Cult Coll 16:51–61. [Google Scholar]
- 115. Jelsbak L, Thomsen LE, Wallrodt I, Jensen PR, Olsen JE. 2012. Polyamines are required for virulence in Salmonella enterica serovar Typhimurium. PLoS One 7:e36149. doi: 10.1371/journal.pone.0036149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Goforth JB, Walter NE, Karatan E. 2013. Effects of Polyamines on Vibrio cholerae virulence properties. PLoS One 8:e60765. doi: 10.1371/journal.pone.0060765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Shah P, Nanduri B, Swiatlo E, Ma Y, Pendarvis K. 2011. Polyamine biosynthesis and transport mechanisms are crucial for fitness and pathogenesis of Streptococcus pneumoniae. Microbiology (Reading) 157:504–515. doi: 10.1099/mic.0.042564-0 [DOI] [PubMed] [Google Scholar]
- 118. Kreger AS. 1984. Cytolytic activity and virulence of Vibrio damsela. Infect Immun 44:326–331. doi: 10.1128/iai.44.2.326-331.1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Lee C-T, Chen I-T, Yang Y-T, Ko T-P, Huang Y-T, Huang J-Y, Huang M-F, Lin S-J, Chen C-Y, Lin S-S, Lightner DV, Wang H-C, Wang AH-J, Wang H-C, Hor L-I, Lo C-F. 2015. The opportunistic marine pathogen Vibrio parahaemolyticus becomes virulent by acquiring a plasmid that expresses a deadly toxin. Proc Natl Acad Sci U S A 112:10798–10803. doi: 10.1073/pnas.1503129112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Taggart JC, Lalanne J-B, Li G-W. 2021. Quantitative control for stoichiometric protein synthesis. Annu Rev Microbiol 75:243–267. doi: 10.1146/annurev-micro-041921-012646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Lee SE, Shin SH, Kim SY, Kim YR, Shin DH, Chung SS, Lee ZH, Lee JY, Jeong KC, Choi SH, Rhee JH. 2000. Vibrio vulnificus has the transmembrane transcription activator ToxRS stimulating the expression of the hemolysin gene vvhA. J Bacteriol 182:3405–3415. doi: 10.1128/JB.182.12.3405-3415.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Murdoch CC, Skaar EP. 2022. Nutritional immunity: the battle for nutrient metals at the host-pathogen interface. Nat Rev Microbiol 20:657–670. doi: 10.1038/s41579-022-00745-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Fouz B, Toranzo AE, Biosca EG, Mazoy R, Amaro C. 1994. Role of iron in the pathogenicity of Vibrio damsela for fish and mammals. FEMS Microbiol Lett 121:181–188. doi: 10.1111/j.1574-6968.1994.tb07097.x [DOI] [PubMed] [Google Scholar]
- 124. Fouz B, Biosca EG, Amaro C. 1997. High affinity iron-uptake systems in Vibrio damsela: role in the acquisition of iron from transferrin. J Appl Microbiol 82:157–167. doi: 10.1111/j.1365-2672.1997.tb03568.x [DOI] [PubMed] [Google Scholar]
- 125. Pajuelo D, Lee C-T, Roig FJ, Hor L-I, Amaro C. 2015. Novel host-specific iron acquisition system in the zoonotic pathogen Vibrio vulnificus. Environ Microbiol 17:2076–2089. [DOI] [PubMed] [Google Scholar]
- 126. Roig FJ, Amaro C. 2009. Plasmid diversity in Vibrio vulnificus biotypes. Microbiology (Reading) 155:489–497. doi: 10.1099/mic.0.023424-0 [DOI] [PubMed] [Google Scholar]
- 127. Ganie HA, Choudhary A, Baranwal S. 2022. Structure, regulation, and host interaction of outer membrane protein U (OmpU) of Vibrio species. Microb Pathog 162:105267. doi: 10.1016/j.micpath.2021.105267 [DOI] [PubMed] [Google Scholar]
- 128. Xi D, Li Y, Yan J, Li Y, Wang X, Cao B. 2020. Small RNA coaR contributes to intestinal colonization in Vibrio cholerae via the two-component system Envz/Ompr. Environ Microbiol 22:4231–4243. doi: 10.1111/1462-2920.14906 [DOI] [PubMed] [Google Scholar]
- 129. Gregory GJ, Morreale DP, Carpenter MR, Kalburge SS, Boyd EF. 2019. Quorum sensing regulators Apha and Opar control expression of the Operon responsible for biosynthesis of the compatible solute Ectoine. Appl Environ Microbiol 85:e01543-19. doi: 10.1128/AEM.01543-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
A list of all differentially expressed genes (DEGs) is provided in Table S2. The complete genome of Pdd RM-71 strain is available under the accession number GCF_001708035.2.