ABSTRACT
Coronavirus genome replication and expression are mediated by the viral replication-transcription complex (RTC) which is assembled from multiple nonstructural proteins (nsp). Among these, nsp12 represents the central functional subunit. It harbors the RNA-directed RNA polymerase (RdRp) domain and contains, at its N terminus, an additional domain called NiRAN which is widely conserved in coronaviruses and other nidoviruses. In this study, we produced bacterially expressed coronavirus nsp12s to investigate and compare NiRAN-mediated NMPylation activities from representative alpha- and betacoronaviruses. We found that the four coronavirus NiRAN domains characterized to date have a number of conserved properties, including (i) robust nsp9-specific NMPylation activities that appear to operate largely independently of the C-terminal RdRp domain, (ii) nucleotide substrate preference for UTP followed by ATP and other nucleotides, (iii) dependence on divalent metal ions, with Mn2+ being preferred over Mg2+, and (iv) a key role of N-terminal residues (particularly Asn2) of nsp9 for efficient formation of a covalent phosphoramidate bond between NMP and the N-terminal amino group of nsp9. In this context, a mutational analysis confirmed the conservation and critical role of Asn2 across different subfamilies of the family Coronaviridae, as shown by studies using chimeric coronavirus nsp9 variants in which six N-terminal residues were replaced with those from other corona-, pito- and letovirus nsp9 homologs. The combined data of this and previous studies reveal a remarkable degree of conservation among coronavirus NiRAN-mediated NMPylation activities, supporting a key role of this enzymatic activity in viral RNA synthesis and processing.
IMPORTANCE There is strong evidence that coronaviruses and other large nidoviruses evolved a number of unique enzymatic activities, including an additional RdRp-associated NiRAN domain, that are conserved in nidoviruses but not in most other RNA viruses. Previous studies of the NiRAN domain mainly focused on severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and suggested different functions for this domain, such as NMPylation/RNAylation of nsp9, RNA guanylyltransferase activities involved in canonical and/or unconventional RNA capping pathways, and other functions. To help resolve partly conflicting information on substrate specificities and metal ion requirements reported previously for the SARS-CoV-2 NiRAN NMPylation activity, we extended these earlier studies by characterizing representative alpha- and betacoronavirus NiRAN domains. The study revealed that key features of NiRAN-mediated NMPylation activities, such as protein and nucleotide specificity and metal ion requirements, are very well conserved among genetically divergent coronaviruses, suggesting potential avenues for future antiviral drug development targeting this essential viral enzyme.
KEYWORDS: NMPylation, coronavirus, replication
INTRODUCTION
Coronaviridae, a large family of plus-strand RNA viruses in the order Nidovirales (1, 2), are subdivided into 3 subfamilies (Orthocoronavirinae, Letovirinae, and Pitovirinae). Because of their role as important human pathogens, two orthocoronaviruses of the genus Betacoronavirus, severe acute respiratory syndrome coronavirus (SARS-CoV) (3–5) and SARS-CoV-2 (6–8), have been characterized extensively over the past few years. Coronaviruses have exceptionally large RNA genomes of approximately 30 kb (9). Their replicase genes encode two large polyproteins, pp1a and pp1ab, that contain a conserved array of enzymes and cofactors (10, 11) which, following proteolytic release from pp1a/pp1ab by virally encoded proteases, assemble into viral replication-transcription complexes (RTC) that direct viral genomic RNA (gRNA) replication and production of a set of subgenomic mRNAs (sgmRNAs). The final processing products released from pp1a and pp1ab are called nonstructural proteins 1 to 16 (nsp1 to nsp16). Most of these proteins are part of the RTC, while a few of them have functions that are not directly involved in viral RNA synthesis and/or modification (12). Over the past 20 years, a large number of studies provided important insight into the enzymatic functions and structures of coronavirus replicative enzymes (reviewed in references 10, 12, and 13). More recently, and especially since the start of the ongoing SARS-CoV-2 pandemic in early 2020, impressive progress has also been made in the structure analysis of larger complexes of key replicative enzymes and their cofactors (10, 13–18). While the majority of enzymatic functions that, presumably, are involved in viral RNA synthesis and/or processing had been identified and characterized by 2014, including RNA-dependent RNA polymerase (RdRp, nsp12) (19), helicase (nsp13) (20), RNA 5′-triphosphatase (nsp13) (21, 22), endoribonuclease (nsp15) (23, 24), 3′-to-5′ exoribonuclease (nsp14) (25), RNA guanine-N7 (nsp14), and ribose 2′-O methyltransferases (nsp16) (26, 27), it remained unknown whether coronaviruses and related nidoviruses also encode a guanylyltransferase (GTase), a key enzyme of the classical pathway of cap synthesis that catalyzes the transfer of a (methylated) GMP to a diphosphorylated RNA 5′ end through formation of a 5′-to-5′ triphosphate linkage. Also, the possible role(s) in viral replication of the N-terminal domain of nsp12 (and its orthologs in other nidoviruses) remained elusive. In 2015, it was shown that this particular domain, which is now referred to as the NiRAN (nidovirus RdRp-associated nucleotidyltransferase), is conserved across all nidovirus families but not in other RNA viruses, indicating an important function that appears to be linked to a specific step in viral replication that is unique to members of the order Nidovirales (28). This study also showed that NiRAN catalyzes self-NMPylation of an arterivirus NiRAN-RdRp, and substitutions of conserved NiRAN residues domains were found to abolish arterivirus (equine arteritis virus [EAV]) and coronavirus (SARS-CoV) replication in cell culture. In a recent study (29), we were able to show that coronavirus NiRAN domains (of HCoV-229E and SARS-CoV-2 nsp12) have nucleotidyltransferase (NMPylation) activities that specifically target nsp9, an RNA-binding protein encoded by ORF1a. The protein is NMPylated at its N terminus through formation of a phosphoramidate bond with the primary N-terminal amine of the nsp9 Asn1 residue (29). Several lines of evidence suggested that the conserved nsp9 N terminus is a biologically relevant target of coronavirus NiRAN-mediated NMPylation activities, with Asn2 (and, to a lesser extent, Asn1) playing critical roles in this reaction (29). This conclusion was supported by (i) data from a mutational analysis of nsp9 N-terminal residues using a bacterial expression system, (ii) the strict conservation of the nsp9 Asn2 residue in viruses from all Orthocoronavirinae genera (29), and (iii) the characterization of HCoV-229E mutants carrying substitutions of selected N-terminal residues of nsp9. The study also revealed that coronavirus NiRAN domains (i) can use different nucleoside triphosphate (NTP) substrates in the nsp9 NMPylation reaction but have a preference for UTP (followed by ATP and GTP) and (ii) require a metal ion cofactor, with Mn2+ ions being strongly preferred over Mg2+ ions. A subsequent study (30) confirmed the substrate specificities established earlier for the SARS-CoV-2 NiRAN (29) but questioned the proposed dependence on Mn2+ ions in this reaction by presenting data suggesting that nsp9 NMPylation proceeds equally efficiently in the presence of Mg2+. The structural basis for complex formation between nsp12 and nsp9 and, more specifically, the binding of the nsp9 N terminus in the NiRAN active site was revealed in a cryo-electron microscopy study using an in vitro-assembled SARS-CoV-2 RTC that included nsp9 (18). In contrast to the biochemical studies mentioned above, the structural study arrived at different conclusions regarding the potential functional significance of the binding of the nsp9 N terminus in the NiRAN active site by suggesting a regulatory role of nsp9 in controlling a NiRAN-mediated RNA guanylyltransferase identified in the same study (18). Evidence for RNA guanylyltransferase activity involving NiRAN was also reported by another laboratory (31). Based on data obtained in these studies (18, 31), NiRAN was proposed to have a canonical GTase activity that produces the cap core structure by transferring GMP to 5′-diphosphorylated RNA provided by the RNA 5′-triphosphatase activity of coronavirus nsp13 (21, 22).
The number of possible NiRAN functions and substrates increased further when Park et al. (32) reported that, in addition to the NMPylation activity described previously, NiRAN is capable of covalently linking a short RNA to the nsp9 Asn1 residue. Furthermore, a large body of data support a model in which (i) RNAylation of nsp9 represents an intermediate step in a noncanonical pathway of 5′ RNA capping and (ii) a GDP molecule, which is suggested to be bound in the NiRAN G pocket and, possibly, provided by the helicase (nsp13)-associated GTPase activity (21), deRNAylates nsp9-pRNA by attacking the phosphoramidate bond, thereby producing GpppRNA and regenerating unmodified nsp9. Methylation reactions by the nsp14 guanine-N7 and nsp16 ribose-2′-O methyltransferase activities would subsequently convert the core cap structure to a fully functional 7MeGpppA2′-O-Me-RNA cap1 structure (32). In support of this model, several other structural studies identified an additional G-specific pocket in the NiRAN active site (33–35). To reconcile the published data supporting these two different models of NiRAN-mediated capping reactions, a possible involvement of NiRAN in alternative RNA guanylylation pathways has been suggested (35). In addition, several laboratories discussed a possible involvement of NiRAN in other functions, including NMPylated nsp9-primed initiation of RNA synthesis (29, 30), while other laboratories reported that NiRAN NMPylates other coronavirus nsps (nsp7 and nsp8) (34, 36) and uses an NMPylated form of nsp8 to prime viral RNA synthesis (37).
As indicated above, partially conflicting information has been reported regarding possible substrates and functions of coronavirus NiRAN domains. This also includes the role of different metal ions for NiRAN-mediated activities and the range of protein substrates that are NMPylated by SARS-CoV-2 NiRAN. To help resolve this partially conflicting information, we decided to extend our research to additional NiRAN homologs of alpha- and betacoronaviruses, seeking to identify conserved enzymatic properties and verify previous conclusions regarding NiRAN-mediated NMPylation of specific protein substrates (including N-terminal nsp9 residues), metal ion requirements, and nucleotide specificities. Our data consistently show that the NMPylation activities of coronavirus nsp12 (NiRAN-RdRp) homologs representing different orthocoronavirus genera act specifically on nsp9 (not on nsp7 and nsp8), use Mn2+ (rather than Mg2+) as the preferred metal ion cofactor, and use different nucleotides as cofactors, yet with a preference for UTP (followed by ATP). The data also show that NiRAN NMPylation activities are sensitive to the presence of additional residues at the nsp9 N terminus but tolerate substitutions of 6 N-terminal residues with those of nsp9 homologs from other corona-, pito-, and letoviruses, provided that Asn2 is retained, confirming the important role of this residue in the binding and appropriate orientation of the nsp9 N terminus in the NiRAN active site.
RESULTS AND DISCUSSION
Nsp9 NMPylation activities of alpha- and betacoronavirus NiRAN domains.
To characterize NiRAN-mediated NMPylation activities of additional alpha- and betacoronaviruses, we produced C-terminally His-tagged forms of nsp12 (~106 to 107 kDa) and nsp9 (~13 kDa) of SARS-CoV and porcine transmissible gastroenteritis virus (TGEV), respectively, in Escherichia coli and purified the proteins as described in Materials and Methods. The proteins were used in an NMPylation assay containing α-32P-labeled NTPs. Following SDS-PAGE, the Coomassie-stained gels were exposed to imaging screens, and the reaction products were analyzed by phosphorimaging. To minimize potential nonspecific protein NMPylation, the reaction mixtures were adjusted to contain very low concentrations of nsp12 (50 nM). Under the conditions used in this experiment (50 nM nsp12, 10-min reaction time, 60-min exposure time), a minor self-NMPylation of nsp12 reported previously was not detectable, while radiolabeled products that comigrated with nsp9 were readily detected, suggesting efficient formation of covalent nsp9-NMP adducts by the recombinant SARS-CoV and TGEV nsp12 proteins. Quantitative analyses revealed that all four NTPs were used as substrates, albeit with different efficiency and a clear preference for UTP (followed by ATP) (UTP > ATP > GTP > CTP) (Fig. 1A and C). These data are consistent with our previous observations for SARS-CoV-2 and HCoV-229E nsp12 (29). To our knowledge, protein UMPylation through formation of a phosphoramidate bond has not been reported previously for other viruses. Also, the preference of coronavirus NiRAN for UTP is noteworthy because UMPylation (in contrast to other NMPylation reactions) is rarely observed in cellular systems, with YidU, a pseudokinase of Salmonella enterica serovar Typhimurium (38), being one of only few examples in the literature. The somewhat relaxed nucleotide substrate specificity, now confirmed for NiRAN domains of 4 alpha- and betacoronaviruses (this study and reference 29), does not appear to be a conserved feature among other nidoviruses, as the NiRAN domain of EAV (family Arteriviridae) was shown to specifically use GTP and UTP as nucleotide substrates (28).
FIG 1.
NiRAN-mediated NMPylation of nsp9 by SARS-CoV and TGEV nsp12. (A and C) Nsp12-mediated NMPylation of nsp9 in the presence of different nucleotides. A 0.05 μM concentration of SARS-CoV nsp12-His6 (A) or TGEV nsp12-His6 (C) was incubated in a standard NMPylation assay with 4 μM SARS-CoV nsp9-His7 (A) or TGEV-His6 (C) in the presence of the indicated [α-32P]NTP (lanes 3 to 6). Protein UMPylation was not detected in reaction mixtures containing nsp9 or nsp12 alone (lanes 1 and 2). (B and D) Nsp9-specific UMPylation activities of recombinant SARS-CoV (B) and TGEV (D) nsp12 variants containing the indicated amino acid substitution(s) in the active sites of the NiRAN (lane 4) or RdRp (lane 5) domain. The left (A and B) and right (C and D) panels show the data obtained for SARS-CoV and TGEV, respectively. The sizes of marker proteins (in kilodaltons) are given on the left, and the position of nsp9 is indicated. The column diagrams depict relative NMPylation activities compared to (UMPylation) activities obtained in reaction mixtures containing UTP. Data obtained in three independent experiments were included in the analysis (means and standard errors of the means [SEM] are given). *, P ≤ 0.05; **, P ≤ 0.01.
Next, we sought to answer the question of whether both the NiRAN and RdRp active sites contribute to the NMPylation activity of nsp12. First, we produced coronavirus nsp12 variants in which the Lys73 (in SARS-CoV) or Lys69 (in TGEV) residues were substituted with alanine (Fig. 2). The corresponding Lys residue of SARS-CoV-2 nsp12 and SelO was previously shown to be involved in NTP binding through interactions with the phosphates (15, 32, 35, 39). In reactions using SARS-CoV nsp12_K73A and TGEV nsp12_K69A, no radioactive signal indicative of nsp9 UMPylation was detected, confirming that the nucleotide transfer activity was mediated by NiRAN (Fig. 1B and D, lanes 4) rather than potentially contaminating bacterial nucleotidyltransferases. The results are consistent with previous studies showing that the corresponding substitution abolished (self-)NMPylation activities of other recombinantly produced arterivirus (EAV nsp9) and coronavirus (SARS-CoV-2 and HCoV-229E nsp12) NiRAN-RdRp proteins, confirming the critical role of this invariant residue for activity (28, 29). In line with this, genetically engineered coronavirus genome RNAs containing this particular Lys-to-Ala substitution (SARS-CoV nsp12_K73A and HCoV-229E nsp12_K67A) failed to support viral RNA synthesis and, as a result, production of infectious virus progeny (28, 29). As another set of controls, we produced nsp12 variants in which two Asp active-site residues in RdRp motif C were each substituted with Ala (Fig. 2). Consistent with data obtained for the EAV NiRAN-RdRp variant containing these two substitutions (40), the SARS-CoV nsp12_DD760/1AA and TGEV nsp12_DD757/8AA variants retained their NMPylation activities, confirming that the NMPylation activity is mediated by NiRAN and does not involve the RdRp active site (Fig. 1B and D, lanes 5).
FIG 2.
Multiple-sequence alignment of coronavirus nsp12 NiRAN and RdRp domains. Shown are nsp12 NiRAN and partial RdRp domains from viruses representing the four recognized genera, Alpha, Beta-, Gamma-, and Deltacoronavirus, of the subfamily Orthocoronavirinae. Conserved amino acid residues that have been substituted with Ala in this study and are known to be part of the active sites of the NiRAN and RdRp domains are indicated. Sequences were aligned using Clustal Omega (52) and converted using ESPript (53). A black background indicates invariant residues. SARS-CoV, severe acute respiratory syndrome coronavirus (NC_004718.3); SARS-CoV-2, severe acute respiratory syndrome coronavirus 2 (NC_045512.2); MERS-CoV, Middle East respiratory syndrome coronavirus (NC_019843); IBV, avian infectious bronchitis virus (NC_001451); PDCoV-HKU15, porcine deltacoronavirus HKU15 (NC_039208); HCoV-229E, human coronavirus 229E (NC_002645.1); TGEV, porcine transmissible gastroenteritis virus (NC_038861.1). The NiRAN domain is highlighted with a gray background. Also indicated is a small segment of the C-terminal RdRp domain including motif C, while the remainder of the RdRp sequence alignment is not shown in this representation.
Previously, we showed that HCoV-229E nsp12 NMPylates nsp9 and has a pronounced specificity for this particular substrate. In contrast to this finding, two recent studies identified two other proteins, nsp7 and nsp8, as possible targets of nsp12-mediated NMPylation activities (34, 36, 37). Given that, in those studies, reaction conditions were used that differed from our standard NMPylation assay, such as much higher concentrations of nsp12 (up to 1 μM) and Mn2+ (up to 6 mM) and longer reaction times (up to 1 h), we adjusted our reaction conditions according to the (different) protocols described in those earlier studies. As potential substrates, we used increasing concentrations of recombinant forms of nsp7 and nsp8 that lacked any terminal tag sequences to minimize the risk of potential structural changes interfering with NMPylation. Under these adjusted reaction conditions and with exposure of the gel for 1 h, no radioactive adduct was detected for either nsp7 or nsp8 (Fig. 3, lanes 1 to 10), while a radiolabeled nsp9 adduct was readily detected (Fig. 3, lane 11). The data confirm previous conclusions on the distinct specificity of coronavirus NiRAN for nsp9 (29, 30) and are strongly supported by recent structural studies of nsp9/nsp12 complexes (32, 33, 35). Based on the combined biochemical and structural evidence, it seems reasonable to suggest that the previously reported NMPylation of nsp7 and/or nsp8 by SARS-CoV-2 nsp12 is nonspecific (and may have been facilitated by the absence of the “correct” substrate [30]). Thus, the biological relevance of these observations remains questionable.
FIG 3.
Protein substrate specificity of SARS-CoV-2 nsp12-mediated UMPylation activity. (Left) SARS-CoV-2 nsp12-His6 was incubated with tag-free SARS-CoV-2 nsp7 or nsp8 using the reaction conditions described by Conti et al. (36), including a high concentration of MnCl2 (6 mM) and incubation at 30°C for 30 min. SARS-CoV-2 nsp12 (0.25 μM) was incubated with increasing concentrations of potential target proteins (nsp7 and nsp8) as indicated (lanes 1 to 8). (Right) A UMPylation assay was performed using the reaction conditions described by Shannon et al. (37). 1 μM SARS-CoV-2 nsp12-His6 was incubated with 6 μM nsp7, nsp8, or nsp9-His6 (as indicated at the top) in the presence of 2 mM MnCl2 at 37°C for 60 min (lanes 9 to 11). Reaction products were separated in Tris-Tricine gels and stained with Coomassie brilliant blue (top panels). Radiolabeled proteins were visualized by phosphorimaging with a 1-h exposure time (bottom panels). Due to the high concentration of nsp9 used in this assay, a minor dimeric form of nsp9 can be detected in the autoradiogram in addition to the major monomeric form of nsp9 (lane 11). Sizes of marker proteins (in kilodaltons) are on the left.
NiRAN transfers one NMP moiety to the N-terminal residue of nsp9.
To validate and extend previous conclusions on the nucleotide substrate specificity in NiRAN-mediated nsp9 NMPylation reactions, we performed competitive NMPylation assays and identified the reaction products by mass spectrometry analyses. SARS-CoV nsp12 and nsp9 were incubated with ATP/GTP/CTP and ATP/GTP/UTP, respectively, in an NMPylation assay and analyzed by deconvoluted intact protein mass spectrometry. Due to the size difference of only 1 Da between CTP and UTP, two separate experiments were necessary to distinguish the nucleotide substrates. As a control, nsp9 was incubated in the presence of all four NTPs but without nsp12. In this case, only non-NMPylated nsp9-His7 was detected at 13,360 Da (calculated, 13,361 Da), confirming that nsp9 has no self-NMPylation activity (Fig. 4A). In reaction mixtures containing nsp12, the spectra showed peaks for nsp9 shifted to the right, corresponding precisely to an increase in size calculated for an nsp9 molecule bound to one NMP. Signals indicating NMPylation of nsp9 at multiple sites were not detected, supporting the site-specific NMPylation of the target protein as previously reported (29, 32). Using competing NTPs in the same reaction, the substrate preference observed previously in separate reactions was perfectly reproduced, with UTP being preferred over other nucleotides (UTP > ATP > GTP > CTP) (Fig. 4B and C). Furthermore, the peak sizes of NMPylated versus non-NMPylated forms of nsp9 in the spectra revealed that, although nsp12 was used at a 10-fold-lower concentration than the nsp9 substrate, there was more NMPylated nsp9 than non-NMPylated nsp9. This indicates that interactions between nsp12 and nsp9 are transient, with one nsp12 molecule having the ability to NMPylate multiple nsp9 molecules. Thus, despite the lower intracellular concentration of nsp12 compared to nsp9 due to the ribosomal frameshift required for nsp12 (but not nsp9) biosynthesis, nsp12 may have the capacity to NMPylate most (if not all) nsp9 molecules at some stage of the replication cycle. The energy required for NMP transfer is most likely provided by NTP hydrolysis and pyrophosphate (PPi) release, a well established mechanism in biochemical reactions. To corroborate previous conclusions on the NMP binding site detected for HCoV-229E nsp9 (29) and SARS-CoV-2 nsp9 (32), we sought to identify the NMP binding site in SARS-CoV nsp9. To this end, GMPylated SARS-CoV nsp9 was cleaved with trypsin, and the N-terminal fragment isolated by nanoflow high-performance liquid chromatography (nano-HPLC) was analyzed using tandem mass spectrometry. Figure 4D provides information on the ions detected in the tandem mass spectrum for the N-terminal fragment NNELSPAVALR. The peak at 998.5661 m/z corresponding to the nonmodified C-terminal fragment ion y9 (NELSPAVALR) shown in the magnified area in Fig. 4E provided the first strong indication that the GMP was bound to the N-terminal Asn of nsp9.
FIG 4.
Evaluation of NiRAN-mediated NMPylation of nsp9 and identification of the NMPylation site by mass spectrometry. SARS-CoV nsp9-His7 was used in the standard NMPylation assay in the presence of all four NTPs without nsp12-His6 (A), in the presence of nsp12 and UTP, ATP, and GTP (B) or in the presence of nsp12 and CTP, ATP, and GTP (C). Shown are deconvoluted mass spectra with the intact nsp9-His7. (D) SARS-CoV nsp9-His7 with a covalently bound GMP was treated with trypsin, and the resulting peptide mixture was separated by nano-HPLC and measured by tandem mass spectrometry. The observed fragments on the N-terminal peptide NNELSPAVALR of nsp9-His7 are shown schematically. (E) Tandem mass spectrum of the GMPylated tryptic N-terminal peptide. The section from 960 to 1,005 m/z is enlarged and displays a peak at 998.5661 m/z (calculated, 998.5629; both in bold), indicating that the y9 fragment containing amino acids 2 to 10 was not GMPylated and providing strong evidence that the GMP moiety was bound to the N-terminal asparagine.
N-terminal residues of nsp9 are critically involved in nsp12-mediated NMPylation.
In an earlier study, we showed that specific residues at the nsp9 N terminus are critically involved in HCoV-229E nsp12-mediated NMPylation. To corroborate this conclusion further, we performed a mutational analysis of other alphacoronavirus (TGEV) or betacoronavirus (SARS-CoV) nsp9 proteins, in which we replaced the two N-terminal Asn residues with Ala or Ser and used the purified proteins to measure UMPylation by the cognate nsp12. Substitution of Asn1 caused a 40% decrease in signal intensity for UMPylated SARS-CoV nsp9_N1A compared to the wild-type protein, while the same substitution had less profound effects on TGEV nsp9 UMPylation (reduction by 20% in nsp9_N1A) (Fig. 5A and B, lanes 2 and 5). In contrast, substitution of Asn2 caused a strong decrease in nsp9 UMPylation for both viruses (Fig. 5A and B, lanes 3 and 6). Similarly profound effects were observed when both N-terminal residues were replaced with Ala (Fig. 5A and B, lanes 4 and 7). The mutational analysis supports our previous conclusion that Asn2 has a key role in coronavirus nsp9 NMPylation and is consistent with the strong conservation of this particular nsp9 residue in viruses from all subfamilies of the family Coronaviridae (29). A cryo-electron microscopy structural study of SARS-CoV-2, which was published at nearly the same time, suggested that interactions of SARS-CoV-2 nsp9 Asn2 with conserved residues may help to appropriately position the nsp9 N terminus in the NiRAN active site (18). However, the latter study failed to confirm NiRAN-mediated NMPylation of SARS-CoV-2 nsp9, which was probably due to the presence of two additional N-terminal residues in the nsp9 construct used in this particular study as suggested by other studies (29, 30, 32). To corroborate this conclusion further, we produced a set of coronavirus nsp9 proteins (derived from SARS-CoV-2, SARS-CoV, and TGEV) that contained either an authentic N terminus or an N terminus with two additional residues (Ser and Gly) and analyzed nsp12-mediated UMPylation of these proteins. With all three coronavirus nsp9 homologs, the presence of two additional N-terminal residues abolished UMPylation of nsp9, while proteins containing an authentic N terminus were UMPylated by the cognate nsp12 (Fig. 5C). Taken together, these data support an essential role for the (length of) the nsp9 N terminus and the Asn2 side chain in nsp9 NMPylation. Although Asn1 is conserved as well (but not to the same extent), this residue appears to have a slightly less critical function. Consistent with this idea, genetically engineered HCoV-229E variants encoding mutant forms of nsp9 with N1A or N1S substitutions were previously shown to be viable in cell culture, while no coronavirus variants encoding nsp9_N2A or nsp9_N2S variants could be recovered. In line with this, recombinant forms of HCoV-229E and SARS-CoV-2 nsp9 in which Asn1 was replaced with Ala or Ser were confirmed to be NMPylated by nsp12, albeit less efficiently (29, 32), while a nonconservative substitution with Asp (nsp9_N1D) abolished NMPylation by the SARS-CoV-2 NiRAN (32), suggesting specific structural constraints for the N-terminal Asn1 residue. In this context, it is worth noting that the nsp8|nsp9 cleavage site differs from all other cleavage sites in coronavirus replicase polyproteins in that Asn is conserved at the P1′ position, resulting in less efficient cleavage of this particular site by the viral main protease Mpro (41, 42). Although the structural and/or functional basis for the conservation of Asn1 remains to be studied in more detail, it is tempting to speculate that Asn1 plays a regulatory role in the proteolytic release of nsp9 to get this protein involved in a specific step in RNA synthesis and/or processing in a timely coordinated manner. Alternatively, Asn1 may be involved in downstream functions and/or control the efficiency of subsequent de-NMPylation/de-RNAylation reactions of NMPylated/RNAylated forms of nsp9. The latter idea is supported by data suggesting that the stability of phosphoramidate bonds between NMP and amino acids depends on the amino acid involved (43).
FIG 5.
Coronavirus nsp12-mediated NMPylation of nsp9 variants with substitutions or addition of specific N-terminal residues. (A) SARS-CoV nsp9-His7 and (B) TGEV nsp9-His6 carrying substitutions of specific N-terminal residues were used in the standard NMPylation assay in the presence of UTP and the cognate coronavirus nsp12-His6. (C) Nsp9 variants (SG-nsp9) of different coronaviruses (as indicated) with each containing two additional N-terminal residues (serine and glycine) were produced and used in UMPylation assays. Wild-type (wt) nsp9 of the respective coronavirus served as a control. Top panels show Coomassie-stained gels after SDS-PAGE, and bottom panels show the corresponding autoradiography. The graphs show relative activities compared to wt protein. Data were obtained in three independent experiments. Signal intensities were analyzed using ImageJ and compared (as percentages) to the signal intensity obtained for the nsp9 wt protein (lane 1). Positions of marker proteins with sizes (in kilodaltons) are indicated on the left. *, P = 0.05; **, P = 0.01; ***, P = 0.001.
Impact of divalent cations on NMPylation activity.
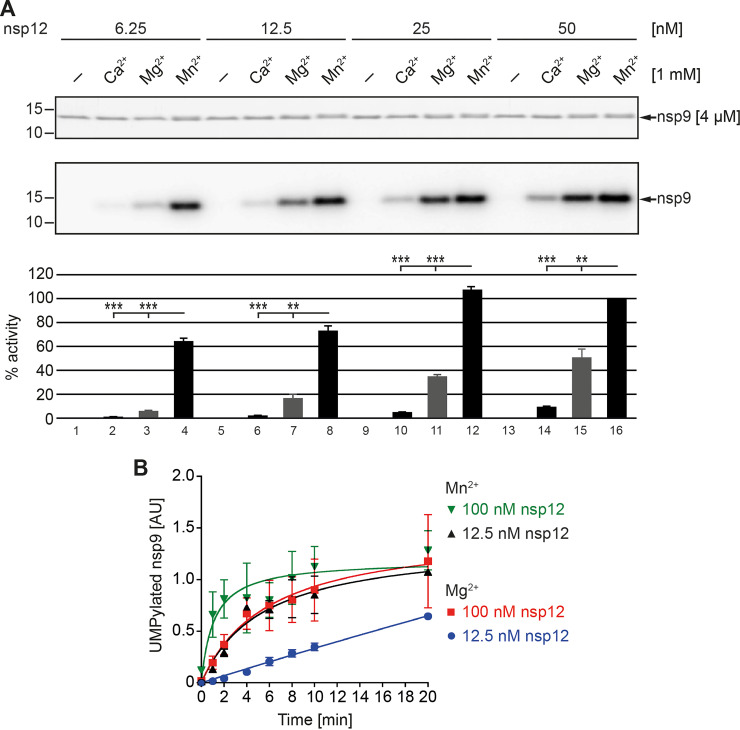
Because of somewhat contradictory results on the role of metal ions for both NiRAN and cellular pseudokinase activities reported in recent studies (28–30, 35, 38, 39), we turned again to the role of metal ions, particularly Mn2+ and Mg2+, in coronavirus nsp12 NMPylation activity using two additional coronavirus NiRAN proteins. nsp12 and nsp9 were incubated in reaction buffer supplemented with [α-32P]UTP and different metal cations at 1 mM. For both SARS-CoV and TGEV, a strong signal for UMPylated nsp9 was observed in the presence of Mn2+, while in the presence of Mg2+, a much weaker signal was detected. For Ca2+ or Zn2+ ions, a near-background signal intensity similar to the negative control was observed (Fig. 6A and C). We also performed an NMPylation assay at much lower Mn2+ concentrations, ranging from 1 mM to 0.1 mM, and did not observe any loss of NMPylation activity even at the lowest concentration tested (Fig. 6B and D), suggesting efficient binding of Mn2+ to the NiRAN active site at very low (close to physiological) concentrations. Overall, these data support our previous conclusion that NMPylation activity is highest in the presence of Mn2+, significantly higher than in the presence of Mg2+ ions. A similar preference for or even dependence on Mn2+ ions was reported in a recent study for SARS-CoV-2 NiRAN-mediated nsp9 RNAylation activity (35).
FIG 6.
Role of metal ions in NMPylation activity. Experiments were performed using SARS-CoV (A and B) and TGEV (C and D) nsp9 and nsp12. (A and C) Standard NMPylation assays were done in the presence of [α-32P]UTP and a 1 mM concentration of the indicated divalent cation (lanes 2 to 5). As a control, the reaction was performed in the absence of metal ions (lane 1). (B and D) To investigate the impact of Mn2+ on NMPylation at more physiological concentrations, Mn2+ was included in the reaction mixture at concentrations ranging from 1 to 0.1 mM. Coomassie-stained protein bands representing nsp9 are shown in the top panels, and corresponding areas of the autoradiograms of the same gel are shown in the middle panels. In the graphs, relative activity data obtained in three independent experiments are shown in percent (mean and SEM). Radioactive signals obtained for nsp9 in the presence of 1 mM Mn2+ were used as a reference and set to 100%. The positions of molecular weight markers (in kilodaltons) are indicated on the left. *, P ≤ 0.05; **, P ≤ 0.01; ***, P ≤ 0.001.
Inspired by recent observations indicating that Mg2+ and Mn2+ ions support NiRAN-mediated UMPylation activity equally strongly, along with suggestions that Mn2+ might alter the geometry of the NiRAN active site and thereby facilitate nonspecific NMPylation of proteins (30), we tested the somewhat different experimental conditions of the two studies as a possible cause of the different conclusions. Differences consisted essentially in a preincubation of the reaction partners before starting the reaction and a different molar ratio between enzyme and substrate (1 μM nsp12 and 10 μM nsp9 in the study by Wang et al. [30] and 0.05 μM nsp12 and 4 μM nsp9 in our protocol). In our experiments, preincubation of the enzyme-substrate mixtures could be excluded as a possible cause (data not shown), suggesting that the different molar ratio of nsp12 to nsp9 was responsible for the different results. To test this possibility, the NMPylation assay was performed with different ratios of nsp12 to nsp9 in the presence of different divalent cations. Ca2+ was included in this experiment, because a crystal structure analysis of Pseudomonas syringae SelO revealed the binding of a Ca2+ ion in the active site of this bacterial pseudokinase (39), whereas, for nidoviruses, no evidence has been obtained to suggest that Ca2+ has a role in NiRAN activity. The substrate (4 μM nsp9) was incubated with increasing concentrations of nsp12, and the production of NMPylated nsp9 was monitored by SDS-PAGE of the reaction products and autoradiography. No significant increase of NMPylated nsp9 was observed in the presence of Ca2+ ions, while in the presence of Mg2+, an increase in radiolabeled nsp9 adducts was observed with increasing concentrations of nsp12. Under these conditions, significantly more radiolabeled reaction product was detected when the reactions were carried out in the presence of Mn2+ ions. Also, maximum accumulation of radiolabeled product was already reached at significantly lower concentration of nsp12 (Fig. 7A). In line with this, kinetics analyses revealed that, using two different concentrations of nsp12 (12.5 nM and 100 nM), accumulation of UMPylated nsp9 product over time was dependent on the type of metal ion used in the reaction. In reactions using the same concentration of enzyme but a different metal ion, more product was detectable in the presence of Mn2+ at each time point (Fig. 7B), and maximum UMPylated product accumulation occurred much faster in the presence of Mn2+ than in reactions carried out in the presence of Mg2+. Taken together, these data lead us to conclude that coronavirus NiRANs can use both Mn2+ and Mg2+ ions as cofactor for the NMPylation reaction but Mn2+ supports this activity much more strongly. The data also lead us to suggest that the similar NMPylation activities observed in another study for SARS-CoV-2 nsp12 in the presence of Mn2+ and Mg2+ (30) were likely due to the relatively low molar ratio between substrate and enzyme, resulting in rapid conversion of the (limited amount of) substrate present, thereby blurring the differences in activity that may have existed in the presence of Mn2+ and Mg2+.
FIG 7.
NMPylation activities at different enzyme-to-substrate ratios in the presence of different metal ion cofactors. (A) Increasing concentrations of SARS-CoV-2 nsp12-His6 were incubated in the standard UMPylation assay with a constant concentration of nsp9-His6 in the presence of Ca2+, Mg2+, or Mn2+ or without metal ion as indicated at the top. The column diagram shows results from three independent experiments (means and SEM), which were analyzed by phosphorimaging using ImageJ. Relative activities (in percent) were determined in relation to the activity obtained for 50 nM nsp12-His6 in the presence of 1 mM Mn2+. Positions of marker proteins with molecular masses (in kilodaltons) are indicated on the left. **, P ≤ 0.01; ***, P ≤ 0.001. (B) nsp12-His6 of SARS-CoV-2 (12.5 nM or 100 nM) was incubated with 4 μM nsp9-His6 in the presence of either Mn2+ or Mg2+ in the NMPylation assay with [α-32P]UTP. At 2-min intervals, reaction aliquots were collected and the reaction was stopped by addition of protein sample buffer and heat inactivation at 90°C. Reaction products obtained at the different time points were separated by SDS-PAGE, and radioactive signal intensities were analyzed by phosphorimaging and calculated as arbitrary units [AU]. The data were obtained in three independent experiments (means and SEM).
NiRAN-mediated nsp9 NMPylation is a conserved mechanism in the Orthocoronavirinae.
To our knowledge, NiRAN-mediated NMPylation of nsp9 has so far been studied for four viruses of the Orthocoronavirinae, the alphacoronaviruses HCoV-229E and TGEV (this study and reference 29) and the closely related betacoronaviruses SARS-CoV and SARS-CoV-2, both of which belong to the same virus species (6, 28–30, 32). As reported earlier (29), the N-terminal tripeptide sequences of coronavirus nsp9s display a high degree of sequence identity. In addition, the identification of a single NMPylation site at the N terminus of nsp9 as confirmed by mass spectrometry and nuclear magnetic resonance spectroscopy (this study and references 29 and 32) suggests that the NiRAN-mediated NMPylation and/or RNAylation of nsp9 (32, 35) is a conserved enzymatic process in coronaviruses and, potentially, other nidoviruses. To answer the question of whether interactions between nsp12 and nsp9 including its N-terminal residues are conserved among genetically diverged coronaviruses to an extent that is sufficient for nsp9-specific NMPylation across genus boundaries, we performed NMPylation assays in which nsp12s of HCoV-229E, SARS-CoV, and TGEV were incubated with either their cognate nsp9 or an nsp9 of another virus. As before, nsp9 UMPylation was examined by phosphorimaging of the reaction products separated by SDS-PAGE. As controls for potential nonspecific UMPylation, mutant forms of nsp9 carrying a substitution of the N-terminal dipeptide Asn-Asn with Ala-Ala were used (compare Fig. 5A and B). As shown in Fig. 8, all wild-type nsp9s were UMPylated by nsp12 from the same or another coronavirus, whereas nsp9s carrying N-terminal Asn-Asn-to-Ala-Ala substitutions were not UMPylated. The data reveal a remarkable degree of conservation of nsp9 and nsp12 and their interactions among viruses representing different coronavirus genera, further supporting the idea that nsp12-mediated NMPylation of nsp9 is an essential and biologically relevant mechanism.
FIG 8.
NiRAN-mediated NMPylation of (noncognate) nsp9 proteins derived from other coronaviruses. The nsp12-His6 of HCoV-229E (lanes 1 to 6), SARS-CoV (lanes 7 to 12), and TGEV (lanes 13 to 18) were combined in the standard NMPylation assay with either their cognate nsp9 or an nsp9 from another coronavirus, as indicated at the top. As controls, mutant forms of the respective nsp9s in which the two N-terminal Asn residues were substituted with two Ala residues were used (compare Fig. 5). (Top) Coomassie-stained nsp9s separated by SDS-PAGE; (bottom) radioactive signals obtained by phosphorimaging. The positions of marker proteins with molecular sizes (in kilodaltons) are indicated on the left. Arrows indicate the positions of recombinant forms of nsp9 of different coronaviruses with slightly different migration in the gel.
Limited conservation of leto- and coronavirus nsp9 N-terminal sequences and role of nsp9 Asn2 in nsp9-specific NMPylation by nsp12.
Together with the subfamily Orthocoronavirinae, the family Coronaviridae contains two other subfamilies, Pitovirinae and Letovirinae, represented by microhyla letovirus 1 (MLeV) and Pacific salmon nidovirus (PsNV), respectively (44, 45). Orthologs of orthocoronavirus nsp9 have been identified in the pito- and letovirus replicase polyproteins, and although proteolytic processing of the replicase polyproteins encoded by these viruses remains to be studied in detail, potential cleavage sites, including those that are used to release the nsp9 ortholog from the pitovirus and letovirus polyproteins, have been identified (29). In contrast to the NNE motif conserved at the N terminus of (ortho)coronavirus nsp9s, the pito- and letovirus nsp9 orthologs have an N-terminal alanine residue (Fig. 9A). Like in all orthocoronaviruses, Asn is found at the second position of the leto- and pitovirus nsp9 orthologs. We sought to answer the question of whether the N-terminal sequence of pito- and letovirus nsp9 orthologs is sufficient to support NMPylation of chimeric coronavirus nsp9s carrying heterologous N-terminal sequences of nsp9 orthologs from other subfamilies. Specifically, we replaced the six N-terminal amino acid residues of SARS-CoV-2 nsp9 with the six N-terminal residues of HCoV-229E nsp9 (nsp9_HCoV-229E|SARS2), MLeV (nsp9_MLeV|SARS2), and PsNV (nsp9_PsNV|SARS2) (Fig. 9A). NMPylation of the recombinant chimeric proteins by SARS-CoV-2 nsp12 was analyzed by microscale thermophoresis using increasing concentrations of the respective nsp9 derivative. All chimeric nsp9 proteins were found to be NMPylated by SARS-CoV-2 nsp12, although the half-maximal effective concentration (EC50) values determined for nsp9_PsNV|SARS2 (6.3 μM) and especially for nsp9_MLeV|SARS2 (845 μM) differed significantly from the protein concentration required for half-maximal NMPylation of the wild-type SARS-CoV-2 nsp9 (SARS2|SARS2) (1.3 μM) (Fig. 9B). In another set of experiments, the chimeric nsp9s, along with MLeV nsp9 and nsp9_229E|SARS2 nsp9, were incubated with nsp12 of SARS-CoV-2, HCoV-229E, and TGEV, respectively. Nsp9_HCoV-229E|SARS2 was similarly efficiently UMPylated by SARS-CoV-2 nsp12 as the cognate wild-type (wt) SARS-CoV-2 nsp9 (SARS2|SARS2), while other chimeric proteins were (much) less efficiently UMPylated, with nsp9_PsNV|SARS2 being more efficiently UMPylated than nsp9_MLeV|SARS2. Consistent results were obtained for all three coronavirus nsp12s used in this experiment. UMPylation was drastically reduced (but still detectable) when the authentic MLeV nsp9 was used as a substrate for nsp12s, indicating limited conservation of structural elements promoting nsp9-nsp12 interactions among viruses from different subfamilies in the family Coronaviridae (Fig. 9C). Based on our NMPylation data obtained for coronavirus nsp9_N1A mutants (Fig. 5A and B), it seems reasonable to suggest that the reduced NMPylation observed for chimeric nsp9 variants carrying leto-/pitovirus-derived N termini is mainly due to the presence of alanine at the N terminus in both cases (29). Unlike the PsNV nsp9 N terminus, which contains a Glu residue at position 3 (as in orthocoronavirus nsp9s), this residue is replaced with Val in the MLeV sequence (Fig. 9A), which likely is another factor contributing to the reduced NMPylation observed for nsp9_MLeV|SARS2 compared to nsp9_PsNV|SARS2 (Fig. 9A). This is supported by previous mutational studies of HCoV-229E nsp9 in which substitution of Glu3 with Ala resulted in decreased UMPylation in vitro and a nonviable phenotype of the corresponding virus, likely due to the failure to appropriately position the three N-terminal residues of nsp9 in the NiRAN active site (29, 35).
FIG 9.
Coronavirus nsp12-mediated NMPylation of nsp9 variants in which six N-terminal residues were substituted with six N-terminal residues of nsp9 homologs of leto- and pitoviruses. (A) N-terminal sequences of the recombinant chimeric nsp9 variants used in this experiment. In three chimeric proteins, the six N-terminal residues of SARS-CoV-2 nsp9 (indicated as SARS2|SARS2) were replaced with the six N-terminal residues of an nsp9 homolog from an alphacoronavirus (HCoV-229E, 229E|SARS2), the letovirus MLeV (MLeV|SARS2), or the pitovirus PsNV (PsNV|SARS2). Residues starting at position 7 (after the pipe symbol [|]) represent the SARS-CoV-2 nsp9 (wt) sequence. MLeV|MLeV represents the wt MLeV nsp9 homolog. Highlighted in gray is the Asn2 residue that was previously shown to be conserved in orthocorona- and letoviruses (29). (B) NMPylation of chimeric nsp9 variants as determined by microscale thermophoresis assays. SARS-CoV-2 nsp9 (SARS2|SARS2) was used as a positive control. EC50 values represent protein substrate concentrations at which half-maximal UMPylation by SARS-CoV-2 nsp12 was detected for this particular substrate. Data from three independent experiments were used for analysis. (C) Chimeric nsp9s and MLeV nsp9 were incubated with the nsp12 of SARS-CoV-2 (lanes 1 to 5), HCoV-229E (lanes 6 to 10), or TGEV (lanes 11 to 15) in a standard UMPylation assay to assess potential NMPylation activities on (noncognate) nsp9 homologs of genetically diverged members of the family Coronaviridae. (D) To corroborate the potential role in NMPylation of the conserved nsp9 Asn2 residue in leto- and pitoviruses, additional proteins in which Asn2 was also replaced with Ala were produced and used in UMPylation assays. (Top) Coomassie-stained nsp9 variants separated by SDS-PAGE; (bottom) autoradiogram of the gel. Positions of marker proteins with molecular masses (in kilodaltons) are shown on the left.
Leto- and pitovirus nsp9 orthologs share an asparagine at the second position with orthocoronavirus nsp9s (Fig. 9A). In coronavirus nsp9, this residue is thought to be essential for positioning the N-terminal residue as a target for NMPylation in the NiRAN active site (18, 32). Consistent with this idea, substitution of Asn2 with Ala significantly reduces coronavirus nsp9 NMPylation (Fig. 5) (29). To investigate whether Asn2 has a similarly important role in leto-/pitoviruses, we replaced the second asparagine in the nsp9 chimeras with Ala and tested these proteins in a standard NMPylation assay. We observed a significant reduction in UMPylation in chimeric proteins in which Asn2 was replaced with Ala, suggesting that, in the context of a pito-/letovirus-derived N terminus, Asn2 has a similarly important function as shown previously for coronaviruses (Fig. 9D). The combined data lead us to conclude that genetically diverged viruses from the three subfamilies of the Coronaviridae use similar (but not identical) mechanisms to position the nsp9 N terminus in the active site of the NiRAN domain, with Asn2 playing a critical role. Coronavirus nsp9s differ from their pito-/letovirus homologs in that they have Asn at the N terminus, which is thought to slow down proteolytic release of the nsp9 N terminus (42) and, as a result, NMPylation (29) (or RNAylation [32]) of nsp9. In contrast, pitovirus and letovirus nsp9 homologs have alanine at the N terminus, one of the canonical small residues (Ala, Ser, and Gly) that are conserved at the P1′ position of most coronavirus Mpro cleavage sites (41). Potential implications of retaining a conventional Mpro cleavage site at the N-terminal nsp9 processing site in viruses of these two subfamilies remain to be studied.
In this study, we used additional nsp9 and nsp12 homologs representing genetically divergent representatives of the family Coronaviridae to corroborate previous conclusions on biochemical properties of NiRAN-mediated NMPylation activities. The data obtained in this work and several other recent studies lead us to a number of conclusions: (i) all alpha- and betacoronavirus nsp12s characterized to date exhibit robust protein NMPylation activities in vitro; (ii) nsp9 is the specific target of this activity; (iii) NiRAN catalyzes the formation of a phosphoramidate bond between the amino group of the N-terminal asparagine and NMP, resulting in the production of (mono-)NMPylated nsp9 and release of PPi; (iv) recent studies showed that, in addition to NMP, NiRAN is able to transfer (specific) ribooligonucleotides to the N terminus of nsp9 using the same chemistry (i.e., formation of a phosphoramidate bond); (v) coronavirus NiRAN domains appear to have conserved substrate preferences for specific nucleotides (in NMPylation reactions) or for a specific 5′-terminal nucleotide of a ribooligonucleotide (in RNAylation reactions); (vi) NMPylation and RNAylation of nsp9 are strongly supported by (or dependent on) Mn2+ ions (however, see below for additional Mg2+-dependent NiRAN activities); (vii) the N-terminal residues of nsp9 are required to appropriately position the nsp9 N terminus in the NiRAN active site, with Asn2 (and, to a lesser extent, Asn1) playing critical roles; (viii) reverse genetics studies provided compelling evidence that viral replication is abolished if conserved residues in the pseudokinase-like active center of the NiRAN domain or N-terminal nsp9 residues are replaced with alanine, suggesting that one or more NiRAN activities involving nsp9 as a substrate or reaction intermediate are essential for coronavirus replication. As indicated above (see the introduction) (Fig. 10), recent research provides strong evidence that an RNAylated form of nsp9 (nsp9-pRNA) that is generated by NiRAN in a Mn2+-dependent reaction represents a reaction intermediate in a noncanonical capping pathway that additionally involves a GDP molecule that is bound in a distinct G pocket of the active center and attacks the phosphoramidate bond in nsp9-pRNA, thereby forming a GpppRNA core cap structure and regenerating nsp9. In contrast to this model, other studies provided evidence to suggest that NiRAN has guanylyltransferase activity that is part of a conventional capping pathway in which GMP (produced from GTP) is linked to a 5′-diphosphorylated RNA to produce GpppA and that does not rely on nsp9 (18, 31). In another study, NiRAN was implicated in two alternative mechanisms to produce the core cap structure (35). Although evidence is accumulating to suggest that NiRAN has a role in RNA capping, this does not preclude a possible involvement of NiRAN’s nsp9-specific NMPylation activities in other functions, including nsp9-pN-primed initiation of minus- and/or plus-strand RNA synthesis, as suggested earlier (29). In this model (Fig. 10), nsp9-pA or nsp9-pU would be provided by the nsp12 NiRAN domain and subsequently used by the nsp12 RdRp domain of the same or another RTC as a primer to initiate RNA synthesis. In this context, it is worth noting that (next to UTP) ATP is the preferred nucleotide substrate for nsp9 NMPylation (this study and reference 29) and that replacement of a 5′-terminal A with other nucleotides strongly reduced nsp9 RNAylation (32), suggesting that NiRAN has specific substrates. As most coronavirus genomes have an A at the 5′ end, AMPylated nsp9 could bind to the 3′-terminal UMP of the minus-strand template and subsequently be used as a primer for plus-strand synthesis. A similar (but not identical) mechanism for the initiation of RNA synthesis is employed by picornaviruses, which are evolutionarily related to the Nidovirales (46, 47). In this case, a diuridylated form of the picornaviral VPg protein (VPg-pUpU) acts as a primer to initiate RNA synthesis by the 3D polymerase (48, 49). Following (protein-primed) initiation of coronavirus RNA synthesis, the nascent nsp9-pRNA strand could be separated, at some stage, from its template by the viral 5′-to-3′ helicase while the helicase-associated GTPase activity that fuels double-stranded RNA unwinding and translocation could provide a GDP molecule that, in a second NiRAN-mediated reaction, attacks the phosphoramidate bond between nsp9 and the 5′-terminal nucleotide of the nascent strand. In a recent study, a similar model was proposed in which NiRAN has a dual function in both RNA synthesis and capping and in which nsp9 gets recycled by cleavage of the nsp9-pRNA phosphoramidate bond (30). In part, this model was based on the observation that formation of the nsp9-pRNA phosphoramidate bond is reversible in the presence of PPi, thereby making nsp9 available for a new cycle. In the noncanonical capping model described above, the role of PPi in recycling nsp9-pRNA is taken by the GDP molecule that attacks this bond in the nsp9-pRNA intermediate to produce the core cap structure GpppRNA and to release an unmodified nsp9. Although many details remain to be validated, these studies provide interesting working hypotheses for future biochemical and structural studies aimed at elucidating the molecular details of the initiation of coronavirus RNA synthesis and capping.
FIG 10.
Proposed model of potential roles of NiRAN-mediated enzymatic activities. Proteins (or protein domains) suggested to mediate specific enzymatic reactions are highlighted in red. NiRAN domains have been shown to NMPylate (A) or RNAylate (B) the N-terminal residue of nsp9 by forming a covalent phosphoramidate bond (29, 32). (C) A GDP molecule bound in the G pocket (33–35) of the NiRAN active site has been suggested to attack this bond (32), thereby releasing unmodified nsp9 and forming a GpppRNA core cap structure that is subsequently methylated by the guanine-N7 methyltransferase (NMT) domain of nsp14 (26), which harbors an additional 3′-to-5′ exoribonuclease domain (ExoN) in its N-terminal part (25). In another reaction mediated by nsp16 (in complex with nsp10), the cap0 structure is converted to a cap1 structure by ribose 2′-O-methylation on the first nucleotide of the RNA (27, 54, 55). nsp10 can also bind to nsp14 (not shown), which is known to enhance ExoN (but not NMT) activity of nsp14 (56). (A) By analogy to the protein-primed initiation of RNA synthesis established for picornaviruses and several other plus-strand RNA viruses (48, 49), NMPylated forms of nsp9 have been speculated to have a role in the initiation of coronavirus RNA synthesis (29, 30), where, for example, nsp9-pU or nsp9-pA could act as a primer to promote the initiation of RNA synthesis by the RdRp domain located in the C-terminal half of nsp12. It should be noted, however, that there is limited information on the molecular mechanisms involved in the initiation of plus- and minus-strand RNA synthesis, and at present, there is no direct evidence to support this hypothesis. Similarly, the mechanisms that control the specific capping of coronavirus plus-strand RNAs as well as the potential role(s) of the coronavirus helicase (nsp13) in this process remain to be studied.
The available structural and functional information on coronavirus NiRAN domains and their protein and RNA targets provide compelling evidence for essential and, possibly, multiple roles of this highly conserved domain in the life cycle of coronaviruses and other nidoviruses. Clearly, the universal conservation of this domain in all nidoviruses (28) makes it a prime target for the development of broad-spectrum drugs against a wide range of viral pathogens in this genetically diverse virus order, including newly emerging coronaviruses, such as Middle East respiratory syndrome coronavirus (MERS-CoV) and SARS-CoV-2.
MATERIALS AND METHODS
Plasmids, mutagenesis, and protein production in E. coli.
For cloning, viral RNA was isolated from Vero E6 cells infected with SARS-CoV or SARS-CoV-2, Huh-7 cells infected with HCoV-229E, or ST cells infected with TGEV. The nsp9 and nsp12 coding sequences of SARS-CoV (GenBank accession number NC_004718.3, pp1ab residues 4118 to 4230 and 4370 to 5301), SARS-CoV-2 (GenBank accession number NC_045512.2, pp1ab residues 4141 to 4253 and 4393 to 5324), TGEV (GenBank accession number NC_038861.1, pp1ab residues 3753 to 3863 and 3999 to 4927), and HCoV-229E nsp12 (GenBank accession number NC_002645.1, pp1ab residues 4069 to 4995) were amplified by reverse transcription-PCR (RT-PCR) and cloned into pASK3-Ub-CHis6 plasmid DNA (50, 51). The resulting expression plasmids encoded the respective full-length proteins fused to an N-terminal ubiquitin tag and a C-terminal His6 tag, with the exception of SARS-CoV nsp9, which contained a His7 tag. Protein expression was under the control of a tetracycline-inducible promoter. E. coli TB1 cells were cotransformed with the appropriate pASK3-Ub-CHis6 plasmid construct and pCGI plasmid DNA encoding the ubiquitin-specific carboxyl-terminal hydrolase 1 (Ubp1) (50) and cultivated in LB medium containing 100 μg/mL ampicillin and 34 μg/mL chloramphenicol. At an optical density at 600 nm (OD600) of 0.6, protein production was induced with 0.2 μg/mL anhydrotetracycline (IBA Lifesciences). After incubation at 18°C for 16 h, cells were harvested by centrifugation and resuspended in lysis buffer (20 mM Tris-HCl [pH 8.0], 500 mM NaCl, 10 mM imidazole, 0.05% Tween 20, 10 mM β-mercaptoethanol, 5% glycerol) containing 100 μg/mL lysozyme and EDTA-free protease inhibitor cocktail (Roche) for 30 min. After sonication, insoluble material was removed by centrifugation. 1 mL nickel nitrilotriacetic acid (Ni-NTA) resin (New England Biolabs [NEB]) was preequilibrated with lysis buffer, mixed with soluble supernatant, and incubated for 1 h on a rotary shaker. After washing with 20 mL lysis buffer, the Ni-NTA resin with the bound proteins was loaded on a chromatography column and proteins were eluted with elution buffer (20 mM Tris-HCl [pH 8.0], 300 mM NaCl, 300 mM imidazole, 0,05% Tween 20, 10 mM β-mercaptoethanol, 5% glycerol). Eluate fractions containing the desired protein were identified by SDS-PAGE and Coomassie staining, pooled, dialyzed against storage buffer (20 mM Tris-HCl [pH 8.0], 150 mM NaCl, 2 mM dithiothreitol [DTT], 40% glycerol), and stored at −20°C until further use. To produce tag-free SARS-CoV-2 nsp7 and nsp8 (pp1ab residues 3860 to 3942 and 3943 to 4140), the respective coding sequences, along with a short linker sequence (SGSG) and a C-terminal His6 tag, were inserted into pASK3-Ub-CHis6 plasmid DNA. Following Ni-NTA affinity chromatography, the purified protein was cleaved with recombinant coronavirus main protease (Mpro) as described further below. Mpro and the C-terminal tag were removed by Ni-NTA affinity chromatography. Fractions containing the desired protein were pooled, dialyzed against storage buffer, and stored at −20°C.
The coding sequence of HCoV-229E nsp9 (GenBank accession number NC_002645.1, pp1a residues 3825 to 3933) was cloned into pMAL-c2X plasmid DNA (NEB). pMAL-c4X plasmid DNA containing the coding sequence of the coronavirus nsp9 homolog of MLeV (GenBank accession number GECV01031551, pp1a residues 1784 to 1887) with a C-terminal His6 tag was synthesized and assembled by Biocat (Heidelberg, Germany). To produce maltose-binding protein (MBP) fusion proteins, E. coli TB1 cells were freshly transformed with the appropriate pMAL plasmid construct. Bacterial cultures were grown in LB medium supplemented with 100 μg/mL ampicillin, and recombinant protein production was induced at an OD600 of 0.6 with 1 mM isopropyl-β-d-thiogalactopyranoside (Roth). After incubation at 18°C for 16 h, bacteria were centrifuged, and the pellet was resuspended in ice-cold MBP buffer (20 mM Tris-HCl [pH 7.5], 200 mM NaCl, 1 mM EDTA, 1 mM DTT) containing 100 μg/mL lysozyme (Roth) and EDTA-free protease inhibitor cocktail (Roche) and lysed for 30 min. Following sonication, insoluble material was removed by centrifugation and the soluble supernatant was mixed with 1 mL amylose resin (NEB) preequilibrated in MBP buffer. Following incubation on a rotating shaker for 2 h, the amylose resin with the bound MBP fusion protein was centrifuged (700 × g for 2 min) and washed twice with 20 mL factor Xa cleavage buffer (25 mM Tris-HCl [pH 8.0], 300 mM NaCl, 5 mM CaCl2, 1 mM DTT). Then, the fusion protein was cleaved with factor Xa (NEB) according to the manufacturer’s instructions. Following centrifugation of the amylose resin, the supernatant containing the desired protein was collected, dialyzed against storage buffer (20 mM Tris-HCl [pH 8.0], 150 mM NaCl, 2 mM DTT, 40% glycerol), and stored at −20°C. The coding sequence of the feline coronavirus Mpro (GenBank accession number DQ010921.1, pp1a residues 8790 to 9695) was cloned into pMAL-c2X (NEB), along with a C-terminal His6 tag. Mpro was expressed as an MBP fusion protein (see above) and additionally purified by Ni-NTA affinity chromatography essentially as described above with minor modifications. Briefly, bacterial cells were pelleted and resuspended in lysis buffer containing 20 mM Tris-HCl (pH 8.0), 300 mM NaCl, 10 mM imidazole, 5% glycerol, 0.05% Tween 20, and 10 mM β-mercaptoethanol with 100 μg/mL lysozyme. Following sonication, insoluble material was removed by centrifugation and the soluble supernatant was loaded on a Ni-NTA column. Nonspecifically bound proteins were removed by several wash steps using a lysis buffer containing 30 mM imidazole, and the fusion protein was eluted in a lysis buffer containing 200 mM imidazole. Eluate fractions containing the desired protein were dialyzed overnight in buffer containing 20 mM Tris-HCl (pH 7.4), 5 mM CaCl2, 200 mM NaCl, and 2 mM DTT. Thereafter, the MBP-Mpro fusion protein was cleaved with factor Xa according to the manufacturer’s instructions. Mpro was purified (and separated from MBP and factor Xa) by Ni-NTA chromatography using an elution buffer containing 20 mM Tris-HCl (pH 7.4), 200 mM NaCl, 200 mM imidazole, and 2 mM DTT. The protein solution was dialyzed against 20 mM Tris-HCl (pH 7.4), 200 mM NaCl, 2 mM DTT, and 5% glycerol, and aliquots of the purified protein were stored at −80°C until further use.
To produce chimeric forms of SARS-CoV-2 nsp9 in which the six N-terminal residues were replaced with the six N-terminal residues of the PsNV or MLeV nsp9 homologs, respectively, the pASK3-Ub-SARS-CoV-2-nsp9-CHis6 expression construct was modified using PCR-based methods. In the resulting two expression plasmids, the codons representing the first six codons of the SARS-CoV-2 nsp9 sequence were replaced with the corresponding codons of PsNV (GenBank accession number MK611985.1) or MLeV (GenBank accession number GECV01031551).
Sequences of oligonucleotides used for cloning, site-directed mutagenesis, and sequencing are available upon request.
NMPylation assay.
NiRAN-mediated NMPylation of nsp9 was analyzed as described previously with some modifications (29). Briefly, NMPylation reaction mixtures (10 μL) were set up with nsp12 (0.05 μM) and nsp9 (4 μM) in reaction buffer containing 50 mM HEPES (pH 8.0), 30 mM NaCl, 1 mM MnCl2, 25 μM NTP, a 0.17 μM concentration of the appropriate [α-32P]NTP (Hartmann Analytic; 3,000 Ci/mmol), 5 mM DTT, and 8% glycerol and incubated at 30°C for 10 min. Where indicated, the reaction conditions were adjusted to previously published protocols as follows. According to the protocol used by Conti et al. (36), the proteins were incubated in reaction buffer containing 50 mM Tris-HCl (pH 8.5), 25 mM NaCl, 6 mM MnCl2, 25 μM UTP, 0.09 μM [α-32P]UTP, 1 mM DTT, and 2% glycerol at 30°C for 30 min. According to the protocol reported by Shannon et al. (37), the proteins were incubated in buffer containing 20 mM HEPES (pH 7.5), 30 mM NaCl, 2 mM MnCl2, 25 μM UTP, 0.09 μM [α-32P]UTP, 1 mM DTT, 2% glycerol at 37°C for 60 min. Concentrations and molar ratios of nsp12 and potential protein substrates used in the respective experiments are given in Fig. 3. Reactions were stopped by the addition of protein sample buffer (62.5 mM Tris-HCl [pH 6.8], 100 mM DTT, 2.5% SDS, 10% glycerol, 0.005% bromophenol blue) and heat denaturation at 90°C for 4 min. Reaction products were separated by SDS-PAGE. Gels were fixed and stained in a solution containing 40% methanol, 10% acetic acid, and 0.05% Coomassie brilliant blue R-250. Radiolabeled proteins were detected by phosphorimaging (Amersham Typhoon IP; Cytiva) after exposure for 1 h. Signals were analyzed using ImageJ software, and statistical significance was calculated using a two-tailed Student’s t test. Modifications in the standard protocol are indicated in the figure legends.
Mass-spectrometric analyses.
To measure masses of intact proteins, 1 μM nsp12 and 10 μM nsp9 of SARS-CoV were incubated with 500 μM concentrations (each) of UTP, ATP, and GTP or CTP, ATP, and GTP in the nucleotidylation assay at 30°C for 60 min. After nucleotidylation, samples were desalted using the Acquity H-class HPLC system equipped with a MassPrep column (Waters) and eluted into the electrospray ionization (ESI) source of a Synapt G2Si mass spectrometer (Waters). Isocratic elution was performed with 5% solvent A (0.05% formic acid) for 2 min, followed by a linear gradient of 95% with solvent B (80% acetonitrile–0.045% formic acid) for the next 8 min and a holding time of 4 min. The temperature of the column was 60°C, and the flow rate was 0.1 mL/min. Positively charged ions within the mass range of 500 to 5,000 m/z were measured, and Glu-fibrinopeptide B was measured every 45 s for automatic correction of mass drift. The averaged spectra were deconvoluted after baseline subtraction and subsequent smoothing with MassLynx instrument software with the MaxEnt1 extension.
HPLC-tandem mass spectrometry was used to identify the binding site of GMP to SARS-CoV nsp9. GMPylated nsp9 was digested overnight at 37°C with sequencing-grade modified trypsin (Serva). The resulting peptides were purified and concentrated with desalting columns (Macherey-Nagel), resolved in 5% acetonitrile–0.1% formic acid, and injected into a C18 PepMap preconcentration column (Thermo Scientific). Automated trapping and desalting of the peptides were performed with solvent A (0.05% formic acid) at a flow rate of 6 μL/min. The separation of the peptides was achieved with a gradient of solvent A and solvent B (80% acetonitrile–0.045% formic acid) at a flow rate of 300 mL/min. Solvent B was maintained for 4 min, followed by a linear gradient to 45% within the next 30 min and another linear gradient to 95% within the next 5 min. The eluate was sprayed directly via a nanoemitter (Proxeon, Denmark) at a voltage of 2,300 V into the heated capillary of an Orbitrap Velos Pro mass spectrometer (Thermo Scientific), which was equipped with an Ultimate nanoRSLC-HPLC system (Dionex) and a custom-made C18 reverse-phase (RP) column (75-μm inside diameter [ID], 50 cm long, filled with ReproSil-Pur 120 C18-AQ 2.4-μm beads from Dr. Maisch). A survey scan with a resolution of 60,000 within the Orbitrap mass analyzer was combined with at least three data-dependent MS/MS scans with a dynamic exclusion for 30 s, either using collision-induced dissociation (CID) with the linear ion trap or using high-energy collisional dissociation (HCD) combined with Orbitrap detection at a resolution of 7,500.
Data analysis was performed using Proteome Discoverer 2.2 (Thermo Scientific) with SEQUEST or Byonic (Bioinformatics Solutions, Inc.), or the data were manually analyzed with Xcalibur (Thermo Scientific).
Microscale thermophoresis assay.
NiRAN-mediated NMPylation of nsp9 chimeric proteins was measured using a Monolith 2020 (NanoTemper Technologies). The nsp9 at a 17.5 μM starting concentration was diluted 15 times in 1:2 steps. Subsequently, each of the 16 samples was incubated with 50 nM SARS-CoV-2 nsp12 and 25 nM aminoallyl-UTP-X-Cy5 (Jena Bioscience) for 10 min at 30°C in NMPylation buffer (see above). The measurements were performed at 70% excitation and medium microscale thermophoresis (MST) power. Potential self-NMPylation of nsp12 or nsp9 was not detected in control reactions using only a single protein under the same conditions. Data were obtained in at least 3 independent experiments and analyzed using MO.Affinity Analysis v3.0.1 software.
ACKNOWLEDGMENTS
The work was supported by grants from the Deutsche Forschungsgemeinschaft (SFB 1021, projects A01 [to J.Z.] and Z03 [to U.L.]; KFO309, project P3, to J.Z.; GRK 2355 to J.Z.), the German Federal Ministry for Education and Research (COVINET and RAPID to J.Z.), the State of Hesse (LOEWE program) through DRUID (Project B02 to J.Z.) and CoroPan (Projects P5 [to J.Z.] and P8 [to R.M.]), and the Pandemics Network Hesse (to J.Z.).
Contributor Information
John Ziebuhr, Email: John.Ziebuhr@viro.med.uni-giessen.de.
Tom Gallagher, Loyola University Chicago, Health Sciences Campus.
REFERENCES
- 1.de Groot RJ, Baker SC, Baric R, Enjuanes L, Gorbalenya AE, Holmes KV, Perlman S, Poon L, Rottier PJM, Talbot PJ, Woo PCY, Ziebuhr J. 2012. Family Coronaviridae, p 806–828. In King AMQ, Adams MJ, Carstens EB, Lefkowitz EJ (ed), Virus taxonomy. Elsevier, Amsterdam, The Netherlands. [Google Scholar]
- 2.de Groot RJ, Cowley JA, Enjuanes L, Faaberg KS, Perlman S, Rottier PJM, Snijder EJ, Ziebuhr J, Gorbalenya AE. 2012. Order Nidovirales, p 785–795. In King AMQ, Adams MJ, Carstens EB, Lefkowitz EJ (ed), Virus taxonomy. Elsevier, Amsterdam, The Netherlands. [Google Scholar]
- 3.Drosten C, Gunther S, Preiser W, van der Werf S, Brodt HR, Becker S, Rabenau H, Panning M, Kolesnikova L, Fouchier RA, Berger A, Burguiere AM, Cinatl J, Eickmann M, Escriou N, Grywna K, Kramme S, Manuguerra JC, Muller S, Rickerts V, Sturmer M, Vieth S, Klenk HD, Osterhaus AD, Schmitz H, Doerr HW. 2003. Identification of a novel coronavirus in patients with severe acute respiratory syndrome. N Engl J Med 348:1967–1976. doi: 10.1056/NEJMoa030747. [DOI] [PubMed] [Google Scholar]
- 4.Ksiazek TG, Erdman D, Goldsmith CS, Zaki SR, Peret T, Emery S, Tong S, Urbani C, Comer JA, Lim W, Rollin PE, Dowell SF, Ling AE, Humphrey CD, Shieh WJ, Guarner J, Paddock CD, Rota P, Fields B, DeRisi J, Yang JY, Cox N, Hughes JM, LeDuc JW, Bellini WJ, Anderson LJ, SARS Working Group . 2003. A novel coronavirus associated with severe acute respiratory syndrome. N Engl J Med 348:1953–1966. doi: 10.1056/NEJMoa030781. [DOI] [PubMed] [Google Scholar]
- 5.Peiris JS, Lai ST, Poon LL, Guan Y, Yam LY, Lim W, Nicholls J, Yee WK, Yan WW, Cheung MT, Cheng VC, Chan KH, Tsang DN, Yung RW, Ng TK, Yuen KY, group Ss . 2003. Coronavirus as a possible cause of severe acute respiratory syndrome. Lancet 361:1319–1325. doi: 10.1016/S0140-6736(03)13077-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gorbalenya AE, Baker SC, Baric RS, de Groot RJ, Drosten C, Gulyaeva AA, Haagmans BL, Lauber C, Leontovich AM, Neuman BW, Penzar D, Perlman S, Poon LLM, Samborskiy DV, Sidorov IA, Sola I, Ziebuhr J, Coronaviridae Study Group of the International Committee on Taxonomy of Viruses . 2020. The species Severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2. Nat Microbiol 5:536–544. doi: 10.1038/s41564-020-0695-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu F, Zhao S, Yu B, Chen YM, Wang W, Song ZG, Hu Y, Tao ZW, Tian JH, Pei YY, Yuan ML, Zhang YL, Dai FH, Liu Y, Wang QM, Zheng JJ, Xu L, Holmes EC, Zhang YZ. 2020. A new coronavirus associated with human respiratory disease in China. Nature 579:265–269. doi: 10.1038/s41586-020-2008-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhou P, Yang XL, Wang XG, Hu B, Zhang L, Zhang W, Si HR, Zhu Y, Li B, Huang CL, Chen HD, Chen J, Luo Y, Guo H, Jiang RD, Liu MQ, Chen Y, Shen XR, Wang X, Zheng XS, Zhao K, Chen QJ, Deng F, Liu LL, Yan B, Zhan FX, Wang YY, Xiao GF, Shi ZL. 2020. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 579:270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gorbalenya AE, Enjuanes L, Ziebuhr J, Snijder EJ. 2006. Nidovirales: evolving the largest RNA virus genome. Virus Res 117:17–37. doi: 10.1016/j.virusres.2006.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Snijder EJ, Decroly E, Ziebuhr J. 2016. The nonstructural proteins directing coronavirus RNA synthesis and processing. Adv Virus Res 96:59–126. doi: 10.1016/bs.aivir.2016.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ziebuhr J. 2005. The coronavirus replicase. Curr Top Microbiol Immunol 287:57–94. doi: 10.1007/3-540-26765-4_3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.V’Kovski P, Kratzel A, Steiner S, Stalder H, Thiel V. 2021. Coronavirus biology and replication: implications for SARS-CoV-2. Nat Rev Microbiol 19:155–170. doi: 10.1038/s41579-020-00468-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Malone B, Urakova N, Snijder EJ, Campbell EA. 2022. Structures and functions of coronavirus replication-transcription complexes and their relevance for SARS-CoV-2 drug design. Nat Rev Mol Cell Biol 23:21–39. doi: 10.1038/s41580-021-00432-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lou Z, Rao Z. 2022. The life of SARS-CoV-2 inside cells: replication-transcription complex assembly and function. Annu Rev Biochem 91:381–401. doi: 10.1146/annurev-biochem-052521-115653. [DOI] [PubMed] [Google Scholar]
- 15.Chen J, Malone B, Llewellyn E, Grasso M, Shelton PMM, Olinares PDB, Maruthi K, Eng ET, Vatandaslar H, Chait BT, Kapoor TM, Darst SA, Campbell EA. 2020. Structural basis for helicase-polymerase coupling in the SARS-CoV-2 replication-transcription complex. Cell 182:1560–1573.E13. doi: 10.1016/j.cell.2020.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang Q, Wu J, Wang H, Gao Y, Liu Q, Mu A, Ji W, Yan L, Zhu Y, Zhu C, Fang X, Yang X, Huang Y, Gao H, Liu F, Ge J, Sun Q, Yang X, Xu W, Liu Z, Yang H, Lou Z, Jiang B, Guddat LW, Gong P, Rao Z. 2020. Structural basis for RNA replication by the SARS-CoV-2 polymerase. Cell 182:417–428.E13. doi: 10.1016/j.cell.2020.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yan L, Zhang Y, Ge J, Zheng L, Gao Y, Wang T, Jia Z, Wang H, Huang Y, Li M, Wang Q, Rao Z, Lou Z. 2020. Architecture of a SARS-CoV-2 mini replication and transcription complex. Nat Commun 11:5874. doi: 10.1038/s41467-020-19770-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yan L, Ge J, Zheng L, Zhang Y, Gao Y, Wang T, Huang Y, Yang Y, Gao S, Li M, Liu Z, Wang H, Li Y, Chen Y, Guddat LW, Wang Q, Rao Z, Lou Z. 2021. Cryo-EM structure of an extended SARS-CoV-2 replication and transcription complex reveals an intermediate state in cap synthesis. Cell 184:184–193.E10. doi: 10.1016/j.cell.2020.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Subissi L, Posthuma CC, Collet A, Zevenhoven-Dobbe JC, Gorbalenya AE, Decroly E, Snijder EJ, Canard B, Imbert I. 2014. One severe acute respiratory syndrome coronavirus protein complex integrates processive RNA polymerase and exonuclease activities. Proc Natl Acad Sci USA 111:E3900–E3909. doi: 10.1073/pnas.1323705111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Seybert A, Hegyi A, Siddell SG, Ziebuhr J. 2000. The human coronavirus 229E superfamily 1 helicase has RNA and DNA duplex-unwinding activities with 5′-to-3′ polarity. RNA 6:1056–1068. doi: 10.1017/s1355838200000728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ivanov KA, Thiel V, Dobbe JC, van der Meer Y, Snijder EJ, Ziebuhr J. 2004. Multiple enzymatic activities associated with severe acute respiratory syndrome coronavirus helicase. J Virol 78:5619–5632. doi: 10.1128/JVI.78.11.5619-5632.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ivanov KA, Ziebuhr J. 2004. Human coronavirus 229E nonstructural protein 13: characterization of duplex-unwinding, nucleoside triphosphatase, and RNA 5′-triphosphatase activities. J Virol 78:7833–7838. doi: 10.1128/JVI.78.14.7833-7838.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bhardwaj K, Guarino L, Kao CC. 2004. The severe acute respiratory syndrome coronavirus Nsp15 protein is an endoribonuclease that prefers manganese as a cofactor. J Virol 78:12218–12224. doi: 10.1128/JVI.78.22.12218-12224.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ivanov KA, Hertzig T, Rozanov M, Bayer S, Thiel V, Gorbalenya AE, Ziebuhr J. 2004. Major genetic marker of nidoviruses encodes a replicative endoribonuclease. Proc Natl Acad Sci USA 101:12694–12699. doi: 10.1073/pnas.0403127101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Minskaia E, Hertzig T, Gorbalenya AE, Campanacci V, Cambillau C, Canard B, Ziebuhr J. 2006. Discovery of an RNA virus 3′→5′ exoribonuclease that is critically involved in coronavirus RNA synthesis. Proc Natl Acad Sci USA 103:5108–5113. doi: 10.1073/pnas.0508200103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen Y, Cai H, Pan J, Xiang N, Tien P, Ahola T, Guo D. 2009. Functional screen reveals SARS coronavirus nonstructural protein nsp14 as a novel cap N7 methyltransferase. Proc Natl Acad Sci USA 106:3484–3489. doi: 10.1073/pnas.0808790106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Decroly E, Imbert I, Coutard B, Bouvet M, Selisko B, Alvarez K, Gorbalenya AE, Snijder EJ, Canard B. 2008. Coronavirus nonstructural protein 16 is a cap-0 binding enzyme possessing (nucleoside-2′O)-methyltransferase activity. J Virol 82:8071–8084. doi: 10.1128/JVI.00407-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lehmann KC, Gulyaeva A, Zevenhoven-Dobbe JC, Janssen GM, Ruben M, Overkleeft HS, van Veelen PA, Samborskiy DV, Kravchenko AA, Leontovich AM, Sidorov IA, Snijder EJ, Posthuma CC, Gorbalenya AE. 2015. Discovery of an essential nucleotidylating activity associated with a newly delineated conserved domain in the RNA polymerase-containing protein of all nidoviruses. Nucleic Acids Res 43:8416–8434. doi: 10.1093/nar/gkv838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Slanina H, Madhugiri R, Bylapudi G, Schultheiß K, Karl N, Gulyaeva A, Gorbalenya AE, Linne U, Ziebuhr J. 2021. Coronavirus replication-transcription complex: vital and selective NMPylation of a conserved site in nsp9 by the NiRAN-RdRp subunit. Proc Natl Acad Sci USA 118:e2022310118. doi: 10.1073/pnas.2022310118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang B, Svetlov D, Artsimovitch I. 2021. NMPylation and de-NMPylation of SARS-CoV-2 nsp9 by the NiRAN domain. Nucleic Acids Res 49:8822–8835. doi: 10.1093/nar/gkab677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Walker AP, Fan H, Keown JR, Knight ML, Grimes JM, Fodor E. 2021. The SARS-CoV-2 RNA polymerase is a viral RNA capping enzyme. Nucleic Acids Res 49:13019–13030. doi: 10.1093/nar/gkab1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Park GJ, Osinski A, Hernandez G, Eitson JL, Majumdar A, Tonelli M, Henzler-Wildman K, Pawłowski K, Chen Z, Li Y, Schoggins JW, Tagliabracci VS. 2022. The mechanism of RNA capping by SARS-CoV-2. Nature 609:793–800. doi: 10.1038/s41586-022-05185-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Malone BF, Perry JK, Olinares PDB, Lee HW, Chen J, Appleby TC, Feng JY, Bilello JP, Ng H, Sotiris J, Ebrahim M, Chua EYD, Mendez JH, Eng ET, Landick R, Gotte M, Chait BT, Campbell EA, Darst SA. 2023. Structural basis for substrate selection by the SARS-CoV-2 replicase. Nature 614:781–787. doi: 10.1038/s41586-022-05664-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shannon A, Fattorini V, Sama B, Selisko B, Feracci M, Falcou C, Gauffre P, El Kazzi P, Delpal A, Decroly E, Alvarez K, Eydoux C, Guillemot JC, Moussa A, Good SS, La Colla P, Lin K, Sommadossi JP, Zhu Y, Yan X, Shi H, Ferron F, Canard B. 2022. A dual mechanism of action of AT-527 against SARS-CoV-2 polymerase. Nat Commun 13:621. doi: 10.1038/s41467-022-28113-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yan L, Huang Y, Ge J, Liu Z, Lu P, Huang B, Gao S, Wang J, Tan L, Ye S, Yu F, Lan W, Xu S, Zhou F, Shi L, Guddat LW, Gao Y, Rao Z, Lou Z. 2022. A mechanism for SARS-CoV-2 RNA capping and its inhibition by nucleotide analog inhibitors. Cell 185:4347–4360.E17. doi: 10.1016/j.cell.2022.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Conti BJ, Leicht AS, Kirchdoerfer RN, Sussman MR. 2021. Mass spectrometric based detection of protein nucleotidylation in the RNA polymerase of SARS-CoV-2. Commun Chem 4:41. doi: 10.1038/s42004-021-00476-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shannon A, Fattorini V, Sama B, Selisko B, Feracci M, Falcou C, Gauffre P, Kazzi PE, Decroly E, Rabah N, Alvarez K, Eydoux C, Guillemot J-C, Debart F, Vasseur J-J, Noel M, Moussa A, Good S, Lin K, Sommadossi J-P, Zhu Y, Yan X, Shi H, Ferron F, Canard B. 2021. Protein-primed RNA synthesis in SARS-CoVs and structural basis for inhibition by AT-527. bioRxiv. doi: 10.1101/2021.03.23.436564. [DOI]
- 38.Yang Y, Yue Y, Song N, Li C, Yuan Z, Wang Y, Ma Y, Li H, Zhang F, Wang W, Jia H, Li P, Li X, Wang Q, Ding Z, Dong H, Gu L, Li B. 2020. The YdiU domain modulates bacterial stress signaling through Mn(2+)-dependent UMPylation. Cell Rep 32:108161. doi: 10.1016/j.celrep.2020.108161. [DOI] [PubMed] [Google Scholar]
- 39.Sreelatha A, Yee SS, Lopez VA, Park BC, Kinch LN, Pilch S, Servage KA, Zhang J, Jiou J, Karasiewicz-Urbańska M, Łobocka M, Grishin NV, Orth K, Kucharczyk R, Pawłowski K, Tomchick DR, Tagliabracci VS. 2018. Protein AMPylation by an evolutionarily conserved pseudokinase. Cell 175:809–821.E19. doi: 10.1016/j.cell.2018.08.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Posthuma CC, Te Velthuis AJW, Snijder EJ. 2017. Nidovirus RNA polymerases: complex enzymes handling exceptional RNA genomes. Virus Res 234:58–73. doi: 10.1016/j.virusres.2017.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ziebuhr J, Snijder EJ, Gorbalenya AE. 2000. Virus-encoded proteinases and proteolytic processing in the Nidovirales. J Gen Virol 81:853–879. doi: 10.1099/0022-1317-81-4-853. [DOI] [PubMed] [Google Scholar]
- 42.Hegyi A, Ziebuhr J. 2002. Conservation of substrate specificities among coronavirus main proteases. J Gen Virol 83:595–599. doi: 10.1099/0022-1317-83-3-595. [DOI] [PubMed] [Google Scholar]
- 43.Jovanovic D, Tremmel P, Pallan PS, Egli M, Richert C. 2020. The enzyme-free release of nucleotides from phosphoramidates depends strongly on the amino acid. Angew Chem Int Ed Engl 59:20154–20160. doi: 10.1002/anie.202008665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bukhari K, Mulley G, Gulyaeva AA, Zhao L, Shu G, Jiang J, Neuman BW. 2018. Description and initial characterization of metatranscriptomic nidovirus-like genomes from the proposed new family Abyssoviridae, and from a sister group to the Coronavirinae, the proposed genus Alphaletovirus. Virology 524:160–171. doi: 10.1016/j.virol.2018.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mordecai GJ, Miller KM, Di Cicco E, Schulze AD, Kaukinen KH, Ming TJ, Li S, Tabata A, Teffer A, Patterson DA, Ferguson HW, Suttle CA. 2019. Endangered wild salmon infected by newly discovered viruses. Elife 8:e47615. doi: 10.7554/eLife.47615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gorbalenya AE, Koonin EV, Donchenko AP, Blinov VM. 1989. Coronavirus genome: prediction of putative functional domains in the non-structural polyprotein by comparative amino acid sequence analysis. Nucleic Acids Res 17:4847–4861. doi: 10.1093/nar/17.12.4847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Koonin EV, Dolja VV, Krupovic M, Varsani A, Wolf YI, Yutin N, Zerbini FM, Kuhn JH. 2020. Global organization and proposed megataxonomy of the virus world. Microbiol Mol Biol Rev 84:e00061-19. doi: 10.1128/MMBR.00061-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Goodfellow I. 2011. The genome-linked protein VPg of vertebrate viruses - a multifaceted protein. Curr Opin Virol 1:355–362. doi: 10.1016/j.coviro.2011.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Paul AV, Wimmer E. 2015. Initiation of protein-primed picornavirus RNA synthesis. Virus Res 206:12–26. doi: 10.1016/j.virusres.2014.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gohara DW, Ha CS, Kumar S, Ghosh B, Arnold JJ, Wisniewski TJ, Cameron CE. 1999. Production of “authentic” poliovirus RNA-dependent RNA polymerase (3Dpol) by ubiquitin-protease-mediated cleavage in Escherichia coli. Protein Expr Purif 17:128–138. doi: 10.1006/prep.1999.1100. [DOI] [PubMed] [Google Scholar]
- 51.Te Velthuis AJ, Arnold JJ, Cameron CE, van den Worm SH, Snijder EJ. 2010. The RNA polymerase activity of SARS-coronavirus nsp12 is primer dependent. Nucleic Acids Res 38:203–214. doi: 10.1093/nar/gkp904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sievers F, Wilm A, Dineen D, Gibson TJ, Karplus K, Li W, Lopez R, McWilliam H, Remmert M, Soding J, Thompson JD, Higgins DG. 2011. Fast, scalable generation of high-quality protein multiple sequence alignments using Clustal Omega. Mol Syst Biol 7:539. doi: 10.1038/msb.2011.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Robert X, Gouet P. 2014. Deciphering key features in protein structures with the new ENDscript server. Nucleic Acids Res 42:W320–W324. doi: 10.1093/nar/gku316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bouvet M, Debarnot C, Imbert I, Selisko B, Snijder EJ, Canard B, Decroly E. 2010. In vitro reconstitution of SARS-coronavirus mRNA cap methylation. PLoS Pathog 6:e1000863. doi: 10.1371/journal.ppat.1000863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chen Y, Su C, Ke M, Jin X, Xu L, Zhang Z, Wu A, Sun Y, Yang Z, Tien P, Ahola T, Liang Y, Liu X, Guo D. 2011. Biochemical and structural insights into the mechanisms of SARS coronavirus RNA ribose 2′-O-methylation by nsp16/nsp10 protein complex. PLoS Pathog 7:e1002294. doi: 10.1371/journal.ppat.1002294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bouvet M, Lugari A, Posthuma CC, Zevenhoven JC, Bernard S, Betzi S, Imbert I, Canard B, Guillemot JC, Lecine P, Pfefferle S, Drosten C, Snijder EJ, Decroly E, Morelli X. 2014. Coronavirus Nsp10, a critical co-factor for activation of multiple replicative enzymes. J Biol Chem 289:25783–25796. doi: 10.1074/jbc.M114.577353. [DOI] [PMC free article] [PubMed] [Google Scholar]