Abstract
Insect bites that cause itch, pain and swelling are very common. The use of concentrated heat for relief of these symptoms may be a promising approach; however, the scientific evidence for efficacy of hyperthermia treatment is sparse. We report here the results of a large real-world study using a randomized control group to assess the efficacy of hyperthermia on insect bites in real-world conditions, specifically considering mosquito bites as the most common type. The study was conducted in a decentralized manner via a smartphone-controlled medical device, heat it®, for treatment of insect bites and stings through application of heat. The application that controls the device was accompanied by additional questionnaires, that collected data related to insect bites, such as itch and pain intensity. Analysis of data from over 12,000 collected treated insect bites, generated by approximately 1,750 participants (42% female, 39 ± 13 years) showed significant inhibition of itch and pain for all investigated insect species (mosquitoes, horseflies, bees and wasps). Mosquito bite-induced itch was reduced by 57% within the first minute and by 81% 5–10 min after treatment, and the overall reduction in itch and pain was more pronounced than in the control group. In conclusion, the results indicate that local application of heat relieves symptoms of insect bites.
SIGNIFICANCE
Bothersome insect bites are common. Concentrated heat is a promising approach for treatment of bites. To improve the sparse scientific evidence-base, a real-world study of 1,750 participants was performed. Using smartphones and heat it®, a medical device for the application of heat to insect bites, participants gathered data for over 12,000 tracked insect bites. The results showed that that itch and pain caused by insect bites is strongly reduced by heat treatment, no matter how long ago the insect bite occurred. These outcomes could be used to improve the care of insect bites.
Key words: insect bites and stings, hyperthermia, pruritus, itch
Virtually everyone, every year, gets bitten or stung by insects. The impact ranges from short-lived itch or pain to life-threating reactions (1, 2) in individuals with severe allergic reactions. In Germany, where the current study was performed, there are numerous biting or stinging insects, including bees, wasps, horse flies, mosquitos, lice, fleas, and bugs (3). Allergic reactions to Hymenoptera stings are clinically the most important, whereas Diptera bites are much more frequent overall. As climate change leads to an expansion of the geographical range of insects (4, 5), the incidence of bites and stings is expected to increase.
Reactions to insect bites and stings are triggered by substances delivered to the dermis by the insect during the bite or sting. Mosquitoes, for example, inject salivary secretions to ensure blood flow on feeding (2), which causes an immediate inflammatory reaction in approximately 75% of individuals (6). Bees, wasps, hornets, and yellow jackets deliver venom to the skin of their victims when they sting. In bees and wasps, the venom contains various proteins, such as melittin, mastoparan, and phospholipase A2, which can directly activate sensory neurones, causing acute pain and itch sensations, and can lead to an immunoglobulin E (IgE)-independent mast cell degranulation and release of histamine and tryptase (7–9).
Pruritic signals are perceived by diverse subpopulations of cutaneous pruriceptive neurones and are transferred via the spinal projection neurones to the brain, where the processing of the itch sensation occurs in multiple brain areas and circuits (10, 11). Itch and pain, distinct sensations with specific receptors and mediators involved, interact with each other. A painful stimulus, such as noxious heat, causes the inhibition of itch signalling (12). Recent studies show that transient noxious heat stimulation in healthy human subjects suppresses experimental histaminergic and non-histaminergic itch (13, 14). In an open cohort-study at German bathing lakes with 146 individuals who experienced insect bites or stings, the use of concentrated heat (51°C for 3 or 6 s) reduced the itch induced by these bites and stings (15).
Mosquito bites are the most frequent insect bites worldwide (16), and itch induced by these and other insects can be quite bothersome. We report here the results of a large real-world study using a randomized controlled group to assess the efficacy of hyperthermia on insect bites in real-world conditions, especially considering mosquito bites as the most frequent type of bites.
MATERIALS AND METHODS
Clinical study
In this decentralized clinical study, a conformité européenne (CE)-certified class IIa medical device (heat it®; Kamedi GmbH, Karlsruhe, Germany) was used. The device is used for the treatment of insect bites and stings by applying concentrated heat from 47°C to 51.5°C for 4–9 s (configurable by the user). The device is powered by a smartphone and controlled via the heat it® app. The study was performed from June to October 2022 among 1,783 heat it® users (42% females, 39 ± 13 years [mean age ± standard deviation; SD]), who were willing to participate and provided informed consent. The data-set contained over 12,000 registered treatments, mainly collected within Germany.
Method of data collection
Via the heat it® app, all users who had a minimum of 10 treatments were asked if they wanted to join the study. For those users who provided consent, basic demographic information was documented. Subsequently, for each treatment, participants were asked for information regarding the insect sting or bite in question. Data collected included itch and pain sensation on a numerical rating scale (NRS 0–10) immediately before treatment, as well as after treatment in 3 repeated questionnaires (issued via push-notifications in the smartphone app) as well as insect-species, body site and estimated time since the sting or bite (Fig. 1). Participants were free to skip or cancel the questionnaires at any time. Technical data collected included the temperature and duration of the chosen treatment, as well as timestamps of the required participants inter-actions, e.g. for plausibility checking (see below) or assignment of itch and pain assessments to points in time.
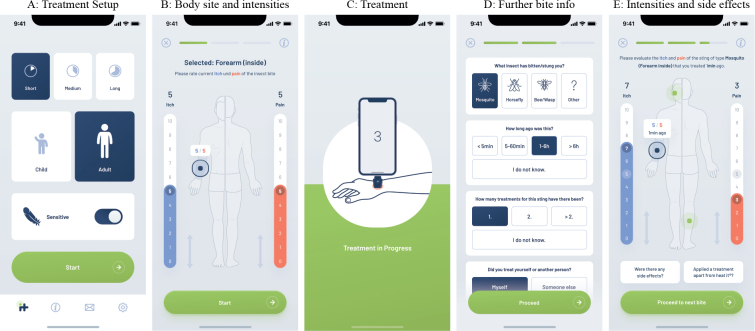
Fig. 1.
App interface for registering an insect bite. (A) The participant sets up the treatment (duration and temperature). (B) The body site with current itch and pain scores of the sting to be treated is recorded by the participant. (C) Treatment is performed. (D) After treatment, some additional information on the treated sting is captured from the participant. (E) The participant is again asked to report the current itch- and pain-sensations. There is also a possibility to report side-effects and/or additional treatments.
Data filtering
Utilizing collected timestamps, data created by participants were subjected to automated checks for plausibility by comparing the time a participant spent on a certain app-screen with realistic values. Thus, if a participant spent too little or too much time completing a questionnaire, the corresponding data was labelled implausible and was not included in the evaluations presented in this report unless explicitly mentioned. This plausibility check was performed only for the first of the post-treatment questionnaires.
Participants were allowed to re-treat a recorded bite before completing all 3 of the post-treatment questionnaires. Approximately 10% of all recorded insect bites contain such repeated treatments. This data was filtered out when considering any relationship between heat treatment and reduction in itch and/or pain intensity in this study.
Control group
This study includes a control group that was generated by asking participants to delay the treatment they were about to perform. Each participant was asked to make such a delay on a randomized basis in approximately 5% of all treatment cases. The question was asked after the participant provided information about the current itch and pain resulting from the insect bite to be treated. Each participant was free to decline the request and treat immediately. However, if the requested delay was accepted, the questionnaires regarding the insect bite were rolled out as normal, with the sole difference that no treatment took place. After all of the questionnaires were completed (approximately 10 min later), subjects were offered the opportunity to treat the bite.
Incentivization
To increase participation in this study, a gamification concept was implemented. Participants were awarded virtual points for each completed questionnaire and badges for good data quality or consistent usage of the app. Participants also gained access to a personalized screen in the app showing registered bites visualized on a body site heatmap. Also, participants were informed that they could win a prize in a raffle, and that the number of raffle tickets they were given was determined by the number of virtual points earned.
Statistical analysis
Statistical analyses were performed with the software SciPy (version 1.9.3) (17). To determine significant differences between independent groups, the Mann–Whitney U test, and for dependent groups the Wilcoxon test, was used, since most of the sampled data was neither normally distributed nor preserved equal variances. Multiple observations from the same participant were summarized into a single data-point by mean averaging for rolling out statistical tests. Descriptive statistics of age of the participants were generated by sampling the age ranges with equity distributions, thus generating a continuous distribution from which mean and SD could be derived.
Effect sizes are common language effect sizes according to (18) (represented by the shorthand CL in this report), calculated with the help of the package Pingouin (19) (version 0.5.3). For convenience, in this study, small, medium and large effect sizes correspond to values of 0.56, 0.64 and 0.71, respectively (18).
RESULTS
Insect bites and stings induce considerable itch and pain
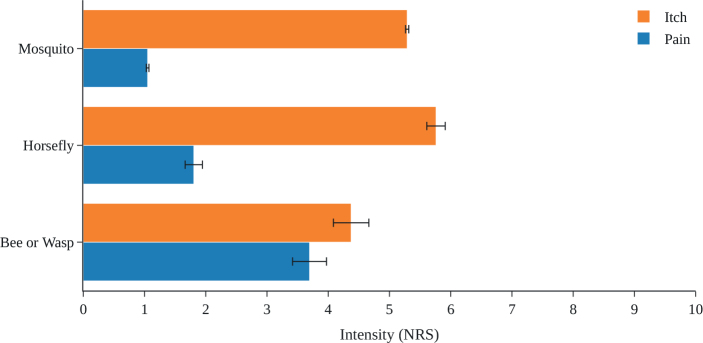
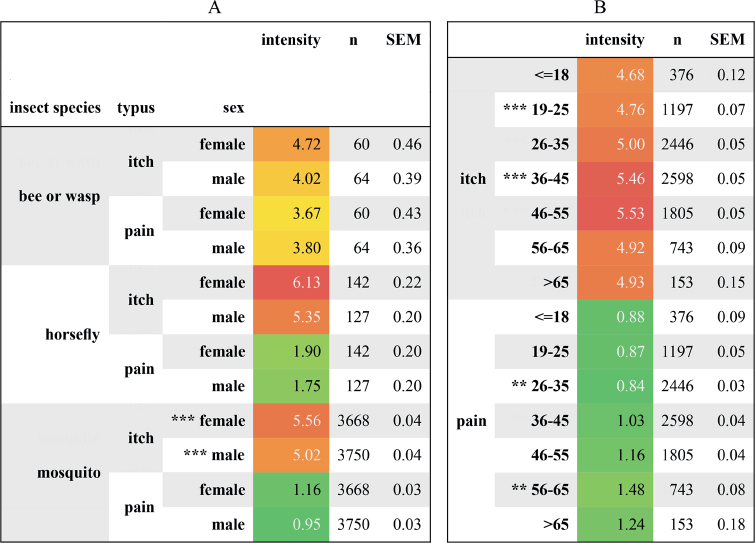
Across a total of 12,076 insect bites or stings in 1,783 affected individuals, 95% of plausible ones were caused by mosquitoes, 3.3% by horse flies, and 1.7% by bees or wasps. One in 5 documented bites or stings were not assigned to a specific type of insect. The mean ± SD itch intensity on a numerical rating scale (NRS 0–10) was highest for horse fly bites (5.8 ± 2.5), followed by mosquito bites (5.3 ± 2.3) and bee or wasp stings (4.4 ± 3.3). The intensity of pain was highest for bee and wasp stings (3.7 ± 3.2) and was lower for horse fly and mosquito bites (1.9 ± 2.3 and 1.1 ± 1.9, respectively) (Fig. 2). Itch and pain responses were largely similar in male and female participants and across age groups, although there was a trend towards increased pain responses with higher age (Fig. 3).
Fig. 2.
Pre-treatment intensities of itch and pain induced by insect bites or stings. Data are shown as mean with standard error of mean (SEM) as error bars. NRS: numerical rating scale.
Fig. 3.
Mean pre-treatment intensities of itch and pain. Participants were asked to rate itch and pain intensities on a numerical rating scale (NRS, 0–10). Mean intensities (with standard error of the mean; SEM) of itch and pain are presented separated by insect species and (A) sex of participants, and (B) age of participants. Colours from light green to dark red indicate milder to more severe ratings. **p < 0.01; ***p < 0.001, CLA = 0.57, (H0:μfemale = μfemale); CLB(itch) = 0.39, CLB(pain) = 0.41 (H0:μ19-25 = μ36-45 and H0:μ26-35 = μ56-65, respectively).
Application of heat reduces itch and pain caused by bites and stings of all insects investigated
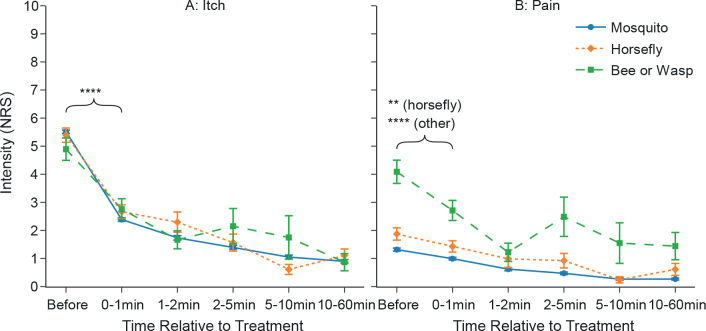
The application of heat to sites of insect bites or stings markedly reduced itch and pain compared with baseline, across all insects investigated. Participants were asked to rate itch and pain before and at various time points after heat application. Compared with the initial values, itch ratings for all insect species decreased significantly within the first minute after treatment (p < 0.0001 each). Itch was reduced by 57% for mosquito bites within the first minute after treatment and by 81% 5–10 min after treatment. Reductions were similar for horse fly bites, with 50% reduction in itch within the first minute and 89% reduction 5–10 min after treatment. Bee or wasp stings reached 44% and 64% itch reduction, respectively (Fig. 4A).
Fig. 4.
Application of concentrated heat reduces itch and pain following insect bites or stings. Data are presented as mean±standard error of the mean (SEM); multiple observations of the same participants were summarized using mean. Data shown as “0–1 min” includes all itch/pain-ratings ranging from directly after the treatment up to and including 1 min after the treatment. **p < 0.01; ****p < 0.0001; effect sizes CLitch: 0.87, 0.77, 0.69 for mosquito, horse fly, bee or wasp; CLpain: 0.53, 0.55, 0.62 (H0:μBefore ≤ μ0-1min). NRS: numerical rating scale.
Pain intensity was also reduced (p < 0.0001 regarding mosquito and bee or wasp stings and p < 0.01 regarding horse fly bites). Evaluating the same time slots as for itch, i.e. within the first minute and 5–10 min after the event, the reductions were 24% and 80% for mosquito bites, 22% and 87% for horse fly bites, and 34% and 62% for bee or wasp stings, respectively.
While intensity ratings for both itch and pain were continuously decreasing over time for mosquito bites, ratings of bee and wasp stings and horse fly bites also decreased, but showed some fluctuation over time (Fig. 4B).
Interestingly, itch reduction after mosquito bites did not depend on the time between the occurrence of the bite and the heat treatment. The reduction was as high for insect bites that occurred less than 5 min before treatment as it was for insect bites that occurred more than 6 h before treatment (Fig. S1).
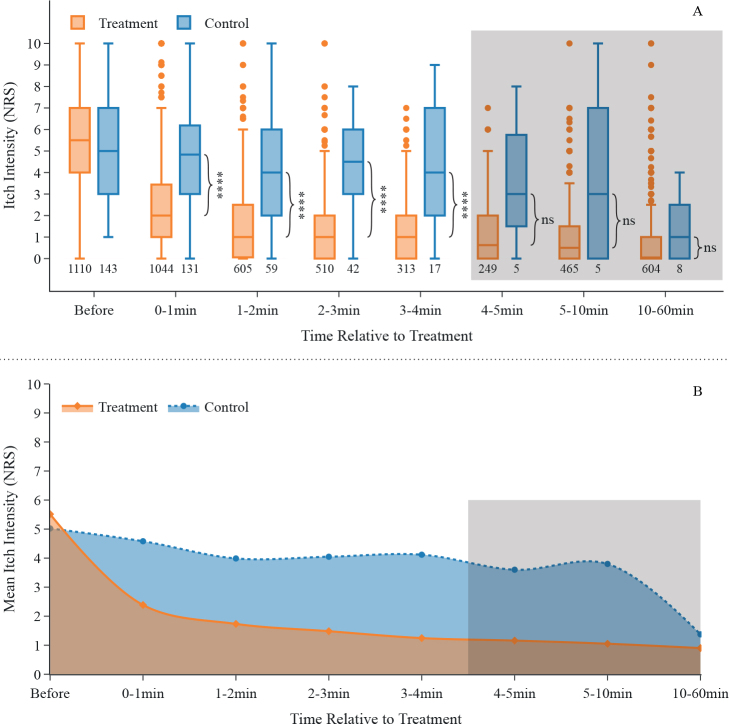
Application of heat results in a faster reduction in itch than in the control group
In approximately 5% of all cases, after a report of current itch and pain of the insect sting or bite was entered, participants were asked on a randomized basis to accept a delayed treatment (by approximately 10 min). Compared with this control group, participants who actively heat-treated their mosquito bite exhibited a 7.1-times greater decrease in pruritus after 1 min. Two min after heat application, the treatment group showed a 4.4-times greater itch-reduction, and 10 min after treatment itch was still reduced by a factor of 2.0 (Fig. 5A). This notable difference between the treatment and control group appears to diminish more than 10 min after treatment. This is in line with the general observation that a non-scratched mosquito bite will eventually stop itching. However, since the control group contains very little data from 4 min onwards, such conclusions cannot be drawn with certainty here. Overall, hyperthermia treatment resulted in a faster reduction and a shorter duration of itch compared with the control group without treatment (Fig. 5B).
Fig. 5.
Application of concentrated heat results in a faster reduction of itch following mosquito bites compared with non-treatment controls. (A) Data are presented as box and whiskers, shown with median, upper and lower quartile, whiskers 1.5 interquartile range; multiple observations of same participants were summarized using mean. Data shown as “0–1 min” includes all itch-ratings ranging from directly after the treatment up to and including 1 min after the treatment. Numbers below the boxes depict numbers of participants in the corresponding group. The last 3 time-bins are greyed out as the control group contains very little data. ****p < 0.0001; ns: p>0.05; effect sizes CL for each time bin, starting with 0–1 min: 0.89, 0.87, 0.80, 0.81, 0.66, 0.70, 0.62 (H0:∆treatment ≤ ∆control for each time group with ∆: reduction of itch intensity compared with before). (B) Area under the curve-plot showing reduction of itch over time for treatment- and control group (itch intensity displayed as mean); multiple observations of same participants were summarized using mean. The last 3 time-bins are greyed out as the control group contains very little data. NRS: numerical rating scale.
Using controlled hyperthermia is safe
Each time the participants were asked to report itch and pain ratings, they were offered the opportunity to indicate side-effects that may have occurred due to the treatment with the device. Across all 48,712 sub-questionnaire data-points (including implausible data), 64 adverse events (0.13%) were documented, of which 58 were linked to reactions to insect bites and/or treatment with the heating device (i.e. sensation of heat, redness of skin, swelling), 2 were not categorizable, and 4 were reports of “extraordinary heat”, which were categorized as potential treatment-associated adverse effects (Table SI).
Those events were carefully followed up to ensure that participants were not harmed by the treatment. To this end, this study reviewed the automatically generated treatment curves of the 4 devices, which all showed standard behaviour of the heating controller with no intervention from the temperature safety switch that triggers at temperatures above 55°C. In addition, the study checked whether the 4 users reported the events via the dedicated “contact” section within the app, which none of them did. Finally, their future treatment behaviour was monitored, and all 4 participants performed 10 or more treatments in the month following the adverse event, indicating that it was not considered severe.
DISCUSSION
This is the first published controlled real-world study of the use of concentrated heat to alleviate itch induced by insect bites or stings. The results demonstrate a significant reduction in itch and pain using local heat application after insect bites or stings, based on data derived from a large data-set with more than 12,000 registered treatments from more than 1,700 individuals.
In the summer months especially, pruritus caused by insect bites and stings can lead to a significant impairment in well-being. Many different methods for itch relief after mosquito bites are described and applied, most of them based either on temperature effects or pain stimuli. For example, the application of cooling saliva, cold from cold objects or ice cubes, or a pain stimulus directly next to the insect bite are recommended as home remedies (20). The targeted application of concentrated heat is also thought to relieve itching by activating heat receptors and inducing a pain stimulus. The use of a heat applicator was reported earlier in an open cohort study at German public bathing lakes (15). This showed that the application of heat resulted in relief of itch, but no control group was included. In experimental studies on the reduction of itch by heat, 2 research groups could also show that the application of targeted and concentrated heat leads to a reduction in histamine- and non-histamine-mediated itch (14, 21). These earlier findings are in line with and support the current results.
The current study was not aimed at the identification and characterization of the underlying mechanisms of action of heat-mediated itch relief. The effects are probably explained, at least in part, by the activation of transient receptor potential cation channel subfamily V member 1 (TRPV1) via C-mechano-insensitive nerve fibres (10, 14).
Limitations
Despite the large data-set and the inclusion of a control group, this study has some limitations. To motivate subjects to participate in the study and to maximize the number of documented treatments, the control group was limited to 5% of the overall treatments. Furthermore, the chosen design of the control group is not blinded. A blinded approach, using a lower temperature heat application, was considered prior to the study. As all participants included in the study were required to have a minimum of 10 treatments, the lower temperature is likely to be almost always identified as the control treatment, thus eliminating the effect of blinding. Furthermore, such an approach would apply only to participants who utilize heat applications with high temperatures, which introduces unwanted mixing of statistical effects. In total, a delayed treatment approach was considered to be superior to the described blinded approach, both in terms of user satisfaction and statistical clearness.
The 10-min delay in treatment in the control group may have caused participants to focus on the itch more than usual and therefore report higher scores. The data collected do not provide insight into the sustainability of the heat treatment beyond a time period of 1 h. Understanding if, and under what circumstances, itch or pain recur after a successfully treated insect bite may be the subject of further research.
Conclusion and future research
This study shows that concentrated heat induced by a heating device reduced pruritus caused by insect bites and stings, even if the insect bites occurred more than 6 h previously. Across more than 12,000 treatments registered, less than 1% of participants reported side-effects. Further investigation of these side-effects showed that none posed a serious risk to the participant.
As has been described by others previously, sensitivity to heat can vary between individuals as well as between body sites (22, 23). Furthermore, the required conditions of noxious heat counter-stimuli (treatment temperature and time) and its influencing factors for achieving an optimized reduction in itch and pain are not understood in detail. The data collected within this study allow for investigation of correlations between treatment settings chosen by users, demographics, individual heat sensitivity, body site and efficacy of treatment.
The data presented here indicate that the combination of the medical device heat it® and the corresponding smartphone app creates a novel means of data generation in real-world settings. The design of the study is highly customizable via the app interface, as is the variety of data collected. Collection of location data for real-time mosquito hotspot maps (a topic that others are working on intensively (24)) is feasible, as is detailed photographic analysis of insect species or symptoms. The large number of users of the device (approximately 250,000 as of December 2022) enables the collection of extensive data-sets, expanding the possibilities of future research.
Supplementary Material
ACKNOWLEDGEMENTS
This study was funded by Kamedi.
Conflicts of interest
MMe is, or recently was, a speaker and/or advisor for and/or has received research funding from AbbVie, Amgen, ArgenX, AstraZeneca, Bayer, Celldex, Celgene, Escient, Galderma, Grünenthal, GSK, Menlo, Novartis, Pfizer, Pharvaris, Regeneron, Roche, Sanofi-Aventis, Teva, Third Harmonic Bio and Viforpharma. CR and LL are co-founders, shareholders and managing directors of Kamedi GmbH. MElberskirch is an employee of Kamedi GmbH. MMa is, or recently was, a speaker and/or advisor for and/or has received research funding from Allakos, Amgen, Aralez, ArgenX, AstraZeneca, Celldex, Centogene, CSL Behring, FAES, Genentech, GIInnovation, GSK, Innate Pharma, Kyowa Kirin, Leo Pharma, Lilly, Menarini, Moxie, Novartis, Pfizer, Roche, Sanofi/Regeneron, Third Harmonic Bio, UCB, and Uriach.
REFERENCES
- 1.Cerpes U, Repelnig ML, Legat FJ. Itch in Hymenoptera sting reactions. Front Allergy 2021; 2: 727776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stibich AS, Carbonaro PA, Schwartz RA. Insect bite reactions: an update. Dermatology 2001; 202: 193–197. [DOI] [PubMed] [Google Scholar]
- 3.Brehler R. Insekten und Spinnentiere als Auslöser toxischer und allergischer Reaktionen in Deutschland. Allergo J 2017; 26: 31–40. [Google Scholar]
- 4.Demain JG. Hymenoptera allergy and anaphylaxis: are warmer temperatures changing the impact? Curr Opin Allergy Clin Immunol 2020; 20: 438–444. [DOI] [PubMed] [Google Scholar]
- 5.Demain JG. Insect migration and changes in venom allergy due to climate change. Immunol Allergy Clin North Am 2021; 41: 85–95. [DOI] [PubMed] [Google Scholar]
- 6.Przybilla B, Ruëff F. Insect Stings. Deutsches Ärzteblatt International. 2012. [cited 2022 Dec 5]. Available from: https://www.aerzteblatt.de/10.3238/arztebl.2012.0238. [DOI] [PMC free article] [PubMed]
- 7.Metz M, Piliponsky AM, Chen CC, Lammel V, Åbrink M, Pejler G, et al. Mast cells can enhance resistance to snake and honeybee venoms. Science 2006; 313: 526–530. [DOI] [PubMed] [Google Scholar]
- 8.Shi N, Szanto TG, He J, Schroeder CI, Walker AA, Deuis JR, et al. Venom composition and pain-causing toxins of the Australian great carpenter bee Xylocopa aruana. Sci Rep 2022; 12: 22168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Du YR, Xiao Y, Lu ZM, Ding J, Xie F, Fu H, et al. Melittin activates TRPV1 receptors in primary nociceptive sensory neurons via the phospholipase A2 cascade pathways. Biochem Biophys Res Commun 2011; 408: 32–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Andersen HH, Elberling J, Lo Vecchio S, Arendt-Nielsen L. Topography of itch: evidence of distinct coding for pruriception in the trigeminal nerve. Itch (Phila) 2017; 2: e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen XJ, Sun YG. Central circuit mechanisms of itch. Nat Commun 2020; 11: 3052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dong X, Dong X. Peripheral and central mechanisms of itch. Neuron 2018; 98: 482–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yosipovitch G, Fast K, Bernhard JD. Noxious heat and scratching decrease histamine-induced itch and skin blood flow. J Invest Dermatol 2005; 125: 1268–1272. [DOI] [PubMed] [Google Scholar]
- 14.Riccio D, Andersen HH, Arendt-Nielsen L. Antipruritic effects of transient heat stimulation on histaminergic and nonhistaminergic itch. Br J Dermatol 2019; 181: 786–795. [DOI] [PubMed] [Google Scholar]
- 15.Mueller C, Grossjohann, Fischer. The use of concentrated heat after insect bites/stings as an alternative to reduce swelling, pain, and pruritus: an open cohort-study at German beaches and bathing-lakes. Clin Cosmet Investig Dermatol 2011; 4: 191–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Seda J, Horrall S. Mosquito Bites. In: StatPearls. Treasure Island (FL: ): StatPearls Publishing; 2022. [cited 2023 Mar 7]. Available from: http://www.ncbi.nlm.nih.gov/books/NBK539915/ [PubMed] [Google Scholar]
- 17.Virtanen P, Gommers R, Oliphant TE, Haberland M, Reddy T, Cournapeau D, et al. SciPy 1.0: Fundamental algorithms for scientific computing in python. Nature Methods 2020; 17: 261–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vargha A, Delaney HD, Vargha A. A Critique and Improvement of the “CL” Common Language Effect Size Statistics of McGraw and Wong. J Educat Behav Stats 2000; 25: 101. [Google Scholar]
- 19.Vallat R. Pingouin: statistics in Python. J Open Source Software 2018; 3: 1026. [Google Scholar]
- 20.Urban & Vogel . Was bei quälenden Mückenstichen hilft. MMW – Fortschritte der Medizin 2015; 157: 7. [DOI] [PubMed] [Google Scholar]
- 21.Yosipovitch G, Duque MI, Fast K, Dawn AG, Coghill RC. Scratching and noxious heat stimuli inhibit itch in humans: a psychophysical study. Br J Dermatol 2007; 156: 629–634. [DOI] [PubMed] [Google Scholar]
- 22.Stevens JC, Choo KK. Temperature sensitivity of the body surface over the life span. Somatosens Mot Res 1998; 15: 13–28. [DOI] [PubMed] [Google Scholar]
- 23.Jankowski KS. Morning types are less sensitive to pain than evening types all day long: chronotype and pain. Eur J Pain 2013; 17: 1068–1073. [DOI] [PubMed] [Google Scholar]
- 24.Juzniz-Zonta Z, Sanpera-Calbet I, Eritja R, Palmer J, Escobar A, Garriga J, et al. Mosquito alert: leveraging citizen science to create a GBIF mosquito occurrence dataset. Gigabyte 2022; 2022: gigabyte54. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.