Abstract
Dietary restriction is known to promote longevity in a variety of eukaryotic organisms. Most studies of dietary restriction have been performed on animals bred for many generations under conditions that differ substantially from their natural environment, raising the possibility that some apparent beneficial effects of dietary restriction are due to adaptation to laboratory conditions. To address this question in an invertebrate model, we determined the effect of dietary restriction by bacterial deprivation on life span in five different wild-derived Caenorhabditis elegans strains and two strains of the related species Caenorhabditis remanei. Longevity was enhanced in each of the wild-derived C. elegans strains, in most cases to a degree similar to that observed in N2, the standard laboratory strain. Both strains of C. remanei were substantially longer lived any of the C. elegans isolates, produced larger brood sizes, and retained the ability to produce offspring for a longer period of time. Dietary restriction failed to increase mean life span in one C. remanei isolate, but significantly increased the maximum life span of both C. remanei strains. Thus, we find no evidence that adaptation to laboratory conditions has significantly altered the aging process in C. elegans under either standard or food-restricted conditions.
Keywords: Calorie restriction, Caloric restriction, Food, Aging, Longevity, Natural environment, Laboratory, Reproduction, Diet
1. Introduction
Dietary restriction (DR), defined as a decrease in food availability in the absence of malnutrition, extends life span in a variety of eukaryotic organisms including the budding yeast Saccharomyces cerevesiae (Jiang et al., 2000; Lin et al., 2000), the nematode Caenorhabditis elegans (Houthoofd et al., 2003; Kaeberlein et al., 2006; Lakowski and Hekimi, 1998; Lee et al., 2006), and rodents (Weindruch et al., 1986). DR is also known to slow many physiological aging processes and retard age-associated pathologies (Weindruch and Walford, 1988). The mechanism(s) by which DR acts to slow aging in laboratory animals is unknown, but may involve modulating the activity of evolutionarily conserved nutrient response pathways, such as those mediated by insulin/IGF-1-like signaling and the target of rapamycin (TOR) kinase (Kennedy et al., 2007).
Most studies involving DR have been performed using organisms bred for many generations under laboratory conditions characterized by an abundant food source, a lack of pathogens and predation, and low temperature variation (Pugh et al., 1999). Laboratory breeding protocols tend to favor individuals exhibiting rapid growth and early reproductive maturity (Harper et al., 2006). Studies comparing laboratory bred mouse strains to recently captured wild strains found that wild mice have less body mass and consume less food than those bred in the laboratory (Austad, 2001; Austad and Kristan, 2003; Bronson, 1984), suggesting that DR protocols may resemble natural feeding levels more closely than do laboratory control diets. Combined with evidence from both mice (Miller et al., 2002) and flies (Linnen et al., 2001; Sgró and Partridge, 2000) that laboratory strains have a reduced life span relative to wild strains, this raises the question of whether the beneficial effects of DR are artifacts of adaptation to a low-hazard, food-rich environment. In this scenario DR may simply return laboratory strains to an environment and life cycle more closely resembling those found in nature. A recent study using wild-derived mice (grandoffspring of mice captured in the wild) found that DR did not increase mean life span but did increase maximum life span of these animals (Harper et al., 2006).
To test the possibility that life span extension from DR in C. elegans is a laboratory artifact, we examined the effect of DR on longevity in wild-derived nematode populations. The natural food source of C. elegans is thought to be exclusively bacterial; however, the effect of dietary restriction in wild nematode populations is difficult to predict because little is known about food availability and feeding levels in the natural environment (Caswell-Chen et al., 2005; Hodgkin and Doniach, 1997; Jovelin et al., 2003). In the laboratory, nematodes are typically maintained on nutrient-agar nematode growth medium (NGM) with Escherichia coli OP50 at a cell density of ~109 cells/cm2 or greater, while natural food sources are presumably not as abundant as these standard laboratory conditions.
To accomplish DR in nematodes, we utilized a optimized reduced bacterial feeding protocol in which animals are maintained in the absence of bacterial food after the fourth day of adulthood; this DR method is hereafter referred to as bacterial deprivation (BD). BD provides maximum life span extension from food restriction under the standard laboratory conditions used in a majority of prior C. elegans studies (Kaeberlein et al., 2006; Lee et al., 2006). Genetic epistasis tests suggest that BD promotes longevity by a mechanism similar to previously described genetic models of DR, such as mutation of eat-2 (Hekimi et al., 1998). Specifically, BD increases life span (1) independently of the FOXO family transcription factor daf-16, (2) independently of the sirtuin sir-2.1, (3) additively with mutation of the insulin-like receptor daf-2, and (4) non-additively with mutation of eat-2 (Kaeberlein et al., 2006; Lee et al., 2006). Although animals subjected to BD are unable to feed on bacterial cells after the fourth day of adulthood, the extreme longevity and motility (foraging) of BD animals indicates that energy reserves are sufficient to maintain health and maximize longevity. Thus, BD meets the formal definition of DR, a reduction in food availability in the absence of malnutrition.
The effect of BD on survival was examined in five wild C. elegans isolates, and two different isolates of the related species, Caenorhabditis remanei. C. remanei is characterized by a female/male mating type, in contrast to C. elegans, which is primarily hermaphroditic (Baird et al., 1992; Brenner, 1974; Geldziler et al., 2006). To induce DR, we utilized a reduced bacterial feeding protocol (bacterial deprivation; BD) that has been shown to optimally increase life span under standard laboratory conditions (Kaeberlein et al., 2006; Lee et al., 2006). BD was found to significantly increase both mean and maximum life span in every strain examined, with the exception of one C. remanei isolate in which maximum, but not mean, life span was increased.
2. Materials and methods
All worm strains used in the study are listed in Table 1. Strains were provided by the Caenorhabditis Genetics Center or obtained from Thomas and propagated at 25 °C on solid nematode growth media (NGM) seeded with the E. coli strain OP50. E. coli OP50 cultures were seeded in liquid Luria broth (LB) from a single bacteria colony, grown overnight at 37 °C, and then concentrated fivefold. Control plates (10 ml NGM agar in 50 mm diameter Petri dishes) were seeded with 100 μl of the concentrated OP50 stock and allowed to dry overnight. The dry bacterial food source was then killed by exposure to a UV-source. Ampicillin was added to the NGM prior to pouring the plates at a concentration of 100 μM to ensure all bacteria were killed and to inhibit contamination by foreign bacteria. Unseeded plates used for BD experiments were also treated with UV and Ampicillin.
Table 1.
Nematode strains used in this study
| Strain | Description | Source |
|---|---|---|
| N2 | Standard C. elegans laboratory strain. | J. Thomas |
| JU262 | C. elegans wild isolate from Le Blanc (Indre, France). Isolated from a vegetable garden garbage pile by M. Felix. Weak Phm phenotype | CGC |
| MY2 | C. elegans wild isolate obtained by H. Schulenburg from compost heap in Roxel, Mnster, North-West Germany. Frozen within 5 generations after isolation; microsatellite genotype EU2. | CGC |
| PS2025 | C. elegans wild isolate. Isolated by J. Demodena from a garden in Altadena, CA. | CGC |
| PX176 | C. elegans wild isolate. Isolated by B. White from compost in Eugene, OR. | CGC |
| PX178 | C. elegans wild isolate. Isolated by B. White from organic soil in Eugene, OR. | CGC |
| EM464 | C. remanei ssp. vulgaris | J. Thomas |
| SB146 | C. remanei ssp. remanei | J. Thomas |
All strains were obtained from the Caenorhabditis Genetics Center (CGC) or were provided by J. Thomas (University of Washington, Seattle).
To initiate life span experiments, 15 hermaphrodites (C. elegans) or 15 females and 5 males (C. remanei) per plate were transferred to NGM + UV-killed E. coli OP50, allowed to lay eggs for 6 h, and then removed. The resulting synchronized population was allowed to reach L4, at which point approximately hermaphrodites (C. elegans) or females (C. remanei) were transferred to NGM + UV-killed OP50 supplemented 50 μM 5-fluorodeoxyuridine (FUDR). Although developmental timing was not quantified, no significant difference in the time from egg to L4 was noted among any of the C. elegans or C. remanei strains examined. On day 4 of adulthood, approximately 1/3 of the cohort was transferred to NGM + UV-killed OP50 + 50 μM FUDR (CF = control fed media) and the remainder were transferred to NGM + 50 μM FUDR (BD media). Animals on CF media were transferred to fresh plates as needed to renew the bacterial food source. Each animal was scored every 2–3 days for movement in response to gentle prodding to determine viability. Animals that crawled off of, or burrowed into, the NGM and did not return to the surface were not included in the data set. Control fed and BD life span analysis was repeated at least twice for each wild-derived nematode strain, with similar results obtained in independent replicate experiments.
The Wilcoxon rank sum test (MATLAB RANKSUM function) was used to determine statistical significance and calculate P-values. Maximum life span was determined by taking the mean age at death of the longest-lived 10% of a given test population.
Brood size assays were initiated in the same manner described above for life span. For C. elegans strains, when the synchronized population reached L4, 10 hermaphrodites were transferred to NGM + UV-killed OP50 (1 animal per plate). For C. remanei, 10 females and 30 males were transferred to NGM + UV-killed OP50 (1 female, 3 males per plate). Each day all worms were transferred to fresh plates. Old plates were stored at 25 °C for 2 days and then scored for number of animals present. Transfers and scoring continued until all worms from a given strain failed to produce progeny for two consecutive days. Animals that crawled off of or burrowed into the NGM agar and did not return to the surface were not included in the data set.
3. Results
Life span was measured for five wild-derived isolates of C. elegans and two strains of C. remanei on control fed (CF) and BD regimens and compared to the most commonly used laboratory strain of C. elegans, N2. Subjecting worms to BD extended both mean and maximum life span relative to CF in all cases, except for the mean life span of the C. remanei strain SB146, which is addressed below (Table 2).
Table 2.
Survival statistics for N2, wild C. elegans strains, and C. remanei strains
| Species | Strain | Brood size | P-value | CF |
BD |
Max LS ratio (BD/CF) | Mean LS ratio (BD/CF) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Max LS | Mean LS | SE | N | Max LS | Mean LS | SE | N | ||||||
| C. elegans | JU262 | 230 ± 24 | 1.7E–10 | 43.0 | 28.6 | 0.92 | 74 | 48.6 | 37.1 | 0.72 | 76 | 1.13 | 1.30 |
| MY2 | 184 ± 7 | 6.7E–16 | 33.1 | 26.1 | 0.40 | 106 | 45.9 | 36.1 | 0.95 | 71 | 1.39 | 1.38 | |
| N2 | 290 ± 10 | 7.8E–23 | 32.8 | 24.5 | 0.38 | 143 | 42.6 | 31.8 | 0.45 | 250 | 1.30 | 1.30 | |
| PS2025 | 210 ± 14 | 0.000 | 36.1 | 28.1 | 0.38 | 143 | 55.0 | 42.6 | 0.87 | 81 | 1.52 | 1.51 | |
| PX176 | 192 ± 7 | 3.8E–23 | 33.7 | 23.7 | 0.52 | 91 | 44.6 | 34.8 | 0.61 | 127 | 1.32 | 1.47 | |
| PX178 | 291 ± 9 | 1.7E–09 | 43.9 | 29.6 | 0.60 | 124 | 47.4 | 36.4 | 0.90 | 76 | 1.08 | 1.23 | |
| C. remanei | EM464f | 469 ± 66 | 1.6E–06 | 70.8 | 44.5 | 2.15 | 44 | 98.0 | 62.7 | 2.76 | 59 | 1.38 | 1.41 |
| SB146f | 409 ± 80 | 0.727 | 47.7 | 37.2 | 1.26 | 30 | 66.3 | 38.7 | 2.83 | 29 | 1.39 | 1.04 | |
All life span (LS) data is given in days from the time of hatching. Maximum life span (Max LS) refers to the mean of the longest-lived 10% of each test group. Brood size indicates average total number of offspring produced per individual adult hermaphrodite (C. elegans) or female (C. remanei) and is shown as average ± standard error. P-value is the result of a Wilcoxon Rank-Sum test of control fed versus BD survival for each strain.
Mean and maximum life span was observed to vary within both species. Several wild C. elegans strains displayed life span characteristics similar to N2 (Fig. 1A). Strain PX176 was particularly comparable to N2, having similar mean and maximum life span under CF and BD conditions. MY2 and N2 animals had similar mean and maximum life span on the CF diet and similar maximum life span on the BD diet, but MY2 exhibited a longer mean life span than N2 in response to BD. Three wild C. elegans strains, JU262, PX178 and PS2025, were long-lived relative to N2 both on the control diet and when subjected to BD (Fig. 1B). Both JU262 and PX178 exhibited a relatively smaller extension of maximum life span by BD (8% and 13%, respectively) compared to the rest of the C. elegans strains tested (averaging 29% extension), while mean life span extension by BD was comparable. Only two strains of C. remanei were tested, and while both were long lived relative to any of the C. elegans strains, EM464 was also significantly longer lived than SB146 (Fig. 2).
Fig. 1.
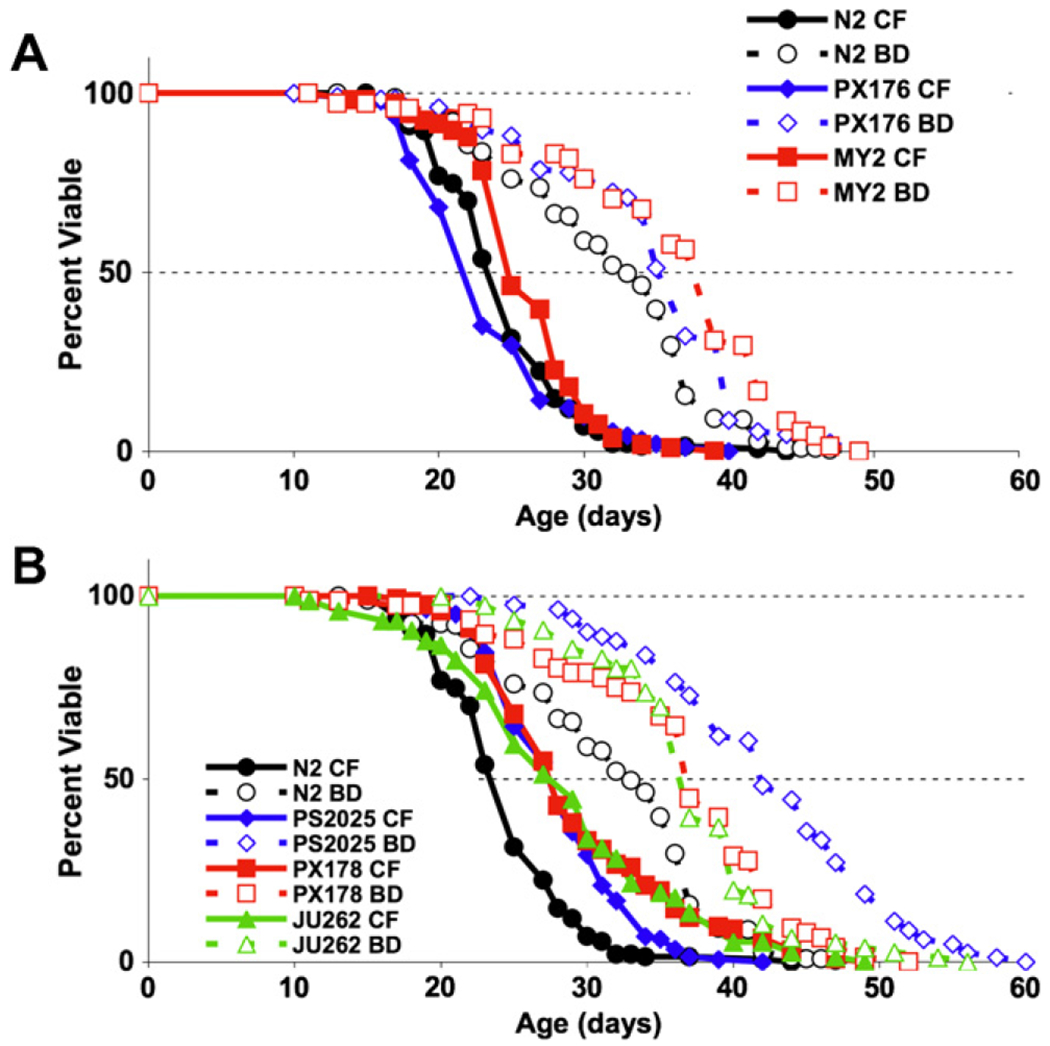
BD extends life span in wild-derived C. elegans strains. (A) Strains MY2 and PX176 exhibit longevity comparable to N2 and significant life span extension in response to BD. (B) BD increases the life spans of strains JU262, PS2025, and PX178, which are longer-lived than N2 on both CF and BD diets.
Fig. 2.

BD increases maximum life span in two C. remanei strains. C. remanei strains EM464 and SB146 are long lived relative to C. elegans N2. EM464 exhibits significant mean and maximum life span extension by BD. BD does not extend mean life span of SB146, but does increase maximum life span.
A substantial strain-dependent variation in the degree of life span extension by BD was observed among the different C. elegans strains tested. The extension of mean life span in the BD experimental groups relative to control groups ranged from 23% for strain PX178 to 51% for strain PS2025, while the extension of maximum life span ranged from 8% for strain PX178 to 52% for strain PS2025 (Table 2). The average extension of mean life span is 37 ± 10% and the average extension of maximum life span is 29 ± 15%. For the two C. remanei strains, maximum life span was extended by a similar degree while the mean life span extension was quite different. Strain EM464 exhibited a robust mean life span extension of 41% on BD relative to CF. In contrast, mean life span of strain SB146 was not significantly extended by BD.
Reproductive capacity and longevity have been linked in several different organisms, with reduced fecundity often, but not always, showing an inverse correlation with life span (Mukhopadhyay and Tissenbaum, 2007; Partridge et al., 2005). We therefore quantified the brood size for each strain to determine whether a correlation between reproduction and longevity could be discerned (Table 2). C. elegans strains MY2 and PX176 – those with similar life span characteristics to N2 – both displayed a substantially smaller brood size than N2 (63% and 66% of N2 brood size, respectively, Fig. 3A). Of the C. elegans strains that were longer lived than N2, PX178 had a nearly identical brood size, while those of JU262 and PS2025 were some-what smaller (79% and 72% of N2 brood size, respectively, Fig. 3B). All three long-lived wild strains displayed an earlier reproductive peak relative to N2. Both C. remanei strains produced larger broods and remained reproductively active longer than the C. elegans strains (Fig. 3C).
Fig. 3.
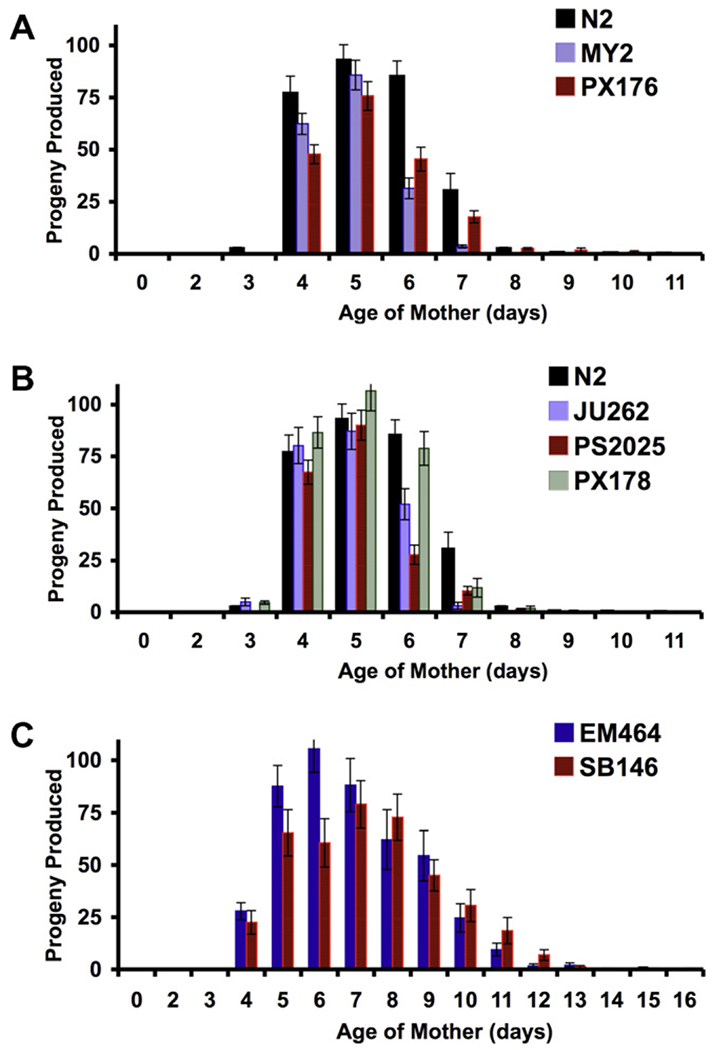
Brood size analysis of wild-derived C. elegans and C remanei strains Number of progeny produced per animal was determined for C. elegans N2 and compared to wild-derived C. elegans isolates (A) MY2 and PX176, (B) JU262, PS2025, and PX178. (C) Number of progeny produced per animal was determined for C. remanei strains EM464 and SB146. Data shown is the mean number of progeny produced for 10 animals. Error bars are standard error of the mean.
4. Discussion
The data presented here demonstrate that DR by bacterial food deprivation extends life span in several wild C. elegans populations obtained from different locations around the world. In all five cases, wild-derived C. elegans exhibited significantly longer life spans when subject to BD. The survival parameters of the different strains varied substantially, but no systematic difference between wild-derived and laboratory-maintained C. elegans was observed. Thus, we find no evidence that adaptation to the laboratory has altered either the survival of C. elegans under standard conditions or the response of C. elegans to food restriction.
The longevity-enhancing effects of BD are also conserved among at least one related nematode species, C. remanei. Strain EM464 shows robust life span extension via BD. Although BD did not significantly increase mean life span in a second C. remanei strain, SB146, maximum life span was significantly increased. The failure of BD to increase mean life span in SB146 is reminiscent of the effect of DR observed in one strain of wild-derived mice (Harper et al., 2006). Analysis of additional C. remanei isolates will be necessary to determine whether EM464 or SB146 represent a typical response to BD for this species.
It is noteworthy that both C. remanei strains examined displayed significantly greater longevity than any of the C. elegans strains. Unlike C. elegans, C. remanei are not hermaphroditic. This difference makes comparative analysis of the brood size experiments presented here difficult, as the C. remanei females were maintained in the presence of males, while C. elegans hermaphrodites were not allowed to mate with males. The life span data, however, was derived solely from female C. remanei maintained in the absence of male animals during adulthood and is directly comparable to the C. elegans life span data. We attempted to measure the response of C. remanei males to BD, but were unable to obtain a sufficient cohort due to the loss of nearly 100% of the animals to foraging up the sides of the Petri dishes, which resulted in desiccation and death.
Both C. elegans and C. remanei showed substantial variations in longevity on a CF diet and in their response to BD. This variation could have many possible roots and should be amenable to further study. There are a number genetic and environmental factors known to modulate nematode life span, including insulin/IGF-1 signaling, mitochondrial function, mRNA translation, and induction of stress response genes. Further exploration of the relative importance of each of these pathways in determining the longevity of different C. elegans isolates would be of interest.
Although laboratory adaptation in C. elegans does not appear to be a significant confounding factor for studies of basic mechanisms of aging in this organism, our data should not be interpreted to demonstrate that life span extension from DR is not specific to laboratory conditions. Particularly in the case of C. elegans, it seems likely that the natural environment experienced by this organism could often be more similar to BD conditions, compared to the extreme abundance of food and nutrients provided under standard laboratory conditions. Further complicating this issue, many C. elegans in the natural environment exist as dauer-arrested larvae, rather than reproductive adults. In the laboratory, animals are able to thrive in food-deprived conditions because they are provided with 5-fluorodeoxyuridine (FUdR), a drug commonly used in longevity studies to prevent eggs from hatching. If FUdR is not provided (such as in the natural environment), then hermaphrodites will die rapidly from internal hatching of eggs, a known starvation response. Additional environmental variables, such as temperature and the presence of pathogenic bacteria or fungi, are also absent in the laboratory and may influence the interplay between nutrient availability and aging.
In summary, the ability of DR to increase life span in some organisms may be influenced by inadvertent selection for growth under laboratory conditions, but in nematodes it appears to be a trait of wild-derived populations. Future studies will be required to address the possibility that nutrient-rich laboratory environments accelerate aging, and DR represents a return to more natural conditions that are compatible with longer life spans. As DR is among the most widely studied and best characterized longevity interventions, efforts to address the relevance of DR in natural populations are of interest.
Acknowledgements
Some strains used in this work were provided by the Caenorhabditis Genetics Center, which is funded by the NIH National Center for Research Resources. We also thank J. Thomas for providing additional strains. We thank K. Steinkraus for helpful discussion of this manuscript. This work was supported by an AFAR Research Grant to M.K.
References
- Austad SN, 2001. Does caloric restriction in the laboratory simply prevent overfeeding and return house mice to their natural level of food intake? SAGE KE; 6. [DOI] [PubMed] [Google Scholar]
- Austad SN, Kristan DM, 2003. Are mice calorically restricted in nature? Aging Cell 2, 201–207. [DOI] [PubMed] [Google Scholar]
- Baird SE, Sutherlin ME, Emmons SW, 1992. Reproductive isolation in Rhabditidae (Nematoda: Secernentea); mechanisms that isolate Six Speciew of three genera. Evolution 46, 585–594. [DOI] [PubMed] [Google Scholar]
- Brenner S, 1974. The genetics of Caenorhabditis elegans. Genetics 77, 71–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronson FH, 1984. Energy allocation and reproduction development in wild and domestic house mice. Biology of Reproduction 31, 83–88. [DOI] [PubMed] [Google Scholar]
- Caswell-Chen EP, Chen J, Lewis EE, Douhan GW, Nadler SA, Carey JR, 2005. Revising the standard wisdom of C. elegans natural history: ecology of longevity. SAGE KE 2005, p. 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geldziler B, Chatterjee I, Kadandale P, Putiri E, Patel R, Singson A, 2006. A comparative study of sperm morphology, cytology and activation in Caenorhabditis elegans, Caenorhabditis remanei and Caenorhabditis briggsae. Development Genes and Evolution 216, 198–208. [DOI] [PubMed] [Google Scholar]
- Harper JM, Leathers CW, Austad SN, 2006. Does caloric restriction extend life in wild mice? Aging Cell 5, 441–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hekimi S, Lakowski B, Barnes TM, Ewbank JJ, 1998. Molecular genetics of life span in C. elegans: how much does it teach us? Trends in Genetics 14, 14–20. [DOI] [PubMed] [Google Scholar]
- Hodgkin J, Doniach T, 1997. Natural variation and copulatory plug formation in Caenorhabditis elegans. Genetics 146, 149–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houthoofd K, Braeckman BP, Johnson TE, Vanfleteren JR, 2003. Life extension via dietary restriction is independent of the Ins/IGF-1 signaling pathway in Caenorhabditis elegans. Experimental Gerontology 38, 947–954. [DOI] [PubMed] [Google Scholar]
- Jiang JC, Jaruga E, Repnevskaya MV, Jazwinski SM, 2000. An intervention resembling caloric restriction prolongs life span and retards aging in yeast. The FASEB Journal 14, 2135–2137. [DOI] [PubMed] [Google Scholar]
- Jovelin R, Ajie BC, Phillips PC, 2003. Molecular evolution and quantitative variation for chemosensory behaviour in the nematode genus Caenorhabditis. Molecular Ecology 12, 1325–1337. [DOI] [PubMed] [Google Scholar]
- Kaeberlein TL, Smith ED, Tsuchiya M, Welton KL, Thomas JH, Fields S, Kennedy BK, Kaeberlein M, 2006. Lifespan extension in Caenorhabditis elegans by complete removal of food. Aging Cell 5, 487–494. [DOI] [PubMed] [Google Scholar]
- Kennedy BK, Steffen KK, Kaeberlein M, 2007. Ruminations on dietary restriction and aging. Cellular and Molecular Life Sciences 64, 1323–1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakowski B, Hekimi S, 1998. The genetics of caloric restriction in Caenorhabditis elegans. Proceedings of the National Academy of Sciences 95, 13091–13096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee GD, Wilson MA, Zhu M, Wolkow CA, Cabo R.d., Ingram DK, Zou S, 2006. Dietary deprivation extends lifespan in Caenorhabditis elegans. Aging Cell 5, 515–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin SJ, Defossez PA, Guarente L, 2000. Requirement of NAD and SIR2 for life-span extension by calorie restriction in Saccharomyces cerevisiae. Science 289, 2126–2128. [DOI] [PubMed] [Google Scholar]
- Linnen C, Tatar M, Promislow D, 2001. Cultural artifacts: a comparison of senescence in natural, laboratory-adapted and artificially selected lines of Drosphila melanogastar. Evolutionary Ecology Research 3, 877–888. [Google Scholar]
- Miller RA, Harper JM, Dysko RC, Durkee SJ, Austad SN, 2002. Longer life spans and delayed maturation in wild-derived mice. Experimental Biology and Medicine 227, 500–508. [DOI] [PubMed] [Google Scholar]
- Mukhopadhyay A, Tissenbaum HA, 2007. Reproduction and longevity: secrets revealed by C. elegans. Trends in Cell Biology 17, 65–71. [DOI] [PubMed] [Google Scholar]
- Partridge L, Gems D, Withers DJ, 2005. Sex and death: what is the connection? Cell 120, 461–472. [DOI] [PubMed] [Google Scholar]
- Pugh TD, Klopp RG, Weindruch R, 1999. Controlling caloric consumption: protocols for rodents and rhesus monkeys. Neurobiology of Aging 20, 157–165. [DOI] [PubMed] [Google Scholar]
- Sgró CM, Partridge L, 2000. Evolutionary responses of the life history of wild-caught Drosophila melanogaster to two standard methods of laboratory culture. The American Naturalist 156, 341–353. [Google Scholar]
- Weindruch R, Walford RL, 1988. The Retardation of Aging and Disease by Dietary Restriction. Charles C. Thomas, Springfield, IL. [Google Scholar]
- Weindruch R, Walford RL, Fligiel S, Guthrie D, 1986. The retardation of aging in mice by dietary restriction: longevity, cancer, immunity and lifetime energy intake. The Journal of Nutrition 116, 641–654. [DOI] [PubMed] [Google Scholar]


