Introduction:
Mastocytosis, a clonal proliferation of mast cells commonly involving the skin and bone marrow, has a varied clinical presentation ranging from cutaneous lesions to systemic disease. Cutaneous mastocytosis is managed symptomatically, but systemic mastocytosis is treated with targeted therapy against the mutated receptor tyrosine kinase c-KIT, the pathogenic driver of mastocytosis. However, there are no guidelines for the treatment of cutaneous mastocytosis refractory to symptomatic management.
We herein report a method to select genetically informed therapy for symptomatic and recalcitrant cutaneous mastocytosis.
Case presentation:
We performed a mutational analysis of dermal mast cells after enrichment by laser capture in a 23-year-old woman with recalcitrant cutaneous mastocytosis. The analysis revealed a aspartic acid to valine substitution at codon 816 (D816V) mutation in the protein c-KIT. Based on these results, we initiated treatment with the multi-kinase/KIT inhibitor midostaurin, a treatment effective against the D816V c-KIT mutation. After 3 months of treatment, the patient exhibited a reduction in the number and size of cutaneous lesions and reported resolution of pruritus and decreased severity of other mast cell-related symptoms.
Discussion:
The treatment of mastocytosis relies heavily on whether the disease is limited to the skin or systemic. However, there are no guidelines for cutaneous mastocytosis that does not respond to symptomatic management. In the present report describing a patient with recalcitrant cutaneous mastocytosis, we describe a strategy in which skin mutational analysis is used to guide the selection of targeted therapy.
Conclusion:
Performing mast cell mutational analyses in the skin provides a means to select targeted therapy for symptomatic and refractory cutaneous mastocytosis.
Keywords: mastocytosis, c-KIT, molecular genetics, targeted therapy, midostaurin
Introduction
Mastocytosis is characterized by a clonal proliferation of mast cells in one or more organ systems and commonly involves the skin or bone marrow.1 The clinical presentation ranges from skin-limited disease to systemic involvement of the bone marrow or other organs.2 Distinguishing between cutaneous and systemic mastocytosis is essential for selection of an appropriate therapeutic approach. Although cutaneous mastocytosis is managed symptomatically, systemic mastocytosis is treated with targeted therapy.
The treatment goal for cutaneous mastocytosis is to control mast cell–related symptoms, including pruritus and flushing.3 The first-line therapy is first-generation and second-generation H1 antihistamines. If patients have refractory symptoms, the antihistamine dose may be increased or H2 antihistamines, oral glucocorticoids, leukotriene antagonists, or phototherapy may be utilized. If mast cell–related symptoms include gastrointestinal manifestations, such as abdominal pain, nausea, or diarrhea, proton pump inhibitors can be added to the treatment regimen.3
Targeted therapy directed toward the receptor tyrosine kinase c-KIT is commonly utilized for systemic mastocytosis because the constitutively activated c-KIT is the driver of pathogenesis in mastocytosis.1 The c-KIT gene product, also known as CD117 or mast cell growth factor receptor, is critical for the development and survival of mast cells. Activating mutations in the c-KIT gene leading to mastocytosis were originally described in 1996.4 With the advent of targeted therapies, imatinib was the first tyrosine kinase inhibitor approved for the treatment of aggressive systemic mastocytosis in adults.5 However, imatinib was found to be sensitive to only a few c-KIT mutations (F522C, V560G) and ineffective toward the most common c-KIT mutation in mastocytosis: aspartic acid to valine substitution at codon 816 (D816V).2,5
Targeted therapies against the D816V mutation did not become available until 2018, when midostaurin, an orally available multi-kinase/KIT inhibitor, was approved for the treatment of D816V-positive aggressive systemic mastocytosis, systemic mastocytosis with an associated hematological neoplasm, and mast cell leukemia.5 Although midostaurin proved effective in reducing cutaneous lesions and mast cell–related symptoms in patients with advanced systemic mastocytosis,6 its use for treatment of cutaneous mastocytosis has never been evaluated, even in patients with recalcitrant symptomatic disease who harbor the D816V mutation. We herein report the successful use of midostaurin for treatment of recalcitrant D816V-positive cutaneous mastocytosis.
Case presentation
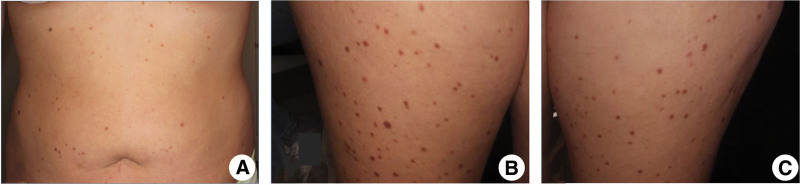
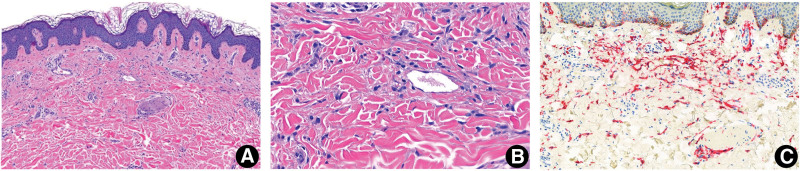
A 23-year-old Caucasian woman presented for evaluation of an 8-year history of multiple red-brown pruritic macules on her trunk and lower extremities (Fig. 1). She noted that the lesions had been previously diagnosed as moles. A review of systems was positive for 3 to 4 episodes of diarrhea daily with intermittent nausea and vomiting. A punch biopsy of a representative lesion on the thigh demonstrated an increased number of CD117-positive mast cells in the dermis (Fig. 2). Cutaneous mastocytosis was diagnosed, and a workup was initiated to rule out systemic involvement.
Figure 1.
Multiple erythematous pruritic reticular macules and patches on the patient’s trunk (A) and lower extremities (B and C) consistent with cutaneous mastocytosis.
Figure 2.
Histopathological features of a urticarial lesion from the patient with pecalcitrant cutaneous mastocytosis. (A and B) A urticarial lesion demonstrates an increased number of mast cells in the dermis (H&E: A, × 50; B, ×100). (C) CD117 detected by tyrosine protein kinase-KIT is positive (× 75).
Laboratory tests revealed a normal tryptase concentration, and the patient’s bone marrow biopsy displayed normal cellularity and absence of c-KIT mutation. Given the patient’s gastrointestinal symptoms, biopsies of the stomach, duodenum, and colon were performed, but examination of the specimens revealed no histologic evidence of mastocytosis. With these results, the patient did not meet the criteria for systemic mastocytosis.2 Diagnosis of cutaneous mastocytosis was confirmed, and the patient received symptomatic treatment with an H1 antihistamine and an H2 antihistamine. However, she continued to be symptomatic and developed new lesions. Multiple H1 antihistamines with dose escalation were utilized, and a leukotriene antagonist was added to the regimen.
Despite treatment escalation and the use of multiple treatment modalities, the patient’s symptoms and lesions did not improve. Because of her recalcitrant symptoms, we considered a treatment approach to directly eliminate mast cells with continuation of the symptomatic management. To identify the appropriate targeted therapy, we performed mutational analysis on the mast cells in the skin biopsy.7 To increase the yield of the skin biopsy, we enriched the mast cell isolation by a laser capture technique. The analysis revealed an A>T substitution at position 2447 on chromosome 4 that resulted in a missense D816V mutation in the protein c-KIT (COSMIC genomic mutation ID COSV55386424). Based on these results, we initiated treatment with midostaurin at 50 mg twice daily. After 1 month of treatment, the patient exhibited a reduction in the number and size of cutaneous lesions and reported resolution of pruritus and decreased severity of other mast cell-related symptoms. At the time of this writing, the patient had continued to tolerate midostaurin for 6 months.
Patient consent declaration
The patient gave the written informed consent about her case publication, including the images and other disease details.
Discussion
The treatment of mastocytosis relies heavily on whether the disease is limited to the skin or systemic. The criteria for cutaneous mastocytosis are the presence of typical small brown monomorphic skin lesions and an increased number of mast cells on histologic examination.8 Systemic disease is diagnosed when aggregates of atypical or spindle-shaped mast cells that express CD25 are found within the bone marrow or other extracutaneous organs. Additional markers of systemic disease include an elevated serum tryptase concentration and detection of c-KIT mutation in the bone marrow, blood, or other extracutaneous organs. As such, the workup for patients with mastocytosis should include measurement of the tryptase concentration, peripheral blood flow cytometry, skin biopsy, and bone marrow biopsy with associated molecular studies. Based on the results of our patient’s workup, we excluded a diagnosis of systemic mastocytosis. The tryptase concentration and peripheral blood flow cytometry results were unremarkable, and the bone marrow and gastrointestinal biopsies showed no mast cells and no c-KIT mutation. Overall, these results indicated that the disease was confined to the skin.
Although imatinib was utilized in one patient with cutaneous mastocytosis,9 our case provides evidence that refractory D816V-positive cutaneous mastocytosis can be treated with midostaurin. Furthermore, we have demonstrated a strategy in which skin mutational analysis is used to guide the selection of targeted therapy. Typically, c-KIT mutational analysis is performed in the bone marrow, and the results determine the choice of systemic therapy. In contrast, mutational analysis of the skin is not routinely performed for clinical use. Clinicians can request that pathologists perform laser capture of mast cells from skin biopsies to increase the DNA yield for analysis. Performing mast cell mutational analyses in the skin provides a means to select targeted therapy for symptomatic and refractory cutaneous mastocytosis. However, the main limitation of this study is that larger scale studies are required to assess the validity of genetically informed therapy for recalcitrant cutaneous mastocytosis and efficacy of midostaurin for D816V-positive cutaneous mastocytosis.
Source of funding
This work was supported by a grant from the National Cancer Institute, USA (No. R03CA252818 to NN).
References
- [1].Tzankov A, Duncavage E, Craig FE, et al. Mastocytosis. Am J Clin Pathol 2021;155(2):239–266. doi:10.1093/ajcp/aqaa183. [DOI] [PubMed] [Google Scholar]
- [2].Pardanani A. Systemic mastocytosis in adults: 2021 Update on diagnosis, risk stratification and management. Am J Hematol 2021;96(4):508–525. doi:10.1002/ajh.26118. [DOI] [PubMed] [Google Scholar]
- [3].Czarny J, Lange M, Ługowska-Umer H, et al. Cutaneous mastocytosis treatment: strategies, limitations and perspectives. Postepy Dermatol Alergol 2018;35(6):541–545. doi:10.5114/ada.2018.77605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Longley BJ, Tyrrell L, Lu SZ, et al. Somatic c-KIT activating mutation in urticaria pigmentosa and aggressive mastocytosis: establishment of clonality in a human mast cell neoplasm. Nat Genet 1996;12(3):312–314. doi:10.1038/ng0396-312. [DOI] [PubMed] [Google Scholar]
- [5].Kasamon YL, Ko CW, Subramaniam S, et al. FDA Approval Summary: midostaurin for the treatment of advanced systemic mastocytosis. Oncologist 2018;23(12):1511–1519. doi:10.1634/theoncologist.2018-0222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Hartmann K, Gotlib J, Akin C, et al. Midostaurin improves quality of life and mediator-related symptoms in advanced systemic mastocytosis. J Allergy Clin Immunol 2020;146(2):356–366.e4. doi:10.1016/j.jaci.2020.03.044. [DOI] [PubMed] [Google Scholar]
- [7].Bodemer C, Hermine O, Palmérini F, et al. Pediatric mastocytosis is a clonal disease associated with D816V and other activating c-KIT mutations. J Invest Dermatol 2010;130(3):804–815. doi:10.1038/jid.2009.281. [DOI] [PubMed] [Google Scholar]
- [8].Hartmann K, Escribano L, Grattan C, et al. Cutaneous manifestations in patients with mastocytosis: Consensus report of the European Competence Network on Mastocytosis; the American Academy of Allergy, Asthma & Immunology; and the European Academy of Allergology and Clinical Immunology. J Allergy Clin Immunol 2016;137(1):35–45. doi:10.1016/j.jaci.2015.08.034. [DOI] [PubMed] [Google Scholar]
- [9].Hoffmann KM, Moser A, Lohse P, et al. Successful treatment of progressive cutaneous mastocytosis with imatinib in a 2-year-old boy carrying a somatic KIT mutation. Blood 2008;112(5):1655–1657. doi:10.1182/blood-2008-03-147785. [DOI] [PubMed] [Google Scholar]