Objective
In AIDS Clinical Trials Group study A5375, a pharmacokinetic trial of levonorgestrel emergency contraception, double-dose levonorgestrel (3 mg, versus standard dose 1.5 mg) offset the induction effects of efavirenz or rifampin on plasma levonorgestrel exposure over 8 h post-dose (AUC0-8h). We characterized the pharmacogenetics of these interactions.
Methods
Cisgender women receiving efavirenz- or dolutegravir-based HIV therapy, or on isoniazid-rifampin for tuberculosis, were followed after a single oral dose of levonorgestrel. Linear regression models, adjusted for BMI and age, characterized associations of CYP2B6 and NAT2 genotypes (which affect plasma efavirenz and isoniazid exposure, respectively) with levonorgestrel pharmacokinetic parameters.
Results
Of 118 evaluable participants, 17 received efavirenz/levonorgestrel 1.5 mg, 35 efavirenz/levonorgestrel 3 mg, 34 isoniazid-rifampin/levonorgestrel 3 mg, and 32 (control group) dolutegravir/levonorgestrel 1.5 mg. There were 73 Black and 33 Asian participants. Regardless of genotype, women on efavirenz and isoniazid-rifampin had higher levonorgestrel clearance. In the efavirenz/levonorgestrel 3 mg group, CYP2B6 normal/intermediate metabolizers had levonorgestrel AUC0-8h values similar to controls, while CYP2B6 poor metabolizers had AUC0-8h values of 40% lower than controls. In the isoniazid-rifampin group, NAT2 rapid/intermediate acetylators had levonorgestrel AUC0-8h values similar to controls, while NAT2 slow acetylators had AUC0-8h values 36% higher than controls.
Conclusion
CYP2B6 poor metabolizer genotypes exacerbate the efavirenz-levonorgestrel interaction, likely by increased CYP3A induction with higher efavirenz exposure, making the interaction more difficult to overcome. NAT2 slow acetylator genotypes attenuate the rifampin-levonorgestrel interaction, likely by increased CYP3A inhibition with higher isoniazid exposure.
Keywords: CYP2B6, efavirenz, emergency contraception, HIV therapy, isoniazid, levonorgestrel, NAT2, pharmacogenetics, rifampin, tuberculosis
Introduction
Tuberculosis and HIV (HIV-1) are leading causes of infection-related deaths worldwide [1]. Women comprise the majority of persons with HIV worldwide [2]. Among women with HIV or tuberculosis, unintended pregnancies are associated with poor maternal and neonatal outcomes [3]. Emergency contraception is safe and effective when used as a single dose soon after sex [4], such that the WHO recommends access to emergency contraception, when needed, to prevent unintended pregnancy [5].
Isoniazid and rifampin are cornerstone medications for treating drug-sensitive tuberculosis, while efavirenz is recommended by the WHO for individuals of childbearing potential as an alternative to dolutegravir in first-line antiretroviral therapy (ART) [6]. Rifampin and efavirenz induce cytochrome (CYP) P450 enzymes that metabolize levonorgestrel [7,8], reducing plasma concentrations and compromising the efficacy of some hormonal contraceptives [9,10], and limiting family planning options. Isoniazid inhibits CYP3A4, CYP2A6, CYP1A2 and CYP2C19 [11], which may increase plasma exposure of drugs metabolized by these isoforms [12]. Dolutegravir is not expected to affect hormonal contraceptive plasma exposure.
Genetic polymorphisms predict increased plasma isoniazid and efavirenz exposure. Isoniazid is metabolized by N-acetyltransferase 2 (NAT2) [13], and NAT2 loss-of-function alleles are frequent [13–15]. One or two copies of such alleles confer intermediate or slow acetylator phenotypes, respectively, and progressively greater isoniazid exposure [13–15]. Increased plasma efavirenz exposure is predicted by frequent CYP2B6 polymorphisms [16].
Study A5375 of the AIDS Clinical Trials Group (ACTG) evaluated the pharmacokinetics and safety of dose- adjusted levonorgestrel emergency contraception in combination with efavirenz-based ART or rifampicin-containing tuberculosis therapy [17]. The trial showed that double-dose levonorgestrel (3 mg rather than standard 1.5 mg dose) effectively compensated for the lowering effects of efavirenz and rifampin-isoniazid on plasma levonorgestrel exposure over 8 h post-dose (AUC0-8h). The present analyses examined whether genetic polymorphisms that impact plasma efavirenz and isoniazid exposure affect drug-drug interactions following an oral dose of levonorgestrel.
Methods
Study population
A5375 (NCT03819114) was a phase II, open-label, partially randomized trial to determine the effects of steady-state efavirenz, isoniazid-rifampin, or dolutegravir on single-dose plasma levonorgestrel pharmacokinetics. Participants were post-menarcheal women at least 16 years of age, that were either living with HIV (without tuberculosis) and receiving either efavirenz- or dolutegravir-based ART or were being treated for tuberculosis (without HIV) with isoniazid and rifampin. Participants were excluded if pregnant, within 6 weeks post-partum, or breastfeeding an infant less than 6 months of age, had received levonorgestrel or other hormonal contraceptives within the prior 30 days, norethisterone enanthate within the prior 60 days, or depo-medroxyprogesterone within the prior 90 days. Institutional review boards of participating institutions approved the study, and participants provided written informed consent.
Procedures
A description of A5375 procedures and primary results are provided elsewhere [17]. Women with HIV and receiving efavirenz-based ART were randomized 1 : 2 to Group A (efavirenz/levonorgestrel 1.5 mg) or group B (efavirenz/levonorgestrel 3.0 mg). Women living with HIV and receiving dolutegravir-based ART were assigned to control group C (dolutegravir/levonorgestrel 1.5 mg). Women being treated for tuberculosis with isoniazid-rifampin were assigned to group D (isoniazid-rifampin/levonorgestrel 3 mg). Participants received a single dose of levonorgestrel on day 0, with or within 2 h of food consumption. Plasma was collected pre-dose and 0.5, 1, 1.5, 2, 3, 4, 6, 8, 24 and 48 h post-dose. Drug concentrations were quantified by validated, quality-controlled, liquid chromatography-tandem mass spectrometry [18–20]. The coefficient of variation of each analyte was less than 15%, and assays met Food and Drug Administration guidance on bioanalytical method validation [21]. Adherence to ART and tuberculosis medications was assessed using a self-report questionnaire [22]. Standard noncompartmental techniques were used to determine pharmacokinetic parameters using Phoenix WinNonLin (Certara) software.
Genetic polymorphisms
Human DNA extracted from whole blood was used to genotype three CYP2B6 polymorphisms (rs3745274, rs28399499, rs4803419) that predict increased plasma efavirenz exposure [16], four NAT2 polymorphisms (rs1801279, rs1801280, rs1799930, rs1799931) that predict increased plasma isoniazid exposure [13–15], one UGT1A1 polymorphism (rs887829) that predicts increased plasma dolutegravir exposure [23,24], and two SLCO1B1 polymorphisms (rs4149032, rs4149056), the former associated in one study with reduced plasma rifampin exposure [25], and the latter with reduced transporter function that impacts statins and other drugs [26]. Genotyping was done in VANTAGE (Vanderbilt Technology for Advanced Genomics) using Taqman (ThermoFisher Scientific, Massachusetts, USA). Genotyping efficiency was 100% for all polymorphisms in all participants except for one individual in whom rs4149032 was indeterminate. All were in Hardy-Weinberg equilibrium (P > 0.05).
Statistical analysis
Primary analyses focused on associations of levonorgestrel clearance with CYP2B6 in the efavirenz arms, and NAT2 in the isoniazid-rifampin arm. We also considered UGT1A1 in the dolutegravir arm and SLCO1B1 in each arm. Associations were assessed by linear regression models using STATA version 17.0 (StataCorp, College Station, Texas, USA). For CYP2B6 and UGT1A1, intermediate and poor metabolizer groups were pairwise evaluated in relation to the reference normal metabolizer group, and intermediate and slow acetylator groups in relation to the reference rapid acetylator group for NAT2. We secondarily assessed associations of levonorgestrel AUC0-8h. Covariates included screening BMI and age. For associations with levonorgestrel clearance among efavirenz recipients, the 1.5 mg and 3 mg levonorgestrel dose groups were combined, with levonorgestrel dose included as a covariate, despite A5375 showing that clearance in these groups did not differ by dose [17]. Analyses were repeated within subgroups based on self-identified race. Geometric mean ratios (GMR) summarized between-group comparisons. The 90% confidence interval (CI) of the GMR was calculated using the pooled variance of the difference and log transformation of pharmacokinetic parameters. Anti-logs of GMR and confidence bounds are reported. Some analyses used the dolutegravir arm as a control, as A5375 showed no apparent interaction between dolutegravir and levonorgestrel [17].
We defined CYP2B6 metabolizer groups based on combinations of three polymorphisms, CYP2B6 516G→T (rs3745274), 983T→C (rs28399499), and 15582C→T (rs4803419) as follows: normal metabolizer (1 : 15582CC-516GG-983TT or 2 : 15582CT-516GG-983TT); intermediate metabolizer (3 : 15582TT-516GG-983TT; 4 : 15582CC-516GT-983TT; 5 : 15582CC-516GG-983CT; 6 : 15582CT-516GT-983TT; or 7 : 15582CT-516GG-983CT); and poor metabolizer (8 : 15582CC-516TT-983TT; 9 : 15582CC-516GT-983CT; 10 : 15582CC-516GG-983CC) [16]. For NAT2, acetylator groups were defined based on combinations of rs1801280 (NAT2*5), rs1799930 (NAT2*6), rs1799931 (NAT2*7), and rs1801279 (NAT2*14), as slow if homozygous for the variant allele at any locus (i.e. AA, CC, AA, AA, respectively), or heterozygous at 2 or more loci; intermediate if heterozygous at a single locus; or rapid if no variant allele at any locus. For UGT1A1 rs887829, participants with CC, CT, and TT genotypes were classified as normal, intermediate, and slow metabolizers, respectively. We did not correct for multiple comparisons, given strong a priori evidence of the pharmacokinetic impact of the polymorphisms on efavirenz and isoniazid. Two-sided statistical tests were used. Clearance and AUC0-8h were natural log transformed.
Results
Participant characteristics
A5375 enrolled 122 cisgender women across seven countries, of whom 118 (97%) were evaluable for genetic associations. Among 52 women in the efavirenz groups, 8 (15.4%) were CYP2B6 poor metabolizers. Among 34 women in the isoniazid-rifampin group, 15 (44.1%) were NAT2 slow acetylators. The median age was lowest in the isoniazid-rifampin group. Median screening BMI was lowest in the isoniazid-rifampin group and efavirenz/levonorgestrel 1.5 mg group, and highest in the dolutegravir group. The baseline characteristics of participants are shown in Table 1.
Table 1.
Baseline characteristics of A5375 participants included in genetic association analyses
| INH-RIFa (n = 34) | EFV-LNG 1.5b (n = 17) | EFV-LNG 3.0c (n = 35) | DTGd (n = 32) | Total (n = 118) | |
|---|---|---|---|---|---|
| Age in years, median (IQRe) | 24.5 (20.8–35.3) | 42 (34.5–45) | 36 (29–42) | 34 (29–40) | 34 (26.8–41.3) |
| Race/ethnicity, n (%) | |||||
| Black | 30 (88.2) | 7 (41.2) | 12 (34.3) | 24 (75) | 73 (61.9) |
| Asian | 2 (5.9) | 9 (52.9) | 20 (57.1) | 2 (6.3) | 33 (28.0) |
| Latina | 2 (5.9) | 1 (5.9) | 3 (8.6) | 4 (12.5) | 10 (8.5) |
| White | - | - | - | 1 (0.9) | 1 (0.9) |
| Multiple | - | - | - | 1 (0.9) | 1 (0.9) |
| Country; n (%) | |||||
| Botswana | 2 (5.9) | - | - | - | 2 (1.7) |
| Brazil | 2 (5.9) | 1 (5.9) | 3 (8.6) | 4 (12.5) | 10 (8.5) |
| Kenya | 4 (11.8) | - | - | - | 4 (3.4) |
| Malawi | 6 (17.7) | - | - | 10 (31.3) | 16 (13.6) |
| South Africa | 18 (52.9) | 7 (41.2) | 12 (34.3) | 2 (6.3) | 39 (33.1) |
| Thailand | 2 (5.9) | 9 (52.9) | 20 (57.1) | 2 (6.3) | 33 (28.0) |
| USA | - | - | - | 14 (43.8) | 14 (11.9) |
| BMIe in kg/m2, median (range) | 21.5 (19.7–24.6) | 20.3 (18.3–27.6) | 23.5 (20.5–26.6) | 25.3 (21.6–28.5) | 23.2 (20.0–26.3) |
| CYP2B6 metabolizer, n (%) | |||||
| Normal | 11 (34.4) | 3 (17.7) | 12 (34.3) | 12 (37.5) | 38 (32.8) |
| Intermediate | 14 (43.8) | 11 (64.7) | 18 (51.4) | 9 (28.1) | 52 (44.8) |
| Poor | 7 (21.9) | 3 (17.7) | 5 (14.3) | 11 (34.4) | 26 (22.4) |
| NAT2 acetylator, n (%) | |||||
| Rapid | 4 (11.8) | 2 (11.8) | 3 (8.6) | 5 (15.6) | 14 (11.9) |
| Intermediate | 15 (44.1) | 9 (52.9) | 21 (60) | 13 (40.6) | 58 (49.2) |
| Slow | 15 (44.1) | 6 (35.3) | 11 (31.4) | 14 (43.8) | 46 (39.0) |
| UGT1A1 metabolizer, n (%) | |||||
| Normal | 11 (34.4) | 8 (47.1) | 19 (54.3) | 5 (15.6) | 43 (37.1) |
| Intermediate | 14 (43.8) | 9 (52.9) | 14 (40) | 22 (68.8) | 59 (50.1) |
| Poor | 7 (21.9) | - | 2 (5.7) | 5 (15.6) | 14 (12.1) |
| SLCO1B1 rs4149032, n (%) | |||||
| CC | 3 (9.4) | 3 (17.7) | 8 (22.9) | 5 (16.1) | 19 (16.4) |
| CT | 14 (43.8) | 3 (17.7) | 14 (40.0) | 12 (38.7) | 43 (37.1) |
| TT | 15 (46.9) | 11 (64.7) | 13 (37.1) | 14 (45.2) | 53 (45.7) |
| SLCO1B1 rs4149056, n (%) | |||||
| CT | 5 (15.6) | 1 (5.9) | 5 (14.3) | 1 (3.1) | 12 (10.3) |
| TT | 27 (84.4) | 16 (94.1) | 30 (85.7) | 31 (96.9) | 104 (89.7) |
INH-RIF, isoniazid-rifampin with 3 mg levonorgestrel (LNG) dose.
EFV-LNG 1.5, efavirenz with 1.5 mg LNG dose.
EFV-LNG 3.0, efavirenz with 3 mg LNG dose.
DTG, dolutegravir with 1.5 mg LNG dose.
IQR, interquartile range.
Genetic associations with levonorgestrel clearance in the efavirenz group
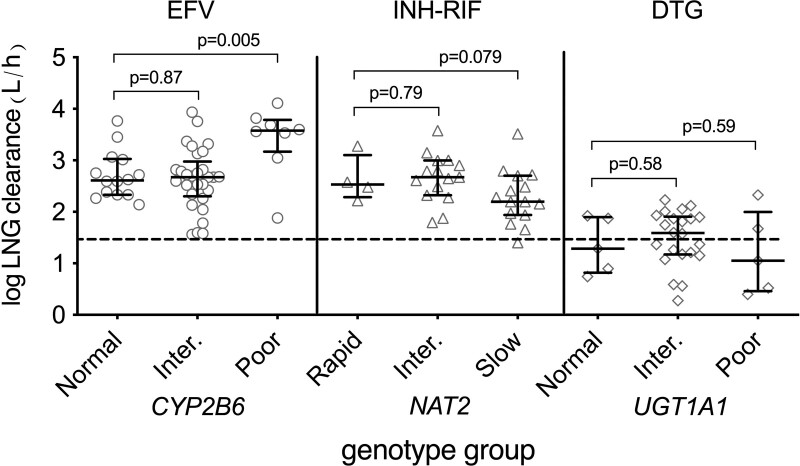
In multivariable analyses among all 52 women in the efavirenz group, CYP2B6 genotype was significantly associated with log levonorgestrel clearance, with CYP2B6 poor metabolizers having the most rapid clearance. Model results are shown in Table 2. This association was driven by CYP2B6 poor metabolizers. The adjusted GMR of clearance in poor versus normal metabolizers was 2.09 (90% CI: 1.47–3.19), and in intermediate versus normal metabolizers was 0.97 (90% CI: 0.71–1.33). Unadjusted log clearance values are shown in Fig. 1 (left section). In multivariable analyses that controlled for CYP2B6 genotype, there was not evidence of association of SLCO1B1 rs4149056 and rs4149032 with levonorgestrel clearance (P > 0.05 for each).
Table 2.
Multivariable models of log levonorgestrel clearance associations with genotype, sex, BMI, and levonorgestrel dose.
| Efavirenz group log clearance β coeff., P-value (n = 52) | Isoniazid-Rifampin group log clearance β coeff., P-value (n = 34) | Dolutegravir group log clearance β coeff., P-value (n = 32) | |||
|---|---|---|---|---|---|
| CYP2B6 genotypea | NAT2 genotypea | UGT1A1 genotypea | |||
| Intermediate | −0.03 to 0.87 | Intermediate | −0.074 to 0.79 | Intermediate | 0.15 to 0.58 |
| Poor | 0.74 to 0.005 | Slow | −0.50 to 0.079 | Poor | −0.19 to 0.59 |
| BMIb | 0.035 to 0.017 | BMI | 0.0001 to 0.99 | BMI | 0.033 to 0.028 |
| Agec | 0.002 to 0.87 | Age | −0.022 to 0.038 | Age | −0.001 to 0.93 |
| Levonorgestrel dosed | 0.051 to 0.67 | - | - | ||
The reference group is normal metabolizer for CYP2B6 and UGT1A1, and rapid acetylator for NAT2.
Per 1 kg/m2
Per year.
Per mg increase in levonorgestrel dose.
Fig. 1.
Relationships between human genetic variants and log levonorgestrel clearance among women in A5375. Left panel: relationship of CYP2B6 metabolizer group with plasma levonorgestrel clearance in women with HIV on efavirenz, who received a single 1.5 mg or 3 mg oral dose of levonorgestrel. Middle panel: relationship of NAT2 acetylator group with plasma levonorgestrel clearance in women being treated for tuberculosis with isoniazid and rifampin, and who received a single 3 mg oral dose of levonorgestrel. Right panel: relationship of UGT1A1 genotype with plasma levonorgestrel clearance in women with HIV on dolutegravir, who received a single 1.5 mg oral dose of levonorgestrel. Error bars indicate the median and interquartile range. The markers are unadjusted for BMI or age. The dashed line indicates median log levonorgestrel clearance (1.47) in the dolutegravir control group. P values from multivariable linear regression are shown. DTG, dolutegravir; EFV, efavirenz; INH, isoniazid; Inter, intermediate; LNG, levonorgestrel; RIF, rifampin.
In multivariable analyses that included only the 29 Asian women in the efavirenz group, CYP2B6 genotype was also significantly associated with levonorgestrel clearance, again driven by CYP2B6 poor metabolizers. Among Asian women, the adjusted GMR of clearance in poor versus normal metabolizers was 2.77 (90% CI: 1.96–3.91), and in intermediate versus normal metabolizers was 0.88 (90% CI: 0.68–1.15).
In multivariable analyses that included only the 19 Black women in the efavirenz group, CYP2B6 poor metabolizers also had the most rapid levonorgestrel clearance, but this was not statistically significant. Among Black women, the adjusted GMR of clearance in poor versus normal metabolizers was 1.96 (90% CI: 0.89–4.31), and in intermediate versus normal metabolizers was 1.76 (90% CI: 0.89–3.48).
Genetic associations with levonorgestrel clearance in the isoniazid-rifampin group
In multivariable analyses among the 34 women in the isoniazid-rifampin group, NAT2 genotype was associated with log levonorgestrel clearance, with NAT2 slow acetylators having the slowest clearance. Model results are shown in Table 2. This was driven by NAT2 slow acetylators. The adjusted GMR of clearance in slow versus rapid acetylators was 0.60 (90% CI: 0.38–0.97), and in intermediate versus rapid acetylators was 0.93 (90% CI: 0.59–1.47). Unadjusted log clearance values are shown in Fig. 1 (middle section). In multivariable analyses that controlled for NAT2 acetylator status, there was no evidence of association between SLCO1B1 rs4149056 and rs4149032 with levonorgestrel clearance (P > 0.05 for each).
In multivariable analyses that included only the 30 Black women in the isoniazid-rifampin group, NAT2 acetylator status was also significantly associated with levonorgestrel clearance, again driven by NAT2 slow acetylators. Among Black women, the adjusted GMR of clearance in slow versus rapid acetylators was 0.56 (90% CI: 0.35–0.88), and in intermediate versus rapid acetylators was 0.88 (90% CI: 0.57–1.37).
Genetic associations with levonorgestrel clearance in the dolutegravir control group
In multivariable analyses among all 32 women in the dolutegravir group, there was no evidence of association between UGT1A1 genotype and levonorgestrel clearance. Model results are shown in Table 2. The adjusted GMR of clearance in TT versus CC was 1.01 (90% CI: 0.63–1.65), and in CT versus CC was 0.85 (90% CI: 0.59–1.24). Unadjusted log clearance values are shown in Fig. 1 (right section). In multivariable analyses that controlled for UGT1A1 genotype, there was no evidence of association between SLCO1B1 rs4149056 and rs4149032 with levonorgestrel clearance (P > 0.05 for each).
Genetic associations with plasma levonorgestrel AUC0-8h
Genetic association with plasma levonorgestrel AUC0-8h were generally consistent with those for clearance. In multivariable analyses among the 17 women in the efavirenz/levonorgestrel 1.5 mg group, CYP2B6 genotype was significantly associated with log AUC0-8h, with CYP2B6 poor metabolizers having the lowest AUC. In these 17 women, the adjusted GMR of AUC0-8h in poor versus normal metabolizers was 0.43 (90% CI: 0.27–0.69), and in intermediate versus normal metabolizers was 1.30 (90% CI: 0.88–1.91). Similarly, among the 35 women in the efavirenz/levonorgestrel 3 mg group, there was a relationship between AUC0-8h and CYP2B6 genotype. In these 35 women, the adjusted GMR of AUC0-8h in poor versus normal metabolizers was 0.59 (90% CI: 0.39–0.88), and in intermediate versus normal metabolizers was 0.95 (90% CI: 0.72–1.27).
Among the 34 women in the rifampin-isoniazid group, NAT2 slow acetylators had the highest levonorgestrel AUC0-8h values, but this was not significantly significant. The adjusted GMR of AUC0-8h in slow versus normal acetylators was 1.31 (90% CI: 0.93–1.86), and in intermediate versus normal acetylators was 1.01 (90% CI: 0.72–1.42). Among the 32 women in the dolutegravir group, a relationship between AUC0-8h and UGT1A1 genotype was not observed, nor in analyses that controlled for rs887829, SLCO1B1 rs4149056 and rs4149032 (P > 0.05 for each).
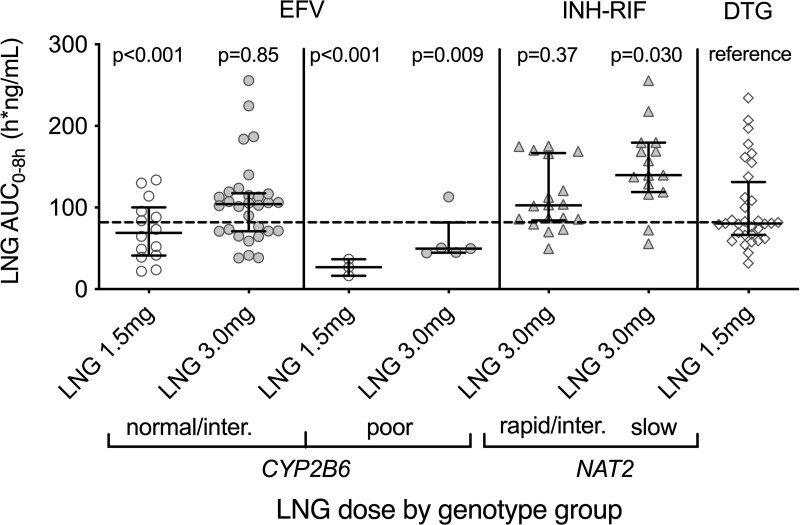
Within each study arm and metabolizer/acetylator group, we considered AUC0-8h values in relation to dolutegravir controls, adjusting for BMI and age. Because levonorgestrel clearance in the efavirenz group was similar in CYP2B6 normal and intermediate metabolizers, we combined these groups for AUC0-8h analyses. Among CYP2B6 normal and intermediate metabolizers in the efavirenz group, levonorgestrel 1.5 mg yielded a lower levonorgestrel AUC0-8h values than in controls (adjusted GMR 0.58; 90% CI: 0.46–0.72), while levonorgestrel 3 mg yielded AUC0-8h values comparable to controls (adjusted GMR 0.98; 90% CI: 0.83–1.16). In contrast, among CYP2B6 poor metabolizers in the efavirenz group, levonorgestrel 1.5 mg yielded AUC0-8h markedly lower than in controls (adjusted GMR 0.23; 90% CI: 0.15, 0.33); Levonorgestrel 3 mg also yielded AUC0-8h lower than in controls (adjusted GMR 0.60; 90% CI: 0.44–0.82), and Cmax values 23% lower than in controls (adjusted GMR 0.77; 90% CI: 0.55–1.06). Unadjusted AUC0-8h values are shown in Fig. 2.
Fig. 2.
Relationships between human genetic variants and levonorgestrel AUC0-8h among women in A5375. Left panel: relationship between levonorgestrel dose and levonorgestrel AUC0-8h among CYP2B6 normal and intermediate metabolizers in women with HIV on efavirenz, and who received a single 1.5 mg or 3 mg oral dose of levonorgestrel. Panel second from left: relationship between levonorgestrel dose and levonorgestrel AUC0-8h among CYP2B6 poor metabolizers in women with HIV on efavirenz, and who received a single 1.5 mg or 3 mg oral dose of levonorgestrel. Panel second from right: relationship between NAT2 genotype and levonorgestrel AUC0-8h among women with tuberculosis on isoniazid and rifampin, and who received a single 3 mg oral dose of levonorgestrel. Right panel: levonorgestrel AUC0-8h in women with HIV on dolutegravir, and who received a single 1.5 mg oral dose of levonorgestrel. Error bars indicate the median and interquartile range. Clear and gray shaded markers represent 1.5 mg or 3 mg doses of levonorgestrel, respectively. The markers are unadjusted for BMI or age. The horizontal dashed line indicates a median AUC0-8h in the dolutegravir control group. P values from multivariable linear regression are shown. DTG, dolutegravir, EFV, efavirenz, INH, isoniazid, Inter, intermediate, inter, intermediate, LNG, levonorgestrel, RIF, rifampin.
Levonorgestrel clearance in the isoniazid-rifampin group was similar in NAT2 rapid and intermediate acetylators. We therefore combined these groups for evaluation of AUC0-8h compared to the control group and adjusted for BMI and age. Among NAT2 rapid and intermediate acetylators in the isoniazid-rifampin group, levonorgestrel 3 mg yielded levonorgestrel AUC0-8h values comparable to controls (adjusted GMR 1.10; 90% CI: 0.92–1.32). In contrast, among NAT2 slow acetylators in the isoniazid-rifampin group, levonorgestrel 3 mg yielded higher levonorgestrel AUC0-8h values than in controls (adjusted GMR 1.36; 90% CI: 1.08–1.71). Unadjusted AUC0-8h values are shown in Fig. 2.
Discussion
Primary analyses from A5375 showed that, among women who received a single dose of levonorgestrel, those on efavirenz-based ART exhibited much faster plasma levonorgestrel clearance compared to a dolutegravir control group, and that doubling the levonorgestrel dose from 1.5 mg to 3.0 mg yielded levonorgestrel AUC0-8h values comparable to controls [17]. The present analyses show that effects of double-dose levonorgestrel to compensate for efavirenz-induced levonorgestrel clearance differ by CYP2B6 genotype. Among CYP2B6 normal and intermediate metabolizers in the efavirenz group, levonorgestrel 1.5 mg yielded levonorgestrel AUC0-8h approximately 42% lower than in controls, and among CYP2B6 poor metabolizers approximately 77% lower than in controls. With double-dose levonorgestrel, among CYP2B6 normal and intermediate metabolizers in the efavirenz group, levonorgestrel 3 mg yielded levonorgestrel AUC0-8h similar to controls, yet among CYP2B6 poor metabolizers, levonorgestrel AUC0-8h remained approximately 40% lower than in controls. These results indicate that among CYP2B6 poor metabolizers receiving efavirenz, a levonorgestrel dose greater than 3 mg might be needed to achieve AUC0-8h values comparable to controls. As Cmax is suggested to be associated with emergency contraceptive effectiveness [27], it is reassuring that levonorgestrel 3 mg yielded Cmax values only 23% lower than in controls among CYP2B6 poor metabolizers in the efavirenz group. However, caution is still warranted, as the pharmacokinetic-pharmacodynamic relationship has not been established.
The pharmacogenetic interaction with efavirenz in this study is complex. In CYP2B6 poor metabolizers, plasma efavirenz exposure is much greater than it is in normal and intermediate metabolizers [16]. The resultant induction of CYP3A4 expression by efavirenz is therefore expected to be greater in poor metabolizers than in normal and intermediate metabolizers, and levonorgestrel clearance by CYP3A4 increased to a greater extent in poor metabolizers. This explains why double-dose levonorgestrel in these CYP2B6 poor metabolizers yields AUC0-8h values that remain lower than in controls, while double-dose levonorgestrel in normal and intermediate metabolizers yields a similar AUC0-8h compared to controls.
Associations of CYP2B6 genotype with levonorgestrel clearance and AUC0-8 among efavirenz recipients were not unexpected. Many contraceptive hormones including levonorgestrel are metabolized by CYP3A isoforms, and greater efavirenz exposure has been shown to increase rates of plasma clearance of some hormonal contraceptives [9,10]. Among women receiving efavirenz-based ART who then received a levonorgestrel-releasing contraceptive implant, CYP2B6 poor metabolizers had more rapid levonorgestrel clearance and lower AUC values [28]. In ACTG study A5316, efavirenz reduced plasma concentrations of etonogestrel and ethinyl estradiol, given as a vaginal ring [9], with CYP2B6 poor metabolizers having the greatest reductions [29]. However, study findings have not been consistent. In ACTG study A5093, which compared pharmacokinetics of depot-medroxyprogesterone acetate (DMPA) and selected ART regimens among women with HIV found no difference in MPA AUC, Cmax or clearance based on CYP2B6 genotype [30,31]. Findings were similarly negative in pharmacogenetic analyses from ACTG study A5338, which studied the pharmacokinetics of DMPA among women with HIV receiving efavirenz-based ART and rifampicin plus isoniazid for tuberculosis co-infection [32]. Another study demonstrated higher plasma MPA AUC values among women receiving efavirenz-based ART than women not on ART [33].
Primary analyses from A5375 showed that, among pre-menopausal women who received a single dose of levonorgestrel, those on isoniazid-rifampin therapy for tuberculosis exhibited much faster levonorgestrel clearance compared to dolutegravir controls, and that a levonorgestrel 3.0 mg double-dose yielded levonorgestrel AUC0-8h values somewhat greater than in controls [17]. The present analyses show that effects of double-dose levonorgestrel to compensate for rifampin-induced levonorgestrel clearance, in the presence of the CYP3A4 inhibitor isoniazid, differ by NAT2 genotype. Among NAT2 normal and intermediate acetylators who received levonorgestrel 3 mg, AUC0-8h was approximately 10% greater than in controls, but among slow acetylators was approximately 36% greater than in controls. Since such levels should provide effective contraception, and because the risk of toxicity following a single dose is low, double-dose levonorgestrel for all women receiving concomitant isoniazid and rifampin is appropriate regardless of NAT2 genotype.
The pharmacogenetic interaction with isoniazid and rifampin in this study is complex. Rifampin potently induces CYP3A4 expression [7], while isoniazid is a mechanism-based inhibitor of CYP3A4 [11]. In individuals receiving both isoniazid and rifampin, induction by rifampin typically dominates the inhibitory effect of isoniazid. In NAT2 normal and intermediate acetylators, plasma isoniazid exposure is relatively low, so isoniazid minimally counteracts the inductive effect of rifampin, and levonorgestrel clearance by CYP3A4 is greatly increased. Double-dose levonorgestrel in these individuals therefore yields AUC0-8h values only slightly higher than in controls. In NAT2 poor metabolizers, plasma isoniazid exposure is greater, so the inhibitory effect of isoniazid on CYP3A4 is greater, which more effectively attenuates the inductive effect of rifampin on levonorgestrel clearance. Double-dose levonorgestrel in these women therefore yields median AUC0-8h values considerably greater than in controls.
The association of NAT2 genotype with levonorgestrel clearance and AUC0-8h was not unexpected. An analogous situation occurs in some patients receiving tuberculosis therapy that includes isoniazid with rifampin, who paradoxically experience increased plasma efavirenz exposure in the presence of CYP2B6 and NAT2 loss-of-function polymorphisms [34–36]. In ACTG trial A5279, which studied rifapentine plus isoniazid for preventing tuberculosis in patients living with HIV [37], NAT2 slow acetylators had higher plasma concentrations not only of efavirenz, but also of rifapentine, its 25-desacetyl rifapentine metabolite, and nevirapine [12], reflecting greater inhibition of CYP isoforms that metabolize these drugs.
The present trial enrolled women of various ancestries. In subgroup analyses, results generally reflected those in the total group. Among 29 Asian women in the efavirenz group, CYP2B6 genotype was significantly associated with levonorgestrel clearance, while among 30 Black women in the isoniazid-rifampin group, NAT2 acetylator status was significantly associated with levonorgestrel clearance. The lack of significant association between CYP2B6 genotype and levonorgestrel clearance among 19 Black women in the efavirenz group may be due to a small sample size. There were too few women in other subgroups to analyze.
Because treatment assignment was not by randomization (except for 1.5 versus 3 mg of levonorgestrel among those taking efavirenz), there were anticipated differences in some baseline characteristics between trial arms (Table 1), including BMI as was reported in the A5375 primary manuscript [17]. Therefore, all comparisons adjusted for BMI, which affects levonorgestrel concentrations during emergency contraception [38,39].
We found no associations between UGT1A1 genotype and levonorgestrel clearance in the dolutegravir group. This was expected, since A5375 primary analyses showed that dolutegravir did not alter levonorgestrel pharmacokinetics in comparison to a historical control [17]. Similarly, we did not find associations between SLCO1B1 genotype and levonorgestrel clearance. A single study from South Africa associated SLCO1B1 rs4149032 with rifampin clearance [25]. The infrequency of SLCO1B1 rs4149056 may have precluded finding associations.
Regarding potential clinical implications for women on efavirenz, while CYP2B6 poor metabolizers who received a levonorgestrel dose of 3 mg did not achieve AUC0-8h comparable to controls, we are reassured that Cmax values were closer to those in controls. In our total sample, 22.4% of women were CYP2B6 poor metabolizers, reflecting that these genotypes are frequent with Asian and African ancestry [40]. Lacking future data, and because results of CYP2B6 genotyping would almost certainly not be available in clinical practice prior to emergency contraception, it is reasonable to recommend double-dose levonorgestrel (3 mg) for all women receiving efavirenz, understanding that some women will have lower Cmax and AUC0-8h values.
The present analysis had limitations. Because A5375 was only partially randomized, pharmacokinetic differences between groups may be affected by factors not captured in our analyses. Because of the modest sample size, we could only study frequent polymorphisms with large effect sizes. Because A5375 did not study rifampin without isoniazid, we cannot know what levonorgestrel exposure would be in such women, although it would almost certainly be lower than with isoniazid-rifampin.
In summary, among cisgender women with HIV and receiving efavirenz-based ART, the pharmacokinetic effect of doubling the levonorgestrel dose for emergency contraception varies by CYP2B6 genotype. Similarly, among women being treated for tuberculosis with isoniazid and rifampin, the effect of doubling the levonorgestrel dose varies by NAT2 genotype. This analysis reinforces the importance of evaluating pharmacogenetics effects on drug-drug interactions.
Acknowledgements
We gratefully acknowledge the patients who participated in trial A5375, and site personnel who contributed to this work, including: Petronella Casey and Penelope Madlala, Durban International CRS (Grant UM1AI69432); Patcharaphan Sugandhavesa and Daralak Tavornprasit, Chiang Mai University HIV Treatment (CMU HIV Treatment) CRS, Grant U01AI069399; Hay Mar Su Lwin and Anchalee Avihingsanon, Thai Red Cross AIDS Research Centre (TRC-ARC) CRS, Grant UM1AI69399; Instituto de Pesquisa Clinica Evandro Chagas (IPEC) CRS; Evans Kachale, Blantyre CRS (Grant 5UM1AI069518); Jaclyn Bennet and Noluthando Mwelase, University of the Witwatersrand Helen Joseph CRS (Grant AI069463, AI068636); Lindee Ganger and Lynne Cornelissen, Family Center for Research with Ubuntu (FAM-CRU) CRS (Grant 5UM1AI069521); Cecelia Kanyama and Cornelius Munyanga; Malawi CRS (Grant 5UM1AI069423); Kenya Medical Research Institute/Walter Reed Project Clinical Research Center (KEMRI/WRP) CRS; Mariam Aziz and Joan A. Swiatek, Rush University CRS (Grant U01 AI069471); Suri Moonsamy and Nazim Akoojee, Soweto ACTG CRS (Grant 5UM1AI069453); Erin Hoffman and Catherine Kronk, Chapel Hill CRS (5UM1AI069423, UL1TR002489); Penn Therapeutics CRS; Unoda A. Chakalisa and Lesedi Tirelo, Gaborone CRS (UM1AI69456); Triniece Pearson and Rachelle Price, Northwestern University CRS (Grant UM1 AI069471, UL1TR001422); Trinity Health and Wellness Center CRS; Jennifer Sullivano and Jamie Nemeth, University of Pittsburgh CRS (UM1AI069494, UL1TR001857); Rebecca Fry and Jessenia Fuentes, Weill Cornell Uptown CRS (Grant UM1 AI069419, UL1 TR002384). In addition, we gratefully acknowledge Karin Klingman for her support during the study conduct.
This work was supported by the National Institute of Allergy and Infectious Diseases [grant number UM1 AI068634, UM1 AI068636 and UM1 AI106701] and the Eunice Kennedy Shriver National Institute of Child Health and Human Development [grant number R01 HD085887 to KS]. Additional support included AI069439, AI110527, AI077505, AI120790, and TR002243 (David Haas). Nana Agyemang was supported by R25 AI164610 (Chandravanu Dash, PI). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The funders of the study oversaw the development and monitoring of the study but had no role in the conduct, analyses, and conclusions of the study.
CG, PFB-Z, and EB, coauthors of this study and former and current medical officers from NIH/NIAID (CG, PFB-Z) and program officer at NIH/ORWH (EB), had a role in the study design, data interpretation, manuscript revision, and intellectual contribution. The views expressed in this article are those of the authors and should not be construed to represent the views of the NIH, the Department of State, or the Department of Health and Human Services.
Conflicts of interest
Scarsi: Organon, LLC. Research support paid to her institution. For the remaining authors, there are no conflicts of interest.
References
- 1.World Health Organization. Global Tuberculosis Report 2022. 2022. https://www.who.int/teams/global-tuberculosis-programme/tb-reports/global-tuberculosis-report-2022. [Accessed 30 January 2023].
- 2.World Health Organization. Tuberculosis in women. 2018. https://www.who.int/publications/m/item/tuberculosis-in-women. [Accessed 12 August 2022].
- 3.Pillay T, Sturm AW, Khan M, Adhikari M, Moodley J, Connolly C, et al. Vertical transmission of Mycobacterium tuberculosis in KwaZulu Natal: impact of HIV-1 co-infection. Int J Tuberc Lung Dis 2004; 8:59–69. [PubMed] [Google Scholar]
- 4.Curtis KM, Tepper NK, Jatlaoui TC, Berry-Bibee E, Horton LG, Zapata LB, et al. U.S. Medical eligibility criteria for contraceptive use, 2016. MMWR Recomm Rep 2016; 65:1–103. [DOI] [PubMed] [Google Scholar]
- 5.World Health Organization. Emergency contraception. 2021. https://www.who.int/news-room/fact-sheets/detail/emergency-contraception. [Accessed 5 April 2022].
- 6.World Health Organization. Consolidated guidelines on HIV prevention, testing, treatment, service delivery and monitoring: recommendations for a public health approach. 2021. https://www.who.int/publications/i/item/9789240031593. [Accessed 8 August 2022]. [PubMed]
- 7.Williamson B, Dooley KE, Zhang Y, Back DJ, Owen A. Induction of influx and efflux transporters and cytochrome P450 3A4 in primary human hepatocytes by rifampin, rifabutin, and rifapentine. Antimicrob Agents Chemother 2013; 57:6366–6369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kobayashi K, Mimura N, Fujii H, Minami H, Sasaki Y, Shimada N, et al. Role of human cytochrome P450 3A4 in metabolism of medroxyprogesterone acetate. Clin Cancer Res 2000; 6:3297–3303. [PubMed] [Google Scholar]
- 9.Scarsi KK, Cramer YS, Rosenkranz SL, Aweeka F, Berzins B, Coombs RW, et al.; AIDS Clinical Trials Group A5316 Study Team. Antiretroviral therapy and vaginally administered contraceptive hormones: a three-arm, pharmacokinetic study. Lancet HIV 2019; 6:e601–e612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Patel RC, Onono M, Gandhi M, Blat C, Hagey J, Shade SB, et al. Pregnancy rates in HIV-positive women using contraceptives and efavirenz-based or nevirapine-based antiretroviral therapy in Kenya: a retrospective cohort study. Lancet HIV 2015; 2:e474–e482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wen X, Wang JS, Neuvonen PJ, Backman JT. Isoniazid is a mechanism-based inhibitor of cytochrome P450 1A2, 2A6, 2C19 and 3A4 isoforms in human liver microsomes. Eur J Clin Pharmacol 2002; 57:799–804. [DOI] [PubMed] [Google Scholar]
- 12.Haas DW, Podany AT, Bao Y, Swindells S, Chaisson RE, Mwelase N, et al. Pharmacogenetic interactions of rifapentine plus isoniazid with efavirenz or nevirapine. Pharmacogenet Genomics 2021; 31:17–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang YS, Chern HD, Su WJ, Wu JC, Lai SL, Yang SY, et al. Polymorphism of the N-acetyltransferase 2 gene as a susceptibility risk factor for antituberculosis drug-induced hepatitis. Hepatology 2002; 35:883–889. [DOI] [PubMed] [Google Scholar]
- 14.Roy PD, Majumder M, Roy B. Pharmacogenomics of anti-TB drugs-related hepatotoxicity. Pharmacogenomics 2008; 9:311–321. [DOI] [PubMed] [Google Scholar]
- 15.Ramachandran G, Swaminathan S. Role of pharmacogenomics in the treatment of tuberculosis: a review. Pharmgenomics Pers Med 2012; 5:89–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Holzinger ER, Grady B, Ritchie MD, Ribaudo HJ, Acosta EP, Morse GD, et al. Genome-wide association study of plasma efavirenz pharmacokinetics in AIDS Clinical Trials Group protocols implicates several CYP2B6 variants. Pharmacogenet Genom 2012; 22:858–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Scarsi KK, Smeaton LM, Podany AT, Olefsky M, Woolley E, Barr E, et al. Pharmacokinetics of dose-adjusted levonorgestrel emergency contraception combined with efavirenz-based antiretroviral therapy or rifampicin-containing tuberculosis regimens. Contraception 2023; 121:109951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cirrincione LR, Penchala SD, Scarsi KK, Podany AT, Winchester LC, Back DJ, et al. Development, validation and utilization of a highly sensitive LC-MS/MS method for quantification of levonorgestrel released from a subdermal implant in human plasma. J Chromatogr B Analyt Technol Biomed Life Sci 2018; 1084:106–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fletcher CV, Brundage RC, Fenton T, Alvero CG, Powell C, Mofenson LM, et al. Pharmacokinetics and pharmacodynamics of efavirenz and nelfinavir in HIV-infected children participating in an area-under-the-curve controlled trial. Clin Pharmacol Ther 2008; 83:300–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Winchester LC, Podany AT, Baldwin JS, Robbins BL, Fletcher CV. Determination of the rifamycin antibiotics rifabutin, rifampin, rifapentine and their major metabolites in human plasma via simultaneous extraction coupled with LC/MS/MS. J Pharm Biomed Anal 2015; 104:55–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.US Food and Drug Administration. Bioanalytical method validation: guidance for industry. 2018. https://www.fda.gov/media/70858/download. [Accessed 12 August 2022].
- 22.Mngqibisa R, Kendall MA, Dooley K, Wu XS, Firnhaber C, McIlleron H, et al.; A5338 Study Team. Pharmacokinetics and pharmacodynamics of depot medroxyprogesterone acetate in African women receiving treatment for human immunodeficiency virus and tuberculosis: potential concern for standard dosing frequency. Clin Infect Dis 2020; 71:517–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen S, St Jean P, Borland J, Song I, Yeo AJ, Piscitelli S, et al. Evaluation of the effect of UGT1A1 polymorphisms on dolutegravir pharmacokinetics. Pharmacogenomics 2014; 15:9–16. [DOI] [PubMed] [Google Scholar]
- 24.Cindi Z, Kawuma AN, Maartens G, Bradford Y, Venter F, Sokhela S, et al. Pharmacogenetics of dolutegravir plasma exposure among Southern Africans living with HIV. J Infect Dis 2022; 226:1616–1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chigutsa E, Visser ME, Swart EC, Denti P, Pushpakom S, Egan D, et al. The SLCO1B1 rs4149032 polymorphism is highly prevalent in South Africans and is associated with reduced rifampin concentrations: dosing implications. Antimicrob Agents Chemother 2011; 55:4122–4127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Link E, Parish S, Armitage J, Bowman L, Heath S, Matsuda F, et al.; SEARCH Collaborative Group. SLCO1B1 variants and statin-induced myopathy--a genomewide study. N Engl J Med 2008; 359:789–799. [DOI] [PubMed] [Google Scholar]
- 27.Edelman AB, Cherala G, Blue SW, Erikson DW, Jensen JT. Impact of obesity on the pharmacokinetics of levonorgestrel-based emergency contraception: single and double dosing. Contraception 2016; 94:52–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Neary M, Lamorde M, Olagunju A, Darin KM, Merry C, Byakika-Kibwika P, et al. The effect of gene variants on levonorgestrel pharmacokinetics when combined with antiretroviral therapy containing efavirenz or nevirapine. Clin Pharmacol Ther 2017; 102:529–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Haas DW, Cramer YS, Godfrey C, Rosenkranz SL, Aweeka F, Berzins B, et al.; AIDS Clinical Trials Group A5316 Study Team. Pharmacogenetic interactions between antiretroviral drugs and vaginally administered hormonal contraceptives. Pharmacogenet Genomics 2020; 30:45–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Watts DH, Park JG, Cohn SE, Yu S, Hitti J, Stek A, et al. Safety and tolerability of depot medroxyprogesterone acetate among HIV-infected women on antiretroviral therapy: ACTG A5093. Contraception 2008; 77:84–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cohn SE, Park JG, Watts DH, Stek A, Hitti J, Clax PA, et al.; ACTG A5093 Protocol Team. Depo-medroxyprogesterone in women on antiretroviral therapy: effective contraception and lack of clinically significant interactions. Clin Pharmacol Ther 2007; 81:222–227. [DOI] [PubMed] [Google Scholar]
- 32.Haas DW, Mngqibisa R, Francis J, McIlleron H, Robinson JA, Kendall MA, et al.; AIDS Clinical Trials Group A5338 Study Team. Pharmacogenetics of interaction between depot medroxyprogesterone acetate and efavirenz, rifampicin, and isoniazid during treatment of HIV and tuberculosis. Pharmacogenet Genomics 2022; 32:24–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nanda K, Amaral E, Hays M, Viscola MA, Mehta N, Bahamondes L. Pharmacokinetic interactions between depot medroxyprogesterone acetate and combination antiretroviral therapy. Fertil Steril 2008; 90:965–971. [DOI] [PubMed] [Google Scholar]
- 34.Kwara A, Lartey M, Sagoe KW, Court MH. Paradoxically elevated efavirenz concentrations in HIV/tuberculosis-coinfected patients with CYP2B6 516TT genotype on rifampin-containing antituberculous therapy. AIDS 2011; 25:388–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dooley KE, Denti P, Martinson N, Cohn S, Mashabela F, Hoffmann J, et al.; TSHEPISO Study Team. Pharmacokinetics of Efavirenz and treatment of HIV-1 among pregnant women with and without tuberculosis coinfection. J Infect Dis 2015; 211:197–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Luetkemeyer AF, Rosenkranz SL, Lu D, Grinsztejn B, Sanchez J, Ssemmanda M, et al.; Adult AIDS Clinical Trials Group A5221 and A5243 Study Teams. Combined effect of CYP2B6 and NAT2 genotype on plasma efavirenz exposure during rifampin-based antituberculosis therapy in the STRIDE study. Clin Infect Dis 2015; 60:1860–1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Swindells S, Ramchandani R, Gupta A, Benson CA, Leon-Cruz J, Mwelase N, et al.; BRIEF TB/A5279 Study Team. One month of rifapentine plus isoniazid to prevent HIV-related tuberculosis. N Engl J Med 2019; 380:1001–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Praditpan P, Hamouie A, Basaraba CN, Nandakumar R, Cremers S, Davis AR, et al. Pharmacokinetics of levonorgestrel and ulipristal acetate emergency contraception in women with normal and obese body mass index. Contraception 2017; 95:464–469. [DOI] [PubMed] [Google Scholar]
- 39.Natavio M, Stanczyk FZ, Molins EAG, Nelson A, Jusko WJ. Pharmacokinetics of the 1.5 mg levonorgestrel emergency contraceptive in women with normal, obese and extremely obese body mass index. Contraception 2019; 99:306–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.National Center for Biotechnology Information. dbSNP homepage. 2022. http://www.ncbi.nlm.nih.gov/SNP/index.html. [Accessed 12 August 2022].