Abstract
There is no currently approved adoptive cellular therapy for solid tumors. Pre-clinical and clinical studies have demonstrated that low-dose radiotherapy (LDRT) can enhance intratumoral T cell infiltration and efficacy. This case report describes a 71-year-old female patient with rectal mucosal melanoma that had developed metastases to liver, lung, mediastinum, axillary nodes, and brain. After systemic therapies had failed, she enrolled in the radiation sub-study of our phase-I clinical trial exploring the safety and efficacy of afamitresgene autoleucel (afami-cel), genetically engineered T cells with a T cell receptor (TCR) targeting the MAGE-A4 tumor antigen in patients with advanced malignancies (NCT03132922). Prior to the infusion of afami-cel, she received concurrent lymphodepleting chemotherapy and LDRT at 5.6 Gy/4 fractions to the liver. Time to partial response was 10 weeks, and duration of overall response was 18.4 weeks. Although the patient progressed at 28 weeks, the disease was well controlled after high-dose radiotherapy to liver metastases and checkpoint inhibitors. As of the last follow-up, she remains alive over two years after LDRT and afami-cel therapy. This report suggests that afami-cel in combination with LDRT safely enhanced clinical benefit. This provides evidence for further exploring the benefit of LDRT in TCR-T cell therapy.
Keywords: adoptive T cell receptor T cell therapy, immunotherapy, low-dose radiotherapy, metastatic melanoma, radiation therapy
Introduction
The most prominent success stories in immunotherapy have been with checkpoint inhibitors (CPI) and adoptive cellular therapy (ACT) such as chimeric antigen receptor T cell therapy (CAR-T) and T cell receptor T cell therapy (TCR-T) [1]. CAR-T has been successful in hematological malignancies, with four Food and Drug Administration approvals [2]; however, solid tumors have proven more challenging [3]. Several factors hamper CAR-T in solid tumors. Solid tumors undergo a metabolic shift that disfavors effector immunocytes while favoring immunosuppressive cell populations [4–15], secrete immunosuppressive factors [e.g. IL-10 and tumor growth factor-β (TGF-β)] [5,12,16], and form a high-density stromal barrier that hinders infiltration of T cells [15]. Altogether, these factors impede T cell infiltration, activation, and effector function. The tumor’s immunosuppressive shield can be penetrated; however, using low-dose radiotherapy (LDRT), facilitates immune cell infiltration into the tumor, reduces TGF-β levels, and decreases the number of suppressor immune cells [17–21].
Moreover, once inside the tumor, T cells often face additional challenges. Solid tumors are often deficient in tumor-associated antigen (TAA) presentation. This may result from low-affinity TCRs for TAAs [22], low levels of antigen-presenting major histocompatibility complex (MHC) on the tumor cell surface [23], or both. CAR-T cells bypass the need for MHC presentation but can only recognize extracellular antigens. In contrast, TCRs can recognize and target both intracellular and extracellular antigens, albeit in an MHC-restricted fashion [24]. Unfortunately, this increases the risk of alloreactive autoimmunity, whereby TCRs reactive towards a given peptide MHC recognize and attack non-tumor cells displaying allelic variations of those MHC molecules. Proper target selection is, therefore, vital.
Melanoma-associated cancer-testis antigen (MAGE)-A4 is an attractive target for T cell therapy because its expression level is higher in solid tumor metastases compared to primary tumors while absent in most normal tissues [25,26]. Afamitresgene autoleucel (afami-cel, formerly ADP-A2M4, and MAGE-A4c1032) is a therapy consisting of autologous T cells expressing a specific peptide-enhanced affinity receptor (SPEAR) with high affinity and specificity towards the MAGE-A4230-239 peptide presented by HLA-A*028. Preclinical studies support the specificity, safety, and antitumor activity of afami-cel [27]. A phase I clinical trial investigating the efficacy and safety of afami-cel in HLA-A*02+ subjects with MAGE-A4+ tumors is ongoing and has demonstrated efficacy in non-small cell lung cancer, head-and-neck cancer, and synovial sarcoma (NCT03132922) [28].
We report the first case of a patient with metastatic mucosal melanoma who demonstrated a durable partial response (PR) after treatment with LDRT plus afami-cel (a sub-study group of this phase-I afami-cel trial, NCT03132922). We hypothesize that the combination of LDRT and afami-cel therapy improved the patient’s response by modulating the stroma, facilitating infiltration of MAGE-A4 T cells, and promoting an immunostimulatory TME that translated into improved antitumor responses. We suggest that this approach may be potentially beneficial to patients with solid tumors.
Case presentation
In March 2017, a 71-year-old woman was diagnosed with stage I invasive mucosal polypoid melanoma of the ano-rectal region that invaded the rectocolonic mucosa and submucosa found incidentally during hemorrhoidectomy. Her medical history included grade 2 hypertension, type I diabetes, grade 2 arthritis, grade 2 adrenal cortical hypofunction (secondary to prior CPI treatment), and early-stage invasive ductal carcinoma of the breast in 2015. Molecular analysis of the tumor revealed BRAF, NRAS, and c-KIT mutations. Supplementary Figure S1, Supplemental Digital Content 1, http://links.lww.com/MR/A305 shows the clinical course of the patient. The patient underwent transanal excision of the tumor followed by anorectal RT (30 Gy/5 fractions). In March 2018, she developed metastases to the lungs, liver, and mediastinal and left axillary lymph nodes. A liver biopsy revealed metastatic mucosal melanoma. The patient was then treated with nanoparticle albumin-bound paclitaxel (Abraxane, Celgene Company, Summit, New Jersey, USA) alone and in combination with one of two CPIs – pembrolizumab first, and then ipilimumab. In October 2018, the enlargement of multiple metastases, as well as new metastasis to the gallbladder, liver, abdomen, pelvis, and right upper back, occurred. The patient continued treatment with nanoparticle albumin-bound paclitaxel and ipilimumab. In May 2019, an additional 4 mm metastasis in the left inferior frontal lobe was found via MRI. In June 2019, the patient discontinued paclitaxel and ipilimumab and began nivolumab. Additionally, she received Leksell Gamma Knife-Stereotactic Radiosurgery (LSK-SRS) (20 Gy) to the brain lesion and RT (20 Gy/5 fractions) to the right hip metastasis.
In September 2019, after eligibility determination and HLA and MAGE-A4 screening, the patient enrolled in the trial (NCT03132922). The patient’s baseline MAGE-A4 expression was 100% 3+ by immunohistochemistry histoscore (Supplementary Figure S2, Supplemental Digital Content 1, http://links.lww.com/MR/A305). The patient underwent leukapheresis and the manufacture of afami-cel soon thereafter. Prior to the infusion of 7.4 × 109 afami-cel, she received concurrent lymphodepleting chemotherapy (fludarabine, 20 mg/m2, 23–26 September 2019, and cyclophosphamide, 600 mg/m2, 23–25 September 2019) and LDRT (1.4 Gy × 4 fractions, 23–26 September 2019) to the liver lesions. Supplementary Figure S3, Supplemental Digital Content 1, http://links.lww.com/MR/A305 shows the LDRT-field and dose-volume histogram. Seven days later, on 30 September 2019, the patient received afami-cel infusion.
Following treatment, she experienced transient lymphodepleting chemotherapy-related grade 3 leukopenia, grade 4 lymphopenia, grade 3 neutropenia, grade 3 anemia, grade 1 nausea, and grade 2 fatigue; and T cell infusion-related grade 1 fatigue, grade 2 fever, grade 1 cytokine release syndrome (CRS), and grade 3 immune effector cell-associated neurotoxicity syndrome (ICANS), which was considered a serious adverse event (SAE) (Supplementary Table S1, Supplemental Digital Content 1, http://links.lww.com/MR/A305). Two other SAEs unrelated to treatment were grade-3 hyperglycemia and grade-3 adrenal insufficiency, both of which resolved within 5 days. The patient received tocilizumab and dexamethasone for CRS and ICANS and filgrastim-sndz for neutropenia. The neutropenia resolved within 28 days, and the ICANS resolved within 3 days.
Six weeks following T cell infusion, CT imaging showed reductions in the size of multiple lesions in the liver, mediastinal lymph nodes, and small bilateral pulmonary and pelvic nodules (Table 1). According to RECIST1.1, the patient achieved best overall response (BOR) – a confirmed PR – at 10 weeks after afami-cel infusion (Fig. 1a). The patient experienced PD at 28 weeks following afami-cel/LDRT treatment and 18.4 weeks after BOR, which was confirmed by CT scan at 33 weeks. Of note, the PR duration within the LDRT (in-field) and no-dose lesion (out-of-field) were 22.7 weeks and 4 weeks, respectively. Two months after PD, the patient enrolled in the salvage RT trial (NCT02710253) and received high-dose RT (HDRT) to the posterior liver (30 Gy/5 fractions) (Supplementary Figure S3, Supplemental Digital Content 1, http://links.lww.com/MR/A305). Meanwhile, she received nivolumab and ipilimumab beginning in July 2020 but changed to nivolumab alone in September 2020 due to diarrhea. The CT scan in September 2020 showed PR again (according to original baseline) in both in-field and out-of-field lesions. At the last follow-up in September 2021, via phone, the patient reported feeling well and has been off treatment since March 2021. Presently, 2 years after afami-cel/LDRT, her disease is well-controlled and requires only surveillance. These results support the safety and efficacy of LDRT in combination with afami-cel therapy and merit further preclinical and clinical studies.
Table 1.
Treatment response
| Baseline, 12 September 2019 | Week 6, 9 November 2019 | Week 10, 9 December 2019 | Week 14, 6 January 2020 | Week 18, 3 February 2020 | Week 28, 16 April 2020 | Week 33, 19 May 2020 | Week 49, 4 September 2020 | |
|---|---|---|---|---|---|---|---|---|
| Non-LDRT lesions | ||||||||
| (1) Left upper lobe nodule | 1.4 | 0.8 | 0 | 0.6 | 1 | 1 | 1.3 | 0.8 |
| (2) Right paratracheal lymph node | 1.6 | 1.3 | 1.1 | 1.3 | 1.4 | 1.1 | 1.1 | 0.9 |
| (3) Peritoneal implant | 5.1 | 4.7 | 4.2 | 5.4 | 4.6 | 4.7 | 5.2 | 3.8 |
| Lesion-specific sum (cm) | 8.1 | 6.8 | 5.3 | 7.3 | 7 | 6.8 | 7.6 | 5.5 |
| Lesion-specific change (%) | ||||||||
| Baseline | −16.0 | −34.6 | −9.9 | −13.6 | −16.0 | −6.2 | −32.1 | |
| Nadir | −16.0 | −22.1 | 37.7 | 32.1 | 28.3 | 43.4 | 3.8 | |
| Lesion-specific response | SD | PR | PD | PD | PD | PD | PR | |
| LDRT lesions | ||||||||
| (1) Segment VIII liver lesion | 7.2 | 3.7 | 2.7 | 2.7 | 2.5 | 3.1 | 3.1 | 2.7 |
| (2) Segment III liver lesion | 5 | 3.8 | 3.7 | 3.8 | 4.3 | 5.3 | 5.1 | 3.8 |
| Lesion-specific sum (cm) | 12.2 | 7.5 | 6.4 | 6.5 | 6.8 | 8.4 | 8.2 | 6.5 |
| Lesion-specific change (%) | ||||||||
| Baseline | −38.5 | −47.5 | −46.7 | −44.3 | −31.1 | −32.8 | −46.7 | |
| Nadir | −38.5 | −14.7 | 1.6 | 6.3 | 31.3 | 28.1 | 1.6 | |
| Lesion-specific response | PR | PR | PR | PR | PD | PD | PR | |
| Overall response | ||||||||
| Overall sum (cm) | 20.3 | 14.3 | 11.7 | 13.8 | 13.8 | 15.2 | 15.8 | 12 |
| Overall change (%) | ||||||||
| Baseline | −29.6 | −42.4 | −32.0 | −32.0 | −25.1 | −22.2 | −40.9 | |
| Nadir | −29.6 | −18.2 | 17.9 | 17.9 | 29.9 | 35.0 | 2.6 | |
| Overall response | SD | PR | PR | PR | PD | PD | PR | |
LDRT, low-dose radiotherapy; PR, partial response; PD, progressive disease; SD, stable disease.
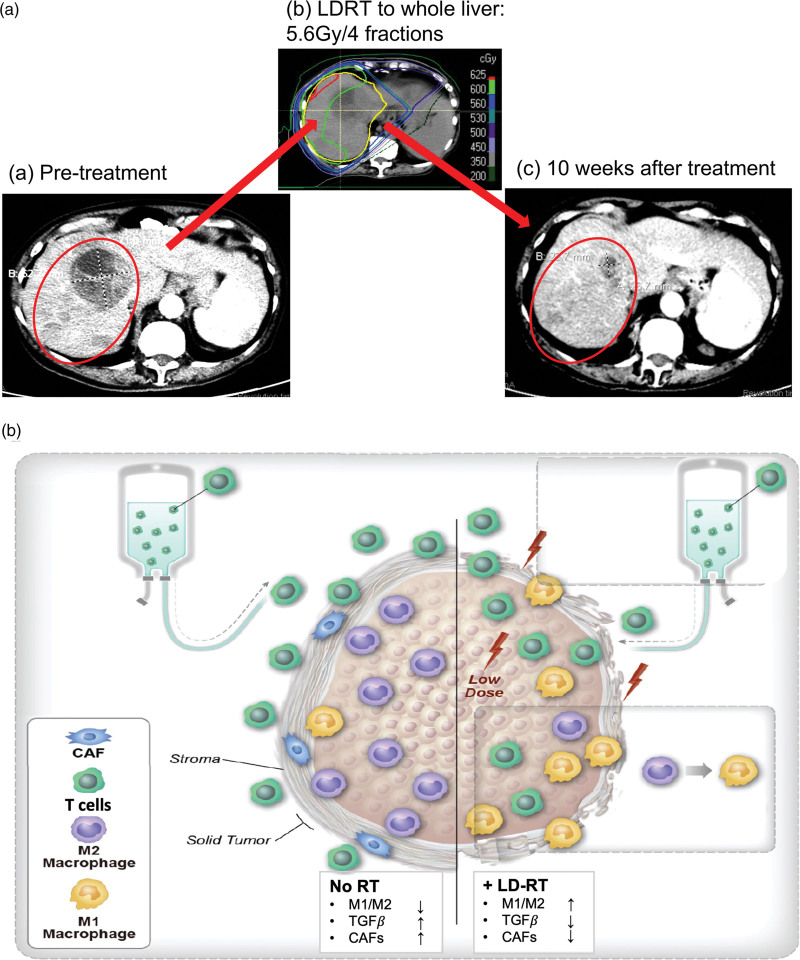
Fig. 1.
(a) Representative images of LDRT lesion response. Baseline CT image prior to treatment (a, 12 September 2019). The representative lesions (red circled) measured 12.2 cm in sum of longest diameter; liver metastases received LDRT (b, 5.6 Gy/4 fractions, 23 September 2019–26 September 2019) 7 days before afami-cel infusion; c, 10 weeks after TCR/LDRT therapy, the patient achieved best overall response with PR and the LDRT lesion shrank to 6.4 cm (-47.5%) on 12 September 2019. (b) Overview of LDRTs effects on the tumor stroma when in combination with T cell therapy. The tumor stroma factors including immunosuppressive M2 macrophages, TGF-β and CAFs inhibit T cell infiltration and activity and limit the effectiveness of T cell therapy for solid tumors (left). Local delivery of LDRT could induce a higher ratio of M1/M2, decrease TGF-β and reduce CAFs, which facilitate effective T cells infiltrating into the tumor and enhance the antitumor effect of T cell therapy (right). CAFs, cancer-associated fibroblasts; LDRT, low-dose radiotherapy; PD, progressive disease; PR, partial response; RT, radiotherapy; TCR, T cell receptor; TGF-β, tumor growth factor-β.
Discussion and conclusions
Identifying a sufficiently specific TAA is a major challenge for a safe and effective ACT. The demonstrated safety and clinical efficacy of afami-cel in various solid tumors supports the therapeutic benefit of engineered TCR-T in patients with malignancies expressing MAGE-A4 [28–30]. According to data from the phase-I trial, afami-cel shows promising efficacy and a manageable safety profile at a dose range of 1.2–10 × 109. During the 2020 American Society of Clinical Oncology Meeting, responses were demonstrated in patients with synovial sarcoma, non-small cell lung, and head-and-neck cancers [28]. In the ongoing phase II SPEARHEAD-1 trial (NCT04044768) [30], the overall response rate was 41.4% for synovial sarcoma patients (n = 12, with two complete responses) and 25.0% (n = 1) for myxoid/round cell liposarcoma patients. The median duration-of-response has not yet been reached (range, 4.3+–38.0+ weeks). Another phase I trial (SURPASS, NCT04044859) with ADP-A2M4CD8, a next-generation SPEAR T cell targeting MAGE-A4, is ongoing.
A variety of factors determine patient responses to ACT [24]. Two potential reasons for failure are the lack of intratumoral penetration and the inhibitory TME. LDRT in combination with ACT has shown promising results in clearing these obstacles. Our lab has recently demonstrated the efficacy of LDRT preclinically and clinically. LDRT improved tumor control, CPI efficacy, and overall survival in mice [18]. Moreover, in a recent clinical trial, we observed that LDRT, when used to complement HDRT, safely promoted effector immune cell infiltration into the tumor [31]. DeSelm et al. demonstrated the benefit of LDRT prior to CAR-T in an in-vivo orthotopic pancreatic cancer cell model [32]. Pancreatic cancer cells in this model were heterogeneous, with only some expressing the CAR target (sLeA). LDRT and ACT resulted in tumor lysis of both antigen-positive and antigen-negative cells. Consistent results were observed in a patient with diffuse large B cell lymphoma with heterogeneous CD19 expression. The patient received palliative RT to the lower leg (4 Gy × 5 fractions) prior to ACT [32]. One year later, the patient’s disease progressed with recurrence at initial disease sites and the development of new lesions. However, the area that received palliative RT remained disease-free.
In this case report, the patient achieved a durable PR after treatment with LDRT in combination with afami-cel therapy. The duration-of-response within the LDRT field was almost three-fold longer compared to the out-of-field lesions. Based on our preclinical and clinical studies [18,31,33], we hypothesize that LDRT modulates the stroma of tumors to facilitate afami-cel T cell infiltration and tumorolysis. The tumor stroma factors including immunosuppressive M2 macrophages, TGF-β, and cancer-associated fibroblasts (CAFs) inhibit T cell infiltration and activity and limit the effectiveness of T cell therapy for solid tumors. Local delivery of LDRT could induce a higher ratio of M1/M2, decrease TGF-β and reduce CAFs, which facilitate effective T cells infiltrating into the tumor and enhance the antitumor effect of T cell therapy (Fig. 1b). This case report supports previous observations in providing evidence for further studies to explore the benefit of LDRT in ACT.
Acknowledgements
The authors thank all investigators and research staff at MD Anderson Cancer Center and Adaptimmune.
This work was funded by Adaptimmune; the National Cancer Institute (via Cancer Center Support Core Grant P30CA016672 to The University of Texas MD Anderson Cancer Center).
Conflicts of interest
J.W.W. reports research support from GlaxoSmithKline, Bristol Meyers Squibb, Merck, Nanobiotix, RefleXion, Alkermes, Artidis, Mavu Pharma, Takeda, Varian, and Checkmate Pharmaceuticals. He serves on the scientific advisory board for Legion Healthcare Partners, RefleXion Medical, MolecularMatch, Merck, AstraZeneca, Aileron Therapeutics, OncoResponse, Checkmate Pharmaceuticals, Mavu Pharma, Alpine Immune Sciences, Ventana Medical Systems, Nanobiotix, China Medical Tribune, GI Innovation, Genentech, and Nanorobotics. He is on Speaking Engagements for Ventana Medical Systems, US Oncology, Alkermes, and Boehringer Ingelheim. He is co-founder of Healios, MolecularMatch, OncoResponse and serves as an advisor to Astra Zeneca, OncoResponse, Merck, MolecularMatch, Incyte, Aileron, and Nanobiotix. J.W.W. holds stock or ownership in Alpine Immune Sciences, Checkmate Pharmaceuticals, Healios, Mavu Pharma, Legion Healthcare Partners, MolecularMatch, Nanorobotics, OncoResponse, and RefleXion. He has accepted honoraria in the form of travel costs from Nanobiotix, RefleXion, Varian, Shandong University, The Korea Society of Radiology, Aileron Therapeutics, and Ventana. J.W.W. has the following patents; MP470 (amuvatinib), MRX34 regulation of PDL1, XRT technique to overcome immune resistance. MD Anderson Cancer Center has a trademark for RadScopalTM. D.S.H. declares grants from AbbVie, Adaptimmune, Aldi-Norte, Amgen, Astra-Zeneca, Bayer, BMS, Daiichi-Sankyo, Deciphera, Eisai, Erasca, Fate Therapeutics, Genentech, Genmab, Infinity, Kite, Kyowa, Lilly, LOXO, Merck, Medimmune, Mirati, Mologen, Navier, NCI-CTEP, Novartis, Numab, Pfizer, Pyramid Bio, SeaGen, Takeda, Turning Point Therapeutics, Verstatem, VM Oncology; Travel, accommodations and expenses from Bayer, Genmab, AACR, ASCO, SITC, Telperian; Consulting, speaker or advisory role from Adaptimmune, Alpha Insights, Acuta, Alkermes, Amgen, Aumbiosciences, Atheneum, Axiom, Barclays, Baxter, Bayer, Boxer Capital, BridgeBio, CDR-life AG, COR2ed, COG, Ecor1, Genentech, Gilead, GLG, Group H, Guidepoint, HCW Precision, Immunogen, Infinity, Janssen, Liberium, Medscape, Numab, Oncologia Brasil, Pfizer, Pharma Intelligence, POET Congress, Prime Oncology, Seattle Genetics, ST Cube, Takeda, Tavistock, Trieza Therapeutics, Turning Point, WebMD, Ziopharm; Other ownership interests from OncoResponse (Founder), and Telperian Inc (Advisor). T.W., H.D., Q.L., P.M.F., and E.N. are Adaptimmune employees. For the remaining authors, there are no conflicts of interest.
Supplementary Material
Footnotes
Supplemental Digital Content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's website, www.melanomaresearch.com.
References
- 1.Waldman AD, Fritz JM, Lenardo MJ. A guide to cancer immunotherapy: from T cell basic science to clinical practice. Nat Rev Immunol 2020; 20:651–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sadeqi Nezhad M, Yazdanifar M, Abdollahpour-Alitappeh M, Sattari A, Seifalian A, Bagheri N. Strengthening the CAR-T cell therapeutic application using CRISPR/Cas9 technology. Biotechnol Bioeng 2021; 118:3691–3705. [DOI] [PubMed] [Google Scholar]
- 3.Beyar-Katz O, Gill S. Advances in chimeric antigen receptor T cells. Curr Opin Hematol 2020; 27:368–377. [DOI] [PubMed] [Google Scholar]
- 4.Reina-Campos M, Moscat J, Diaz-Meco M. Metabolism shapes the tumor microenvironment. Curr Opin Cell Biol 2017; 48:47–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jarnicki AG, Lysaght J, Todryk S, Mills KH. Suppression of antitumor immunity by IL-10 and TGF-beta-producing T cells infiltrating the growing tumor: influence of tumor environment on the induction of CD4+ and CD8+ regulatory T cells. J Immunol 2006; 177:896–904. [DOI] [PubMed] [Google Scholar]
- 6.Chen W, Jin W, Hardegen N, Lei KJ, Li L, Marinos N, et al. Conversion of peripheral CD4+CD25-naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J Exp Med 2003; 198:1875–1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Husain Z, Huang Y, Seth P, Sukhatme VP. Tumor-derived lactate modifies antitumor immune response: effect on myeloid-derived suppressor cells and NK cells. J Immunol 2013; 191:1486–1495. [DOI] [PubMed] [Google Scholar]
- 8.Rodriguez PC, Quiceno DG, Zabaleta J, Ortiz B, Zea AH, Piazuelo MB, et al. Arginase I production in the tumor microenvironment by mature myeloid cells inhibits T cell receptor expression and antigen-specific T cell responses. Cancer Res 2004; 64:5839–5849. [DOI] [PubMed] [Google Scholar]
- 9.Fletcher M, Ramirez ME, Sierra RA, Raber P, Thevenot P, Al-Khami AA, et al. l-Arginine depletion blunts antitumor T cell responses by inducing myeloid-derived suppressor cells. Cancer Res 2015; 75:275–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang F, Wang H, Wang X, Jiang G, Liu H, Zhang G, et al. TGF-β induces M2-like macrophage polarization via SNAIL-mediated suppression of a pro-inflammatory phenotype. Oncotarget 2016; 7:52294–52306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Colegio OR, Chu NQ, Szabo AL, Chu T, Rhebergen AM, Jairam V, et al. Functional polarization of tumour-associated macrophages by tumour-derived lactic acid. Nature 2014; 513:559–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ghiringhelli F, Puig PE, Roux S, Parcellier A, Schmitt E, Solary E, et al. Tumor cells convert immature myeloid dendritic cells into TGF-beta-secreting cells inducing CD4+CD25+ regulatory T cell proliferation. J Exp Med 2005; 202:919–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bader JE, Voss K, Rathmell JC. Targeting metabolism to improve the tumor microenvironment for cancer immunotherapy. Mol Cell 2020; 78:1019–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mariathasan S, Turley S, Nickles D, Castiglioni A, Yuen K, Wang Y. TGFβ attenuates tumour response to PD-L1 blockade by contributing to exclusion of T cells. Nature 2018; 22: 544–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Menon H, Ramapriyan R, Cushman TR, Verma V, Kim HH, Schoenhals JE, et al. Role of radiation therapy in modulation of the tumor stroma and microenvironment. Front Immunol 2019; 10:193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ghiringhelli F, Ménard C, Terme M, Flament C, Taieb J, Chaput N, et al. CD4+CD25+ regulatory T cells inhibit natural killer cell functions in a transforming growth factor-beta-dependent manner. J Exp Med 2005; 202:1075–1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Klug F, Prakash H, Huber PE, Seibel T, Bender N, Halama N, et al. Low-dose irradiation programs macrophage differentiation to an iNOS+/M1 phenotype that orchestrates effective T cell immunotherapy. Cancer Cell 2013; 24:589–602. [DOI] [PubMed] [Google Scholar]
- 18.Barsoumian HB, Ramapriyan R, Younes AI, Caetano MS, Menon H, Comeaux NI, et al. Low-dose radiation treatment enhances systemic antitumor immune responses by overcoming the inhibitory stroma. J ImmunoTher Cancer 2020; 8:e000537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu R, Xiong S, Zhang L, Chu Y. Enhancement of antitumor immunity by low-dose total body irradiation is associated with selectively decreasing the proportion and number of T regulatory cells. Cell Mol Immunol 2010; 7:157–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pandey R, Shankar BS, Sharma D, Sainis KB. Low dose radiation induced immunomodulation: effect on macrophages and CD8+ T cells. Int J Radiat Biol 2005; 81:801–812. [DOI] [PubMed] [Google Scholar]
- 21.Zhou L, Zhang X, Li H, Niu C, Yu D, Yang G, et al. Validating the pivotal role of the immune system in low-dose radiation-induced tumor inhibition in Lewis lung cancer-bearing mice. Cancer Med 2018; 7:1338–1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aleksic M, Liddy N, Molloy PE, Pumphrey N, Vuidepot A, Chang KM, et al. Different affinity windows for virus and cancer-specific T cell receptors: implications for therapeutic strategies. Eur J Immunol 2012; 42:3174–3179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cheng J, Zhao L, Zhang Y, Qin Y, Guan Y, Zhang T, et al. Understanding the mechanisms of resistance to CAR T cell therapy in malignancies. Front Oncol 2019; 9:1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rath JA, Arber C. Engineering strategies to enhance TCR-based adoptive T cell therapy. Cells 2020; 9:1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tio D, Kasiem FR, Willemsen M, van Doorn R, van der Werf N, Hoekzema R, et al. Expression of cancer/testis antigens in cutaneous melanoma: a systematic review. Melanoma Res 2019; 29:349–357. [DOI] [PubMed] [Google Scholar]
- 26.Ishihara M, Kageyama S, Miyahara Y, Ishikawa T, Ueda S, Soga N, et al. MAGE-A4, NY-ESO-1 and SAGE mRNA expression rates and co-expression relationships in solid tumours. BMC Cancer 2020; 20:606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sanderson JP, Crowley DJ, Wiedermann GE, Quinn LL, Crossland KL, Tunbridge HM, et al. Preclinical evaluation of an affinity-enhanced MAGE-A4-specific T cell receptor for adoptive T cell therapy. Oncoimmunology 2020; 9:1682381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hong DS, Tine BAV, Olszanski AJ, Johnson ML, Liebner DA, Trivedi T, et al. Phase I dose escalation and expansion trial to assess the safety and efficacy of ADP-A2M4 SPEAR T cells in advanced solid tumors. J Clin Oncol 2020; 38(15_suppl):102. [Google Scholar]
- 29.Araujo DM, Mihaela DM, Agulnik M, D'Angelo SP, Blay J-Y, Strauss SJ, et al. SPEARHEAD-1: a phase II trial of ADP-A2M4 SPEAR T cells in patients with advanced synovial sarcoma or myxoid/round cell liposarcoma. J Clin Oncol 2021; 38(suppl 15):TPS11569. [Google Scholar]
- 30.D’Angelo SP, Tine BAV, Attia S, Blay J-Y, Strauss SJ, Morales CMV, et al. SPEARHEAD-1: a phase 2 trial of afamitresgene autoleucel (Formerly ADP-A2M4) in patients with advanced synovial sarcoma or myxoid/round cell liposarcoma. J Clin Oncol 2021; 3939(suppl 15):11504. [Google Scholar]
- 31.Patel RR, He K, Barsoumian HB, Chang JY, Tang C, Verma V, et al. High-dose irradiation in combination with non-ablative low-dose radiation to treat metastatic disease after progression on immunotherapy: results of a phase II trial. Radiother Oncol 2021; 162:60–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.DeSelm C, Palomba ML, Yahalom J, Hamieh M, Eyquem J, Rajasekhar VK, et al. Low-dose radiation conditioning enables CAR T cells to mitigate antigen escape. Mol Ther 2018; 26:2542–2552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Menon H, Chen D, Ramapriyan R, Verma V, Barsoumian HB, Cushman TR, et al. Influence of low-dose radiation on abscopal responses in patients receiving high-dose radiation and immunotherapy. J ImmunoTher Cancer 2019; 7:237. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.