Abstract
In this study, we aimed to evaluate the association of early anxious behavior with serotonin, dopamine, and their metabolites in a 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) murine model of Parkinson’s disease. Forty C57BL/6 male mice were randomly divided into the control group (n = 20) and the model group (n = 20). Mice in the model group were injected intraperitoneally with MPTP. The light-dark box (LDB) and elevated plus-maze were used to monitor anxious behavior. The association of early anxious behavior with neurotransmitters in the prefrontal cortex, hippocampus, and striatum was evaluated. In our murine model, MPTP induced a decreased level of 5-hydroxytryptamine and its metabolite 5-hydroxyindoleacetic acid (5-HIAA) in the prefrontal cortex, hippocampus, and striatum (all P < 0.05); however, it only induced a decreased level of dopamine and its metabolite homovanillic acid (HVA) in the striatum (both P < 0.001), with a negative correlation in the hippocampus and a positive correlation in the cortex and striatum. In the LDB, 5-hydroxytryptamine levels in the cortex and dopamine and HVA levels in the striatum were negatively correlated with anxious behavior. Moreover, in the elevate plus-maze, 5-hydroxytryptamine and 5-HIAA in the cortex and dopamine and HVA in the striatum were positively correlated with the ratio of the time spent in open arms. In the murine model of early Parkinson’s disease, the balance between dopamine and 5-hydroxytryptamine systems varied among brain regions. The depletion of 5-hydroxytryptamine in the cortex and dopamine in the striatum may be associated with anxiety behaviors in MPTP-treated mice.
Keywords: anxiety, dopamine, 1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine, mice, Parkinson’s disease, serotonin
Introduction
Parkinson’s disease is a neurodegenerative disorder characterized by impairment in motor function that includes resting tremors, bradykinesia, and balance dysfunction; however, severe nonmotor symptoms, such as olfactory deficits, memory loss, impaired executive function, depression, and anxiety, occur in 30–60% of patients [1,2]. Induction of Parkinson’s disease by 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) administration is widely accepted as an animal model of Parkinson’s disease; however, whether this model induces nonmotor symptoms of Parkinson’s disease remains a debated issue [3–5].
Anxiety, a very common nonmotor symptom of Parkinson’s disease, often appears in the early stage of Parkinson’s disease and persists through the mid and late stages of the disease. Anxiety seriously affects the quality of life of patients with Parkinson’s disease. Previous studies have indicated that the etiology of anxiety disorders, in general, is related to genetic and neurobiochemical factors, combined with life stress events [6,7]. Although the etiology of anxiety symptoms in patients with Parkinson’s disease remains unclear, Parkinson’s disease exerts pervasive effects on the brain, including the basolateral amygdala, nucleus accumbens, frontal cortex, and raphe nucleus. Serotonin (5-hydroxytryptamine) and dopamine may play a central role in regulating emotional behavior. In general, disturbances in 5-hydroxytryptamine neurotransmission can lead to depression, anxiety, and memory impairment. Moreover, 5-hydroxytryptamine 4 receptor agonists can promote the extinction of situational fear. MPTP-treated mice are an accepted animal model to evaluate nonmotor symptoms of Parkinson’s disease [8]. Furthermore, MPTP-treated male C57BL/6 mice have been shown to develop associative memory impairment and conditioned fear [9] which are associated with a depletion of dopamine and 5-hydroxytryptamine levels in the striatum, frontal cortex, and amygdala. Whether the depletion of these neurotransmitters is associated with anxiety symptoms in Parkinson’s disease, however, is unclear [3]. Accordingly, in this study, we aimed to evaluate the association between changes in neurotransmitter levels in the brain and anxiety behaviors in a murine model of early-stage Parkinson’s disease induced by acute MPTP administration. Due to the limitations of material sampling techniques, we selected the hippocampus, prefrontal cortex, and basal ganglia as brain regions for neurotransmitter measurement.
Materials and methods
Laboratory animals and ethics statement
Forty male C57BL/6J mice with body weights, ranging from 20 g to 25 g, were maintained on a 12 : 12-h light/dark cycle and an ambient temperature of 22 °C. They were fed ad libitum. Animals were divided evenly into two groups by a numerical random table, namely, model groups and one control group, with 20 mice in each group. All procedures were carried out in accordance with the Medical Sciences guidelines for animal handling of Mianyang Central Hospital affiliated with the Medical College of University of Electronic Science and Technology of China.
Model preparation
For the mice in the model group, MPTP (CAS: 23007-85-4, Sigma-Aldrich, Massachusetts, USA) was diluted in saline to a concentration of 1 mg/ml and injected intraperitoneally at a dose of 20 mg/kg four times a day (injection during the day at 2-h intervals) for two consecutive days. Mice in the control group received an equal volume of saline, intraperitoneally injected [10,11]. Three days after the last MPTP injection, the anxiety behaviors of the mice in both groups were assessed using the light-dark box (LDB) and elevated plus-maze (EPM) tests.
Anxiety behavior assessment
Light-dark box test
The LDB, 44 cm × 21 cm × 21 cm, was based on Crawley’s design [12,13]. The light and dark compartments were set at 2/3 and 1/3 of the LDB, respectively, with both sides connected by an opening 13 cm × 5 cm in size. Prior to testing in the LDB, animals were provided with a 1-hr period to acclimate to the testing room. After this period, the incandescent lamp above the light compartment was turned on and the mice were allowed to explore the box freely for 5 min. Time, in seconds (s), spent in the dark compartment and the frequency of leaving the dark compartment (i.e. the number of times a mouse moved from the dark to the light compartment) were calculated as indices of anxiety.
Elevated plus-maze test
The EPM was based on the experimental design described by Lister [14]. After a 30-min period to acclimate to the test room, mice were placed in the central area of the maze, facing an open arm, and were allowed to freely explore the maze for 5 min. The following EPM parameters were calculated for analysis: open arm entry, defined as the number of times a mouse entered an open arm, based on the entry of all four paws into the arm, with the end of an entry defined as the exit of any paw from the arm; open arm time, defined as the time spent in all open arms; closed arm entry, defined as the number of times a mouse entered a closed arm; and closed arm time, defined as the time spent in all closed arms. The following two ratios were also calculated: OEr, calculated as the ratio of open-arm entries ; and OTr, calculated as the ratio of the time in open arms . The OEr and OTr have previously been used as effective indicators of anxiety behaviors in mice [15].
HPLC
After the behavioral tests, the mice were quickly sacrificed and the bilateral hippocampus, prefrontal cortex, and striatum were isolated. The levels of dopamine and its metabolite, homovanillic acid (HVA), and 5-hydroxytryptamine and its metabolite, 5-hydroxyindoleacetic acid (5-HIAA), in these brain regions, were quantified using HPLC. Briefly, tissue samples of selected brain structures were homogenized in 0.1 mmol/l of ice-cold HClO4. The homogenates were centrifuged twice at 10 000 × g for 10 min at 4 °C. After centrifugation, the supernatant was filtered through a 0.22 μm membrane. The obtained aliquots (40 μl) were injected into the HPLC system, The emission wavelength is 330 nm and the excitation wavelength is 280 nm. Column temperature was maintained at 35 °C. An external standard containing dopamine, HVA, 5-hydroxytryptamine, and 5-HIAA at concentrations of 1 mg/ml was used. Mobile phase A: chromatographic pure methanol, ultrasonic degassing for 20 min. Mobile phase B: 0.1 mol/L sodium acetate (containing 0.1 mmol/l ethylenediamine tetraacetic acid), ultrapure water configuration, pH 5.0 with acetic acid, ultrasonic degassing for 20 min. Mobile phase C: ultrapure water, ultrasonic degassing for 20 min. Mobile phase D: 90% pure water and 10% isopropyl alcohol. Adjust the container intake, mix mobile phases A and B in A certain proportion, filter with 0.22 μm microporous filter, ultrasonic gas for 20 min, close exhaust valve, sample quantity of 40 μl/time, and finally mix column with 90% mobile phase B + 10% mobile phase A, then gradient elution, mobile phase D siphon discharge at a rate of three drops per minute. After each sample determination, the samples were rinsed with mobile phase C for 20 min and then with mobile phase A for 10 min. The concentration of dopamine, HVA, 5-hydroxytryptamine, and 5-HIAA in the sample solution were calculated according to the measured peak area.
Statistical analysis
All data were tested as normal distribution by the Shapiro–Wilk test or QQ plot and expressed as the mean ± SD, and the differences between the two groups were evaluated using the independent sample t-test. The differences among different brain regions in the same experimental group were analyzed by one-way analysis of variance, and the multiple comparisons were performed by Bonferroni correction. The associations between anxiety behaviors and neurotransmitter levels were evaluated using Pearson correlation. P values < 0.05 with two-sided test were considered significant. Statistical analyses were performed using SPSS 21.0 software (SPSS, Inc., an IBM Company) and the MedCalc application (MedCalc Software Ltd).
Results
Anxiety behaviors in the light-dark box test
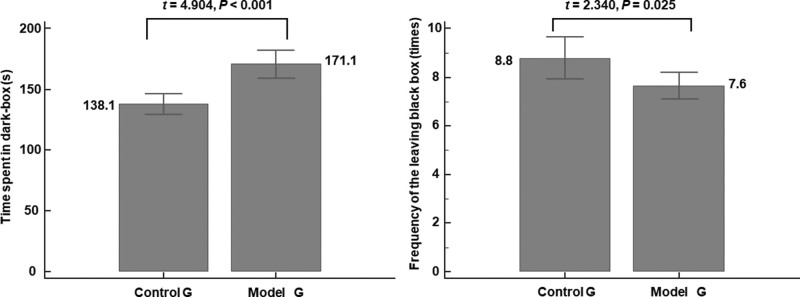
Significant anxiety symptoms appeared in the model group. We calculated the time spent in the dark box and the frequency of leaving the dark compartment, which are important behavioral indicators of anxiety in mice. The results showed that the time spent in the dark box was significantly longer in the model group than in the control group (171.1 ± 23.6 vs. 138.1 ± 18.6, respectively, t = 4.904, P < 0.001, Fig. 1a). The frequency of leaving the dark compartment was lower for the model group than the control group (7.6 ± 1.2 vs. 8.8 ± 1.9, respectively, t = −2.340, P = 0.025, Fig. 1b). These findings are indicative of higher anxiety in the model group than in control group.
Fig. 1.
Anxiety behaviors in the LDB test. Significant anxiety symptoms appeared in the model group. Compared to the control group, (a) the time mice spent in the dark compartment is significantly longer (*P < 0.01) and (b) the frequency of mice leaving the dark compartment was significantly lower for the model group (*P < 0.05). LDB, light-dark box.
Anxiety behaviors in the elevated plus-maze test
Significant anxiety symptoms appeared in the model group. We observed open arm entry, open arm time, closed arm entry, and closed arm time, and calculated the OEr and OTr, which are important behavioral indicators of anxiety in mice. Regarding the EPM data, while there was no difference in the OEr between the model group and control group (0.212 ± 0.086 vs. 0.188 ± 0.054, respectively, t = 1.024, P = 0.312), the OTr was significantly lower for the control group than model group (0.078 ± 0.042 vs. 0.118 ± 0.048, respectively, t = −2.817, P = 0.008). These findings are indicative of higher anxiety in the model group than in the control group, consistent with the findings on the LDB test.
Neurotransmitter levels determined by HPLC
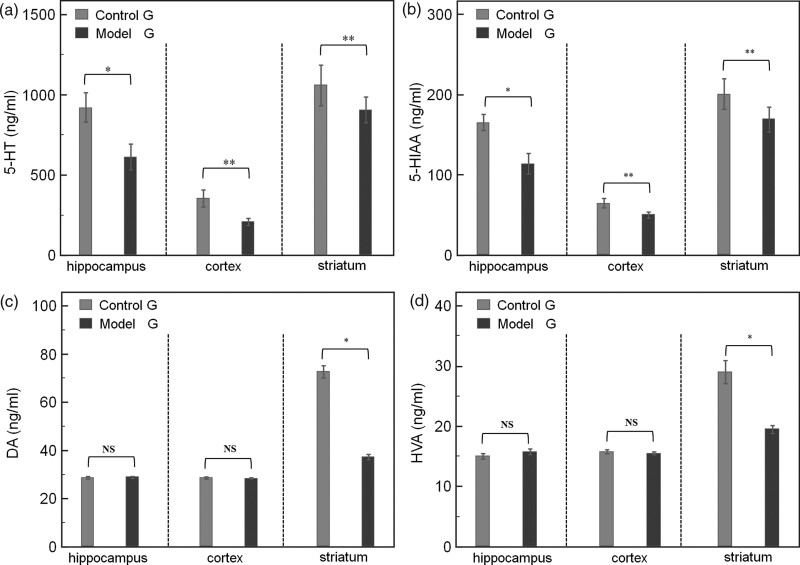
Dopamine and its metabolite HVA were significantly decreased in the striatum in the model group, and 5-hydroxytryptamine and metabolite 5-HIAA were significantly decreased in all brain regions. We measured the neurotransmitters most associated with anxiety behavior, 5-hydroxytryptamine and its metabolite, 5-HIAA, and dopamine and its metabolite, HVA. With regards to neurotransmitter levels, 5-hydroxytryptamine levels of the model group (Fig. 2a) were lower than that of the control group in the hippocampus, prefrontal cortex, and striatum (hippocampus: 613.0 ± 167.5 vs. 920.6 ± 190.5, t = −5.422, P < 0.001; prefrontal cortex: 210.3 ± 47.2 vs. 355.2 ± 110.2, t = −5.405, P < 0.001; striatum: 904.6 ± 171.8 vs. 1059.4 ± 268.2, t = −2.173, P = 0.036). Similarly, 5-HIAA levels did so (Fig. 2b) (hippocampus: 114.0 ± 27.9 vs. 165.5 ± 21.9, t = −6.489, P < 0.001; prefrontal cortex: 50.2 ± 7.7 vs. 65.2 ± 12.5, t = −4.579, P < 0.0011; striatum: 69.1 ± 33.7 vs. 201.0 ± 41.2, t = −2.687, P = 0.011). These findings are indicative of nonspecific damage to 5-hydroxytryptamine systems in the MPTP model of Parkinson’s disease; however, levels of dopamine and its metabolite, HVA, in the model group were lower than that of the control group only in the striatum (Fig. 2c and d) (dopamine: 37.2 ± 2.5 vs. 72.7 ± 5.5, t = −26.366, P < 0.001; HVA, 19.5 ± 1.4 vs. 29.0 ± 4.0, t = −9.942, P < 0.001); however, levels of dopamine and HVA in the hippocampus and prefrontal cortex were comparable between the two groups. These findings are indicative of the highly selective damage caused by MPTP to dopamine systems.
Fig. 2.
Neurotransmitter levels determined by HPLC. Dopamine and its metabolite HVA were significantly decreased in the striatum in the model group, and 5-hydroxytryptamine and metabolite 5-HIAA were significantly decreased in all brain regions. Compared to the control group, the 5-hydroxytryptamine levels in the hippocampus, prefrontal cortex, and striatum are significantly lower in the (a) model group (*P < 0.01 and **P < 0.05, respectively). The 5-HIAA levels in the hippocampus, prefrontal cortex, and striatum are significantly lower in the model group than in (b) the control group (*P < 0.01 and **P < 0.05, respectively). The (c, *P < 0.01) dopamine and (d, *P < 0.01) HVA levels in the striatum are significantly lower in the model group than in the control group. 5-HIAA, 5-hydroxyindoleacetic acid; HVA, homovanillic acid.
Correlation between neurotransmitter levels
In the model group, 5-hydroxytryptamine (F = 121.524, P < 0.001), HIAA (F = 121.524, P < 0.001), dopamine (F = 121.524, P < 0.001), and HVA (F = 121.524, P < 0.001) had different concentration levels in the brain. Pairwise comparisons with Bonferroni correction, 5-hydroxytryptamine (|tB| = 6.519–15.524, all P < 0.001) and its metabolite 5-HIAA (|tB| = 6.794–14.663, all P < 0.001) were statistically different between any two sites. While the concentration levels of dopamine and its metabolite HVA were higher in the striatum than in the hippocampus (tB = 16.359 and 11.280, both P < 0.001) and the cortical (tB = 17.274 and 11.787, both P < 0.001); however, there was no difference between the hippocampus and the cortex (tB = 0.915 and 0.507, both P = 1.000). In the control group, 5-hydroxytryptamine (F = 18.702, P < 0.001), HIAA (F = 19.319, P < 0.001), dopamine (F = 35.753, P < 0.001), and HVA (F = 31.938, P < 0.001) matched the model group in different parts of the brain. Pairwise comparisons with Bonferroni correction, 5-hydroxytryptamine (|tB| = 2.794–6.057, all P < 0.05) and its metabolite 5-HIAA (|tB| = 2.899–6.211, all P < 0.05) were statistically different between any two sites. While the concentration levels of dopamine and its metabolite HVA were higher in the striatum than in the hippocampus (tB = 8.113 and 6.121, both P < 0.001) and cortical (tB = 6.057 and 3.863, both P < 0.001); however, there was no difference between the hippocampus and the cortex (|tB| = 1.992 and 2.244, both P = 0.154 and 0.086).
We analyzed the relationship between 5-hydroxytryptamine and dopamine in different brain regions and the relationship between 5-HIAA and HVA. Significant correlations between neurotransmitters were presented as follows: the correlation between 5-HIAA and HVA levels was negative in the hippocampus (r = −0.323, t = −2.107, P = 0.042) but positive in the prefrontal cortex (r = 0.351, t = 2.314, P = 0.026) and striatum (r = 0.703, t = 6.090, P < 0.001). Overall, 5-hydroxytryptamine levels were positively correlated with dopamine levels only in the striatum (r = 0.386, t = 2.580, P = 0.014). These findings indicate that the balance between dopamine and 5-hydroxytryptamine systems varies among brain regions in the MPTP model, with a negative correlation in the hippocampus and a positive correlation in the prefrontal cortex and striatum.
Correlation between anxiety behaviors and neurotransmitter levels
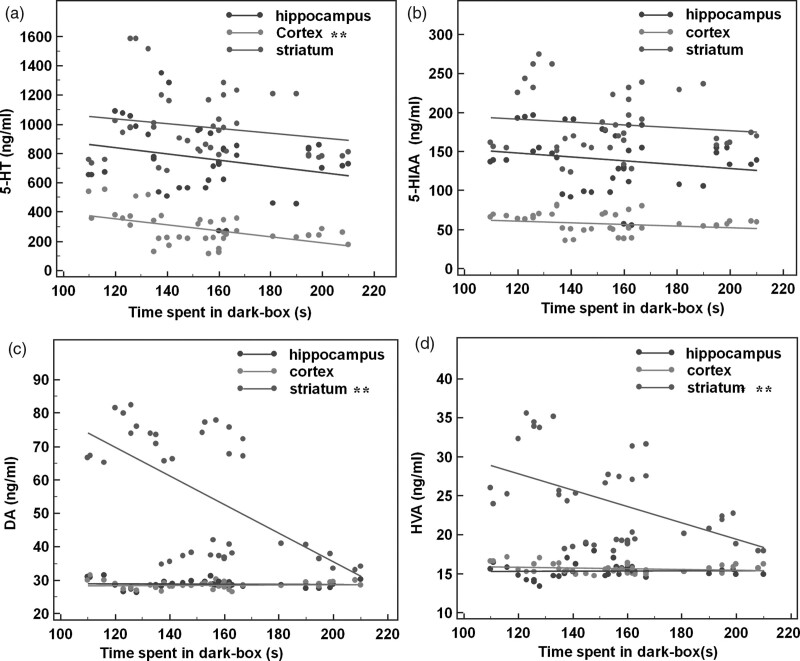
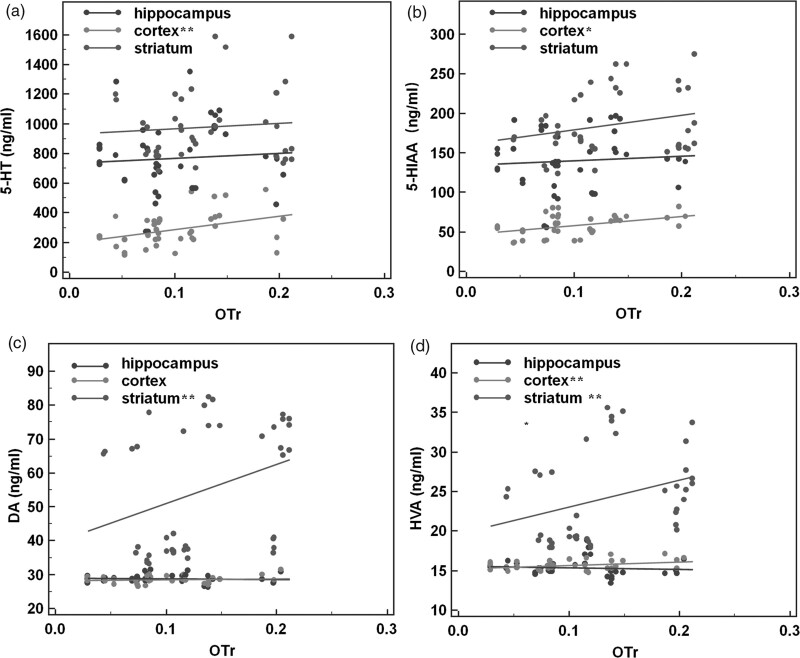
Depletion of 5-hydroxytryptamine in the cortex and dopamine in the striatum may be associated with anxiety behaviors. We analyzed the relationship between core indicators of anxious behaviors (the time spent in the dark box and OTr) and neurotransmitters (5-hydroxytryptamine, 5-HIAA, dopamine, HVA). The correlation between neurotransmitter and anxiety behaviors of mice in the LDB test or EPM test was analyzed by the Pearson correlation. On the LDB test, the time spent in the dark box had no association with any neurotransmitter in the hippocampus (all P > 0.05) but negatively correlated with the 5-hydroxytryptamine level in the prefrontal cortex (r = −0.485, t = −3.551, P = 0.001, Fig. 3a), as well as dopamine (r = −0.650, t = −4.883, P < 0.001, Fig. 3c) and HVA (r = −0.519, t = −3.566, P = 0.001, Fig. 3d) levels in the striatum. Therefore, time spent in the dark increased with the depletion of 5-hydroxytryptamine in the cortex and dopamine in the striatum. On the EPM test, OTr was also not associated with any neurotransmitter in the hippocampus (all P > 0.05) but positively correlated with levels of 5-hydroxytryptamine (r = 0.392, t = 2.624, P = 0.013, Fig. 4a), 5-HIAA (r = 0.442, t = 3.040, P = 0.004, Fig. 4b), and HVA (r = 0.356, t = 2.351, P = 0.024, Fig. 4d) in the prefrontal cortex, as well as dopamine (r = 0.334, t = 2.183, P = 0.035, Fig. 4c) and HVA (r = 0.323, t = 2.1024, P = 0.042, Fig. 4d) in the striatum. These findings indicate that the depletion of 5-hydroxytryptamine in the cortex and dopamine in the striatum is associated with a decrease in the OTr.
Fig. 3.
Correlation between anxiety behaviors in the LDB test and neurotransmitter levels. Depletion of 5-hydroxytryptamine in the cortex and dopamine in the striatum may be associated with anxiety behaviors. (a) The 5-hydroxytryptamine level in the prefrontal cortex is inversely correlated with the time mice spent in the dark box (r = −0.485, P < 0.01). (b) The 5-HIAA level in the prefrontal cortex did not correlate with the time mice spent in the dark box. The (c) dopamine (r = −0.65, P < 0.01) and (d) HVA (r = −0.519, P < 0.01, **P < 0.05) levels in the striatum inversely correlated with the time mice spent in the dark box. 5-HIAA, 5-hydroxyindoleacetic acid; HVA, homovanillic acid, LDB, light-dark box.
Fig. 4.
Correlation between anxiety behaviors in the EPM test and neurotransmitter levels. Depletion of 5-hydroxytryptamine in the cortex and dopamine in the striatum may be associated with anxiety behaviors. In the hippocampus, there was no correlation between neurotransmitter levels and OTr (all P > 0.05). In the prefrontal cortex, (a) 5-hydroxytryptamine (r = 0.392, P < 0.05), (b) 5-HIAA (r = 0.442, P < 0.01), and (d) HVA (r = 0.356, P < 0.05) levels correlated positively with OTr. In the striatum, (c) dopamine (r = 0.334, P < 0.05) and (d) HVA (r = 0.323, P < 0.05) levels both correlated positively with OTr (*P < 0.01, **P < 0.05). EPM, elevated plus-maze; 5-HIAA, 5-hydroxyindoleacetic acid; HVA, homovanillic acid; OTr, ratio of time entering open arms.
Discussion
Parkinson’s disease is a very common neurodegenerative disease. The motor disorders of Parkinson’s disease are well understood, and various drug treatment options with demonstrated high efficacy are available to manage these symptoms; however, nonmotor symptoms of Parkinson’s disease, such as cognitive, behavioral, emotional, sleep, and autonomic disorders, are often ignored by clinicians, although these symptoms have an impact on the prognosis of Parkinson’s disease and can influence treatment outcomes for motor symptoms. Of these nonmotor symptoms, anxiety is predominant and is associated with significant negative impacts on activities of daily living and quality of life of patients with Parkinson’s disease. Clinically, most patients with Parkinson’s disease-related anxiety disorders do not receive timely treatment. If more physicians understood the role of anxiety in Parkinson’s disease, more patients might be offered psychotherapy or drug treatment, which may improve their quality of life [16–18]. Evaluation of the mechanisms underlying Parkinson’s disease-related anxiety is difficult in humans and, thus, the use of animal models is indispensable. The murine MPTP model is an accepted model of Parkinson’s disease, including anxiety behaviors, although the underlying mechanisms of Parkinson’s disease-related anxiety in this model have remained unclear [19,20]. The acute murine model of Parkinson’s disease we used in this study, which consists of two consecutive days of MPTP administration, is one of the innovations of our study design to better assess behaviors of anxiety in early Parkinson’s disease [21,22].
Various anxiety disorders share a common neurobiological basis. Although impairment of the 5-hydroxytryptamine system in anxiety disorders has previously been demonstrated, the involvement of the dopamine system has been poorly studied. Anxiety in Parkinson’s disease is a specific category of anxiety disorder as it depends on the biochemical effects of both the 5-hydroxytryptamine and dopamine systems [23–25].
The neurobiological basis of anxiety behaviors is complex, involving multiple extra-basal brain regions (the amygdala, nucleus accumbens, frontal cortex, and raphe nucleus) and the neural transfer networks connecting these regions. Dopamine and 5-hydroxytryptamine receptors are widely present in these networks and play important roles in the development of anxiety behaviors. Among these receptors, 5-hydroxytryptamine 1A receptors are most densely distributed in the limbic system, whereas dopamine and 5-hydroxytryptamine 1B receptors are mainly found in the basal ganglia and 5-hydroxytryptamine 2, 3, and 4 receptors are concentrated in the neocortex, posterior limbus, and hippocampus, respectively. By contrast, 5-hydroxytryptamine 5, 6, and 7 receptors are distributed in the cerebral cortex, limbic system, hypothalamus, thalamus, brainstem, and other brain regions [26]. On the basis of the currently available sampling technology in our laboratory, we selected the hippocampus, prefrontal cortex, and striatum as the brain regions to measure the levels of dopamine, HVA, 5-hydroxytryptamine, and 5-HIAA neurotransmitters.
Some studies have suggested that anxiety disorder may be a determinant factor in the quality of life of patients with Parkinson’s disease. A retrospective cohort study of a large sample population showed that individuals with an anxious personality are more likely to be negatively affected by Parkinson’s disease, indicating that anxiety symptoms may be an independent risk factor for Parkinson’s disease [27]. As anxiety symptoms and Parkinson’s disease course may influence one another, it is important to elucidate Parkinson’s disease-associated anxiety symptoms. In this study, we used established behavioral testing tools (LDB and EPM) to evaluate the anxiety behaviors in our murine model of Parkinson’s disease [28]. Our findings showed that mice in the model group spent a significantly longer time in the dark compartment (Fig. 1a) and a decreased frequency of leaving this compartment (Fig. 1b) compared to animals in the control group. Moreover, the OTr was significantly lower in the model group than in the control group. Therefore, behaviors of anxiety in the model group were observed on both the LDB and EPM tests, which is consistent with the clinical behaviors of anxiety in patients with Parkinson’s disease.
The interaction of 5-hydroxytryptamine with dopaminergic neurons is critical when studying the mechanisms of various neuroactive drugs, including antipsychotics [29]. Studies have shown that the 5-hydroxytryptamine system is involved in the anxiety symptoms of patients with Parkinson’s disease [30], but the mechanisms of specific 5-hydroxytryptamine systems in the pathogenesis of Parkinson’s disease-related anxiety have not previously been described. Similarly, while it is generally accepted that dopamine depletion in patients with Parkinson’s disease causes motor symptoms, the role of the dopamine system in Parkinson’s disease-related anxiety disorders has not been previously described. Accordingly, we designed our study to address this gap in knowledge, evaluating the change in concentrations of dopamine, HVA, 5-hydroxytryptamine, and 5-HIAA neurotransmitters in various brain regions and anxiety behaviors in an MPTP murine model of early Parkinson’s disease.
The content of dopamine and HVA in the striatum was significantly lower in the model group than in the control group. This finding validates our MTPT mouse model of Parkinson’s disease to study Parkinson’s disease-associated changes in behavior. MPTP is a meperidine derivative and a potent neurotoxin that selectively damages nigrostriatal dopaminergic neurons in the basal ganglia. Because the dopamine and 5-hydroxytryptamine systems in the limbic system are interdependent, changes in dopamine levels in these nuclei can affect 5-hydroxytryptamine levels and vice versa. This bidirectional interaction may be involved in the generation of anxiety behaviors [31,32].
In our study, depletion of 5-hydroxytryptamine and its metabolite, 5-HIAA, was observed in the hippocampus, cortex, and striatum. The toxic effects of MPTP should be considered in the interpretation of this finding, highlighting the importance of evaluating the role of changes in 5-hydroxytryptamine neurotransmitter levels in the anxiety behaviors of Parkinson’s disease mice. In clinical practice, selective serotonin reuptake inhibitors (SSRIs) are the most commonly used anxiolytic drugs, which indirectly increase 5-hydroxytryptamine concentrations in the brain, thereby improving the anxiety symptoms of patients. Decreased 5-hydroxytryptamine concentrations are associated with the occurrence of anxiety behaviors [33], and a similar depletion of 5-hydroxytryptamine appears to induce anxiety behaviors in mice.
Pearson’s correlation coefficients suggested that 5-HIAA and HVA concentrations were negatively correlated in the hippocampus, whereas 5-HIAA and HVA contents were positively correlated in the cortex and striatum. Given that the balance between the dopamine system and the 5-hydroxytryptamine system affects brain regions differently, this relationship may affect the anxiety behavior of mice. The 5-hydroxytryptamine level in the cortex was negatively correlated with the time mice spent in the dark compartment of the LDB, suggesting that 5-hydroxytryptamine depletion in the cortex of mice may increase the time they spend in the dark box and aggravate the anxiety behaviors. Furthermore, intrastriatal dopamine and HVA levels were negatively correlated with the time mice stayed in the dark compartment. This suggests that decreases in striatal dopamine concentrations may increase the time mice spend in the dark compartment of the LDB and aggravate anxiety behaviors in mice. These findings show that in addition to changes in the 5-hydroxytryptamine system, depletion of the dopamine system is also involved in the mechanisms causing anxiety behaviors. It is likely that imbalances between the 5-hydroxytryptamine and dopamine systems in the hippocampus, cortex, and striatum lead to anxiety behaviors in mice and that the balance between these systems varies among brain regions. A reduction in 5-hydroxytryptamine concentration in the cortex (i.e. a relative increase in dopamine concentration) may be involved in the mechanisms leading to anxiety disorders. Similarly, in the hippocampus and striatum, decreased 5-hydroxytryptamine levels (i.e. a relative decrease in dopamine levels) may be involved in mechanisms underlying anxiety disorders. These findings are similar to the results of previously published studies [34,35].
Pearson’s correlation coefficients between anxiety behaviors in behavioral tests and neurotransmitter levels were consistent for the EPM and LDB tests. In the cortex, 5-hydroxytryptamine, 5-HIAA, and HVA concentrations were positively correlated with the OTr. In the striatum, dopamine and HVA levels were both positively correlated with the OTr. This suggests that 5-hydroxytryptamine depletion in the cortex and depletion of dopamine in the striatum are associated with decreases in the OTr. The findings of both behavioral assessments indicate that anxiety behaviors of mice are related to the decreases in 5-hydroxytryptamine and dopamine concentrations in different brain regions. These findings require further verification.
Previous studies have reported that the murine MPTP model of Parkinson’s disease affects both dopamine and 5-hydroxytryptamine levels in brain regions, including the hippocampus [36]. Therefore, our study design considered both the 5-hydroxytryptamine and the dopamine systems on anxiety behaviors. SSRIs, commonly used to treat anxiety disorders, are thought to exert their therapeutic effect by blocking 5-hydroxytryptamine reuptake via the serotonin transporter system. In clinical practice, SSRIs have poor effects on some patients with anxiety disorders for whom low-dose dopamine receptor antagonists alleviate anxiety symptoms [37]. This suggests that dopaminergic mechanisms may also be important as the 5-hydroxytryptamine and dopamine systems have bidirectional influences on each other. Animal studies have shown that 5-hydroxytryptamine agonists and SSRIs increase extracellular dopamine levels in the striatum, hypothalamus, and prefrontal cortex and that dopamine depletion abrogates the antidepressant effects of SSRIs. This provides new insights into key therapeutic mechanisms of SSRIs, including biochemical effects on dopamine neurotransmission [38]. It might be possible to design neurotransmitter modulators that selectively target specific brain regions to alleviate anxiety symptoms without affecting motor symptoms in patients with Parkinson’s disease.
This study had several limitations. Firstly, we did not monitor additional behavioral data such as latency time, transition, movements in the light, movements in dark, time in light, time in dark, and rears. Secondly, two consecutive days of administration of MPTP developing anxiety behaviors is not confirmed by previous studies. Finally, we did not measure the neurochemicals in anxiety-related brain regions like the amygdala. Further studies are needed to improve and verify these limitations.
Conclusion
In a murine MPTP model of early Parkinson’s disease, the balance between the dopamine and 5-hydroxytryptamine systems varied among brain regions. Depletion of 5-hydroxytryptamine in the cortex and dopamine in the striatum may be associated with anxiety behaviors in MPTP-treated mice; however, these results are limited to MPTP-treated mice. To verify that such biochemical changes in the brain are consistent in Parkinson’s disease-related anxiety disorders, we need additional animal experiments and clinical evidence.
Acknowledgements
This work was supported by a Sichuan Provincial Health and Family Planning Commission grant (number 17PJ088). S.Y., S.Z., and H.H. contributed to the conception and design of the study. J.S. organized the database. Y.Y. performed the statistical analysis. R.Y. wrote the first draft of the manuscript. Y.Z., Y.Y., S.X., and L.H. wrote sections of the manuscript. J.S. reviewed and revised the manuscripts. All authors contributed to manuscript revision and read and approved the submitted version. The data that support the findings of this study are available from the corresponding author upon reasonable request.
Conflicts of interest
There are no conflicts of interest.
Footnotes
Dr. Yuwei Yang and Dr. Jian Shi contributed equally.
References
- 1.Liguori S, Moretti A, Palomba A, Paoletta M, Gimigliano F, De Micco R, et al. Non-motor impairments affect walking kinematics in Parkinson disease patients: a cross-sectional study. NeuroRehabilitation 2021; 49:481–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Polo-Morales A, Alcocer-Salas A, Rodríguez-Violante M, Pinto-Solís D, Solís-Vivanco R, Cervantes-Arriaga A. Association between somatization and nonmotor symptoms severity in people with Parkinson disease. J Geriatr Psychiatry Neurol 2021; 34:60–65. [DOI] [PubMed] [Google Scholar]
- 3.Vucković MG, Wood RI, Holschneider DP, Abernathy A, Togasaki DM, Smith A, et al. Memory, mood, dopamine, and serotonin in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-lesioned mouse model of basal ganglia injury. Neurobiol Dis 2008; 32:319–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maegawa H, Niwa H. Generation of mitochondrial toxin rodent models of Parkinson’s disease using 6-OHDA, MPTP, and rotenone. Methods Mol Biol 2021; 2322:95–110. [DOI] [PubMed] [Google Scholar]
- 5.Pathania A, Garg P, Sandhir R. Impaired mitochondrial functions and energy metabolism in MPTP-induced Parkinson’s disease: comparison of mice strains and dose regimens. Metab Brain Dis 2021; 36:2343–2357. [DOI] [PubMed] [Google Scholar]
- 6.Martínez-Martín P, Damián J. Parkinson disease: depression and anxiety in Parkinson disease. Nat Rev Neurol 2010; 6:243–245. [DOI] [PubMed] [Google Scholar]
- 7.Overton PG, Coizet V. The neuropathological basis of anxiety in Parkinson’s disease. Med Hypotheses 2020; 144:110048. [DOI] [PubMed] [Google Scholar]
- 8.Han NR, Kim YK, Ahn S, Hwang TY, Lee H, Park HJ. A comprehensive phenotype of non-motor impairments and distribution of alpha-synuclein deposition in parkinsonism-induced mice by a combination injection of MPTP and probenecid. Front Aging Neurosci 2020; 12:599045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ishii T, Kinoshita KI, Muroi Y. Serotonin 5-HT4 receptor agonists improve facilitation of contextual fear extinction in an MPTP-induced mouse model of Parkinson’s disease. Int J Mol Sci 2019; 20:5340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Campolo M, Filippone A, Biondo C, Mancuso G, Casili G, Lanza M, et al. TLR7/8 in the pathogenesis of Parkinson’s disease. Int J Mol Sci 2020; 21:9384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Campolo M, Paterniti I, Siracusa R, Filippone A, Esposito E, Cuzzocrea S. TLR4 absence reduces neuroinflammation and inflammasome activation in Parkinson’s diseases in vivo model. Brain Behav Immun 2019; 76:236–247. [DOI] [PubMed] [Google Scholar]
- 12.Crawley J, Goodwin FK. Preliminary report of a simple animal behavior model for the anxiolytic effects of benzodiazepines. Pharmacol Biochem Behav 1980; 13:167–170. [DOI] [PubMed] [Google Scholar]
- 13.Kosari-Nasab M, Rabiei A, Doosti MH, Salari AA. Adolescent silymarin treatment increases anxiety-like behaviors in adult mice. Behav Pharmacol 2014; 25:325–330. [DOI] [PubMed] [Google Scholar]
- 14.Lister RG. The use of a plus-maze to measure anxiety in the mouse. Psychopharmacology (Berl) 1987; 92:180–185. [DOI] [PubMed] [Google Scholar]
- 15.Pellow S, Chopin P, File SE, Briley M. Validation of open:closed arm entries in an elevated plus-maze as a measure of anxiety in the rat. J Neurosci Methods 1985; 14:149–167. [DOI] [PubMed] [Google Scholar]
- 16.Maetzler W. Comment: why do nondopaminergic features in Parkinson disease matter. Neurology 2014; 82:417. [DOI] [PubMed] [Google Scholar]
- 17.Khatri DK, Choudhary M, Sood A, Singh SB. Anxiety: an ignored aspect of Parkinson’s disease lacking attention. Biomed Pharmacother 2020; 131:110776. [DOI] [PubMed] [Google Scholar]
- 18.Zhang Q, Yang X, Song H, Jin Y. Cognitive behavioral therapy for depression and anxiety of Parkinson’s disease: a systematic review and meta-analysis. Complement Ther Clin Pract 2020; 39:101111. [DOI] [PubMed] [Google Scholar]
- 19.AlShimemeri S, Di LDG, Fox SH. MPTP Parkinsonism and implications for understanding Parkinson’s disease. Mov Disord Clin Pract 2022; 9:42–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ma Y, Rong Q. Effect of different MPTP administration intervals on mouse models of Parkinson’s disease. Contrast Media Mol Imaging 2022; 2022:2112146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bell IR, Amend D, Kaszniak AW, Schwartz GE, Peterson JM, Stini WA, et al. Trait shyness in the elderly: evidence for an association with Parkinson’s disease in family members and biochemical correlates. J Geriatr Psychiatry Neurol 1995; 8:16–22. [PubMed] [Google Scholar]
- 22.Weiner WJ. Early diagnosis of Parkinson’s disease and initiation of treatment. Rev Neurol Dis 2008; 5:46–53; quiz 54. [PubMed] [Google Scholar]
- 23.Stein DJ, Stahl S. Serotonin and anxiety: current models. Int Clin Psychopharmacol 2000; 15(Suppl 2):S1–S6. [DOI] [PubMed] [Google Scholar]
- 24.Forbes EJ, Byrne GJ, O’Sullivan JD, Yang J, Marsh R, Dissanayaka NN. Defining atypical anxiety in Parkinson’s disease. Mov Disord Clin Pract 2021; 8:571–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Strawn JR, Lu L, Peris TS, Levine A, Walkup JT. Research review: pediatric anxiety disorders – what have we learnt in the last 10 years. J Child Psychol Psychiatry 2021; 62:114–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sharp SI, Ballard CG, Ziabreva I, Piggott MA, Perry RH, Perry EK, et al. Cortical serotonin 1A receptor levels are associated with depression in patients with dementia with Lewy bodies and Parkinson’s disease dementia. Dement Geriatr Cogn Disord 2008; 26:330–338. [DOI] [PubMed] [Google Scholar]
- 27.Bower JH, Grossardt BR, Maraganore DM, Ahlskog JE, Colligan RC, Geda YE, et al. Anxious personality predicts an increased risk of Parkinson’s disease. Mov Disord 2010; 25:2105–2113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Campos AC, Fogaça MV, Aguiar DC, Guimarães FS. Animal models of anxiety disorders and stress. Revista brasileira de psiquiatria (Sao Paulo, Brazil: 1999) 2013; 35:S101–S111. [DOI] [PubMed] [Google Scholar]
- 29.De Deurwaerdère P, Di GG. Serotonin in health and disease. Int J Mol Sci 2020; 21:3500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang X, Xu J, Wang Q, Ding D, Wu L, Li Y, et al. Chronic stress induced depressive-like behaviors in a classical murine model of Parkinson’s disease. Behav Brain Res 2021; 399:112816. [DOI] [PubMed] [Google Scholar]
- 31.Mitra N, Mohanakumar KP, Ganguly DK. Dissociation of serotoninergic and dopaminergic components in acute effects of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine in mice. Brain Res Bull 1992; 28:355–364. [DOI] [PubMed] [Google Scholar]
- 32.Deo N, Redpath G. Serotonin receptor and transporter endocytosis is an important factor in the cellular basis of depression and anxiety. Front Cell Neurosci 2021; 15:804592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hudd TR, Blake CS, Rimola-Dejesus Y, Nguyen TT, Zaiken K. A case report of serotonin syndrome in a patient on selective serotonin reuptake inhibitor (SSRI) monotherapy. J Pharm Pract 2020; 33:206–212. [DOI] [PubMed] [Google Scholar]
- 34.Nikolaus S, Antke C, Beu M, Müller HW. Cortical GABA, striatal dopamine and midbrain serotonin as the key players in compulsive and anxiety disorders – results from in vivo imaging studies. Rev Neurosci 2010; 21:119–139. [DOI] [PubMed] [Google Scholar]
- 35.DeGroot SR, Zhao-Shea R, Chung L, Klenowski PM, Sun F, Molas S, et al. Midbrain dopamine controls anxiety-like behavior by engaging unique interpeduncular nucleus microcircuitry. Biol Psychiatry 2020; 88:855–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sy HN, Wu SL, Wang WF, Chen CH, Huang YT, Liou YM, et al. MPTP-induced dopaminergic degeneration and deficits in object recognition in rats are accompanied by neuroinflammation in the hippocampus. Pharmacol Biochem Behav 2010; 95:158–165. [DOI] [PubMed] [Google Scholar]
- 37.Crapanzano C, Damiani S, Guiot C. Quetiapine in the anxiety dimension of mood disorders: a systematic review of the literature to support clinical practice. J Clin Psychopharmacol 2021; 41:436–449. [DOI] [PubMed] [Google Scholar]
- 38.Hjorth OR, Frick A, Gingnell M, Hoppe JM, Faria V, Hultberg S, et al. Expectancy effects on serotonin and dopamine transporters during SSRI treatment of social anxiety disorder: a randomized clinical trial. Transl Psychiatry 2021; 11:559. [DOI] [PMC free article] [PubMed] [Google Scholar]