Premenopausal stage women with either vasomotor or non-vasomotor menopausal symptoms have significantly higher prevalence of poor cardiovascular health metrics, compared to those without any menopausal symptoms.
Key Words: Climacteric women, Ideal cardiovascular health metrics, Menopause symptoms, Premenopausal stage, STRAW-10, Vasomotor symptoms
Abstract
Objective
We examined the association between menopause symptoms and the prevalence of ideal cardiovascular health (CVH) metrics among premenopausal women.
Methods
This cross-sectional study comprised 4,611 premenopausal women aged 42 to 52 years. Data for CVH metrics were collected during health screening examinations. Menopause symptoms were measured using the Korean version of the Menopause-Specific Quality of Life questionnaire. For vasomotor, psychosocial, physical, and sexual symptoms, participants were divided into absent or symptomatic groups, further divided into tertiles (range, 0-7; 7 being the most bothersome). Ideal CVH metrics were defined according to the American Heart Association Life Simple 7 metrics, except dietary component. Cardiovascular health metrics were scored from 0 (unhealthy) to 6 (healthy) and classified as poor (0-2), intermediate (3-4), and ideal (5-6). Multinomial logistic regression models were used to estimate the prevalence ratios for intermediate and poor CVH metrics using ideal CVH as the reference.
Results
The overall and 4 menopause-specific quality of life domain scores were significantly associated with poorer CVH metrics scores in a dose-response manner (P < 0.05). After adjusting for age, parity, education level, anti-Mullerian hormone levels, and alcohol intake, women with the most bothersome degree for vasomotor, psychosocial, physical, and sexual symptoms had significantly higher prevalence of poor CVH metrics, with corresponding prevalence ratios (95% confidence interval) of 2.90 (1.95-4.31), 2.07 (1.36-3.15), 3.01 (1.19-7.65), and 1.66 (1.15-2.39), respectively, compared with those without each vasomotor, psychosocial, physical, and sexual symptom.
Conclusions
Premenopausal stage women with either vasomotor or nonvasomotor menopausal symptoms have significantly higher prevalence of poor CVH metrics, compared with those without any menopausal symptoms.
Cardiovascular disease (CVD) is the main cause of mortality worldwide and a significant public health burden.1 In 2010, the American Heart Association established ideal cardiovascular health (CVH) metrics based on seven modifiable risk factors and lifestyle behaviors: body mass index (BMI), physical activity, smoking status, blood pressure (BP), diet, fasting glucose, and cholesterol levels.2 According to some previous studies, adherence to ideal CVH metrics was significantly associated with a lower risk of CVD and mortality and subclinical CVD and other non-CVD-related disorders,3,4 despite their initial design for CVD prevention. People with average or optimal CVH behaviors had decreased risks of cancer, chronic kidney diseases, pneumonia, and chronic obstructive pulmonary disease, compared with those with inadequate habits. As non-CVDs account for a large portion of healthcare costs worldwide, improvement in CVH by targeting individuals with low CVH metrics scores might reduce long-term healthcare expenditures.
Menopause and menopausal transition are critical periods for women.5,6 During menopausal transition, women experience several menopause symptoms, such as hot flashes, night sweats, anxiety, depression, sleep disorders, memory impairment, a lack of energy, and other physical and psychological symptoms, due to hormonal fluctuations.7 Menopause is a known major risk factor for CVD in middle-aged women,8 because the prevalence and incidence of CVD markedly increase in women after menopause compared with those before menopause.9 Several observational studies have suggested that endogenous estrogen withdrawal caused by menopause might reduce the protective pattern of CVD risk in women.10,11 The onset timing, duration, severity, and types of menopause symptoms vary among women in menopausal transition.12 Although studies have revealed associations between vasomotor symptoms, other psychological menopausal symptoms, and increased CVD risk,12 the relationship between comprehensive menopausal symptoms and ideal CVH metrics is unknown. Our previous study focused on the association of ideal CVH metrics with development of early-onset vasomotor symptoms among premenopausal women.13 However, determining whether the association between menopausal symptoms and CVD metrics is limited to vasomotor symptoms or also related to other menopausal symptoms can help identify the relevant preventive measure among women in menopausal transition to lower their risks of CVD events. By figuring out which aspects of menopausal symptoms are mostly associated with CVH, we can help target women with specific symptoms at high risk of cardiovascular disease. Therefore, we investigated the association of vasomotor and other menopause symptoms with the ideal CVH metrics among premenopausal stage, Korean women.
METHODS
Study population
In this cohort study designed to evaluate changes in physical and psychosocial aspects across menopausal stages in middle-aged Korean women, participants were enrolled from 2014 to 2018 from the Kangbuk Samsung Health Study, a cohort study of Korean adults who had annual or biennial comprehensive health checkups at the Kangbuk Samsung Hospital Total Healthcare Center clinics.13 The eligibility criteria for recruitment were as follows: (1) women aged 42 to 52 years; (2) no history of oophorectomy, hysterectomy, or hormone therapy (HT); (3) at least one menstrual period in the 3 months preceding the health examinations and no amenorrhea lasting ≥60 d (premenopause or early menopause transition); and (4) no history of a chronic disease that may affect menstrual cycles (malignancy, renal failure, and hypothyroidism or hyperthyroidism). For study participants' enrollments, well-trained researchers received informed consents of this cohort study from women who visited Kangbuk Samsung Hospital Total Healthcare Center clinics for their health check-ups. If they agreed to participate in the cohort study, we asked the participants to fill out additional questionnaires for assessing their subjective menopausal symptoms. Among the 5,230 women initially enrolled, 194 who withdrew from the study were excluded. For the analysis in the present study, 289 who were in their early menopausal transition stages at baseline were further excluded to focus on exclusively premenopausal stage women. For the present study to address the association between domain-specific menopausal symptoms and the ideal CVH among premenopausal stage women, we restricted to exclusively premenopausal stage women at baseline who completed Menopause-Specific Quality of Life (MENQOL) questionnaire. Women with a history of coronary disease, heart disease, and stroke (n = 23) and no information on MENQOL (n = 17) and factors of ideal CVH metrics (n = 96) were also excluded (Fig. 1). Finally, a total of 4,611 women were included in this study.
FIG. 1.
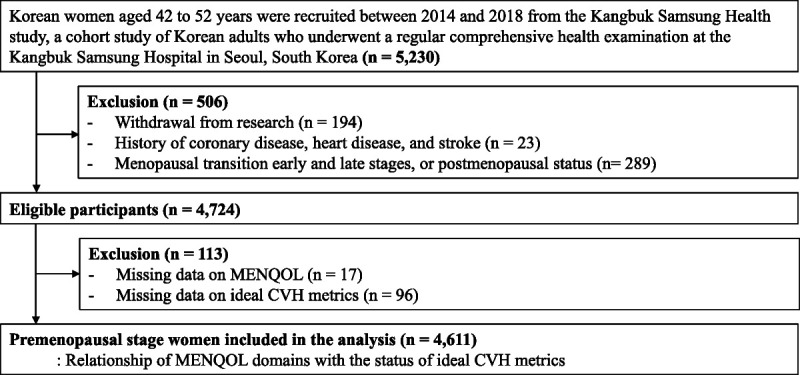
Flow diagram for the selection of the study population.
This study was approved by the institutional review board of Kangbuk Samsung Hospital (no. KBSMC 2022-06-037). All participants signed a written informed consent form. Every method in the cohort study was conducted in accordance with the relevant rules and regulations.
Measurements
We collected data on demographic and clinical features, health-related behaviors, and reproductive factors using standardized, structured, and self-administered questionnaires. Among health-related habits, smoking status was classified as never, former, or current smoker. Alcohol consumption was divided into two groups: <10 g/d, the cutoff of light drinking for women,14 and ≥10 g/d. Furthermore, education status was dichotomized as less than a university degree and equal to or greater than a university degree. Physical activity was measured using the validated Korean version of the International Physical Activity Questionnaire short form.15 We defined parity as the number of pregnancies, including livebirths and stillbirths. Diet was assessed using a 103-item self-administered food frequency questionnaire validated for use in Korea and designed to measure dietary patterns over the past year.16
We categorized menopausal stages according to the criteria of the Stages of Reproductive Aging Workshop (STRAW) +10: (1) premenopause, which do not meet any criteria of the following later stages including early and late transition stage or postmenopausal stage; (2) early menopausal transition, defined as a persistent difference of ≥7 days in length of consecutive cycles; (3) late menopausal transition, defined as amenorrhea of ≥60 days; and (4) postmenopause, defined as amenorrhea of ≥12 months.17
Participants' height, weight, and body composition were measured by trained nurses while donning a lightweight gown with no shoes. Body mass index was calculated by dividing an individual's body weight by their height squared. After a 5-minute rest in a sitting position, BP was measured three times using an automatic BP equipment (5300P, Welch Allyn, Skaneateles, NY). Blood samples were gathered from the antecubital vein after at least 10 hours of fasting. Anti-Mullerian hormone (AMH) was measured using an Elecsys AMH Plus instrument on the Cobas e801 immunoassay analyzer (Roche Diagnostics, Tokyo, Japan). Serum total cholesterol and triglyceride levels were determined using an enzymatic colorimetric assay. High- and low-density lipoprotein cholesterol concentrations were directly measured using a homogenous enzymatic colorimetric assay. Serum fasting glucose levels were measured using the hexokinase method on Modular DPP systems (Roche Diagnostics, Tokyo, Japan) until 2015, and the Cobas 8000 c702 (Roche Diagnostics, Rotkreuz, Switzerland) thereafter. Glycated hemoglobin A1c levels were determined using a turbidimetric inhibition immunoassay and the Cobas Integra 800 (Roche Diagnostics, Rotkreuz, Switzerland) until January 2018 and the Cobas 8000 c513 (Roche Diagnostics, Rotkreuz, Switzerland) thereafter (RRID:AB_2909460 and AB_2909459).
Assessment of menopause symptoms
Menopause symptoms were measured using the Korean version of the MENQOL, which was designed to assess the influence of menopause-related items on women's quality of life.5,18,19 This questionnaire comprises 29 items in four domains, including vasomotor (3 items), psychosocial (7 items), physical (16 items), and sexual (3 items) domains20; hot flashes, night sweats, and sweating symptoms for vasomotor domain; dissatisfaction with life, anxiety, poor memory, accomplishing less than usual, feeling depressed, impatience, and a desire to be alone for psychosocial domain; flatulence, tiredness, difficulty sleeping, drying skin, bloating, involuntary urination when laughing or coughing for physical domain; and changes in sexual desire, vaginal dryness during intercourse, and avoiding intimacy for sexual domain.20 Participants reported whether they had experienced menopause symptoms in the previous month and evaluated their severity and bothersome, ranging from “no bother at all” (0) to “extremely bothersome” (6) on a seven-point Likert scale. In this statistical analysis, the raw MENQOL values were recoded to an eight-point grading system, including zero: “No” was graded as zero, and “Yes, but not bothered at all” was recoded as one. The bothersome degree ranged from 1 to 6 and was changed from 2 to 7.13 The mean scores were calculated within each domain. Participants were categorized into four groups, including the absent symptom group and, if a symptom was present, tertiles (tertiles 1-3), based on the distribution of MENQOL scores among women with the presence of symptoms. We compared present-tertile 1, present-tertile 2, and present-tertile 3 versus the absent symptom group as the reference to investigate the association between overall and each MENQOL domain with the ideal CVH metrics.
Definition of ideal CVH metrics
The American Heart Association Life Simple 7 metrics defined ideal CVH metrics in 2010.2 The ideal CVH metrics consist of the following ideal health behaviors and factors: (1) smoking: never or former smoker; (2) BMI <23.0 kg/m2; (3) physical activity: ≥150 min/wk of moderate-intensity physical activity, ≥75 min/wk of vigorous-intensity physical activity, or ≥150 min/wk of moderate- or vigorous-intensity physical activity; (4) total cholesterol <200 mg/dL; (5) BP <120/80 mm Hg; (6) fasting glucose levels <100 mg/dL; and (7) diet: 4 or 5 healthy dietary elements of 5 healthy dietary components, including fruits and vegetables (≥450 g/d), fish (≥198 g/wk), fiber-rich whole grains (≥85 g/d), sodium (<1,500 mg/d), and sugar-sweetened beverages (≤1 L/wk).2,21 Each ideal CVH metric was scored one point, and the number of ideal CVH metrics for each participant was summed to calculate the ideal CVH score (range, 0-7 points). Of note, diet information was only available for a subset of participants (n = 1,430) and was obtained using a food frequency questionnaire in which information on whole grains was unavailable. Thus, the CVH metrics without diet factor was used, ranging from 0 to 6 points, and was categorized into three groups of poor (0-2), intermediate (3-4), and ideal (5-6) CVH metrics as an outcome. The cutoffs were selected based on previous articles, which have used ideal CVH metrics.22,23
Statistical analysis
The demographic and clinical characteristics of the study population are presented as mean values with standard deviations (SD) and numbers with percentages. For continuous variables with skewed distribution, data are presented as median values with interquartile ranges. Multinomial logistic regression analyses were performed to estimate the prevalence ratios (PRs) with 95% confidence intervals (CIs) for prevalent intermediate or poor CVH metrics according to the severity of overall menopause symptoms, including vasomotor, psychosocial, physical, and sexual domains, using ideal CVH metrics as the reference. For the linear trend test, the number of each MENQOL category was included as a continuous variable in the model. In the adjusted models, we selected potential confounders based on previous studies, which were age, parity,24 education level,25 alcohol consumption,26 and AMH levels. Confounding variables were chosen for inclusion in the multivariable models if they met the following criteria: (1) were associated with the outcome (intermediate or poor CVH metrics), and (2) were associated with the exposure (menopausal symptoms), but (3) were not intermediate variables in the causal pathway between the exposure (menopausal symptoms) and the outcome (intermediate or poor CVH metrics). Anti-Mullerian hormone levels reflect ovarian reserve and can be used to predict the time to final menstrual period among late reproductive-age women.27,28 All statistical analyses were conducted using Stata version 17.0 (Stata Corp LP, College Station, TX) and R 4.2.1 (R Foundation for Statistical Computing, Vienna, Austria). Statistical significance was defined as a two-sided P value less than 0.05.
RESULTS
Table 1 lists the demographic and clinical characteristics of the 4,611 participants according to severity of overall menopausal symptoms. The mean age was 44.86 ± 2.46 years. The mean overall MENQOL score was 1.50 ± 1.08 at baseline. In the MENQOL domains, the mean scores for vasomotor, psychosocial, physical, and sexual symptoms were 0.5 ± 0.9, 1.3 ± 1.3, 1.8 ± 1.2, and 1.3 ± 1.5, respectively. The proportions of women with poor CVH status, high education levels, and current smokers significantly increased as overall menopausal symptoms became more severe. According to the trend test across absent, present-T1, present-T2, present-T3, total and LDL cholesterol, and triglyceride levels were significantly elevated, and the proportion of those with high physical activity was significantly decreased.
Table 1.
Demographic and clinical characteristics of study participants according to severity of overall menopausal symptoms
| Characteristics | Total participants (n = 4,611) |
Overall MENQOL categories | P for trend | |||
|---|---|---|---|---|---|---|
| Absent | Present-T1 (<0.9) | Present-T2 (0.9- < 1.9) | Present-T3 (≥1.9) | |||
| Overall MENQOL scorea | 1.50 ± 1.08 | 142 (3.1) | 1,494 (32.4) | 1,486 (32.2) | 1,489 (32.3) | <0.001 |
| Mean vasomotor domain | 0.47 ± 0.93 | 0 | 0.09 ± 0.32 | 0.32 ± 0.60 | 1.07 ± 1.29 | <0.001 |
| Mean psychosocial domain | 1.32 ± 1.27 | 0 | 0.31 ± 0.38 | 1.08 ± 0.65 | 2.68 ± 1.14 | <0.001 |
| Mean physical domain | 1.81 ± 1.20 | 0 | 0.70 ± 0.37 | 1.70 ± 0.43 | 3.20 ± 0.79 | <0.001 |
| Mean sexual domain | 1.32 ± 1.50 | 0 | 0.34 ± 0.66 | 1.10 ± 1.09 | 2.65 ± 1.55 | <0.001 |
| Ideal CVH metrics | 4.45 ± 1.09 | 4.67 ± 1.02 | 4.56 ± 1.06 | 4.43 ± 1.07 | 4.34 ± 1.14 | <0.001 |
| Poor (0-2) | 222 (4.8) | 4 (2.8) | 57 (3.8) | 66 (4.4) | 95 (6.4) | <0.001 |
| Intermediate (3-4) | 1,955 (42.4) | 48 (33.8) | 594 (39.8) | 657 (44.2) | 656 (44.1) | |
| Ideal (5-6) | 2,434 (52.8) | 90 (63.4) | 843 (56.4) | 763 (51.4) | 738 (49.6) | |
| Age, years | 44.86 ± 2.46 | 44.63 ± 2.51 | 44.85 ± 2.48 | 44.83 ± 2.42 | 44.93 ± 2.46 | 0.424 |
| BMI, kg/m2 | 22.43 ± 2.98 | 21.96 ± 2.82 | 22.10 ± 2.81 | 22.48 ± 2.96 | 22.76 ± 3.13 | <0.001 |
| Parity, % | 4,095 (88.8) | 128 (90.1) | 1,328 (88.9) | 1,314 (88.4) | 1,325 (89.0) | 0.935 |
| Anti-Mullerian hormone, ng/mL | 0.63 [0.23, 1.30] | 0.71 [0.25, 1.35] | 0.60 [0.23, 1.29] | 0.67 [0.24, 1.36] | 0.62 [0.22, 1.29] | 0.996 |
| High educationb, % | 3,657 (79.3) | 104 (73.2) | 1,170 (78.3) | 1,185 (79.7) | 1,198 (80.5) | 0.040 |
| Amounts of alcohol intake, g/day | 5.00 ± 9.02 | 4.71 ± 7.38 | 4.58 ± 7.76 | 4.81 ± 8.72 | 5.48 ± 9.94 | 0.045 |
| High alcohol intakesc, % | 558 (12.1) | 17 (12.0) | 174 (11.7) | 161 (10.8) | 206 (13.8) | 0.313 |
| Current smokersd, % | 91 (2.0) | 1 (0.7) | 27 (1.8) | 18 (1.2) | 45 (3.0) | 0.003 |
| High physical activitye, % | 2,210 (47.9) | 79 (55.6) | 779 (52.1) | 715 (48.1) | 637 (42.8) | <0.001 |
| Diabetes, % | 91 (2.0) | 4 (2.9) | 21 (1.4) | 27 (1.8) | 39 (2.6) | 0.093 |
| Hypertension, % | 230 (5.0) | 5 (3.6) | 65 (4.4) | 68 (4.6) | 92 (6.2) | 0.078 |
| SBP, mmHg | 103.80 ± 11.66 | 103.91 ± 10.90 | 103.53 ± 11.45 | 103.71 ± 11.45 | 104.15 ± 12.14 | 0.524 |
| DBP, mmHg | 66.80 ± 9.18 | 67.73 ± 8.59 | 66.54 ± 9.02 | 66.67 ± 9.10 | 67.10 ± 9.46 | 0.215 |
| Fasting glucose, mg/dl | 93.14 ± 12.48 | 94.13 ± 12.99 | 92.46 ± 10.83 | 93.27 ± 13.84 | 93.59 ± 12.52 | 0.058 |
| Total cholesterol, mg/dl | 192.52 ± 30.41 | 184.67 ± 30.85 | 191.66 ± 30.15 | 193.56 ± 30.24 | 193.10 ± 30.68 | 0.005 |
| LDL-C mg/dl | 118.98 ± 28.78 | 109.97 ± 27.65 | 117.76 ± 28.22 | 120.18 ± 28.59 | 119.87 ± 29.45 | 0.000 |
| HDL-C, mg/dl | 66.71 ± 15.84 | 68.64 ± 15.54 | 67.53 ± 16.15 | 66.70 ± 15.81 | 65.71 ± 15.54 | 0.007 |
| Triglyceride, mg/dl | 75 [58, 101] | 76 [59, 98] | 71 [56, 95] | 75 [58, 101] | 78 [59, 106] | <0.001 |
| hs-CRP, mg/L | 0.03 [0.02, 0.06] | 0.03 [0.02, 0.06] | 0.03 [0.02, 0.05] | 0.03 [0.02, 0.07] | 0.03 [0.02, 0.06] | 0.564 |
Data are presented as means ± standard deviations, medians[interquartile range], or numbers(percentages).
BMI, body mass index; CVH, cardiovascular health; DBP, diastolic blood pressure; HDL-C, high-density lipoprotein cholesterol; hsCRP, high-sensitive C-reactive protein; MENQOL, menopause-specific quality of life; LDL-C, low-density lipoprotein cholesterol; SBP, systolic blood pressure; T, tertiles.
aAmong MENQOL questionnaire, higher values indicate worse symptoms, which were ranged from 0 (absent) to 7 (being the most bothersome) points.
bhigh education was defined as equal to or greater than a university degree.
chigh alcohol intakes was defined as ≥10 g/day.
dSmoking status was classified as never, former, or current smoker.
eHigh physical activity inlcuded moderate and high intensity defind by the validated Korean version of the International Physical Activity Questionnaire short form.
The prevalence of each MENQOL item and each symptom score, if the symptom is present, are listed in Table 2. For the vasomotor domain, the most prevalent symptom was sweating (22.3%), followed by hot flashes (18.8%). In the psychosocial domain, the most and least common symptoms were poor memory (71.9%) and dissatisfaction with own personal life (31.1%), respectively. For physical domain, the most common symptom was feeling tired or worn out (78.0%), while the least common symptom was increased facial hair (16.6%). In the sexual domain, the prevalence of vaginal dryness during intercourse and avoiding intimacy were 47.1%, and 45.6%, respectively. Among the four domains, women who experienced bothersome physical symptoms constituted the highest proportion.
Table 2.
Frequency distribution and scores of the MENQOL items
| Domains | No. total | No. of cases (%) | Scorea (range, 1-7) Mean ± SD |
|---|---|---|---|
| Vasomotor | |||
| (1) Hot flashes | 4,589 | 863 (18.8) | 2.6 ± 1.2 |
| (2) Night sweats | 4,599 | 643 (14.0) | 2.2 ± 1.2 |
| (3) Sweating | 4,574 | 1,018 (22.3) | 2.8 ± 1.4 |
| Psychosocial | |||
| (4) Dissatisfaction with my personal life | 4,595 | 1,429 (31.1) | 2.6 ± 1.2 |
| (5) Feeling anxious or nervous | 4,594 | 1,894 (41.2) | 2.7 ± 1.1 |
| (6) Poor memory | 4,602 | 3,309 (71.9) | 3.0 ± 1.2 |
| (7) Accomplishing less than usual | 4,601 | 2,792 (60.7) | 2.9 ± 1.2 |
| (8) Feeling depressed, down or blue | 4,594 | 1,780 (38.8) | 2.6 ± 1.2 |
| (9) Being impatient with other people | 4,602 | 1,958 (42.6) | 2.7 ± 1.1 |
| (10) Feeling of wanting to be alone | 4,599 | 1,970 (42.8) | 2.8 ± 1.3 |
| Physical | |||
| (11) Flatulence(wind) or gas pains | 4,593 | 2,033 (44.3) | 2.8 ± 1.3 |
| (12) Aching muscles and joints | 4,596 | 2,736 (59.5) | 3.1 ± 1.3 |
| (13) Feeling tired or worn out | 4,600 | 3,589 (78.0) | 3.3 ± 1.3 |
| (14) Difficulty sleeping | 4,589 | 1,731 (37.7) | 2.8 ± 1.4 |
| (15) Aches in the back of neck or head | 4,601 | 3,001 (65.2) | 3.3 ± 1.4 |
| (16) Decrease in physical strength | 4,599 | 3,289 (71.5) | 3.2 ± 1.3 |
| (17) Decrease in stamina | 4,599 | 3,033 (66.0) | 3.2 ± 1.2 |
| (18) Lack of energy | 4,601 | 3,025 (65.8) | 3.1 ± 1.2 |
| (19) Drying skin | 4,608 | 3,528 (76.6) | 3.3 ± 1.3 |
| (20) Weight gain | 4,592 | 3,197 (69.6) | 3.3 ± 1.4 |
| (21) Increased facial hair | 4,589 | 762 (16.6) | 1.9 ± 1.1 |
| (22) Changes in the appearance, texture, or tone of the skin | 4,605 | 3,587 (77.9) | 3.2 ± 1.2 |
| (23) Feeling bloated | 4,600 | 2,768 (60.2) | 3.1 ± 1.3 |
| (24) Low backache | 4,599 | 1,899 (41.3) | 3.0 ± 1.4 |
| (25) Frequent urination | 4,599 | 2,376 (51.7) | 3.1 ± 1.3 |
| (26) Involuntary urination when laughing or coughing | 4,605 | 2,403 (52.2) | 2.8 ± 1.4 |
| Sexual | |||
| (27) Change in sexual desire | 4,609 | 1,928 (41.8) | 2.9 ± 1.3 |
| (28) Vaginal dryness during intercourse | 4,590 | 2,162 (47.1) | 3.1 ± 1.4 |
| (29) Avoiding intimacy | 4,594 | 2,093 (45.6) | 2.9 ± 1.3 |
Data are presented as means ± standard deviations, medians [interquartile range], or numbers (percentages).
MENQOL, menopause-specific quality of life; SD, standard deviation.
aScore ranges from 1 to 7, including only women with menopausal symptoms.
Table 3 presents the results from the multinomial logistic regression model, which was used to compare the PRs for intermediate or poor CVH metrics to the ideal group across the severity groups of each MENQOL domain. In the analysis, groups without any menopause symptoms of each MENQOL domain were considered as references. After adjusting for age, parity, education level, AMH concentrations, and alcohol intakes, the PRs (95% CIs) for having intermediate CVH metrics in the highest tertile category of vasomotor, psychosocial, and physical domains were 1.63 (1.32-2.02), 1.25 (1.04-1.50), and 1.72 (1.21-2.43), respectively, compared with those without symptoms. Moreover, women in the highest tertile score group (most bothersome symptoms) versus absent symptom group had significantly higher PRs (95% CIs) for prevalent poor CVH status in all MENQOL domains, with corresponding PR (95% CI) of 2.90 (1.95-4.31) for vasomotor domain, 2.07 (1.36-3.15) for psychosocial domain, 3.01 (1.19-7.65) for physical domain, and 1.66 (1.15-2.39) for sexual domain. In all MENQOL domains, there were significantly increasing trends of the prevalence of poor CVH metrics with increasing symptom severity (P < 0.05). Among the MENQOL domains, the vasomotor domain showed the highest association with poor CVH metrics. In the analyses to examine the continuous relationship of MENQOL symptoms with ideal CVH metrics using multivariable-adjusted linear regression models, the mean scores of all domains were significantly and negatively associated with the scores of ideal CVH metrics (Supplementary Table 1, http://links.lww.com/MENO/B134).
Table 3.
Cross-sectional association between the MENQOL domains and lower scores of ideal CVH metrics (n = 4,611)
| No. total | Intermediate scores (3-4) of CVH metrics vs. Ideal (5-6) | Poor scores (0-2) of CVH metrics vs. Ideal (5-6) | ||||||
|---|---|---|---|---|---|---|---|---|
| No. cases | Crude PR (95% CI) |
Fully-adjusted PR (95% CI) |
No. cases | Crude PR (95% CI) |
Fully-adjusted PR (95% CI) |
|||
| Overall MENQOL | ||||||||
| Absent | 142 | 48 | Reference | Reference | 4 | Reference | Reference | |
| Present-T1 (<0.9) | 1,494 | 594 | 1.32 (0.92-1.90) | 1.30 (0.90-1.88) | 57 | 1.52 (0.54-4.29) | 1.51 (0.53-4.30) | |
| Present-T2 (0.9- < 1.9) | 1,488 | 658 | 1.61 (1.12-2.33) | 1.62 (1.12-2.25) | 66 | 1.95 (0.69-5.47) | 2.01 (0.71-5.71) | |
| Present-T3 (≥1.9) | 1,487 | 655 | 1.67 (1.16-2.40) | 1.64 (1.13-2.37) | 95 | 2.90 (1.04-8.06) | 2.90 (1.03-8.15) | |
| P for trend | <0.001 | <0.001 | <0.001 | <0.001 | ||||
| per 1 SD increase | 1.15 (1.08-1.22) | 1.15 (1.08-1.22) | 1.44 (1.27-1.64) | 1.44 (1.27-1.64) | ||||
| Vasomotor | ||||||||
| Absent | 3,243 | 1,282 | Reference | Reference | 128 | Reference | Reference | |
| Present-T1 (<1.1) | 601 | 294 | 1.52 (1.27-1.82) | 1.48 (1.24-1.78) | 31 | 1.61 (1.07-2.43) | 1.54 (1.01-2.33) | |
| Present-T2 (1.1- < 1.7) | 339 | 166 | 1.58 (1.25-2.00) | 1.54 (1.21-1.94) | 23 | 2.20 (1.37-3.53) | 2.09 (1.29-3.37) | |
| Present-T3 (≥1.7) | 428 | 213 | 1.74 (1.41-2.15) | 1.63 (1.32-2.02) | 40 | 3.27 (2.22-4.82) | 2.90 (1.95-4.31) | |
| P for trend | <0.001 | <0.001 | <0.001 | <0.001 | ||||
| per 1 SD increase | 1.23 (1.16-1.31) | 1.20 (1.13-1.28) | 1.52 (1.37-1.70) | 1.46 (1.31-1.63) | ||||
| Psychosocial | ||||||||
| Absent | 904 | 358 | Reference | Reference | 35 | Reference | Reference | |
| Present-T1 (<0.9) | 1,387 | 600 | 1.18 (0.99-1.40) | 1.16 (0.97-1.38) | 59 | 1.18 (0.77-1.82) | 1.16 (0.75-1.79) | |
| Present-T2 (0.9- < 2.1) | 1,202 | 508 | 1.12 (0.94-1.34) | 1.12 (0.93-1.34) | 49 | 1.11 (0.71-1.74) | 1.1 (0.70-1.73) | |
| Present-T3 (≥2.1) | 1,118 | 489 | 1.27 (1.06-1.52) | 1.25 (1.04-1.50) | 79 | 2.10 (1.38-3.18) | 2.07 (1.36-3.15) | |
| P for trend | 0.030 | 0.042 | <0.001 | <0.001 | ||||
| per 1 SD increase | 1.08 (1.02-1.15) | 1.08 (1.01-1.15) | 1.29 (1.14-1.46) | 1.29 (1.14-1.47) | ||||
| Physical | ||||||||
| Absent | 164 | 56 | Reference | Reference | 5 | Reference | Reference | |
| Present-T1 (<1.2) | 1,513 | 594 | 1.27 (0.90-1.78) | 1.25 (0.89-1.77) | 56 | 1.34 (0.52-3.41) | 1.33 (0.52-3.43) | |
| Present-T2 (1.2- < 2.3) | 1,510 | 663 | 1.56 (1.11-2.19) | 1.58 (1.12-2.23) | 64 | 1.68 (0.66-4.28) | 1.76 (0.69-4.53) | |
| Present-T3 (≥2.3) | 1,424 | 642 | 1.72 (1.22-2.43) | 1.72 (1.21-2.43) | 97 | 2.92 (1.16-7.34) | 3.01 (1.19-7.65) | |
| P for trend | <0.001 | <0.001 | <0.001 | <0.001 | ||||
| per 1 SD increase | 1.16 (1.09-1.23) | 1.17 (1.10-1.24) | 1.45 (1.27-1.65) | 1.46 (1.28-1.67) | ||||
| Sexual | ||||||||
| Absent | 1,876 | 781 | Reference | Reference | 82 | Reference | Reference | |
| Present-T1 (<1.4) | 1,035 | 444 | 1.06 (0.91-1.24) | 1.04 (0.89-1.22) | 48 | 1.09 (0.75-1.58) | 1.07 (0.74-1.57) | |
| Present-T2 (1.4- < 2.7) | 905 | 396 | 1.09 (0.92-1.28) | 1.07 (0.91-1.27) | 36 | 0.94 (0.63-1.41) | 0.93 (0.61-1.40) | |
| Present-T3 (≥2.7) | 795 | 334 | 1.07 (0.90-1.27) | 1.05 (0.88-1.25) | 56 | 1.71 (1.19-2.45) | 1.66 (1.15-2.39) | |
| P for trend | 0.323 | 0.478 | 0.019 | 0.031 | ||||
| per 1 SD increase | 1.04 (0.98-1.10) | 1.03 (0.97-1.09) | 1.19 (1.04-1.35) | 1.17 (1.03-1.34) | ||||
Fully adjusted for age, parity, education level, anti-Mullerian hormone levels, and amount of alcohol intake.
CI, confidence interval; CVH, cardiovascular health; MENQOL, menopause-specific quality of life; PR, prevalence ratio; SD, standard deviation; T, tertiles.
DISCUSSION
In this cross-sectional study of premenopausal stage women, physical and psychosocial symptoms were identified to be relatively more common than other menopause symptoms. Furthermore, higher bothersome menopausal symptoms, including vasomotor, psychosocial, physical, and sexual domains, were significantly associated with a higher prevalence of poor CVH metrics in a dose-response manner. Furthermore, in our study even in exclusively menopausal women, the climacteric symptoms were prevalent which were similarly observed in prior studies.29,30 The onset of climacteric symptoms, contrary to the previous belief that these symptoms (eg, vasomotor symptoms) occur near the final menstrual period, recent studies have demonstrated that VMS are commonly observed in the premenopausal and early menopausal transition stages and may persist long after menopause.31-33 Moreover, the prevalence of psychosocial, physical, and sexual symptoms in premenopausal women was similar with another previous study.
Few studies investigated whether menopause symptoms, measured using the MENQOL questionnaire, influenced the risk of poor CVH status. A systematic review evaluated the associations of menopause symptoms, including vasomotor symptoms, depression, insomnia, or panic attacks, with CVD, coronary heart disease, and stroke.12 Women with vasomotor symptoms and other menopausal symptoms had higher relative risks for CVD than those without such symptoms. One of the previous studies found that the incidence of coronary heart disease was significantly higher (approximately 44%) in symptomatic menopausal women than in asymptomatic menopausal women.34 According to a previous cross-sectional study, women with hot flashes or night sweats had significantly higher likelihood of adverse CVD risk factors, such as high BP and BMI, and abnormal lipid concentrations.35 In the Study of Women's Health Across the Nation, which comprised 600 participants without clinical CVD, women with vasomotor symptoms in the previous 2 weeks were found to have poorer features of subclinical CVD, including endothelial dysfunction and aortic calcification, than those without vasomotor symptoms.36 One possible explanation for this result is the potential effect of estrogen deficit during menopausal transition on the association between menopausal symptoms and decline in ideal CVH scores37; this is because a decline in estrogen may lead to worse menopausal symptoms and aggravate CVD risk factors, such as obesity, adverse lipid profiles, high fasting glucose levels, and high BP. On the other hand, large randomized controlled trials38-40 have reported that they could not find beneficial effects of HT on CVD events. Although these trials had some limitations to prove the null association of HT with CVD risks, such as age ranges of study participants, initiated timing of HT among menopausal stage, and durations of HT use, there were still controversial results between observational and randomized controlled trial studies.
In a previous cross-sectional study of a multiethnic population of healthcare workers (mean age, 43 y), depression, life dissatisfaction, and anxiety were significantly associated with increased odds of women having poor CVH metrics.41 A previous cross-sectional study involving 4,313 Chinese adults aged 18 to 65 years revealed that suboptimal health status, characterized by fatigue, lack of energy, and general weakness, was associated with reduced ideal CVH scores.42,43 Furthermore, another previous study of 325 postmenopausal women (mean age, 55.9 y) demonstrated that the changes in sexual desire and avoiding intimacy, assessed by the MENQOL, were positively associated with adverse metabolic conditions, such as fasting hyperglycemia.44 Accordingly, women who had experienced more menopausal symptoms were more likely to have adverse changes in ideal CVH metrics. However, as most of the abovementioned studies were conducted with women of various ages, regardless of menopause, only few studies have focused on perimenopausal or middle-aged women. In the present study exclusively of the premenopausal stage, middle-aged women, menopausal symptoms were relatively common, and both vasomotor and other symptoms were associated with poor CVH metrics, extending their association with the premenopausal stage.
This study had several limitations. As only self-reported questionnaires were used to evaluate the MENQOL and ideal CVH metrics, including smoking status, physical activity, and alcohol consumption, misclassification could have occurred. Based on the ideal CVH metrics, dietary components were excluded because these items were only available to a small percentage of participants. In addition, the questionnaire did not include the assessment for whole-grain intake, which is equivalent to brown rice in Korea. We could not measure reproductive hormones, such as estrogen or follicle stimulating hormone. Although only premenopausal women were included in this study, interindividual differences in reproductive hormones may have led to heterogeneity, which may affect the association of menopausal symptoms with ideal CVH metrics. However, when AMH concentrations, a proxy measure of ovarian reserve, were included to control for other unmeasured reproductive hormones, the results were still statistically significant before and after adjusting for AMH levels. Nevertheless, the possibility of residual confounding cannot be excluded from the observed associations. Finally, because our study cohort consisted of middle-aged Korean women, our findings may not be applicable to other ethnic groups. However, to our knowledge, this is the first study to explore the association of the severity of menopause symptoms, measured using the validated tool for menopause-specific symptoms, with lower scores of CVH metrics. Owing to the prospective design, large sample size of premenopausal women from a well-characterized cohort, and the use of well-standardized clinical, lifestyle, and laboratory variables in this study, we could control for various potential confounders.
CONCLUSIONS
In this cross-sectional study of Korean women in the premenopausal stage, women with menopausal symptoms in all domains, including vasomotor, psychosocial, physical, and sexual symptoms, had a significantly higher prevalence of poor CVH status. Thus, women with bothersome menopausal symptoms, even in the premenopausal stage, may benefit from relevant surveillance of CV risk factors and management as well as measures to reduce discomfort from those symptoms. Further research is required to confirm the role of menopausal symptoms in CVD risk and demonstrate that the management strategies targeted for menopause symptoms of premenopausal and middle-aged women of diverse ethnicities can improve CV health and subsequent CVD complications.
Supplementary Material
Footnotes
Funding/support: This work was supported by the Research Program funded by the Korea Centers for Disease Control and Prevention (grant numbers 2014ER630200, 2017ER630200, 2020ER710200, 2020ER710201, 2020ER710202, and 2023ER060500).
Financial disclosure/conflicts of interest: None reported.
Authors’ contributions: H.R.C. conceived the study, collected and analyzed the data, designed the analytic strategy, and drafted the manuscript. Y.C. and S.R. planned, designed, and directed this study and ensured quality assurance and control. Y.K., R.K., and G.L. collected the data and conducted the literature review. J.K., M.J.K., Y.C., K.H.K., H.K., J.C., Y.H., J.P., Z.D., H.Y.P., and E.G. interpreted the results and conducted critical revision of the manuscript. All authors read and approved the final manuscript.
Supplemental digital content is available for this article. Direct URL citations are provided in the HTML and PDF versions of this article on the journal’s Website (www.menopause.org).
Contributor Information
Hye Rin Choi, Email: hrchoi7542@gmail.com.
Yejin Kim, Email: reenya273@gmail.com.
Yoosun Cho, Email: misslonghorn46@gmail.com.
Min-Jung Kwon, Email: mjkkmd@skku.edu.
Jeonggyu Kang, Email: jg1980.kang@samsung.com.
Ria Kwon, Email: ria.kwon@samsung.com.
Ga-Young Lim, Email: gayoung.lim@samsung.com.
Kye-Hyun Kim, Email: khmd.kim@samsung.com;kdmd.kim@samsung.com.
Hoon Kim, Email: obgyhoon@gmail.com.
Yun Soo Hong, Email: hong.yunsoo@jhu.edu.
Jihwan Park, Email: jhpark@jhu.edu.
Di Zhao, Email: dizhao@jhu.edu.
Juhee Cho, Email: jh1448.cho@samsung.com.
Eliseo Guallar, Email: eguallar@jhu.edu.
Hyun-Young Park, Email: mdhypark@gmail.com.
REFERENCES
- 1.Members WG Mozaffarian D Benjamin EJ, et al. Heart disease and stroke statistics-2016 update: a report from the American Heart Association. Circulation 2016;133:e38–e360. doi: 10.1161/CIR.0000000000000350 [DOI] [PubMed] [Google Scholar]
- 2.Lloyd-Jones DM Hong Y Labarthe D, et al. Defining and setting national goals for cardiovascular health promotion and disease reduction: the American Heart Association's strategic Impact Goal through 2020 and beyond. Circulation 2010;121:586–613. doi: 10.1161/CIRCULATIONAHA.109.192703 [DOI] [PubMed] [Google Scholar]
- 3.Guo L, Zhang S. Association between ideal cardiovascular health metrics and risk of cardiovascular events or mortality: a meta-analysis of prospective studies. Clin Cardiol 2017;40:1339–1346. doi: 10.1002/clc.22836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Michos ED, Khan SS. Further understanding of ideal cardiovascular health score metrics and cardiovascular disease. Expert Rev Cardiovasc Ther 2021;19:607–617. doi: 10.1080/14779072.2021.1937127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Williams RE, Levine KB, Kalilani L, Lewis J, Clark RV. Menopause-specific questionnaire assessment in US population-based study shows negative impact on health-related quality of life. Maturitas 2009;62:153–159. doi: 10.1016/j.maturitas.2008.12.006 [DOI] [PubMed] [Google Scholar]
- 6.Greendale GA, Lee NP, Arriola ER. The menopause. Lancet 1999;353:571–580. doi: 10.1016/S0140-6736(98)05352-5 [DOI] [PubMed] [Google Scholar]
- 7.Cohen LS, Soares CN, Joffe H. Diagnosis and management of mood disorders during the menopausal transition. Am J Med 2005;118(suppl 12B(12)):93–97. doi: 10.1016/j.amjmed.2005.09.042 [DOI] [PubMed] [Google Scholar]
- 8.Cagnacci A, Cannoletta M, Palma F, Zanin R, Xholli A, Volpe A. Menopausal symptoms and risk factors for cardiovascular disease in postmenopause. Climacteric 2012;15:157–162. doi: 10.3109/13697137.2011.617852 [DOI] [PubMed] [Google Scholar]
- 9.Merz AA, Cheng S. Sex differences in cardiovascular ageing. Heart 2016;102:825–831. doi: 10.1136/heartjnl-2015-308769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Allison MA Manson JE Langer RD, et al. Oophorectomy, hormone therapy, and subclinical coronary artery disease in women with hysterectomy: the Women's Health Initiative coronary artery calcium study. Menopause 2008;15(4 Pt 1):639–647. doi: 10.1097/gme.0b013e31816d5b1c [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Agrinier N Cournot M Dallongeville J, et al. Menopause and modifiable coronary heart disease risk factors: a population based study. Maturitas 2010;65:237–243. doi: 10.1016/j.maturitas.2009.11.023 [DOI] [PubMed] [Google Scholar]
- 12.Muka T Oliver-Williams C Colpani V, et al. Association of vasomotor and other menopausal symptoms with risk of cardiovascular disease: a systematic review and meta-analysis. PloS One 2016;11:e0157417. doi: 10.1371/journal.pone.0157417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Choi HR Chang Y Kim Y, et al. Ideal cardiovascular health metrics and risk of incident early-onset vasomotor symptoms among premenopausal women. J Clin Endocrinol Metabol 2022;107:2666–2673. doi: 10.1210/clinem/dgac327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fernandez-Sola J. Cardiovascular risks and benefits of moderate and heavy alcohol consumption. Nat Rev Cardiol 2015;12:576–587. doi: 10.1038/nrcardio.2015.91 [DOI] [PubMed] [Google Scholar]
- 15.Craig CL Marshall AL Sjostrom M, et al. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc 2003;35:1381–1395. doi: 10.1249/01.MSS.0000078924.61453.FB [DOI] [PubMed] [Google Scholar]
- 16.Ahn Y Kwon E Shim JE, et al. Validation and reproducibility of food frequency questionnaire for Korean genome epidemiologic study. Eur J Clin Nutr 2007;61:1435–1441. doi: 10.1038/sj.ejcn.1602657 [DOI] [PubMed] [Google Scholar]
- 17.Harlow SD Gass M Hall JE, et al. Executive summary of the Stages of Reproductive Aging Workshop+10: addressing the unfinished agenda of staging reproductive aging. J Clin Endocrinol Metabol 2012;97:1159–1168. doi: 10.1210/jc.2011-3362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Park JH, Bae SH, Jung YM. Validity and reliability of the Korean version of the Menopause-Specific Quality of Life [in Korean]. J Korean Acad Nurs 2020;50:487–500. doi: 10.4040/jkan.20049 [DOI] [PubMed] [Google Scholar]
- 19.Sydora BC, Fast H, Campbell S, Yuksel N, Lewis JE, Ross S. Use of the Menopause-Specific Quality of Life (MENQOL) questionnaire in research and clinical practice: a comprehensive scoping review. Menopause 2016;23:1038–1051. doi: 10.1097/GME.0000000000000636 [DOI] [PubMed] [Google Scholar]
- 20.Lewis JE, Hilditch JR, Wong CJ. Further psychometric property development of the Menopause-Specific Quality of Life questionnaire and development of a modified version, MENQOL-Intervention questionnaire. Maturitas 2005;50:209–221. doi: 10.1016/j.maturitas.2004.06.015 [DOI] [PubMed] [Google Scholar]
- 21.Kim JY Ko YJ Rhee CW, et al. Cardiovascular health metrics and all-cause and cardiovascular disease mortality among middle-aged men in Korea: the Seoul male cohort study. J Prev Med Public Health 2013;46:319–328. doi: 10.3961/jpmph.2013.46.6.319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Isiozor NM, Kunutsor SK, Voutilainen A, Kurl S, Kauhanen J, Laukkanen JA. Ideal cardiovascular health and risk of acute myocardial infarction among Finnish men. Atherosclerosis 2019;289:126–131. doi: 10.1016/j.atherosclerosis.2019.08.024 [DOI] [PubMed] [Google Scholar]
- 23.Gaye B Tajeu GS Vasan RS, et al. Association of changes in cardiovascular health metrics and risk of subsequent cardiovascular disease and mortality. J Am Heart Assoc 2020;9:e017458. doi: 10.1161/JAHA.120.017458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ogunmoroti O Osibogun O Kolade OB, et al. Multiparity is associated with poorer cardiovascular health among women from the Multi-Ethnic Study of Atherosclerosis. Am J Obstet Gynecol 2019;221:631. e1–631. e16. doi: 10.1016/j.ajog.2019.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Janković S, Stojisavljević D, Janković J, Erić M, Marinković J. Association of socioeconomic status measured by education, and cardiovascular health: a population-based cross-sectional study. BMJ Open 2014;4:e005222. doi: 10.1136/bmjopen-2014-005222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ogunmoroti O Osibogun O McClelland RL, et al. Alcohol type and ideal cardiovascular health among adults of the Multi-Ethnic Study of Atherosclerosis. Drug Alcohol Depend 2021;218:108358. doi: 10.1016/j.drugalcdep.2020.108358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Freeman EW, Sammel MD, Lin H, Gracia CR. Anti-mullerian hormone as a predictor of time to menopause in late reproductive age women. J Clin Endocrinol Metab 2012;97:1673–1680. doi: 10.1210/jc.2011-3032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Finkelstein JS Lee H Karlamangla A, et al. Antimullerian hormone and impending menopause in late reproductive age: the study of women's health across the nation. J Clin Endocrinol Metab. 2020;105:e1862–e1871 doi: 10.1210/clinem/dgz283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Randolph JF Jr. Sowers M Bondarenko I, et al. The relationship of longitudinal change in reproductive hormones and vasomotor symptoms during the menopausal transition. J Clin Endocrinol Metabol 2005;90:6106–6112. doi: 10.1210/jc.2005-1374 [DOI] [PubMed] [Google Scholar]
- 30.Reed SD Lampe JW Qu C, et al. Premenopausal vasomotor symptoms in an ethnically diverse population. Menopause 2014;21:153–158. doi: 10.1097/GME.0b013e3182952228 [DOI] [PubMed] [Google Scholar]
- 31.Avis NE Crawford SL Greendale G, et al. Duration of menopausal vasomotor symptoms over the menopause transition. JAMA Intern Med 2015;175:531–539. doi: 10.1001/jamainternmed.2014.8063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Col NF, Guthrie JR, Politi M, Dennerstein L. Duration of vasomotor symptoms in middle-aged women: a longitudinal study. Menopause 2009;16:453–457. doi: 10.1097/gme.0b013e31818d414e [DOI] [PubMed] [Google Scholar]
- 33.Freeman EW, Sammel MD, Lin H, Liu Z, Gracia CR. Duration of menopausal hot flushes and associated risk factors. Obstet Gynecol 2011;117:1095–1104. doi: 10.1097/AOG.0b013e318214f0de [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Huang CH, Li CL, Kor CT, Chang CC. Menopausal symptoms and risk of coronary heart disease in middle-aged women: a nationwide population-based cohort study. PloS One 2018;13:e0206036. doi: 10.1371/journal.pone.0206036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gast GC, Samsioe GN, Grobbee DE, Nilsson PM, van der Schouw YT. Vasomotor symptoms, estradiol levels and cardiovascular risk profile in women. Maturitas 2010;66:285–290. doi: 10.1016/j.maturitas.2010.03.015 [DOI] [PubMed] [Google Scholar]
- 36.Thurston RC, Sutton-Tyrrell K, Everson-Rose SA, Hess R, Matthews KA. Hot flashes and subclinical cardiovascular disease: findings from the Study of Women's Health Across the Nation Heart Study. Circulation 2008;118:1234–1240. doi: 10.1161/CIRCULATIONAHA.108.776823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Matthews KA Crawford SL Chae CU, et al. Are changes in cardiovascular disease risk factors in midlife women due to chronological aging or to the menopausal transition? J Am Coll Cardiol 2009;54:2366–2373. doi: 10.1016/j.jacc.2009.10.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hulley S Grady D Bush T, et al. Randomized trial of estrogen plus progestin for secondary prevention of coronary heart disease in postmenopausal women. Heart and Estrogen/progestin Replacement Study (HERS) Research Group. JAMA 1998;280:605–613. doi: 10.1001/jama.280.7.605 [DOI] [PubMed] [Google Scholar]
- 39.Rossouw JE Anderson GL Prentice RL, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women's Health Initiative randomized controlled trial. JAMA 2002;288:321–333. doi: 10.1001/jama.288.3.321 [DOI] [PubMed] [Google Scholar]
- 40.Viscoli CM, Brass LM, Kernan WN, Sarrel PM, Suissa S, Horwitz RI. A clinical trial of estrogen-replacement therapy after ischemic stroke. N Engl J Med 2001;345:1243–1249. doi: 10.1056/NEJMoa010534 [DOI] [PubMed] [Google Scholar]
- 41.Mathews L Ogunmoroti O Nasir K, et al. Psychological factors and their association with ideal cardiovascular health among women and men. J Womens Health 2018;27:709–715. doi: 10.1089/jwh.2017.6563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yan YX Liu YQ Li M, et al. Development and evaluation of a questionnaire for measuring suboptimal health status in urban Chinese. J Epidemiol 2009;19:333–341. doi: 10.2188/jea.je20080086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang Y Liu X Qiu J, et al. Association between ideal cardiovascular health metrics and suboptimal health status in Chinese population. Sci Rep 2017;7:14975. doi: 10.1038/s41598-017-15101-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chedraui P, Hidalgo L, Chavez D, Morocho N, Alvarado M, Huc A. Menopausal symptoms and associated risk factors among postmenopausal women screened for the metabolic syndrome. Arch Gynecol Obstet 2007;275:161–168. doi: 10.1007/s00404-006-0239-7 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


