Objective
This study aimed to investigate the effect of oleracein E (OE) in improving 2,4,6-trinitrobenzene sulfonic acid (TNBS)-induced ulcerative colitis (UC).
Methods
Lipopolysaccharide (LPS) was used to induce a UC cell model, and TNBS was used to induce a UC rat model. ELISA was performed to assess the levels of inflammatory factors (IL-1β, TNF-α, and IL-6). Moreover, the activities of catalase (CAT), myeloperoxidase (MPO), and malonaldehyde (MDA) were detected by kits. Western blotting was performed to assess related proteins of the Nrf2/HO-1 signaling pathway, tight junction protein (ZO-1, Occludin, and claudin-2) expression levels, and apoptosis-related proteins (Bcl2, Bax, and cleaved caspase 3). Flow cytometry was used to analyze ROS levels. The morphology of colon tissues and the apoptosis of cells were detected by HE and TUNEL staining, respectively.
Results
OE significantly increased the activity of CAT and decreased the activity of MPO in LPS-induced Caco-2 cells and TNBS-induced UC rats. However, the levels of IL-1β, IL-6, and TNF-α were markedly reduced both in vivo and in vitro. In addition, OE significantly increased the levels of Nrf2/HO-1 signaling pathway-related proteins and tight junction proteins and inhibited cell apoptosis. HE staining showed that OE significantly decreased the severity of acute TNBS-induced colitis in rats.
Conclusion
OE may exert a regulatory effect on ameliorating intestinal barrier injury and reducing inflammation and oxidative stress levels by activating the Nrf2/HO-1 pathway.
Keywords: intestinal epithelial barrier, Nrf2/HO-1, oleracein E, oxidative stress, ulcerative colitis
Introduction
Ulcerative colitis (UC) is a chronic idiopathic inflammatory bowel disease (IBD) confined to the colon. The annual incidence rate of UC is approximately 10–20 per 100 000 people. The symptoms of most patients with UC can be relieved, while a few have no response to first-line or second-line clinical drug treatment. Quite a few of these patients have experienced adverse reactions to the current treatment [1,2]. Therefore, new treatments for UC are constantly being sought. As a medicinal and edible herb that is widely used in traditional medicine, Portulaca oleracea L. has been shown to possess many properties, such as antibacterial, anti-inflammatory, hemostatic, antioxidant, antiaging, neuroprotective, hypoglycemic, and lipid-lowering functions [3]. As shown in previous reports, Portulaca oleracea L. exerts a protective effect on IBD, and its mechanism may be related to reducing inflammatory reactions and oxidative stress and promoting the repair of the intestinal mucosa [4–6]. However, the active ingredients and mechanism of Portulaca in the treatment of IBD remain unclear.
IBD is characterized by high-level inflammation and a loss of intestinal barrier integrity, and impaired intestinal epithelial barrier function is an important local pathological feature of UC [7,8]. Because the intestinal inflammatory process is often accompanied by an increase in the level of oxidative stress and oxidative stress further damages the epithelial barrier, oxidative stress plays an important role in the functional damage to the epithelial barrier in individuals with UC [9].
Oleracein E (OE) is a phenolic alkaloid first isolated from the alcohol extract of Portulaca oleracea L. and was identified as a tetrahydroisoquinoline with strong antioxidant activity [10,11]. OE is considered a neuroprotective agent that protects against oxidative stress-induced nerve injury [12,13]. In addition, OE has been reported to improve cardiac function by downregulating the expression of proteins in the MAPK signaling pathway in an experimental acute myocardial infarction model [14]. However, the role of OE in UC and other IBD s remains unclear. Nuclear Factor E2-related Factor 2 (Nrf2) is a cytoprotective factor that regulates the expression of antioxidant, anti-inflammatory, and detoxification genes. After activation, Nrf2 translocates to the nucleus and activates downstream pathways, thus inhibiting oxidative stress [15,16]. As one of the most important endogenous protective systems, the Nrf2/HO-1 signaling pathway is the most classic pathway of Nrf2 and functions as an antioxidant pathway [17,18]. Researchers have not clearly determined whether OE improves intestinal epithelial barrier function and alleviates UC by reducing inflammation and inhibiting oxidative stress through the activation of the Nrf2/HO-1 pathway.
In the present study, we aimed to verify whether OE exerts a protective effect on the intestinal barrier in a UC model. Thus, the effect of OE on an experimental UC model induced by 2,4,6-trinitrobenzene sulfonic acid (TNBS) and lipopolysaccharide (LPS)-induced Caco-2 cell injury was detected.
Materials and methods
Ethics statement
The study was approved and supervised by the Department of Scientific Research, Kunming Yan’an Hospital. All protocols were conducted in accordance with the ethical guidelines of the 1975 Declaration of Helsinki. The animal experiments described in this study were performed in compliance with the Laboratory Animal Management of National Animal Science and Technology Commission’s Regulations.
Animals and establishment of the UC model
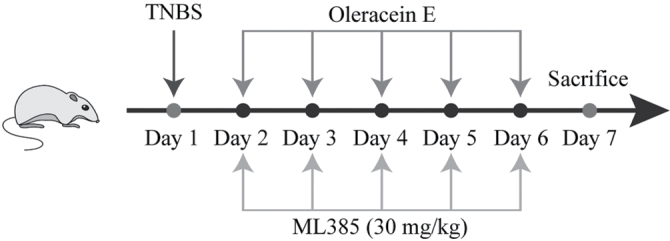
SPF male SD rats (180–200 g) were purchased from Nanjing Junke Biological Engineering Co., Ltd. After 7 days of adaptive feeding in a room temperature (25 °C) environment with constant humidity (50% ± 5%), rats were randomly divided into 5 groups (n = 5): control, model, low-dose (5 mg/kg of OE), medium-dose (10 mg/kg of OE), and high-dose (20 mg/kg of OE) groups. The UC rat model was established using the method described below. Rats were fasted for 24 h before modeling but were allowed free access to drinking water. After mild anesthesia was induced with ether, 20 mg of TNBS (100 mg/kg, St. Louis, Missouri, USA) was dissolved in 0.8 ml of 50% ethanol, and the mixed solution was slowly injected into 7–8 cm of the proximal end of the descending colon, and the rats were maintained in the vertical position for 60 s. The rats in the blank group were injected with the same amount of physiological saline using the same method. After 24 h, 70% or more of the rats had diarrhea, blood in their stool, and perianal filth, which indicated that the model was successful. On the second day of modeling, the groups were intragastrically administered the indicated treatments; the OE groups were administered the corresponding dose of OE solution for 5 days, whereas the blank group and the model group were administered the corresponding volume of tap water. Rats were humanely killed on day 7 under deep anesthesia. Finally, the colon was removed for subsequent experiments.
Schematic diagram of modeling and treatment.
Disease activity index score monitoring
The disease activity status and weight of the rats were observed each day, the stool characteristics and blood in the stool were observed, and whether there was occult blood in the stool was detected with an occult blood kit. According to the reference [19], the disease activity index (DAI) score is 0 to 4 points from light to heavy according to the changes in the characteristics of mice (body weight change rate, stool characteristics, and presence or absence of blood in stool). DAI = (body weight change rate + stool characteristics + whether or not blood in stool)/3.
Cell culture
Caco-2 cells were obtained from the American Type Culture Collection (Manassas, Virginia, USA). Cell lines were maintained at 37 °C and 5% CO2 in DMEM (Gibco, Logan, Utah, USA) containing 10% FBS and 1% penicillin/streptomycin. Caco-2 cells were then induced by LPS (1 μg/ml) to construct a UC model [20].
Determination of myeloperoxidase, catalase, and malonaldehyde
After the rats were anesthetized with ether, they were humanely killed, and all colon tissue was quickly collected. The tissue was homogenized by adding 10% of the tissue homogenate in the homogenization medium at 4 °C in sterile physiological saline and thoroughly grinding the tissue in an ice bath. Centrifugation was performed at 3500 rpm for 10 min at 4 °C, and the supernatant was collected. The levels of myeloperoxidase (MPO), catalase (CAT), and malonaldehyde (MDA) in the colon tissue homogenate were measured, and all operations were performed according to the instructions provided with the kits (Beyotime, China).
Hematoxylin and eosin staining
The sigmoid colon tissues of the rats were harvested and fixed with 4% paraformaldehyde for 24 h, and paraffin sections (4 μm thickness) were prepared. After dewaxing with xylene and rehydration in a graded series of ethanol solutions, the sections were stained with hematoxylin for 5 min, differentiated in hydrochloric acid/ethanol for 30 s and eosin for 2 min. After dehydration, clearing, and mounting procedures, sections were photographed under a microscope. According to the reference [19], the histopathological score was evaluated from 0 to 4 points according to the degree of colonic tissue lesion (inflammation, lesion depth, crypt destruction and lesion range), and the final histopathological score was the sum of 4 items.
TUNEL staining
Paraffin sections of rat sigmoid colon tissues were deparaffinized in xylene for 5 min and then washed with ethanol (100, 95, 90, 80, and 70%) for 3 min at each step using the descending gradient method, followed by 2 washes with PBS. Colon tissues were treated with protease K for 30 min, washed twice with PBS, immersed in blocking solution, and incubated for 10 min (room temperature) before two rinses with PBS. The sections were incubated with a mixture of 2 μl of TdT and 48 μl of dUTP solution (Beyotime, China) in a humidified chamber (1 h, room temperature). Following PBS washes (three times), the secondary antibody and DAB chromogenic agent were used for photography under a microscope.
Flow cytometry
The degree of apoptosis was assessed by flow cytometry using an Annexin V-FITC/PI Apoptosis Detection Kit (BD Biosciences, Newark, New Jersey, USA) and analyzed with FlowJo software.
ELISA
The levels of the inflammatory cytokines TNF-α (Abcam, Cambridge, UK), IL-6 (Abcam), and IL-1β (Abcam) in the colon were measured using corresponding ELISA kits according to the manufacturer’s instructions. Absorbance was determined with a microplate spectrophotometer (BioTeke, Beijing, China).
Western blotting
Protein extraction kits were used to extract colon proteins. Briefly, colon tissues were homogenized in precooled saline and centrifuged (10 000 g, 4 °C) for 30 min. The extracted proteins were quantified with a Bestbio BCA Assay Kit (Shanghai, China), electrophoretically separated on SDS–PAGE gels and transferred to polyvinyl difluoride membranes (Millipore, Billerica, Massachusetts, USA). After blocking with 5% BSA and washing three times, the membranes were incubated with primary or secondary antibodies according to the experimental protocol. The band intensities were calculated using ImageJ software. Working dilutions of the antibodies were prepared as follows: claudin-2 (Abcam, 1:3000), ZO-1 (Abcam, 1:3000), occludin (Abcam, 1:3000), Bax (Abcam, 1:15000), Bcl2 (Abcam, 1:3000), Nrf2 (Abcam, 1:1500), and HO-1 (Abcam, 1:3000). Lamin B and β-actin antibodies were used to normalize the samples for equal loading.
Reactive oxygen species assay
Reactive oxygen species (ROS) were measured using a reactive oxygen species analysis kit (Beyotime). Cells were suspended in medium containing 10 μmol/L DCFH-DA and incubated at 37 °C for 30 min. ROS levels were then measured by flow cytometry (CytoFlex; Beckman Coulter, Bria, California, USA).
Statistical analysis
All data are presented as the means ± SEM. Differences between two or multiple groups were evaluated using a two-tailed Student’s t-test or one-way analysis of variance. Statistical analyses were performed using GraphPad Prism 7.0 software (GraphPad Software, Inc., California, USA). P ≤ 0.05 indicated a statistically significant difference.
Results
OE improves TNBS-induced UC in rats
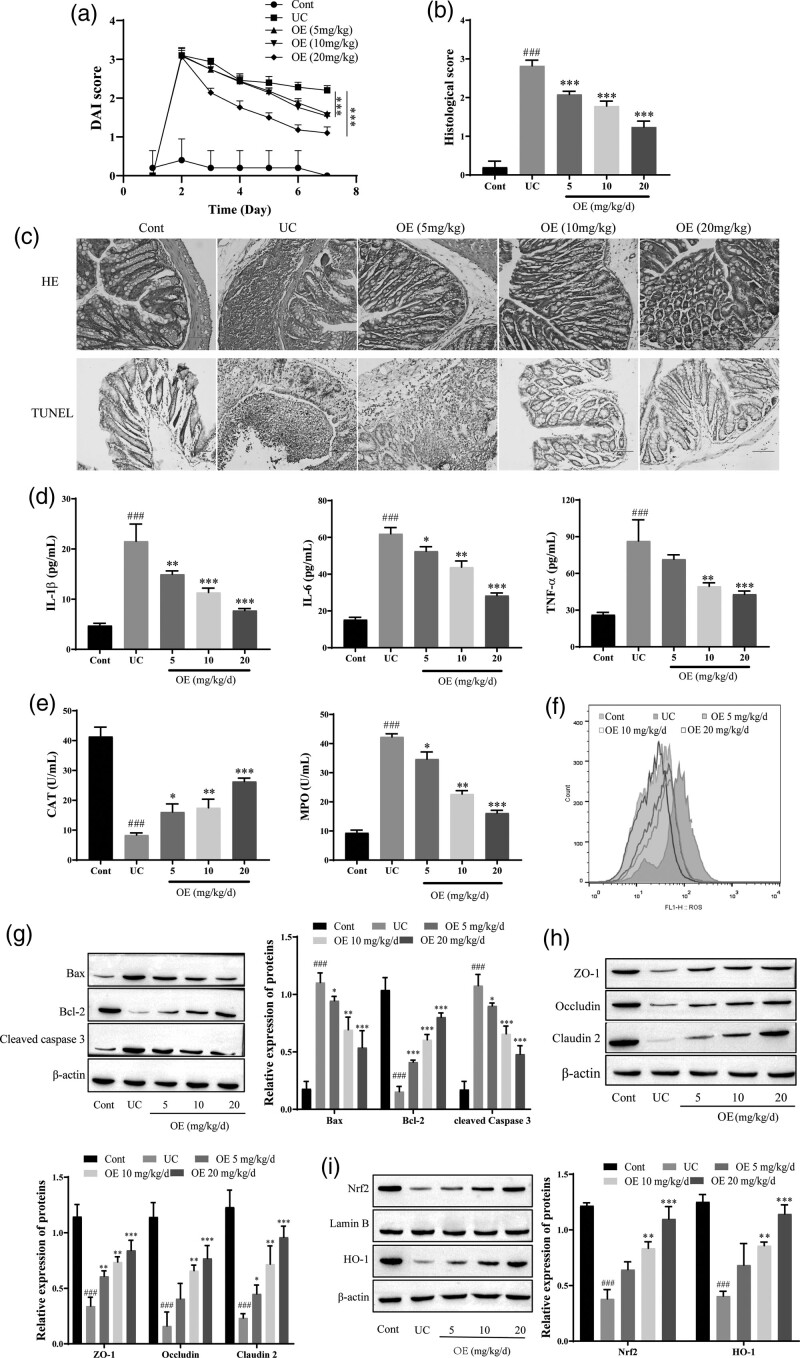
Histological features were assessed using hematoxylin and eosin (HE) staining to investigate the effect of OE on TNBS-induced UC in rats, as shown in Fig. 1. Compared with the control group, the DAI score in the model group was significantly higher, and the DAI score was significantly reduced by OE administration (Fig. 1a). The colonic structures in the UC group exhibited marked necrosis with epithelial damage, detachment or exfoliation, submucosal infiltration of inflammatory cells, and crypt hyperplasia compared to those in the control group. In contrast, the administration of OE (5 mg/kg/d, 10 mg/kg/d, or 20 mg/kg/d) significantly attenuated the structural collapse and infiltration of inflammatory cells in the colon (Fig. 1b). TUNEL staining revealed decreased apoptosis after OE treatment (Fig. 1c). The levels of these proinflammatory cytokines were significantly increased in the colon of the UC group; treatment with OE dose-dependently reversed the production of proinflammatory cytokines in the UC group. Notably, 20 mg/kg/d OE exerted a therapeutic effect and induced levels of these cytokines that were comparable to those in normal colon tissue (Fig. 1d). OE treatment significantly increased the activity of the antioxidant CAT, decreased MPO activity, and reduced ROS production, and 20 mg/kg/d OE exerted a greater effect (Fig. 1e–f). The expression of Bcl2 was significantly reduced in the UC group compared with the control group, while the levels of the Bax and cleaved caspase 3 proteins were significantly increased. The expression of the Bcl2 protein was significantly increased, while the levels of the Bax and cleaved caspase 3 proteins were significantly decreased after OE treatment (Fig. 1g). Intestinal mucosal healing is associated with tight junction proteins and apoptosis of cells. Compared with the control group, the expression of the tight junction proteins ZO-1, Occludin, and Claudin-2 was significantly decreased in the UC group. The expression of the tight junction proteins ZO-1, Occludin, and Claudin-2 was significantly increased after OE treatment (Fig. 1h). In addition, compared with UC group, the expression of Nrf2 and HO-1 was significantly increased in the NC group, and the expression of these proteins was increased by OE treatment. Based on these observations, the amelioration of TNBS-induced UC in rats by OE may be related to Nrf2/HO-1 (Fig. 1i).
Fig. 1.
Effect of OE on TNBS-induced increases in tissue levels of inflammatory factors, intestinal epithelial cell apoptosis, and oxidative stress levels in UC rats. (a) Disease activity index (DAI) scores. (b) HE staining and histological score for the degree of damage in the rat colon (scale bar = 100 µm). (c) TUNEL for histomorphological changes in rats with UC (scale bar = 100 µm). (d) ELISA for IL-6, IL-1β, and TNF-α. (e) The contents of MPO and CAT were determined by kits. (f) Flow cytometry analysis of ROS levels. (g) Western blots showing the levels of Bax, Bcl2, and cleaved caspase 3. (h) Western blots showing the levels of the tight junction proteins ZO-1, Occludin, and Claudin-2. (i) Western blots showing the levels of Nrf2 and HO-1. Compared with the NC group, ###P ≤ 0.001; Compared with the UC group, *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001.
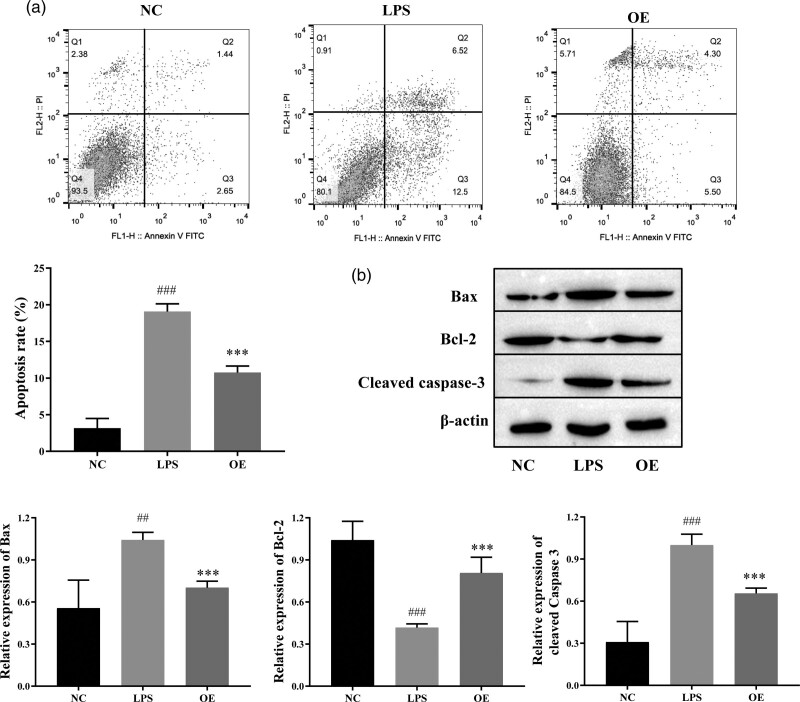
OE blocked the apoptosis of LPS-induced Caco-2 cells
An LPS-induced Caco-2 cell model was used to investigate the molecular mechanism of OE treatment in IBD. The apoptosis rate of LPS-treated Caco-2 cells was significantly increased compared with that of the control group, while the apoptosis rate of LPS-treated Caco-2 cells was reduced by OE treatment (Fig. 2a). The Western blots showed that the levels of Bax and cleaved caspase 3 were increased in the LPS group, while the expression of Bcl2 was significantly decreased compared with the control group. The levels of Bax and cleaved caspase 3 were decreased in the OE-treated group compared with the LPS group, while the expression of Bcl2 was significantly increased (Fig. 2b). These results suggest that OE inhibits apoptosis of LPS-induced Caco-2 cells.
Fig. 2.
Effect of OE on LPS-induced Caco-2 cell apoptosis. (a) Flow cytometry for cell apoptosis. (B–E) Western blot for the levels of apoptotic proteins Bax, Bcl2, and cleaved caspase 3. Compared with the NC group, ##P ≤ 0.01, ###P ≤ 0.001; compared with the LPS group, ***P ≤ 0.001.
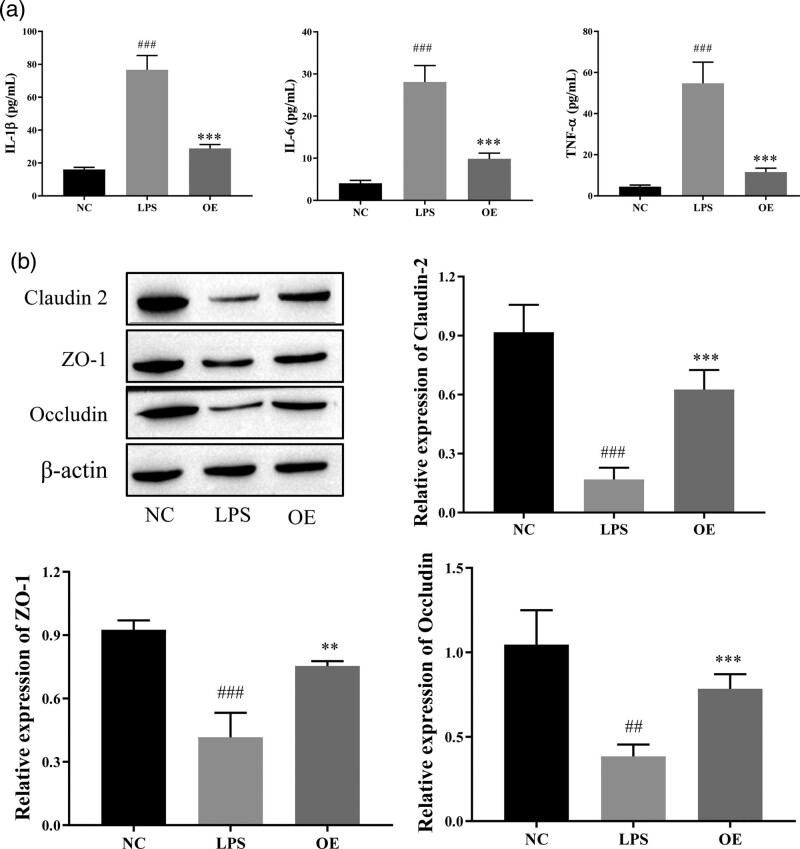
OE reduced cytokine levels and ameliorated colonic mucosal damage in LPS-induced Caco-2 cells
We measured the levels of proinflammatory factors using ELISA to investigate the effect of OE on inflammation in Caco-2 cells and intestinal epithelial barrier function at the cellular level. The levels of the proinflammatory factors IL-1β, IL-6, and TNF-α were significantly reduced in the OE-treated group compared with the LPS group (Fig. 3a). This finding indicates that OE inhibits inflammation in Caco-2 cells and tends to alleviate UC. Next, the expression levels of tight junction proteins were detected by using Western blotting, and significantly higher levels of the claudin-2, ZO-1, and occludin proteins were detected in the OE group than the LPS group (Fig. 3b). Based on this finding, OE promotes intestinal mucosal healing. Taken together, OE repairs damage in individuals with UC by inhibiting intestinal mucosal inflammation and promoting the healing of the damaged intestinal mucosal barrier.
Fig. 3.
Effect of OE on LPS-induced inflammatory factors and tight junction protein TJs in Caco-2 cells. (a) ELISA for the expression of the inflammatory factors IL-1β, IL-6 and TNF-α. (b) Western blot for the expression of the tight junction proteins ZO-1, Claudin-2, and Occludin. Compared with the NC group, ##P ≤ 0.01, ###P ≤ 0.001; compared with the LPS group, **P ≤ 0.01, ***P ≤ 0.001.
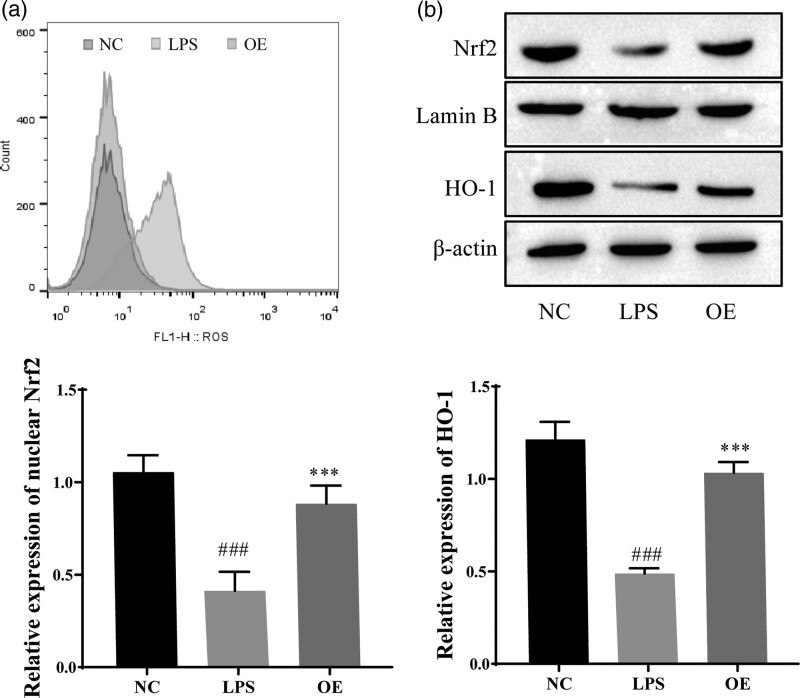
OE inhibits oxidative stress in LPS-induced Caco-2 cells by activating the Nrf2/HO-1 pathway
We verified the effect of OE on LPS-induced oxidative stress in Caco-2 cells. As shown in Fig. 4, ROS levels were detected using flow cytometry, and the results showed that OE treatment decreased ROS levels in LPS-treated Caco-2 cells. Thus, OE inhibited oxidative stress in a UC model (Fig. 4a). The expression levels of Nrf2 and HO-1 were detected using western blotting, and the results showed significantly decreased expression of Nrf2 and HO-1 in the LPS group compared with the NC group, and Nrf2 and HO-1 expression were increased by OE treatment (Fig. 4b). This finding indicates that OE may inhibit LPS-induced oxidative stress by activating the Nrf2/HO-1 pathway.
Fig. 4.
Effect of OE on LPS-induced oxidative stress in Caco-2 cells. (a) Flow cytometry analysis of ROS levels in cells. (b) Western blots for Nrf2, Lamin B, and HO-1 expression. #P < 0.05 compared with the NC ###P ≤ 0.001; Compared with the LPS group, ***P ≤ 0.001.
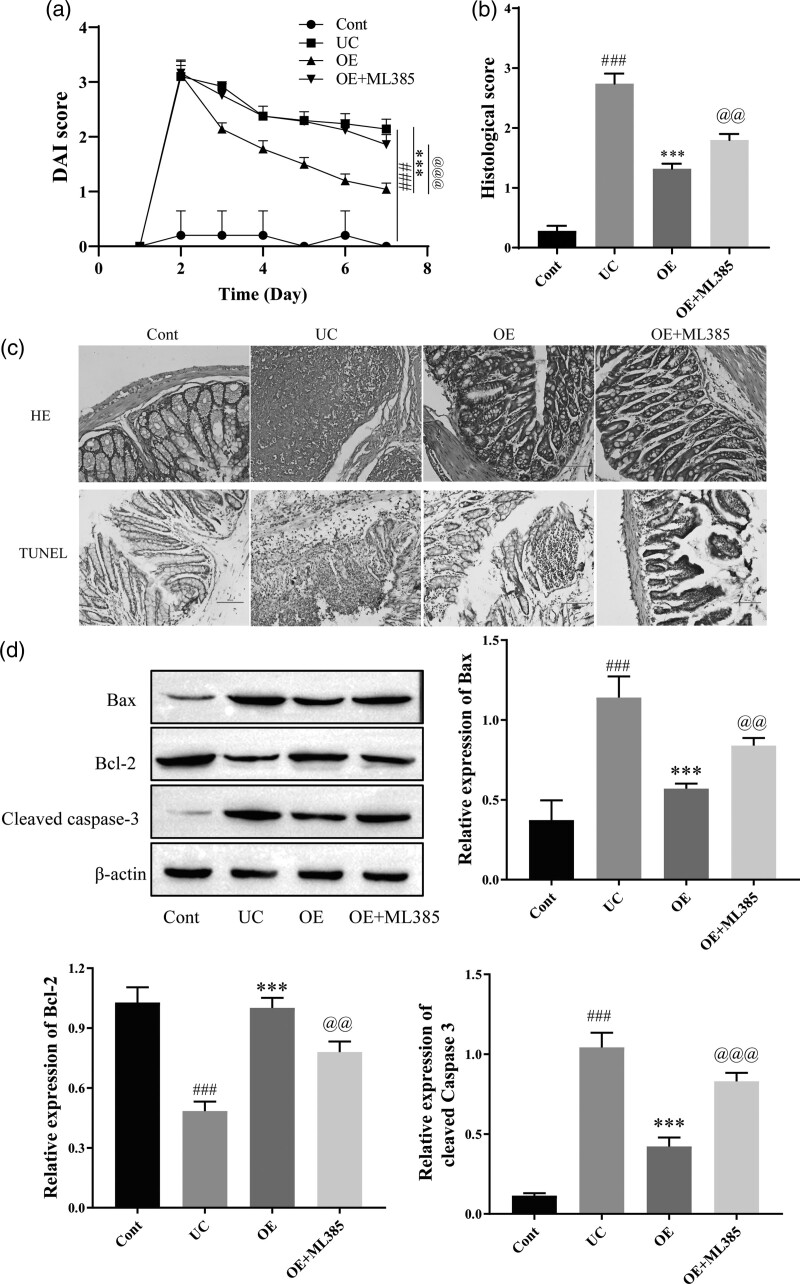
OE inhibits apoptosis and alleviates TNBS-induced tissue damage in rats via the Nrf2/HO-1 pathway
As described above, OE inhibited the inflammatory response, oxidative stress, and intestinal epithelial barrier damage in LPS-induced Caco-2 cells and TNBS-induced UC mouse models. We focused on the Nrf2/HO-1 signaling pathway to further elucidate the underlying mechanisms. Therefore, we used ML385 (Nrf2 inhibitor) in subsequent experiments. Compared with the control group, the DAI score of the UC group was significantly higher. OE treatment reduced the DAI score, and the addition of ML385 reversed the effect of OE (Fig. 5a). UC colonic tissue damage was significantly alleviated by OE treatment, and the therapeutic effect of OE was inhibited when ML385 was administered (Fig. 5b). The TUNEL assay results confirmed OE inhibited inflammation-induced cell apoptosis, ML385 reversed above result (Fig. 5c). Subsequently, we examined the expression of apoptosis-related proteins. The results showed a significant increase in the levels of cleaved caspase 3 and Bax and a significant decrease in the level of Bcl2 in the UC group. Cleaved caspase 3 and Bax levels were significantly decreased after OE treatment, while Bcl2 expression was significantly increased. The addition of ML385 to OE treatment reversed the inhibitory effect of OE on apoptotic cells (Fig. 5d). Thus, OE alleviates tissue damage by activating the Nrf2/HO-1 signaling pathway, effectively inhibiting colon cell apoptosis.
Fig. 5.
The effect of OE on TNBS-induced apoptotic protein expression and histomorphology in rats by the Nrf2/HO-1 signaling pathway. (a) Disease activity index (DAI) scores. (b) HE staining and histological score for the extent of damage in the rat colon (scale bar = 100 µm). (c) HE and TUNEL staining for histomorphological changes in rats with UC (scale bar = 100 µm). (d) Western blots for the expression of apoptosis-related proteins Bax, Bcl2, and cleaved caspase 3. Compared with the control group, ###P ≤ 0.001; compared with the UC group, ***P ≤ 0.001; compared with the OE group, @@P ≤ 0.01, @@@P ≤ 0.001.
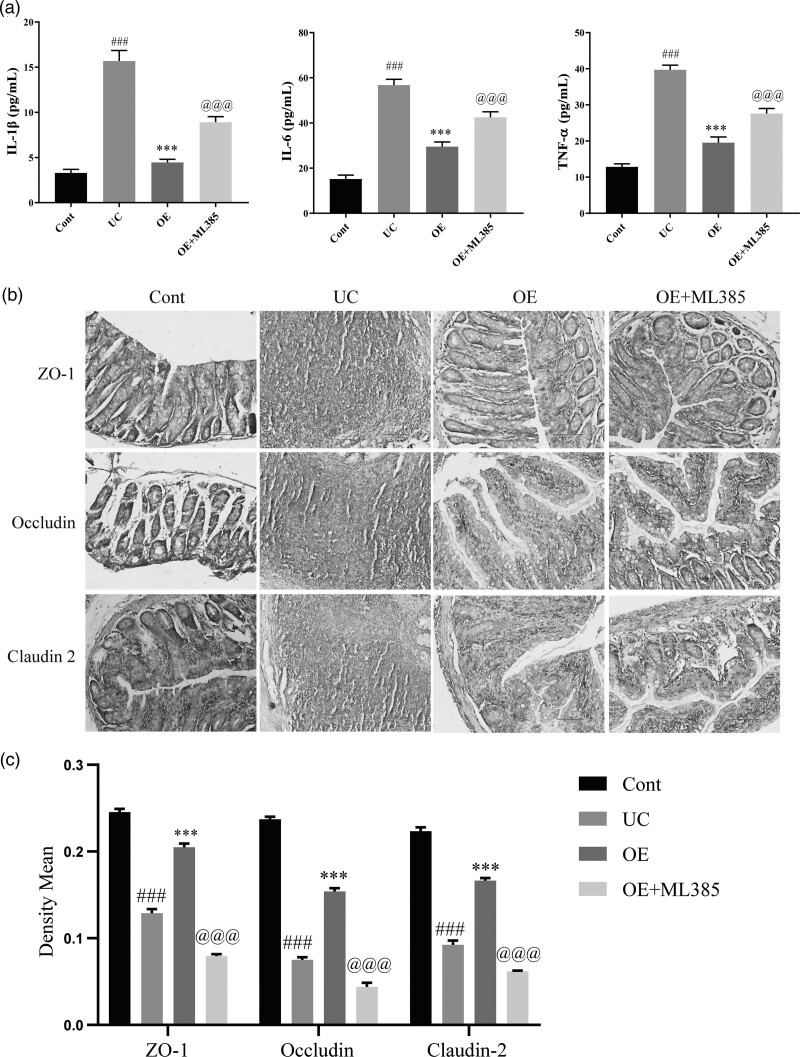
OE inhibits inflammation and promotes the repair of TNBS-induced intestinal epithelial damage in rats via the Nrf2/HO-1 signaling pathway
Epithelial barrier damage is one of the hallmarks of colonic inflammation in individuals with UC. We verified the effect of the Nrf2/HO-1 signaling pathway on the colonic epithelial barrier in UC by measuring the levels of the inflammatory factors IL-1β, IL-6, and TNF-α by ELISA. The levels of the inflammatory factors IL-1β, IL-6, and TNF-α were significantly higher in the UC group than in the control group, while significantly lower levels of these inflammatory factors were detected after OE treatment. However, the inhibitory effect of OE on these inflammatory factors was significantly reversed after the administration of ML385 (Fig. 6a). Subsequently, we assessed the effect of OE on epithelial barrier damage in animals with UC by performing immunohistochemical staining to detect tight junction protein expression, and OE exerted a significant reparative effect on colonic epithelial damage. However, when the ML385 inhibitor was administered with OE, it inhibited the reparative effect of OE on colonic epithelial damage (Fig. 6b and c). Based on this finding, OE inhibits inflammatory protein expression and promotes the repair of UC intestinal epithelial injury through the Nrf2/HO-1 signaling pathway.
Fig. 6.
The effect of OE on TNBS-induced inflammation and epithelial barrier damage in rats by the Nrf2/HO-1 signaling pathway. (a) ELISA for the levels of IL-6, IL-1β, and TNF-α. (b) Immunohistochemical staining for changes in colon morphology (scale bar = 100 µm). (c) Mean optical density values of immunohistochemical staining. Compared with the control group, ###P ≤ 0.001; Compared with the UC group, ***P ≤ 0.001; compared with the OE group, @@@P ≤ 0.001.
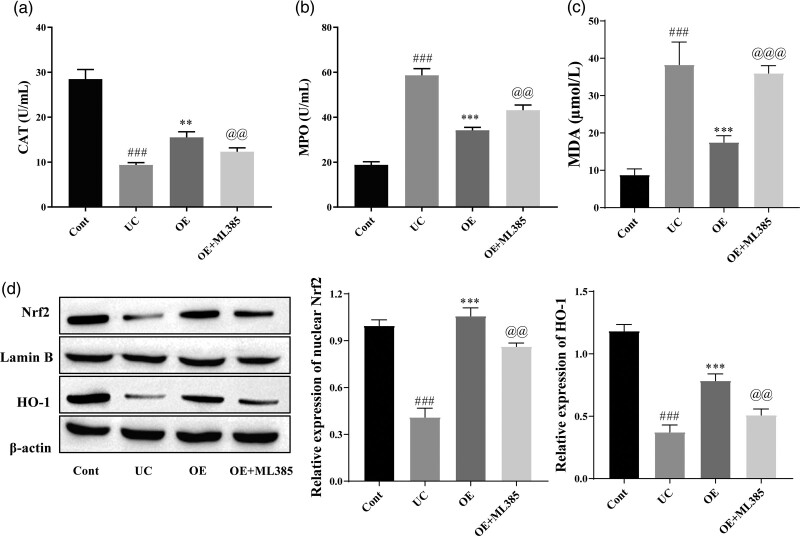
OE inhibits TNBS-induced oxidative stress in rats via the Nrf2/HO-1 signaling pathway
According to the experiments described above, OE inhibits inflammation and promotes intestinal epithelial tissue repair via the Nrf2/HO-1 signaling pathway. Therefore, we further evaluated and confirmed the role of the Nrf2/HO-1 signaling pathway in oxidative stress in a model of UC. As shown in Fig. 7, CAT and MPO levels were measured using kits. CAT levels were significantly increased and MPO and MDA levels were significantly decreased in the OE group compared with the UC group, and the effect of OE on CAT, MPO, and MDA levels was reversed after treatment with ML385 (Fig. 7a–c). Western blot results showed significantly increased levels of Nrf2 and HO-1 in the OE-treated group compared with the UC group, and their levels were significantly reduced after the administration of ML385 (Fig. 7d). In summary, OE ameliorated UC by inhibiting oxidative stress through the Nrf2/HO-1 pathway.
Fig. 7.
The effect of OE on TNBS-induced oxidative stress in rats by the Nrf2/HO-1 signaling pathway. (a, b and c) Kits for detecting CAT, MPO, and MDA levels. (d) Western blots for Nrf2 and HO-1 expression. Compared with the control group, ###P ≤ 0.001; compared with the UC group, **P ≤ 0.01, ***P ≤ 0.001; compared with the OE group, @@P ≤ 0.01, @@@P ≤ 0.001.
Discussion
UC is a persistently worsening inflammatory disease of the intestine that is harmful to an individual’s mental and physical health and may increase the risk of colon cancer [21]. Current medications used to improve the clinical symptoms and inflammation associated with UC include immunosuppressants, aminosalicylates, corticosteroids, and other biological agents. However, these drugs may also produce side effects. Therefore, research seeking to develop safer, effective, and targeted UC therapies is imminent. Amaranthus extract has been shown to treat dextran sulfate sodium-induced UC. Therefore, in this study, we aimed to explore the molecular mechanism by which OE exerts its protective effect on UC. OE inhibited inflammation and oxidative stress in LPS-stimulated Caco-2 cells and rats TNBS-induced colitis models to promote the repair of intestinal epithelial damage with an optimal effect at 20 mg/kg/d. Second, OE exerted its effects by activating the Nrf2/HO-1 signaling pathway.
Inflammatory responses regulated by multiple proinflammatory factors play a key role in the progression of UC. In-vivo and in-vitro experiments showed that the levels of inflammatory factors (IL-1β, IL-18, IL-6, TNF-α) in individuals with UC correlate with the severity of inflammation [22]. IL-1β, a cytokine produced by activated macrophages, is an important mediator of the inflammatory response, and increased IL-1β production causes an autoimmune process that damages colonic tissue [23]. IL-6 is a proinflammatory factor that causes colonic damage, leading to inflammation. TNF-α is a multifunctional cytokine that induces apoptosis, proliferation, differentiation, and inflammation [24]. In our TNBS-induced UC animal model and LPS-treated Caco-2 cells, IL-6, TNF-α, and IL-1β levels were significantly increased, further confirming the key roles of these proinflammatory cytokines in the pathogenesis of intestinal inflammation. Administration of OE significantly inhibited the expression of proinflammatory factors, suggesting that OE alleviates UC by suppressing inflammatory factor expression.
Based on accumulating evidence, oxidative stress is involved in various types of tissue damage associated with UC. ROS, MPO, and CAT are markers of oxidative stress. Oxidative stress occurs when ROS accumulate, and the balance of the intracellular oxidant-antioxidant system is disrupted when ROS production exceeds the scavenging capacity of the antioxidant system [25]. MPO is a cytotoxic enzyme in neutrophils that is associated with tissue damage in many inflammatory processes [26]. CAT is an antioxidant enzyme responsible for the conversion of hydrogen peroxide into oxygen and water, and reduced CAT activity increases oxidative stress. The results of our current study confirm that OE treatment significantly reduces MPO activity and increases the production of the antioxidant enzyme CAT compared to UC tissues while removing ROS. Thus, OE alleviates UC by inhibiting oxidative stress. In addition, oxidative stress induces apoptosis through several pathways [27], and we examined apoptosis in TNBS-induced UC mouse tissues and LPS-treated Caco-2 cells using western blotting, flow cytometry, and TUNEL assays. OE effectively inhibited apoptosis in UC colon tissues and LPS-stimulated Caco-2 cells. This finding was presented in a study by El Sayed et al. [28]. showed that ‘protection against TNBS-induced colitis in rats can be achieved through the regulation of oxidative stress and apoptosis-related proteins’.
Impaired intestinal mucosal barrier function is an important feature to assess the extent of UC, and barrier function depends on the integrity of the mucosal layer, which is determined by the expression and assembly of tight junction proteins. The tight junction complex consists of transmembrane proteins (e.g. occludin and the claudin family) and linker proteins (e.g. ZO-1 and claudin-2) [29]. In general, increased levels of ZO-1 and Occludin and decreased levels of Claudin-2 promote the repair of intestinal epithelial barrier damage. As shown in our results, OE increased the expression of the tight junction proteins ZO-1, Claudin-2 and Occludin in UC tissues and Caco-2 cells. OE affects intestinal epithelial barrier damage repair by regulating the expression of tight junction proteins.
Nrf2 is a redox-sensitive transcription factor that plays a key role in oxidative stress. Nrf2 is located in the cytoplasm under normal conditions. Upon the exposure of cells to oxidative stress, it translocates to the nucleus to induce the expression of antioxidant molecules. HO-1 is a recognized antioxidant gene located downstream of Nrf2 [30]. Dong et al. [31]. showed that activation of the Nrf2/HO-1 signaling pathway protects cells from HO-induced oxidative stress. Our study explored whether OE plays a role in UC through the Nrf2/HO-1 signaling pathway. OE directly inhibited the expression of proinflammatory factors, alleviated oxidative stress, and promoted intestinal epithelial barrier repair in a TNBS-induced UC rats model and LPS-stimulated Caco-2 cells through activation of the Nrf2/HO-1 signaling pathway.
Conclusions
OE may improve intestinal barrier damage and reduce inflammation and oxidative stress levels by activating the Nrf2/HO-1 pathway, thereby alleviating UC. Our study may provide a potential molecular basis for future UC treatment.
Acknowledgements
This work was supported by the Yunnan Provincial Key Laboratory of Tumor Immune Prevention and Control (No. 2017DG004), the Kunming-Medical Joint Special Project of the Yunnan Provincial Department of Science and Technology (No. 202001AY070001-261), and the Kunming Municipal Health Commission Health Research Project (No. 2019-03)-03-002), supported by the Kunming Yan’an Hospital In-Hospital Project (No. yyky018-029), and the Kunming Health Science and Technology Talent Training Project (No. 2021-SW (backup)-07).
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Conflicts of interest
There are no conflicts of interest.
Footnotes
Yun Huang and Yu Su contributed equally to the writing of this article.
References
- 1.Neurath MF, Leppkes M. Resolution of ulcerative colitis. Semin Immunopathol 2019; 41:747–756. [DOI] [PubMed] [Google Scholar]
- 2.Iheozor-Ejiofor Z, Kaur L, Gordon M, Baines PA, Sinopoulou V, Akobeng AK. Probiotics for maintenance of remission in ulcerative colitis. Cochrane Database Syst Rev 2020; 3:Cd007443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rahimi VB, Ajam F, Rakhshandeh H, Askari VR. A pharmacological review on Portulaca oleracea L.: focusing on anti-inflammatory, anti- oxidant, immuno-modulatory and antitumor activities. J Pharmacopuncture 2019; 22:7–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yang X, Yan Y, Li J, Tang Z, Sun J, Zhang H, et al. Protective effects of ethanol extract from Portulaca oleracea L on dextran sulphate sodium-induced mice ulcerative colitis involving anti-inflammatory and antioxidant. Am J Transl Res 2016; 8:2138–2148. [PMC free article] [PubMed] [Google Scholar]
- 5.Kong R, Luo H, Wang N, Li J, Xu S, Chen K, et al. Portulaca extract attenuates development of dextran sulfate sodium induced colitis in mice through activation of PPARγ. PPAR Res 2018; 2018:6079101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim Y, Lim HJ, Jang HJ, Lee S, Jung K, Lee SW, et al. Portulaca oleracea extracts and their active compounds ameliorate inflammatory bowel diseases in vitro and in vivo by modulating TNF-α, IL-6 and IL-1β signalling. Food Res Int 2018; 106:335–343. [DOI] [PubMed] [Google Scholar]
- 7.Yao D, Dong M, Dai C, Wu S. Inflammation and inflammatory cytokine contribute to the initiation and development of ulcerative colitis and its associated cancer. Inflamm Bowel Dis 2019; 25:1595–1602. [DOI] [PubMed] [Google Scholar]
- 8.Soroosh A, Rankin CR, Polytarchou C, Lokhandwala ZA, Patel A, Chang L, et al. miR-24 is elevated in ulcerative colitis patients and regulates intestinal epithelial barrier function. Am J Pathol 2019; 189:1763–1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.John LJ, Fromm M, Schulzke JD. Epithelial barriers in intestinal inflammation. Antioxid Redox Signal 2011; 15:1255–1270. [DOI] [PubMed] [Google Scholar]
- 10.Yang ZJ, Zheng YN, Xiang L. [Study on chemical constituents of Portulaca oleracea]. Zhong Yao Cai 2007; 30:1248–1250. [PubMed] [Google Scholar]
- 11.Yang Z, Liu C, Xiang L, Zheng Y. Phenolic alkaloids as a new class of antioxidants in Portulaca oleracea. Phytother Res 2009; 23:1032–1035. [DOI] [PubMed] [Google Scholar]
- 12.Sun H, He X, Liu C, Li L, Zhou R, Jin T, et al. Effect of Oleracein E, a Neuroprotective Tetrahydroisoquinoline, on Rotenone-induced Parkinson’s disease cell and animal models. ACS Chem Neurosci 2017; 8:155–164. [DOI] [PubMed] [Google Scholar]
- 13.Li L, Jiao Y, Jin T, Sun H, Li S, Jin C, et al. Phenolic alkaloid oleracein E attenuates oxidative stress and neurotoxicity in AlCl(3)-treated mice. Life Sci 2017; 191:211–218. [DOI] [PubMed] [Google Scholar]
- 14.Zhao F, Bai R, Li J, Feng X, Jiao S, Wuken S, et al. Meconopsis horridula Hook. f. & Thomson extract and its alkaloid oleracein E exert cardioprotective effects against acute myocardial ischaemic injury in mice. J Ethnopharmacol 2020; 258:112893. [DOI] [PubMed] [Google Scholar]
- 15.Tonelli C, Chio IIC, Tuveson DA. Transcriptional regulation by Nrf2. Antioxid Redox Signal 2018; 29:1727–1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bellezza I, Giambanco I, Minelli A, Donato R. Nrf2-Keap1 signaling in oxidative and reductive stress. Biochim Biophys Acta Mol Cell Res 2018; 1865:721–733. [DOI] [PubMed] [Google Scholar]
- 17.Li B, Nasser MI, Masood M, Adlat S, Huang Y, Yang B, et al. Efficiency of Traditional Chinese medicine targeting the Nrf2/HO-1 signaling pathway. Biomed Pharmacother 2020; 126:110074. [DOI] [PubMed] [Google Scholar]
- 18.Loboda A, Damulewicz M, Pyza E, Jozkowicz A, Dulak J. Role of Nrf2/HO-1 system in development, oxidative stress response and diseases: an evolutionarily conserved mechanism. Cell Mol Life Sci 2016; 73:3221–3247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang T, Mei Y, Dong W, Wang J, Huang F, Wu J. Evaluation of protein arginine deiminase-4 inhibitor in TNBS- induced colitis in mice. Int Immunopharmacol 2020; 84:106583. [DOI] [PubMed] [Google Scholar]
- 20.Guo H, Zhang L, Wang Y, He X. Mechanisms of HuR in regulation of epithelial cell apoptosis in rat ulcerative colitis. Cell Signal 2021; 82:109957. [DOI] [PubMed] [Google Scholar]
- 21.Pandurangan AK, Esa NM. Signal transducer and activator of transcription 3 - a promising target in colitis-associated cancer. Asian Pac J Cancer Prev 2014; 15:551–560. [DOI] [PubMed] [Google Scholar]
- 22.Kim JM. [Inflammatory bowel diseases and inflammasome]. Korean J Gastroenterol 2011; 58:300–310. [DOI] [PubMed] [Google Scholar]
- 23.Cho EJ, Shin JS, Noh YS, Cho YW, Hong SJ, Park JH, et al. Anti-inflammatory effects of methanol extract of Patrinia scabiosaefolia in mice with ulcerative colitis. J Ethnopharmacol 2011; 136:428–435. [DOI] [PubMed] [Google Scholar]
- 24.Vivinus-Nébot M, Frin-Mathy G, Bzioueche H, Dainese R, Bernard G, Anty R, et al. Functional bowel symptoms in quiescent inflammatory bowel diseases: role of epithelial barrier disruption and low-grade inflammation. Gut 2014; 63:744–752. [DOI] [PubMed] [Google Scholar]
- 25.Manga P, Elbuluk N, Orlow SJ. Recent advances in understanding vitiligo. F1000Res 2016; 5:2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shen J, Cheng J, Zhu S, Zhao J, Ye Q, Xu Y, et al. Regulating effect of baicalin on IKK/IKB/NF-kB signaling pathway and apoptosis-related proteins in rats with ulcerative colitis. Int Immunopharmacol 2019; 73:193–200. [DOI] [PubMed] [Google Scholar]
- 27.Zhou X, Ren M, Yang J, Pan H, Yu M, Ji F. Curcumin improves epithelial barrier integrity of Caco-2 monolayers by inhibiting endoplasmic reticulum stress and subsequent apoptosis. Gastroenterol Res Pract 2021; 2021:5570796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.El Sayed NS, Sayed AS. Protective effect of methylene blue on TNBS-induced colitis in rats mediated through the modulation of inflammatory and apoptotic signalling pathways. Arch Toxicol 2019; 93:2927–2942. [DOI] [PubMed] [Google Scholar]
- 29.Li H, Chen X, Liu J, Chen M, Huang M, Huang G, et al. Ethanol extract of Centella asiatica alleviated dextran sulfate sodium-induced colitis: Restoration on mucosa barrier and gut microbiota homeostasis. J Ethnopharmacol 2021; 267:113445. [DOI] [PubMed] [Google Scholar]
- 30.Lee MS, Lee B, Park KE, Utsuki T, Shin T, Oh CW, et al. Dieckol enhances the expression of antioxidant and detoxifying enzymes by the activation of Nrf2-MAPK signalling pathway in HepG2 cells. Food Chem 2015; 174:538–546. [DOI] [PubMed] [Google Scholar]
- 31.An R, Li D, Dong Y, She Q, Zhou T, Nie X, et al. Methylcobalamin protects melanocytes from H(2)O(2)-Induced oxidative stress by activating the Nrf2/HO-1 pathway. Drug Des Devel Ther 2021; 15:4837–4848. [DOI] [PMC free article] [PubMed] [Google Scholar]