Abstract
Introduction Preeclampsia, a multifactorial disease with pathophysiology not yet fully understood, is a major cause of maternal and perinatal morbidity and mortality, especially when preterm. The diagnosis is performed when there is an association between arterial hypertension and proteinuria or evidence of severity. There are unanswered questions in the literature considering the timing of delivery once preterm preeclampsia has been diagnosed, given the risk of developing maternal complications versus the risk of adverse perinatal outcomes associated with prematurity. The objective of this systematic review is to determine the best timing of delivery for women diagnosed with preeclampsia before 37 weeks of gestation.
Methods Systematic literature review, performed in the PubMed database, using the terms preeclampsia, parturition and timing of delivery to look for studies conducted between 2014 and 2017. Studies that compared the maternal and perinatal outcomes of women who underwent immediate delivery or delayed delivery, in the absence of evidence of severe preeclampsia, were selected.
Results A total of 629 studies were initially retrieved. After reading the titles, 78 were selected, and their abstracts, evaluated; 16 were then evaluated in full and, in the end, 6 studies (2 randomized clinical trials and 4 observational studies) met the inclusion criteria. The results were presented according to gestational age range (< 34 weeks and between 34 and 37 weeks) and by maternal and perinatal outcomes, according to the timing of delivery, considering immediate delivery or expectant management. Before 34 weeks, the maternal outcomes were similar, but the perinatal outcomes were significantly worse when immediate delivery occurred. Between 34 and 37 weeks, the progression to severe maternal disease was slightly higher among women undergoing expectant management, however, with better perinatal outcomes.
Conclusions When there is no evidence of severe preeclampsia or impaired fetal well-being, especially before 34 weeks, the pregnancy should be carefully surveilled, and the delivery, postponed, aiming at improving the perinatal outcomes. Between 34 and 37 weeks, the decision on the timing of delivery should be shared with the pregnant woman and her family, after providing information regarding the risks of adverse outcomes associated with preeclampsia and prematurity.
Keywords: preeclampsia, delivery, prematurity, preterm preeclampsia
Abstract
Resumo
Introdução A pré-eclâmpsia, doença multifatorial e com fisiopatologia ainda não totalmente estabelecida, é importante causa de morbimortalidade materna e perinatal, especialmente quando pré-termo. O diagnóstico é realizado quando há associação entre hipertensão arterial e proteinúria ou evidência de gravidade. Existem questionamentos na literatura se, frente ao diagnóstico de pré-eclâmpsia, a resolução da gravidez deve ser imediata ou postergada, considerando o risco de desenvolvimento de complicações maternas versus os resultados perinatais associados à prematuridade. O objetivo desta revisão sistemática é estabelecer o melhor momento de resolução da gestação em mulheres com pré-eclâmpsia antes das 37 semanas.
Metodologia Revisão sistemática da literatura, realizada na base de dados PubMed, usando os termos preeclampsia parturition e timing of delivery, para encontrar estudos feitos entre 2014 a 2017. Foram selecionados estudos que comparassem os desfechos maternos e perinatais de mulheres submetidas a resolução imediata ou a postergação do parto, na ausência de evidência de pré-eclâmpsia grave.
Resultados Foram localizados 629 artigos; após leitura dos títulos, 78 foram selecionados. Realizada avaliação dos seus resumos, 16 foram avaliados na integralmente e, finalmente, 6 estudos preencheram os requisitos de inclusão (2 ensaios clínicos randomizados e 4 estudos observacionais). Os resultados foram apresentados conforme a faixa de idade gestacional (≤ 34 semanas, e entre 34 e 37 semanas) e a avaliação dos desfechos maternos e perinatais. Antes das 34 semanas, os resultados maternos foram semelhantes; entretanto, os desfechos perinatais foram significativamente piores quando houve resolução imediata. Entre 34 e 37 semanas, a progressão para doença materna grave foi discretamente maior entre as mulheres submetidas a conduta expectante; entretanto, os desfechos perinatais foram melhores quando o parto foi postergado.
Conclusões Na ausência de evidências de pré-eclâmpsia grave ou de prejuízo da vitalidade fetal, o parto deve ser postergado, principalmente antes das 34 semanas, com vigilância materna e fetal rigorosas. Entre 34 e 37 semanas, a decisão deve ser compartilhada com a gestante e sua família, após esclarecimento sobre os desfechos adversos associados à pré-eclâmpsia e prematuridade.
Palavras-Chave: pré-eclâmpsia, parto, prematuridade, pré-eclâmpsia pré-termo
Introduction
Hypertensive disorders occur in 2 to 8% of pregnancies,1 and are a major cause of maternal morbidity and mortality, accounting for 25.7% of maternal deaths in Latin America.2 In Brazil, preeclampsia is the leading cause of severe maternal morbidity, and its primary complication, eclampsia, is the most prevalent cause of maternal death.3 Preeclampsia can present with various conditions ranging from mild forms of hypertension, sometimes with no need for antihypertensive drugs, to severe forms with possible serious complications, which require intensive care.4
Gestational hypertension is defined as the occurrence of systolic blood pressure levels > 140 mm Hg or diastolic blood pressure > 90 mm Hg.5 Preeclampsia is traditionally diagnosed when, associated with gestational hypertension at a gestational age ≥ 20 weeks, significant proteinuria appears (≥ 0.3 g in a 24-hour urine test), or there is end organ damage: renal (serum creatinine > 1.1 mg/dL), hepatic (transaminases > 70 U/L, bilirubin > 1.2 mg/dL), cerebral (headache, seizures, posterior reversible encephalopathy syndrome [PRES]), hematologic (thrombocytopenia < 100,000/mm3 or lactic dehydrogenase ≥ 600 U/L), or pulmonary (pulmonary edema, dyspnea not attributable to another cause).6
Although it has been recognized as a disease for over one hundred years, the pathophysiology of preeclampsia is not yet fully understood.7 Previous studies have demonstrated histopathological differences between placentas of healthy women and those of women with preeclampsia,8 and, for several decades, it has been understood that the disease is mediated by endothelial dysfunction due to placental malperfusion.9 The hypothetical “two-stage” model suggests that a poorly perfused placenta (stage 1: without clinical symptoms) due to inadequate uterine spiral artery remodeling releases antiangiogenic factors into the circulation (stage 2), and this determines the clinical manifestation of preeclampsia.10 Delivery, and consequent removal of the placenta, is the only effective treatment.7
Modern obstetric practices recommend taking maternal and fetal conditions into account in maternal-fetal care, to ensure the safe monitoring of the pregnancies and achieve the best outcomes for both mothers and newborns. Preeclampsia, a disease with a high association with morbidity and mortality,3 11 is a classic example of this dichotomous situation, in which it is sometimes necessary to postpone the delivery to protect maternal health.12 Approximately 13% of cases of preeclampsia develop before 34 weeks of gestation, and 32% develop between 34 and 37 weeks,13 and are associated with high risk of maternal complications,14 as well as a high rate of medically-indicated premature births.15 16 17
While immediate delivery in cases of preeclampsia at over 37 weeks of gestation improves the maternal and perinatal outcomes,18 19 the scientific evidence is still inconclusive regarding the best timing of delivery in the event preterm preeclampsia is diagnosed, due to complications related to prematurity. The goal of the expectant management is to reduce the impact of medically-indicated premature births. However, maternal and fetal surveillance is necessary, and the presence of any serious signs should lead to delivery regardless of the gestational age.18 19
The objective of this review is to provide an update on the available evidence on the best timing of delivery once preterm preeclampsia has been diagnosed.
Methods
A systematic literature review was performed to investigate the best timing of delivery for women who develop preeclampsia before a gestational age of 37 weeks. The review considered 3 years, from January 2014 to February 2017, and we used the PubMed database. The Medical Subject Headings selected were preeclampsia, parturition and timing of delivery, combined in the following way: preeclampsia AND (parturition OR timing of delivery). Observational studies and clinical trials were included; systematic reviews, guidelines and meta-analyses were excluded. The search was limited to studies published in English or Portuguese.
The research protocol for this review was created in accordance with the patient, intervention, comparison and outcome (PICO) strategy.20 For the research protocol, the “patients” were defined as women who developed preeclampsia before 37 weeks of gestation; “intervention” was defined as delivery within 24 hours of the diagnosis of preeclampsia; a “comparison” was made in relation to the expectant management; and the “outcomes” considered were the maternal (progression to severe preeclampsia, eclampsia, hemolysis, elevated liver enzyme levels, and low platelet levels (HELLP) syndrome, other serious maternal morbidities, or maternal death) and fetal (prenatal death, admission to an intensive care unit [ICU], or respiratory disorders) outcomes. The current study followed all recommendations of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Statement.21
The search identified a total of 629 studies. After an analysis of the titles by 2 independent reviewers, 78 articles were selected. After carefully reading the abstracts of these articles, 16 were selected to be fully read and, afterwards, 6 were included in the analysis of this review, 4 of which were observational studies,13 14 22 23 and 2, clinical trials24 25 (Fig. 1).
Fig. 1.
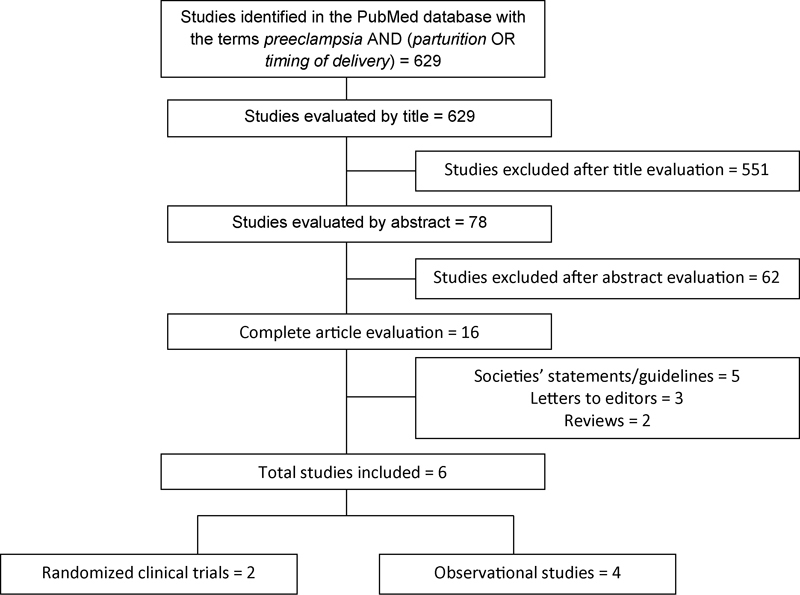
Flowchart for the selection of articles for the systematic review.
A brief description of each of the studies included is presented in Table 1. The results are shown according to the gestational age of preeclampsia onset (before 34 weeks and between 34 and 37 weeks).
Table 1. Studies included in this systematic review, with presentation of the type of study, number of participants and main findings.
| Authors, year, country | Type of study | Partic. | Main findings |
|---|---|---|---|
| Owens et al, 2014, USA25 | RCT | 183 | Expectant management: 75 cases; immediate delivery: 94 cases. Progression to severe preeclampsia: 41% versus 3% (expectant management versus immediate delivery: significant difference). Most common diagnostic criterion of severe preeclampsia: severe hypertension (> 160 × 110 mm Hg). Newborn with weight below the 10th percentile: 15% versus 20% (expectant management versus immediate delivery: no significant difference). Admission to neonatal ICU: 19% versus 21% (expectant management versus immediate delivery: no significant difference). Respiratory distress syndrome: 8% versus 12% (expectant management versus immediate delivery: no significant difference). |
| Broekhuijsen et al, 2015, Holland24 | RCT | 703 | Expectant management: 351 cases; immediate delivery: 352 cases. Occurrence of maternal complications: 3.1% versus 1.1% (expectant management versus immediate delivery: no significant difference). HELLP syndrome: 2% versus 1% (expectant management versus immediate delivery: no significant difference). Occurrence of perinatal complications: 1.7% versus 5.7% (expectant management versus immediate delivery: significant difference; relative risk: 3.3). Admission to neonatal ICU: 3.7% versus 7.4% (expectant management versus immediate delivery: significant difference; relative risk: 2.0). Transient tachypnea: 1.7% versus 5.7% (expectant management versus immediate delivery: significant difference; relative risk: 3.3). |
| Lisonkova et al., 2014, USA14 | RO | 670,120 | Increased incidence of preeclampsia in the studied period (2000 to 2008), from 2.9% to 3.1%. Early-onset preeclampsia = 0.3%, and late-onset = 2.7%, with a 4.5% increase in the incidence of early-onset preeclampsia. The maternal mortality rateb among women with early-onset preeclampsia was 4 times higher than for those with with late-onset (42.1 versus 11.2). Severe maternal morbidityc was higher among women with early-onset preeclampsia (12.2 versus 5.5). Increased risk of cardiovascular, respiratory, neurological, kidney and liver complications among women with early-onset compared with late-onset preeclampsia. |
| Pettit et al, 2015, Australia13 | RO | 696 | Early; late; term. Incidence: 13%; 32%; 55%. Cesarean section rate: 70%; 55%; 44%. Severe hypertension: 43%; 35%; 31%. Fetal growth restriction: 32%; 18%; 15%. Perinatal mortality:a 80, 10, 5 |
| Helou et al, 2016, Australia22 | RO | 516 | Incidence of preeclampsia: 3.5%. Mean gestational age at delivery among women with preeclampsia: 36 weeks. Fetal growth restriction: 21.7% Admission to neonatal ICU: 28.5% In 77% of the cases of late-onset preeclampsia, the women underwent expectant management, with progression to severe preeclampsia in 5.2% of the cases. |
| McKinney et al, 2016, USA23 | RO | 199 | Incidence of fetal growth restriction in women with late-onset preeclampsia: 31%. Progression to severe preeclampsia: 51.1% (among normal fetuses) and 42.9% (among fetuses with growth restriction). The interval to delivery was significantly shorter in women with fetal growth restriction (median [interquartile range] of 3 [1.6] days versus normal growth, 5 [2.12] days; p < 0.001). |
Abbreviations: HELLP, hemolysis, elevated liver enzyme levels, and low platelet levels; ICU, intensive care unit; Partic., number of participants; RCT, randomized clinical trial; RO, retrospective observational.
Notes: aPer thousand births;
per group of 100,000 births;
for any severe maternal morbidity, per group of 100 births.
Results
Preeclampsia Onset Prior to 34 Weeks
The observational studies showed that women with early-onset preeclampsia had more severe conditions upon developing the disease,13 and were more frequently subject to serious maternal morbidities such as renal failure, sepsis, the need for a hysterectomy or blood transfusion, and increased risk of maternal death,14 with higher cesarean rates (70%).13 When preeclampsia was diagnosed before 30 weeks, there was an association with fetal growth restriction (FGR), and the interval to delivery was shorter.23 Progression to severe preeclampsia occurred in almost half (43%) of the women under expectant management.13
On perinatal outcomes, the observational studies presented a high incidence of FGR,14 leading to lower birth weights among women with preeclampsia; almost 1/3 (32%) were below the 10th percentile in weight for their gestational age. There was also a high rate of perinatal mortality (80/1,000 live births).13
Onset of Preeclampsia between 34 and 37 Weeks
Among the women who developed preeclampsia between 34 and 37 weeks, the results of the observational studies had a wide range of findings: the progression to severe forms of the disease was as high as 51% and 35%13 23 in 2 of the considered studies, and as low as 5% in the other.22 The association with the risk of complications, even though significantly lower when compared with the women with early-onset preeclampsia, was only observed in one of the studies evaluated.14 Among the clinical trials, one of them, with 703 women, showed that the option of expectant management added 5 to 10 days to the gestation, with no significant increase in maternal complications, such as HELLP syndrome, thromboembolic events, placental abruption, eclampsia and maternal death.24 Another study involving 169 women found a significant difference in the progression to other severe forms of the disease among women under expectant management (41% versus 3%).25
Considering the perinatal outcomes, one of the observational studies identified significant weight gain at birth in the comparison between expectant management and immediate delivery, as well as a lower need for ICU admission and decreased risk of perinatal death.13 One of the clinical trials indicated similar perinatal outcomes in the two groups,25 while another showed an increased risk of significant neonatal complications for newborns who were delivered immediately compared with cases in which expectant management was adopted.24
Discussion
Women with preterm preeclampsia had a greater risk of medically-indicated premature birth; however, if there were no signs of severe maternal complications or impaired fetal well-being during close surveillance, delaying delivery when preeclampsia is diagnosed before 37 weeks (and especially in cases of early-onset preeclampsia) improved the neonatal outcomes, without major harm to the maternal health.
Previous systematic reviews with a similar scope and different temporal profiles have confirmed the low number of clinical trials that compare expectant management and immediate delivery in relation to the maternal and perinatal outcomes of preterm preeclampsia.26 27 Conducting clinical trials for diseases with high morbidity and mortality and low frequency, as is the case of early-onset preeclampsia, can be challenging, due to the difficulties in implementing inclusion protocols and carrying out the studies, in addition to the ethical issues involved. In this context, it is important to consider the need for further studies, preferably multicenter ones, including various high and low-income settings.
Expectant management is possible for cases of preeclampsia when the gestational age at diagnosis is < 37 weeks, and the major goal is improving perinatal outcomes, especially reducing neonatal complications; however, the management should be reevaluated if there are signs of progression to severe forms of preeclampsia, or if there is evidence of impaired fetal well-being (Table 2). The occurrence of severe preeclampsia is diagnosed in the presence of any of the following criteria: systolic blood pressure ≥ 160 mm Hg; diastolic blood pressure ≥ 110 mm Hg, with no response to treatment after 4 hours; eclampsia (seizures); and persistence, worsening or association of laboratory markers, such as platelet counts < 100,000/mm3, aspartate transaminase (AST) > 70 U/L, and creatinine > 1.1 mg/dL. Fetal growth restriction, oligohydramnios, changes in the umbilical artery observed by Doppler assessment, and non-reassuring cardiotocography are indications of impaired fetal well-being, and demand surveillance. Clinical symptoms such as pulmonary edema and right hypochondrium pain not attributable to other causes, or brain disorders, such as headaches, and visual disorders (scotomas, obnubilation) are also considered reasons for interrupting the expectant management. In the absence of such clinical signs or laboratory or ultrasound findings, the delivery should reach term (37 weeks).18
Table 2. How to talk with pregnant mothers and their families about the risks, benefits and uncertainties of immediate delivery versus expectant management when preterm preeclampsia is diagnosed (adapted from Chappell, Milne e Shennan, 2015)18 .
| • Explain what is happening with her and her baby; clarify that she is not guilty of the occurrence of preeclampsia, which can happen to anyone. Explain that the team is available to answer her questions. Go over the symptoms that she must immediately report: headache, vomiting, abdominal pain, changes in vision such as blurred vision or flashes, sweating of the face, feet or hands, and reduced fetal movement. • Explain why she is being hospitalized and the importance of staying, to improve outcomes in case of emergency. • Inform her that preeclampsia is generally resolved with delivery and removal of the placenta. Say that as long as it is receiving nutrients, the best place for the baby is inside the mother, but if the situation changes, delivering the baby and receiving neonatal care may be better. • Explain the two possibilities: labor induction or cesarean section (if indicated) in the next 24 hours, or remaining in the hospital until 37 weeks or before, if there are any changes in her clinical condition. At 37 weeks, delivery is the best option. |
Early-onset preeclampsia, before 34 weeks, occurs less frequently than the late-onset (after 37 weeks) form of the disease, but it is associated with higher maternal morbidity and mortality.14 Early-onset preeclampsia is more frequently observed in women suffering from chronic hypertension,28 and is also more frequent among primiparous women and those with diabetes.14 In women with previous hypertension, diagnosing preeclampsia can be challenging. Therefore, initial screening for proteinuria, seeking any pregestational disease, and knowledge of the initial blood pressure levels are important at the beginning of the antenatal care.28 Furthermore, women who develop early-onset preeclampsia are at a greater risk of developing several serious complications, such as acute renal failure, respiratory morbidities, cardiomyopathies, sepsis and a chance of maternal death nine times higher.14
Preeclampsia that develops between 34 and 37 weeks, also called preterm late-onset preeclampsia, is more frequent than early-onset preeclampsia, but with reduced severity.13 14 Women aged between 20 and 34 years are the most prone, as well as those with diabetes and those with male fetuses.14 Smoking is associated with a lower incidence of this condition in this gestational age range.14
In countries with socioeconomic development similar to Brazil, hypertensive disorders are the major reason for medically-indicated premature births, with increased rates of cesarean section (c-section).29 Nevertheless, clinical trials have shown that preterm labor induction, when indicated, could reduce the occurrence of c-sections by ∼ 40%.24 25 Therefore, when preeclampsia occurs, the route of delivery should preferably be vaginal. Induced labor should be considered when there is maternal clinical stability, good fetal well-being, a favorable cervix, and the gestational age is > 32 weeks. A multidisciplinary approach is required, with the support of the anesthesia and neonatal teams.18
The decision toward immediate delivery should not be mistaken with an emergency or sudden delivery. It is important to establish that, when choosing immediate delivery, the studies considered “immediate” a delivery within 24 hours, prioritizing the maternal clinical condition, including the use of magnesium sulfate, intravenous antihypertensive drugs, or corticosteroid therapy, when indicated, as well as a programmed transfer to a center with maternal and neonatal ICUs.18 24 25
Current data support the idea that early-onset preeclampsia may be a more serious condition than late-onset preeclampsia. However, when it is possible to delay the timing of the delivery beyond 34 weeks, there is a considerable improvement in perinatal outcomes. The study by Pettit et al, which had a significant number of cases (n = 696),13 showed that the women who developed early-onset preeclampsia had a mean gestational age at delivery of 33 weeks, compared with 38 weeks for late-onset (term preeclampsia), with higher incidence of c-sections (70%) among women with early-onset preeclampsia. The perinatal mortality rate in this group was 80/1,000, but dropped to 10/1,000 in cases of preterm late-onset preeclampsia (between 34–37 weeks), and to 5/1,000 in cases of term preeclampsia (≥ 37 weeks).
In cases of early-onset preeclampsia with FGR, it is essential that women be followed in a hospital with the resources to monitor the fetal well-being, and that is also equipped with a neonatal ICU. In such situations, the decision to transfer the patient or to use corticoids for fetal lung maturity should not be delayed, since the interval between diagnosis and delivery is usually very short, even among expectant management cases.23
A systematic review with a meta-analysis of clinical studies conducted up to 2014 was published in 2017.30 It showed that the maternal outcomes did not worsen, despite a significant difference in the occurrence of placental abruption in women who underwent expectant management after the diagnosis of early-onset preeclampsia. In terms of perinatal outcomes, the mortality rate in both groups was similar; however, there was a higher occurrence of complications, such as the need for ventilatory support, intraventricular hemorrhage and hypoxic-ischemic encephalopathy among the newborns of women who underwent an elective delivery before 34 weeks of gestation for severe preeclampsia compared with those undergoing expectant management. The results highlight the importance of larger studies to draw more definitive conclusions, especially considering the maternal outcomes. A similar conclusion was also reached in the latest Cochrane review on the subject.26
To evaluate preeclampsia outcomes and the ideal timing of delivery for women between the 34th and 37th weeks of gestation, a Dutch group conducted a major study (HYPITAT-II)24 that included ∼ 700 women randomized into 2 groups: scheduled birth (labor induction or c-section) within 24 hours of the diagnosis of preeclampsia, and expectant management until 37 weeks of gestation. The women included in this study had been diagnosed with gestational hypertension or preeclampsia, with no evidence of severe features, and adequate fetal well-being. The maternal outcomes, including thromboembolic events, pulmonary edema, HELLP syndrome, eclampsia, placental abruption and maternal death, were similar between the two groups, with a mean period of seven days between diagnosis and birth among the expectant management group. In terms of perinatal outcomes (Apgar score < 7 at the 5th minute, cord blood acidosis, neonatal ICU admission, perinatal death, neonatal infection, hypoglycemia, transient tachypnea, meconium aspiration syndrome, pneumothorax, periventricular leukomalacia, intraventricular hemorrhage, seizures, and necrotizing enterocolitis), there was a 3.3 times greater relative risk among women who underwent immediate delivery in comparison to those who had the delivery postponed. Thus, the authors established that there is no justification for immediate delivery when there is no severe maternal or fetal condition, given the perinatal complications resulting from prematurity.
An American study published in 201425 with a design similar to the Dutch study,24 but with far fewer included cases (169), showed a progression to severe preeclampsia in 41% of the women under expectant management, compared with only 3% of those who underwent immediate delivery. Considering the perinatal outcomes, the birth weight reduction was only around 300 g in the cases of immediate delivery, with no difference in the other studied outcomes. Therefore, the authors suggested that the timing of the delivery should be immediate, and that expectant management should only be considered in selected cases. Nevertheless, the small number of women, associated with the single-center nature of the study, is a weak point of the study that must be considered.
As in the American study,25 the study that preceded the HYPITAT-II,24 HYPITAT-I,19 provided evidence that expectant management in cases of preeclampsia after 36 weeks heightened the risk of occurrence of maternal complications, without improvement in fetal outcomes. HYPITAT-II, which evaluated more women at more institutions, did not confirm the findings of the initial study.
Two meta-analyses27 30 published in 2017 demonstrated that immediate delivery, if preeclampsia is diagnosed after 34 weeks, reduces the occurrence of maternal complications, including HELLP syndrome. With respect to the perinatal outcomes, after 34 weeks, 1 meta-analyses30 did not observe neonatal or fetal deaths in either group. However, it did not evaluate the occurrence of various significant complications among the newborns. The other meta-analysis27 stated that there were large differences among the studies that were selected, which did not enable further statistical evaluations, but the analysis showed a greater occurrence of respiratory distress and need for admission to an ICU among newborns whose birth occurred immediately after the diagnosis of preeclampsia compared with those whose mothers were under expectant management.
According to various studies, it is prudent to consider admission to a hospital that has intensive neonatal and adult care for women diagnosed with preeclampsia between 34 and 37 weeks, and close maternal and fetal surveillance, considering the risks of progression of the disease. Referral centers should provide adequate training on how to deal with severe cases, with special attention regarding how to provide adequate guidance to the woman and her family, considering information about outcomes and follow-up, sharing responsibility in the decision-making, especially between 34 and 37 weeks of gestation. Awareness of the relevant symptoms that should be promptly communicated to the care team is also key (Table 2).18
As far as the follow-up of the women who choose the expectant management, the recommendations are blood pressure monitoring at least every six hours, and laboratorial tests every two days or less, at the physician's discretion. The fetal well-being evaluation should entail an ultrasound with Doppler velocimetry at the time of admission and then weekly, or at shorter intervals, at the physician's discretion. A cardiotocography should also be performed for fetal well-being assessment.18
The use of magnesium sulfate to prevent eclampsia has already been substantiated, and recent meta-analyses have proven its role in reducing seizures and potentially decreasing maternal mortality.31 This effect has been confirmed in studies outside the controlled environments of clinical trials, indicating that its use in the daily practice is associated with a reduction in eclampsia cases.32 Nevertheless, there are known obstacles to its use, such as difficulty obtaining it in primary care facilities and lack of knowledge, or lack of well-established clinical protocols for its use,33 which impacts on its routine use to decrease preeclampsia complications.
More recently, studies showed that magnesium sulfate serves as a neuroprotective medication for fetuses in cases of imminent premature birth, especially under 32 weeks, reducing the occurrence of cerebral palsy.34 35 36 No maternal complications associated with its use have been observed. The recommendation is to administer a loading dose of 4 g, followed by 1 g every hour. The infusion of magnesium sulfate, when used for fetal neuroprotection, should be stopped immediately after birth.
The clinical use of biomarkers for risk prediction, preclinical diagnosis, and to determine cases more prone to develop clinical complications is being extensively studied, with important breakthroughs in the last decade due to a better understanding of the pathophysiology of preeclampsia.37 The role of angiogenic and antiangiogenic biomarkers is unfolding, and technologies that enable their adequate and precise assessment are being developed, so that the results will ensure the predictive, diagnostic and prognostic approaches.38 The main biomarkers that have been studied are placental growth factor (PlGF), soluble fms-like tyrosine kinase-1 (sFlt-1), and pregnancy-associated plasma protein A (PAPP-A). All are highly sensitive in diagnosing early-onset preeclampsia, but demonstrate worse results for identifying late-onset preeclampsia.39 Many studies to determine the best timing and how to implement those tests in the daily clinical practice are currently ongoing.40 41 42 Future studies, and the association of biomarkers with ultrasound findings, promise to improve the prediction of preeclampsia and its early management with measures that can reduce the morbidity and mortality associated with the disease.39
Preeclampsia can be a devastating event for many women, but most survive and may choose to become pregnant again. There is conflicting information in the literature regarding the chance of preeclampsia recurring in future gestations. Individual studies have shown that the occurrence of preeclampsia substantially increases the risk of recurrence in later gestations and, in such cases, the disease can develop earlier, with more severe complications.43 44 However, a meta-analysis based on individual participant data (IPD) showed that the rate of recurrence of preeclampsia was 21%, with this risk increasing if there was HELLP syndrome or FGR in the previous gestation. The study found that, in the event of recurrence, the forms of preeclampsia were less severe. However, it is relevant to note that few of the considered studies were from low or middle income settings.45 It is essential, therefore, that these women receive proper counseling considering the risks, taking into account their personal medical history, and how the disease manifested and progressed in the previous gestation. Among cases of severe early-onset preeclampsia, other underlying conditions should also be investigated postpartum, such as antiphospholipid syndrome, and interventions such as low dose aspirin and calcium (in places of low dietary intake) should also be performed.46
In addition, women who have survived preeclampsia are at risk of developing cardiovascular diseases in the long term. A literature review published in 2015 evaluating the risk of acute myocardial infarction and strokes in women who had preeclampsia showed a greater risk of such outcomes, as well as an increased risk of developing chronic hypertension,47 reinforcing that preeclampsia is a disease of endothelial dysfunction, which may not be resolved at the end of pregnancy.48 Therefore, the literature provides evidence that preeclampsia is a risk factor for future cardiovascular disease, and that women who developed it should receive special care, similar to that provided for other risk factors, such as hypertension, dyslipidemia, obesity and smoking.49 In the postpartum period, women with a history of preeclampsia should be informed about the interventions that have an impact on such risk factors, such as physical exercise and quitting smoking. If preeclampsia is understood as an endothelial disease, it also implies increased risks of developing chronic kidney disease during life, and this should also be assessed inj the postpartum period.50 51
The main recommendations found through this systematic review regarding the timing of delivery in women with early-onset preeclampsia are presented in Table 3, while the recommendations for those who developed preeclampsia between 34 and 37 weeks are presented in Table 4.
Table 3. Timing of delivery recommendations for women diagnosed with preeclampsia before 34 weeks.
| • Early-onset preeclampsia is less frequent than late-onset preeclampsia, but is more severe. • It occurs more frequently in primiparas, and is associated with maternal comorbidities, such as chronic hypertension and diabetes. • The difference between uncontrolled chronic hypertension and superimposed preeclampsia depends on early, adequate and quality antenatal care, with an investigation of the complications associated with hypertension, which should be performed as soon as possible, checking whether any target organs have been affected, such as: the kidneys (urea dosage, creatinine and proteinuria) the heart (electrocardiogram, chest X-ray and cardiological assessment, with other tests, if necessary) and the eyes (eye exam to identify hypertensive retinopathy). • Women with early-onset preeclampsia are more prone to serious complications during pregnancy and the postpartum period, as well as to an increased risk of death. • Early-onset preeclampsia is an important determining factor for medically-indicated preterm birth, with increased perinatal mortality arising from this condition. • The latency time between the diagnosis of early-onset preeclampsia and the timing of the delivery is generally short (less than one week); therefore, measures such as antenatal corticosteroid therapy for fetal lung maturity and transfer to hospital units with more support (maternal and neonatal intensive care units) should not be delayed. • There is no definitive evidence in the literature on the best timing of delivery. The decision upon expectant management should consider close maternal and fetal surveillance, and if there is impaired maternal or fetal health, delivery should be performed, with adequate measures for better clinical support, such as the use of magnesium sulfate to prevent eclampsia. |
Table 4. Timing of delivery recommendations for women diagnosed with preeclampsia between 34 and 37 weeks.
| • Although late-onset preeclampsia is more frequent, it is less serious than early-onset preeclampsia, and generally affects younger women (aged between 21 and 34 years). • Evidence suggests that expectant management is not associated with the development of major maternal complications. • Immediate delivery increases the risk of perinatal complications, especially those associated with prematurity, but it does not increase neonatal mortality. • The decision on the timing of delivery should be shared with the patient and her family, and expectant management may be an option when there are no serious maternal conditions or impaired fetal well-being. • Women with late-onset preeclampsia should be treated in a hospital environment, under strict clinical, laboratory and ultrasound surveillance. • Clinical surveillance should be performed daily, with subsequent control of blood pressure (at least every six hours); the symptoms of severity should be clearly explained to the patient and immediately considered by the care team. • The laboratory assessment should be serial (two times per week), or whenever the patient's clinical condition or symptoms worsen, searching for renal function, hepatic or hematological damages. • The fetal well-being assessment should entail an ultrasound with Doppler velocimetry and cardiotocography at the time of admission and at least weekly, or at shorter intervals, depending on each case. • Decompensated blood pressure, laboratory abnormalities (platelets < 100,000; aspartate transaminase > 70 U/L; creatinine > 1.1 mg/dL), clinical symptoms (epigastric or right hypochondrium pain, or neurological symptoms), or evidence of impaired fetal well-being determine immediate delivery. • At 37 weeks, delivery should be performed, with possible labor induction. |
Conclusions
Preterm preeclampsia accounts for 3% to 25% of the cases of preeclampsia, but it is associated with serious maternal and perinatal complications.14 52 Obstetricians must be able to diagnose its occurrence and provide optimal and timely care to the mother and fetus, which includes a decision regarding the best timing of delivery. It is essential that this care be provided at a facility that has maternal and neonatal intensive care support. In cases of preterm preeclampsia, the studies examined in this review found that the expectant management is associated with better perinatal results. Therefore, it is suggested that the delivery be delayed when there are no maternal or fetal complications. Strict surveillance is important, and the decision should be shared with the mother, after providing her with clear information about the disease. New clinical trials, preferably multicenter ones, with standardization of the outcomes studied and inclusion of high- and low-income settings, are necessary to confirm the findings presented to date.
Acknowledgments
National Specialized Preeclampsia Committee of the Brazilian Federation of Gynecology and Obstetrics Associations: Ricardo Cavalli, Sergio Hofmeister de Almeida Martins Costa, Leandro Gustavo de Oliveira, Elias Ferreira de Melo Júnior, Francisco Lázaro Pereira de Souza, Henri Augusto Korkes, Ione Rodrigues Brum, José Geraldo Lopes Ramos, Maria Laura Costa do Nascimento, Maria Rita de Figueiredo Lemos Bortolotto, Mário Dias Corrêa Junior, and Nelson Sass.
Conflicts of Interest The authors declare no conflicts of interest.
Contributions
The theme of this systematic review was defined by the Specialized Preeclampsia Committee. JPG and MLC performed the systematic literature review (independent assessment), and extracted data from each article included. FGS acted as a third reviewer, when there were any issues concerning the selection of the studies. JPG wrote the first version of the manuscript. MLC, MAP and FGS reviewed the first version, and then incorporated the suggestions from the other co-authors and members of the Specialized Preeclampsia Committee. All authors read and agreed with the final version of the article.
This revision is part of the Project Series, Guidelines and Recommendations of the Federação Brasileira das Associações de Ginecologia e Obstetrícia – FEBRASGO, elaborated by the Specialized National Committees in Hypertension in Pregnancy.
References
- 1.Duley L. The global impact of pre-eclampsia and eclampsia. Semin Perinatol. 2009;33(03):130–137. doi: 10.1053/j.semperi.2009.02.010. [DOI] [PubMed] [Google Scholar]
- 2.Say L, Chou D, Gemmill A et al. Global causes of maternal death: a WHO systematic analysis. Lancet Glob Health. 2014;2(06):e323–e333. doi: 10.1016/S2214-109X(14)70227-X. [DOI] [PubMed] [Google Scholar]
- 3.Giordano JC, Parpinelli MA, Cecatti JG et al. The burden of eclampsia: results from a multicenter study on surveillance of severe maternal morbidity in Brazil. PLoS One. 2014;9(05):e97401. doi: 10.1371/journal.pone.0097401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tranquilli AL, Dekker G, Magee L et al. The classification, diagnosis and management of the hypertensive disorders of pregnancy: A revised statement from the ISSHP. Pregnancy Hypertens. 2014;4(02):97–104. doi: 10.1016/j.preghy.2014.02.001. [DOI] [PubMed] [Google Scholar]
- 5.Lowe SA, Brown MA, Dekker GA et al. Guidelines for the management of hypertensive disorders of pregnancy 2008. Aust N Z J Obstet Gynaecol. 2009;49(03):242–246. doi: 10.1111/j.1479-828X.2009.01003.x. [DOI] [PubMed] [Google Scholar]
- 6.Steegers EA, von Dadelszen P, Duvekot JJ, Pijnenborg R.Pre-eclampsia Lancet 2010376(9741):631–644. [DOI] [PubMed] [Google Scholar]
- 7.Phipps E, Prasanna D, Brima W, Jim B. Preeclampsia: Updates in Pathogenesis, Definitions, and Guidelines. Clin J Am Soc Nephrol. 2016;11(06):1102–1113. doi: 10.2215/CJN.12081115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brosens I. A study of the spiral arteries of the decidua basalis in normotensive and hypertensive pregnancies. J Obstet Gynaecol Br Commonw. 1964;71:222–230. doi: 10.1111/j.1471-0528.1964.tb04270.x. [DOI] [PubMed] [Google Scholar]
- 9.Roberts JM, Taylor RN, Musci TJ, Rodgers GM, Hubel CA, McLaughlin MK. Preeclampsia: an endothelial cell disorder. Am J Obstet Gynecol. 1989;161(05):1200–1204. doi: 10.1016/0002-9378(89)90665-0. [DOI] [PubMed] [Google Scholar]
- 10.Roberts JM, Hubel CA.The two stage model of preeclampsia: variations on the theme Placenta 200930(Suppl A):S32–S37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Souza JP, Gülmezoglu AM, Vogel Jet al. Moving beyond essential interventions for reduction of maternal mortality (the WHO Multicountry Survey on Maternal and Newborn Health): a cross-sectional study Lancet 2013381(9879):1747–1755. [DOI] [PubMed] [Google Scholar]
- 12.Newman MG, Robichaux AG, Stedman CM et al. Perinatal outcomes in preeclampsia that is complicated by massive proteinuria. Am J Obstet Gynecol. 2003;188(01):264–268. doi: 10.1067/mob.2003.84. [DOI] [PubMed] [Google Scholar]
- 13.Pettit F, Mangos G, Davis G, Henry A, Brown MA. Pre-eclampsia causes adverse maternal outcomes across the gestational spectrum. Pregnancy Hypertens. 2015;5(02):198–204. doi: 10.1016/j.preghy.2015.02.002. [DOI] [PubMed] [Google Scholar]
- 14.Lisonkova S, Sabr Y, Mayer C, Young C, Skoll A, Joseph KS. Maternal morbidity associated with early-onset and late-onset preeclampsia. Obstet Gynecol. 2014;124(04):771–781. doi: 10.1097/AOG.0000000000000472. [DOI] [PubMed] [Google Scholar]
- 15.Vogel JP, Souza JP, Mori R et al. Maternal complications and perinatal mortality: findings of the World Health Organization Multicountry Survey on Maternal and Newborn Health. BJOG. 2014;121 01:76–88. doi: 10.1111/1471-0528.12633. [DOI] [PubMed] [Google Scholar]
- 16.Delorme P, Goffinet F, Ancel PY et al. Cause of Preterm Birth as a Prognostic Factor for Mortality. Obstet Gynecol. 2016;127(01):40–48. doi: 10.1097/AOG.0000000000001179. [DOI] [PubMed] [Google Scholar]
- 17.Goldenberg RL, Culhane JF, Iams JD, Romero R.Epidemiology and causes of preterm birth Lancet 2008371(9606):75–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chappell LC, Milne F, Shennan A. Is early induction or expectant management more beneficial in women with late preterm pre-eclampsia? BMJ. 2015;350:h191. doi: 10.1136/bmj.h191. [DOI] [PubMed] [Google Scholar]
- 19.Koopmans CM, Bijlenga D, Groen Het al. Induction of labour versus expectant monitoring for gestational hypertension or mild pre-eclampsia after 36 weeks' gestation (HYPITAT): a multicentre, open-label randomised controlled trial Lancet 2009374(9694):979–988. [DOI] [PubMed] [Google Scholar]
- 20.O'Sullivan D, Wilk S, Michalowski W, Farion K. Using PICO to align medical evidence with MDs decision making models. Stud Health Technol Inform. 2013;192:1057. [PubMed] [Google Scholar]
- 21.Liberati A, Altman DG, Tetzlaff J et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700. doi: 10.1136/bmj.b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Helou A, Walker S, Stewart K, George J.Management of pregnancies complicated by hypertensive disorders of pregnancy: Could we do better? Aust N Z J Obstet Gynaecol 2016 [DOI] [PubMed]
- 23.McKinney D, Boyd H, Langager A, Oswald M, Pfister A, Warshak CR. The impact of fetal growth restriction on latency in the setting of expectant management of preeclampsia. Am J Obstet Gynecol. 2016;214(03):3950–3.95E9. doi: 10.1016/j.ajog.2015.12.050. [DOI] [PubMed] [Google Scholar]
- 24.Broekhuijsen K, van Baaren GJ, van Pampus MGet al. Immediate delivery versus expectant monitoring for hypertensive disorders of pregnancy between 34 and 37 weeks of gestation (HYPITAT-II): an open-label, randomised controlled trial Lancet 2015385(9986):2492–2501. [DOI] [PubMed] [Google Scholar]
- 25.Owens MY, Thigpen B, Parrish MR et al. Management of preeclampsia when diagnosed between 34-37 weeks gestation: deliver now or deliberate until 37 weeks? J Miss State Med Assoc. 2014;55(07):208–211. [PubMed] [Google Scholar]
- 26.Churchill D, Duley L, Thornton JG, Jones L. Interventionist versus expectant care for severe pre-eclampsia between 24 and 34 weeks' gestation. Cochrane Database Syst Rev. 2013;(07):CD003106. doi: 10.1002/14651858.CD003106.pub2. [DOI] [PubMed] [Google Scholar]
- 27.Cluver C, Novikova N, Koopmans CM, West HM. Planned early delivery versus expectant management for hypertensive disorders from 34 weeks gestation to term. Cochrane Database Syst Rev. 2017;1:CD009273. doi: 10.1002/14651858.CD009273.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Seely EW, Ecker J. Chronic hypertension in pregnancy. Circulation. 2014;129(11):1254–1261. doi: 10.1161/CIRCULATIONAHA.113.003904. [DOI] [PubMed] [Google Scholar]
- 29.Morisaki N, Togoobaatar G, Vogel JP et al. Risk factors for spontaneous and provider-initiated preterm delivery in high and low Human Development Index countries: a secondary analysis of the World Health Organization Multicountry Survey on Maternal and Newborn Health. BJOG. 2014;121 01:101–109. doi: 10.1111/1471-0528.12631. [DOI] [PubMed] [Google Scholar]
- 30.Wang Y, Hao M, Sampson S, Xia J. Elective delivery versus expectant management for pre-eclampsia: a meta-analysis of RCTs. Arch Gynecol Obstet. 2017;295(03):607–622. doi: 10.1007/s00404-016-4281-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Duley L, Gülmezoglu AM, Henderson-Smart DJ, Chou D. Magnesium sulphate and other anticonvulsants for women with pre-eclampsia. Cochrane Database Syst Rev. 2010;(11):CD000025. doi: 10.1002/14651858.CD000025.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McDonald SD, Lutsiv O, Dzaja N, Duley L. A systematic review of maternal and infant outcomes following magnesium sulfate for pre-eclampsia/eclampsia in real-world use. Int J Gynaecol Obstet. 2012;118(02):90–96. doi: 10.1016/j.ijgo.2012.01.028. [DOI] [PubMed] [Google Scholar]
- 33.Lotufo FA, Parpinelli MA, Osis MJ, Surita FG, Costa ML, Cecatti JG. Situational analysis of facilitators and barriers to availability and utilization of magnesium sulfate for eclampsia and severe preeclampsia in the public health system in Brazil. BMC Pregnancy Childbirth. 2016;16:254. doi: 10.1186/s12884-016-1055-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jacquemyn Y, Zecic A, Van Laere D, Roelens K. The use of intravenous magnesium in non-preeclamptic pregnant women: fetal/neonatal neuroprotection. Arch Gynecol Obstet. 2015;291(05):969–975. doi: 10.1007/s00404-014-3581-1. [DOI] [PubMed] [Google Scholar]
- 35.Locatelli A, Consonni S, Ghidini A. Preterm labor: approach to decreasing complications of prematurity. Obstet Gynecol Clin North Am. 2015;42(02):255–274. doi: 10.1016/j.ogc.2015.01.004. [DOI] [PubMed] [Google Scholar]
- 36.Pryde PG, Mittendorf R. Using prophylactic, but not tocolytic, magnesium sulfate to reduce cerebral palsy related to prematurity: what dose, and what about infant mortality? J Perinat Med. 2011;39(04):375–378. doi: 10.1515/jpm.2011.036. [DOI] [PubMed] [Google Scholar]
- 37.Acharya A, Brima W, Burugu S, Rege T. Prediction of preeclampsia-bench to bedside. Curr Hypertens Rep. 2014;16(11):491. doi: 10.1007/s11906-014-0491-3. [DOI] [PubMed] [Google Scholar]
- 38.Hagmann H, Thadhani R, Benzing T, Karumanchi SA, Stepan H. The promise of angiogenic markers for the early diagnosis and prediction of preeclampsia. Clin Chem. 2012;58(05):837–845. doi: 10.1373/clinchem.2011.169094. [DOI] [PubMed] [Google Scholar]
- 39.Anderson UD, Gram M, Åkerström B, Hansson SR. First trimester prediction of preeclampsia. Curr Hypertens Rep. 2015;17(09):584. doi: 10.1007/s11906-015-0584-7. [DOI] [PubMed] [Google Scholar]
- 40.Klein E, Schlembach D, Ramoni A et al. Influence of the sFlt-1/PlGF Ratio on Clinical Decision-Making in Women with Suspected Preeclampsia. PLoS One. 2016;11(05):e0156013. doi: 10.1371/journal.pone.0156013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Verlohren S, Galindo A, Schlembach D et al. An automated method for the determination of the sFlt-1/PIGF ratio in the assessment of preeclampsia. Am J Obstet Gynecol. 2010;202(02):1610–1.61E13. doi: 10.1016/j.ajog.2009.09.016. [DOI] [PubMed] [Google Scholar]
- 42.Verlohren S, Herraiz I, Lapaire O et al. The sFlt-1/PlGF ratio in different types of hypertensive pregnancy disorders and its prognostic potential in preeclamptic patients. Am J Obstet Gynecol. 2012;206(01):580–5.8E9. doi: 10.1016/j.ajog.2011.07.037. [DOI] [PubMed] [Google Scholar]
- 43.Giannubilo SR, Landi B, Ciavattini A. Preeclampsia: what could happen in a subsequent pregnancy? Obstet Gynecol Surv. 2014;69(12):747–762. doi: 10.1097/OGX.0000000000000126. [DOI] [PubMed] [Google Scholar]
- 44.Melamed N, Hadar E, Peled Y, Hod M, Wiznitzer A, Yogev Y. Risk for recurrence of preeclampsia and outcome of subsequent pregnancy in women with preeclampsia in their first pregnancy. J Matern Fetal Neonatal Med. 2012;25(11):2248–2251. doi: 10.3109/14767058.2012.684174. [DOI] [PubMed] [Google Scholar]
- 45.van Oostwaard MF, Langenveld J, Schuit E et al. Recurrence of hypertensive disorders of pregnancy: an individual patient data metaanalysis. Am J Obstet Gynecol. 2015;212(05):6240–6.24E19. doi: 10.1016/j.ajog.2015.01.009. [DOI] [PubMed] [Google Scholar]
- 46.Costa ML. Preeclampsia: Reflections on How to Counsel About Preventing Recurrence. J Obstet Gynaecol Can. 2015;37(10):887–893. doi: 10.1016/s1701-2163(16)30023-8. [DOI] [PubMed] [Google Scholar]
- 47.Lee G, Tubby J. Preeclampsia and the risk of cardiovascular disease later in life--A review of the evidence. Midwifery. 2015;31(12):1127–1134. doi: 10.1016/j.midw.2015.09.005. [DOI] [PubMed] [Google Scholar]
- 48.Charlton F, Tooher J, Rye KA, Hennessy A. Cardiovascular risk, lipids and pregnancy: preeclampsia and the risk of later life cardiovascular disease. Heart Lung Circ. 2014;23(03):203–212. doi: 10.1016/j.hlc.2013.10.087. [DOI] [PubMed] [Google Scholar]
- 49.Seely EW, Tsigas E, Rich-Edwards JW. Preeclampsia and future cardiovascular disease in women: How good are the data and how can we manage our patients? Semin Perinatol. 2015;39(04):276–283. doi: 10.1053/j.semperi.2015.05.006. [DOI] [PubMed] [Google Scholar]
- 50.Facca TA, Kirsztajn GM, Sass N. Preeclampsia (marker of chronic kidney disease): from genesis to future risks. J Bras Nefrol. 2012;34(01):87–93. [PubMed] [Google Scholar]
- 51.van der Graaf AM, Toering TJ, Faas MM, Lely AT. From preeclampsia to renal disease: a role of angiogenic factors and the renin-angiotensin aldosterone system? Nephrol Dial Transplant. 2012;27 03:iii51–iii57. doi: 10.1093/ndt/gfs278. [DOI] [PubMed] [Google Scholar]
- 52.Browne JL, Vissers KM, Antwi E et al. Perinatal outcomes after hypertensive disorders in pregnancy in a low resource setting. Trop Med Int Health. 2015;20(12):1778–1786. doi: 10.1111/tmi.12606. [DOI] [PubMed] [Google Scholar]


