Abstract
Introduction Ventriculomegaly (VM) is one the most frequent anomalies detected on prenatal ultrasound. Magnetic resonance imaging (MRI) may enhance diagnostic accuracy and prediction of developmental outcome in newborns.
Purpose The aim of this study was to assess the correlation between ultrasound and MRI in fetuses with isolated mild and moderate VM. The secondary aim was to report the neurodevelopmental outcome at 4 years of age.
Methods Fetuses with a prenatal ultrasound (brain scan) diagnosis of VM were identified over a 4-year period. Ventriculomegaly was defined as an atrial width of 10–15 mm that was further divided as mild (10.1–12.0 mm) and moderate (12.1–15.0 mm). Fetuses with VM underwent antenatal as well as postnatal follow-ups by brain scan and MRI. Neurodevelopmental outcome was performed using the Griffiths Mental Development Scales and conducted, where indicated, until 4 years into the postnatal period.
Results Sixty-two fetuses were identified. Ventriculomegaly was bilateral in 58% of cases. A stable dilatation was seen in 45% of cases, progression was seen in 13%, and regression of VM was seen in 4.5% respectively. Fetal MRI was performed in 54 fetuses and was concordant with brain scan findings in 85% of cases. Abnormal neurodevelopmental outcomes were seen in 9.6% of cases.
Conclusion Fetuses in whom a progression of VM is seen are at a higher risk of developing an abnormal neurodevelopmental outcome. Although brain scan and MRI are substantially in agreement in defining the grade of ventricular dilatation, a low correlation was seen in the evaluation of VM associated with central nervous system (CNS) or non-CNS abnormalities.
Keywords: fetus, ventriculomegaly, ultrasound, magnetic resonance imaging, neurodevelopmental outcome
Abstract
Resumo
Introdução Ventriculomegalia (VM) é uma das anomalias mais frequente no ultrassom pre-natal. Ressonâncias magnéticas (RM) melhoram a precisão do diagnóstico e previsão do desenvolvimento em recém-nascidos.
Objetivo A proposta deste estudo foi avaliar a correlação entre ultrassom e RM em fetos com leve e moderada VM isolada. O objetivo secundário foi reportar o resultado neurológico na idade de 4 anos.
Métodos Fetos com diagnóstico pré-natal pelo ultrassom de VM foram identificados na idade de 4 anos. Ventriculomegalia foi definida como medida do átrio do ventrículo lateral entre 10–15 mm, a qual foi subdividida em leve (10,1–12,0 mm) e moderada (12,1–15,0 mm). Fetos com VM foram seguidos nos períodos pré-natal e pós-natal por ultrassom e RM. O resultado neurológico foi realizado usando a escala de desenvolvimento mental de Griffiths, quando indicada, até a idade de 4 anos.
Resultados Sessenta e dois fetos foram identificados. Ventriculomegalia bilateral ocorreu sem 58% dos casos. Uma dilatação estável foi observada em 45%, progressiva em 13% e regressiva em 4,5% dos casos, respectivamente. Ressonância magnética fetal foi realizada em 54 fetos, e foi concordante com os achados do ultrassom em 85% dos casos. Desenvolvimento neurológico anormal foi observado em 9,6% dos casos.
Conclusão Fetos nos quais ocorreu progressão da VM são de alto risco para desenvolvimento neurológico anormal. Apesar do ultrassom e da RM mostrarem substancial concordância na definição do grau de dilatação ventricular, uma baixa correlação foi vista na avaliação da VM associada ou não com anomalias do sistema nervoso central.
Palavras-chave: feto, ventriculomegalia, ultrassom, ressonância magnética, desenvolvimento neurológico
Introduction
Ventriculomegaly (VM) is one of the most common brain abnormalities observed at prenatal ultrasound (brain scan).1 The prevalence of VM varies between 0.3 and 10 per 1,000 births, depending on the technique used for the evaluation of one or both ventricles.2 Mild and moderate VM, defined as an atrial width of 10–15 mm between 15 and 40 weeks of gestation, may be associated with neural and extraneural malformations, fetal infections and chromosomal anomalies.3 Traditionally, the diagnosis of VM is based on prenatal brain scans during the second and the third trimesters of pregnancy. Ventriculomegaly may be isolated or associated with cranial and/or extracranial malformations, resulting in poorer prognosis in such cases. Therefore, the introduction of magnetic resonance imaging (MRI) in fetuses with a prenatally brain scan diagnosis of VM will reduce misclassified cases, thus providing a more accurate prediction of the neurodevelopmental outcome.4
The objective of this study was to compare the correlation between prenatal and postnatal brain scans with fetal and postnatal MRIs and to report the neurodevelopmental outcome at 4 years of age in infants with isolated mild and moderate VM.
Methods
The study was conducted between January 2007–2010 and approved by the local Health Board Authority of the Guastalla Civil Hospital, Reggio Emilia, Italy. Inclusion criteria were fetuses with an ultrasound diagnosis (brain scan) of isolated borderline VM at the second and third trimesters of pregnancy in whom both fetal and postnatal MRIs were available. Ventriculomegaly was defined as an atrial width of 10–15 mm that was further divided as mild (10.1–12.0 mm) and moderate (12.1–15.0 mm).5 Ultrasound criteria of regression and/or progression of VM were classified as < 2 mm or > 2 mm of lateral ventricle (LV) measurement at the follow-up brain scan, respectively.
Fetal karyotype and TORCH analysis were evaluated in all women. Sixty-two consecutive fetuses met the study criteria. Clinical follow-up was performed at 6–48 months into the postnatal period. Transfontanelle ultrasound and MRI, electroencephalogram (EEG), Griffith test, and an ophthalmological examination were performed. Fetal and post-natal MRI were all performed at a single center and diagnosed by an expert neuroradiologist using the T2-weighted single-shot fast spin echo (SSFSE) technique, while T1-weighted images were arranged at the time of the postnatal follow-up. The MRI equipment was a 1.5 Tesla Signa Twin Speed superconducting system (GE, Milwaukee, WI, US) using an 8-element phased array surface coil. Standard SSFSE imaging was performed in the fetal sagittal, coronal, and axial planes. The T2-weighted MRI settings were: repetition time (TR)/single-shot echo time (TE) = 3,000/180 milliseconds; matrix, 320 × 256; 4 mm slice; field of view [FOV] = 340 mm. Axial T1-weighted sections were also acquired in gradient-echo 2D (TR/TE = 11/5 m; T1 = 100 milliseconds; 5 mm slice; matrix, 256 × 224; FOV = 340 mm; number of excitations [NEX], 3). Infinite/90; bandwidth, 32 kHz; FOV = 30 × 30 cm; matrix, 256 × 192; gap, 1.5 mm; NEX, 0.5; refocusing pulse of less than 180 degrees; slice thickness, 4 mm; and 0.6 second per slice, echo-train length, 72; 1 signal acquired. In addition, T1 axial sections weighted on the subcallosal plane were also performed with a slice of 5 mm and TR/TE= 4,000/minimum; matrix, 128 × 128; FOV = 280 mm, all axis; b max = 600. Griffiths Mental Development Scales measure development trends that are significant for intelligence, or indicative of functional mental growth in babies and young children from birth to the developmental age of 8 years. Within the 0–2-year scales, a profile is obtained from 5 subscales examining locomotor, personal-social, language, eye-and-hand coordination and performance. In the 2–8-year scales, this profile is expanded to add a Practical Reasoning (General Quotient) subscale.
The neurodevelopmental outcome was collected from 12–48 months using a clinical questionnaire developed by Pediatric Neurologists and conducted by telephone interview.
Results
Sixty-two consecutives fetuses with a prenatal ultrasound diagnosis of mild and moderate isolated ventriculomegaly were identified. The mean maternal age was 32 years (range 21–41) while the mean gestational age at diagnosis was 25.6 (range 18.1–35.1) weeks of gestation. The male to female ratio was 1.9. Fetal karyotype was obtained in 33 cases (53%) by amniocentesis, identifying 1 fetus with trisomy 21. TORCH analysis resulted negative in 57 (92%) cases. At the brain scan, 58% of cases of VM were bilateral, and 23% of fetuses had a moderate VM. Follow-up using ultrasound demonstrated a stable VM in 45% of cases, resolution of VM in 36%, regression in 4.5%, and progression in 13% of cases. Fetal MRI was performed in 54 cases (87%), 31 of which at a gestational age lower than than 25 weeks. Overall, MRI was concordant with ultrasound in 85% of VM cases. Six newborns (9.6%) were found to have an abnormal neurodevelopmental outcome at 4 years of age. In 7 cases (n = 7/54, 13%), the fetal MRI detected associated cerebral abnormalities that were undiagnosed by antenatal brain scan: 3 cases of white matter anomalies (1 case of micro/lissencephaly, 1 case of reduced posterior white matter and 1 case of abnormal gyration). In the 4 remaining cases, VM was associated with cerebellar vermian hypoplasia with widened supratentorial-subarachnoid spaces, 2 cases of intraventricular hemorrhage and 1 case of reduced antero-posterior diameters of the cerebral hemispheres. A postnatal transfontanellar scan was performed in 36 cases while, MRI was performed in 9 cases. Table 1 shows the findings by brain scan, fetal and postnatal scans, as well as MRI in six cases with an adverse neurodevelopment outcome (Figs. 1 2 3 4).
Table 1. Correlation between prenatal ultrasound, fetal and postnatal ultrasounds and magnetic resonance imaging in six cases with adverse neurodevelopment outcomes.
| MA | GA | Karyotype | US (Brain Scan) | Fetal MRI | Pregnancy outcome | Postnatal outcome |
|---|---|---|---|---|---|---|
| 31 | 22.1 | 46,XX | Bilateral borderline VM US follow-up: - 29 wks: RLV 12.5mm/LLV 11,3 mm - 33 wks: RLV13mm/LLV 12 mm |
SVD at 40 wks MRI: hippocampus anomaly |
Nystagmus, visual impairment | |
| 35 | 21 | 46,XX | Unilateral borderline VM (11 mm) | − | IOL at 38 wks for PE | Mild neurodevelopmental delay |
| 35 | 35 | 46,XX | Unilateral moderate VL (14 mm) | Bilateral VL; decreased volume of the posterior white matter | IOL at 38 wks for PE. Microcephaly, deep hypotonia; cyst of the thalamocaudate sulcus, hypoplastic pons, slightly rotated cerebellar vermis | West syndrome, visual impairment, moderate neuromotor impairment |
| 34 | 22 | 46,XY | Bilateral, mild borderline VM (11 mm) US follow-up: – 26 wks: 13 mm/11 mm – 30 wks: 15 mm/11 mm – 34, 36, 38 wks: 7 mm/11 mm |
Bilateral, mild borderline VM (10.2 mm) II MRI 33 wks: right hydrocephalus (LV 17 mm); mild left VL (11 mm) |
SVD at 40 wks MRI: stable LV, abnormal periventricular neuronal migration |
Epilepsy, hypermetropy |
| 37 | 22 | 46,XX | Unilateral mild VL (11.5 mm) | Mild unilateral VL | C-s at 41 wks; bilateral, mild-moderate neurosensory hypoacusis | Mild neuromotor delay |
| 28 | 33.2 | ND | Bilateral, borderline VM (11 mm), anti HPA-1 Ab-positive; negative antiplatelet Ab US follow-up 36 wks: RLV 12 mm, left hydrocephalus (LV 16 mm) associated with septum at the level of the occipital horn; choroid plexus cyst |
Bilateral VM (RLV, 15 mm; LLV 11mm); septum of the occipital horn; cyst of the thalamocaudate sulcus; distorted choroid plexus | SVD at 40 wks Brain Scan: ventricular dilatation; CMV reinfection MRI: VM, increased signal of the temporal white matter and increased ADC, abnormal white matter migration, septum in the left occipital horn, abnormal hyppocampus gyration |
Mild neuromotor impairment |
Abbreviations: Ab, antibody; ADC, apparent diffusion coefficient; anti-HPA, anti-human platelet antigen; CMV, cytomegalovirus; C-s, cesarean section; GA, gestational age; IOL, induction of labor; LV, lateral ventricle, LLV, left lateral ventricle; MA, maternal age; MRI, magnetic resonance imaging; ND, not done; PE, preeclampsia; RLV, right lateral ventricle; SVD, spontaneous vaginal delivery; US, ultrasound; VM, ventriculomegaly; wks, weeks of gestation.
Fig. 1.
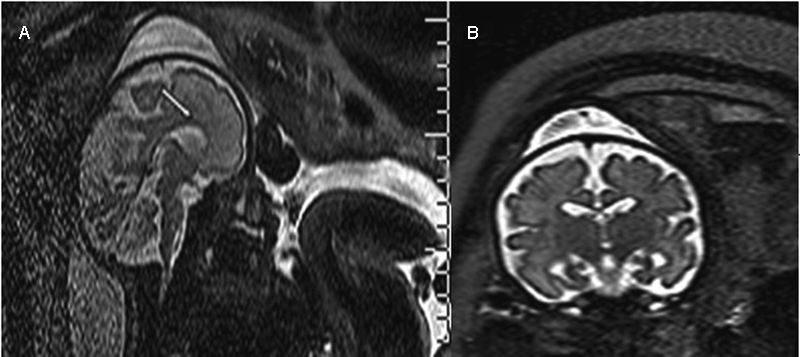
T2-weighted MRI performed in a fetus at 31.3 weeks of gestation showing a thin corpus callosum (white arrow) (A) associated with mild macrocrania (B).
Fig. 2.
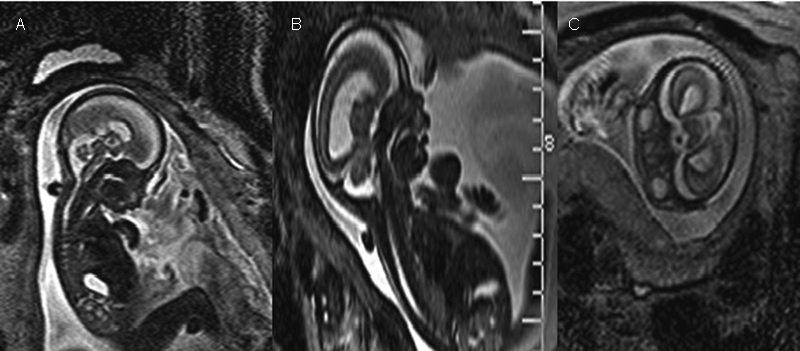
T2-weighted MRI performed in a fetus at 28.4 weeks of gestation: brachycephaly (A), subcuticular frontal edema (B) and hypertelorism (C) were detected.
Fig. 3.
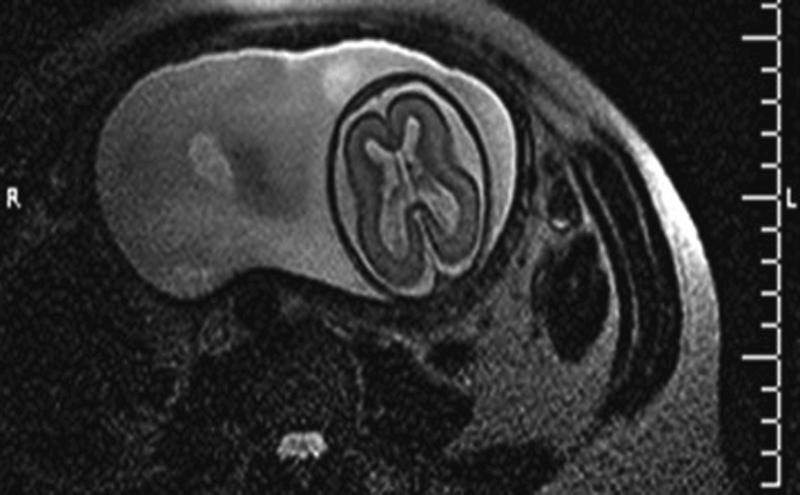
T2-weighted MRI performed in a fetus at 30.5 weeks of gestation documenting reduced opercularization associated with increased periencephalic liquoral spaces.
Fig. 4.
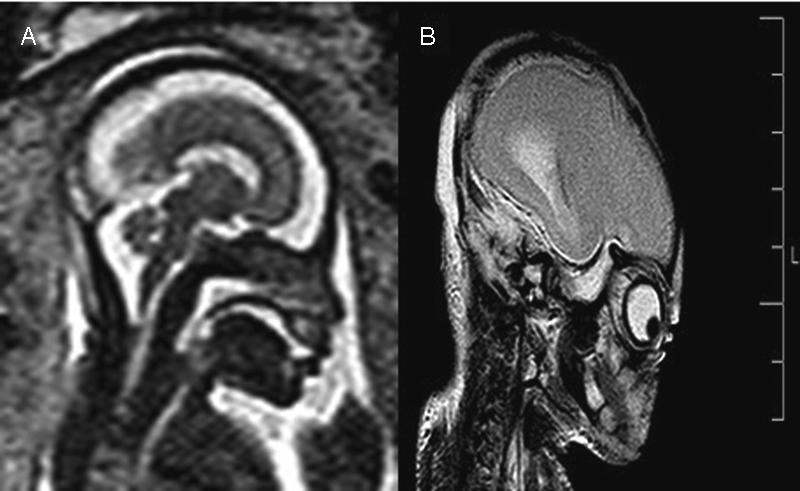
T2-weighted MRI performed in a fetus at 29.3 weeks of gestation: microcephaly with microlissencephaly was prompted (A). Microlissencephaly was excluded following a postmortem MRI (B).
Discussion
Evaluation of the LVs is an essential part of the standard sonographic examination of the fetus. Normal-sized ventricles provide reassurance of the normal development of the cerebrum, while increased LVs > 15 mm are highly associated with major cerebral anomalies.6
The mean diameter of the atria of the LVs in normal fetuses is 7.6 + 0.6 mm throughout the pregnancy.7 The prevalence of mild and moderate VM has been reported to vary from 1.48 to 22 per 1,000 live births in the low- and in high-risk pregnancy population respectively.6 Dilatation of the LVs can variably be associated with dilatation of the 3rd and 4th ventricles,5 and an asymmetric enlargement of the LVs is defined as a difference as a width of ≥ 2 mm between the two LVs.8
Ventriculomegaly recognizes multifactorial causes and may be seen isolated or associated with CNS or non-CNS malformations, fetal infections and/or chromosomal anomalies, and may occur because of primary overproduction of cerebrospinal fluid (CBF), as seen in cases of choroid plexus papilloma,9 or secondary to obstructions (communicating) of the CBF caused by the narrowing or forking of the aqueduct of Sylvius,10 Dandy–Walker complex with obstruction of the foramina of Luschka and Magendie,11 or caused by intraventricular hemorrhage,12 13 14 infections3 15 16 17 or by extrinsic processes like tumors,12 arachnoid cysts or Chiari type II malformations.
Disorders of neuronal proliferation (megalencephaly, microcephaly), neuronal migration (schizencephaly, lissencephaly), agenesis of the corpus callosum (ACC), holoprosencephaly,13 14 18 19 20 21 vascular insults, or porencephaly (secondary to destructive processes of the cerebral white matter)2 may also be considered. Congenital viral infections and common viable trisomy are associated in 3 to 15% of cases, depending on maternal age and background risk.3 16 19 Multiple congenital malformations in fetuses with VM are calculated to range from 10 to 76%, with the lowest incidence seen for mild VM and highest incidence for moderate VM.3 13 14 15 16 18 19 20 21 22
Our series showed that 23% of fetuses had moderate VM, and that 58% of borderline VMs were bilateral. The prenatal brain scan follow-up demonstrated resolution in 36%, regression in 4.5%, stable dilatation in 45% and progression in 13% of cases. Brain scan findings were in agreement with those of the fetal MRI in 85% of cases. In 8 fetuses (15%), the MRI revealed additional information that were of critical impact on counseling, antenatal management and care planning by the multispecialist team. Postnatal follow-up conducted using Griffiths Mental Development Scales revealed 6 cases (11.7%) with abnormal neurodevelopmental outcomes.
Our findings are in keeping with those of Lam and Kumar,23 who reported 306 cases of VM in which it was demonstrated that the majority of fetuses with mild VM normalized, whereas the majority of moderate cases of VM remained stable (46% in the mild group and 26% in the moderate group resolved; only 1 case in the severe group progressed); the rate of progression of VM increased with severity. Infants with progression of VM are at a higher risk of subsequent neurodevelopmental delay than those with non-progression,4 even though mild VM is associated with an incidence of perinatal and neonatal deaths comparable to those of the general population.3 6 15 17 24
Valsky et al25 showed that the MRI could detect additional findings in a woman with increased maternal body mass index (8.3%) and in 2 cases (5.5%) with bleeding in germinal centers, while in the series of Benacerraf et al,26 the MRI revealed additional information (14% of the cases) in a case of cerebellar hypoplasia and in a case of enlarged cisterna magna. When the brain scan was compared with the MRI, Levine et al27 demonstrated a change in the final diagnosis in 23% of cases identified as abnormal at ultrasound, a change in counselling in 41% of cases, and a change in patient management in 13.5% of cases respectively. Similarly, Salomon et al28 concluded that the use of the fetal MRI modified obstetric management in 6% of cases, studying 185 third trimester fetuses with mild VM. Furthermore, in fetuses with apparently isolated mild VM, Ouahba et al4 showed that the MRI diagnosed major cerebral anomalies in 9% of cases, including cortical malformations, absence of the septum pellucidum, partial agenesis of the corpus callosum and agenesis of the cerebellar vermis.
Fetal MRI was diagnostic in 47.3% of cases of VM associated with CNS anomalies, while the brain scan could only detect 32.7% of cases. When VM associated with non-CNS anomalies was considered, the MRI diagnosed 18.2% of cases compared with 14.5% diagnosed by ultrasound.29
Parazzini et al,1 30 on the contrary, reported disagreement between ultrasound and fetal MRI in 27.3% of 179 fetuses with isolated mild VM at ultrasound; MRI provided additional information in a fetus with bilateral frontal schizencephaly and agenesis of the septum pellucidum, and in a fetus with isolated agenesis of the septum pellucidum. As one of the main advantages of the MRI over the ultrasound is analysis of gyration, MRI study at 30 and 32 weeks of gestation should be the most appropriate time period to perform the investigation.28 31
Prenatal brain scan diagnosis of mild, isolated VM carries a 12–13% rate of false negative results; this must be taken into consideration when counselling parents-to-be.32 Fetuses with mild, isolated VM have good prognosis, although an overall 11% risk of neurodevelopmental delay in fetuses with isolated mild and moderate VMs is seen.32
A follow-up by brain scan between 28 and 34 weeks of gestation should be indicated in all cases of prenatally ultrasound diagnosis of VM,33 as the risk of progression of VM (defined as an increase in the ventricular measurement of more than 3 mm) is of ∼ 16%.32 In addition, infants with progression of VM are at a higher risk of subsequent neurodevelopmental delay than those with non-progression.4 Ultrasound and MRI are substantially in agreement in defining the degree of VM (either isolated or with associated anomalies), although a low correlation between ultrasound and MRI in the evaluation of VM associated either with CNS or non-CNS anomalies has been seen. Magnetic Resonance Imaging may be a useful complementary diagnostic investigation in the detection of hemorrhagic foci, porencephaly, cortical and subependymal tubers, midline anomalies and callosal dysgenesis, as well as posterior fossa abnormalities. From 25 weeks onwards, the MRI may add additional information about cortical development and maturation, and is more accurate in detecting white matter pathology compared with the brain scan.34 Nonetheless, fetal MRI has a limited role over ultrasound in assessing the size of the cerebral ventricles, except for cases where fetal position and calvarial ossification cause reverberation artifacts and shadowing.35
In our series, the fetal MRI was performed at a mean gestational age of 31 weeks, 4 days, and allowed to detect, compared with the prenatal brain scan, the following CNS pathologies: microcephaly and microlissencephaly; suspected interventricolar septi; brachycephaly; impaired cortical gyration; cyst of the thalamocaudate sulcus; abnormal periventricular neuronal migration; increased signal of the temporal white matter and increased apparent diffusion coefficient (ADC); abnormal white matter migration; septum in the left occipital horn; and abnormal hippocampus gyration. Following the analysis of the medical records and results from our series, we developed a clinical-diagnostic flowchart to be used in cases of prenatally detected isolated mild and moderate VMs (Fig. 5). This clinical flowchart may aid antenatal care management. Specifically, fetal karyotype, TORCH analysis, search for anti-human platelet antigen (anti-HPA) antibodies (justified only if there is a suspicion of intracranial hemorrhage on imaging) and a thorough fetal ultrasound examination should be recommended; the fetal MRI may integrate the brain scan, especially when performed in the third trimester of pregnancy, enhancing prenatal counselling and antenatal care management by the multispecialist team. In addition, the postnatal MRI may also allow the diagnosis of associated cerebral abnormalities undiscovered antenatally. The clinical limitations of the MRI are represented by elevated costs, availability and decreased sensitivity at a gestational age < 25 weeks of gestation.
Fig. 5.
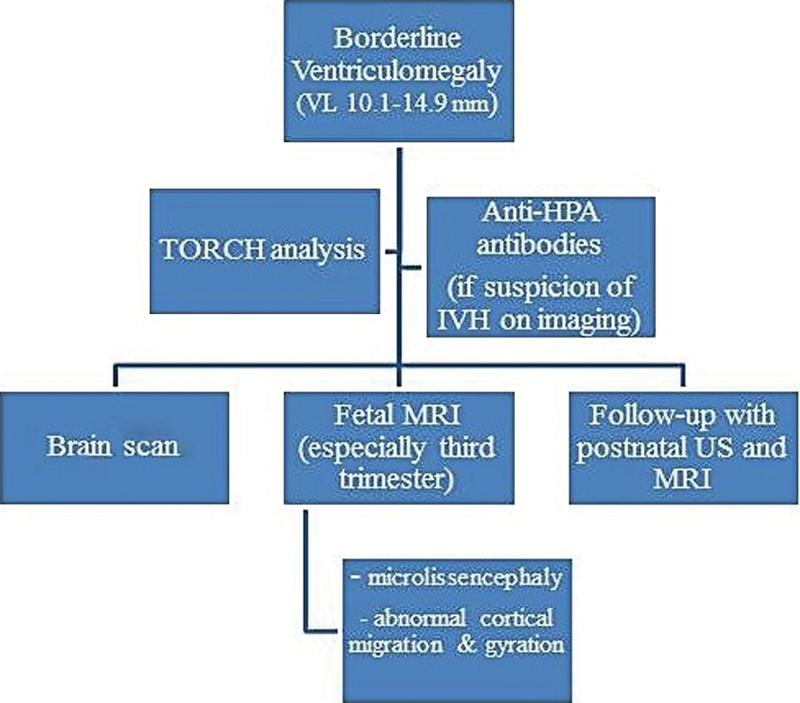
Clinical-diagnostic flowchart to be used in cases of prenatally diagnosed mild and moderate ventriculomegaly. Abbreviations: anti-HPA, anti-human platelet antigen; IVH, intraventricular hemorrhage; MRI, magnetic resonance imaging; US, ultrasound; TORCH, toxoplasmosis, others, rubella, cytomegalovirus, herpes virus; VL, ventriculomegaly.
Note
Study performed in the Prenatal Diagnostic Unit, Department of Obstetrics & Gynecology, Guastalla Civil Hospital, AUSL Reggio Emilia, Italy.
References
- 1.Parazzini C, Righini A, Doneda C. et al. Is fetal magnetic resonance imaging indicated when ultrasound isolated mild ventriculomegaly is present in pregnancies with no risk factors? Prenat Diagn. 2012;32(8):752–757. doi: 10.1002/pd.3896. [DOI] [PubMed] [Google Scholar]
- 2.Pilu G, Hobbins J C. Sonography of fetal cerebrospinal anomalies. Prenat Diagn. 2002;22(4):321–330. doi: 10.1002/pd.310. [DOI] [PubMed] [Google Scholar]
- 3.Vergani P, Locatelli A, Strobelt N. et al. Clinical outcome of mild fetal ventriculomegaly. Am J Obstet Gynecol. 1998;178(2):218–222. doi: 10.1016/s0002-9378(98)80003-3. [DOI] [PubMed] [Google Scholar]
- 4.Ouahba J, Luton D, Vuillard E. et al. Prenatal isolated mild ventriculomegaly: outcome in 167 cases. BJOG. 2006;113(9):1072–1079. doi: 10.1111/j.1471-0528.2006.01050.x. [DOI] [PubMed] [Google Scholar]
- 5.Levene M I, Chervenak F A, Whittle M. London: Churchill Livingstone; 2001. Fetal and neonatal neurology and neurosurgery. 3rd ed. [Google Scholar]
- 6.Signorelli M, Tiberti A, Valseriati D. et al. Width of the fetal lateral ventricular atrium between 10 and 12 mm: a simple variation of the norm? Ultrasound Obstet Gynecol. 2004;23(1):14–18. doi: 10.1002/uog.941. [DOI] [PubMed] [Google Scholar]
- 7.Cardoza J D, Goldstein R B, Filly R A. Exclusion of fetal ventriculomegaly with a single measurement: the width of the lateral ventricular atrium. Radiology. 1988;169(3):711–714. doi: 10.1148/radiology.169.3.3055034. [DOI] [PubMed] [Google Scholar]
- 8.Kinzler W L, Smulian J C, McLean D A, Guzman E R, Vintzileos A M. Outcome of prenatally diagnosed mild unilateral cerebral ventriculomegaly. J Ultrasound Med. 2001;20(3):257–262. doi: 10.7863/jum.2001.20.3.257. [DOI] [PubMed] [Google Scholar]
- 9.Nejat F, Kazmi S S, Ardakani S B. Congenital brain tumors in a series of seven patients. Pediatr Neurosurg. 2008;44(1):1–8. doi: 10.1159/000110655. [DOI] [PubMed] [Google Scholar]
- 10.D'Addario V, Pinto V, Di Cagno L, Pintucci A. Sonographic diagnosis of fetal cerebral ventriculomegaly: an update. J Matern Fetal Neonatal Med. 2007;20(1):7–14. doi: 10.1080/14767050601036188. [DOI] [PubMed] [Google Scholar]
- 11.Whitelaw A Neonatal hydrocephalus - clinical assessment and non surgical treatment London: Churchill Livingstone; 2001. p. 739–61. [Google Scholar]
- 12.Sherer D M, Onyeije C I. Prenatal ultrasonographic diagnosis of fetal intracranial tumors: a review. Am J Perinatol. 1998;15(5):319–328. doi: 10.1055/s-2007-993951. [DOI] [PubMed] [Google Scholar]
- 13.Fong K W, Ghai S, Toi A, Blaser S, Winsor E J, Chitayat D. Prenatal ultrasound findings of lissencephaly associated with Miller-Dieker syndrome and comparison with pre- and postnatal magnetic resonance imaging. Ultrasound Obstet Gynecol. 2004;24(7):716–723. doi: 10.1002/uog.1777. [DOI] [PubMed] [Google Scholar]
- 14.Malinger G, Kidron D, Schreiber L. et al. Prenatal diagnosis of malformations of cortical development by dedicated neurosonography. Ultrasound Obstet Gynecol. 2007;29(2):178–191. doi: 10.1002/uog.3906. [DOI] [PubMed] [Google Scholar]
- 15.Pilu G, Falco P, Gabrielli S, Perolo A, Sandri F, Bovicelli L. The clinical significance of fetal isolated cerebral borderline ventriculomegaly: report of 31 cases and review of the literature. Ultrasound Obstet Gynecol. 1999;14(5):320–326. doi: 10.1046/j.1469-0705.1999.14050320.x. [DOI] [PubMed] [Google Scholar]
- 16.Gaglioti P, Danelon D, Bontempo S, Mombrò M, Cardaropoli S, Todros T. Fetal cerebral ventriculomegaly: outcome in 176 cases. Ultrasound Obstet Gynecol. 2005;25(4):372–377. doi: 10.1002/uog.1857. [DOI] [PubMed] [Google Scholar]
- 17.Graham E, Duhl A, Ural S, Allen M, Blakemore K, Witter F. The degree of antenatal ventriculomegaly is related to pediatric neurological morbidity. J Matern Fetal Med. 2001;10(4):258–263. doi: 10.1080/714052753. [DOI] [PubMed] [Google Scholar]
- 18.Pastorino D, Prefumo F, Rossi A. et al. Apparently isolated borderline ventriculomegaly and lissencephaly. Prenat Diagn. 2007;27(5):483–484. doi: 10.1002/pd.1726. [DOI] [PubMed] [Google Scholar]
- 19.Nicolaides K H, Berry S, Snijders R J, Thorpe-Beeston J G, Gosden C. Fetal lateral cerebral ventriculomegaly: associated malformations and chromosomal defects. Fetal Diagn Ther. 1990;5(1):5–14. doi: 10.1159/000263529. [DOI] [PubMed] [Google Scholar]
- 20.Mercier A, Eurin D, Mercier P Y, Verspyck E, Marpeau L, Marret S. Isolated mild fetal cerebral ventriculomegaly: a retrospective analysis of 26 cases. Prenat Diagn. 2001;21(7):589–595. doi: 10.1002/pd.88. [DOI] [PubMed] [Google Scholar]
- 21.Morris J E, Rickard S, Paley M N, Griffiths P D, Rigby A, Whitby E H. The value of in-utero magnetic resonance imaging in ultrasound diagnosed foetal isolated cerebral ventriculomegaly. Clin Radiol. 2007;62(2):140–144. doi: 10.1016/j.crad.2006.06.016. [DOI] [PubMed] [Google Scholar]
- 22.Breeze A C, Alexander P M, Murdoch E M, Missfelder-Lobos H H, Hackett G A, Lees C C. Obstetric and neonatal outcomes in severe fetal ventriculomegaly. Prenat Diagn. 2007;27(2):124–129. doi: 10.1002/pd.1624. [DOI] [PubMed] [Google Scholar]
- 23.Lam S J, Kumar S. Evolution of fetal ventricular dilatation in relation to severity at first presentation. J Clin Ultrasound. 2014;42(4):193–198. doi: 10.1002/jcu.22124. [DOI] [PubMed] [Google Scholar]
- 24.Gaglioti P, Oberto M, Todros T. The significance of fetal ventriculomegaly: etiology, short- and long-term outcomes. Prenat Diagn. 2009;29(4):381–388. doi: 10.1002/pd.2195. [DOI] [PubMed] [Google Scholar]
- 25.Valsky D V Ben-Sira L Porat S et al. The role of magnetic resonance imaging in the evaluation of isolated mild ventriculomegaly J Ultrasound Med 2004234519–523., quiz 525–526 [DOI] [PubMed] [Google Scholar]
- 26.Benacerraf B R, Shipp T D, Bromley B, Levine D. What does magnetic resonance imaging add to the prenatal sonographic diagnosis of ventriculomegaly? J Ultrasound Med. 2007;26(11):1513–1522. doi: 10.7863/jum.2007.26.11.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Levine D, Barnes P D, Robertson R R, Wong G, Mehta T S. Fast MR imaging of fetal central nervous system abnormalities. Radiology. 2003;229(1):51–61. doi: 10.1148/radiol.2291020770. [DOI] [PubMed] [Google Scholar]
- 28.Salomon L J, Ouahba J, Delezoide A L. et al. Third-trimester fetal MRI in isolated 10- to 12-mm ventriculomegaly: is it worth it? BJOG. 2006;113(8):942–947. doi: 10.1111/j.1471-0528.2006.01003.x. [DOI] [PubMed] [Google Scholar]
- 29.Manganaro L, Savelli S, Francioso A. et al. Role of fetal MRI in the diagnosis of cerebral ventriculomegaly assessed by ultrasonography. Radiol Med (Torino) 2009;114(7):1013–1023. doi: 10.1007/s11547-009-0434-2. [DOI] [PubMed] [Google Scholar]
- 30.Parazzini C, Righini A, Doneda C, Rustico M, Lanna M, Triulzi F. Response to “Is fetal magnetic resonance imaging indicated when ultrasound isolated mild ventriculomegaly is present in pregnancies with no risk factors?”. Prenat Diagn. 2014;34(9):919. doi: 10.1002/pd.4378. [DOI] [PubMed] [Google Scholar]
- 31.Garel C, Alberti C. Coronal measurement of the fetal lateral ventricles: comparison between ultrasonography and magnetic resonance imaging. Ultrasound Obstet Gynecol. 2006;27(1):23–27. doi: 10.1002/uog.2666. [DOI] [PubMed] [Google Scholar]
- 32.Melchiorre K, Bhide A, Gika A D, Pilu G, Papageorghiou A T. Counseling in isolated mild fetal ventriculomegaly. Ultrasound Obstet Gynecol. 2009;34(2):212–224. doi: 10.1002/uog.7307. [DOI] [PubMed] [Google Scholar]
- 33.Baffero G M, Crovetto F, Fabietti I. et al. Prenatal ultrasound predictors of postnatal major cerebral abnormalities in fetuses with apparently isolated mild ventriculomegaly. Prenat Diagn. 2015;35(8):783–788. doi: 10.1002/pd.4607. [DOI] [PubMed] [Google Scholar]
- 34.Griffiths P D, Reeves M J, Morris J E. et al. A prospective study of fetuses with isolated ventriculomegaly investigated by antenatal sonography and in utero MR imaging. AJNR Am J Neuroradiol. 2010;31(1):106–111. doi: 10.3174/ajnr.A1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cardoen L, De Catte L, Demaerel P. et al. The role of magnetic resonance imaging in the diagnostic work-up of fetal ventriculomegaly. Facts Views Vis Obgyn. 2011;3(3):159–163. [PMC free article] [PubMed] [Google Scholar]


