Abstract
Objective To evaluate the correlation between upper limb functionality and quality of life in women with five-year survival following breast cancer surgical treatment. The secondary objective was to evaluate the function of the ipsilateral upper limb and the quality of life in relation to the type of surgery and the presence of pain.
Methods The Disabilities of Arm, Shoulder and Hand (DASH), and the Functional Assessment of Cancer Therapy – Breast plus Arm Morbidity (FACTB + 4) questionnaires were used to evaluate upper limb function and quality of life respectively. Data distribution was verified by the Shapiro-Wilk test. Pearson's correlation coefficient was used for the parametric variables, and Spearman's rank correlation coefficient was used for the distribution of non-parametric variables. The statistical significance was set at 5% (p < 0.05).
Results The study included 30 patients, with a mean age of 51.23 (±8.72) years. The most common complications were: pain (50%), adherence (33.3%), and nerve lesion (20.0%). There was a moderate negative correlation between the instruments DASH and FACTB + 4 (total score), r = -0.634, and a strong negative correlation between the DASH and the FACTB + 4 arm subscale, r = -0.829. The scores of both questionnaires showed significant difference on the manifestation of pain. However, there was no significant difference found when comparing the scores considering the type of surgery performed.
Conclusions Five years after surgery, the patients showed regular functionality levels on the ipsilateral upper limb and decreased quality of life, especially in the group manifesting pain.
Keywords: mastectomy, range of motion, quality of life, upper limb functionality
Abstract
Resumo
Objetivo Avaliar se há correlação entre a funcionalidade e a qualidade de vida em pacientes com sobrevida de cinco anos submetidas ao tratamento cirúrgico para câncer de mama e, secundariamente, avaliar a função do membro superior homolateral à cirurgia, e a qualidade de vida em função do tipo de cirurgia mamária e da presença de dor.
Métodos Foram utilizados os questionários DASH e FACTB + 4 para avaliar a função do membro superior e a qualidade de vida respectivamente. Os dados foram submetidos ao teste de normalidade de Shapiro-Wilk. O coeficiente de correlação de Pearson foi utilizado para as variáveis com distribuição paramétrica e, para as variáveis com distribuição não paramétrica, o coeficiente de correlação de Spearman. Adotou-se o nível de significância de 5% (p < 0,05).
Resultados Foram incluídas 30 pacientes, com média de idade de 51,23 ( ± 8,72) anos. As complicações mais incidentes foram: dor (50%), aderência cicatricial (33,3%), e lesão nervosa (20,0%). Foi observada correlação negativa de magnitude moderada entre os instrumentos DASH e FACTB + 4 (pontuação total), r = -0,634, e de magnitude forte entre o DASH e a subescala braço do FACTB + 4, r = -0,829. As pontuações dos questionários apresentaram diferença significativa em função da presença de dor. Entretanto, não foi observada diferença significativa quando comparadas as pontuações com relação ao tipo de cirurgia.
Conclusões Após cinco anos de cirurgia, as pacientes apresentaram grau regular de funcionalidade do membro homolateral à cirurgia e diminuição na qualidade de vida relacionada à saúde, principalmente no grupo que relatava presença de dor.
Palavras-chave: mastectomia, amplitude de movimento articular, qualidade de vida, funcionalidade de membro superior
Introduction
Breast cancer (BC) is currently the neoplasm with the highest incidence and is the most common cause of cancer-related deaths among women worldwide.1 2 In Brazil, it represents the second leading cancer-related cause of death among women, after non-melanoma skin carcinoma.3 Between the years 2014 and 2015, there was an estimated 57,120 new cases in Brazil, which represents an incidence of 56.1 cases per 100,000 women.4
The primary treatment choice for BC is surgery (radical or conservative), and the other options are radiation, chemotherapy, hormonal therapy and targeted therapy as adjuvant treatments.5 Despite the advances in surgical techniques, the procedure is still associated with a high prevalence of complications on the ipsilateral upper limb, and more than half of the patients who undergo axillary clearance present post-surgery comorbidities.6
The main complications related to surgery are sensitivity impairment, seroma production, altered ventilatory capacity, lymphedema, axillary cording, reduced strength on the upper limb, reduced range of motion (ROM) of the arm and shoulder, and pain.7 8 Pain itself is the main physical/functional impairment reported by patients,9 with a prevalence of up to 60%.9 10
Functionality involves the interrelation of the personal aspects of an individual (physical and emotional) with the surroundings (the environment and the engagement on activities).11 The morbidities resulting from BC treatment create a negative impact on upper limb functionality, affecting daily activities; when added to altered body image and emotional disturbances such as anxiety and depression, that can affect the quality of life of women who have undergone treatment.12 13 Associated with the increasing survival rate in women with BC, this fact has led to a large number of studies about quality of life related to health.14 15
However, the majority of the studies focuses on the evaluation of comorbidities and their effects on functionality and quality of life up to two years after surgery.16 17 These data should be complemented by longer studies of five and ten years following surgical treatment to obtain a better definition of the comorbidities and tof he impact on function and quality of life on this population.18 19 20
For the reasons presented before, this study has the primary objective of investigating a possible correlation between functionality and quality of life in patients with five-year survival who underwent surgical treatment for BC. The secondary goal is to evaluate surgical ipsilateral upper limb function and quality of life related to the type of surgery and pain.
Methods
This is a cross-sectional, observational analytical study with a quantitative approach, completed between February and April 2015 at the Physical Therapy ambulatory of Maternidade Carmela Dutra (MCD), in the city of Florianópolis, Brazil.
The study population consisted of women living in Florianópolis submitted to surgery for BC treatment according to the International Statistical Classification of Diseases and Related Health Problems 10th Revision (ICD-10), sectionC50 (‘Malignant neoplasm of breast’), who were part of the Hospital Cancer Registry (HCR) at MCD between the years 2009 and 2010. The study excluded patients with other primary cancer types, metastasized cancer, and with an indication for nodulectomy surgery with no lymph node resection or sentinel lymph node biopsy. Subjects with cognitive impairments who were unable to complete the questionnaires were also excluded. The protocol was approved by the Ethics and Human Subject Research Committees at Universidade Estadual de Santa Catarina and MCD (CAAE 39722314.70000.0118). All participants signed an informed consent form as per the Helsinki Declaration and the Brazilian National Health Council Resolution n. 466, from December 2012. The Participants' identities and data confidentiality were protected.
Data Collection
All participants completed an initial survey containing: personal information, gynecological and obstetrics history, cancer history, daily habits and general questions related to the diagnosis and the physical therapy treatment for axillary cording. The participants then underwent a physical exam to investigate possible adherence, breast sensitivity through Semmes-Weinstein monofilament and ipsilateral arm lymphedema as per the Simplified Clinical Classification for Lymphedema.21 The McGill questionnaire was used to evaluate pain.22
Stature and body mass were verified by Sanny® (American Medical do Brasil Ltda, São Bernardo do Campo, Brazil) stadiometer and digital bioimpedance scale Ironman Segmental Body Composition Monitor (Tanita Corporation, 14-2, 1-Chome, Maeno-Cho, Itabashi-Ku, Tokyo, Japan) model BC-558 respectively. All measurements were performed barefoot, standing erect with the head aligned. The body mass index (BMI) was calculated according to World Health Organization (WHO) guidelines.23
The participants completed the Disabilities of Arm, Shoulder and Hand (DASH) and the Functional Assessment of Cancer Therapy – Breast plus Arm Morbidity (FACTB + 4) questionnaires, which were applied by a single examiner, and translated and culturally adapted for the Brazilian population.24 25
The DASH questionnaire is an instrument used to evaluate physical function. It verifies the impact of disability and the symptoms of upper extremities as a functional unit, and measures the difficulty or inability to perform specific activities. It consists of 30 items, which are divided in physical function, symptoms and social function. Each item is rated on a scale from 1–5. The total score ranges from 0–100 points; the higher the score, the more severe the disability.24 Scores between the 25–75th quartiles are indicative of some disability, while scores lower than the 25th quartile represent minimal or no disability, and those greater than the 75th quartile indicate a high disability level.26
The FACTB + 4 questionnaire was developed to assess quality of life in patients with breast cancer, and it was validated for the study population.27 It consists of 36 questions: 27 of them refer to quality of life in general; 9 relate to specific problems faced by women with breast cancer; and 4 relate to upper limb morbidity. This instrument is divided in six scales, which may be scored separately: physical well-being; social/family well-being; emotional well-being; functional well-being; breast cancer subscale; and arm subscale. Each one has scores that range from 0–4. The total score ranges from 0 to 164 points, and high scores are associated with a better quality of life.28
Statistical Analysis
The statistical analyses were performed using the Statistical Package for the Social Sciences (IBM-SPSS, Inc., Chicago, IL, USA – licensed for use at Universidade Estadual de Santa Catarina ) software, version 20.0, and presented as descriptive statistics (mean and standard deviation) and frequencies. Data distribution was verified using the Shapiro-Wilk test, and the correlation was used to describe the degree and direction of the relationship between the variables. Pearson's correlation coefficient was used for the parametric variables, and Spearman's rank correlation coefficient for the non-parametric ones. Levene's test was used to analyze the homogeneity of variance. The comparison of the group means was done using the Student's t-test for parametric variables and the Wilcoxon–Mann–Whitney test for non-parametric variables. The statistical significance was set at 5% (p < 0.05).
Results
The mean time to complete both questionnaires was 30 minutes. Thirty patients were included; they had a mean age of 51.23 ( ± 8.72) years, and a mean BMI of 28.18 (±5.06), characterizing an overweight population. The majority of the patients were married or on a stable union (73.3%), were caucasians (93.3%), and had less than 8 years of schooling (60.0%). Aside from that, 36.7% of them were ex-smokers, and 16.7% were smokers. Half of the participants did not practice any physical activity. Table 1 shows the participants' demographics and habits.
Table 1. Participants' demographics and daily life activities.
| Variables | Mean (SD) |
|---|---|
| Age (years) | 51.23 (8.72) |
| BMI (Kg/m) | 28.18 (5.06) |
| Variables | N (%) |
| Marital status | |
| Single | 1 (3.3) |
| Married/stable union | 22 (73.3) |
| Widow | 2 (6.7) |
| Separated | 5 (16.7) |
| Race | |
| White | 28 (93.3) |
| African American | 1 (3.3) |
| Brown | 1 (3.3) |
| Educational level | |
| < 8 years | 18 (60.0) |
| > 8 years | 12 (40.0) |
| Smoking status | |
| Non-smoker | 14 (46.7) |
| Ex-smoker | 11 (36.7) |
| Smoker | 5 (16.7) |
| Physical Activity | |
| Inactive | 15 (50.0) |
| Eventual | 4 (13.3) |
| Regular | 11 (36.7) |
| Unknown | 3 (10.0) |
Abbreviations: BMI, Body Mass Index; SD, Standard deviation.
Note: N = 30.
The progression of the disease and the frequency of the complications are shown in Table 2. Early stage diagnosis occurred in 83.3% of the participants, and the predominant histological type was ductal infiltrating (66.7%). Conservative surgery was performed in 53.3% of the patients, and 70.0% of this group did not have either early or late breast reconstruction performed after surgery. The most frequent complications were: pain (50%), adherence (33.3%), nerve lesion (20.0%) and lymphedema (13.3%). Axillary cording was presented by 13.3% of the participants; half of them underwent treatment, and half progressed with spontaneous resolution with no specific interventions.
Table 2. Disease progression and frequency of post-surgery complications.
| Variables | N (%) |
|---|---|
| Stage | |
| Early | 25 (83.3) |
| Advanced | 2 (6.7) |
| Unknown | 3 (10.0) |
| Histology | |
| Intraductal in situ | 1 (3.3) |
| Invasive Lobular | 5 (16.7) |
| Ductal infiltrative | 20 (66.7) |
| Other | 4 (13.3) |
| Surgery type | |
| Conservative | 14 (46.7) |
| Non-conservative | 16 (53.3) |
| Reconstructive surgery | |
| No | 21 (70.0) |
| Yes | 7 (23.3) |
| Unknown | 2 (6.7) |
| Scar adherence | |
| No | 20 (66.7) |
| Yes | 10 (33.3) |
| Cording | |
| No | 26 (86.7) |
| Yes | 4 (13.3) |
| Cording treatment | |
| Treated | 2 (50.0) |
| Spontaneous recovery | 2 (50.0) |
| Nerve lesion | |
| No | 24 (80.0) |
| Yes | 6 (20.0) |
| Lymphedema | |
| No | 26 (86.7) |
| Yes | 4 (13.3) |
| Pain | |
| No | 15 (50.0) |
| Yes | 15 (50.0) |
Note: N = 30.
Table 3 illustrates the mean, minimal, maximal and standard deviation data for the scores on both questionnaires and their individual domains. The mean score for the DASH was 41.03 (±22.27) points, and the total score for the FACTB + 4 was 92.40 (±17.27), with scores of 19.8 and 19.6 for the additional concerns and the physical domains respectively.
Table 3. Mean, minimum and maximum scores and standard deviation of the FACTB + 4 and DASH questionnaires.
| Questionnaire (variable) | Minimum | Maximum | Mean | SD |
|---|---|---|---|---|
| DASH | 2.00 | 76.00 | 41.03 | 22.27 |
| FACTB + 4 (physical) | 9.00 | 28.00 | 19.60 | 5.49 |
| FACTB + 4 (social/family) | 10.00 | 27.00 | 18.23 | 4.61 |
| FACTB + 4 (emotional) | 8.00 | 26.00 | 17.43 | 4.93 |
| FACTB + 4 (functional) | 3.00 | 26.00 | 17.13 | 4.46 |
| FACTB + 4 (AC) | 6.00 | 29.00 | 19.80 | 5.52 |
| FACTB + 4 (total) | 54.00 | 124.00 | 92.40 | 17.27 |
Abbreviations: AC, additional concerns; DASH, Disabilities of Arm, Shoulder and Hand; FACTB + 4 Functional Assessment of Cancer Therapy – Breast plus Arm Morbidity; SD, standard deviation.
N = 30.
There was a negative correlation between the DASH and the total score of the FACTB + 4, between the physical domain of the FACTB + 4 and the DASH, and a high correlation coefficient between the DASH and the upper limb subscale of the FACTB + 4. Only the social/family domain of the FACTB + 4 did not show a significant statistical correlation with any other variables. The detailed correlation analysis and the more relevant data are presented in Table 4 and Fig. 1.
Table 4. Correlation between the DASH and the FACTB + 4 domains.
| DASH | |||
|---|---|---|---|
| R | R2 | p | |
| FACTB + 4 (social/family) | 0.003 | 0.000 | 0.988 |
| FACTB + 4 (emotional) | −0.495 | 0.245 | 0.005 |
| FACTB + 4 (physical) | −0.720 | 0.518 | 0.000 |
| FACTB + 4 (functional) | −0.425 | 0.180 | 0.019 |
| FACTB + 4 (AC) | −0.462 | 0.213 | 0.010 |
| FACTB + 4 (total) | −0.634 | 0.401 | 0.000 |
| FACTB + 4 (arm subscale) | −0.829 | 0.687 | 0.000 |
Abbreviations: AC, additional concerns; DASH, Disabilities of Arm, Shoulder and Hand; FACTB + 4 Functional Assessment of Cancer Therapy – Breast plus Arm Morbidity; r, correlation coefficient; R2 , coefficient of determination.
Fig. 1.
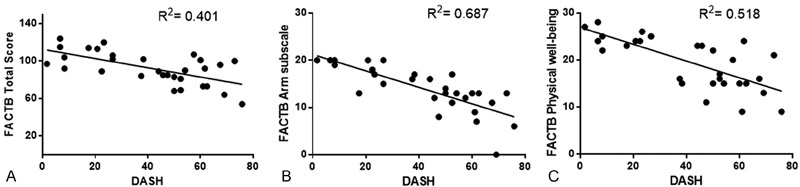
Correlation between the DASH and FACTB + 4 scores. (A) Pearson's correlation analysis between the DASH and the total score of the FACTB + 4. Correlation coefficient = -0.634; coefficient of determination (R2) = 0.401; p = 0.000. (B) Spearman's coefficient correlation between the DASH and the arm subscale of the FACTB + 4. Correlation coefficient = -0.829; R2 = 0.687; p = 0.000. (C) Spearman's coefficient correlation between the DASH and the physical domain of the FACTB + 4. Correlation coefficient = -0.720; R2= 0.518; p = 0.000.
The analysis of the scores of both questionnaires and their domains in reference to the type of surgery performed (conservative or non-conservative) showed no statistical significant difference. However, when considering the presence or absence of pain, there was an influence on the score for both questionnaires (p < 0.05) (Table 5).
Table 5. Mean score values and comparison tests between the mean values on the FACTB + 4 domains and on the DASH in reference to pain.
| Questionnaire (variable) | (SD) | (SD) | p |
|---|---|---|---|
| No pain (N = 15) | With pain (N = 15) | ||
| DASH | 25.87 (19.42) | 56.19 (12.07) | 0.000* |
| FACTB + 4 (social/family) | 18.53 (4.96) | 17.93 (4.40) | 0.728 |
| FACTB + 4 (emotional) | 19.60 (3.98) | 15.27 (4.95) | 0.014* |
| FACTB + 4 (physical) | 22.87 (4.50) | 16.33 (4.38) | 0.001* |
| FACTB + 4 (functional) | 18.87 (3.42) | 15.40 (4.81) | 0.032* |
| FACTB + 4 (AC) | 21.80 (5.29) | 17.80 (5.16) | 0.045* |
| FACTB + 4 (total) | 101.87 (14.94) | 82.93 (14.23) | 0.001* |
| FACTB + 4 (arm subscale) | 16.33 (4.54) | 12.06 (4.47) | 0.015* |
Abbreviations: AC, additional concerns; DASH, Disabilities of Arm, Shoulder and Hand; FACTB + 4 Functional Assessment of Cancer Therapy – Breast plus Arm Morbidity; SD, Standard deviation.
Note: *Significance level of 5% (p < 0.05).
Discussion
The correlation found between the DASH and the total score of the FACTB + 4 was -0.634, and the one between the DASH and the physical domain of the FACTB + 4 was -0.720. These findings indicate a moderate correlation among the variables. Still, a strong correlation (r = -0.829) between the arm subscale of the FACTB + 4 and the DASH was observed, with a coefficient of determination indicating that 68.7% of the score variability on the FACTB + 4 arm subscale is related to the variability of the DASH.
The strong correlation between the DASH and the FACTB + 4 arm subscale, and the moderate correlation between the DASH and the physical domain of the FACTB + 4 could be explained by the fact that both are related to physical aspects and symptoms such as pain, edema, reduced ROM, rigidity and paresthesia of the ipsilateral arm. According to a study conducted by Fernandes,29 the DASH does not correlate with instruments of distinct concepts. Thus, when a correlation is performed between the DASH score and the total score of the FACTB + 4 (which presents domains not related to physical function, such as emotional well-being and family/social well-being), there is a decrease in the magnitude of the correlation.
The subjects presented, on average, moderate upper limb dysfunction (41.3 points) according to the DASH, and decreased quality of life (92.04 points) according to the FACTB + 4. These results are similar to those of other studies17 30 31 that showed a significant correlation between the morbidity of the arm and reduced quality of life after BC treatment. The present study also confirms the findings of the study conducted by Fangel et al,12 which showed a moderate correlation between the physical domain on the 30-item European Organization for Research and Treatment of Cancer Core Quality of Life Questionnaire ( EORTC-QLQ-C30) and function.
In agreement with other study findings,32 but diverging from other authors20 as well, when the questionnaire scores were analyzed according to the type of surgery (conservative or non-conservative), there was no significant difference. This could be explained by the fact that the extent of the procedure (with or without lymph node dissection) could have more influence on comorbidities than the type of surgery itself.32 33 The presence of positive lymph nodes indicates more aggressive surgical procedures, systemic therapy and longer radiation treatment, which contributes to increased functional impairment.34
On the other hand, when the comparison is performed considering the presence or absence of pain on the ipsilateral arm, there was a significant difference on the total score of the FACTB + 4 (101.87 no pain; 82.93 with pain) and, above all, on the DASH (25.87 no pain; 56.19 with pain). Some studies mention pain as the most incident comorbidity directly related to the worsening of upper extremity function and/or a worse quality of life.5 16 33 34 The onset of pain may occur immediately after surgery, or it may occur as a consequence of radiation, 5 and the pain can endure for a long period of time. The intercostobrachial nerve lesion is considered the main cause of pain,33 and some other causes may be myofascial pain syndrome and axillary cord.8 35 Milder symptoms, such as pain and paresthesia, are common between two and five years after the axillary lymphadenectomy.36
The present study showed a lower percentage of comorbidities (pain, adherence, nerve lesion and lymphedema) related to BC treatment compared with those found in the literature.25 One of the main reasons for this finding is that most studies focus on comorbidities up to two years post-surgery,9 17 34 37 and rarely on the data about the prevalence of comorbidities after five or more years.25 36
Apart from the surgical treatment, another important factor associated with upper limb morbidities is radiation. Radiation causes adverse effects such as pain, fatigue, fibrosis, sensitive changes and cutaneous impairment, like radiodermititis.5 19 Sensitivity changes are associated with the intercostobrachial nerve lesion caused during the surgical procedure, or caused by other therapies, such as radiation.32
Sensorial damage and pain may influence the accomplishment of functional tasks due to the inhibitory muscle effect.38 Levy et al34 suggested that pain, sensitivity changes, fatigue and weakness may coexist and have a significant cumulative effect, contributing to long-term functional morbidity. Furthermore, changes on the axillary region due to the dissection of the lymph nodes, as well as scar retraction, tissue fibrosis, stiffness, thoracic muscle hypotrophy and hypomobility contribute to decreased shoulder ROM and function.38 39 According to Fangel et al,12 the dysfunctions resulting from BC treatment lead to changes in the routines of the patients, at the family and professional levels, which may affect their self-esteem and, consequently, their quality of life.
Many symptoms and dysfunctions are not measured by the conventional clinical methods. The use of questionnaires completes this gap, for they contribute to the recognition of functional and emotional problems, aid in the description of a group and in the evaluation of the results of an intervention, and that refines the clinical results.14 40 Moreover, questionnaires are low-cost, convenient and easy tools that allow the identification of the needs of the patients; they also assist the physical therapist in terms of rehabilitation planning and selection of the adequate therapies.12
Even though the sample size was not calculated in advance, the power of all correlations with α = 0.05 was 95%.41
The main limitation of the present study was the lack of follow-up during the five-year survival, which would enable a yearly report of the quality of life and function in this group. Also, the results refer to a group treated at a highly recognized facility, which may not reflect the general population.
Conclusion
There was a moderate negative correlation between the total scores of the DASH and FACTB + 4 questionnaires and a strong correlation between the DASH and the arm subscale of the FACT + 4.
The participants presented on average regular function based on the DASH, and decreased quality of life based on the FACTB + 4. Those who presented pain had worse ipsilateral upper limb function and worse quality of life compared with the participants with no pain. There was no significant difference between groups when considering the type of surgery performed.
Acknowledgments
The study was conducted at Universidade do Estado de Santa Catarina, Maternidade Carmela Dutra (Florianópolis), with funds from Programa de Educação pelo Trabalho para a Saúde/Vigilância em Saúde (PET/VS) – 2013/2015, of the Brazilian Health and Education Ministries.
References
- 1.World Health Organization [Internet]. Breast cancer: prevention and control 2014. [cited 2014 Oct 26]. Available from: <http://www.who.int/cancer/detection/breastcancer/en/>
- 2.Torre L A, Bray F, Siegel R L, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65(02):87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 3. Brasil. Ministério da Saúde. Instituto Nacional de Câncer José Alencar Gomes da Silva (INCA) [Internet]. Tipos de câncer: mama. 2014. [citado 2014 Out 08]. Disponível em: <http://www2.inca.gov.br/wps/wcm/connect/tiposdecancer/site/home/mama/cancer_mama>
- 4. Brasil. Ministério da Saúde. Instituto Nacional de Câncer José Alencar Gomes da Silva. Coordenação de Prevenção e Vigilância [Internet] Estimativa 2014: incidência de câncer no Brasil. Rio de Janeiro: INCA; 2014. [citado 2014 Out 26]. Disponível em: http://www.saude.sp.gov.br/resources/ses/perfil/gestor/homepage/outros-destaques /estimativa-de-incidencia-de-cancer-2014/estimativa_cancer_24042014.pdf
- 5.Bezerra T S, Rett M T, Mendonça A CR, Santos D E, Prado V M, De Santana J M. Hipoestesia, dor e incapacidade no membro superior após radioterapia adjuvante no tratamento para câncer de mama. Rev Dor. 2012;13(04):320–326. [Google Scholar]
- 6.Assis M R, Marx A G, Magna L A, Ferrigno I SV. Late morbidity in upper limb function and quality of life in women after breast cancer surgery. Braz J Phys Ther. 2013;17(03):236–243. doi: 10.1590/s1413-35552012005000088. [DOI] [PubMed] [Google Scholar]
- 7.Nascimento S L, Oliveira R R, Oliveira M MF, Amaral M TP. Complicações e condutas fisioterapêuticas após cirurgia por câncer de mama: estudo retrospectivo. Fisioter Pesqui. 2012;19(03):248–255. [Google Scholar]
- 8.Yeung W M, McPhail S M, Kuys S S. A systematic review of axillary web syndrome (AWS) J Cancer Surviv. 2015;9(04):576–598. doi: 10.1007/s11764-015-0435-1. [DOI] [PubMed] [Google Scholar]
- 9.Barranger E, Dubernard G, Fleurence J, Antoine M, Darai E, Uzan S. Subjective morbidity and quality of life after sentinel node biopsy and axillary lymph node dissection for breast cancer. J Surg Oncol. 2005;92(01):17–22. doi: 10.1002/jso.20343. [DOI] [PubMed] [Google Scholar]
- 10.Schreiber K L, Martel M O, Shnol H et al. Persistent pain in postmastectomy patients: comparison of psychophysical, medical, surgical, and psychosocial characteristics between patients with and without pain. Pain. 2013;154(05):660–668. doi: 10.1016/j.pain.2012.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Organização Mundial da Saúde. Direção Geral da Saúde [Internet] Classificação internacional de funcionalidade, incapacidade e saúde. Lisboa; 2004. [citado 2014 Out 10]. Disponível em: <http://www.inr.pt/uploads/docs/cif/CIF_port_%202004.pdf>
- 12.Fangel L MV, Panobianco M S, Kebbe L M, Almeida A M, Gozzo T O.Qualidade de vida e desempenho de atividades cotidianas após tratamento das neoplasias mamárias Acta Paul Enferm 2013260193–100.pp. [Google Scholar]
- 13.Lotti R CB, Barra A A, Dias R C, Makluf A SD. Impacto do tratamento de câncer de mama na qualidade de vida. Rev Bras Cancerol. 2008;54(04):367–371. [Google Scholar]
- 14.Majewski J M, Lopes A DF, Davoglio T, Leite J CC. Quality of life of women recovering from breast cancer after being subjected to mastectomies compared with those who had conservative surgery: a review of the literature. Cien Saude Colet. 2012;17(03):707–716. doi: 10.1590/s1413-81232012000300017. [DOI] [PubMed] [Google Scholar]
- 15.Ganz P A. Assessing the quality and value of quality-of-life measurement in breast cancer clinical trials. J Natl Cancer Inst. 2011;103(03):196–199. doi: 10.1093/jnci/djq542. [DOI] [PubMed] [Google Scholar]
- 16.Velloso F SB, Barra A A, Dias R C. Functional performance of upper limb and quality of life after sentinel lymph node biopsy of breast cancer. Rev Bras Fisioter. 2011;15(02):146–153. doi: 10.1590/s1413-35552011000200010. [DOI] [PubMed] [Google Scholar]
- 17.Rietman J S, Geertzen J HB, Hoekstra H J et al. Long term treatment related upper limb morbidity and quality of life after sentinel lymph node biopsy for stage I or II breast cancer. Eur J Surg Oncol. 2006;32(02):148–152. doi: 10.1016/j.ejso.2005.11.008. [DOI] [PubMed] [Google Scholar]
- 18.Nascimento de Carvalho F, Bergmann A, Koifman R J. Functionality in women with breast cancer: the use of International Classification of Functioning, Disability and Health (ICF) in clinical practice. J Phys Ther Sci. 2014;26(05):721–730. doi: 10.1589/jpts.26.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang E J, Park W B, Seo K S, Kim S W, Heo C Y, Lim J Y. Longitudinal change of treatment-related upper limb dysfunction and its impact on late dysfunction in breast cancer survivors: a prospective cohort study. J Surg Oncol. 2010;101(01):84–91. doi: 10.1002/jso.21435. [DOI] [PubMed] [Google Scholar]
- 20.Arndt V, Stegmaier C, Ziegler H, Brenner H. Quality of life over 5 years in women with breast cancer after breast-conserving therapy versus mastectomy: a population-based study. J Cancer Res Clin Oncol. 2008;134(12):1311–1318. doi: 10.1007/s00432-008-0418-y. [DOI] [PubMed] [Google Scholar]
- 21.Miller A J, Bruna J, Beninson J. A universally applicable clinical classification of lymphedema. Angiology. 1999;50(03):189–192. doi: 10.1177/000331979905000302. [DOI] [PubMed] [Google Scholar]
- 22.Castro C ES. São Carlos: Universidade Federal de São Carlos; 1999. A formulação linguística da dor: versão brasileira do questionário McGill de dor [dissertação] [Google Scholar]
- 23. World Health Organization. Obesity: preventing and managing the global epidemic: Report of a World Health Organization Consultation Geneva: WHO; 2000. (WHO Obesity Technical Report Series, 284). [PubMed] [Google Scholar]
- 24.Orfale A G, Araújo P MP, Ferraz M B, Natour J. Translation into Brazilian Portuguese, cultural adaptation and evaluation of the reliability of the Disabilities of the Arm, Shoulder and Hand Questionnaire. Braz J Med Biol Res. 2005;38(02):293–302. doi: 10.1590/s0100-879x2005000200018. [DOI] [PubMed] [Google Scholar]
- 25.Paim C R. Belo Horizonte: Universidade Federal de Minas Gerais; 2008. Complicações e qualidade de vida em pacientes submetidas a biopsia de linfonodo sentinela ou a linfadenectomia axilar no câncer de mama [dissertação] [Google Scholar]
- 26.Thomas-Maclean R L, Hack T, Kwan W, Towers A, Miedema B, Tilley A. Arm morbidity and disability after breast cancer: new directions for care. Oncol Nurs Forum. 2008;35(01):65–71. doi: 10.1188/08.ONF.65-71. [DOI] [PubMed] [Google Scholar]
- 27.Michels F AS, Latorre M RDO, Maciel M S. Validação e reprodutibilidade do questionário FACT-B+4 de qualidade de vida específico para câncer de mama e comparação dos questionários IBCSG, EORTC-BR23 e FACT-B+4. Cad Saude Colet. 2012;20(03):321–328. [Google Scholar]
- 28.Oliveira I S, Costa L CM, Manzoni A CT, Cabral C MN. Assessment of the measurement properties of quality of life questionnaires in Brazilian women with breast cancer. Braz J Phys Ther. 2014;18(04):372–383. doi: 10.1590/bjpt-rbf.2014.0045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fernandes M R. Correlation between functional disability and quality of life in patients with adhesive capsulitis. Acta Ortop Bras. 2015;23(02):81–84. doi: 10.1590/1413-78522015230200791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Góis M C, Furtado P R, Ribeiro S O, Lisboa L L, Viana E SR, Micussi M TABC.Amplitude de movimento e medida de independência funcional em pacientes mastectomizadas com linfadenectomia axilar Rev Ciênc Méd (Campinas) 201221(1–6):111–118. [Google Scholar]
- 31.Hayes S C, Johansson K, Stout N Let al. Upper-body morbidity after breast cancer: incidence and evidence for evaluation, prevention, and management within a prospective surveillance model of care Cancer 2012118(8, Suppl)2237–2249. [DOI] [PubMed] [Google Scholar]
- 32.Albuquerque V T, Bezerra L MA, D'Oliveira G DF, Melo G F. Goiânia: Pontifícia Universidade Católica de Goiás; 2013. Funcionalidade de membros superiores em mulheres após cirurgia para câncer de mama [monografia]. . [Google Scholar]
- 33.Lahoz M A, Nyssen S M, Correia G N, Garcia A PU, Driusso P. Capacidade funcional e qualidade de vida em mulheres pós-mastectomizadas. Rev Bras Cancerol. 2010;56(04):423–430. [Google Scholar]
- 34.Levy E W, Pfalzer L A, Danoff J et al. Predictors of functional shoulder recovery at 1 and 12 months after breast cancer surgery. Breast Cancer Res Treat. 2012;134(01):315–324. doi: 10.1007/s10549-012-2061-1. [DOI] [PubMed] [Google Scholar]
- 35.Stubblefield M D, Keole N. Upper body pain and functional disorders in patients with breast cancer. PM R. 2014;6(02):170–183. doi: 10.1016/j.pmrj.2013.08.605. [DOI] [PubMed] [Google Scholar]
- 36.Warmuth M A, Bowen G, Prosnitz L R et al. Complications of axillary lymph node dissection for carcinoma of the breast: a report based on a patient survey. Cancer. 1998;83(07):1362–1368. doi: 10.1002/(sici)1097-0142(19981001)83:7<1362::aid-cncr13>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 37.Kootstra J, Hoekstra-Weebers J EHM, Rietman H et al. Quality of life after sentinel lymph node biopsy or axillary lymph node dissection in stage I/II breast cancer patients: a prospective longitudinal study. Ann Surg Oncol. 2008;15(09):2533–2541. doi: 10.1245/s10434-008-9996-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Borstad J D, Szucs K A. Three-dimensional scapula kinematics and shoulder function examined before and after surgical treatment for breast cancer. Hum Mov Sci. 2012;31(02):408–418. doi: 10.1016/j.humov.2011.04.002. [DOI] [PubMed] [Google Scholar]
- 39.Camargo M C, Marx A G. São Paulo: Roca; 2000. Reabilitação física no câncer de mama. [Google Scholar]
- 40.Osoba D. Health-related quality of life and cancer clinical trials. Ther Adv Med Oncol. 2011;3(02):57–71. doi: 10.1177/1758834010395342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hulley S B, Cummings S R, Browner W S, Grady D G. Porto Alegre: Art Med; 2015. Delineando a pesquisa clínica. 4a ed. [Google Scholar]


