Abstract
Introduction The placenta, translates how the fetus experiences the maternal environment and is a principal influence on birth weight (BW).
Objective To explore the relationship between placental growth measures (PGMs) and BW in a public maternity hospital.
Methods Observational retrospective study of 870 singleton live born infants at Hospital Maternidad Sardá, Universidad de Buenos Aires, Argentina, between January 2011 and August 2012 with complete data of PGMs. Details of history, clinical and obstetrical maternal data, labor and delivery and neonatal outcome data, including placental measures derived from the records, were evaluated. The following manual measurements of the placenta according to standard methods were performed: placental weight (PW, g), larger and smaller diameters (cm), eccentricity, width (cm), shape, area (cm2), BW/PW ratio (BPR) and PW/BW ratio (PBR), and efficiency. Associations between BW and PGMs were examined using multiple linear regression.
Results Birth weight was correlated with placental weight (R2 = 0.49, p < 0.001), whereas gestational age was moderately correlated with placental weight (R2 = 0.64, p < 0.001). By gestational age, there was a positive trend for PW and BPR, but an inverse relationship with PBR (p < 0.001). Placental weight alone accounted for 49% of birth weight variability (p < 0,001), whereas all PGMs accounted for 52% (p < 0,001). Combined, PGMs, maternal characteristics (parity, pre-eclampsia, tobacco use), gestational age and gender explained 77.8% of BW variations (p < 0,001). Among preterm births, 59% of BW variances were accounted for by PGMs, compared with 44% at term. All placental measures except BPR were consistently higher in females than in males, which was also not significant. Indices of placental efficiency showed weakly clinical relevance.
Conclusions Reliable measures of placental growth estimate 53.6% of BW variances and project this outcome to a greater degree in preterm births than at term. These findings would contribute to the understanding of the maternal–placental programming of chronic diseases.
Keywords: Placenta, placental weight, placental characteristics, birth weight, gestational age
Abstract
Resumo
Introdução A placenta traduz como o feto experimenta o ambiente materno, além de ser a principal influência sobre o peso ao nascer (PN).
Objetivo Explorar a relação entre medidas de crescimento da placenta (MCPs) e PN em uma maternidade pública.
Métodos Estudo retrospectivo observacional de 870 recém-nascidos vivos únicos na Maternidade Sardá, Universidade de Buenos Aires, Argentina, entre janeiro de 2011 e agosto de 2012 com os dados completos das MCPs. Foram avaliados dados da história clínica e obstétrica materna, trabalho de parto e resultados neonatais, incluindo medidas da placenta derivadas dos registros médicos. Foram realizadas as seguintes medições manuais da placenta: peso da placenta (PP, g), diâmetros maior e menor (cm), excentricidade, espessura (cm), forma, área (cm2), razões PN/PP e PP/PN e eficiência. Associações entre PN e MCPs foram examinadas por meio de regressão linear múltipla.
Resultados Peso ao nascer foi correlacionado com peso placentário (R2 = 0,49, p < 0,001), enquanto idade gestacional foi moderadamente correlacionada com peso placentário (R2 = 0,64, p < 0,001). Por idade gestacional, houve uma tendência positiva para a relação PP e PN/PP, mas uma relação inversa com a razão PP/PN (p < 0,001). Somente peso da placenta respondeu por 49% da variabilidade do peso ao nascer (p < 0,001), ao passo que todas as MCPs foram responsáveis por 52% (p < 0,001). Combinados, MCPs, características maternas (paridade, pré-eclâmpsia, fumo), idade gestacional e sexo explicaram 77,8% da variação do peso ao nascer (p < 0,001). Entre nascimentos pré-termo, 59% da variância do PN foi contabilizada pelas MCPs, em comparação com 44% a termo. Todas as medidas placentárias, exceto a razão PN/PP, foram consistentemente maiores em mulheres do que em homens, mas não significativas. Índices de eficiência placentária mostraram fraca relevância clínica.
Conclusões medidas confiáveis de crescimento placentário estimam 53,6% da variância do peso ao nascer, e projetam esse resultado a um maior grau em nascimentos pré-termo do que a termo. Estes resultados contribuiriam para a compreensão da programação materno-placentária de doenças crónicas.
Palavras-chave: Placenta, peso da placenta, características da placenta, peso ao nascer, idade gestacional
Introduction
Genetic, environmental and socioeconomic factors1 2 influence birth weight (BW), as well as illnesses that occur during pregnancy, such as infections, hypertensive disorders and diabetes mellitus.3
The placenta, to a large degree, translates how the fetus experiences the maternal environment, and genetic influences aside, is the main factor to influence BW. The growth of the placenta is directly related to its functional efficiency as the only fetal source of both nutrients and oxygen. Placental weight (PW) is one way to characterize placental size, but it is a collection of many dimensions of growth, including its surface area and its width.
The placental surface area reflects the laterally expanding growth of the chorionic disc (measured by disc shape and chorionic disc diameters), whereas disc width reflects the arborization of the villous and vascular nutrient exchange surface. Placental weight and other placental growth measures (PGMs: larger and smaller diameters, disc width) are routinely collected in pathology laboratories across the world, but limited data regarding these placental dimensions in the Latin American population are available.
These PGMs were designed to capture different aspects of the placenta that, first of all, relate to placental function, theoretically influencing BW by different mechanisms; secondly, PGMs are conventionally considered to have different ‘critical periods of development’,4 and, finally, they are most relevant to the ‘fetal origins’ hypothesis, which posits that a wide variety of lifelong health risks are influenced by the fetal experience.
Additionally, PW and other PGMs were found to be predictive of maternal disease, obstetric outcome, perinatal morbidity and mortality and childhood growth and development.5
The aim of this study was to explore the relationships of PGMs with BW in a Latin American population.
Methods
This was an observational, retrospective study on 875 singleton live born infants at the Department of Obstetrics and Gynecology of Hospital Maternidad Sardá, Universidad of Buenos Aires, Argentina, between January 2011 and August 2012 with complete data for placental measures. Details of history, clinical and obstetrical maternal data, labor and delivery and neonatal outcome data, including placental measures derived from the records, were evaluated. Exclusion criteria were gestational age (GA) less than 22+0 weeks and over 42+6 weeks, twin gestation, PW lower than 100 g or higher than 2,500 g.
Chronic hypertension was defined as the presence of elevated blood pressure (> 140/90 mm Hg) before pregnancy or before the 20th week of gestation. Preeclampsia was defined as hypertension presenting beyond 20 weeks of gestation with > 300 mg protein in a 24-hour urine collection. Eclampsia was defined as the occurrence of seizures in a pregnant woman with preeclampsia.6 Women were diagnosed as having gestational diabetes according to the criteria of the World Health Organization (WHO) protocol.7 The diagnosis of clinical chorioamnionitis was based on a maternal temperature of > 38°C with or without uterine tenderness and/or foul vaginal discharge with no other cause of fever. Gestational age was ascertained by menstrual dates and early ultrasound measurements. Small for gestational age was defined when the BW for GA and sex were less than the ten percentile according to a local intrauterine growth chart.8
Preparation of the Placenta
After delivery, the placenta was placed in a self-sealing plastic bag and stored in a refrigerator; the date and time of birth were added to the label.9 A series of manual measurements of the placenta's gross structure were then made in accordance with standard methods used in pathology examinations.10
Placental weights (g) were taken using an electronic scale; cord and membranes were cut before weighing. Leary et al suggested that the fetal weight/placental weight correlation does not change when placentas are weighed trimmed compared when they are weighed untrimmed.11 The longest diameter of the surface (length) was measured using a transparent plastic ruler placed on the surface and recorded in centimeters. The diameter perpendicular to the length was defined as the smallest diameter, and was measured in the same way. Chorionic plate eccentricity was calculated as the ratio of the largest to the smallest diameters of the chorionic plate. Eccentricity measures the relative asymmetry of normal placental growth, and may mark the uterine constraint of placental growth. Placental width at the center of the chorionic disc was measured by piercing the disc with a needle onto which millimeter marks were inscribed, and analyzed in units of 0.1 cm.12 Placental shape was defined as the difference between the larger and smaller diameters: oval placenta ≥ 3 cm; round placenta < 3 cm.13 Placental area (cm2) was calculated assuming an elliptical surface, using the formula length x breadth x π/4.14 The placental weight (g) to birth weight (g)ratio ([PW/BW], PBR) , a proxy for placental efficiency (“efficiency 1”), was calculated as a percentage ([PW/BW] x 100).15 The birth weight (g) to placental weight (g) ratio ([BW/PW], BPR) has been used to indicate fetal nutrition adequacy.16 Finally, 2 other measures of placental efficiency were calculated using the ratios of length (“efficiency 2”) and breadth (“efficiency 3”) to BW.13
The placental measurements were obtained blinded from pregnancy and childbirth data.
Statistical Analysis
Sexual dimorphism and differences between term and preterm were explored for all placental measures. As the PGMs are all measures of the same organ, they are necessarily interdependent. As there was no strong correlation among the PGMs (data not shown), unadjusted and multiple linear regression techniques explored the associations of PGMs to BW, and present the point estimates of effects with 95% confidence intervals (95%CI) and R-squared.
For the regression models, the following criteria were predefined. Firstly, when a PGM included the dependent variable (BW) as well as any other predictors, it was excluded from the adjusted models. Secondly, the goodness of fit of each regression model was carefully assessed according to Royston and Wright.17 This resulted in four placental predictors (placental weight, largest and smallest diameters and disc width). Thirdly, three models were predefined: a)unadjusted included each PGMs; b) model 1 adjusted for all PGMs, and c) the full model included PGMs, maternal characteristics (gestational age [weeks], parity, pre-eclampsia, tobacco use) and infant gender.
A sample size of 47 subjects to obtain a statistical power of 80% with α set at 0.05 and an expected adjusted coefficient of determination of 0.40 was calculated (as this seemed to possibly reveal a clinically relevant prediction of BW).
The Stata 12 software (College Station, Texas, USA) was used for all statistical calculations, and significance was set at a 0.05 level.
The study was submitted and approved by the Research Ethics Committee of Hospital Maternidad Sardá.
Results
None of the exclusion criteria were applied, leaving a final analytical sample of 870 mothers and their corresponding placentas for analysis. The mean maternal age was 24 years (range 13–45 years); the level of education, 10.1 years (standard deviation, SD, 3.0 years); primiparity, 24.5%; smoking during pregnancy, 12.6%; gestational diabetes, 4.9%; chronic hypertension, 3.3%; pre-eclampsia, 8.7%; eclampsia, 0.9%; clinical chorioamnionitis, 7.9%; and fetal growth restriction, 13.0%. Infants' characteristics included male gender (55.3%), a mean GA at birth of 35.6 weeks (22.2–42.5 weeks), and a mean BW of 2,581 g (380–4,860 g); 6.3% (n = 55) of the infants were born at ≤ 28 gestational weeks; 8.7% (n = 76) at 29–32 weeks; 36.6% (n = 319) at 33–36 weeks; and 48.2% (n = 420) at ≥ 37 weeks. Small for gestational age represented 18.8% of the sample, and it was greater for term (31.2%) than preterm neonates (8.3%), whereas congenital anomalies were present in 7.1%. The elective cesarean section rate was of 40.7%, and the vaginal birth rate, 43.2%; the remainder was induced labor.
A description of the placental variables is presented in Table 1. Birth weight was correlated with PW (r = 0.70, R2 = 0.49, p < 0.001), but there was a wide variation in PW for any given BW, suggesting that there are large differences in placental efficiency. For example, babies weighing around 3,000 g had placentas ranging from 300 to 700 g in weight. (Fig. 1) Birth weight was weakly correlated with other placental measures, ranging from -0.11 (efficiency 2) to 0.47 for the chorionic plate area (all p < 0.001).
Table 1. Placental characteristics.
| Characteristic | na | Mean | SD | Range |
|---|---|---|---|---|
| Weight (g) | 870 | 432 | 120 | 110–838 |
| Gestational age (w): | ||||
| 22–28 | 55 | 266 | 81.9** | |
| 29–32 | 76 | 350 | 90.5 | |
| 33–36 | 319 | 428 | 101.4 | |
| 37–42 | 420 | 474 | 115.6 | |
| Weight z scoreb | 870 | −1.1 | 0.9 | −5.0–2.1 |
| Term neonates | 420 | −1.4 | 0.9 | −5.0–1.3* |
| Preterm neonates | 450 | −0.9 | 0.8 | −4.3–2.1* |
| Largest diameter of placenta (cm) | 862 | 16.6 | 2.5 | 5.0–29.0 |
| Smallest diameter of placenta (cm) | 862 | 12.4 | 2.9 | 3.0–23.0 |
| Chorionic plate eccentricityc | 862 | 1.4 | 0.4 | 0.8–5.6 |
| Disc thickness (cm) | 820 | 3.0 | 0.6 | 1.0–7.0 |
| Chorionic plate area (cm2) | 862 | 164.8 | 55.8 | 15.7–397 |
| Placental shape | 870 | 4.1 | 2.6 | −2–18 |
| Oval (≥ 3 cm): 612/870 (70.4%) | ||||
| Round (< 3): 258/870 (29.6%) | ||||
| BPR | 870 | 5.9 | 1.5 | 2.1–23.5 |
| Term neonates | 420 | 6.6 | 1.4 | 2.2–23.5* |
| Preterm neonates | 450 | 5.4 | 1.3 | 2.1–10.8* |
| Gestational age (w): | ||||
| 22–28 | 55 | 3.7 | 1.0** | |
| 29–32 | 76 | 4.8 | 0.9 | |
| 33–36 | 319 | 5.8 | 1.1 | |
| 37–42 | 420 | 6.6 | 1.5 | |
| PBR | 870 | 17.6 | 5.1 | 4.2 - 46.0 |
| Term neonates | 420 | 15.5 | 3.5 | 4.1–44.6* |
| Preterm neonates | 450 | 19.5 | 5.6 | 9.1–46.0* |
| Gestational age (w): | ||||
| 22–28 | 55 | 28.4 | 6.8** | |
| 29–32 | 76 | 21.5 | 4.3 | |
| 33–36 | 319 | 17.5 | 3.7 | |
| 37–42 | 420 | 15.5 | 3.5 | |
| Efficiency 1d | 866 | 176.8 | 50.4 | 42.5–460 |
| Efficiency 2d | 855 | 7.1 | 2.9 | 1.4–31.5 |
| Efficiency 3d | 815 | 1.3 | 0.6 | 0.4–6.4 |
Abbreviations: BPR, birth weight to placental weight ratio; PBR, placental weight to birth weight ratio; SD, standard deviation; w, weeks.
The denominator, when specified, indicates that there were some missing values.
Source: Thompson et al., 2007. There were no available values for 22–23 weeks of gestational age.
Ratio of larger to smaller diameter.
Ratio of placental weight (1), length (2) and breadth (3) to birth weight (efficiency).
t-test, p < 0.001.
ANOVA, p < 0.001.
Fig. 1.
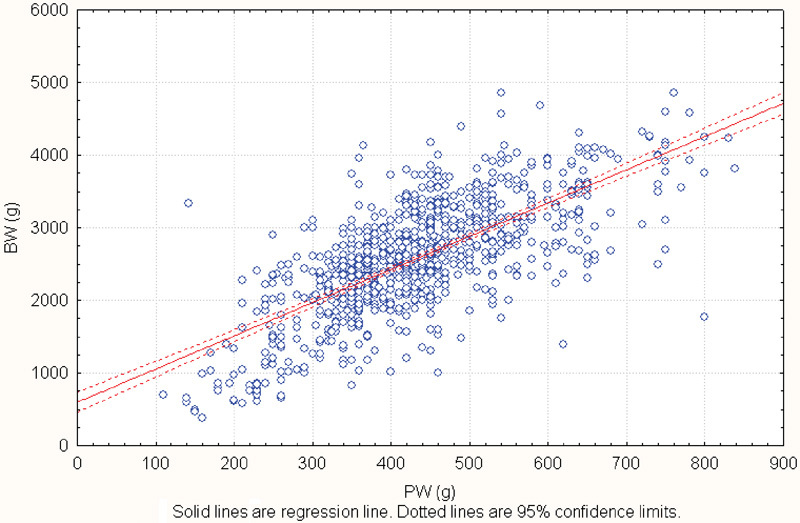
Birth weight according to placental weight. r = 0.704 (p < 0.001), BW = 569.63 + 4.57*PW. Abbreviations: BW, birth weight; PW, placental weight.
Gestational age was strongly correlated with PW (r = 0.80, R2 = 0.64, p < 0.001), but there was a wide variation in PW for any given GA For example: babies around 36 weeks had placentas ranging from 200 to 700 g in weight. Combined, PW and GA reached 76.8% of BW variability (p < 0.001).
Significant differences between term and preterm placentas were only observed for weight z score, BPR and PBR (data not shown). When examined by GA intervals, there was a positive trend for BPR (Fig. 2), but an inverse relationship with PBR (data not shown, all p < 0.001). Two-thirds of placental shapes were oval. Furthermore, all placental measures except BPR were consistently higher in females than in males, which was also not significant.
Fig. 2.
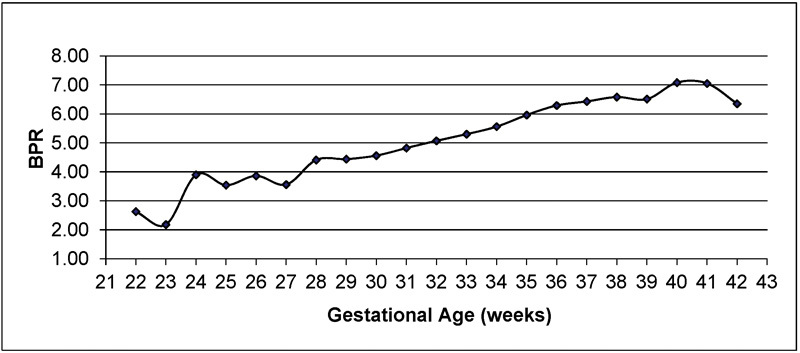
Birth weight to placental weight ratio (BPR) according to gestational age. Mean BPR increases with gestational age. Results were obtained from all placentas submitted to the Department of Pathology at Hospital Maternidad Sardá in 2011–2012. Data are similar to those of a previously published study (Benirschke, Kaufmann; 2000).4 There appear to be three tiers of significantly increasing workload (relatively increased fetal demand based on weight). The interval between 28–37 weeks of gestation is the most pronounced, followed by a plateau until 39 weeks, and then another increase until 41 weeks of gestation.
Comparing univariable and multivariable point estimates of effect (Table 2) revealed important results: R-squared values increased steadily from the unadjusted model (5–49%) to 52% in the four placental growth models, whereas the multivariable model, including the maternal characteristics, GA, sex and the PGMs, accounted for 78% of BW variability (regression equation: BW (g) = −3186.667 + 2.47*PW + 14.9*largest diameter + 5.52*smallest diameter + 17.19* disc width [p < 0.001]). In the full model, only placental weight and largest diameter retained an independent effect. Additionally, when excluding smallest diameter and disc width from the model, there was a minimal change in the adjusted R-squared value.
Table 2. Point estimates of effects for regression model of placental measures predicting birth weight (g).
| Unadjusted models | Multivariate models# | |||||
|---|---|---|---|---|---|---|
| Point estimate (95%CI) |
R-squared | Model 1a | Full Modelb | |||
| Point estimate (95%CI) |
Adjusted R-squared |
Point estimate (95%CI) |
Adjusted R-squared |
|||
| Placental weight (g) | 4.5 (4.2–4.8) |
0.49*** | 3.8 (3.4–4.3)*** |
0.52*** | 2.4 (2.1– 2.7)*** |
0.78*** |
| Largest diameter (cm) | 142.4 (1237–161.1) |
0.21*** | 23.3 (3.4–43.2)** |
16.4 (2.7– 30.9)** |
||
| Smallest diameter (cm) | 115.8 (99.0–132.6) |
0.18*** | 27.8 (11.6–44.1)*** |
5.1 (−6.0–16.3) |
||
| Disc width (cm) | 299.1 (210.7–387.5) |
0.05*** | 61.3 (−7.9–130.5) |
17.1 (−30.7–65.1) |
||
Abbreviation: 95%CI, 95% confidence interval.
Predictors include placental measures (placental weight, largest diameter, smallest diameter and disc width), gestational age (weeks), parity, pre-eclampsia, tobacco use and infant gender.
Model 1: adjusted for all placental measures (no adjustments for maternal variables, gestational age and sex).
Full Model: adjusted for all placental measures, maternal variables, gestational age and sex.
Significant at the 0.05 level.
Significant at the 0.001 level.
For preterm and term neonates, placental weight alone accounted for 52.5% and 37.4% of BW variances respectively (p < 0.001).
Table 3 shows regression models of placental measures predicting BW according to GA. Overall, an inverse relationship between GA and the adjusted R-squared of all placental measures were observed; the adjusted R-squared for the PGMs were smaller at or over 33 weeks of gestation compared with < 33 weeks.
Table 3. Point estimates of effects for regression model of placental measures predicting birth weight (g) according to gestational age.
| Measure | Gestational age | |||||
|---|---|---|---|---|---|---|
| ≤ 32 w (n = 131) | 33–36 w (n = 319) | 37–42 w (n = 420) | ||||
| Point estimate (95%CI) |
Adjusted R-squared* |
Point estimate (95%CI) |
Adjusted R-squared* |
(95%CI) | Adjusted R-squared* |
|
| Placental weight (g) | 2.20 (1.59–2.82) |
0.84** | (1.72–2.62) | 0.53** | 2.65 (2.62–3.04) |
0.49** |
| Large diameter (cm) | −17.90 (−39.14–3.32) |
14.7 (−3.25–32.69) |
15.27 (−4.52–35.06) |
|||
| Small diameter (cm) | 19.55 (1.92–37.17) |
5.85 (−9.74–21.46) |
8.32 (−8.23–24.88) |
|||
| Disk width (cm) | −36.25 (−103.7–31.2) |
−14.31 (−50.83–22.20) |
−4.90 (−40.67–30.85) |
|||
Abbreviation: 95%CI, 95% confidence interval.
Predictors include placental measures, parity, preeclampsia, tobacco use and infant gender.
p < 0.001.
Discussion
To our knowledge, this is the most comprehensive description of PGMs and their relation to BW in a selected population reported to date in Latin America, and it may provide a useful guide for future analyses of placental gross anatomy.
Although the BW of male neonates showed a marginal difference from that of females (58.3 g, p = 0.499), an opposite effect was observed for placental measures, suggesting that the growth of female placentas may not be the same as that of male placentas.
One hypothesis that explains this fact would be the “Feminine Eco-stability Hypothesis”, which holds that the female sex is less sensitive to external factors that modulate ontogenetic development; in contrast, the male sex would be more negatively affected by environmental factors.18
The ratio of fetal to placental weight (or its inverse) is used as an endpoint measure of placental nutrient transport efficiency across species.19 A small BPR was associated with higher odds of small for gestational age (SGA) infants, and may serve as an indicator suggestive of adverse intrauterine environment.20 Our BPR findings increased with GA, suggesting a more dynamic growth during prematurity, in agreement with a previous study.21
The PBR has been associated with health throughout the lifespan, and has been shown to decrease with GA.22 The present study confirms these results and reveals values in a comparable range with other studies.15 23 Moreover, an elevated PBR may be an expression of a relatively inefficient placenta with reduced ability to maintain fetal growth, and thus can be used as a predictor of hypertension in adulthood for assumed lack of “placental functional efficiency”.24
In our study, we also measured placental efficiency using the ratios of the length and breadth of the surface to BW in addition to the customary PBR.25 Our findings are in agreement with a previous study,13 but in an attempt to define the mechanisms for this from our data, a subgroup analysis was run; in a multiple regression analysis, all indices were weakly associated with several maternal characteristics, GA and sex. Only the ratio of the length to BW showed clinical relevance, indicated by the adjusted R-squared (62%, p < 0.001). This suggests that when placental growth across the major axis in early pregnancy is adequate, there is increased thickening of the placenta, reflecting a different aspect of the control of the placental exchange surface.
In all regression analyses, we focused on the adjusted R-squared rather than on the magnitude of the β coefficients, because R-squared changes would be unaffected by collinearity.
Surprisingly, our results showed a higher R-squared for prediction of BW compared with other studies, although it was methodologically different.26 27 28
After adjustment for maternal characteristics, GA and infant gender, 77% of BW variances were accounted for, suggesting ‘partial mediation’ of their effects on BW by the feature of the four placental measures.
Table 2 shows that only placental weight and largest diameter retained an independent effect on BW after adjustment for mother and infant conditions, in consonance with another study.26 Barker et al14 observed that placental growth along the major axis (a proxy of volume) is qualitatively different from growth along the minor axis, and postulated that the minor axis is more important for nutrient transfer to the fetus, partially explaining our results.
We speculate that one of the main reasons for the comparatively low explanatory power of smallest diameter and disc thickness is that these measures have limitations, and incompletely capture the variability that characterizes the human placenta.
The difference in prediction of BW using PGMs in preterm versus term (80.8% versus 49.8% respectively) denotes different abilities of PGMs to account for BW across pregnancy, and suggests that PGMs are more powerful at gestational ages when they are considered to be more dynamic, which occurs prior to the third trimester.4 In other words, PGMs will have their strongest associations with BW variances when they themselves are changing.
Chorionic plate measures complement the ability of PW to account for BW variances.
These novels PGMs are related to both GA and BW at delivery, but their relations are not identical. In the present study, PGMs accounted for 25.8% of GA variances and 52.7% of BW variances. One explanation might be that growth parameters naturally change across gestation, independent of any environmental effectors.12
These measures are highly reproducible, and may clarify the complex interrelations among mother, placenta and fetus to maintain pregnancy and support fetal growth. The reliance on R-squared is sufficient to demonstrate the power of these novel measures.
While sample size was relatively large, the placental examinations were performed at the request of attending physicians. Consequently, our study was derived from a convenience sample in which preterm birth, SGA and congenital anomalies were overrepresented. Therefore, our findings may not be generalized to the general population. However, they do closely mirror the hospital registry because they present similar demographic, clinical and obstetrical maternal data.
Moreover, these data are cross-sectional, and longitudinal studies would be necessary to validate the results. However, longitudinal studies of a cohort of placentas to register morphometry in real time throughout pregnancy are very difficult to perform; they could be developed using ultrasound or magnetic resonance imaging, considering that their estimates have an error of 10 to 20%.29 On the other hand, information on placental morphometry is rarely collected and evaluated in Latin America. These findings would contribute to the understanding of the maternal–placental programming of chronic disease.
As it was a retrospective analysis, a variety of health behaviors and environmental exposures has not been controlled for. Nevertheless, to ensure an accurate and complete report of this observational study, the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement was followed.30
In conclusion, we have demonstrated that 52% of the variability of BW may be accounted for by the effects of four simple placental measures. In association with well-appreciated maternal and infant influences, this figure raised to 78%.
References
- 1.England L J, Kendrick J S, Wilson H G, Merritt R K, Gargiullo P M, Zahniser S C. Effects of smoking reduction during pregnancy on the birth weight of term infants. Am J Epidemiol. 2001;154(8):694–701. doi: 10.1093/aje/154.8.694. [DOI] [PubMed] [Google Scholar]
- 2.Andersson S W, Niklasson A, Lapidus L, Hallberg L, Bengtsson C, Hulthén L. Sociodemographic characteristics influencing birth outcome in Sweden, 1908-1930. Birth variables in the Population Study of Women in Gothenburg. J Epidemiol Community Health. 2000;54(4):269–278. doi: 10.1136/jech.54.4.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ananth C V, Peedicayil A, Savitz D A. Effect of hypertensive diseases in pregnancy on birthweight, gestational duration, and small-for-gestational-age births. Epidemiology. 1995;6(4):391–395. doi: 10.1097/00001648-199507000-00011. [DOI] [PubMed] [Google Scholar]
- 4.Benirschke K Kaufmann P Placental shape aberrations New York: Springer-Verlag; 2000. p. 399–418. [Google Scholar]
- 5.Risnes K R, Romundstad P R, Nilsen T I, Eskild A, Vatten L J. Placental weight relative to birth weight and long-term cardiovascular mortality: findings from a cohort of 31,307 men and women. Am J Epidemiol. 2009;170(5):622–631. doi: 10.1093/aje/kwp182. [DOI] [PubMed] [Google Scholar]
- 6.Report of the National High Blood Pressure Education Program Working Group on High Blood Pressure in Pregnancy. Am J Obstet Gynecol. 2000;183(1):S1–S22. [PubMed] [Google Scholar]
- 7.World Health Organization . Geneva: WHO; 1999. Definition, diagnosis and classification of diabetes mellitus and its complications. Report of a WHO consultation. Part 1: Diagnosis and classification of diabetes mellitus. [Google Scholar]
- 8.Urquia M L Alazraqui M Spinelli H G Frank J W [Reference birthweights for the Argentine population by multiplicity of birth, sex, and gestational age] Rev Panam Salud Publica 2011292108–119. Spanish [PubMed] [Google Scholar]
- 9.Bendon R W Sander C M Examination of the placenta Philadelphia: Churchill Livingstone; 1999. p. 27–47. [Google Scholar]
- 10.Lewis S Benirschke K Overview of placental pathology and justification for examination of the placenta Philadelphia: Churchill Livingstone; 1999. p. 1–26. [Google Scholar]
- 11.Leary S D, Godfrey K M, Greenaway L J, Davill V A, Fall C H. Contribution of the umbilical cord and membranes to untrimmed placental weight. Placenta. 2003;24(2–3):276–278. doi: 10.1053/plac.2002.0888. [DOI] [PubMed] [Google Scholar]
- 12.Salafia C M, Maas E, Thorp J M, Eucker B, Pezzullo J C, Savitz D A. Measures of placental growth in relation to birth weight and gestational age. Am J Epidemiol. 2005;162(10):991–998. doi: 10.1093/aje/kwi305. [DOI] [PubMed] [Google Scholar]
- 13.Winder N R, Krishnaveni G V, Veena S R. et al. Mother's lifetime nutrition and the size, shape and efficiency of the placenta. Placenta. 2011;32(11):806–810. doi: 10.1016/j.placenta.2011.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barker D J, Thornburg K L, Osmond C, Kajantie E, Eriksson J G. The surface area of the placenta and hypertension in the offspring in later life. Int J Dev Biol. 2010;54(2–3):525–530. doi: 10.1387/ijdb.082760db. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Burkhardt T, Schäffer L, Schneider C, Zimmermann R, Kurmanavicius J. Reference values for the weight of freshly delivered term placentas and for placental weight-birth weight ratios. Eur J Obstet Gynecol Reprod Biol. 2006;128(1–2):248–252. doi: 10.1016/j.ejogrb.2005.10.032. [DOI] [PubMed] [Google Scholar]
- 16.Thame M, Osmond C, Bennett F, Wilks R, Forrester T. Fetal growth is directly related to maternal anthropometry and placental volume. Eur J Clin Nutr. 2004;58(6):894–900. doi: 10.1038/sj.ejcn.1601909. [DOI] [PubMed] [Google Scholar]
- 17.Royston P, Wright E M. Goodness-of-fit statistics for age-specific reference intervals. Stat Med. 2000;19(21):2943–2962. doi: 10.1002/1097-0258(20001115)19:21<2943::aid-sim559>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 18.Lampl M, Gotsch F, Kusanovic J P. et al. Sex differences in fetal growth responses to maternal height and weight. Am J Hum Biol. 2010;22(4):431–443. doi: 10.1002/ajhb.21014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fowden A L Sferruzzi-Perri A N Coan P M Constancia M Burton G J Placental efficiency and adaptation: endocrine regulation J Physiol 2009587(Pt 14):3459–3472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Luque-Fernandez M A, Ananth C V, Jaddoe V W. et al. Is the fetoplacental ratio a differential marker of fetal growth restriction in small for gestational age infants? Eur J Epidemiol. 2015;30(4):331–341. doi: 10.1007/s10654-015-9993-9. [DOI] [PubMed] [Google Scholar]
- 21.Wallace J M, Bhattacharya S, Horgan G W. Gestational age, gender and parity specific centile charts for placental weight for singleton deliveries in Aberdeen, UK. Placenta. 2013;34(3):269–274. doi: 10.1016/j.placenta.2012.12.007. [DOI] [PubMed] [Google Scholar]
- 22.Heinonen S, Taipale P, Saarikoski S. Weights of placentae from small-for-gestational age infants revisited. Placenta. 2001;22(5):399–404. doi: 10.1053/plac.2001.0630. [DOI] [PubMed] [Google Scholar]
- 23.Little W A. The significance of placental/fetal weight ratios. Am J Obstet Gynecol. 1960;79:134–137. doi: 10.1016/0002-9378(60)90372-0. [DOI] [PubMed] [Google Scholar]
- 24.Williams L A, Evans S F, Newnham J P. Prospective cohort study of factors influencing the relative weights of the placenta and the newborn infant. BMJ. 1997;314(7098):1864–1868. doi: 10.1136/bmj.314.7098.1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Misra D P, Salafia C M, Miller R K, Charles A K. Non-linear and gender-specific relationships among placental growth measures and the fetoplacental weight ratio. Placenta. 2009;30(12):1052–1057. doi: 10.1016/j.placenta.2009.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Salafia C M, Zhang J, Charles A K. et al. Placental characteristics and birthweight. Paediatr Perinat Epidemiol. 2008;22(3):229–239. doi: 10.1111/j.1365-3016.2008.00935.x. [DOI] [PubMed] [Google Scholar]
- 27.Coall D A, Charles A K, Salafia C M. Gross placental structure in a low-risk population of singleton, term, first-born infants. Pediatr Dev Pathol. 2009;12(3):200–210. doi: 10.2350/08-02-0413.1. [DOI] [PubMed] [Google Scholar]
- 28.Pala H G, Artunc-Ulkumen B, Koyuncu F M, Bulbul-Baytur Y. Three-dimensional ultrasonographic placental volume in gestational diabetes mellitus. J Matern Fetal Neonatal Med. 2016;29(4):610–614. doi: 10.3109/14767058.2015.1012066. [DOI] [PubMed] [Google Scholar]
- 29.Avni R, Neeman M, Garbow J R. Functional MRI of the placenta—From rodents to humans. Placenta. 2015;36(6):615–622. doi: 10.1016/j.placenta.2015.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.von Elm E Altman D G Egger M Pocock S J Gøtzsche P C Vandenbroucke J P; STROBE Initiative. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies BMJ 20073357624806–808. [DOI] [PMC free article] [PubMed] [Google Scholar]


