Abstract
Preeclampsia (PE) is a significant gestational disorder that causes complications in 3–5% of all human pregnancies. Apart from the immediate risks and complications for mother and fetus, both additionally carry elevated lifelong risks for specific complications. Offspring of PE pregnancies (PE-F1) have higher risks for hypertension, stroke and cognitive impairment compared with well-matched offspring (F1) from uncomplicated pregnancies. Prior to the clinical onset of PE, placental angiokines secreted into the maternal plasma are deviated. In many PE patients this includes deficits in placental growth factor (PGF). Our laboratory found that mice genetically-deleted for PGF (PGF − / − ) have altered cerebrovascular and brain neurological development detectable from midgestation to adulthood. We hypothesized that the PGF deficits seen in human PE, deviate fetal cerebrovascular and neurological development in a manner that impairs cognitive functions and elevates stroke risk. Here we summarize the initial analytical outcomes from a pilot study of 8–10 year old male and female PE-F1s and matched controls. Our studies were the first to report magnetic resonance imaging (MRI), magnetic resonance angiography (MRA) and functional brain region assessment by eye movement control and clinical psychometric testing in PE-F1s. Further studies in larger cohorts are essential to define whether there are image-based biomarkers that describe unique anatomical features in PE-F1 brains.
Keywords: preeclampsia, PGF, fetal brain, MRI, cognitive development
Abstract
Resumo
A pré-eclampsia (PE) é importante doença gravídica complicando 3–5% de todas as gestações humanas. Além dos riscos imediatos e complicações para a mãe e o feto, a PE associa-se a outros riscos materno-fetais elevados em longo prazo. Nascituros de gestações complicadas por PE (PE-F1) apresentam maiores riscos de desenvolver hipertensão, acidente vascular cerebral e disfunção cognitiva em comparação com prole (F1) de gestações sem complicações. Antes do aparecimento clínico da PE, angiocitocinas placentárias secretadas no plasma materno apresentam-se alteradas. Em muitos pacientes com PE, isso inclui valores plasmáticos reduzidos de Fator de Crescimento Placentário (PGF). Nosso laboratório identificou que camundongos geneticamente não produtores de PGF (PGF − / − ) apresentam alterações vasculares e de desenvolvimento cerebral detectáveis do período gestacional à idade adulta. Nossa hipótese é que os déficits de PGF identificados em mulheres que desenvolveram PE podem desviar o desenvolvimento neurológico e vascular cerebral fetal, de maneira a prejudicar funções cognitivas, elevando o risco de AVC. Aqui resumimos os resultados analíticos iniciais de um estudo piloto com crianças do sexo masculino e feminino de 8–10 anos de idade nascidas de mães que tiveram PE (PE-F1s) comparadas com crianças controle pareadas por idade e sexo. Nossos estudos são os primeiros a relatar a ressonância magnética (RNM), a angiorressonância e a avaliação funcional do cérebro pelo controle de movimento dos olhos e pelo teste clínico psicotécnico em PE-F1s. Estudos adicionais em coortes maiores são essenciais para definir se há biomarcadores com base em imagens que possam descrever características anatômicas únicas em cérebros de crianças PE-F1.
Palavras-chave: pré-eclâmpsia, PGF, cérebro fetal, RNM, desenvolvimento cognitivo
Introduction
Preeclampsia (PE) is a significant clinical gestational disorder occurring in 3–5% of all human pregnancies, and is among the leading causes of maternal and fetal morbidity and mortality.1 PE accounts for up to 12% of all annual global maternal deaths,2 and up to 25% of all annual global fetal and neonatal deaths.3 PE by definition is new-onset hypertension (> 140/90 mmHg) and at least one of: proteinuria (> 300 mg/day), thrombocytopenia (< 105/uL), renal insufficiency (serum creatinine >1.1 mg/dL), impaired liver function, pulmonary edema, headaches or visual disturbances after the 20th week of gestation.4 The systemic presentation of PE has been best explained as systemic vascular inflammation.5 Numerous disturbances in the levels of angiogenic molecules precede and accompany clinical signs, such as low placental growth factor (PGF) and elevation of the soluble form of its receptor (sFLT1).6 7
Beyond the immediate gestational complications of PE, numerous long-term maternal complications have been identified.8 9 10 Women who have experienced PE have significantly increased risks of developing future cardiovascular risk factors such as dyslipidemia,11 hypertension12 and metabolic disease.13 PE at least doubles the risk of future heart disease,14 15 elevating lifetime risks for coronary artery disease,16 cardiovascular disease and stroke.17
This review explores the impact of preeclampsia upon the brain of offspring from preeclamptic gestations. While PE's impact on brain vascular and neurological development occurs during fetal life, postnatal brain assessments are used to study the legacy of the preeclamptic gestation in the offspring. Preliminary outcomes of a novel pilot investigation that we undertook in 8–10-year-old children born from singleton PE gestations (PE-F1) are also discussed.18 These studies were the first to report Magnetic Resonance Imaging (MRI), Magnetic Resonance Angiography (MRA) and functional brain region assessment using eye movement control testing in PE-F1s.19 A mechanistic pathway for PE-induced deviations in brain development is proposed from a mouse model of PGF deficiency. Finally, some ideas regarding screening and future approaches for potential therapeutic interventions are introduced, should further studies of larger populations support the pilot study findings.
Methods
The MEDLINE database to March 2016 was searched for articles published in English between 1990–2016 that focused on preeclampsia but additionally mentioned brain function or cognition of offspring. The search terms used were: preeclampsia, cognition, cognitive tests, fetal, brain, offspring, children, newborn, and eye movements (or any of its synonyms). This database screen identified 277 publications. After reading the title and/or abstract, many of these publications were judged to be relevant and were not included in this review. A total of 57 articles were relevant and provided the basis for this review.
Preeclampsia (PE) and Effects on PE-F1s
Offspring born to PE pregnancies (PE-F1s) exhibit elevated lifetime risks for several health disorders and impaired functional capacities across multiple body systems, including the cardiovascular, endocrine and neurological systems.20 21 22 In particular, PE-F1s from the Helsinki Birth Cohort23 are reported to have deficits in cognitive function and elevated stroke risk.24 25 26
During childhood and young adulthood, PE-F1s display a body mass index (BMI) 0.6 kg/m2 higher than children born to uncomplicated pregnancies.27 These PE-F1s also display 2.5 mmHg higher systolic and 1.4 mmHg higher diastolic blood pressure during the same timeframe.27 These increases in pressure translate to an approximate 2-fold increased risk of stroke in adulthood.26
PE-F1s are also more likely to experience cerebrovascular and cognitive related disorders than offspring born to non-PE pregnancies matched for gestation length and current age or maternal hypertension during the index pregnancy.28 As children, PE-F1s exhibit deficits in several cognitive function domains,28 including verbal reasoning.29 They also score lower for total intelligence quotient (IQ)30 and mental development indices (MDI).31 32 As these children move through adolescence and adulthood, deficits in verbal and arithmetic reasoning persist.28 During adulthood and up to old age, PE-F1s display more depressive symptoms and higher rates of cognitive decline.24 Table 1 summarizes key findings related to the PE-F1 brain and cognition that have been published to date.
Table 1. Brain and cognition related main findings previously reported in humans.PE-F1s.
| Author (year) | Findings in PE-F1s |
|---|---|
| Many et al30(2003) | Lower IQ scores |
| Kajantie et al26(2009) | Smaller head circumferences at birth; elevated risk of stroke |
| Tuovinen et al24(2010) | Higher rates of depression |
| Whitehouse et al29(2012) | Reduced verbal ability |
| Tuovinen et al23(2012) | Greater cognitive decline at old ages |
| Morsing and Maršál32(2014) | Lower mean verbal IQ (VIQ) and lower full scale IQ (FSIQ) |
| Rätsep et al18(2015) | Enlarged brain regional volumes in five regions (cerebellum, temporal lobe, brainstem and right and left amygdala) |
| Rätsep et al19(2016) | Deficits in working memory and visuospatial processing |
Abbreviations: PE- F1, offspring of preeclampsia pregnancies; IQ, intelligence quotient; VIQ, verbal intelligence quotient; FSIQ, lower full scale intelligence quotient.
Preeclampsia (PE) and Placenta Growth Factor (PGF) - A Close Relationship
During human pregnancy, several angiogenic factors become expressed at increased levels to support the growth, development and viability of the conceptus through an uncomplicated pregnancy.33 In particular, vascular endothelial growth factor (VEGF) and its related family member, placental growth factor (PGF), are highly expressed at predictable times over pregnancy.33 A deficiency in maternal plasma PGF has been linked to an increased likelihood of PE.6 The primary source for the gestational elevation of angiokines is the placenta.34
PGF serves as a biomarker predictive of PE, particularly when combined with clinical factors such as blood pressure35 36 37 or other angiogenic markers such as soluble fms-like tyrosine kinase-1 (sFLT)38 and soluble endoglin (sENG).6 35 39 In addition, low maternal levels of PGF in early to mid-pregnancy are thought to be a marker for distinguishing between two types of PE.36
Mouse studies suggest that the normal gestational roles of PGF are to optimize vascular development within decidua basalis and to sustain normal maternal cardiac function in late gestation.40 41 In mice genetically engineered to be PGF-deficient (PGF − / − ), our laboratory identified reduced and aberrant vascular branching in the decidua basalis during early pregnancy (gestation days (GD) 6.5–9.5 of the term 19 day mouse gestation)40 and enlarged labyrinthine vascular spaces in late placentas (GD 15.5–18.5). The latter finding was interpreted as a negative feature resulting from reduced vascular branching into capillaries in the placental exchange region.40
Pilot Study Brain and Cognitive Outcomes for PE-F1s
A recent pilot study18 used 8–10 year old children (n = 20) whose mothers were part of a research cohort, the Preeclampsia Network (PE-NET).13 Five boys and five girls whose mothers' pregnancies were complicated by PE (n = 10) were matched by sex, gestation and current age with children who experienced normal pregnancies (n = 10). All pregnancies were singleton. All of the children were assessed by the same protocol, which included clinical cognitive testing (NEPSY II), eye movement control tests and Magnetic Resonance Imaging and Angiography (MRI/MRA) data collection sequences.18 The consented and assented children underwent MRI/MRA to evaluate their brain structural and vascular anatomy. The PE-F1s exhibited enlarged brain regional volumes in five regions (cerebellum, temporal lobe, brainstem and right and left amygdalae) when compared with their cohort-matched controls.18 Diffusion Tension Image (DTI) analysis suggests further alterations (manuscript in preparation).
Time of flight (TOF) MRA analysis data revealed significant differences in the occipital and parietal lobes. In both the occipital and parietal lobes, the mean vessel radius was significantly shorter in the PE group (control: 0.50 ± 0.01 mm versus PE: 0.45 ± 0.01 mm, p = 0.004; control: 0.55 ± 0.01 mm versus PE 0.52 ± 0.01 mm, p = 0.025 respectively).18 To understand the significance of the MRA findings, an MRI sequence for Arterial Spin Labeling (ASL) could be conducted, and would determine if brain perfusion is lower in regions with smaller caliber blood vessels.
Psychometric testing outcomes revealed overall deficits in working memory and visuospatial processing amongst PE-F1s.19 These deficits appear to correlate with the anatomic alterations identified in our MRI analysis within the occipital lobe, parietal lobe, cerebellum and brain stem. Eye movement control impairments also correlate with several of the regions that showed structural deviation in PE-F1s.19
Plasma samples collected at term from the mothers of 12 study participants were available for PGF quantification by enzyme linked immunosorbent assay. Samples from the PE mothers had significantly lower levels of PGF than samples from the control mothers (control: 221.0 ± 46.6 pg/mL versus PE: 37.2 ± 21.5 pg/mL, p = 0.024). This suggests that at least some of the PE-F1 study participants had aberrant placental PGF production. The PGF overall data were non-correlative and do not indicate that fetal PGF levels were lower than normal. The data also do not exclude the possibilities that other angiogenic or neurodevelopmental pathways provide a primary etiology to explain the findings of this pilot study. Power calculations based on these data estimate 76 pairs of PE-F1 and control children would be needed within a single time window of PE (that is, term or late preterm) to validate the observed anatomic differences.
Preeclampsia-deviated Brain Development – A Proposed Pathway
Fetal brain undergoes great constitutional change and growth during development to build the structural, vascular and neurological anatomy that supports future autonomic and cognitive functions. Because the brain requires oxygen and nutrients provided by circulation, development of the fetal cerebral circulation is coincident with brain neural development, and both tissues share many common molecular pathways.42 Axon induction, guidance and arterial specification all use VEGF and PGF.43
Fetal human brain development begins with the formation of the neural tube at gestational age (GA)3–4 weeks.44 Following neural tube closure, the rostral portion differentiates into three vesicles that eventually form the forebrain, midbrain and hindbrain.44 Simultaneously, the fetal cerebral vasculature forms. Six pairs of brachial arches, each containing primitive branchial arch arteries, are present at approximately GA18 days.45 The internal carotid arteries, which supply the anterior portion of the developing fetal brain, begin to form at GA24 days. At GA28 days, each internal carotid artery splits into anterior and posterior divisions, driven by the formation of the brain stem and the occipital lobe.45 46 Once the main branches of the cerebral arterial tree have formed, the fetal brain utilizes this blood supply to grow rapidly. Neurons begin to migrate along glial cell scaffolds, and the brain folds into gyri and sulci.47 In circumstances of maternal stress and uteroplacental nutrient deprivation, human fetal brain development takes priority over other tissues, with evidence of head sparing effects.48
PGF-deficient mice (PGF − / − ) display altered fetal brain vascular development by mid pregnancy (gestation day 10.5).40 The earliest deviations are in vessels of the hindbrain, then during development of the Circle of Willis (CW) and, in the early postpartum interval, the growing vascular plexus of the retina.49 50 These vascular alterations include narrower lumens, deficient collateral branching and atypical crossovers, deviations that persist into adulthood. Neuronal tissue structure is unlikely to be normal in these mice because adult differences in regional brain volumes have been detected by MRI, and behavior of the mice is atypical. Vascular alterations may underlie these neurological tissue changes, or they may be due to absence of PGF in the neurons themselves.50 PGF has been studied as a member in the cytokine network of Wallerian Degeneration.51 After injury, PGF − /− mice have decreased Schwann cell proliferation and present less macrophage invasion than matched inbred controls, resulting in poorer functional recovery.51
Overall, these mouse studies suggest PGF plays a crucial role in neurovascular development, and that its deficiency contributes to long-term neuropathology.50 Since PGF appears to occur at all stages of brain development,50 it is unclear exactly when the phase of suboptimal human gestational PGF occurs, but reports have indicated it to be around the end of the first trimester.7 Overlapping of timeframes for suboptimal placental PGF production and CW development occur in humans.50 The Circle of Willis is formed between the first and second months of pregnancy (40–55 days),52 and low PGF levels are present in maternal plasma in the majority of women who develop PE during the first trimester53 and as early as week 7.
If genetic and/or epigenetic mechanisms down-regulate PGF prior to embryonic gastrulation, the derivatives of both inner cell mass and trophectoderm cell lineages would be expected to have similar PGF deficient phenotypes. Therefore, vascular alterations in F1s are to be expected from PGF-insufficient PE pregnancies. To date, PGF levels across gestation have been followed in human placenta and maternal plasma but not the fetus.38 54
We hypothesized that the mechanisms deviating placenta-derived angiokines in the circulations of women who progress to PE are established prior to blastocyst formation, and are as equally expressed in the tissue derivatives of the inner cell mass, that is, the fetus6 33 35 37 39 (Fig. 1), as in the placenta. This hypothesis implicates deviations in fetal synthesis of PGF or other angiokines, rather than maternal hypertension or deviated placental angiokine synthesis as potential mechanisms that could compromise brain vascular development, brain structure and cognitive functions of PE-F1s. This hypothesis additionally suggests that deviations in PE-F1 vascular development are widespread and not restricted to the brain. This may be a component of the elevated cardiovascular disease risk reported in PE-F1s.
Fig. 1.
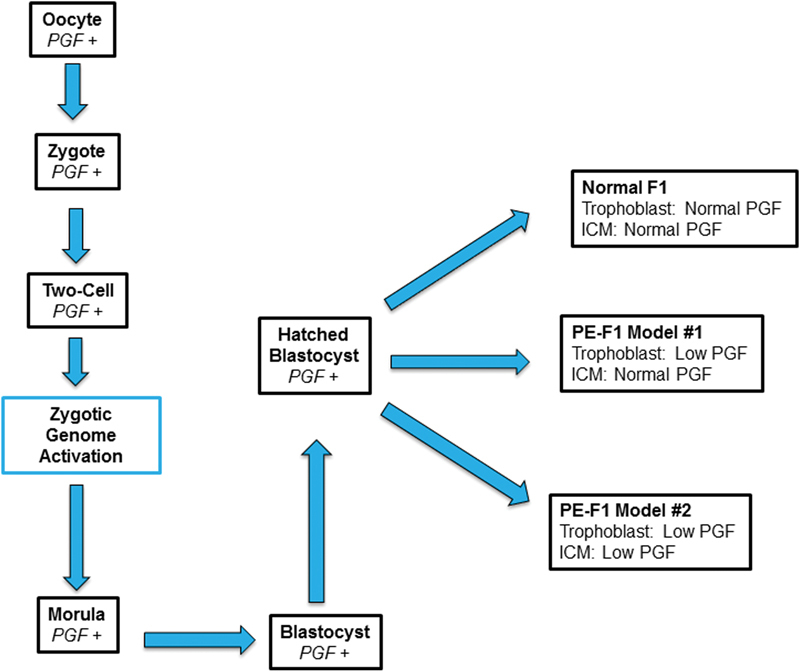
PGF Expression During Human Preimplantation Development. PGF is expressed by the Oocyte, Zygote, Two-cell Embryo and, after Zygotic Genome Activation, by the Morula and Blastocyst. At the window of implantation, hatched Blastocysts will show one of three patterns for PGF expression. Normal F1: In Normal pregnancies – Normal Expression of PGF on ICM and in Trophoblast PE-F1 Model #1 (Classic PE): Low PGF in Trophoblast and thus in maternal plasma over gestation but PGF levels are normal in ICM and its derivative tissues (includes membranes and embryo/fetus) PE-F1 Model #2 (Our hypothesis): Low PGF in both Trophoblast and thus in maternal plasma over gestation, and PGF levels are low in ICM and its derivative tissues (includes membranes and embryo/fetus) Abbreviations: PGF, placental growth factor; PE, preeclampsia; ICM, inner cell mass.
Significance
The combination of MRI studies with neurocognitive tests is a proven approach to identify and link deviations in brain structure with cognition for several childhood conditions.55 56 57 Data from the detailed pilot studies that combined MRI/MRA, eye movement control and cognitive function tests of 8–10 year old PE-F1s18 19 strongly suggest that extension of this approach to PE-F1s will identify consistent vascular and neuroanatomic anomalies, that is, an image phenotype of the PE-F1 brain. This phenotype may explain the cognitive deviations and elevated stroke risk that have been reported in pediatric or adult PE-F1 populations.
The neurocognitive subtests chosen for the pilot study were those used for children participating in NeuroDevNet's studies. NeuroDevNet is a Canadian Centers of Excellence Consortium (http://www.neurodevnet.ca/) that studies neurological brain development and function in children with Fetal Alcohol Spectrum Disorder, Cerebral Palsy and Autism Spectrum Disorder. Selection of these testing paradigms gives the additional exciting possibility for future research outcome comparisons between PE-F1s and children with other major neurodevelopmental disorders.56 57
Further work to define image-based biomarkers describing a unique PE-F1 brain anatomy could lead to personalized interventions and therapies aimed at preventing the development of fetal brain aberrations or their postnatal amelioration. For both the parents and the PE-F1 child, knowledge of the impacts of a PE gestational complication may lead to brain-region specific enhanced educational support to improve the child's typical academic and social progress, and to the consideration of stroke prevention prophylaxis at younger ages. The relatively small sample size and racial homogeneity (Caucasian) of the pilot study participants are weaknesses.
Conclusion
Gestations that include fetal exposure to PE appear to elevate risk for altering cerebral vascular and neuroanatomy during development. Such changes during fetal life may explain the postnatal findings of elevated risks for stroke and specific deviations in cognitive functioning, including visual spatial processing and memory. A large, appropriately controlled, cohort study is needed to validate the current findings. Characterization of a PE-F1 “brain imaging signature” could eventually help identify individuals who may need enhanced educational or medical support.
Acknowledgments
We thank Drs. Nils Forkert (University of Calgary), James Reynolds, Graeme Smith, Matthew Rätsep, and Mr. Brandon Maser (Queen's University) for their helpful discussions and collaboration. We additionally thank all of the children and parents who participated in the study conducted at Queen's University. This study was supported by Awards from the Harry Botterell Foundation for the Neurological Sciences Award and the Garfield Kelly Cardiovascular Research Development Fund from Kingston General Hospital Foundation, the Canada Research Chairs Program (PWS; BAC), and a Postdoctoral Training Award (Estágio Sênior, Grant 99999.002771/2015–02) from CAPES, Brazil (EAFF).
References
- 1.American College of Obstetricians and Gynecologists; Task Force on Hypertension in Pregnancy . Hypertension in pregnancy. Report of the American College of Obstetricians and Gynecologists' Task Force on Hypertension in Pregnancy. Obstet Gynecol. 2013;122(5):1122–1131. doi: 10.1097/01.AOG.0000437382.03963.88. [DOI] [PubMed] [Google Scholar]
- 2.Duley L. The global impact of pre-eclampsia and eclampsia. Semin Perinatol. 2009;33(3):130–137. doi: 10.1053/j.semperi.2009.02.010. [DOI] [PubMed] [Google Scholar]
- 3.Lo J O, Mission J F, Caughey A B. Hypertensive disease of pregnancy and maternal mortality. Curr Opin Obstet Gynecol. 2013;25(2):124–132. doi: 10.1097/GCO.0b013e32835e0ef5. [DOI] [PubMed] [Google Scholar]
- 4.Tranquilli A L, Dekker G, Magee L. et al. The classification, diagnosis and management of the hypertensive disorders of pregnancy: A revised statement from the ISSHP. Pregnancy Hypertens. 2014;4(2):97–104. doi: 10.1016/j.preghy.2014.02.001. [DOI] [PubMed] [Google Scholar]
- 5.Ahmed A, Ramma W. Unravelling the theories of pre-eclampsia: are the protective pathways the new paradigm? Br J Pharmacol. 2015;172(6):1574–1586. doi: 10.1111/bph.12977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Torry D S Wang H S Wang T H Caudle M R Torry R J Preeclampsia is associated with reduced serum levels of placenta growth factor Am J Obstet Gynecol 1998179(6 Pt 1):1539–1544. [DOI] [PubMed] [Google Scholar]
- 7.Levine R J, Maynard S E, Qian C. et al. Circulating angiogenic factors and the risk of preeclampsia. N Engl J Med. 2004;350(7):672–683. doi: 10.1056/NEJMoa031884. [DOI] [PubMed] [Google Scholar]
- 8.Lykke J A, Langhoff-Roos J, Sibai B M, Funai E F, Triche E W, Paidas M J. Hypertensive pregnancy disorders and subsequent cardiovascular morbidity and type 2 diabetes mellitus in the mother. Hypertension. 2009;53(6):944–951. doi: 10.1161/HYPERTENSIONAHA.109.130765. [DOI] [PubMed] [Google Scholar]
- 9.Melchiorre K, Sutherland G R, Liberati M, Thilaganathan B. Preeclampsia is associated with persistent postpartum cardiovascular impairment. Hypertension. 2011;58(4):709–715. doi: 10.1161/HYPERTENSIONAHA.111.176537. [DOI] [PubMed] [Google Scholar]
- 10.Mongraw-Chaffin M L, Cirillo P M, Cohn B A. Preeclampsia and cardiovascular disease death: prospective evidence from the child health and development studies cohort. Hypertension. 2010;56(1):166–171. doi: 10.1161/HYPERTENSIONAHA.110.150078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Magnussen E B, Vatten L J, Myklestad K, Salvesen KÅ, Romundstad P R. Cardiovascular risk factors prior to conception and the length of pregnancy: population-based cohort study. Am J Obstet Gynecol. 2011;204(6):5260–5.26E10. doi: 10.1016/j.ajog.2011.02.016. [DOI] [PubMed] [Google Scholar]
- 12.Magnussen E B, Vatten L J, Lund-Nilsen T I, Salvesen K A, Davey Smith G, Romundstad P R. Prepregnancy cardiovascular risk factors as predictors of pre-eclampsia: population based cohort study. BMJ. 2007;335(7627):978. doi: 10.1136/bmj.39366.416817.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Smith G N, Walker M C, Liu A. et al. A history of preeclampsia identifies women who have underlying cardiovascular risk factors. Am J Obstet Gynecol. 2009;200(1):580–5.8E9. doi: 10.1016/j.ajog.2008.06.035. [DOI] [PubMed] [Google Scholar]
- 14.Rich-Edwards J W, Fraser A, Lawlor D A, Catov J M. Pregnancy characteristics and women's future cardiovascular health: an underused opportunity to improve women's health? Epidemiol Rev. 2014;36:57–70. doi: 10.1093/epirev/mxt006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sattar N, Greer I A. Pregnancy complications and maternal cardiovascular risk: opportunities for intervention and screening? BMJ. 2002;325(7356):157–160. doi: 10.1136/bmj.325.7356.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Irgens H U, Reisaeter L, Irgens L M, Lie R T. Long term mortality of mothers and fathers after pre-eclampsia: population based cohort study. BMJ. 2001;323(7323):1213–1217. doi: 10.1136/bmj.323.7323.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bushnell C, Chireau M. Preeclampsia and Stroke: Risks during and after Pregnancy. Stroke Res Treat. 2011;2011:858134. doi: 10.4061/2011/858134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rätsep M T Paolozza A Hickman A F et al. Brain structural and vascular anatomy is altered in offspring of pre-eclamptic pregnancies: a pilot study AJNR Am J Neuroradiol 2015; [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rätsep M T, Hickman A F, Maser B. et al. Impact of preeclampsia on cognitive function in the offspring. Behav Brain Res. 2016;302:175–181. doi: 10.1016/j.bbr.2016.01.030. [DOI] [PubMed] [Google Scholar]
- 20.Lazdam M, de la Horra A, Pitcher A. et al. Elevated blood pressure in offspring born premature to hypertensive pregnancy: is endothelial dysfunction the underlying vascular mechanism? Hypertension. 2010;56(1):159–165. doi: 10.1161/HYPERTENSIONAHA.110.150235. [DOI] [PubMed] [Google Scholar]
- 21.Seidman D S, Laor A, Gale R, Stevenson D K, Mashiach S, Danon Y L. Pre-eclampsia and offspring's blood pressure, cognitive ability and physical development at 17-years-of-age. Br J Obstet Gynaecol. 1991;98(10):1009–1014. doi: 10.1111/j.1471-0528.1991.tb15339.x. [DOI] [PubMed] [Google Scholar]
- 22.Tenhola S, Rahiala E, Martikainen A, Halonen P, Voutilainen R. Blood pressure, serum lipids, fasting insulin, and adrenal hormones in 12-year-old children born with maternal preeclampsia. J Clin Endocrinol Metab. 2003;88(3):1217–1222. doi: 10.1210/jc.2002-020903. [DOI] [PubMed] [Google Scholar]
- 23.Tuovinen S, Räikkönen K, Pesonen A K. et al. Hypertensive disorders in pregnancy and risk of severe mental disorders in the offspring in adulthood: the Helsinki Birth Cohort Study. J Psychiatr Res. 2012;46(3):303–310. doi: 10.1016/j.jpsychires.2011.11.015. [DOI] [PubMed] [Google Scholar]
- 24.Tuovinen S, Räikkönen K, Kajantie E. et al. Depressive symptoms in adulthood and intrauterine exposure to pre-eclampsia: the Helsinki Birth Cohort Study. BJOG. 2010;117(10):1236–1242. doi: 10.1111/j.1471-0528.2010.02634.x. [DOI] [PubMed] [Google Scholar]
- 25.Tuovinen S, Eriksson J G, Kajantie E. et al. Maternal hypertensive disorders in pregnancy and self-reported cognitive impairment of the offspring 70 years later: the Helsinki Birth Cohort Study. Am J Obstet Gynecol. 2013;208(3):2000–2.0E11. doi: 10.1016/j.ajog.2012.12.017. [DOI] [PubMed] [Google Scholar]
- 26.Kajantie E, Eriksson J G, Osmond C, Thornburg K, Barker D J. Pre-eclampsia is associated with increased risk of stroke in the adult offspring: the Helsinki birth cohort study. Stroke. 2009;40(4):1176–1180. doi: 10.1161/STROKEAHA.108.538025. [DOI] [PubMed] [Google Scholar]
- 27.Davis E F, Lazdam M, Lewandowski A J. et al. Cardiovascular risk factors in children and young adults born to preeclamptic pregnancies: a systematic review. Pediatrics. 2012;129(6):e1552–e1561. doi: 10.1542/peds.2011-3093. [DOI] [PubMed] [Google Scholar]
- 28.Tuovinen S, Eriksson J G, Kajantie E, Räikkönen K. Maternal hypertensive pregnancy disorders and cognitive functioning of the offspring: a systematic review. J Am Soc Hypertens. 2014;8(11):832–470. doi: 10.1016/j.jash.2014.09.005. [DOI] [PubMed] [Google Scholar]
- 29.Whitehouse A J, Robinson M, Newnham J P, Pennell C E. Do hypertensive diseases of pregnancy disrupt neurocognitive development in offspring? Paediatr Perinat Epidemiol. 2012;26(2):101–108. doi: 10.1111/j.1365-3016.2011.01257.x. [DOI] [PubMed] [Google Scholar]
- 30.Many A, Fattal A, Leitner Y, Kupferminc M J, Harel S, Jaffa A. Neurodevelopmental and cognitive assessment of children born growth restricted to mothers with and without preeclampsia. Hypertens Pregnancy. 2003;22(1):25–29. doi: 10.1081/PRG-120016791. [DOI] [PubMed] [Google Scholar]
- 31.Spinillo A, Iasci A, Capuzzo E, Egbe T O, Colonna L, Fazzi E. Two-year infant neurodevelopmental outcome after expectant management and indicated preterm delivery in hypertensive pregnancies. Acta Obstet Gynecol Scand. 1994;73(8):625–629. doi: 10.3109/00016349409013455. [DOI] [PubMed] [Google Scholar]
- 32.Morsing E, Maršál K. Pre-eclampsia- an additional risk factor for cognitive impairment at school age after intrauterine growth restriction and very preterm birth. Early Hum Dev. 2014;90(2):99–101. doi: 10.1016/j.earlhumdev.2013.12.002. [DOI] [PubMed] [Google Scholar]
- 33.Carmeliet P, Ferreira V, Breier G. et al. Abnormal blood vessel development and lethality in embryos lacking a single VEGF allele. Nature. 1996;380(6573):435–439. doi: 10.1038/380435a0. [DOI] [PubMed] [Google Scholar]
- 34.Charnock-Jones D S. Soluble flt-1 and the angiopoietins in the development and regulation of placental vasculature. J Anat. 2002;200(6):607–615. doi: 10.1046/j.1469-7580.2002.00063.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lambert-Messerlian G, Eklund E E, Chien E K. et al. Use of first or second trimester serum markers, or both, to predict preeclampsia. Pregnancy Hypertens. 2014;4(4):271–278. doi: 10.1016/j.preghy.2014.07.001. [DOI] [PubMed] [Google Scholar]
- 36.Powers R W, Roberts J M, Plymire D A. et al. Low placental growth factor across pregnancy identifies a subset of women with preterm preeclampsia: type 1 versus type 2 preeclampsia? Hypertension. 2012;60(1):239–246. doi: 10.1161/HYPERTENSIONAHA.112.191213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Staff A C, Benton S J, von Dadelszen P. et al. Redefining preeclampsia using placenta-derived biomarkers. Hypertension. 2013;61(5):932–942. doi: 10.1161/HYPERTENSIONAHA.111.00250. [DOI] [PubMed] [Google Scholar]
- 38.Zeisler H, Llurba E, Chantraine F. et al. Predictive value of the sFlt-1:PIGF ratio in women with suspected preeclampsia. N Engl J Med. 2016;374(1):13–22. doi: 10.1056/NEJMoa1414838. [DOI] [PubMed] [Google Scholar]
- 39.Brennan L J, Morton J S, Davidge S T. Vascular dysfunction in preeclampsia. Microcirculation. 2014;21(1):4–14. doi: 10.1111/micc.12079. [DOI] [PubMed] [Google Scholar]
- 40.Rätsep M T, Carmeliet P, Adams M A, Croy B A. Impact of placental growth factor deficiency on early mouse implant site angiogenesis. Placenta. 2014;35(9):772–775. doi: 10.1016/j.placenta.2014.07.006. [DOI] [PubMed] [Google Scholar]
- 41.Aasa K L, Zavan B, Luna R L. et al. Placental growth factor influences maternal cardiovascular adaptation to pregnancy in mice. Biol Reprod. 2015;92(2):44. doi: 10.1095/biolreprod.114.124677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rätsep M T Hickman A F Croy B A The Elsevier trophoblast research award lecture: Impacts of placental growth factor and preeclampsia on brain development, behaviour, and cognition Placenta 2016; [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 43.Quaegebeur A, Lange C, Carmeliet P. The neurovascular link in health and disease: molecular mechanisms and therapeutic implications. Neuron. 2011;71(3):406–424. doi: 10.1016/j.neuron.2011.07.013. [DOI] [PubMed] [Google Scholar]
- 44.Tau G Z, Peterson B S. Normal development of brain circuits. Neuropsychopharmacology. 2010;35(1):147–168. doi: 10.1038/npp.2009.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Menshawi K, Mohr J P, Gutierrez J. A functional perspective on the embryology and anatomy of the cerebral blood supply. J Stroke. 2015;17(2):144–158. doi: 10.5853/jos.2015.17.2.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kathuria S, Gregg L, Chen J, Gandhi D. Normal cerebral arterial development and variations. Semin Ultrasound CT MR. 2011;32(3):242–251. doi: 10.1053/j.sult.2011.02.002. [DOI] [PubMed] [Google Scholar]
- 47.Lenroot R K, Giedd J N. Brain development in children and adolescents: insights from anatomical magnetic resonance imaging. Neurosci Biobehav Rev. 2006;30(6):718–729. doi: 10.1016/j.neubiorev.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 48.Baker J, Workman M, Bedrick E, Frey M A, Hurtado M, Pearson O. Brains versus brawn: an empirical test of Barker's brain sparing model. Am J Hum Biol. 2010;22(2):206–215. doi: 10.1002/ajhb.20979. [DOI] [PubMed] [Google Scholar]
- 49.Rätsep M T, Felker A M, Kay V R, Tolusso L, Hofmann A P, Croy B A. Uterine natural killer cells: supervisors of vasculature construction in early decidua basalis. Reproduction. 2015;149(2):R91–R102. doi: 10.1530/REP-14-0271. [DOI] [PubMed] [Google Scholar]
- 50.Luna R L, Kay V R, Rätsep M T. et al. Placental growth factor deficiency is associated with impaired cerebral vascular development in mice. Mol Hum Reprod. 2016;22(2):130–142. doi: 10.1093/molehr/gav069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chaballe L, Close P, Sempels M. et al. Involvement of placental growth factor in Wallerian degeneration. Glia. 2011;59(3):379–396. doi: 10.1002/glia.21108. [DOI] [PubMed] [Google Scholar]
- 52.Van Overbeeke J J, Hillen B, Tulleken C A. A comparative study of the circle of Willis in fetal and adult life. The configuration of the posterior bifurcation of the posterior communicating artery. J Anat. 1991;176:45–54. [PMC free article] [PubMed] [Google Scholar]
- 53.Romero R, Nien J K, Espinoza J. et al. A longitudinal study of angiogenic (placental growth factor) and anti-angiogenic (soluble endoglin and soluble vascular endothelial growth factor receptor-1) factors in normal pregnancy and patients destined to develop preeclampsia and deliver a small for gestational age neonate. J Matern Fetal Neonatal Med. 2008;21(1):9–23. doi: 10.1080/14767050701830480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cetin I Mazzocco M I Giardini V et al. PlGF in a clinical setting of pregnancies at risk of preeclampsia and/or intrauterine growth restriction J Matern Fetal Neonatal Med 2016191–6. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 55.Paolozza A, Treit S, Beaulieu C, Reynolds J N. Response inhibition deficits in children with Fetal Alcohol Spectrum Disorder: relationship between diffusion tensor imaging of the corpus callosum and eye movement control. Neuroimage Clin. 2014;5:53–61. doi: 10.1016/j.nicl.2014.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Paolozza A, Munn R, Munoz D P, Reynolds J N. An in-depth analysis of saccade metrics in children with fetal alcohol spectrum disorder (FASD) Int J Dev Neurosci. 2015;47(A):57–58. [Google Scholar]
- 57.Paolozza A, Munn R, Munoz D P, Reynolds J N. Eye movements reveal sexually dimorphic deficits in children with fetal alcohol spectrum disorder. Front Neurosci. 2015;9:76. doi: 10.3389/fnins.2015.00076. [DOI] [PMC free article] [PubMed] [Google Scholar]


