Abstract
In the last decade, it has become increasingly recognized that a balanced gut microbiota plays an important role in maintaining the health of the host. Numerous clinical and preclinical studies have shown that changes in gut microbiota composition are associated with a variety of neurological diseases, e.g., Parkinson's disease, Alzheimer's disease, and myasthenia gravis. However, the underlying molecular mechanisms are complex and remain unclear. Behavioral phenotypes can be transmitted from humans to animals through gut microbiota transplantation, indicating that the gut microbiota may be an important regulator of neurological diseases. However, further research is required to determine whether animal-based findings can be extended to humans and to elucidate the relevant potential mechanisms by which the gut microbiota regulates neurological diseases. Such investigations may aid in the development of new microbiota-based strategies for diagnosis and treatment and improve the clinical management of neurological disorders. In this review, we describe the dysbiosis of gut microbiota and the corresponding mechanisms in common neurological diseases, and discuss the potential roles that the intestinal microbiome may play in the diagnosis and treatment of neurological disorders.
Keywords: Gut microbiota, Gut–brain axis, Neurological diseases, Parkinson's disease, Alzheimer's disease, Autism spectrum disorder, Cerebrovascular disease
Introduction
The gastrointestinal tract is the most complex microecosystem in the human body. It consists of approximately 10 times more bacterial cells than human cells in the body, and 150 times more bacterial genes than human genes. The gut microbiome is increasingly recognized as the “second genome” that contributes to exogenous biotransformation in the host. The gut microbiota is composed mainly of bacteria, with smaller proportions of fungi, viruses, archaea, and protozoa. Complex and active interactions exist between these gut microorganisms, which jointly affect the balance of gut microecology. The gut microbiota has a variety of biological functions, such as xenobiotic metabolism, fermentation of dietary fiber, vitamin synthesis, pathogen defense, and immune regulation.[1,2] Although the gut microbial community will remain relatively stable over time, many factors may influence the composition and relative abundance of different microbes, including genetics, age, regions, lifestyles, drugs, infections, and diet, among which diet is the most influential and modifiable factor.[3] For example, a fiber-deprived diet reduces the diversity of the microbiota in the colon and disrupts longitudinal and lateral gradients in microbiotic composition, which is characterized by the loss of distal colon microbiota and a reduction in the adjacent mucosal community.[4] Long term high-fat diets can reduce or eliminate the number of beneficial bacteria in the gut, such as Alistipes and Bacteroides spp., while increasing the number of harmful bacteria, including Faecalibacterium spp. Dietary fat may induce disturbances in the arachidonic acid and lipopolysaccharide (LPS) biosynthesis pathways, and in addition elevate inflammatory responses.[5] Thus, the gut microbiota–host symbiotic relationship plays an important role in maintaining human health.
The gut microbiota has an important impact on the function of the host's intestinal mucosa. Communication between the gut microbiome and mucosa promotes immune system maturation and forms a key immunological barrier to defend against pathogens. Dysbiosis of the gut microbiota can induce intestinal inflammatory responses and the occurrence of various intestinal diseases. In the past, it was believed that the brain was less affected by gut microorganisms because of physical distance and the existence of the blood–brain barrier (BBB). However, the human body is a unified whole; therefore, in addition to the proximal effect on the gut, the question remained whether the gut microbiota had an impact on the function of distal organs. In 2011, Neufeld et al[6] were the first to report a reduction in anxiety-like behavior and changes in central neurochemicals in gut microbiota–absent mice, suggesting a potential role for gut microbiota in brain function and behavior. At present, with the advancement of the Human Microbiome Project, it has become clear that there is a close connection between the brain and peripheral systems. An increasing number of studies have found that the gut microbiota affects brain function and may be involved in the regulation of various brain diseases, which brings us new and significant opportunities to study neurological diseases. In 2012, the concept of a microbiota–gut–brain (MGB) axis was formally proposed,[7] which revealed pathways by which the gastrointestinal tract affects the brain and demonstrated that the gastrointestinal tract is closely connected with the brain via gut microbial carriers. More recently, studies have been focused on the regulation of brain function by the gut microbiota and how the microbiome influences neurological diseases. These studies provide new insight into the diagnosis and treatment of neurological diseases.
MGB axis
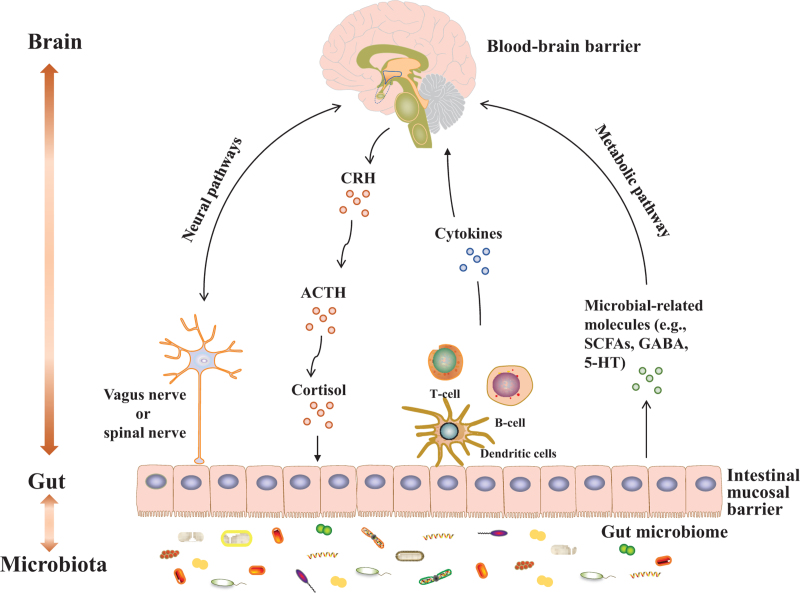
Accumulating data have confirmed bidirectional communication and regulation between the gut microbiome and brain through the MGB axis. However, what are the exact pathways through which pathogenic factors within the gut microbiota affect brain function? Current studies have identified the following five communication pathways [Figure 1]:
Figure 1.
The MGB axis. 5-HT: 5-hydroxytryptophan; ACTH: Adrenocorticotrophin; CRH: Corticotropin releasing hormone; GABA: γ-aminobutyric acid; MGB: Microbiota–gut–brain; SCFAs: Short chain fatty acids.
-
(1)
The first is the neural pathway. There are neuroanatomical structures between the brain and gut, and many intestinal nerves are distributed in the intestinal wall. Intestinal information can be transmitted to the brain through the intestinal nerves and the vagus nerve.[8,9] In turn, the brain, as the center of the body, can also regulate intestinal functions through the descending vagus nerve. The vagus nerve is an important part of the MGB axis and a key node for information transmission between the gut and the brain.
-
(2)
The neuroendocrine pathway consists of the hypothalamic–pituitary–adrenal (HPA) axis and is an important component of the neuroendocrine system and also a major pathway in gut–brain communication.[10] Stress leads to changes in gut microbial composition and functions via activation of the HPA axis.[11] Dysfunction of the HPA axis plays a pivotal role in the pathogenesis of neuropsychiatric diseases.
-
(3)
The third is the inflammatory signaling pathway. Gut-derived inflammatory factors, such as tumor necrosis factor-α (TNF-α), interferon-γ (IFN-γ), and interleukin (IL)-6, can disrupt BBB integrity and participate in the development of brain diseases after entering into systemic circulation via a damaged gut mucosal barrier.[12] Inflammation-induced activity of the HPA axis promotes the secretion of glucocorticoids, which in turn leads to activation of intestinal function and increased production of pro-inflammatory factors. Moreover, activated enteric immune cells, including T helper 17 (Th17) and natural killer cells, can translocate into the brain and induce neuroinflammation. Stress response to neuroinflammation changes the gut microbial composition, which then stimulates enteric immune cells, and microbiota-derived metabolites act as regulators in this bidirectional relay of inflammatory signals. In addition, intestinal inflammatory information triggers brain functions through the afferent vagus nerve, while efferent vagus nerve signaling to enteric immune cells suppresses intestinal inflammation.[13] These inflammatory signaling routes across the MGB axis play an important roles in regulating gut microbiota-related brain diseases.
-
(4)
Microbial-derived metabolites can regulate host brain function in a variety of ways.[14] Short chain fatty acids (SCFAs), such as acetate, propionate, and butyrate, are the most frequently assessed metabolites in microbe–host interactions and play an important role in regulating host immunity and metabolism. Gut-derived SCFAs can influence intestinal barrier integrity, regulate enteric immunity, and enter systemic circulation to modulate peripheral inflammatory responses, neuroinflammation, and HPA axis activity, and protect the BBB. These routes may be involved in the mechanisms by which SCFAs directly or indirectly regulate brain structure and function.[15] Tryptophan is the most studied amino acid, and its downstream metabolites (e.g., indole, 5-hydroxytryptophan [5-HT], and kynurenine) have extensive effects on host function. Bile acids are modified by the gut microbiota and participate in the regulation of various aspects of host function. Microbiota-modified bile acids may induce intestinal inflammation potentially through bile acid-activated receptors,[16] which contribute to brain disease-related neuroinflammation. Neurotransmitters (e.g., serotonin, γ-aminobutyric acid [GABA], and catecholamines) produced in the gut are key modulators of gut microbiota–host interactions.
-
(5)
The gut mucosal barrier and BBB are natural barriers in the MGB axis. The gut mucosal barrier is composed of multiple layers that contain the commensal microbiota and gut mucosal immune system, and acts as the physical and functional interaction zone between the gut microbiota and host.[17] The BBB plays a key role in maintaining brain homeostasis. The gut microbiota and their derived metabolites have important effects on BBB integrity and brain function.[18] Certain physical and chemical factors or stress can damage barrier functions and increase permeability, which then allows harmful factors to enter the brain and eventually promote disease development.[19]
The interactions between the gut microbiota and host remain unclear. The development of animal models and new technologies provide important research tools for an in-depth understanding of the role of the MGB axis in disease. At present, gut microbiota research mainly includes the use of germ-free (GF) animals (usually rodents), antibiotic-treated animals, or fecal microbiota transplantation (FMT) into animals. First, the GF animal is a very important model organism for understanding gut microbiota–host relationships.[20] GF animals are not exposed to any microbes after birth, and this model provides a valuable tool for an in-depth understanding of the function of microorganisms in shaping the behavior, physiology, and neurobiology of the host. By comparing GF animals with specific-pathogen free (SPF) animals, we can evaluate how gut microbiota influences brain function and behaviors from “none of microbiota” and “full of microbiota” perspectives. Second, the antibiotic-treated animal model is an effective pharmacological tool that can be used to study the effects of gut microbiota dysbiosis on brain functions and behaviors. Antibiotic treatment can be carried out at any stage of life, thus providing greater temporal flexibility and microbial specificity compared with the GF animal model. Another advantage of antibiotic treatment is that it can better mimic human clinical conditions. The flexibility and specificity of antibiotic treatment make this model an important tool for evaluating the function of the MGB axis and a key element for future research in this field. Third, the FMT animal model is established by transplanting the fecal microbiota from one individual into another by oral gavage. In preclinical studies, FMT has been successfully used to construct animal models of brain diseases, including schizophrenia,[21,22] major depressive disorder (MDD),[23,24] and autism spectrum disorder (ASD).[25] Therefore, FMT provides a good tool for a further understanding of how the gut microbiota affects host functions.
Currently, a variety of techniques are available to detect the gut microbiota and these include fluorescence-based quantitative polymerase chain reaction, 16S ribosomal RNA amplicon sequencing, metagenomics, metatranscriptomics, metaproteomics, and microbial microarrays. Although the composition and potential of the gut microbiota can be revealed by the above methods, the in situ functions of the microbiota remain unclear. Approximately 70% of gut microorganisms are difficult to culture in vitro, resulting in a limited understanding of their microbiology. The fluorescent–amino acid labeling strategy is a novel in vivo labeling method for microbiota, which allows the in situ visualization of spatial distributions of the gut microbiota that cannot be cultured in vitro and identifies the internal and external factors affecting metabolic activities.[26] This quantitative three-dimensional imaging strategy for native gut microbiota will enable further understanding of host–microbiota interactions. Additionally, gut-on-a-chip models, which are based on microfluidic technology, can be used to simulate the three-dimensional structure of the gut,[27] reveal the in situ function of gut microbiota, and provide new insight into how the gut microbiota affects host function.
Gut microbiota and neurodevelopment
In humans, the brain develops rapidly during the first 3 years after birth. Development includes neuronal migration, axonal growth, synaptogenesis, and myelination, which are necessary to establish neural networks that act as the morphological basis of brain function. Of interest, the development of the gut microbiota parallels the development of the brain,[28] which suggests a possible relationship between gut microbiota and neurodevelopment. Neonatal antibiotics have been shown to induce dysbiosis of gut microbial development, which subsequently impaired myelination in the prefrontal cortex region and contributed to cognitive developmental disability.[29] The gut microbiota influences the neurodevelopment at an early stage of life and this influence continues into adulthood.[30] Previous findings indicated that gut microbial signals can be transmitted to the central nervous system to influence neurodevelopment (e.g., BBB formation, microglial maturation) by activating the vagus nerve, producing and releasing microbiota-derived metabolites into the circulatory system, and affecting the immune system and hormone release.[28,31] A previous study that included 89 infants found that cognitive development at 2 years of age, which was assessed using the Mullen Scales of Early Learning, was significantly related to the gut microbial composition, and lower Mullen scores were associated with higher α-diversity of the gut microbiota.[32] Resting-state functional magnetic resonance imaging of 39 1-year-old human infants indicated that amygdala-mid/forebrain, anterior cingulate-insular, and sensorimotor-parietal functional connectivity were significantly associated with α-diversity of the gut microbiota.[33] Multiple studies have examined the role of the MGB axis in neurodevelopmental processes. High-fat diets in early life lead to impaired hippocampal development and cognitive deficits in mice by depleting the gut commensal bacterium Akkermansia muciniphila.[34] GF animals provide the strongest model for understanding the effects of gut microbiota on neurodevelopment. Absence of the gut microbiome in GF mice results in increased hippocampal volume and neurogenesis, increased myelination and myelin gene expression in the prefrontal cortex, and altered microglia maturation and activity.[35] Furthermore, increased permeability of the BBB was observed in GF mice,[36] and loss of integrity of the BBB allowed immune cells and many bacterial components to migrate to the brain, which subsequently caused neuroinflammation. These neurobiological changes are relevant to various neurological diseases.
Gut microbiota and neurological diseases
As shown in [Table 1], dysbiosis of the gut microbiota has been shown to participate in the pathogenesis of multiple major neurological diseases,[35] including migraine, cerebrovascular disease, Alzheimer's disease (AD), Parkinson's disease (PD), multiple sclerosis (MS), neuromyelitis optica spectrum disorders (NMOSD), myasthenia gravis (MG), epilepsy, ASD, and MDD.
Table 1.
Summary of the MGB axis in neurological diseases.
| Abundance of microbiota | |||||
| Neurological diseases | Increased | Decreased | Pathogenesis | Gut microbiota-based diagnosis | Gut microbiota-targeting intervention |
| Migraine | Phylum: Firmicutes Genus: Streptococcus[38] and Pseudomonas[38] Species: Rothia mucilaginosa[38] Haemophilus parainfluenzae,[38] Blautia hydrogenotrophica,[39] Clostridium spp.,[39] Eggerthella lenta,[39] Flavonifractor plautii,[39] and Ruminococcus gnavus[39] |
Genus: Faecalibacterium[39] Species: Faecalibacterium prausnitzii,[39]Bifidobacterium adolescentis,[39]Methanobrevibacter smithii,[39]Bacteroides spp.,[39]Clostridium sp. L2_50,[39]Coprococcus catus,[39]Eubacterium hallii,[39]Eubacterium ramulus,[39]Odoribacter splanchnicus,[39]Prevotella copri,[39]Ruminococcus callidus,[39]Ruminococcus champanellensis,[39]Ruminococcus obeum,[39] and Sutterella wadsworthensis[39] |
Gut microbiota dysbiosis contributes to migraine via metabolic dysfunctions and insufficient SCFA synthesis[39]; Gut microbiota dysbiosis contributes to migraine-like pain via TNF-α upregulation,[40,41]∗ c-Fos upregulation,[41]∗ and calcitonin gene-related peptide downregulation[41] in the trigeminal nociceptive system | – | Probiotic Lactobacillus casei Shirota relieves migraine symptoms at 4 months follow-up[42]; a multispecies probiotic containing Bifidobacterium spp. and Lactobacillus spp. induces a ≥2 days reduction in migraine days in 12/31 patients in the probiotics group vs. 7/29 in the placebo group[43]; a multispecies probiotic containing 14 strains of Bifidobacterium spp., Lactobacillus spp., and Streptococcus spp. is effective to improve migraine headache in both chronic and episodic migraineurs[44] |
| ICH | Phylum: Firmicutes/Bacteroidetes[49] Family: Nocardiaceae,[46]∗ Helicobacteraceae,[46]∗ Veillonellaceae,[46]∗ Bacteroidaceae,[46]∗ and Akkermansiaceae[46]∗ Genus: Bacteroides,[49] Acidaminococcus,[49] and Bacteroides ovatus[49] Species: Bacteroides fragilis[49] |
Phylum: Firmicutes[46]∗ and Verrucomicrobia[49] Family: Barnesiellaceae[46]∗ and Moraxellaceae[46]∗ Genus: Prevotella,[49]Akkermansia,[49] and Blautia[49] Species: Prevotella copri,[49]Akkermansia muciniphila,[49] and Ruminococcus callidus[49] |
Gut microbiota dysbiosis regulates ICH outcomes via destruction of the gut mucosa, delayed small intestinal motility, intestinal barrier dysfunction, T cell translocation, and increased inflammatory responses and oxidative stress[45,46]∗ | – | Transplantation of healthy fecal microbiome ameliorates the ICH-induced neurobehavioral impairment in the subacute phase and later phases[46]∗ |
| Cerebral infarction | Phylum: Proteobacteria[52] and Bacteroidetes[53]∗ Family: Enterobacteriaceae,[51] Ruminococcaceae,[51] Veillonellaceae,[51] and Lachnospiraceae[51] Genus: Escherichia/Shigella,[52]Peptoniphilus,[52]Ezakiella,[52]Enterococcus,[52] and Prevotella[53]∗ Species: Escherichia coli[51]∗ |
Phylum: Firmicutes,[52] Bacteroidetes,[52] and Firmicutes[53]∗ Family: Bacteroidaceae[51] and Prevotellaceae[51] Genus: Blautia,[52]Subdoligranulum,[52]Bacteroides,[52]Anaerostipes,[52]Faecalibacterium,[53]∗Streptococcus,[53]∗Lactobacillus,[53]∗ and Oscillospira[53]∗ |
Gut microbiota dysbiosis contributes to stroke via gut–immune–brain axis[52]; ischemic stroke rapidly triggers gut microbiome dysbiosis with Enterobacteriaceae overgrowth that in turn exacerbates brain infarction by accelerating systemic inflammation[51]∗; in addition, gut microbiota dysbiosis regulates cerebral infarction by intestinal mucosal barrier disruption and systemic inflammation activation[53]∗ | Enterobacteriaceae is an independent risk factor for stroke patients with poor primary outcomes[51] | Reconstructing the gut microbiota by transplanting fecal bacteria rich in SCFAs and supplementing with butyric acid were found to be effective treatments for cerebral ischemic stroke;[54]∗ a prebiotic and a cocktail of four SCFA-producers (Lactobacillus fermentum, Clostridium symbiosum, Faecalibacterium prausnitzii, and Bifidobacterium longum) alleviate poststroke neurological deficits and inflammation, and elevated gut, brain and plasma SCFA concentrations in aged stroke mice[55]∗ |
| MCI | Phylum: Bacteroidetes[59] Class: Bacteroidia[59] Order: Bacteroidales[59] Family: Veillonellaceae[59] and Bacteroidaceae[59] Genus: Escherichia,[58]Lactobacillus,[58]Escherichia/Shigella[56]Bacteroides,[59] and Prevotella[64] |
Genus: Bacteroides,[58]. Blautia,[59]Faecalibacterium,[64]Anaerostipes,[64] and Ruminoccocus[64] Species: Eubacterium rectale[56] and Bacteroides fragilis[56] |
Gut microbiota dysbiosis contributes to MCI and/or AD via peripheral inflammatory activation,[56] blood-brain barrier impairment, neuroinflammation promotion, and neurodegeneration,[58] as well as intrinsic brain activity alterations[59] | Using the cutoff values from random forest models with all different genera fecal inputs, 28 of 30 patients with MCI could be identified correctly with a sensitivity of 93%[58] | – |
| AD | Phylum: Actinobacteria and Verrucomicrobia Family: 13 bacteria (e.g., Bifidobacteriaceae, Verrucomicrobiaceae, Coriobacteriaceae, Erysipelotrichaceae, Enterococcaceae, Corynebacteriaceae, etc.) Genus: six genera (Dorea, Lactobacillus, Streptococcus, Bifidobacterium, Blautia, and Escherichia),[58] 16 genera (e.g., Bifidobacterium, Akkermansia, Clostridium, Enterococcus, Eggerthella, Olsenella, etc.)[60] |
Phylum: Firmicutes Family: Ruminococcaceae, Lachnospiraceae, and Clostridiaceae Genus: five genera (Alistipes, Bacteroides, Parabacteroides, Sutterella, and Paraprevotella),[58] eight genera (e.g., Faecalibacterium, Roseburia, Dialister, Romboutsia, Coprococcus, Butyricicoccus, etc.)[60] |
The biosynthesis and the metabolism of fatty acids might participate in the mechanisms by which gut microbiota regulates AD-related changes through immunomodulatory[60] suppression of astrocyte activation around Aβ plaques was also observed[61] | – | A Bifidobacterium spp. probiotic alleviates AD-related pathological changes and behaviors[62]∗; a fermented milk product with probiotic containing Bifidobacterium animalis subsp Lactis, Streptococcus thermophiles, Lactobacillus bulgaricus, and Lactococcus lactis subsp Lactis improves the brain activity of AD patients[63]; specific strains of Faecalibacterium prausnitzii improves Aβ-induced cognitive impairment in Alzheimer's-type dementia[64]∗ |
| PD | Phylum: Verrucomicrobia[65] and Proteobacteria[65] Family: Verrucomicrobiaceae,[65] Enterobacteriaceae,[65] Christensenellaceae,[65] Lactobacillaceae,[65,70] Coriobacteriaceae,[65,70] Bifidobacteriaceae,[65,70] Desulfovibrionaceae,[70] Rikenellaceae,[70] and Oscillospiraceae[70] Genus: Akkermansia,[65]Oscillospira,[65]Desulfovibrio,[70]Oscillibacter,[70]Lactobacillus,[70]Alistipes,[70]Bifidobacterium,[70] and Collinsella[70] Species: Bifidobacterium dentium[70] and Bifidobacterium longum[70] |
Family: Lachnospiraceae[65] Genus: Roseburia[65,70] and Ruminococcus[65] |
Gut microbiota regulates the formation of α-Syn that was influenced by ubiquitin system, the upregulation of the xenobiotic degradation pathways,[65] and the gut-to-brain spread of α-synucleinopathy[67,68] and the neuroinflammation,[68] as well as the δ-secretase elicited apoptosis in nigral dopaminergic neurons,[69] which participate in the pathogenesis of PD; gut microbiota–derived epitope peptides involves in the pathogenesis of PD via abnormalities in immunity and glutamate and propionate metabolism[70] | – | – |
| MS | Phylum: Actinobacteria[72,75] and Verrucomicrobia[73] Family: Christensenellaceae,[72] Sphingobacteriaceae,[74] Caulobacteraceae,[74] and Pseudomonadaceae[74] Genus: Bilophilia,[72]Bifidobacterium,[72]Desulfovibrio,[72]Methanobrevibacter,[73]Akkermansia,[73]Psuedomonas,[74]Mycoplana,[74]Haemophilus,[74]Blautia,[74]Dorea,[74] and Pedobacter[74] |
Family: Lachnospiraceae,[72] Ruminococcaceae,[72] Coriobacteriaceae,[74] and Porphyromonadaceae,[74] Genus: Butyricimonas,[73]Slackia,[73]Collinsella,[73]Parabacteroides,[74]Adlercreutzia,[74]Prevotella,[74,75]Collinsella,[74]Faecalibacterium,[75] and Anaerostipes[75] |
Gut microbiota involves in the pathogenesis of PD via the abnormalities in glutathione metabolism and LPS biosynthesis,[72] the dendritic cell maturation, interferon signaling and NF-kB signaling pathways,[73] as well as fatty acid biosynthesis[74] and inflammatory response[75] | – | A probiotic mixture VSL3 containing strains of Lactobacillus, Bifidobacterium, and Streptococcus spp.-induced anti-inflammatory peripheral immune response that is associated with improved disease outcome in MS patients[76,77]; a mixture of human gut-derived 17 Clostridia strains and their metabolite, butyrate, improves the clinical outcome of EAE via inducing lower histopathological features in the CNS and enhanced anti-inflammatory responses[78]∗; gut microbiota-derived PA improved clinical outcomes of MS by an immunomodulatory mechanism[79] |
| NMOSD | Genus: Flavonifractor,[80]Streptococcus,[80,81,83]Shigella,[81]Lachnoclostridium,[82]Oscillibacter,[83] and Veillonella[83] Species: Clostridium bolteae,[82]Alistipes,[81,84]Haemophilus,[81,84]Butyricimonas,[81,84] and Rothia[81,84] |
Genus: Faecalibacterium,[80,81]Lachnospiracea_incertae_sedis,[80]Prevotella,[80]Blautia,[80]Roseburia,[80]Romboutsia,[80]Coprococcus,[80]Fusicatenibacter,[80]Clostridium,[81,84]Parabacteroides,[81,84]Oxalobacter,[81,84] and Burkholderia[81,84] | Gut microbiota involves in the pathogenesis of NMOSD via the abnormalities in inflammatory response,[81,82] and the disruption in intestinal barrier[84] | These microbial biomarkers have predictive power to distinguish NMOSD from controls with a sensitivity of 93%[80]; nine genus-level microbial biomarkers identify the NMOSD patients from controls with a sensitivity of 97%[83] | – |
| MG | 34 increased OTUs[87]: Bacteroidaceae (9 OTUs), Lachnospiraceae (9 OTUs), Veillonellaceae (3 OTUs), and Prevotellaceae (3 OTUs) Species: 16 species[89] |
46 decreased OTUs[87]: Lachnospiraceae (23 OTUs), Ruminococcaceae (8 OTUs), Erysipelotrichaceae (4 OTUs), Peptostreptococcaceae (3 OTUs), and Clostridiaceae_1 (3 OTUs) Genus: Clostridium[88] Species: Fusobacterium prausnitzii[88] and nine species[89] |
Gut microbiota involves in the pathogenesis of NMOSD via the disturbances in amino acid metabolism, nucleotide metabolism, and microbial metabolism pathways,[87] as well as the SCFAs’ production[89] | A marker panel composed of four genera (Clostridiaceae, Lachnospiraceae, Erysipelotrichaceae, and Bacteroidaceae) and six fecal metabolites (leucine, cytosine, oxalic acid, N-acetyltryptophan, d-glyceric acid, and xanthine) discriminates between MG patients and healthy controls with 100% accuracy[87]; a microbial panel composed of five species (Fusobacterium mortiferum, Prevotella stercorea, Prevotella copri, Megamonas funiformis, and Megamonas hypermegale) distinguishes the MG patients from healthy controls with a sensitivity of 94% in cross-validation and 84% in the independent validation cohort[89] | Treatment with Bifidobacterium and Lactobacillus probiotic strains alleviates MG-associated symptoms in EAMG and EAE models by inflammatory regulation[90]∗ |
| Epilepsy | Phylum: Proteobacteria[91] Genus: Campylobacter,[91]Delftia,[91]Haemophilus,[91]Lautropia,[91]Neisseria,[91] and Cronobacter[94] |
Phylum: Firmicutes,[91] Bacteroidetes,[91] and Actinobacteria[91] Genus: Blautia,[91]Coprococcus,[91]Faecalibacterium,[91]Ruminococcus,[91]Bacteroides,[91,94]Parabacteroides,[91]Bifidobacterium,[91,94]Collinsella,[91] and Prevotella[94] |
Gut microbiota dysbiosis participates in the etiology of epilepsy via autoimmune mechanisms and inflammation[91] | Transplantation of the KD-associated microbiota, and treatment with Akkermansia and Parabacteroides, each reduces spontaneous recurrent seizures[93]∗; transplantation of fecal microbiota from healthy volunteers cures epilepsy in a case with Crohn's disease[97] | |
| ASD | Phylum: Bacteroidetes[98] Family: Lachnospiraceae[25]∗ Genus: Akkermansia[25]∗ and Sutterella[25]∗ Species: Eisenbergiella tayi,[25]∗Veillonella parvula,[100] and Lactobacillus rhamnosus[100] |
Phylum: Firmicutes[98] Genus: Streptococcus,[98]Veillonella,[98]Escherichia,[98]Actinomyces,[98]Parvimonas,[98]Bulleidia,[98] and Peptoniphilus[98] Species: Bacteroides ovatus,[25]∗Parabacteroides merdae,[25]∗Bifidobacterium longum,[100] and Prevotella copri[100] |
Maternal intestinal bacteria promote Th17 cell differentiation to release IL-17A that subsequently induces autism in offspring mice via influencing neurodevelopment[99]∗; in patients, gut microbiota dysbiosis participates in the pathogenesis of ASD via increase in intestinal permeability, abnormalities in metabolic pathways,[98] deficiency in microbial detoxification, glutathione depletion, and mitochondrial dysfunction.[100] Microbial metabolite p-cresol regulates ASD core behavioral symptoms via suppression of dopaminergic neurons’ activity[102] | Microbiota-associated detoxification enzymes discriminate ASD from control subjects with a diagnostic power of 88%[100] | Administration of microbial-derived metabolites, taurine and 5-aminovaleric acid, improves the ASD-like behaviors in these mice[25]∗; Transplantation of fecal microbiota from healthy controls to ASD patients relieves the ASD symptoms, and Eubacterium coprostanoligenes can enhance the FMT response[103] |
| MDD | Family: Actinomycineae,[24,105] Coriobacterineae,[24] Lactobacillaceae,[24] Streptococcaceae,[24,105] Eubacteriaceae,[24] Lachnospiraceae,[24,105] Ruminococcaceae,[24,105] Porphyromonadaceae,[105] Clostridiaceae,[105] Erysipelotrichaceae,[105] and Enterobacteriaceae,[109] Genus: Clostridium[106,108,110]Holdemania,[106]Adlercreutzia,[106]Eggerthella,[106,107]Parabacteroides,[106]Streptococcus,[106–108,110]Ruminococcus,[106]Bifidobacterium,[106–108]Blautia,[106]Alistipes,[109]Bacteroides,[108]Oscillibacter,[108]Prevotella,[110] and Klebsiella,[110] Species: Bacteroides stercoris CAG:120,[114]Bacteroides stercoris,[114]Bacteroides dorei,[114]Bacteroides vulgatus,[114]Bacteroides fragilis,[114]Bacteroides thetaiotaomicron,[114]Bacteroides eggerthii,[114] and Bacteroides ovatus[114] |
Family: Bacteroidaceae,[24] Rikenellaceae,[24,105] Lachnospiraceae,[24] Acidaminococcaceae,[24] Veillonellaceae,[24] Sutterellaceae,[24,105] Prevotellaceae,[105] Oscillospiraceae,[105] and Enterobacteriaceae,[105] Genus: Megamonas,[106] Sutterella,[106]Prevotella,[106]Faecalibacterium,[109]Bifidobacterium,[111] and Lactobacillus[111] Species: Blautia obeum,[114]Blautia sp. GD8,[114]Blautia wexlerae,[114]Blautia sp. Marseille-P2398,[114] and Blautia sp. CAG:237[114] |
Gut microbiota dysbiosis participates in the pathogenesis of MDD through regulating many molecular metabolic pathways, e.g., carbohydrate, GABA, phenylalanine, tryptophan,[24,114] glycerophospholipids, and sphingolipids metabolism,[118] as well as the CREB, Ras/MAPK signaling pathways,[119–122] and endocannabinoid signaling pathway[123] | 26 differential OTUs belonging mainly to the Lachnospiraceae (8 OTUs), Bacteroidaceae (7 OTUs), Pseudomonadaceae (3 OTUs), and Ruminococcaceae (3 OTUs) distinguishes the subjects with MDD from those with HC with a high diagnostic accuracy of 96%[113]; a combinatorial marker panel of these six biomarkers discriminates MDD from healthy controls with high classification power in both the discovery (AUC = 0.98) and validation sets (AUC = 0.90)[114] | Oral administration of Lactobacillus plantarum PS128 relives the depressive symptoms of MDD patients[115,116]; the encapsulated microbial therapeutic (MET-2) containing 40 strains of bacteria reduces mean MADRS and GAD-7 scores in MDD patients[117] |
The references indicated with an asterisk (∗) represent studies conducted with animals.
α-Syn: α-synuclein; Aβ: Amyloid β-protein; AD: Alzheimer's disease; ASD: Autism spectrum disorder; AUC: Area under curve; CNS: Central nervous system; CREB: cAMP-response element binding protein; EAE: Experimental autoimmune encephalomyelitis; EAMG: Experimental autoimmune myasthenia gravis; FMT: Fecal microbiota transplantation; GABA: γ-aminobutyric acid; GAD-7: Generalized anxiety disorder; HC: Healthy control; ICH: Intracerebral hemorrhage; IL: Interleukin; KD: Ketogenic diet; LPS: Lipopolysaccharide; MADRS: Montgomery-Asberg depression rating scale; MAPK: Mitogen-activated protein kinase; MCI: Mild cognitive impairment; MDD: Major depressive disorder; MG: Myasthenia gravis; MGB: Microbiota–gut–brain; MS: Multiple sclerosis; NF-κB: Nuclear factor kappa beta; NMOSD: Neuromyelitis optica spectrum disorders; OTUs: Operational taxonomic units; PA: Propionic acid; PD: Parkinson's disease; SCFAs: Short chain fatty acids; Th17: T helper 17; TNF-α: Tumor necrosis factor-α; –: Not reported.
Gut microbiota and migraine
Migraine is a common disabling neurological headache disorder. Epidemiological studies have shown that migraines occur more frequently in patients with gastrointestinal disorders, including those with Helicobacter pylori infection, celiac disease, irritable bowel syndrome, gastroparesis, and hepatobiliary disease, than in the general population.[37]H. pylori infection is strongly associated with the severity of migraines. Nitrates are identified as common triggers for migraine headaches. A higher abundance of nitrate-, nitrite-, and nitric oxide-reducing bacterial taxa (Haemophilus parainfluenzae and Rothia mucilaginosa) were identified in the oral and fecal samples of migraineurs than in those of non-migraineurs.[38] A metagenome-wide association study identified a decrease in the α-diversity of gut microbiota in migraineurs at both the genus and species levels, and Clostridium spp. were enriched in migraineurs, while beneficial species, including Bifidobacterium adolescentis, Faecalibacterium prausnitzii, and Methanobrevibacter smithii, were enriched in non-migraineurs.[39] In addition, both gut microbiota deprivation in GF mice and gut microbiota dysbiosis in broad-spectrum antibiotic-treated mice significantly prolonged nitroglycerin-induced migraine-like pain compared with that in control mice,[40] suggesting a potential role for gut microbiota in the pathogenesis of migraine. Moreover, significant nitroglycerin-induced hyperalgesia was observed in GF mice colonized with gut microbiota from SPF mice. More severe hyperalgesia was also identified in mice transplanted with gut microbiota from migraine patients than in mice receiving microbiota from non-migrainous, healthy controls. These results highlight the involvement of the gut microbiota in the normal mechanical pain sensation and pathogenesis of migraine.[41] A randomized double-blind clinical trial found that the probiotic bacterium Lactobacillus casei Shirota significantly alleviated symptoms of vestibular migraine at a 4-month follow-up compared with placebo.[42] After a 12-week treatment with a probiotic containing multiple species belonging to Bifidobacterium and Lactobacillus genera, the frequency and intensity of episodic migraines were reduced in clinical patients.[43] Similar therapeutic efficacy of a probiotic containing 14 bacterial strains, such as Bifidobacterium infantis Protexin (PXN) 27, Lactobacillus rhamnosus PXN 54, Bacillus subtilis PXN 21, and Streptococcus thermophilus PXN 66, was shown for both episodic and chronic migraine interventions.[44]
Gut microbiota and cerebrovascular disease
Cerebrovascular diseases are the most common neurological diseases, among which intracerebral hemorrhage (ICH) is the most serious type with a mortality rate of 40% to 60%. Hematoma expansion is the key cause of death in ICH; thus, early identification and intervention are important to reduce the incidence of death. A previous study demonstrated that gut microbiota dysbiosis damaged the gut structure and barrier function in ICH patients, resulting in the migration of T cells from the gut to the brain via the MGB axis; infiltration of T cells in the area surrounding the cerebral hematoma played a key role in ICH-induced hyperactivated inflammatory responses and oxidative stress,[45,46] which may be associated with hematoma expansion in ICH patients.[47,48] It remains to be determined whether we can intervene in the process of early hematoma expansion by regulating the structure and function of gut microbiota. Compared with that in healthy controls, the relative abundance of Bacteroides, Acidaminococcus, and Bacteroidesovatus spp. and Bacteroides fragilis was increased in ICH patients, while the abundance of Verrucomicrobia, Prevotella, Akkermansia, and Blautia spp. and Prevotella copri, Akkermansia muciniphila, and Ruminococcus callidus was decreased.[49] Cavernous angioma (CA) is a rare genetic neurovascular disease that causes cerebral hemorrhage. In 2020, researchers found that patients with CA had increased abundance of the Gram-negative bacterium Odoribacter splanchnicus and decreased abundance of Gram-positive Bifidobacterium adolescentis and Faecalibacterium prausnitzii. The combination of these three species provided good sensitivity (92%) and specificity (67%) in CA diagnosis based on the receiver operating characteristic (ROC) curves.[50] Such imbalances of gut microbiota in CA patients suggested increased production of LPS, which can enter the brain, act on blood vessel walls, and promote progression of disease.[50]
Cerebral infarction, also known as cerebral ischemic stroke, is a common type of cerebrovascular disease. Brain ischemia has been reported to rapidly impair intestinal barrier function and lead to gut dysbiosis with overgrowth of Enterobacteriaceae spp., resulting in activation of systemic inflammation, which in return exacerbated cerebral infarction.[51] Previously, a prospective case-control study showed a decrease in obligate anaerobic gut bacteria in stroke patients (e.g., Anaerostipes and Subdoligranulum spp.), as well as an enrichment of trimethylamine-producing bacteria and a loss of butyrate-producing bacteria.[52] Persistent gut microbiota dysbiosis; intestinal mucosa impairment; and increased plasma levels of LPS, TNF-α, IFN-γ, and IL-6 were observed after cerebral infarction in cynomolgus monkeys. SCFAs are important microbial-derived immune modulators and can inhibit LPS-induced inflammatory responses; SCFAs levels were significantly decreased at 6 and 12 months after cerebral infarction in these monkeys.[53] A previous study showed that administration of non-absorbable antibiotics led to reduced cerebral infarct volume and neurological impairment by altering the composition of the gut microbiota.[54] Transplantation of SCFA-rich fecal microbiota and butyric acid supplementation were effective treatments for cerebral ischemic stroke.[54] Interestingly, aged post-stroke mice that received fecal samples from young mice (2–3 months old) showed significant behavioral recovery and reduced brain/intestinal inflammation, which was because of the higher levels of SCFAs in the feces of the younger mice.[55] Furthermore, neurological deficit scores in post-stroke mice were significantly improved after treating with a prebiotic and a cocktail of four SCFA-producing bacteria, which included Lactobacillus fermentum, Clostridium symbiosum, Faecalibacterium prausnitzii, and Bifidobacterium longum.[55]
Gut microbiota and AD
AD is a neurodegenerative disease characterized by progressive cognitive and behavioral impairment. AD is the most common type of dementia in aged adults. Clinical studies have identified a decrease in anti-inflammatory Eubacterium rectale and Bacteroides fragilis and an increase in pro-inflammatory Escherichia/Shigella spp. in older patients with cognitive impairment and brain amyloidosis.[56,57] AD-related alterations in gut microbiota have been observed in patients with mild cognitive impairment (MCI), which is a predementia state, suggesting that changes occur in the gut microbiota before the onset of AD. A diagnostic model based on differential gut microbiota composition at the genus level was able to distinguish between patients with MCI and controls with a 93% (28/30) sensitivity rate.[58] Compared with healthy controls, patients with MCI showed different “gut microbiota–intrinsic brain activity-cognitive function interaction” patterns.[59] In AD patients, the α-diversity of gut microbiota was significantly decreased. The abundance of butyrate-producing genera, e.g., Faecalibacterium, was significantly decreased, which was positively correlated with the severity of AD symptoms. In contrast, the abundance of lactate-producing genera, e.g., Bifidobacterium, was increased, which was negatively correlated with clinical symptoms. This gut microbiota dysbiosis in AD patients alters fatty acid metabolism and folate biosynthesis pathways, which then promote immunomodulatory dysfunction.[60] In addition, the gut microbiota is associated with pathological changes in amyloid β-protein (Aβ) in AD. A recent study found an increase in Aβ plaques in 3-month-old APPSWE/PS1ΔE9 mice (model for Aβ amyloidosis) that received gut microbiota from 16-month-old APPSWE/PS1ΔE9 mice, while such pathological changes were not observed in wild-type mice. These findings suggested that although gut microbiota dysbiosis itself cannot induce Aβ plaques, it can promote the development of AD in a genetically predisposed AD background.[61] Treatment with a Bifidobacterium spp. probiotic formulation (SLAB51) in mice alleviated the progression of AD by changing the gut microbiota composition and its metabolites; restoring impaired neuronal proteolytic pathways, such as autophagy and the ubiquitin proteasome system; and reducing Aβ aggregation.[62] In a clinical trial, administration of fermented milk containing Bifidobacterium and Lactobacillus spp. significantly improved the cognitive, sensory, and emotional functions of AD patients.[63] In addition, specific strains of Faecalibacterium prausnitzii improved cognitive impairment in an AD mouse model through mechanisms related to oxidative stress and mitochondrial function, suggesting that F. prausnitzii may be used for microbiota-targeted therapy in patients with AD.[64]
Gut microbiota and PD
PD is a common neurodegenerative disease in the elderly. The increase in life expectancy worldwide is accompanied by a significant increase in the incidence of PD. Previous data showed changes in the composition of the gut microbiota of patients with PD, including a lower relative abundance of Lachnospiraceae spp. The decreased abundance of Lachnospiraceae spp. and increased abundance of Lactobacillaceae and Christensenellaceae spp. were related to the severity of clinical symptoms, ie, postural instability, gait disturbances, and cognitive impairment.[65] These microbial changes in PD may be used as early biomarkers for PD.[66] The aggregation of α-synuclein (α-Syn) plays a key role in the pathogenesis of PD. Gastrointestinally injected pathological α-Syn fibrils can be widely spread to the brain through the vagus nerve and induce PD-like symptoms in mice, suggesting an important role for the MGB axis in the pathogenesis of PD.[67] After transplanting the fecal microbiota from PD patients into Thy1-α-Syn (α-Syn overexpressing) transgenic mice, motor symptoms were aggravated, suggesting that α-Syn overexpression (genetics) and gut microbiota dysbiosis (environment) jointly influence disease outcome.[68] It has been demonstrated that δ-secretase is involved in the cleavage of α-Syn and Tau proteins and mediates their fibrillization and retrograde transmission from the gut to the brain. The activation of δ-secretase promotes the formation of α-Syn/Tau complexes and elicits apoptosis in nigral dopaminergic neurons, which are associated with motor dysfunction and cognitive impairment in PD.[69] Recent studies have found that gut microbiome-derived epitope peptides were significantly different between PD patients and healthy controls and were related to abnormal inflammatory responses and biosynthesis of glutamate and propionate. Changes in these epitope peptides aggravated the pathology of PD and may be potential biomarkers for PD.[70]
Gut microbiota and MS
MS is an autoimmune disease triggered by environment–gene interactions. The pathology of MS is mainly characterized by glial reaction, inflammation, and demyelination.[71] Increasing evidence indicates that the gut microbiota can play both a protective and pathogenic role in the progression of MS. A previous study based on the 16S ribosomal RNA sequencing found that the relative abundance of butyrate-producing bacteria (Ruminococcaceae and Lachnospiraceae spp.) was decreased in pediatric MS patients compared with that in healthy controls, while levels of Christensenellaceae, Bilophilia, Bifidobacterium, and Desulfovibrio spp. were increased, suggesting a pro-inflammatory milieu in the gut of children with MS.[72] In addition, significant increases in Methanobrevibacter, Akkermansia, Blautia, and Dorea genera and decreases in Butyricimonas, Parabacteroides, and Prevotella genera were also observed in MS patients compared with those in controls.[73,74] Immunomodulatory therapy increased Prevotella and Sutterella spp. and reduced Sarcina spp.[73] A similar decrease in the relative abundance of Prevotella was observed in a separate study.[75] Treatment of MS patients with a probiotic containing strains of Lactobacillus, Bifidobacterium, and Streptococcus spp. changed the structure and composition of the gut microbiota, enhanced antioxidant capacity, and induced an anti-inflammatory response in the periphery that was characterized by a reduced proportion of CD14high/CD16low monocytes; these changes improved the patients’ psychological status and clinical symptoms.[76,77] Treatment of experimental autoimmune encephalomyelitis in mice with a mixture of 17 strains of Clostridium spp. derived from human fecal microbiota increased the level of butyrate in the sera, reduced demyelination and astrocyte/microglial reactivity, inhibited axonal damage, and improved outcomes.[78] The level of microbiota-derived propionic acid (PA) was reduced in the serum and feces of patients with MS.[79] Oral supplementation of PA significantly increased the proportion of functionally competent regulatory T cells and the level of anti-inflammatory cytokines (e.g., IL-10) and decreased pro-inflammatory T helper 1 and 17 cells in MS patients, thus preventing disease progression and suggesting a potential immunomodulatory treatment role for PA in MS.[79]
Gut microbiota and NMOSD
NMOSD represent a group of autoimmune demyelinating diseases of the central nervous system that mainly involve the optic nerve and spinal cord. Emerging evidence has suggested an important role for gut microbiota in the pathogenesis of NMOSD. Previous studies have identified an increased abundance of Flavonifractor and Streptococcus spp. and a decreased abundance of several butyrate-producing bacteria, including Faecalibacterium, Coprococcus, and Prevotella spp., in NMOSD patients compared with that of healthy controls. The combination of seven candidate microbial markers differentiated between NMOSD patients and healthy controls with a sensitivity rate of 93%.[80] In addition, the abundance of Streptococcus spp. was positively correlated with NMOSD severity, while the levels of microbiota-produced SCFAs were significantly decreased in NMOSD patients and negatively correlated with disease severity, especially for acetate and butyrate.[81] A higher level of Clostridium bolteae was identified in aquaporin 4 (AQP4)-IgG + NMOSD patients compared with that in AQP4-IgG- patients, suggesting that this microorganism may be causally related to the immunopathogenesis of NMOSD in susceptible individuals.[82] Specific novel microbial biomarkers for predicting AQP4-IgG+ and AQP4-IgG− NMOSD patients have been identified in disease-specific parameters.[83] In addition, a study of sigmoid mucosal biopsies from NMOSD patients proposed that mucosal microbiota imbalance and inflammatory cell activation allowed pathogens to cross the damaged intestinal barrier and participate in the pathogenesis of NMOSD.[84] Variations of several gut microorganisms, especially Vagococcus and Anaerobiospirillum spp., and their metabolites (e.g., bile acids), correlated with NMOSD recurrence through CXCL13-induced activation of the CXCR5+ CD4+ follicular T helper cell pathway.[85]
Gut microbiota and MG
MG is an autoimmune disease involving neuromuscular junctions and is characterized by systemic skeletal muscle weakness. The gut microbiota is the central regulator of the host immune system, and its dysbiosis may be involved in the pathogenesis of central and peripheral autoimmune diseases.[86] Previous studies have found a reduction in the α-diversity of gut microbiota in patients with MG. The abundance of Bacteroidaceae, Veillonellaceae, and Prevotellaceae spp. was increased, and Ruminococcaceae, Erysipelotrichaceae, Peptostreptococcaceae, Clostridiaceae, and Clostridium spp. and Fusobacterium prausnitzii were decreased in MG patients compared with those in healthy controls; changes in the abundance of Lachnospiraceae spp. and fecal metabolites were also observed, which were mainly related to disturbances in amino acid, nucleotide, and microbial metabolic pathways.[87,88] A previous study identified a marker panel that was composed of four genera (Clostridiaceae, Lachnospiraceae, Erysipelotrichaceae, and Bacteroidaceae) and six significantly related fecal metabolites (leucine, cytosine, oxalic acid, N-acetyltryptophan, d-glyceric acid, and xanthine). This panel was able to discriminate between MG patients and healthy controls with 100% accuracy, which was more accurate than microbial or metabolic markers alone.[87] Another metagenome-wide association study built a microbial panel composed of five species (Fusobacterium mortiferum, Prevotella stercorea, Prevotella copri, Megamonas funiformis, and Megamonas hypermegale).[89] This panel gained an area under the curve of 94% in the discovery cohort and 84% in the validation cohort. Dysbiosis of the gut microbiota in MG reduces SCFA production, which suggests that altered gut microbiota may play a vital role in the pathogenesis of MG by reducing SCFAs.[89] Treatment with Bifidobacterium and Lactobacillus spp. can alleviate MG-associated symptoms by increasing the serum levels of transforming growth factor-β and the percentages of regulatory T cells in peripheral blood.[90]
Gut microbiota and epilepsy
Epilepsy is a common chronic neurological disease characterized by recurrent, episodic, and transient brain dysfunction caused by excessive synchronous neuronal discharge. The gut microbiota may regulate the etiology of epilepsy. Compared with those in healthy controls, the genera Delftia, Lautropia, Campylobacter, Haemophilus, and Neisseria were increased in patients with epilepsy, while Blautia, Faecalibacterium, Parabacteroides, and Bifidobacterium spp. were decreased.[91] Various antiepileptic drugs, such as valproate and clobazam, have been found to influence the composition of the gut microbiota in animal models and vice versa.[92] The ketogenic diet (KD) is a high-fat, low-carbohydrate diet, which is an auxiliary therapeutic diet for epilepsy patients. Based on a mouse model of refractory epilepsy, Olson et al[93] found that the KD significantly suppressed seizures and rapidly changed the composition of the gut microbiota in mice within 4 days, and this change was characterized by an increase in the abundance of Akkermansia muciniphila and Parabacteroides sp. The anti-epileptic effects of the KD were not observed in GF and antibiotic-treated SPF mice; however, after co-administration of Akkermansia muciniphila and Parabacteroides sp., anti-seizure effects of the KD were restored in antibiotic-treated mice, suggesting a modulatory role for the gut microbiota in KD treatment for epilepsy.[93] Results of 16S rDNA sequencing of fecal samples from 14 epileptic and 30 healthy infants found that, compared with that in healthy controls, the abundance of Proteobacteria spp. was significantly increased in epileptic infants,[94] and the KD relieved epilepsy symptoms by decreasing Proteobacteria and Firmicutes spp. and increasing Bacteroides, Bifidobacterium, and Prevotella spp.[94,95] In addition, Lindefeldt et al[96] found that the relative abundance of Bifidobacterium sp., Eubacterium rectale, and Dialister sp. were decreased in fecal samples from children with severe epilepsy after 3 months of KD treatment, while Escherichia coli was increased. Reconstructing the gut microbiota of epilepsy patients by transplanting fecal microbiota from healthy volunteers prevented the relapse of seizures.[97]
Gut microbiota and ASD
ASD is a complex neurodevelopmental disorder, which is mainly characterized by dysfunction of social communication and repetitive stereotypical behaviors. The incidence of ASD has been gradually increasing over recent decades. There is a sex difference in ASD incidence, with a male-to-female ratio of approximately 4.5:1. Gastrointestinal diseases are present in 30% to 70% of children with ASD and represent the most common comorbidity. A previous study that focused on the changes in the gut microbiota composition of ASD patients found that the proportion of Bacteroidetes/Firmicutes spp. and the relative abundance of Sutterella, Odoribacter, and Butyricimonas spp. were significantly increased, while the relative abundance of Veillonella and Streptococcus spp. was significantly decreased. Further functional analysis showed that the abundance of butyrate- and lactate-producing bacteria was reduced in ASD patients compared with that in healthy controls, which may lead to an increase in intestinal permeability. In addition, the activation of arginine–ornithine and ether lipid metabolism and the inhibition of steroid hormone biosynthesis, glycosaminoglycan degradation, and lipoic acid metabolism may participate in the pathogenesis of ASD.[98] Moreover, Kim et al[99] found that maternal intestinal bacteria from mice that promoted Th17 cell differentiation and IL-17A release subsequently induced autism in offspring. Recently, Sharon et al[25] transplanted fecal microbiota from ASD patients into GF mice and demonstrated that the adult offspring of these mice displayed hallmark autistic behaviors, including decreased locomotion and communication and repetitive behavior. Changes in the gut microbiome of ASD mice were vertically and stably transmitted to offspring. In addition, upregulated and extensive alternative splicing of ASD-related genes in the brains of the offspring was observed, and decreased microbial-related small molecules, such as taurine and 5-aminovaleric acid, in the colon contents were also identified. Administration of taurine and 5-aminovaleric acid significantly improved the ASD-like behaviors in these mice. A recent study found a decrease in the relative abundance of intestinal protective bacteria in ASD patients, which led to decreased expression of ASD-associated detoxifying enzymes, deficiency in microbial detoxification, glutathione depletion, mitochondrial dysfunction, and ultimately the onset of ASD.[100] These differentially expressed, microbial-related detoxifying enzymes have demonstrated good diagnostic efficiency for ASD with an area under the ROC curve of 0.88.[100] In addition, the level of the gut microbial metabolite p-cresol is increased in ASD patients.[101] Mice exposed to p-cresol presented with ASD core behavioral symptoms, such as stereotypical and impaired social behaviors, and decreased activity of dopaminergic neurons in the ventral tegmental area.[102] Additionally, transplantation of fecal microbiota from healthy humans to ASD patients significantly improved ASD symptoms by altering the gut microbiota and serum levels of neurotransmitters, and this study also indicated that Eubacterium coprostanoligenes may be a potential regulator of FMT treatment response in children with ASD.[103]
Gut microbiota and MDD
MDD is a severe mental disorder with a high prevalence and suicide rate, which seriously endangers a patient's daily life and creates a heavy burden on the patient's family and society. Accumulating studies have demonstrated a close relationship between gut microbiota dysbiosis and the onset of MDD. Although the composition of the gut microbiota in MDD is inconsistent across studies,[24,104–111] a higher abundance of bacteria from the phylum Actinobacteria and Eggerthella spp. and a lower abundance of Bacteroidetes, Prevotellaceae, Coprococcus, Faecalibacterium, and Sutterella spp. are the most consistent findings in MDD patients compared with those in healthy controls.[112] The most recent studies found specific changes in Bacteroidetes in MDD patients[113] that were characterized by an increase in nine Bacteroidetes strains and a decrease in five Brucella strains,[114] which provided a foundation for the development of microbial-based MDD diagnostic strategy. After transplanting fecal microbiota from MDD patients into GF mice, the remodeled mice displayed significant depressive-like behaviors, such as an increase in immotility time in the forced swimming and tail suspension tests. The gut microbiota composition in the mice was consistent with that in the MDD patients who provided the fecal microbiota.[24] Similar findings were reported by Kelly et al[23] who found that antibiotic-treated rats showed anhedonia after receiving fecal microbiota from MDD patients. These findings confirmed that gut microbiota dysbiosis plays a causal role in the onset of MDD. Etiology-based intervention is an effective method to promote the prevention and treatment of diseases. Oral administration of Lactobacillus plantarum PS128 led to a significant remission in depressive symptoms of MDD patients.[115,116] Furthermore, the encapsulated microbial therapeutic MET-2, which contains 40 strains of bacteria, was effective in reducing the mean Montgomery–Åsberg Depression Rating Scale and Generalized Anxiety Disorder-7 scores in MDD patients.[117]
Previous studies have found that gut microbiota dysbiosis may participate in the pathogenesis of MDD by regulating multiple molecular metabolic pathways, e.g., carbohydrate, GABA, phenylalanine, and tryptophan.[24,114] Clinically-based findings provide good evidence for MDD analysis; however, clinical samples are susceptible to confounding factors, such as drugs, diet, and geography. Compared with that in rodents, the gut microbiome composition in non-human primates has greater similarity with humans and is less affected by these confounding factors, which are difficult to exclude clinically. Based on a naturally-occurring depression in monkeys, it was discovered that gut microbiota dysbiosis induced glycerophospholipid and sphingolipid metabolic alterations in the gut–brain axis, which mediated the depressive-like behaviors.[118] In addition, the gut microbiota dysbiosis regulated the depressive-like behaviors by disrupting the cAMP-response element binding protein (CREB) and Ras/mitogen-activated protein kinase (MAPK) signaling pathways.[119–122] Another study reported that gut microbiota dysbiosis induced depressive-like behaviors by decreasing the endocannabinoid signaling pathway, and selectively enhancing the central endocannabinoid alleviated the depressive-like behaviors.[123]
Diagnosis and treatment of neurological diseases based on gut microbiota
The gut microbiota is a dynamic sensor in vivo that can monitor minuscule changes in the human body and accurately reflect human health status. Because gut microbiota dysbiosis and derived markers correlate with disease severity, studies based on gut microecology may provide new insight into the diagnosis and treatment of neurological diseases. A growing number of studies have constructed prediction and diagnosis models of diseases based on gut microbiotic dysbiosis and derived molecular changes. However, diagnostic strategies based on the gut microbiota require further optimization to standardize the processes and reduce the differences between laboratories. In addition, the validation of microbial-derived markers based on the large clinical cohorts, optimization of analysis and processing of microbiome data, and further understanding of specific microbial-related gut–brain molecular mechanisms in different disease states are crucial for developing diagnostic strategies for diseases related to the gut microbiota and its derived metabolites. In-depth studies of the MGB axis facilitate the identification of new intervention targets for the treatment of brain diseases, the recognition of “gut treatment of brain disease,” selection of personalized treatments depending on the specific changes in gut microbiota, and use of probiotics, prebiotics, and microbial drugs to regulate the balance of gut microbiota and promote accurate clinical transformation. At present, three main methods are used to regulate gut microbiota, which are outlined below.
The first method is FMT. FMT refers to the transplantation of fecal microbiota from healthy individuals into the gut of patients to reconstruct the overall gut microecology and provide disease treatment. FMT has been used to treat various clinical conditions, including recurrent Clostridioides difficile infection, ulcerative colitis, Crohn's disease, hepatic encephalopathy, and autism. More than 70 hospitals in China have carried out FMT to treat various diseases. Zhang et al[124] developed the washed microbiota transplantation (WMT) modification based on an intelligent fecal microbiota separation system and strict quality control. The WMT protocol provides better quality control, microbiota enrichment precision, and clinical treatment safety than traditional FMT.
The second method is the use of probiotics and prebiotics. Probiotics are living microorganisms that are beneficial to the host when a suitable amount is ingested. The first generation of probiotics was developed from a limited number of species of which the most common belonged to Lactobacillus and Bifidobacterium spp. At present, probiotics are mainly used as dietary supplements in food to regulate the gut microbiota and improve intestinal function and have not been developed strictly in accordance with the standards of drug development. Prebiotics are the “food” that promote the growth of probiotics in the gut. Probiotics and prebiotics would be included in the “do not harm, may be helpful” category and are intended to act as a supplementary tool for improving human health.
The third method is the use of live biotherapeutic products, which are products containing live microorganisms (e.g., bacteria) that can be used to prevent and treat human diseases and indications and are also known as second-generation probiotics. In clinical practice, live biotherapeutic products are used as drugs and must have a clear clinical indication. At present, clinically approved live biotherapeutic products are limited and include Mutaflor, which was approved in Germany as a maintenance therapy during remission of ulcerative colitis. MIYAIRI 588 was approved in Japan to improve symptoms caused by gut microbiotic dysbiosis. SK08 viable powder containing Bacteroides fragilis was approved in China for treatment of irritable bowel syndrome and ulcerative colitis. In a phase three clinical trial, oral administration of SER-109, which is composed of purified Firmicutes spores, was superior to placebo in reducing the risk of recurrent C. difficile infection.[125] A similar effect was observed with the microbiota-based investigational therapeutic RBX2660 for C. difficile infection.[126] Additionally, EXE-346, which is composed of eight live strains of probiotics with fixed proportions, has been approved as an orphan drug by the US Food and Drug Administration. These live biotherapeutic products will provide new clinical options for disease treatment.
Future prospects
In this review, we evaluated a large number of preclinical and clinical studies that have confirmed the involvement of gut microbiota dysbiosis in the pathogenesis of neurological diseases and carried out a preliminary exploration of the underlying molecular mechanisms in the MGB axis. Animal models provide useful tools to explore the role of gut microbiota in the onset of neurological disease. However, extending these findings to the diagnosis and treatment of neurological diseases in humans is still a substantial challenge. The obvious biological differences between animals and humans and the complexity, individuality, and dynamics of gut microbiota in humans aggravate this challenge. The gut is estimated to contain approximately 1 × 1015 bacteria. Only 1500 bacterial species have been isolated thus far, which indicates that our research in the gut microbial field is still in its infancy. Clarification of gut microbiota composition and the effects of various confounders on microbial characteristics under healthy conditions is the first step to determine whether gut microbiota dysbiosis plays a pathogenic role in the onset of disease. Future investigations must consider the confounding factors that influence gut microbial composition and function as well as the bidirectionality of gut microbiota–host interactions under healthy and diseased states. Examination of key gut microbiota components and their derived markers and revealing the underlying gut–brain molecular mechanisms will be essential in the development of gut microbiota-based diagnosis and treatment strategies for neurological diseases. Beginning with the discovery of gut microbiota, the recognition of gut microbiota–host interactions and the use of gut microbiota as treatments are promising approaches to promote human health. However, the clinical application of gut microbiota-based diagnostic and treatment strategies for diseases will require rigorous evaluation that will provide guidance for future research.
Funding
This study was supported by the National Key R&D Program of China (No. 2017YFA0505700), the Non-Profit Central Research Institute Fund of the Chinese Academy of Medical Sciences (No. 2019PT320002), the Natural Science Foundation Project of China (No. 81820108015), the China Postdoctoral Science Foundation (Nos. 2020TQ0393, 2020M683634XB, and 2021M693926), and the Chongqing Science and Technology Commission (Nos. cstc2021jcyj-bsh0026 and cstc2021jcyj-bsh0034).
Conflicts of interest
None.
Footnotes
How to cite this article: Liu L, Wang H, Chen X, Xie P. Gut microbiota: a new insight into neurological diseases. Chin Med J 2023;136:1261–1277. doi: 10.1097/CM9.0000000000002212
References
- 1.Cummings JH, Macfarlane GT. Role of intestinal bacteria in nutrient metabolism. JPEN J Parenter Enteral Nutr 1997; 21:357–365. doi: 10.1177/0148607197021006357. [DOI] [PubMed] [Google Scholar]
- 2.Lavelle A, Sokol H. Gut microbiota-derived metabolites as key actors in inflammatory bowel disease. Nat Rev Gastroenterol Hepatol 2020; 17:223–237. doi: 10. 1038/s41575-019-0258-z. [DOI] [PubMed] [Google Scholar]
- 3.Tao J, Li S, Gan RY, Zhao CN, Meng X, Li HB. Targeting gut microbiota with dietary components on cancer: effects and potential mechanisms of action. Crit Rev Food Sci Nutr 2020; 60:1025–1037. doi: 10.1080/10408398.2018.1555789. [DOI] [PubMed] [Google Scholar]
- 4.Riva A, Kuzyk O, Forsberg E, Siuzdak G, Pfann C, Herbold C, et al. A fiber-deprived diet disturbs the fine-scale spatial architecture of the murine colon microbiome. Nat Commun 2019; 10:4366.doi: 10.1038/s41467-019-12413-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wan Y, Wang F, Yuan J, Li J, Jiang D, Zhang J, et al. Effects of dietary fat on gut microbiota and faecal metabolites, and their relationship with cardiometabolic risk factors: a 6-month randomised controlled-feeding trial. Gut 2019; 68:1417–1429. doi: 10.1136/gutjnl-2018-317609. [DOI] [PubMed] [Google Scholar]
- 6.Neufeld KM, Kang N, Bienenstock J, Foster JA. Reduced anxiety-like behavior and central neurochemical change in germ-free mice. Neurogastroenterol Motil 2011; 23:255–264. doi: 10.1111/j.1365-2982.2010.01620.x. [DOI] [PubMed] [Google Scholar]
- 7.Cryan JF, Dinan TG. Mind-altering microorganisms: the impact of the gut microbiota on brain and behaviour. Nat Rev Neurosci 2012; 13:701–712. doi: 10.1038/nrn3346. [DOI] [PubMed] [Google Scholar]
- 8.Han W, Tellez LA, Perkins MH, Perez IO, Qu T, Ferreira J, et al. A neural circuit for gut-induced reward. Cell 2018; 175:665–678. doi: 10.1016/j.cell.2018.08.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kaelberer MM, Buchanan KL, Klein ME, Barth BB, Montoya MM, Shen X, et al. A gut-brain neural circuit for nutrient sensory transduction. Science 2018; 361:eaat5236.doi: 10.1126/science.aat5236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Luo Y, Zeng B, Zeng L, Du X, Li B, Huo R, et al. Gut microbiota regulates mouse behaviors through glucocorticoid receptor pathway genes in the hippocampus. Transl Psychiatry 2018; 8:187.doi: 10.1038/s41398-018-0240-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dinan TG, Cryan JF. Regulation of the stress response by the gut microbiota: implications for psychoneuroendocrinology. Psychoneuroendocrinology 2012; 37:1369–1378. doi: 10.1016/j.psyneuen.2012.03.007. [DOI] [PubMed] [Google Scholar]
- 12.Merola B, Longobardi S, Colao A, Di Somma C, Ferone D, Rossi E, et al. Hypothalamic-pituitary-adrenal axis in neuropsychiatric disorders. Ann N Y Acad Sci 1994; 741:263–270. doi: 10.1111/j.1749-6632.1994.tb23109.x. [DOI] [PubMed] [Google Scholar]
- 13.Agirman G, Yu KB, Hsiao EY. Signaling inflammation across the gut-brain axis. Science 2021; 374:1087–1092. doi: 10.1126/science.abi6087. [DOI] [PubMed] [Google Scholar]
- 14.McCarville JL, Chen GY, Cuevas VD, Troha K, Ayres JS. Microbiota metabolites in health and disease. Annu Rev Immunol 2020; 38:147–170. doi: 10.1146/annurev-immunol-071219-125715. [DOI] [PubMed] [Google Scholar]
- 15.O’Riordan KJ, Collins MK, Moloney GM, Knox EG, Aburto MR, Fülling C, et al. Short chain fatty acids: microbial metabolites for gut-brain axis signalling. Mol Cell Endocrinol 2022; 546:111572.doi: 10.1016/j.mce.2022.111572. [DOI] [PubMed] [Google Scholar]
- 16.Thibaut MM, Bindels LB. Crosstalk between bile acid-activated receptors and microbiome in entero-hepatic inflammation. Trends Mol Med 2022; 28:223–236. doi: 10.1016/j.molmed.2021.12.006. [DOI] [PubMed] [Google Scholar]
- 17.Camara-Lemarroy CR, Metz L, Meddings JB, Sharkey KA, Wee Yong V. The intestinal barrier in multiple sclerosis: implications for pathophysiology and therapeutics. Brain 2018; 141:1900–1916. doi: 10.1093/brain/awy131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Parker A, Fonseca S, Carding SR. Gut microbes and metabolites as modulators of blood-brain barrier integrity and brain health. Gut Microbes 2020; 11:135–157. doi: 10.1080/19490976.2019.1638722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ait-Belgnaoui A, Durand H, Cartier C, Chaumaz G, Eutamene H, Ferrier L, et al. Prevention of gut leakiness by a probiotic treatment leads to attenuated HPA response to an acute psychological stress in rats. Psychoneuroendocrinology 2012; 37:1885–1895. doi: 10.1016/j.psyneuen.2012.03.024. [DOI] [PubMed] [Google Scholar]
- 20.Williams SCP. Gnotobiotics. Proc Natl Acad Sci U S A 2014; 111:1661.doi: 10.1073/pnas.1324049111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zheng P, Zeng B, Liu M, Chen J, Pan J, Han Y, et al. The gut microbiome from patients with schizophrenia modulates the glutamate-glutamine-GABA cycle and schizophrenia-relevant behaviors in mice. Sci Adv 2019; 5:eaau8317.doi: 10.1126/sciadv.aau8317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhu F, Guo R, Wang W, Ju Y, Wang Q, Ma Q, et al. Transplantation of microbiota from drug-free patients with schizophrenia causes schizophrenia-like abnormal behaviors and dysregulated kynurenine metabolism in mice. Mol Psychiatry 2020; 25:2905–2918. doi: 10.1038/s41380-019-0475-4. [DOI] [PubMed] [Google Scholar]
- 23.Kelly JR, Borre Y, O’Brien C, Patterson E, El Aidy S, Deane J, et al. Transferring the blues: depression-associated gut microbiota induces neurobehavioural changes in the rat. J Psychiatr Res 2016; 82:109–118. doi: 10.1016/j.jpsychires.2016.07.019. [DOI] [PubMed] [Google Scholar]
- 24.Zheng P, Zeng B, Zhou C, Liu M, Fang Z, Xu X, et al. Gut microbiome remodeling induces depressive-like behaviors through a pathway mediated by the host's metabolism. Mol Psychiatry 2016; 21:786–796. doi: 10.1038/mp.2016.44. [DOI] [PubMed] [Google Scholar]
- 25.Sharon G, Cruz NJ, Kang DW, Gandal MJ, Wang B, Kim YM, et al. Human gut microbiota from autism spectrum disorder promote behavioral symptoms in mice. Cell 2019; 177:e1600–e1618. doi: 10.1016/j.cell.2019.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang W, Zhang N, Du Y, Gao J, Li M, Lin L, et al. Three-dimensional quantitative imaging of native microbiota distribution in the gut. Angew Chem Int Ed Engl 2021; 60:3055–3061. doi: 10.1002/anie.202010921. [DOI] [PubMed] [Google Scholar]
- 27.Ashammakhi N, Nasiri R, Barros NR, Tebon P, Thakor J, Goudie M, et al. Gut-on-a-chip: current progress and future opportunities. Biomaterials 2020; 255:120196.doi: 10.1016/j.biomaterials.2020.120196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang S, Harvey L, Martin R, van der Beek EM, Knol J, Cryan JF, et al. Targeting the gut microbiota to influence brain development and function in early life. Neurosci Biobehav Rev 2018; 95:191–201. doi: 10.1016/j.neubiorev.2018.09.002. [DOI] [PubMed] [Google Scholar]
- 29.Keogh CE, Kim DHJ, Pusceddu MM, Knotts TA, Rabasa G, Sladek JA, et al. Myelin as a regulator of development of the microbiota-gut-brain axis. Brain Behav Immun 2021; 91:437–450. doi: 10.1016/j.bbi.2020.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Agirman G, Hsiao EY. SnapShot: the microbiota-gut-brain axis. Cell 2021; 184:2524e–e12524. doi: 10.1016/j.cell.2021.03.022. [DOI] [PubMed] [Google Scholar]
- 31.Sampson TR, Mazmanian SK. Control of brain development, function, and behavior by the microbiome. Cell Host Microbe 2015; 17:565–576. doi: 10.1016/j.chom.2015.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Carlson AL, Xia K, Azcarate-Peril MA, Goldman BD, Ahn M, Styner MA, et al. Infant gut microbiome associated with cognitive development. Biol Psychiatry 2018; 83:148–159. doi: 10.1016/j.biopsych.2017.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gao W, Salzwedel AP, Carlson AL, Xia K, Azcarate-Peril MA, Styner MA, et al. Gut microbiome and brain functional connectivity in infants-a preliminary study focusing on the amygdala. Psychopharmacology (Berl) 2019; 236:1641–1651. doi: 10.1007/s00213-018-5161-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang Y, Zhong Z, Wang B, Xia X, Yao W, Huang L, et al. Early-life high-fat diet-induced obesity programs hippocampal development and cognitive functions via regulation of gut commensal Akkermansia muciniphila. Neuropsychopharmacology 2019; 44:2054–2064. doi: 10.1038/s41386-019-0437-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cryan JF, O’Riordan KJ, Sandhu K, Peterson V, Dinan TG. The gut microbiome in neurological disorders. Lancet Neurol 2020; 19:179–194. doi: 10.1016/S1474-4422(19)30356-4. [DOI] [PubMed] [Google Scholar]
- 36.Braniste V, Al-Asmakh M, Kowal C, Anuar F, Abbaspour A, Tóth M, et al. The gut microbiota influences blood-brain barrier permeability in mice. Sci Transl Med 2014; 6:263ra158.doi: 10.1126/scitranslmed.3009759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cámara-Lemarroy CR, Rodriguez-Gutierrez R, Monreal-Robles R, Marfil-Rivera A. Gastrointestinal disorders associated with migraine: a comprehensive review. World J Gastroenterol 2016; 22:8149–8160. doi: 10.3748/wjg.v22.i36.8149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gonzalez A, Hyde E, Sangwan N, Gilbert JA, Viirre E, Knight R. Migraines are correlated with higher levels of nitrate-, nitrite-, and nitric oxide-reducing oral microbes in the American Gut Project Cohort. mSystems 2016; 1:e105–e116. doi: 10.1128/mSystems.00105-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen J, Wang Q, Wang A, Lin Z. Structural and functional characterization of the gut microbiota in elderly women with migraine. Front Cell Infect Microbiol 2019; 9:470.doi: 10.3389/fcimb.2019.00470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tang Y, Liu S, Shu H, Yanagisawa L, Tao F. Gut microbiota dysbiosis enhances migraine-like pain via tnfα upregulation. Mol Neurobiol 2020; 57:461–468. doi: 10.1007/s12035-019-01721-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kang L, Tang W, Zhang Y, Zhang M, Liu J, Li Y, et al. The gut microbiome modulates nitroglycerin-induced migraine-related hyperalgesia in mice. Cephalalgia 2021; 3331024211050036.doi: 10.1177/03331024211050036. [DOI] [PubMed] [Google Scholar]
- 42.Qi X, Fan G, Jia H. The probiotic Shirota attenuates symptoms of vestibular migraine: a randomised placebo-controlled double-blind clinical trial. Benef Microbes 2020; 11:469–476. doi: 10.3920/BM2020.0058. [DOI] [PubMed] [Google Scholar]
- 43.de Roos NM, van Hemert S, Rovers JMP, Smits MG, Witteman BJM. The effects of a multispecies probiotic on migraine and markers of intestinal permeability-results of a randomized placebo-controlled study. Eur J Clin Nutr 2017; 71:1455–1462. doi: 10.1038/ejcn.2017.57. [DOI] [PubMed] [Google Scholar]
- 44.Martami F, Togha M, Seifishahpar M, Ghorbani Z, Ansari H, Karimi T, et al. The effects of a multispecies probiotic supplement on inflammatory markers and episodic and chronic migraine characteristics: a randomized double-blind controlled trial. Cephalalgia 2019; 39:841–853. doi: 10.1177/0333102418820102. [DOI] [PubMed] [Google Scholar]
- 45.Cheng Y, Zan J, Song Y, Yang G, Shang H, Zhao W. Evaluation of intestinal injury, inflammatory response and oxidative stress following intracerebral hemorrhage in mice. Int J Mol Med 2018; 42:2120–2128. doi: 10.3892/ijmm.2018.3755. [DOI] [PubMed] [Google Scholar]
- 46.Yu X, Zhou G, Shao B, Zhou H, Xu C, Yan F, et al. Gut microbiota dysbiosis induced by intracerebral hemorrhage aggravates neuroinflammation in mice. Front Microbiol 2021; 12:647304.doi: 10.3389/fmicb.2021.647304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Di Napoli M, Parry-Jones AR, Smith CJ, Hopkins SJ, Slevin M, Masotti L, et al. C-reactive protein predicts hematoma growth in intracerebral hemorrhage. Stroke 2014; 45:59–65. doi: 10.1161/STROKEAHA.113.001721. [DOI] [PubMed] [Google Scholar]
- 48.Zheng GR, Chen B, Shen J, Qiu SZ, Yin HM, Mao W, et al. Serum myeloperoxidase concentrations for outcome prediction in acute intracerebral hemorrhage. Clin Chim Acta 2018; 487:330–336. doi: 10.1016/j.cca.2018.10.026. [DOI] [PubMed] [Google Scholar]
- 49.Li Y, Xu H, Wang J, Zheng F, Deng L, Li J, et al. Analysis of bacterial flora structure of patients with cerebral hemorrhage due to hyperactivity of liver-yang by 16S rRNA gene sequencing technique (in Chinese). Chin J Exp Tradit Med Formulae 2019; 25:83–88. [Google Scholar]
- 50.Polster SP, Sharma A, Tanes C, Tang AT, Mericko P, Cao Y, et al. Permissive microbiome characterizes human subjects with a neurovascular disease cavernous angioma. Nat Commun 2020; 11:2659.doi: 10.1038/s41467-020-16436-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Xu K, Gao X, Xia G, Chen M, Zeng N, Wang S, et al. Rapid gut dysbiosis induced by stroke exacerbates brain infarction in turn. Gut 2021; gutjnl–2020–323263. doi: 10.1136/gutjnl-2020-323263. [DOI] [PubMed] [Google Scholar]
- 52.Haak BW, Westendorp WF, van Engelen TSR, Brands X, Brouwer MC, Vermeij J-D, et al. Disruptions of anaerobic gut bacteria are associated with stroke and post-stroke infection: a prospective case-control study. Transl Stroke Res 2021; 12:581–592. doi: 10.1007/s12975-020-00863-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chen Y, Liang J, Ouyang F, Chen X, Lu T, Jiang Z, et al. Persistence of gut microbiota dysbiosis and chronic systemic inflammation after cerebral infarction in cynomolgus monkeys. Front Neurol 2019; 10:661.doi: 10.3389/fneur.2019.00661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chen R, Xu Y, Wu P, Zhou H, Lasanajak Y, Fang Y, et al. Transplantation of fecal microbiota rich in short chain fatty acids and butyric acid treat cerebral ischemic stroke by regulating gut microbiota. Pharmacol Res 2019; 148:104403.doi: 10.1016/j.phrs.2019.104403. [DOI] [PubMed] [Google Scholar]
- 55.Lee J, d’Aigle J, Atadja L, Quaicoe V, Honarpisheh P, Ganesh BP, et al. Gut microbiota–derived short-chain fatty acids promote poststroke recovery in aged mice. Circ Res 2020; 127:453–465. doi: 10.1161/circresaha.119.316448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cattaneo A, Cattane N, Galluzzi S, Provasi S, Lopizzo N, Festari C, et al. Association of brain amyloidosis with pro-inflammatory gut bacterial taxa and peripheral inflammation markers in cognitively impaired elderly. Neurobiol Aging 2017; 49:60–68. doi: 10.1016/j.neurobiolaging.2016.08.019. [DOI] [PubMed] [Google Scholar]
- 57.Mancuso C, Santangelo R. Alzheimer's disease and gut microbiota modifications: the long way between preclinical studies and clinical evidence. Pharmacol Res 2018; 129:329–336. doi: 10.1016/j.phrs.2017.12.009. [DOI] [PubMed] [Google Scholar]
- 58.Li B, He Y, Ma J, Huang P, Du J, Cao L, et al. Mild cognitive impairment has similar alterations as Alzheimer's disease in gut microbiota. Alzheimers Dement 2019; 15:1357–1366. doi: 10.1016/j.jalz.2019.07.002. [DOI] [PubMed] [Google Scholar]
- 59.Liu P, Jia XZ, Chen Y, Yu Y, Zhang K, Lin YJ, et al. Gut microbiota interacts with intrinsic brain activity of patients with amnestic mild cognitive impairment. CNS Neurosci Ther 2021; 27:163–173. doi: 10.1111/cns.13451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ling Z, Zhu M, Yan X, Cheng Y, Shao L, Liu X, et al. Structural and functional dysbiosis of fecal microbiota in chinese patients with alzheimer's disease. Front Cell Dev Biol 2020; 8:634069.doi: 10.3389/fcell.2020.634069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang M, Cao J, Gong C, Amakye WK, Yao M, Ren J. Exploring the microbiota-Alzheimer's disease linkage using short-term antibiotic treatment followed by fecal microbiota transplantation. Brain Behav Immun 2021; 96:227–238. doi: 10.1016/j.bbi.2021.06.003. [DOI] [PubMed] [Google Scholar]
- 62.Bonfili L, Cecarini V, Berardi S, Scarpona S, Suchodolski JS, Nasuti C, et al. Microbiota modulation counteracts Alzheimer's disease progression influencing neuronal proteolysis and gut hormones plasma levels. Sci Rep 2017; 7:2426.doi: 10.1038/s41598-017-02587-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tillisch K, Labus J, Kilpatrick L, Jiang Z, Stains J, Ebrat B, et al. Consumption of fermented milk product with probiotic modulates brain activity. Gastroenterology 2013; 144:1394–1401. doi: 10.1053/j.gastro.2013.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ueda A, Shinkai S, Shiroma H, Taniguchi Y, Tsuchida S, Kariya T, et al. Identification of strains for gut microbiome-based intervention in Alzheimer's-type dementia. Cell Rep Med 2021; 2:100398.doi: 10.1016/j.xcrm.2021.100398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Barichella M, Severgnini M, Cilia R, Cassani E, Bolliri C, Caronni S, et al. Unraveling gut microbiota in Parkinson's disease and atypical parkinsonism. Mov Disord 2019; 34:396–405. doi: 10.1002/mds.27581. [DOI] [PubMed] [Google Scholar]
- 66.Wang Q, Luo Y, Chaudhuri KR, Reynolds R, Tan E-K, Pettersson S. The role of gut dysbiosis in Parkinson's disease: mechanistic insights andtherapeutic options. Brain 2021; 144:2571–2593. doi: 10.1093/brain/awab156. [DOI] [PubMed] [Google Scholar]
- 67.Kim S, Kwon SH, Kam TI, Panicker N, Karuppagounder SS, Lee S, et al. Transneuronal propagation of pathologic α-synuclein from the gut to the brain models parkinson's disease. Neuron 2019; 103:627–641. doi: 10.1016/j.neuron.2019.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sampson TR, Debelius JW, Thron T, Janssen S, Shastri GG, Ilhan ZE, et al. Gut microbiota regulate motor deficits and neuroinflammation in a model of parkinson's disease. Cell 2016; 167:1469–1480. doi: 10.1016/j.cell.2016.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ahn EH, Kang SS, Liu X, Chen G, Zhang Z, Chandrasekharan B, et al. Initiation of Parkinson's disease from gut to brain by δ-secretase. Cell Res 2020; 30:70–87. doi: 10.1038/s41422-019-0241-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Li Z, Lu G, Li Z, Wu B, Luo E, Qiu X, et al. Altered actinobacteria and firmicutes phylum associated epitopes in patients with parkinson's disease. Front Immunol 2021; 12:632482.doi: 10.3389/fimmu.2021.632482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Reich DS, Lucchinetti CF, Calabresi PA. Multiple Sclerosis. N Engl J Med 2018; 378:169–180. doi: 10.1056/NEJMra1401483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tremlett H, Fadrosh DW, Faruqi AA, Zhu F, Hart J, Roalstad S, et al. Gut microbiota in early pediatric multiple sclerosis: a case-control study. Eur J Neurol 2016; 23:1308–1321. doi: 10.1111/ene.13026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jangi S, Gandhi R, Cox LM, Li N, von Glehn F, Yan R, et al. Alterations of the human gut microbiome in multiple sclerosis. Nat Commun 2016; 7:12015.doi: 10.1038/ncomms12015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chen J, Chia N, Kalari KR, Yao JZ, Novotna M, Paz Soldan MM, et al. Multiple sclerosis patients have a distinct gut microbiota compared to healthy controls. Sci Rep 2016; 6:28484.doi: 10.1038/srep28484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Miyake S, Kim S, Suda W, Oshima K, Nakamura M, Matsuoka T, et al. Dysbiosis in the gut microbiota of patients with multiple sclerosis, with a striking depletion of species belonging to clostridia XIVa and IV clusters. PLoS One 2015; 10:e0137429.doi: 10.1371/journal.pone.0137429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tankou SK, Regev K, Healy BC, Cox LM, Tjon E, Kivisakk P, et al. Investigation of probiotics in multiple sclerosis. Mult Scler 2018; 24:58–63. doi: 10.1177/1352458517737390. [DOI] [PubMed] [Google Scholar]
- 77.Tankou SK, Regev K, Healy BC, Tjon E, Laghi L, Cox LM, et al. A probiotic modulates the microbiome and immunity in multiple sclerosis. Ann Neurol 2018; 83:1147–1161. doi: 10.1002/ana.25244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Calvo-Barreiro L, Eixarch H, Cornejo T, Costa C, Castillo M, Mestre L, et al. Selected clostridia strains from the human microbiota and their metabolite, butyrate, improve experimental autoimmune encephalomyelitis. Neurotherapeutics 2021; 18:920–937. doi: 10.1007/s13311-021-01016-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Duscha A, Gisevius B, Hirschberg S, Yissachar N, Stangl GI, Eilers E, et al. Propionic acid shapes the multiple sclerosis disease course by an immunomodulatory mechanism. Cell 2020; 180:1067–1080. doi: 10.1016/j.cell.2020.02.035. [DOI] [PubMed] [Google Scholar]
- 80.Shi Z, Qiu Y, Wang J, Fang Y, Zhang Y, Chen H, et al. Dysbiosis of gut microbiota in patients with neuromyelitis optica spectrum disorders: a cross sectional study. J Neuroimmunol 2020; 339:577126.doi: 10.1016/j.jneuroim.2019.577126. [DOI] [PubMed] [Google Scholar]
- 81.Gong J, Qiu W, Zeng Q, Liu X, Sun X, Li H, et al. Lack of short-chain fatty acids and overgrowth of opportunistic pathogens define dysbiosis of neuromyelitis optica spectrum disorders: a Chinese pilot study. Mult Scler 2019; 25:1316–1325. doi: 10.1177/1352458518790396. [DOI] [PubMed] [Google Scholar]
- 82.Pandit L, Cox LM, Malli C, D’Cunha A, Rooney T, Lokhande H, et al. Clostridium bolteae is elevated in neuromyelitis optica spectrum disorder in India and shares sequence similarity with AQP4. Neurol Neuroimmunol Neuroinflamm 2021; 8:e907.doi: 10.1212/NXI.0000000000000907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zhang J, Xu YF, Wu L, Li HF, Wu ZY. Characteristic of gut microbiota in southeastern Chinese patients with neuromyelitis optica spectrum disorders. Mult Scler Relat Disord 2020; 44:102217.doi: 10.1016/j.msard.2020.102217. [DOI] [PubMed] [Google Scholar]
- 84.Cui C, Tan S, Tao L, Gong J, Chang Y, Wang Y, et al. Intestinal barrier breakdown and mucosal microbiota disturbance in neuromyelitis optical spectrum disorders. Front Immunol 2020; 11:2101.doi: 10.3389/fimmu.2020.02101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Cheng X, Zhou L, Li Z, Shen S, Zhao Y, Liu C, et al. Gut microbiome and bile acid metabolism induced the activation of CXCR5+ CD4+ T follicular helper cells to participate in neuromyelitis optica spectrum disorder recurrence. Front Immunol 2022; 13:827865.doi: 10.3389/fimmu.2022.827865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Levy M, Kolodziejczyk AA, Thaiss CA, Elinav E. Dysbiosis and the immune system. Nat Rev Immunol 2017; 17:219–232. doi: 10.1038/nri.2017.7. [DOI] [PubMed] [Google Scholar]
- 87.Zheng P, Li Y, Wu J, Zhang H, Huang Y, Tan X, et al. Perturbed microbial ecology in myasthenia gravis: evidence from the gut microbiome and fecal metabolome. Adv Sci (Weinh) 2019; 6:1901441.doi: 10.1002/advs.201901441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Qiu D, Xia Z, Jiao X, Deng J, Zhang L, Li J. Altered gut microbiota in myasthenia gravis. Front Microbiol 2018; 9:2627.doi: 10.3389/fmicb.2018.02627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Liu P, Jiang Y, Gu S, Xue Y, Yang H, Li Y, et al. Metagenome-wide association study of gut microbiome revealed potential microbial marker set for diagnosis of pediatric myasthenia gravis. BMC Med 2021; 19:159.doi: 10.1186/s12916-021-02034-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Consonni A, Cordiglieri C, Rinaldi E, Marolda R, Ravanelli I, Guidesi E, et al. Administration of bifidobacterium and lactobacillus strains modulates experimental myasthenia gravis and experimental encephalomyelitis in Lewis rats. Oncotarget 2018; 9:22269–22287. doi: 10.18632/oncotarget.25170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Şafak B, Altunan B, Topçu B, Eren Topkaya A. The gut microbiome in epilepsy. Microb Pathog 2020; 139:103853.doi: 10.1016/j.micpath.2019.103853. [DOI] [PubMed] [Google Scholar]
- 92.Iannone LF, Gómez-Eguílaz M, Citaro R, Russo E. The potential role of interventions impacting on gut-microbiota in epilepsy. Expert Rev Clin Pharmacol 2020; 13:423–435. doi: 10.1080/17512433.2020.1759414. [DOI] [PubMed] [Google Scholar]
- 93.Olson CA, Vuong HE, Yano JM, Liang QY, Nusbaum DJ, Hsiao EY. The gut microbiota mediates the anti-seizure effects of the ketogenic diet. Cell 2018; 173:1728–1741. doi: 10.1016/j.cell.2018.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Xie G, Zhou Q, Qiu CZ, Dai WK, Wang HP, Li YH, et al. Ketogenic diet poses a significant effect on imbalanced gut microbiota in infants with refractory epilepsy. World J Gastroenterol 2017; 23:6164–6171. doi: 10.3748/wjg.v23.i33.6164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zhang Y, Zhou S, Zhou Y, Yu L, Zhang L, Wang Y. Altered gut microbiome composition in children with refractory epilepsy after ketogenic diet. Epilepsy Res 2018; 145:163–168. doi: 10.1016/j.eplepsyres.2018.06.015. [DOI] [PubMed] [Google Scholar]
- 96.Lindefeldt M, Eng A, Darban H, Bjerkner A, Zetterström CK, Allander T, et al. The ketogenic diet influences taxonomic and functional composition of the gut microbiota in children with severe epilepsy. NPJ Biofilms Microbiomes 2019; 5:5.doi: 10.1038/s41522-018-0073-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.He Z, Cui BT, Zhang T, Li P, Long CY, Ji GZ, et al. Fecal microbiota transplantation cured epilepsy in a case with Crohn's disease: the first report. World J Gastroenterol 2017; 23:3565–3568. doi: 10.3748/wjg.v23.i19.3565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zhang M, Ma W, Zhang J, He Y, Wang J. Analysis of gut microbiota profiles and microbe-disease associations in children with autism spectrum disorders in China. Sci Rep 2018; 8:13981.doi: 10.1038/s41598-018-32219-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kim S, Kim H, Yim YS, Ha S, Atarashi K, Tan TG, et al. Maternal gut bacteria promote neurodevelopmental abnormalities in mouse offspring. Nature 2017; 549:528–532. doi:10.1038/nature23910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zhang M, Chu Y, Meng Q, Ding R, Shi X, Wang Z, et al. A quasi-paired cohort strategy reveals the impaired detoxifying function of microbes in the gut of autistic children. Sci Adv 2020; 6:eaba3760.doi: 10.1126/sciadv.aba3760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Needham BD, Adame MD, Serena G, Rose DR, Preston GM, Conrad MC, et al. Plasma and fecal metabolite profiles in autism spectrum disorder. Biol Psychiatry 2021; 89:451–462. doi: 10.1016/j.biopsych.2020.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Bermudez-Martin P, Becker JAJ, Caramello N, Fernandez SP, Costa-Campos R, Canaguier J, et al. The microbial metabolite p-Cresol induces autistic-like behaviors in mice by remodeling the gut microbiota. Microbiome 2021; 9:157.doi: 10.1186/s40168-021-01103-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Li N, Chen H, Cheng Y, Xu F, Ruan G, Ying S, et al. Fecal microbiota transplantation relieves gastrointestinal and autism symptoms by improving the gut microbiota in an Open-Label Study. Front Cell Infect Microbiol 2021; 11:759435.doi: 10.3389/fcimb.2021.759435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Chen JJ, Zheng P, Liu YY, Zhong XG, Wang HY, Guo YJ, et al. Sex differences in gut microbiota in patients with major depressive disorder. Neuropsychiatr Dis Treat 2018; 14:647–655. doi: 10.2147/NDT.S159322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Chen Z, Li J, Gui S, Zhou C, Chen J, Yang C, et al. Comparative metaproteomics analysis shows altered fecal microbiota signatures in patients with major depressive disorder. Neuroreport 2018; 29:417–425. doi: 10.1097/WNR.0000000000000985. [DOI] [PubMed] [Google Scholar]
- 106.Chung YE, Chen HC, Chou HL, Chen IM, Lee MS, Chuang LC, et al. Exploration of microbiota targets for major depressive disorder and mood related traits. J Psychiatr Res 2019; 111:74–82. doi: 10.1016/j.jpsychires.2019.01.016. [DOI] [PubMed] [Google Scholar]
- 107.Lai WT, Zhao J, Xu SX, Deng WF, Xu D, Wang MB, et al. Shotgun metagenomics reveals both taxonomic and tryptophan pathway differences of gut microbiota in bipolar disorder with current major depressive episode patients. J Affect Disord 2021; 278:311–319. doi: 10.1016/j.jad.2020.09.010. [DOI] [PubMed] [Google Scholar]
- 108.Rong H, Xie XH, Zhao J, Lai WT, Wang MB, Xu D, et al. Similarly in depression, nuances of gut microbiota: evidences from a shotgun metagenomics sequencing study on major depressive disorder versus bipolar disorder with current major depressive episode patients. J Psychiatr Res 2019; 113:90–99. doi: 10.1016/j.jpsychires.2019.03.017. [DOI] [PubMed] [Google Scholar]
- 109.Jiang H, Ling Z, Zhang Y, Mao H, Ma Z, Yin Y, et al. Altered fecal microbiota composition in patients with major depressive disorder. Brain Behav Immun 2015; 48:186–194. doi: 10.1016/j.bbi.2015.03.016. [DOI] [PubMed] [Google Scholar]
- 110.Lin P, Ding B, Feng C, Yin S, Zhang T, Qi X, et al. Prevotella and Klebsiella proportions in fecal microbial communities are potential characteristic parameters for patients with major depressive disorder. J Affect Disord 2017; 207:300–304. doi: 10.1016/j.jad.2016.09.051. [DOI] [PubMed] [Google Scholar]
- 111.Aizawa E, Tsuji H, Asahara T, Takahashi T, Teraishi T, Yoshida S, et al. Possible association of Bifidobacterium and Lactobacillus in the gut microbiota of patients with major depressive disorder. J Affect Disord 2016; 202:254–257. doi: 10.1016/j.jad.2016.05.038. [DOI] [PubMed] [Google Scholar]
- 112.Simpson CA, Diaz-Arteche C, Eliby D, Schwartz OS, Simmons JG, Cowan CSM. The gut microbiota in anxiety and depression - a systematic review. Clin Psychol Rev 2021; 83:101943.doi: 10.1016/j.cpr.2020.101943. [DOI] [PubMed] [Google Scholar]
- 113.Zheng P, Yang J, Li Y, Wu J, Liang W, Yin B, et al. Gut microbial signatures can discriminate unipolar from bipolar depression. Adv Sci (Weinh) 2020; 7:1902862.doi: 10.1002/advs.201902862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Yang J, Zheng P, Li Y, Wu J, Tan X, Zhou J, et al. Landscapes of bacterial and metabolic signatures and their interaction in major depressive disorders. Sci Adv 2020; 6:eaba8555.doi: 10.1126/sciadv.aba8555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Chen HM, Kuo PH, Hsu CY, Chiu YH, Liu YW, Lu ML, et al. Psychophysiological effects of PS128 in patients with major depressive disorder: a preliminary 8-week open trial. Nutrients 2021; 13:3731.doi: 10.3390/nu13113731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ho YT, Tsai YC, Kuo TBJ, Yang CCH. Effects of PS128 on depressive symptoms and sleep quality in self-reported insomniacs: a randomized, double-blind, placebo-controlled pilot trial. Nutrients 2021; 13:2820.doi: 10.3390/nu13082820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Chinna Meyyappan A, Forth E, Milev R. Microbial ecosystem therapeutic-2 intervention in people with major depressive disorder and generalized anxiety disorder: Phase 1, Open-Label Study. Interact J Med Res 2022; 11:e32234.doi: 10.2196/32234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Zheng P, Wu J, Zhang H, Perry SW, Yin B, Tan X, et al. The gut microbiome modulates gut-brain axis glycerophospholipid metabolism in a region-specific manner in a nonhuman primate model of depression. Mol Psychiatry 2020; 26:2380–2392. doi: 10.1038/s41380-020-0744-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Liu L, Wang H, Yu Y, Zeng B, Rao X, Chen J, et al. Microbial regulation of a lincRNA-miRNA-mRNA network in the mouse hippocampus. Epigenomics 2020; 12:1377–1387. doi: 10.2217/epi-2019-0307. [DOI] [PubMed] [Google Scholar]
- 120.Wang H, Liu L, Rao X, Zeng B, Yu Y, Zhou C, et al. Integrated phosphoproteomic and metabolomic profiling reveals perturbed pathways in the hippocampus of gut microbiota dysbiosis mice. Transl Psychiatry 2020; 10:346.doi: 10.1038/s41398-020-01024-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Huang C, Yang X, Zeng B, Zeng L, Gong X, Zhou C, et al. Proteomic analysis of olfactory bulb suggests CACNA1E as a promoter of CREB signaling in microbiota-induced depression. J Proteomics 2019; 194:132–147. doi: 10.1016/j.jprot.2018.11.023. [DOI] [PubMed] [Google Scholar]
- 122.Liu L, Wang H, Rao X, Yu Y, Li W, Zheng P, et al. Comprehensive analysis of the lysine acetylome and succinylome in the hippocampus of gut microbiota-dysbiosis mice. J Adv Res 2021; 30:27–38. doi: 10.1016/j.jare.2020.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Chevalier G, Siopi E, Guenin-Macé L, Pascal M, Laval T, Rifflet A, et al. Effect of gut microbiota on depressive-like behaviors in mice is mediated by the endocannabinoid system. Nat Commun 2020; 11:6363.doi: 10.1038/s41467-020-19931-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Zhang T, Lu G, Zhao Z, Liu Y, Shen Q, Li P, et al. Washed microbiota transplantation vs. manual fecal microbiota transplantation: clinical findings, animal studies and in vitro screening. Protein Cell 2020; 11:251–266. doi: 10.1007/s13238-019-00684-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Feuerstadt P, Louie TJ, Lashner B, Wang EEL, Diao L, Bryant JA, et al. SER-109, an oral microbiome therapy for recurrent infection. N Engl J Med 2022; 386:220–229. doi: 10.1056/NEJMoa2106516. [DOI] [PubMed] [Google Scholar]
- 126.Langdon A, Schwartz DJ, Bulow C, Sun X, Hink T, Reske KA, et al. Microbiota restoration reduces antibiotic-resistant bacteria gut colonization in patients with recurrent Clostridioides difficile infection from the open-label PUNCH CD study. Genome Med 2021; 13:28.doi: 10.1186/s13073-021-00843-9. [DOI] [PMC free article] [PubMed] [Google Scholar]