PURPOSE
The aim of this study was to assess the cost-effectiveness of using next-generation sequencing (NGS) versus single-gene testing (SgT) for the detection of genetic molecular subtypes and oncogenic markers in patients with advanced non–small-cell lung cancer (NSCLC) in the setting of Spanish reference centers.
METHODS
A joint model combining decision tree with partitioned survival models was developed. A two-round consensus panel was performed to describe clinical practice of Spanish reference centers, providing data on testing rate, prevalence of alterations, turnaround times, and treatment pathways. Treatment efficacy data and utility values were obtained from the literature. Only direct costs (euros, 2022), obtained from Spanish databases, were included. A lifetime horizon was considered, so a 3% discount rate for future costs and outcomes was considered. Both deterministic and probabilistic sensitivity analyses were performed to assess uncertainty.
RESULTS
A target population of 9,734 patients with advanced NSCLC was estimated. If NGS was used instead of SgT, 1,873 more alterations would be detected and 82 more patients could potentially be enrolled in clinical trials. In the long term, using NGS would provide 1,188 additional quality-adjusted life-years (QALYs) in the target population compared with SgT. On the other hand, the incremental cost of NGS versus SgT in the target population was €21,048,580 euros for a lifetime horizon (€1,333,288 for diagnosis phase only). The obtained incremental cost-utility ratios were €25,895 per QALY gained, below the standard cost-effectiveness thresholds.
CONCLUSION
Using NGS in Spanish reference centers for the molecular diagnosis of patients with metastatic NSCLC would be a cost-effective strategy over SgT.
INTRODUCTION
Advances in biomarkers, targeted therapies (TTs), and immuno-oncology have transformed the clinical management of patients with advanced non–small-cell lung cancer (NSCLC).1 Currently, there are highly effective TTs against mutations in oncogenic drivers such as the exon 20 insertions epidermal growth factor receptor gene (EGFR), anaplastic lymphoma receptor kinase gene (ALK), ROS proto-oncogene 1 (ROS1), B-Raf proto-oncogene (BRAF), neurotrophic tyrosine kinase gene (NTRK), or mesenchymal-epithelial transition (MET). In addition, investigational therapies for Kirsten rat sarcoma viral oncogene (KRAS) and human epidermal growth factor receptor 2 (HER2) mutations have shown promising results.1-4
CONTEXT
Key Objective
Next-generation sequencing (NGS) is being widely used for the molecular diagnosis of cancer, substituting the sequential single-gene detection. It is a costly technology, and its implementation is not widespread in Spain. To our knowledge, our study is the first to evaluate the efficiency of using NGS in diagnosing patients with non–small-cell lung cancer (NSCLC) from the perspective of Spanish reference centers.
Knowledge Generated
NGS is a cost-effective strategy compared with single-gene testing (SgT) in the molecular diagnosis of patients with metastatic NSCLC. More targetable genomic alterations could be detected by NGS, and therefore, more patients could potentially be treated with targeted therapies or enrolled in specific clinical trials.
Relevance
Our findings suggest that adopting NGS in Spanish reference centers for diagnosis of patients with NSCLC would be a cost-effective strategy over SgT.
The use of small molecular tyrosine kinase inhibitors and immunotherapy has led to unprecedented survival benefits in selected patients with NSCLC.5 With that known, the main goal in the clinical management of lung cancer is to individualize the most effective course of treatment for a patient as different drugs are available and can be used in combination or sequentially to overcome resistance mechanisms.6
Consequently, the European Society for Medical Oncology recommends the routine use of next-generation sequencing (NGS) technology to identify tumor samples in advanced nonsquamous NSCLC, prostate cancers, ovarian cancers, and cholangiocarcinoma.7 This technique has already been included in the latest updates of the National Consensus guidelines of the Spanish Society of Pathology and the Spanish Society of Medical Oncology (SEOM).8
NGS can either be directed toward the whole genome or exome of the patient (comprehensive NGS panel) or toward specific genes through a predetermined gene panel (targeted NGS panel). The last one represents a choice for daily clinical practice: providing faster results, lower cost, and higher sensitivity with lower detection thresholds.9 Given the relatively recent development of NGS, there is limited evidence regarding the economics of using NGS compared with other testing strategies in real-world clinical practice.10 To generate evidence on this topic, a cost-effectiveness analysis was recently performed comparing NGS versus single sequential testing in the molecular assessment of advanced NSCLC, using a center in southern Spain as a pilot. The results of this pilot analysis showed that using NGS provides significant benefits in terms of alterations detected, treatment with TTs, and clinical trial enrollment.11
The aim of this study is to assess the cost-effectiveness of using the NGS panel versus single-gene testing (SgT), in both the short and long term, for the detection of genetic molecular subtypes and oncogenic markers in patients with advanced NSCLC, in the setting of Spanish reference centers.
METHODS
Model Structure and Data Collection
Using a previous model from the pilot analysis11 as a starting point, a joint model combining a decision tree model with partitioned survival models (PSMs) was developed.
The decision tree model covers the diagnostic phase since the patient is diagnosed with NSCLC and molecular analysis is required, until the results of the patient's molecular profile are obtained. On the basis of these diagnostic results, treatment is assigned, and PSMs are used to assess long-term cost and health consequences. Through this joint model, NGS was compared versus SgT where EGFR, ALK, and ROS1 are determined individually but in parallel, and after them, the rest of the biomarkers are determined sequentially, as shown in Figure 1. Expression of PD-L1 is assessed by immunohistochemistry in both arms. The panel of experts agreed that this is the standard procedure in Spanish reference centers.
FIG 1.
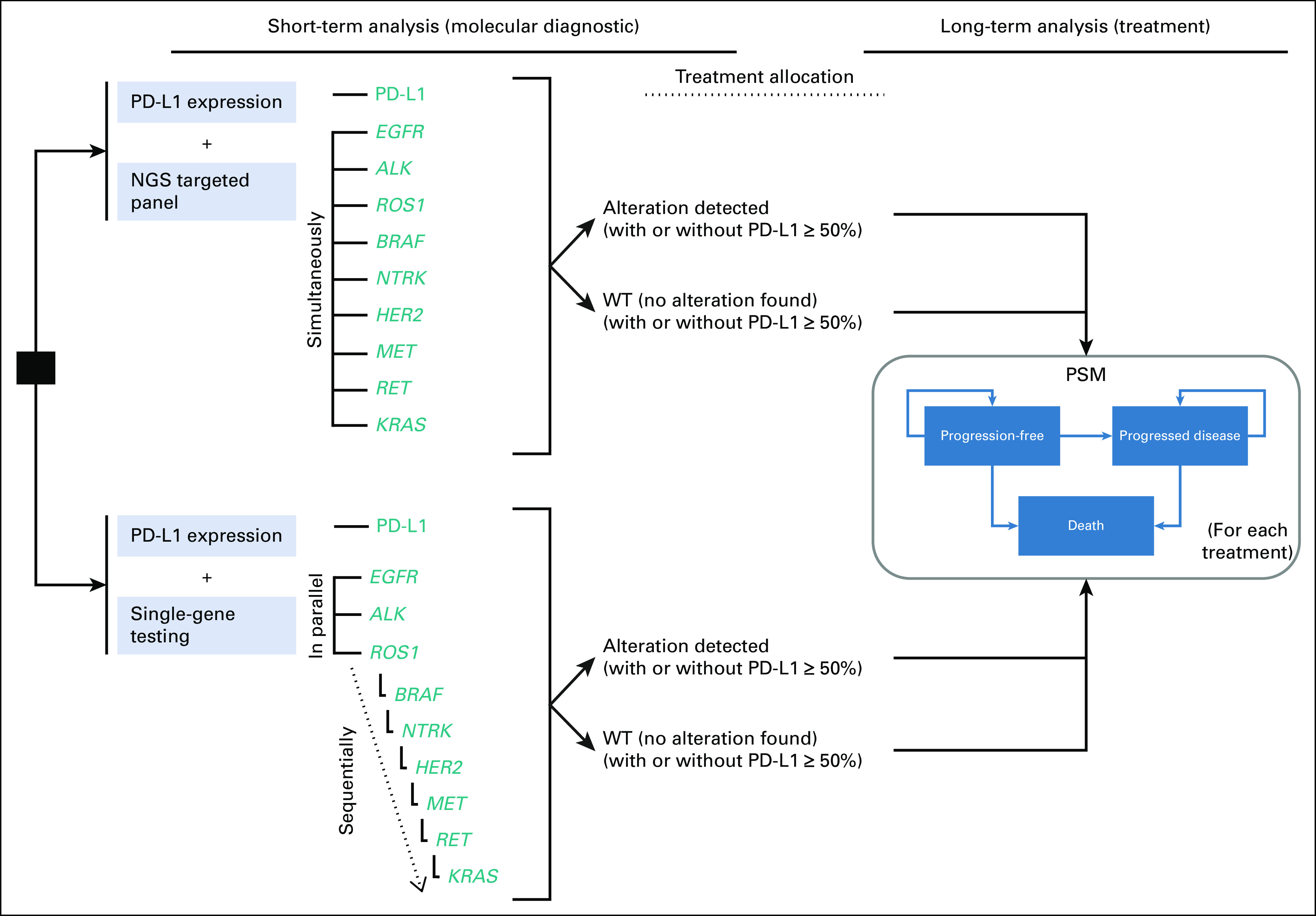
Diagram of the model. ALK, anaplastic lymphoma receptor kinase gene; BRAF, B-Raf proto-oncogene, serine/threonine kinase; EGFR, epidermal growth factor receptor gene; HER2, human epidermal growth factor receptor 2 gene; KRAS, KRAS proto-oncogene; MET, MET proto-oncogene; NTRK, Neurotrophic tyrosine receptor kinase gene; PSM, partitioned survival models; RET, RET proto-oncogene; ROS1, ROS proto-oncogene 1, receptor tyrosine kinase; WT, wild-type.
The PSM uses monthly cycles, and the analysis was performed using a lifetime horizon, so a 3% discount rate for future costs and health outcomes was applied following Spanish guidelines.12,13 The analysis was performed from the perspective of Spanish reference centers, so only direct medical costs were considered.
Validation and Data Collection
The model design and main assumptions were validated by a group of experts, who also provided information on their usual clinical practice through a two-round consensus panel. The panel of experts was integrated by 12 Spanish clinical experts (oncologists, pathologists, and molecular biologists) from different reference hospitals in various Spanish regions. In the first round, a questionnaire was used to ask the experts about clinical aspects such as the testing rate and prevalence of the biomarkers, turnaround times, treatment pathways, or efficacy data. After the analysis of the first-round responses, all the results were presented to the expert panel in a meeting where consensus was reached for all the variables.
Target Population
The hypothetical cohort of patients was defined as those newly diagnosed with advanced or metastatic NSCLC, nonsquamous histology or squamous histology who were never smokers, with unknown genomic alteration status.
Therefore, this target population was sized as shown in Table S1, Data Supplement. First, according to SEOM, around 30,948 patients were diagnosed with incident lung cancer in 2022 and 85% (26,306 patients) would be NSCLC.7,14 At diagnosis, we considered that 54.50% of patients with NSCLC are stage IIIB-IV.15 Finally, only patients with nonsquamous histology and who are never smokers with squamous NSCLC were considered.16,17 Thus, the target population is composed of 9,734 theoretical patients with advanced or metastatic NSCLC with nonsquamous histology and who are never smokers with squamous tumors that would be diagnosed and treated in 1 year.
Decision Tree Parameters
The testing rate in the SgT and the prevalence of alterations (and PD-L1 expression) in the biomarkers included in the analysis are the main variables in the decision tree model. In addition, the probability of requiring a rebiopsy in the case of tissue exhaustion, time to results, and staff costs were variables included in the diagnostic phase represented by the decision tree model.
The testing rate, defined as the percentage in which determination is finally performed, and the positivity rate of alterations in the biomarkers are shown in Table S2, Data Supplement. The figures in Table S2, Data Supplement, considered in the base case of the analysis are the averages obtained from the two-round consensus panel. Alternatively, a scenario where the perspective (reference centers) is broadened using testing rates reported in the Spanish database LungPath was assessed.18
As shown in Figure 1, PD-L1 expression is determined in parallel to both comparators (NGS and SgT), and since PD-L1 expression can be found simultaneously with a biomarker alteration, the model estimates the PD-L1 overexpression (TPS ≥ 50%) in both wild-type (WT) patients and concomitantly patients with a biomarker alteration. No strong associations between PD-L1 expression and NSCLC gene mutations, beyond EGFR and KRAS, were found in the literature.19 Therefore, overall PD-L1 overexpression (Table S2, Data Supplement) is considered to apply equally to WT patients and patients with any alteration, except in EGFR+ patients where an odds ratio (OR) of 0.09 is applied20 and in KRASG12C patients where an OR of 0.34 is applied.21
In line with the pilot,11 the probability of requiring a rebiopsy because of tissue exhaustion was included in the model following the approach described by Pennel et al10
To calculate the time to results for both the NGS panel and SgT, the specific time required for each diagnostic task (for both technicians and physicians) and the working days required were obtained from the two-round consensus panel (Table S3, Data Supplement).
Regarding the cost inputs for the decision tree model, the unit cost for each single test and the cost of the NGS panel were also obtained from the averages from the two-round consensus panel, so they are representative of the Spanish reference centers (Table S4, Data Supplement). In addition, to establish the cost per hour for technicians and physicians, the gross annual salary was obtained from the average of four Spanish Autonomous Communities.22-25
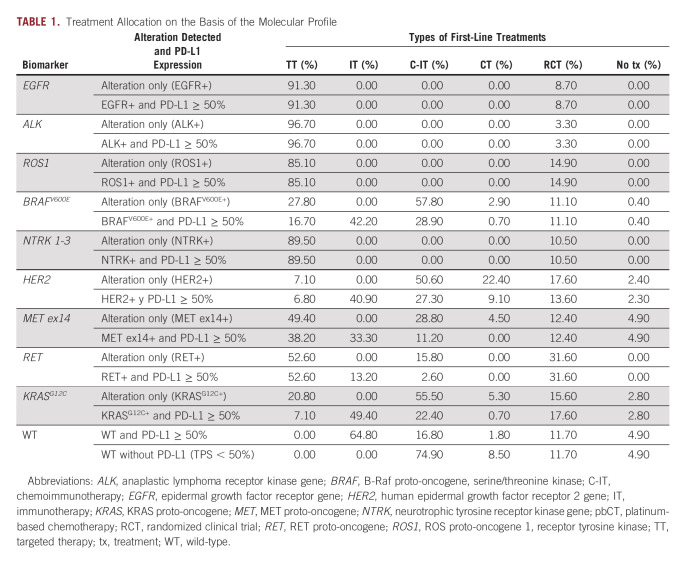
After obtaining molecular results and PD-L1 results provided by the decision tree model, a first-line treatment is assigned. Table 1 shows the treatment allocation obtained from the two-round consensus panel for the following groups of treatments: TTs, immunotherapies, chemoimmunotherapies, enrollment in randomized clinical trials (RCT), and no treatment.
TABLE 1.
Treatment Allocation on the Basis of the Molecular Profile
The distribution in Table 1 represents the perspective of reference centers and is considered representative of clinical practice in Spain. It should be noted that reference centers have a greater capacity to recruit patients for RCT, so a more representative scenario of small-medium centers where enrollment in RCT is not available is explored within the sensitivity analysis.
PSM Parameters
PSM is commonly used in oncology since it allows long-term extrapolation on the basis of progression-free survival (PFS) and overall survival (OS) curves without the need for transition probabilities.26
In the absence of individualized patient data for all treatments, it was considered to fit exponential models to lambda parameters obtained from median PFS and OS (Table S5, Data Supplement), in line with previous studies.11,27,28 Therefore, cost and utilities are assigned to PSM health states, and the proportion of patients in each state is estimated using exponential PFS and OS curves.
The following direct costs were considered in the long-term analysis: drug acquisition and administration costs of first-line treatments, treatment-related adverse events (trAEs), health care costs associated with disease management, and drug costs of subsequent treatments (after first-line progression).
All the drug costs are expressed as the exfactory price considering the corresponding deductions as per Royal Decree Law 08/2010,29,30 and vial sharing was assumed for intravenous treatment. Industry-sponsored drugs administered in RCT entail no cost to the hospital. At the time of the analysis, some of the treatments included were not reimbursed in Spain, so access was available through compassionate use or foreign medications. So, for trastuzumab deruxtecan and sotorasib, prices from France were considered, for larotrectinib, German price was considered, and for capmatinib, parity price to pralsetinib was assumed since prices from other European countries were not found.
TrAEs of grade ≥3 with a frequency of ≥5% in the respective RCT of first-line treatments were included in the model.
Regarding the health care costs associated with disease management, Table S6, Data Supplement, shows the health resources consumption (for both PFS and PD health states) obtained from the two-round consensus panel and their unit cost obtained from the Spanish health care database eSalud.31
In addition, subsequent treatments (or best supportive care) after progression to first-line treatment were considered and are detailed in Table S7, Data Supplement.
Finally, utility values for PFS and PD health states (0.71 [0.67-0.76] and 0.67 [0.59-0.75], respectively) were obtained from the literature.32
Sensitivity Analysis
To assess the robustness of the base case results, several deterministic and probabilistic sensitivity analyses were performed.
First, alternative scenarios were explored, modifying some model assumptions or data sources:
1. National perspective including small-medium centers: Testing rate form LungPath database and without enrollment in RCT
2. SgT sequence: BRAFV600E is determined in parallel to EGFR, ALK, and ROS1
3. No second-line included
In addition, a one-way sensitivity analysis was performed by modifying the various parameters individually by ±20% with respect to the base case value.
Finally, a probabilistic sensitivity analysis (PSA) was also performed. According to international recommendations, 1,000 simulations were run by second-order Monte Carlo methodology, modifying the model variables simultaneously with a given distribution.33 Probabilities were modified by a beta distribution. OR values obtained from the studies by Huynh et al20 and Jeanson et al21 were modified following a log-normal distribution. Turnaround times and working days were modified using a beta distribution. Median PFS and OS were modified following a normal distribution. Utility values were modified using a beta distribution. All unit costs were modified according to a gamma distribution.
RESULTS
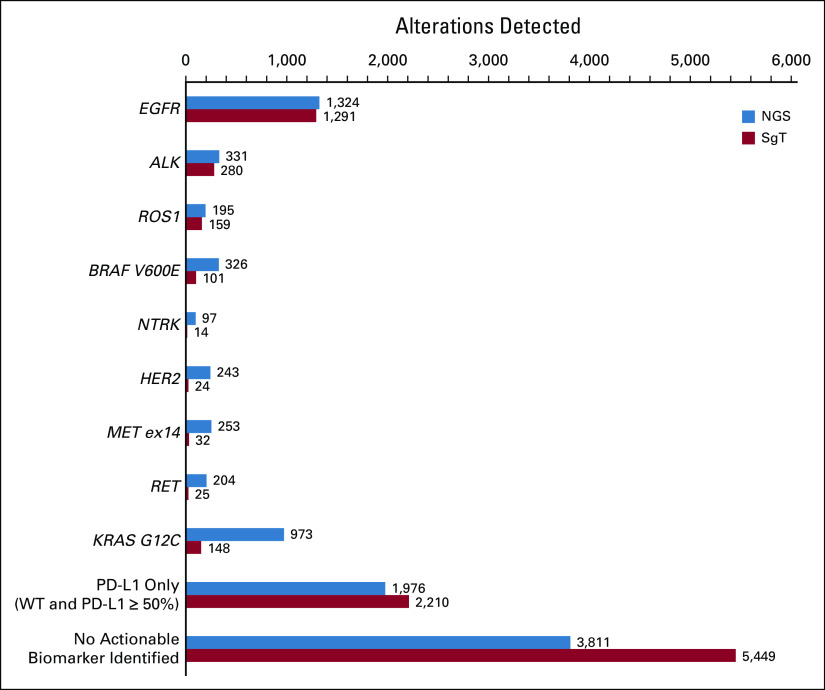
The short-term results of the diagnostic phase yielded by the decision tree show that for a potential target population of 9,734 patients, 1,873 more alterations could be detected (Fig 2) and 82 more patients could be enrolled in RCT using NGS instead of SgT.
FIG 2.
Short-term results: alterations detected in the target population. ALK, anaplastic lymphoma receptor kinase gene; BRAF, B-Raf proto-oncogene, serine/threonine kinase; EGFR, epidermal growth factor receptor gene; HER2, human epidermal growth factor receptor 2 gene; KRAS, KRAS proto-oncogene; MET, MET proto-oncogene; NGS, next-generation sequencing; NTRK, neurotrophic tyrosine receptor kinase gene; RET, RET proto-oncogene; ROS1, ROS proto-oncogene 1, receptor tyrosine kinase; SgT, single-gene testing; WT, wild-type.
Although NGS allows more alterations to be detected and more patients to be enrolled in RCT, it also entails a higher cost in the short term. The cost of the diagnostic phase using NGS in the target population is €1,333,288 higher than using SgT (€6,836,672 v €5,503,384, respectively).
Regarding the time to results, we found that using the NGS panel, complete results would be available in 10.00 working days, whereas obtaining results using SgT depends on whether it is required to complete the whole sequence. EGFR, ALK, and ROS1 results would be available in 5.93 days on average, but time to results for the last biomarker in the sequence (KRAS) would be 15.48 days (Figure S1, Data Supplement). These results would be representative of Spanish reference centers, and it is to be expected that in small- to medium-sized centers, the estimated times will be substantially higher.
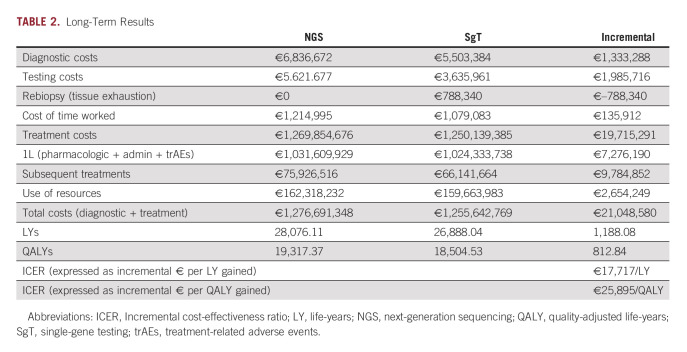
Long-term results for a lifetime horizon obtained from the PSM are shown in Table 2.
TABLE 2.
Long-Term Results
Using NGS instead of SgT in the molecular diagnosis of patients with advanced NSCLC would increase almost 1,200 life-years (LY) in the estimated target population over a lifetime horizon. Results in quality-adjusted life-years (QALYs) are shown graphically in Figure S2, Data Supplement. The incremental cost associated with the use of NGS is higher in the long-term analysis than in the diagnostic phase as more patients are treated with TTs for a longer time.
The comparison of costs and QALYs through the incremental cost-utility ratio (ICUR) shows that using NGS in Spanish reference centers would be cost-effective as it was below the cost-effectiveness thresholds commonly considered.34,35
Sensitivity Analysis
Scenario analyses performed show that the model is less sensitive to changes in the sequence of the SgT arm than to changes in treatment allocation, with a significant increase in cost-effectiveness ratios when enrollment in RCT is not considered. Specifically, the scenario representing a national perspective yields an ICUR of €45,910/QALY, the scenario without including second-line treatment yields an ICUR of €13,857/QALY, and the scenario where BRAF is determined in parallel to EGFR, ALK, and ROS1 yields an ICUR of €25,473 /QALY.
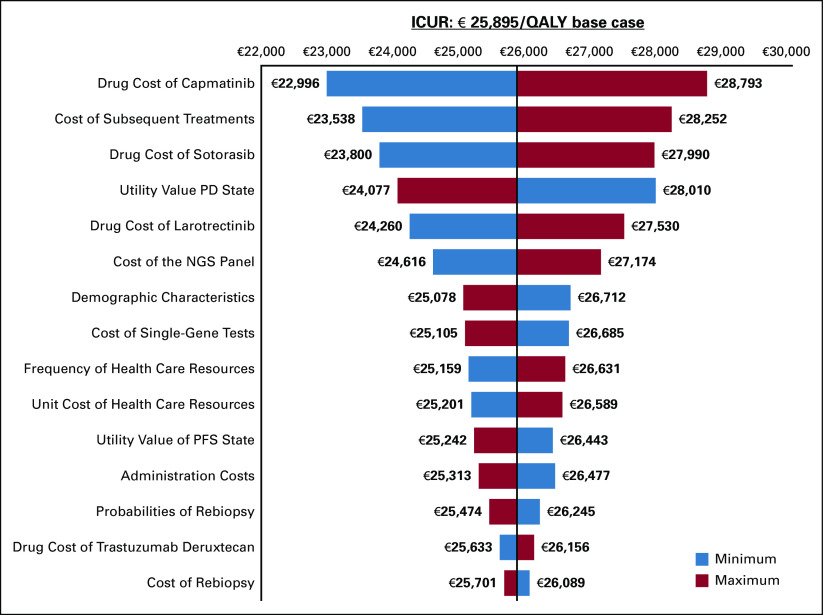
The one-way sensitivity analysis is represented using a tornado diagram (Fig 3). Minimum and maximum variations on the price assumptions of sotorasib and capmatinib, together with variations on subsequent treatments, show the greatest impact on the base case ICUR.
FIG 3.
One-way sensitivity analysis, represented by tornado diagrams. ICUR, incremental cost-utility ratio; NGS, next-generation sequencing; PD, progressed disease; PFS, progression-free survival.
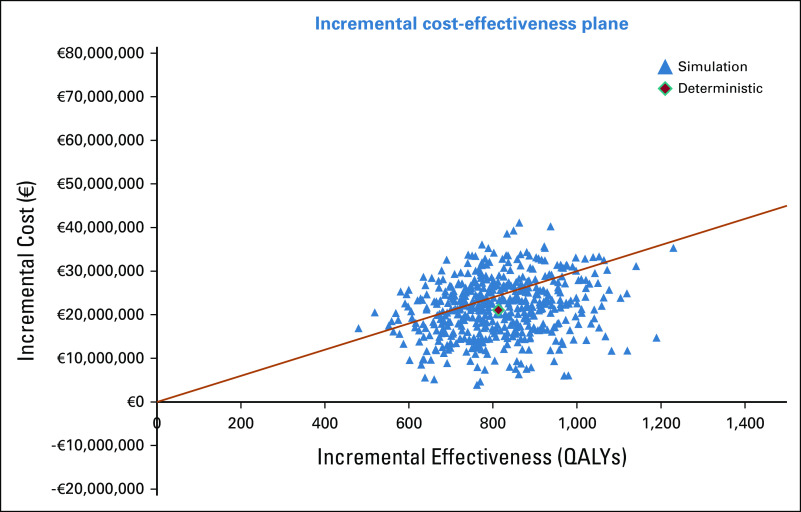
Finally, the results of the PSA are represented graphically in an incremental cost-effectiveness plane (Fig 4).
FIG 4.
PSA results, represented by a cost-effectiveness plane. PSA, probabilistic sensitivity analysis; QALYs, quality-adjusted life-years.
DISCUSSION
NGS testing should be the preferred strategy to ensure maximum testing yield to optimize tissue availability, turnaround time, and costs of molecular testing.36,37 In addition, recent works are studying the relationship between comprehensive molecular genotyping and OS in patients with NSCLC.38 However, the cost savings associated with the use of NGS are in question.37,39,40 Therefore, we aimed to assess the cost-effectiveness of using the NGS panel instead of SgT for the molecular diagnosis of patients with advanced NSCLC from the perspective of reference centers in Spain. Thus, this study provides continuity and broadens the perspective of the pilot study performed in a single center in southern Spain.11 To this end, a broad multidisciplinary panel of experts from several Spanish reference centers was formed, and the cost-effectiveness model developed presents some improvements over the one used in the pilot project. Specifically, the long-term results, an exploratory analysis in the previous pilot, are now more robust and include health care costs associated with disease management, trAEs costs, and subsequent treatment costs after progression to first-line treatment.
The results obtained in our study show that implementing NGS in Spanish reference centers, covering a potential target population of 9,734 patients, would have a clear benefit in terms of LY gained (1,188.08 LY, 812.84 QALYs) since more patients would be candidates for receiving the best possible treatment according to the genetic profile of their tumor. This clinical benefit outweighs the higher cost associated with NGS (mainly because of the higher cost of TTs), as shown by ICURs obtained that are below the usual cost-effectiveness thresholds.34,35,41
Short-term results covering only the diagnostic phase are even more favorable for NGS since first-line treatments account for most of the cost overruns associated with NGS versus SgT. Another immediate benefit of using NGS in Spanish reference centers is that a greater number of patients could be candidates for RCT participation, which means substantial savings for the center, as several studies have shown,42,43 and more importantly, a potential benefit for the patient. In addition, in terms of time to results, the use of NGS allows having complete results for all mutations in 10 working days on average. Although by SgT, the results of ALK, EGFR, and ROS1 performed in parallel could be available sooner (5.93 working days), if the last biomarker in the sequence needs to be determined, time to results would rise to 15.48 working days on average. Therefore, the more the biomarkers incorporated into routine diagnostics, the greater the benefit of using NGS in the time to results.
Several cost analyses and economic evaluations of NGS have been performed in the past few years, as reported in two systematic reviews of the literature.37,44,45
The study performed by Weymann et al focused on economic evaluations of NGS published between 2000 and 2016, aiming to characterize the availability and scope of economic evidence. Among the 55 studies identified, only five economic evaluations were performed on lung cancer. They highlight not only the significant increase in economic evaluations of NGS published between 2014 and 2016 but also the differences in cost-effectiveness results across studies according to the methodology adopted, comparator selected, and funding source.45
Zheng et al37 conducted a literature review to assess the diagnostic and economic value of NGS versus SgT in NSCLC biomarker testing. In the relevant literature focused on the comparison of NGS versus single testing (n = 14), 10 were economic evaluations, but only six of them assessed the cost-effectiveness of NGS. Despite the differences in methodology between the studies, NGS led to a greater proportion of patients assigned to targeted therapy and increased LY gained while being cost-neutral or cost-saving. NGS was generally found to be cost-effective at typical thresholds. It is worth mentioning that one of the studies identified in the literature review was a cost analysis conducted in Spain and reported that NGS implementation was feasible and could be performed at a reasonable cost because NGS is a multiplexed molecular diagnostic tool able to overcome the limitations of current molecular diagnosis in advanced cancer, allowing an improved and economically sustainable molecular profiling.44
None of the economic evaluations identified in these literature reviews have used a joint model focused on implementing NGS in reference centers.
Similar to all theoretical models, our study has some limitations such as the rigidity inherent to pharmacoeconomic models or the lack of inclusion of small- to medium-sized centers to obtain an enhanced/improved national perspective.
The testing rate and the prevalence of alterations in the selected biomarkers were obtained from direct consultation and therefore reflected the clinical practice of the experts' centers, so there is an inherent limitation to the source of these data. It was assumed that the prevalence of alterations in the selected biomarkers is the same regardless of the diagnostic method, so the different testing rates determine the greater number of alternations detected with NGS (assumed 100%) since specificity and sensitivity variables are not included in the model.
In line with this limitation, the present analysis does consider a lower overexpression of PD-L1 in EGFR+ and KRASG12C+ patients.20,21 PD-L1 overexpression may be associated with alterations of some other biomarker besides EGFR and KRAS, but no studies have reported statically significant differences on the basis of TPS > 50% for PD-L1.
Regarding the time to results, for the sake of simplicity, the ‘batch’ effect has not been considered. In addition to this limitation, it should be considered that the days of sending and/or receiving samples to other centers are not considered since the analysis is conducted from the perspective of reference centers. In addition, the possible effects on health outcomes of starting first-line treatment earlier or later were not incorporated into the analysis because of lack of evidence.
The long-term analysis presents some limitations also. Since a lifetime horizon was considered, extrapolations of the survival curves are necessary. In the absence of individualized patient data for each treatment included in the model, the utilization of exponential parametric models fitted to median PFS and OS was considered appropriate to avoid bias between treatments. In addition, it has been necessary to anticipate the price of some treatments not yet available in Spain, so that the price at which they will be reimbursed may differ from the one we assumed.
To overcome these limitations, we conducted several sensitivity analyses that measured the associated uncertainty and confirmed the results' robustness.
In conclusion, our analysis strengthens the results shown in the pilot conducted in a south Spanish single center and shows how, from the perspective of reference centers, NGS would be a cost-effective strategy in the molecular diagnosis of patients with NSCLC over SgT.
Edurne Arriola
Consulting or Advisory Role: Sanofi, Lilly, Boehringer Ingelheim
Speakers' Bureau: AstraZeneca, Roche, MSD Oncology, Bristol Myers Squibb Foundation
Travel, Accommodations, Expenses: Takeda, AstraZeneca, AstraZeneca
Reyes Bernabé
Consulting or Advisory Role: Roche, AstraZeneca, Bristol Myers Squibb/Celgene
Travel, Accommodations, Expenses: AstraZeneca
Rosario García Campelo
Consulting or Advisory Role: Roche/Genentech, MSD Oncology, AstraZeneca, Bristol Myers Squibb, Pfizer, Novartis, Takeda, Boehringer Ingelheim, Janssen Oncology
Speakers' Bureau: Roche, AstraZeneca, Bristol Myers Squibb, Pfizer, Novartis, Takeda, Boehringer Ingelheim, MSD Oncology, Sanofi/Aventis, Janssen Oncology, Amgen, Lilly
Travel, Accommodations, Expenses: Roche/Genentech, MSD Oncology, Pfizer
Fernando López-Ríos
Honoraria: Roche, Thermo Fisher Scientific, AstraZeneca, MSD, Bayer, BMS, Janssen, Lilly, Pfizer, Takeda
Research Funding: Thermo Fisher Scientific, Roche, Lilly
Laura Mezquita
Consulting or Advisory Role: Roche, Takeda
Speakers' Bureau: Bristol Myers Squibb, AstraZeneca, Takeda, Roche
Research Funding: Bristol Myers Squibb, Boehringer Ingelheim, Inivata, Stilla, Amgen
Travel, Accommodations, Expenses: Roche, AstraZeneca, Takeda
Jon Zugazagoitia
Honoraria: AstraZeneca Spain, Bristol Myers Squibb/Celgene, Pfizer, Roche/Genentech, NanoString Technologies, Guardant Health, Sanofi/Regeneron
Consulting or Advisory Role: AstraZeneca, Pfizer, Bristol Myers Squibb/Celgene, Novartis, Guardant Health, Sanofi/Regeneron
Speakers' Bureau: Bristol Myers Squibb/Celgene, Pfizer, Roche, MSD Oncology, AstraZeneca, NanoString Technologies, Guardant Health
Research Funding: AstraZeneca (Inst), Roche/Genentech (Inst)
Travel, Accommodations, Expenses: Bristol Myers Squibb/Celgene, Roche/Genentech
Natalia Arrabal
Employment: Roche
J. Francisco Garcia
Employment: Roche
David Carcedo
Consulting or Advisory Role: Roche (Inst)
Enrique de Álava
Honoraria: Roche, Amgen Astellas BioPharma
Consulting or Advisory Role: Pfizer
Travel, Accommodations, Expenses: PharmaMar
No other potential conflicts of interest were reported.
PRIOR PRESENTATION
The preliminary results of this analysis were presented as an oral presentation at the annual congress of the Spanish Society of Medical Oncology, Madrid, Spain, October 18-21, 2022.
SUPPORT
Supported by Roche Farma SA Roche Farma SA. played no role in the design of the study; collection, analysis, and interpretation of data; and writing the manuscript.
DATA SHARING STATEMENT
Qualified researchers may request access to individual patient-level data through the clinical study data request platform (https://vivli.org/). Further details on Roche's criteria for eligible studies are available here (https://vivli.org/members/ourmembers/). For further details on Roche's Global Policy on the Sharing of Clinical Information and how to request access to related clinical study documents, see https://www.roche.com/research_and_development/who_we_are_how_we_work/clinical_trials/our_commitment_to_data_sharing.htm.
AUTHOR CONTRIBUTIONS
Conception and design: Natalia Arrabal, David Carcedo
Administrative support: Natalia Arrabal, J. Francisco García
Collection and assembly of data: Edurne Arriola, Reyes Bernabé, Rosario García Campelo, Michele Biscuola, Ana Belén Enguita, Fernando López-Ríos, Rafael Martínez, Laura Mezquita, Sarai Palanca, María Jesús Pareja, Jon Zugazagoitia, Enrique de Álava
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/po/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Edurne Arriola
Consulting or Advisory Role: Sanofi, Lilly, Boehringer Ingelheim
Speakers' Bureau: AstraZeneca, Roche, MSD Oncology, Bristol Myers Squibb Foundation
Travel, Accommodations, Expenses: Takeda, AstraZeneca, AstraZeneca
Reyes Bernabé
Consulting or Advisory Role: Roche, AstraZeneca, Bristol Myers Squibb/Celgene
Travel, Accommodations, Expenses: AstraZeneca
Rosario García Campelo
Consulting or Advisory Role: Roche/Genentech, MSD Oncology, AstraZeneca, Bristol Myers Squibb, Pfizer, Novartis, Takeda, Boehringer Ingelheim, Janssen Oncology
Speakers' Bureau: Roche, AstraZeneca, Bristol Myers Squibb, Pfizer, Novartis, Takeda, Boehringer Ingelheim, MSD Oncology, Sanofi/Aventis, Janssen Oncology, Amgen, Lilly
Travel, Accommodations, Expenses: Roche/Genentech, MSD Oncology, Pfizer
Fernando López-Ríos
Honoraria: Roche, Thermo Fisher Scientific, AstraZeneca, MSD, Bayer, BMS, Janssen, Lilly, Pfizer, Takeda
Research Funding: Thermo Fisher Scientific, Roche, Lilly
Laura Mezquita
Consulting or Advisory Role: Roche, Takeda
Speakers' Bureau: Bristol Myers Squibb, AstraZeneca, Takeda, Roche
Research Funding: Bristol Myers Squibb, Boehringer Ingelheim, Inivata, Stilla, Amgen
Travel, Accommodations, Expenses: Roche, AstraZeneca, Takeda
Jon Zugazagoitia
Honoraria: AstraZeneca Spain, Bristol Myers Squibb/Celgene, Pfizer, Roche/Genentech, NanoString Technologies, Guardant Health, Sanofi/Regeneron
Consulting or Advisory Role: AstraZeneca, Pfizer, Bristol Myers Squibb/Celgene, Novartis, Guardant Health, Sanofi/Regeneron
Speakers' Bureau: Bristol Myers Squibb/Celgene, Pfizer, Roche, MSD Oncology, AstraZeneca, NanoString Technologies, Guardant Health
Research Funding: AstraZeneca (Inst), Roche/Genentech (Inst)
Travel, Accommodations, Expenses: Bristol Myers Squibb/Celgene, Roche/Genentech
Natalia Arrabal
Employment: Roche
J. Francisco Garcia
Employment: Roche
David Carcedo
Consulting or Advisory Role: Roche (Inst)
Enrique de Álava
Honoraria: Roche, Amgen Astellas BioPharma
Consulting or Advisory Role: Pfizer
Travel, Accommodations, Expenses: PharmaMar
No other potential conflicts of interest were reported.
REFERENCES
- 1. Yang SR, Schultheis AM, Yu H, et al. Precision medicine in non-small cell lung cancer: Current applications and future directions. Semin Cancer Biol. 2022;84:184–198. doi: 10.1016/j.semcancer.2020.07.009. [DOI] [PubMed] [Google Scholar]
- 2. Rebuzzi SE, Zullo L, Rossi G, et al. Novel emerging molecular targets in non-small cell lung cancer. Int J Mol Sci. 2021;22:2625. doi: 10.3390/ijms22052625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Russo A, Lopes AR, McCusker MG, et al. New targets in lung cancer (excluding EGFR, ALK, ROS1) Curr Oncol Rep. 2020;22:48. doi: 10.1007/s11912-020-00909-8. [DOI] [PubMed] [Google Scholar]
- 4. Tan AC, Lai GGY, Tan GS, et al. Utility of incorporating next-generation sequencing (NGS) in an Asian non-small cell lung cancer (NSCLC) population: Incremental yield of actionable alterations and cost-effectiveness analysis. Lung Cancer. 2020;139:207–215. doi: 10.1016/j.lungcan.2019.11.022. [DOI] [PubMed] [Google Scholar]
- 5. Majeed U, Manochakian R, Zhao Y, et al. Targeted therapy in advanced non-small cell lung cancer: Current advances and future trends. J Hematol Oncol. 2021;14:108. doi: 10.1186/s13045-021-01121-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Fumagalli C, Barberis M. Diagnostic and predictive biomarkers in lung cancer. Cancers. 2021;13:2577. doi: 10.3390/cancers13112577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mosele F, Remon J, Mateo J, et al. Recommendations for the use of next-generation sequencing (NGS) for patients with metastatic cancers: A report from the ESMO precision medicine working group. Ann Oncol. 2020;31:1491–1505. doi: 10.1016/j.annonc.2020.07.014. [DOI] [PubMed] [Google Scholar]
- 8. Garrido P, Conde E, de Castro J, et al. Updated guidelines for predictive biomarker testing in advanced non-small-cell lung cancer: A National Consensus of the Spanish Society of Pathology and the Spanish Society of Medical Oncology. Clin Transl Oncol. 2020;22:989–1003. doi: 10.1007/s12094-019-02218-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Esagian SM, Grigoriadou G, Nikas IP, et al. Comparison of liquid-based to tissue-based biopsy analysis by targeted next generation sequencing in advanced non-small cell lung cancer: A comprehensive systematic review. J Cancer Res Clin Oncol. 2020;146:2051–2066. doi: 10.1007/s00432-020-03267-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pennell NA, Mutebi A, Zhou Z-Y, et al. Economic impact of next-generation sequencing versus single-gene testing to detect genomic alterations in metastatic non–small-cell lung cancer using a decision analytic model JCO Precision Oncol 10.1200/PO.18.00356, 2019 [DOI] [PubMed] [Google Scholar]
- 11. de Alava E, Pareja MJ, Carcedo D, et al. Cost-effectiveness analysis of molecular diagnosis by next-generation sequencing versus sequential single testing in metastatic non-small cell lung cancer patients from a south Spanish hospital perspective. Expert Rev Pharmacoeconomics Outcomes Res. 2022;22:1033–1042. doi: 10.1080/14737167.2022.2078310. [DOI] [PubMed] [Google Scholar]
- 12.Puig-Junoy J, Oliva-Moreno J, Trapero-Bertrán M, et al. Guía y recomendaciones para la realización y presentación de evaluaciones económicas y análisis de impacto presupuestario de medicamentos en el ámbito del CatSalut. Generalitat de Catalunya. Departament de Salut. Servei Català de la Salut. Barcelona; 2014. https://catsalut.gencat.cat/web/.content/minisite/catsalut/proveidors_professionals/medicaments_farmacia/farmaeconomica/caeip/gaeip_publica_castellano_octubre2014_catsalut.pdf [Google Scholar]
- 13. López Bastida J, Oliva J, Antoñanzas F, et al. Propuesta de guía para la evaluación económica aplicada a las tecnologías sanitarias. Gaceta Sanitaria. 2010;24:154–170. doi: 10.1016/j.gaceta.2009.07.011. [DOI] [PubMed] [Google Scholar]
- 14.SEOM . Las cifras del cáncer en España 2022. https://seom.org/images/LAS_CIFRAS_DEL_CANCER_EN_ESPANA_2022.pdf [Google Scholar]
- 15. Vidal J, Clavé S, de Muga S, et al. Assessment of ALK status by FISH on 1000 Spanish non-small cell lung cancer patients. J Thorac Oncol. 2014;9:1816–1820. doi: 10.1097/JTO.0000000000000361. [DOI] [PubMed] [Google Scholar]
- 16. de Castro J, Tagliaferri P, de Lima VCC, et al. Systemic therapy treatment patterns in patients with advanced non-small cell lung cancer (NSCLC): PIvOTAL study. Eur J Cancer Care (Engl) 2017;26:e12734. doi: 10.1111/ecc.12734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Provencio M, Carcereny E, Rodríguez-Abreu D, et al. Lung cancer in Spain: Information from the Thoracic Tumors Registry (TTR study) Transl Lung Cancer Res. 2019;8:461–475. doi: 10.21037/tlcr.2019.08.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Salas C, Martín-López J, Martínez-Pozo A, et al. Real-world biomarker testing rate and positivity rate in NSCLC in Spain: Prospective Central Lung Cancer Biomarker Testing Registry (LungPath) from the Spanish Society of Pathology (SEAP) J Clin Pathol. 2021;75:193–200. doi: 10.1136/jclinpath-2020-207280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Liu Y, Wu A, Li X, et al. A retrospective analysis of eleven gene mutations, PD-L1 expression and clinicopathological characteristics in non-small cell lung cancer patients. Asian J Surg. 2022;45:367–375. doi: 10.1016/j.asjsur.2021.06.030. [DOI] [PubMed] [Google Scholar]
- 20. Huynh TG, Morales-Oyarvide V, Campo MJ, et al. Programmed cell death ligand 1 expression in resected lung adenocarcinomas: Association with immune microenvironment. J Thorac Oncol. 2016;11:1869–1878. doi: 10.1016/j.jtho.2016.08.134. [DOI] [PubMed] [Google Scholar]
- 21. Jeanson A, Tomasini P, Souquet-Bressand M, et al. Efficacy of immune checkpoint inhibitors in KRAS-mutant non-small cell lung cancer (NSCLC) J Thorac Oncol. 2019;14:1095–1101. doi: 10.1016/j.jtho.2019.01.011. [DOI] [PubMed] [Google Scholar]
- 22.CatSalut 2022. https://governobert.gencat.cat/es/transparencia/Funcio-publica/empleats-publics/taules-retributives/ Tablas retributivas. Gobierno abierto.
- 23.GVA 2022. http://www.san.gva.es/web/dgrhs/retribuciones-personal-iiss Retribucions personal IISS - Conselleria de Sanitat Universal i Salut Pública.
- 24.B.O.C.M. 2022. https://www.comunidad.madrid/transparencia/sites/default/files/open-data/downloads/bocm-20220128-23.pdf ORDEN de 21 de enero de 2022, de la Consejería de Economía, Hacienda y Empleo, por la que se dictan Instrucciones para la Gestión de las Nóminas del Personal de la Comunidad de Madrid para 2022. Madrid.
- 25.Servicio Andaluz de Salud 2022. https://ws050.juntadeandalucia.es/verificarFirma/ Actualización de retribuciones compensaciones por la participación de los Centros Hospitalarios en el Programa de Detección, Extracción y Trasplantes de Órganos y Tejidos.
- 26.YHEC 2016. https://yhec.co.uk/glossary/cost-effectiveness-acceptability-curve-ceac/ Cost-effectiveness acceptability curve (CEAC)—YHEC—York Health Economics Consortium.
- 27. Nadal E, Bautista D, Cabezón-Gutiérrez L, et al. Clinical and economic impact of current ALK rearrangement testing in Spain compared with a hypothetical no-testing scenario. BMC Cancer. 2021;21:689. doi: 10.1186/s12885-021-08407-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rojo F, Conde E, Torres H, et al. Clinical and economic impact of ‘ROS1-testing’ strategy compared to a ‘no-ROS1-testing’ strategy in advanced NSCLC in Spain. BMC Cancer. 2022;22:292. doi: 10.1186/s12885-022-09397-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.B.O.E. 2010. https://www.boe.es/diario_boe/txt.php?id=BOE-A-2010-8228 Real Decreto-Ley 8/2010, de 20 de mayo, por el que se adoptan medidas extraordinarias para la reducción del déficit público. 45070–45128.
- 30.C.G.C.O.F. 2022. Consejo General de Colegios Oficiales de Farmacéuticos. Portal Farma, BotPLUS.
- 31.Gisbert R, Brosa M.2018. http://www.oblikue.com/bddcostes/ Healthcare cost database eSalud.
- 32. Chouaid C, Agulnik J, Goker E, et al. Health-related quality of life and utility in patients with advanced non–small-cell lung cancer: A prospective cross-sectional patient survey in a real-world setting. J Thorac Oncol. 2013;8:997–1003. doi: 10.1097/JTO.0b013e318299243b. [DOI] [PubMed] [Google Scholar]
- 33. Briggs AH. Handling uncertainty in cost-effectiveness models. Pharmacoeconomics. 2000;17:479–500. doi: 10.2165/00019053-200017050-00006. [DOI] [PubMed] [Google Scholar]
- 34.WHO 2022. https://www.who.int/news-room/questions-and-answers/item/who-choice-frequently-asked-questions WHO-CHOICE.
- 35. Sacristán JA, Oliva J, Campillo-Artero C, et al. What is an efficient health intervention in Spain in 2020? Gaceta Sanitaria. 2020;34:189–193. doi: 10.1016/j.gaceta.2019.06.007. [DOI] [PubMed] [Google Scholar]
- 36. de Maglio G, Pasello G, Dono M, et al. The storm of NGS in NSCLC diagnostic-therapeutic pathway: How to sun the real clinical practice. Crit Rev Oncol Hematol. 2022;169:103561. doi: 10.1016/j.critrevonc.2021.103561. [DOI] [PubMed] [Google Scholar]
- 37. Zheng Y, Vioix H, Liu FX, et al. Diagnostic and economic value of biomarker testing for targetable mutations in non-small-cell lung cancer: A literature review. Future Oncol. 2022;18:505–518. doi: 10.2217/fon-2021-1040. [DOI] [PubMed] [Google Scholar]
- 38. Aggarwal C, Marmarelis ME, Hwang W-T, et al. Association of comprehensive molecular genotyping and overall survival in patients with advanced non-squamous non-small cell lung cancer. J Clin Oncol. 2022;40 suppl 16; abstr 9022. [Google Scholar]
- 39. Vanderpoel J, Stevens AL, Emond B, et al. Total cost of testing for genomic alterations associated with next-generation sequencing versus polymerase chain reaction testing strategies among patients with metastatic non-small cell lung cancer. J Med Econ. 2022;25:457–468. doi: 10.1080/13696998.2022.2053403. [DOI] [PubMed] [Google Scholar]
- 40. Bruno R, Fontanini G. Next generation sequencing for gene fusion analysis in lung cancer: A literature review. Diagnostics. 2020;10:521. doi: 10.3390/diagnostics10080521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Vallejo-Torres L, García-Lorenzo B, Serrano-Aguilar P. Estimating a cost-effectiveness threshold for the Spanish NHS. Health Econ. 2018;27:746–761. doi: 10.1002/hec.3633. [DOI] [PubMed] [Google Scholar]
- 42. D’Ambrosio F, de Feo G, Botti G, et al. Clinical trials and drug cost savings for Italian health service. BMC Health Serv Res. 2020;20:1089. doi: 10.1186/s12913-020-05928-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Mañes-Sevilla M, Romero-Jiménez R, Herranz-Alonso A, et al. Drug cost avoidance in clinical trials of breast cancer. J Oncol Pharm Pract. 2019;25:1099–1104. doi: 10.1177/1078155218775193. [DOI] [PubMed] [Google Scholar]
- 44. Simarro J, Murria R, Pérez-Simó G, et al. Development, implementation and assessment of molecular diagnostics by next generation sequencing in personalized treatment of cancer: Experience of a public reference healthcare hospital. Cancers. 2019;11:1196. doi: 10.3390/cancers11081196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Weymann D, Pataky R, Regier DA. Economic evaluations of next-generation precision oncology: A critical review. JCO Precis Oncol. 2018 doi: 10.1200/PO.17.00311. 10.1200/PO.17.00311 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Qualified researchers may request access to individual patient-level data through the clinical study data request platform (https://vivli.org/). Further details on Roche's criteria for eligible studies are available here (https://vivli.org/members/ourmembers/). For further details on Roche's Global Policy on the Sharing of Clinical Information and how to request access to related clinical study documents, see https://www.roche.com/research_and_development/who_we_are_how_we_work/clinical_trials/our_commitment_to_data_sharing.htm.