PURPOSE
Genomic profiling programs have been implemented to apply next-generation sequencing (NGS) for facilitating trial enrollment. SCRUM-Japan GI-SCREEN is a large-scale genomic profiling program in advanced gastrointestinal cancers using a validated genomic assay with the goal of facilitating enrollment in targeted clinical trials, generating real-world data, and performing clinicogenomic analysis for biomarker discovery.
PATIENTS AND METHODS
Genotyping of tumor tissue samples from 5,743 patients with advanced gastrointestinal cancers enrolled in GI-SCREEN was centrally performed with NGS. Patients were enrolled in matched trials of targeted agents affiliated with GI-SCREEN on the basis of genotyping results.
RESULTS
A total of 11 gastrointestinal cancers were included, with colorectal cancer being the most common. The median age ranged from 59 to 70.5 years across cancer types. Patients enrolled after initiation of first-line treatment had significantly longer overall survival (OS) than that before treatment initiation with a median survival time difference of 8.9 months and a hazard ratio (HR) ranging from 0.25 to 0.73 across cancer types, demonstrating an immortal time bias. One hundred and forty-nine patients received matched therapies in clinical trials on the basis of their identified alterations. Among patients with colorectal cancer harboring actionable alterations, the median OS was significantly longer in patients who received matched therapies in trials than in those who did not (HR, 0.52; 95% CI, 0.26 to 1.01; P = .049). Cancer-specific pathway alterations were significantly associated with shorter survival and related to primary resistance to matched trial therapies.
CONCLUSION
Our genomic profiling program led to patient enrollment in targeted clinical trials and improved survival of patients with colorectal cancer who received matched therapies in clinical trials. To avoid immortal time bias, precautions are needed when using data from patients who have undergone NGS testing after initiation of the evaluated treatment line.
INTRODUCTION
The importance of personalized treatment has greatly increased to improve clinical care for patients with cancer. Comprehensive genomic profiling using next-generation sequencing (NGS) has been widely accepted for certain types of cancers.1 Although large-scale genomic profiling programs have been implemented to apply clinical sequencing to precision oncology by facilitating clinical trial enrollment,2-4 the survival benefit of these profiling programs remains unknown. Since NGS testing is generally performed on patients receiving systemic therapy,5 the evaluation of the benefit of treatments in clinical trials on the basis of clinical sequencing must carefully address bias caused by an immortal time, the follow-up period during which death or the study outcome cannot occur.6 Nevertheless, the impact of immortal time bias is not well understood in genomic profiling programs. Biomarker exploration is another potential purpose of genomic profiling studies.
CONTEXT
Key Objective
Using a large-scale genomic profiling program (SCRUM-Japan GI-SCREEN), we evaluated the immortal time bias, survival benefit of patients who were enrolled in targeted clinical trials, and genomic alterations associated with prognosis in gastrointestinal cancers.
Knowledge Generated
Patients who underwent genomic profiling after the initiation of first-line treatment had a longer overall survival (OS) than those before first-line treatment, demonstrating an immortal time bias. Matched therapies in clinical trials improved OS in patients with colorectal cancer harboring actionable alterations. Cancer-specific pathway alterations were significantly associated with shorter survival and related to primary resistance to matched therapies in clinical trials.
Relevance
A large-scale genomic profiling program could allow patient enrollment in targeted clinical trials and improve survival of patients who received matched therapies in clinical trials.
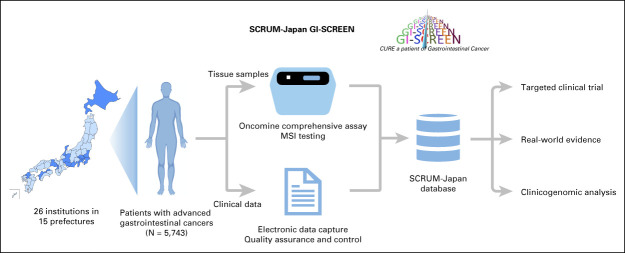
One example of a genomic profile program is the Cancer Genome Screening Project for Individualized Medicine in Japan (SCRUM-Japan), which included patients with advanced solid tumors through a collaboration among medical institutions across Japan and aimed to realize cancer precision medicine.7,8 GI-SCREEN, one component of SCRUM-Japan, profiles genomic alterations of advanced gastrointestinal cancers using a validated genomic assay and collects quality-assured clinical data with the goal of (1) facilitating enrollment in targeted clinical trials, (2) generating regulatory-grade real-world data, and (3) performing high-level clinicogenomic analysis for biomarker discovery (Fig 1).
FIG 1.
Study schema of SCRUM-Japan GI-SCREEN. Patients with advanced gastrointestinal cancers were enrolled in the SCRUM-Japan GI-SCREEN involving 26 core cancer institutions in Japan. Tumor tissue samples from the enrolled patients were analyzed using the Oncomine Comprehensive Assay. Clinicopathologic information and efficacy data of systemic therapy were collected using an electronic data capture system and were finalized by completion of the resolution of autogenerated and manually added queries from the SCRUM-Japan Data Center. The collected data were used to assign patients to targeted clinical trials, generate real-world evidence, and perform clinicogenomic analysis. MSI, microsatellite instability.
To address these issues, we aimed to evaluate the survival benefit of targeted clinical trials and explore the genomic alterations in oncogenic signaling pathways, their impact on survival and their relationship with the efficacy of targeted clinical trials by using GI-SCREEN, a large-scale qualified clinicogenomic data set of advanced gastrointestinal cancers.
PATIENTS AND METHODS
Study Design
SCRUM-Japan GI-SCREEN is a nationwide tumor tissue cancer genomic profiling study involving 26 core cancer institutions in Japan8 that primarily aim to accelerate development and improve care by matching patients to suitable clinical trials. The key inclusion criteria were as follows: (1) histopathologically confirmed unresectable or metastatic gastrointestinal cancer, (2) receipt (or planned receipt) of systemic therapy, (3) age 20 years and older, (4) an Eastern Cooperative Oncology Group performance status score of 0-1, (5) adequate organ function, and (6) available tumor tissue. All eligible patients provided written informed consent and were enrolled immediately after consent, either before the initiation of the first-line treatment or during the first-line or subsequent treatment line. Genotyping of archival or fresh tumor tissue samples from enrolled patients was centrally performed using the Oncomine Comprehensive Assay (OCA; Thermo Fisher Scientific, Waltham, MA). Details regarding testing are provided in the Data Supplement.
The SCRUM-Japan GI-SCREEN was initiated in February 2015, and enrollment was completed in April 2019, before the initiation of the subsequent SCRUM-Japan study, MONSTAR-SCREEN.7 GI-SCREEN CRC for colorectal cancer and GI-SCREEN Non-CRC for noncolorectal gastrointestinal cancers were conducted in accordance with the Declaration of Helsinki and the Japanese Ethical Guidelines for Medical and Health Research Involving Human Subjects. All study protocols were approved by the institutional review board of each participating institution and registered in the University Hospital Medical Information Network (UMIN) Clinical Trials Registry (protocol IDs: UMIN000016343 for GI-SCREEN CRC and UMIN000016344 for GI-SCREEN Non-CRC).
Clinical Data Collection and Matching of Patients to Clinical Trials
Clinicopathologic information and efficacy data of systemic therapy of the patients enrolled in the GI-SCREEN were collected using an electronic data capture system. Clinical data were updated annually. All data were finalized by combining the autogenerated and manually added queries from the SCRUM-Japan Data Center. The clinical data and genotyping results were stored in a clinical-grade database and used for integrated clinicogenomic analysis.
The patients were enrolled in matched clinical trials of targeted agents affiliated with GI-SCREEN on the basis of their genotyping results. The affiliated trials comprised company-sponsored and investigator-initiated trials. If a patient's matched trial was unavailable at patient’s institution, they were referred to the institution conducting the trial through the SCRUM-Japan GI-SCREEN network. Enrolled patients were treated according to the protocol of each clinical trial. Deidentified efficacy data were used for the survival analysis of targeted therapies in clinical trials.
Outcomes and Statistical Analysis
The primary end points were the prevalence of genomic alterations and the association between genomic alterations and survival outcomes. The response to the targeted agents was assessed per Response Evaluation Criteria in Solid Tumors version 1.1 by local site investigators. In this study, progression-free survival (PFS) was estimated for each matched therapy in clinical trials and defined as the time from the date of initiation of the matched therapy to the date of disease progression according to investigator assessment or death from any cause. Overall survival (OS) was estimated from the date of initiation of the first-line treatment to the date of death. The Kaplan-Meier method was used to estimate survival rates, and the treatment groups were compared using the log-rank test. Multivariate analysis for OS according to clinical factors or pathway alterations was conducted using the Cox proportional hazards model with calculation of the hazard ratio (HR) and 95% CI. All available clinical factors and pathways were included in the multivariate analysis. Data from patients who had never received systemic therapy were excluded from survival analysis. To assess the immortal time bias, the OS was compared between patients enrolled after versus before the initiation of first-line treatment for each cancer type. This analysis for immortal time bias was conducted post hoc to evaluate the factors associated with the long OS of patients enrolled in the GI-SCREEN. No sample size calculation was performed because the present study was observational in nature. Statistical analyses were performed using SAS (version 9.4). The data cutoff for the analyses was November 30, 2020. All P-values of <0.05 were considered statistically significant.
RESULTS
Patient Characteristics
Of the 5,743 patients with advanced gastrointestinal cancers enrolled in the GI-SCREEN between February 2015 and April 2019, clinical data were available for 5,737 (99.9%) patients (Data Supplement). The baseline characteristics of the patients with each cancer type are summarized in the Data Supplement. The median age ranged from 59 to 70.5 years across cancer types. More males were enrolled than females in all cancer types excluding appendiceal cancer.
Impact of Immortal Time Bias on OS Time in Real-World Data
Using data from 5,389 patients for whom survival outcome data were available (median follow-up time, 25.0 months; total number of events, 3,806), we analyzed OS and found that our cohort had a better OS than those in previous pivotal trials of first-line treatment. For example, the median OS of patients with metastatic colorectal cancer in GI-SCREEN was 39.6 months (95% CI, 37.4 to 41.1 months), which was notably longer than the median OS of 27-30 months reported in recent pivotal trials9-11 (Fig 2A). The long OS observed in this cohort may be because more than 80% of the patients included in GI-SCREEN were enrolled after the initiation of first-line treatment, suggesting the presence of immortal time bias (because GI-SCREEN would not include patients who died before genomic profiling, Fig 2B). To address this hypothesis, we compared the OS of patients enrolled after versus before the initiation of first-line treatment for each cancer type, excluding those with anal canal cancer since no patients were enrolled before treatment initiation. The comparative analysis showed that patients enrolled after the initiation of first-line treatment had a longer OS with a median difference in survival time of 8.9 months and a HR ranging from 0.25 to 0.73 across cancer types (Fig 2C). For example, the median OS was 41.2 months (95% CI, 39.7 to 42.8 months) and 28.0 months (95% CI, 25.7 to 32.1 months) in patients with metastatic colorectal cancer enrolled after and before the initiation of first-line treatment, respectively (HR, 0.63; 95% CI 0.55 to 0.72; P < .0001). This trend persisted in the multivariate analysis (Data Supplement). The median OS of patients who were enrolled before treatment initiation (treatment-naïve patients) was closer to that in previous trials9-11 (Fig 2A).
FIG 2.
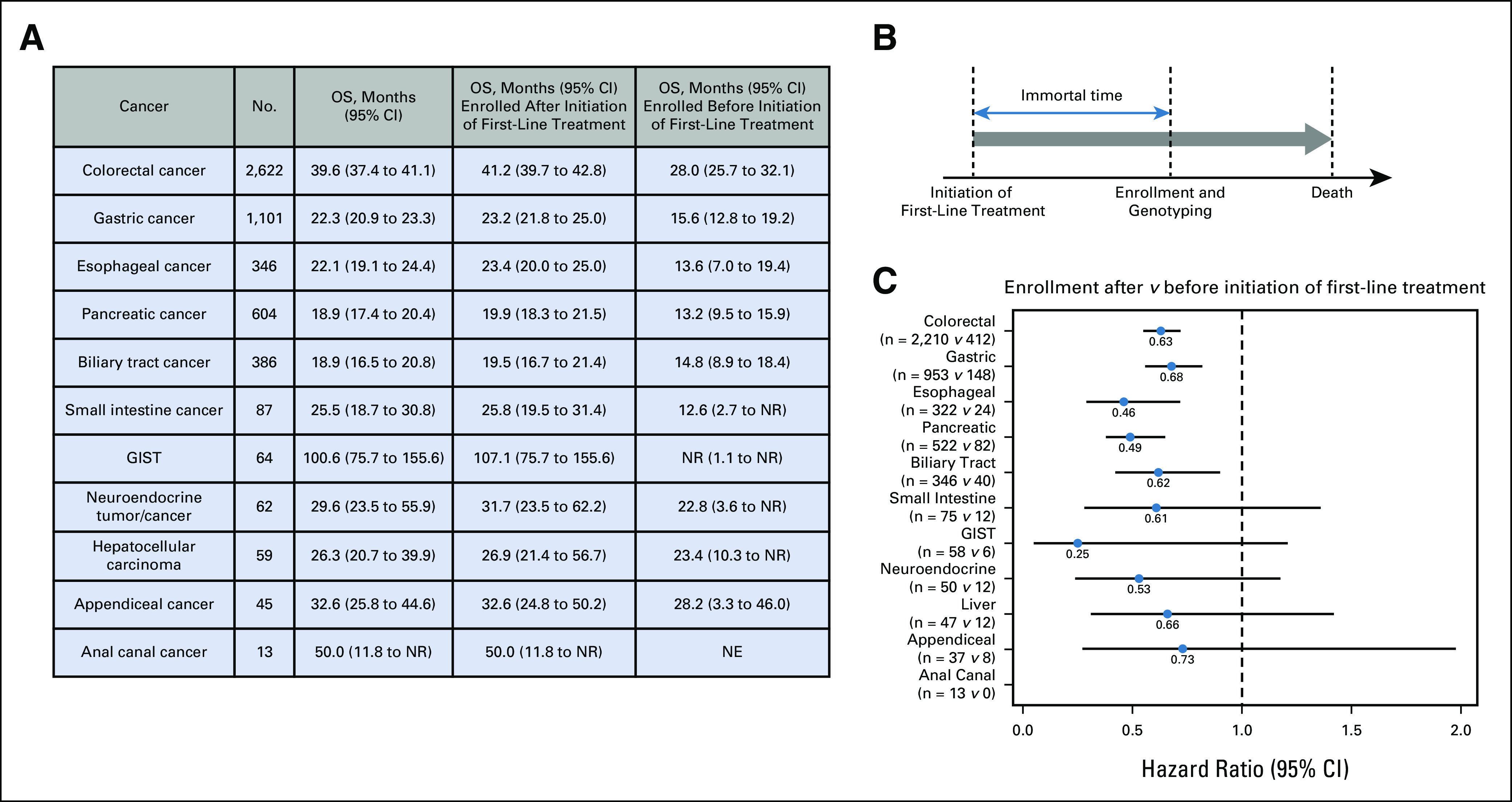
Immortal time bias of OS. (A) OS of patients enrolled in the overall population and patients enrolled after and before the initiation of first-line treatment. (B) Schematic of immortal time bias in clinical sequencing studies. Patients who died before genomic profiling would never have been enrolled. (C) Hazard ratio of the OS of patients enrolled after versus before the initiation of first-line treatment. GIST, gastrointestinal stromal tumor; NE, not evaluated; NR, not reached; OS, overall survival.
Outcome of Patients Enrolled in Targeted Clinical Trials
Of the 5,620 patients with tissue samples, 4,598 (82%) had conclusive OCA results. Actionable alterations classified as tier 1 or 2 were identified at different frequencies, ranging from 7.5% in liver cancer to 89.3% in pancreatic cancer (Fig 3A). This implies a different utility of comprehensive genomic profiling across gastrointestinal cancers.
FIG 3.
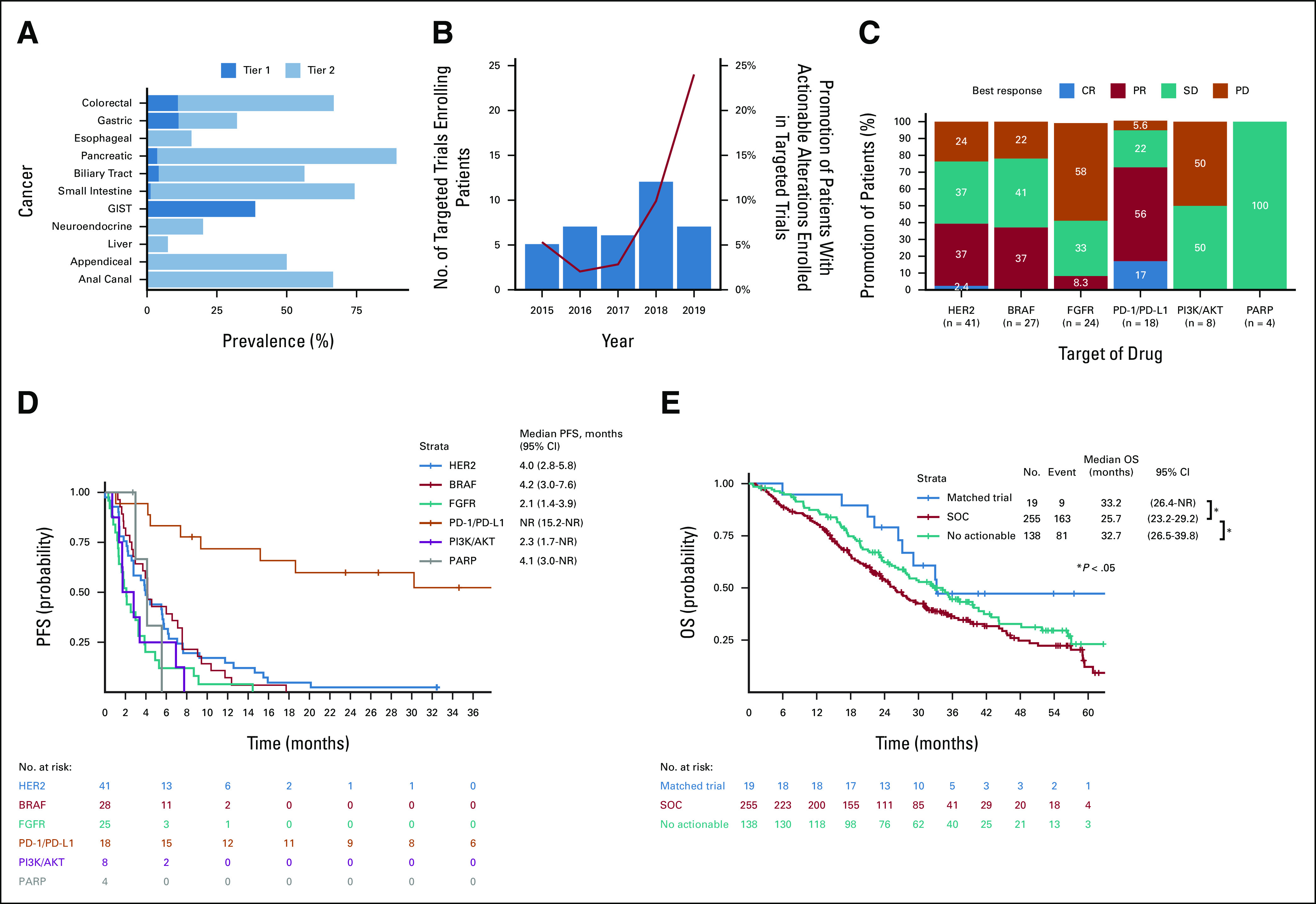
Efficacy of matched therapies in patients enrolled in affiliated clinical trials. (A) The prevalence of patients with tier 1 or 2 genomic alterations as the most actionable alterations. (B) Number of targeted trials enrolling patients in GI-SCREEN (box plot) and proportion of patients enrolled in a matched clinical trial among those with actionable alterations (line plot). (C) Number of patients who had tumor responses according to RECIST v1.1 by matched therapies in the matched clinical trials. (D) Kaplan-Meier curves of PFS of patients receiving matched therapies in matched clinical trials. (E) Kaplan-Meier curves of OS of treatment-naïve patients with metastatic colorectal cancer harboring actionable alterations receiving matched therapies in matched clinical trials and SOC and no actionable alterations. CR, complete response; OS, overall survival; PD, progressive disease; PFS, progression-free survival; PR, partial response; RECIST, Response Evaluation Criteria in Solid Tumors; SD, stable disease; SOC, standard of care.
As of the data cutoff date, 149 patients were enrolled in clinical trials on the basis of the identified alterations and had received matched trial therapies. Among these patients, the most common cancer type was colorectal cancer, followed by biliary tract, esophageal, and gastric cancers (Data Supplement), and the most frequently targeted altered genes were BRAF, ERBB2, FGF/FGFR, microsatellite instability-high (MSI-H), and PIK3CA (Data Supplement). The proportion of patients with actionable alterations enrolled in trials reached approximately 10% as the number of targeted clinical trials affiliated with the GI-SCREEN increased in 2018 (Fig 3B). Among the matched therapies administered to at least four patients, the objective response rate was high for therapies targeting PD-1/PD-L1 (72%), HER2 (39%), and BRAF (37%), whereas few or no responses were achieved with therapies targeting FGFR, PI3K/AKT, and poly (ADP-ribose) polymerase (PARP) (Fig 3C). The median PFS was not reached in patients receiving PD-1–/PD-L1–targeted therapy and was at least 4 months in those receiving therapies targeting HER2, BRAF, and PARP (Fig 3D). To evaluate the OS benefit of matched therapy in clinical trials on the basis of identified biomarkers in patients with colorectal cancer, we compared the OS between patients with actionable alterations who received matched trial therapy and those who did not. In this analysis, patients enrolled in GI-SCREEN after initiation of first-line treatment were excluded to eliminate the effect by immortal time bias shown above. Among treatment-naïve patients with colorectal cancer harboring actionable alterations that excluded the immortal time bias, the median OS was significantly longer in patients who received matched therapies in clinical trials than in those who did not receive matched therapy (HR 0.52; 95% CI, 0.26 to 1.01; P = .049; Fig 3E).
Genomic Alterations in Oncogenic Signaling Pathways and Survival Outcome
To explore the potential oncogenic signaling pathways related to the prognosis in gastrointestinal cancers, we evaluated genomic alterations related to 10 canonical oncogenic signaling pathways curated through a combination of computational methods and expert review in The Cancer Genome Atlas project.12 For each tumor type, the fraction of samples with at least one pathogenic alteration (mutation, amplification, or gene fusion) in each of the 10 oncogenic signaling pathways was computed. Receptor tyrosine kinase (RTK)/RAS (15.0%-89.5%) and p53 (8.2%-75.5%) pathway alterations were most frequently involved in gastrointestinal cancers (Fig 4A). KRAS mutation was the most frequently identified RTK-/RAS-related alteration in colorectal, pancreatic, biliary tract, small intestine, appendiceal, and anal canal cancers and neuroendocrine tumors/cancers. The lowest prevalence of genomic alterations in the 10 pathways analyzed was observed in patients with gastrointestinal stromal tumors and with hepatocellular carcinoma (45% and 55%, respectively).
FIG 4.
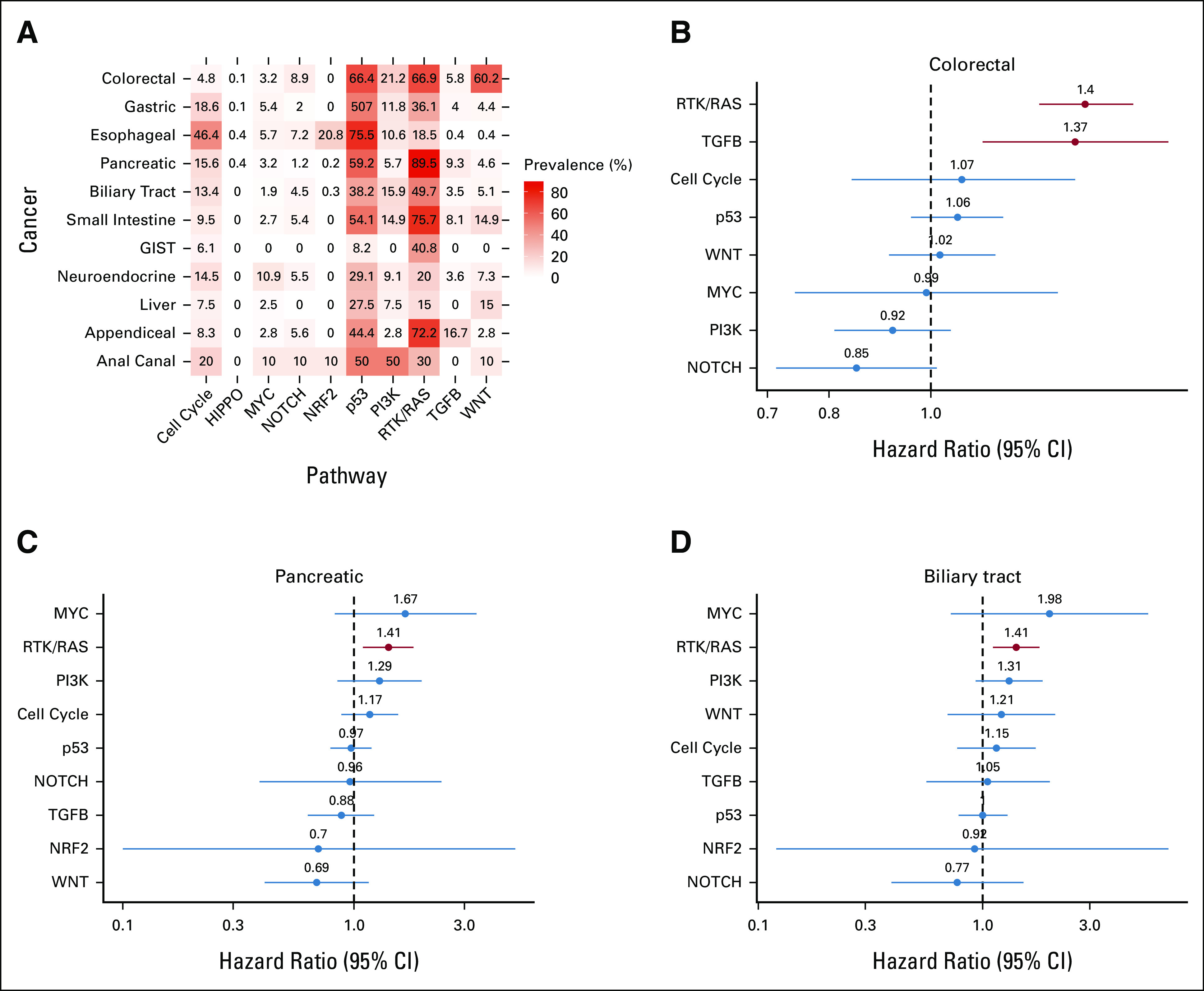
Association of pathway alterations and survival outcomes in gastrointestinal cancers. (A) The prevalence of patients with genomic alterations related to each oncogenic signaling pathway. Forest plot of OS according to each pathway alteration in (B) colorectal cancer, (C) pancreatic cancer, and (D) biliary tract cancer. OS, overall survival.
A multivariate analysis showed that the pathways significantly associated with shorter OS were the RTK/RAS and TGFB pathways in colorectal cancer, the NOTCH and WNT pathways in gastric cancer, the RTK/RAS pathway in pancreatic and biliary tract cancers, the cell cycle and MYC pathway in neuroendocrine tumors, the p53 pathway in hepatocellular carcinoma, and the p53 and TGFB pathways in appendiceal cancer (P < .05; Figs 4B-4D and Data Supplement). The relationship between OS and each genomic alteration involving the RTK/RAS pathway showed that most genomic alterations were associated with a worse prognosis in colorectal, pancreatic, and biliary tract cancers (Figs 4B-4D). Fusions in RTK-/RAS-related genes, such as ALK, NTRK3, RET, and ROS1, were strong negative prognostic factors for patients with colorectal cancer (HR, >2). The relationships in other pathways are shown in the Data Supplement.
To evaluate whether oncogenic signaling pathways affected the efficacy of targeted trials, we performed multivariate analysis to assess the association of pathway alterations with PFS of patients who received matched therapies in the matched trials. PI3K pathway alterations in HER2-targeted therapies (HR, 13.2; P = .003) and MYC pathway alterations in FGFR-targeted therapies (HR, 54.6; P = .022) were significantly associated with shorter PFS (Data Supplement).
DISCUSSION
Although global genomic profiling programs have been implemented to facilitate patient enrollment in targeted clinical trials, it remains unclear whether these programs improve patient outcomes. Our evaluation of large-scale qualified clinicogenomic data from SCRUM-Japan GI-SCREEN showed the effect of immortal time bias on survival in a real-world setting and the relationship of oncogenic signaling pathway alterations with prognosis and the efficacy of matched therapies in clinical trials. The SCRUM-Japan GI-SCREEN collaborative network, which achieved 99.9% completeness of clinical data, made this high-quality study possible, whereas electronic health record–based real-world databases generally miss clinical data in 30%-40% of the population.13,14
The impact of immortal time biases has been unclear in genomic profiling programs targeting patients with advanced cancers who underwent NGS testing primarily aimed at assessing eligibility for clinical trials of targeted agents during their treatment course. Our evaluation revealed that patients who underwent NGS testing after the initiation of first-line treatment had a longer OS than those who underwent NGS testing before first-line treatment, with a median difference in survival time of 8.9 months and HRs of 0.25-0.73 for different cancer types. Since most clinical trials enroll patients who have completed standard therapies, NGS is generally performed for patients receiving systemic therapy and are likely to be eligible for clinical trials.5 Therefore, the real-world population with available NGS results does not include patients who did not undergo NGS testing for reasons such as deterioration of their condition after the initiation of systemic therapy, which may cause immortal time bias. Indeed, the difference in the OS observed between patients who underwent NGS before and after the initiation of systemic therapy was significant and thus cannot be ignored. Although studies evaluating survival outcomes using real-world data have increased,15,16 real-world data of patients receiving NGS have been used in few studies, in which OS was longer than that in clinical trials as seen in the GI-SCREEN program.17,18 To avoid immortal time bias, precautions are needed when using data from patients who have undergone NGS testing after the initiation of the evaluated treatment line.
The most important aim of SCRUM-Japan GI-SCREEN is the systemic application of comprehensive genomic profiling to enroll patients with advanced gastrointestinal cancers harboring actionable alterations for whom few treatment options are available. Patients who received matched therapies in clinical trials on the basis of the actionable alterations identified in GI-SCREEN had an objective response rate and a PFS comparable with those achieved in pivotal clinical trials.19-25 Furthermore, the median OS of patients with colorectal cancer who had actionable alterations and received matched therapy in clinical trials was approximately 7 months longer than that of patients with actionable alterations receiving unmatched therapy in the treatment-naïve population. This survival benefit of molecularly matched therapy may support comprehensive genomic profiling using NGS for patients with advanced gastrointestinal cancers to improve their survival. Although the efficacy of a part of these matched therapies is already known because some drugs used in trials, such as immune checkpoint inhibitors for MSI-H disease and encorafenib plus cetuximab for BRAF V600E–mutant colorectal cancer, have been approved, the utility of genomic profiling in our data can be applied in current standard of care and screening for clinical trials. In this population, patients without actionable alterations had a comparable prognosis with those with actionable alterations receiving matched therapy, possibly because of the lack of resistance to systemic therapy because of actionable alterations and the survival benefit of anti-EGFR therapy in patients with RAS wild-type colorectal cancer.
Our pathway-level analysis successfully identified the biologic signaling pathways that are truly prognostically relevant in each cancer type by classifying genomic alterations with similar functions into 10 oncogenic signaling pathways. The RTK/RAS pathway was the significant pathway relevant to poor prognosis in colorectal, pancreatic, and biliary tract cancers. This underlies the significance of a strategy targeting downstream proteins in this pathway, such as KRAS G12C, developed recently, in addition to conventional antibody drugs against RTK proteins.26 Pathway analysis also showed the potential resistance mechanism against molecularly targeted therapy: the PI3K pathway for HER2-targeted therapy and the MYC pathway for FGFR-targeted therapy. The involvement of pathway alterations in resistance to targeted therapy warrants further evaluation of the mechanisms and potential combination strategies to overcome resistance.
An important limitation of this study was that the survival analyses were conducted retrospectively. The biomarker for the efficacy of targeted therapy was explored using data on genomic alterations of archival tumor tissue samples collected before clinical trials. Nevertheless, the efficacy of the matched therapy and the association between pathway alterations and prognosis were consistent with those reported in previous studies. Patients who received matched therapy had a limited number of events because of the analysis only for those who were enrolled before initiation of systemic treatment. The survival benefit of genomic profiling needs to be further evaluated. In addition, pathway analysis targeted only genomic alterations but not other molecular abnormalities, such as transcriptomic and proteomic alterations. To address this, we recently launched a new comprehensive molecular profiling study, MONSTAR-SCREEN-2 (UMIN000043899), in which multiomics analyses are performed for patients with solid tumors to evaluate the biologic behavior of an accurate multilayered network of oncogenic signaling pathways.7
In conclusion, our nationwide large-scale genomic profiling program led to patient enrollment in targeted clinical trials and improved survival of patients with colorectal cancer who received matched therapies in clinical trials. Although the proportion of patients enrolled in trials was low in total, it had increased as the number of trials affiliated with GI-SCREEN was expanded, suggesting that genotyping screening and availability of clinical trials are crucial to accelerating clinical trial enrollment. As novel types of treatments, such as antibody drug conjugates, immunotherapy, and cell therapy, have been developed, the demand for the latest diagnostic technologies has increased. The SCRUM-Japan platform has applied liquid biopsy technologies and multiomics approach to develop such therapies. These ongoing studies will reinforce the utility of molecular profiling programs in the new era of cancer therapy and uncover the detailed cross-talk mechanisms among oncogenic signal transduction pathways in the future.
ACKNOWLEDGMENT
The authors would like to thank all the patients and their families who participated in this study, all investigators, research nurses, study coordinators, and all the National Cancer Center Hospital East Translational Research Support Section members. This study was supported by grants from the National Cancer Center Research and Development Fund (31-A-5 to A. Ohtsu) and the SCRUM-Japan Funds (http://www.scrum-japan.ncc.go.jp/index.html).
Yoshiaki Nakamura
Honoraria: Chugai Pharma, Guardant Health AMEA, Merck
Research Funding: Taiho Pharmaceutical (Inst), Guardant Health (Inst), Genomedia (Inst), Chugai Pharma (Inst), Guardant Health (Inst), Seattle Genetics (Inst), Roche (Inst)
Riu Yamashita
Consulting or Advisory Role: Takeda
Wataru Okamoto
Honoraria: Chugai Pharma, Ono Pharmaceutical, Bristol-Myers Squibb Japan, Yakult Honsha, Lilly Japan, Thermo Fisher Scientific, Takeda, Novartis, Taiho Pharmaceutical
Consulting or Advisory Role: Daiichi Sankyo
Research Funding: Janssen Oncology (Inst)
Yoshito Komatsu
Honoraria: Lilly Japan, Taiho Pharmaceutical, Chugai Pharma, Takeda, Bayer Yakuhin, Bristol-Myers Squibb Co, Sanofi/Aventis, Merck, Yakult Honsha, Ono Pharmaceutical, Nipro Corporation, Moroo Co, Asahi Kasei, Mitsubishi Tanabe Pharma, Otsuka, Medical Review Co., Ltd, Daiichi Sankyo
Research Funding: MSD K.K, Taiho Pharmaceutical, Yakult Honsha, Bayer Yakuhin, DAIICHI SANKYO CO., Ltd, Ono Pharmaceutical, NanoCarrier, Eisai, Sanofi/Aventis, Sysmex, Shionogi, IQVIA, Parexel International Corporation, Astellas Pharma, Mediscience Planning, Sumitomo Dainippon Pharma Co., Ltd, A2 Healthcare, Incyte, Lilly (Inst), Nipro Corporation (Inst), BeiGene (Inst)
Satoshi Yuki
Honoraria: Chugai Pharma, Takeda, Lilly Japan, Bayer Yakuhin, Taiho Pharmaceutical, Bristol-Myers Squibb Japan, Ono Pharmaceutical, Merck, MSD K.K
Makoto Ueno
Honoraria: Taiho Pharmaceutical, Yakult Honsha, AstraZeneca, MSD, Ono Pharmaceutical, SERVIER, Chugai Pharma, Incyte, Takeda, Novartis
Consulting or Advisory Role: Novocure
Research Funding: Taiho Pharmaceutical (Inst), Eisai (Inst), AstraZeneca (Inst), Ono Pharmaceutical (Inst), MSD (Inst), Incyte (Inst), Astellas Pharma (Inst), Chugai Pharma (Inst), Delta-Fly Pharma (Inst), JPH Clinical Development (Inst), Chiome Bioscience (Inst)
Ken Kato
Honoraria: Lilly, BMS, Ono Pharmaceutical
Consulting or Advisory Role: Ono Pharmaceutical, BeiGene, MSD, Oncolys BioPharma, Bayer
Speakers' Bureau: Ono Pharmaceutical, Bristol-Myers Squibb Japan, MSD
Research Funding: Ono Pharmaceutical (Inst), Shionogi (Inst), MSD Oncology (Inst), BeiGene (Inst), Chugai Pharma (Inst), Bayer (Inst), AstraZeneca (Inst), Taiho Pharmaceutical (Inst)
Hiroya Taniguchi
Honoraria: Bayer, Sanofi, Takeda, Chugai Pharma, Taiho Pharmaceutical, Lilly Japan, Merck Serono, Yakult Honsha, Medical & Biological Laboratories Co., Ltd, Bristol-Myers Squibb Japan, MSD K.K, Novartis, Daiichi Sankyo, Mitsubishi Tanabe Pharma, Nippon Kayaku, Ono Yakuhin
Research Funding: Dainippon Sumitomo Pharma (Inst), Array BioPharma (Inst), MSD Oncology (Inst), Ono Pharmaceutical (Inst), Daiichi Sankyo (Inst), Sysmex (Inst), Novartis (Inst), Takeda (Inst)
Yoshinori Kagawa
Consulting or Advisory Role: Taiho Pharmaceutical, Merck, Lilly
Speakers' Bureau: Lilly, Sanofi, Takeda, Merck, Taiho Pharmaceutical, MSD, Chugai Pharma, Yakult Pharmaceutical, Bayer, Ono Pharmaceutical
Research Funding: Ono Pharmaceutical
Tadamichi Denda
Honoraria: Ono Pharmaceutical
Speakers' Bureau: Daiichi-Sankyo, Ono Pharmaceutical
Research Funding: Ono Pharmaceutical (Inst), Amgen (Inst)
Hiroki Hara
Honoraria: Chugai Pharma, Taiho Pharmaceutical, Merck Serono, Yakult Honsha, Lilly, Ono Pharmaceutical, Takeda, Bristol-Myers Squibb, Bayer, Asahi Kasei, MSD, Daiichi Sankyo/UCB Japan
Consulting or Advisory Role: Ono Pharmaceutical, MSD, Boehringer Ingelheim, Bristol-Myers Squibb Japan, Chugai Pharma
Research Funding: AstraZeneca (Inst), Merck Serono (Inst), MSD (Inst), Ono Pharmaceutical (Inst), Taiho Pharmaceutical (Inst), Boehringer Ingelheim (Inst), Dainippon Sumitomo Pharma (Inst), Daiichi Sankyo (Inst), BeiGene (Inst), Astellas Pharma (Inst), Bayer (Inst), Amgen (Inst), Chugai Pharma (Inst), Janssen Oncology (Inst), ALX Oncology (Inst), Eisai (Inst), Bristol-Myers Squibb Japan (Inst)
Taito Esaki
Honoraria: Lilly, Taiho Pharmaceutical, Daiichi Sankyo, Chugai Pharma
Research Funding: Daiichi Sankyo (Inst), MSD (Inst), Novartis (Inst), Ono Pharmaceutical (Inst), Astellas Pharma (Inst), Lilly (Inst), Bayer (Inst), Nihon Kayaku (Inst), Amgen Astellas BioPharma (Inst), Parexel (Inst), IQVIA (Inst), Quintiles (Inst), Eisai (Inst), Pfizer (Inst), Chugai Pharma (Inst), Syneos Health (Inst), Asahi Kasei pharma (Inst), Amgen (Inst), Dainippon Sumitomo (Inst), Dainippon Sumitomo (Inst)
Toshikazu Moriwaki
Speakers' Bureau: Taiho Pharmaceutical, Chugai Pharma, Yakult Honsha, Takeda, Merck Serono, Lilly Japan, Bayer Yakuhin, Ono Pharmaceutical
Research Funding: Taiho Pharmaceutical (Inst), MSD (Inst), Takeda (Inst), Yakult Honsha (Inst), Asahi Kasei (Inst), Isofol Medical (Inst)
Yu Sunakawa
Honoraria: Taiho Pharmaceutical, Chugai Pharma, Takeda, Bayer Yakuhin, Bristol-Myers Squibb Japan, Lilly Japan, Merck, Sysmex, MSD K.K, Ono Pharmaceutical, Daiichi Sankyo, Guardant Health, Incyte
Consulting or Advisory Role: Bristol-Myers Squibb Japan, MSD K.K, Daiichi Sankyo, Merck
Research Funding: Taiho Pharmaceutical, Takeda, Chugai Pharma, Lilly Japan, Sanofi, Otsuka
Eiji Oki
Speakers' Bureau: Chugai Pharma, Lilly Japan, Takeda, Ono Pharmaceutical, Bristol-Myers Squibb Japan, Taiho Pharmaceutical
Research Funding: Guardant Health
Fumio Nagashima
Speakers' Bureau: Taiho Pharmaceutical, Ono Pharmaceutical, Merck Serono, Takeda, Chugai Pharma, Yakult Honsha, Sumitomo Dainippon, Kyowa Hakko Kirin, Janssen
Research Funding: Taiho Pharmaceutical (Inst), Ono Pharmaceutical (Inst), OncoTherapy Science (Inst), Merck Serono (Inst), Zeria Pharmaceutical (Inst), Lilly Japan (Inst), Takeda (Inst), Chugai Pharma (Inst), Yakult Pharmaceutical (Inst), Sumitomo Dainippon (Inst), Daiichi Sankyo (Inst), Shionogi (Inst), Novartis (Inst), J-Pharma (Inst), Bristol-Myers Squibb (Inst), Kyowa Hakko Kirin (Inst), Mochida Pharmaceutical Co. Ltd (Inst), Astellas Pharma (Inst), Bayer (Inst), MSD (Inst), Eisai (Inst), NanoCarrier (Inst), Janssen (Inst), Baxalta/Shire (Inst)
Tomohiro Nishina
Honoraria: Taiho Pharmaceutical, Chugai Pharma, Lilly, Merck Serono, Takeda, Bristol-Myers Squibb, Ono Pharmaceutical
Research Funding: Taiho Pharmaceutical (Inst), Chugai Pharma (Inst), Dainippon Sumitomo Pharma (Inst), Lilly Japan (Inst), Merck Serono (Inst), MSD (Inst), Daiichi Sankyo (Inst), Ono Pharmaceutical (Inst), Eisai (Inst)
Taroh Satoh
Honoraria: Chugai Pharma, Merck Serono, Bristol-Myers Squibb, Takeda, Yakult Honsha, Lilly, Bayer Yakuhin, Ono Pharmaceutical, Merck, Astellas Pharma, Taiho Pharmaceutical, Nihon Kayaku, Daiichi-Sankyo
Consulting or Advisory Role: Bayer Yakuhin, Lilly, Ono Pharmaceutical, Takara Bio, Merck Serono, Nihon Kayaku
Research Funding: Yakult Honsha (Inst), Chugai Pharma (Inst), Ono Pharmaceutical (Inst), Sanofi (Inst), Lilly (Inst), Daiichi Sankyo (Inst), Merck (Inst), Merck Serono (Inst), Gilead Sciences (Inst), Dainippon Sumitomo Pharma (Inst), IQVIA (Inst)
Hisato Kawakami
Honoraria: Chugai/Roche, Taiho Pharmaceutical, Ono Pharmaceutical, Bristol-Myers Squibb Japan, Yakult Pharmaceutical, Takeda, MSD K.K, Merck Serono, Lilly Japan, Daiichi Sankyo, Bayer
Consulting or Advisory Role: Taiho Pharmaceutical, Bristol-Myers Squibb Japan, Ono Pharmaceutical, Lilly Japan, Daiichi Sankyo Co. Ltd
Research Funding: Chugai Pharma (Inst), Daiichi Sankyo (Inst), Eisai (Inst), Kobayashi Pharmaceutical (Inst)
Kensei Yamaguchi
Consulting or Advisory Role: Bristol-Myers Squibb Japan, Daiichi Sankyo
Speakers' Bureau: Chugai Pharma, Bristol-Myers Squibb Japan, Takeda, Taiho Pharmaceutical, Lilly, Ono Pharmaceutical, Daiichi Sankyo, Merck
Research Funding: Ono Pharmaceutical (Inst), Taiho Pharmaceutical (Inst), Daiichi Sankyo (Inst), Lilly (Inst), Gilead Sciences (Inst), Yakult Honsha (Inst), Chugai Pharma (Inst), Boehringer Ingelheim (Inst), Eisai (Inst), MSD Oncology (Inst), Sanofi (Inst), Bristol-Myers Squibb (Inst)
Takeshi Kato
Honoraria: Chugai Pharma, Ono Pharmaceutical, Takeda, Lilly Japan, Asahi Kasei
Research Funding: Chugai Pharma
Akihito Tsuji
Speakers' Bureau: Chugai Pharma, Taiho Pharmaceutical, Merck Serono, Takeda Pharmaceutical Company Limited, Lilly Japan, Bristol-Myers Squibb Corporation, Sanofi
Research Funding: Taiho Pharmaceutical Co., Ltd (Inst), Sanofi (Inst), Ono Pharmaceutical (Inst)
Hisateru Yasui
Honoraria: Taiho Pharmaceutical, Chugai Pharma, Bristol-Myers Squibb Japan, Daiichi Sankyo, Lilly Japan, Yakult Honsha, Bayer Yakuhin, Takeda, Ono Pharmaceutical
Research Funding: MSD (Inst), Ono Pharmaceutical (Inst), Astellas Pharma (Inst), AstraZeneca (Inst), Daiichi Sankyo (Inst)
Masahiro Goto
Honoraria: Daiichi Sankyo Company, Limited, Ono Pharmaceutical, Taiho Pharmaceutical, MSD K.K, Takeda, Sumitomo Dainippon Pharma Co., Ltd, Yakult Pharmaceutical, Eli Lilly Japan K.K
Research Funding: Chugai Pharma, Taiho Pharmaceutical, Nippon Kayaku
Yasuo Hamamoto
Consulting or Advisory Role: Dainippon Sumitomo Pharma, Chugai Pharma
Research Funding: Ono Pharmaceutical, Bristol-Myers Squibb Japan, MSD Oncology, Lilly Japan
Masashi Wakabayashi
Honoraria: Nihon Medi-Physics
Kohei Shitara
Honoraria: Bristol-Myers Squibb, Takeda, Janssen
Consulting or Advisory Role: Lilly, Bristol-Myers Squibb, Takeda, Pfizer, Ono Pharmaceutical, MSD, Taiho Pharmaceutical, Novartis, AbbVie, GlaxoSmithKline, Daiichi Sankyo, Boehringer Ingelheim, Amgen, Astellas Pharma, Guardant Health
Research Funding: MSD (Inst), Daiichi Sankyo (Inst), Taiho Pharmaceutical (Inst), Chugai Pharma (Inst), Ono Pharmaceutical (Inst), Astellas Pharma (Inst), Eisai (Inst), Amgen (Inst)
Hideaki Bando
Honoraria: Taiho Pharmaceutical, Chugai Pharma, Lilly Japan, Ono Pharmaceutical
Katsuya Tsuchihara
Honoraria: Chugai Pharma, Takeda, Illumina, Eisai, Boehringer Ingelheim Seiyaku, Bayer Yakuhin, Gilead Sciences
Atsushi Ohtsu
Employment: Celgene, AstraZeneca
Honoraria: Taiho Pharmaceutical, Ono Pharmaceutical
Research Funding: Bristol-Myers Squibb
Takayuki Yoshino
Honoraria: Chugai Pharma, Merck, Bayer Yakuhin, Ono Pharmaceutical, MSD K.K
Research Funding: MSD (Inst), Daiichi Sankyo Company, Limited (Inst), Ono Pharmaceutical (Inst), Taiho Pharmaceutical (Inst), Amgen (Inst), Sanofi (Inst), Pfizer (Inst), Genomedia (Inst), Sysmex (Inst), Nippon Boehringer Ingelheim (Inst), Chugai Pharma (Inst)
No other potential conflicts of interest were reported.
DATA SHARING STATEMENT
A data sharing statement provided by the authors is available with this article at DOI https://doi.org/10.1200/PO.22.00653.
AUTHOR CONTRIBUTIONS
Conception and design: Yoshiaki Nakamura, Wataru Okamoto, Yoshinori Kagawa, Eiji Oki, Takeshi Kato, Kohei Shitara, Katsuya Tsuchihara, Atsushi Ohtsu, Takayuki Yoshino
Administrative support: Takeshi Kato, Hideaki Bando, Katsuya Tsuchihara, Izumi Miki, Hiroko Ichiki
Provision of study materials or patients: Yoshiaki Nakamura, Yoshito Komatsu, Ken Kato, Yoshinori Kagawa, Hiroki Hara, Yu Sunakawa, Taroh Satoh, Kensei Yamaguchi, Koushiro Ohtsubo, Takeshi Kato, Hideaki Bando, Izumi Miki
Collection and assembly of data: Yoshiaki Nakamura, Wataru Okamoto, Yoshito Komatsu, Satoshi Yuki, Makoto Ueno, Ken Kato, Hiroya Taniguchi, Yoshinori Kagawa, Tadamichi Denda, Hiroki Hara, Taito Esaki, Toshikazu Moriwaki, Yu Sunakawa, Eiji Oki, Fumio Nagashima, Tomohiro Nishina, Taroh Satoh, Hisato Kawakami, Kensei Yamaguchi, Koushiro Ohtsubo, Takeshi Kato, Yosuke Horita, Akihito Tsuji, Hisateru Yasui, Masahiro Goto, Yasuo Hamamoto, Kohei Shitara, Hideaki Bando, Katsuya Tsuchihara, Izumi Miki, Hiroko Ichiki, Takayuki Yoshino
Data analysis and interpretation: Yoshiaki Nakamura, Riu Yamashita, Yoshito Komatsu, Hiroya Taniguchi, Hiroki Hara, Taroh Satoh, Masashi Wakabayashi, Takashi Ikeno, Hideaki Bando, Atsushi Ohtsu, Takayuki Yoshino
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/po/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Yoshiaki Nakamura
Honoraria: Chugai Pharma, Guardant Health AMEA, Merck
Research Funding: Taiho Pharmaceutical (Inst), Guardant Health (Inst), Genomedia (Inst), Chugai Pharma (Inst), Guardant Health (Inst), Seattle Genetics (Inst), Roche (Inst)
Riu Yamashita
Consulting or Advisory Role: Takeda
Wataru Okamoto
Honoraria: Chugai Pharma, Ono Pharmaceutical, Bristol-Myers Squibb Japan, Yakult Honsha, Lilly Japan, Thermo Fisher Scientific, Takeda, Novartis, Taiho Pharmaceutical
Consulting or Advisory Role: Daiichi Sankyo
Research Funding: Janssen Oncology (Inst)
Yoshito Komatsu
Honoraria: Lilly Japan, Taiho Pharmaceutical, Chugai Pharma, Takeda, Bayer Yakuhin, Bristol-Myers Squibb Co, Sanofi/Aventis, Merck, Yakult Honsha, Ono Pharmaceutical, Nipro Corporation, Moroo Co, Asahi Kasei, Mitsubishi Tanabe Pharma, Otsuka, Medical Review Co., Ltd, Daiichi Sankyo
Research Funding: MSD K.K, Taiho Pharmaceutical, Yakult Honsha, Bayer Yakuhin, DAIICHI SANKYO CO., Ltd, Ono Pharmaceutical, NanoCarrier, Eisai, Sanofi/Aventis, Sysmex, Shionogi, IQVIA, Parexel International Corporation, Astellas Pharma, Mediscience Planning, Sumitomo Dainippon Pharma Co., Ltd, A2 Healthcare, Incyte, Lilly (Inst), Nipro Corporation (Inst), BeiGene (Inst)
Satoshi Yuki
Honoraria: Chugai Pharma, Takeda, Lilly Japan, Bayer Yakuhin, Taiho Pharmaceutical, Bristol-Myers Squibb Japan, Ono Pharmaceutical, Merck, MSD K.K
Makoto Ueno
Honoraria: Taiho Pharmaceutical, Yakult Honsha, AstraZeneca, MSD, Ono Pharmaceutical, SERVIER, Chugai Pharma, Incyte, Takeda, Novartis
Consulting or Advisory Role: Novocure
Research Funding: Taiho Pharmaceutical (Inst), Eisai (Inst), AstraZeneca (Inst), Ono Pharmaceutical (Inst), MSD (Inst), Incyte (Inst), Astellas Pharma (Inst), Chugai Pharma (Inst), Delta-Fly Pharma (Inst), JPH Clinical Development (Inst), Chiome Bioscience (Inst)
Ken Kato
Honoraria: Lilly, BMS, Ono Pharmaceutical
Consulting or Advisory Role: Ono Pharmaceutical, BeiGene, MSD, Oncolys BioPharma, Bayer
Speakers' Bureau: Ono Pharmaceutical, Bristol-Myers Squibb Japan, MSD
Research Funding: Ono Pharmaceutical (Inst), Shionogi (Inst), MSD Oncology (Inst), BeiGene (Inst), Chugai Pharma (Inst), Bayer (Inst), AstraZeneca (Inst), Taiho Pharmaceutical (Inst)
Hiroya Taniguchi
Honoraria: Bayer, Sanofi, Takeda, Chugai Pharma, Taiho Pharmaceutical, Lilly Japan, Merck Serono, Yakult Honsha, Medical & Biological Laboratories Co., Ltd, Bristol-Myers Squibb Japan, MSD K.K, Novartis, Daiichi Sankyo, Mitsubishi Tanabe Pharma, Nippon Kayaku, Ono Yakuhin
Research Funding: Dainippon Sumitomo Pharma (Inst), Array BioPharma (Inst), MSD Oncology (Inst), Ono Pharmaceutical (Inst), Daiichi Sankyo (Inst), Sysmex (Inst), Novartis (Inst), Takeda (Inst)
Yoshinori Kagawa
Consulting or Advisory Role: Taiho Pharmaceutical, Merck, Lilly
Speakers' Bureau: Lilly, Sanofi, Takeda, Merck, Taiho Pharmaceutical, MSD, Chugai Pharma, Yakult Pharmaceutical, Bayer, Ono Pharmaceutical
Research Funding: Ono Pharmaceutical
Tadamichi Denda
Honoraria: Ono Pharmaceutical
Speakers' Bureau: Daiichi-Sankyo, Ono Pharmaceutical
Research Funding: Ono Pharmaceutical (Inst), Amgen (Inst)
Hiroki Hara
Honoraria: Chugai Pharma, Taiho Pharmaceutical, Merck Serono, Yakult Honsha, Lilly, Ono Pharmaceutical, Takeda, Bristol-Myers Squibb, Bayer, Asahi Kasei, MSD, Daiichi Sankyo/UCB Japan
Consulting or Advisory Role: Ono Pharmaceutical, MSD, Boehringer Ingelheim, Bristol-Myers Squibb Japan, Chugai Pharma
Research Funding: AstraZeneca (Inst), Merck Serono (Inst), MSD (Inst), Ono Pharmaceutical (Inst), Taiho Pharmaceutical (Inst), Boehringer Ingelheim (Inst), Dainippon Sumitomo Pharma (Inst), Daiichi Sankyo (Inst), BeiGene (Inst), Astellas Pharma (Inst), Bayer (Inst), Amgen (Inst), Chugai Pharma (Inst), Janssen Oncology (Inst), ALX Oncology (Inst), Eisai (Inst), Bristol-Myers Squibb Japan (Inst)
Taito Esaki
Honoraria: Lilly, Taiho Pharmaceutical, Daiichi Sankyo, Chugai Pharma
Research Funding: Daiichi Sankyo (Inst), MSD (Inst), Novartis (Inst), Ono Pharmaceutical (Inst), Astellas Pharma (Inst), Lilly (Inst), Bayer (Inst), Nihon Kayaku (Inst), Amgen Astellas BioPharma (Inst), Parexel (Inst), IQVIA (Inst), Quintiles (Inst), Eisai (Inst), Pfizer (Inst), Chugai Pharma (Inst), Syneos Health (Inst), Asahi Kasei pharma (Inst), Amgen (Inst), Dainippon Sumitomo (Inst), Dainippon Sumitomo (Inst)
Toshikazu Moriwaki
Speakers' Bureau: Taiho Pharmaceutical, Chugai Pharma, Yakult Honsha, Takeda, Merck Serono, Lilly Japan, Bayer Yakuhin, Ono Pharmaceutical
Research Funding: Taiho Pharmaceutical (Inst), MSD (Inst), Takeda (Inst), Yakult Honsha (Inst), Asahi Kasei (Inst), Isofol Medical (Inst)
Yu Sunakawa
Honoraria: Taiho Pharmaceutical, Chugai Pharma, Takeda, Bayer Yakuhin, Bristol-Myers Squibb Japan, Lilly Japan, Merck, Sysmex, MSD K.K, Ono Pharmaceutical, Daiichi Sankyo, Guardant Health, Incyte
Consulting or Advisory Role: Bristol-Myers Squibb Japan, MSD K.K, Daiichi Sankyo, Merck
Research Funding: Taiho Pharmaceutical, Takeda, Chugai Pharma, Lilly Japan, Sanofi, Otsuka
Eiji Oki
Speakers' Bureau: Chugai Pharma, Lilly Japan, Takeda, Ono Pharmaceutical, Bristol-Myers Squibb Japan, Taiho Pharmaceutical
Research Funding: Guardant Health
Fumio Nagashima
Speakers' Bureau: Taiho Pharmaceutical, Ono Pharmaceutical, Merck Serono, Takeda, Chugai Pharma, Yakult Honsha, Sumitomo Dainippon, Kyowa Hakko Kirin, Janssen
Research Funding: Taiho Pharmaceutical (Inst), Ono Pharmaceutical (Inst), OncoTherapy Science (Inst), Merck Serono (Inst), Zeria Pharmaceutical (Inst), Lilly Japan (Inst), Takeda (Inst), Chugai Pharma (Inst), Yakult Pharmaceutical (Inst), Sumitomo Dainippon (Inst), Daiichi Sankyo (Inst), Shionogi (Inst), Novartis (Inst), J-Pharma (Inst), Bristol-Myers Squibb (Inst), Kyowa Hakko Kirin (Inst), Mochida Pharmaceutical Co. Ltd (Inst), Astellas Pharma (Inst), Bayer (Inst), MSD (Inst), Eisai (Inst), NanoCarrier (Inst), Janssen (Inst), Baxalta/Shire (Inst)
Tomohiro Nishina
Honoraria: Taiho Pharmaceutical, Chugai Pharma, Lilly, Merck Serono, Takeda, Bristol-Myers Squibb, Ono Pharmaceutical
Research Funding: Taiho Pharmaceutical (Inst), Chugai Pharma (Inst), Dainippon Sumitomo Pharma (Inst), Lilly Japan (Inst), Merck Serono (Inst), MSD (Inst), Daiichi Sankyo (Inst), Ono Pharmaceutical (Inst), Eisai (Inst)
Taroh Satoh
Honoraria: Chugai Pharma, Merck Serono, Bristol-Myers Squibb, Takeda, Yakult Honsha, Lilly, Bayer Yakuhin, Ono Pharmaceutical, Merck, Astellas Pharma, Taiho Pharmaceutical, Nihon Kayaku, Daiichi-Sankyo
Consulting or Advisory Role: Bayer Yakuhin, Lilly, Ono Pharmaceutical, Takara Bio, Merck Serono, Nihon Kayaku
Research Funding: Yakult Honsha (Inst), Chugai Pharma (Inst), Ono Pharmaceutical (Inst), Sanofi (Inst), Lilly (Inst), Daiichi Sankyo (Inst), Merck (Inst), Merck Serono (Inst), Gilead Sciences (Inst), Dainippon Sumitomo Pharma (Inst), IQVIA (Inst)
Hisato Kawakami
Honoraria: Chugai/Roche, Taiho Pharmaceutical, Ono Pharmaceutical, Bristol-Myers Squibb Japan, Yakult Pharmaceutical, Takeda, MSD K.K, Merck Serono, Lilly Japan, Daiichi Sankyo, Bayer
Consulting or Advisory Role: Taiho Pharmaceutical, Bristol-Myers Squibb Japan, Ono Pharmaceutical, Lilly Japan, Daiichi Sankyo Co. Ltd
Research Funding: Chugai Pharma (Inst), Daiichi Sankyo (Inst), Eisai (Inst), Kobayashi Pharmaceutical (Inst)
Kensei Yamaguchi
Consulting or Advisory Role: Bristol-Myers Squibb Japan, Daiichi Sankyo
Speakers' Bureau: Chugai Pharma, Bristol-Myers Squibb Japan, Takeda, Taiho Pharmaceutical, Lilly, Ono Pharmaceutical, Daiichi Sankyo, Merck
Research Funding: Ono Pharmaceutical (Inst), Taiho Pharmaceutical (Inst), Daiichi Sankyo (Inst), Lilly (Inst), Gilead Sciences (Inst), Yakult Honsha (Inst), Chugai Pharma (Inst), Boehringer Ingelheim (Inst), Eisai (Inst), MSD Oncology (Inst), Sanofi (Inst), Bristol-Myers Squibb (Inst)
Takeshi Kato
Honoraria: Chugai Pharma, Ono Pharmaceutical, Takeda, Lilly Japan, Asahi Kasei
Research Funding: Chugai Pharma
Akihito Tsuji
Speakers' Bureau: Chugai Pharma, Taiho Pharmaceutical, Merck Serono, Takeda Pharmaceutical Company Limited, Lilly Japan, Bristol-Myers Squibb Corporation, Sanofi
Research Funding: Taiho Pharmaceutical Co., Ltd (Inst), Sanofi (Inst), Ono Pharmaceutical (Inst)
Hisateru Yasui
Honoraria: Taiho Pharmaceutical, Chugai Pharma, Bristol-Myers Squibb Japan, Daiichi Sankyo, Lilly Japan, Yakult Honsha, Bayer Yakuhin, Takeda, Ono Pharmaceutical
Research Funding: MSD (Inst), Ono Pharmaceutical (Inst), Astellas Pharma (Inst), AstraZeneca (Inst), Daiichi Sankyo (Inst)
Masahiro Goto
Honoraria: Daiichi Sankyo Company, Limited, Ono Pharmaceutical, Taiho Pharmaceutical, MSD K.K, Takeda, Sumitomo Dainippon Pharma Co., Ltd, Yakult Pharmaceutical, Eli Lilly Japan K.K
Research Funding: Chugai Pharma, Taiho Pharmaceutical, Nippon Kayaku
Yasuo Hamamoto
Consulting or Advisory Role: Dainippon Sumitomo Pharma, Chugai Pharma
Research Funding: Ono Pharmaceutical, Bristol-Myers Squibb Japan, MSD Oncology, Lilly Japan
Masashi Wakabayashi
Honoraria: Nihon Medi-Physics
Kohei Shitara
Honoraria: Bristol-Myers Squibb, Takeda, Janssen
Consulting or Advisory Role: Lilly, Bristol-Myers Squibb, Takeda, Pfizer, Ono Pharmaceutical, MSD, Taiho Pharmaceutical, Novartis, AbbVie, GlaxoSmithKline, Daiichi Sankyo, Boehringer Ingelheim, Amgen, Astellas Pharma, Guardant Health
Research Funding: MSD (Inst), Daiichi Sankyo (Inst), Taiho Pharmaceutical (Inst), Chugai Pharma (Inst), Ono Pharmaceutical (Inst), Astellas Pharma (Inst), Eisai (Inst), Amgen (Inst)
Hideaki Bando
Honoraria: Taiho Pharmaceutical, Chugai Pharma, Lilly Japan, Ono Pharmaceutical
Katsuya Tsuchihara
Honoraria: Chugai Pharma, Takeda, Illumina, Eisai, Boehringer Ingelheim Seiyaku, Bayer Yakuhin, Gilead Sciences
Atsushi Ohtsu
Employment: Celgene, AstraZeneca
Honoraria: Taiho Pharmaceutical, Ono Pharmaceutical
Research Funding: Bristol-Myers Squibb
Takayuki Yoshino
Honoraria: Chugai Pharma, Merck, Bayer Yakuhin, Ono Pharmaceutical, MSD K.K
Research Funding: MSD (Inst), Daiichi Sankyo Company, Limited (Inst), Ono Pharmaceutical (Inst), Taiho Pharmaceutical (Inst), Amgen (Inst), Sanofi (Inst), Pfizer (Inst), Genomedia (Inst), Sysmex (Inst), Nippon Boehringer Ingelheim (Inst), Chugai Pharma (Inst)
No other potential conflicts of interest were reported.
REFERENCES
- 1. Mosele F, Remon J, Mateo J, et al. Recommendations for the use of next-generation sequencing (NGS) for patients with metastatic cancers: A report from the ESMO Precision Medicine Working Group. Ann Oncol. 2020;31:1491–1505. doi: 10.1016/j.annonc.2020.07.014. [DOI] [PubMed] [Google Scholar]
- 2. Zehir A, Benayed R, Shah RH, et al. Mutational landscape of metastatic cancer revealed from prospective clinical sequencing of 10,000 patients. Nat Med. 2017;23:703–713. doi: 10.1038/nm.4333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Flaherty KT, Gray RJ, Chen AP, et al. Molecular landscape and actionable alterations in a genomically guided cancer clinical trial: National Cancer Institute Molecular Analysis for Therapy Choice (NCI-MATCH) J Clin Oncol. 2020;38:3883–3894. doi: 10.1200/JCO.19.03010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Park KH, Choi JY, Lim AR, et al. Genomic landscape and clinical utility in Korean advanced pan-cancer patients from prospective clinical sequencing: K-MASTER program. Cancer Discov. 2022;12:938–948. doi: 10.1158/2159-8290.CD-21-1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hilal T, Nakazawa M, Hodskins J, et al. Comprehensive genomic profiling in routine clinical practice leads to a low rate of benefit from genotype-directed therapy. BMC Cancer. 2017;17:602. doi: 10.1186/s12885-017-3587-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Brown S, Lavery JA, Shen R, et al. Implications of selection bias due to delayed study entry in clinical genomic studies. JAMA Oncol. 2022;8:287–291. doi: 10.1001/jamaoncol.2021.5153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Nakamura Y, Fujisawa T, Taniguchi H, et al. SCRUM-Japan GI-SCREEN and MONSTAR-SCREEN: Path to the realization of biomarker-guided precision oncology in advanced solid tumors. Cancer Sci. 2021;112:4425–4432. doi: 10.1111/cas.15132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Nakamura Y, Taniguchi H, Ikeda M, et al. Clinical utility of circulating tumor DNA sequencing in advanced gastrointestinal cancer: SCRUM-Japan GI-SCREEN and GOZILA studies. Nat Med. 2020;26:1859–1864. doi: 10.1038/s41591-020-1063-5. [DOI] [PubMed] [Google Scholar]
- 9. Cremolini C, Loupakis F, Antoniotti C, et al. FOLFOXIRI plus bevacizumab versus FOLFIRI plus bevacizumab as first-line treatment of patients with metastatic colorectal cancer: Updated overall survival and molecular subgroup analyses of the open-label, phase 3 TRIBE study. Lancet Oncol. 2015;16:1306–1315. doi: 10.1016/S1470-2045(15)00122-9. [DOI] [PubMed] [Google Scholar]
- 10. Venook AP, Niedzwiecki D, Lenz HJ, et al. Effect of first-line chemotherapy combined with cetuximab or bevacizumab on overall survival in patients with KRAS wild-type advanced or metastatic colorectal cancer: A randomized clinical trial. JAMA. 2017;317:2392–2401. doi: 10.1001/jama.2017.7105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cremolini C, Antoniotti C, Rossini D, et al. Upfront FOLFOXIRI plus bevacizumab and reintroduction after progression versus mFOLFOX6 plus bevacizumab followed by FOLFIRI plus bevacizumab in the treatment of patients with metastatic colorectal cancer (TRIBE2): A multicentre, open-label, phase 3, randomised, controlled trial. Lancet Oncol. 2020;21:497–507. doi: 10.1016/S1470-2045(19)30862-9. [DOI] [PubMed] [Google Scholar]
- 12. Sanchez-Vega F, Mina M, Armenia J, et al. Oncogenic signaling pathways in The Cancer Genome Atlas. Cell. 2018;173:321–337.e10. doi: 10.1016/j.cell.2018.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Becker T, Weberpals J, Jegg AM, et al. An enhanced prognostic score for overall survival of patients with cancer derived from a large real-world cohort. Ann Oncol. 2020;31:1561–1568. doi: 10.1016/j.annonc.2020.07.013. [DOI] [PubMed] [Google Scholar]
- 14. Rivera DR, Henk HJ, Garrett-Mayer E, et al. The friends of cancer research real-world data collaboration pilot 2.0: Methodological recommendations from oncology case studies. Clin Pharmacol Ther. 2022;111:283–292. doi: 10.1002/cpt.2453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Griffith SD, Miksad RA, Calkins G, et al. Characterizing the feasibility and performance of real-world tumor progression end points and their association with overall survival in a large advanced non-small-cell lung cancer data set. JCO Clin Cancer Inform. 2019 doi: 10.1200/CCI.19.00013. 10.1200/CCI.19.00013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rugo HS, Dieras V, Cortes J, et al. Real-world survival outcomes of heavily pretreated patients with refractory HR+, HER2-metastatic breast cancer receiving single-agent chemotherapy-a comparison with MONARCH 1. Breast Cancer Res Treat. 2020;184:161–172. doi: 10.1007/s10549-020-05838-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hendifar A, Blais EM, Wolpin B, et al. Retrospective case series analysis of RAF family alterations in pancreatic cancer: Real-world outcomes from targeted and standard therapies. JCO Precis Oncol. 2021 doi: 10.1200/PO.20.00494. 10.1200/PO.20.00494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kim JH, Yoon S, Lee DH, et al. Real-world utility of next-generation sequencing for targeted gene analysis and its application to treatment in lung adenocarcinoma. Cancer Med. 2021;10:3197–3204. doi: 10.1002/cam4.3874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Nakamura Y, Okamoto W, Kato T, et al. Circulating tumor DNA-guided treatment with pertuzumab plus trastuzumab for HER2-amplified metastatic colorectal cancer: A phase 2 trial. Nat Med. 2021;27:1899–1903. doi: 10.1038/s41591-021-01553-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Siena S, Di Bartolomeo M, Raghav K, et al. Trastuzumab deruxtecan (DS-8201) in patients with HER2-expressing metastatic colorectal cancer (DESTINY-CRC01): A multicentre, open-label, phase 2 trial. Lancet Oncol. 2021;22:779–789. doi: 10.1016/S1470-2045(21)00086-3. [DOI] [PubMed] [Google Scholar]
- 21. Shitara K, Bang YJ, Iwasa S, et al. Trastuzumab deruxtecan in previously treated HER2-positive gastric cancer. N Engl J Med. 2020;382:2419–2430. doi: 10.1056/NEJMoa2004413. [DOI] [PubMed] [Google Scholar]
- 22. Kopetz S, Grothey A, Yaeger R, et al. Encorafenib, binimetinib, and cetuximab in BRAF V600e-mutated colorectal cancer. N Engl J Med. 2019;381:1632–1643. doi: 10.1056/NEJMoa1908075. [DOI] [PubMed] [Google Scholar]
- 23. Subbiah V, Lassen U, Elez E, et al. Dabrafenib plus trametinib in patients with BRAF(V600E)-mutated biliary tract cancer (ROAR): A phase 2, open-label, single-arm, multicentre basket trial. Lancet Oncol. 2020;21:1234–1243. doi: 10.1016/S1470-2045(20)30321-1. [DOI] [PubMed] [Google Scholar]
- 24. Le DT, Kim TW, Van Cutsem E, et al. Phase II open-label study of pembrolizumab in treatment-refractory, microsatellite instability-high/mismatch repair-deficient metastatic colorectal cancer: KEYNOTE-164. J Clin Oncol. 2020;38:11–19. doi: 10.1200/JCO.19.02107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Marabelle A, Le DT, Ascierto PA, et al. Efficacy of pembrolizumab in patients with noncolorectal high microsatellite instability/mismatch repair-deficient cancer: Results from the phase II KEYNOTE-158 study. J Clin Oncol. 2020;38:1–10. doi: 10.1200/JCO.19.02105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Fakih MG, Kopetz S, Kuboki Y, et al. Sotorasib for previously treated colorectal cancers with KRAS(G12C) mutation (CodeBreaK100): A prespecified analysis of a single-arm, phase 2 trial. Lancet Oncol. 2022;23:115–124. doi: 10.1016/S1470-2045(21)00605-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
A data sharing statement provided by the authors is available with this article at DOI https://doi.org/10.1200/PO.22.00653.