Abstract
BACKGROUND
B-cell maturation antigen (BCMA)–directed chimeric antigen receptor (CAR) T-cell therapies have generated responses in patients with advanced myeloma, but relapses are common. G protein–coupled receptor, class C, group 5, member D (GPRC5D) has been identified as an immunotherapeutic target in multiple myeloma. Preclinical studies have shown the efficacy of GPRC5D-targeted CAR T cells, including activity in a BCMA antigen escape model.
METHODS
In this phase 1 dose-escalation study, we administered a GPRC5D-targeted CAR T-cell therapy (MCARH109) at four dose levels to patients with heavily pretreated multiple myeloma, including patients with relapse after BCMA CAR T-cell therapy.
RESULTS
A total of 17 patients were enrolled and received MCARH109 therapy. The maximum tolerated dose was identified at 150×106 CAR T cells. At the 450×106 CAR T-cell dose, 1 patient had grade 4 cytokine release syndrome and immune effector cell–associated neurotoxicity syndrome (ICANS), and 2 patients had a grade 3 cerebellar disorder of unclear cause. No cerebellar disorder, ICANS of any grade, or cytokine release syndrome of grade 3 or higher occurred in the 12 patients who received doses of 25×106 to 150×106 cells. A response was reported in 71% of the patients in the entire cohort and in 58% of those who received doses of 25×106 to 150×106 cells. The patients who had a response included those who had received previous BCMA therapies; responses were observed in 7 of 10 such patients in the entire cohort and in 3 of 6 such patients who received 25×106 to 150×106 cells.
CONCLUSIONS
The results of this study of a GPRC5D-targeted CAR T-cell therapy (MCARH109) confirm that GPRC5D is an active immunotherapeutic target in multiple myeloma. (Funded by Juno Therapeutics/Bristol Myers Squibb; ClinicalTrials.gov number, NCT04555551.)
Autologous T cells that are engineered to express a chimeric antigen receptor (CAR) targeting a tumor-associated antigen (CAR T-cell therapy) have shown promising efficacy in multiple hematologic cancers.1 In multiple myeloma, studies of B-cell maturation antigen (BCMA) CAR T-cell therapy have shown deep responses in patients with advanced disease, with promising rates and durations of response. In a pivotal phase 2 study, 73% of the patients who received idecabtagene vicleucel (ide-cel) had a response, and the median progression-free survival was 8.8 months.2 Ciltacabtagene autoleucel (cilta-cel) was similarly evaluated in a phase 1b–2 study; 98% of the patients had a response, and the median progression-free survival had not been reached after a median follow-up of 27.7 months.3,4 These results led to the Food and Drug Administration approval of both products for the treatment of relapsed or refractory multiple myeloma in patients receiving four or more previous lines of therapy.
However, in contrast to CD19-directed CAR T-cell therapies for acute lymphoblastic leukemia and B-cell lymphoma,5–8 BCMA-directed CAR T-cell therapies have not generated survival curves with a plateau in patients with multiple myeloma, and most patients are likely to have an eventual relapse. Patients who have a relapse after BCMA-directed therapies have limited treatment options, and therapies with new targets or mechanisms of action are needed. G protein–coupled receptor, class C, group 5, member D (GPRC5D) is an orphan G protein–coupled receptor of unclear function in human tissue.9,10 This receptor is expressed in several myeloma cell lines and in bone marrow plasma cells from patients with multiple myeloma. Substantial expression in normal tissue is limited to plasma cells, and low expression is seen in a subset of cells in the hair follicles of the skin and hard keratinizing tissue.9–12
In preclinical studies of multiple myeloma cell lines and mouse models of myeloma, Smith et al.9 found in vitro and in vivo antitumor efficacy of GPRC5D CAR T cells in multiple myeloma, including in a BCMA antigen escape model. On the basis of these promising preclinical results, we designed a phase 1 dose-escalation study of MCARH109, a second-generation CAR T-cell therapy with a human B-cell–derived GPRC5D single-chain variable fragment, a 4–1BB costimulatory domain, and a CD3ζ signaling domain. This GPRC5D CAR T-cell therapy was administered to patients with relapsed or refractory multiple myeloma, including patients who had received BCMA-directed therapies.
METHODS
STUDY DESIGN AND PATIENTS
This open-label phase 1 study was conducted at Memorial Sloan Kettering Cancer Center (MSKCC). Key eligibility criteria included an age of 18 years or older; a diagnosis of relapsed or refractory multiple myeloma with three or more previous lines of treatment, including proteasome inhibitors, immunomodulatory agents, and anti-CD38 antibody–based therapies; measurable disease (defined as any of the following: a serum level of monoclonal protein of ≥0.5 g per deciliter, a urinary level of monoclonal protein of ≥200 mg per 24 hours, a serum level of involved free light chains of ≥10 mg per deciliter with an abnormal free light-chain ratio, plasma cell infiltration in bone marrow of ≥30% in patients with nonsecretory myeloma, or one or more biopsy-proven extramedullary plasmacytomas measuring ≥2 cm in diameter); an Eastern Cooperative Oncology Group performance-status score of 0 or 1 (on a 5-point scale in which higher scores reflect greater disability); and adequate organ function. Patients with previous treatment with CAR T-cell therapy or bispecific antibodies were eligible, and baseline GPRC5D expression in the bone marrow was not required for enrollment. The details of the study design and eligibility requirements are provided in the study protocol, available with the full text of this article at NEJM.org.
Patient apheresis products were collected, and CD4+ and CD8+ cells were selected and separately cryopreserved. CD4+ and CD8+ T cells, in a 1:1 ratio, were activated and transduced with a lentiviral vector containing the GPRC5D CAR and subsequently expanded (see the Methods section in the Supplementary Appendix, available at NEJM.org).
Bridging therapy after apheresis was allowed but was stopped at least 2 weeks before lymphodepleting chemotherapy. All the patients needed to have measurable disease before starting lymphodepletion, which comprised cyclophosphamide (300 mg per square meter of body-surface area per day) and fludarabine (30 mg per square meter per day) for 3 consecutive days. Two days after the lymphodepletion regimen was completed, the MCARH109 infusion was administered. The dose escalation included four levels: 25×106, 50×106, 150×106, and 450×106 total CAR T cells; all the patients were followed until disease progression, and long-term follow-up was continued until death or withdrawal of consent.
STUDY OVERSIGHT
The study was approved by the MSKCC institutional review board and was conducted in accordance with the principles of the Declaration of Helsinki and International Council on Harmonisation guidelines for Good Clinical Practice. Written informed consent was obtained from all the patients before apheresis and before the start of treatment. All the authors had access to the data and were involved in the analysis of the results and vouch for the completeness and accuracy of the data and for the adherence of the study to the protocol.
END POINTS AND STUDY PROCEDURES
The primary end point of the study was the safety of MCARH109. Toxic effects were graded according to National Cancer Institute Common Terminology Criteria for Adverse Events, version 5.0, and cytokine release syndrome and immune effector cell–associated neurotoxicity syndrome (ICANS) were graded according to the American Society for Transplantation and Cellular Therapy consensus guidelines.13 Key secondary end points included clinical response according to the standard International Myeloma Working Group response assessment criteria14 and minimal residual disease in bone marrow, assessed by means of multicolor flow cytometry (sensitivity, 1 in 105) at specified time points.15 Exploratory end points included quantification of MCARH109 in the blood by quantitative polymerase-chain-reaction assay, MCARH109 immunophenotyping, and GPRC5D immunohistochemical analysis of bone marrow and plasmacytoma samples at baseline and at the time of disease progression. (For details on exploratory end points, see the Methods section in the Supplementary Appendix.)
STATISTICAL ANALYSIS
This phase 1 study followed a standard 3+3 dose-escalation design. The percentage of patients with a response was reported along with an exact 95% confidence interval. The definitions of dose-limiting toxic effects are outlined in the protocol. The duration of response (partial response or better) was estimated with the use of Kaplan–Meier methods because no patient died while in remission. Correlative analyses are limited by the phase 1 nature of this study and are therefore descriptive and exploratory.
RESULTS
STUDY DESIGN AND PATIENT CHARACTERISTICS
Between September 15, 2020, and June 16, 2021, a total of 19 patients were enrolled and completed apheresis (Fig. S1 in the Supplementary Appendix). Two patients completed apheresis but discontinued the study before treatment: 1 patient withdrew consent before the start of MCARH109 manufacturing, and 1 patient was withdrawn owing to rapid progression of disease after the manufacturing of the product. MCARH109 was successfully manufactured for all 18 apheresis products, and this report includes results from the 17 patients who received MCARH109 therapy (Table 1 and Table S1). Table S2 shows the preinfusion characteristics of each patient’s product, as well as the prespecified criteria for releasing the product for treatment.
Table 1.
Characteristics of the Patients at Baseline.*
| Characteristic | 25×106 CAR T cells (N = 3) | 50×106 CAR T cells (N = 3) | 150×106 CAR T cells (N = 6) | 450×106 CAR T cells (N = 5) | Total (N = 17) |
|---|---|---|---|---|---|
| Median age (range) — yr | 60 (38–76) | 50 (39–56) | 59 (40–74) | 65 (63–73) | 60 (38–76) |
| Male sex — no. (%) | 2 (67) | 3 (100) | 4 (67) | 4 (80) | 13 (76) |
| High-risk cytogenetic feature — no. (%)† | 3 (100) | 2 (67) | 3 (50) | 5 (100) | 13 (76) |
| Extramedullary plasmacytoma — no. (%) | 3 (100) | 1 (33) | 4 (67) | 0 | 8 (47) |
| Nonsecretory myeloma — no. (%) | 2 (67) | 0 | 1 (17) | 0 | 3 (18) |
| Previous lines of therapy — median (range) | 6 (6–8) | 5 (4–8) | 7 (5–14) | 6 (5–12) | 6 (4–14) |
| Disease refractory to last line of therapy — no. (%) | 3 (100) | 3 (100) | 5 (83) | 3 (60) | 14 (82) |
| Penta-exposed — no. (%)‡ | 3 (100) | 3 (100) | 6 (100) | 5 (100) | 17 (100) |
| Triple-refractory disease — no. (%)§ | 3 (100) | 3 (100) | 6 (100) | 4 (80) | 16 (94) |
| Previous autologous transplantation — no. (%) | 3 (100) | 3 (100) | 6 (100) | 5 (100) | 17 (100) |
| Previous allogeneic transplantation — no. (%) | 0 | 2 (67) | 1 (17) | 0 | 3 (18) |
| Previous BCMA therapy — no. (%)¶ | 1 (33) | 1 (33) | 4 (67) | 4 (80) | 10 (59) |
| Previous CAR T-cell therapy — no. (%) | 0 | 1 (33) | 3 (50) | 4 (80) | 8 (47) |
| Bridging therapy — no. (%) | 3 (100) | 3 (100) | 6 (100) | 4 (80) | 16 (94) |
| Disease refractory to bridging therapy — no./total no. (%) | 3/3 (100) | 3/3 (100) | 5/6 (83) | 4/5 (80) | 15/16 (94) |
BCMA denotes B-cell maturation antigen, and CAR chimeric antigen receptor.
High-risk cytogenetic features included del(17p), t(4;14), t(14;16), and 1q gain.
Penta-exposed patients were those who had received previous treatment with two proteasome inhibitors, two immunomodulatory drugs, and one anti-CD38 antibody.
Triple-refractory disease was defined as refractory to a proteasome inhibitor, an immunomodulatory drug, and an anti-CD38 antibody.
Included are BCMA-targeted antibody–drug conjugates, bispecific antibodies, and CAR T-cell therapies.
The median age of the patients was 60 years (range, 38 to 76), and the median number of previous lines of therapy was 6 (range, 4 to 14). All the patients were penta-exposed (previous treatment with two proteasome inhibitors, two immunomodulatory drugs, and one anti-CD38 antibody), and 16 patients (94%) had triple-refractory disease (refractory to a proteasome inhibitor, an immunomodulatory drug, and an anti-CD38 antibody). A total of 10 patients (59%) had received previous treatment with BCMA-targeted therapies, including 8 (47%) who received previous BCMA CAR T-cell therapy; the median time from the last BCMA therapy to MCARH109 infusion was 16.4 months (range, 4.4 to 36.6). Of these 10 patients, 9 (90%) had had an objective response to the previous BCMA therapy, and 2 patients had disease refractory to the BCMA therapy (1 patient who had a response subsequently had progression). All the patients had received treatment with high-dose melphalan and had undergone an autologous stem-cell transplantation, and 3 patients (18%) had previously undergone an allogeneic transplantation. A total of 14 patients (82%) had disease refractory to their last line of therapy, and 16 (94%) received bridging therapy after leukapheresis; 15 of 16 patients (94%) had disease refractory to bridging therapy. Three patients (18%) had non-secretory myeloma at baseline, and 8 (47%) had extramedullary plasmacytoma at baseline. A total of 13 patients (76%) had one or more high-risk cytogenetic features, defined by the presence of 1q gain, del(17p), t(4;14), or t(14;16). The full details of previous treatments administered and responses are provided in Table S3.
SAFETY AND MAXIMUM TOLERATED DOSE OF MCARH109
All the patients had one or more adverse events that emerged or worsened after MCARH109 infusion. Cytokine release syndrome was seen in 15 patients (88%) and was of grade 1 or 2 in all patients except for 1 patient who had received the highest dose level (450×106 CAR T cells) and had a grade 4 event (Table 2). This same patient had grade 4 ICANS and grade 4 macrophage activation syndrome constituting a dose-limiting toxic effect. No other patients had ICANS or macrophage activation syndrome. A total of 9 patients (53%) received tocilizumab and 4 (24%) received dexamethasone for the management of cytokine release syndrome or ICANS. The patient with grade 4 cytokine release syndrome, macrophage activation syndrome, and ICANS also received siltuximab and anakinra. Two additional patients who received the dose of 450×106 CAR T cells had a grade 3 cerebellar disorder that was considered by the investigator to be possibly related to MCARH109 and constituted dose-limiting toxic effects for this dose. Hematologic toxic effects, suspected to be secondary to lymphodepleting chemotherapy, were the most common grade 3 or 4 events, with grade 3 or higher neutropenia, thrombocytopenia, and anemia seen in 16 patients (94%), 11 patients (65%), and 6 patients (35%), respectively. Nonhematologic grade 3 or higher events were uncommon (Table 2). Infections were noted in 3 patients (18%), including 2 patients (12%) with grade 3 events (bacterial infection and parvovirus infection). Time-limited on-target, off-tumor toxic effects that were related to GPRC5D expression in the skin and keratinized tissue manifested as grade 1 nail changes, including nail loss in 11 patients (65%) (at all four dose levels), grade 1 rash in 3 patients (18%), and grade 1 dysgeusia or dry mouth in 2 patients (12%). The median time from MCARH109 infusion to nail changes was 3.3 months (range, 1.1 to 6.0), and nail changes resolved in 10 of 11 patients (91%) without any interventions. Rash was treated with topical glucocorticoids or observation alone, and the symptoms resolved; the dysgeusia resolved in both patients without any interventions.
Table 2.
Adverse Events.*
| Adverse Event | Any Grade | Grade 1 | Grade 2 | Grade 3 | Grade 4 |
|---|---|---|---|---|---|
| number (percent) | |||||
| Cytokine release syndrome | 15 (88) | 7 (41) | 7 (41) | 0 | 1 (6) |
| Nail changes | 11 (65) | 11 (65) | 0 | 0 | 0 |
| Fatigue | 7 (41) | 6 (35) | 1 (6) | 0 | 0 |
| Nausea | 4 (24) | 4 (24) | 0 | 0 | 0 |
| Infections | 3 (18) | 0 | 1 (6) | 2 (12) | 0 |
| Rash | 3 (18) | 3 (18) | 0 | 0 | 0 |
| Cerebellar disorder | 2 (12) | 0 | 0 | 2 (12) | 0 |
| Dysgeusia | 2 (12) | 2 (12) | 0 | 0 | 0 |
| Immune effector cell–associated neurologic syndrome | 1 (6) | 0 | 0 | 0 | 1 (6) |
| Macrophage activation syndrome | 1 (6) | 0 | 0 | 0 | 1 (6) |
| Pruritus | 1 (6) | 0 | 1 (6) | 0 | 0 |
| Pain | 1 (6) | 0 | 1 (6) | 0 | 0 |
| Bleeding | 1 (6) | 0 | 1 (6) | 0 | 0 |
| Dry mouth | 1 (6) | 1 (6) | 0 | 0 | 0 |
| Dizziness | 1 (6) | 1 (6) | 0 | 0 | 0 |
| Allergic reaction | 1 (6) | 1 (6) | 0 | 0 | 0 |
| Lymphocyte count decreased | 17 (100) | 0 | 0 | 0 | 17 (100) |
| Neutropenia | 17 (100) | 0 | 0 | 5 (29) | 12 (71) |
| White-cell count decreased | 17 (100) | 0 | 0 | 5 (29) | 12 (71) |
| Thrombocytopenia | 15 (88) | 3 (18) | 1 (6) | 7 (41) | 4 (24) |
| Hypocalcemia | 15 (88) | 1 (6) | 10 (59) | 3 (18) | 1 (6) |
| Anemia | 15 (88) | 1 (6) | 7 (41) | 7 (41) | 0 |
| Hypoalbuminemia | 14 (82) | 6 (35) | 8 (47) | 0 | 0 |
| Elevated AST level | 11 (65) | 8 (47) | 0 | 2 (12) | 1 (6) |
| Elevated partial-thromboplastin time | 10 (59) | 9 (53) | 1 (6) | 0 | 0 |
| Elevated ALT level | 7 (41) | 3 (18) | 3 (18) | 1 (6) | 0 |
| Hypokalemia | 6 (35) | 6 (35) | 0 | 0 | 0 |
| Decreased fibrinogen | 6 (35) | 2 (12) | 3 (18) | 1 (6) | 0 |
| INR increased | 5 (29) | 3 (18) | 2 (12) | 0 | 0 |
| Hypomagnesemia | 3 (18) | 3 (18) | 0 | 0 | 0 |
| Elevated creatinine level | 3 (18) | 2 (12) | 0 | 1 (6) | 0 |
| Hypernatremia | 3 (18) | 3 (18) | 0 | 0 | 0 |
| Elevated alkaline phosphatase level | 2 (12) | 2 (12) | 0 | 0 | 0 |
| Hyperkalemia | 1 (6) | 0 | 0 | 0 | 0 |
Shown are events that were considered by the investigator to be possibly, probably, or definitely related to lymphodepleting chemotherapy or MCARH109. ALT denotes alanine aminotransferase, AST aspartate aminotransferase, and INR international normalized ratio.
CEREBELLAR TOXIC EFFECTS AFTER MCARH109 INFUSION
Among the patients who received the 450×106 CAR T-cell dose, dizziness developed in one patient at 2.1 weeks after the MCARH109 infusion, and unsteady gait developed in another patient at 5.6 months after the infusion. Neurologic examination in both patients initially showed wide-based gait and saccadic eye movements consistent with labyrinthitis or vestibular neuritis; however, symptoms gradually evolved to include difficulty with visual fixation, appendicular and truncal ataxia, and dysarthria. Cognition, motor strength, and tactile sensation were unaffected. The two patients, at 6.5 and 8.4 months, respectively, had a grade 3 cerebellar disorder that was considered to be a dose-limiting toxic effect for the dose of 450×106 CAR T cells.
Magnetic resonance imaging (MRI) of the brain that was performed within 6 weeks after symptom onset in both patients was negative for any abnormal contrast enhancement, T2-weighted fluid-attenuated inversion recovery (FLAIR) signal change, or cerebellar atrophy. Repeat contrast-enhanced MRI of the brain plus positron-emission tomography of the brain after 4 months remained unrevealing. Analysis of the cerebrospinal fluid (CSF) ruled out infectious or inflammatory causes. In one of the two patients, flow cytometric assessment of CSF for CAR T cells was able to be performed at week 24, and CAR T cells were detectable at a very low level (see the Supplementary Appendix). Both patients received multiple treatments, including oral glucocorticoids, high-dose methylprednisolone, intravenous immunoglobulin, and meclizine, and their symptoms stabilized. The two patients, at 7.7 and 10.8 months of follow-up, respectively, have an ongoing grade 3 cerebellar disorder. The baseline characteristics of these two patients as compared with all other patients in the study are provided in Table S4, and a timeline of these symptoms, diagnostic testing performed, and treatments administered are outlined in Figure S2A and S2B. With a minimum follow-up of 6 months (or until withdrawal of consent or progression of disease), no other patients (15 patients) have had similar symptoms.
To better understand the potential mechanism of these symptoms, we analyzed the Allen Brain Atlas, which includes microarray data from six healthy human brains.16–18 GPRC5D expression is low throughout the brain (samples rarely reach the log2 >5 threshold defined by the Atlas to indicate presence or absence).19 GPRC5D expression was found to be specifically enriched in the inferior olivary nucleus (Fig. S3A, S3B, and S3C), a structure located in the medulla oblongata that relays motor and sensory signals from the spinal cord to the cerebellum and regulates motor coordination.
ANTIMYELOMA EFFICACY OF MCARH109
Clinical responses were noted across all four dose levels evaluated in the study. The percentage of patients who had a response across all dose levels was 71% (95% confidence interval [CI], 44 to 90), including 12 patients (71%) who had a partial response or better, 10 (59%) who had a very good partial response or better, and 6 (35%) who had a complete response or stringent complete response (Table 3). Among the 12 patients who received the doses that did not produce unacceptable side effects (25×106 to 150×106 CAR T cells), 7 (58%; 95% CI, 28 to 85) had an objective response. Flow cytometry for minimal residual disease in bone marrow was negative at one or more time points in 8 of 12 patients (67%) with a partial response or better. The median duration of response was 7.8 months (95% CI, 5.7 to not reached) in the entire cohort and was also 7.8 months (95% CI, 4.6 to not reached) in patients who received 25×106 to 150×106 CAR T cells. A partial response or better was noted in 7 of 10 patients (70%) who had received previous BCMA-targeted therapies and received MCARH109 across all four dose levels and in 3 of 6 patients (50%) treated at doses of 25×106 to 150×106 cells (Table 3). This included 6 of 8 patients (75%) and 2 of 4 patients (50%), respectively, with previous CAR T-cell therapy.
Table 3.
Clinical Responses in All Patients and in Patients with or without Previous BCMA-Directed Therapies.
| Response | All Patients | Previous BCMA Therapies | No Previous BCMA Therapies | |||
|---|---|---|---|---|---|---|
| All Dose Levels (N = 17) | 25×106–150×106 CAR T Cells (N = 12) | All Dose Levels (N = 10) | 25×106–150×106 CAR T Cells (N = 6) | All Dose Levels (N = 7) | 25×106–150×106 CAR T Cells (N = 6) | |
| number (percent) | ||||||
| Partial response or better | 12 (71) | 7 (58) | 7 (70) | 3 (50) | 5 (71) | 4 (67) |
| Very good partial response or better | 10 (59) | 5 (42) | 6 (60) | 2 (33) | 4 (57) | 3 (50) |
| Complete response or better | 6 (35) | 3 (25) | 4 (40) | 2 (33) | 2 (29) | 1 (17) |
| Negativity for MRD in bone marrow* | 8 (47) | 6 (50) | 3 (30) | 2 (33) | 5 (71) | 4 (67) |
Negativity for minimal residual disease (MRD) in bone marrow was assessed by means of 10-color flow cytometry with a sensitivity of 1 in 105 at 4 weeks after CAR T-cell therapy, at the occurrence of a complete response, and as clinically indicated.
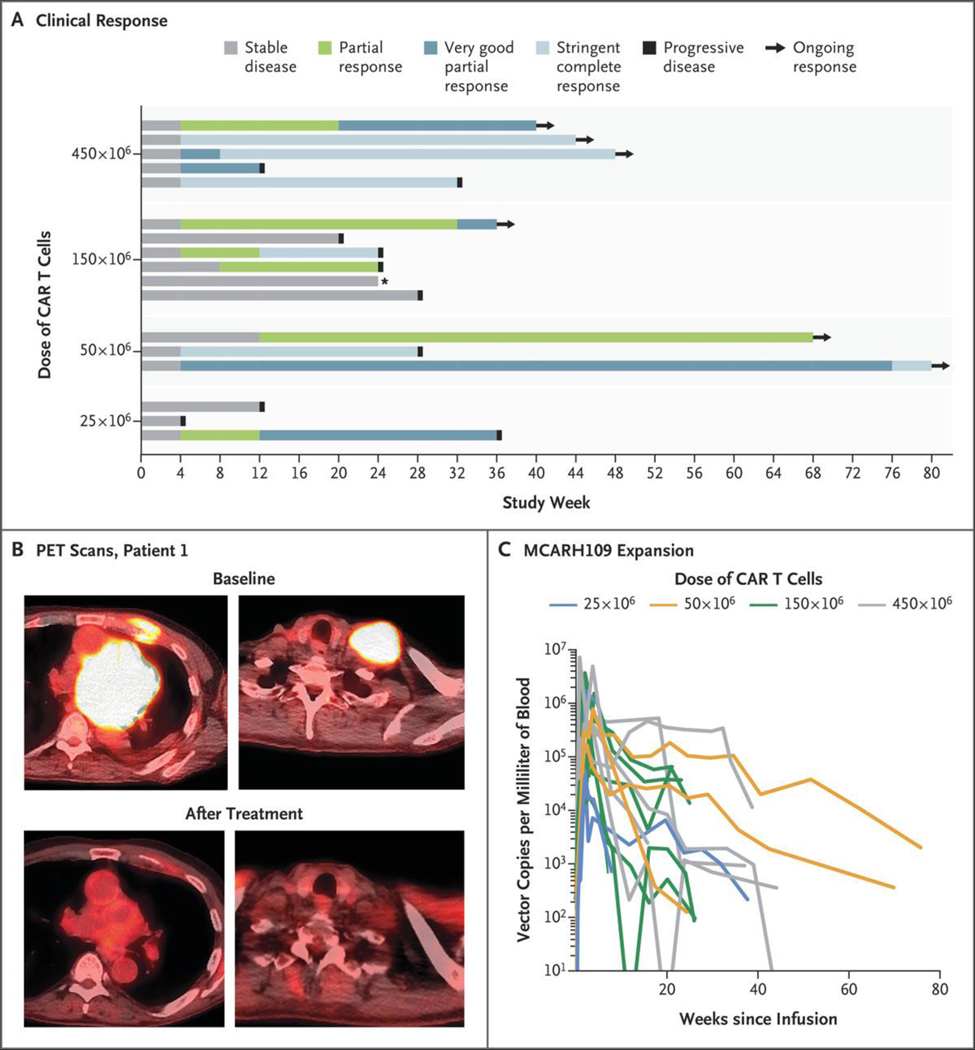
With a median follow-up of 10.1 months (95% CI, 8.5 to not reached), 6 of the 12 patients (50%) with a partial response or better remain progression-free, with 2 patients completing more than 1 year of follow-up after MCARH109 infusion (Fig. 1A and 1B).
Figure 1. Clinical Responses to GPRC5D-Targeted Chimeric Antigen Receptor (CAR) T-Cell Therapy.
Panel A shows a swimmer’s plot of clinical responses over time. The star indicates a patient who discontinued the study without progression of disease. Panel B shows positron-emission tomographic (PET) scans at baseline and after treatment in Patient 1, who received the dose of 25×106 CAR T cells. Panel C shows MCARH109 expansion over time among all the patients. GPRC5D denotes G protein–coupled receptor, class C, group 5, member D.
MCARH109 EXPANSION AND PERSISTENCE IN PERIPHERAL BLOOD
We noted robust expansion of MCARH109 in the peripheral blood across all four dose levels evaluated in the study (Fig. 1C). The median peak expansion in the peripheral blood was 445,170 vector copies per milliliter (range, 33,944 to 6,925,200), and the median time to peak was 14 days (range, 7 to 111). The median area under the curve during the first 28 days after MCARH109 infusion (AUC0–28 days) was 5,039,204 copies per milliliter (range, 339,357 to 52,246,713) with a dose-dependent response. The peak expansion and AUC0–28 days appeared to be related to the dose of MCARH109 administered (Fig. S4A and S4B). Persistent MCARH109 was detected in the peripheral blood at 4 weeks in 17 of 17 patients (100%), at 24 weeks in 11 of 11 patients (100%), and at 52 weeks in 1 of 2 patients (50%).
IMMUNOHISTOCHEMICAL ANALYSIS
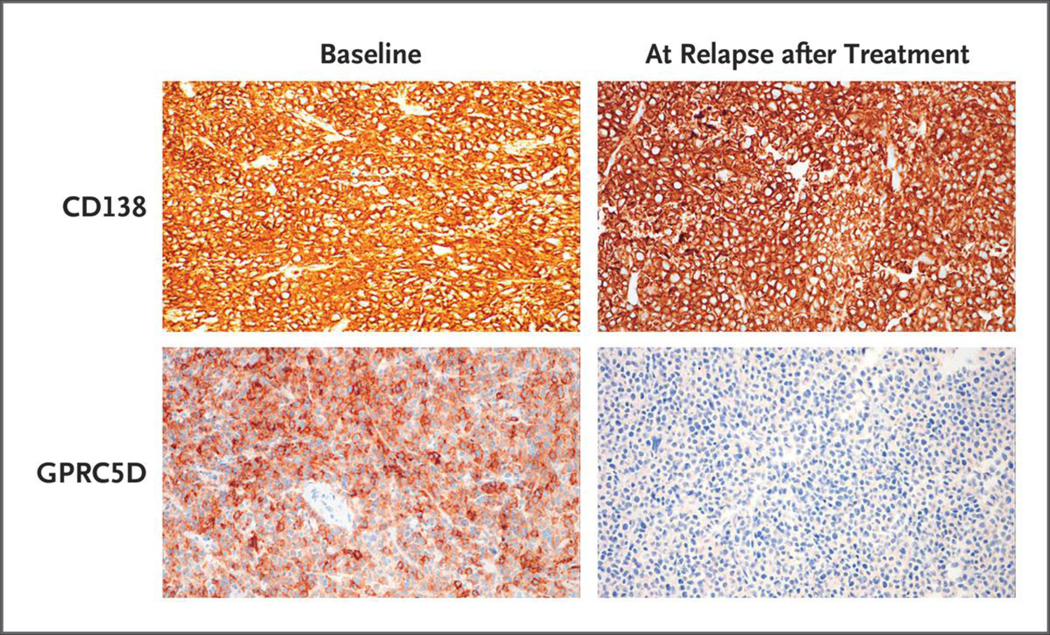
A total of 15 of 17 patients (88%) had GPRC5D expression in their baseline bone marrow or plasmacytoma biopsy, including 11 of 12 patients (92%) with an objective response to MCARH109 and 4 of 5 patients (80%) who did not have a response. Six patients had progression after an initial response, of whom 4 (67%) had no GPRC5D expression at the time of progression and 2 (33%) had decreased expression (defined as reduced staining intensity or proportion of GPRC5D-positive plasma cells as compared with baseline) (Fig. 2).
Figure 2. Loss of GPRC5D on Immunohistochemical Analysis at Relapse after MCARH109 Infusion.
GPRC5D and CD138 immunohistochemical analysis of plasmacytoma tissue samples from Patient 15 at baseline and at relapse after MCARH109 infusion (dose level, 150×106 CAR T cells) shows loss of GPRC5D expression at the time of relapse. The patient had a best response of stringent complete response.
DISCUSSION
This study of the GPRC5D-directed CAR T-cell therapy MCARH109 involved patients with relapsed or refractory multiple myeloma, including those with previous BCMA-directed therapies. We conservatively initiated the study with a starting dose of 25×106 CAR T cells. Even at this low dose, we found that GPRC5D was an active target for multiple myeloma. At the highest dose evaluated (450×106 CAR T cells), 3 of 5 patients had dose-limiting toxic effects, including 2 patients with persistent cerebellar disorder. The occurrence of cerebellar symptoms in these 2 patients who received 450×106 CAR T cells will require additional studies to better understand the cause and management. The exact cause of these symptoms is unclear but could possibly be related to low-level expression of GPRC5D in the cerebellum or the inferior olivary nucleus. Reports of delayed Parkinsonism-like neurologic toxic effects have also been reported in 1 to 5% of patients treated with cilta-cel20,21 and is attributed to potential expression of BCMA in the basal ganglia.21 No dose-limiting toxic effects or cerebellar symptoms were observed in patients with longer follow-up who received the dose levels of 25×106 to 150×106 cells (12 patients [71%]). As expected, on the basis of normal tissue expression, on-target, off-tumor toxic effects included transient rash, dysgeusia, and nail changes, all of which were limited to grade 1 or 2 events. As compared with the bispecific GPRC5D T-cell engager therapy (talquetamab),22,23 the frequency and severity of rash and dysgeusia were lower with MCARH109. Nail changes seen with MCARH109 were also largely reversible and did not lead to intervention.
Clinical responses were noted at all dose levels evaluated, with 71% of the patients having a response. At the maximum tolerated dose of 150×106 cells and lower doses, the percentage of patients with a response was 58%, and the median duration of response was 7.8 months. Responses were seen in patients who are typically excluded from other studies of CAR T-cell therapy, including those with nonsecretory myeloma (18%), previous allogeneic hematopoietic stem-cell transplantation (18%), extramedullary disease (47%), and previous BCMA-directed therapies (59%). The study was particularly enriched for patients with previous BCMA CAR T-cell therapy (47%); MCARH109 had a promising likelihood of response in these patients, with no discernible differences in clinical response according to previous CAR T-cell treatment.
All clinical responses were associated with robust expansion of MCARH109 in the peripheral blood and persistence across all doses evaluated. We assessed GPRC5D expression by immunohistochemical analysis in bone marrow or plasmacytoma biopsies at baseline and at the time of progression. Only 2 patients had low or no expression of GPRC5D at baseline; all 6 patients who had a relapse after an initial objective response had decreased GPRC5D (2 of 6 patients) or loss of GPRC5D (4 of 6 patients) at the time of progression. These results are based on small numbers, and the sensitivity of the assay to detect GPRC5D expression, particularly at low levels, has not been established. Additional studies are needed to understand the mechanisms of relapse after GPRC5D-targeted therapies, including the role of antigen escape.
MCARH109 is a GPRC5D-targeted CAR T-cell therapy for multiple myeloma. A maximum tolerated dose of 150×106 CAR T cells has been defined. A high likelihood of response was noted across all dose levels and particularly among patients with limited or no other therapeutic options such as those with relapses or disease refractory to previous BCMA-therapies, including anti-BCMA CAR T-cell therapies. These results provide support that GPRC5D is a new and effective target for the immunotherapy of myeloma, consistent with the results reported for talquetamab, a bispecific T-cell engager targeting GPRC5D.22,23 GPRC5D as an additional new target in myeloma offers the opportunity to explore several unanswered questions, including the role of targeting alternate antigens as opposed to sequential BCMA-directed therapies in patients with relapse after BCMA therapy and the role of BCMA- and GPRC5D-directed combination therapies to target low-antigen-density reservoirs of relapse and induce deeper and longer durations of response. A multiinstitutional study of GPRC5D-targeted CAR T-cell therapy is currently enrolling patients (ClinicalTrials.gov number, NCT04674813).
Supplementary Material
Acknowledgments
Supported by Juno Therapeutics/Bristol Myers Squibb. All the authors employed by Memorial Sloan Kettering Cancer Center (MSKCC) were supported by a National Cancer Institute (NCI) Core Grant (P30 CA008748), and Dr. Smith was supported by NCI grant K08CA241400.
We thank all the patients who participated in the study as well as their families and caregivers; the clinical and research teams within the Myeloma, Cellular Therapy, and Bone Marrow Transplant services at MSKCC; Katherin Shaw, Ph.D., for assistance with Figure 1A; Rafael Irizarry, Ph.D., chair of the Department of Data Sciences at Dana-Farber Cancer Institute, for his advice and mentorship regarding the Allen Brain Atlas statistical analyses; and Ivan Kotchetkov, M.D., and Karlo Perica, M.D., of MSKCC for their advice and discussions regarding the cerebellar disorder noted in the study.
Footnotes
Disclosure forms provided by the authors are available with the full text of the article at NEJM.org. In addition, MSKCC has institutional financial interests relative to Juno Therapeutics/Bristol Myers Squibb.
A data sharing statement provided by the authors is available with the full text of this article at NEJM.org.
REFERENCES
- 1.Shah UA, Mailankody S. Emerging immunotherapies in multiple myeloma. BMJ 2020; 370:m 3176. [DOI] [PubMed] [Google Scholar]
- 2.Munshi NC, Anderson LD Jr, Shah N, et al. Idecabtagene vicleucel in relapsed and refractory multiple myeloma. N Engl J Med 2021; 384: 705–16. [DOI] [PubMed] [Google Scholar]
- 3.Berdeja JG, Madduri D, Usmani SZ, et al. Ciltacabtagene autoleucel, a B-cell maturation antigen-directed chimeric antigen receptor T-cell therapy in patients with relapsed or refractory multiple myeloma (CARTITUDE-1): a phase 1b/2 open-label study. Lancet 2021; 398: 314–24. [DOI] [PubMed] [Google Scholar]
- 4.Martin T, Usmani SZ, Berdeja JG, et al. Ciltacabtagene autoleucel, an anti-B-cell maturation antigen chimeric antigen receptor T-cell therapy, for relapsed/refractory multiple myeloma: CARTITUDE-1 2-year follow-up. J Clin Oncol 2022. June 4 (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chong EA, Ruella M, Schuster SJ. Five-year outcomes for refractory B-cell lymphomas with CAR T-cell therapy. N Engl J Med 2021; 384: 673–4. [DOI] [PubMed] [Google Scholar]
- 6.Park JH, Rivière I, Gonen M, et al. Long-term follow-up of CD19 CAR therapy in acute lymphoblastic leukemia. N Engl J Med 2018; 378: 449–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Neelapu SS, Locke FL, Bartlett NL, et al. Axicabtagene ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. N Engl J Med 2017; 377: 2531–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maude SL, Laetsch TW, Buechner J, et al. Tisagenlecleucel in children and young adults with B-cell lymphoblastic leukemia. N Engl J Med 2018;378: 439–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smith EL, Harrington K, Staehr M, et al. GPRC5D is a target for the immunotherapy of multiple myeloma with rationally designed CAR T cells. Sci Transl Med 2019; 11(485): eaau7746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pillarisetti K, Edavettal S, Mendonça M, et al. A T-cell-redirecting bispecific G-protein-coupled receptor class 5 member D x CD3 antibody to treat multiple myeloma. Blood 2020; 135: 1232–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cohen Y, Gutwein O, Garach-Jehoshua O, Bar-Haim A, Kornberg A. GPRC5D is a promising marker for monitoring the tumor load and to target multiple myeloma cells. Hematology 2013; 18:348–51. [DOI] [PubMed] [Google Scholar]
- 12.Atamaniuk J, Gleiss A, Porpaczy E, et al. Overexpression of G protein-coupled receptor 5D in the bone marrow is associated with poor prognosis in patients with multiple myeloma. Eur J Clin Invest 2012; 42:9 53–60. [DOI] [PubMed] [Google Scholar]
- 13.Lee DW, Santomasso BD, Locke FL, et al. ASTCT consensus grading for cytokine release syndrome and neurologic toxicity associated with immune effector cells. Biol Blood Marrow Transplant 2019; 25: 625–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kumar S, Paiva B, Anderson KC, et al. International Myeloma Working Group consensus criteria for response and minimal residual disease assessment in multiple myeloma. Lancet Oncol 2016; 17(8): e328–e346. [DOI] [PubMed] [Google Scholar]
- 15.Roshal M, Flores-Montero JA, Gao Q, et al. MRD detection in multiple myeloma: comparison between MSKCC 10-color single-tube and EuroFlow 8-color 2-tube methods. Blood Adv 2017; 1: 728–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Allen Institute for Brain Science. Gene search for “GPRC5D OR POU4F1 OR TNFRSF17,” microarray data, Allen human brain atlas. 2022. (https://human.brain-map.org/microarray/search/show?exact_match=false&search_term=GPRC5D%20OR%20POU4F1%20OR%20TNFRSF17&search_type=gene&page_num=0).
- 17.Allen Institute for Brain Science. Mi-croarray data, Allen human brain atlas. 2022. (https://human.brain-map.org).
- 18.Hawrylycz MJ, Lein ES, Guillozet-Bongaarts AL, et al. An anatomically comprehensive atlas of the adult human brain transcriptome. Nature 2012; 489: 391–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Allen Institute for Brain Science. Technical white paper: microarray data normalization. March 2013. (https://help.brain-map.org/download/attachments/2818165/Normalization_WhitePaper.pdf?version=1&modificationDate=136183650 2191&api=v2). [Google Scholar]
- 20.Cohen AD, Parekh S, Santomasso BD, et al. Incidence and management of CAR-T neurotoxicity in patients with multiple myeloma treated with ciltacabtagene autoleucel in CARTITUDE studies. Blood Cancer J 2022; 12: 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Van Oekelen O, Aleman A, Upadhyaya B, et al. Neurocognitive and hypokinetic movement disorder with features of parkinsonism after BCMA-targeting CAR-T cell therapy. Nat Med 2021; 27: 2099–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chari A, Berdeja JG, Oriol A, et al. 290 A phase 1, first-in-human study of talquetamab, a G protein-coupled receptor family C group 5 member D (GPRC5D) x CD3 bispecific antibody, in patients with relapsed and/or refractory multiple myeloma (RRMM). Presented at the 62nd ASH Annual Meeting and Exposition, virtual, December 5–8, 2020. (https://ash.confex.com/ash/2020/webprogram/Paper133873.html). [Google Scholar]
- 23.Krishnan AY, Minnema MC, Berdeja JG, et al. Updated phase 1 results from MonumenTAL-1: first-in-human study of talquetamab, a G protein-coupled receptor family C group 5 member D x CD3 bispecific antibody, in patients with relapsed/refractory multiple myeloma. Blood 2021; 138:Suppl 1: 158 (https://ashpublications.org/blood/article/138/Supplement%201/158/478074/Updated-Phase-1-Results-from-MonumenTAL-1-First-in). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.