INTRODUCTION
KRASG12C mutations occur in about 14% of non–small-cell lung cancers (NSCLCs)1 and are responsive to selective KRASG12C inhibitors (G12Ci), including sotorasib (AMG510) and adagrasib (MRTX849).2,3 Sotorasib is the first approved drug for metastatic KRASG12C-mutant NSCLC, with a response rate of 28%-37% and median progression-free survival of 5.6-6.8 months.2,4 Thus far, in published trials of sotorasib, patients with active, untreated brain metastases were excluded from participation, and therefore, little is known on the central nervous system (CNS) efficacy of sotorasib. Because brain metastases commonly occur in patients with KRAS-mutant NSCLC and significantly affect morbidity and mortality,5 we sought to better characterize the incidence of brain metastases in patients with KRASG12C-mutant NSCLC and assess preliminary CNS efficacy in patients with active brain metastases who received commercial-use sotorasib.
METHODS
Patients with de novo stage IV NSCLC and available KRAS mutational status who had consented to a correlative research study (Dana-Farber/Harvard Cancer Center protocol #02-180) at the Dana-Farber Cancer Institute, as well as to the publication of the information reported hereby, between September 2013 and December 2021 were included in this study. Institutional Review Board approved Dana-Farber/Harvard Cancer Center protocol #02-180. Patients provided written consent. Clinicopathological data were collected from electronic medical records. Genomic alterations and tumor mutational burden were assessed on all NSCLCs which successfully underwent comprehensive tumor genomic profiling with the OncoPanel platform as previously described.6 Intracranial disease was assessed by magnetic resonance imaging (MRI) and reviewed by a dedicated radiologist (M.N.) and radiation oncologist (A.A.) according to response assessment in neuro-oncology brain metastases (RANO-BM) criteria in patients with at least one target CNS lesion (≥ 5 mm) and/or nontarget lesions. For nontarget lesions, cases with neither a complete response nor progressive disease were categorized as stable disease.7 Survival estimates were performed by the Kaplan-Meier method and multiple comparisons correction used the Benjamini-Hochberg procedure. P-values < .05 and Q-values < 0.05 (for multiple comparisons analyses) were considered significant.
RESULTS
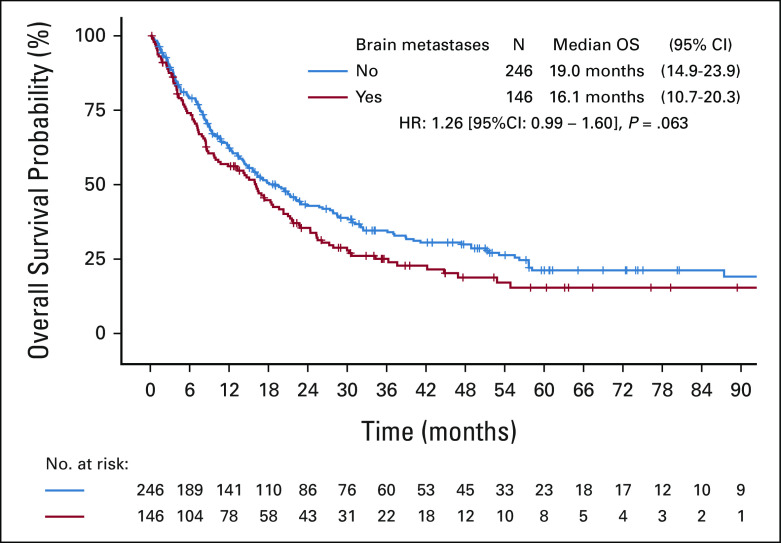
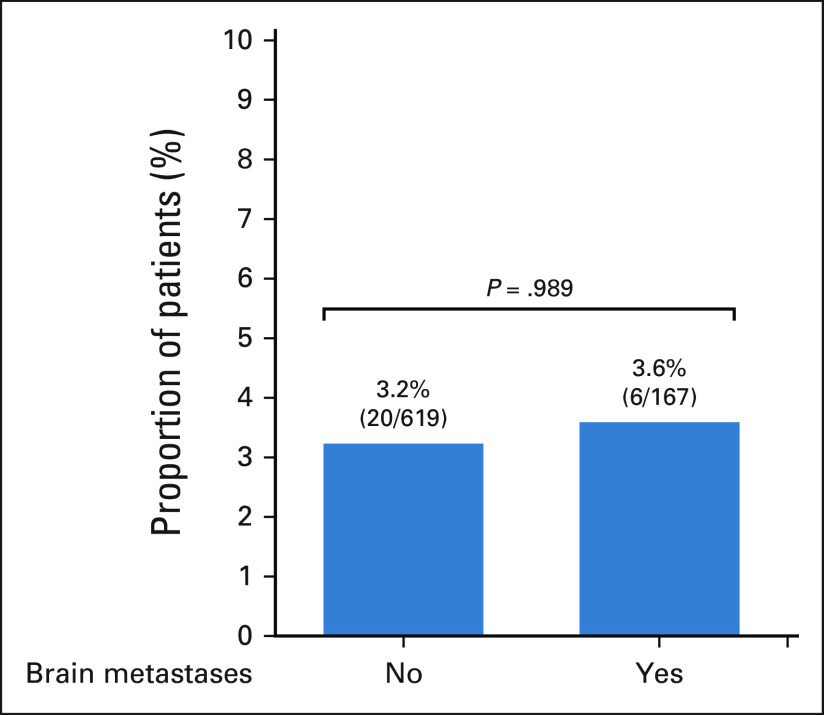
We identified 899 patients with a diagnosis of stage IV KRAS-mutant NSCLC. Among these patients, 35.1% (N = 316) had synchronous brain metastases, defined as those occurring within 3 months of initial pathological diagnosis of NSCLC. There was no significant difference in the incidence of brain metastases in patients with KRASG12C-mutant NSCLC (N = 146/392, 37.2%) compared with patients with KRASnon-G12C–mutant NSCLC (N = 170/507, 33.5%, P = .26, Fig 1A). Overall survival in KRAS-mutant NSCLC was significantly longer in patients without versus with brain metastases at the time of metastatic diagnosis (16.0 v 13.2 months, respectively; P = .017, Fig 1B). A similar trend was observed when restricting this analysis to patients with KRASG12C-mutant NSCLC, although not statistically significant (19.0 v 16.1 months, respectively; P = .063; Appendix Fig A1).
FIG 1.
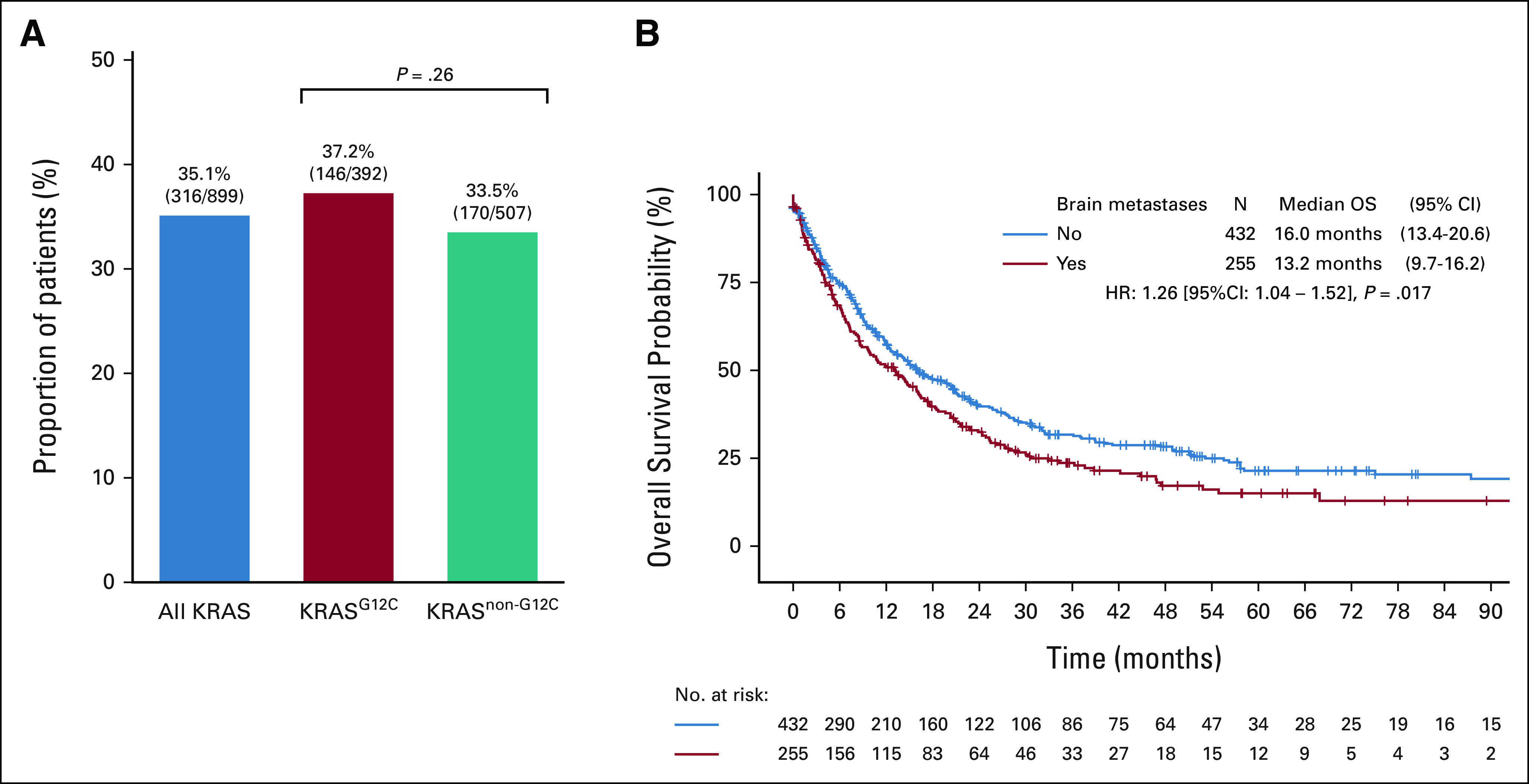
(A) Prevalence of synchronous brain metastases at stage IV diagnosis in patients with KRAS-mutant NSCLC. (B) Kaplan-Meier estimates of OS of patients with stage IV KRAS-mutant NSCLC by presence of synchronous brain metastases at diagnosis. NSCLC, non–small-cell lung cancer; OS, overall survival.
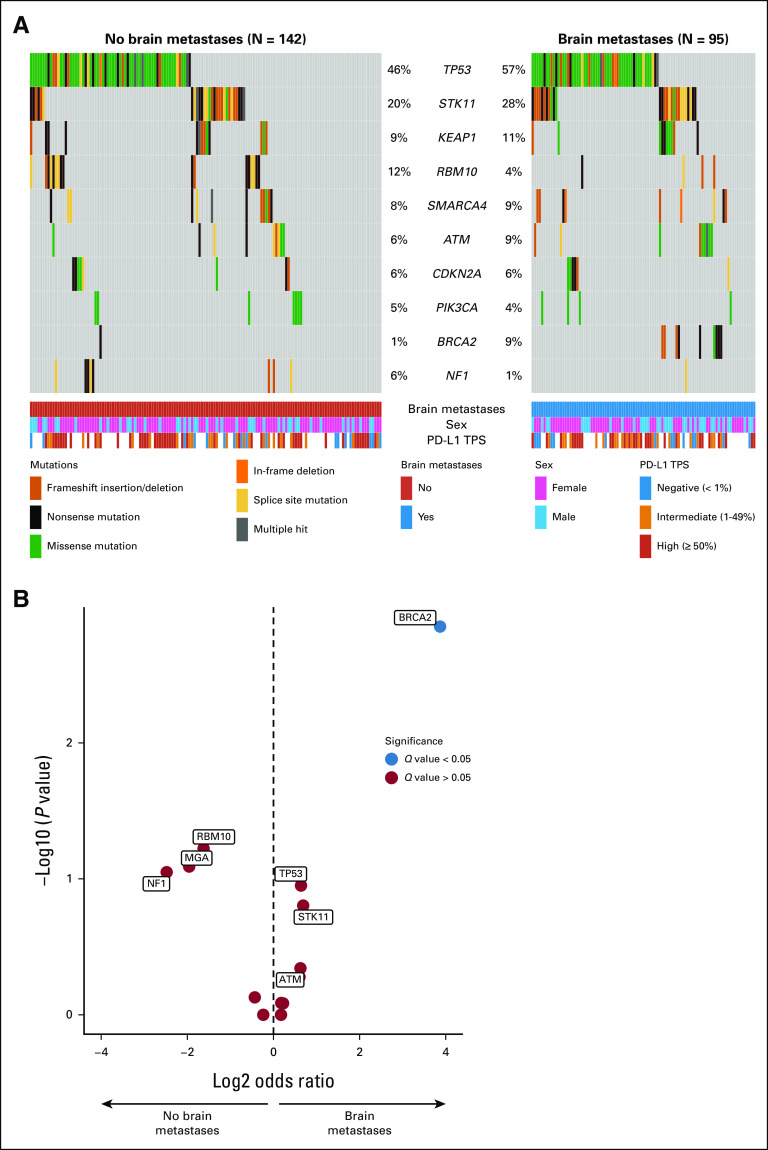
When comparing KRASG12C-mutant NSCLCs without versus with synchronous brain metastases at metastatic diagnosis, there was no difference in the programmed cell death ligand 1 tumor proportion score (median, 40% v 20%, respectively; P = .222) or tumor mutational burden (median, 9.89 v 10.65 mutations/megabase, respectively; P = .324, Appendix Figs A2A and A2B). To determine if there were any genomic differences in KRASG12C-mutant NSCLCs without versus with brain metastases, we performed a gene enrichment analysis which showed no significant difference in the frequency of TP53, STK11, or KEAP1 alterations after correcting for multiple comparisons (Appendix Figs A3A and A3B). There was an enrichment in BRCA2 mutations in NSCLCs with brain metastases (N = 9/95 [9.5% CI] v N = 1/142 [0.7%]; odds ratio, 0.07; 95% CI, 0.01 to 0.51; Q = 0.021); however, the number of overall cases with pathogenic BRCA2 alterations in our cohort was small, and an association of BRCA2 mutation with the presence of brain metastases in KRASG12C-mutant NSCLC was not observed in the publicly available Memorial Sloan Kettering–Metastatic Events and Tropisms cohort data (Appendix Fig A4).8
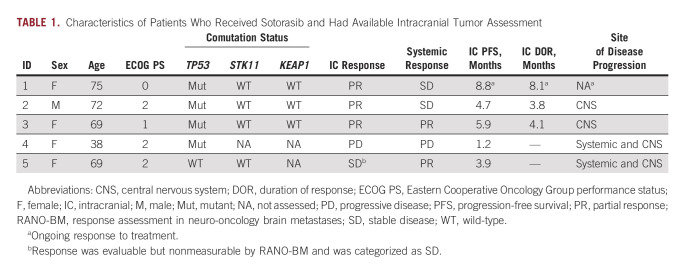
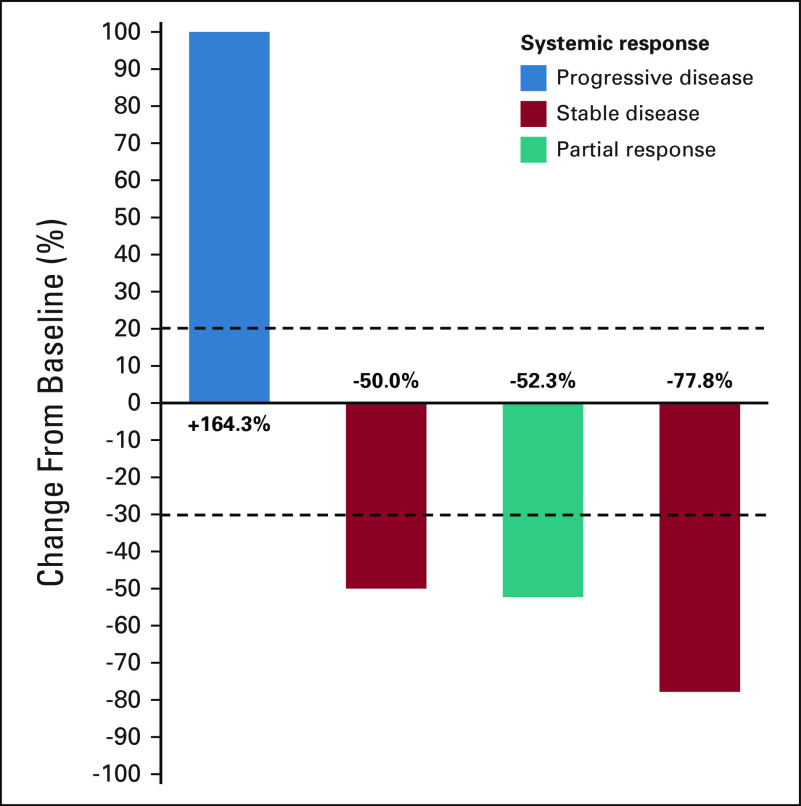
Because brain metastases frequently occur in patients with NSCLC and are associated with worse survival, we sought to examine whether there was preliminary evidence of intracranial activity in patients with KRASG12C-mutant NSCLC and active, untreated brain metastases who received commercial-use sotorasib. Among all patients with stage IV KRAS-mutant NSCLC, we identified six patients who developed active, untreated brain metastases (at any point in their disease course) before sotorasib initiation, with a median overall survival of not reached (8.7 to not estimable) at a median follow-up time of 8.8 months (95% CI, 7.8 to not estimable), relative to the start date of sotorasib treatment. All patients had baseline MRI immediately before sotorasib initiation. One patient died 3 weeks after starting sotorasib because of disease progression and did not have further systemic or intracranial imaging; the remaining five patients had at least one subsequent MRI while on sotorasib, and their outcomes are summarized in Table 1. Of them, patient 5 had five nonmeasurable lesions by RANO-BM criteria at baseline (< 5 × 5 mm). After sotorasib treatment, four of five lesions completely resolved while one decreased in size but was still present on subsequent MRI (1.5 mm slice thickness). This response was categorized as stable disease as this patient had nonmeasurable disease at baseline. The remaining four patients had measurable disease by RANO-BM criteria. Confirmed intracranial disease response to treatment was observed in three of four patients (Fig 2), with a median duration of response of 4.1 months (95% CI, 3.9 to not estimable), and a median intracranial progression-free survival of 4.7 months (95% CI, 3.9 to not estimable). Patient 1 is still on sotorasib with an ongoing intracranial response 7 months on treatment. Patient 2 had objective response in the brain and systemic disease improvement that did not reach RECIST criteria for partial response, followed by intracranial disease progression with controlled systemic disease. Patient 3 had initial objective response both intracranially and extracranially, followed by isolated CNS progression but continued systemic response. Finally, patient 4 had primary extracranial and intracranial disease progression. We describe two illustrative cases of patients with untreated brain metastases from KRASG12C-mutant NSCLC who achieved intracranial disease response to sotorasib.
TABLE 1.
Characteristics of Patients Who Received Sotorasib and Had Available Intracranial Tumor Assessment
FIG 2.
Waterfall plot of changes from baseline in intracranial disease burden of patients with KRASG12C-mutant NSCLC with active untreated brain metastases at the time of sotorasib start and with measurable disease according to RANO-BM criteria. Systemic response by RECIST v. 1.1. criteria is also shown. NSCLC, non–small-cell lung cancer; RANO-BM, response assessment in neuro-oncology brain metastases.
Patient 1
A 75-year-old woman with a history of tobacco use presented with metastatic KRASG12C-mutant lung adenocarcinoma progressing after multiple lines of cytotoxic chemotherapy and immune checkpoint inhibition; her most recent therapy before sotorasib was vinorelbine. After a brain MRI demonstrated multiple supratentorial and infratentorial enhancing lesions (greatest 10 × 6 mm in the right lateral cerebellum) with no significant mass effect or hemorrhage (Fig 3A), the patient was started on sotorasib 960 mg by mouth once daily. After 6 weeks of treatment, a brain MRI showed intracranial partial response with a decrease in size of all lesions, followed by further improvement at subsequent scans (Fig 3A). Treatment was still ongoing 8.8 months after sotorasib initiation, with minimal adverse effects.
FIG 3.
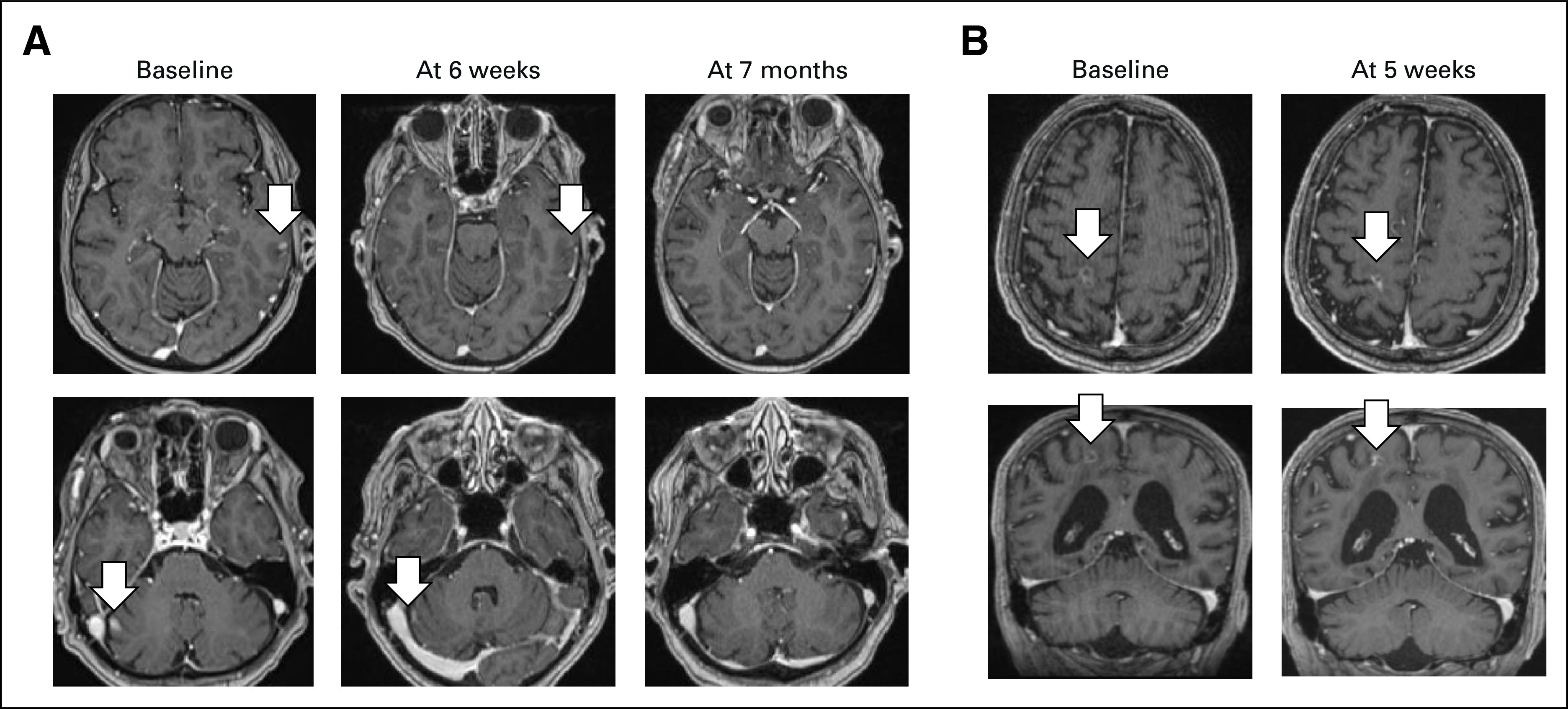
Two illustrative cases of patients with untreated brain metastases from KRASG12C-mutant NSCLC who achieved intracranial disease response to sotorasib. (A) 75-year-old woman showing partial response 6 weeks after treatment start and ongoing at 7 months. (B) 72-year-old man achieving partial response at 5 weeks from treatment start. NSCLC, non–small-cell lung cancer.
Patient 2
A 72-year-old man with a history of tobacco use was diagnosed with stage IV KRASG12C-mutant lung adenocarcinoma with liver, bone, and brain metastases. After treatment with first-line chemoimmunotherapy, a brain MRI showed the appearance of new brain lesions and enlargement of previously detected brain metastases; the largest one was a 12-mm lesion in the right precentral gyrus (Fig 3B). The patient was started on sotorasib 960 mg by mouth once daily. An intracranial partial response was observed at the first brain MRI performed 5 weeks after treatment start (Fig 3B) which was confirmed on a subsequent MRI 2 months later. The right precentral gyrus lesion was progressing 4 months later and was treated with stereotactic radiosurgery (20 Gy in one fraction). The patient continued treatment with sotorasib which is still ongoing 8 months after treatment start, with good tolerance, except for grade 2 elevation of liver function tests.
DISCUSSION
In patients with KRASG12C-mutant NSCLC, the lifetime incidence of brain metastases is approximately 40%,9 and effective therapy for CNS disease represents an important unmet need in this population. Tyrosine kinase inhibitors of epidermal growth factor receptor (EGFR) or anaplastic lymphoma kinase, for example, are highly effective against intracranial metastases, with CNS response rates reaching up to 70% with the EGFR inhibitor osimertinib10 and 60%-80% with the anaplastic lymphoma kinase inhibitors alectinib or lorlatinib.11,12 With the development of novel KRASG12C inhibitors with potential CNS activity in NSCLC, the decision of whether to offer local CNS treatment modalities such as radiation or surgery before initiation of a G12Ci should be discussed by a multidisciplinary team. Adagrasib demonstrated activity in preclinical models of brain metastases,9 and recent data presented from the KRYSTAL-1 study of adagrasib in patients with KRASG12C-mutant NSCLC showed an intracranial objective response rate of 31.6% among 19 evaluable patients with active, untreated brain metastases.13 Although a post hoc analysis of the CodeBreaK100 study reported on the intracranial activity of sotorasib in patients with stable and treated brain metastases,14 patients with active, untreated brain metastases were not included in this analysis. Recently, an intracranial response to sotorasib was described in a patient with KRASG12C-mutant NSCLC and active untreated brain metastases.15
Although our study is limited by the small sample size and the lack of brain metastasis animal models and correlative analyses from patient cerebrospinal fluid, this retrospective series demonstrates preliminary intracranial activity of sotorasib in patients with KRASG12C-mutant NSCLC and untreated brain metastases. Prospective studies are needed to more accurately quantify the efficacy of sotorasib in this population. Although CNS responses were observed, the duration of intracranial disease control for some patients was relatively short, highlighting the need to further investigate CNS penetration of sotorasib and to develop improved strategies for treating brain metastases in patients with KRASG12C-mutant NSCLC.
APPENDIX
FIG A1.
Kaplan-Meier estimates of overall survival of patients with stage IV KRASG12C-mutant NSCLC by presence of synchronous brain metastases at diagnosis. NSCLC, non–small-cell lung cancer.
FIG A2.
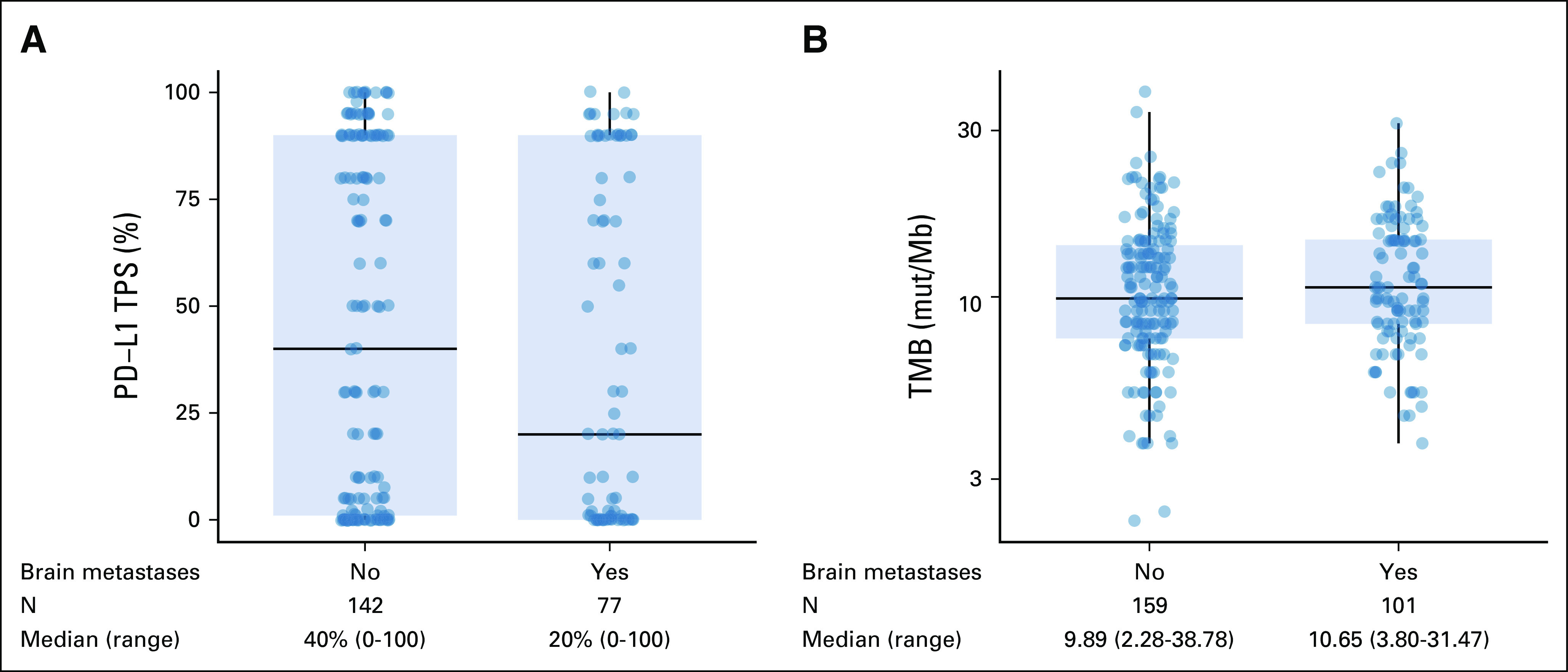
Comparison of (A) PD-L1 TPS and (B) TMB in stage IV KRASG12C NSCLC with and without brain metastases at diagnosis. NSCLC, non–small-cell lung cancer; PD-L1, programmed cell death ligand 1; TMB, tumor mutational burden; TPS, tumor proportion score.
FIG A3.
(A) Oncoprint showing frequency of the 10 most commonly mutated genes in our cohort of stage IV KRASG12C-mutant NSCLC without and with brain metastases at diagnosis. (B) Volcano plot of gene enrichment analysis in stage IV KRASG12C NSCLC without v with brain metastases at diagnosis. NSCLC, non–small-cell lung cancer; PD-L1, programmed cell death ligand 1.
FIG A4.
Comparison of the prevalence of BRCA2 mutations in stage IV KRASG12C-mutant NSCLC with and without brain metastases in the MSK-MET cohort. MSK-MET, Memorial Sloan Kettering–Metastatic Events and Tropisms; NSCLC, non–small-cell lung cancer.
Ayal Aizer
Consulting or Advisory Role: Novartis, NH TherAGUIX, Seattle Genetics
Research Funding: Varian Medical Systems, NH TherAGUIX
Biagio Ricciuti
Consulting or Advisory Role: Regeneron
Lynette M. Sholl
Stock and Other Ownership Interests: Moderna Therapeutics
Consulting or Advisory Role: Genentech (Inst), Lilly (Inst), AstraZeneca
Research Funding: Roche/Genentech (Inst), Bristol Myers Squibb (Inst)
Mizuki Nishino
Consulting or Advisory Role: Daiichi Sankyo, AstraZeneca
Research Funding: Toshiba (Inst), AstraZeneca (Inst), Daiichi Sankyo (Inst)
Mark M. Awad
Consulting or Advisory Role: Genentech, Merck, Pfizer, Boehringer Ingelheim, AbbVie, AstraZeneca/MedImmune, Clovis Oncology, Nektar, Bristol Myers Squibb, ARIAD, Foundation Medicine, Syndax, Novartis, Blueprint Medicines, Maverick Therapeutics, Achilles Therapeutics, Neon Therapeutics, Hengrui Therapeutics, Gritstone Bio, Archer, Mirati Therapeutics, NextCure, EMD Serono, AstraZeneca, Panasonic, Instil Bio, Gilead Sciences, Janssen Oncology
Research Funding: Genentech/Roche (Inst), Lilly (Inst), AstraZeneca (Inst), Bristol Myers Squibb (Inst)
Open Payments Link: https://openpaymentsdata.cms.gov/physician/1127368
No other potential conflicts of interest were reported.
SUPPORT
Elva J. and Clayton L. McLaughlin Fund for Lung Cancer Research to M.M.A.
AUTHOR CONTRIBUTIONS
Conception and design: Giuseppe Lamberti, Mark M. Awad
Financial support: Mark M. Awad
Administrative support: Mark M. Awad
Collection and assembly of data: Giuseppe Lamberti, Ayal Aizer, Biagio Ricciuti, Joao V. Alessi, Federica Pecci, Shu-Chi Tseng, Lynette M. Sholl, Mizuki Nishino
Data analysis and interpretation: Giuseppe Lamberti, Ayal Aizer, Biagio Ricciuti
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/po/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Ayal Aizer
Consulting or Advisory Role: Novartis, NH TherAGUIX, Seattle Genetics
Research Funding: Varian Medical Systems, NH TherAGUIX
Biagio Ricciuti
Consulting or Advisory Role: Regeneron
Lynette M. Sholl
Stock and Other Ownership Interests: Moderna Therapeutics
Consulting or Advisory Role: Genentech (Inst), Lilly (Inst), AstraZeneca
Research Funding: Roche/Genentech (Inst), Bristol Myers Squibb (Inst)
Mizuki Nishino
Consulting or Advisory Role: Daiichi Sankyo, AstraZeneca
Research Funding: Toshiba (Inst), AstraZeneca (Inst), Daiichi Sankyo (Inst)
Mark M. Awad
Consulting or Advisory Role: Genentech, Merck, Pfizer, Boehringer Ingelheim, AbbVie, AstraZeneca/MedImmune, Clovis Oncology, Nektar, Bristol Myers Squibb, ARIAD, Foundation Medicine, Syndax, Novartis, Blueprint Medicines, Maverick Therapeutics, Achilles Therapeutics, Neon Therapeutics, Hengrui Therapeutics, Gritstone Bio, Archer, Mirati Therapeutics, NextCure, EMD Serono, AstraZeneca, Panasonic, Instil Bio, Gilead Sciences, Janssen Oncology
Research Funding: Genentech/Roche (Inst), Lilly (Inst), AstraZeneca (Inst), Bristol Myers Squibb (Inst)
Open Payments Link: https://openpaymentsdata.cms.gov/physician/1127368
No other potential conflicts of interest were reported.
REFERENCES
- 1. Biernacka A, Tsongalis PD, Peterson JD, et al. The potential utility of re-mining results of somatic mutation testing: KRAS status in lung adenocarcinoma. Cancer Genet. 2016;209:195–198. doi: 10.1016/j.cancergen.2016.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Skoulidis F, Li BT, Dy GK, et al. Sotorasib for lung cancers with KRAS p.G12C mutation. N Engl J Med. 2021;384:2371–2381. doi: 10.1056/NEJMoa2103695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Jänne PA, Riely GJ, Gadgeel SM, et al. Adagrasib in non–small-cell lung cancer harboring a KRASG12C mutation. N Engl J Med. 2022;387:120–131. doi: 10.1056/NEJMoa2204619. [DOI] [PubMed] [Google Scholar]
- 4. Johnson ML, de Langen A, Waterhouse DM, et al. LBA10—Sotorasib versus docetaxel for previously treated non-small cell lung cancer with KRAS G12C mutation: CodeBreaK 200 phase III study. Ann Oncol. 2022;33:S1417–S1418. [Google Scholar]
- 5. Vassella E, Kashani E, Zens P, et al. Mutational profiles of primary pulmonary adenocarcinoma and paired brain metastases disclose the importance of KRAS mutations. Eur J Cancer. 2021;159:227–236. doi: 10.1016/j.ejca.2021.10.006. [DOI] [PubMed] [Google Scholar]
- 6. Garcia EP, Minkovsky A, Jia Y, et al. Validation of OncoPanel: A targeted next-generation sequencing assay for the detection of somatic variants in cancer. Arch Pathol Lab Med. 2017;141:751–758. doi: 10.5858/arpa.2016-0527-OA. [DOI] [PubMed] [Google Scholar]
- 7. Lin NU, Lee EQ, Aoyama H, et al. Response assessment criteria for brain metastases: Proposal from the RANO group. Lancet Oncol. 2015;16:e270–e278. doi: 10.1016/S1470-2045(15)70057-4. [DOI] [PubMed] [Google Scholar]
- 8. Nguyen B, Fong C, Luthra A, et al. Genomic characterization of metastatic patterns from prospective clinical sequencing of 25,000 patients. Cell. 2022;185:563–575.e11. doi: 10.1016/j.cell.2022.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sabari JK, Velcheti V, Shimizu K, et al. Activity of adagrasib (MRTX849) in brain metastases: Preclinical models and clinical data from patients with KRASG12C-mutant non-small cell lung cancer. Clin Cancer Res. 2022;28:3318–3328. doi: 10.1158/1078-0432.CCR-22-0383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wu YL, Ahn MJ, Garassino MC, et al. CNS efficacy of osimertinib in patients with T790M-positive advanced non–small-cell lung cancer: Data from a randomized phase III trial (Aura3) J Clin Oncol. 2018;36:2702–2709. doi: 10.1200/JCO.2018.77.9363. [DOI] [PubMed] [Google Scholar]
- 11. Lin JJ, Jiang GY, Joshipura N, et al. Efficacy of alectinib in patients with ALK-positive NSCLC and symptomatic or large CNS metastases. J Thorac Oncol. 2019;14:683–690. doi: 10.1016/j.jtho.2018.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Solomon BJ, Bauer TM, Ignatius Ou SH, et al. Post hoc analysis of lorlatinib intracranial efficacy and safety in patients with ALK-positive advanced non-small-cell lung cancer from the phase III CROWN study. J Clin Oncol. 2022;40:3593–3602. doi: 10.1200/JCO.21.02278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sabari JK, Spira AI, Heist RS, et al. Activity of adagrasib (MRTX849) in patients with KRAS G12C-mutated NSCLC and active, untreated CNS metastases in the KRYSTAL-1 trial. J Clin Oncol. 2022;40:LBA9009. 17_suppl. [Google Scholar]
- 14. Ramalingam S, Skoulidis F, Govindan R, et al. P52.03 efficacy of sotorasib in KRAS p.G12C-mutated NSCLC with stable brain metastases: A post-hoc analysis of CodeBreaK 100. J Thorac Oncol. 2021;16:S1123. [Google Scholar]
- 15. Koster K-L, Appenzeller C, Lauber A, et al. Sotorasib shows intracranial activity in patients with KRAS G12C-mutated adenocarcinoma of the lung and untreated active brain metastases. Case Rep Oncol. 2022;15:720–725. doi: 10.1159/000525341. [DOI] [PMC free article] [PubMed] [Google Scholar]