Abstract
PURPOSE
Selpercatinib, a highly selective, potent RET inhibitor with CNS activity, demonstrated sustained antitumor responses and intracranial activity in patients with RET-altered advanced non–small-cell lung cancer (NSCLC) in the global LIBRETTO-001 and Chinese LIBRETTO-321 trials. We report a prospective case series based on updated data from patients with brain metastases at baseline in LIBRETTO-321.
MATERIALS AND METHODS
We included patients with advanced NSCLC and brain metastasis with a centrally confirmed KIF5B/CCDC6/NCOA4-RET fusion. Patients with previously treated or untreated CNS metastases were included if asymptomatic or neurologically stable. Patients received oral selpercatinib 160 mg, twice daily, until progression. Objective systemic and intracranial response was independently assessed per RECIST v1.1. The data cutoff (DCO) was March 31, 2022.
RESULTS
In total, 8/26 (31%) patients were included: 1/8 (13%) had previous brain surgery but no previous systemic therapy and 3/8 (38%) had received brain radiotherapy. Best overall systemic response was partial response (PR) in 6/8 patients (75%) and stable disease (SD) in 2/8 (25%). Among patients with measurable baseline CNS lesions, 4/5 (80%) achieved a confirmed intracranial response (3/5 PRs and 1/5 complete response [CR]). The best overall intracranial response was CR in 3/8 (38%), PR in 3/8 (38%), and SD in 1/8 (13%) and nonprogressive disease/non-CR in 1/8 (13%); 2/8 patients (25%) had CNS-only disease progression. The duration of treatment was 2.8-24.0 months, and 5/8 patients (63%) had treatment ongoing at DCO. Of 8 patients, 5 (63%) had grade ≥3 treatment-related adverse events (TRAEs) requiring dose modification. There were no treatment discontinuations because of TRAEs.
CONCLUSION
Selpercatinib demonstrated clinically meaningful and durable intracranial activity in Chinese patients with brain metastases from RET-altered NSCLC, consistent with the global LIBRETTO-001 trial.
Selpercatinib had intracranial activity in Chinese patients with brain metastases from RET-altered NSCLC.
INTRODUCTION
RET, a proto-oncogene encoding a receptor tyrosine kinase, is affected by oncogenic gene fusion events in 0.6%-2.0% of non–small-cell lung cancers (NSCLCs) in Chinese patients.1-7 RET-altered NSCLC has a propensity to metastasize to the CNS, with almost half of patients with stage IV tumors developing brain metastases during the course of their disease.8
CONTEXT
Key Objective
Does selpercatinib demonstrate intracranial activity in Chinese patients with brain metastasis from RET-altered advanced non–small-cell lung cancer (NSCLC)?
Knowledge Generated
Treatment with selpercatinib resulted in clinically meaningful intracranial activity, with objective systemic and intracranial responses observed. Systemically, 6/8 patients achieved a partial response (PR) and 2/8 had stable disease (SD). Intracranially, 3/8 patients demonstrated a complete response (CR), 3/8 a PR, and 1/8 had SD; 1/8 patients had nonprogressive disease/non-CR and 2/8 patients had CNS-only disease progression.
Relevance
These results are consistent with those reported in the primary findings from the global LIBRETTO-001 and Chinese LIBRETTO-321 trials and suggest selpercatinib is a promising treatment for Chinese patients with brain metastases from RET-altered NSCLC.
Selpercatinib (formerly known as LOXO-292) is a highly selective and potent RET inhibitor with CNS activity.9 It has been approved in multiple countries for the treatment of RET-altered advanced or metastatic lung or thyroid cancers10 after positive results from the global phase I/II LIBRETTO-001 trial.11-13 The global LIBRETTO-001 trial was complemented by results from the phase II LIBRETTO-321 trial conducted in Chinese patients with RET fusion–positive NSCLC.14 In both the global and Chinese trials, selpercatinib showed manageable toxicity and was generally well tolerated.11,14 Given the high incidence of brain metastasis in patients with RET fusion–positive NSCLC, selpercatinib was designed to penetrate the blood-brain barrier.11 Selpercatinib has demonstrated intracranial activity in patients with brain metastases from RET-altered NSCLC in case reports9,15 and preplanned subanalyses of the LIBRETTO-001 and LIBRETTO-321 trials.11,12,14
To provide further information on the intracranial and systemic activity of selpercatinib in Chinese patients with RET fusion–positive NSCLC and brain metastases, we report a post hoc case series on the basis of updated data from the eight patients with brain metastases at baseline included in the LIBRETTO-321 trial.
MATERIALS AND METHODS
Patients
As reported previously,14 LIBRETTO-321 is a multicenter, open-label, Phase II trial conducted to evaluate selpercatinib in Chinese patients with advanced RET-altered solid tumors (ClinicalTrials.gov identifier: NCT04280081). The trial was performed in accordance with the Declaration of Helsinki and other national and local regulations. The protocol received institutional review board/independent ethics committee approval at each site. Patients provided written informed consent for their data to be presented in this article.
Adults with advanced NSCLC were eligible to enroll into Cohort 1 of the LIBRETTO-321 trial on the basis of detection of a RET alteration by local laboratory (KIF5B-RET, CCDC6-RET, NCOA4-RET, or other fusion) or central laboratory (KIF5B/CCDC6/NCOA4-RET only) in tumor tissue. All patients with brain metastasis were centrally confirmed as RET fusion–positive (KIF5B/CCDC6/NCOA4-RET only). RET fusions were detected in a certified local laboratory by polymerase chain reaction (PCR) or next-generation sequencing (NGS) and/or at a central laboratory using the AmoyDx 9-in-1 PCR assay (Amoy Diagnostics Co, Ltd, Shanghai, China). Eligible patients had progression on, or intolerance to, standard therapy (chemotherapy and/or immunotherapy) or declined/were deemed unsuitable for such therapy. Patients with CNS metastases were eligible if they were asymptomatic or neurologically stable for ≥2 weeks. This is a post hoc report of the CNS-response population included in LIBRETTO-321, defined as all selpercatinib-treated patients with confirmed RET fusion–positive NSCLC with independent review committee (IRC)–assessed CNS metastases at baseline.
Treatment and Assessments
Patients received selpercatinib 160 mg, orally, twice daily, until disease progression, unacceptable toxicity, withdrawal of consent, or death. Tumor assessments were performed at baseline and week 4 (optional), week 8, then every 8 weeks until week 48, and every 12 weeks thereafter. All patients with RET fusion–positive NSCLC underwent baseline brain imaging with magnetic resonance imaging (MRI) or, if MRI was contraindicated, contrast-enhanced computed tomography (CT). For patients with CNS metastases at baseline, brain imaging was repeated at each tumor assessment. Responses were confirmed ≥4 weeks after the response was first observed.
Objective response was assessed by IRC and investigators according to the RECIST v1.1. Objective intracranial response was evaluated by IRC per RECIST v1.1 in the CNS-response population. Adverse events (AEs) were monitored until 28 days after the last selpercatinib dose and graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events v5.0.
Case Series
The present case series reports demographics, clinical characteristics (including disease and treatment history), and updated efficacy and safety outcomes with selpercatinib for the eight patients in the CNS-response population (Table 1). The data cutoff (DCO) was March 31, 2022.
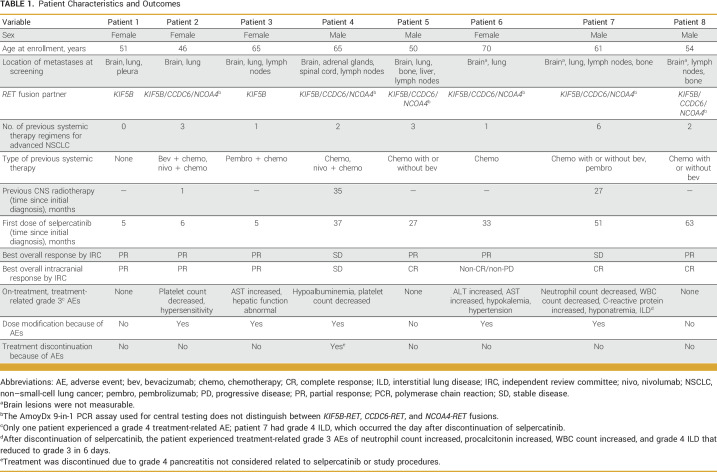
TABLE 1.
Patient Characteristics and Outcomes
RESULTS
Patient 1
A 51-year-old female patient presenting with gait instability, dizziness, nausea, and headache was found to have bilateral cerebellar and left parietal lesions on brain MRI. Palliative resection of the cerebellar masses was performed, and pathologic assessment of the surgical specimen supported a diagnosis of brain metastasis from a poorly differentiated lung adenocarcinoma. Further lesions were identified in the lung and pleura by CT (stage IVB). The patient was enrolled into LIBRETTO-321 on the basis of detection of a KIF5B-RET fusion (K15;R12) in brain tumor tissue by NGS (OncoScreen Focus CDx Tissue Kit; Burning Rock Biotech, Guangzhou, China) at a local laboratory and subsequently centrally confirmed. After the initiation of first-line therapy with selpercatinib, the best overall response by IRC was partial response (PR) and the best change in sum of target lesion diameters (SOLD) was −86%. A PR was evident from the first assessment at week 4 and was maintained throughout follow-up (Fig 1). The best intracranial response was a PR, which was also observed from week 4 until week 88 (Figs 2A-2D). There were no grade ≥3 AEs or dose modifications because of AEs. As of March 2022, the patient remains on treatment with a total treatment duration of 24.0 months.
FIG 1.
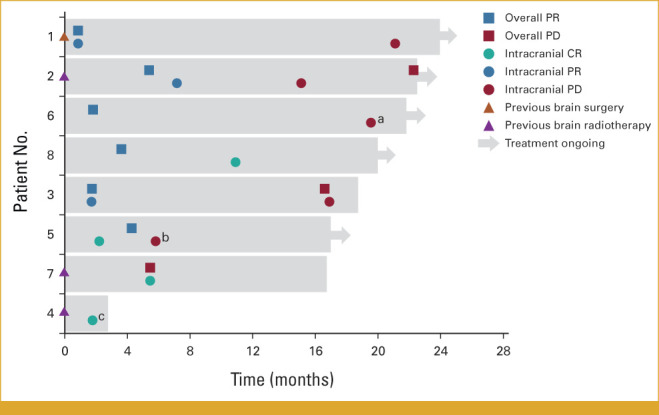
Swimmer plot showing duration of treatment and overall and intracranial response according to IRC assessment. Since CNS and whole-body images were reviewed by separate teams, patients may have experienced an intracranial PD without having a systemic PD. aIntracranial progression characterized by the growth of nontarget lesions and appearance of a new lesion. bIntracranial progression characterized by the growth of target lesions only. cIntracranial CR was not confirmed before treatment was discontinued. CR, compete response; IRC, independent review committee; PD, progressive disease; PR, partial response.
FIG 2.
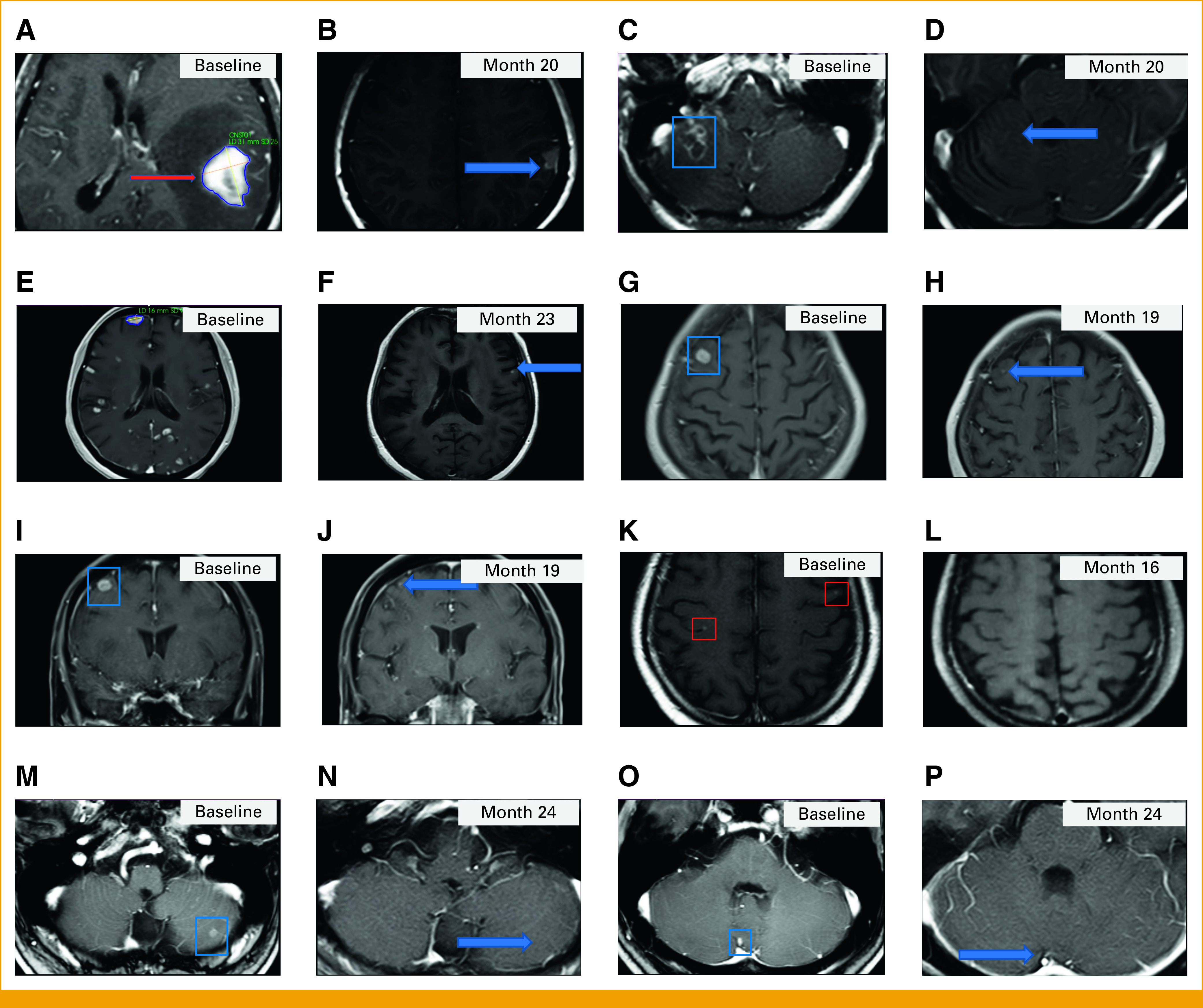
Postcontrast T1-weighted MRI brain images. Patient 1: A 31 mm × 25 mm enhancing left parietal lesion (A) at baseline (red arrow) that was absent at month 2 and still absent (B) at month 20 (the blue arrow shows a hyperintense cavity) and an enhancing right cerebellar lesion (C) at baseline that was absent from month 8 and still absent (D) at month 20 (the blue arrow shows the expected location of the lesion). Patient 2: Multiple brain metastases (E) at baseline and a decrease in size and number of the enhancing brain metastases (F) at month 23, including resolution of the enhancing right frontal lesion. The blue arrow in (F) points to a tiny enhancing lesion. Patient 3: (G) Axial and (I) coronal baseline images demonstrating a 13 mm × 10 mm enhancing right frontal lesion and demonstrating a decrease in size of the right frontal lesion compared with baseline (H and J) at month 19. Blue arrows in (H) and (J) point to the smaller on study enhancing right frontal lesion. Patient 7: A small bilateral frontal enhancing lesions (K) at baseline demonstrating resolution (L) at month 16. Patient 8: Enhancing left cerebellar lesions (M and O) at baseline that were absent from month 2 and 10, respectively, with no visible lesions in the same locations at (N and P) month 24 (blue arrows show the expected locations of the lesions). The same T1-weighted imaging sequence was used to acquire all images. MRI, magnetic resonance imaging.
Patient 2
A 46-year-old female patient presented with lower right limb numbness accompanied by a burning sensation in the arch of the foot. Although CT showed no signs of metastasis in the lumbar spine, contrast-enhanced cranial MRI revealed multiple foci in the cerebellar hemispheres; the temporal, occipital, frontal, and parietal lobes; and the posterior horn of the left lateral ventricle. Given the prevalence of brain metastasis in lung cancer, plain chest CT was conducted and showed a nodule in the lower right lung lobe. Lung biopsy pathology indicated a diagnosis of NSCLC with adenocarcinoma histology (stage IVA). In addition to WBRT (30 Gy in 10 fractions), the patient received one cycle of bevacizumab plus carboplatin and pemetrexed, followed by one cycle of nivolumab plus carboplatin and pemetrexed. Thereafter, treatment was switched to nivolumab plus carboplatin and docetaxel for a further approximately 2 months, with a best overall response of stable disease (SD). However, approximately 4 months after the start of treatment, brain MRI indicated progression of the existing brain lesions.
After central confirmation of a RET fusion, the patient enrolled in LIBRETTO-321 and received selpercatinib, with a best overall response by IRC of PR, best change in SOLD of −38%, and best intracranial response by IRC of PR (Figs 2E and 2F). A CT scan after 22.4 months showed systemic progression because of the presence of new lesions; however, the intracranial PR was ongoing. Treatment-related AEs included grade 3 platelet count decreased, grade 3 hypersensitivity (managed with treatment interruption for 16 days and dose reduction to 40 mg twice daily, with a subsequent re-escalation to 160 mg twice daily in stepwise increments), and grade 2 hypoalbuminemia that resolved to grade 1 after treatment interruption for 7 days. The patient is still receiving postprogression selpercatinib as of March 2022, with a total treatment duration of 22.5 months.
Patient 3
A 65-year-old female patient presented with cough, expectoration, and occasional mild dyspnea. Computed tomography and positron emission tomography CT revealed a mass in the lower right lung lobe, with right hilar and mediastinal lymph node enlargement. After a lung biopsy, a diagnosis of stage IVB NSCLC with adenocarcinoma histology was established. The patient received a single cycle of pembrolizumab plus carboplatin and pemetrexed and achieved a PR, but the disease progressed after approximately 1 month, with the new onset of multiple nodules in the right cerebellar hemisphere and frontal lobe on cranial MRI.
The patient enrolled in LIBRETTO-321 on the basis of detection of a KIF5B-RET fusion (K15;R12) in local NGS tissue testing (550 gene assay for solid tumors; Anhui Anlong Gene Technology Co, Ltd, Hefei, China). The presence of a RET fusion was subsequently centrally confirmed. Best overall response by IRC was PR (best change in SOLD: −68%), and best intracranial response was PR, both of which were evident from the first on-treatment assessment after 8 weeks. The intracranial PR was maintained at all subsequent assessments (Figs 2G-2J). Treatment was continued beyond progression of extracranial disease after 16.6 months until a second unconfirmed progression event, characterized by the investigator as the growth of extracranial lesions and appearance of new kidney and bone lesions after 18.6 months, at which point selpercatinib was discontinued. Treatment-related AEs included grade 3 hepatic function abnormality (managed with treatment interruption for 16 days and dose reduction to 80 mg twice daily), grade 3 AST increased (managed with treatment interruption for 7 days and further dose reduction to 40 mg twice daily), and grade 2 gamma-glutamyl transferase increased (managed with dose interruption for 11 days). The dose was subsequently re-escalated, first to 80 mg twice daily and then 120 mg twice daily. The patient died of lung cancer approximately 3.5 months after discontinuing selpercatinib.
Patient 4
A 62-year-old male patient presenting with an occasional productive cough was found to have a mass in the lower right lung on chest CT. Radical resection was performed, and pathologic examination of the surgical specimen established a diagnosis of early NSCLC with adenocarcinoma histology (stage IA). The disease recurred approximately 1.5 years later, with interlobar fissure lymphadenopathy on CT scan. Initial systemic treatment was nab-paclitaxel plus cisplatin, followed by nivolumab plus pemetrexed with or without cisplatin, which was discontinued due to immune-related creatinine increase. The patient subsequently presented with intracranial metastases and was treated with brain radiotherapy (40 Gy).
After central detection of a RET fusion, the patient enrolled in LIBRETTO-321. At screening, lesions were detected in the right adrenal gland, spinal cord and lymph nodes by CT and in the cerebellum and frontal and temporal lobes of the brain by contrast-enhanced MRI (stage IVB). The patient had not experienced progression of intracranial metastasis after radiotherapy. The patient underwent a single postbaseline assessment at week 8, with best overall response by IRC of SD (best change in SOLD: −27%). Intracranial response by IRC at the same time point was an unconfirmed CR. Treatment-related grade 3 AEs of hypoalbuminemia and platelet count decreased were reported, and grade 1/2 hypersensitivity led to dose modification, specifically treatment interruption for 5 days, followed by dose reduction to 40 mg twice daily, which was subsequently re-escalated to 80 mg twice daily. Treatment was discontinued due to grade 4 pancreatitis not considered related to selpercatinib or study procedures after 2.8 months.
Patient 5
A left lung mass was detected by chest CT in a 48-year-old male patient undergoing colectomy. CT-guided lung biopsy and imaging assessments confirmed a diagnosis of stage IV NSCLC with adenocarcinoma histology, which had metastasized to regional lymph nodes and distant tissues. The patient received a total of 11 cycles of bevacizumab plus nedaplatin and pemetrexed or carboplatin plus nab-paclitaxel over approximately 2 years, after which enlargement of the left lung lesion on CT indicated disease progression. On the basis of central detection of a RET fusion, the patient was enrolled into LIBRETTO-321. Baseline MRI revealed multiple brain metastases in the cerebellum, corpus callosum, and the occipital, frontal, parietal, and temporal lobes while CT scans showed lesions in the lymph nodes, liver, thoracic and lumbar vertebrae, and pubic bone. Best overall response to selpercatinib by IRC was PR (best change in SOLD: −46%), which was first observed at the second assessment at week 19 and maintained in all subsequent scans. Best intracranial response by IRC was CR, which was observed at the first assessment at week 10 and maintained until intracranial disease progression after 8.0 months. There were no treatment-related grade ≥3 AEs or dose modifications because of AEs. As of March 2022, the patient is still receiving treatment (total duration: 17.0 months) and remains in systemic PR.
Patient 6
A lung mass was detected during physical examination in a 68-year-old female patient, who subsequently underwent lobectomy of the upper right lung. Postoperative pathology indicated a poorly differentiated lung adenocarcinoma with lymph node metastases (stage IIB), for which adjuvant pemetrexed plus carboplatin was administered. After approximately 14 months, the patient relapsed with multiple distant metastases and was treated with pemetrexed monotherapy, but disease progression occurred approximately 6 months later.
The patient was enrolled in LIBRETTO-321 on the basis of central detection of a RET fusion. Leptomeningeal and frontal lobe metastases were identified by brain MRI during baseline assessments (stage IVB). After the initiation of selpercatinib, the patient achieved a best overall response by IRC of PR (best change in SOLD: −51%), which was observed at the first postbaseline assessment and sustained through subsequent assessments. Brain lesions were nonmeasurable; therefore, best intracranial response was non-CR/nonprogressive disease. Intracranial progression occurred after 19.6 months, with the growth of existing brain metastases and appearance of a new leptomeningeal lesion. Treatment-related grade 3 AEs included ALT and AST increased, which were managed by treatment interruption of 21 days and dose reduction to 80 mg twice daily, followed by treatment interruption of 9 days and dose reduction to 40 mg twice daily. Other treatment-related grade 3 AEs were hypokalemia and hypertension (managed with omission of a single selpercatinib dose). As of March 2022, the patient is still receiving postprogression selpercatinib (total duration: 21.8 months), with ongoing systemic PR.
Patient 7
A 57-year-old male patient presented with goiter. Pathologic analysis of fine-needle aspirates from the thyroid and cervical lymph nodes suggested a diagnosis of pulmonary adenocarcinoma with thyroid metastases. CT revealed an obstructive mass in the left lower lung, with enlargement of left hilar and mediastinal lymph nodes, and lung biopsy confirmed the presence of adenocarcinoma NSCLC (stage IVA). The patient received first-line pemetrexed plus cisplatin and pemetrexed maintenance, followed by second-line pembrolizumab in a clinical trial, with a best response of SD in both treatment lines. At this point, cranial MRI revealed a small nodule in the brain, which resolved after brain radiotherapy (18 Gy; Figs 2K and 2L). The patient received further chemotherapy (with or without bevacizumab), again with a best response of SD, but the disease ultimately progressed.
After central detection of a RET fusion, the patient enrolled in LIBRETTO-321 and presented with small de novo bilateral nodules in the frontal lobe on cranial MRI during screening, which were not measurable. Metastasis in the thoracic spine was identified by CT (stage IVB). Best overall response to selpercatinib by IRC was SD, with best change in SOLD of −52%. An unconfirmed PR was observed by investigator assessment. Best intracranial response was CR, which was first observed after 5.5 months and maintained at all subsequent assessments. Although a new pleural lesion was identified by IRC after 5.5 months, radiographic progression was not observed by investigator assessment and treatment was continued for a total of 16.7 months until clinical progression. Treatment-related grade 3 AEs were neutrophil count decreased, WBC count decreased (managed with dose interruption for 8 days), C-reactive protein increased, hyponatremia (managed with dose interruption), and interstitial lung disease, all of which were ongoing for 2 days at the time of discontinuation because of disease progression, and after discontinuation of selpercatinib, neutrophil count increased, procalcitonin increased, WBC increased, hypoxia, C-reactive protein increased, and grade 4 interstitial lung disease that reduced to grade 3 after 6 days. The patient died of lung cancer 1 month after discontinuing selpercatinib.
Patient 8
A 48-year-old male patient underwent radical thoracoscopic resection of a mass in the lower right lung lobe. Postoperative pathology indicated a poorly to moderately differentiated adenocarcinoma with regional nodal metastasis (stage IIB), for which four cycles of adjuvant pemetrexed plus cisplatin were administered. The disease recurred after approximately 5 years with metastasis in the periosseous soft tissue of the tibia and the liver. First-line treatment for metastatic disease was carboplatin plus pemetrexed (with or without bevacizumab), with a best response of SD, but hepatic lesions progressed after approximately 3 months.
The patient was then enrolled in LIBRETTO-321 on the basis of central detection of a RET fusion. During screening assessments, CT revealed an enlarged mediastinal lymph node and a metastasis in a thoracic vertebra, while cranial MRI showed bilateral cerebellar nodules, which were not measurable (stage IVB). After the initiation of selpercatinib, the best overall response by IRC was a PR, which was first observed after 3.6 months and maintained throughout subsequent follow-up, with best change in SOLD of −38%. The best intracranial response was CR, which was evident from 10.9 months and sustained thereafter (Figs 2M-2P). No treatment-related grade ≥3 AEs or dose modifications because of treatment-related AEs occurred. As of July 2022, the patient is continuing to receive selpercatinib (total duration: 24.0 months), with systemic PR and CNS CR both ongoing.
DISCUSSION
The present case series demonstrates the durable intracranial and systemic activity of the CNS-penetrant, selective RET inhibitor selpercatinib in eight Chinese patients with RET fusion–positive NSCLC and brain metastases at baseline included in the LIBRETTO-321 trial. Compared with a previous analysis,14 the present data set shows an increase in the rate of IRC-assessed intracranial response (from 63% to 75%) and intracranial CR (from 25% to 38%) because of the deepening of intracranial response from SD to CR with additional duration of treatment in a patient with nonmeasurable brain disease. Intracranial responses were ongoing at DCO in 3/5 patients (60%) with ongoing treatment, and the intracranial duration of response (DOR) ranged from 0+ to 21.1 months (+ indicates censoring). Furthermore, CNS responses were observed irrespective of previous treatment with chemotherapy with or without immunotherapy and/or brain radiotherapy, as well as in a treatment-naïve patient with previous brain surgery. However, it should be noted that the CNS responses were more evident in those patients who had received radiotherapy. We also report, to our knowledge, the first data on systemic response to selpercatinib in patients with brain metastases, which was attained by 6/8 patients (75%). This proportion is similar to the overall response rates (ORRs) reported in the overall populations of patients with RET fusion–positive NSCLC in the LIBRETTO-001 and LIBRETTO-321 trials,12,14 suggesting consistent overall efficacy irrespective of the presence of CNS lesions. Selpercatinib had a manageable safety profile in patients with brain metastases, with no discontinuations because of treatment-related AEs.
Our findings are consistent with previous reports of intracranial responses to selpercatinib in patients with RET fusion–positive NSCLC from clinical trials and clinical practice.12,16,17 In particular, among 26 patients with measurable brain metastases at baseline in the global LIBRETTO-001 study, an intracranial response was attained by 22 patients (85%), of whom seven achieved an intracranial CR (27%).12 After 25.8 months of follow-up, the median intracranial DOR was 9.4 months and the 1-year DOR rate was 36%. Intracranial responses to selpercatinib have also been described in patients with RET mutation–positive medullary thyroid cancer, RET fusion–positive congenital mesoblastic nephroma, and RET-amplified recurrent glioblastoma multiforme.18-20
Strengths of this study include the screening of all patients for brain metastases at baseline and the regular follow-up and preplanned assessment of intracranial responses by IRC as a secondary end point of the trial. However, since follow-up brain imaging was not required for patients without CNS disease at baseline, this case series did not assess the potential for selpercatinib to delay the development of brain metastasis. Further limitations include the relatively small number of patients with brain metastasis, the exclusion of patients with symptomatic brain metastases and/or neurologic instability, and the current immaturity of progression-free and overall survival data. Finally, the central PCR assay did not distinguish between KIF5B, CCDC6, and NCOA4 fusion partners. However, responses to selpercatinib were observed irrespective of RET fusion partner in LIBRETTO-001,11 and real-world data also indicate similar effectiveness of selpercatinib between patients with the two most common types of fusions (KIF5B-RET or CCDC6-RET).17
In conclusion, selpercatinib demonstrated clinically meaningful and durable intracranial activity in eight Chinese patients with brain metastases from RET fusion–positive NSCLC, which was consistent with the global LIBRETTO-001 trial. Selpercatinib is a promising treatment for both extracranial and intracranial disease in this patient population.
ACKNOWLEDGMENT
Medical writing support was provided by Mark Dyson, DPhil (Berlin, Germany), on behalf of Rude Health Consulting and Jake Burrell, PhD (Rude Health Consulting), and paid for by Eli Lilly and Company.
Jianying Zhou
Consulting or Advisory Role: Hansoh Pharma
Research Funding: Hansoh Pharma (Inst)
Yuping Sun
Honoraria: Roche, Pfizer, AstraZeneca
Consulting or Advisory Role: AstraZeneca, Roche
Research Funding: Roche, Pfizer
Lin Wu
Speakers' Bureau: AstraZeneca, Roche, Bristol-Myers Squibb, MSD, Pfizer, Lilly, Boehringer Ingelheim, Merck, Innovent Biologics, Hengrui Pharmaceutical
Ye Guo
Honoraria: Merck Serono, Roche, MSD, BMS, BeiGene
Consulting or Advisory Role: Merck Serono, MSD, Roche, Janssen Oncology
Shao Jingxin
Employment: Lilly China
Stock and Other Ownership Interests: Lilly Medical
Honoraria: Lilly China
Wanli Zhang
Employment: Lilly
Travel, Accommodations, Expenses: Lilly
Shun Lu
Consulting or Advisory Role: AstraZeneca, Pfizer, Boehringer Ingelheim, Hutchison MediPharma, Simcere, Zai Lab, GenomiCare, Yuhan, Roche, Menarini, InventisBio Co. Ltd
Speakers' Bureau: AstraZeneca, Roche, Hansoh Pharma, Hengrui Therapeutics
Research Funding: AstraZeneca (Inst), Hutchison MediPharma (Inst), BMS (Inst), Hengrui Therapeutics (Inst), BeiGene (Inst), Roche (Inst), Hansoh (Inst), Lilly Suzhou Pharmaceutical Co (Inst)
No other potential conflicts of interest were reported.
SUPPORT
Supported by Eli Lilly and Company.
CLINICAL TRIAL INFORMATION
AUTHOR CONTRIBUTIONS
Conception and design: Shao Jingxin, Shun Lu
Provision of study materials or patients: Ying Cheng, Dingzhi Huang, Jianying Zhou, Chengzhi Zhou, Yuping Sun, Lin Wu, Ye Guo, Shun Lu
Collection and assembly of data: Ying Cheng, Dingzhi Huang, Jianying Zhou, Chengzhi Zhou, Yuping Sun, Lin Wu, Ye Guo, Shun Lu
Data analysis and interpretation: Shao Jingxin, Wanli Zhang, Shun Lu
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/po/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Jianying Zhou
Consulting or Advisory Role: Hansoh Pharma
Research Funding: Hansoh Pharma (Inst)
Yuping Sun
Honoraria: Roche, Pfizer, AstraZeneca
Consulting or Advisory Role: AstraZeneca, Roche
Research Funding: Roche, Pfizer
Lin Wu
Speakers' Bureau: AstraZeneca, Roche, Bristol-Myers Squibb, MSD, Pfizer, Lilly, Boehringer Ingelheim, Merck, Innovent Biologics, Hengrui Pharmaceutical
Ye Guo
Honoraria: Merck Serono, Roche, MSD, BMS, BeiGene
Consulting or Advisory Role: Merck Serono, MSD, Roche, Janssen Oncology
Shao Jingxin
Employment: Lilly China
Stock and Other Ownership Interests: Lilly Medical
Honoraria: Lilly China
Wanli Zhang
Employment: Lilly
Travel, Accommodations, Expenses: Lilly
Shun Lu
Consulting or Advisory Role: AstraZeneca, Pfizer, Boehringer Ingelheim, Hutchison MediPharma, Simcere, Zai Lab, GenomiCare, Yuhan, Roche, Menarini, InventisBio Co. Ltd
Speakers' Bureau: AstraZeneca, Roche, Hansoh Pharma, Hengrui Therapeutics
Research Funding: AstraZeneca (Inst), Hutchison MediPharma (Inst), BMS (Inst), Hengrui Therapeutics (Inst), BeiGene (Inst), Roche (Inst), Hansoh (Inst), Lilly Suzhou Pharmaceutical Co (Inst)
No other potential conflicts of interest were reported.
REFERENCES
- 1. Mulligan LM. RET revisited: Expanding the oncogenic portfolio. Nat Rev Cancer. 2014;14:173–186. doi: 10.1038/nrc3680. [DOI] [PubMed] [Google Scholar]
- 2. Meng H, Guo X, Sun D, et al. Genomic profiling of driver gene mutations in Chinese patients with non-small cell lung cancer. Front Genet. 2019;10:1008. doi: 10.3389/fgene.2019.01008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cai W, Su C, Li X, et al. KIF5B-RET fusions in Chinese patients with non-small cell lung cancer. Cancer. 2013;119:1486–1494. doi: 10.1002/cncr.27940. [DOI] [PubMed] [Google Scholar]
- 4. Zhang K, Chen H, Wang Y, et al. Clinical characteristics and molecular patterns of RET-rearranged lung cancer in Chinese patients. Oncol Res. 2019;27:575–582. doi: 10.3727/096504018X15344979253618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wang R, Hu H, Pan Y, et al. RET fusions define a unique molecular and clinicopathologic subtype of non-small-cell lung cancer. J Clin Oncol. 2012;30:4352–4359. doi: 10.1200/JCO.2012.44.1477. [DOI] [PubMed] [Google Scholar]
- 6. Tsuta K, Kohno T, Yoshida A, et al. RET-rearranged non-small-cell lung carcinoma: A clinicopathological and molecular analysis. Br J Cancer. 2014;110:1571–1578. doi: 10.1038/bjc.2014.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xu C, Wang W, Wang D, et al. 415P—Real-world fusion landscape of RET gene fusions and its response to cabozantinib in Chinese non-small cell lung cancer (NSCLC) using next generation sequencing Annals of Oncology S1386-S14062020 [Google Scholar]
- 8. Drilon A, Lin JJ, Filleron T, et al. Frequency of brain metastases and multikinase inhibitor outcomes in patients with RET-rearranged lung cancers. J Thorac Oncol. 2018;13:1595–1601. doi: 10.1016/j.jtho.2018.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Subbiah V, Velcheti V, Tuch BB, et al. Selective RET kinase inhibition for patients with RET-altered cancers. Ann Oncol. 2018;29:1869–1876. doi: 10.1093/annonc/mdy137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bradford D, Larkins E, Mushti SL, et al. FDA approval summary: Selpercatinib for the treatment of lung and thyroid cancers with RET gene mutations or fusions. Clin Cancer Res. 2021;27:2130–2135. doi: 10.1158/1078-0432.CCR-20-3558. [DOI] [PubMed] [Google Scholar]
- 11. Drilon A, Oxnard GR, Tan DSW, et al. Efficacy of selpercatinib in RET fusion-positive non-small-cell lung cancer. N Engl J Med. 2020;383:813–824. doi: 10.1056/NEJMoa2005653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Drilon A, Subbiah V, Gautschi O, et al. 27P Durability of efficacy and safety with selpercatinib in patients (pts) with RET fusion+ non-small cell lung cancer (NSCLC) Ann Oncol. 2022;33:S43. [Google Scholar]
- 13. Wirth LJ, Sherman E, Robinson B, et al. Efficacy of selpercatinib in RET-altered thyroid cancers. N Engl J Med. 2020;383:825–835. doi: 10.1056/NEJMoa2005651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lu S, Cheng Y, Huang D, et al. Efficacy and safety of selpercatinib in Chinese patients with advanced RET fusion-positive non-small-cell lung cancer: A phase II clinical trial (LIBRETTO-321) Ther Adv Med Oncol. 2022;14:17588359221105020. doi: 10.1177/17588359221105020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Guo R, Schreyer M, Chang JC, et al. Response to selective RET inhibition with LOXO-292 in a patient with RET fusion-positive lung cancer with leptomeningeal metastases. JCO Precis Oncol. 2019;3:1–6. doi: 10.1200/PO.19.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Subbiah V, Gainor JF, Oxnard GR, et al. Intracranial efficacy of selpercatinib in RET fusion-positive non-small cell lung cancers on the LIBRETTO-001 trial. Clin Cancer Res. 2021;27:4160–4167. doi: 10.1158/1078-0432.CCR-21-0800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Illini O, Hochmair MJ, Fabikan H, et al. Selpercatinib in RET fusion-positive non-small-cell lung cancer (SIREN): A retrospective analysis of patients treated through an access program. Ther Adv Med Oncol. 2021;13:17588359211019675. doi: 10.1177/17588359211019675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Andreev-Drakhlin A, Cabanillas M, Amini B, et al. Systemic and CNS activity of selective RET inhibition with selpercatinib (LOXO-292) in a patient with RET-mutant medullary thyroid cancer with extensive CNS metastases. JCO Precis Oncol. 2020;4:1302–1306. doi: 10.1200/PO.20.00096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ortiz MV, Gerdemann U, Raju SG, et al. Activity of the highly specific RET inhibitor selpercatinib (LOXO-292) in pediatric patients with tumors harboring RET gene alterations. JCO Precis Oncol. 2020;4:341–347. doi: 10.1200/PO.19.00401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen A, Czech C, Morgan K, et al. INNV-11. Complete response to selpercatinib in a patient with recurrent glioblastoma and RET amplification. Neuro Oncol. 2021;23(suppl 6):vi107. doi: 10.6004/jnccn.2022.7030. [DOI] [PubMed] [Google Scholar]