PURPOSE
Increasing utilization of comprehensive genomic profiling (CGP) and a growing number of targeted agents (TAs) have led to substantial improvements in outcomes among patients with cancer with actionable mutations. We sought to evaluate real-world experience with off-label TAs among Veterans who underwent CGP.
METHODS
The National Precision Oncology Program database and VA Corporate Data Warehouse were queried to identify patients who underwent CGP between February 2019 and December 2021 and were prescribed 1 of 73 TAs for malignancy. OncoKB annotations were used to select patients who received off-label TAs based upon CGP results. Chart abstraction was performed to review response, toxicities, and time to progression.
RESULTS
Of 18,686 patients who underwent CGP, 2,107 (11%) were prescribed a TA and 169 (0.9%) were prescribed a total of 183 regimens containing off-label TAs for variants in 31 genes. Median age was 68 years and 83% had prior systemic therapy, with 28% receiving three or more lines. Frequency of off-label TA prescriptions was highest for patients undergoing CGP for thyroid (8.6%) and breast (7.6%) cancers. Most patients harbored alterations in BRCA1/BRCA2/ATM (22.5%), ERBB2 (19.5%), and BRAF (19.5%). Among the 160 regimens prescribed > 4 weeks, 43 (27%) led to response. Median progression-free survival and overall survival were 5.3 (4.2-6.5) and 9.7 (7.5-11.9) months, respectively. Patients with OncoKB level 2/3A/3B annotations had longer median progression-free survival (5.8 [4.5-7] months v 3.7 [1.6-7.7] months; hazard ratio, 0.45; 95% CI, 0.24 to 0.82; P = .01) compared with those receiving level 4 treatments.
CONCLUSION
Although administration of off-label TAs is infrequent after CGP, more than one quarter of treatment regimens led to response. TAs associated with level 4 annotations lead to worse outcomes than TAs bearing higher levels of evidence.
BACKGROUND
The increasing availability of comprehensive genomic profiling (CGP) and the expanding number of targeted agents (TAs) have driven a myriad of opportunities to provide targeted cancer care to patients in routine oncology practice. For medical oncologists reviewing CGP results, identifying a mutation bearing level 1 therapeutic level of evidence (LOE), defined as a gene variant recognized by the Food and Drug Administration (FDA) for predicting response to an FDA-approved drug for a patient's specific indication, is most exciting.1 Unfortunately, rates of mutations with level 1 LOE occur in a minority of patients, and biomarkers with lower LOE for TAs are more frequently observed. Nevertheless, for patients harboring advanced cancers for which traditional systemic therapies have limited benefit, prescribing off-label TAs based upon the results of CGP offers attractive options to medical oncologists.
CONTEXT
Key Objective
Does off-label administration of targeted agents (TAs) lead to promising real-world outcomes among patients with cancers bearing actionable mutations? This study sought to evaluate overall response rate and survival outcomes based upon OncoKb level of evidence for TAs administered within a large-scale precision oncology program.
Knowledge Generated
Of the 18,686 Veterans who underwent comprehensive genomic profiling, 169 were prescribed 183 unique regimens including at least one molecular-guided TA. Most patients harbored mutations in BRCA1/BRCA2/ATM, ERBB2, and BRAF. Among regimens administered for > 4 weeks, the overall response rate was 26.9%. Longer progression-free survival was observed among patients receiving regimens bearing OncoKB level 2/3A/3B evidence compared with those receiving level 4 treatments.
Relevance
Prescribing off-label TAs for which there is biological rationale but no clinical evidence of response (ie, OncoKB level 4 annotations) is associated with worse outcomes compared with patients treated with therapies supported by clinical evidence.
The National Cancer Institute and ASCO have sought to assess targeted approaches in a tumor-agnostic fashion by enrolling patients in several arms of the MATCH and TAPUR trials, respectively.2-4 Through these comprehensive studies, patients bearing specific biomarkers can receive TAs, and drugs associated with improved outcomes may be FDA-approved across malignancies. Regardless, given the marked number of mutations that may be targeted and the numbers of patients who are not candidates for trials or are not cared for at sites offering basket clinical trials, a large volume of patients are treated with off-label TAs based upon CGP results in routine oncology practice. As such, investigators have recently reported outcomes with off-label TAs at single institutions, with median duration of treatment ranging between 3.5 and 3.8 months.5,6
In July 2016, the US Department of Veterans Affairs (VA) launched the National Precision Oncology Program (NPOP) to support the completion of CGP among Veteran patients with malignancies through external vendor laboratories.7,8 Consequently, VA medical oncologists across the country may submit tumor and plasma samples for CGP and review the results with VA precision oncology experts. The resulting collection of Veterans who have undergone CGP provides the opportunity to evaluate the administration of off-label TAs across a substantial number of patients in real-world practice. Reporting administrations of off-label TAs associated with high rates of response for specific cancers or across cancers bearing companion gene variants could encourage the use of such targeted approaches in both investigational and clinical practice.
Consequently, the primary aim of the current study was to assess the objective response rate (ORR) among Veterans with solid tumors or hematologic malignances treated with off-label TA regimens. The secondary aims were to evaluate the progression-free survival (PFS), overall survival (OS), and toxicity associated with these regimens. Outcomes were further categorized based upon therapeutic LOE for the prescribed off-label TAs after inputting disease histology and gene variants in OncoKB, a precision oncology knowledge database updated in real time.1 The authors hypothesized that the ORR for Veterans would be approximately 25%, in line with previously reported results.6,9 Furthermore, patients with OncoKB level 2 evidence for TAs would have superior outcomes to those with lower LOE.
METHODS
Included Subjects
Patients who were prescribed off-label TAs based upon CGP for either solid or liquid tumors between February 2019 and December 2021 were selected for inclusion. Patients who were treated with off-label TAs for more than 4 weeks were eligible for outcomes assessments.
Data Source and Ethical Considerations
A list of 73 anticancer TAs and their FDA-approved indications was initially compiled after review of the National Cancer Institute's website in December 2021 (Data Supplement, online only).10 Hormonal therapies, such as androgen-deprivation therapy, and immune checkpoint inhibitors (ICI) were excluded. The VA Corporate Data Warehouse, a repository comprising data from VA clinical and administrative systems, was then accessed in December 2021 to identify Veterans who were prescribed a TA at any time for any indication.11 Thereafter, patients prescribed TAs were cross-matched with those in the NPOP database who had a sample undergo CGP using one of the following Foundation Medicine panels between February 2019 and December 2021: FoundationOne CDx (324 genes), FoundationOne Liquid CDx (324 genes), or FoundationOne Heme (406 genes for DNA sequencing, 265 for RNA sequencing).12 Patients who had undergone CGP and were prescribed one of the eligible TAs were then reviewed by one author (V.V.), and patients who were prescribed only TAs for an FDA-approved indication that did not require a companion biomarker detectable by CGP were excluded (Data Supplement).
Each patient's OncoTree diagnostic code and gene variants were then assessed via OncoKB in March 2022. LOE for TAs were retrieved from OncoKB (Data Supplement), and patients with TA prescriptions matching any OncoKB LOE were identified. Patients bearing gene variants associated with OncoKB level 2-4 evidence for prescribed TAs were selected. Patients with gene variants associated with level 1 evidence for prescribed TAs were excluded, unless other TAs were otherwise prescribed related to gene variants bearing level 2-4 evidence. Patients who were prescribed off-label TAs that were not associated with any LOE for detected gene variants were excluded. Finally, the VA's Joint Legacy Viewer, a platform providing access to patient health information and documentation recorded from any VA site across the health care system, was additionally accessed to complete chart abstraction in April 2022.13,14
This study was formally approved by the Durham VA Medical Center Institutional Review Board as a research study and the New Mexico VA Medical Center Institutional Review Board as a quality improvement study.
Data Collection
From the VA Corporate Data Warehouse, structured TA prescription data and demographic data, including age, race, sex, and death dates, were extracted. From the NPOP database, disease histology and dates and results of CGP were obtained. From OncoKB, LOE for prescribed off-label TAs were acquired. From Joint Legacy Viewer, oncology provider visit notes, imaging and pathology reports, and laboratory results were reviewed by three authors (V.V., E.K., and M.P.). From oncology provider notes, off-label TA prescriptions and treatment durations were confirmed, and associated toxicities, disease stage at the time of treatment, dates of clinical progression, and previous, concurrent, and future systemic therapies administered were recorded. From imaging reports, dates of radiographic progression were obtained for patients with solid tumors. The presence of increasing size of tumors and/or new tumor(s) was used to identify radiographic progression as per RECIST 1.1 criteria.15 From laboratory results and pathology reports, response to therapy was obtained for patients with hematologic malignancies.
Data Analysis
Baseline demographics, disease features, off-label TA regimens, toxicities leading to adjustments in TA prescriptions, targeted gene variants and corresponding OncoKB LOE, and subsequent systemic agents were reported for all patients receiving at least one dose of off-label therapy. Treatment-altering toxicities were categorized by those resulting in treatment breaks, dose reductions, and/or discontinuations (DCs). Outcome assessments were conducted for patients who received off-label TAs for more than 28 days. The ORR and median time to response were computed. For regimens that could not be assessed for response despite > 28 days of treatment, patients were considered nonresponders for ORR computation. Kaplan-Meier (KM) curves were further constructed to compute median PFS and OS and their 95% CI. A composite PFS end point was assessed for all regimens administered and was defined as the time interval between drug initiation and the earliest of clinical progression, radiographic progression, or death due to progression. For the PFS KM curve, patients who discontinued therapy or died due to drug toxicity or a noncancer etiology, were lost to follow-up, or had not progressed by April 15, 2022, the completion date for chart abstraction, were censored. For the OS KM curve, patients who were lost to follow-up or had not passed away by April 15, 2022, were censored. For patients receiving more than one off-label TA regimen, only the first regimen was included for the OS analysis. Regimens were then separated by OncoKB LOE, and PFS and OS KM curves were constructed based upon the same. Given that the KM curves indicated substantially worse outcomes with TAs bearing level 4 evidence, median PFS were compared between patients receiving level 2/3A/3B therapies and those receiving level 4 therapies using a Cox proportional hazards model. The hazard ratio and 95% CI were computed for PFS for patients bearing superior OncoKB LOE, with P < .05 indicating significance.
RESULTS
Selected Patients
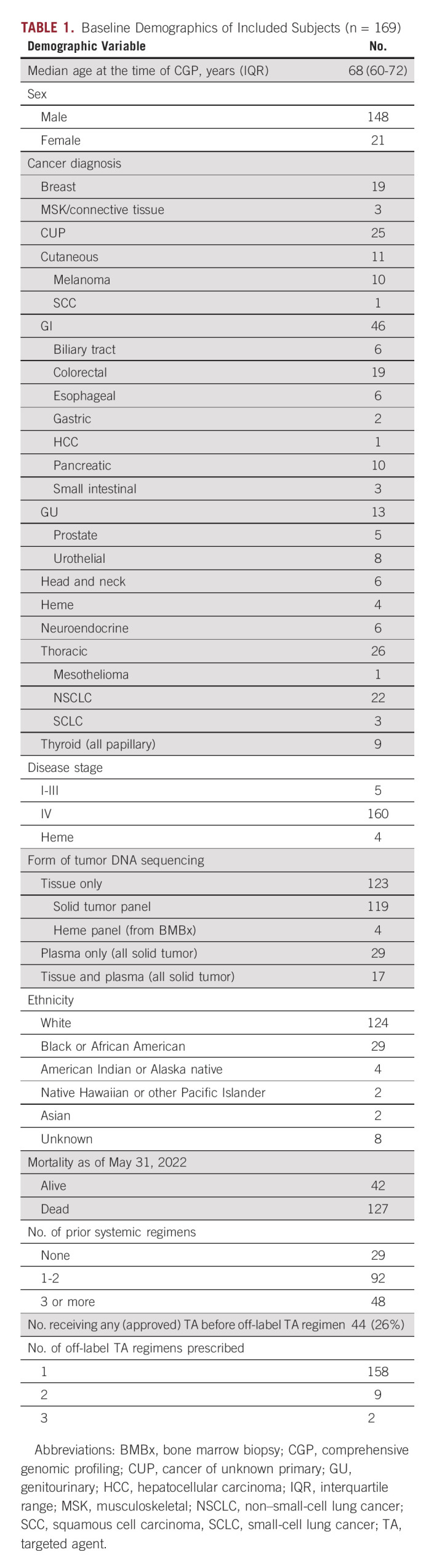
A total of 71,886 patients were prescribed 1 of 73 prespecified TAs, 18,686 patients underwent CGP between February 2019 and December 2021, and 2,107 patients received TAs and underwent CGP in that time frame (Fig 1). After exclusion of 749 patients who received TAs that were FDA-approved regardless of CGP results (Data Supplement), 617 patients (3.3% of all completing CGP) were prescribed OncoKB level 1 therapies, 169 (0.9%) were prescribed 183 level 2-4 therapies, and 572 patients did not have level 1-4 evidence for their detected gene variants or were not prescribed relevant TAs for their CGP results. Of the 169 patients receiving level 2-4 therapies, the median age was 68 (range, 60-72) years, 148 (88%) were male, and 140 (83%) had received prior systemic therapy (Table 1). Off-label TAs were most prescribed for patients with gastrointestinal (47; 27.8%), thoracic (26; 15.4%), and unknown primary (cancer of unknown primary, 25; 14.8%) cancers. Rates of off-label TA prescriptions were highest for patients undergoing CGP for thyroid (8.6%), breast (7.6%), and small intestinal (3.3%) cancers.
FIG 1.
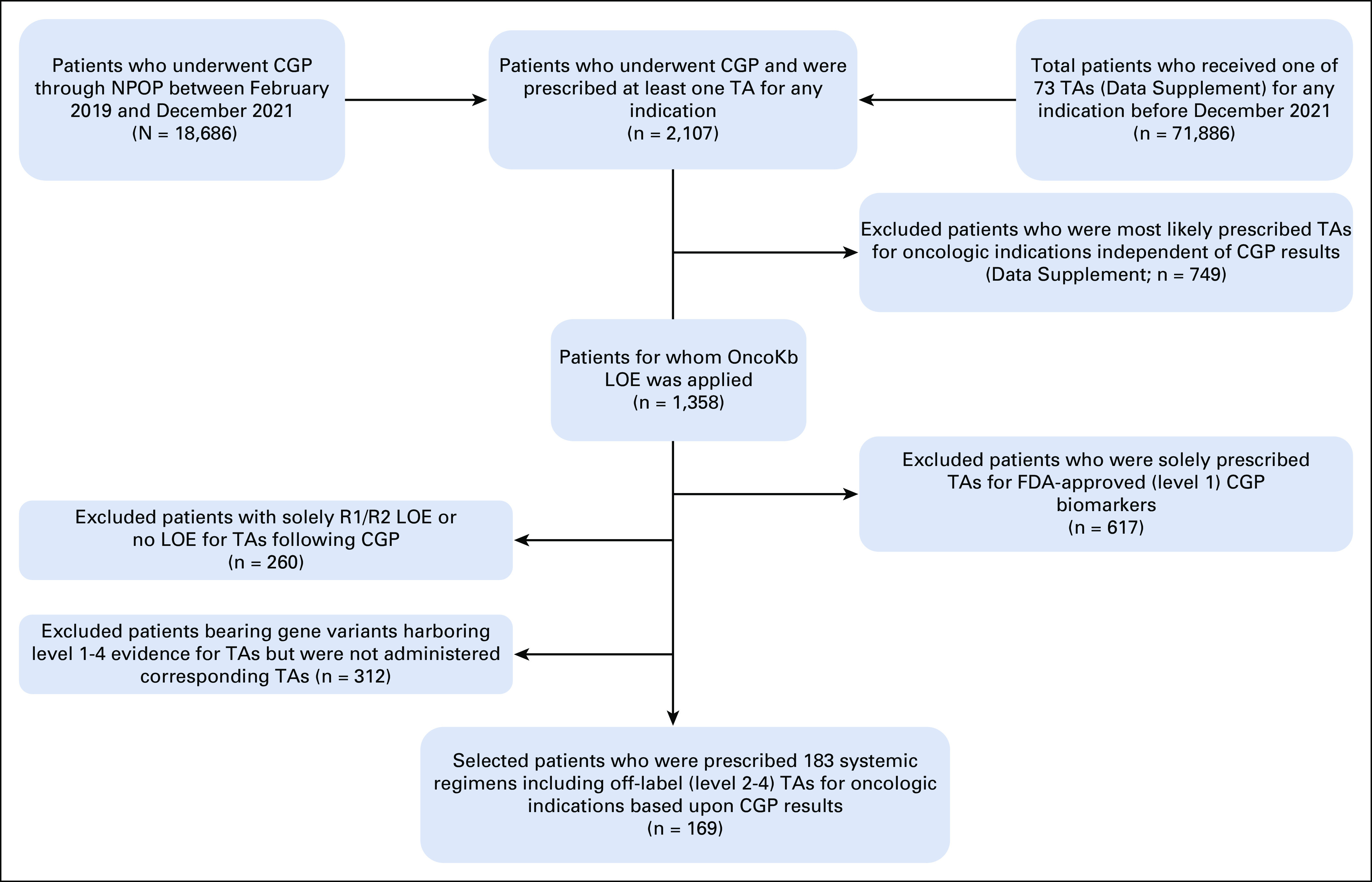
Study selection flowchart. CGP, comprehensive genomic profiling; FDA, Federal Drug Administration; LOE, level of evidence; NPOP, National Precision Oncology Program; TA, targeted agent.
TABLE 1.
Baseline Demographics of Included Subjects (n = 169)
Actionable Gene Variants and LOE
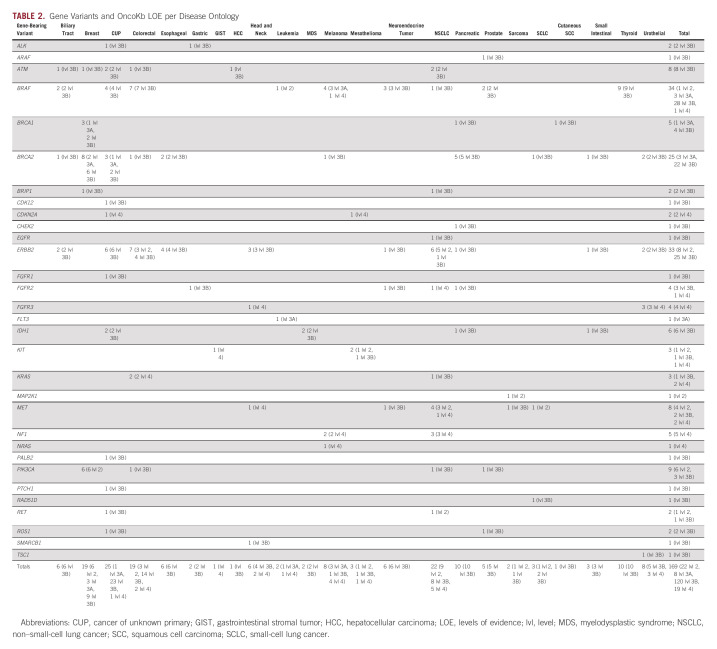
The most common variants involved BRAF (33 patients, 36 regimens), ERBB2 (33 patients, 38 regimens), and ATM/BRCA1/BRCA2 (38 patients, 39 regimens; Table 2). Of the 183 total prescribed regimens, 24 (13.1%) carried level 2 evidence for at least one TA prescribed, 7 (3.8%) carried at least 3A evidence, 130 (71%) carried at least 3B evidence, and 22 (12.0%) carried no LOE > 4. Twelve regimens included two concurrent off-label TAs bearing different LOE. Three regimens included off-label TAs that were not associated with LOE per OncoKB; these regimens were included as the other TA within the regimens carried evidence for use. Of the 36 regimens targeting BRAF mutations, 29 were directed toward V600E mutations, of which 1 regimen carried level 2 evidence and 28 carried level 3B evidence for at least one TA prescribed. For the 39 regimens directed toward ERRB2 gene variants, nine had level 2 evidence and 28 had level 3B evidence for at least one TA prescribed. For the 38 regimens targeting ATM/BRCA1/BRCA2 gene variants, three harbored level 3A evidence for use and 36 harbored level 3B evidence.
TABLE 2.
Gene Variants and OncoKb LOE per Disease Ontology
Off-Label TA Regimens Prescribed and Toxicities
The most frequently administered off-label TAs included 41 (22.4%) olaparib prescriptions, 38 (20.7%) trastuzumab-based (trastuzumab, ado-trastuzumab, and trastuzumab deruxtecan) prescriptions, and 27 (14.8%) trametinib prescriptions (Data Supplement). Thirty-three regimens (18%) included two off-label TAs prescribed, and all combinatorial off-label therapies targeted one singular gene variant per patient. Sixteen patients received chemotherapy, nine received hormonal therapy, nine received FDA-approved TAs, and four received ICI concurrently. Fifty-three regimens (28.9%) lead to toxicities leading to adjustments in off-label TA dosing, with 30 regimens (16.4%) requiring DC. Further systemic therapy was administered after 58 off-label TA regimens (31.7%), including 24 future treatments (13.1%) that included traditional cytotoxic chemotherapy.
Clinical Outcomes With Off-Label TAs
Of the 160 regimens prescribed to 148 patients administered for more than 28 days, the ORR was 26.9% (43 regimens), with eight regimens (5.0%) achieving CR. The median time to response was 2.9 (range, 2.5-3.1) months. The ORR for regimens targeting (25) BRAF V600E mutations, (36) ERBB2 mutations, and (34) ATM/BRCA1/BRCA2 mutations were 32%, 22.2%, and 17.6%, respectively. Notably, high ORRs were observed for patients receiving therapy directed toward PIK3CA mutations (10 regimens received > 28 days, 40% ORR) and MET mutations (eight regimens, 57.1% ORR). The ORR for 143 regimens administered for more than 28 days bearing OncoKB level 2/3A/3B for at least one TA prescribed was 26.5%. The ORR for 17 regimens harboring no more than level 4 evidence was 29.4%.
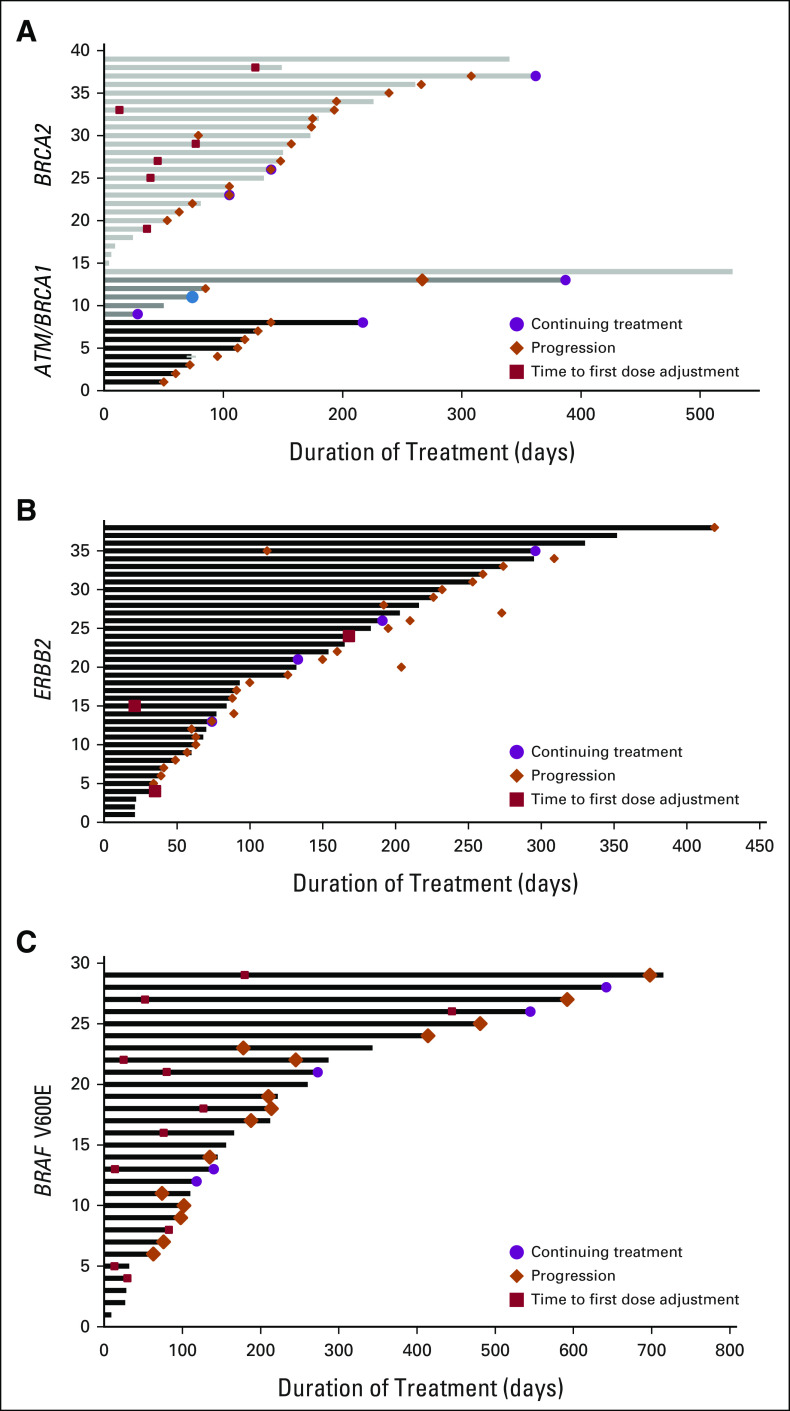
For all 160 regimens administered more than 28 days, the median PFS was 5.3 (range, 4.2-6.5) months (Fig 2). One patient was lost to follow-up. From onset of administration of first off-label regimen for these 148 unique patients, the median OS was 9.7 (range, 7.5-11.9) months. Patients with OncoKB level 2/3A/3B annotations had longer PFS (hazard ratio, 0.45; 95% CI, 0.24 to 0.82; P = .01) compared with patients bearing no more than level 4 annotations. The OS also appeared to be longer (10.7 [8.4-13.8] months v 4.5 [2.0-5.9] months), although a statistical comparison for OS was not undertaken, given the limited number of patients. The swimmer's plot conveying treatment duration, time to progression, and time to treatment-adjusting toxicities for patients harboring BRAF V600E, ERBB2, and ATM/BRCA1/BRCA2 mutations is conveyed in Figure 3.
FIG 2.
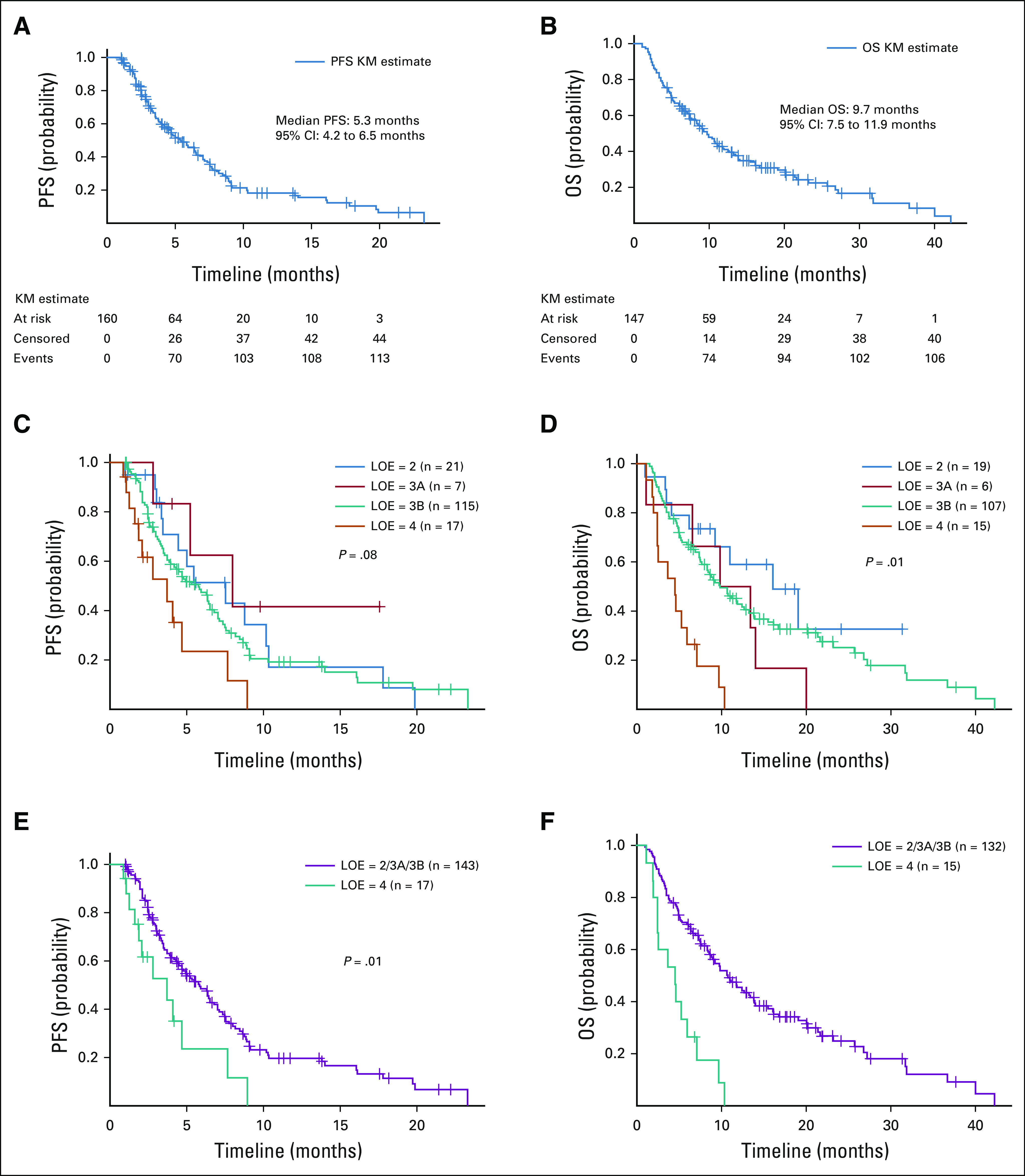
(A) PFS and (B) OS curves for all patients who were treated with off-label TAs for more than 4 weeks. (C) PFS and (D) OS curves based upon OncoKB therapeutic LOE. The median PFS was 7.5 months (95% CI, 3.5 to 10.3) for LOE = 2 group, 8.0 months (95% CI, 2.8 to NR) for LOE = 3A group, 5.8 months (95% CI, 3.9 to 6.5) for LOE = 3B group, and 3.7 months (95% CI, 1.6 to 7.7) for LOE = 4 group. The median OS was 16.1 months (95% CI, 6.2 to NR) for LOE = 2 group, 13.4 months (95% CI, 1.1 to 20.1) for LOE = 3A group, 9.9 months (95% CI, 7.5 to 13.2) for LOE = 3B group, and 4.5 months (95% CI, 2.0 to 5.9) for LOE = 4 group. (E) PFS and (F) OS curves for LOE two groups: 2/3A/3B versus 4. The median PFS was 5.8 months (95% CI, 4.5 to 7.0) for LOE = 2/3A/3B group and 3.7 months (95% CI, 1.6 to 7.7) for LOE = 4 group. The HR was 2.24 (95% CI, 1.22 to 4.11; P = .01) for being in group 4 compared with in group 2/3A/3B. The HR was 0.45 (95% CI, 0.24 to 0.82; P = .01) for being in group 2/3A/3B compared with in group 4. The median OS was 10.7 months (95% CI, 8.4 to 13.8) for LOE = 2/3A/3B group and 4.5 months (95% CI, 2.0 to 5.9) for LOE = 4 group. HR, hazard ratio; LOE, level of evidence; NR, not reached; OS, overall survival; PFS, progression-free survival; TA, targeted agent.
FIG 3.
Swimmer's plots for off-label TA regiments administered to patients with (A) ATM/BRCA1/BRCA2, (B) ERBB2, and (C) BRAF V600E mutations. Each entry refers to an off-label regimen targeting patients with the companion mutations. Time to first dose adjustment refers to reduction in dose, treatment break, or treatment discontinuation. TA, targeted agent.
DISCUSSION
To our knowledge, this study includes the largest cohort of patients treated with molecular-guided off-label TAs in real-world practice. The observed ORR of 26.5% was in general accord with the findings of the MyPathway basket trial (23%), a nonrandomized phase IIa prospective study originally assessing response among patients bearing mutations in one of four genes or pathways with targeted therapies approved for other indications, and a recent single-institutional retrospective study (28.6%) assessing patients treated with off-label therapies for variants within at least 21 different genes.6,9 Notably, the reported ORR increased after removing nonevaluable patients (35.1%) in the latter study. The patients in our study harbored actionable variants within 31 unique targeted genes, and consequently, our findings indicate that response remains acceptably high with an off-label therapeutic approach despite the inclusion of an expansive diverse set of gene variants. The ORR in our study reflects only patients who were treated for more than 28 days, were prescribed off-label TAs, and had gene variants annotated by OncoKB with a LOE.
Apart from OncoKB, LOE or tiers of evidence are increasingly being offered by informatics platforms and cancer societies to prioritize treatment recommendations after CGP.16,17 In our study, patients prescribed TAs bearing OncoKB level 4 evidence had substantially worse PFS compared with patients treated with therapies with level 2/3A/3B evidence, albeit ORR was similar between the two groups. Although the classification schema varies across the available precision oncology information databases, our findings suggest that gene variant annotations recommending administration of off-label TAs simply based upon biological evidence likely lead to worse outcomes compared with annotations supported by some clinical evidence. Of note, about one third of patients with BRAF V600E achieved response, and median PFS among these patients was 5.9 months. Given the outcomes observed among patients treated with off-label dabrafenib and trametinib across cancers within subprotocol H of the MATCH trial (ORR, 38%) and across rare cancers within the Rare Oncology Agnostic Research basket study (ORR range, 33%-56%), the FDA recently approved the use of these two drugs in the second line for all patients with advanced cancers bearing V600E mutations with no excellent therapeutic alternatives in June 2022.18-21 Consequently, patients in our study bearing this mutation would now harbor level 1 evidence for treatment, which would adjust the overall PFS and OS for all included patients and for patients classified as those with level 2/3A/3B annotations. Of note, sotorasib was recently granted accelerated approval for patients with non–small-cell lung cancer bearing KRAS G12C mutations based upon a single-arm phase II study, which would have classified one level 3B treatment regimen to a level 1 regimen in our study.22
Clinical trials have increasingly sought to evaluate outcomes among patients treated with a combination of systemic agents representing diverse sets of therapeutic mechanisms.23-25 As a result, TAs are frequently assessed in conjunction with other TAs, ICIs, or with chemotherapy, and multimodal therapy often conveys improved outcomes with acceptable toxicity. Across the 64 regimens involving more than one systemic agent in our study, 52 were evaluable for outcomes, of which 30.7% (16) achieved response. Although 11 of the 64 regimens were discontinued because of toxicity, further systemic therapy was administered after six of these 11 regimens, thereby indicating that toxicities were not entirely detrimental to further drug administrations. In fact, 40% of all patients receiving multimodal therapy received further systemic therapy after DC of off-label TAs. These collective findings suggest that in real-world practice, clinicians are often comfortable with combining molecular-guided off-label TAs with either other off-label TAs or approved drugs from other classes, and that toxicities are often acceptable with these combinatorial approaches. Specifically, of all 183 regimens administered, 16 (8.7%) regimens included chemotherapy, with 11 (6.0%) containing more than one chemotherapeutic agent. Thirteen of these 16 regimens were for patients bearing ERBB2 mutations, among whom only one patient discontinued treatment because of toxicity. These prescription patterns reflect the familiarity among oncology providers with administering anti-HER2 therapies across cancer types in combination with chemotherapy, likely because of the widespread use of anti-HER2 agents among patients with HER2-positive breast and gastric cancers. Interestingly, of all 38 regimens directed toward ERBB2 mutations, 23 included multiagent therapies, 10 included dual-HER2 blockade, and 11 included newer-generation anti-HER2 therapies (ado-trastuzumab, lapatinib, neratinib, trastuzumab deruxtecan, and trastuzumab emtansine). Investigators designing future clinical trials should consider evaluating the newer anti-HER2 agents across all patients with solid tumors with ERBB2 pathogenic genetic variants, given evidence of efficacy in multiple tumor types and manageable toxicity profiles.
Our study has limitations. First, only patients who were prescribed TAs based upon gene mutations discovered through NPOP and bearing OncoKB level 2-4 evidence were included. Veterans with tumors bearing actionable variants detected through precision testing external to NPOP were excluded. Additionally, Veterans who may have been prescribed off-label molecular-guided therapy for variants that were not annotated by OncoKB were excluded. Second, only off-label TAs administered for > 4 weeks were included for outcomes assessments. It is possible that patients progressed with treatment < 4 weeks. However, for our patient population, which frequently bears several comorbidities and socioeconomic restrictions, we felt that most patients who were treated for < 4 weeks were discontinued for reasons other than lack of response, and consequently, were not evaluable. Third, the retrospective evaluation of real-world practices reflects the limitations from variability in response and toxicity evaluation and documentation in the clinical record. Furthermore, multimodal systemic agents were often administered concurrently, thereby preventing determination of each agent's contribution to outcomes and toxicities. Forth, a small number of patients with liquid tumors were included in our analyses, which may convolute the overall results, given the heterogeneity of patients selected. Finally, although our work evaluated patients bearing variants in 31 unique genes, the majority (61%) of patients had variants of only three gene groups: BRAF V600E, ERBB2, and ATM/BRCA1/BRCA2.
In conclusion, more than onequarter of systemic regimens containing molecular-guided, off-label TAs lead to objective tumor response among patients bearing diverse disease ontology and genomics. Patients specifically harboring MET and PIK3CA mutations have promising response rates, and basket trials should prospectively evaluate these patients in a tumor-agnostic fashion. In real-world practice, off-label TAs are often concurrently administered with other FDA-approved or off-label TAs, particularly among patients bearing ERBB2 mutations, and rates of DC because of toxicities are relatively low despite a high rate of prior systemic therapies. Clinicians should be wary that prescribing off-label TAs for which there is biological rationale but no clinical evidence of response (ie, OncoKB level 4 annotations) is associated with worse outcomes compared with patients treated with therapies supported by clinical evidence.
Vishal Vashistha
Employment: UnitedHealthcare (I)
Research Funding: IBM (Inst)
Other Relationship: IBM
Uncompensated Relationships: IBM
Evangelia Katsoulakis
Stock and Other Ownership Interests: AbbVie, Novavax, Pfizer, Intuitive Surgical, Candel Therapeutics
Research Funding: Advantagene Local Site PI (Inst)
Sara Ahmed
Stock and Other Ownership Interests: PierianDx
Michael J. Kelley
Research Funding: Novartis (Inst), AstraZeneca (Inst), Bristol Myers Squibb (Inst), Regeneron (Inst), Genentech (Inst)
Other Relationship: IBM (Inst)
Open Payments Link: https://openpaymentsdata.cms.gov/physician/827136
No other potential conflicts of interest were reported.
Footnotes
V.V. and E.K. contributed equally to this work.
AUTHOR CONTRIBUTIONS
Conception and design: Vishal Vashistha, Evangelia Katsoulakis, Sara Ahmed, Michael J. Kelley
Collection and assembly of data: Vishal Vashistha, Evangelia Katsoulakis, Aixia Guo, Meghan Price
Data analysis and interpretation: Vishal Vashistha, Evangelia Katsoulakis, Aixia Guo, Meghan Price, Michael J. Kelley
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/po/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Vishal Vashistha
Employment: UnitedHealthcare (I)
Research Funding: IBM (Inst)
Other Relationship: IBM
Uncompensated Relationships: IBM
Evangelia Katsoulakis
Stock and Other Ownership Interests: AbbVie, Novavax, Pfizer, Intuitive Surgical, Candel Therapeutics
Research Funding: Advantagene Local Site PI (Inst)
Sara Ahmed
Stock and Other Ownership Interests: PierianDx
Michael J. Kelley
Research Funding: Novartis (Inst), AstraZeneca (Inst), Bristol Myers Squibb (Inst), Regeneron (Inst), Genentech (Inst)
Other Relationship: IBM (Inst)
Open Payments Link: https://openpaymentsdata.cms.gov/physician/827136
No other potential conflicts of interest were reported.
REFERENCES
- 1. Chakravarty D, Gao J, Phillips SM, et al. OncoKB: A precision oncology knowledge base. JCO Precis Oncol. 2017 doi: 10.1200/PO.17.00011. 10.1200/PO.17.00011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Flaherty KT, Gray RJ, Chen AP, et al. Molecular landscape and actionable alterations in a genomically guided cancer clinical trial: National Cancer Institute Molecular Analysis For Therapy Choice (NCI-MATCH) J Clin Oncol. 2020;38:3883–3894. doi: 10.1200/JCO.19.03010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mangat PK, Halabi S, Bruinooge SS, et al. Rationale and design of the Targeted Agent and Profiling Utilization Registry (TAPUR) study. JCO Precis Oncol. 2018 doi: 10.1200/PO.18.00122. 10.1200/PO.18.00122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Andrews A. ASCO and NCI launch largest precision medicine trials using real-world evidence. Am Health Drug Benefits. 2015;8:37. [PMC free article] [PubMed] [Google Scholar]
- 5. Cobain EF, Wu YM, Vats P, et al. Assessment of clinical benefit of integrative genomic profiling in advanced solid tumors. JAMA Oncol. 2021;7:525–533. doi: 10.1001/jamaoncol.2020.7987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Upadhyay VA, Johnson BE, Landman AB, Hassett MJ. Real-world analysis of off-label use of molecularly targeted therapy in a large academic medical center cohort. JCO Precis Oncol. 2022 doi: 10.1200/PO.21.00232. 10.1200/PO.21.00232 [DOI] [PubMed] [Google Scholar]
- 7. Kelley MJ. VA national precision oncology program. Fed Pract. 2020;37:S22–S27. doi: 10.12788/fp.0037. suppl 4; abstr 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Poonnen PJ, Duffy JE, Hintze B, et al. Genomic analysis of metastatic solid tumors in veterans: Findings from the VHA national precision oncology program. JCO Precis Oncol. 2019;3 doi: 10.1200/PO.19.00075. suppl 15; abstr 3074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hainsworth JD, Meric-Bernstam F, Swanton C, et al. Targeted therapy for advanced solid tumors on the basis of molecular profiles: Results from MyPathway, an open-label, phase IIa multiple basket study. J Clin Oncol. 2018;36:536–542. doi: 10.1200/JCO.2017.75.3780. [DOI] [PubMed] [Google Scholar]
- 10.2022. https://www.cancer.gov/about-cancer/treatment/types/targeted-therapies/approved-drug-list Targeted Therapy Drug List by Cancer Type—NCI.
- 11. Price LE, Shea K, Gephart S. The veterans affairs’s corporate data warehouse: Uses and implications for nursing research and practice. Nurs Adm Q. 2015;39:311–318. doi: 10.1097/NAQ.0000000000000118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.https://www.foundationmedicine.com/portfolio Compare Our Tests | Foundation Medicine.
- 13. Yuan Y, Price M, Schmidt DF, et al. Integrated health record viewers and reduction in duplicate medical imaging: Retrospective observational analysis. JMIR Med Inform. 2022;10:e32168. doi: 10.2196/32168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Legler A, Price M, Parikh M, et al. Effect on VA patient satisfaction of provider’s use of an integrated viewer of multiple electronic health records. J Gen Intern Med. 2019;34:132–136. doi: 10.1007/s11606-018-4708-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 16. Mateo J, Chakravarty D, Dienstmann R, et al. A framework to rank genomic alterations as targets for cancer precision medicine: The ESMO scale for clinical actionability of molecular targets (ESCAT) Ann Oncol. 2018;29:1895–1902. doi: 10.1093/annonc/mdy263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kancherla J, Rao S, Bhuvaneshwar K, et al. Evidence-based network approach to recommending targeted cancer therapies. JCO Clin Cancer Inform. 2020;4:71–88. doi: 10.1200/CCI.19.00097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Salama AKS, Li S, Macrae ER, et al. Dabrafenib and trametinib in patients with tumors with BRAFV600E mutations: Results of the NCI-MATCH trial subprotocol H. J Clin Oncol. 2020;38:3895–3904. doi: 10.1200/JCO.20.00762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Subbiah V, Lassen U, Élez E, et al. Dabrafenib plus trametinib in patients with BRAFV600E-mutated biliary tract cancer (ROAR): A phase 2, open-label, single-arm, multicentre basket trial. Lancet Oncol. 2020;21:1234–1243. doi: 10.1016/S1470-2045(20)30321-1. [DOI] [PubMed] [Google Scholar]
- 20. Wen PY, Stein A, van den Bent M, et al. Dabrafenib plus trametinib in patients with BRAFV600E-mutant low-grade and high-grade glioma (ROAR): A multicentre, open-label, single-arm, phase 2, basket trial. Lancet Oncol. 2022;23:53–64. doi: 10.1016/S1470-2045(21)00578-7. [DOI] [PubMed] [Google Scholar]
- 21. Subbiah V, Kreitman RJ, Wainberg ZA, et al. Dabrafenib plus trametinib in patients with BRAF V600E-mutant anaplastic thyroid cancer: Updated analysis from the phase II ROAR basket study. Ann Oncol. 2022;33:406–415. doi: 10.1016/j.annonc.2021.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.https://www.nejm.org/doi/full/10.1056/NEJMoa2103695 Sotorasib for Lung Cancers With KRAS p. G12C Mutation | NEJM. [DOI] [PMC free article] [PubMed]
- 23. Tan AC, Bagley SJ, Wen PY, et al. Systematic review of combinations of targeted or immunotherapy in advanced solid tumors. J Immunother Cancer. 2021;9:e002459. doi: 10.1136/jitc-2021-002459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lopez JS, Banerji U. Combine and conquer: Challenges for targeted therapy combinations in early phase trials. Nat Rev Clin Oncol. 2017;14:57–66. doi: 10.1038/nrclinonc.2016.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Boshuizen J, Peeper DS. Rational cancer treatment combinations: An urgent clinical need. Mol Cell. 2020;78:1002–1018. doi: 10.1016/j.molcel.2020.05.031. [DOI] [PubMed] [Google Scholar]